Abstract
Background and purpose:
Synaptic deficiency is generally accepted to be involved in major depression, and accordingly classic antidepressants exert their effects through enhancing synaptic efficiency. Hypericin is one of the major active constituents of extracts of St. John's Wort (Hypericum perforatum L.) with antidepressive actions, but little is known about its therapeutic mechanisms. Our aim was to explore whether hypericin has a modulatory effect on neuronal action potential (AP) duration by acting on voltage-gated ion channels.
Experimental approach:
We used voltage-clamp and current-clamp techniques in a whole-cell configuration to study primary cultures of neonatal rat hippocampal neurones. We measured the effects of extracellularly applied hypericin on AP duration as well as on voltage-gated Na+, IA and IK currents.
Key results:
Extracellularly applied hypericin dose-dependently increased AP duration but barely affected its amplitude. Further analysis revealed that hypericin inhibited both transient IA and delayed rectifier IK potassium currents. In contrast, hypericin exerted no significant effect on both Na+ peak current and its decay kinetics.
Conclusions and implications:
Extracellularly applied hypericin increased AP duration, which might be ascribed to its effect on IA and IK currents. As a small increase in AP duration could lead to a dramatic increase in synaptic efficiency, our results imply that hypericin might exert its antidepressant effects by enhancing presynaptic efficiency.
Keywords: St. John's Wort extracts, hypericin, Na+ current, IA current, IK current, action potential duration
Introduction
Major depression occurs worldwide as a severe medical and economic problem, often leading to disability in well over 50% of patients (Kessler et al., 2003; Spijker et al., 2004). In depressed patients, both overall mortality (Cuijpers and Smit, 2002) and suicide risk (Bostwick and Pankratz, 2000) were reported to be substantially increased. Fifty years of studies on the action of antidepressant drugs led to a general belief that a deficiency of monoaminergic neurotransmission is involved in major depression, and clinically available antidepressants, including the monoamine oxidase inhibitors and tricyclic antidepressants, exert their effects by enhancing functions of the monoaminergic system (Charney and Manji, 2004; Berton and Nestler, 2006). Although the available antidepressants have proved to be generally safe and effective, side effects are nonetheless a serious problem, and most importantly, more than half of all the depressed patients showed more or less resistance to these drugs (Berton and Nestler, 2006). At the same time, aqueous alcoholic extracts of St. John's Wort (Hypericum perforatum L.) were well documented in numerous clinical studies and meta-analyses to have antidepressant efficacy comparable to that of classic antidepressants in mild-to-moderate depression, with fewer side effects (Linde et al., 2005; Clement et al., 2006). Their mechanism of action in the treatment of major depression, however, remains largely unknown.
Hyperforin and hypericin are generally believed to be the major therapeutically active constituents of St. John's Wort extracts, and both compounds have shown significant antidepressant effects in behavioural models of major depression in rodents (Butterweck et al., 1997; 1998; 2003; Zanoli et al., 2002). In vitro studies revealed that St. John's Wort extracts potently inhibited the synaptosomal reuptake of 5-HT, noradrenaline and dopamine. Most of this inhibition is believed to be due to hyperforin (Muller et al., 1998; Mennini and Gobbi, 2004; Butterweck and Schmidt, 2007) and whether hypericin, the other major component, plays a role in enhancing synaptic efficiency is still an open question.
In addition to neurotransmitter reuptake, another crucial determinant of synaptic strength is presynaptic transmitter release, which is largely dependent on somatic and presynaptic action potential (AP) duration (Wheeler et al., 1996; Sabatini and Regehr, 1997; Geiger and Jonas, 2000; Atwood and Karunanithi, 2002; King and Meriney, 2005; Bean, 2007). Indeed, dendritic AP is closely related to somatic APs (Stuart et al., 1997) and synaptic efficiency could be dramatically increased by a small increase in AP duration, not only in presynaptic structures (Sabatini and Regehr, 1997; Qian and Saggau, 1999; Bean, 2007) but also in somata (Chalazonitis et al., 1987; Wheeler et al., 1996; Yazejian et al., 1997; King and Meriney, 2005). For example, broad AP waveforms applied to somata of chick ciliary ganglion neurons are more effective at activating calcium current than brief APs (King and Meriney, 2005). Intracellular recordings from CA3 somata showed that changes in somatic AP duration could alter synaptic transmission (Wheeler et al., 1996). Several different ion conductances collectively mediate an AP in a voltage- and time-dependent manner. The rising phase of AP was largely attributable to explosive activation of inward currents of voltage-gated Na+ channels (Hodgkin and Huxley, 1952) and slow decay kinetics of Na+ currents were found to contribute to broadening of APs (Geiger and Jonas, 2000). In rat hippocampal pyramidal neurons, two classes of voltage-gated K+ channels (nomenclature follows Alexander et al., 2008) played a major role in spike repolarization: the transient IA current which normally provides most of the repolarizing drive, and the delayed rectifying K+ channels (IK) (Bean, 2007) providing a delayed but sustained repolarizing force. In this study, we show at first that hypericin did significantly increase the AP duration in primary cultures of neonatal rat hippocampal neurons. Then, to find the ionic mechanism of the increase in AP duration, we investigated the effect of hypericin on Na+, IA and IK currents in these neurons.
Methods
Cell culture
All animal care and experimental protocols were approved by the animal research ethical committee (Institute of Biophysics, CAS, Beijing, China). Hippocampal neurons were acutely isolated and cultured according to previously published protocols (Brewer et al., 1993; Chen et al., 2005) with slight modifications. Briefly, newborn Sprague-Dawley rats (within 24 h postnatal; Weitonglihua Animal Center, Beijing, China) were decapitated and isolated hippocampi were incubated in 0.25% trypsin-EDTA (GIBCO) for 7 min at 37°C. After enzymatic treatment, hippocampal tissues were gently triturated in Dulbecco's modified Eagle's medium (Life Technologies) supplemented with 10% fetal bovine serum (Hyclone, Logan, Utah) using a fire-polished Pasteur pipette. The dissociated hippocampal cells were plated at a density of 2 × 105 cells·cm−2 onto poly-L-lysine (0.05 mg·mL−1, Sigma, St. Louis, MO, USA) overnight-coated glass coverslips and then incubated in a humidified atmosphere of 5% CO2 at 37°C. The medium was changed 7 h later to Neurobasal™-A Medium supplemented with 2% B27 (GIBCO) and 0.5 mM glutamine without antibiotics addition. After that, half of the culture medium was replaced with fresh culture medium every 3 days.
Electrophysiology
Whole-cell voltage- and current-clamp recordings were performed on primary cultures of hippocampal pyramidal neurons between 6 and 10 days in vitro by using a EPC-10 patch-clamp amplifier (HEKA, Germany). All experiments were conducted at room temperature. Pipette and membrane capacitances, and serial resistance were compensated automatically. Series resistance compensation of 50–80% was employed routinely to reduce the voltage error. Offset potentials were nullified directly before formation of the seal. Transients and leakage currents were recorded and digitally subtracted offline in all experiments using averaged records with hyperpolarizing impulses (100 ms voltage steps at −90 mV) that activated no currents. A program package Pulse + Pulsefit (HEKA, Germany) was used for data acquisition and analysis. The extracellular solution for all the recordings was Hanks' balanced salts solution (Sigma; in mM): 1.3 CaCl2, 0.8 MgSO4, 5.4 KCl, 0.4 KH2PO4, 136.9 NaCl, 0.3 Na2PO4, 10 D-glucose and 4.2 NaHCO3. The intracellular solution for AP and K+ current recording contained (in mM): 155 KCl, 2 NaCl, 0.1 CaCl2, 1 EGTA, 2 MgATP and 10 HEPES at pH 7.4. The intracellular solution for Na+ current recording contained (in mM): 150 CsCl, 0.1 CaCl2, 2.5 EGTA, 2 MgATP and 10 HEPES, pH 7.4. The K+ currents were evoked by depolarizing voltage steps from −110 to +70 mV in 20 mV increments with duration of 200 ms. The IK current was derived by applying a 50 ms prepulse to −50 mV immediately prior to each depolarization step in order to inactivate the IA current. Subtraction of IK currents from those without this inactivating prepulse yielded IA. Na+ currents were evoked by depolarizing voltage steps from −100 mV to +20 mV in 10 mV increments with duration of 12 ms. K+ current evoked at +30 mV and the maximal Na+ current of all traces were analysed. The steady-state outward IK current was measured as mean value in a range from 85% to 95% of the current trace. The decay time constant of both IA and Na+ currents was derived by fitting a single exponential to current traces evoked at +30 mV and −30 mV respectively. APs were evoked by passing long depolarizing pulses (100 ms) of increasing magnitude (−20 to 130 pA in 50 pA steps) through the patch electrode (Xi and Xu 1996; Heflin and Cook, 2007; Yu et al., 2008). AP amplitudes were measured from the resting potential, and AP duration were defined as the width at half of the AP amplitude (Geiger and Jonas, 2000; Bean, 2007; Johnston et al., 2009). It has been reported that the photoactive hypericin exhibits enhanced in vitro cytotoxicity on light activation (Theodossiou et al., 2008). Therefore, we compared experimental results in the dark and under weak green light, but no significant difference was found. So, most of the experiments were performed under the weak green light.
Data analysis
All the data are mean ± SE for at least three experiments. Data were analysed statistically by one-way analysis of variance (anova) followed by the Bonferroni test for multiple comparisons. The criterion for a significant difference was P < 0.05.
Materials
Hypericin was purchased from Sigma-Aldrich (St. Louis, MO, USA). It was dissolved in dimethyl sulphoxide (DMSO) as a stock solution and diluted into extracellular solution to different concentrations as required. The amount of DMSO was not more than 0.5% per assay.
Results
Effect of hypericin on AP
Current clamp in a whole-cell configuration was employed to investigate the effects of hypericin on AP waveform in primary cultures of hippocampal pyramidal neurons. At 1.0 µM, extracellularly applied hypericin caused a slight but noticeable increase in AP duration in all cells that generated a single AP. As shown in Figure 1A, AP duration increased from 2.10 ms before application of hypericin to 2.75 ms after application of 1.0 µM hypericin. In contrast, the corresponding AP amplitude was barely affected. The AP duration, relative to that in control cultures with no interventions, decreased to 0.96 after application of DMSO, the solvent of hypericin, and increased concentration-dependently after application of hypericin (0.1–1.0 µM; Figure 1B). The corresponding relative AP amplitudes were not changed over this concentration range (Figure 1C). These results indicated that hypericin only increased the AP duration without affecting its amplitude.
Figure 1.

Effect of hypericin on action potential waveforms. (A) Action potential traces showing effect of 1.0 µM hypericin on AP waveform (representative of six cells). The AP was evoked from the same cell by a 100 ms depolarizing step of 130 pA. AP duration (B) and AP amplitude (C) relative to control values (=1.0) after different concentrations of hypericin. AP amplitude was measured from the holding potential and AP duration was defined as the width at half of the AP amplitude. **P < 0.01, ***P < 0.001, significantly different from control; one-way anova). AP, action potential.
Effect of hypericin on Na+ currents
To analyse the ionic mechanisms involved in these effects of hypericin, we tested the effect of hypericin on the ion channels that contribute to the AP in neurons. First, voltage clamp of whole-cell configuration was employed to investigate the effects of hypericin on voltage-gated Na+ currents in our system. As illustrated in Figure 2A, extracellularly applied 1.0 µM hypericin exerted no significant effects on Na+ peak current or its decay kinetics. The mean values of the ratio (I/I0) of the whole-cell Na+ peak current before (I0) and after (I) application of hypericin (0.1–1.0 µM) were unchanged from control (DMSO only) values (Figure 2B). The corresponding values of the ratio (τ/τ0) of the decay time constant of Na+ current evoked at −30 mV before (τ0) and after (τ) application were similarly unaffected (Figure 2C). These results are compatible with the overall effects of hypericin on AP, which showed no effect on the rising phase and amplitude of the AP.
Figure 2.
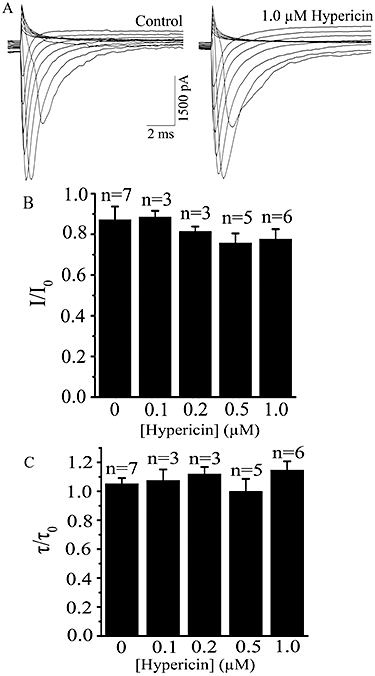
Effects of hypericin on the whole-cell peak Na+ currents. (A) Current traces showing effect of extracellularly applied 1.0 µM hypericin on whole-cell Na+ currents (representative of seven cells). The currents was evoked by voltage steps from −100 mV to +20 mV in 10 mV increments for 12 ms. (B) Summary data of the ratio (I/I0) of the whole-cell Na+ peak currents before (I0) and after (I) application of different concentrations of hypericin. (C) Summary data of the ratio (τ/τo) of the decay time constant of Na+ current evoked at −30 mV before (τ0) and after (τ) application of different concentrations of hypericin. The decay time constant of Na+ current was derived by fitting a single exponential. There are no significant differences between control and different hypericin concentration groups in both (B) and (C) (P > 0.05, one-way anova).
Effect of hypericin on K+ currents
Our experiments showed that the falling phase of APs was significantly altered by application of hypericin, suggesting that many voltage-dependent K+ channels could be involved. Therefore, we tested the action of hypericin on the outward K+ currents, which could be split into two major components: a fast transient IA and a delayed rectifier IK current (Klee et al., 1995). As shown in Figure 3A and B, extracellularly applied 1.0 µM hypericin significantly inhibited IA peak current. Normalized IA current traces evoked at +30 mV clearly showed that hypericin accelerated the decay kinetics of the IA currents. The ratio (I/I0) value of the whole-cell IA peak current evoked at +30 mV before (I0) and after (I) treatment was concentration-dependently decreased after application of hypericin (0.1–1.0 µM) (Figure 3B). Moreover, the corresponding ratios (τ/τ0) of the decay time constant of the IA current were also decreased after application of hypericin (Figure 3C). Thus hypericin dose-dependently suppressed the IA peak current and slowed its decay kinetics.
Figure 3.

Effects of hypericin on peak IA current and its decay kinetics. (A) Whole-cell IA current traces(representative of seven cells) for the control (left panel) and in the presence of 1.0 µM hypericin (middle panel). The currents were evoked by voltage steps from −110 to +70 mV in 20 mV increments for 200 ms and derived from subtraction (see Methods). Right panel: Normalized IA current traces evoked at +30 mV showing effects of 1.0 µM hypericin on its decay kinetics. (B) Ratios (I/I0) of the whole-cell IA peak current evoked at −30 mV before (I0) and after (I) application of different concentrations of hypericin. (C) Summary data of the ratio (τ/τo) of the decay time constant of the IA current evoked at +30 mV before (τ0) and after (τ) application of different concentrations of hypericin. The decay time constant of IA current was derived by fitting a single exponential function. *P < 0.05, **P < 0.01, ***P < 0.001, significantly different from control; one-way anova.
In addition, extracellularly applied 1.0 µM hypericin also significantly inhibited the IK current (Figure 4A) and over the concentration range used here (Figure 4B).
Figure 4.

Effects of hypericin on IK currents. (A) Current traces showing effects of 1.0 µM hypericin on whole-cell IK currents (representative of seven cells). The currents were evoked by voltage steps from −110 to +70 mV in 20 mV increments for 150 ms following a prepulse to −50 mV for 50 ms to inactivate the IA current. (B) Summary data of the ratio (I/Io) of the whole-cell IK current evoked at +30 mV before (I0) and after (I) application of different concentrations of hypericin. ***P < 0.001, significantly different from control; one-way anova. The steady-state outward IK current (+30 mV) was measured as mean value in a range from 85% to 95% of the current trace.
Discussion and conclusions
Hypericin is a highly lipophilic molecule with a rigid planar configuration. It has been recently shown that hypericin can preferentially incorporate and partition into ordered raft domains of membrane systems (Ho et al., 2009). Ion channels are embedded in the lipid environment of the plasma membrane. They can be modulated by dynamic alterations in the microenvironment of the membrane (Ordway et al., 1989; Barrantes, 2002; Tillman and Cascio, 2003). For example, it has been shown that lipid soluble molecules can regulate many K+ channels, such as TRAAK channels (K2P4.1, Maingret et al., 2000), inward rectifier GIRK channels (Kir3, Huang et al., 1998), Ca2+-dependent BKCa channels (KCa1.1, Chi and Qi, 2006) and voltage-dependent K+ (Kv) channels (Schmidt and Mackinnon, 2008). Therefore, because hypericin is able to partition into lipid membranes, we suggest this compound might regulate the IA and IK channels by directly interacting with the channels or indirectly modulate the channels through its modification of the physical properties of the lipid bilayer. Clearly, further study is necessary to support this suggestion.
Although St. John's Wort extracts are well known to have antidepressant efficacy comparable to that of classic antidepressants (Linde et al., 2005; Clement et al., 2006), their antidepressant mechanisms remain largely unknown. One mechanism proposed is that St. John's Wort extracts (mainly due to hyperforin) in vitro potently inhibited the synaptosomal reuptake of 5-HT, noradrenaline and dopamine (Muller et al., 1998; Mennini and Gobbi, 2004; Butterweck and Schmidt, 2007), which might lead to enhanced synaptic efficiency and consequently to antidepressant effects. In our present study, we found that extracellularly applied hypericin increased AP duration in hippocampal neurons and that this effect of hypericin might be explained by its modulation of voltage-gated K+ currents. Our results are compatible with earlier work showing that hypericin prolonged AP duration in cardiac myocytes which could be attributed to its ability to increase L-type Ca2+ channel (CaV1) conductance consequent on a decreased cellular cGMP level via inhibition of soluble guanylate cyclase (Sauviat et al., 2007). As a small increase in AP duration can lead to a dramatic increase in synaptic efficiency (Wheeler et al., 1996; Sabatini and Regehr, 1997; Qian and Saggau, 1999; King and Meriney, 2005; Bean, 2007), our results showing increased AP duration in neurons could similarly lead to a prolonged presynaptic AP duration and enhanced synaptic efficiency. Our study therefore, shows another activity of St. John's Wort extracts exerted by hypericin, one of its major constituents, and which could be highly relevant to the clinically observed anti-depressant effects of these extracts.
Acknowledgments
This work was partly supported by the National Basic Research Program of China (2005CB522804) and by the Research Foundation of Shenzhen. We thank Ms M.Y. Huang for technical assistance.
Glossary
Abbreviations:
- AP
action potential
- DMSO
dimethyl sulphoxide
Conflict of interest
The authors state no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC), 3rd edn. Br J Pharmacol. 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwood HL, Karunanithi S. Diversification of synaptic strength: presynaptic elements. Nat Rev Neurosci. 2002;3:497–516. doi: 10.1038/nrn876. [DOI] [PubMed] [Google Scholar]
- Barrantes FJ. Lipid matters: nicotinic acetylcholine receptor-lipid interactions. Mol Membr Biol. 2002;19:277–284. doi: 10.1080/09687680210166226. [DOI] [PubMed] [Google Scholar]
- Bean BP. The action potential in mammalian central neurons. Nat Rev Neurosci. 2007;8:451–465. doi: 10.1038/nrn2148. [DOI] [PubMed] [Google Scholar]
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- Bostwick JM, Pankratz VS. Affective disorders and suicide risk: a reexamination. Am J Psychiatry. 2000;157:1925–1932. doi: 10.1176/appi.ajp.157.12.1925. [DOI] [PubMed] [Google Scholar]
- Brewer GJ, Torricelli JR, Evege EK, Price PJ. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J Neurosci Res. 1993;35:567–576. doi: 10.1002/jnr.490350513. [DOI] [PubMed] [Google Scholar]
- Butterweck V, Schmidt M. St. John's wort: role of active compounds for its mechanism of action and efficacy. Wien Med Wochenschr. 2007;157:356–361. doi: 10.1007/s10354-007-0440-8. [DOI] [PubMed] [Google Scholar]
- Butterweck V, Wall A, Lieflander-Wulf U, Winterhoff H, Nahrstedt A. Effects of the total extract and fractions of Hypericum perforatum in animal assays for antidepressant activity. Pharmacopsychiatry. 1997;30(Suppl. 2):117–124. doi: 10.1055/s-2007-979531. [DOI] [PubMed] [Google Scholar]
- Butterweck V, Petereit F, Winterhoff H, Nahrstedt A. Solubilized hypericin and pseudohypericin from Hypericum perforatum exert antidepressant activity in the forced swimming test. Planta Med. 1998;64:291–294. doi: 10.1055/s-2006-957437. [DOI] [PubMed] [Google Scholar]
- Butterweck V, Christoffel V, Nahrstedt A, Petereit F, Spengler B, Winterhoff H. Step by step removal of hyperforin and hypericin: activity profile of different Hypericum preparations in behavioral models. Life Sci. 2003;73:627–639. doi: 10.1016/s0024-3205(03)00314-x. [DOI] [PubMed] [Google Scholar]
- Chalazonitis A, Peterson ER, Crain SM. Nerve growth factor regulates the action potential duration of mature sensory neurons. Proc Natl Acad Sci USA. 1987;84:289–293. doi: 10.1073/pnas.84.1.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney DS, Manji HK. Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Sci STKE. 2004;2004:re5. doi: 10.1126/stke.2252004re5. [DOI] [PubMed] [Google Scholar]
- Chen X, Chi S, Liu M, Yang W, Wei T, Qi Z, et al. Inhibitory effect of ganglioside GD1b on K+ current in hippocampal neurons and its involvement in apoptosis suppression. J Lipid Res. 2005;46:2580–2585. doi: 10.1194/jlr.M500252-JLR200. [DOI] [PubMed] [Google Scholar]
- Chi S, Qi Z. Regulatory effect of sulphatides on BKCa channels. Br J Pharmacol. 2006;149:1031–1038. doi: 10.1038/sj.bjp.0706947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement K, Covertson CR, Johnson MJ, Dearing K. St. John's wort and the treatment of mild to moderate depression: a systematic review. Holist Nurs Pract. 2006;20:197–203. doi: 10.1097/00004650-200607000-00008. [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Smit F. Excess mortality in depression: a meta-analysis of community studies. J Affect Disord. 2002;72:227–236. doi: 10.1016/s0165-0327(01)00413-x. [DOI] [PubMed] [Google Scholar]
- Geiger JR, Jonas P. Dynamic control of presynaptic Ca2+ inflow by fast-inactivating K+ channels in hippocampal mossy fiber boutons. Neuron. 2000;28:927–939. doi: 10.1016/s0896-6273(00)00164-1. [DOI] [PubMed] [Google Scholar]
- Heflin SJ, Cook PB. Narrow and wide field amacrine cells fire action potentials in response to depolarization and light stimulation. Vis Neurosci. 2007;24:197–206. doi: 10.1017/S095252380707040X. [DOI] [PubMed] [Google Scholar]
- Ho YF, Wu MH, Cheng BH, Chen YW, Shih MC. Lipid-mediated preferential localization of hypericin in lipid membranes. Biochim Biophys Acta. 2009;1788:1287–1295. doi: 10.1016/j.bbamem.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- Johnston J, Postlethwaite M, Forsythe ID. The impact of synaptic conductance on action potential waveform: evoking realistic action potentials with a simulated synaptic conductance. J Neurosci Methods. 2009 doi: 10.1016/j.jneumeth.2009.06.025. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) Jama. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- King JD, Jr, Meriney SD. Proportion of N-type calcium current activated by action potential stimuli. J Neurophysiol. 2005;94:3762–3770. doi: 10.1152/jn.01289.2004. [DOI] [PubMed] [Google Scholar]
- Klee R, Ficker E, Heinemann U. Comparison of voltage-dependent potassium currents in rat pyramidal neurons acutely isolated from hippocampal regions CA1 and CA3. J Neurophysio. 1995;74:1982–1995. doi: 10.1152/jn.1995.74.5.1982. [DOI] [PubMed] [Google Scholar]
- Linde K, Mulrow CD, Berner M, Egger M. St John's wort for depression. Cochrane Database Syst Rev. 2005 doi: 10.1002/14651858.CD000448.pub2. (4): CD000448. DOI: 10.1002/14651858.CD000448.pub3. [DOI] [PubMed] [Google Scholar]
- Maingret F, Patel AJ, Lesage F, Lazdunski M, Honore E. Lysophospholipids open the two-pore domain mechano-gated K+ channels TREK-1 and TRAAK. J Biol Chem. 2000;275:10128–10133. doi: 10.1074/jbc.275.14.10128. [DOI] [PubMed] [Google Scholar]
- Mennini T, Gobbi M. The antidepressant mechanism of Hypericum perforatum. Life Sci. 2004;75:1021–1027. doi: 10.1016/j.lfs.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Muller WE, Singer A, Wonnemann M, Hafner U, Rolli M, Schafer C. Hyperforin represents the neurotransmitter reuptake inhibiting constituent of hypericum extract. Pharmacopsychiatry. 1998;31(Suppl. 1):16–21. doi: 10.1055/s-2007-979341. [DOI] [PubMed] [Google Scholar]
- Ordway RW, Walsh JV, Jr, Singer JJ. Arachidonic acid and other fatty acids directly activate potassium channels in smooth muscle cells. Science. 1989;244:1176–1179. doi: 10.1126/science.2471269. [DOI] [PubMed] [Google Scholar]
- Qian J, Saggau P. Modulation of transmitter release by action potential duration at the hippocampal CA3-CA1 synapse. J Neurophysiol. 1999;81:288–298. doi: 10.1152/jn.1999.81.1.288. [DOI] [PubMed] [Google Scholar]
- Sabatini BL, Regehr WG. Control of neurotransmitter release by presynaptic waveform at the granule cell to Purkinje cell synapse. J Neurosci. 1997;17:3425–3435. doi: 10.1523/JNEUROSCI.17-10-03425.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauviat MP, Colas A, Chauveau MJ, Drapier JC, Négrerie M. Hypericin activates L-type Ca2+ channels in cardiac myocytes. J Nat Prod. 2007;70:510–514. doi: 10.1021/np060309h. [DOI] [PubMed] [Google Scholar]
- Schmidt D, Mackinnon R. Voltage-dependent K+ channel gating and voltage sensor toxin sensitivity depend on the mechanical state of the lipid membrane. Proc Natl Acad Sci USA. 2008;105:19276–19281. doi: 10.1073/pnas.0810187105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spijker J, Graaf R, Bijl RV, Beekman AT, Ormel J, Nolen WA. Functional disability and depression in the general population. Results from the Netherlands Mental Health Survey and Incidence Study (NEMESIS) Acta Psychiatr Scand*. 2004;110:208–214. doi: 10.1111/j.1600-0447.2004.00335.x. [DOI] [PubMed] [Google Scholar]
- Stuart G, Spruston N, Sakmann B, Hausser M. Action potential initiation and backpropagation in neurons of the mammalian CNS. Trends Neurosci. 1997;20:125–131. doi: 10.1016/s0166-2236(96)10075-8. [DOI] [PubMed] [Google Scholar]
- Theodossiou TA, Papakyriakou A, Hothersall JS. Molecular modeling and experimental evidence for hypericin as a substrate for mitochondrial complex III; mitochondrial photodamage as demonstrated using specific inhibitors. Free Radic Biol Med. 2008;45:1581–1590. doi: 10.1016/j.freeradbiomed.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Tillman TS, Cascio M. Effects of membrane lipids on ion channel structure and function. Cell Biochem Biophys. 2003;38:161–190. doi: 10.1385/CBB:38:2:161. [DOI] [PubMed] [Google Scholar]
- Wheeler DB, Randall A, Tsien RW. Changes in action potential duration alter reliance of excitatory synaptic transmission on multiple types of Ca2+ channels in rat hippocampus. J Neurosci. 1996;16:2226–2237. doi: 10.1523/JNEUROSCI.16-07-02226.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi XZ, Xu ZC. The effect of neurobiotin on membrane properties and morphology of intracellularly labeled neurons. J Neurosci Methods. 1996;65:27–32. doi: 10.1016/0165-0270(95)00140-9. [DOI] [PubMed] [Google Scholar]
- Yazejian B, Digregorio DA, Vergara JL, Poage RE, Meriney SD, Grinnell AD. Direct measurements of presynaptic calcium and calcium-activated potassium currents regulating neurotransmitter release at cultured Xenopus nerve-muscle synapses. J Neurosci. 1997;17:2990–3001. doi: 10.1523/JNEUROSCI.17-09-02990.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Shu Y, McCormick DA. Cortical action potential backpropagation explains spike threshold variability and rapid-onset kinetics. J Neurosci. 2008;28:7260–7272. doi: 10.1523/JNEUROSCI.1613-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoli P, Rivasi M, Baraldi C, Baraldi M. Pharmacological activity of hyperforin acetate in rats. Behav Pharmacol. 2002;13:645–651. doi: 10.1097/00008877-200212000-00006. [DOI] [PubMed] [Google Scholar]


