Abstract
Purpose
Angiogenesis, which plays an important role in tumor growth and metastasis, is regulated by a balance between angiogenic stimulators and inhibitors. Pigment epithelium-derived factor (PEDF), a secreted glycoprotein is an important inhibitor of angiogenesis. Although the precise mechanisms by which PEDF exerts its actions remain poorly understood, there is growing evidence supporting the role of PEDF as a candidate antitumor agent. In this study, we investigated the role of PEDF in breast cancer.
Methods
We investigated the correlation of PEDF protein levels with cancer progression and prognosis in patients with invasive ductal breast cancer (IDC). We used immunohistochemistry in a cohort of 119 breast cancer patients to examine the expression of PEDF protein with an anti-PEDF antibody and to measure the microvessel density (MVD) with an anti-CD34 antibody.
Results
PEDF was an endogenous inhibitor of angiogenesis in endothelial cells. Decreased intratumoral expression of PEDF was associated with a higher microvessel density (MVD), a more metastatic phenotype, and poorer clinical outcome. PEDF was positive in 43.7% patients. Patients with low PEDF expression had a significantly higher MVD count when compared with patients with high PEDF expression. In univariate and multivariate analysis, PEDF was an independent prognostic factor.
Conclusion
The inverse correlation between PEDF expression and MVD in human breast cancer suggests that low PEDF expression is associated with angiogenesis in breast cancer. PEDF expression is therefore a potentially useful prognostic marker for breast cancer.
Keywords: Pigment epithelium-derived factor, Angiogenesis, Breast cancer
Introduction
Breast cancer is one of the most common malignancies in women, representing 23% of all cancers in women. An estimated 1.15 million women worldwide are diagnosed with breast cancer. With 411,000 women expected to die of breast cancer in a year (Parkin et al. 2005), breast cancer ranks second only to lung cancer in mortality. The histological tumor type, grade, tumor size, lymph node involvement, steroid hormone receptor expression and HER-2 status all dictate the prognosis of the disease and choice of treatment. However, assessment of these clinical and pathological features is not sufficient to fully capture the heterogeneous clinical course of breast cancer (Weigelt et al. 2005), making it necessary to continue the search for new biomarkers associated with invasion and metastases.
Angiogenesis, the process of forming new blood vessels from existing vascular networks, is a critical event which is essential for the growth and persistence of solid tumors and their metastasis (Jimenez and Volpert 2001). Tumor tissue microvascularity is an independent prognostic indicator of the biological aggressiveness of breast cancer (Viacava et al. 2004; Cao et al. 2004). Quantification of angiogenesis, using microvessel density (MVD) as a parameter, is considered to be a valuable prognostic indicator of breast cancer aggressiveness (Dhakal et al. 2009). Antibodies against CD34, which is predominantly found in endothelial cells, have proven to be particularly reliable in assessing MVD (Teo et al. 2003). There are currently no studies investigating the expression of anti-angiogenic factors in breast cancer tissue.
Pigment epithelium-derived factor (PEDF), a 50-kDa secreted glycoprotein, was first identified and isolated from the conditioned media of primary human fetal retinal pigment epithelial cells (Tombran-Tink and Johnson 1989). It was initially identified as an effective neurotrophic factor, but was later discovered to have potent anti-angiogenic activity, far greater than any other known endogenously produced factor (Dawson et al. 1999). Although it belongs to the serine protease inhibitor family, PEDF does not inhibit proteases. It is known to exert its antitumor activity through varied mechanisms in order to inhibit tumor proliferation, cellular invasion, migration, differentiation, and tumor angiogenesis (Ek and Choong 2006).
Immunohistochemistry results previously demonstrated that in addition to having a strong correlation with MVD, PEDF expression also showed a strong correlation with clinical pathological factors and prognosis in ductal pancreatic adenocarcinoma patients (Uehara et al. 2004). Decreased intratumoral expression of PEDF was shown to be associated with a higher MVD, a more metastatic phenotype, and poorer clinical outcome. Although PEDF has been shown to be expressed in breast cancer tissue (Cai et al. 2006a), the prognostic significance of its expression in breast cancer has not been examined to date. To our knowledge, ours is the first study to immunohistochemically determine PEDF expression in invasive breast cancer and to correlate its expression with MVD, clinicopathological features, and survival in a large number of patients with invasive breast cancer.
Materials and methods
Patients and tissue specimens
This study used archival material from the Department of Pathology at the Tumor Hospital Affiliated to Harbin Medical University. Breast cancer tissue specimens were obtained from patients undergoing primary mastectomies at our institution from January 1, 2000 to December 31, 2002. Tumor tissues and benign breast tissues removed from the same patients were examined diagnostically by pathologists, and the benign tissues were confirmed to be free from tumor deposits. The most important inclusion criterion was presence of primary, unilateral, and operable infiltrating ductal carcinoma. Among exclusion criteria were distant metastasis at the time of diagnosis, locally advanced disease, inflammatory carcinoma, and synchronous bilateral of carcinoma in situ. Tumor size at the largest diameter of the invasive carcinoma was measured in millimeters by the pathologist. All archival tumor blocks of each tumor were initially assessed by hematoxylin and eosin (H&E) staining to select a tumor block with an invasive carcinoma and to include the tumor border and a cross-sectional area as large as possible. Four 4-μm-thick sections from the paraffin blocks were mounted on a ChenMate slide. Morphology and protein expression were evaluated in consecutive sections. Normal mammary parenchyma obtained from 30 women who underwent breast reduction was also analyzed. Histological classification of tumors was based on the WHO criteria. (Table 1). All protocols were reviewed and approved by the Ethical Committee of Harbin Medical University, Harbin, China. Written consent was obtained from all participating patients.
Table 1.
Patient demographics: clinical characteristics of the patients with breast cancer
| Clinical data | No. of cases |
|---|---|
| Patients | |
| Normal | 30 |
| Tumor | 119 |
| Age (years) | |
| Median | 49 |
| Range | 29–85 |
| Lymph node metastasis | |
| Negative | 54 |
| Positive | 65 |
| TNM stage | |
| I | 26 |
| II | 50 |
| III | 39 |
| IV | 4 |
| Histological grading | |
| G1 | 13 |
| G2 | 70 |
| G3 | 36 |
| Tumor size (cm) | |
| Mean | 2.8 |
| Median | 3 |
| Range | 1–6 |
| NPI | |
| 1 | 24 |
| 2 | 52 |
| 3 | 43 |
| Survival status | |
| Good prognosis | 49 |
| Poor prognosis | 70 |
| Estrogen receptor | |
| Negative | 47 |
| Positive | 72 |
| Progesterone receptor | |
| Negative | 55 |
| Positive | 64 |
Follow-up
Clinical and pathological records of all patients on the study were reviewed periodically. Patients were followed regularly for 5 years at the Tumor Hospital Affiliate of the Harbin Medical University. Clinical records were obtained from the departments providing follow-up care to ten patients on the study who moved to other parts of the country. All patients were followed until death or the study closing date (October 10, 2008). Disease-free survival (DFS) measured the first recurrence at any site and overall survival (OS) measured death from any case were the two assessments used for prognostic analyses.
Immunohistochemistry
Immunohistochemical staining was performed using the EnVision + System-HRP. Sections were incubated overnight at 4°C in primary anti-PEDF antibody (monoclonal mouse anti-human PEDF antibody; Chemicon International, Temecula, Calif) diluted to 1:200. The Envision kit (USCN Life science, USA), which uses DAB as a chromogen, was utilized to reveal antibody binding. All sections were counterstained with hematoxylin, dehydrated, and mounted with a coverslip. For negative controls, the primary antibody was substituted with PBS in order to confirm the specificity of the primary antibody.
Evaluation of labeling
Immunohistochemical evaluation of PEDF expression was carried out independently by two pathologists. Semiquantitative expression levels were based on staining intensity and distribution. Staining intensity was scored as follows: 0 (no staining); 1 (weak staining); 2 (moderate staining); 3 (strong staining). The positively stained area (distribution) was expressed as the percentage of the whole area under evaluation and scored as follows: 0 (no staining); 1 (1–25% positive cells); 2 (26–50% positive cells); 3 (51–75% positive cells); 4 (76–100% positive cells). Overall expression was then graded as low expression (score 0–2), intermediate expression (score 3–5), and high expression (score 6–7). Patients were classified into two groups according to PEDF expression. Patients exhibiting high expression were classified as PEDF positive and the remainder as PEDF negative.
Microvessel staining and counting
The degree of angiogenesis was determined by calculating MVD. The staining process was similar to that used for PEDF. A polyclonal antibody against CD34 antigen (polyclonal mouse anti-human CD34 antibody; QBEnd/10, Santa Cruz Biotechnology, USA) was used at a dilution of 1:50 (antibody diluent (Dako-Cytomation, USA) in order to detect intratumoral microvessels. Angiogenesis was estimated with the clinical data and prognostic outcome blinded. MVD assessment in all the 119 cases was carried out by two independent investigators, according to a modified version of the International Consensus Report (Vermeulen et al. 2002). Areas with a higher density of CD34-positive cells and cell clusters relative to adjacent areas were classified as ‘hot spots’. The slides were initially screened at low power to identify the areas with the highest number of microvessels or vascularity hot spots. Microvessels were counted in a sufficiently extended field (400× magnification). The MVD was calculated as the mean value of microvessels in the ten most vascularized areas.
Statistical analyses
All analyses were performed using the statistical software SPSS 13.0. Correlations between PEDF immunoreactivity and clinicopathological variables were evaluated by the X2 test. The Kaplan–Meier method was used to estimate overall survival. Correlation of survival differences with PEDF expression was analyzed by the log-rank test. The influence of different variables on survival was assessed using Cox univariate and multivariate regression analysis. Risk ratios and their 95% confidence interval (CI) were recorded for each marker. The level of significance was set at P < 0.05.
Results
Clinical results
A total of 119 patients with a mean age of 51 (range 29–85; median age 49) were enrolled in the study. The mean follow-up period was 59 months (range 3–169 months; median 60 months). Fifty-six patients (47.1%) died, and 63 patients (52.9%) were alive at the time of study completion. Twenty-six patients (21.8%) were at stage I, 50 patients (42%) were at stage II, 39 patients (32.8%) were at stage III, and 4 patients (3.5%) were at stage IV. Lymph node metastases were present in 65 patients (54.6%) and absent in 54 (46.4%) patients. Thirteen patients were classified as grade I, 70 were grade II, and the remaining patients were grade III.
Immunohistochemical pattern of PEDF expression in normal tissue and breast carcinoma
Breast tumor tissue exhibited dramatically lower levels of PEDF protein expression when compared with normal breast tissue. PEDF was detected in the cytoplasm of some epithelial cells (Fig. 1a). In 18 of the 30 normal controls (60%), we demonstrated PEDF reactivity in approximately 25% of the epithelial cells. Of the 119 breast cancer specimens, we found high levels of staining in 52 samples (43.7%), medium levels of staining in 30 samples (25.2%) and low levels of staining in 37 samples (31.1%). Since high levels of staining were classified as PEDF positivity, our results meant that 52 of our patients were classified as PEDF positive, and the rest (n = 67) were PEDF negative. We also showed positive PEDF staining in 14 (53.8%) of the 26 stage I patients, 19 (38%) of the 50 stage II patients, 18 (46.2%) of the 39 stage III patients, and in none of the stage IV patients. In contrast, PEDF was positive in 3 of the 13 grade I tumors (23.1%), 30 of the 70 grade II tumors (42.9%), and 19 of the 36 grade III tumors (52.8%). These data demonstrate that the frequency and intensity of PEDF expression was much higher in low-grade than in high-grade breast cancer.
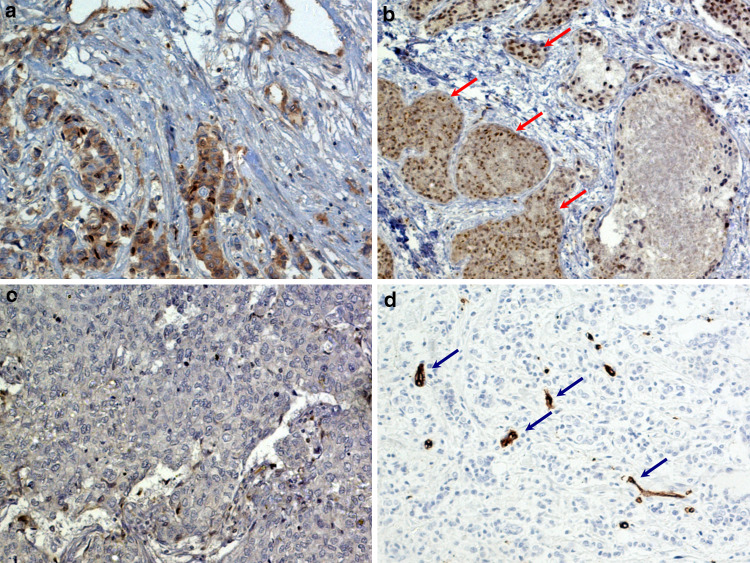
Fig. 1.
Cytoplasmic localization of PEDF in epithelial cells (a). PEDF-positive cells (red arrows) in an invasive ductal breast cancer specimen (b). PEDF-negative specimen (c). CD34-positive endothelial cells of blood vessels in tumor tissue (d). Strongly immunoreactive areas in the tumor with high vessel density are marked with blue arrows
MVD
We demonstrated that CD34 antigen was localized in the cytoplasm and cellular membrane of vascular endothelial cells in breast cancer tissue. MVD ranged from 2 to 59, with a mean of 29.2 (Fig. 1d).
Correlation between PEDF expression and MVD
We found a significant association between PEDF expression and MVD by the Mann–Whitney U test. The median MVD was 24.6 ± 4.18 (mean ± SD) in PEDF-positive specimens and 32.8 ± 7.12 in PEDF-negative specimens (Table 2).
Table 2.
Correlation between PEDF and microvascular density
| PEDF expression | Cases | Mean ± SD | Standard error | P value |
|---|---|---|---|---|
| Negative | 67 | 32.8 ± 7.12 | 1.18 | <0.0005 |
| Positive | 52 | 24.6 ± 4.18 | 0.82 |
PEDF expression in relation to prognosis
We used the Nottingham prognostic index (NPI) (Haybittle et al. 1982) as an indicator to evaluate the patient’s prognosis. NPI range is from 2.2 to 7.2 with a median survival value of 4.5. Patients with a good prognosis formed the NPI-1 group (n = 24), with an NPI of 3.4. Patients with a moderate prognosis formed the NPI-2 group (n = 52), with an NPI of 3.4–5.4 and patients with a poor prognosis formed the NPI-3 group (n = 43), with an NPI of 5.4. We found no significant association between PEDF expression levels and NPI status using the Kruskal–Wallis test (P = 0.7763).
PEDF expression and survival status
We used an average 5-year follow-up period to assess the survival of breast cancer patients in the context of PEDF expression levels. Patients were divided into two groups on the basis of their prognosis. The good prognosis group (n = 49) comprised patients who remained disease-free, and the poor prognosis group (n = 70) comprised patients who had recurrence, metastasis to a distant site, or had died as a result of breast cancer. Our results demonstrated that patients with a poor prognosis had low levels of PEDF expression (P = 0.0134) (Table 3).
Table 3.
Correlation of PEDF expression with prognosis
| Variables | No. of cases | Pigment epithelium-derived factor | ||||
|---|---|---|---|---|---|---|
| Negative (n = 67) | Positive (n = 52) | Score range | Median value | P | ||
| NPI | ||||||
| 1 | 24 | 15 | 9 | 2.6–3.2 | 2.6 | 0.7763 |
| 2 | 52 | 28 | 24 | 3.8–4.8 | 4.4 | |
| 3 | 43 | 24 | 19 | 5.5–6.4 | 5.6 | |
| Survival status | ||||||
| Good | 49 | 21 | 28 | 0.0134 | ||
| Poor | 70 | 46 | 24 | |||
Correlation between PEDF expression and various clinicopathological features
Correlations between PEDF expression and various clinicopathological features are summarized in Table 4. We did not find a significant correlation between PEDF expression and age, menopausal status, adjuvant treatment, lymph node metastasis, histopathological grading, pathological tumor-node metastasis stage, estrogen receptor expression, or progesterone receptor expression. PEDF expression, however, was closely associated with tumor size. Twenty-six of the 47 patients (55.3%) whose tumor size was ≤2 cm had a significantly higher incidence (P = 0.0389) of PEDF-positive expression than patients (36.1%; 26 of 72 patients) with a tumor size >2 cm. We found a trend toward lower PEDF expression in tumors from older and postmenopausal patients, although these associations were not significant. We found no significant associations between any of the adjuvant treatment modalities and PEDF expression.
Table 4.
Correlation between PEDF expression and various clinicopathological features
| Variables | No. of cases | Pigment epithelium derived factor | P | |
|---|---|---|---|---|
| Negative (n = 67) | Positive (n = 52) | |||
| Age (years) | ||||
| <50 | 61 | 34 | 27 | 0.8986 |
| ≥50 | 58 | 33 | 25 | |
| Menopausal status | ||||
| Premenopausal | 52 | 29 | 23 | 0.9177 |
| Postmenopausal | 67 | 38 | 29 | |
| Lymph node metastasis | ||||
| Negative | 54 | 32 | 22 | 0.5534 |
| Positive | 65 | 35 | 30 | |
| Tumor size (cm) | ||||
| ≤2.0 | 47 | 21 | 26 | 0.0389 |
| >2.0 | 72 | 46 | 26 | |
| Histopathological grade | ||||
| G1 | 13 | 10 | 3 | 0.1122 |
| G2, G3 | 106 | 57 | 49 | |
| Pathological stage (TNM) | ||||
| I | 26 | 12 | 14 | 0.2379 |
| II, III, IV | 93 | 55 | 38 | |
| ER status | ||||
| Negative | 47 | 26 | 21 | 0.8613 |
| Positive | 72 | 41 | 31 | |
| PR status | ||||
| Negative | 55 | 35 | 20 | 0.1349 |
| Positive | 64 | 32 | 32 | |
| Adjuvant treatment | ||||
| None | 38 | 34 | 14 | 0.3018 |
| Therapy (chemotherapy, endocrine therapy, chemo + endocrine, radiation therapy) | 81 | 43 | 38 | |
Univariate and multivariate analyses of PEDF expression and clinicopathological variables
Univariate analyses of overall survival using Cox regression analysis identified PEDF-positive expression (P = 0.0093), lymph node metastasis (P = 0.0417), tumor diameter (P = 0.0081), pathological tumor-node metastasis stage (P = 0.0311), and PR (progesterone receptor) status (P = 0.0005) as significant prognostic predicators. Age, menopausal status, histopathological grade and ER (estrogen receptor) status had no prognostic value. Multivariate analysis was performed on the same set of patients for PEDF expression and pathological predictors for survival time using the Cox regression model. The results indicated that PEDF positivity (risk ratio 2.203; P = 0.0062) and PR status (risk ratio 2.832; P = 0.0002) were independent favorable prognostic factors. Lymph node metastasis also had an independent unfavorable prognostic factor (risk ratio 2.074; P = 0.0105) (Table 5).
Table 5.
Prognostic factors in the Cox proportional hazards model
| Variables | Risk ratio | Univariate | P | Risk ratio | Multivariate | P |
|---|---|---|---|---|---|---|
| 95% Confidence interval | 95% Confidence interval | |||||
| Age (years) | ||||||
| <50/≥50 | 0.994 | (0.589–1.679) | 0.9825 | |||
| Menopausal status | ||||||
| Premenopausal/postmenopausal | 1.088 | (0.642–1.843) | 0.7539 | |||
| Pathological stage | ||||||
| I/II, III, IV | 2.391 | (1.083–5.281) | 0.0311 | |||
| Lymph node metastasis | ||||||
| Negative/positive | 1.77 | (1.022–3.091) | 0.0417 | 2.074 | (1.186–3.625) | 0.0105 |
| Tumor size (cm) | ||||||
| ≤2.0/>2.0 | 2.226 | (1.231–4.026) | 0.0081 | |||
| Histopathological grading | ||||||
| G1/G2, G3 | 3.999 | (0.974–16.412) | 0.0543 | |||
| ER status | ||||||
| Negative/positive | 1.61 | (0.952–2.724) | 0.0758 | |||
| PR status | ||||||
| Negative/positive | 2.641 | (1.533–4.551) | 0.0005 | 2.832 | (1.633–4.912) | 0.0002 |
| Pigment epithelium-derived factor | ||||||
| Positive/negative | 2.108 | (1.202–3.697) | 0.0093 | 2.203 | (1.252–3.877) | 0.0062 |
Kaplan–Meier survival analysis
Kaplan–Meier survival curves are shown in Fig. 2. Among the 119 study patients, PEDF-positive patients showed higher survival rates when compared with PEDF-negative patients (log-rank test, P = 0.008; Fig. 2a). PEDF-positive patients also had significantly higher disease-free survival rates than PEDF-negative patients (log-rank test, P = 0.002; Fig. 2b). The survival curves were significantly separated in TNM II, III patients, and PEDF-positive patients survived longer than PEDF-negative patients (Fig 3a, b). We also showed a significant difference in MVD between these two patient groups (log-rank test, P = 0.025; Fig. 4a; log-rank test, P = 0.0012; Fig. 4b).
Fig. 2.
Kaplan–Meier analysis for overall survival based on PEDF expression in breast cancer patients (log-rank test, P = 0.008 (a) Kaplan–Meier analysis for DFS based on PEDF expression in patients with breast cancer (log-rank test, P = 0.002 (b). A PEDF-positive patients (n = 52). B PEDF-negative patients (n = 67)
Fig. 3.
The survival curves are significantly separated in TNM II, III patients, and the PEDF-positive patients exhibit longer survival times when compared with PEDF-negative patients. a TNM II patients, A, PEDF-positive patients (n = 20). B PEDF-negative patients (n = 30); b TNM III patients, A, PEDF-positive patients (n = 18). B PEDF-negative patients (n = 21)
Fig. 4.
Kaplan–Meier analysis for overall survival based on MVD status in patients with breast cancer (log-rank test, P = 0.025 (a) Kaplan–Meier analysis for DFS based on MVD status in patients with breast cancer (log-rank test, P = 0.012 (b) The difference in OS between patients with low MVD (A, n = 54) and high MVD (B, n = 65) is significant
Discussion
Tumor growth, invasiveness, and metastasis all depend on their ability to continuously stimulate the growth of new capillary blood vessels (Hanahan and Weinberg 2000). PEDF functions as an anti-angiogenic factor to inhibit tumor growth. Exogenous or cell surface PEDF also directly interacts with extracellular matrix collagen to prevents tumor cell migration (Zhang et al. 2006b) and is thought to contribute to the maintenance of an appropriate angiogenic balance throughout the course of development (Fernandez-Garcia et al. 2007).
To the best of our knowledge, our study is the first to demonstrate a strong correlation between decreased PEDF expression and the progression of breast cancer. We used immunohistochemistry to demonstrate that 52 of 119 patients (43%) were PEDF positive. PEDF protein levels were dramatically lower in breast cancer tissue compared with normal breast tissue. Our results agreed with an earlier study showing localization of PEDF expression in the cytoplasm of endothelial cells (Cai et al. 2006a). We showed that tumor size was closely correlated with PEDF expression (P = 0.0389), confirming Folkman’s theory that tumors are dormant yet viable, unable to grow beyond 2–3 mm3 in size in the absence of neovascularization (Folkman 1971). Our data showed that PEDF-positive patients had higher overall survival rates and disease-free survival rates when compared with PEDF-negative patients, suggesting that endogenous angiogenic inhibitors are essential for maintaining angiogenic homeostasis in breast tissue.
Clinical studies previously demonstrated the association between low PEDF expression and increased MVD, greater metastatic potential, and a poorer clinical outcome in several tumors (Uehara et al. 2004; Zhang et al. 2006a). PEDF was also previously found in effusions from patients with breast cancer (Bard et al. 2004). However, the relationship between PEDF expression and MVD in breast cancer has not been previously characterized. MVD was previously used to quantitate tumor angiogenesis in invasive breast cancer and provided an independent assessment of tumor prognosis (Offersen et al. 2003). High MVD is a predictor of high risk of metastasis and shorter survival in solid tumors such as nasopharyngeal carcinoma (Guang-Wu et al. 2000), gastric carcinoma (Ohta et al. 2003), and pancreatic carcinoma (Seo et al. 2000). Based on these reports, we investigated the association of PEDF expression with MVD and looked at its correlation with prognosis in breast carcinoma. Our results agree with previous reports showing that the extent of tumor angiogenesis is a critical factor in determining tumor growth potential (Weir et al. 2003) and suggested that PEDF expression was significantly correlated with MVD and disease prognosis. However, it is not yet clear whether the diagnosis of breast cancer can be improved by the measurement of PEDF expression in combination with any other biomarker/s.
A number of angiogenic factors, most of which are induced by tumor cells dictate the process of tumor angiogenesis. However, the surrounding stroma tissue which was previously thought to be passive structural element, eliciting an immune response in an attempt to reject the tumor, is now recognized as an active participant and contributor to tumor progression by indirectly inducing angiogenic factors (Blacque and Worrall 2002). Contrary to previous studies (Cai et al. 2006a), we demonstrated by immunohistochemistry that PEDF was present in stromal cells of normal breast tissues.
The monoclonal antibodies cetuximab (Erbitux) and bevacizumab (Avastin), which target the epidermal growth factor receptor (EGFR) and VEGF, respectively, have gained regulatory approval (Goldberg 2005; Kerr 2004) in the treatment of breast cancer. Hypoxia and the pro-angiogenic factor VEGF are known to downregulate PEDF through proteolytic degradation (Notari et al. 2005). PEDF regulation is complex in light of the fact that although it is downregulated by VEGF, it is also known to be a potent inhibitor of VEGF via the regulated intramembrane proteolysis of VEGFR-1 (Cai et al. 2006b).
In conclusion, our results demonstrated a significant correlation between low levels of PEDF protein and adverse prognostic factors such as lymph node metastasis and survival status in breast cancer. We also demonstrated a positive correlation between PEDF protein expression levels, MVD, and tumor size of breast carcinoma. Based on the inverse correlation between PEDF expression levels and survival in human breast cancer patients, we suggest that suppression of PEDF expression plays a major role in promoting angiogenesis in breast cancer, and that in addition to being used as a prognostic marker for breast carcinoma, PEDF could emerge as an attractive new drug target in the treatment of breast cancer.
Acknowledgments
The authors thank Dr. Hong-Fei Ji, Yi Hong-Li, and Wei-Xu, for their contribution to experiments and data. This work was supported by project grants from the Science Foundation of Department of Public Health in China (Code WKJ2007-3-001).
Conflict of interest statement
None of the authors had any conflict of interest.
Footnotes
The authors D. Zhou and S.-Q. Cheng are contributed equally to this article.
Contributor Information
Guo-Qiang Zhang, Email: 6-fu@163.com.
Da Pang, Phone: +86-451-86298613, FAX: +86-451-86663760, Email: pangdasir0451@yahoo.cn.
References
- Bard MP, Hegmans JP, Hemmes A et al (2004) Proteomic analysis of exosomes isolated from human malignant pleural effusions. Am J Respir Cell Mol Biol 31:114–121 [DOI] [PubMed] [Google Scholar]
- Blacque OE, Worrall DM (2002) Evidence for a direct interaction between the tumor suppressor serpin, maspin, and types and collagen. J Biol Chem 277:10783–10788 [DOI] [PubMed] [Google Scholar]
- Cai J, Jiang WG, Grant M, Boulton M (2006a) Pigment epithelium-derived factor (PEDF) inhibits angiogenesis via regulated intracellular proteolysis of VEGFR1. J Biol Chem 281:3604–3613 [DOI] [PubMed] [Google Scholar]
- Cai J, Parr C, Watkins G (2006b) Decreased pigment epithelium-derived factor expression in human breast cancer progression. Clin Cancer Res 12(11):3510–3517 [DOI] [PubMed] [Google Scholar]
- Cao Y, Paner GP, Kahn LB, Rajan PB (2004) Noninvasive carcinoma of the breast angiogenesis and cell proliferation. Arch Parhol Lab Med 128:893–896 [DOI] [PubMed] [Google Scholar]
- Dawson DW, Volper OV, Gills P, Crawford SE, Xu H, Benedict W et al (1999) Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science 285:245–248 [DOI] [PubMed] [Google Scholar]
- Dhakal HP, Bassarova A, Naume B et al (2009) Breast carcinoma vascularity: a comparison of manual microvessel count and Chalkley count. Histol Histopathol 24:1049–1059 [DOI] [PubMed] [Google Scholar]
- Ek ET, Choong PF (2006) The role of high-dose therapy and autologous stem cell transplantation for pediatric bone and soft tissue sarcomas. Expert Rev Anticancer Ther 6(2):225–237 [DOI] [PubMed] [Google Scholar]
- Fernandez-Garcia NL, Volpert OV, Jimenez B (2007) Pigment epithelium-derived factor as a multifunctional antitumor factor. J Mol Med 85(1):15–22 [DOI] [PubMed] [Google Scholar]
- Folkman J (1971) Tumor angiogenesis: therapeutic implication. N Engl J Med 285:1182–1186 [DOI] [PubMed] [Google Scholar]
- Goldberg RM (2005) Cetuximab. Nat Rev Drug Discov (suppl 1):10–11 [DOI] [PubMed]
- Guang-Wu H, Sunagawa M, Jie-Em L et al (2000) The relationship between microvessel density, the expression of vascular endothelial growth factor (VEGF), and the extension of nasopharyngeal carcinoma. Laryngoscope 110:2066–2069 [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA (2000) The hallmarkers of cancer. Cell 100:57–70 [DOI] [PubMed] [Google Scholar]
- Haybittle JL, Blamey RW, Elston CW et al (1982) A prognostic index in primary breast cancer. Br J Cancer 45:361–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez B, Volpert OV (2001) Mechanistic insights on the inhibition of tumor angiogenesis. J Mol Med 78:663–672 [DOI] [PubMed] [Google Scholar]
- Kerr DG (2004) Targeting angiogenesis in cancer: clinical development of bevacizumab. Nat Clin Pract Oncol 1:39–43 [DOI] [PubMed] [Google Scholar]
- Notari L, Miller A, Martinez A, Amaral J, Ju M, Robinson G, Smith LE, Becerra SP (2005) Pigment epithelium-derived factor is a substrate for matrix metalloproteinase type 2 and type 9: implications for downregulation in hypoxia. Invest Ophthalmol Vis Sci 46:2736–2747 [DOI] [PubMed] [Google Scholar]
- Offersen BV, Borre M, Overgaard J (2003) Quantification of angiogenesis as a prognostic marker I human carcinomas: a critical evaluation of histopathological methods for estimation of vascular density. Eur J Cancer 39:881–890 [DOI] [PubMed] [Google Scholar]
- Ohta M, Konno H, Tanaka T et al (2003) The significance of circulating vascular endothelial growth factor (VEGF) protein in gastric cancer. Cancer Lett 192:215–225 [DOI] [PubMed] [Google Scholar]
- Parkin DM, Brey F, Ferlay J, Pisani P (2005) Global cancer statistics 2002. CA Cancer J Clin 55(2):74–108 [DOI] [PubMed] [Google Scholar]
- Seo Y, Baba H, Fukuda T, Takashima M, Sugimachi K (2000) High expression of vascular endothelial growth factor is associated with liver metastasis and a poor prognosis for patients with ductal pancreatic adenocarcinoma. Cancer 88:2239–2245 [DOI] [PubMed] [Google Scholar]
- Teo NB, Shoker BS, Jarvis C, Martin L, Sloane JP, Holcombe C (2003) Angiogenesis and invasive recurrence in ductal carcinoma in situ of the breast. Eur J Cancer 39:38–44 [DOI] [PubMed] [Google Scholar]
- Tombran-Tink J, Johnson LV (1989) Neuronal differentiation of retinoblastoma cells induced by medium conditioned by human RPE cells. Invest Ophthalmol Vis Sci 30:1700–1707 [PubMed] [Google Scholar]
- Uehara H, Miyamoto M, Kato K et al (2004) Expression of pigment epithelium-derived factor decrease liver metastasis and correlates with favorable prognosis for patients with ductal pancreatic adenocarcinoma. Cancer Res 64:3533–3537 [DOI] [PubMed] [Google Scholar]
- Vermeulen PB, Gasparini G, Fox SB, Colpaert C, Marson LP, Gion M, Belien JAM, de Waal RMW, Van Marck E, Magnani E, Weidner N, Harris AL, Dirix LY (2002) Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. Eur J Cancer 38(12):1564–1579 [DOI] [PubMed] [Google Scholar]
- Viacava P, Naccarato AG, Bocci G, Fanelli G, Aretini P, Lonobile A, Evangelista G, Montruccoli G, Bevilacqua G (2004) Angiogenesis and VEGF expression in pre-invasive lesions of the human breast. J Pathol 204:140–146 [DOI] [PubMed] [Google Scholar]
- Weigelt B, Wessels LF, Bosma AJ, Glas AM, Nuyten DS, He YD, Dai H, Peterse JL, van’t Veer LJ (2005) No common denominator for breast cancer lymph node metastasis. Br J Cancer 93:924–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir HK, Thun MJ, Hankey BF, Rises LA, Howe HL, Wingo PA, Jemal A, Ward E, Anderson RN, Edwards BK (2003) Annual report to the nation on the status of cancer, 1975–2000, featuring the uses of surveillance data for cancer prevention and control. J Natl Cancer Inst 95:1276–1299 [DOI] [PubMed] [Google Scholar]
- Zhang LJ, Chen JF, Ke Y, Mansel RE, Jiang WG (2006a) Expression of pigment epithelial derived factor is reduced in non-small cell lung cancer and is linked to clinical outcome. Int J Mol Med 17:937–944 [PubMed] [Google Scholar]
- Zhang SX, Wang JJ, Gao G, Shao C, Mott R, Ma JX (2006b) Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. FASEB J 20:323–325 [DOI] [PubMed] [Google Scholar]