Abstract
We have recently demonstrated that microglia and astrocytes express nucleotide-binding oligomerization domain-2 (NOD2), a novel cytosolic pattern recognition receptor for bacterial motifs, and we have shown that this intracellular receptor is essential for glial responses to Gram-negative pathogens. Here, we demonstrate that intact Staphylococcus aureus, a major Gram-positive causative agent of brain abscesses, activates the transcription factor NF-kB and is a potent stimulus for inflammatory cytokine production in primary murine microglia and astrocytes. Interestingly, we demonstrate that NOD2 is essential for maximal glial responses to intact S. aureus, but not cellular lysates. As such, this data indicates that NOD2 plays an important role in initiating inflammatory mediator production by resident brain cells following S. aureus infection and we suggest that this cytosolic receptor acts in conjunction with cell surface pattern recognition receptors to elicit maximal glial responses.
Keywords: microglia, astrocytes, Gram-positive bacteria, abscess, NLR, cytokines
Introduction
Staphylococcus aureus is a Gram-positive organism that is one of the main causative agents of brain abscesses in humans [4, 21, 28]. Brain abscess is a significant clinical problem despite recent advances in both detection and therapy due to the appearance of antibiotic resistant strains of S. aureus and its ubiquitous nature. Such infections are associated with an intense inflammatory host response within the central nervous system (CNS) [21, 22, 35] and a high degree of mortality [4, 21, 28]. However, the means by which S. aureus elicits immune activation in the CNS have not been fully defined.
While the brain has traditionally been viewed as a “victim organ” of infiltrating leukocytes, it has become increasingly apparent that resident glial cells such as microglia and astrocytes play an important role in the initiation and/or progression of immune responses following pathogen invasion [6]. Gram-positive bacterial cell walls and cellular components that serve as ligands for Toll-like pattern recognition receptors (TLR) can induce inflammatory mediator production by glial cells [1, 15, 25, 30]. Importantly, S. aureus has been demonstrated to elicit immune functions in both microglia and astrocytes [13, 22]. Factors produced by glia following S. aureus challenge include the signature inflammatory cytokines IL-6 and TNF-α [13, 24, 22], indicating that these CNS cells play an important role in brain abscess development and associated tissue damage.
We have recently demonstrated that astrocytes and microglia constitutively express nucleotide-binding oligomerization domain-2 (NOD2) [36, 37], a member of the nucleotide-binding domain leucine-rich repeat region-containing family of proteins (NLR) that functions as a cytosolic sensor for bacterial peptidoglycan motifs [17, 18, 20]. Importantly, we have recently confirmed that NOD2 mediates the ability of muramyl dipeptide to augment TLR-induced inflammatory cytokine production by primary astrocytes and microglia, and showed that this receptor is an important component in their responses to the Gram-negative bacterial pathogens Neisseria meningitidis and Borrelia burgdorferi [8]. In the present study, we demonstrate that NOD2 is essential for the maximal responses of microglia and astrocytes to viable S. aureus.
Materials and Methods
Isolation of primary murine glia
Mouse neonatal brain microglia and astrocytes were isolated from wild type C57BL/6j mice (NOD2+/+) and NOD2-deficient B6.129S1-Nod2tm1Flv/J mice (NOD2-/-) (Jackson Laboratory, Bar Harbor, Maine) as described previously [2, 7, 8, 31-33, 36, 37] and in accordance with federal guidelines and institutional animal use policies. Our glial isolation protocols have previously been demonstrated to yield 95% authentic microglia due to their characteristic morphology and the presence of the microglial cell surface markers CD11b and F4/80 as determined by immunocytometry [31], and greater than 97% authentic astrocytes due to their characteristic morphology and the presence of the astrocyte marker, glial fibrillary acidic protein in the absence CD11b as determined by confocal microscopy [2].
Culture of S. aureus and preparation of antigen lysates
Confluent glial cell layers were exposed to a S. aureus clinical isolate (UAMS-1; ATCC 49230) at multiplicities of infection (MOI) of between 25:1 and 250:1 bacteria to cells in media without antibiotics for 120 min at 37°C. This MOI range was selected based upon our earlier studies in other murine cell types demonstrating maximal induction of cytokine production over this range. Cells were subsequently washed and supplemented with 25 μg/ml gentamicin to kill remaining extracellular bacteria. In some experiments, glia were exposed to bacterial lysates prepared from an equal number of S. aureus pulsed three times with a cell sonicator and boiled for 3 minutes.
Quantification of glial IL-6 and TNF-α secretion
IL-6 and TNF-α secretion was quantified by specific capture ELISA as described previously [7, 8, 31, 33].
Western blot analysis of NF-kB activation
Nuclear protein extracts were prepared and immunoblot analyses were performed for the presence of the NF-kBp65 subunit (RelA) as previously described [31, 32]. Densitometric analysis of Western blots was performed using ImageJ software. Results are presented as mean values +/- SEM of arbitrary densitometric units corrected for background intensity and normalized to protein loading.
Statistical analyses
All results are presented as the mean +/- SD or SEM as indicated and tested by Student's t test or one-way ANOVA with Tukey's post hoc test as appropriate, using commercially available software (Sigma Stat; Systat Software, San Jose, CA). Results were considered to be statistically significant at a probability of < 0.05.
Results
NOD2 expression is required for maximal NF-kB activation in glia following S. aureus infection
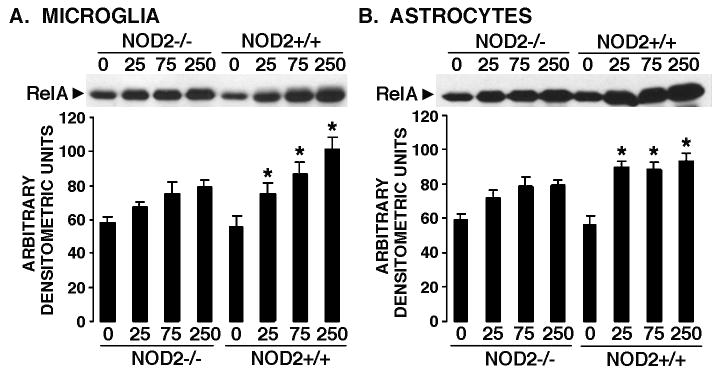
To begin to define the role of NOD2 in S. aureus-mediated glial inflammatory responses, primary microglia and astrocytes derived from NOD2+/+ and NOD2-/- mice were untreated or infected with viable S. aureus (MOI of 25:1, 75:1 or 250:1). At 2 hours following infection we assessed the activation of the pivotal proinflammatory transcription factor, NF-kB, by measuring nuclear translocation of the RelA subunit. As shown in Figure 1A, NOD2+/+ derived microglia exposed to S. aureus demonstrated a significant elevation in nuclear RelA levels following bacterial challenge (p < 0.05). Importantly, exposure of microglia derived from NOD2-/- animals to S. aureus failed to elicit such an effect (Figure 1A).
Figure 1.
Activation of NF-kB following exposure to S. aureus is reduced in NOD2 deficient glia. Microglia (Panel A) or astrocytes (Panel B) (2 × 106 cells per well) from NOD2+/+ and NOD2-/- animals were untreated (0) or exposed to viable S. aureus (MOI, of 25:1, 75:1, 250:1). At 2 hrs following challenge, nuclear protein isolates were assayed for the presence of NF-kB p65 (RelA) by immunoblot analysis. Nuclear RelA levels were quantified by densitometric analyses and normalized to protein loading. Average values +/- SEM are shown for each treatment group composed of three separate in vitro cell populations. Asterisks indicate statistically significant differences from corresponding uninfected cells (p < 0.05).
Consistent with our previous work employing Gram-negative pathogens [7, 8, 33], astrocytes respond to the Gram-positive organism S. aureus with a significant increase in nuclear RelA translocation (Figure 1B). Interestingly, astrocytes derived from NOD2-/- mice failed to demonstrate a significant increase in the nuclear levels of this pro-inflammatory signaling molecule following bacterial challenge (Figure 1B).
S. aureus-induced cytokine expression by isolated glia is mediated in part by NOD2
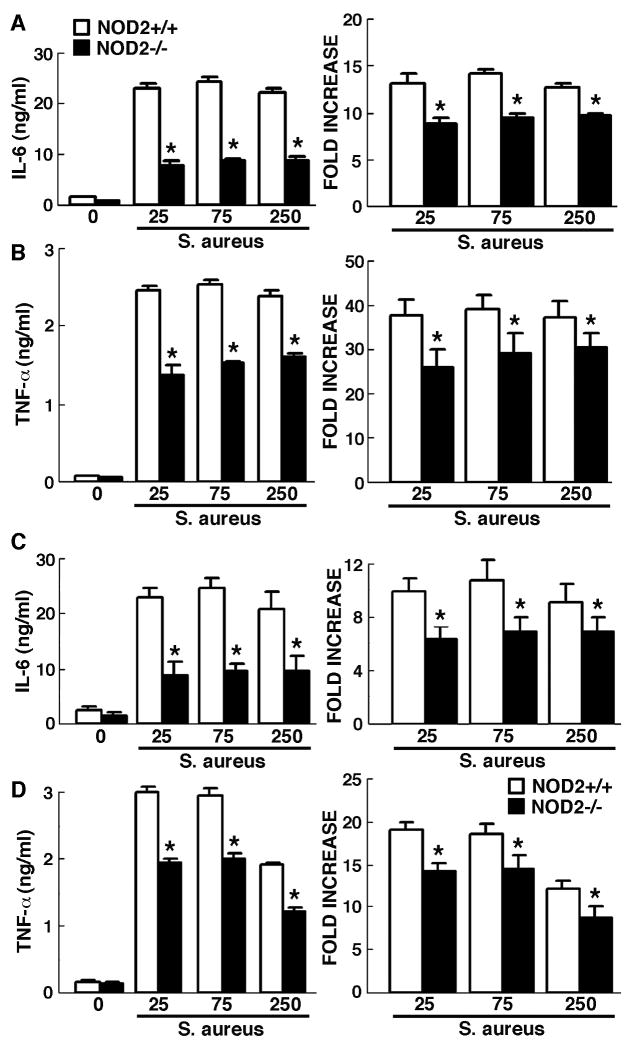
To further define the importance of NOD2 in S. aureus-mediated glial inflammatory responses, we have assessed the production of the inflammatory cytokines, IL-6 and TNF-α, by primary glia derived from NOD2+/+ and NOD2-/- mice following S. aureus infection (MOI of 25:1, 75:1 or 250:1). As shown in Figures 2A and 2B, microglia derived from NOD2+/+ animals exposed to S. aureus produced large amounts of IL-6 (Panel A) and TNF-α (Panel B) at 24 hours following infection. Importantly, NOD2-/- derived microglia produced significantly less of these cytokines following S. aureus challenge than NOD2+/+ cells both in terms of absolute cytokine levels in the culture supernatant, and as fold increases in cytokine production (Figures 2A and 2B).
Figure 2.
Inflammatory cytokine responses of glia to intact S. aureus are significantly lower in the absence of NOD2 expression. Microglia (Panels A and B) and astrocytes (Panels C and D) (2 × 106 cells per well) from NOD2+/+ and NOD2-/- animals were untreated or exposed to viable S. aureus (MOI, of 25:1, 75:1, 250:1). At 24 hrs following challenge culture supernatants were assayed for IL-6 (Panel A) or TNF-α (Panel B) content. Data are presented as the supernatant concentrations (Left panels) and as fold increases over levels in unstimulated cells (Right panels) and are the means of triplicate determinations of samples from three experiments +/- SD. Asterisks indicate significant differences between cells derived from NOD2+/+ and NOD2-/- animals (p < 0.05).
Similarly, astrocytes demonstrated robust inflammatory cytokine production following exposure to S. aureus (Figures 2C and 2D), consistent with the findings of other researchers [13]. Interestingly, NOD2-/- derived astrocytes produced significantly lower concentrations and fold increases over basal levels of both IL-6 and TNF-α at 24 hours following bacterial challenge than NOD2+/+ derived cells (Figures 2C and 3D).
NOD2 is not required for maximal glial responses to S. aureus lysates
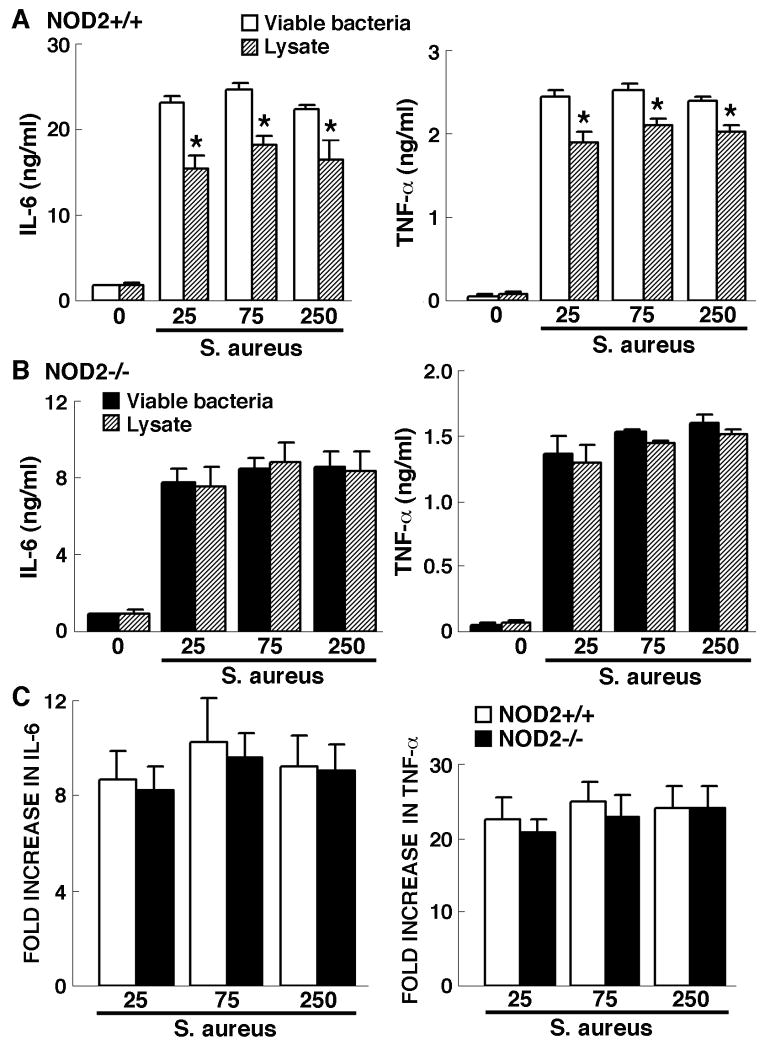
To further explore the role of NOD2 in the inflammatory responses of resident CNS cells, we investigated the ability of S. aureus lysates to induce IL-6 and TNF-α production by glia. As shown in Figure 3A, while S. aureus lysates elicit significant IL-6 and TNF-α production by NOD2+/+ derived microglia, such production is significantly lower than that induced by an equal number of intact viable bacteria. Interestingly, this difference was not apparent in NOD2-/- derived microglia where lysates and intact bacteria elicited almost identical levels of IL-6 and TNF-α production (Figure 3B). Furthermore, inflammatory cytokine production by NOD2-/- derived microglia following exposure to S. aureus lysates was not significantly different from that produced by identically treated NOD2+/+ derived cells (Figure 3C).
Figure 3.
Microglial cytokine responses to S. aureus lysates are significantly smaller than those elicited by intact bacteria and are not dependent on the expression of NOD2. Microglia (2 × 106 cells per well) from NOD2+/+ (Panels A and C) or NOD2-/- (Panels B and C) animals were untreated or exposed to either viable S. aureus (MOI, of 25:1, 75:1, 250:1) or lysates derived from an equal number of bacteria. At 24 hrs following challenge culture supernatants were assayed for the presence of IL-6 and TNF-α. Data are presented as the supernatant concentrations (Panels A and B) and as fold increases over levels in unstimulated cells (Panel C) and are the means of triplicate determinations of samples from three separate experiments +/- SD. Asterisks indicate statistically significant differences in cytokine production between cells treated with intact bacteria or bacterial lysates (p < 0.05).
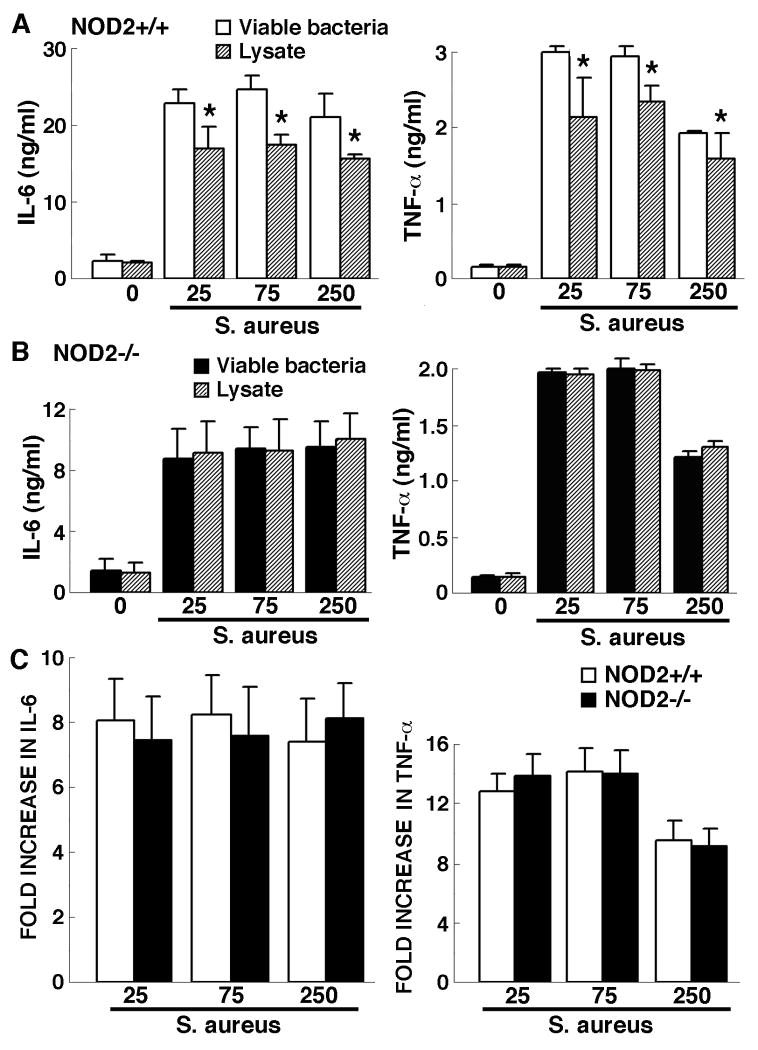
Similarly, S. aureus lysates elicited significantly less inflammatory cytokine production by astrocytes than an equal number of intact bacteria (Figure 4A). Again, this disparity was absent in parallel experiments utilizing NOD2-/- derived astrocytes (Figure 4B). Furthermore, increases in IL-6 and TNF-α production by NOD2-/- cells following exposure to S. aureus lysates were not significantly different from those induced in similarly treated NOD2+/+ derived cells (Figure 4C). Taken together, these data indicate that viable S. aureus is a more potent stimulus for inflammatory cytokine production by wild type mice-derived glial cells than mixed bacterial components and this disparity is mediated by NOD2.
Figure 4.
Astrocyte cytokine responses to S. aureus lysates are significantly smaller than those elicited by intact bacteria and are not dependent on the expression of NOD2. Astrocytes (2 × 106 cells per well) from NOD2+/+ (Panels A and C) or NOD2-/- (Panels B and C) animals were untreated or exposed to either viable S. aureus (MOI, of 25:1, 75:1, 250:1) or lysates derived from an equal number of bacteria. At 24 hrs following challenge culture supernatants were assayed for the presence of IL-6 and TNF-α. Data are presented as the supernatant concentrations (Panels A and B) and as fold increases over levels in unstimulated cells (Panel C) and are the means of triplicate determinations of samples from three separate experiments +/- SD. Asterisks indicate statistically significant differences in cytokine production between cells treated with intact bacteria or bacterial lysates (p < 0.05).
Discussion
A growing body of evidence suggests that resident glial cells play an important role in the initiation/progression of CNS inflammation following infection. To accomplish this role, CNS cells such as microglia and astrocytes express multiple microbial pattern recognition receptors including TLRs to perceive bacterial pathogens and to initiate CNS inflammation [3, 5, 11, 23, 29, 31]. Accordingly, our laboratory and others have demonstrated the ability of microglia and astrocytes to produce key inflammatory cytokines including TNF-α and IL-6 in response to TLR ligands and the Gram-negative bacterial CNS pathogens, N. meningitidis and B. burgdorferi [2, 3, 5, 9, 13, 22, 24, 27, 31, 32, 40].
In the present study, we demonstrate that mixed antigen lysates prepared from the Gram-positive organism, S. aureus, are potent stimuli for both microglia and astrocytes. We show that bacterial lysate preparations elicit the production of significant quantities of TNF-α and IL-6 by glial cells. These findings are in agreement with the results of others demonstrating that lipoteichoic acid derived from S. aureus can elicit MAP kinase activation and inflammatory mediator production by isolated rat microglia [26]. Similarly, heat killed S. aureus and cell wall components have been demonstrated to induce cytokine production by murine astrocytes [13]. Importantly, in the present study we report that acute in vitro exposure to viable intact S. aureus results in significantly greater inflammatory immune production by both microglia and astrocyte than that elicited by lysate preparations derived from an equal number of bacteria.
NLR proteins such as NOD2 have been identified as cytosolic pattern recognition receptors that play a role in the initiation of inflammatory host immune responses to bacterial challenge [10, 38]. NOD2 appears to function as an intracellular receptor for a minimal motif common to all bacterial peptidoglycans [14, 17, 39]. Previously, we have characterized the expression of NOD2 and its downstream effector molecule Rip2 kinase in primary cultures of murine glial cells [36, 37]. More recently, we have shown that exposure of microglia and astrocytes to bacterial lysates induces the association of NOD2 with Rip2 kinase, and demonstrated that this cytosolic receptor underlies the ability of MDP to augment TLR-induced inflammatory responses of microglia and astrocytes [8]. Furthermore, we have demonstrated that this cytosolic receptor is required for maximal inflammatory immune responses of primary glia cells to the Gram-negative bacteria, N. meningitidis and B. burgdorferi, and shown that NOD2 is a significant contributor to the progression of inflammatory CNS damage caused by these organisms following in vivo administration [8].
In the present study, we have assessed the relative importance of NOD2 in the immune responses of isolated cultures of primary glia cells to Gram-positive staphylococci. We show that activation of the pivotal inflammatory transcriptional activator NF-kB and production of key inflammatory cytokines in both astrocytes and microglia following challenge with intact S. aureus are significantly reduced in the absence of NOD2 expression. Taken together, these studies suggest that NOD2 could be an important component in the development of the damaging inflammation associated with brain abscesses.
The present study indicates that the glial immune responses to Gram-positive bacterial components are significantly smaller than those elicited by intact S. aureus. Interestingly, we have found that inflammatory cytokine production by either microglia or astrocytes following exposure to staphylococcal lysates is unaffected by the absence of NOD2 expression. This finding is in contrast to the results obtained using intact S. aureus and our previous studies in which we demonstrated that glial responses to Gram-negative bacterial lysates are significantly attenuated in cells derived from NOD2 deficient animals [8]. However, the present findings are in agreement with previously reported effects of intact S. aureus on astrocytes and microglia. While TLR2 has been implicated in the initiation of CNS inflammation following Gram-positive bacterial infections based upon the observed attenuation of glial responses to S. aureus in the absence of TLR2, CD14, and/or MyD88 expression [11-13, 23] and the ability of specific TLR2 ligands to mimic meningitis disease pathology [19], it is apparent that glial cells employ additional mechanisms to perceive such pathogens. Esen and coworkers [13] have previously demonstrated that TLR2 expression is required for a portion, but not all, of the inflammatory response of murine astrocytes to S. aureus challenge. Importantly, these investigators have also shown that TLR2 and its co-receptor, CD14, are required for microglial responses to bacterial components but not intact S. aureus [13, 23].
Based upon these observations and our present findings, we suggest that maximal glial responses to intact Gram-positive bacterial pathogens require the involvement of cytosolic NOD2 protein. While the notion that resident glial cells internalize S. aureus is controversial, such a hypothesis is supported by the documented ability of this organism to invade a variety of other cell types including endothelial cells, epithelial cells, and immune cells [34]. As such, we propose a scenario in which S. aureus is perceived by microglia and astrocytes via TLRs and NOD2 in a cooperative manner. In this scenario, extracellular bacteria and/or their products are recognized by cell surface pattern recognition receptors such as TLR2 while internalized intact bacteria are perceived by NOD2, and the signaling pathways initiated by these interactions act in synergy to maximize inflammatory glial responses.
Acknowledgments
Supported by grants to IM from the NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brandenburg LO, Varoga D, Nicolaeva N, Leib SL, Wilms H, Podschun R, Wruck CJ, Schröder JM, Pufe T, Lucius R. Role of glial cells in the functional expression of LL-37/rat cathelin-related antimicrobial peptide in meningitis. J Neuropathol Exp Neurol. 2008;67:1041–1054. doi: 10.1097/NEN.0b013e31818b4801. [DOI] [PubMed] [Google Scholar]
- 2.Bowman CC, Rasley A, Tranguch SL, Marriott I. Cultured astrocytes express toll-like receptors for bacterial products. Glia. 2003;43:281–291. doi: 10.1002/glia.10256. [DOI] [PubMed] [Google Scholar]
- 3.Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61:1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- 4.Calfee DP, Wispelwey B. Brain abscess. Semin Neurol. 2000;20:353–360. doi: 10.1055/s-2000-9397. [DOI] [PubMed] [Google Scholar]
- 5.Carpentier PA, Begolka WS, Olson JK, Elhofy A, Karpus WJ, Miller SD. Differential activation of astrocytes by innate and adaptive immune stimuli. Glia. 2005;49:360–374. doi: 10.1002/glia.20117. [DOI] [PubMed] [Google Scholar]
- 6.Chauhan VS, Marriott I. Bacterial infections of the central nervous system: a critical role for resident glial cells in the initiation and progression of inflammation. Curr Immunol Rev. 2007;3:133–143. [Google Scholar]
- 7.Chauhan VS, Sterka DG, Jr, Gray DL, Bost KL, Marriott I. Neurogenic exacerbation of microglial and astrocyte responses to Neisseria meningitidis and Borrelia burgdorferi. J Immunol. 2008;180:8241–8249. doi: 10.4049/jimmunol.180.12.8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chauhan VS, Sterka DG, Jr, Furr SR, Young AB, Marriott I. NOD2 plays an important role in the inflammatory responses of microglia and astrocytes to bacterial CNS pathogens. Glia. 2009;57:414–423. doi: 10.1002/glia.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- 10.Dziarski R, Gupta D. The peptidoglycan recognition proteins (PGRPs) Genome Biol. 2006;7:232. doi: 10.1186/gb-2006-7-8-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esen N, Kielian T. Recognition of Staphylococcus aureus-derived peptidoglycan (PGN) but not intact bacteria is mediated by CD14 in microglia. J Neuroimmunol. 2005;170:93–104. doi: 10.1016/j.jneuroim.2005.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esen N, Kielian T. Central role for MyD88 in the responses of microglia to pathogen-associated molecular patterns. J Immunol. 2006;176:6802–6811. doi: 10.4049/jimmunol.176.11.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esen N, Tanga FY, DeLeo JA, Kielian T. Toll-like receptor 2 (TLR2) mediates astrocyte activation in response to the Gram-positive bacterium Staphylococcus aureus. J Neurochem. 2004;88:746–758. doi: 10.1046/j.1471-4159.2003.02202.x. [DOI] [PubMed] [Google Scholar]
- 14.Fournier B, Philpott DJ. Recognition of Staphylococcus aureus by the innate immune system. Clin Microbiol Rev. 2005;18:521–540. doi: 10.1128/CMR.18.3.521-540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freyer D, Weih M, Weber JR, Bürger W, Scholz P, Manz R, Ziegenhorn A, Angestwurm K, Dirnagl U. Pneumococcal cell wall components induce nitric oxide synthase and TNF-alpha in astroglial-enriched cultures. Glia. 1996;16:1–6. doi: 10.1002/(SICI)1098-1136(199601)16:1<1::AID-GLIA1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, Philpott DJ, Sansonetti PJ. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278:8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 18.Girardin SE, Travassos LH, Herve M, Blanot D, Boneca IG, Philpott DJ, Sansonetti PJ, Mengin-Lecreulx D. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J Biol Chem. 2003;278:41702–41708. doi: 10.1074/jbc.M307198200. [DOI] [PubMed] [Google Scholar]
- 19.Hoffmann O, Braun JS, Becker D, Halle A, Freyer D, Dagand E, Lehnardt S, Weber JR. TLR2 mediates neuroinflammation and neuronal damage. J Immunol. 2007;190:28–23. doi: 10.4049/jimmunol.178.10.6476. [DOI] [PubMed] [Google Scholar]
- 20.Inohara N, Ogura Y, Fontalba A, Gutierrez O, Pons F, Crespo J, Fukase K, Inamura S, Kusumoto S, Hashimoto M, Foster SJ, Moran AP, Fernandez-Luna JL, Nunez G. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn's disease. J Biol Chem. 2003;278:5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 21.Kielian T. Immunopathogenesis of brain abscess. J Neuroinflammation. 2004;1:16. doi: 10.1186/1742-2094-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kielian T. Microglia and chemokines in infectious diseases of the nervous system: views and reviews. Front Biosci. 2004;9:732–750. doi: 10.2741/1266. [DOI] [PubMed] [Google Scholar]
- 23.Kielian T, Esen N, Bearden ED. Toll-like receptor 2 (TLR2) is pivotal for recognition of S. aureus peptidoglycan but not intact bacteria by microglia. Glia. 2005;49:567–576. doi: 10.1002/glia.20144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kielian T, Mayes P, Kielian M. Characterization of microglial responses to Staphylococcus aureus: effects on cytokine, costimulatory molecule, and Toll-like receptor expression. J Neuroimmunol. 2002;130:86–99. doi: 10.1016/s0165-5728(02)00216-3. [DOI] [PubMed] [Google Scholar]
- 25.Kim YS, Täuber MG. Neurotoxicity of glia activated by gram-positive bacterial products depends on nitric oxide production. Infect Immun. 1996;64:3148–3153. doi: 10.1128/iai.64.8.3148-3153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinsner A, Boveri M, Hareng L, Brown GC, Coecke S, Hartung T, Bal-Price A. Highly purified lipoteichoic acid induced pro-inflammatory signalling in primary culture of rat microglia through Toll-like receptor 2: selective potentiation of nitric oxide production by muramyl dipeptide. J Neurochem. 2006;99:596–607. doi: 10.1111/j.1471-4159.2006.04085.x. [DOI] [PubMed] [Google Scholar]
- 27.Lieberman AP, Pitha PM, Shin HS, Shin ML. Production of tumor necrosis factor and other cytokines by astrocytes stimulated with lipopolysaccharide or a neurotropic virus. Proc Natl Acad Sci USA. 1989;86:6348–6352. doi: 10.1073/pnas.86.16.6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathisen GE, Johnson JP. Brain abscess. Clin Infect Dis. 1997;25:763–779. doi: 10.1086/515541. [DOI] [PubMed] [Google Scholar]
- 29.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 30.Prinz M, Kann O, Draheim HJ, Schumann RR, Kettenmann H, Weber JR, Hanisch UK. Microglial activation by components of gram-positive and -negative bacteria: distinct and common routes to the induction of ion channels and cytokines. J Neuropathol Exp Neurol. 1999;58:1078–1089. doi: 10.1097/00005072-199910000-00006. [DOI] [PubMed] [Google Scholar]
- 31.Rasley A, Anguita J, Marriott I. Borrelia burgdorferi induces inflammatory mediator production by murine microglia. J Neuroimmunol. 2002;130:22–31. doi: 10.1016/s0165-5728(02)00187-x. [DOI] [PubMed] [Google Scholar]
- 32.Rasley A, Marriott I, Halberstadt CR, Bost KL, Anguita J. Substance P augments Borrelia burgdorferi-induced prostaglandin E2 production by murine microglia. J Immunol. 2004;172:5707–5713. doi: 10.4049/jimmunol.172.9.5707. [DOI] [PubMed] [Google Scholar]
- 33.Rasley A, Tranguch SL, Rati DM, Marriott I. Murine glia express the immunosuppressive cytokine, interleukin-10, following exposure to Borrelia burgdorferi or Neisseria meningitidis. Glia. 2006;53:583–592. doi: 10.1002/glia.20314. [DOI] [PubMed] [Google Scholar]
- 34.Sinha B, Fraunholz M. Staphylococcus aureus host cell invasion and post-invasion events. Int J Med Microbiol. 2009;77:3611–3625. doi: 10.1016/j.ijmm.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 35.Stenzel W, Soltek S, Miletic H, Hermann MM, Korner H, Sedgwick JD, Schluter D, Deckert M. An essential role for tumor necrosis factor in the formation of experimental murine Staphylococcus aureus-induced brain abscess and clearance. J Neuropathol Exp Neurol. 2005;64:27–36. doi: 10.1093/jnen/64.1.27. [DOI] [PubMed] [Google Scholar]
- 36.Sterka D, Jr, Marriott I. Characterization of nucleotide-binding oligomerization domain (NOD) protein expression in primary murine microglia. J Neuroimmunol. 2006;179:65–75. doi: 10.1016/j.jneuroim.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 37.Sterka D, Jr, Rati DM, Marriott I. Functional expression of NOD2, a novel pattern recognition receptor for bacterial motifs, in primary murine astrocytes. Glia. 2006;53:322–330. doi: 10.1002/glia.20286. [DOI] [PubMed] [Google Scholar]
- 38.Strober W, Murray PJ, Kitani A, Watanabe T. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol. 2006;6:9–20. doi: 10.1038/nri1747. [DOI] [PubMed] [Google Scholar]
- 39.Takada H, Uehara A. Enhancement of TLR-mediated innate immune responses by peptidoglycans through NOD signaling. Curr Pharm Des. 2006;12:4163–4172. doi: 10.2174/138161206778743510. [DOI] [PubMed] [Google Scholar]
- 40.Takeshita S, Takeshita F, Haddad DE, Janabi N, Klinman DM. Activation of microglia and astrocytes by CpG oligodeoxynucleotides. Neuroreport. 2001;12:3029–3032. doi: 10.1097/00001756-200110080-00010. [DOI] [PubMed] [Google Scholar]