Abstract
Cow’s milk contains high levels of estrogens, progesterone and insulin-like growth factor 1 (IGF-1), all of which are associated with breast cancer. We investigated whether prepubertal milk exposure affects mammary gland development and carcinogenesis in rats. Sprague Dawley rats were given either whole milk or tap water to drink from postnatal day (PND) 14 to PND 35, and thereafter normal tap water. Mammary tumorigenesis was induced by administering 7,12-dimethylbenz[a]anthracene (DMBA) on PND 50. Milk exposure increased circulating E2 levels on PND 25 by 10-fold (p<0.001) and accelerated vaginal opening, which marks puberty onset, by 2.5 days (p<0.001). However, rats exposed to milk before puberty exhibited reduced carcinogen-induced mammary carcinogenesis; i.e., their tumor latency was longer (p<0.03) and incidence was lower (p<0.05) than in the controls. On PND 25 and 50, mammary glands of the milk exposed rats had significantly less terminal end buds (TEBs) than the tap water exposed controls (p<0.019). ER-α protein levels were elevated in the TEBs and lobules of milk rats, compared to rats given tap water (p<0.019), but no changes in cyclin D1 expression, cell proliferation or apoptosis were seen. IGF-1 mRNA levels were reduced on PND 50 in the mammary glands of rats exposed to milk at puberty. Our results suggest that drinking milk before puberty reduces later risk of developing mammary cancer in rats. This might be mediated by a reduction in the number of TEBs and lower expression of IGF-1 mRNA in the mammary glands of milk-exposed animals.
Keywords: Cow’s milk, breast cancer, prepubertal exposure, animal model
Introduction
Bovine milk and dairy products are part of a daily diet for many people. However, milk contains detectable to high levels of various hormones and growth factors that have been proposed to be associated with increased breast cancer risk, including estrogens, progesterone, leptin and insulin like growth factors (IGFs) 1–4. Animal 5–8 and some human studies 9–12 indicate that milk increases breast cancer risk; however, most studies have reported no change in risk being associated with milk intake 13–15 and some have reported a protective effect 16–18. The protective effect was seen mainly against premenopausal breast cancer 16;17. Childhood milk or dairy consumption in humans has been associated with a reduced breast cancer risk 17;19–21; this intake may confer protection against both pre- and postmenopausal breast cancer 20;21.
A contributing factor to the conflicting results regarding milk consumption and breast cancer risk could potentially be a difference in response to hormones present in milk, depending on the developmental stage of the breast at the time of exposure. It has been suggested that timing of exposures to estrogenic compounds, including endogenous hormones and those originating from the diet, determines whether they increase, reduce or have no effect on breast cancer risk 22. In animal models, for example, in utero estrogenic exposures increase breast cancer risk, and also increase the number of terminal end buds (TEBs) that are the targets of malignant transformation in the rodent mammary gland, and delay their differentiation 23. Prepubertal estrogenic exposures, in contrast, reduce mammary cancer risk and are associated with a reduction in the number of TEBs, a reduction in cell proliferation and increased apoptosis within the TEBs 24;25.
Animal studies investigating the role of whole, low-fat or non-fat milk on mammary tumorigenesis have all focused on the effect of milk exposure after treatment with the mammary carcinogen 7,12-dimethylbenz(a)anthracene (DMBA) 5–8. In these studies, milk feeding was initiated 24 hours to one week after DMBA administration and continued for 20 weeks. Milk was found to increase mammary tumor incidence, number of tumors and tumor volume regardless of the milk fat content, when compared to rats that were given tap water or liquid which was energy and nutrient balanced with milk.
In the present study we asked whether prepubertal cow’s milk consumption affected carcinogen-induced mammary tumorigenesis and whether it was associated with changes in biomarkers previously linked to altered breast cancer risk; i.e, mammary gland morphology, cell proliferation and apoptosis, and expression of estrogen receptor (ER) α and insulin like growth factor (IGF)-1 in the mammary gland.
Material and methods
Animals and treatments
Pregnant Sprague Dawley dams were obtained from Charles River Laboratories (Wilmington, MA) and housed individually in standard rat plexiglass cages, at a constant temperature and humidity, under a 12-hour light-dark cycle. All animals were fed the AIN93G (American Institute of Nutrition) semipurified diet ad lib throughout the experiment. The day after the pups were born, male pups were removed and females were cross-fostered to avoid any litter effect. Each nursing dam had a total of 10 female pups. The study was performed in accordance with the appropriate institutional and federal regulations.
When pups were 14 days of age, dams were divided to two groups (6 dams per group, n = 60 female pups per group) that were given either tap water (control rats) or commercial whole milk containing 4% fat, purchased from the local supermarket (milk-exposed rats). Fresh milk was provided for the milk group every day. Since a previous study found no differences in body weight or mammary tumorigenesis between rats given tap water or a liquid which had a nutrient composition similar to that of milk 7, we chose to give tap water for the control group.
After weaning at postnatal day (PND) 22, the pups were housed three per cage and continued on the same liquid they received before weaning until PND 35; all rats received tap water from that age onwards.
Since pups nurse until they are weaned, although they start consuming food pellets at about PND 16 and may also occasionally drink directly from the drinking bottle, rat pups in the present study were exposed to cow’s milk during the first exposure week mainly through their dam. However, after weaning at PND 21 they consumed the cow’s milk or water directly from the drinking bottle, for a total of 2 more weeks.
Serum estradiol level
At PND 25, 5 control and 8 milk exposed rat pups from each group were sacrificed and blood was collected by cardiac puncture. Serum was separated and kept at −80° C until use. The level of 17β-estradiol (E2) was determined using a EIA kit from Alpco Diagnostics (Windham, NH) according to the manufacturer’s instructions.
Puberty onset, vaginal opening
From PND 25 to 42, rats were examined daily to evaluate vaginal opening (VO). VO typically occurs in the Sprague-Dawley rat around PND 32–34 and represents the initial stage of attaining sexual maturity. Rats were recorded positive for VO when the vagina exhibited complete canalization and patency.
Mammary gland morphology
Mammary gland morphology is indicative of the level of susceptibility to develop mammary cancer. In particular, we and others have found that an increase in the number of terminal end buds (TEBs) proceeds an increase in the risk of developing mammary tumors (reviewed in 23). Changes in mammary gland morphology between milk and tap-water exposed rats were assessed on PND 25 and 50 in the 4th mammary glands from six to eight rats per group.
Analysis of mammary epithelial structures in whole mounts was based on visual evaluation and computer-assisted image analysis. We have developed a visual scale to assess growth patterns of mammary epithelial cells 26. In this study, the following characteristics of the coded mammary glands were evaluated double-blindly using a 5-point scale for a density of (0: no structures detected, 5: numerous structures) (i) alveolar structures between the lymph node and periphery of the epithelial tree and (ii) lobular structures between nipple and lymph node. In addition, the number of TEBs at the distal periphery of the epithelial tree (defined as zone C by Russo & Russo 27) was counted.
Immunohistochemistry
We determined cell proliferation and apoptosis, and the expression of ER-α and cyclin D1 in the mammary glands of 50-day-old rats. Six rats per group were used and their third left mammary gland were fixed in 10% buffered formalin overnight at 4 0C, dehydrated with graded ethanol and embedded in paraffin. The embedded tissue was sectioned (5 μm) and mounted on silane-coated glass slides. Embedding and mounting were performed at the histopathology laboratory, Lombardi Comprehensive Cancer Center (http://lombardi.georgetown.edu/research/resources/histopathology.htm).
Cell proliferation – PCNA assay
Using PCNA (proliferating cell nuclear antigen) immunohistochemistry, cell proliferation was determined. Unless otherwise noted, all materials for the PCNA assay were provided in the Vectastain Elite ABC Kit (Vector Laboratories, Burlingame, CA). Sections were deparaffinized in xylene, hydrated through graded alcohols and heated in the microwave for antigen retrieval in Antigen Retrieval Solution for 20 minutes. Sections were then incubated 15 minutes in 3% H2O2 for 15 minutes to block endogenous peroxides. Sections were washed in phosphate-buffered saline (PBS) containing 0.1% Triton X-100 for 20 minutes to reduce nonspecific binding, and blocked with Vectastain Blocking Serum for 20 minutes. Tissue sections were incubated overnight at 4°C with the primary antibody against PCNA (Santa Cruz Biotechnology, Inc.) at a dilution of 1:700. After several washes, sections were treated with secondary antibody (biotinylated, anti-rabbit IgG) for one hour at room temperature, followed by treatment with avidin and biotinylated horseradish peroxidase complex for 30 minutes at room temperature. Sections were washed, and antigen-antibody complex was visualized by incubation with the chromogen, 3,3′-diaminobenzidine (DAB) for 1 minute, then washed and counterstained for 45 sec with Vector’s Hematoxylin QS Nuclear Counterstain. Proliferation index was determined by calculating the percentage of cells that had positive PCNA staining (only darkly stained cells were counted) separately in TEBs, lobule-alveolar structures (LAs) and ducts among 1000 cells per structure and per gland. Slides were blindly evaluated with help of the Image Tool software.
Apoptosis - TUNEL (terminal deoxynuclotidyl transferase dUTP nick end labeling) assay
The nuclei containing degraded DNA in mammary gland sections were stained by using the TUNEL assay, an in situ apoptosis ApopTag Peroxidase detection kit (Chemicon, S7101), as recommended by the manufacturer and as described previously 28. The proportion of cells undergoing apoptosis was determined by calculating the percentage of apoptotic cells through both positive staining and histological evaluation of at least 1000 cells per structure (TEBs, LAs or ducts).
ER-α and cyclin D1 protein expression
For determination of ER-α and cyclin D1 protein expression, mammary tissue sections were initially handled as described in the PCNA assay. These sections were then incubated overnight at 4°C with primary antibodies against ER-α (MC-20, rabbit polyclonal IgG) at the ratio of 1:100, or cyclin D1 (DCS-6, mouse monoclonal IgG) (Santa Cruz Biotechnology, Inc.) at the ratio of 1:700. After several washes, sections were treated with secondary antibody (biotinylated, anti-goat IgG and anti-mouse for ER-α and cyclin D1), and the following procedure was identical to the previously described procedure for PCNA staining.
IGF-1 mRNA expression
The third right mammary glands from six 50-day-old rats per group were obtained for real time PCR analysis of IGF-1 mRNA expression. Mammary tissue was collected and immediately placed on dry-ice. The RNA was purified using the RNeasy Lipid Tissue Mini Kit (Quiagen, Valencia, CA) according to the manufacturer’s instructions. cDNA was reverse transcribed from 50 μg/ml of total input RNA using Taqman Reverse Transcription Kit as described by the manufacturer (Applied Biosystems, Foster City, CA). Real time PCR was performed with ABI Prism 7900 Sequence Detection System with PCR master Mix, primers and probes for IGF-1 (Rn_00710306_m1) (Applied Biosystems, Foster City, CA). The probe was conjugated to 6-carboxy-flourescein phosphoradidite (FAM dye) at the 5′ labelled end with a non-fluorescent quencher located at the 3′ labelled end. The 18S RNA from Applied Biosystems was used as an endogenous control. All assays were run on 384 well plates in triplicate for the target gene and the endogenous control. Results were assessed by relative quantification of gene expression using the ΔΔCT method.
Mammary tumorigenesis
At PND 50, 49 rats in the control group and 24 in the milk group were given 10 mg of 7,12-dimethylbenz[a]anthracene (DMBA) (Sigma Chemical Co., St. Louis, MO) by oral gavage. In our prior studies, 10 mg DMBA was shown to induce tumors in approximately two-thirds of the control group and thus enables assessment of both reduction and increase in tumorigenicity 24. Starting at 6 weeks after DMBA administration, animals were examined for tumors by palpation once a week. Tumor growth was measured using a caliper and the length, width, and height of each tumor was recorded. The endpoints for data analysis were (i) latency to tumor appearance, (ii) the number of animals with tumors (tumor incidence) and (iii) the number of tumors per animal (tumor multiplicity). Animals were sacrificed when the tumor burden was approximately 10% of the total body weight. All remaining animals, including those that did not develop tumors were sacrificed 18 weeks after DMBA administration.
Statistical analyses
The results of serum estradiol levels, IGF-1 mRNA, and some mammary tumor endpoints (latency and multiplicity) were analyzed using the t-test. The number of proliferating or apoptotic cells, and protein levels of ERα and cyclin D1 were determined using two-way ANOVA, with milk exposure and mammary epithelial structures (lobules, TEBs, and ducts) as independent variables. Mammary gland morphology at the ages of 25 and 50 days (density of alveolar buds and lobules, and number of TEBs was also analysed using two-way ANOVA. Kaplan-Meier curves were used to compare differences in vaginal opening and tumor incidence, followed by the log-rank test. All tests were performed using the SPSS SigmaStat software, and differences were considered significant if the p-value was less than 0.05.
Results
Serum estradiol levels and body weight
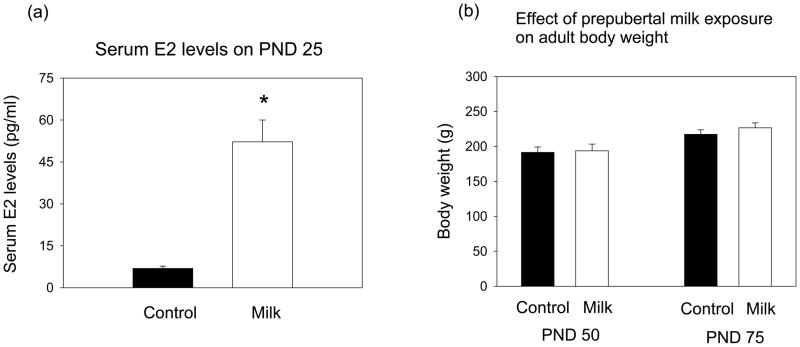
Serum E2 levels, measured on postnatal day (PND) 25, were higher in the rats exposed to milk from PND 14 onwards than in the control rats consuming tap water throughout life (t=4.49, df=11, p<0.001) (Fig. 1a).
FIGURE 1.
(a) Serum estradiol levels on postnatal day (PND) 25 (n = 5–8/group). Significantly different from each other: *p<0.05. (b) Body weight on PND 50 and 75 (n = 4–6/group, means ± SEM shown). Control and milk-exposed rats do not differ from each other.
Body weights were determined on PND 50 and 75. No differences between the control and milk exposed rats were seen (Fig. 1b).
Vaginal opening
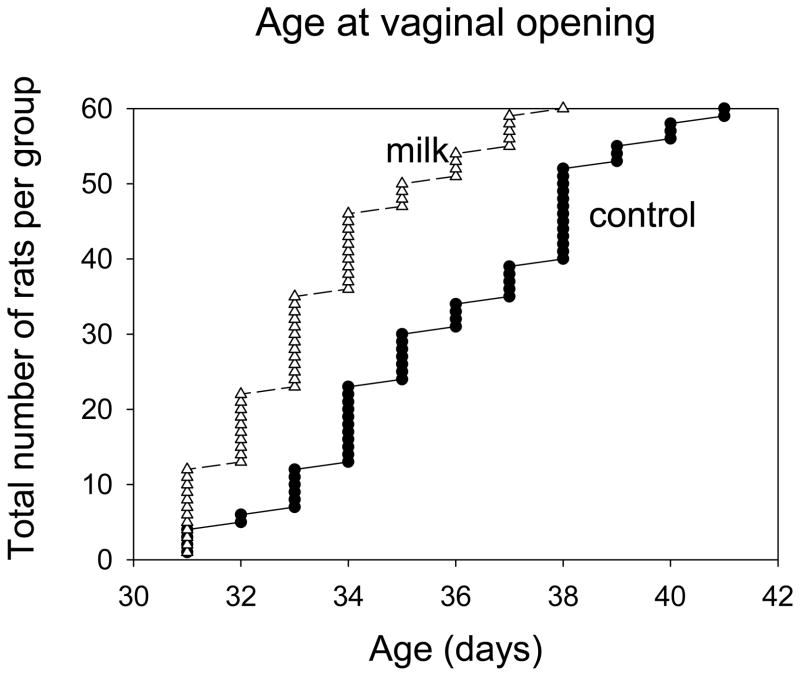
Prepubertally milk-exposed rats exhibited earlier puberty onset, determined by assessing the age of vaginal opening, compared to the control group (Log Rank = 23.755, p<0.001) (Fig. 2). The age at which 50% (30 of 60 rats) of the rats showed vaginal opening was 35.5 and 33.0 for the control and milk groups, respectively.
FIGURE 2.
Effects of milk exposure on vaginal opening (n =60 per group). Rats were exposed to milk or tap water between PND 14 to 35, and then all shifted to tap water. Vaginal opening occurred significantly earlier in the milk group: p<0.001
Mammary tumorigenesis
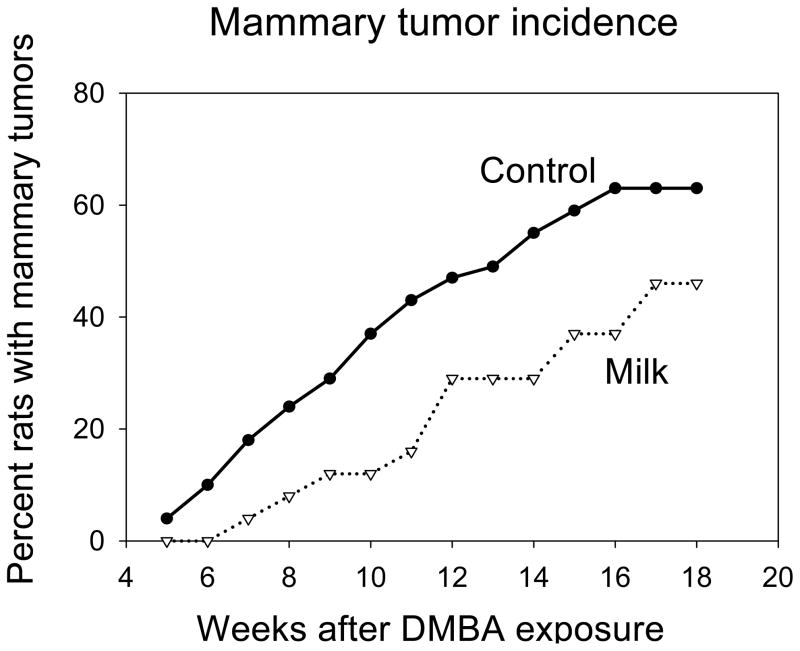
Tumor latency (the time between DMBA administration and appearance of the first detectable tumor per animal) was longer in the milk exposed rats than in the controls rats (t=2.19, df=71, p<0.03) (TABLE I). The proportion of rats per group that developed tumors (tumor incidence) was lower in the milk exposed animals than in the control group (Log Rank=3.84, p<0.05) (Fig. 3). The average number of tumors per animal (multiplicity) was lower in the rats that had milk during prepubertal life than in the controls, but the difference failed to reach statistical significance (t=1.62, df=71, p<0.10) (TABLE I).
TABLE I.
Effects of pre-pubertal milk exposure on DMBA-induced mammary tumorigenesis. Tumor latency, tumor multiplicity, tumor incidence. Results are means± SEM unless else is noted.
| Control n=49 | Milk n=24 | p-value | |
|---|---|---|---|
| Tumor latency, weeks from DMBA exposure | 13.0 ± 0.7 | 15.4 ± 0.8 | 0.03 |
| Tumor multiplicity, number per rat | 1.5 ± 0.3 | 0.8 ± 0.2 | 0.10 |
| Final tumor incidence (percentile) | 63 | 46 | 0.02 |
FIGURE 3.
Effects of prepubertal milk exposure on tumor incidence (n = 49 in the control and n=24 in the milk group). Milk-exposed rats exhibited significantly lower mammary tumor incidence: p<0.05.
Mammary gland morphology
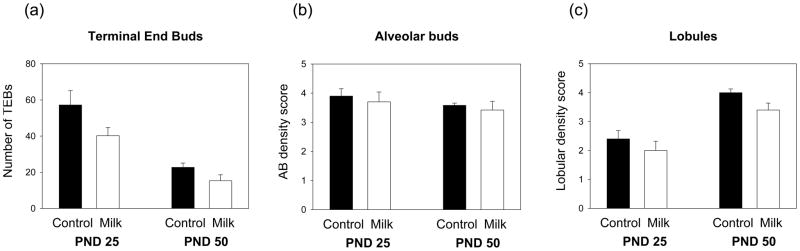
Total number of TEBs were counted in the mammary glands of 25- and 50-day-old rats. The data indicated that mammary glands of rats exposed to milk before puberty contained significantly less TEBs than the glands of tap water controls (F(1,18)=6.67, p<0.019) (Fig. 4a). In accordance with previous reports 27, mammary glands of 25-day-old rats contained significantly more TEBs than the glands of the 50-day-old rats (F(1,18)=39.72, p<0.001).
FIGURE 4.
Effects of prepubertal milk exposure on mammary gland morphology on PND 25 and 50. (a) Total number of terminal end buds (TEBs), and (b) density of alveolar buds and (c) lobules assessed visually using a scale between 0–5, are shown. Milk exposed rats had less TEBs (p<0.019) and lower lobular density (p<0.060) than the controls. In addition, the number of TEBs was lower (p<0.001) and the density of lobules was higher (p<0.001) on PND 50 than on PND 25. Values are expressed as mean ± SEM, n = 5–6 per group.
The densities of alveolar buds and lobules in the mammary epithelial tree were determined blindly using a visual scale. No differences in alveolar bud density were noted between the rats exposed to tap water or those consuming milk before puberty (Fig. 4b). Density of lobules, however, was affected by age, with mammary glands obtained on PND 50 containing significantly more lobules than the glands obtained on PND 25 (F(1,18)=37.81, p<0.001) (Fig. 4c). In addition, the milk-exposed rats tended to have lower lobular density than the control rats (F(1,18)=4.02, p<0.060).
Cell proliferation and apoptosis
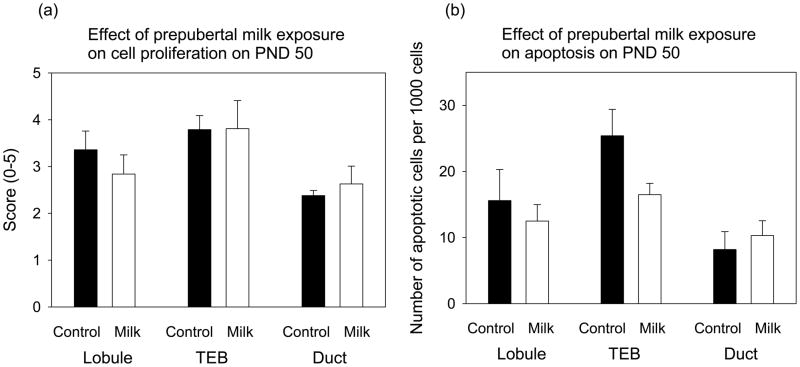
Prepubertal milk exposure did not affect mammary cell proliferation, assessed by PCNA staining, or the number of apoptotic cells, assessed using TUNEL, in lobulo-alveolar structures, TEBs or ducts, when compared to the control rats and determined on PND 50 (Fig. 5). Different epithelial structures, however, exhibited significantly different levels of PCNA staining (F(2,30)=5.26, p<0.011) or apoptotic cells (F(2,27)=7.68, p<0.002). TEBs contained more proliferating cells and cells which underwent apoptosis than ducts did (p<0.008 and p<0.002, respectively).
FIGURE 5.
Effect of prepubertal milk exposure on mammary gland (a) cell proliferation (PCNA staining), assessed on a visual scale from 0–5, and (b) the number of apoptotic cells per 1000 cells in terminal end buds (TEB), lobulo-alveolar structures (Lobule) and ducts. Each value represents the mean ± SEM, n = 5–6 rats/group. Milk group did not differ from the tap water controls, but when compared to ducts, TEBs contained more proliferating cells (p<0.008) and cells which underwent apoptosis (p<0.002).
ER-α and cyclin D1 expression
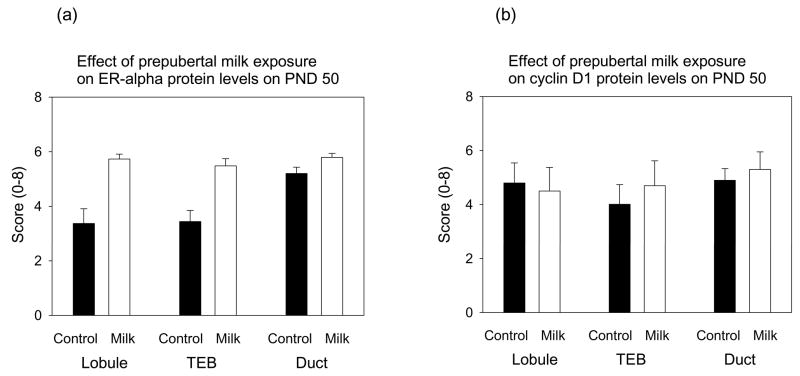
Estrogen receptor (ER) α protein levels were determined by immunohistochemistry and quantitated using a visual scale which took into account the percentile of cells stained positive (score 0–5) and staining intensity (score 0–3). When these scores were combined, the mammary glands of milk exposed rats on PND 50 contained more ER-positive cells than the glands of the control rats (F(1,27)=33.83, p<0.001) (Fig. 6a). This difference was seen in the lobules and TEBs, but not in the ducts (F for interaction F(2,27)=3.70, p<0.038). In the control rats, the first two structures contained fewer ER-α positive cells than the ducts (F(2,27)=5.50, p<0.01), whilst no differences across different structures were seen in the milk-exposed group.
FIGURE 6.
Effects of prepubertal milk exposure on (a) ER-α and (b) cyclin D1 expression determined using immunohistochemistry assessed in terminal end buds (TEB’s), lobulo-alveolar structures (Lobules) and ducts. Each value represents the mean ± SEM, n = 5–6 rats/group. Compared to tap water controls, milk-exposed rats expressed significantly higher levels of ER-α (p<0.001). In addition, ducts of control rats expressed significantly more ER-α than the lobules or TEBs (p<0.01); this was not seen in the milk group (interaction: p<0.038).
Cyclin D1 protein levels were not affected by prepubertal milk exposure (Fig. 6b), and neither was the expression different in the different epithelial structures.
IGF-1 mRNA expression
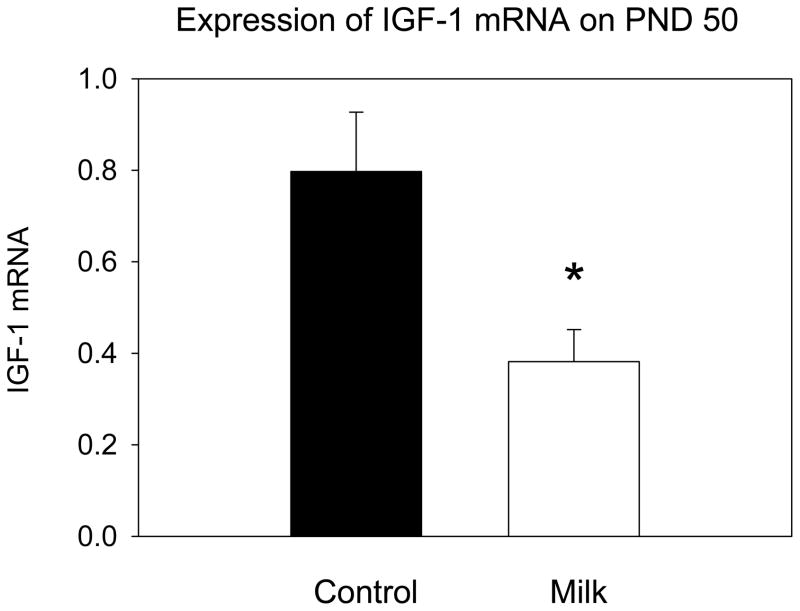
Milk intake during prepuberty was associated with a significantly reduced IGF-1 mRNA expression in the mammary glands on PND 50 determined using real time PCR (t=2.58, df=9, p<0.03) (Fig. 7).
FIGURE 7.
IGF-1 mRNA levels in the mammary glands of 50 day-old rats measured by RT-PCR. Values are expressed as mean ± SEM, n = 5–6 per group, significantly different from the control group: * p<0.05.
Discussion
The only source of nutrition for newborn mammals is species-specific milk, obtained via nursing. After weaning, milk is not consumed, except by humans who continue to consume milk and dairy products throughout their life. In addition, the constituents of cow, human and rat milk are all different 29;30. We found that cow’s milk intake before puberty onset reduced the risk of developing mammary tumors in rats. This result is in line with human studies suggesting that childhood consumption of cow’s milk is associated with a reduced breast cancer risk 17;19–21. Previous animal studies have shown that milk exposure in adulthood promotes the growth of carcinogen-induced mammary tumors 5–8. Findings from human studies are inconsistent 9–11;13–18. These conflicting results in rats and women may reflect the fact that the effects of milk are dependent on the age when cow’s milk is consumed.
It is becoming increasingly clear that the age when an individual is exposed to various dietary compounds or hormones determines how they affect breast cancer risk 22. For example, an exposure to estrogens before 24 and shortly after puberty onset 31, particularly at the levels which mimic high pregnancy estrogenic environment 32, provides a life-long protection against mammary tumorigenesis in animal models. In contrast, estrogenic exposures after mammary tumor initiation promote the malignant growth 33;34. Milk contains high levels of estrogens and other hormones and growth factors 35. Milk obtained from non-pregnant cows and cows during the first pregnancy trimester contains 0–60 ng/L estrogens (free and conjugated), and during the second and third trimester the levels increase to above 1600 ng/L 36. Calculations for a typical Western dairy herd, done by using the dynamic SimHerd model 37, show that 42 % of commercial milk is produced by pregnant cows, of which half is from cows in their second and third trimester. However, because of the high levels of estrogens in late pregnancy, estrogen levels in commercial milk are high 35. Estrogen levels are not dependent on milk’s fat content due to conjugated estrogens, which compromise most of these hormones, being stored in the aqueous fraction of milk 4;35.
We measured circulating E2 levels on PND 25 and found that the levels were 10-fold higher in the milk-group than in the tap-water drinking controls. However, since vaginal opening indicating puberty onset occurred on average 2.5 days earlier in the milk-exposed pups than in the controls (PND 33 vs 35.5), and we did not measure the content of estrogens in the milk, it is possible that the increase reflected at least partly the approaching onset of ovarian estrogen production. Nevertheless, our finding is in line with a previous study indicating that adult rats consuming milk had higher circulating levels of estradiol and estrone than rats kept on tap water 6. Further, consumption of milk in humans has been reported to lead to an increase in E2 levels38. It is therefore possible that reduced susceptibility to mammary tumorigenesis in rats, and perhaps in humans, who consumed milk before puberty, is caused by an increase in prepubertal estrogenic activity.
In addition of reducing later mammary cancer risk, prepubertal estrogenic exposures, including an exposure to genistein which is a phytochemical in soy with estrogenic properties, alter mammary gland morphology and expression of ER-α 24;39. Changes in the mammary gland include a reduction in the number of TEBs 23. We found that prepubertal milk exposure also reduced TEBs. Since TEBs are the sites of malignant transformation in a rodent mammary gland27, and over 90% of human breast cancers originate from a similar structure in the human breast, called terminal ductal lobular units (TDLUs) 40;41, prepubertal milk exposure may reduce later mammary cancer risk by eliminating structures which give rise to cancer.
The role of ER-α in affecting breast cancer risk remains to be determined. Although binding of E2 to the ER-α and the subsequent activation of this receptor induces estrogen-mediated increase in breast cancer cell proliferation 33;34, and some studies suggest that high levels of ER-α expression in the normal mammary tissue are predictive of high breast cancer risk42, some studies link high mammary ER-α expression to low breast cancer risk 43. These contrasting findings may reflect multiple roles of this receptor in the mammary gland. On one hand, estrogens promote growth via ER-α, but on the other hand, ER-α is expressed in the differentiated luminal mammary cells 44 and not in mammary stem cells which are proposed to be the cell of origin of breast cancer 45. We found that ER-α protein levels were significantly higher on PND 50 in the mammary glands of rats exposed to milk before puberty onset. This increase was not associated with any significant changes in mammary cell proliferation, suggesting that down-stream targets of ER-α activation by milk do not include genes which are linked to increased cell proliferation. Consistent with this conclusion, no changes in mammary cyclin D1 expression was seen between the milk and control groups. Thus, the increase in ER-α expression by milk may reflect increased population of differentiated luminal cells.
Epidemiological studies indicate that women who entered puberty early exhibit increased breast cancer risk. It therefore seems contradictory that prepubertal estrogenic exposures which reduce breast cancer risk, accelerate puberty onset 24, also found here in rats drinking milk and exhibiting elevated E2 levels. However, since puberty onset is determined by multiple factors, some of which may be present in the milk and/or altered in an individual consuming milk, the origins of earlier vaginal opening in the present study is not known. Further, we have previously proposed that the link between early puberty onset and increased breast cancer risk reflect in utero hormonal environment 22 which can both accelerate puberty onset and increase later mammary tumorigenesis, and not pubertal exposures.
Milk contains insulin like growth factors, including both IGF-1 and IGF-2 46. The levels are further increased in cows given recombinant bovine growth hormone to improve milk yield47. Children who consume milk have higher circulating levels of IGF-1 than those who do not 48–50. When IGF-1 levels have been measured in adult individuals who consumed milk during childhood but not regularly thereafter, it has been found that their levels are reduced, when compared to non-milk drinkers 51;52. We did not measure circulating IGF-1 levels in the current study, but if they are reduced in adulthood, this could explain a reduction in mammary tumorigenesis in the rats drinking milk before puberty. IGF-1 might play a role in the etiology of premenopausal breast cancer 53. Further, transgenic mice over-expressing IGF-1 in the mammary gland show increased susceptibility to carcinogen induced mammary tumorigenesis 54. IGF-1 also is a potent mitogen in ER-positive breast cancer cell lines 55. IGF-1 enhances cancer progression through ERα activation via the mitogen activated protein kinase (MAPK) pathway56, the MAPK pathway being a key player in inducing cell proliferation closely linked to breast cancer 57.
IGF-1 is expressed both in the stroma and epithelium, where it plays a role in mediating the proliferation of epithelial cells and in inducing normal ductal branching, respectively 58. IGF-1 also is important for TEB formation 59. We determined the expression of IGF-1 mRNA in the mammary glands of milk exposed and control rats, and found that the expression was significantly reduced on PND 50. This finding is in agreement with lower number of TEBs in the milk-exposed rats, suggesting that the observed reduction in mammary tumorigenesis in the prepubertally milk exposed rats may be related to the down-regulation of IGF-1 mRNA in the mammary gland.
In summary, we found that pre-pubertal intake of cow’s milk reduces later susceptibility to develop mammary tumors. The protective effect may have been caused by an increase in prepubertal estrogenic environment which is known to reduce later mammary cancer risk in rats24. The protective effect might also be related to a long-lasting reduction in mammary IGF-1 expression and the number of TEBs.
Acknowledgments
The authors wish to thank Dr. Walter C. Willett at Harvard School of Public Health for providing the idea for this study and for his comments concerning the manuscript. This work was supported by a grant from National Cancer Institute (U54 CA000970).
Abbreviations
- DMBA
7,12-dimethylbenz[a]anthracene
- E2
17β-estradiol
- ER
estrogen receptor
- IGF-1
insulin like growth factor
- MAPK
mitogen activated protein kinase
- PND
postnatal day
- TEB
terminal end bud
- VO
vaginal opening
Footnotes
Novelty and impact: Previous studies have shown that milk promotes mammary tumorigenesis when consumed after cancer has been initiated. Our study is the first to investigate the effect of prepubertal intake of cow’s milk on mammary tumorigenesis in an animal model. The results indicated a protective effect by prepubertal milk exposure. In addition, we found that the mammary glands of milk-exposed rats contained less targets for malignant transformation; i.e., TEBs, and expressed lower levels of IGF-1. These findings contribute to a better understanding of the association between milk consumption and breast cancer by suggesting that the age when milk is consumed determines how it affects the risk.
Reference List
- 1.Malekinejad H, Scherpenisse P, Bergwerff AA. Naturally occurring estrogens in processed milk and in raw milk (from gestated cows) J Agric Food Chem. 2006;54:9785–91. doi: 10.1021/jf061972e. [DOI] [PubMed] [Google Scholar]
- 2.Purup S, Vestergaard M, Pedersen O, Sejrsen K. Biological activity of bovine milk on proliferation of human intestinal cells. J Dairy Res. 2007;74:58–65. doi: 10.1017/S0022029906002093. [DOI] [PubMed] [Google Scholar]
- 3.Parola R, Macchi E, Fracchia D, Sabbioni A, Avanzi D, Motta M, Accornero P, Baratta M. Comparison between plasma and milk levels of leptin during pregnancy and lactation in cow, a relationship with beta-lactoglobulin. J Anim Physiol Anim Nutr (Berl) 2007;91:240–246. doi: 10.1111/j.1439-0396.2007.00698.x. [DOI] [PubMed] [Google Scholar]
- 4.Courant F, Antignac JP, Maume D, Monteau F, Andre F, Le Bizec B. Determination of naturally occurring oestrogens and androgens in retail samples of milk and eggs. Food Addit Contam. 2007;24:1358–66. doi: 10.1080/02652030701329637. [DOI] [PubMed] [Google Scholar]
- 5.Ma DF, Katoh R, Zhou H, Wang PY. Promoting effects of milk on the development of 7,12-dimethylbenz(a)anthracene (DMBA)-induced mammary tumors in rats. Acta Histochem Cytochem. 2007;40:61–67. doi: 10.1267/ahc.07008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qin LQ, Xu JY, Wang PY, Ganmaa D, Li J, Wang J, Kaneko T, Hoshi K, Shirai T, Sato A. Low-fat milk promotes the development of 7,12-dimethylbenz(A)anthracene (DMBA)-induced mammary tumors in rats. Int J Cancer. 2004;110:491–96. doi: 10.1002/ijc.20172. [DOI] [PubMed] [Google Scholar]
- 7.Qin LQ, Xu JY, Tezuka H, Li J, Arita J, Hoshi K, Sato A. Consumption of commercial whole and non-fat milk increases the incidence of 7,12-dimethylbenz(a)anthracene-induced mammary tumors in rats. Cancer Detect Prev. 2007;31:339–43. doi: 10.1016/j.cdp.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 8.Zhou H, Qin LQ, Tang FL, Ma DF, Wang PY, Wang Y. Effect of milk on the 7,12-dimethylbenz[a]-anthracene-induced mammary tumor model in rat. Food Chem Toxicol. 2007;45:1868–72. doi: 10.1016/j.fct.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 9.Gaard M, Tretli S, Loken EB. Dietary fat and the risk of breast cancer: a prospective study of 25,892 Norwegian women. Int J Cancer. 1995;63:12–17. doi: 10.1002/ijc.2910630104. [DOI] [PubMed] [Google Scholar]
- 10.Ewertz M, Gill C. Dietary factors and breast-cancer risk in Denmark. Int J Cancer. 1990;46:779–84. doi: 10.1002/ijc.2910460505. [DOI] [PubMed] [Google Scholar]
- 11.Le MG, Moulton LH, Hill C, Kramar A. Consumption of dairy produce and alcohol in a case-control study of breast cancer. J Natl Cancer Inst. 1986;77:633–36. doi: 10.1093/jnci/77.3.633. [DOI] [PubMed] [Google Scholar]
- 12.Cho E, Spiegelman D, Hunter DJ, Chen WY, Stampfer MJ, Colditz GA, Willett WC. Premenopausal fat intake and risk of breast cancer. J Natl Cancer Inst. 2003;95:1079–85. doi: 10.1093/jnci/95.14.1079. [DOI] [PubMed] [Google Scholar]
- 13.Moorman PG, Terry PD. Consumption of dairy products and the risk of breast cancer: a review of the literature. Am J Clin Nutr. 2004;80:5–14. doi: 10.1093/ajcn/80.1.5. [DOI] [PubMed] [Google Scholar]
- 14.Parodi PW. Dairy product consumption and the risk of breast cancer. J Am Coll Nutr. 2005;24:556S–68S. doi: 10.1080/07315724.2005.10719504. [DOI] [PubMed] [Google Scholar]
- 15.Al SW, Salhab M, Mokbel K. Dairy products and breast cancer risk: a review of the literature. Int J Fertil Womens Med. 2005;50:244–49. [PubMed] [Google Scholar]
- 16.Shin MH, Holmes MD, Hankinson SE, Wu K, Colditz GA, Willett WC. Intake of dairy products, calcium, and vitamin d and risk of breast cancer. J Natl Cancer Inst. 2002;94:1301–11. doi: 10.1093/jnci/94.17.1301. [DOI] [PubMed] [Google Scholar]
- 17.Hjartaker A, Laake P, Lund E. Childhood and adult milk consumption and risk of premenopausal breast cancer in a cohort of 48,844 women - the Norwegian women and cancer study. Int J Cancer. 2001;93:888–93. doi: 10.1002/ijc.1409. [DOI] [PubMed] [Google Scholar]
- 18.van’t VP, Dekker JM, Lamers JW, Kok FJ, Schouten EG, Brants HA, Sturmans F, Hermus RJ. Consumption of fermented milk products and breast cancer: a case-control study in The Netherlands. Cancer Res. 1989;49:4020–4023. [PubMed] [Google Scholar]
- 19.Shu XO, Jin F, Dai Q, Wen W, Potter JD, Kushi LH, Ruan Z, Gao YT, Zheng W. Soyfood intake during adolescence and subsequent risk of breast cancer among Chinese women. Cancer Epidemiol Biomarkers Prev. 2001;10:483–88. [PubMed] [Google Scholar]
- 20.Pryor M, Slattery ML, Robinson LM, Egger M. Adolescent diet and breast cancer in Utah. Cancer Res. 1989;49:2161–67. [PubMed] [Google Scholar]
- 21.Hislop TG, Goldman AJ, Elwood JM, Brauer G, Kan L. Childhood and recent eating patterns and risk of breast cancer. Cancer Detect Prev. 1986;9:47–58. [PubMed] [Google Scholar]
- 22.de Assis S, Hilakivi-Clarke L. Timing of dietary estrogenic exposures and breast cancer risk. Ann N Y Acad Sci. 2006;1089:14–35. doi: 10.1196/annals.1386.039. [DOI] [PubMed] [Google Scholar]
- 23.Hilakivi-Clarke L. Nutritional modulation of terminal end buds: its relevance to breast cancer prevention. Curr Cancer Drug Targets. 2007;7:465–74. doi: 10.2174/156800907781386641. [DOI] [PubMed] [Google Scholar]
- 24.Cabanes A, Wang M, Olivo S, de Assis S, Gustafsson JA, Khan G, Hilakivi-Clarke L. Prepubertal estradiol and genistein exposures up-regulate BRCA1 mRNA and reduce mammary tumorigenesis. Carcinogenesis. 2004;25:741–48. doi: 10.1093/carcin/bgh065. [DOI] [PubMed] [Google Scholar]
- 25.Hilakivi-Clarke L, Shajahan A, Yu B, de AS. Differentiation of mammary gland as a mechanism to reduce breast cancer risk. J Nutr. 2006;136:2697S–9S. doi: 10.1093/jn/136.10.2697S. [DOI] [PubMed] [Google Scholar]
- 26.Hilakivi-Clarke L, Cho E, Raygada M, Kenney N. Alterations in mammary gland development following neonatal exposure to estradiol, transforming growth factor alpha, and estrogen receptor antagonist ICI 182,780. J Cell Physiol. 1997;170:279–89. doi: 10.1002/(SICI)1097-4652(199703)170:3<279::AID-JCP9>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 27.Russo J, Russo IH. Biological and molecular bases of mammary carcinogenesis. Lab Invest. 1987;57:112–37. [PubMed] [Google Scholar]
- 28.Olivo SE, Hilakivi-Clarke L. Opposing effects of prepubertal low and high fat n-3 polyunsaturated fatty acid diets on rat mammary tumorigenesis. Carcinogenesis. 2005;26:1563–72. doi: 10.1093/carcin/bgi118. [DOI] [PubMed] [Google Scholar]
- 29.Keen CL, Lonnerdal B, Clegg M, Hurley LS. Developmental changes in composition of rat milk: trace elements, minerals, protein, carbohydrate and fat. J Nutr. 1981;111:226–36. doi: 10.1093/jn/111.2.226. [DOI] [PubMed] [Google Scholar]
- 30.Roberts HR, Pettinati JD, Bucek W. A comparative study of human, cow, sow, and rat milk using paper chromatography. J Dairy Sci. 1954;37:538–45. [Google Scholar]
- 31.Nandi S, Guzman RC, Yang J. Hormones and mammary carcinogenesis in mice, rats, and humans: a unifying hypothesis. Proc Natl Acad Sci U S A. 1995;92:3650–3657. doi: 10.1073/pnas.92.9.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guzman RC, Yang J, Rajkumar L, Thordarson G, Chen X, Nandi S. Hormonal prevention of breast cancer: Mimicking the protective effect of pregnancy. Proc Natl Acad Sci USA. 1999;96:2520–2525. doi: 10.1073/pnas.96.5.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clarkson TB, Appt SE, Wood CE, Cline JM. Lessons to be learned from animal studies on hormones and the breast. Maturitas. 2004;49:79–89. doi: 10.1016/j.maturitas.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 34.Soderqvist G, von SB. Lessons to be learned from clinical studies on hormones and the breast. Maturitas. 2004;49:90–96. doi: 10.1016/j.maturitas.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Farlow DW, Xu X, Veenstra TD. Quantitative measurement of endogenous estrogen metabolites, risk-factors for development of breast cancer, in commercial milk products by LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:1327–34. doi: 10.1016/j.jchromb.2009.01.032. [DOI] [PubMed] [Google Scholar]
- 36.Malekinejad H, Scherpenisse P, Bergwerff AA. Naturally occurring estrogens in processed milk and in raw milk (from gestated cows) J Agric Food Chem. 2006;54:9785–91. doi: 10.1021/jf061972e. [DOI] [PubMed] [Google Scholar]
- 37.Ostergaard S, Chagunda MG, Friggens NC, Bennedsgaard TW, Klaas IC. A stochastic model simulating pathogen-specific mastitis control in a dairy herd. J Dairy Sci. 2005;88:4243–57. doi: 10.3168/jds.S0022-0302(05)73111-8. [DOI] [PubMed] [Google Scholar]
- 38.Maruyama K, Oshima T, Ohyama K. Exposure to exogenous estrogen through intake of commercial milk produced from pregnant cows. Pediatr Int. 2009 doi: 10.1111/j.1442-200X.2009.02890.x. [DOI] [PubMed] [Google Scholar]
- 39.Warri A, Saarinen NM, Makela SI, Hilakivi-Clarke L. The role of early life genistein exposures in modifying breast cancer risk. Br J Cancer. 2008 doi: 10.1038/sj.bjc.6604321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cardiff RD. Are the TDLU of the human the same as the LA of mice? J Mammary Gland Biol Neoplasia. 1998;3:3–5. doi: 10.1023/a:1018714016205. [DOI] [PubMed] [Google Scholar]
- 41.Russo J, Hu YF, Yang X, Russo IH. Developmental, cellular, and molecular basis of human breast cancer. J Natl Cancer Inst Monogr. 2000:17–37. doi: 10.1093/oxfordjournals.jncimonographs.a024241. [DOI] [PubMed] [Google Scholar]
- 42.Khan SA, Rogers MA, Khurana KK, Meguid MM, Numann PJ. Estrogen receptor expression in benign breast epithelium and breast cancer risk. J Natl Cancer Inst. 1998;90:37–42. doi: 10.1093/jnci/90.1.37. [DOI] [PubMed] [Google Scholar]
- 43.Lagiou P, Georgila C, Samoli E, Lagiou A, Zourna P, Minaki P, Vassilarou D, Papadiamandis I, Sfikas C, Kalapothaki V, Sekeris CE, Trichopoulos D. Estrogen alpha and progesterone receptor expression in the normal mammary epithelium in relation to breast cancer risk. Int J Cancer. 2009;124:440–442. doi: 10.1002/ijc.23899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sleeman KE, Kendrick H, Robertson D, Isacke CM, Ashworth A, Smalley MJ. Dissociation of estrogen receptor expression and in vivo stem cell activity in the mammary gland. J Cell Biol. 2007;176:19–26. doi: 10.1083/jcb.200604065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stingl J, Caldas C. Molecular heterogeneity of breast carcinomas and the cancer stem cell hypothesis. Nat Rev Cancer. 2007;7:791–99. doi: 10.1038/nrc2212. [DOI] [PubMed] [Google Scholar]
- 46.Collier RJ, Miller MA, McLaughlin CL, Johnson HD, Baile CA. Effects of recombinant bovine somatotropin (rbST) and season on plasma and milk insulin-like growth factors I (IGF-I) and II (IGF-II) in lactating dairy cows. Domest Anim Endocrinol. 2008;35:16–23. doi: 10.1016/j.domaniend.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 47.Collier RJ, Miller MA, Hildebrandt JR, Torkelson AR, White TC, Madsen KS, Vicini JL, Eppard PJ, Lanza GM. Factors affecting insulin-like growth factor-I concentration in bovine milk. J Dairy Sci. 1991;74:2905–11. doi: 10.3168/jds.S0022-0302(91)78473-7. [DOI] [PubMed] [Google Scholar]
- 48.Rogers I, Emmett P, Gunnell D, Dunger D, Holly J. Milk as a food for growth? The insulin-like growth factors link. Public Health Nutr. 2006;9:359–68. doi: 10.1079/phn2006853. [DOI] [PubMed] [Google Scholar]
- 49.Gunnell D, Oliver SE, Peters TJ, Donovan JL, Persad R, Maynard M, Gillatt D, Pearce A, Hamdy FC, Neal DE, Holly JM. Are diet-prostate cancer associations mediated by the IGF axis? A cross-sectional analysis of diet, IGF-I and IGFBP-3 in healthy middle-aged men. Br J Cancer. 2003;88:1682–86. doi: 10.1038/sj.bjc.6600946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heaney RP, McCarron DA, wson-Hughes B, Oparil S, Berga SL, Stern JS, Barr SI, Rosen CJ. Dietary changes favorably affect bone remodeling in older adults. J Am Diet Assoc. 1999;99:1228–33. doi: 10.1016/S0002-8223(99)00302-8. [DOI] [PubMed] [Google Scholar]
- 51.Ben-Shlomo Y, Holly J, McCarthy A, Savage P, Davies D, Davey SG. Prenatal and postnatal milk supplementation and adult insulin-like growth factor I: long-term follow-up of a randomized controlled trial. Cancer Epidemiol Biomarkers Prev. 2005;14:1336–39. doi: 10.1158/1055-9965.EPI-04-0908. [DOI] [PubMed] [Google Scholar]
- 52.van der Pols JC, Bain C, Gunnell D, Smith GD, Frobisher C, Martin RM. Childhood dairy intake and adult cancer risk: 65-y follow-up of the Boyd Orr cohort. Am J Clin Nutr. 2007;86:1722–29. doi: 10.1093/ajcn/86.5.1722. [DOI] [PubMed] [Google Scholar]
- 53.Schernhammer ES, Holly JM, Pollak MN, Hankinson SE. Circulating levels of insulin-like growth factors, their binding proteins, and breast cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14:699–704. doi: 10.1158/1055-9965.EPI-04-0561. [DOI] [PubMed] [Google Scholar]
- 54.de Ostrovich KK, Lambertz I, Colby JK, Tian J, Rundhaug JE, Johnston D, Conti CJ, DiGiovanni J, Fuchs-Young R. Paracrine overexpression of insulin-like growth factor-1 enhances mammary tumorigenesis in vivo. Am J Pathol. 2008;173:824–34. doi: 10.2353/ajpath.2008.071005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee AV, Yee D. Insulin-like growth factors and breast cancer. Biomed Pharmacother. 1995;49:415–21. doi: 10.1016/0753-3322(96)82678-3. [DOI] [PubMed] [Google Scholar]
- 56.Kashima H, Shiozawa T, Miyamoto T, Suzuki A, Uchikawa J, Kurai M, Konishi I. Autocrine stimulation of IGF1 in estrogen-induced growth of endometrial carcinoma cells: involvement of the mitogen-activated protein kinase pathway followed by up-regulation of cyclin D1 and cyclin E. Endocr Relat Cancer. 2009;16:113–22. doi: 10.1677/ERC-08-0117. [DOI] [PubMed] [Google Scholar]
- 57.Santen RJ, Song RX, McPherson R, Kumar R, Adam L, Jeng MH, Yue W. The role of mitogen-activated protein (MAP) kinase in breast cancer. J Steroid Biochem Mol Biol. 2002;80:239–56. doi: 10.1016/s0960-0760(01)00189-3. [DOI] [PubMed] [Google Scholar]
- 58.Loladze AV, Stull MA, Rowzee AM, Demarco J, Lantry JH, III, Rosen CJ, LeRoith D, Wagner KU, Hennighausen L, Wood TL. Epithelial-specific and stage-specific functions of insulin-like growth factor-I during postnatal mammary development. Endocrinology. 2006;147:5412–23. doi: 10.1210/en.2006-0427. [DOI] [PubMed] [Google Scholar]
- 59.Kleinberg DL, Feldman M, Ruan W. IGF-I: an essential factor in terminal end bud formation and ductal morhogenesis. J Mammary Gland Biol Neoplasia. 2000;5:7–17. doi: 10.1023/a:1009507030633. [DOI] [PubMed] [Google Scholar]