Abstract
AIMS
Udenafil is a phosphodiesterase 5 inhibitor used for the treatment of erectile dysfunction. It is metabolized to DA-8164, a major metabolite, by CYP3A4. This study was performed to investigate the effect of ketoconazole, a known CYP3A4 inhibitor, on the pharmacokinetics of udenafil.
METHODS
An open-label, two-period, fixed-sequence crossover study was performed in 12 healthy male volunteers. They received a single 100-mg oral dose of udenafil. Following a 5-day interval, 400 mg of ketoconazole was administered once a day for three consecutive days. On day 3 of ketoconazole treatment, a second 100 mg of udenafil was dosed concomitantly. Blood samples were collected at time points up to 48 h without ketoconazole treatment and up to 72 h with ketoconazole co-administration. The plasma concentration of udenafil was determined using liquid chromatography–tandem mass spectrometry.
RESULTS
Following ketoconazole co-administration, the mean Cmax and AUClast of udenafil (95% confidence interval) increased 1.9-fold (1.60, 2.27) and 3.2-fold (2.82, 3.63), respectively. The median time to reach the Cmax was delayed in the co-administrated treatment, while the mean terminal elimination half-life (t1/2) remained relatively unchanged regardless of ketoconazole co-administration. The metabolic AUC ratio (AUClast of DA-8164/AUClast of udenafil) was 1.71 when udenafil was administered alone, and the value decreased to 0.19 when udenafil was dosed in the presence of ketoconazole. Regarding safety assessments, no clinically significant difference or serious adverse event was observed.
CONCLUSIONS
The systemic exposure of udenafil increased significantly when it was administered with ketoconazole. Dose adjustment may be required when these drugs are used together.
Keywords: ketoconazole, pharmacokinetics, phosphodiesterase type 5 inhibitor, udenafil
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Udenafil is a phosphodiesterase 5 inhibitor used for the treatment of erectile dysfunction.
Udenafil is safe and well tolerated in healthy subjects, and effective as treatment for erectile dysfunction.
In vitro studies have demonstrated that CYP3A4 is the major enzyme responsible for the metabolism of udenafil.
WHAT THIS STUDY ADDS
The pharmacokinetic characteristics of udenafil in the presence of ketoconazole, a potent CYP3A4 inhibitor, were determined in healthy Korean volunteers.
Systemic exposure of udenafil was significantly increased when it was administered with ketoconazole.
Introduction
Udenafil (Zydena®; Dong-A Pharmaceutical Co., Seoul, Korea) is a potent and selective phosphodiesterase (PDE) 5 inhibitor [1], reported to be safe and effective as an oral treatment for erectile dysfunction [1, 2]. Following oral administration, udenafil reached a peak concentration at 0.8–1.3 h and was eliminated with a half-life of 7.3–12.1 h [1]. A steady state was reached at 5 days with only slight accumulation, and urinary excretion of unchanged udenafil was <12% [1, 3]. The therapeutic dose of udenafil is 100 mg, and is not to be administered more than once a day.
Udenafil is metabolized primarily by CYP3A4 to its active N-dealkylated metabolite, DA-8164, which has approximately half the pharmacological activity compared with that of the parent compound [4]. This study aimed to evaluate the drug interaction between udenafil and ketoconazole in healthy subjects.
Methods
An open-label, two-period, fixed-sequence crossover study was conducted in healthy male volunteers. Each volunteer gave written informed consent before being enrolled. The study protocol was approved by the Institutional Review Board of Seoul National University Hospital.
Subjects received a single 100-mg oral dose of udenafil on day 1 after overnight fasting and maintained a fasting state until 4 h after drug administration. A 400-mg dose of ketoconazole was administered once a day on the morning of days 6, 7 and 8. Subjects were kept from eating food for 1 h before and after drug administration on days 6 and 7. On day 8, overnight-fasted subjects received 100 mg of udenafil again approximately 1 h after ketoconazole dosing.
Following a single dose of udenafil, blood samples were collected: before, and 0.5, 1, 1.5, 2, 2.5, 3, 4, 6, 8, 12, 24, 32 and 48 h after dosing. Following the second dose of udenafil, which was administered after the co-administration of ketoconazole, an additional blood sample was drawn at 72 h after udenafil dosing to ensure complete characterization of the pharmacokinetic profile of udenafil even if co-administration of ketoconazole increased the t1/2 of udenafil.
Udenafil and DA-8164 concentrations in plasma were determined using liquid chromatography–tandem mass spectrometry [5, 6]. The lower limit of quantification was 2 ng ml−1, and the method was validated over the range 2–2000 ng ml−1. The accuracy for within- and between-runs ranged from 101.0% to 104.1% and from 94.8% to 97.4%, respectively, and the precision for within- and between-runs ranged from 2.3% to 5.3% and from 5.6% to 9.2%.
Pharmacokinetic parameters were determined by noncompartmental methods using WinNonlin® (Version 5.0; Pharsight Corp., Mountain View, CA, USA). The metabolic AUC ratio of udenafil was calculated as the AUClast of DA-8164 divided by that of udenafil after correcting the AUC values by molecular weights of udenafil (MW 516.66) and DA-8164 (MW 405.4).
The log-transformed Cmax, AUClast, AUC∞ and other pharmacokinetic variables were compared between the values from the two treatments using the paired t-test. These differences were estimated with geometric mean and 95% confidence interval (CI). Tmax was examined using the Wilcoxon signed rank test based on matched pairs. All statistical analyses were performed using SPSS® 12.0 software (SPSS, Seoul, Korea).
Adverse events (AEs) were monitored by asking general health-related questions and subjects' self-reporting. Physical examinations, 12-lead ECGs, laboratory tests including clinical chemistry, haematology and urinalysis were performed at predetermined intervals.
Results
Thirteen volunteers were enrolled, and one subject withdrew informed consent before drug administration. Twelve volunteers completed the study. Their mean age (range) was 24.3 years (23–27) and the mean weight (range) was 68.8 kg (58.1–80.8), with individual body weight values within 80–120% of ideal body weight.
Udenafil, administered alone, was rapidly absorbed with a Cmax of 310.3 ± 93.8 ng ml−1, which was attained in approximately 1.0 h (range 1.0–2.5) after a single oral dose of 100 mg udenafil (Figure 1). The AUClast was 2051.5 ± 379.6 ng h−1 ml−1. When udenafil was administered with ketoconazole, the Cmax and AUClast increased to 574.4 ± 124.3 ng ml−1 and 6581.2 ± 1404.5 ng h−1 ml−1, respectively. The median Tmax (range) was delayed to 1.5 h (1.0–4.0) after co-administration of ketoconazole. The t1/2 remained around 12 h regardless of the co-administration of ketoconazole (Figure 1, Table 1).
Figure 1.
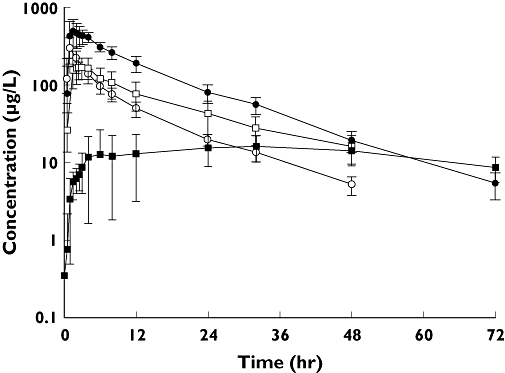
Mean concentration–time profiles of udenafil alone (○), and with ketoconazole (•), N-dealkylated udenafil alone (□) and with ketoconazole ( ) after oral administration of udenafil 100 mg alone and in combination with ketoconazole 400 mg to 12 healthy male volunteers
) after oral administration of udenafil 100 mg alone and in combination with ketoconazole 400 mg to 12 healthy male volunteers
Table 1.
Mean plasma pharmacokinetic parameters of a single 100-mg oral dose of udenafil with and without co-administration of ketoconazole 400 mg
| Parameter | Udenafil alone | Udenafil + ketoconazole | Geometric mean ratio (95% CI) |
|---|---|---|---|
| Cmax (ng ml−1)* | 310.3 (93.8) | 574.4 (124.3) | 1.9 (1.60, 2.27) |
| AUClast (ng h−1 ml−1)* | 2051.5 (379.6) | 6581.2 (1404.5) | 3.20 (2.82, 3.63) |
| AUC∞ (ng h−1 ml−1)* | 2142.6 (395.1) | 6682.0 (1420.5) | 3.11 (2.73, 3.55) |
| t1/2 (h) | 11.9 (1.5) | 12.7 (1.6) | – |
| Tmax (h)†‡ | 1.0 (1.0–2.5) | 1.5 (1.0–4.0) | – |
| CL/F (l h−1)* | 48.7 (12.4) | 15.6 (3.3) | – |
| Metabolic AUC ratio*§ | 1.71 (0.53) | 0.19 (0.09) | – |
P < 0.0001, comparison of values based on the paired t-test.
P < 0.05, comparison of values based on the Wilcoxon signed rank test.
Tmax values are presented as the median (minimum–maximum).
The metabolic AUC ratio of udenafil was calculated as the AUClast of DA-8164 divided by that of udenafil; each AUC was corrected by molecular weight of udenafil and DA-8164. Values represent the arithmetic mean ± SD except for the Tmax.
In the presence of ketoconazole, the mean Cmax and AUClast of udenafil increased 1.9-fold (95% CI 1.60, 2.27) and 3.2-fold (2.82, 3.63), respectively, compared with udenafil alone. The metabolic AUC ratios were 1.71 ± 0.53 (mean ± SD) in udenafil alone and 0.19 ± 0.09 in the co-administration (Table 1).
Despite the higher systemic exposure of udenafil with ketoconazole co-administration, no clinically significant or consistent trends of change were observed in terms of laboratory tests, vital signs or ECGs. None of the subjects developed any serious AE.
Discussion
The study has demonstrated the effects of the co-administration of ketoconazole on the pharmacokinetics of udenafil in humans. The result affirmed previous studies that udenafil was metabolized by CYP3A4 and ketoconazole inhibited hepatic and intestinal CYP3A4 [7, 8]. In the presence of ketoconazole, the systemic exposure of udenafil was increased and the metabolic AUC ratio was decreased. The clearance (CL/F) was significantly decreased with ketoconazole, although the half-lives of two treatments were similar. These findings suggested a predominant contribution of intestinal CYP3A4 inhibition by ketoconazole.
The inhibition of intestinal CYP3A by ketoconazole extended beyond the residence time of 3.5 h [9, 10] and the Tmax of udenafil was 0.8–1.3 h [1]. Thus a 1-h interval between ketoconazole and udenafil dosing was speculated to result in a maximum inhibitory effect.
Udenafil was also demonstrated to be a substrate of P-glycoprotein (P-gp) by recent studies using Caco-2 cells [7, 11]. P-gp inhibitors including cyclosporin and verapamil increased the influx and decreased the efflux of udenafil [11]. Ketoconazole is known to be an inhibitor of not only CYP3A4 but also P-gp [12]. Therefore the co-administration of ketoconazole apparently increases the oral bioavailability and AUC of udenafil through inhibition of both intestinal CYP3A4 and P-gp.
The effect of 400 mg ketoconazole on the pharmacokinetics of udenafil was less when compared with other PDE5 inhibitors metabolized by CYP3A4, such as vardenafil and tadalafil [13]. Co-administration of a 200-mg oral dose of ketoconazole in healthy volunteers resulted in 10.0-fold increase in the AUC of a 5-mg dose of vardenafil [14]. In the case of tadalafil, a 400-mg dose of ketoconazole increased the AUC of a 20-mg dose of tadalafil 4.1-fold [14].
In conclusion, this study has demonstrated that the systemic exposure of udenafil was increased significantly when it was administered with ketoconazole. Dose adjustment may be required when udenafil is administered with ketoconazole or other drugs that potently alter the activity of CYP3A4.
Competing interests
None to declare.
K-H.S. is supported by a training programme grant of the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A070001). This study was sponsored by Dong-A Pharmaceutical Company, Seoul, Korea.
REFERENCES
- 1.Kim BH, Lim HS, Chung JY, Kim JR, Lim KS, Sohn DR, Cho JY, Yu KS, Shin SG, Paick JS, Jang IJ. Safety, tolerability and pharmacokinetics of udenafil, a novel PDE-5 inhibitor, in healthy young Korean subjects. Br J Clin Pharmacol. 2008;65:848–54. doi: 10.1111/j.1365-2125.2008.03107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Paick JS, Kim SW, Yang DY, Kim JJ, Lee SW, Ahn TY, Choi HK, Suh JK, Kim SC. The efficacy and safety of udenafil, a new selective phosphodiesterase type 5 inhibitor, in patients with erectile dysfunction. J Sex Med. 2008;5:946–53. doi: 10.1111/j.1743-6109.2007.00723.x. [DOI] [PubMed] [Google Scholar]
- 3.Amakye D, Ward J, Bryson S, Han K. DA-8159-phase I studies to investigate the safety and pharmacokinetics in healthy male Caucasian subjects. Clin Pharmacol Ther. 2004;75:P86–P86. [Google Scholar]
- 4.Ji HY, Lee HW, Kim HH, Kim DS, Yoo M, Kim WB, Lee HS. Role of human cytochrome P450 3A4 in the metabolism of DA-8159, a new erectogenic. Xenobiotica. 2004;34:973–82. doi: 10.1080/00498250400010898. [DOI] [PubMed] [Google Scholar]
- 5.Shim HJ, Lee EJ, Jung YH, Kim SH, Yoo M, Kwon JW, Kim WB, Lee MG. Determination of a new phosphodiesterase V inhibitor, DA-8159, in plasma and urine by high-performance liquid chromatography. J Pharm Biomed Anal. 2002;30:527–33. doi: 10.1016/s0731-7085(02)00397-7. [DOI] [PubMed] [Google Scholar]
- 6.Cho JY, Lim HS, Yu KS, Shim HJ, Jang IJ, Shin SG. Sensitive liquid chromatography assay with ultraviolet detection for a new phosphodiesterase V inhibitor, DA-8159, in human plasma and urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;795:179–86. doi: 10.1016/s1570-0232(03)00565-8. [DOI] [PubMed] [Google Scholar]
- 7.Kehrer DF, Mathijssen RH, Verweij J, de Bruijn P, Sparreboom A. Modulation of irinotecan metabolism by ketoconazole. J Clin Oncol. 2002;20:3122–9. doi: 10.1200/JCO.2002.08.177. [DOI] [PubMed] [Google Scholar]
- 8.Jakate AS, Roy P, Patel A, Abramowitz W, Persiani S, Wangsa J, Kapil R. Effect of azole antifungals ketoconazole and fluconazole on the pharmacokinetics of dexloxiglumide. Br J Clin Pharmacol. 2005;60:498–507. doi: 10.1111/j.1365-2125.2005.02465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibbs MA, Baillie MT, Shen DD, Kunze KL, Thummel KE. Persistent inhibition of CYP3A4 by ketoconazole in modified Caco-2 cells. Pharm Res. 2000;17:299–305. doi: 10.1023/a:1007550717526. [DOI] [PubMed] [Google Scholar]
- 10.Madsen JL. Effects of gender, age, and body mass index on gastrointestinal transit times. Dig Dis Sci. 1992;37:1548–53. doi: 10.1007/BF01296501. [DOI] [PubMed] [Google Scholar]
- 11.Ji HY, Shim HJ, Yoo M, Park ES, Lee HS. Transport of a new erectogenic udenafil in Caco-2 cells. Arch Pharm Res. 2007;30:1168–73. doi: 10.1007/BF02980254. [DOI] [PubMed] [Google Scholar]
- 12.Wang EJ, Lew K, Casciano CN, Clement RP, Johnson WW. Interaction of common azole antifungals with P glycoprotein. Antimicrob Agents Chemother. 2002;46:160–5. doi: 10.1128/AAC.46.1.160-165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ku HY, Ahn HJ, Seo KA, Kim H, Oh M, Bae SK, Shin JG, Shon JH, Liu KH. The contributions of cytochromes P450 3A4 and 3A5 to the metabolism of the phosphodiesterase type 5 inhibitors sildenafil, udenafil, and vardenafil. Drug Metab Dispos. 2008;36:986–90. doi: 10.1124/dmd.107.020099. [DOI] [PubMed] [Google Scholar]
- 14.Gupta M, Kovar A, Meibohm B. The clinical pharmacokinetics of phosphodiesterase-5 inhibitors for erectile dysfunction. J Clin Pharmacol. 2005;45:987–1003. doi: 10.1177/0091270005276847. [DOI] [PubMed] [Google Scholar]


