Abstract
Purpose: Accelerated partial breast irradiation via interstitial balloon brachytherapy is a fast and effective treatment method for certain early stage breast cancers. The radiation can be delivered using a conventional high-dose rate (HDR) 192Ir gamma-emitting source or a novel electronic brachytherapy (eBx) source which uses lower energy x rays that do not penetrate as far within the patient. A previous study [A. Dickler, M. C. Kirk, N. Seif, K. Griem, K. Dowlatshahi, D. Francescatti, and R. A. Abrams, “A dosimetric comparison of MammoSite high-dose-rate brachytherapy and Xoft Axxent electronic brachytherapy,” Brachytherapy 6, 164–168 (2007)] showed that the target dose is similar for HDR 192Ir and eBx. This study compares these sources based on the dose received by healthy organs and tissues away from the treatment site.
Methods: A virtual patient with left breast cancer was represented by a whole-body, tissue-heterogeneous female voxel phantom. Monte Carlo methods were used to calculate the dose to healthy organs in a virtual patient undergoing balloon brachytherapy of the left breast with HDR 192Ir or eBx sources. The dose-volume histograms for a few organs which received large doses were also calculated. Additional simulations were performed with all tissues in the phantom defined as water to study the effect of tissue inhomogeneities.
Results: For both HDR 192Ir and eBx, the largest mean organ doses were received by the ribs, thymus gland, left lung, heart, and sternum which were close to the brachytherapy source in the left breast. eBx yielded mean healthy organ doses that were more than a factor of ∼1.4 smaller than for HDR 192Ir for all organs considered, except for the three closest ribs. Excluding these ribs, the average and median dose-reduction factors were ∼28 and ∼11, respectively. The volume distribution of doses in nearby soft tissue organs that were outside the PTV were also improved with eBx. However, the maximum dose to the closest rib with the eBx source was 5.4 times greater than that of the HDR 192Ir source. The ratio of tissue-to-water maximum rib dose for the eBx source was ∼5.
Conclusions: The results of this study indicate that eBx may offer lower toxicity to most healthy tissues, except nearby bone. TG-43 methods have a tendency to underestimate dose to bone, especially the ribs. Clinical studies evaluating the negative health effects caused by irradiating healthy organs are needed so that physicians can better understand when HDR 192Ir or eBx might best benefit a patient.
Keywords: electronic brachytherapy, partial breast irradiation, Monte Carlo, phantom
INTRODUCTION
Accelerated partial breast irradiation (APBI) with brachytherapy as the sole therapeutic treatment is an option for certain breast cancers. Variations in this treatment modality include the interstitial technique in which an array of catheters are inserted through the breast in the area around the excision cavity, and the intracavitary technique in which a balloonlike applicator is implanted within the excision cavity.1 The intracavitary technique was introduced with the approval of the MammoSite single lumen balloon (Hologic Inc., Bedford, MA) by the U.S. Food and Drug Administration in May 2002 for boost treatment of early stage breast cancer. This APBI method is available to patients with small, localized tumors and begins with breast conserving surgery such as lumpectomy.2 MammoSite therapy utilizes a spherical balloon (4–6 cm in diameter) attached to a catheter, which is placed directly inside the lumpectomy cavity during surgery and is then inflated with a solution of saline and contrast agent. A high-dose rate (HDR) 192Ir remote afterloader inserts a radioactive source into the balloon center to deliver a therapeutic dose to cover the 10 mm margin of the lumpectomy cavity. Currently the dose is delivered in ten fractions administered twice a day for five days—A very short period compared to several weeks of external beam therapy. This short treatment time is due in part to the fact that radiation is delivered from within the planning target volume (PTV), thereby sparing healthy tissues to a greater degree.3 After the last treatment fraction, the balloon is deflated and removed from the patient. Preliminary results from clinical trials indicate that breast conserving surgery in conjunction with balloon brachytherapy can lead to good cosmetic outcomes, comparable to the interstitial approach.4, 5, 6, 7, 8
At the present, balloon brachytherapy is conventionally delivered with a HDR 192Ir source, but it can also be delivered with an electronic brachytherapy (eBx) source involving a miniaturized x-ray tube.9, 10, 11, 12 The 192Ir radionuclide emits several gamma rays with energies up to 1.4 MeV that can easily penetrate through a large portion of the patient’s body. On the other hand, eBx devices such as the model S700 Xoft Axxent x-ray source (Xoft Inc., Fremont, CA) utilize 50 kVp x rays in the range of 20–50 keV, which can be selected by changing the applied electrical voltage. For initial clinical usage, the model S700 source has been operated at a tube voltage of 50 kVp and a tube current of 0.3 mA.11 Tube voltages other than 50 kVp are not clinically available at this time. Photons are produced by thermionic electrons from the cathode which are accelerated to the tungsten anode for conversion to bremsstrahlung x rays. The cylindrical model S700 source has a 5.4 mm outer diameter and includes a cooling sheath through which water flows to prevent the device from overheating.11 Unlike radionuclides which emit radiation upon disintegration, eBx sources can be turned off instantly to provide an important safety advantage for both the patient and medical treatment team. The features of this new device have garnered great interest and debate within the medical physics community regarding its potential use as an alternative to conventional HDR brachytherapy.13, 14, 15
There have been few studies comparing the patient dose received from these two different brachytherapy sources. To our knowledge, no study has examined heterogeneous patient models. A previous study by Dickler et al.16 in 2007 found that HDR 192Ir and eBx balloon breast brachytherapy provide similar PTV coverage, but with eBx yielding slightly larger high-dose volumes near the source. Dickler et al.16 also compared doses received by organs at risk (OARs) for treatments involving the HDR 192Ir and eBx sources and concluded that the latter was associated with smaller doses to the ipsilateral breast, ipsilateral lung, and heart. As several studies have reported evidence that link breast cancer patients undergoing radiotherapy to an increased risk for heart disease and lung cancer,17, 18, 19, 20 the findings by Dickler et al.16 suggest that eBx may be superior in terms of healthy tissue toxicity. The study by Dickler et al.16 demonstrated the need to take into account OARs in the evaluation and comparison of these two brachytherapy sources. However, it did not consider radiation dose to other organs located beyond the treatment volume where dose is dependent on the photon energy. Furthermore, this study was based on AAPM TG-43 dosimetry methods,21, 22, 23 which assume precalculated dose distributions in a spherical water phantom that were then superimposed on partial-body patient images. This method is fast and practical for the clinic, but is strictly valid only for a homogeneous water phantom of the same size and shape used for its derivation. As such, Dickler et al.16 could only estimate the dose to three healthy tissues during the brachytherapy procedure and did not account for differences in the radiation attenuation of bone, fat, muscle, and lung due to their elemental composition and densities. The dosimetric influence of tissue heterogeneities has been studied by many groups, and has been found to be potentially important at low energy, where the photoelectric effect is the dominant photon interaction.24
The current study was inspired by the hypothesis that additional information on how healthy organs beyond the treatment site are irradiated may be critical for evaluating the efficacy of eBx. The Monte Carlo method was employed to simulate detailed radiation transport within a three-dimensional (3D) patient represented by a whole-body, heterogeneous adult female computational phantom. Irradiation scenarios for HDR 192Ir and an eBx source were carefully defined in the Monte Carlo code so that the absorbed doses received by many organs throughout the body could be calculated. This paper describes the Monte Carlo simulations and evaluates organ doses attributed to each source type. These data can help physicians select the most appropriate source type for each patient.
MATERIALS AND METHODS
RPI-adult female phantom
In order to perform Monte Carlo radiation dose calculations for a realistic brachytherapy breast cancer treatment case, the anatomy of a female patient must first be defined in a Monte Carlo code environment. For this work, the female patient was represented by the triangular mesh-based, whole-body RPI-adult female computational phantom, shown in Fig. 1, which was recently developed at Rensselaer.25, 26 As this phantom is based on triangular meshes, which are often used in the computer graphics and animation industries, it has the key feature of being able to adjust its body height, bodyweight, posture, and organ size. A version of this phantom, with a height of 163 cm and weight of 60 kg, was used in this study to represent the reference woman specified by the International Commission on Radiological Protection (ICRP).27 In addition, the RPI-adult female phantom consists of over 140 organs and tissues, which were adjusted to agree within 0.5% with the ICRP reference woman organ mass data. As most Monte Carlo codes do not currently accept mesh geometries directly, the mesh-based RPI-adult female reference phantom was converted to voxel geometry with the aid of an in-house developed software so that it could be adopted into Monte Carlo dose calculations. The resulting phantom geometry was formatted as a 3D array of cubic voxels with side length 2.5 mm that each represented a specific tissue in the body or surrounding air. The 3D array consisted of approximately 24×106 voxels and had a height, width, and depth of 164, 61.5, and 37.5 cm, respectively. Memory limitations and computational efficiency restricted the use of higher resolution voxels to represent a whole-body patient. Nonetheless, the process of converting the mesh-based phantom into 2.5 mm voxels resulted in a less than 0.5% change in the volume of most organs. Larger volume differences were observed for small organs such as the eye lenses (∼3%). The skin was described by artificially reducing the density of a single layer of voxels overlaying the phantom’s body to 0.395 g∕cm3. This density value forced the mass of the phantom’s skin to the ICRP reference value of 2.3 kg, while at the same time compensated for the unrealistically large volume that arose because of the inability of the 2.5 mm voxels to describe such a thin structure. The 2.5 mm voxel size of the phantom used in this study is deemed satisfactory because the effects of this choice of resolution on the calculated doses are expected to be small compared to patient-to-patient variations.
Figure 1.
Half-skinned trimetric view of the RPI-adult female phantom showing the detailed internal bone, muscle, and organ structures.
Monte Carlo calculations
The voxelized phantom along with the appropriate tissue density and elemental composition data were imported into the Monte Carlo N-Particle eXtended (MCNPX) 2.5.0 code.28 Photon transport through the phantom was simulated for two different treatment scenarios: HDR 192Ir and 50 kVp eBx balloon brachytherapy using the model S700 source. Electrons were not tracked, but upon generation their energy was accounted for through local deposition. For both HDR 192Ir and eBx, a 4.4 cm diameter balloon filled with water (1 g∕cm3) was inserted into a lumpectomy cavity which was defined in the left breast of the RPI-adult female phantom (Fig. 2). Details on the modeling of these two treatments are described in Secs. 2B1, 2B2, 2B3, 2B4. The balloon was placed in the virtual patient such that there was a margin larger than 5 mm from the surface of the balloon to the skin, lungs, heart, and ribs.2 The MCNPX F6 tally was used to generate a track length estimate of energy deposition (or kerma) in selected organs for both treatment methods. Dose-volume histograms (DVHs) were generated for a few organs by tallying energy deposition in individual voxels. Additional simulations were run with all the tissues (but not the surrounding air) in the virtual patient set to water (1 g∕cm3) for the purpose of studying the differences between the TG-43 and the Monte Carlo tissue-heterogeneous dosimetry approaches. As MCNPX provides dose results per photon, conversion factors were applied to convert the data for appropriate comparison. These factors assumed that a total of 34 Gy was delivered in ten treatment fractions to a PTV located 1 cm beyond the balloon surface in water. In other words, 3.4 Gy is administered 3.2 cm from the source center during one treatment fraction. Enough particle histories (typically >108) were run so that the statistical uncertainties (k=1) of the Monte Carlo doses were below 1% for most organs, except for a few small organs located far from the treatment site.
Figure 2.
Isodose lines for 100%, 50%, 25%, and 5% of the 34 Gy prescription dose are shown on top of a grayscale image depicting the various organs within the phantom: (a) HDR 192Ir in tissue; (b) 50 kVp eBx in tissue; (c) HDR 192Ir in water; and (d) 50 kVp eBx in water. The brachytherapy balloon is shown in the left breast.
HDR 192Ir treatment modeling
The radionuclide 192Ir emits gamma rays and beta particles.29 The source is usually encapsulated in stainless steel or nitinol, which shields most of the beta particles. The remaining beta particles are stopped in the brachytherapy balloon. However, the capsule does not readily attenuate the gamma rays.30 The HDR 192Ir source was modeled as an isotropic gamma-ray point source placed at the balloon center. 192Ir emits many different gamma rays, but only the nine gamma rays with the largest abundances were considered. The maximum gamma-ray energy considered was 612.5 keV and the average in-vacuum photon energy was 372 keV.
In the clinic, the MammoSite balloon is filled with saline and an iodine contrast agent that assists with radiographic (x ray) or computed tomography localization. For simplicity, in this study the MammoSite balloon device was modeled as a sphere of water. Balloon contrast and geometric details such as the silicone balloon wall and the nylon catheter were not considered. The 192Ir spectrum is not significantly perturbed by the balloon contrast. A previous study found that there was a 1.6% reduction in the dose rate at the prescription distance relative to water for a 4 cm balloon with a contrast concentration of 10%.31 Variation in organ doses among patients is greater than 1.6%.
eBx treatment modeling
For the eBx treatment scenario, the tube voltage of the S700 source was set to the customary value of 50 kVp. As the internal geometric details of the device are proprietary, it was not feasible to simulate the bremsstrahlung production process directly via electrons. Instead, the eBx source was modeled as an isotropic point source located in the balloon center emitting the 50 kVp photon spectrum, which was experimentally measured and reported previously in the literature by Rivard et al.11 The average photon energy of the spectrum is 27 keV. In reality the eBx source is small, but finite in size and emits photons with a slight forward polar anisotropy.11 However, the isotropic point source approximation is adequate for the calculation of organ doses beyond the treatment site.
The balloon device used with the eBx source is similar to the MammoSite balloon, with the major difference being that no contrast agent is introduced into the liquid filling the balloon because this would significantly alter the energy profile. Instead, the wall of the eBx balloon is impregnated with barium sulfate contrast for which the average attenuation across the various balloon sizes is approximately 6%.32 For simplicity, the eBx balloon was modeled in this study as a sphere of water. Geometric details of the eBx balloon and attenuation of the source due to the barium sulfate wall contrast were ignored. It should be noted that Rivard et al.11 showed that the radial dose function g(r) in water for the 50 kVp eBx source changes by about 3% per 0.1 cm for 2<r<3 cm. Hence, modeling the eBx balloon as a sphere of water is a reasonable approximation because variations in balloon size and the effects of patient-specific geometry are larger than the spectral influences the barium sulfate wall contrast would cause.
Benchmarking in water
In order to establish a baseline for comparing the HDR 192Ir and eBx sources, the Monte Carlo source models were benchmarked against data in the literature by calculating their radial dose functions g(r) in liquid water (1 g∕cm3). Concentric spheres were defined in the Monte Carlo code to calculate the dose rate as a function of r, the radial distance from the source. Data were gathered for 0.1<r≤10 cm with a 0.05 cm step size. g(r) was calculated from these results by normalizing the data to the dose rate at r=1 cm and then multiplying a point-source geometry function. The validity of the assumptions used to model the brachytherapy sources in the Monte Carlo code were verified by comparing the calculated g(r) against data reported in the literature for the Nucletron HDR 192Ir V2 source33 (model 105.002, Nucletron Corp., Veenendal, the Netherlands) and the model S700 source.11 Enough particle histories (∼5×106) were run to ensure that statistical uncertainties (k=1) of the Monte Carlo g(r) were below 0.5%.
Study limitations
The organ doses received by a patient undergoing balloon brachytherapy of the breast are strongly dependent on the source-organ geometry. Hence, the absolute organ doses reported in this work correspond specifically to a patient with the anatomy of the RPI-adult female phantom who has received a total of 34 Gy to water at the PTV edge. The treatment site considered in this work was located approximately in the center of the left breast of the RPI-adult female. The minimum, maximum, median, and mean distances between the brachytherapy source and selected organs are listed in Table 1. It is noted that the actual organ doses received by a specific patient may vary from those calculated in this work because of differences in the location of the radiation source within the breast and because of differences in the relative positions, sizes, and shapes of the internal organs. This study considers a treatment involving a single dwell position in the center of the balloon. Hence, the organ dose distribution may differ from treatment plans which use multiple dwell positions to irradiate an irregularly shaped PTV region. In addition, in clinical situations sometimes air bubbles may form adjacent to the balloon device after implantation. This study did not account for these undesired air bubbles which, depending on their location, can result in a less homogeneous PTV dose. Furthermore, the results of this study involving a computational female phantom may not be generalizable to all women and for all breast sizes and shapes. Nevertheless, the organ dose distribution data presented in this paper for the two different brachytherapy sources are expected to be representative of a wide range of clinical conditions.
Table 1.
Summary of the organ masses and volumes for the RPI-adult female phantom as well as the minimum, mean, median, and maximum distances of these organs from the brachytherapy source located in the center of the left breast. The mean organ doses of the heterogeneous tissue phantom were calculated using the Monte Carlo code for 34 Gy treatments involving the HDR 192Ir and 50 kVp eBx sources. Organ dose data are also listed for a phantom with all tissues defined to be water. The type A uncertainties (k=1) for these results are given in percent.
| Organ name | Mass (g) | Volume (cm3) | Distance (cm) Organ to balloon center | Average organ dose (Gy) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tissue Phantom | Water Phantom | |||||||||||||
| Min. | Mean | Median | Max. | HDR 192Ir | Type A (%) | 50 kVp eBx | Type A (%) | HDR 192Ir | Type A (%) | 50 kVp eBx | Type A (%) | |||
| Adrenal gland, left | 6.49×100 | 6.36×100 | 20.4 | 21.5 | 21.5 | 22.4 | 7.00×10−1 | 2.0×10−1 | 1.17×10−1 | 3.8×10−1 | 3.87×10−1 | 2.7×10−1 | 1.59×10−2 | 1.0×100 |
| Adrenal gland, right | 6.49×100 | 6.36×100 | 24.4 | 25.8 | 25.8 | 26.9 | 2.40×10−1 | 3.5×10−1 | 7.77×10−3 | 1.5×100 | 2.14×10−1 | 3.7×10−1 | 4.58×10−3 | 2.0×100 |
| Brain | 1.30×103 | 1.25×103 | 28.6 | 34.7 | 34.7 | 40.7 | 8.24×10−2 | 1.1×10−1 | 1.36×10−3 | 5.8×10−1 | 8.57×10−2 | 1.1×10−1 | 3.46×10−3 | 3.6×10−1 |
| Esophagus | 3.50×101 | 3.40×101 | 14.3 | 17.9 | 16.9 | 23.9 | 7.59×10−1 | 8.0×10−2 | 1.17×10−1 | 1.6×10−1 | 7.16×10−1 | 9.0×10−2 | 6.73×10−2 | 2.2×10−1 |
| Eye lens, left | 2.06×10−1 | 1.88×10−1 | 29.9 | 30.2 | 30.3 | 30.6 | 1.94×10−1 | 1.6×100 | 1.15×10−2 | 3.9×100 | 2.03×10−1 | 1.6×100 | 2.04×10−2 | 3.1×100 |
| Eye lens, right | 2.06×10−1 | 1.88×10−1 | 31.2 | 31.6 | 31.6 | 32.0 | 1.29×10−1 | 2.0×100 | 1.65×10−3 | 1.0×101 | 1.47×10−1 | 2.0×100 | 9.11×10−3 | 4.8×100 |
| Gall bladder, wall | 8.01×100 | 7.78×100 | 16.7 | 19.3 | 19.4 | 21.8 | 5.89×10−1 | 1.3×10−1 | 5.26×10−2 | 3.3×10−1 | 5.72×10−1 | 1.3×10−1 | 3.43×10−2 | 4.1×10−1 |
| Heart, contents | 3.70×102 | 3.49×102 | 7.4 | 11.5 | 11.6 | 14.9 | 2.43×100 | 3.0×10−2 | 8.48×10−1 | 4.0×10−2 | 2.37×100 | 3.0×10−2 | 5.56×10−1 | 5.0×10−2 |
| Heart, wall | 2.50×102 | 2.38×102 | 6.7 | 11.9 | 12.3 | 15.6 | 2.44×100 | 3.0×10−2 | 9.76×10−1 | 3.0×10−2 | 2.37×100 | 3.0×10−2 | 6.13×10−1 | 4.0×10−2 |
| Kidney, left | 1.50×102 | 1.43×102 | 18.4 | 22.2 | 22.2 | 25.8 | 5.46×10−1 | 7.0×10−2 | 7.58×10−2 | 1.4×10−1 | 3.62×10−1 | 8.6×10−2 | 1.46×10−2 | 32×10−1 |
| Kidney, right | 1.26×102 | 1.20×102 | 23.2 | 26.8 | 26.8 | 29.9 | 2.05×10−1 | 1.2×10−1 | 5.89×10−3 | 5.3×10−1 | 1.92×10−1 | 1.2×10−1 | 3.75×10−3 | 6.6×10−1 |
| Large intestine | 3.60×102 | 3.46×102 | 20.3 | 31.0 | 30.5 | 44.1 | 1.38×10−1 | 8.0×10−2 | 5.44×10−3 | 3.0×10−1 | 1.24×10−1 | 8.6×10−2 | 2.59×10−3 | 4.2×10−1 |
| Liver | 1.40×103 | 1.33×103 | 12.2 | 23.1 | 23.7 | 31.7 | 3.73×10−1 | 5.0×10−2 | 3.51×10−2 | 1.0×10−1 | 3.81×10−1 | 5.0×10−2 | 2.52×10−2 | 1.2×10−1 |
| Lung, left | 4.22×102 | 1.69×103 | 3.0 | 12.3 | 12.7 | 18.7 | 3.07×100 | 2.0×10−2 | 1.90×100 | 2.0×10−2 | 2.74×100 | 2.0×10−2 | 1.02×100 | 2.0×10−2 |
| Lung, right | 5.28×102 | 2.11×103 | 10.8 | 19.4 | 19.5 | 26.2 | 6.40×10−1 | 3.0×10−2 | 8.50×10−2 | 7.0×10−2 | 5.93×10−1 | 3.0×10−2 | 4.48×10−2 | 8.0×10−2 |
| Ovary, left | 5.49×100 | 5.28×100 | 28.8 | 30.1 | 30.1 | 31.7 | 1.38×10−1 | 5.7×10−1 | 3.19×10−3 | 2.8×100 | 1.13×10−1 | 6.3×10−1 | 1.37×10−3 | 4.1×100 |
| Ovary, right | 5.49×100 | 5.28×100 | 30.4 | 31.7 | 31.6 | 33.3 | 1.05×10−1 | 5.8×10−1 | 1.38×10−3 | 3.9×100 | 9.48×10−2 | 6.3×10−1 | 8.41×10−4 | 5.0×100 |
| Pancreas | 1.20×102 | 1.14×102 | 19.4 | 22.4 | 22.1 | 26.6 | 4.13×10−1 | 1.0×10−1 | 3.04×10−2 | 2.7×10−1 | 3.53×10−1 | 1.1×10−1 | 1.33×10−2 | 4.1×10−1 |
| Pituitary gland | 5.95×10−1 | 5.78×10−1 | 31.4 | 31.9 | 31.9 | 32.3 | 1.31×10−1 | 1.2×100 | 2.31×10−3 | 5.7×100 | 1.34×10−1 | 1.2×100 | 5.80×10−3 | 4.0×100 |
| Rib2, left | 1.80×101 | 1.37×101 | 6.5 | 14.9 | 16.0 | 20.6 | 1.82×100 | 7.9×10−2 | 1.33×100 | 1.1×10−1 | 1.74×100 | 8.7×10−2 | 4.85×10−1 | 1.0×10−1 |
| Rib3, left | 1.77×101 | 1.34×101 | 3.4 | 13.3 | 13.8 | 20.0 | 3.79×100 | 5.0×10−2 | 5.91×100 | 5.0×10−2 | 3.73×100 | 5.7×10−2 | 2.11×100 | 4.6×10−2 |
| Rib4, left | 1.79×101 | 1.39×101 | 2.9 | 12.9 | 13.5 | 19.6 | 4.69×100 | 4.3×10−2 | 8.46×100 | 4.4×10−2 | 4.60×100 | 4.6×10−2 | 3.00×100 | 3.8×10−2 |
| Rib5, left | 2.17×101 | 1.66×101 | 4.5 | 13.5 | 13.8 | 19.3 | 2.85×100 | 4.6×10−2 | 3.44×100 | 5.7×10−2 | 2.71×100 | 4.6×10−2 | 1.11×100 | 5.3×10−2 |
| Salivary gland, left | 3.50×101 | 3.33×101 | 23.8 | 26.2 | 26.2 | 28.6 | 3.59×10−1 | 1.6×10−1 | 6.21×10−2 | 2.7×10−1 | 3.50×10−1 | 1.7×10−1 | 6.12×10−2 | 2.8×10−1 |
| Salivary gland, right | 3.50×101 | 3.33×101 | 25.9 | 28.3 | 28.4 | 30.8 | 1.79×10−1 | 2.3×10−1 | 7.73×10−3 | 7.5×10−1 | 1.80×10−1 | 2.4×10−1 | 1.26×10−2 | 6.2×10−1 |
| Skin | 2.31×103 | 5.84×103 | 2.9 | 53.5 | 44.0 | 127.6 | 3.24×10−1 | 1.0×10−2 | 1.34×10−1 | 1.0×10−2 | 3.21×10−1 | 1.0×10−2 | 1.25×10−1 | 1.0×10−2 |
| Small intestine | 8.80×102 | 8.46×102 | 21.5 | 31.1 | 31.1 | 40.1 | 1.21×10−1 | 1.0×10−1 | 3.70×10−3 | 3.7×10−1 | 1.10×10−1 | 1.1×10−1 | 1.83×10−3 | 5.3×10−1 |
| Spinal cord | 2.80×101 | 2.69×101 | 17.8 | 24.8 | 22.5 | 38.2 | 3.78×10−1 | 1.0×10−1 | 2.30×10−2 | 2.9×10−1 | 3.39×10−1 | 1.1×10−1 | 1.74×10−2 | 3.6×10−1 |
| Spleen | 1.30×102 | 1.23×102 | 16.2 | 20.2 | 20.1 | 25.0 | 6.29×10−1 | 9.0×10−2 | 1.04×10−1 | 1.6×10−1 | 4.61×10−1 | 1.1×10−1 | 2.58×10−2 | 3.1×10−1 |
| Sternum | 4.91×101 | 4.49×101 | 6.5 | 10.2 | 10.1 | 15.0 | 2.81×100 | 5.8×10−2 | 9.69×10−1 | 6.5×10−2 | 2.98×100 | 5.8×10−2 | 8.94×10−1 | 6.8×10−2 |
| Stomach, wall | 1.40×102 | 1.35×102 | 13.5 | 18.2 | 17.7 | 27.2 | 8.32×10−1 | 5.0×10−2 | 1.56×10−1 | 8.0×10−2 | 7.33×10−1 | 5.0×10−2 | 6.65×10−2 | 1.2×10−1 |
| Thymus | 2.00×101 | 1.94×101 | 5.7 | 8.7 | 8.8 | 11.5 | 4.14×100 | 6.0×10−2 | 1.72×100 | 7.0×10−2 | 4.29×100 | 6.0×10−2 | 1.60×100 | 7.0×10−2 |
| Thyroid | 1.71×101 | 1.63×101 | 17.2 | 19.1 | 19.0 | 22.0 | 4.24×10−1 | 2.0×10−1 | 3.50×10−2 | 5.3×10−1 | 4.52×10−1 | 2.0×10−1 | 3.06×10−2 | 5.4×10−1 |
| Tongue | 6.02×101 | 5.73×101 | 22.0 | 24.2 | 24.1 | 26.8 | 3.48×10−1 | 1.4×10−1 | 4.85×10−2 | 2.8×10−1 | 3.41×10−1 | 1.5×10−1 | 4.44×10−2 | 2.8×10−1 |
| Trachea | 8.00×100 | 7.77×100 | 12.2 | 15.2 | 14.7 | 20.1 | 1.13×100 | 1.2×10−1 | 2.16×10−1 | 2.1×10−1 | 1.07×100 | 1.2×10−1 | 1.32×10−1 | 2.7×10−1 |
| Urinary bladder, wall | 4.00×101 | 3.85×101 | 38.9 | 42.3 | 42.4 | 45.6 | 2.60×10−2 | 3.3×10−1 | 1.02×10−4 | 4.2×100 | 2.24×10−2 | 3.6×10−1 | 5.57×10−5 | 5.7×100 |
| Uterus | 7.99×101 | 7.61×101 | 28.2 | 31.5 | 31.4 | 35.0 | 1.12×10−1 | 2.6×10−1 | 1.53×10−3 | 1.5×100 | 9.82×10−2 | 2.8×10−1 | 9.80×10−4 | 2.0×100 |
RESULTS
Benchmarking in water
Figure 3 depicts g(r) for the HDR 192Ir and eBx sources with comparison to published results. For the HDR 192Ir source, the percent difference between g(r) and results by Daskalov et al.33 at r=1.5, 2, 3, 5, 7, and 10 cm were 0.08%, 0.03%, 0.3%, 1.5%, 3.2%, and 8.0%, respectively. When correcting for differences in phantom size and subsequent scattering conditions, these differences were <1%.34 For the eBx source, the percent difference between g(r) and measured results by Rivard et al.11 at r=1.5, 2, 3, 5, and 7 cm were 1.2%, 3.4%, 5.2%, 7.0%, and 3.5%, respectively. The observed differences were within the dosimetric uncertainties described by Rivard et al.,11 who found the average standard deviation of repeated measurements of g(r) at r=3, 5, and 7 cm to be 4.5%, 5.3%, and 6.3%, respectively. Experimental eBx g(r>7 cm) data were unavailable. Therefore, the data in Fig. 3 indicate that our isotropic point-source models for the HDR 192Ir and eBx sources were appropriate. As g(r) was calculated for both sources using a pointwise geometry function, Fig. 3 also demonstrates that at small radial distances (r≤7 cm), the dose rate due to the HDR 192Ir source falls off with r2 because its g(r) is approximately constant. On the other hand, the g(r) for the eBx source falls off roughly as r3. These results are consistent with previous data.14
Figure 3.
The radial dose functions for the HDR 192Ir and 50 kVp eBx sources used in this study. The data are in good agreement with experimental and Monte Carlo calculated data found in the literature (Refs. 11, 33).
Mean organ dose
Table 1 summarizes the doses to several organs in the RPI-adult female phantom which are adjacent and away from the target for the HDR 192Ir and eBx sources. Data are listed for the heterogeneous tissue phantom and the homogeneous water phantom for a treatment in which 34 Gy was delivered to water at the PTV outer edge (r=3.2 cm). Isodose lines for the two sources in the tissue and water phantoms are shown in Fig. 2 for an axial slice through the center of the balloon. The PTV is represented in Fig. 3 by the region of tissue between radial distances of 2.2 and 3.2 cm. The largest doses observed were for organs such as the ribs, thymus gland, left lung, heart, and sternum which were among the closest organs to the brachytherapy source located at the center of the left breast. The distribution of organ doses shows a complex pattern depending on the source type (HDR 192Ir vs eBx), phantom heterogeneity (tissue vs water), organ location, organ size, and organ shape.
HDR 192Ir vs eBx
The ratios of HDR 192Ir to eBx mean organ dose were calculated for the tissue and water phantoms and were compared in Fig. 4. These ratios were greater for the homogeneous water phantom for most, but not all, of the organs considered.
Figure 4.
Comparison of the organ doses received by the RPI-adult female phantom during a balloon brachytherapy of the left breast involving the HDR 192Ir and 50 kVp eBx sources. The results are shown as a ratio of the HDR 192Ir doses relative to the eBx doses for both the tissue and water phantoms.
For the tissue-heterogeneous phantom, the mean organ doses for the eBx source were smaller than those of the HDR 192Ir source for all organs considered except the ipsilateral third, fourth, and fifth ribs (i.e., rib3, rib4, and rib5) because the low-energy x rays were less penetrating. When these three ribs were excluded, the dose-reduction factors for the remaining organs were all greater than a factor of ∼1.4. The average dose-reduction factor for the remaining organs was ∼28 with a median of ∼11. This ratio was greater than 50 for certain organs (e.g., brain) that were far from the source. Of those organs which received smaller mean doses with eBx, the smallest mean dose-reductions were observed for ipsilateral rib2 and the left lung and were 1.4 and 1.6, respectively. The dose to the third through fifth ipsilateral ribs was greater for the eBx source as compared to HDR 192Ir source because of the larger mass attenuation coefficient of bone at low energy attributed to increased photoelectric absorption. The eBx dose enhancement factors for these three ribs were 1.6, 1.8, and 1.2, respectively. The mean dose to the ipsilateral rib2 was smaller for eBx than HDR 192Ir, presumably because of an averaging effect that arises because this rib was further from the source than the others considered.
For the water phantom, the mean organ doses for the eBx source were smaller than those of the HDR 192Ir source for all organs considered. As expected, dose enhancement to the closest ribs was not observed because this phantom was comprised of only of water. The mean dose-reduction observed over all organs considered with the eBx source was a factor of ∼33 with a median reduction of ∼15. The dose-reduction factors for the third, fourth, and fifth ipsilateral ribs were the smallest of all organs considered and were 1.8, 1.5, and 2.4, respectively.
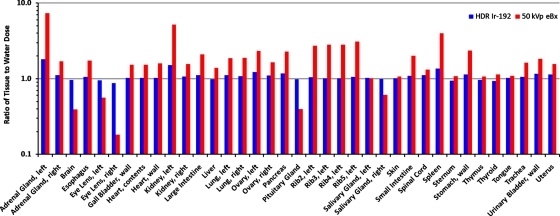
Tissue vs water phantom
The organ dose ratios in the tissue vs water phantom were calculated for the HDR 192Ir and eBx sources, and were compared in Fig. 5. For most organs, the mean dose in tissue was higher than in water. However, the mean dose in tissue was less than or approximately equal to that in water for some organs. For example, the brain, which was shielded by the skull, had a decrease in mean organ dose compared to the water phantom in which no high Z shielding occurred. For the HDR 192Ir source, the mean, median, and standard deviation of the tissue-to-water dose ratios were 1.09, 1.05, and 0.2, respectively. For the eBx source, these values were 1.9, 1.6, and 1.4. As expected, only small differences in the mean organ dose were observed for the HDR 192Ir source in tissue and water and much larger differences between tissue and water dose were observed for the lower energy eBx source. The average tissue-to-water mean organ dose ratio for the four ipsilateral ribs was 1.03 and 2.9 for the HDR 192Ir and eBx sources, respectively.
Figure 5.
Comparison of the organ doses received by the RPI-adult female phantom with tissue heterogeneities and with all tissues defined as water. The results are shown as a ratio of the tissue phantom doses relative to the water phantom doses for both the HDR 192Ir and 50 kVp eBx sources.
Dose-volume histograms
DVHs were generated for a few of the organs in the tissue and water phantoms which received high doses: Left rib4, thymus, left lung, and heart. These results, shown in Fig. 6 and summarized in Table 2, illustrate that the volume distribution of doses are greatly improved with eBx for the nearby soft tissue organs, but not for nearby bone. The maximal tissue dose in rib4 was ∼41 Gy for HDR 192Ir and ∼222 Gy for eBx. The maximum eBx doses for the sternum and the second through fifth ipsilateral ribs were higher than those for HDR 192Ir by factors of 2.4, 2.4, 3.7, 5.4, and 4.1, respectively. The maximum dose to the left lung was also higher for the eBx source by a factor of 1.08. Small portions of left rib4 and the left lung were within the 3.2 cm prescription distance, but outside the 5 mm balloon surface margin. The maximum eBx dose for the thymus, heart, and right lung was smaller than when using HDR 192Ir by factors of 1.4, 1.2, and 3.7, respectively.
Figure 6.
Dose-volume histograms for the tissue and water phantoms. (a) Left lung (ipsilateral); (b) heart (wall and contents); (c) rib4 (ipsilateral); and (d) thymus. The dose-volume histogram of rib4 for the HDR 192Ir source in the water phantom is not shown because it is indistinguishable from the tissue phantom histogram shown in plot (c).
Table 2.
Percentage of organ volumes receiving various doses for the HDR 192Ir and eBx sources.
| Dose (Gy) | Left lung (ispilateral) | Dose (Gy) | Heart (wall and contents) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Tissue | Water | Tissue | Water | ||||||
| HDR 192Ir | 50 kVp eBx | HDR 192Ir | 50 kVp eBx | HDR 192Ir | 50 kVp eBx | HDR 192Ir | 50 kVp eBx | ||
| 3.4 | 24.3%±4.2% | 12.5%±1.2% | 21.2%±2.4% | 7%±0.3% | 0.51 | 100%±0% | 55%±1.6% | 100%±0% | 38.7%±1% |
| 5.1 | 12.7%±1.9% | 7.5%±0.6% | 12%±1.3% | 4.4%±0.2% | 1.02 | 99.2%±0.5% | 28.5%±0.9% | 99.8%±0.3% | 14.8%±0.4% |
| 6.8 | 7.8%±1.1% | 5%±0.4% | 7.6%±0.8% | 2.9%±0.2% | 1.7 | 67.3%±2.1% | 14%±0.5% | 66.9%±2.5% | 4.8%±0.2% |
| 8.5 | 5.1%±0.7% | 3.5%±0.3% | 5.1%±0.6% | 2%±0.1% | 3.4 | 18%±0.9% | 2.7%±0.1% | 15.8%±1% | 0%±0% |
| 10.2 | 3.5%±0.5% | 2.5%±0.2% | 3.5%±0.4% | 1.5%±0.1% | 5.1 | 4.1%±0.3% | 0.33%±0.03% | 3.1%±0.3% | 0%±0% |
| Dose (Gy) | Rib4 (ipsilateral) | Dose (Gy) | Thymus | ||||||
| Tissue | Water | Tissue | Water | ||||||
| HDR 192Ir | 50 kVp eBx | HDR 192Ir | 50 kVp eBx | HDR 192Ir | 50 kVp eBx | HDR 192Ir | 50 kVp eBx | ||
| 5.1 | 22.4%±0.3% | 20%±0.1% | 22.7%±0% | 15.2%±0.1% | 1.02 | 100%±0% | 60.8%±0.8% | 100%±0% | 60.5%±0.7% |
| 6.8 | 18.5%±0.1% | 18%±0% | 18.7%±0.1% | 12.8%±0% | 1.7 | 100%±0% | 32.7%±0.5% | 100%±0% | 32%±0.6% |
| 10.2 | 13.5%±0.1% | 15.5%±0% | 13.5%±0% | 9.7%±0.2% | 3.4 | 56.9%±1.2% | 12.1%±0.2% | 61.9%±0.6% | 8.6%±0.2% |
| 13.6 | 10.2%±0.1% | 13.6%±0.1% | 10.2%±0% | 7.5%±0% | 5.1 | 23.8%±0.9% | 3.7%±0.1% | 25.5%±0.4% | 1.4%±0.1% |
| 17.0 | 7.6%±0.1% | 11.9%±0% | 7.9%±0.1% | 5.6%±0.1% | 6.8 | 10%±0.6% | 0.3%±0.1% | 10.4%±0.1% | 0%±0% |
The HDR 192Ir DVHs for the tissue and water phantoms were very similar, illustrating that this source is not greatly affected by tissue heterogeneities due to the high photon energy. For eBx, DVHs in water were less than for tissue, suggesting that TG-43 methods underestimate healthy organ dose for this source. The maximum eBx dose to the thymus, heart, left lung, and right lung were underestimated by factors of 1.3, 1.8, 1.06, and 1.2, respectively. For the second through fifth ribs, these factors were 4.6, 3.7, 4.9, and 5.3, respectively. Similarly, a recent study involving images of eight patients undergoing intracavitary breast brachytherapy with a 50 kVp eBx source found that the TG-43 approach underestimated the maximum dose to nearby rib bone by a factor of ∼5.35
In a retrospective study involving imaging data from 15 patients, Dickler et al.16 found that the proportion of ipsilateral lung volume receiving 30% of the prescription dose or 10.2 Gy (%V30) was 3.7%, versus 1.1% for HDR 192Ir and 50 kVp eBx sources, respectively, with variations across the 15 patients of ±2.3% and ±0.8%, respectively. Dickler et al.16 found that the portion of the heart volume receiving 5% of the prescription dose (1.7 Gy or %V5) was 59.2%±14.9% vs 9.4%±9.8%. In this study, for the tissue phantom, the %V30 of the ispilateral lung was determined to be 3.5%±0.5% vs 2.5%±0.2% for HDR 192Ir and 50 kVp eBx, respectively. For the water phantom these values were 3.5%±0.4% and 1.5%±0.1%, respectively. Similarly, this study found that the heart %V5 for the tissue phantom was 67.3%±2.1% for HDR 192Ir vs 14.0%±0.5% for 50 kVp eBx. The heart %V5 in the water phantom was 66.9%±2.5% and 4.8%±0.2%, respectively. These results are consistent with clinical values reported by Dickler et al.16
DISCUSSION
The clinical effect of a reduced dose to nearby healthy soft tissues and an enhanced dose to nearby ribs due to eBx is not certain. As some studies have suggested, there may be a link between low doses to the heart and lungs during radiotherapy to heart disease and lung cancer, dose-reductions to these organs afforded by eBx could prove clinically relevant.17, 18, 19, 20 Regardless, the ALARA or “as low as reasonably achievable” precautionary principle of radiation protection suggests that eBx optimizes dose to nearby healthy soft tissue, even though this principle does not technically apply to patients whose irradiation is medically justified.36 Issues associated with irradiation of healthy organs by scattered radiation outside the treatment volume for external beam and image-guided procedures (e.g., cone-beam CT) have become a topic of discussion.37, 38 Although three active AAPM task groups (TG-75, TG-158, and TG-180) concern with secondary dose to organs away from the target volume, brachytherapy has been excluded from these on-going efforts thus far in part due to a lack of organ dose data.
On the other hand, Dickler et al.16 have shown that eBx provides a less homogeneous PTV dose and that the mean PTV volume receiving >200% of the target dose slightly exceeded the levels of some patients with fat necrosis.39 Furthermore, this work has shown that eBx provides a greater dose to the nearby bone, especially the ribs. HDR 192Ir brachytherapy may be better for some patients if the treatment site is sufficiently close to a rib and rib dose is a concern. It seems that the optimal source choice for balloon brachytherapy may depend on patient geometry and treatment location, perhaps with eBx being more advantageous as the distance between the source and ribs increases. This work has demonstrated that eBx is ideal for healthy organs outside the prescription distance where the dose rate is lower than for HDR 192Ir. Otherwise, small portions of these healthy organs, such as the left lung in this study, will receive higher dose with eBx than they would with HDR 192Ir.
This work has also shown that TG-43 treatment planning methods, which do not account for tissue heterogeneities, systematically underestimate the dose to most nearby healthy organs. It should specifically be noted that eBx provides a higher dose to nearby ribs which were among the organs for which TG-43 methods were found to provide the least accurate dose estimates. Therefore, new treatment planning methods which can account for tissue heterogeneities are important for routine use of eBx—Such advances are underway.40
CONCLUSION
This paper reports the first detailed assessment of doses to multiple organs from both HDR 192Ir and eBx balloon breast brachytherapy. Previous work by others on this topic considered only a few organs and utilized a partial-body water phantom.16 This paper is important because it utilizes a whole-body, tissue-heterogeneous phantom and a well-benchmarked Monte Carlo code to calculate organ doses for a virtual patient undergoing brachytherapy with either source. The organ dose data show that eBx leads to a reduction in the mean dose to healthy organs by a factor greater than 1.4, except for nearby ribs. The mean dose enhancement factor for the closest rib was 1.8. The maximum dose to the closest rib with the eBx source was 5.4 times greater than that of the HDR 192Ir source. A previous study has shown that target volume dose was similar for HDR 192Ir and eBx brachytherapy sources. The significantly lower doses to many nearby healthy organs delivered by eBx, as reported here, suggest that eBx may be superior in terms of normal tissue sparing for some patients. Future work should focus on investigating how the organ doses change when the right breast is treated, and the sensitivity of the organ doses to the treatment location and phantom size∕shape. The dose to nearby ribs as a function of source-to-rib distance should be given careful study. These factors may play an important role in determining when using HDR 192Ir or eBx brachytherapy is most beneficial. Further clinical trials are necessary to improve the understanding of the benefits and risks associated with eBx.
ACKNOWLEDGMENTS
The development of the RPI-Adult Female phantom was funded in part by a grant from the National Cancer Institute (Grant No. R01CA116743). Mr. Mille gratefully acknowledges support from the Burton J. Moyer Memorial Fellowship (2009-2010) offered jointly by the Health Physics Society (HPS) and the Northern California Chapter of the HPS.
This work was presented in part on 26 July 2009 at the John R. Cameron Young Investigator Symposium of the 51st annual meeting of the American Association of Physicists in Medicine.
References
- Belkacémi Y., Hannoun-Lévi J. M., and Lartigau E., in New Technologies in Radiation Oncology, edited by Schlegel W., Bortfeld T., and Grosu A. -L. (Springer, New York, 2006), pp. 397–407. 10.1007/3-540-29999-8_31 [DOI] [Google Scholar]
- Smith B. D., Arthur D. W., Buchholz T. A., Haffty B. G., Hahn C. A., Hardenbergh P. H., Julian T. B., Marks L. B., Todor D. A., Vicini F. A., Whelan T. J., White J., Wo J. Y., and Harris J. R., “Accelerated partial breast irradiation consensus statement from the American society for radiation oncology (ASTRO),” Int. J. Radiat. Oncol., Biol., Phys. 74, 987–1001 (2009). [DOI] [PubMed] [Google Scholar]
- Stewart A. J., O’Farrell D. A., Cormack R. A., Hansen J. L., Khan A. J., Mutyala S., and Devlin P. M., “Dose volume histogram analysis of normal structures associated with accelerated partial breast irradiation delivered by high dose rate brachytherapy and comparison with whole breast external beam radiotherapy fields,” Radiat. Oncol. 3(39) (2008). 10.1186/1748-717X-3-39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkison J. B., Kuske R. R., Quiet C. A., Beriwal S., and Patel R. R., “Five-year results of accelerated partial breast irradiation for ductal carcinoma in situ treated by interstitial multicatheter high-dose-rate brachytherapy or MammoSite,” Brachytherapy 8, 107–108 (2009). 10.1016/j.brachy.2009.03.013 [DOI] [Google Scholar]
- Adkison J. B., Patel R. R., Beriwal S., Quiet C. A., and Kuske R. R., “Five-year results for accelerated partial breast irradiation using MammoSite balloon brachytherapy: The first 100 patients,” Brachytherapy 7, 104 (2008). 10.1016/j.brachy.2008.02.371 [DOI] [Google Scholar]
- Benitez P. R., Streeter O., and Vicini F., “Preliminary results and evaluation of MammoSite balloon brachytherapy for partial breast irradiation for pure ductal carcinoma in situ: A phase II clinical study,” Am. J. Surg. 192, 427–433 (2006). 10.1016/j.amjsurg.2006.06.013 [DOI] [PubMed] [Google Scholar]
- Vicini F. A., Beitsch P. D., Quiet C. A., Keleher A., Garcia D., Snider H. C., Gittleman M. A., Zannis V. J., Kuerer H., Whitacre E. B., Whitworth P. W., Fine R. E., Haffty B. G., and Arrambide L. S., “First analysis of patient demographics, technical reproducibility, cosmesis, and early toxicity: Results of the American Society of Breast Surgeons MammoSite breast brachytherapy trial,” Cancer 104, 1138–1148 (2005). 10.1002/cncr.21289 [DOI] [PubMed] [Google Scholar]
- Shah N. M., Tenenholz T., Arthur D., DiPetrillo T., Bornstein B., Cardarelli G., Zheng Z., Rivard M. J., Kaufman S., and Wazer D. E., “MammoSite and interstitial brachytherapy for accelerated partial breast irradiation: Factors affecting toxicity and cosmesis,” Cancer 101, 727–734 (2004). 10.1002/cncr.20424 [DOI] [PubMed] [Google Scholar]
- Chiu-Tsao S., Rusch T. W., Axelrod S., Tsao H., and Harrison L., “Dose response of GafChromic XR-T film to a new electronic brachytherapy source,” Radiother. Oncol. 71(S2), S84 (2004). [Google Scholar]
- Rusch T. W., Davis S. D., DeWerd L. A., Burnside R. R., Axelrod S., and Rivard M. J., “Characterization of a new miniature x-ray source for electronic brachytherapy,” Med. Phys. 31, 1807 (2004). [Google Scholar]
- Rivard M. J., Davis S. D., DeWerd L. A., Rusch T. W., and Axelrod S., “Calculated and measured brachytherapy dosimetry parameters in water for the Xoft Axxent x-ray source: An electronic brachytherapy source,” Med. Phys. 33, 4020–4032 (2006). 10.1118/1.2357021 [DOI] [PubMed] [Google Scholar]
- Hiatt J., Cardarelli G., Hepel J., Wazer D., and Sternick E., “A commissioning procedure for breast intracavitary electronic brachytherapy systems,” J. Appl. Clin. Med. Phys. 9, 58–68 (2008). 10.1120/jacmp.v9i3.2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt R. W., Thomadsen B. R., and Orton C. G., “Miniature x-ray tubes will ultimately displace Ir-192 as the radiation sources of choice for high dose rate brachytherapy,” Med. Phys. 35, 815–817 (2008). 10.1118/1.2836415 [DOI] [PubMed] [Google Scholar]
- Holt R. W. and Rivard M. J., “Electronic brachytherapy: Comparisons with external-beam and high-dose-rate 192Ir brachytherapy,” J. Am. Coll. Radiol. 5, 221–223 (2008). 10.1016/j.jacr.2007.12.001 [DOI] [PubMed] [Google Scholar]
- Sternick E. S., Todor D. A., and Orton C. G., “Intensity modulated electronic brachytherapy will soon become the brachytherapy treatment of choice for irregularly shaped tumor cavities or those closely bounded by critical structures,” Med. Phys. 36, 681–683 (2009). 10.1118/1.3075768 [DOI] [PubMed] [Google Scholar]
- Dickler A., Kirk M. C., Seif N., Griem K., Dowlatshahi K., Francescatti D., and Abrams R. A., “A dosimetric comparison of MammoSite high-dose-rate brachytherapy and Xoft Axxent electronic brachytherapy,” Brachytherapy 6, 164–168 (2007). 10.1016/j.brachy.2007.01.005 [DOI] [PubMed] [Google Scholar]
- Darby S. C., McGale P., Talyor C. W., and Peto R., “Long-term mortality from heart disease and lung cancer after radiotherapy for early breast cancer: Prospective cohort study of about 300000 women in US SEER cancer registries,” Lancet Oncol. 6, 557–565 (2005). 10.1016/S1470-2045(05)70251-5 [DOI] [PubMed] [Google Scholar]
- Schultz-Hector S. and Trott K. R., “Radiation-induced cardiovascular diseases: Is the epidemiologic evidence compatible with the radiobiologic data?,” Int. J. Radiat. Oncol., Biol., Phys. 67, 10–18 (2007). [DOI] [PubMed] [Google Scholar]
- Taylor C. W., Nisbet A., McGale P., and Darby S. C., “Cardiac exposures in breast cancer radiotherapy: 1950s-1990s,” Int. J. Radiat. Oncol., Biol., Phys. 69, 1484–1495 (2007). [DOI] [PubMed] [Google Scholar]
- McGale P. and Darby S. C., “Commentary: A dose-response relationship for radiation-induced heart disease—current issues and future prospects,” Int. J. Epidemiol. 37, 518–523 (2008). 10.1093/ije/dyn067 [DOI] [PubMed] [Google Scholar]
- Nath R., Anderson L. L., Luxton G., Weaver K. A., Williamson J. F., and Meigooni A. S., “Dosimetry of interstitial brachytherapy sources: Recommendations of the AAPM Radiation Therapy Committee Task Group No. 43,” Med. Phys. 22, 209–234 (1995). 10.1118/1.597458 [DOI] [PubMed] [Google Scholar]
- Rivard M. J., Coursey B. M., DeWerd L. A., Hanson W. F., Huq M. S., Ibbott G. S., Mitch M. G., Nath R., and Williamson J. F., “Update of AAPM Task Group No. 43 Report: A revised AAPM protocol for brachytherapy dose calculations,” Med. Phys. 31, 633–674 (2004). 10.1118/1.1646040 [DOI] [PubMed] [Google Scholar]
- Rivard M. J., Butler W. M., DeWerd L. A., Huq M. S., Ibbott G. S., Meigooni A. S., Melhus C. S., Mitch M. G., Nath R., and Williamson J. F., “Supplement to the 2004 update of the AAPM Task Group No. 43 Report,” Med. Phys. 34, 2187–2205 (2007). 10.1118/1.2736790 [DOI] [PubMed] [Google Scholar]
- Rivard M. J., Venselaar J. L. M., and Beaulieu L., “The evolution of brachytherapy treatment planning,” Med. Phys. 36, 2136–2153 (2009). 10.1118/1.3125136 [DOI] [PubMed] [Google Scholar]
- Na Y. H., Zhang J., Ding A., and Xu X. G., in Handbook of Anatomical Models for Radiation Dosimetry, edited by Xu X. G. and Eckerman K. F. (Taylor & Francis, Boca Raton, 2009), Chap. 14, pp. 347–375. [Google Scholar]
- Zhang J., Na Y. H., Caracappa P. F., and Xu X. G., “RPI-AM and RPI-AF, a pair of mesh-based, size-adjustable adult male and female computational phantoms using ICRP-89 parameters and their calculations for organ doses from monoenergetic photon beams,” Phys. Med. Biol. 54, 5885–5908 (2009). 10.1088/0031-9155/54/19/015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Commission on Radiological Protection, “ICRP Publication 89: Basic anatomical and physiological data for use in radiological protection: Reference values,” Ann. ICRP 32(3–4) (2002). [PubMed] [Google Scholar]
- Hendricks J. S.et al. , “MCNPX extensions, version 2.5.0,” LLNL Report No. LA-UR-05–2675, LLNL, Los Alamos, NM, 2005.
- NUDAT 2.5, National Nuclear Data Center, Brookhaven National Laboratory, http://www.nndc.bnl.gov/nudat2/index.jsp (last accessed 9 January 2010).
- Xu X. G., “Effective dose for patients undergoing coronary and femoral intravascular radiotherapy involving an HDR 192Ir source,” Radiat. Prot. Dosim. 115, 289–293 (2005). 10.1093/rpd/nci201 [DOI] [PubMed] [Google Scholar]
- Kassas B., Mourtada F., Horton J. L., and Lane R. G., “Contrast effects on dosimetry of a partial breast irradiation system,” Med. Phys. 31, 1976–1979 (2004). 10.1118/1.1763006 [DOI] [PubMed] [Google Scholar]
- Xoft Inc., “Axxent Controller Operator Manual-Model 110,” Xoft Inc., Sunnyvale, CA, 2009.
- Daskalov G. M., Löffler E., and Williamson J. F., “Monte-Carlo aided dosimetry of a new high dose-rate brachytherapy source,” Med. Phys. 25, 2200–2208 (1998). 10.1118/1.598418 [DOI] [PubMed] [Google Scholar]
- Granero D., Perez-Calatayud J., Pujades-Claumarchirant M. C., Ballester F., Melhus C. S., and Rivard M. J., “Equivalent phantom sizes and shapes for brachytherapy dosimetric studies of 192Ir and 137Cs,” Med. Phys. 35, 4872–4877 (2008). 10.1118/1.2982140 [DOI] [PubMed] [Google Scholar]
- Buo B., Papanikolaou N., and Shi C., “Monte Carlo based plan verification for electronic brachytherapy: The dosimetric influence of patient boundary and tissue inhomogeneities,” Int. J. Radiat. Oncol., Biol., Phys. 75, S120–S121 (2009). [Google Scholar]
- International Commission on Radiological Protection, “ICRP Publication 103: The 2007 Recommendations of the International Commission of Radiological,” Ann. ICRP 37(2–4), 1–332 (2007). [DOI] [PubMed] [Google Scholar]
- Xu X. G., Bednarz B., and Paganetti H., “A review of dosimetry studies on external-beam radiation treatment with respect to second cancer induction,” Phys. Med. Biol. 53, R193–R241 (2008). 10.1088/0031-9155/53/13/R01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. J., Balter J., Balter S., J. A.BenComo, Jr., Das I. J., Jiang S. B., Ma C. -M., Olivera G. H., Rodebaugh R. F., Ruchala K. J., Shirato H., and Yin F. -F., “The management of imaging dose during image-guided radiotherapy: Report of the AAPM Task Group 75,” Med. Phys. 34, 4041–4063 (2007). 10.1118/1.2775667 [DOI] [PubMed] [Google Scholar]
- Wazer D. E., Kaufman S., Cuttino L., DiPetrillo T., and Arthur D. W., “Accelerated partial breast irradiation: An analysis of variables associated with late toxicity and long-term cosmetic outcome after high-dose-rate interstitial brachytherapy,” Int. J. Radiat. Oncol., Biol., Phys. 64, 489–495 (2006). 10.1016/j.ijrobp.2005.06.028 [DOI] [PubMed] [Google Scholar]
- Poon E. and Verhaegen F., “A CT-based analytical dose calculation method for HDR 192Ir brachytherapy,” Med. Phys. 36, 3982–3994 (2009). 10.1118/1.3184695 [DOI] [PubMed] [Google Scholar]