Abstract
Purpose
The side-to-side (SS) tendon suture technique was designed to function as a repair that permits immediate post-operative activation and mobilization of a transferred muscle. This study was designed to test the strength and stiffness of the SS technique against a variation of the Pulvertaft (PT) repair technique.
Methods
Flexor digitorum superficialis (FDS) and flexor digitorum profundus (FDP) tendons were harvested from four fresh cadavers and used as a model system. Seven SS and six PT repairs were performed using the FDS as the donor and the FDP as the recipient tendon. For SS repairs, the FDS was woven through one incision in the FDP, and was joined with four cross-stitch running sutures down both sides, and one double-loop suture at each tendon free end; for PT repairs, FDS was woven through three incisions in FDP, joined with a double-loop suture at both ends of the overlap, and four evenly spaced mattress sutures between the ends. Tendon repairs were placed in a tensile testing machine, pre-conditioned and tested to failure.
Results
There were no statistically significant differences in cross-sectional area (p=0.99) or initial length (p=0.93) between SS and PT repairs. Therefore, all comparisons between methods were made using measures of loads and deformations, rather than stresses and strains.. All failures occurred in the repair region, rather than at the clamps. However, failure mechanisms were different between the two techniques—PT repairs failed by the suture knots either slipping or pulling through the tendon material, followed by the FDS tendon pulling through the FDP tendon; SS repairs failed by shearing of fibers within the FDS. Load at first failure (p < 0.01), ultimate load (p < 0.001), and repair stiffness (p < 0.05) were all significantly different between SS and PT techniques; in all cases the mean value for SS was higher than for PT.
Discussion
The SS repair, using a cross-stitch suture technique, was significantly stronger and stiffer compared to the PT repair using a mattress suture technique. This suggests that using SS repairs could enable patients to load the repair soon after surgery. Ultimately, this should reduce the risk of developing adhesions and result in improved functional outcome and fewer complications in the acute post-operative period. Future work will address the specific mechanisms (for example, suture-throw technique, tendon-weave technique) that underlie the improved strength and stiffness of the SS repair.
Keywords: early mobilization, flexor tendon, muscle, tendon transfer, tetraplegia
Introduction
The long-term goal of tendon transfer surgery is restoration of lost function. Previous studies have established that early controlled activity and motion reduce the incidence of adhesion formation, improve range of motion, and reduce post-operative recovery time (1–3). Further, early activation and loading of the muscle-tendon unit significantly improves tensile strength (4), vascularity and cellularity (5) of tendon end-to-end repair sites in model systems. However, while the benefits of early motion following tendon repair are supported by basic scientific and clinical studies, traditionally many authors have advocated a period of immobilization following tendon transfer surgery (6–10) to ensure that the repair is strong enough to withstand forces and motion without being compromised. More recent reports have advocated early active mobilization of transferred muscles (11–12). Thus, prerequisite for early return to activity is a strong and stiff repair that enables efficient load transfer through the repair, across the joint(s) of interest and into the bony insertion, with a minimal risk of repair site failure. The side-to-side (SS) repair technique was developed to achieve these goals, and motivated this study comparing the mechanical properties of the SS with a variation of the Pulvertaft (PT) (13) repair technique in a model system where tendon size, suture distance and overlap area were standardized. The Pulvertaft suture technique was not well defined in the original publication and consequently has been varied and applied in different manners; therefore a specific variation will be tested here. The two techniques, as tested in the current study, differ in the following respects: 1) the SS consists of a single weave of the donor tendon through the recipient, whereas the PT consists of multiple weaves of the donor tendon through the recipient; 2) the SS repair is stabilized using a cross-stitch suture method, compared to the use of mattress sutures in the PT repair. Our comparisons assessed the mechanical properties of the repair techniques, thus simulating the ‘time-zero,’ or immediate post-surgical state of the repairs.
Materials and Methods
Flexor digitorum profundus (FDP) and flexor digitorum superficialis (FDS) tendons were harvested from the second to fifth digits of single arms of four fresh human cadavers (below elbow amputation specimens). Of the 32 total tendons, four were used in pilot testing, and data from two others were lost in a computer malfunction (leaving 26 tendons for experimental testing; two tendons were sutured together for each mechanical test, thereby enabling 13 test specimens). The mean (±standard deviation) age upon death was 85.0 ± 11.9 years. Tendons were soaked in phosphate-buffered saline and frozen for approximately one week immediately following harvest. At the time of testing, tendons were thawed and repairs were performed with the FDS tendon serving as the donor and the FDP tendon as the recipient. Seven SS repairs and six PT repairs were performed by an experienced hand surgeon (Table 1 displays a comparison between the two repair techniques). Ethibond green braided 3-0 polyester suture (Ethicon, Inc., Somerville, NJ, USA) was used for all repairs. For the PT repair, the FDS was woven through three incisions (two horizontal and one vertical) in FDP and was stabilized with a double-loop suture at both ends of the overlap, with four evenly spaced mattress sutures between the ends (Fig. 1). Mattress sutures were applied with two connection points between the tendons, one at the top of the loop and a second where the stitch was completed. This provided the PT repair with a total of ten suture points connecting the tendons. For the SS repair, the FDS was woven through one incision in the FDP, and was stabilized with four cross-stitch running sutures down both sides (eight total cross-stitches), and one double-loop suture at each tendon free end (Fig. 1). This provided the SS repair with a total of ten suture points connecting the tendons. Each connection point referred to for the SS and PT repairs indicates a strand of suture piercing through and directly interacting with both the donor and recipient tendons, thereby connecting the two tendons together. The length of the overlap region was standardized between the two techniques and equated to 29.4 ±1.8 mm for SS and 29.7 ±1.4 mm for PT (p = 0.99). Clinically, a minimum overlap region of 50 mm is recommended for the SS repair (11); the smaller overlap length was used here due to limitations imposed by the mechanical testing apparatus. However, since the comparison was made between equivalent lengths, we did not consider this a fatal flaw in the experiment. Tendon cross-sectional area was calculated using the following equation (14):
where ρ=tendon density (0.00112 g/mm3) (15), mass and length were measured from small sections of the tendon free-ends that were weighed.
Table 1.
Comparison of Side-to-Side and Pulvertaft repair techniques. Number of connection points indicates the number of times the suture makes a physical connection between the two tendons. The amount of suture material was not quantified and therefore a specific value cannot be attributed.
| Variable | Side-to-Side | Pulvertaft Weave |
|---|---|---|
| Number of weaves | 1 | 3 |
| Amount of overlap (cm) | 3 | 3 |
| Number of connection points | 10 | 10 |
| Type of stitch | cross-stitch, double-loop | mattress, double-loop |
| Amount of suture material | more | less |
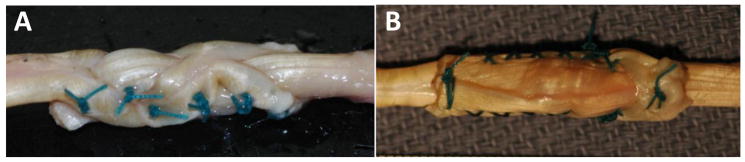
Figure 1.
A) Pulvertaft (PT) repair consists of the FDS weaving through three incisions in the FDP, one double-loop suture at each tendon free-end, and four mattress sutures evenly spaced between (note that mattress sutures were made with two connection points between the tendons, one at the top of the loop and a second where the stitch was completed); B) Side-to-side (SS) repair consists of the FDS inserting through one incision in the FDP, four cross-stitch running sutures back and forth down both sides, and one double-loop suture at each tendon free-end.
All mechanical tests were carried out using a tensile testing machine (Instron Model 1122, Norwood, MA, USA). Clamps secured the tendons on each side of the repair, and specimens were mounted in a vertical orientation. Two small incisions were made at each free end of the tendons, and gauze was wrapped through these incisions and around the free ends to provide more holding strength within the clamps. Specimens were immersed in phosphate-buffered saline solution throughout the test. Slack length of the overall structure was established as the length just prior to the initiation of load resistance based on the electronic noise of the force transducer. Repairs were tested in tension at a displacement rate of 10 mm/min. First, repairs were pre-conditioned with five consecutive cycles of 5% clamp-to-clamp displacement. At the end of the pre-conditioning cycles, repairs were allowed to stress-relax for approximately 25 seconds, and then were elongated to failure. Peak loads in the pre-conditioning cycles were always less than loads of first failure detection.
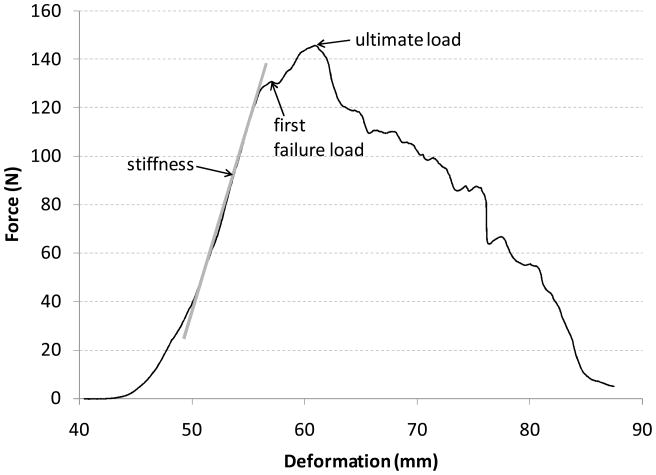
Repair deformation was quantified by video-tracking elastin dye lines placed on either side of the repair region. Variables measured were: peak load during each of the five preconditioning cycles, load of first failure (first negative inflection of force during the failure test), ultimate load (highest force achieved during the failure test), and repair stiffness (slope of the linear region of the load-deformation curve) (Fig. 2). Statistical comparisons between SS and PT repair techniques were made using non-parametric Mann-Whitney U tests with a significance level (α) of 0.05.
Figure 2.
Representative force-deformation curve for a PT repair. Stiffness was calculated as the slope of the linear portion of the force-deformation curve.
Results
There were no statistically significant differences in the cross-sectional area (p=0.99) or initial deformation (distance between tracked elastin lines at slack length, p=0.93) between SS and PT repairs. Therefore, all statistical comparisons were made between non-normalized loads and deformations (as opposed to stress and strain, which would be normalized to cross-sectional area and initial length, respectively).
All failures occurred in the repair region, rather than at the clamps or within the tendon substance. PT repairs failed with suture knots either slipping and/or pulling through the tendon material, followed by the FDS tendon pulling through the FDP tendon; SS repairs failed by the longitudinal shearing of fibers within the FDS, whereby fibers that were locked down with the running sutures stayed attached to the FDP, and adjacent, non-locked down fibers sheared away with the FDS.
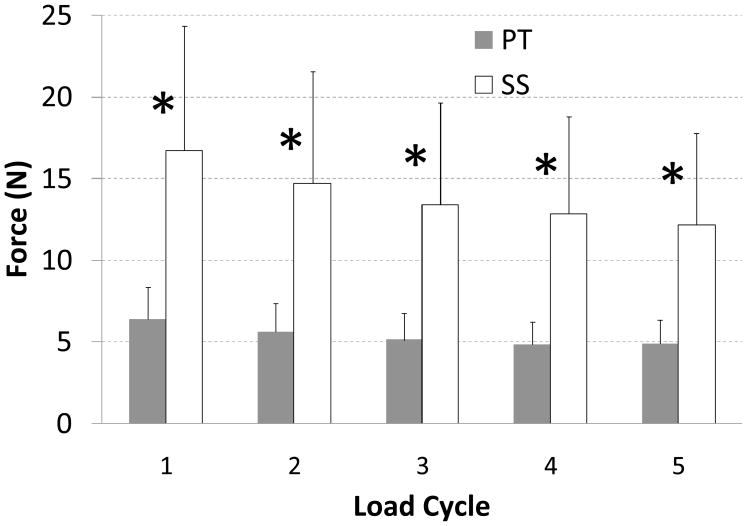
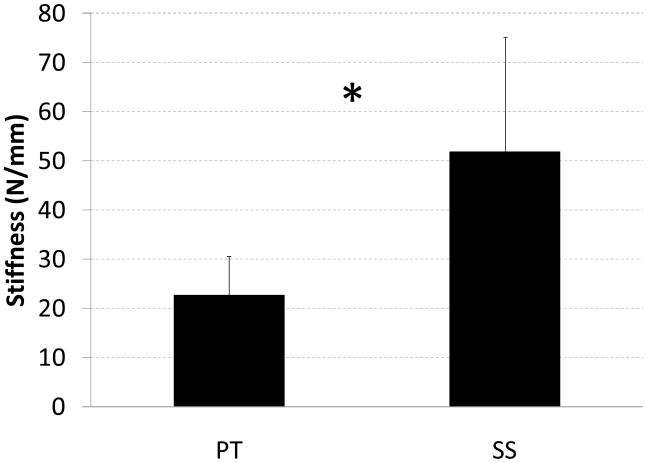
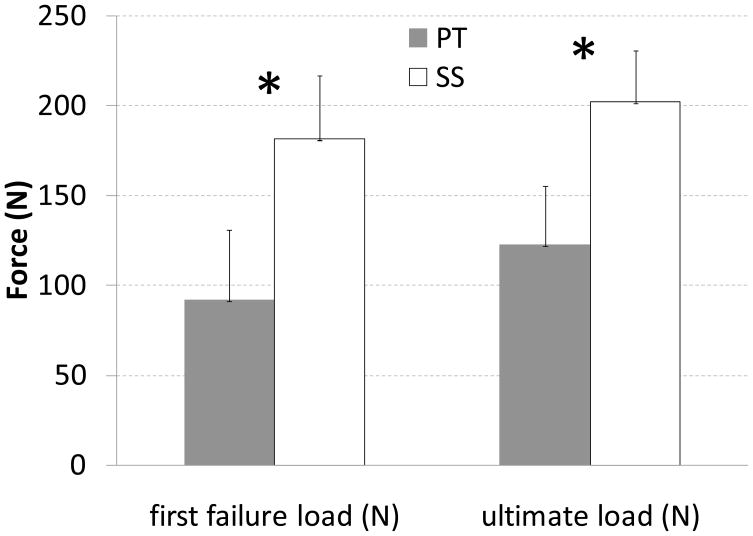
Peak load during each of the conditioning displacement cycles (range p = 0.005 to p = 0.01, Fig. 3), load at first failure (p = 0.001), ultimate load (p = 0.001), and repair stiffness (p = 0.001) were all significantly different between SS and PT techniques; in all cases the mean value for SS was higher than for PT (Figs. 3–5; Table 2).
Figure 3.
Mean load (N) reached at each the peak of each of the five conditioning cycles. A statistically significant difference (*) was found between SS and PT repairs at each cycle. Standard deviation bars are shown.
Figure 5.
Mean stiffness (N/mm) for SS and PT repair techniques. The asterisk indicates a statistically significant difference between the two repair types. Standard deviation bars are shown.
Table 2.
First failure load (N), ultimate load (N) and stiffness (N/mm) for each specimen.
| Specimen # | Repair Type | First Fail Load (N) | Ultimate Load (N) | Stiffness (N/mm) |
|---|---|---|---|---|
| 1 | PT | 145 | 162 | 25 |
| 2 | PT | 69 | 116 | 32 |
| 3 | PT | 131 | 146 | 19 |
| 4 | PT | 96 | 96 | 30 |
| 5 | PT | 55 | 140 | 20 |
| 6 | PT | 56 | 75 | 11 |
| 7 | SS | 180 | 180 | 30 |
| 8 | SS | 248 | 256 | 97 |
| 9 | SS | 205 | 209 | 33 |
| 10 | SS | 148 | 221 | 54 |
| 11 | SS | 159 | 184 | 65 |
| 12 | SS | 175 | 184 | 45 |
| 13 | SS | 154 | 180 | 38 |
Discussion
This in-vitro human cadaveric study demonstrated that the method of tendon repair used for musculotendinous transfer may influence the immediate strength of repair and, therefore, the ability to pursue post-operative rehabilitation protocols which utilize early motion. The main result of this study was that the SS suture method tested here produced significantly stronger and stiffer repairs compared to the tested variation of the PT repair. Originally, the SS technique was designed to provide sufficient mechanical strength to permit immediate contractile use of a transferred muscle after surgery (11). Traditional clinical guidelines advocated a minimum of three weeks of immobilization after surgery (6–10), but more recent guidelines have advocated early active mobilization of tendon repairs, thereby increasing the need to implement a strong repair. In recent years, in tetraplegia surgery, the immediate post-operative activation of a transferred muscle using the SS repair has been implemented successfully in hundreds of clinical cases (11). The current study provides mechanical justification that, at the time of repair, the SS repair using a cross-stitch technique is indeed stronger than this variation of the PT repair, using a mattress stitch technique, thereby providing a larger safety margin that provides assurance to surgeons who promote immediate loading of the repair site. This study is limited as it was not designed to isolate the specific mechanisms (for example, suture-throw technique, tendon-weave technique) underlying the difference in strength and stiffness; future work will need to address this question.
Mean failure loads for both SS and PT repairs were greater than the estimated maximum isometric force that can be generated by the FDS muscle group (16–17). Average first detected failure loads in the current study were 182 N and 92 N for SS and PT repairs, respectively, which provide safety factors (the relative difference between the estimated maximum load a muscle can produce and the strength of its tendon) of 2.64 and 1.33 times the estimated 69 N maximum load (calculated based on the architecture of these muscles (17)). This would appear to establish adequate margins of safety for both repair techniques. However, it is important to note that biological changes to tendon material immediately after surgery occur that can affect tendon repair strength (18). Previous work demonstrated that tensile strength of in vivo end-to-end tendon repairs actually decreased for several days after surgery, before healing and strengthening takes effect (18), although more recent work has found no change in strength over the first three weeks post-repair (19). From a safety perspective, the time course for the healing of tendon repairs needs to be considered, such that if strength declines during the first post-operative days, safety factors of the repairs may be compromised. It should be noted that strength of the transferred muscle will generally decline also due to post-operative atrophy (20). Thus, the time-varying nature of both tendon repair strength and muscular strength must be considered over the rehabilitation process. It is important to note that there exists no evidence as to whether such time-varying changes differ between the repair techniques studied here, and future work will need to address this issue. The much greater safety factor for the SS repair, in comparison to the PT repair, should be beneficial to maintain the repair failure threshold above the muscular applied loads during the early post-operative period.
Relatively little mechanical testing has been done to examine the tensile strength of repair techniques used in tendon transfers (for example, a Medline search using combinations of key words: tendon, transfer, strength, repair, weave; uncovered five papers (21–25) examining the mechanical strength of overlapping tendon to tendon repair techniques). Further, because of differences between studies regarding surgical techniques, suture material used, and testing procedures, it is often difficult to make direct comparisons. Three recent papers, with methods comparable to those of the current study, quantified the tensile strength of the PT repair technique. The ultimate load in each of these papers (106 ±13 N, Kulikov et al. (18); 102 ±6 N, De Smet et al. (19); 128 N (no standard deviation reported), Gabuzda et al. (20)) was comparable to the ultimate load for this technique quantified here (122 ±33 N). Interestingly, Gabuzda et al. (20) compared PT weave using both mattress sutures and cross-stitch sutures, in identical locations, to join the tendons together. They found that the cross-stitches increased ultimate load of the repair by approximately 72% to 220 N. This load is comparable to the ultimate load documented here for the SS repair technique (202 ±29 N). Thus, it would appear that cross-stitch sutures significantly increase the strength of a tendon repair. Of the previously discussed studies, only Kulikov et al. (18) quantified PT repair stiffness, and reported a value of 11 ±1 N/mm, much lower than the stiffness documented here for either the PT (23 ± 8 N/mm) or SS (52 ± 23 N/mm) repair techniques. The large difference in PT stiffness may simply result from variations of the PT technique employed between studies, or in how deformation was measured. In the current study, deformation was measured across the repair site by tracking the movement of elastin dye lines on either side of the repair; Kulikov et al. (18) did not explicitly describe their measurement of deformation, but it appears that it included the deformation of both the repair region as well as the tendon material on either side of the repair region, which would yield a lower stiffness value. In the current study, the SS was significantly stiffer than the PT repair, which may be beneficial as it permits more efficient load transfer from donor muscle to recipient tendon, and ultimately to the bony insertion site.
The SS technique consists of the donor tendon inserted through a single incision on the recipient, one double-loop suture at both ends of the overlap, and running cross-stitch sutures down both sides (Fig. 1). The PT technique consists of the donor tendon weaving through three incisions on the recipient, with a double-loop suture at both ends of the overlap, and four mattress sutures evenly spaced between the two end sutures. Observation of the failure modes in each case demonstrated a consistent finding: the load resisted by the PT repair increased until one of the six stabilizing sutures failed, either by a knot slipping or by suture pull-out from the tendon material, thus causing an immediate drop in the load (first failure load). The resisted load then quickly recovered and increased until a second suture location failed, causing a second immediate drop in the load, which most often did not recover (ultimate load). Thus, it appeared that the sutures were loaded in an unbalanced manner, creating stress concentrations at individual suture/tissue interfaces. The SS repairs failed in an entirely different manner. The tendon material of the donor tendon appeared to separate longitudinally and slide apart, with the fibers locked with the running sutures staying attached to the recipient tendon, and the adjacent non-locked fibers pulling away with the donor. Thus, the running cross-stitch sutures acted to distribute the load over a wider suture-tissue interface, thus reducing stress concentration at individual sutures.
Numerous reports document that early passive (3) and active (1,2) mobilization of a transferred muscle reduces the incidence of adhesion formation, improves the recovery of joint range of motion and reduces post-operative recovery time. Tendon end-to-end repair models have been shown to benefit from early motion and loading with improved tensile strength (4), vascularity and cellularity (5). A strong surgical repair is required to enable a patient to activate a transferred muscle and load the repair with a minimum risk of damaging the repair. The SS suture technique appears to meet these requirements based at time-zero or immediately post-repair, both from the mechanical evidence demonstrated here and from clinical experience (13). There are a number of differences between the SS and PT repair techniques (Table 1), and our conclusions are limited to the specific variations tested here. Previous work (23) has shown that the primary difference improving the strength of the SS repair may be the use of cross-stitch versus mattress sutures. Thus, stabilizing the PT repair with cross-stitch sutures may greatly improve its strength and stiffness, potentially matching that of the SS technique demonstrated here. Future work will be designed to specifically test this hypothesis.
Figure 4.
Mean first failure and ultimate loads (N) for SS and PT repair techniques. Asterisks indicate a statistically significant difference between the two repair types. Standard deviation bars are shown.
Acknowledgments
The authors would like to acknowledge Mr. Robert Healey for help with the data collection, Prof. David Amiel for the use of material testing equipment, NSERC Canada for Post-doctoral funding (S.H.M. Brown), Swedish Research Council grant 11200, and NIH grant HD050837.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Silfverskiöld KL, May EJ. Early active mobilization after tendon transfers using mesh reinforced suture techniques. J Hand Surg. 1995;20B:291–300. doi: 10.1016/s0266-7681(05)80081-6. [DOI] [PubMed] [Google Scholar]
- 2.Rath S. Immediate postoperative active mobilization versus immobilization following tendon transfer for claw deformity correction in the hand. J Hand Surg. 2008;33A:232–240. doi: 10.1016/j.jhsa.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 3.Doi K, Hattori Y, Yamazaki H, Wahegaonkar AL, Addosooki A, Watanabe M. Importance of early passive mobilization following double free gracilis muscle transfer. Plast Reconstr Surg. 2008;121:2037–2045. doi: 10.1097/PRS.0b013e3181706f3c. [DOI] [PubMed] [Google Scholar]
- 4.Gelberman RH, Woo SL, Lothringer K, Akeson WH, Amiel D. Effects of early intermittent passive mobilization on healing canine flexor tendons. J Hand Surg. 1982;7A:170–175. doi: 10.1016/s0363-5023(82)80083-x. [DOI] [PubMed] [Google Scholar]
- 5.Gelberman RH, Amiel D, Gonsalves M, Woo S, Akeson WH. The influence of protected passive mobilization on the healing of flexor tendons: a biochemical and microangiographic study. Hand. 1981;13:120–128. doi: 10.1016/s0072-968x(81)80051-4. [DOI] [PubMed] [Google Scholar]
- 6.Smith RJ. Tendon Transfers of the Hand and Forearm. Boston: Little, Brown and Company; 1987. pp. 313–317. [Google Scholar]
- 7.Sarris I, Darlis NA, Sotereanos DG. Tendon transfers for radial nerve paralysis. In: Strickland JW, Graham TJ, editors. Master Techniques in Orthopaedic Surgery: The Hand. 2. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 199–208. [Google Scholar]
- 8.Trumble TE. Principles of Hand Surgery & Therapy. Philadelphia: W.B. Saunders Company; 2000. Brachial Plexus Injuries; pp. 297–312. [Google Scholar]
- 9.Kozin SH, Ciocco R, Speakman T. Tendon transfers for brachial plexus palsy. In: Macklin EJ, Callahan AD, Skirven TM, et al., editors. Rehabilitation of the Hand & Upper Extremity. St Louis: Mosby Inc; 2002. pp. 832–853. [Google Scholar]
- 10.Peljovich AE, Kucera KA, Gonzalez-Hernandez E, Keith MW. Rehabilitation of the hand and upper extremity in tetraplegia. In: Macklin EJ, Callahan AD, Skirven TM, et al., editors. Rehabilitation of the Hand & Upper Extremity. St Louis: Mosby Inc; 2002. pp. 854–876. [Google Scholar]
- 11.Fridén J, Reinholdt C. Current concepts in reconstruction of hand function in tetraplegia. Scand J Surg. 2008;97:341–346. doi: 10.1177/145749690809700411. [DOI] [PubMed] [Google Scholar]
- 12.Rath S. Immediate active mobilization versus immobilization for opposition tendon transfer in the hand. J Hand Surg. 2006;31A:754–759. doi: 10.1016/j.jhsa.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 13.Pulvertaft RG. Tendon grafts for flexor tendon injuries in the fingers and thumb. A study of technique and results. J Bone J Surg. 1956;38B:175–194. doi: 10.1302/0301-620X.38B1.175. [DOI] [PubMed] [Google Scholar]
- 14.Loren GJ, Lieber RL. Tendon biomechanical properties enhance human wrist muscle specialization. J Biomech. 1995;28:791–799. doi: 10.1016/0021-9290(94)00137-s. [DOI] [PubMed] [Google Scholar]
- 15.Ker RF. Dynamic tensile properties of the plantaris tendon of sheep (ovis aries) J Exp Biol. 1981;93:283–302. doi: 10.1242/jeb.93.1.283. [DOI] [PubMed] [Google Scholar]
- 16.Lieber RL, Jacobson MD, Fazeli BM, Abrams RA, Botte MJ. Architecture of selected muscles of the arm and forearm: anatomy and implications for tendon transfer. J Hand Surg. 1992;17:787–798. doi: 10.1016/0363-5023(92)90444-t. [DOI] [PubMed] [Google Scholar]
- 17.Ward SR, Loren GJ, Lundberg S, Lieber RL. High stiffness of human digital flexor tendons is suited for precise finger positional control. J Neurophysiol. 2006;96:2815–2818. doi: 10.1152/jn.00284.2006. [DOI] [PubMed] [Google Scholar]
- 18.Mason ML, Allen HS. The rate of healing of tendons: an experimental study of tensile strength. Ann Surg. 1941;113:424–456. doi: 10.1097/00000658-194103000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyer MI, Gelberman RH, Burns ME, Dinopoulos H, Hofem R, Silva MJ. Intrasynovial flexor tendon repair: an experimental study comparing low and high levels of in vivo force during rehabilitation in canines. J Bone J Surg. 2001;83-A:891–899. [PubMed] [Google Scholar]
- 20.Gutowski KA, Orenstein HH. Restoration of elbow flexion after brachial plexus injury: the role of nerve and muscle transfers. Plast Reconstr Surg. 2000;106:1348–1359. doi: 10.1097/00006534-200011000-00020. [DOI] [PubMed] [Google Scholar]
- 21.Kulikov YI, Dodd S, Gheduzzi S, Miles AW, Giddins GE. An in vitro biomechanical study comparing the spiral linking technique against the pulvertaft weave for tendon repair. J Hand Surg. 2007;32E:377–381. doi: 10.1016/J.JHSB.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 22.De Smet L, Schollen W, Degreef I. In vitro biomechanical study to compare the double-loop technique with the Pulvertaft weave for tendon anastomosis. Scand J Plast Reconstr Surg Hand Surg. 2008;42:305–307. doi: 10.1080/02844310802401330. [DOI] [PubMed] [Google Scholar]
- 23.Gabuzda GM, Lovallo JL, Nowak MD. Tensile strength of the end-weave flexor tendon repair. J Hand Surg. 1994;19B:397–400. doi: 10.1016/0266-7681(94)90098-1. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka T, Zhao C, Ettema AM, Zobitz ME, An KN, Amadio PC. Tensile strength of a new suture for fixation of tendon grafts when using a weave technique. J Hand Surg. 2006;31A:982–986. doi: 10.1016/j.jhsa.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Kim SH, Chung MS, Baek GH, Lee YH, Lee S, Gong HS. A loop-tendon suture for tendon transfer or graft surgery. J Hand Surg. 2007;32A:367–372. doi: 10.1016/j.jhsa.2006.12.007. [DOI] [PubMed] [Google Scholar]