Abstract
Enterotoxigenic Escherichia coli (ETEC) is responsible for 280 million to 400 million episodes of diarrhea and about 380,000 deaths annually. Epidemiological data suggest that ETEC strains which secrete heat-stable toxin (ST), alone or in combination with heat-labile toxin (LT), induce the most severe disease among children in developing countries. This makes ST an attractive target for inclusion in an ETEC vaccine. ST is released upon colonization of the small intestine and activates the guanylate cyclase C receptor, causing profuse diarrhea. To generate a successful toxoid, ST must be made immunogenic and nontoxic. Due to its small size, ST is nonimmunogenic in its natural form but becomes immunogenic when coupled to an appropriate large-molecular-weight carrier. This has been successfully achieved with several carriers, using either chemical conjugation or recombinant fusion techniques. Coupling of ST to a carrier may reduce toxicity, but further reduction by mutagenesis is desired to obtain a safe vaccine. More than 30 ST mutants with effects on toxicity have been reported. Some of these mutants, however, have lost the ability to elicit neutralizing immune responses to the native toxin. Due to the small size of ST, separating toxicity from antigenicity is a particular challenge that must be met. Another obstacle to vaccine development is possible cross-reactivity between anti-ST antibodies and the endogenous ligands guanylin and uroguanylin, caused by structural similarity to ST. Here we review the molecular and biological properties of ST and discuss strategies for developing an ETEC vaccine that incorporates immunogenic and nontoxic derivatives of the ST toxin.
According to the World Health Organization, enterotoxigenic Escherichia coli (ETEC) is responsible for 280 million to 400 million episodes of diarrhea, many of which lead to malnutrition, and about 380,000 deaths annually. Most of the victims are children less than 5 years of age living in developing countries. This makes ETEC one of the most important enteropathogens in impoverished children. ETEC is also considered the most common cause of travelers' diarrhea (63).
ETEC is transmitted by the fecal-oral route, whereupon it colonizes the small intestine. Adhesion to the intestinal epithelium is mediated by colonization factor antigens (CFs), which are fimbriae or fibrillae, filamentous protein structures on the bacterial surface (11). To date, 25 distinct CFs have been identified (61). The essential ETEC secretogenic virulence factors are the heat-stable (ST) and heat-labile (LT) enterotoxins. Both elicit net secretion of ions and water, resulting in watery diarrhea, in the most serious cases producing a profuse cholera-like condition. ETEC can produce two distinct heat-stable toxins, STa/STI and STb/STII, which are unrelated structurally, functionally, and immunologically (18). Only the former, hereinafter referred to as “ST,” is thought to play an important role in human disease and will be discussed in this context. LT is a large (84,000 Da), immunogenic oligotoxin related to cholera toxin in sequence, structure, and mechanism (48). In contrast, ST is a small polypeptide (2,000 Da) which is nonimmunogenic in its natural form. Epidemiological data suggest that strains secreting ST, with or without LT, induce the most severe disease among children of developing countries (61, 62). Two ST variants are known: the 19-amino-acid STh (synonyms, STIb and STaII), found in human ETEC strains, and the 18-amino-acid STp (synonyms, STIa and STaI), isolated from human and porcine strains. STh and STp are very similar, with 14 identical residues, including the cysteines of the 3 disulfide pairs (34, 72).
A variety of strategies have been pursued in attempts to develop a vaccine against ETEC. The most promising vaccine candidate to date is a killed whole-cell vaccine comprising 5 different ETEC strains which express the most prevalent CFs, coadministered with recombinant cholera toxin (CT) B subunit (CT-B). This approach exploits the immunological cross-reactivity between CT and LT (8, 48). Although efficacious against more-serious diarrhea among American travelers to Guatemala, this vaccine was not protective in Egyptian children (45, 61).
An epidemiological study in Guinea-Bissau found that antigens other than the CFs may contribute substantially to the acquisition of natural anticolonizing immunity (58). This interpretation suggests that other surface antigens should be considered for inclusion in an ETEC vaccine. In addition, ST is present in approximately 75% of all clinical ETEC isolates and seems to be associated with more-serious illness than LT, which makes it a very attractive candidate to include as a component of an ETEC vaccine (61).
The main challenges for making a vaccine component from ST are to engineer the molecule so as to render it immunogenic while abolishing toxicity without losing protective epitopes. Such a mutant variant is often referred to as an ST toxoid. Here we review the molecular and biological properties of ST and discuss strategies for developing an ETEC vaccine that incorporates immunogenic and nontoxic derivatives of the toxin.
ST BIOLOGY
Heat-stable toxin biogenesis.
The heat-stable toxins of ETEC are encoded on transmissible plasmids (47, 55) and are expressed as 72-amino-acid prepropeptides (33). The presequence (amino acids 1 to 19) is a signal peptide that directs translocation of the prepropolypeptide across the inner membrane, mediated by the Sec machinery (36). Outer membrane (OM) translocation is accompanied by cleavage of the propeptide (STh amino acids 20 to 53; STp, 20 to 54), generating the mature ST (STh, 54 to 72; STp, 55 to 72); OM translocation is believed to require the TolC channel (67, 68), though this has not been directly demonstrated. The periplasmic thiol:disulfide interchange protein DsbA is required for the formation of the three disulfide bridges found in the mature ST (66).
ST toxicity in the gut.
ETEC colonization of the small intestine is facilitated by CF-mediated mucosal adhesion. Thereupon, the colonizing strain expresses its enterotoxins, though expression levels do not seem to be influenced by colonization (46). In the intestine, ST binds to and activates the intestinal brush border guanylate-cyclase-C (GC-C) receptor, which is the receptor of the endogenous ligands guanylin and uroguanylin (15, 43, 56, 57). ST activates GC-C when present in submicromolar concentrations and is known to bind to the receptor at nanomolar concentrations (10). The immediate result of ST-mediated GC-C receptor activation is an increase in intracellular messenger cyclic GMP (cGMP). cGMP in turn mediates decreased absorption of sodium and chloride ions and increased secretion of bicarbonate and chloride ions, ultimately resulting in watery diarrhea (56). In normal physiology, the GC-C receptor plays an important role in fluid homeostasis, pH control, and electrolyte balance, regulated by the endogenous ligands (15). Mutant mice that lack the GC-C receptor are viable and resistant to ST, and they are protected against ETEC diarrhea, underscoring the pivotal role of GC-C in ST-induced diarrhea (44).
Structure and function of ST.
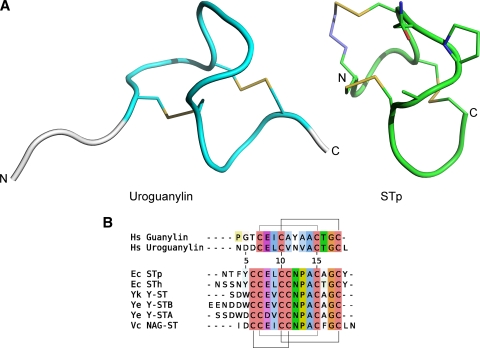
The ETEC heat-stable enterotoxins closely resemble guanylin and uroguanylin both in sequence and in three-dimensional structure (Fig. 1). The endogenous GC-C ligands have two disulfide bridges in a 1-3/2-4 pattern, and the STs have three, forming a 1-4/2-5/3-6 pattern. The two cystines in guanylin and uroguanylin, which are structurally equivalent to the 2-5 and 3-6 cystines of ST, allow the peptide to form two distinct interconvertible topologies, of which only one conformer is able to activate the GC-C receptor (29). Interestingly, the third cystine found in the ST peptides seems to lock the conformation in the active form.
FIG. 1.
Structures and sequence alignment of guanylate cyclase C receptor ligands. (A) Structures of the A form of human uroguanylin (left, PDB:1UYA) and the toxic domain (residues 6 to 18) of STp (right, PDB:1ETN). The N and C termini are marked. The two structures have a similar fold, and the part of the uroguanylin structure that corresponds to the ST structure is shown in cyan. The structures share two disulfide bridges (Cys7-Cys15 and Cys10-Cys19, shown as yellow sticks), and ST has an additional one (Cys6-Cys11). Note that Cys6 in ST is replaced with 5-beta-mercaptopropionate (shown as blue/yellow stick). The proposed GC-C receptor-interacting residues of ST (STp, Asn11-Pro12-Ala13) are shown as sticks. (B) Sequence alignment of the human GC-C ligands (top) and bacterial GC-C ligands (bottom). Disulfide bonds are marked with lines. Residue numbering is according to STh. The species abbreviations are as follows: Hs, Homo sapiens; Ec, Escherichia coli; Yk, Yersinia kristensenii; Ye, Yersinia enterocolitica; Vc, Vibrio cholerae.
The amino acid sequence from the first to last cysteine of ST forms the so-called toxic domain and has been reported to contain all the properties necessary for full biological activity (1, 72). However, an STh variant lacking the four N-terminal residues was reported to have a 10-fold reduction of potency compared to full-length ST, suggesting that the residues outside the toxic domain may also be required for full potency (60).
The structure of a synthetic, fully toxic analog of the effector domain of STp, where the first cysteine was replaced with β-mercaptopropinoic acid, has been solved by X-ray crystallography (34) (Fig. 1A). This analysis revealed that the backbone forms a right-handed spiral comprising three β-turns in a U-shaped configuration, tightly connected by the three disulfide linkages. Interestingly, the core of the structure does not seem to be stabilized by a prominent hydrophobic core. The importance of the conserved cystines for ST function had previously been elucidated by mutagenesis: disruption of the 2-5 and 3-6 cystines (Cys6Ala and Cys17Ala STp mutants), which are shared with the endogenous ligands, resulted in a complete loss of toxicity (31, 51). In contrast, toxicity was diminished but not abolished by breaking the ST-specific 1-4 cystine (Cys5Ala STp mutant). The functional effects of the cystine-breaking mutants probably reflect their important structural role.
Mutagenesis studies suggest that the region consisting of the three residues of the second β-turn (STp, Asn11-Pro12-Ala13) (Fig. 1B), which is completely conserved among the bacterial GC-C ligands, is important for interaction with the GC-C receptor (34). As would be predicted, mutation of these three residues (32, 60, 70, 71, 73) more or less diminishes toxin activity (Fig. 1 and 2). Furthermore, structural analysis revealed that the three residues protrude as a patch, thus creating a possible site of interaction with the GC-C receptor (34). One weakly toxic (STp, Ala13Gly) mutant and one nontoxic (STp, Ala13Leu) mutant showed only minor structural differences compared to the fully toxic analog (42). This observation further corroborates the functional importance of this conserved patch.
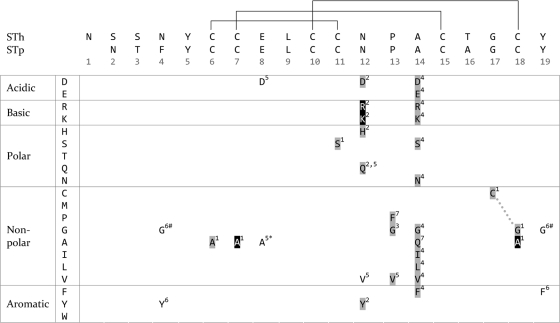
FIG. 2.
ST mutants and effects on toxicity reported in peer-reviewed publications. The top of the figure shows an alignment of the STh and STp sequences, with the positions of the disulfide bonds marked with lines (residue numbering is according to the STh sequence). Below the alignment is an amino acid matrix, where the residues are grouped according to physicochemical properties (leftmost column). Only residues that have been mutated and tested for effect on toxicity are shown, and the following scheme is used: no shading, no effect on toxicity; gray shading, reduced toxicity; black shading, nontoxic. All mutations affect single amino acid residues, except one double mutation, which is marked with a dashed line. Two additional double mutants are not shown in the figure: STh (Pro13Gly Ala14Leu) (2) and STh (Asn12Tyr Tyr19Asn) (20, 72). The first was reported to be nontoxic but was not included since it is unclear whether the effect of toxicity was due to the change in residues or a result of the fusion construct. The latter was not included since it gave an effect similar to that of the Asn12Tyr single mutant. The ST variants used in the different reports are as follows (numbers correspond to superscript numbers in the matrix, and references are given in parentheses): 1, STp (31);, 2, STp (32); 3, STh (60); 4, STp (71); 5, STp (69); 6, STp (70); 7, STp (73). Notes: the Glu8Ala mutant (*) had an effect on DsbA-dependent disulfide bond formation; both the Phe4Gly and Tyr19Gly mutants (#) affected translocation of the ST across the outer membrane.
ST VACCINOLOGY
ST made immunogenic through chemical conjugation to carriers.
Due to its small size, ST is nonimmunogenic in its natural form, and hence, the first step toward the generation of an ST toxoid is to couple it to an appropriate carrier. The first report of a successful attempt to make ST immunogenic was chemical conjugation of ST to porcine immunoglobulin G (21). Rats immunized with this ST-IgG conjugate were protected against ST-only-producing ETEC when tested using the ligated ileal loop assay (19). In the next step toward developing an ST vaccine, synthetic ST was conjugated to the B subunit of the heat-labile enterotoxin (LT-B) and shown to be immunogenic in both rats and rabbits. These immunized animals were protected in challenge experiments with ST and ST-producing ETEC (22, 23, 27). Interestingly, the chemical coupling of ST to LT-B reduced the toxicity more than 600-fold (22). LT-B is an attractive carrier since it provides immunization against LT as well as having the ability to target the conjugate to the GM1 ganglioside (30). After showing protection in animals, the ST-LT-B conjugate was also tested by peroral immunization of human volunteers, and Klipstein and coworkers were able to show a strong antitoxin response in both serum (IgG) and jejunal aspirates (IgA) (25). A completely synthetically produced peptide vaccine consisting of ST and 26 amino acids of LT-B yielded similar results in human volunteers (24). In both cases, the jejunal aspirates neutralized ST in the suckling mouse assay (12), indicating that it is also possible to mount an immunogenic response to ST in humans. It is worth noting that the synthetic ST toxin used by Klipstein and colleagues was based on the STp sequence published by Chan and Giannella (5), which was later discovered to have two interchanged residues (STp, Asn11Tyr and Tyr18Asn) (54). However, this mutant ST peptide apparently had the same biological and immunological properties as native ST (16, 27). In spite of the apparent successful generation of both a chemical conjugate and a synthetic ST vaccine candidate, these promising studies have not been further pursued.
One advantage of chemical conjugation is that it provides the opportunity to attain high hapten-to-carrier ratios, which may be important for achieving sufficiently high titers of anti-ST antibodies for effective neutralization in vivo. The results obtained with ST-LT-B chemical conjugates are included in Table 1. In addition to porcine IgG and LT-B, ST has been chemically conjugated to bovine serum albumin (17) and cholera toxin B (CT-B) (52).
TABLE 1.
Promising human vaccine candidatesa
| Researcher (publication period) | Vaccine molecule | ST description | Carrier | Subjects | Adm. | Toxicityb | Neutralizationc | Protection upon challenge | Reference(s) |
|---|---|---|---|---|---|---|---|---|---|
| Klipstein (1983-1985) | Synthetic ST, chem. conjug. | STp (N11Y Y18N) | LT-B, porcine IgG | Rats, rabbits, humansd | i.p., p.o. | Residuale | Yes | Yes, ST and ETECf | 22, 23, 24 |
| Houghten (1985-1986) | Completely synthetic | STp (N11Y Y18N) | Synthetic LT-B(58-83)-STp | Rats, humansd | p.o. | None | Yes | Yes, ETECg | 16, 25 |
| Sanchez (1988-1990) | Recombinant with mutation | STh (6-15) (C7A) | CT-B | Rabbits | s.c. | None | NR | NR | 39, 40, 41 |
| Clements (1990-1993) | Recombinant | STh C-term: V | LT-B | Mice | i.p, p.o.f | None | Yes | NR | 4, 9 |
| Lowenadler (1991) | Recombinant | STp | ZZ | Rabbits | s.c. | Reduced | Yes | NR | 28 |
| Pereira (2001) | Recombinant with mutations | STh, STh (C7A C18A) | Flagellin | Mice | i.p., i.m., p.o. | NR | Yes | NR | 35 |
| Zhang (2009) | Recombinant with mutations | STp (P12F), STp (A13Q) | pLT (R192G) | Rabbits | i.m. | Residualg | Yesh | NR | 73 |
Abbreviations: NR, not reported; SMA, suckling mouse assay; chem. conjug., chemical conjugation; Adm., administration; i.p., intraperitoneal; s.c., subcutaneous; p.o., peroral; i.m., intramuscular; C-term, C terminus.
As determined by testing in SMA, except where otherwise noted.
Reduction of ST activity in SMA, except where otherwise noted.
No challenge performed in human volunteers.
Testing in human volunteers reporting no clinical symptoms.
Challenge in rats and rabbits.
Challenge only in rats.
fExpressed in S. Dublin.
gToxicity testing in T84 cells and porcine ligated loop.
Testing in T84 cells only.
Genetic fusion of ST to carriers.
Recombinant techniques have the advantage of a simple and cheap mode of toxoid production, providing precisely defined and homogeneous proteins that can be delivered by live bacterial vectors (9). The first carrier to which ST was genetically fused was the A subunit of the heat-labile enterotoxin (LT-A), and the LT-A-ST fusion protein was shown to react with an ST monoclonal antibody (41). In later studies, ST has been fused to a number of different carriers in various ways, including LT-B (4, 9, 14), pLT(Arg192Gly) (73), CT-B (39, 40), the outer membrane protein OmpC (38), the ZZ moiety derived from Staphylococcus aureus protein A (28), the major subunit ClpG of Escherichia coli CS31A fimbriae (2), Salmonella flagellin (35), and green fluorescent protein (GFP) (65). The most successful genetic fusions of ST to suitable carriers, from a human vaccine perspective, are summarized in Table 1.
The first successful attempt at rendering ST immunogenic by genetic fusion was the coupling of a fragment of STh (positions 6 to 15), carrying a Cys7Ala mutation, to the N terminus of CT-B (40). This study was followed by experiments where various ST-related fragments, including the full sequence of STh, were fused to the N and C termini of CT-B, resulting in several fusion proteins that elicited ST-specific immune responses in rabbits (39). The obtained level of anti-ST antibodies only partially neutralized ST in challenge experiments with suckling mice. It is also worth noting that all fusion proteins were recognized by one of two neutralizing monoclonal antibodies (MAb) against ST, whereas a free ST C terminus was required for recognition by the second neutralizing MAb. However, the fusions retained some toxicity (A.-M. Svennerholm, personal communication). The CT-B component of the currently licensed cholera vaccine contributes to its protection against LT-ETEC diarrhea (6-8). A CT-B-ST vaccine could accordingly give protection against both ETEC enterotoxins.
Due to the success of using LT-B in chemical conjugation constructs, it was also chosen as a carrier for genetic fusions to ST (4, 9). When ST was fused directly to the C terminus of LT-B, no anti-ST antibodies were elicited in immunized mice (9). The insertion of a seven-amino-acid linker, however, rendered ST immunogenic. Furthermore, the resulting antibodies, when mixed with ST, were able to neutralize ST toxicity in suckling mice (9). In a follow-up study, a Salmonella enterica serovar Dublin strain that expressed a slightly different LT-B-ST fusion construct with an eight-amino-acid linker was used to immunize mice orally (4). The results were rather puzzling, since neither sera nor mucosal secretions exhibited reactivity to native ST in an enzyme-linked immunosorbent assay (ELISA), but they were both able to neutralize native ST in suckling mice. Interestingly, the genetic fusions of ST to LT-B also rendered ST nontoxic when assessed in the suckling mouse assay (9).
More recently, the genetic fusions of the mutants STp(Pro12Phe) and STp(Ala13Gln) to porcine LT(Arg192Gly) were reported to eliciting neutralizing antibodies in rabbits after intramuscular immunization (73). ST has also been genetically coupled to the S. aureus protein A ZZ fragment (28) and Salmonella flagellin (35). The ZZ-ST fusion peptide, where ST was fused to the N terminus of ZZ, elicited an immune response in rabbits with serum levels that were able to neutralize native ST in suckling mice. The toxicity of ZZ-ST was lower than that of native ST, and a polymeric form of ZZ-ST was about 100 times less toxic than the monomeric fusion protein (28). When ST was inserted into the hypervariable region of the Salmonella flagellin, a good antigenic response was observed only after two of the disulfide bonds were disrupted by substitution of cysteine residues (STh, Cys7Ala Cys18Ala) (35). The flagellin-ST fusion protein was delivered using an attenuated Salmonella strain. These genetic fusion experiments show that the mode of presenting ST epitopes is important for proper antigenic recognition and that minor changes can have profound effects on immunogenicity.
The ability to attain a high hapten-to-carrier ratio by genetic fusion, as opposed to chemical conjugation, is limited. However, recombinant constructs of ST-CT-B and ST-LT-B have been reported to pentamerize, thereby creating a 5:1 hapten-to-carrier ratio (37, 40).
Enhancing ST antigenicity.
One very interesting result obtained with ST chemically conjugated to LT-B suggests that it is possible not only to render ST immunogenic but also to increase its immunogenicity through proper manipulation of the polypeptide (26). By manipulating the conditions used to promote disulfide bond formation in synthetically produced ST, Klipstein et al. were able to isolate hyperantigenic ST variants.
Detoxifying ST.
Detoxification of ST can be achieved, at least in part, by genetic fusion or chemical conjugation, but it has often been combined with mutagenesis (2, 35, 39). Several mutants with effects on toxicity have been reported and are summarized in Fig. 2.
The previously mentioned reduction or complete loss of toxicity resulting from mutations of the conserved cysteines emphasizes the important structural role of the disulfide bridges (31, 51). The next residue that was targeted for mutagenesis was Asn11 of STp. Six different amino acids were substituted for Asn11 (Fig. 2), and all six ST mutants were recognized by anti-ST antibodies, suggesting that the variants have structural conformations similar to that of native ST. Two of the variants, Asn11Lys and Asn11Arg, were apparently nontoxic, and the other four showed reduced toxicity (32). One substitution in the same position, namely, Asn11Val, was later reported not to affect toxicity (70).
Mutations of the two conserved amino residues immediately following Asn11 in the sequence, Pro12 and Ala13, showed results similar to those for the Asn11 mutants (60, 71, 73). All published substitutions in these two positions have reduced toxicity (Fig. 2).
One challenge in the development of an ST vaccine is to separate toxicity from antigenicity (2). Due to the small size of ST, it is likely that single-amino-acid substitutions which reduce toxicity may also impinge on the resulting toxoid's ability to induce neutralizing antibodies. The literature reports substitutions that do not effect antigenicity (32), as well as others that clearly disrupt the ability to elicit neutralizing immune responses to the native toxin (3). The discovery of a neutralizing MAb that is specific for the N terminus of the molecule which does not include the toxic domain is encouraging in this respect (53). ST variants with greatly reduced toxicity, resulting from the mutation of two cysteines (STp, Cys6Ala and Cys17Ala), are also promising, since they are still recognized by a neutralizing antibody (51). Finally, the recently published STp(Pro12Phe) and STp(Ala13Gln) vaccine candidates also retained antigenicity while showing greatly reduced toxicity in GC-C receptor cell-based and porcine ligated loop assays (73).
ST vaccine delivery.
Most published studies use antibody responses in serum as a measure of immunogenicity and antitoxin response. There is good evidence, however, that a strong mucosal antibody response with production of secretory IgA is needed for prevention of ETEC disease (50). Oral delivery of the vaccine is therefore a natural first approach, presenting antigens directly to the gut-associated lymphoid tissue. An oral vaccine is also desirable, because it can be administrated easily to children without the need for injections. Attempts at expressing ST in attenuated bacteria intended for oral delivery include use of attenuated Salmonella, Shigella, and Lactobacillus reuteri (4, 65, 74). Alternative routes to elicit mucosal responses could include transgenic foods or nasal or rectal administration. Some evidence suggests that transcutaneous immunization can induce secretory IgA, as can parenteral priming followed by mucosal boost (13). Most published animal experiments have used oral or intraperitoneal administration, with a few studies administering the vaccine candidate by subcutaneous or intramuscular injections.
Vaccine challenges and safety issues.
A successful ST vaccine must be able to elicit a strong immune response to ST in the gut. Provided this can be achieved, there are still concerns as to whether an ST-based vaccine can be efficacious (49). The molar ratio of anti-ST antibody needed for neutralization of the toxin in the suckling mouse assay seems to be approximately 1:1 (53). As far as we are aware, there are no estimates of the amount of ST toxin produced during an ETEC infection in humans, and a concern is that the gut might be unable to produce the amounts of anti-ST antibodies required to effectively neutralize ST. However, even if it may be impossible to achieve a complete neutralization of ST, an ST vaccine may give partial protection against the ST toxin and hence effectively reduce the ETEC disease burden.
A particular safety issue that must be addressed prior to clinical evaluation of a vaccine candidate is the possibility of cross-reactivity between anti-ST antibodies and endogenous peptides of the guanylin family. Since the endogenous GC-C ligands and ST have similar structures and activate the same receptor, cross-reactivity due to epitope mimicry may occur. The ideal ST vaccine should elicit neutralizing antibodies that bind specifically to ST epitopes not shared by the guanylin peptides. It should be noted, however, that cross-reactivity shown in vitro does not necessarily imply that a vaccine will induce clinical autoimmune disease (64). Conversely, lack of demonstrable cross-reactivity in vitro does not exclude autoimmune reactions. We are not aware of any publications that have addressed these important ST vaccine safety aspects.
CONCLUSIONS
The most promising ETEC vaccine to date is a killed whole-cell vaccine comprising five different ETEC strains, which express the most prevalent CFs, coadministered with recombinant cholera toxin B subunit (8, 48). The information reviewed here suggests that a multivalent ETEC vaccine can be improved by adding an ST toxoid component. Since ST is present in approximately three-quarters of symptomatic ETEC infections (61), an ST toxoid-containing vaccine has the potential of covering a broad range of ETEC infections. Even if an ST vaccine does not induce full protection against the ST toxin, it could still substantially reduce the severity of ETEC infections, as has been demonstrated for cholera vaccines (50, 59).
To summarize, a successful ST vaccine should consist of a nontoxic ST mutant coupled, either by chemical conjugation or by recombinant fusion, to a carrier, in order to evoke an adequate immune response. Despite the fact that only a few residues have been subject to extensive substitution experiments and the exact mechanism of interaction between ST and the GC-C receptor remains unknown, several promising toxoid candidates have been developed. These results encourage further research on ST as a vaccine target.
Acknowledgments
We thank Hans Steinsland for providing valuable comments on the manuscript and Ann-Mari Svennerholm for providing supplementary information on her experiments with ST mutants.
This work was supported by the Research Council of Norway, GLOBVAC program, grant number 185872/S50.
Editor: A. T. Maurelli
Footnotes
Published ahead of print on 15 March 2010.
REFERENCES
- 1.Aimoto, S. S., H. H. Watanabe, H. H. Ikemura, Y. Y. Shimonishi, T. T. Takeda, Y. Y. Takeda, and T. T. Miwatani. 1983. Chemical synthesis of a highly potent and heat-stable analog of an enterotoxin produced by a human strain of enterotoxigenic Escherichia coli. Biochem. Biophys. Res. Commun. 112:320-326. [DOI] [PubMed] [Google Scholar]
- 2.Batisson, I., and M. D. Vartanian. 2000. Contribution of defined amino acid residues to the immunogenicity of recombinant Escherichia coli heat-stable enterotoxin fusion proteins. FEMS Microbiol. Lett. 192:223-229. [DOI] [PubMed] [Google Scholar]
- 3.Batisson, I., M. D. Vartanian, B. Gaillard-Martinie, and M. Contrepois. 2000. Full capacity of recombinant Escherichia coli heat-stable enterotoxin fusion proteins for extracellular secretion, antigenicity, disulfide bond formation, and activity. Infect. Immun. 68:4064-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cárdenas, L., and J. D. Clements. 1993. Development of mucosal protection against the heat-stable enterotoxin (ST) of Escherichia coli by oral immunization with a genetic fusion delivered by a bacterial vector. Infect. Immun. 61:4629-4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chan, S. K., and R. A. Giannella. 1981. Amino acid sequence of heat-stable enterotoxin produced by Escherichia coli pathogenic for man. J. Biol. Chem. 256:7744-7746. [PubMed] [Google Scholar]
- 6.Clemens, J. D., J. R. Harris, D. A. Sack, J. Chakraborty, F. Ahmed, B. F. Stanton, M. U. Khan, B. A. Kay, N. Huda, M. R. Khan, et al. 1988. Field trial of oral cholera vaccines in Bangladesh: results of one year of follow-up. J. Infect. Dis. 158:60-69. [DOI] [PubMed] [Google Scholar]
- 7.Clemens, J. D., D. A. Sack, J. R. Harris, J. Chakraborty, M. R. Khan, B. F. Stanton, B. A. Kay, M. U. Khan, M. Yunus, W. Atkinson, et al. 1986. Field trial of oral cholera vaccines in Bangladesh. Lancet ii:124-127. [DOI] [PubMed] [Google Scholar]
- 8.Clemens, J. D., D. A. Sack, J. R. Harris, J. Chakraborty, P. K. Neogy, B. Stanton, N. Huda, M. U. Khan, B. A. Kay, M. R. Khan, et al. 1988. Cross-protection by B subunit-whole cell cholera vaccine against diarrhea associated with heat-labile toxin-producing enterotoxigenic Escherichia coli: results of a large-scale field trial. J. Infect. Dis. 158:372-377. [DOI] [PubMed] [Google Scholar]
- 9.Clements, J. D. 1990. Construction of a nontoxic fusion peptide for immunization against Escherichia coli strains that produce heat-labile and heat-stable enterotoxins. Infect. Immun. 58:1159-1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crane, M. R., M. Hugues, P. D. O'Hanley, and S. A. Waldman. 1992. Identification of two affinity states of low affinity receptors for Escherichia coli heat-stable enterotoxin: correlation of occupation of lower affinity state with guanylate cyclase activation. Mol. Pharmacol. 41:1073-1080. [PubMed] [Google Scholar]
- 11.Gaastra, W., and A. M. Svennerholm. 1996. Colonization factors of human enterotoxigenic Escherichia coli (ETEC). Trends Microbiol. 4:444-452. [DOI] [PubMed] [Google Scholar]
- 12.Giannella, R. A. 1976. Suckling mouse model for detection of heat-stable Escherichia coli enterotoxin: characteristics of the model. Infect. Immun. 14:95-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glenn, G. M., S. A. Frech, R. T. Kenney, and L. R. Ellingsworth. 2005. Transcutaneous immunization: antigen and adjuvant delivery to the skin. Drugs Pharm. Sci. 155:769-788. [Google Scholar]
- 14.Guzman-Verduzco, L. M., and Y. M. Kupersztoch. 1987. Fusion of Escherichia coli heat-stable enterotoxin and heat-labile enterotoxin B subunit. J. Bacteriol. 169:5201-5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasegawa, M., and Y. Shimonishi. 2005. Recognition and signal transduction mechanism of Escherichia coli heat-stable enterotoxin and its receptor, guanylate cyclase C. J. Pept. Res. 65:261-271. [DOI] [PubMed] [Google Scholar]
- 16.Houghten, R. A., J. M. Ostresh, and F. A. Klipstein. 1984. Chemical synthesis of an octadecapeptide with the biological and immunological properties of human heat-stable Escherichia coli enterotoxin. Eur. J. Biochem. 145:157-162. [DOI] [PubMed] [Google Scholar]
- 17.Kauffman, P. E. 1981. Production and evaluation of antibody to the heat-stable enterotoxin from a human strain of enterotoxigenic Escherichia coli. Appl. Environ. Microbiol. 42:611-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy, D. J., R. N. Greenberg, J. A. Dunn, R. Abernathy, J. S. Ryerse, and R. L. Guerrant. 1984. Effects of Escherichia coli heat-stable enterotoxin STb on intestines of mice, rats, rabbits, and piglets. Infect. Immun. 46:639-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klipstein, F. A., and R. F. Engert. 1979. Protective effect of active immunization with purified Escherichia coli heat-labile enterotoxin in rats. Infect. Immun. 23:592-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klipstein, F. A., R. F. Engert, and J. D. Clements. 1982. Development of a vaccine of cross-linked heat-stable and heat-labile enterotoxins that protects against Escherichia coli producing either enterotoxin. Infect. Immun. 37:550-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klipstein, F. A., R. F. Engert, and J. D. Clements. 1981. Protection in rats immunized with Escherichia coli heat-stable enterotoxin. Infect. Immun. 34:637-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klipstein, F. A., R. F. Engert, J. D. Clements, and R. A. Houghten. 1983. Protection against human and porcine enterotoxigenic strains of Escherichia coli in rats immunized with a cross-linked toxoid vaccine. Infect. Immun. 40:924-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klipstein, F. A., R. F. Engert, J. D. Clements, and R. A. Houghten. 1983. Vaccine for enterotoxigenic Escherichia coli based on synthetic heat-stable toxin crossed-linked to the B subunit of heat-labile toxin. J. Infect. Dis. 147:318-326. [DOI] [PubMed] [Google Scholar]
- 24.Klipstein, F. A., R. F. Engert, and R. A. Houghten. 1986. Immunisation of volunteers with a synthetic peptide vaccine for enterotoxigenic Escherichia coli. Lancet i:471-472. [DOI] [PubMed] [Google Scholar]
- 25.Klipstein, F. A., R. F. Engert, and R. A. Houghten. 1985. Mucosal antitoxin response in volunteers to immunization with a synthetic peptide of Escherichia coli heat-stable enterotoxin. Infect. Immun. 50:328-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klipstein, F. A., R. F. Engert, and R. A. Houghten. 1984. Properties of cross-linked toxoid vaccines made with hyperantigenic forms of synthetic Escherichia coli heat-stable toxin. Infect. Immun. 44:268-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klipstein, F. A., R. F. Engert, and R. A. Houghten. 1983. Properties of synthetically produced Escherichia coli heat-stable enterotoxin. Infect. Immun. 39:117-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowenadler, B. 1991. A recombinant Escherichia coli heat-stable enterotoxin (STa) fusion protein eliciting anti-STa neutralizing antibodies. FEMS Microbiol. Lett. 82:271. [DOI] [PubMed] [Google Scholar]
- 29.Marx, U. C., J. Klodt, M. Meyer, H. Gerlach, P. Rosch, W. G. Forssmann, and K. Adermann. 1998. One peptide, two topologies: structure and interconversion dynamics of human uroguanylin isomers. J. Pept. Res. 52:229-240. [DOI] [PubMed] [Google Scholar]
- 30.Moss, J., J. C. Osborne, P. H. Fishman, S. Nakaya, and D. C. Robertson. 1981. Escherichia coli heat-labile enterotoxin. Ganglioside specificity and ADP-ribosyltransferase activity. J. Biol. Chem. 256:2861-2865. [PubMed] [Google Scholar]
- 31.Okamoto, K., K. Okamoto, J. Yukitake, Y. Kawamoto, and A. Miyama. 1987. Substitutions of cysteine residues of Escherichia coli heat-stable enterotoxin by oligonucleotide-directed mutagenesis. Infect. Immun. 55:2121-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okamoto, K., K. Okamoto, J. Yukitake, and A. Miyama. 1988. Reduction of enterotoxic activity of Escherichia coli heat-stable enterotoxin by substitution for an asparagine residue. Infect. Immun. 56:2144-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okamoto, K., and M. Takahara. 1990. Synthesis of Escherichia coli heat-stable enterotoxin STp as a pre-pro form and role of the pro sequence in secretion. J. Bacteriol. 172:5260-5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ozaki, H., T. Sato, H. Kubota, Y. Hata, Y. Katsube, and Y. Shimonishi. 1991. Molecular structure of the toxin domain of heat-stable enterotoxin produced by a pathogenic strain of Escherichia coli. A putative binding site for a binding protein on rat intestinal epithelial cell membranes. J. Biol. Chem. 266:5934-5941. [PubMed] [Google Scholar]
- 35.Pereira, C. M., B. E. Guth, M. E. Sbrogio-Almeida, and B. A. Castilho. 2001. Antibody response against Escherichia coli heat-stable enterotoxin expressed as fusions to flagellin. Microbiology 147:861-867. [DOI] [PubMed] [Google Scholar]
- 36.Rasheed, J. K., L. M. Guzman-Verduzco, and Y. M. Kupersztoch. 1990. Two precursors of the heat-stable enterotoxin of Escherichia coli: evidence of extracellular processing. Mol. Microbiol. 4:265-273. [DOI] [PubMed] [Google Scholar]
- 37.Rosales-Mendoza, S., A. G. Alpuche-Solis, R. E. Soria-Guerra, L. Moreno-Fierros, L. Martinez-Gonzalez, A. Herrera-Diaz, and S. S. Korban. 2009. Expression of an Escherichia coli antigenic fusion protein comprising the heat labile toxin B subunit and the heat stable toxin, and its assembly as a functional oligomer in transplastomic tobacco plants. Plant J. 57:45-54. [DOI] [PubMed] [Google Scholar]
- 38.Saarilahti, H. T., E. T. Palva, J. Holmgren, and J. Sanchez. 1989. Fusion of genes encoding Escherichia coli heat-stable enterotoxin and outer membrane protein OmpC. Infect. Immun. 57:3663-3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sanchez, J., S. Johansson, B. Lowenadler, A. M. Svennerholm, and J. Holmgren. 1990. Recombinant cholera toxin B subunit and gene fusion proteins for oral vaccination. Res. Microbiol. 141:971-979. [DOI] [PubMed] [Google Scholar]
- 40.Sanchez, J., A. M. Svennerholm, and J. Holmgren. 1988. Genetic fusion of a non-toxic heat-stable enterotoxin-related decapeptide antigen to cholera toxin B-subunit. FEBS Lett. 241:110-114. [DOI] [PubMed] [Google Scholar]
- 41.Sanchez, J., B. E. Uhlin, T. Grundstrom, J. Holmgren, and T. R. Hirst. 1986. Immunoactive chimeric ST-LT enterotoxins of Escherichia coli generated by in vitro gene fusion. FEBS Lett. 208:194-198. [DOI] [PubMed] [Google Scholar]
- 42.Sato, T., H. Ozaki, Y. Hata, Y. Kitagawa, Y. Katsube, and Y. Shimonishi. 1994. Structural characteristics for biological activity of heat-stable enterotoxin produced by enterotoxigenic Escherichia coli: X-ray crystallography of weakly toxic and nontoxic analogs. Biochemistry 33:8641-8650. [DOI] [PubMed] [Google Scholar]
- 43.Schulz, S., C. K. Green, P. S. Yuen, and D. L. Garbers. 1990. Guanylyl cyclase is a heat-stable enterotoxin receptor. Cell 63:941-948. [DOI] [PubMed] [Google Scholar]
- 44.Schulz, S., M. J. Lopez, M. Kuhn, and D. L. Garbers. 1997. Disruption of the guanylyl cyclase-C gene leads to a paradoxical phenotype of viable but heat-stable enterotoxin-resistant mice. J. Clin. Invest. 100:1590-1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sizemore, D. R., K. L. Roland, and U. S. Ryan. 2004. Enterotoxigenic Escherichia coli virulence factors and vaccine approaches. Expert Rev. Vaccines 3:585-595. [DOI] [PubMed] [Google Scholar]
- 46.Sjoling, A., F. Qadri, M. Nicklasson, Y. A. Begum, G. Wiklund, and A. M. Svennerholm. 2006. In vivo expression of the heat stable (estA) and heat labile (eltB) toxin genes of enterotoxigenic Escherichia coli (ETEC). Microbes Infect. 8:2797-2802. [DOI] [PubMed] [Google Scholar]
- 47.So, M., and B. J. McCarthy. 1980. Nucleotide sequence of the bacterial transposon Tn1681 encoding a heat-stable (ST) toxin and its identification in enterotoxigenic Escherichia coli strains. Proc. Natl. Acad. Sci. U. S. A. 77:4011-4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Svennerholm, A.-M., and J. Tobias. 2008. Vaccines against enterotoxigenic Escherichia coli. Expert Rev. Vaccines 7:795-804. [DOI] [PubMed] [Google Scholar]
- 49.Svennerholm, A. M., C. Åhren, and M. Jertborn. 1997. Oral inactivated vaccines against enterotoxigenic Escherichia coli, p. 865-873. In M. M. Levine, G. C. Woodrow, J. B. Kaper, and G. S. Cobon (ed.), New generation vaccines. Marcel Dekker, Inc., New York, NY.
- 50.Svennerholm, A. M., and J. J. Holmgren. 1995. Oral vaccines against cholera and enterotoxigenic Escherichia coli diarrhea. Adv. Exp. Med. Biol. 371B:1623-1628. [PubMed] [Google Scholar]
- 51.Svennerholm, A. M., M. Lindblad, B. Svennerholm, and J. Holmgren. 1988. Synthesis of nontoxic, antibody-binding Escherichia coli heat-stable enterotoxin (ST (a)) peptides. FEMS Microbiol. Lett. 55:23-28. [Google Scholar]
- 52.Svennerholm, A. M., M. Wikstrom, M. Lindblad, and J. Holmgren. 1986. Monoclonal antibodies against Escherichia coli heat-stable toxin (STa) and their use in a diagnostic ST ganglioside GM1-enzyme-linked immunosorbent assay. J. Clin. Microbiol. 24:585-590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takeda, T., G. B. Nair, K. Suzuki, H. X. Zhe, Y. Yokoo, P. D. Mol, W. Hemelhof, J. P. Butzler, Y. Takeda, and Y. Shimonishi. 1993. Epitope mapping and characterization of antigenic determinants of heat-stable enterotoxin (STh) of enterotoxigenic Escherichia coli by using monoclonal antibodies. Infect. Immun. 61:289-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thompson, M. R., and R. A. Giannella. 1985. Revised amino acid sequence for a heat-stable enterotoxin produced by an Escherichia coli strain (18D) that is pathogenic for humans. Infect. Immun. 47:834-836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turner, S. M., R. R. Chaudhuri, Z. D. Jiang, H. DuPont, C. Gyles, C. W. Penn, M. J. Pallen, and I. R. Henderson. 2006. Phylogenetic comparisons reveal multiple acquisitions of the toxin genes by enterotoxigenic Escherichia coli strains of different evolutionary lineages. J. Clin. Microbiol. 44:4528-4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Uzzau, S., and A. Fasano. 2000. Cross-talk between enteric pathogens and the intestine. Cell. Microbiol. 2:83-89. [DOI] [PubMed] [Google Scholar]
- 57.Vaandrager, A. B. 2002. Structure and function of the heat-stable enterotoxin receptor/guanylyl cyclase C. Mol. Cell. Biochem. 230:73-83. [PubMed] [Google Scholar]
- 58.Valentiner-Branth, P., H. Steinsland, T. K. Fischer, M. Perch, F. Scheutz, F. Dias, P. Aaby, K. Mølbak, and H. Sommerfelt. 2003. Cohort study of Guinean children: incidence, pathogenicity, conferred protection, and attributable risk for enteropathogens during the first 2 years of life. J. Clin. Microbiol. 41:4238-4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Loon, F. P. L., J. D. Clemens, J. Chakraborty, M. R. Rao, B. A. Kay, D. A. Sack, M. Yunus, M. Ali, A. M. Svennerholm, and J. Holmgren. 1996. Field trial of inactivated oral cholera vaccines in Bangladesh: results from 5 years of follow-up. Vaccine 14:162-166. [DOI] [PubMed] [Google Scholar]
- 60.Waldman, S. A., and P. O'Hanley. 1989. Influence of a glycine or proline substitution on the functional properties of a 14-amino-acid analog of Escherichia coli heat-stable enterotoxin. Infect. Immun. 57:2420-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walker, R. I., D. Steele, T. Aguado, and the Ad Hoc ETEC Technical Expert Committee. 2007. Analysis of strategies to successfully vaccinate infants in developing countries against enterotoxigenic E. coli (ETEC) disease. Vaccine 25:2545-2566. [DOI] [PubMed] [Google Scholar]
- 62.Wolf, M. K. 1997. Occurrence, distribution, and associations of O and H serogroups, colonization factor antigens, and toxins of enterotoxigenic Escherichia coli. Clin. Microbiol. Rev. 10:569-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.World Health Organization. 2006. Future directions for research on enterotoxigenic Escherichia coli vaccines for developing countries. Wkly. Epidemiol. Rec. 81:97. [PubMed] [Google Scholar]
- 64.Wraith, D. C., M. Goldman, and P. H. Lambert. 2003. Vaccination and autoimmune disease: what is the evidence? Lancet 362:1659-1666. [DOI] [PubMed] [Google Scholar]
- 65.Wu, C.-M., and T.-C. Chung. 2007. Mice protected by oral immunization with Lactobacillus reuteri secreting fusion protein of Escherichia coli enterotoxin subunit protein. FEMS Immunol. Med. Microbiol. 50:354-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamanaka, H., M. Kameyama, T. Baba, Y. Fujii, and K. Okamoto. 1994. Maturation pathway of Escherichia coli heat-stable enterotoxin I: requirement of DsbA for disulfide bond formation. J. Bacteriol. 176:2906-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamanaka, H., T. Nomura, Y. Fujii, and K. Okamoto. 1997. Extracellular secretion of Escherichia coli heat-stable enterotoxin I across the outer membrane. J. Bacteriol. 179:3383-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yamanaka, H., T. Nomura, Y. Fujii, and K. Okamoto. 1998. Need for TolC, an Escherichia coli outer membrane protein, in the secretion of heat-stable enterotoxin I across the outer membrane. Microb. Pathog. 25:111-120. [DOI] [PubMed] [Google Scholar]
- 69.Yamanaka, H., T. Nomura, and K. Okamoto. 1998. Involvement of glutamic acid residue at position 7 in the formation of the intramolecular disulfide bond of Escherichia coli heat-stable enterotoxin Ip in vivo. Microb. Pathog. 24:145-154. [DOI] [PubMed] [Google Scholar]
- 70.Yamanaka, H., and K. Okamoto. 2000. Mutation of aromatic amino acid residues located at the amino- and carboxy-termini of Escherichia coli heat-stable enterotoxin Ip reduces the efficiency of the toxin to cross the outer membrane. Microbiol. Immunol. 44:481-488. [DOI] [PubMed] [Google Scholar]
- 71.Yamasaki, S., T. Sato, Y. Hidaka, H. Ozaki, H. Ito, T. Hirayama, Y. Takeda, T. Sugimura, A. Tai, and Y. Shimonishi. 1990. Structure-activity relationship of Escherichia coli heat-stable enterotoxin: role of Ala residue at position 14 in toxin-receptor interaction. Bull. Chem. Soc. Jpn. 63:2063-2070. [Google Scholar]
- 72.Yoshimura, S., H. Ikemura, H. Watanabe, S. Aimoto, Y. Shimonishi, S. Hara, T. Takeda, T. Miwatani, and Y. Takeda. 1985. Essential structure for full enterotoxigenic activity of heat-stable enterotoxin produced by enterotoxigenic Escherichia coli. FEBS Lett. 181:138-142. [DOI] [PubMed] [Google Scholar]
- 73.Zhang, W., C. Zhang, D. H. Francis, Y. Fang, D. Knudsen, J. P. Nataro, and D. C. Robertson. 2010. Genetic fusions of LTAB and STa toxoids of porcine enterotoxigenic Escherichia coli (ETEC) elicited neutralizing anti-LT and anti-STa antibodies. Infect. Immun. 78:316-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zheng, J. P., Z. S. Zhang, S. Q. Li, X. X. Liu, S. L. Yuan, P. Wang, D. W. Zhan, L. C. Wang, and C. F. Huang. 2005. Construction of a novel Shigella live-vector strain coexpressing CS3 and LTB/STm of enterotoxigenic E.coli. World J. Gastroenterol. 11:3411-3418. [DOI] [PMC free article] [PubMed] [Google Scholar]