Abstract
From the sea hares belonging to two genera, Aplysia and Dolabella, a variety of new cytotoxic substances were isolated as minute constituents: their chemical structures were determined and their cytotoxicity was evaluated. Regarding the highly cytotoxic substances, further chemical and biological studies were performed that included their asymmetric chemical synthesis and elucidation of biological characteristics such as antitumor activity.
Keywords: cytotoxic substances, sea hare, isolation, chemical structure, chemical synthesis, antitumor activity
1. Introduction
A large number of bioactive substances have been isolated and characterized from marine organisms such as microorganisms, algae, sponges, tunicates, coelenterates, echinoderms, bryozoans, and mollusks.1) Sea hares are shell-less and slow-moving marine mollusks that feed on a variety of algae and sponges. Sea hares, which are not eaten by other marine animals, have been postulated to have chemical defense substances. The poisonous properties of the sea hare secretions were already known in the Roman times. Sea hares have been known to be a rich source of bioactive substances, which have generally been isolated as minute constituents of sea hares and are considered to be of dietary origin and/or to be produced by symbiotic microbes.2)
Since the notable report on the isolation of aplysin, a bromosesquiterpene, by Yamamura and Hirata,3) chemical constituents of the sea hare of the genus Aplysia were examined extensively to yield various compounds, most of which were halogenated. The Pettit group investigated the bioactive constituents of the sea hare of the genus Dolabella collected in the Indian Ocean, resulting in the isolation of fifteen novel peptide- and depsipeptide-type compounds named dolastatins 1–15, most of which were cytostatic and antineoplastic.4)
We intensively examined the cytotoxic constituents of the sea hares of two species, Aplysia kurodai and Dolabella auricularia, collected off the coast of the Shima peninsula, Mie prefecture, Japan, and isolated a number of cytotoxic substances. In the present article, the authors comprehensively describe the research results of chemistry (structure, synthesis, etc.) and bioactivity (cytotoxicity, antitumor activity, target biomolecule, etc.) regarding thirty-two cytotoxic substances isolated from the two species of sea hares, A. kurodai and D. auricularia mentioned above: these results were obtained by the authors’ group during the period between 1990 and 2008. Furthermore, this article includes the related research results up to 2009 that were reported by a number of domestic and overseas research groups. Previously, the reviews published by the authors (19975) and 20006)) described the chemical and bioactive aspects of the compounds obtained from these two species of sea hares: the references up to 1998 were covered. Another review published in 20097) dealt with only aplyronines isolated from A. kurodai and covered the references up to 2007. Besides the aforementioned reviews by the authors, a review by Kamiya and co-workers is available regarding the bioactive compounds obtained from these sea hares.8)
2. Cytotoxic substances from the sea hare Aplysia kurodai
2.1. Aplyronine A and aplyronines B–H
2.1.1. Isolation
Originally, aplyronine A (1) was isolated by eight-step chromatographic separation of the lipophilic extract of the sea hare A. kurodai guided by cytotoxicity assay using human tumor (HeLa S3) cells.9),10) Subsequently, we developed a more efficient method for the isolation of 1, which enabled us to isolate not only a considerable quantity of 1 but also seven minor congeners, aplyronines B (2) and C (3)9),10) and D–H (4–8)6),7) (Scheme 1).
Scheme 1.
Isolation procedure for aplyronines A-H (1–8).
2.1.2. Structures
The gross structure of aplyronine A (1) was elucidated on the basis of detailed spectral (IR, NMR, and mass) analysis.9),10) The absolute stereostructure of 1, possessing seventeen asymmetric centers, was determined by NMR spectral analysis and the asymmetric synthesis of the five fragments obtained from chemical degradation of aplyronine A (1).7),9)–13) Aplyronine A (1) was revealed to be an inseparable mixture of four diastereomers as to two amino acid esters: this was confirmed by the asymmetric chemical synthesis of 1 as a diastereomeric mixture of the amino acid esters with the same ratios as in the case of natural 1.14)–16)
The structural elucidation of aplyronines B–H (2–8) was performed by means of the spectral analysis, which led us to deduce their structures (Fig. 1).6),7),9),10) Among them, the structures of aplyronines B (2) and C (3) were confirmed by the asymmetric chemical synthesis of 2 and 3, respectively.16),17) The structural diversity of aplyronines except for aplyronine E (5) originates in variation of the amino acid residues as well as difference in their locations.
Fig. 1.
Structures and cytotoxicity of aplyronines A-H (1–8).
2.1.3. Asymmetric chemical synthesis of aplyronines A–C
The authors’ group achieved the asymmetric chemical synthesis of aplyronine A (1) (Scheme 2), which brought on the following results.14)–16) First, the asymmetric synthesis of 1 unambiguously confirmed its absolute stereostructure. Secondly, while the supply of 1 from the natural source was scarce, the synthesis provided a sufficient amount of 1, which enabled further biological tests. Thirdly, the structure-activity relationships of 1 were examined by employing the analogs and intermediates obtained in the course of the synthesis of 1. The asymmetric chemical synthesis of aplyronines B (2) and C (3) was also performed.16),17) Although the efforts have been made to synthesize aplyronines worldwide, chemical synthesis of aplyronines has not been reported so far except for our synthesis.
Scheme 2.
Outline of the asymmetric chemical synthesis of aplyronine A (1).
2.1.4. Cytotoxicity
Aplyronines A–H (1–8) exhibit strong cytotoxicity against HeLa S3 cells in vitro (Fig. 1).6),7) Five among eight aplyronines (1, 4, 5, 6, 7) are particularly strong cytotoxins. The structural feature common to these five aplyronines is the presence of the methylated serine ester moiety at C7 of the macrolide part of the molecule: aplyronine A (1) is more cytotoxic (ca. 50 times) than aplyronine C (3) that lacks the methylated serine ester group. Furthermore, the location of the methylated serine ester group in the molecule affects the cytotoxicity: aplyronine A (1) is considerably more cytotoxic than aplyronine B (2) or aplyronine H (8).
2.1.5. Antitumor activity
Aplyronine A (1) exhibited potent antitumor activity in vivo against five tumor cell lines6),7),9) (Table 1). The activity was dose-dependent. Particularly, the antitumor activity against P388 leukemia, Lewis lung carcinoma and Ehrlich carcinoma is noteworthy. It is remarkable that all the mice employed for the testing survived after the end of the experimental term (60 days) in the case of Lewis lung carcinoma. Aplyronine A (1) is regarded as a candidate for the development of antitumor agents.18)
Table 1.
Antitumor activity of aplyronine A (1)
| Tumor | Routea) | Dose (mg/kg/day) | Median survival time (days) | Test/Control (%) | Number of survivors after 60 days |
|---|---|---|---|---|---|
| P388 leukemia | i.p. | 0.08 | 59.9 | 545 | 4 / 6 |
| 0.04 | 46.0 | 418 | 2 / 6 | ||
| 0.02 | 17.3 | 157 | 0 / 6 | ||
| 0.01 | 14.4 | 131 | 0 / 6 | ||
| Controls | – | 11.0 | 100 | 0 / 7 | |
|
| |||||
| Colon C26 carcinoma | i.p. | 0.08 | 40.0 | 255 | 0 / 6 |
| 0.04 | 39.0 | 248 | 1 / 6 | ||
| 0.02 | 25.0 | 159 | 1 / 6 | ||
| 0.01 | 21.0 | 134 | 0 / 6 | ||
| Controls | – | 15.7 | 100 | 0 / 10 | |
|
| |||||
| Lewis lung carcinoma | i.p. | 0.08 | 9.3 | 86 | 0 / 6 |
| 0.04 | 60.1 | 556 | 6 / 6 | ||
| 0.02 | 59.9 | 555 | 4 / 6 | ||
| 0.01 | 29.0 | 269 | 2 / 6 | ||
| Controls | – | 10.8 | 100 | 0 / 8 | |
|
| |||||
| B16 melanoma | i.p. | 0.08 | 10.1 | 43 | 0 / 6 |
| 0.04 | 46.8 | 201 | 0 / 6 | ||
| 0.02 | 43.0 | 185 | 0 / 6 | ||
| 0.01 | 35.0 | 152 | 0 / 6 | ||
| Controls | – | 23.3 | 100 | 1 / 9 | |
|
| |||||
| Ehrlich carcinoma | i.p. | 0.08 | 12.0 | 80 | 0 / 6 |
| 0.04 | 59.7 | 398 | 2 / 6 | ||
| 0.02 | 33.0 | 220 | 1 / 6 | ||
| 0.01 | 21.8 | 145 | 0 / 6 | ||
| Controls | – | 15.0 | 100 | 0 / 8 | |
Schedule: i.p. days 1–5. Aplyronine A (1) was dissolved in DMSO (0.08 mg/mL) and then diluted with a physiological solution of NaCl.
2.1.6. The target biomolecule: actin
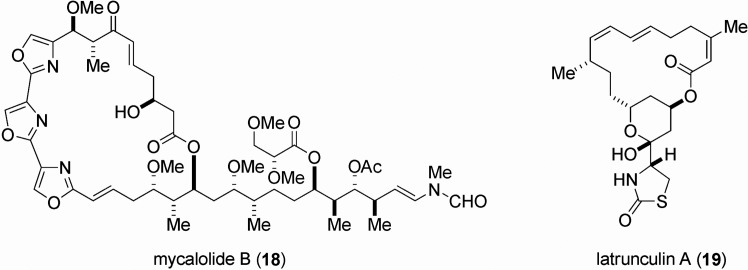
The target biomolecule of aplyronine A (1) was found to be actin, which is one of the most abundant and common proteins in the cytoskeleton.19) Aplyronine A (1) was shown to form a 1:1 complex with globular actin (G-actin, monomer), and to inhibit polymerization of G-actin to fibrous action (F-actin, polymer). Aplyronine A (1) proved to depolymerize F-actin to G-actin by severing. Mycalolide B (18),20),21) a cytotoxic substance isolated from a marine sponge by Fu-setani and co-workers, was reported to depolymerize F-actin by severing and to form a 1:1 complex with G-actin.22) The side chain portion of aplyronine A (1) was deduced to participate in its binding toward actin, because of the structural similarity concerning the side chain part for both compounds, aplyronine A (1) and mycalolide B (18). Latrunculins [e.g., latrunculin A (19)] were the first actin-binding substances isolated from a marine source.23),24) Since then, a considerable number of cytotoxic marine natural products that interact with actin have been identified. For details regarding actin-targeting natural products, reviews are available.25),26)
2.1.7. Structure-activity relationships
Bioactivity of eighteen synthetic analogs and natural aplyronines was examined.16),27),28) Selected data about cytotoxicity and actin depolymerizing activity of these compounds are illustrated in Fig. 2.
Fig. 2.
Structure-activity relationships of aplyronine A (1) and its analogs.
Structure-cytotoxicity relationship. In order for aplyronine A (1) to reveal strong cytotoxicity, the combination of the macrolide ring part and the side chain part is essential, because either the analog 21 (the macrolide moiety alone) or the analog 23 (the side chain moiety alone) showed extremely weak cytotoxicity (Fig. 2). Regarding the functional groups in 1, the presence of the trimethylserine ester, the conjugated diene, and two hydroxyl groups was necessary for the strong cytotoxicity of 1.
Structure-actin depolymerizing activity relationship. The side chain moiety of 1 was shown to play a key role in the actin depolymerizing activity, because the synthetic analog 23 only consisting of the side chain portion of 1 revealed relatively strong activity and the synthetic analog 22 without the side chain was totally inactive (Fig. 2). Further chemical evidence about the interaction between aplyronine A (1) and actin was obtained by photoaffinity labeling experiments, which employed probe 24 containing the side chain portion of 1.29) Since aplyronine A (1) competitively inhibited the binding of the probe 24 to actin, the probe 24 proved to bind to actin specifically at the binding site of aplyronine A (1).
Relation between cytotoxicity and actin depolymerizing activity. As described above, the side chain portion of aplyronine A (1) is essential for both cytotoxicity and actin depolymerizing activity. On the other hand, cytotoxicity of 1 is markedly influenced by the trimethylserine ester, the conjugated diene and two hydroxyl groups, whereas these functional groups affect actin depolymerizing activity to a quite small extent.
2.1.8. Structure of the actin-aplyronine A complex
The three-dimensional structure of the actin-aplyronine A (1) complex (referred to as the AA complex) was determined by X-ray crystallographic analysis, providing details about the molecular interactions between actin and aplyronine A (1) (Fig. 3).30)
Fig. 3.
(a) Structure of the actin-aplyronine A (1) complex; (b) the same complex rotated by ca. 90°.
Aplyronine A (1) binds to the hydrophobic cleft composed of subdomains 1 and 3 of actin by intercalating the side chain of 1 into actin. The hydrophobic interactions between the cleft of actin and aplyronine A (1) play a key role in the actin depolymerizing activity. The macrolide part of aplyronine A (1) binds to subdomain 3 of actin in the AA complex: the conformation of the macrolide part of 1 within subdomain 3 is stabilized by the interactions involving a hydrogen bond between the hydroxyl group at C9 of 1 and Glu334 of actin. One important feature of the structure of the AA complex is that the trimethylserine moiety protrudes toward the bulk solvent region from the molecular surface of actin and does not interact with actin (Fig. 3(b)).
These structural characteristics are considered to be associated with the cytotoxicity of aplyronine A (1). Actually, the cytotoxicity of aplyronine C (3) (which lacks the trimethylserine moiety) and aplyronine A diacetate (20) (which has no hydroxyl group at C9) is weak (Fig. 2).
The cytotoxic analogs of aplyronine A (1) necessarily possess actin depolymerizing activity, whereas the analogs having actin depolymerizing activity are not necessarily cytotoxic. The above findings suggest that aplyronine A (1) first binds to actin to form the actin-aplyronine A (1) complex, which secondly binds to another biomolecule in order to reveal cytotoxicity: the protruding trimethylserine ester group of 1 would play an important role in the second stage.
2.2. Aplaminones
Three cytotoxic alkaloids, aplaminone (25), neoaplaminone (26) and neoaplaminone sulfate (27) were isolated from the sea hare A. kurodai, and their structures were elucidated by the spectral analysis (Fig. 4).31) The absolute stereostructures of 25 and 26 were determined by the chemical method.32) These substances showed cytotoxicity against HeLa S3 cells in vitro [IC50 (μg/mL): 0.28 for 25, 1.6 × 10–7 for 26, and 0.51 for 27].
Fig. 4.
Cytotoxic substances (25–27) from the sea hare A. kurodai and highly cytotoxic substances (28–31) from the sea hare D. auricularia.
3. Cytotoxic substances from the sea hare Dolabella auricularia
We performed the bioassay-guided fractionation of the methanol extract of the sea hare Dolabella auricularia and isolated various cytotoxic substances, most of which were minute constituents of the sea hare: some of them were obtained in sub-milligram quantities. Cytotoxicity of these compounds was evaluated and listed in Table 2. Among the isolated substances, aurilide (28), dolastatin H (29), isodolastatin H (30) and doliculide (31) were highly cytotoxic, further studies of which were performed in detail.
Table 2.
Cytotoxicity of substances from the sea hare D. auricularia
| Substance | IC50 (μg/mL) HeLa S3 cells |
|---|---|
| aurilide (28) | 0.011 |
| dolastatin H (29) | 0.0022 |
| isodolastatin H (30) | 0.0016 |
| doliculide (31) | 0.005 |
|
| |
| dolastatin C (32) | 17.0 |
| dolastatin D (33) | 2.2 |
| dolastatin E (34) | 22.0 |
| dolastatin G (35) | 1.0 |
| isodolastatin G (36) | 5.3 |
| dolastatin I (37) | 12.0 |
| dolabellin (38) | 6.1 |
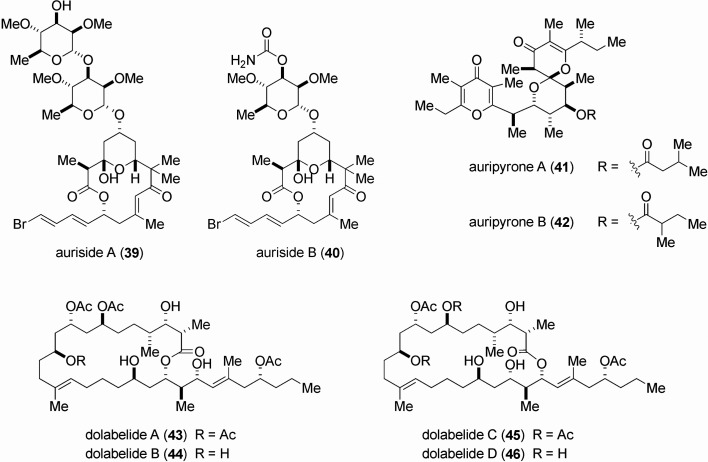
| auriside A (39) | 0.17 |
| auriside B (40) | 1.2 |
| auripyrone A (41) | 0.26 |
| auripyrone B (42) | 0.48 |
| dolabelide A (43) | 6.3 |
| dolabelide B (44) | 1.3 |
| dolabelide C (45) | 1.9 |
| dolabelide D (46) | 1.5 |
| aurilol (47) | 4.3 |
| auriculol (48) | 6.7 |
3.1. Aurilide
Aurilide (28) was isolated as a trace constituent: 0.5 mg from 262 kg of the sea hare.33) The isolation procedure involved two-step solvent partition and subsequent ten-step chromatographic separation. The gross structure of aurilide (28) was determined by the spectral analysis, and the absolute stereostructure of the peptide part was elucidated by chiral HPLC analysis of the component amino acids and isoleucic acid obtained by acid hydrolysis of 28. The absolute stereostructure of a new dihydroxy fatty acid in 28 was elucidated by the asymmetric chemical synthesis. Thus, the absolute stereostructure of aurilide was determined to be a 26-membered cyclodepsipeptide as shown in 28 (Fig. 4).33) The asymmetric chemical synthesis of aurilide (28) was achieved, which not only confirmed its stereostructure but also supplied the ample amount of 28 on a gram scale.34) The scarcity of the natural sample of 28 prevented the evaluation of its cytotoxicity. Thus, cytotoxicity of aurilide (28) was estimated by employing the synthetic sample and was shown to be strong against HeLa S3 cells with an IC50 value of 11 ng/mL (Table 2). Interestingly, 6-epi-aurilide obtained by chemical synthesis was almost non-cytotoxic (IC50 > 4 μg/mL).35) The NCI’s human cancer cell panel indicated that aurilide (28) showed a high level of cytotoxicity (the mean panel GI50 concentration was 0.12 μg/mL), and that 28 was selectively active against lung, ovarian, renal and prostate cancer cell lines.35) Aurilide (28) was not cytocidal but cytostatic against human leukemia cell lines. Aurilide (28) showed unusually high in vivo antitumor activity in the NCI’s hollow-fiber assays, but did not have significant antitumor activity owing to toxicity in the in vivo human tumor xenograft tests.
Solid phase library synthesis of aurilide (28) and its analogs was achieved by Takahashi, Doi and coworkers in 2003.36) The synthesis of the aurilide analogs was performed and the structure-cytotoxicity relationship was investigated in 2008.37)
Aurilides B and C were isolated from a marine cyanobacterium and their stereostructures were determined by Gerwick and co-workers in 2006.38) Structurally, aurilides B and C are closely related to aurilide (28). Cytotoxicity of aurilides B and C against various cancer cell lines was evaluated.38)
In 2004, Nakao, Scheuer and co-workers isolated kulokekahilide-2, a cytotoxin structurally related to aurilide (28), from a Hawaiian marine mollusk and elucidated its structure,39) which was revised by Ki-mura and co-workers in 2008.40)
3.2. Dolastatin H and isodolastatin H
Dolastatin H (29) and isodolastatin H (30) were isolated in trace amounts: 0.3 mg each from 33 kg of the sea hare.41) On the basis of the spectroscopic analysis, these compounds were shown to be peptide esters that were closely related to dolastatin 10 (Fig. 4).42) The structural feature of these compounds is that 3-phenylpropan-1,2-diol is attached through the ester linkage to the C-terminus of a tetrapeptide containing unusual amino acids. The asymmetric chemical synthesis of dolastatin H (29) and isodolastatin H (30) was executed, which unambiguously determined the absolute stereostructures of both compounds, 29 and 30.41) Owing to the scarcity of the natural samples, cytotoxicity of 29 and 30 was evaluated by employing the synthetic samples. Dolastatin H (29) and isodolastatin H (30) showed strong cytotoxicity against HeLa S3 cells with IC50 values of 2.2 and 1.6 ng/mL, respectively (Table 2). The C2 epimers of 29 and 30 are less cytotoxic (IC50: 20 and 29 ng/mL, respectively) than 29 and 30 themselves.41) These findings suggest that cytotoxicity of dolastatins H (29) and isodolastatin H (30) is slightly affected by the C-terminal stereostructures of theses peptide esters. The in vivo antitumor activity of 29 and 30 was examined. While no significant activity was shown for dolastatin H (29), isodolastatin H (30) exhibited antitumor activity with a T/C value of 141% against P388 leukemia (intraperitoneal tumor inoculation-intravenous drug administration). This anti-tumor activity for 30 is a little weaker than that for dolastatin 10, a well-known potent antitumor agent (T/C, 155% against P388 leukemia).
Scheuer and co-workers isolated malevamide D, an isodolastatin H (30) analog, from a marine cyanobacterium and elucidated its structure in 2002.43) Malevamide D demonstrated cytotoxicity against four cancer cell lines in the subnanomolar range.43)
3.3. Doliculide
Doliculide (31) was isolated as a minute constituent: 8.2 mg from 33 kg of the sea hare. The structure including stereochemistry was elucidated by means of the spectroscopic and chemical methods to be a cyclodepsipeptide of mixed peptide-polyketide biogenesis (Fig. 4).44) The asymmetric chemical synthesis of doliculide (31) was achieved, which unambiguously confirmed its absolute stereostructure.45),46) Doliculide (31) was shown to be highly cytotoxic (Table 2). Doliculide (31) phosphate showed antitumor effect to a considerable extent against Lu-61 xenograft in nude mice. In the course of the asymmetric chemical synthesis of 31, analogs of doliculide (31) were synthesized, which were utilized to examine the structure-activity relation of 31. One example is the finding that 11-epi-doliculide was 1000-fold less cytotoxic than doliculide (31): this fact suggested that the conformation of the 16-membered dilactam-lactone ring would be important for cytotoxicity, because the conformation of doliculide (31) was significantly different from that of 11-epi-doliculide.
Doliculide (31) is structurally related to cyclodepsipeptides such as geodiamolides47),48) and jaspamide (jasplakinolide).49),50)
The asymmetric chemical synthesis of doliculide (31) was also achieved by the Ghosh group in 200151) and by the Hanessian group in 2004.52) Hamel, Ghosh and co-workers reported that doliculide (31) arrested cells at the G2/M phase of the cell cycle by interfering with normal actin assembly.53)
3.4. Peptides and depsipeptides
In addition to the strong cytotoxins (28–31) described above, we isolated cytotoxic peptides and depsipeptides as minute constituents of the sea hare D. auricularia as follows (Fig. 5).
Fig. 5.
Cytotoxic peptides and depsipeptides from the sea hare D. auricularia.
Dolastatin C (32) is a linear depsipeptide, the structure of which was elucidated by chemical and spectral methods and was confirmed by the asymmetric chemical synthesis.54) Dolastatin C (32) was shown to be weakly cytotoxic (Table 2). Dolastatin D (33) is a cyclodepsipeptide. The structure of 33 was determined by chemical and spectral means and was confirmed by the asymmetric chemical synthesis.55) Dolastatin D (33) exhibited moderate cytotoxicity (Table 2). Dolastatin E (34) is a cyclic hexapeptide that contains a thiazoline moiety labile to be epimerized.56) The gross structure of 34 was elucidated by spectral data,56) and the stereostructure was determined by the asymmetric chemical synthesis.57) Dolastatin E (34) showed weak cytotoxicity (Table 2).
Dolastatin G (35) is a 35-membered depsipeptide that contains two new fatty acids.58) The stereo-structure of 35 was established by spectral data and the chemical method58) and was confirmed by the asymmetric chemical synthesis.59) Nordolastatin G (36) is a congener of dolastatin G (35): its structure was disclosed by chemical correlation with dolastatin G (35) and by asymmetric chemical synthesis.58),59) Both compounds (35, 36) were shown to be moderately cytotoxic (Table 2). Moore, Paul and co-workers isolated lyngbyastatin 2 and norlyngbyastatin 2, analogs of dolastatin G (35) and nordolastatin G (36), from a marine cyanobacterium and elucidated their structures by the NMR spectroscopic method in 1999.60) Dolastatin I (37) is a cyclic hexapeptide, the structure of which was determined by spectral analysis:61) the stereostructure was established by the chiral HPLC analytical method and was confirmed by the asymmetric chemical synthesis.61),62) Cytotoxicity of 37 was weak (Table 2). Dolabellin (38) is a bisthiazole substance. Its gross structure was elucidated by spectral analysis, and subsequently the stereostructure was determined by means of the chemical method, which was confirmed by the asymmetric chemical synthesis.63) Cytotoxicity of 38 was shown to be moderate (Table 2).
Gerwick, Scheuer and co-workers isolated hectochlorin, a potent stimulator of actin assembly, from a marine cyanobacterium and determined its structure in 2002.64) Structurally, hectochlorin is closely related to dolabellin (38).
3.5. Polyketides
As minute constituents of the sea hare D. auricularia, we isolated the following cytotoxic polyketides (Fig. 6).
Fig. 6.
Cytotoxic polyketides from the sea hare D. auricularia.
Aurisides A (39) and B (40) were separated in sub-milligram quantities: 0.8 mg of 39 and 0.7 mg of 40 from 278 kg of the sea hare.65) First, their gross structures were elucidated to be macrolide glycosides by virtue of spectroscopic analysis, and secondly, their stereostructures were determined on the basis of spectral analysis in conjunction with chemical degradation experiments.65) Whereas cytotoxicity of auriside A (39) was shown to be relatively strong, auriside B (40) was moderately cytotoxic (Table 2). Paterson and co-workers achieved the asymmetric chemical synthesis of aurisides A (39) and B (40) in 2005.66) We executed the asymmetric chemical synthesis of both substances (39, 40) in 2006.67),68)
Two closely related polypropionates, auripyrones A (41) and B (42) were isolated as minute constituents: 1.0 mg of 41 and 1.7 mg of 42 from 452 kg of the sea hare.69) The gross structures of both substances (41, 42) were determined by spectral analysis. Relative stereochemistry of 41 and 42 except for the ester part of 42 was established by the NMR spectral analysis. Auripyrones A (41) and B (42) were shown to be relatively strong cytotoxins (Table 2). The Perkins group performed the asymmetric chemical synthesis of auripyrone A (41) in 2006, which established the absolute stereostructure.70) Subsequently, in 2009, the Jung group achieved the chemical synthesis of auripyrone A (41).71)
Dolabelides A (43), B (44), C (45) and D (46) are closely related macrolides with moderate cytotoxicity (Table 2), and their stereostructures were determined by a combination of the chemical and spectroscopic means.72),73) Leighton and co-workers achieved the asymmetric chemical synthesis of dolabelide D (46) in 2006.74)
3.6. Terpenoids
Concerning cytotoxic terpenoids, two substances were isolated as minute constituents of the sea hare (Fig. 7).
Fig. 7.
Cytotoxic terpenoids from the sea hare D. auricularia.
Aurilol (47) is a bromotriterpene, and the structure including the absolute stereochemistry of the five asymmetric centers among ten was determined by spectroscopic and chemical means.75) Subsequently, Morimoto and co-workers elucidated the complete stereostructure of 47 and performed the asymmetric chemical synthesis of 47 in 2005.76) Auriculol (48) is a highly oxygenated squalene, and the stereostructure was determined by means of the spectroscopic and chemical methods.77) Both aurilol (47) and auriculol (48) exhibited moderate cytotoxicity (Table 2).
4. Concluding remarks
From the sea hares of two genera, Aplysia and Dolabella, a variety of new cytotoxic substances were isolated in minute amounts: their chemical structures were determined and their cytotoxicity against tumor cells was evaluated. Furthermore, chemical and biological studies were performed in detail concerning the highly cytotoxic substances such as aplyronine A (1), aurilide (28), dolastatin H (29) and isodolastatin H (30), some of which exhibited promising in vivo antitumor activity. Since the supply of cytotoxic substances from the natural source was scarce, chemical synthesis of these substances was achieved, which, in some cases, provided the ample amounts necessary for the elucidation of their biological characteristics.
Acknowledgments
The authors would like to thank the co-workers for their dedicated contribution to this project. This work was financially supported by Grants-in-Aid for Scientific Research and for Scientific Research on Priority Area from Ministry of Education, Culture, Sports, Science and Technology, and by the Naito Foundation, the Fujisawa Foundation, the Shorai Foundation for Science and Technology and Ono Pharmaceutical Co., Ltd.
Biographies
Profile
Kiyoyuki Yamada completed his Ph.D. degree at Nagoya University, Japan, under the direction of Professor Yoshimasa Hirata in 1962. He joined the faculty at Nagoya University in 1961, and did postdoctoral work at Stanford University with Professor Eugene E. van Tamelen from 1962 to 1964. He was promoted to Associate Professor at Nagoya University in 1966 and then to Professor in 1979. His research interests are in the fields of natural products chemistry and bio-organic chemistry. He received the Chemical Society of Japan Award in 1996 and the Naito Foundation Research Prize in 1997. Since 1997 he has been Professor Emeritus at Nagoya University.

Profile
Makoto Ojika completed his Ph.D. degree in 1987 under the direction of Professor Kiyoyuki Yamada at Nagoya University. He became Assistant Professor at Nagoya University in 1982, and was a postdoctoral fellow at Columbia University with Professor Koji Nakanishi in 1988–1989. He was promoted to Associate Professor (Graduate School of Bioagricultural Sciences) at Nagoya University in 1995 and then to Professor in 2001. His research interests are in the fields of organic and bio-organic chemistry on natural products. He was awarded the Chemical Society of Japan Award for Young Chemists in 1990.

Profile
Hideo Kigoshi completed his doctoral work in 1989 under the direction of Professor Kiyoyuki Yamada at Nagoya University. He was a postdoctoral fellow with Professor Elias J. Corey (Harvard University) in 1990–1991. He became Assistant Professor at Nagoya University in 1984 and was promoted to Associate Professor in 1994. He moved to University of Tsukuba in 2000 as Professor of Chemistry. His research interests lie in the field of chemistry and chemical biology of the bioactive natural products. He received the Chemical Society of Japan Award for Young Chemists (1993) and the Inoue Research Award for Young Scientists (1993).

Profile
Kiyotake Suenaga received his Ph.D. degree in 1997 from Nagoya University under the direction of Professor Kiyoyuki Yamada. He became Assistant Professor at Nagoya University in 1995. He moved to University of Shizuoka in 2001 and then to University of Tsukuba in 2003. He was appointed as Associate Professor (Faculty of Science and Technology) at Keio University in 2006. His research interests span the fields of natural products chemistry and bio-organic chemistry. He was awarded the Inoue Research Award for Young Scientists in 1998 and the Chemical Society of Japan Award for Young Chemists in 2003.

References
- 1).Blunt J.W., Copp B.R., Hu W.P., Munro M.H.G., Northcote P.T., Princep M.R. (2009) Marine natural products. Nat. Prod. Rep. 26, 170–244 [DOI] [PubMed] [Google Scholar]
- 2).Harrigan G.G., Goetz G. (2002) Symbiotic and dietary marine microalgae as a source of bioactive molecules-experience from natural products research. J. Appl. Phycol. 14, 103–108 [Google Scholar]
- 3).Yamamura S., Hirata Y. (1963) Structure of aplysin and aplysinol, naturally occurring bromo compounds. Tetrahedron 19, 1485–1496 [Google Scholar]
- 4).Pettit G.R. (1997) The dolastatins. InProgress in the Chemistry of Organic Natural Products. Vol. 70 (eds. Herz W., Kirby G.W., Moore R.E., Steglich W., Tamm Ch.). Springer, Wien/New York, pp. 1–79 [Google Scholar]
- 5).Yamada K., Kigoshi H. (1997) Bioactive compounds from the sea hares of two genera: Aplysia and Dolabella. Bull. Chem. Soc. Jpn. 70, 1479–1489 [Google Scholar]
- 6).Yamada K., Ojika M., Kigoshi H, Suenaga K. (2000) Cytotoxic substances from opisthobranch mollusks. InDrugs from the Sea (ed. Fusetani N.). Karger, Basel, pp. 59–73 [Google Scholar]
- 7).Yamada K., Ojika M., Kigoshi H., Suenaga K. (2009) Aplyronine A, a potent antitumor macrolide of marine origin, and the congeners aplyronines B–H: chemistry and biology. Nat. Prod. Rep. 26, 27–43 [DOI] [PubMed] [Google Scholar]
- 8).Kamiya H., Sakai R, Jimbo M. (2006) Bioactive molecules from sea hares. InMolluscs: from Chemoecological Study to Biotechnological Application (eds. Cimino G., Gavagnin M.). Springer-Verlag, Berlin/Heidelberg, pp. 215–240 [Google Scholar]
- 9).Yamada K., Ojika M., Ishigaki T., Yoshida Y., Ekimoto H., Arakawa M. (1993) Aplyronine A, a potent antitumor substance and the congeners aplyronines B and C isolated from the sea hare Aplysia kurodai. J. Am. Chem. Soc. 115, 11020–11021 [Google Scholar]
- 10).Ojika M., Kigoshi H., Yoshida Y., Ishigaki T., Nisiwaki M., Tsukada I., et al. (2007) Aplyronine A, a potent antitumor macrolide of marine origin, and the congeners aplyronines B and C: isolation, structures, and bioactivities. Tetrahedron 63, 3138–3167 [Google Scholar]
- 11).Ojika M., Kigoshi H., Ishigaki T., Yamada K. (1993) Further studies on aplyronine A, an antitumor substance isolated from the sea hare Aplysia kurodai. Tetrahedron Lett. 34, 8501–8504 [Google Scholar]
- 12).Ojika M., Kigoshi H., Ishigaki T., Nisiwaki M., Tsukada I., Mizuta K., et al. (1993) Studies on the stereochemistry of aplyronine A: determination of the stereochemistry of the C21–C34 fragment. Tetrahedron Lett. 34, 8505–8508 [Google Scholar]
- 13).Ojika M., Kigoshi H., Ishigaki T., Tsukada I., Tsuboi T., Ogawa T., et al. (1994) Absolute stereochemistry of aplyronine A, a potent antitumor substance of marine origin. J. Am. Chem. Soc. 116, 7441–7442 [Google Scholar]
- 14).Kigoshi H., Ojika M., Ishigaki T., Suenaga K., Mutou T., Sakakura A., et al. (1994) Total synthesis of aplyronine A, a potent antitumor substance of marine origin. J. Am. Chem. Soc. 116, 7443–7444 [Google Scholar]
- 15).Kigoshi H., Ojika M., Suenaga K., Mutou T., Hirano J., Sakakura A., et al. (1994) Synthetic studies on aplyronine A, a potent antitumor substance of marine origin: stereocontrolled synthesis of the C21–C34 segment. Tetrahedron Lett. 35, 1247–1250 [Google Scholar]
- 16).Kigoshi H., Suenaga K., Mutou T., Ishigaki T., Atsumi T., Ishiwata H., et al. (1996) Aplyronine A, a potent antitumor substance of marine origin, aplyronines B and C, and artificial analogs: total synthesis and structure-cytotoxicity relationships. J. Org. Chem. 61, 5326–5351 [Google Scholar]
- 17).Suenaga K., Ishigaki T., Sakakura A., Kigoshi H., Yamada K. (1995) Absolute stereochemistry and synthesis of aplyronines B and C, the congeners of aplyronine A, a potent antitumor substance of marine origin. Tetrahedron Lett. 36, 5053–5056 [Google Scholar]
- 18).Crews P., Gerwick W.H., Schmitz F.J., France D., Bair K.W., Wright A.E., et al. (2003) Molecular approaches to discover marine natural product anticancer leads – an update from a drug discovery group collaboration. Pharm. Biol. 41(Supplement), 39–52 [Google Scholar]
- 19).Saito S., Watabe H., Ozaki H., Kigoshi H., Yamada K., Fusetani N, et al. (1996) Novel actin depolymerizing macrolide aplyronine A. J. Biochem. 120, 552–555 [DOI] [PubMed] [Google Scholar]
- 20).Fusetani N., Yasumuro K., Matsunaga S., Hashimoto K. (1989) Bioactive marine metabolites. Part 28. Mycalolides A-C, hybrid macrolides of ulapualides and halichondramide, from a sponge of the genus Mycale. Tetrahedron Lett. 30, 2809–2812 [Google Scholar]
- 21).Matsunaga S., Liu P., Celatka C.A., Panek J.S., Fusetani N. (1999) Relative and absolute stereochemistry of mycalolides, bioactive macrolides from the marine sponge Mycale magellanica. J. Am. Chem. Soc. 121, 5605–5606 [Google Scholar]
- 22).Saito S., Watabe H., Ozaki H., Fusetani N., Karaki H. (1994) Mycalolide B, a novel actin-depolymerizing agent. J. Biol. Chem. 269, 29710–29714 [PubMed] [Google Scholar]
- 23).Kashman Y., Groweiss A., Shmueli U. (1980) Latrunculin, a new 2-thiazolidinone macrolide from the marine sponge Latrunculia magnifica. Tetrahedron Lett. 21, 3629–3632 [Google Scholar]
- 24).Spector I., Shocher N.R., Kashman Y., Groweiss A. (1983) Latrunculins: novel marine toxins that disrupt microfilament organization in cultured cells. Science 219, 493–495 [DOI] [PubMed] [Google Scholar]
- 25).Fenteany G., Zhu S. (2003) Small-molecule inhibitors of actin dynamics and cell motility. Curr. Top. Med. Chem. 3, 593–616 [DOI] [PubMed] [Google Scholar]
- 26).Allingham J.S., Klenchin V.A., Rayment I. (2006) Actin-targeting natural products: structures, properties and mechanisms of action. Cell. Mol. Life Sci. 63, 2119–2134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Suenaga K., Kamei N., Okugawa Y., Takagi M., Akao A., Kigoshi H., et al. (1997) Cytotoxicity and actin depolymerizing activity of aplyronine A, a potent antitumor macrolide of marine origin, and the natural and artificial analogs. Bioorg. Med. Chem. Lett. 7, 269–274 [Google Scholar]
- 28).Kigoshi H., Suenaga K., Takagi M., Akao A., Kanematsu K., Kamei N., et al. (2002) Cytotoxicity and actin-depolymerizing activity of aplyronine A, a potent antitumor macrolide of marine origin, and its analogs. Tetrahedron 58, 1075–1102 [Google Scholar]
- 29).Kuroda T., Suenaga K., Sakakura A., Handa T., Okamoto K., Kigoshi H. (2006) Study of the interaction between actin and antitumor substance aplyronine A with a novel fluorescent photoaffinity probe. Bioconjugate Chem. 17, 524–529 [DOI] [PubMed] [Google Scholar]
- 30).Hirata K., Muraoka S., Suenaga K., Kuroda T., Kato K., Tanaka H., et al. (2006) Structure basis for antitumor effect of aplyronine A. J. Mol. Biol. 356, 945–954 [DOI] [PubMed] [Google Scholar]
- 31).Kigoshi H., Imamura Y., Yoshikawa K., Yamada K. (1990) Three new cytotoxic alkaloids, aplaminone, neoaplaminone and neoaplaminone sulfate from the marine mollusk Aplysia kurodai. Tetrahedron Lett. 31, 4911–4914 [Google Scholar]
- 32).Kigoshi H., Adachi Y., Yoshikawa K., Yamada K. (1992) Absolute stereochemistry of aplaminone and neoaplaminone, cytotoxic bromodopamines from a marine mollusc: enantioselective synthesis of debromoneoaplaminone. Tetrahedron Lett. 33, 4195–4198 [Google Scholar]
- 33).Suenaga K., Mutou T., Shibata T., Itoh T., Kigoshi H., Yamada K. (1996) Isolation and stereostructure of aurilide, a novel cyclodepsipeptide from the Japanese sea hare Dolabella auricularia. Tetrahedron Lett. 37, 6771–6774 [Google Scholar]
- 34).Mutou T., Suenaga K., Fujita T., Itoh T., Takada N., Hayamizu K, et al. (1997) Enantioselective synthesis of aurilide, a cytotoxic 26-membered cyclodepsipeptide of marine origin. Synlett, 199–201 [Google Scholar]
- 35).Suenaga K., Mutou T., Shibata T., Itoh T., Fujita T., Takada N., et al. (2004) Aurilide, a cytotoxic depsipeptide from the sea hare Dolabella auricularia: isolation, structure determination, synthesis, and biological activity. Tetrahedron 60, 8509–8527 [Google Scholar]
- 36).Takahashi T., Nagamiya H., Doi T., Griffiths P.G., Bray A.M. (2003) Solid phase library synthesis of cyclic depsipeptides: aurilide and aurilide analogues. J. Combi. Chem. 5, 414–428 [DOI] [PubMed] [Google Scholar]
- 37).Suenaga K., Kajiwara S., Kuribayashi S., Handa T., Kigoshi H. (2008) Synthesis and cytotoxicity of aurilide analogs. Bioorg. Med. Chem. Lett. 18, 3902–3905 [DOI] [PubMed] [Google Scholar]
- 38).Han B., Gross H., Goeger D.E., Mooberry S.I., Gerwick W.H. (2006) Aurilides B and C, cancer cell toxins from a Papua New Guinea collection of the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 69, 572–575 [DOI] [PubMed] [Google Scholar]
- 39).Nakao Y., Yoshida W.Y., Takada Y., Kimura J., Yang L., Mooberry S.L., et al. (2004) Kulokekahilide-2, a cytotoxic depsipeptide from a cephalaspidean mollusk Philinopsis speciosa. J. Nat. Prod. 67, 1332–1340 [DOI] [PubMed] [Google Scholar]
- 40).Takada Y., Umehara M., Nakao Y., Kimura J. (2008) Revised absolute stereochemistry of natural kulokekahilide-2. Tetrahedron Lett. 49, 1163–1165 [Google Scholar]
- 41).Sone H., Shibata T., Fujita T., Ojika M., Yamada K. (1996) Dolastatin H and isodolastatin H, potent cytotoxic peptides from the sea hare Dolabella auricularia: isolation, stereostructures, and synthesis. J. Am. Chem. Soc. 118, 1874–1880 [Google Scholar]
- 42).Pettit G.R., Kamano Y., Herald C.L., Tuinman A.A., Boettner F.E., Kizu H., et al. (1987) The isolation and structure of a remarkable marine animal antineoplastic constituent: dolastatin 10. J. Am. Chem. Soc. 109, 6883–6885 [Google Scholar]
- 43).Horgen F.D., Kazmierski E.B., Westenburg H.E., Yoshida W.Y., Scheuer P.J. (2002) Malevamide D: isolation and structure determination of an isodolastatin H analogue from the marine cyanobacterium Symploca hydnoides. J. Nat. Prod. 65, 487–491 [DOI] [PubMed] [Google Scholar]
- 44).Ishiwata H., Nemoto T., Ojika M., Yamada K. (1994) Isolation and stereostructure of doliculide, a cytotoxic cyclodepsipeptide from the Japanese sea hare Dolabella auricularia. J. Org. Chem. 59, 4710–4711 [Google Scholar]
- 45).Ishiwata H., Sone H., Kigoshi H., Yamada K. (1994) Total synthesis of doliculide, a potent cytotoxic cyclodepsipeptide from the Japanese sea hare Dolabella auricularia. J. Org. Chem. 59, 4712–4713 [Google Scholar]
- 46).Ishiwata H., Sone H., Kigoshi H., Yamada K. (1994) Enantioselective total synthesis of doliculide, a potent cytotoxic cyclodepsipeptide of marine origin and structure-cytotoxicity relationships of synthetic doliculide congeners. Tetrahedron 50, 12853–12882 [Google Scholar]
- 47).Chan W.R., Tinto W.F., Manchand P.S., Todaro L.J. (1987) Stereostructures of geodiamolides A and B, novel cyclodepsipeptides from the marine sponge Geodia sp. J. Org. Chem. 52, 3091–3093 [Google Scholar]
- 48).Dilip de Silva E., Andersen R.J., Allen T.M. (1990) Geodiamolides C to F, new cytotoxic cyclodepsipeptides from the marine sponge Pseudaxinyssa sp. Tetrahedron Lett. 31, 489–492 [Google Scholar]
- 49).Zabriskie T.M., Klocke J.A., Ireland C.M., Marcus A.H., Molinski T.F., Faulkner D.J., et al. (1986) Jaspamide, a modified peptide from a Jaspis sponge, with insecticidal and antifungal activity. J. Am. Chem. Soc. 108, 3123–3124 [Google Scholar]
- 50).Crews P., Manes L.V., Boehler M. (1986) Jasplakinolide, a cyclodepsipeptide from the marine sponge, Jaspis sp. Tetrahedron Lett. 27, 2797–2800 [Google Scholar]
- 51).Ghosh A.K., Liu C. (2001) Total synthesis of antitumor depsipeptide (−)-doliculide. Org. Lett. 3, 635–638 [DOI] [PubMed] [Google Scholar]
- 52).Hanessian S., Mascitti V., Giroux S. (2004) Total synthesis of the cytotoxic cyclodepsipeptide (−)-doliculide: The “ester” effect in acyclic 1,3-induction of deoxypropionates. Proc. Natl. Acad. Sci. USA 101, 11996–12001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53).Bai R., Covell D.G., Liu C., Ghosh A.K., Hamel E. (2002) (−)-Doliculide, a new macrocyclic depsipeptide enhancer of actin assembly. J. Biol. Chem. 277, 32165–32171 [DOI] [PubMed] [Google Scholar]
- 54).Sone H., Nemoto T., Ojika M., Yamada K. (1993) Isolation, structure, and synthesis of dolastatin C, a new depsipeptide from the sea hare Dolabella auricularia. Tetrahedron Lett. 34, 8445–8448 [Google Scholar]
- 55).Sone H., Nemoto T., Ishiwata H., Ojika M., Yamada K. (1993) Isolation, structure, and synthesis of dolastatin D, a cytotoxic cyclic depsipeptide from the sea hare Dolabella auricularia. Tetrahedron Lett. 34, 8449–8452 [Google Scholar]
- 56).Ojika M., Nemoto T., Nakamura M., Yamada K. (1995) Dolastatin E, a new cyclic hexapeptide isolated from the sea hare Dolabella auricularia. Tetrahedron Lett. 36, 5057–5058 [Google Scholar]
- 57).Nakamura M., Shibata T., Nakane K., Nemoto T., Ojika M, Yamada K. (1995) Stereochemistry and total synthesis of dolastatin E. Tetrahedron Lett. 36, 5059–5062 [Google Scholar]
- 58).Mutou T., Kondo T., Ojika M., Yamada K. (1996) Isolation and stereostructures of dolastatin G and nordolastatin G, cytotoxic 35-membered cyclodepsipeptides from the Japanese sea hare Dolabella auricularia. J. Org. Chem. 61, 6340–6346 [DOI] [PubMed] [Google Scholar]
- 59).Mutou T., Kondo T., Shibata T., Ojika M., Kigoshi H., Yamada K. (1996) Synthesis of dolastatin G and nordolastatin G, cytotoxic 35-membered cyclodepsipeptides of marine origin. Tetrahedron Lett. 37, 7299–7302 [Google Scholar]
- 60).Luesch H., Yoshida W.Y., Moore R.E., Paul V.J. (1999) Lyngbyastatin 2 and norlyngbyastatin 2, analogues of dolastatin G and nordolastatin G from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 62, 1702–1706 [DOI] [PubMed] [Google Scholar]
- 61).Sone H., Kigoshi H., Yamada K. (1997) Isolation and stereostructure of dolastatin I, a cytotoxic cyclic hexapeptide from the Japanese sea hare Dolabella auricularia. Tetrahedron 53, 8149–8154 [Google Scholar]
- 62).Kigoshi H., Yamada S. (1999) Synthesis of dolastatin I, a cytotoxic cyclic hexapeptide from the sea hare Dolabella auricularia. Tetrahedron 55, 12301–12308 [Google Scholar]
- 63).Sone H., Kondo T., Kiryu M., Ishiwata H., Ojika M., Yamada K. (1995) Dolabellin, a cytotoxic bisthiazole metabolite from the sea hare Dolabella auricularia: structural determination and synthesis. J. Org. Chem. 60, 4774–4781 [Google Scholar]
- 64).Marquez B.L., Watts K.S., Yokochi A., Roberts M.A., Verdier-Pinard P., Jimenez J.I., et al. (2002) Structure and absolute stereochemistry of hectochlorin, a potent stimulator of actin assembly. J. Nat. Prod. 65, 866–871 [DOI] [PubMed] [Google Scholar]
- 65).Sone H., Kigoshi H., Yamada K. (1996) Aurisides A and B, cytotoxic macrolide glycosides from the Japanese sea hare Dolabella auricularia. J. Org. Chem. 61, 8956–8960 [DOI] [PubMed] [Google Scholar]
- 66).Paterson I., Florence G.J., Heimann A.C., Mackay A.C. (2005) Stereocontrolled total synthesis of (−)-aurisides A and B. Angew. Chem. Int. Ed. 44, 1130–1133 [DOI] [PubMed] [Google Scholar]
- 67).Sone H., Suenaga K., Bessho Y., Kondo T., Kigoshi H, Yamada K. (1998) Synthesis of the aglycon of aurisides A and B, cytotoxic macrolide glycosides of marine origin. Chem. Lett., 85–86 [Google Scholar]
- 68).Suenaga K., Hoshino H., Yoshii T., Mori K., Sone H., Bessho Y., et al. (2006) Enantioselective synthesis of aurisides A and B, cytotoxic macrolide glycosides of marine origin. Tetrahedron 62, 7687–7698 [Google Scholar]
- 69).Suenaga K., Kigoshi H., Yamada K. (1996) Auripyrones A and B, cytotoxic polypropionates from the sea hare Dolabella auricularia: isolation and structures. Tetrahedron Lett. 37, 5151–5154 [Google Scholar]
- 70).Lister T., Perkins M.V. (2006) Total synthesis of auripyrone A. Angew. Chem. Int. Ed. 45, 2560–2564 [DOI] [PubMed] [Google Scholar]
- 71).Jung M.E., Salehi-Rad R. (2009) Total synthesis of auripyrone A using a tandem non-aldol aldol/ Paterson aldol process as a key step. Angew. Chem. Int. Ed. 48, 8766–8769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72).Ojika M., Nagoya T., Yamada K. (1995) Dolabelides A and B, cytotoxic 22-membered macrolides isolated from the sea hare Dolabella auricularia. Tetrahedron Lett. 36, 7491–7494 [Google Scholar]
- 73).Suenaga K., Nagoya T., Shibata T., Kigoshi H., Yamada K. (1997) Dolabelides C and D, cytotoxic macrolides isolated from the sea hare Dolabella auricularia. J. Nat. Prod. 60, 155–157 [Google Scholar]
- 74).Park P.K., O’Malley S.J., Schmidt D.R., Leighton J.L. (2006) Total synthesis of dolabelide D. J. Am. Chem. Soc. 128, 2796–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75).Suenaga K., Shibata T., Takada N., Kigoshi H., Yamada K. (1998) Aurilol, a cytotoxic bromotriterpene isolated from the sea hare Dolabella auricularia. J. Nat. Prod. 61, 515–518 [Google Scholar]
- 76).Morimoto Y., Nishikawa Y., Takaishi M. (2005) Total synthesis and complete assignment of the stereostructure of a cytotoxic bromotriterpene polyether (+)-aurilol. J. Am. Chem. Soc. 127, 5806–5807 [DOI] [PubMed] [Google Scholar]
- 77).Kigoshi H., Itoh T., Ogawa T., Ochi K., Okada M., Suenaga K., et al. (2001) Auriculol, a cytotoxic oxygenated squalene from the Japanese sea hare Dolabella auricularia: isolation, stereostructure, and synthesis. Tetrahedron Lett. 42, 7461–7464 [Google Scholar]