Abstract
Hepatitis C virus (HCV), a member of the Flaviviridae family, is a single-stranded positive-sense RNA virus that infects >170 million people worldwide and causes acute and chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Despite its ability to block the innate host response in infected hepatocyte cell lines in vitro, HCV induces a strong type 1 interferon (IFN) response in the infected liver. The source of IFN in vivo and how it is induced are currently undefined. Here we report that HCV-infected cells trigger a robust IFN response in plasmacytoid dendritic cells (pDCs) by a mechanism that requires active viral replication, direct cell-cell contact, and Toll-like receptor 7 signaling, and we show that the activated pDC supernatant inhibits HCV infection in an IFN receptor-dependent manner. Importantly, the same events are triggered by HCV subgenomic replicon cells but not by free virus particles, suggesting the existence of a novel cell-cell RNA transfer process whereby HCV-infected cells can activate pDCs to produce IFN without infecting them. These results may explain how HCV induces IFN production in the liver, and they reveal a heretofore unsuspected aspect of the innate host response to viruses that can subvert the classical sensing machinery in the cells they infect, and do not infect or directly activate pDCs.
Keywords: innate immune response, TLR7, Toll-like receptor, hepatocyte
Hepatitis C virus (HCV), a member of the Flaviviridae family, is a single-stranded positive-sense RNA virus that causes acute and chronic hepatitis, cirrhosis, and hepatocellular carcinoma (1). Type 1 interferons (IFNα/β) play critical roles in the defense against virus infection. The HCV NS3/4A protease strongly inhibits type 1 IFN induction in infected cells by cleaving a key intermediate in the double-stranded RNA (dsRNA) signaling pathway (2–4). Nonetheless, HCV strongly induces IFN-stimulated gene (ISG) expression in the infected liver (5–7). The discrepancy between these observations suggests that type 1 IFNs may be produced by liver cells other than infected hepatocytes. Plasmacytoid dendritic cells (pDCs) are a highly specialized subset of dendritic cells that produce type 1 IFNs in response to microbial stimuli (8, 9) and are abundant in the HCV-infected liver (10). Although HCV has been reported to suppress pDC numbers and function (11–13), their role in the control of HCV infection has not been examined. Here we show that pDCs produce large amounts of type 1 IFN via Toll-like receptor 7 (TLR7) signaling that is induced by direct cell-to-cell contact with HCV-infected cells. Importantly, these events require viral RNA replication but not virion formation in the stimulator cells. These results could explain how IFN is produced during natural HCV infection, and they reveal a host response mechanism to HCV and possibly other viruses that do not infect or directly activate pDCs.
Results
Robust IFN Production by Cocultivation of pDCs with HCV-Infected Cells.
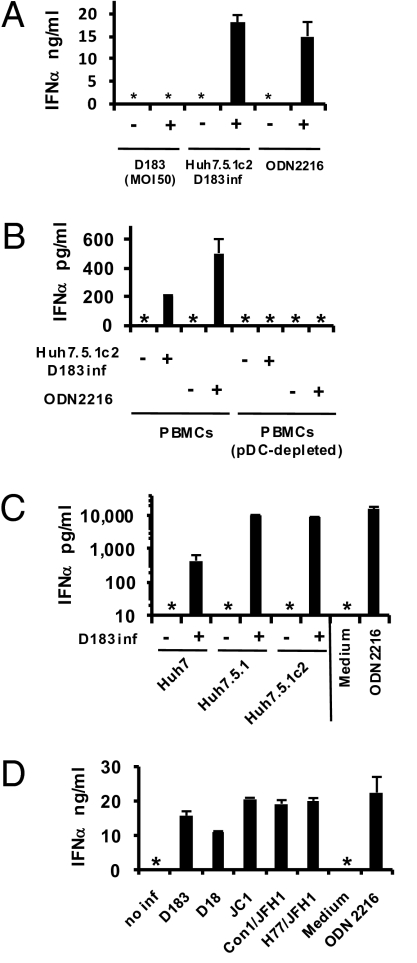
We asked whether highly purified human peripheral blood-derived pDCs (Fig. S1A) produce IFNα in response to HCV-infected cells. As shown in Fig. 1A, pDCs produced >15 ng/mL of IFNα after 24 h of coculture with Huh7.5.1c2 cells (14) that were infected by HCV JFH1/D183 virus (D183) (15), comparable to their response to ODN2216, a potent Toll-like receptor 9 (TLR9) agonist (16). In contrast, highly concentrated, gradient-purified D183 virus could not induce pDC IFNα secretion (<12.5 pg/mL), even at a multiplicity of infection (MOI) of 50 infectious virus particles (∼50,000 HCV RNA-positive particles per pDC) for 24 h at 37 °C (Fig. 1A). Monocyte-derived macrophages and dendritic cells did not produce IFNα or IFNβ in response to infected cells (Fig. S1B Left and Middle), although both produced IL-6 in response to lipopolysaccharide and poly-IC (Fig. S1B Right). The amount of secreted IFNα depended on the number of HCV-infected cells and pDCs (Fig. S1C). Total, but not pDC-depleted, peripheral blood lymphomononuclear cells (PBMCs) produced IFNα when cocultured with D183-infected Huh7.5.1c2 cells (Fig. 1B), indicating that pDCs are the source of IFNα in the PBMC population. Importantly, D183-infected Huh7 and Huh7.5.1 cells also induced IFNα (Fig. 1C) and IFNβ production (Fig. S1D) by pDCs, as did Huh7.5.1c2 cells infected by JFH1/D18 (D18) virus (15) and by chimeric HCV strains (17) based on JFH1 (Fig. 1D and Fig. S1E).
Fig. 1.
IFNα production by coculture of pDCs and HCV-infected cells. (A) pDCs were inoculated with purified D183 virus at an MOI of 50 or cocultured with D183-infected (3 days) or uninfected Huh7.5.1c2 cells. After 24 h, IFNα production was assessed by ELISA. ODN2216 was included at 1 μM. (B) PBMCs (2 × 105) or a pDC-depleted fraction of PBMCs were cocultured (24 h) with D183-infected (3 days) or uninfected Huh7.5.1c2 cells. IFNα production was assessed by ELISA. (C) pDCs were cocultured (24 h) with D183-infected (7 days) or uninfected Huh7, Huh7.5.1, or Huh7.5.1c2 cells at an MOI of 0.01. IFNα production was assessed by ELISA. (D) pDCs were cocultured (24 h) with Huh7.5.1c2 cells infected (7 days) or not with the indicated HCV strains at an MOI of 0.01. IFNα production was assessed by ELISA. n = 3, mean values ± SD (A–C); n = 2, mean values ± average deviation (AVEDEV) (D). No inf, not infected; inf, infected; *, not detected, <12.5 pg/mL.
Kinetics of pDC IFN Induction and Establishment of an Antiviral State.
The IFN-inducing ability of the HCV-infected Huh7.5.1c2 cells correlated directly with their HCV RNA content (Fig. 2A Left and Fig. S2A), and it was suppressed in parallel with the reduction of HCV RNA by the specific HCV NS3/4A serine protease inhibitor BILN2061 (Fig. 2A Right). Because phagocytosis of virus-infected apoptotic cells induces the maturation of conventional DCs (18, 19), we determined whether type 1 IFN production was induced by apoptotic Huh7.5.1c2 cells. As shown in Fig. S2B, no apparent cytopathic effect could be detected by TUNEL assay in D183-infected cells up to day 3 postinoculation, when virtually all of the cells were HCV-positive and could strongly trigger pDC IFN production (Fig. 2A). In addition, their IFN-inducing activity was abolished by radiation-induced apoptosis (Fig. S2C) and by freeze-thaw-induced lysis (Fig. S2D). These results indicate that production of the activating signal and/or the signal transfer process requires viable cells and is not induced by phagocytosis of dying cells. IFNα secretion by pDCs was rapid, starting between 4 and 12 h of coculture and increasing in a time-dependent manner with kinetics similar to ODN2216 stimulation (Fig. 2B). Accordingly, ISGs (MxA, ISG15, and ISG56) were induced at the mRNA (Fig. 2C) and protein level (Fig. S2E), suggesting establishment of an antiviral state. Importantly, supernatant from the activated pDCs prevented HCV infection as efficiently as 100 U/mL of recombinant IFNα, and its antiviral effect was neutralized by anti-IFNα/β receptor antibody (Fig. 2D).
Fig. 2.
Kinetics of IFNα production by pDCs. (A) pDCs were cocultured (24 h) with D183-infected Huh7.5.1c2 cells as indicated (left) or D183-infected (3 days) Huh7.5.1c2 cells that were treated with BILN2061 (1 μM) before coculture as indicated (right). IFNα secretion and intracellular HCV RNA were assessed by ELISA and RT-qPCR, respectively. (B) pDCs were cocultured with D183-infected (3 days) or uninfected Huh7.5.1c2 cells or stimulated by ODN2216 (1 μM) for the indicated periods of time and IFNα production was assessed by ELISA. (C) Cocultured cell mixtures in B were analyzed for MxA, ISG15, and ISG56 mRNA induction by RT-qPCR. Results are displayed as fold induction relative to uninfected cells. (D) Huh7.5.1c2 cells (2.5 × 104) were treated or not with 10 μg/mL anti-IFNα/β receptor antibody (IFNα/β R Ab) (1 h), followed by incubation (2 h) with recombinant IFNα (100 U/mL), control media, or supernatants that were harvested 24 h after coculture of pDCs and D183-infected (3 days) Huh7.5.1c2 cells (inf sup). Then cells were inoculated with D183 virus at an MOI of 0.1. After 48 h, levels of intracellular HCV RNA were monitored by RT-qPCR. Results are displayed as the percentage of HCV RNA in the media control in the absence of anti-IFNα/β receptor antibody. n = 3, mean values ± SD (A–C); n = 2, mean values ± AVEDEV (D). Inf, infected.
IFN Is Produced Only by pDCs in Cocultures.
To determine which of the cocultured cell populations produce type 1 IFN, we compared the IFNα and IFNβ mRNA content of mixed pDC-Huh7.5.1c2 cocultures with the corresponding pDC-enriched and pDC-depleted subpopulations (Fig. 3A). In addition, we monitored intracellular IFNα protein content by FACS analysis of the cocultured cells using pDC-specific and IFNα-specific antibodies (Fig. 3B). IFNα and IFNβ mRNA were detected in the mixed-cell and the pDC-enriched populations but not in the pDC-depleted fraction (Fig. 3A). Similarly, intracellular IFNα protein was only detected in the pDC-gated fraction present in the mixed-cell cultures (Fig. 3B). Collectively, these results indicate that the IFNs were produced exclusively by the pDCs. Interestingly, only a minor subset (≈10%) of pDCs was activated by the infected Huh7.5.1c2 cells (Fig. 3B Lower Right). Whether this reflects differential IFN responsiveness of pDC subsets or differences in the magnitude or kinetics of the IFN response that the pDCs produce remains to be determined.
Fig. 3.
Contribution of HCV-infected cells to type 1 IFN induction. pDCs were cocultured with D183-infected (3 days) or uninfected Huh7.5.1c2 cells. (A) After 14 h, pDCs were enriched as described in Materials and Methods, and RNA from pDC-enriched and pDC-depleted fractions were subjected to IFNα and IFNβ mRNA analysis by RT-qPCR. Results are displayed as the percentage of expression in cocultured cell mixtures before pDC enrichment (n = 2, mean values ± AVEDEV). (B) After 14 h of incubation individually or together with pDCs, D183-infected or uninfected Huh7.5.1c2 cells and cocultured mixtures were stained for IFNα, CD123, and BDCA-2. The results for the total mixed-cell population and for cells gated on cell size, granularity, CD123 positivity, and BDCA-2 positivity (i.e., pDCs) are displayed in the left and right panels, respectively. D183-infected Huh7.5.1c2 cells and mixed cultures are shown in the upper and lower panels, respectively. Note that the pDC-gated population of cocultured IFNα-producing cells does not contain Huh7.5.1c2 cells (compare right upper and lower panels) and that IFNα-producing cells in the mixed population mirror the same population in the pDC-gated fraction (compare left and right lower panels). Inf, infected.
Mechanism of pDC IFN Production Triggered by HCV-Infected Cells.
Next, we examined the mechanism whereby HCV-infected cells trigger pDC IFNα production. Importantly, IFNα production was completely abrogated when pDCs and HCV-infected cells were separated in a transwell plate, indicating that direct cell-to-cell contact is required for pDC IFNα production (Fig. 4A). Neutralizing anti-IFNα/β receptor antibody partially inhibited pDC IFNα production (Fig. 4A), implying that an IFN-mediated autoamplification loop enhances IFN production in this system, either by autocrine or paracrine mechanisms or by prolonging pDC survival (Fig. S3A and SI Discussion). HCV envelope glycoproteins (E1/E2) mediate viral attachment to cellular receptors. However, neutralizing anti-HCV E2 antibody (20) did not inhibit pDC IFNα production, indicating that receptor-mediated entry of HCV into pDCs is not required (Fig. 4A). Importantly, Huh7.5.1c2 cells that stably or transiently replicate JFH1 subgenomic replicons (SGR) that do not produce virus particles also triggered robust pDC IFNα production (Fig. 4B), indicating that pDC IFN production can occur in the absence of viral structural proteins and virion production. In contrast, Huh7.5.1c2 cells transfected with a replication-defective (GND-mutant) replicon (Fig. 4B), GND-mutant genomic JFH1 RNA, or negative-strand JFH1 RNA (Fig. S3B) did not induce IFNα, even though they contained comparable levels of total-input HCV RNA as determined by reverse-transcription real-time quantitative PCR (RT-qPCR) (Fig. 4B and Fig. S3B).
Fig. 4.
Mechanisms of type 1 IFN production by pDCs. (A) pDCs were cocultured (24 h) with D183-infected (2 days) Huh7.5.1c2 cells in the presence or absence of 10 μg/mL anti-IFNα/β receptor antibody or 10 μg/mL anti-HCV E2 antibody, or they were separated by a transwell insert, and IFNα production was assessed by ELISA. (B) pDCs were cocultured (24 h) with Huh7.5.1c2 cells transfected 48 h before coculture with 10 μg in vitro synthesized RNA of wild-type (WT) or replication-defective GND mutant of JFH1 subgenomic replicon (SGR), or Huh7.5.1c2 cells bearing JFH1 SGR. IFNα production and HCV RNA was assessed by ELISA (upper panel) and RT-qPCR (lower panel), respectively. (C) pDCs were preincubated with 0.175 μM IRS661, control ODN, or media for 30 min, and cocultured with D183-infected (2 days) Huh7.5.1c2 cells or stimulated by resiquimod (50 ng/mL) or ODN2216 (1 μM). After 24 h, IFNα production was assessed by ELISA. Results are displayed as the percentage of IFNα produced in the absence of IRS661 or control ODN. (D) pDCs were transfected with 10 μg/mL in vitro synthesized genomic JFH1 RNA formulated with 12.5 μL/mL DOTAP in the presence or absence of 0.175 μM IRS661 or control ODN (lanes 3–5), or stimulated by the same amount of genomic JFH1 RNA or DOTAP alone (lanes 1 and 2). pDCs were also transfected with 10 μg/mL total Huh7 cellular RNA formulated with 12.5 μL/mL DOTAP (lane 6). After 24 h, IFNα production was assessed by ELISA. (E) D183-infected (2 days) Huh7.5.1c2 cells were cocultured (20 h) with pDCs in a chamber slide. Cells were stained for HCV NS3 (red) and TLR7 (green) (left) or for IFNα (yellow), TLR7 (green), and F-actin (red) (right). Nuclei were stained with Hoechst dye (blue). A side view (lower panels) demonstrates the relative location of pDCs and D183-infected cells. Note that the right upper panel reveals three pDCs attached to a single D183-infected cell, only one of which produces IFNα. n = 3 (A–D), mean values ± SD. Inf, infected; rep, replicon; *, not detected.
To examine the nature of the viral RNA present in these cells, we repeated the transfection experiments and performed northern blot analysis to detect the HCV RNA species present in the Huh7.5.1c2 cells at the start and end of the coculture period (i.e., 24 and 40 h after transfection). As shown in Fig. S3C, full-length genomic HCV RNA was only detected in Huh7.5.1c2 cells that had been transiently or stably transfected with wild-type replication-competent JFH RNA or infected with D183 virus, and only those cells triggered pDC IFNα production. In contrast, full-length genomic HCV RNA was not detected in cells transfected with the replication-deficient (i.e., GND-mutant or negative-strand) HCV RNA when coculture was initiated, even though they contained similar amounts of total HCV RNA by qPCR (Fig. 4B and Fig. S3B). Moreover, the total amount of HCV RNA in these cells decreased during the coculture interval (Fig. S3C). These results suggest that pDC IFNα production requires active viral replication in the stimulator cells, either as a mechanism to induce pDC IFNα production or to provide a sufficient amount of the appropriate HCV RNA species needed for triggering pDC IFNα production.
pDCs are known to produce IFN if they are infected by RNA or DNA viruses via TLR7 or TLR9 activation, respectively (21). In the current experiments, the pDCs were activated by HCV RNA-producing cells, not by free HCV particles, raising the possibility of cell-cell transfer of HCV RNA to pDCs and activation of the TLR7-mediated pathway. As shown in Fig. 4C, IRS661, a specific inhibitor of TLR7-mediated signaling (22, 23), inhibited pDC IFNα production induced by both HCV-infected cells and the TLR7 agonist resiquimod but not by the TLR9 agonist ODN2216, indicating that HCV-infected cells trigger pDC IFN production via TLR7. This was supported by the ability of inhibitors of endosomal TLR signaling to abrogate IFNα production (Fig. S3D Upper) without commensurate suppression of HCV RNA (Fig. S3D Lower). Interestingly, pDCs transfected with in vitro synthesized genomic HCV RNA but not total cellular Huh7 RNA produced IFNα in a TLR7-dependent manner (Fig. 4D), indicating that HCV RNA can trigger IFNα production if it is delivered directly into the pDCs. Importantly, HCV negative-strand RNA was not detected in the wild-type HCV RNA-transfected pDCs (Fig. S4A), and pDCs still produced IFNα when transfected with wild-type HCV RNA in the presence of BILN2016 or the viral polymerase inhibitor 2-methyladenosine or when transfected with the GND-mutant genomic HCV RNA (Fig. S4B). Furthermore, transfected genomic HCV RNA of positive and negative polarity triggered pDC IFNα production (Fig. S4C). Together, these results indicate that IFN production was not triggered by HCV RNA replication in the pDCs.
Consistent with the results of the transwell experiment (Fig. 4A), confocal microscopic analysis of cocultured D183-infected Huh7.5.1c2 cells and pDCs revealed very close physical association of the two cell types (Fig. 4E Bottom) with TLR7 restricted to the pDC population (Fig. 4E, green) and HCV NS3 protein and F-actin restricted to Huh7.5.1c2 cells (Fig. 4E, red in left and right panels, respectively). Consistent with the FACS results (Fig. 3B), only some of the attached pDCs contained detectable amounts of IFNα under these conditions (Fig. 4E, yellow in right panels). Collectively, these results and the results shown in Fig. 2A strongly suggest that HCV RNA is transferred from infected cells to pDCs in which it triggers IFNα production by activating TLR7.
IFN-Inducing Ability Is Not Restricted to HCV-Replicating Cells.
Extending the generality of this cell-cell activation process, Huh7.5.1c2 cells that replicate a noncytopathic, subgenomic Venezuelan equine encephalitis virus (VEE) replicon (24) also triggered pDC IFNα production (Fig. 5A) in a TLR7-dependent manner (Fig. 5B). However, pDCs were not activated by Borna disease virus (BDV) -infected Huh7.5.1c2 cells, even though they contained similar levels of viral RNA as the HCV replicon cells (Fig. 5A). These results indicate that this mechanism is triggered by some but not all RNA virus-infected cells.
Fig. 5.
pDC IFN production triggered by VEE replicon cells. (A) pDCs (1 × 105) were cocultured (24 h) with 2 × 105 Huh7.5.1c2 cells bearing the JFH1 or VEE subgenomic replicon or BDV-infected Huh7.5.1c2 cells. IFNα production and intracellular viral RNA contents were assessed by ELISA (upper) and by RT-qPCR (lower), respectively. (B) pDCs, preincubated with 0.175 μM IRS661, control ODN, or media for 30 min, were cocultured (24 h) with 2 × 105 Huh7.5.1c2 cells bearing the JFH1 or VEE subgenomic replicon. pDCs (6 × 104 and 2 × 104) were cocultured with VEE and JFH1 replicon cells, respectively. IFNα production was assessed by ELISA. Results are displayed as the percentage of IFNα produced in the absence of IRS661 or control ODN. n = 3, mean values ± SD. Inf, infected; rep, replicon; *, not detected.
pDCs Isolated from HCV-Infected Patients Sense HCV-Infected Cells and Produce IFN.
pDCs from HCV-infected patients have been reported to be functionally impaired (11, 13). Thus, we asked whether pDCs isolated from HCV-infected patients produce IFN in response to D183-infected Huh7.5.1c2 cells. As shown in Fig. S5, pDCs from three different HCV-infected patients produced IFNα at levels that were comparable with pDCs from the 40 healthy donors we analyzed over the course of this study. These results indicate that the ability of pDCs from HCV-infected patients to produce IFN after cocultivation with HCV-infected cells is not impaired.
Discussion
The present study demonstrates that pDCs produce large quantities of type 1 IFN by direct sensing of HCV-infected cells via a TLR7-mediated pathway. Importantly, active HCV RNA replication is required for this process but virus-particle assembly and free virus particles are not. This mechanism appears to be different from that of other viruses, such as respiratory syncytial virus, influenza virus, and HIV, that trigger pDC IFNα production either by direct infection or virus stimulation (22, 23, 25–27). Although pDC sensing of HCV-infected cells apparently only occurs if those cells are replicating the viral RNA, the pDCs can produce IFN if they are directly transfected by genomic HCV RNA of positive or negative polarity. Thus, a host-cell mechanism must exist that can transfer HCV RNA from infected cells into pDCs. This mechanism is not limited to HCV, however, because pDC IFNα production can also be triggered by cells that replicate a noncytopathic subgenomic VEE replicon. Additional experiments designed to determine the RNA transfer mechanism and to detect the ability of yet other viruses to induce pDC IFN production are clearly warranted.
Although the polyuridine region in the 3′ UTR of HCV has been shown to act as a pathogen-associated molecular pattern (PAMP) (28) and TLR7 recognizes uridine-rich sequences (29), a polyuridine stretch is not an absolute requirement for pDC IFN production in our system because the VEE subgenomic replicon does not contain a polyuridine region. More experiments will be needed to determine the specific PAMP motif(s) recognized by this pDC sensing mechanism.
Only a subset of pDCs produce type 1 IFN in response to HCV-infected cells under the conditions of our experiments. This could reflect the existence of pDC subsets that are either responsive or refractory to the signal transferred from the HCV RNA-replicating cells or to the time required for the signal to be transferred to the pDCs, especially if the pDCs do not all form an active conjugate with HCV-infected cells at the same time.
Interestingly, the mechanism is restricted to interactions between human hepatocytes and human pDCs because D183-infected Huh7.5.1c2 cells did not trigger IFN production by murine pDCs (Fig. S6), and neither immortalized mouse hepatocytes nor human HeLa or HEK293 cells bearing JFH1 subgenomic replicons triggered IFNα production by human pDCs despite their abundant HCV RNA content (Table S1). It also appears to require highly differentiated hepatocytes because, despite many attempts, human hepatoblastoma-derived HepG2 cells that replicate the JFH1 subgenomic replicon did not trigger pDC IFNα production, even though their HCV RNA content was similar to the H77c RNA content of the Huh7 cells harboring a genotype 1a (H77c) replicon that triggered pDC IFNα production (Table S1). Because HepG2 hepatoblastoma cells are relatively less well differentiated than Huh7 hepatoma cells (30, 31), the transfer of HCV RNA into pDCs could require cellular factors that are restricted to well-differentiated human hepatocytes that actively replicate HCV RNA. Because pDCs are prevalent in the HCV-infected liver (10) and pDCs from HCV-infected patients respond normally to HCV-infected cells (Fig. S5), pDC-hepatocyte interactions such as those described herein could occur during natural HCV infection, reflecting a novel aspect of hepatic immunity.
For the host to combat viruses such as HCV that do not induce IFN production by the cells they infect and do not directly infect or stimulate pDCs, it must possess other mechanisms to induce type 1 IFN during those infections. The current observations suggest that sensing of viral replication in infected cells apparently represents an alternative option for pDCs to respond to viruses such as HCV. The current observations also help resolve a puzzling contradiction in which the ability of HCV to induce type 1 IFN and ISGs in the infected liver (5–7) coexists with its remarkable ability to block dsRNA signaling in infected cells (2–4). The host apparently avoids this viral evasion strategy via the ability of uninfected pDCs to sense and be activated by the infected cells. Despite this remarkable defense mechanism, however, the host only rarely defeats HCV infection, suggesting that additional viral evasion mechanisms remain to be discovered.
Materials and Methods
Cells, Replicons, and Viruses.
Huh7, Huh7.5.1, and Huh7.5.1c2 cells derived from Huh7.5.1 cells with improved infection efficiency were described previously (14, 32). Full-length genomic JFH1 RNA and wild-type or replication-defective subgenomic JFH1 replicons were previously described (32, 33). To produce in vitro transcribed JFH1 negative-strand RNA, an oligonucleotide duplex encoding the T3 promoter was inserted into XbaI and SbfI in pUC-vJFH1, yielding pUC-vJFH1-T3. pUC-vJFH1-T3 was linearized with EcoRI, and the linearized DNA was purified and used as a template for T3-driven in vitro transcription (MEGAscript; Ambion). Transfection of in vitro synthesized JFH1 RNA constructs and establishment of replicon cells were performed as previously described (34). Huh7 cells bearing the H77c subgenomic replicon (35) were provided by Dr. Stanley M. Lemon (University of Texas Medical Branch, Galveston, TX). HepG2 (36), HeLa (37), and HEK293 (37) cells bearing the JFH1 subgenomic replicon were provided by Dr. Takaji Wakita (National Institute of Infectious Diseases, Tokyo, Japan). Mouse MMH1-1 cells, provided by Dr. Marco Tripodi (Università La Sapienza, Rome, Italy), bearing the JFH1 subgenomic replicon were generated de novo as previously described (38). All these replicon cells were maintained in the presence of 500 μg/mL G418. A plasmid containing a subgenomic VEE replicon (5′VEErep/S/GFP/Pac), provided by Dr. Ilya Frolov (University of Texas Medical Branch, Galveston, TX) via Dr. Charles M. Rice (Rockefeller University, New York, NY), and the generation of in vitro synthesized VEE replicon RNA were previously described (24). To establish Huh7.5.1c2 cells that stably replicate this VEE subgenomic replicon, in vitro synthesized VEE replicon RNA was transfected into Huh7.5.1c2 cells as described above for the JFH1 replicon (34). GFP-positive cells were selected and maintained in the presence of 10 μg/mL puromycin. BDV (He80) was provided by Dr. Juan Carlos de la Torre (The Scripps Research Institute, La Jolla, CA). Huh7.5.1c2 cells were infected with BDV at an MOI of 1 and cocultured with pDCs after 5 days of infection. JFH1/D18 virus (D18) and JFH1/D183 virus (D183), a wild-type and a cell culture–adapted JFH1 strain, respectively, were described previously (15). Jc1, Con1/JFH1, and H77/JFH1 infectious chimeric HCV constructs were described previously (17). In brief, the JFH1 core NS2 region was replaced with the corresponding sequences from J6CF, Con1, and H77. All infectious chimeric viruses were produced by transfection of the respective in vitro synthesized genomic HCV RNA into Huh7.5.1 cells, and virus stocks containing 104–105 focus-forming units/mL were prepared as described previously (32).
Reagents.
ODN2216 and resiquimod were purchased from InvivoGen and Novartis, respectively. TLR7-specific antagonist IRS661 (5′-TGCTTGCAAGCTTGCAAGCA-3′) and control ODN (5′-TCCTGCAGGTTAAGT-3′) were synthesized on a phosphorothionate backbone by MWG Biotech. DOTAP Liposomal Transfection Reagent was purchased from Roche Applied Science and used according to the manufacturer's instructions. Other reagents are described in SI Materials and Methods.
Preparation of pDCs.
pDCs were isolated from 450 mL of blood from 40 healthy adult human volunteers after informed consent was obtained according to procedures approved by the Scripps Research Institute Human Research Committee. Three chronically HCV-infected patients whose sera were anti-HCV antibody- and HCV RNA-positive were also studied. In those cases, pDCs were isolated from 100 mL of blood. PBMCs were isolated using Ficoll-Hypaque density centrifugation, and pDCs were positively selected from PBMCs using BDCA-4 magnetic beads (Miltenyi Biotec) according to the manufacturer's instructions. The typical yields of PBMCs and pDCs were 500 × 106 and 2 × 106 cells, respectively. The purity of pDCs was >95% as assessed by FACS staining (CD123+, BDCA-2+). Isolated pDCs were cultured in RPMI medium 1640 (Mediatech) supplemented with 10% fetal calf serum (GIBCO), 10 mM Hepes, 100 U/mL penicillin, 100 mg/mL streptomycin, 2 mM L-glutamine, nonessential amino acids, and 1 mM sodium pyruvate. Unless otherwise indicated, 2 × 104 pDCs were placed in 96-well round-bottom plates with a final volume of 200 μL/well for further experiment.
Coculture Experiment.
Huh7, Huh7.5.1, or Huh7.5.1c2 cells were infected or not with the indicated HCV strain at an MOI of 0.1 unless otherwise indicated, and cultured for the indicated periods of time at which point they were washed with PBS, trypsinized, and resuspended in RPMI medium 1640 (Mediatech). Unless otherwise indicated, 2 × 105 HCV-infected or uninfected cells were placed in 96-well round-bottom plates in a 37 °C/5% CO2 incubator for coculture experiments. In some experiments, 2 × 105 indicated stable replicon cells bearing JFH1, H77c, or VEE subgenomic replicon or BDV-infected Huh7.5.1c2 cells were cocultured with pDCs in the same way. At the indicated time points, supernatants were collected and stored at –80 °C for ELISA, and cells were lysed in TRIzol (Invitrogen) for RNA analysis. To separate pDCs from Huh7.5.1c2 cells in the mixed-cell coculture, cells were harvested after 14 h of coculture using Versene (GIBCO), and pDCs were enriched as described above. After two rounds of positive selection, pDC-enriched and pDC-depleted fractions were subjected to RT-qPCR analysis.
ELISA.
Cytokine levels in the cell-culture supernatant were measured using commercially available ELISA kits for human IFNα, human IFNβ, murine IFNα (PBL), and human IL-6 (R&D Systems), following the manufacturers’ instructions.
Materials and methods for the preparation of highly purified virus, flow cytometry, cell viability analysis, TUNEL assay, preparation of MoMacrophage, MoDC, and murine pDCs, RNA analysis, western and northern blotting, and immunofluorescence are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank our colleagues Marlene Dreux and Luca G. Guidotti for helpful suggestions and reviewing the manuscript, and Christina Whitten-Bauer, Josan Chung, and Bryan Boyd for technical support. We also thank Dr. Stanley M. Lemon (University of Texas Medical Branch, Galveston, TX) for Huh7 cells bearing an H77c subgenomic replicon, Dr. Takaji Wakita (National Institute of Infectious Diseases, Tokyo, Japan) for HepG2, HeLa, and HEK293 cells bearing the JFH1 subgenomic replicon, Dr. Ilya Frolov (University of Texas Medical Branch, Galveston, TX) and Dr. Charles M. Rice (Rockefeller University, New York, NY) for the subgenomic VEE replicon (5′VEErep/S/GFP/Pac) plasmid construct, Dr. Juan Carlos de la Torre (The Scripps Research Institute, La Jolla, CA) for BDV (He80), Dr. Dennis R. Burton (The Scripps Research Institute, La Jolla, CA) for recombinant monoclonal human anti-HCV E2 antibody (C1), Dr. Ganes C. Sen (The Lerner Research Institute, Cleveland Clinic, Cleveland, OH) for rabbit polyclonal anti-ISG56 antibody, and Dr. Weidong Zhong (Gilead Sciences, Foster City, CA) for 2-methyladenosine. This work was funded by NIH Grants R01-CA108304 and AI079043. K.T. was supported by the Japan Society for the Promotion of Science Postdoctoral Fellowships for Research Abroad. U.G. was supported by The Irvington Institute Fellowship Program of the Cancer Research Institute. This is manuscript no. 19585-IMS from The Scripps Research Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/1002301107/DCSupplemental.
References
- 1.Chisari FV. Unscrambling hepatitis C virus-host interactions. Nature. 2005;436:930–932. doi: 10.1038/nature04076. [DOI] [PubMed] [Google Scholar]
- 2.Meylan E, et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 3.Loo YM, et al. Viral and therapeutic control of IFN-β promoter stimulator 1 during hepatitis C virus infection. Proc Natl Acad Sci USA. 2006;103:6001–6006. doi: 10.1073/pnas.0601523103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng G, Zhong J, Chisari FV. Inhibition of dsRNA-induced signaling in hepatitis C virus-infected cells by NS3 protease-dependent and -independent mechanisms. Proc Natl Acad Sci USA. 2006;103:8499–8504. doi: 10.1073/pnas.0602957103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bigger CB, et al. Intrahepatic gene expression during chronic hepatitis C virus infection in chimpanzees. J Virol. 2004;78:13779–13792. doi: 10.1128/JVI.78.24.13779-13792.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su AI, et al. Genomic analysis of the host response to hepatitis C virus infection. Proc Natl Acad Sci USA. 2002;99:15669–15674. doi: 10.1073/pnas.202608199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bigger CB, Brasky KM, Lanford RE. DNA microarray analysis of chimpanzee liver during acute resolving hepatitis C virus infection. J Virol. 2001;75:7059–7066. doi: 10.1128/JVI.75.15.7059-7066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu YJ. IPC: Professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. doi: 10.1146/annurev.immunol.23.021704.115633. [DOI] [PubMed] [Google Scholar]
- 9.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 10.Lau DT, et al. Interferon regulatory factor-3 activation, hepatic interferon-stimulated gene expression, and immune cell infiltration in hepatitis C virus patients. Hepatology. 2008;47:799–809. doi: 10.1002/hep.22076. [DOI] [PubMed] [Google Scholar]
- 11.Kanto T, et al. Reduced numbers and impaired ability of myeloid and plasmacytoid dendritic cells to polarize T helper cells in chronic hepatitis C virus infection. J Infect Dis. 2004;190:1919–1926. doi: 10.1086/425425. [DOI] [PubMed] [Google Scholar]
- 12.Shiina M, Rehermann B. Cell culture-produced hepatitis C virus impairs plasmacytoid dendritic cell function. Hepatology. 2008;47:385–395. doi: 10.1002/hep.21996. [DOI] [PubMed] [Google Scholar]
- 13.Ulsenheimer A, et al. Plasmacytoid dendritic cells in acute and chronic hepatitis C virus infection. Hepatology. 2005;41:643–651. doi: 10.1002/hep.20592. [DOI] [PubMed] [Google Scholar]
- 14.Pedersen IM, et al. Interferon modulation of cellular microRNAs as an antiviral mechanism. Nature. 2007;449:919–922. doi: 10.1038/nature06205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhong J, et al. Persistent hepatitis C virus infection in vitro: Coevolution of virus and host. J Virol. 2006;80:11082–11093. doi: 10.1128/JVI.01307-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krug A, et al. Identification of CpG oligonucleotide sequences with high induction of IFN-α/β in plasmacytoid dendritic cells. Eur J Immunol. 2001;31:2154–2163. doi: 10.1002/1521-4141(200107)31:7<2154::aid-immu2154>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 17.Pietschmann T, et al. Construction and characterization of infectious intragenotypic and intergenotypic hepatitis C virus chimeras. Proc Natl Acad Sci USA. 2006;103:7408–7413. doi: 10.1073/pnas.0504877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ignatius R, et al. Canarypox virus-induced maturation of dendritic cells is mediated by apoptotic cell death and tumor necrosis factor α secretion. J Virol. 2000;74:11329–11338. doi: 10.1128/jvi.74.23.11329-11338.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ebihara T, Shingai M, Matsumoto M, Wakita T, Seya T. Hepatitis C virus-infected hepatocytes extrinsically modulate dendritic cell maturation to activate T cells and natural killer cells. Hepatology. 2008;48:48–58. doi: 10.1002/hep.22337. [DOI] [PubMed] [Google Scholar]
- 20.Law M, et al. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat Med. 2008;14:25–27. doi: 10.1038/nm1698. [DOI] [PubMed] [Google Scholar]
- 21.Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 22.Barrat FJ, et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J Exp Med. 2005;202:1131–1139. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang JP, et al. Flavivirus activation of plasmacytoid dendritic cells delineates key elements of TLR7 signaling beyond endosomal recognition. J Immunol. 2006;177:7114–7121. doi: 10.4049/jimmunol.177.10.7114. [DOI] [PubMed] [Google Scholar]
- 24.Petrakova O, et al. Noncytopathic replication of Venezuelan equine encephalitis virus and eastern equine encephalitis virus replicons in mammalian cells. J Virol. 2005;79:7597–7608. doi: 10.1128/JVI.79.12.7597-7608.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hornung V, et al. Replication-dependent potent IFN-α induction in human plasmacytoid dendritic cells by a single-stranded RNA virus. J Immunol. 2004;173:5935–5943. doi: 10.4049/jimmunol.173.10.5935. [DOI] [PubMed] [Google Scholar]
- 26.Hardy AW, Graham DR, Shearer GM, Herbeuval JP. HIV turns plasmacytoid dendritic cells (pDC) into TRAIL-expressing killer pDC and down-regulates HIV coreceptors by Toll-like receptor 7-induced IFN-α. Proc Natl Acad Sci USA. 2007;104:17453–17458. doi: 10.1073/pnas.0707244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beignon AS, et al. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saito T, Owen DM, Jiang F, Marcotrigiano J, Gale M., Jr Innate immunity induced by composition-dependent RIG-I recognition of hepatitis C virus RNA. Nature. 2008;454:523–527. doi: 10.1038/nature07106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science. 2004;303:1529–1531. doi: 10.1126/science.1093616. [DOI] [PubMed] [Google Scholar]
- 30.Olsavsky KM, et al. Gene expression profiling and differentiation assessment in primary human hepatocyte cultures, established hepatoma cell lines, and human liver tissues. Toxicol Appl Pharmacol. 2007;222:42–56. doi: 10.1016/j.taap.2007.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seow TK, Liang RC, Leow CK, Chung MC. Hepatocellular carcinoma: From bedside to proteomics. Proteomics. 2001;1:1249–1263. doi: 10.1002/1615-9861(200110)1:10<1249::AID-PROT1249>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 32.Zhong J, et al. Robust hepatitis C virus infection in vitro. Proc Natl Acad Sci USA. 2005;102:9294–9299. doi: 10.1073/pnas.0503596102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kato T, et al. Efficient replication of the genotype 2a hepatitis C virus subgenomic replicon. Gastroenterology. 2003;125:1808–1817. doi: 10.1053/j.gastro.2003.09.023. [DOI] [PubMed] [Google Scholar]
- 34.Krieger N, Lohmann V, Bartenschlager R. Enhancement of hepatitis C virus RNA replication by cell culture-adaptive mutations. J Virol. 2001;75:4614–4624. doi: 10.1128/JVI.75.10.4614-4624.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yi M, Lemon SM. Adaptive mutations producing efficient replication of genotype 1a hepatitis C virus RNA in normal Huh7 cells. J Virol. 2004;78:7904–7915. doi: 10.1128/JVI.78.15.7904-7915.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Date T, et al. Genotype 2a hepatitis C virus subgenomic replicon can replicate in HepG2 and IMY-N9 cells. J Biol Chem. 2004;279:22371–22376. doi: 10.1074/jbc.M311120200. [DOI] [PubMed] [Google Scholar]
- 37.Kato T, et al. Nonhepatic cell lines HeLa and 293 support efficient replication of the hepatitis C virus genotype 2a subgenomic replicon. J Virol. 2005;79:592–596. doi: 10.1128/JVI.79.1.592-596.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uprichard SL, Chung J, Chisari FV, Wakita T. Replication of a hepatitis C virus replicon clone in mouse cells. Virol J. 2006;3:89. doi: 10.1186/1743-422X-3-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.