Abstract
Purpose
We examined whether Survivin expression is associated with an increased risk of metastasis in prostate cancer.
Methods and Materials
A total of 205 patients with T1 (23%) and T2 (77%) prostate cancer were treated with conventional external beam radiation therapy from 1991 to 1993 at the Massachusetts General Hospital. Of the patients, 62 had adequate and suitable-stained tumor material for Survivin analysis. Median follow-up was 102 months (range, 5–127 months). Distant failure was determined on the basis of clinical criteria. In preclinical studies, replication-deficient adenovirus encoding phosphorylation-defective Survivin Thr34 → Ala dominant-negative mutant pAd-S(T34A) or short hairpin RNA (shRNA) was used to inhibit Survivin in prostate cancer models, and the cell motility, morphology, and metastasis were investigated.
Results
Our correlative data on men with early-stage (T1/T2) prostate cancers treated at Massachusetts General Hospital by definitive radiotherapy indicated that overexpression of Survivin (positive staining in ≥10% cells) was associated with a significantly increased risk for the subsequent development of distant metastasis (p = 0.016) in the univariate analysis. In the multivariate analysis, overexpression of Survivin remained an independent predictor of distant metastasis (p = 0.008). The inhibition of Survivin dramatically inhibited invasiveness of prostate cancer cells in the in vitro invasion assay and spontaneous metastasis in the Dunning prostate cancer in vivo model. Furthermore, attenuation of Survivin resulted in changes in the microtubule cytoskeleton, loss of cellular polarity, and loss of motility.
Conclusions
This study suggests that Survivin may be a potentially important prognostic marker and promising therapeutic target in metastatic prostate cancer.
Keywords: Survivin, Prostate cancer, Metastasis
INTRODUCTION
Metastatic prostate cancer is one of the leading causes of cancer death in men. An estimated 186,320 new cases will occur in the US during 2008, and 28,660 men are expected to die from the disease (1). Although there have been significant advances in the early detection and treatment of localized prostate cancer, invasion and metastasis, the key determinant of lethality in the disease, still represents a major clinical challenge. Therefore, identification of the molecular mechanisms that lead to distant metastasis is critical for improvement of the treatments for metastatic prostate cancer and for development of strategies to prevent primary prostate cancer from metastasizing (2–4).
Survivin, the smallest member of the inhibitor of apoptosis (IAP) family, is a 142-amino acid, 16.5-kDa protein coded by a single-copy gene on the human 17q25 chromosome. The two main reasons for considering Survivin as an attractive therapeutic target in cancer are its differential expression in tumors vs. normal tissues and its potential requirement for maintaining cancer-cell viability (5, 6). Survivin has not been found to be expressed in normal secretory epithelium of the prostate but has been found to be strongly expressed in prostate cancer cells (7). Furthermore, Survivin has been found to be expressed in biologically aggressive prostate cancers with higher Gleason scores and metastasis to the regional lymph node (8, 9). We have found that IGFR1-mediated upregulation of Survivin via PI3K signaling may represent an important mechanism of regulation of its expression in prostate cancer cells and resistance to antiandrogen therapy. Antagonism of Survivin appears to greatly enhance the sensitivity of prostate cancer cells to antiandrogen therapy and chemotherapy (10, 11). In this present study, we investigate the role of Survivin in mediating prostate cancer metastasis.
METHODS AND MATERIALS
Patients and treatment
Between November 1991 and February 1993, 205 patients with T1 to T2 prostate cancer were treated with conventional external beam radiation therapy at the Massachusetts General Hospital (12). No patient had conformal radiation and none received either neoaduvant or adjuvant androgen deprivation therapy. The median and modal dose to the prostate was 68.4 Gy, ranging from 64 to 72.6 Gy. All patients were subsequently followed either by the radiation oncologist, urologist, medical oncologist or primary care physician with regular prostate-specific antigen (PSA) testing and clinical evaluations at 3- to 12-month intervals. Scans were obtained and repeat prostate biopsies were performed only if the PSA and clinical examination warranted. Median number of PSA values obtained in follow-up for those without failure was 10, which represents a minimum. Uninformative PSA values, included those measured in the first 2 years while the PSA was declining; when multiple serial values were identical, only the first and last were kept, were not recorded. Of 205 patients, 62 had adequate staining for Survivin.
Definition of endpoints
Overall survival was defined as death of any cause. Cause-specific survival was defined as death from prostate cancer. Local failure was defined as a palpable recurrence, positive rebiopsy result, or malignant cells identified on a transurethral resection of the prostate. Distant failure was determined on the basis of clinical criteria, with confirmation of bony metastases by a positive bone scan or radiograph. Soft tissue metastases were confirmed by computed tomography. Biochemical relapse-free survival was determined using the American Society for Therapeutic Radiology and Oncology (ASTRO) consensus criteria. Because of concerns that this definition may under call failure, we also looked at the outcome using three increases in PSA as the definition of failure but without backdating as the official definition demands. The aim was to avoid backdating into an earlier time when the denominator in the actuarial calculation is larger and the impact of each individual failure event correspondingly lessened (13).
Immunohistochemical technique
The immunohistochemical staining of Survivin was described previously (14).
Formalin-fixed paraffin-embedded tumor samples were analyzed by Z.M. and L-Y.K using ACIS (Clarient Chromavision Inc., San Juan Capistrano, CA). The slides were scanned at 10× objective to give a pixel-to-tissue area ratio of 1:1 (1 pixel, 1 μm2). Using the Threshold Tool, brown and blue pixels were masked in each slide to derive the tumor tissue area and exclude all white areas. Where possible, at least six regions of tumor cells were gated either by freehand around each tumor gland or by using a 40× circle if more than 90% of the region of interest contained tumor cells. Where there was heterogeneity in staining, the tumor area with the highest intensity of staining was chosen. Nuclear Survivin mean index percentage was calculated by the proprietary software. It was the number of brown pixels divided by the total number of pixels (brown plus blue) in a gated region, taken as a percentage. Median values were used as cut points for subsequent correlation with clinical outcome.
Cell culture
LNCaP, PC3, and DU145 cell lines were obtained from American Type Culture Collection (Manassas, VA). The Dunning rat prostate cancer cell line AT6.3 was described previously (15–18). The human prostate cancer cells LNCaP and PC3 were maintained in RPMI 1640 supplemented with 10% fetal bovine serum (Hyclone, Logan, UT) and penicillin (50 IU/ml)/streptomycin (50 μg/ml; Life Technologies, Gaithersburg, MD). Medium for DU-145 cells was the same as above, except that Dulbecco’s modified Eagle’s medium (DMEM) was substituted for RPMI 1640. AT6.3 cells were maintained in the same media as the LNCaP and PC3 cells, with the addition of 0.1 μg/ml of dexamethasone. All cells were seeded on laminin-coated plates (Sigma, St. Louis, MO) for all immunocytochemistry and cell motility experiments.
Adenoviral preparation
The adenoviruses used for this study were provided generously by Dr. Dario Altieri and are described fully elsewhere (10, 11, 19, 20). The pAd-S(T34A) constructs contain a mutant Thr34 → Ala mutant Survivin cDNA, which has been previously demonstrated to function as a dominant-negative construct. The pAd-S(WT) vector contains the wild-type Survivin cDNA, which can be used to overexpress wild-type Survivin; the pAd-(Empty) contains an empty vector and was used as controls for several experiments. In all, 1 ml of viral supernatant was used to infect 3 × 106 to 5 × 106 293 cells. Viruses were harvested at 2- to 3-day intervals, which was repeated three to five times with a total of 5 × 108 packaging cells, and viral particles were purified by CsCl banding. Green fluorescence forming units (GFU) were estimated by serial dilution of the viral stock in transduced 293 cells.
In vitro invasion assay
The invasion assay was done using BD Biocoat invasion chambers with growth factor reduced Matrigel in 24-well format (BD Biosciences, San Jose, CA) (21). LNCaP, PC-3 and DU-145 cells infected with replication-deficient adenoviruses encoding wild-type Survivin [pAd-S(WT)], a phosphorylation-defective Survivin Thr34 → Ala dominant negative mutant [pAd-S(T34A)], or control vector pAd-(Empty) were suspended in serum-free RPMI 1640 at a concentration of 1 × 105 cells/ml, and 0.5 ml of each was added to the invasion chambers in quadruplicate. RPMI 1640 (0.75 ml) supplemented with 10% fetal bovine serum was added to each well of the plate to act as a chemoattractant and the plates were placed in an incubator for 18 h. Cells that invaded through the insert were stained with Crystal Violet, and eight high-power fields were counted per insert.
In vivo spontaneous metastasis assay
To determine whether Survivin expression is associated with metastatic potential of prostate cancer cells, the in vivo metastasis assays were carried out using the Dunning prostate cancer model. Male 4-to 6-week-old CB17 Severe Combined Immunodeficient (SCID) mice were injected subcutaneously in the flank with 2 × 105 AT6.3 cells, and tumor were allowed to reach a volume of approximately 100 to 150 mm3. Tumors were injected with pAd-(Empty), pAd-S(WT), and pAd-S(T34A) in three sites (5 × 108 GFU/site). At the experimental endpoint (28 days postinoculation), mice were euthanized, their lungs excised and formalin fixed, and the number of lung metastases (>1 mm) counted.
Short hairpin RNA-mediated knockdown of Survivin
Commercial available short hairpin RNA (shRNA) constructs were obtained as bacterial glycerol stocks (Sigma) and used to silence Survivin. AT6.3 cells were transduced with 2.56 × 105 TU/ml virus and polybrene (8 μg/ml) (Chemicon, Pittsburgh, PA). Twenty-four hours later, antibiotic selection (1.3 μmol/l puromycin) was initiated for 10 days. AT6.3 cells transduced with nontarget shRNA (Sigma) served as a control. Stable silencing of Survivin in AT6.3 was determined Western blot.
Western blot analysis
The immunoblot of Survivin was described previously (10).
Motility experiments
Motility experiments were done on laminin-coated plates. In vitro wound healing assays were done on confluent AT6.3 empty and Survivin shRNA clones. The media on the confluent cells was replaced with RPMI 1640 with 0.5% fetal bovine serum media and an area of cells was scraped off using a rubber-tipped cell scraper. Light-microscopic images were taken at Time 0, 24 h, and 48 h.
Immunofluorescence
Cells seeded on laminin-coated plates were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. Cells were probed for mouse monoclonal α-tubulin (Molecular Probes, Eugene OR). The secondary antibody was antimouse Alexa 568 (Molecular Probes). Cells were counterstained with 4′,6-diamidino-2-phenylindole (DAPI; Sigma) to stain the nuclei. Fluorescent images were taken using confocal microscope.
Statistical analysis
For clinical data, actuarial analyses were performed using the Kaplan-Meier method, and differences between curves were assessed using the log-rank method (22, 23). Multivariate analysis was performed using the Cox proportional hazards regression model (24). In in vitro and in vivo studies, data were expressed as mean ± SD, with significance determined by Student’s two-tailed t test.
RESULTS
Survivin expression is associated with an increased risk of distant metastasis in prostate cancer patients
As the first step to test the hypothesis that Survivin is associated with metastatic behavior of human prostate cancer, we examined whether Survivin expression was associated with an increased risk of distant metastasis in men with T1/T2 prostate cancers treated by definitive radiotherapy at Massachusetts General Hospital who have been clinically followed for 10 years or longer. In all, 62 patients had adequate and suitably stained tumor material for Survivin analysis. Pretreatment characteristics of the 62 assessable patients are shown in Table 1. The median age was 74 years (range, 51–90 years). The median age at diagnosis was 74 years (range, 51–90 years). Median follow-up was 102 months (range, 5–127 months). All patients had T1 to T2 tumors; 23% were T1 and 77% T2. The Gleason score was ≤6 in 63%. Pretreatment PSA data was available in all patients and the median pretreatment PSA was 11 (range, 1.5–661). Of the patients, 48% had a pretreatment value of <10. There was no significant association of any standard prognostic feature with percent Survivin staining (data not shown). Figure 1A demonstrates representative stained slides showing Survivin strong and weak immunostaining.
Table 1.
Pretreatment characteristics of the assessable patients (N = 62)
| Characteristic | n | % |
|---|---|---|
| T-stage | ||
| 1 | 15 | 23 |
| 2 | 47 | 77 |
| Gleason score | ||
| 6 | 39 | 63 |
| ≥7 | 23 | 37 |
| PSA | ||
| ≤4 | 8 | 13 |
| 4–10 | 22 | 35 |
| 10–20 | 23 | 37 |
| >20 | 9 | 15 |
| Survivin (%) | ||
| <10 | 37 | 60 |
| ≥10 | 25 | 40 |
Abbreviation: PSA = prostate-specific antigen.
Fig. 1.
(a) Representative stained slides showing Survivin strong immunostaining (left) and Survivin weak immunostaining (right). (b) Kaplan-Meier curve demonstrating that higher level of Survivin expression (≥10%) was associated with an increased risk of distant metastasis in men with T1/T2 prostate cancers treated by definitive radiotherapy at Massachusetts General Hospital.
The results of the univariate analysis revealed a significantly increased risk for the subsequent development of distant metastasis (Fig. 1b). Compared with those with Survivin staining <10%, those who had Survivin staining ≥10% showed a significant decrease in metastasis-free survival (p = 0.016). There was no significant difference in overall survival, cause-specific survival, local control, or biochemical relapse free survival (data not shown). The results of the multivariate analysis can be found in Table 2. Survivin expression and Gleason score were included in a logistic regression model. The p values were 0.05 and 0.03, respectively. For this model, the sensitivity was 29%, specificity 92%, positive predictive value 50%, negative predictive value 81%, and the area under the receiver operator curve was 0.73.
Table 2.
Multivariate analysis for distant metastasis-free survival
| Characteristic | p Value |
|---|---|
| Survivin expression | 0.05 |
| Gleason score | 0.03 |
Survivin promotes the in vitro invasion of prostate cancer cells
Given that higher levels of Survivin expression are associated with a significantly increased risk for the subsequent development of distant metastasis in our correlative data, we investigated whether Survivin is involved in the regulation of metastatic behavior of prostate cancer cells in vitro. Invasion through the ECM is a crucial step in tumor metastasis. Matrigel can be used in vitro as a reconstituted basement membrane that provides a biologically active ECM (25). Human prostate cell lines PC-3, DU-145, and LNCaP were infected with replication-deficient adenoviruses encoding either wild-type Survivin [pAd-S(WT)], to examine Survivin overexpression effects, or a phosphorylation-defective Survivin Thr34 → Ala dominant negative mutant [pAd-S(T34A)], to examine Survivin inactivation effects. As shown in Fig. 2, overexpression of Survivin in pAd-S(WT) arms caused a significant increase in invasive ability in all cell lines as compared with pAd-(Empty) arms. In contrast, inhibition of Survivin resulted in 50% to 70% suppression of invasion in pAd-S(T34A) arms in all cell lines. MTT assay and Trypan blue exclusion assay were performed under the conditions we used in the invasion assay and showed that there were no significant changes of cell viability among different arms (data not shown), indicating that the effect of Survivin on cell invasiveness was not due to its effect on the cell survival. These results suggested that Survivin expression is associated with increased invasiveness of prostate cancer cells in vitro.
Fig. 2.

Matrigel invasion assay on DU-145, LNCaP and PC-3 cells infected with adenoviruses encoding pAd-(Empty), pAd-S(WT), or pAd-S(T34A). The invasion of cells infected with pAd-(Empty) was set as 1.
Survivin is associated with spontaneous metastasis in the Dunning prostate cancer model
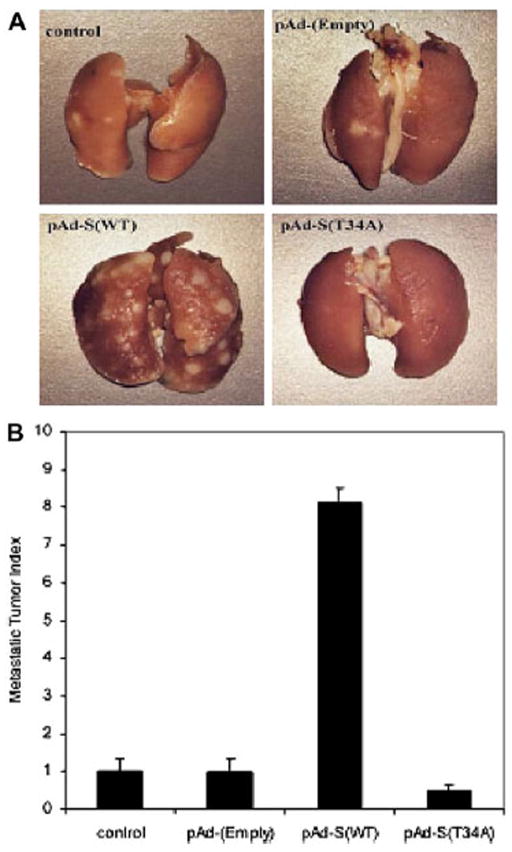
Because our data implicated Survivin in motility and invasion in prostate cancer cells in vitro, it seemed likely that Survivin would be associated with metastasis in vivo. To examine the role of Survivin in metastasis, we performed in vivo spontaneous metastasis experiments using the Dunning prostate cancer model. AT6.3 is a highly metastatic, anaplastic, androgen-independent rat prostatic cancer cell line that was established from a lung metastasis in the Dunning R3327 rat model. Male CB17 SCID mice were injected subcutaneously in the flank with AT6.3 cells. When tumors reached a volume of approximately 100 to 150 mm3, adenoviruses encoding pAd-(Empty), pAd-S(WT), or pAd-S(T34A) were injected intratumorally. Seven weeks later, mice were killed and lung surface metastases were counted. Overexpression of Survivin in pAd-S(WT) arm caused an eightfold increase in metastatic ability as compared with control or pAd-(Empty) arms. In contrast, inhibition of Survivin resulted in 50% suppression of lung metastasis in pAd-S(T34A) arm (Fig. 3). This indicates that Survivin may accelerate spontaneous metastasis in the Dunning prostate cancer model.
Fig. 3.
Spontaneous metastasis assay in the Dunning prostate cancer model with intratumoral injection of adenoviruses encoding pAd-(Empty), pAd-S(WT), and pAd-S(T34A). (a) Representative lungs from different treatment arms. (b) Quantification of the meta-static lesions on the lungs. The metastasis in pAd-(Empty) treated arm was set as 1.
Knockdown of Survivin with shRNA in AT6.3 cells results in a loss of motility of the cells through effects on the cytoskeleton
To further implicate Survivin in cell motility, we used a shRNA strategy to stably knock down Survivin in AT6.3 cells. Figure 4A shows the knockdown efficiency. Nontargeting shRNA (Lane1) and one of the Survivin shRNA constructs (Lane 4) were used in subsequent studies. A wound healing assay was performed to compare their effects on cell motility. Cells transfected with nontargeting shRNA were capable of migrating into and repopulating the cleared region of the laminin-coated plates in 48 h. In contrast, the Survivin stably knockdown AT6.3 cells could no longer migrate into the cleared region, and even after 48 h, the cleared region still remained mostly unpopulated (Fig. 4b). These results suggest that Survivin is necessary for the motility of these metastatic prostate cells on extracellular matrix, similar to the effects that we had observed in human prostate cancer cells.
Fig. 4.
(a) Western blot showing Survivin levels in AT6.3 cells transfected with nontargeting shRNA (Lane 1) and different Survivin shRNA constructs (Lanes 2–5). Nontargeting ShRNA (Lane 1) and one of the Survivin shRNA constructs (Lane 4) were used in subsequent studies. (b) Wound healing assay on AT6.3 cells transfected with nontargeting shRNA or Survivin shRNA. (c) Immunofluorescent staining of α-tubulin in AT6.3 cells transfected with nontargeting shRNA (left) or Survivin shRNA (right). Arrows indicate MTOCs in these cells.
Immunocytochemical staining for α-tubulin demonstrated that expression of the Survivin shRNA in AT6.3 cells resulted in extensive changes in the cytoskeleton, especially evident in microtubules (Fig. 4c). AT6.3 clones with scrambled shRNA seeded on laminin-coated plates showed a unique polarized morphology typical for migrating cells: a broad, flat lamella extending in the direction of migration (the leading edge) and a narrow, retracting tail at the rear of the cell (the trailing edge) (26, 27). There also was an apparent microtubule-organizing center (MTOC) present in front of the nucleus facing the leading edge, as is often seen in motile cells (28, 29). In contrast, when AT6.3 clones expressing Survivin shRNA were seeded on laminin-coated plates, the cells seemed to be flatter and larger, and a loss of cell polarity. The MTOC was also abnormally localized near the nucleus in these cells, with a disorganized microtubule network, further suggesting a loss of polarity. These results illustrate the role of Survivin in polarization and microtubule organization of the AT6.3 prostate cancer cells.
DISCUSSION
Although approximately 75% of prostate cancers are now readily detectable at an early and treatable stage with the introduction of the prostate-specific antigen (PSA) screening test, nearly 30% of men treated by radical prostatectomy suffer from recurrent disease, and the prognosis for men with metastatic disease remains poor. Androgen ablation is the most commonly used therapy in the treatment of advanced metastatic prostate cancer, but while most patients initially respond to this therapy, almost all ultimately relapse and die from androgen-independent prostate cancer and metastasis. Clinicians presently face two major challenges: (1) the lack of definitive markers to determine whether gland-localized malignancy will go on to develop metastatic disease; and (2) even with effective systemic adjuvant therapies, treatment is at best palliative. Although several metastasis-related genes have been identified in prostate cancer, including CD44, NM23, MKK4, KiSS1 protein metastin, BrMS1, and KAI1 (4, 30, 31), few have thus been proved effective in clinical trials (32, 33). Therefore, a greater focus on developing reliable metastatic biomarkers and therapeutic targets holds considerable promise and potential in improving the prognosis of prostate cancer patients.
Growing evidence indicates that Survivin is involved in regulating cell division and modulating apoptosis (34). During mitosis, Survivin localizes to two main subcellular pools. One pool of Survivin localizes to the kinetochores of metaphase chromosomes. In this pool, Survivin acts as a subunit of the chromosomal passenger complex and targets other chromosomal passenger proteins including Aurora kinase B, inner centromere protein antigens (INCENP), and Borealin, to kinetochores (35). A second pool of Survivin directly assembles on polymerized microtubules. This pool of Survivin stabilizes the microtubules, thus contributing to spindle formation. The role of Survivin in apoptosis inhibition has a similar degree of complexity. Upon activation of proapoptotic cell signaling, Survivin is released from the mitochondria in the cytosol and inhibits active caspase-9. This function requires association with the hepatitis B X-interacting protein and/or with X-linked IAP and is inhibited by Smac/Diablo (36). Although Survivin has aroused keen interest in disparate areas of basic and translational research, few studies focus on its role in mediating cancer metastasis. The interaction of Survivin with the microtubules stirred our interest in examining any possible correlation with distant metastasis both clinically, as well as preclinically. To our knowledge, the work presented here is the first study showing the role of Survivin in promotion of cancer metastasis.
We presented the following evidence to support the hypothesis that Survivin is a novel metastasis-associated gene in prostate cancer. (1) Survivin expression was associated with an increased risk of distant metastasis in men with T1/T2 prostate cancers treated by definitive radiotherapy at Massachusetts General Hospital. (2) Survivin promotes the invasiveness of human prostate cancer cell lines in Matrigel invasion assay. (3) Survivin accelerates spontaneous metastasis in the Dunning prostate cancer model. The key finding of this work is the increased risk of distant metastasis in T1/T2 prostate cancer patients with higher level of Survivin expression. The consistency of our clinical correlative and preclinical data is noteworthy, as the latter not only confirms our clinical observations but also highlights potential underlying mechanisms by which Survivin can enhance distant metastases in prostate cancer. Our findings extended the current vision regarding the role of Survivin in cancer progression and could provide a rational basis for using Survivin as a prognostic marker to predict the metastatic risk as well as a potential therapeutic target to improve the treatment of advanced prostate cancer. This study represents a retrospective correlative analysis performed on a relatively small patient population. Our data should be viewed with some caution because of the small dataset, low statistical power, and potential cut-point bias. A larger independent cohort of prostate cancer patients should be investigated to clarify the potential role of Survivin in prostate cancer metastasis.
The potential mechanism by which Survivin mediates prostate cancer metastasis was also investigated in our preclinical prostate cancer models. Metastasis is a sequential cascade of multiple cellular events involving local invasion, intravasation into the circulation, survival and transport in the circulation, extravasation from the bloodstream, and growth in the metastatic site (37–39). Progression through these stages requires changes in cellular phenotype, such as cellular motility, antiapoptotic capability, and expression of adhesion molecules, matrix metalloproteinases, angiogenetic factors, etc. Effective blockage of even one of the requisite steps could result in the abrogation of clinically relevant metastasis (4, 40). We found that Survivin may directly affect cell motility. Inhibition of Survivin in AT6.3 cells resulted in a loss of motility in an in vitro wound assay on laminin-coated plates. Next we focused our study on the molecular changes underlying Survivin-mediated cell migration. It has been reported that Survivin is a regulator of microtubule dynamics (41). Cells microinjected with a polyclonal antibody to Survivin exhibited flattened metaphase spindles that were severely depleted of microtubules, whereas overexpression of Survivin hyperstabilized microtubules and shortened metaphase pole-to-pole distance, suggestive of depressed microtubule dynamics (42). Data from Survivin knockout mice are consistent with these findings (43). Reorganization of the microtubule network is one of the primary mechanisms of cell motility and essential for the intrinsic cell polarization and directional cell migration. We found that attenuation of Survivin in AT6.3 cells led to extensive changes in the cytoskeleton, especially evident in microtubules. In mock-infected AT6.3 cells, endogenous Survivin appeared to maintain polarization of the microtubules outwards from the centrosomes forming a dense meshwork toward the plasma membrane. In contrast, the cell polarization and microtubule network in Survivin knockdown AT6.3 cells was disrupted, which is characteristic with nonmigratory cells. These results illustrate the importance of Survivin in the microtubule organization necessary for motility of the AT6.3 prostate cancer cells.
CONCLUSION
This work provides the first evidence of involvement of Survivin in prostate cancer metastases in preclinical models as well as in T1/T2 prostate cancers patients treated by definitive radiotherapy at Massachusetts General Hospital. Survivin appears to promote metastasis in prostate cancer by regulating microtubule organization, cell polarity, and cell motility. Our study indicates that Survivin could represent a potentially new biomarker and therapeutic target for metastatic prostate cancer.
Acknowledgments
Support for this work was provided by The Ohio State University/ Arthur G. James Comprehensive Cancer Center and National Institutes of Health/National Cancer Institute RO1CA108633 (both to A.C.).
Footnotes
Note—An online CME test for this article can be taken at http://asro.astro.org under Continuing Education.
Conflict of interest: none.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Chakravarti A, Zhai GG. Molecular and genetic prognostic factors of prostate cancer. World J Urol. 2003;21:265–274. doi: 10.1007/s00345-003-0362-z. [DOI] [PubMed] [Google Scholar]
- 3.Isaacs JT. Molecular markers for prostate cancer metastasis. Developing diagnostic methods for predicting the aggressiveness of prostate cancer. Am J Pathol. 1997;150:1511–1521. [PMC free article] [PubMed] [Google Scholar]
- 4.Gopalkrishnan RV, Kang DC, Fisher PB. Molecular markers and determinants of prostate cancer metastasis. J Cell Physiol. 2001;189:245–256. doi: 10.1002/jcp.10023. [DOI] [PubMed] [Google Scholar]
- 5.Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- 6.Li F. Survivin study: What is the next wave? J Cell Physiol. 2003;197:8–29. doi: 10.1002/jcp.10327. [DOI] [PubMed] [Google Scholar]
- 7.McEleny KR, Watson RW, Coffey RN, et al. Inhibitors of apoptosis proteins in prostate cancer cell lines. Prostate. 2002;51:133–140. doi: 10.1002/pros.10061. [DOI] [PubMed] [Google Scholar]
- 8.Kishi H, Igawa M, Kikuno N, et al. Expression of the survivin gene in prostate cancer: Correlation with clinicopathological characteristics, proliferative activity and apoptosis. J Urol. 2004;171:1855–1860. doi: 10.1097/01.ju.0000120317.88372.03. [DOI] [PubMed] [Google Scholar]
- 9.Shariat SF, Lotan Y, Saboorian H, et al. Survivin expression is associated with features of biologically aggressive prostate carcinoma. Cancer. 2004;100:751–757. doi: 10.1002/cncr.20039. [DOI] [PubMed] [Google Scholar]
- 10.Zhang M, Latham DE, Delaney MA, et al. Survivin mediates resistance to antiandrogen therapy in prostate cancer. Oncogene. 2005;24:2474–2482. doi: 10.1038/sj.onc.1208490. [DOI] [PubMed] [Google Scholar]
- 11.Zhang M, Mukherjee N, Bermudez RS, et al. Adenovirus-mediated inhibition of survivin expression sensitizes human prostate cancer cells to paclitaxel in vitro and in vivo. Prostate. 2005;64:293–302. doi: 10.1002/pros.20263. [DOI] [PubMed] [Google Scholar]
- 12.Zietman AL, Chung CS, Coen JJ, et al. 10-Year outcome for men with localized prostate cancer treated with external radiation therapy: Results of a cohort study. J Urol. 2004;171:210–214. doi: 10.1097/01.ju.0000100980.13364.a6. [DOI] [PubMed] [Google Scholar]
- 13.Gretzer MB, Trock BJ, Han M, et al. A critical analysis of the interpretation of biochemical failure in surgically treated patients using the American Society for Therapeutic Radiation and Oncology criteria. J Urol. 2002;168:1419–1422. doi: 10.1016/S0022-5347(05)64464-3. [DOI] [PubMed] [Google Scholar]
- 14.Zhang M, Ho A, Hammond EH, et al. Prognostic value of survivin in locally advanced prostate cancer: Study based on RTOG 8610. Int J Radiat Oncol Biol Phys. 2009;73:1033–1042. doi: 10.1016/j.ijrobp.2008.06.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strock CJ, Park JI, Nakakura EK, et al. Cyclin-dependent kinase 5 activity controls cell motility and metastatic potential of prostate cancer cells. Cancer Res. 2006;66:7509–7515. doi: 10.1158/0008-5472.CAN-05-3048. [DOI] [PubMed] [Google Scholar]
- 16.Hamano M, Kuramochi H, Nihei N, et al. Mechanisms of metastasis suppression by introduction of human chromosome 10 into rat prostate cancer. Asian J Androl. 2002;4:123–129. [PubMed] [Google Scholar]
- 17.Gao AC, Lou W, Dong JT, et al. CD44 is a metastasis suppressor gene for prostatic cancer located on human chromosome 11p13. Cancer Res. 1997;57:846–849. [PubMed] [Google Scholar]
- 18.Bussemakers MJ, van Moorselaar RJ, Giroldi LA, et al. Decreased expression of E-cadherin in the progression of rat prostatic cancer. Cancer Res. 1992;52:2916–2922. [PubMed] [Google Scholar]
- 19.Ambrosini G, Adida C, Sirugo G, et al. Induction of apoptosis and inhibition of cell proliferation by survivin gene targeting. J Biol Chem. 1998;273:11177–11182. doi: 10.1074/jbc.273.18.11177. [DOI] [PubMed] [Google Scholar]
- 20.Chakravarti A, Zhai GG, Zhang M, et al. Survivin enhances radiation resistance in primary human glioblastoma cells via caspase-independent mechanisms. Oncogene. 2004;23:7494–7506. doi: 10.1038/sj.onc.1208049. [DOI] [PubMed] [Google Scholar]
- 21.Zhang M, Altuwaijri S, Yeh S. RRR-alpha-tocopheryl succinate inhibits human prostate cancer cell invasiveness. Oncogene. 2004;23:3080–3088. doi: 10.1038/sj.onc.1207435. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan ELMP. Non parametric estimation from incomplete observations. J Am stat assoc. 1958;53:457–816. [Google Scholar]
- 23.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 24.Cox DR. Regression models and life tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 25.Iwamoto Y, Sugioka Y. Use of a reconstituted basement membrane to study the invasiveness of tumor cells. Adv Exp Med Biol. 1992;324:141–149. doi: 10.1007/978-1-4615-3398-6_14. [DOI] [PubMed] [Google Scholar]
- 26.Ridley AJ, Schwartz MA, Burridge K, et al. Cell migration: Integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 27.Vicente-Manzanares M, Webb DJ, Horwitz AR. Cell migration at a glance. J Cell Sci. 2005;118:4917–4919. doi: 10.1242/jcs.02662. [DOI] [PubMed] [Google Scholar]
- 28.Luders J, Stearns T. Microtubule-organizing centres: A reevaluation. Nat Rev Mol Cell Biol. 2007;8:161–167. doi: 10.1038/nrm2100. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez OC, Schaefer AW, Mandato CA, et al. Conserved microtubule-actin interactions in cell movement and morphogenesis. Nat Cell Biol. 2003;5:599–609. doi: 10.1038/ncb0703-599. [DOI] [PubMed] [Google Scholar]
- 30.Winter SF, Cooper AB, Greenberg NM. Models of metastatic prostate cancer: A transgenic perspective. Prostate Cancer Prostatic Dis. 2003;6:204–211. doi: 10.1038/sj.pcan.4500655. [DOI] [PubMed] [Google Scholar]
- 31.Kauffman EC, Robinson VL, Stadler WM, et al. Metastasis suppression: The evolving role of metastasis suppressor genes for regulating cancer cell growth at the secondary site. J Urol. 2003;169:1122–1133. doi: 10.1097/01.ju.0000051580.89109.4b. [DOI] [PubMed] [Google Scholar]
- 32.Bhandari MS, Petrylak DP, Hussain M. Clinical trials in metastatic prostate cancer—has there been real progress in the past decade? Eur J Cancer. 2005;41:941–953. doi: 10.1016/j.ejca.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Gutman S, Kessler LG. The US Food and Drug Administration perspective on cancer biomarker development. Nat Rev Cancer. 2006;6:565–571. doi: 10.1038/nrc1911. [DOI] [PubMed] [Google Scholar]
- 34.Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- 35.Lens SM, Vader G, Medema RH. The case for Survivin as mitotic regulator. Curr Opin Cell Biol. 2006;18:616–622. doi: 10.1016/j.ceb.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 36.Mita AC, Mita MM, Nawrocki ST, et al. Survivin: Key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin Cancer Res. 2008;14:5000–5005. doi: 10.1158/1078-0432.CCR-08-0746. [DOI] [PubMed] [Google Scholar]
- 37.Bogenrieder T, Herlyn M. Axis of evil: Molecular mechanisms of cancer metastasis. Oncogene. 2003;22:6524–6536. doi: 10.1038/sj.onc.1206757. [DOI] [PubMed] [Google Scholar]
- 38.Jiang WG, Puntis MC, Hallett MB. Molecular and cellular basis of cancer invasion and metastasis: Implications for treatment. Br J Surg. 1994;81:1576–1590. doi: 10.1002/bjs.1800811107. [DOI] [PubMed] [Google Scholar]
- 39.Woodhouse EC, Chuaqui RF, Liotta LA. General mechanisms of metastasis. Cancer. 1997;80:1529–1537. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1529::aid-cncr2>3.3.co;2-#. [DOI] [PubMed] [Google Scholar]
- 40.Robinson VL, Kauffman EC, Sokoloff MH, et al. The basic biology of metastasis. Cancer Treat Res. 2004;118:1–21. doi: 10.1007/978-1-4419-9129-4_1. [DOI] [PubMed] [Google Scholar]
- 41.Altieri DC. The case for survivin as a regulator of microtubule dynamics and cell-death decisions. Curr Opin Cell Biol. 2006;18:609–615. doi: 10.1016/j.ceb.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 42.Giodini A, Kallio MJ, Wall NR, et al. Regulation of microtubule stability and mitotic progression by survivin. Cancer Res. 2002;62:2462–2467. [PubMed] [Google Scholar]
- 43.Okada H, Bakal C, Shahinian A, et al. Survivin loss in thymocytes triggers p53-mediated growth arrest and p53-independent cell death. J Exp Med. 2004;199:399–410. doi: 10.1084/jem.20032092. [DOI] [PMC free article] [PubMed] [Google Scholar]