Abstract
OBJECTIVES
Human X-linked hypophosphatemia (XLH) and its murine homologue, Hyp are caused by inactivating mutations in PHEX gene. The protein encoded by PHEX gene is an endopeptidase whose physiological substrate(s) has not been identified. Dentin matrix protein 1 (DMP1) and dentin sialophosphoprotein (DSPP), two members of the Small Integrin-Binding LIgand, N-linked Glycoprotein (SIBLING) family are proteolytically processed. It has been speculated that PHEX endopeptidase may be responsible for the proteolytic cleavage of DMP1 and DSPP. To test this hypothesis and to analyse the distribution of SIBLING proteins in the predentin/dentin complex and mandible of Hyp mice, we compared the expression of four SIBLING proteins, DMP1, DSPP, bone sialoprotein (BSP) and osteopontin (OPN) between Hyp and wild-type mice.
METHODS
These SIBLING proteins were analysed by protein chemistry and immunohistochemistry.
RESULTS
(1) Dentin matrix protein 1 and DSPP fragments are present in the extracts of Hyp predentin/dentin and bone; (2) the level of DMP1 proteoglycan form, BSP and OPN is elevated in the Hyp bone.
CONCLUSIONS
The PHEX protein is not the enzyme responsible for the proteolytic processing of DMP1 and DSPP. The altered distribution of SIBLING proteins may be involved in the pathogenesis of bone and dentin defects in Hyp and XLH.
Keywords: dentin matrix protein 1, dentin sialophosphoprotein, bone sialoprotein, osteopotin, X-linked hypophosphatemic rickets
Introduction
Human X-linked hypophosphatemic rickets (XLH), caused by mutations in the PHEX gene (phosphate regulating gene with endopeptidase activity on the X chromosome), is characterized by hypomineralization of bone and dentin, widening of osteoid seam and predentin, growth retardation and impaired renal reabsorption of phosphates (Glorieux et al, 1973; Holm et al, 1997). As treatment with vitamin D is not effective, this disease has also been referred to as vitamin D-resistant hypophosphatemic rickets (Abe et al, 1988). Along with manifestations in the skeleton, patients with XLH often have recurrent spontaneous formation of periradicular abscesses affecting multiple non-caries primary and permanent teeth (Pereira et al, 2004; Baroncelli et al, 2006; Batra et al, 2006; Boukpessi et al, 2006). The major histopathological findings in the craniofacial region of XLH patients include the widening of predentin, abnormal cementum and loss of alveolar bone (Murayama et al, 2000). The animal model that resembles the human XLH is the Hyp mice. Hyp mice were produced naturally due to spontaneous mutations in the rodent PHEX gene (Eicher et al, 1976; Beck et al, 1997; Rowe et al, 1997). Hyp mice have been shown to have rickets and osteomalacia, with a decreased mineral content and increased mineral crystal size in their long bones (Boskey et al, 1991), hypomineralized dentin and enlarged predentin (Abe et al, 1989; Boukpessi et al, 2006). Recently, a study showed that the overexpression of the PHEX transgene under a collagen type I promoter corrected the teeth defects in 9-month-old hypophosphatemic mice, but phenotypes in long bone could not be rescued (Boskey et al, 2009).
The extracellular matrix (ECM) of bone and dentin contains type I collagen and non-collagenous proteins (NCPs). The Small Integrin-Binding LIgand, N-linked Glycoprotein (SIBLING) family is one category of NCPs, which includes dentin matrix protein 1 (DMP1), dentin sialophosphoprotein (DSPP), bone sialoprotein (BSP) and osteopontin (OPN) (Fisher et al, 2001). The genes encoding the SIBLING family members are in tandem on human chromosome 4 and their proteins share some common characteristics such as the abundance of acidic amino acid residues, the presence of the integrin-binding Arg–Gly–Asp tripeptide, and similar types of post-translational modifications (e.g. phosphorylation and glycosylation) (Fisher et al, 2001). In mineralized tissues, the SIBLING family members are believed to play important roles in the mineralization of bone and dentin (Qin et al, 2004; Huang et al, 2008b).
Dentin matrix protein 1 is an acidic phosphoprotein that is expressed in dentin, bone and cementum at a relatively high level (George et al, 1993; Macdougall et al, 1998), and in some non-mineralized tissues at a lower level (Terasawa et al, 2004). The importance of DMP1 for osteogenesis and dentinogenesis has been demonstrated by gene ablation experiments in mice (Ye et al, 2004, 2005) and by human genetic studies (Feng et al, 2006). In the ECM of bone and dentin, DMP1 mainly occurs as the proteolytically processed 37 kDa (DMP1 N-terminal) and 57 kDa (DMP1 C-terminal) fragments, which originate from the N-terminal and C-terminal regions of the DMP1 amino acid sequence respectively (Qin et al, 2003b). The proteolytical processing of DMP1 occurs at the N-termini of four aspartyl (Asp) residues. In addition to the 37 kDa form, the N-terminal fragment of DMP1 also occurs as a proteoglycan (referred to as DMP1-PG), in which a single glycosaminoglycan chain is attached to the core protein (Qin et al, 2006). Recently, the full-length form of DMP1 was detected at a concentration much lower than its processed fragments in the bone and dentin (Huang et al, 2008a). The distribution of DMP1 N- and DMP1 C-terminal fragments in dentin are different: the DMP1 N-terminal is located in the unmineralized predentin while the DMP1 C-terminal is mainly found in the mineralized dentin, which suggest that these two fragments may play different roles in dentinogenesis (Maciejewska et al, 2009a). Based on the available data, it is believed that the proteolytic processing of the full-length DMP1 into the N-terminal and C-terminal fragments is an activation step, which is essential for the formation of healthy bone and teeth (Qin et al, 2007).
Dentin sialophosphoprotein, originally thought to be a dentin-specific protein, is now found in the bone, cementum and some non-mineralized tissues at a level remarkably lower than in the dentin. (D’souza et al, 1997; Qin et al, 2002; Baba et al, 2004a; Fisher et al, 2004). DSPP in the dentin and bone is present as the proteolytically processed fragments: dentin sialoprotein (DSP) and dentin phosphoprotein (DPP), which originate from the N-terminal and C-terminal regions of the DSPP amino acid sequence respectively (Macdougall et al, 1997). The major cleavage sites of DSPP are also at the N-termini of aspartyl residues (Macdougall et al, 1997; Qin et al, 2001; Ritchie and Li, 2001), as in the case of DMP1 processing. DSPP mutation studies in humans and gene ablation experiments in mice have demonstrated that DSPP and/or its processed fragments (DSP and DPP) are critical for the mineralization of dentin (Xiao et al, 2001; Zhang et al, 2001; Sreenath et al, 2003; Suzuki et al, 2009) and bone (Verdelis et al, 2008). It has been hypothesized that the proteolytic processing of DSPP into DSP and DPP is an activation step, which may play a critical role in dentinogenesis and/or osteogenesis (Qin et al, 2004). Based on the observations that DMP1 and DSPP are proteolytically processed in a similar manner (i.e. involving the cleavages at the N-termini of aspartyl residues), it is tempting to believe that these two precursor proteins may be proteolytically activated by the same protease.
Bone sialoprotein is primarily found in bone, cementum and tertiary dentin (Ganss et al, 1999; Moses et al, 2006). The biological functions of BSP in the mineralized tissues are complex. Studies showed that BSP acts as a nucleator for the formation of initial hydroxyapatite (HA) crystals, and then, as this mineral grows on the collagen matrix, it acts as an inhibitor in directing the growth of the crystals (Qin et al, 2004). BSP-null mice display minor phenotypic changes: they are a little smaller than their wild-type counterparts (Malaval et al, 2009).
Osteopontin is present abundantly in non-mineralized tissues as well as in the mineralized tissues. In the mineralized tissues, OPN is mainly found in the bone, cementum, predentin, and tertiary dentin (Sodek et al, 2000; Moses et al, 2006). Both in vitro and in vivo studies have shown that OPN is an effective inhibitor of HA formation and growth (Boskey et al, 1993, 2002).
A previous in vitro study described the cleavage of recombinant full-length DMP1 by bone morphogenetic protein-1(BMP-1)/Tolloid-like proteinases (Steiglitz et al, 2004). Several in vitro studies showed that the PHEX protein (an endopeptidase) preferentially cleaves peptide bonds at the N-termini of aspartyl residue (Lipman et al, 1998; Boileau et al, 2001; Sabbagh et al, 2003; Campos et al, 2003). As stated above, DMP1 and DSPP are cleaved at the N-terminal peptide bands of aspartyl residues. PHEX is primarily expressed in osteoblasts, osteocytes and odontoblasts (Du et al, 1996; Guo and Quarles, 1997; Lipman et al, 1998; Ruchon et al, 1998; Thompson et al, 2002), and these are the cells that synthesize DMP1 and DSPP. Additionally, the phenotypic changes in the skeleton and teeth of DMP1-null and DSPP-null mice resemble those in the Hyp mice. These observations have led to a speculation that PHEX endopeptidase may be also responsible for the proteolytic cleavage of DMP1 and DSPP (Qin et al, 2004). To test this hypothesis and to analyse the SIBLING family members in the predentin/dentin complex and bone of Hyp mice, we compared the expression and distribution of DMP1, DSPP, BSP and OPN in the predentin/dentin complex and bone between the Hyp and wild-type (WT) mice.
Materials and methods
Tissue acquisition
WT (C57BL/6) mice and Hyp (C57BL/6-PhexHyp–2J) mice at the ages of 5, 10, 15 weeks were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). The mandible from the 5, 10, 15-week-old mice were used for immunohistochemical (IHC) staining. Ten-week-old mice were used for protein chemistry analysis. Hyp mice used for histology and IHC analyses were male. For protein chemistry analyses, 10 male and 10 female Hyp mice were used (total, 20 Hyp mice), as it was very difficult to raise sufficient number of male mice for these analyses. The animal protocol was approved by the Animal Welfare Committee of Baylor College of Dentistry of the Texas A & M University System Health Science Center.
Extraction and separation of NCPs from the predentin/dentin complex and long bone of hyp mice
Twenty 10-week-old WT and Hyp mice were used for the extraction of NCPs from the predentin/dentin complex and bone. As, it is too difficult to obtain sufficient amounts of bone from the mouse mandible for protein chemistry analyses, the mouse long bone was used for the extraction of NCPs. The relative quantity of SIBLING proteins in the long bone extracts was compared between the Hyp and WT mice; the quantitative difference of the SIBLING family members between the long bone of the two types of mice is expected to similar to that between the mandibles of these two types of mice. The extraction and separation of NCPs from the predentin/dentin complex and bone was performed as previously described (Qin et al, 2001). Briefly, the predentin/dentin complex or bone was placed in 4 M guanidium-HCl (Gdm-HCl)/0.5 M EDTA solution (pH 7.2) containing proteinase inhibitors for 48 h. The extracts were subjected to a Q-Sepharose (Amersham Biosciences, Uppsala, Sweden) ion-exchange chromatography with a gradient ranging 0.1–0.8 M NaCl in 6 M urea solution (pH 7.4); the quantity of total protein was measured for normalization to make sure that exactly same amount of total protein was loaded onto the Q-Sepharose column. The NCPs from the predentin/dentin complex and the long bone were eluted into 120 sequential fractions, each being in 0.5 ml of 6 M urea solution.
Stains-All staining and Western immunoblotting
Sixty microliter of sample from each chromatographic fraction was loaded onto 5–15% gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Stains-All staining and Western immunoblotting were performed to evaluate the SIBLING family members. Stains-All staining was used for detecting all four SIBLING family members eluted from the ion-exchange chromatography. For detection of DMP1 by Western immunoblotting, two types of anti-DMP1 antibodies (Ab) were used: the anti-DMP1-N-9B6.3 monoclonal Ab (Qin et al, 2006) was used at a dilution of 1:1000; while the purified anti-DMP1-C-857 polyclonal Ab (Maciejewska et al, 2009b) was used at a dilution of 1:2000. For detection of DSP, the anti-DSP polyclonal Ab (Butler et al, 1992) was used at a dilution of 1:2000. For detection of BSP, the anti-BSP-10D9.2 monoclonal Ab (Huang et al, 2008b) was used at dilution of 1:2000. For detection of OPN, the anti-OPN monoclonal Ab (Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used at a dilution of 1:1000. For the secondary Ab, the alkaline phosphate-conjugated anti-mouse IgG or anti-rabbit IgG (Sigma-Aldrich, Louis, MO, USA) with a dilution of 1:5000 was employed. Finally, blots were incubated in the chemiluminescent substrate CDP-star (Ambion, Austin, TX, USA) for 5 min and exposed to X-ray films.
Immunohistochemistry
Immunohistochemistry was performed to analyse the difference in the expression and distribution of these SIBLING family members in the predentin/dentin complex and the mandibular bone between the WT and Hyp mice. Mice were perfused with 4% paraformaldehyde in 0.1 M phosphate buffer. The mandibles were demineralized by 8% EDTA (pH 7.4) at 4°C. Tissues were processed for paraffin embedding, and serial sections were prepared. For the detection of DMP1, we used the anti-DMP1-C-8G10.3 (Baba et al, 2004b) and the purified anti-DMP1-N-859 (Huang et al, 2008a) antibodies; both were used at a dilution of 1:800. For the detection of DSP, the anti-DSP-2C12.3 Ab (Baba et al, 2004a) was used at a dilution of 1:800. For the detection of BSP and OPN, antibodies were identical to those used in the Western immunoblotting analyses, and the dilution was 1:500. All IHC experiments were performed using ABC kit and DAB kit (Vector Laboratories Inc., Burlingame, CA, USA), following the manufacturer’s instructions.
Table 1 is a summary of the antibodies used in this study. Detailed information regarding the characteristics of these antibodies can be found in the previous publications.
Table 1.
Antibodies used in this study
| Antibody | Antibody type | Immunizing antigen | Immunoreactivity in Western blotting | Immunoreactivity in immunohistochemistry |
|---|---|---|---|---|
| Anti-DMP1-N-9B6.3a | Monoclonal | 37 kDa (N-terminal) | Yes | Very weak |
| Anti-DMP1-N-859b | Polyclonal | Oligopeptide (residues 101–121) | Yes | Yes |
| Anti-DMP1-C-8G10.3c | Monoclonal | 57 kDa (C-terminal) | No | Yes |
| Anti-DMP1-C-857d | Polyclonal | Oligopeptide (residues 471–485) | Yes | Yes |
| Anti-DSP-2C12.3e | Monoclonal | Purified rat dentin DSP | No | Yes |
| Anti-DSPf | Polyclonal | Purified rat dentin DSP | Yes | Yes |
| Anti-BSP-10D9.2g | Monoclonal | Purified rat bone BSP | Yes | Yes |
| Anti-OPNh | Monoclonal | Mouse recombinant OPN | Yes | Yes |
Anti-DMP1-N-9B6.3 (Qin et al, 2006) was used to detect the N-terminal fragment of DMP1 by Western immunoblotting analysis.
Anti-DMP1-N-859 (Huang et al, 2008a) was used to detect the N-terminal fragment of DMP1 by immunohistochemistry analysis in this study.
Anti-DMP1-C-8G10.3 (Baba et al, 2004b) was used to detect the C-terminal fragment of DMP1 by immunohistochemistry analysis in this study.
Anti-DMP1-C-857 (Maciejewska et al, 2009b) was used to detect the C-terminal fragment of DMP1 by Western immunoblotting analysis.
Anti-DSP-2C12.3 (Baba et al, 2004a) was used to detect DSP by immunohistochemistry analysis.
Anti-DSP (Butler et al, 1992) was used to detect DSP by Western immunoblotting analysis in this study.
Anti-BSP-10D9.2 (Huang et al, 2008b) was used to detect BSP by immunohistochemistry and Western immunoblotting analyses in this study.
Anti-OPN (Santa Cruz) was used to detect OPN by immunohistochemistry and Western immunoblotting analyses in this study.
Results
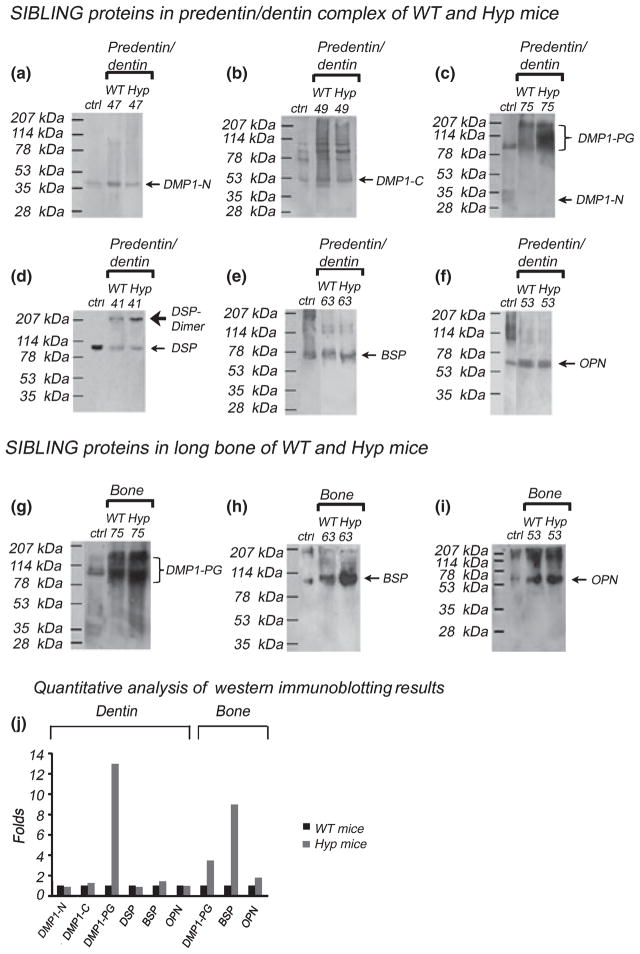
The Q-Sepharose ion-exchange chromatography separated the Gdm-HCl/EDTA extracts of the predentin/dentin complex (Figure 1) and long bone (data not shown) of WT mice and Hyp mice into 120 fractions. Each of the chromatographic fractions that might potentially contain any of the four SIBLING family members was assayed by Stains-All staining and Western immunoblotting. Stains-All staining and Western immunoblotting analyses revealed that all of these four SIBLING members were present in the extracts from the predentin/dentin complex. In this report, the Stains-All staining of fractions 41 through 85 from the predentin/dentin extracts was presented to illustrate these four SIBLING family members along with other NCPs (Figure 2). To illustrate the presence of the DMP1 N- and DMP1 C-terminal fragments, DSP, BSP and OPN in the predentin/dentin complex, Western immunoblotting for a representative fraction was shown (Figure 3a–f). In the bone extracts, the fragments of DMP1, BSP and OPN were detected, and the Western immunoblotting results for DMP1-PG, BSP and OPN in a representative fraction were shown (Figure 3g–i).
Figure 1.
Chromatographic separation of non-collagenous proteins (NCPs) extracted from the predentin/dentin complex of WT (a) and Hyp (b) mice. NCPs in the predentin/dentin complex of the WT and Hyp mice were extracted by 4 M Gdm-HCl/0.5 M EDTA. The Q-Sepharose column separated NCPs from the predentin/dentin complex of the WT (a) and Hyp mice (b) into 120 fractions. Each fraction contained 0.5 ml of 6 M urea solution. Note that the separation profile for the NCPs from the WT mice was similar, but not identical to that from the Hyp mice. The slight differences in the elution profile between the WT and Hyp mice might be due to the fact that the Hyp mice had more predentin, and thus might contain greater amounts of proteoglycans including DMP1-PG which eluted in later fractions (after fraction 65)
Figure 2.
Stains-All staining for chromatographic fractions 41–85 from the predentin/dentin complex of the WT (a) and Hyp (b) mice. Digits at the top of each figure represent fraction numbers of the Q-Sepharose chromatography. MW refers to molecular weight markers. The blue protein bands around 57 and 37 kDa in fractions 45–57 (more prominent in fraction 49) represent DMP1 C- and DMP1 N-terminal fragments respectively. The blue-stained, broad protein bands in fractions 51–57 migrating between the 78 and 114 kDa molecular weight markers represent DPP. The light-blue protein bands in fractions 41–47, migrating at ~100 kDa (close to the 114 kDa molecular weight marker), represent DSP. BSP, migrating just above the 78 kDa molecular weight marker, eluted in fractions 61–85. Minor amounts of DPP co-eluted with BSP in the fractions 61–69; the protein bands between the 78 and 114 kDa molecular weight markers in these fractions represent a mixture of DPP and BSP. The identity of DMP1 fragments, DSP and BSP was confirmed by Western immunoblotting analyses; Western immunoblotting results for a representative chromatographic fraction for each of these molecules are shown in Figure 3
Figure 3.
Western immunoblotting for the SIBLING family members in the extracts from the predentin/dentin complex and the long bone of WT and Hyp mice. A representative fraction is shown, to illustrate the Western immunoblotting results for the SIBLING members. As appropriate antibodies against DPP are not available, we could not carry out Western immunoblotting for DPP. (a–f) Results for the SIBLING members extracted from the predentin/dentin complex. (g–i) Results for the SIBLING members extracted from the long bone. (a, c) Western immunoblotting using the anti-DMP1-N-terminal-9B6.3 monoclonal Ab. Positive control (Ctrl): 1 μg of the N-terminal fragment of DMP1. The core protein of the DMP1-N terminal fragment (37 kDa) (a) and DMP1-PG (c) were detected by the anti-DMP1-N-terminal-9B6.3 Ab. Although there was no difference for the core protein of the DMP1 N-terminal fragment (37 kDa) between the WT and Hyp mice, DMP1-PG was more abundant in the predentin/dentin of the Hyp mice. (b) Western immunoblotting using the anti-DMP1-C-terminal-857 polyclonal Ab. Ctrl: 1 μg of COOH-terminal fragment of DMP1. Note significant difference in the quantity was observed for the DMP1 C-terminal fragment between the WT and Hyp mice. (d) Western immunoblotting using the anti-DSP polyclonal Ab. Ctrl: 0.5 μg of DSP isolated from rat dentin. The expression level of DSP in the dentin of the Hyp mice was similar to WT mice. (e) Western immunoblotting using the anti-BSP-10D9.3 monoclonal Ab. Ctrl: 0.5 μg of BSP isolated from rat long bone. The quantity of BSP in the predentin/dentin of Hyp mice was similar to WT mice. (f) Western immunoblotting using the anti-OPN monoclonal Ab. Ctrl: 1 μg of OPN isolated from rat long bone. The quantity of OPN in the predentin/dentin of Hyp mice was similar to WT mice. (g) Western immunoblotting using the anti-DMP1-N-terminal-9B6.3 monoclonal Ab. Ctrl: 1 μg of DMP1 isolated from the rat long bone. The extracellular matrix (ECM) of the long bone of the Hyp mice had more DMP1-PG than in the WT mice. (h) Western immunoblotting using the anti-BSP-10D9.3 monoclonal Ab. Positive control (Ctrl): 0.5 μg of BSP isolated from the rat long bone. The ECM of the long bone of the Hyp mice had more BSP than the WT mice. (i) Western immunoblotting using the anti-OPN monoclonal Ab. Ctrl: 1 μg of OPN isolated from the rat long bone. The ECM of the long bone of the Hyp mice had more OPN than the WT mice. (j) Quantitative analyses of Western immunoblotting results for SIBLING proteins extracted from the dentin and bone of Hyp and WT mice. For normalization, the quantity of any of the SIBLING proteins from the WT was set as 1, while the quantity of each protein from the Hyp mice was expressed as relative folds to that from the WT mice. The amount of DMP1-PG in the Hyp mouse dentin was more than 12-fold greater than the WT mice. Also note that there was more than eight times of BSP in the Hyp mouse bone than in the WT. The data represent calculations from three separate Western immunoblots that agreed closely
Identification of the DMP1 and DSPP fragments in the extracts from the predentin/dentin complex and bone of hyp mice
In the predentin/dentin extracts of Hyp mice, both the DMP1 N-terminal (37 kDa, blue-stained bands in fractions 45–61) and DMP1 C-terminal (57 kDa, blue-stained bands in fractions 47–61) were visualized by Stains-All staining (Figure 2b1,b2; most prominent in fraction 49); the identity of these protein bands as DMP1 fragments was further confirmed by Western immunoblotting (Figure 3a,b). The amount of DMP1 fragments in the predentin/dentin complex of WT and Hyp mice were similar. In addition, these DMP1 fragments were also detected in the long bone extracts of Hyp mice (data not shown). Similar to the observations about DMP1, the processed fragments of DSPP were observed; DSP (~95 kDa bands in fractions 41–47) and DPP (broad protein bands around 90 kDa in fractions 51–57) were visualized in the dentin extracts from Hyp mice by Stains-All staining (Figure 2b). The identity of DSP in these fractions was further confirmed by Western immunoblotting analyses (Figure 3d). Please note that there is no higher titer, specific anti-DPP Ab that can be used for detecting DPP by Western immunoblotting. These results showed that normal amounts of the proteolytically processed fragments of DMP1/DSPP are present in the predentin/dentin complex of the Hyp mice.
Other SIBLING family members in the Gdm-HCl/EDTA extracts of the predentin/dentin complex of the WT and hyp mice
Stains-All staining revealed the presence of the DMP1 N-terminal, DMP1 C-terminal, DSP, DPP and BSP in the extracts of the predentin/dentin complex (Figure 2). In fractions 59–85, the gradually rising blue smears above the 78 kDa molecular weight marker contained DMP1-PG extracted from the predentin/dentin complex of the WT (Figure 2a) and Hyp mice (Figure 2b), which were confirmed by Western immunoblotting using the anti-DMP1-N-9B6.3 Ab (Figure 3c). Although the predentin/dentin complex of Hyp mice appeared to have DMP1 N-terminal and DMP1 C-terminal fragments that were similar in quantity to the WT mice (Figure 3a,b), the predentin/dentin complex of Hyp mice clearly had more DMP1-PG than the WT mice (Figure 3c). DSP, the blue bands between 78 and 114 kDa molecular weight markers mainly eluted in fractions 41 through 47 (Figure 2a,b). No obvious difference regarding DSP was seen between the WT and Hyp mice (Figure 3d). The most abundant NCP in dentin ECM, DPP, eluted primarily in fractions 51 through 57 (Figure 2a,b, the broad, blue-stained protein bands migrating between 78 and 114 kDa molecular weight markers). BSP was clearly visualized by Stains-All staining in fractions 71–85 (Figure 2, blue band just above the 78 kDa marker); in fractions 59–69 BSP co-eluted with minor amounts of DPP. The identity of BSP was confirmed by Western immunoblotting (Figure 3e). OPN, migrating between 53 and 78 kDa, co-eluted with the DMP1 N- and DMP1 C-terminal fragments in fractions 49–57(Figure 3f). There was no significant difference for BSP and OPN between the predentin/dentin extracts of the WT and Hyp mice.
The SIBLING family members in the Gdm-HCl/EDTA extracts from bone ECM of the WT and hyp mice
Similar analyses were performed for the SIBLING family members extracted from the ECM of bone. Using Western immunoblotting, the DMP1 fragments, BSP and OPN were identified in the ECM extract of long bone from the Hyp mice. The quantity of DMP1-PG, BSP and OPN was dramatically elevated in the Hyp mice compared with the WT mice (Figure 3g–i). For the DMP1 N-terminal fragment (core protein) or the DMP1 C-terminal fragment, we did not observe significant differences in quantity between the WT and Hyp mice (data not shown). As the expression level of DSPP is extremely low (Qin et al, 2002), we did not compare the DSPP fragments in the bone extracts between the WT and Hyp mice.
The differences of SIBLING proteins in the extracts from the dentin and bone of WT and Hyp mice were quantitatively compared (Figure 3j). For these quantitative analyses, the image analysis software Quantity One (Bio-Rad, Hercules, CA, USA) was used to calculate the areas and gray scales (darkness intensity) of SDS-PAGE bands recognized by specific antibodies. As shown in Figure 3j, the amount of DMP1-PG in the Hyp mouse tooth was more than 12-fold greater than in the WT mice, while BSP in the Hyp mouse bone was approximately eight times more than in the WT.
H&E staining and IHC analyses of the SIBLING family members in the predentin/dentin complex and the mandibular bone of the hyp mice
The predentin of Hyp mice was wider than that of the WT mice in all age groups (Figure 4b,e,h,k). For the 5-week-old group, the predentin of the Hyp mice was approximately three times wider than the WT (Figure 4m). The alveolar bone and the mandibular body in the Hyp mice contained remarkably more osteoid (Figure 4f,i,l; osteoid was indicated by arrowheads) than those in the WT mice (Figure 4c). Compared with the WT mice, the thickness of the mandible increased remarkably in the Hyp mice; this increase in thickness was due to the excess accumulation of osteoid in the bone of Hyp mice. There were also greater number of osteocytes in the mandible of Hyp mice than in the WT mice; and the lacunae of osteocytes were larger in the Hyp mice (Figure 4c,f,i,l).
Figure 4.
H&E staining for the predentin/dentin complex and the mandible from 5-week-old WT mice and 5, 10, 15-week-old Hyp mice. Column 1, H&E staining for the mandible at a lower magnification; column 2, H&E staining for the predentin/dentin complex; column 3, H&E staining for the mandible at a higher magnification. a–c, 5-week-old WT mice; d–f, 5-week-old Hyp mice; g–i, 10-week-old Hyp mice; j–l, 15-week-old Hyp mice. The two arrows (two bars) in figure b, e, h, and k were used to indicate the width of predentin. In comparison with the WT mice, the predentin of Hyp mice was wider (compare b with e, h, and k). The mandible of Hyp mice contained greater number of osteocytes and more osteoid (arrowheads) than the WT mice (compare c with f, i and l). (m) Quantity analyses to compare the width of predentin (H&E staining) between the WT and Hyp mice. For normalization, the width of predentin of the WT mice was set as 1; the predentin width of the Hyp mice was expressed as relative folds to that of the WT mice. Note that the predentin of the Hyp mouse was approximately three times wider than the WT mice. The results were obtained from three specimens of three animals, and for each specimen, the predentin width was measured at five points and an average was calculated
In the teeth, these SIBLING family members were mainly observed in the predentin/dentin and cementum. The distribution of the SIBLING family members in the predentin/dentin was similar between the WT and Hyp mice, and showed the following general characteristics: the DMP1 N-terminal was mainly localized in the predentin (Figure 5a,b), while the DMP1 C-terminal was mainly found in the dentin (Figure 5e,f); DSP was observed in the predentin and dentin (Figure 5i,j); BSP and OPN were found more abundantly in the cementum (Figure 5m,n,q,r).
Figure 5.
Immunohistochemical (IHC) staining for the predentin/dentin complex and the mandible of 5-week-old WT and Hyp mice. Column 1: predentin/dentin of 5-week-old WT mice; column 2, predentin/dentin of 5-week-old Hyp mice; column 3, mandible of 5-week-old WT mice; column 4, mandible of 5-week-old Hyp mice. (a–d) IHC for the DMP1 N-terminal; e–h, IHC for the DMP1 C-terminal; i–l, IHC for DSP; m–p, IHC for BSP; q–t, IHC for OPN. Red arrows were used to indicate a relatively weak IHC staining and black arrows were used for a relatively strong IHC staining. In the predentin/dentin complex of both the WT and Hyp mice, the signal for the DMP1 N-terminal was mainly observed in the predentin (PD in a, b) whereas the DMP1 C-terminal was primarily located in the dentin (e, f). In the mandible, a relatively strong signal for the DMP1 N-terminal was observed in the lower border region of the mandible in the Hyp mice while the signal for the N-terminal was generally weak in the mandible of the WT mice (compare c with d). In the Hyp mice, the signal for the N-terminal was also strong in the osteoid or osteoid-like tissues in the sponge bone regions of the alveolar bone. In the WT mice, the signal for the DMP1 C-terminal was strong in the lower border region (cortical bone) of the mandible, but was weak in the spongy bone area (g). In the Hyp mice, the staining for the DMP1 C-terminal was weak in the lower border region of the mandible (h) in which the signal for the N-terminal fragment was strong. The distribution of DSP was similar in the predentin and dentin between the WT and Hyp mice (i, j). In the alveolar bone of the Hyp mice, DSP was more abundant in the osteoid or osteoid-like regions surrounding osteocytes (k, l), and appeared more abundant in the Hyp than in the WT mice. In the tooth, a relatively strong signal for BSP was observed in the cementum (c) in the WT and Hyp mice (m, n). The IHC staining for BSP was stronger in the mandible of the Hyp mice than in the WT mice, especially in the lower border region of the mandible of the Hyp mice (o, p) in which a strong signal for the DMP1 N-terminal was seen. In the tooth, the signal for OPN was strong in the cementum of both the WT and Hyp mice (q, r). The IHC staining for OPN in the alveolar bone of Hyp mice was stronger than in the WT mice. OPN was mainly observed in the areas containing the newly formed bone in the WT mice, but in the Hyp mice, the whole areas of the alveolar bone, especially the osteoid/osteoid-like regions surrounding the osteocytes, displayed strong IHC staining (s, t)
The distribution of the SIBLING family members in the alveolar bone and the mandibular body of the Hyp mice showed features distinct from those of the WT mice (Figure 5: a black arrow was used for a relatively strong signal and a red arrow for a relatively weak signal). In the matrices of the alveolar bone and the mandibular body of the WT mice, the signal for the DMP1 N-terminal is significantly weaker (Figure 5c) than that for the DMP1 C-terminal (Figure 5g); this difference in the distribution of the two fragments is particularly prominent in the highly mineralized cortical bone area (lower border region). The distribution of these DMP1 fragments in the Hyp mice was the opposite: the DMP1 N-terminal fragment was abundant in the matrix, especially in the newly formed osteoid (around osteocytes) and the poorly mineralized, lower border region of the mandible (under normal condition in WT mice this region was an area made of cortical bone) (Figure 5d), while the DMP1 C-terminal fragment was primarily localized in the mineralized spongy bone areas, not in the lower border region of the mandible (Figure 5h). Similar as in the distribution of the DMP1 N-terminal fragment, the level of DSP (the N-terminal fragment of DSPP), was elevated in the osteoid and the poorly mineralized areas of the alveolar bone and the mandibular body in the Hyp mice (Figure 5l). In either the WT (Figure 5k) or Hyp (Figure 5l) mice, DSP was mainly found in the matrices surrounding the lacunae containing osteocytes. The signal for BSP in the alveolar bone and the mandibular body of the Hyp mice (Figure 5p) was remarkably stronger than in those of the WT mice (Figure 5o). The signal for BSP was especially strong in the osteoid surrounding the osteocytes and in the matrix in the lower border region of the mandible. The expression of OPN was also elevated in the alveolar bone and the mandibular body of the Hyp mice (Figure 5t). In the WT mice, OPN was mainly found in the inner surface of the cortical bone and osteoid area (Figure 5s), while in the Hyp mice, OPN was distributed in the whole areas of the alveolar bone and the mandibular body, especially in the osteoid or osteoid-like matrices surrounding the osteocytes (Figure 5t).
Discussion
Based on (1) the PHEX endopeptidase has a strong preference for cleaving peptide bonds at the N-termini of aspartyl residues and the conversion of DMP1 and DSPP into their corresponding fragments involved the cleavages of peptide bonds at the N-termini of aspartyl residues (Qin et al, 2001, 2003b), (2) the PHEX endopeptidase is expressed by the osteogenic and dentinogenic cells that synthesize DMP1 and DSPP, and (3) the similarities between the phenotypic changes of the Hyp mice and those of the DMP1-null and DSPP-null mice, it has been hypothesized that the PHEX endopeptidase may be the enzyme responsible for the proteolytic activation of DMP1 and DSPP (Qin et al, 2004). This study showed that the N- and C-terminal fragments of DMP1 and DSPP are present in the predentin/dentin and bone of Hyp mice, with the quantities similar to (or more than) those in the WT mice. These findings indicate that DMP1 and DSPP are properly processed in the PHEX-deficient mice. A recent in vitro study using co-transfection of full-length DMP1 with PHEX into HEK-293 cells showed that the co-expression of PHEX had no effect on the processing of DMP1, indicating that PHEX does not cleave DMP1 (Lu et al, 2009). Taken together, we believe that PHEX protein is unlikely to be responsible for the proteolytic processing of DMP1 and DSPP.
This study revealed that the expression and distribution of the four SIBLING family members, DMP1, DSPP, BSP and OPN in the bone are different between the Hyp and the WT mice. Protein chemistry analyses showed that the extracts from the ECM of the predentin/dentin and osteoid/bone complexes in the Hyp mice had greater amount of DMP1-PG than in the WT mice, while no significant change in the core protein (37 kDa) fragment was found between the two types of mice. IHC staining using antibodies against the N-terminal fragment of DMP1 revealed that the signal for the N-terminal fragment of DMP1 was stronger in the alveolar bone and the mandibular body of Hyp mice than in the WT mice; the strong signal for the DMP1 N-terminal was particularly prominent in the osteoid of the alveolar bone and in the superficial portion of the mandibular body that was hypomineralized (osteoid-like). As protein chemistry analysis showed a significant increase of DMP1-PG in the bone of Hyp mice while the core protein (37 kDa) fragment remained unchanged, the strong IHC signal for the N-terminal fragment of DMP1 in the Hyp mice must be due to the greater amount of DMP1-PG in the bone matrix. Previous studies showed that DMP1-PG is almost exclusively present in the predentin (nearly absent in the mineralized dentin), suggesting that DMP1-PG may be an inhibitor for dentin mineralization during dentinogenesis (Maciejewska et al, 2009a). It has been speculated that DMP1-PG, along with other proteoglycans, is removed before mineralization occurs, and thus very little or no DMP1-PG remains in the mineralized dentin (Maciejewska et al, 2009a). Osteoid, the precursor of mineralized bone, resembles predentin (the precursor of mineralized dentin). However, under normal conditions, it is difficult to evaluate the differences in the expression/distribution of the SIBLING family members between osteoid and bone using light microscopy approaches, as the osteoid seam is very thin and boundary between the osteoid and bone is not as well demarcated as that between the predentin and dentin. In the Hyp mice, the osteoid seam is much wider and can be clearly distinguished from the mineralized bone; thus the Hyp mice are a good model for evaluating the differential distribution of these DMP1 fragments (perhaps also for other molecules) between the osteoid and bone. Maciejewska et al (2009b) showed that the N- and C-terminal fragments of DMP1 are distributed differently in the different layers of the cartilage of the femur growth plate, but were unable to reveal whether or not there are any differences in the distribution of these DMP1 fragments between the osteoid and bone in normal rats (i.e. under physiological conditions). The present study showed clearly that DMP1-PG is predominantly present in the osteoid of the alveolar bone and the mandibular body in the Hyp mice. As the bone of Hyp mice has greater amount of osteoid, it is not surprising to see that the extracts from the osteoid/bone complex of the Hyp mice has more DMP1-PG than the WT mice. The data obtained in this study regarding the distribution of DMP1-PG is in agreement with those from a previous study demonstrating the DMP1-PG is primarily distributed in the predentin. Taken together, it is tempting to speculate that as a biomineralization inhibitor, the increase of DMP1-PG may be a contributing factor in the pathogenesis of Hyp or its human analogue XLH; one of the major abnormalities of these diseases is hypomineralization. It should be noted that DMP1-PG is the major form of the DMP1 N-terminal fragment in the ECM of predentin/dentin and bone, and that DMP1-PG appears to be a major proteoglycan in the bone and dentin (Qin et al, 2007; Huang et al, 2008b).
Unlike the N-terminal fragment that primarily exists as the proteoglycan form, the DMP1 C-terminal fragment is not glycosylated, but is highly phosphorylated (Qin et al, 2006). Two in vitro studies showed that purified DMP1 C-terminal fragment alone (Tartaix et al, 2004) or in the presence of type I collagen (Gajjeraman et al, 2007) accelerates the nucleation of HA crystals. Additionally, transgenic mouse studies showed that the expression of the DMP1 C-terminal rescued the hypomineralization phenotypes of bone and dentin in the DMP1-null mice (Lu et al, 2009). These data suggest that the DMP1 C-terminal fragment promotes the mineralization of bone and dentin. In this study, a strong signal for the DMP1 C-terminal was observed in the cortical bone of the mandibular body in the WT mice; the cortical bone, especially in the superficial part of the mandible, is more highly mineralized than in the spongy bone localized in the center of the mandible. In the WT mice, the signal for this fragment was remarkably weaker in the spongy bone than in the cortical bone of the mandible. In the Hyp mice, the lower border region of the mandible had a lower level of mineralization, showing osteoid-like structure; the signal for the DMP1 C-terminal was weaker in these osteoid-like structures than in the trabecular bone matrix in the inside portion of the mandible (spongy bone area, Figure 5h). These observations are in agreement with the purported roles of the DMP1 C-terminal fragment as a promoter for the mineralization of bone and dentin. Taken together, these data indicate a potential role of the DMP1 N-terminal and C-terminal fragments in the development process of human XLH and its murine homologue Hyp.
In addition to the fragments of DMP1, other three SIBLING family members also showed altered distribution in the alveolar bone and the mandibular body of Hyp mice. DSP, the N-terminal fragment of DSPP, increased in the alveolar bone of Hyp mice. Similar to the DMP1 N-terminal fragment, DSP also mainly occurs as a proteoglycan (Qin et al, 2003a; Yamakoshi et al, 2005; Sugars et al, 2006). It is likely that the strong signal visualized by the anti-DSP antibodies in the alveolar bone of Hyp mice primarily represents the proteoglycan form of DSP. The expression of BSP was elevated in the bone of Hyp mice; a particularly strong signal for BSP was observed in the superficial portion of the mandibular body that was hypomineralized (osteoid- like) and where the signal for the DMP1 N-terminal was strong. OPN was mainly seen in the unmineralized osteoid and the remodeling area of the cortical bone in the WT mice; while in the Hyp mice, the level of OPN was elevated in the alveolar bone matrix, and the strong signal was more prominent in the matrix around osteocytes.
It should be noted that in this study, both male and female Hyp mice were used for protein chemistry analyses. The phenotypical changes in the skeleton of the male Hyp mice are more obvious than the female (Boskey et al, 2009). Thus, if only male mice had been selected for the protein chemistry analyses in this investigation, we might have observed greater differences in the level of DMP1-PG, BSP and OPN between the Hyp and WT. Nevertheless, as the results from the protein chemistry analyses agreed closely with the findings from the immunohistochemcial staining, the use of both male and female mice for the protein chemistry analyses did not affect our conclusion that the levels of these molecules are elevated in the Hyp mice.
In summary, in the PHEX-deficient mice, all of these four SIBLING family members had altered expression and distribution in the bone. DMP1-PG, DSP, BSP and OPN are increased, while the DMP1 C-terminal fragment is decreased in the osteoid and hypomineralized area of the mandible of the Hyp mice. These observations indicate that the altered expression and distribution of these four SIBLING family members may be involved in the pathogenesis of the bony and dental defects in the human XLH and its murine homologue, Hyp. Clearly, future studies are needed to investigate the specific relationships between the abnormal expression/distribution of the SIBLING family members and the defects of the mineralized tissues. Such studies may provide clues for explaining the loss of alveolar bone and the spontaneous formation of periradicular abscesses in the XLH patients.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (2007, Grant No. 30672334- c03031102), and the USA National Institutes of Health Grant DE 005092 (to CQ).
References
- Abe K, Ooshima T, Tong SML, Yasufuku Y, Sobue S. Structural deformities of deciduous dentin in patients with hypophosphatemic vitamin D-resistant rickets. Oral Surg Oral Med Oral Pathol. 1988;65:191–198. doi: 10.1016/0030-4220(88)90165-x. [DOI] [PubMed] [Google Scholar]
- Abe K, Ooshima T, Masatomi Y, Sobue S, Moriwaki Y. Microscopic and crystallographic examinations of the dentin of the X-linked hypophosphatemic mouse. J Dent Res. 1989;68:1519–1524. doi: 10.1177/00220345890680111001. [DOI] [PubMed] [Google Scholar]
- Baba O, Qin C, Brunn JC, et al. Detection of dentin sialoprotein in rat periodontium. Eur J Oral Sci. 2004a;112:163–170. doi: 10.1111/j.0909-8836.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- Baba O, Qin C, Brunn JC, Wygant JN, Mcintyre BW, Butler WT. Colocalization of dentin matrix protein 1 and dentin sialoprotein at late stages of rat molar development. Matrix Biol. 2004b;23:371–379. doi: 10.1016/j.matbio.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Baroncelli GI, Angiolini M, Ninni E, Galli V, Saggese R, Giuca MR. Prevalence and pathogenesis of dental and periodontal lesions in children with X-linked hypophosphatemic rickets. Eur J Paediatr Dent. 2006;7:61–66. [PubMed] [Google Scholar]
- Batra P, Tejani Z, Mars M. X-linked hypophosphatemia: dental and histologic findings. J Can Dent Assoc. 2006;72:69–72. [PubMed] [Google Scholar]
- Beck L, Soumounou Y, Martel J, et al. Pex/PEX Tissue distribution and evidence for a deletion in the 30region of the Pex gene in X-linked hypophosphatemic mice. J Clin Invest. 1997;99:1200–1209. doi: 10.1172/JCI119276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau G, Tenenhouse HS, Desgroseillers L, Crine P. Characterization of PHEX endopeptidase catalytic activity: identification of parathyroid-hormone-related peptide107-139 as a substrate and osteocalcin, PPi and phosphate as inhibitors. Biochem J. 2001;355:707–713. doi: 10.1042/bj3550707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boskey AL, Gilder H, Neufeld E, Ecarot B, Glorieux FH. Phospholipid changes in the bones of the hypophosphatemic mouse. Bone. 1991;12:345–351. doi: 10.1016/8756-3282(91)90021-a. [DOI] [PubMed] [Google Scholar]
- Boskey Al, Maresca M, Ullrich W, Doty SB, Butler WT, Prince CW. Osteopontin-hydroxyapatite interactions in vitro: inhibition of hydroxyapatite formation and growth in a gelatin gel. Bone Miner. 1993;22:147–159. doi: 10.1016/s0169-6009(08)80225-5. [DOI] [PubMed] [Google Scholar]
- Boskey Al, Spevak L, Paschalis E, Doty SB, Mckee MD. Osteopontin deficiency increases mineral content and mineral crystallinity in mouse bone. Calcif Tissue Int. 2002;71:145–154. doi: 10.1007/s00223-001-1121-z. [DOI] [PubMed] [Google Scholar]
- Boskey Al, Frank A, Fujimoto Y, et al. The PHEX transgene corrects mineralization defects in 9-month-old hypophosphatemic mice. Calcif Tissue Int. 2009;84:126–137. doi: 10.1007/s00223-008-9201-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukpessi T, Septier D, Bagga S, Garabedian M, Goldberg M, Chaussain-Miller C. Dentin alteration of deciduous teeth in human hypophosphatemic rickets. Calcif Tissue Int. 2006;79:294–300. doi: 10.1007/s00223-006-0182-4. [DOI] [PubMed] [Google Scholar]
- Butler WT, Bhown M, Brunn JC, et al. Isolation, characterization and immunolocalization of a 53-kDal dentin sialoprotein (DSP) Matrix. 1992;12:343–351. doi: 10.1016/s0934-8832(11)80030-2. [DOI] [PubMed] [Google Scholar]
- Campos M, Couture C, Hirata IY, et al. Human recombinant endopeptidase PHEX has a strict S1’ specificity for acidic residues and cleaves peptides derived from fibroblast growth factor-23 and matrix extracellular phosphoglycoprotein. Biochem J. 2003;12:271–279. doi: 10.1042/BJ20030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’souza RN, Cavender A, Sunavala G, et al. Gene expression patterns of murine dentin matrix protein 1 (Dmp1) and dentin sialophosphoprotein (DSPP) suggest distinct developmental functions in vivo. J Bone Miner Res. 1997;12:2040–2049. doi: 10.1359/jbmr.1997.12.12.2040. [DOI] [PubMed] [Google Scholar]
- Du L, Desbarats M, Viel J, Glorieux FH, Cawthorn C, Ecarot B. cDNA cloning of the murine Pex gene implicated in X-linked hypophosphatemia and evidence for expression in bone. Genomics. 1996;36:22–28. doi: 10.1006/geno.1996.0421. [DOI] [PubMed] [Google Scholar]
- Eicher EM, Southard JL, Scriver C, Glorieux FH. Hypophosphatemia: mouse model for human familial hypophosphatemic (vitamin D-resistant) rickets. Proc Natl Acad Sci USA. 1976;73:4667–4671. doi: 10.1073/pnas.73.12.4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng JQ, Ward LM, Liu S, et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1230–1235. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher LW, Torchia DA, Fohr B, Young MF, Fedarko NS. Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem Biophys Res Commun. 2001;280:460–465. doi: 10.1006/bbrc.2000.4146. [DOI] [PubMed] [Google Scholar]
- Fisher LW, Jain A, Tayback M, Fedarko NS. Small integrin binding ligand N-linked glycoprotein gene family expression in different cancers. Clin Cancer Res. 2004;10:8501–8511. doi: 10.1158/1078-0432.CCR-04-1072. [DOI] [PubMed] [Google Scholar]
- Gajjeraman S, Narayanan K, Hao J, Qin C, George A. Matrix macromolecules in hard tissues control the nucleation and hierarchical assembly of hydroxyapatite. J Biol Chem. 2007;282:1193–1204. doi: 10.1074/jbc.M604732200. [DOI] [PubMed] [Google Scholar]
- Ganss B, Kim RH, Sodek J. Bone sialoprotein. Crit Rev Oral Biol Med. 1999;10:79–98. doi: 10.1177/10454411990100010401. [DOI] [PubMed] [Google Scholar]
- George A, Sabsay B, Simonian P, Veis A. Characterization of a novel dentin matrix acidic phosphoprotein. Implications for induction of biomineralization. J Biol Chem. 1993;268:12624–12630. [PubMed] [Google Scholar]
- Glorieux FH, Holick MF, Scriver CR, DeLuca HF. X-linked hypophosphataemic rickets: inadequate therapeutic response to 1, 25-dihydroxycholecalciferol. Lancet. 1973;2:287–289. doi: 10.1016/s0140-6736(73)90793-9. [DOI] [PubMed] [Google Scholar]
- Guo R, Quarles LD. Cloning and sequencing of human PEX from a bone cDNA library: evidence for its developmental stage-specific regulation in osteoblasts. J Bone Miner Res. 1997;12:1009–1017. doi: 10.1359/jbmr.1997.12.7.1009. [DOI] [PubMed] [Google Scholar]
- Holm IA, Huang X, Kunkel LM. Mutational analysis of the PEX gene in patients with X-linked hypophosphatemic rickets. Am J Hum Genet. 1997;60:790–797. [PMC free article] [PubMed] [Google Scholar]
- Huang B, Maciejewska I, Sun Y, et al. Identification of full-length dentin matrix protein 1 in dentin and bone. Calcif Tissue Int. 2008a;82:401–410. doi: 10.1007/s00223-008-9140-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Sun Y, Maciejewska I, et al. Distribution of SIBLING proteins in the organic and inorganic phases of rat dentin and bone. Eur J Oral Sci. 2008b;116:104–112. doi: 10.1111/j.1600-0722.2008.00522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman ML, Panda D, Bennett HP, et al. Cloning of human PEX cDNA. Expression, subcellular localization, and endopeptidase activity. J Biol Chem. 1998;273:13729–13737. doi: 10.1074/jbc.273.22.13729. [DOI] [PubMed] [Google Scholar]
- Lu Y, Qin C, Xie Y, Bonewald LF, Feng JQ. Studies of the DMP1 57-kDa functional domain both in vivo and in vitro. Cells Tissues Organs. 2009;189:175–185. doi: 10.1159/000151727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdougall M, Simmons D, Luan X, Nydegger J, Feng J, Gu T. Dentin phosphoprotein and dentin sialoprotein are cleavage products expressed from a single transcript coded by a gene on human chromosome 4. Dentin phosphoprotein DNA sequence determination. J Biol Chem. 1997;272:835–842. doi: 10.1074/jbc.272.2.835. [DOI] [PubMed] [Google Scholar]
- Macdougall M, Gu T, Luan X, Simmons D, Chen J. Identification of a novel isoform of mouse dentin matrix protein 1: spatial expression in mineralized tissues. J Bone Miner Res. 1998;13:422–431. doi: 10.1359/jbmr.1998.13.3.422. [DOI] [PubMed] [Google Scholar]
- Maciejewska I, Cowan C, Svoboda K, Butler WT, D’Souza R, Qin C. The NH2-terminal and COOH-terminal fragments of dentin matrix protein 1 (DMP1) localize differently in the compartments of dentin and growth plate of bone. J Histochem Cytochem. 2009a;57:155–166. doi: 10.1369/jhc.2008.952630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciejewska I, Qin D, Huang B, et al. Distinct compartmentalization of dentin matrix protein 1 fragments in mineralized tissues and cells. Cells Tissues Organs. 2009b;189:186–191. doi: 10.1159/000151372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaval L, Monfoulet L, Fabre T, et al. Absence of bone sialoprotein (BSP) impairs cortical defect repair in mouse long bone. Bone. 2009;45:853–861. doi: 10.1016/j.bone.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Moses K, Butler WT, Qin C. Immunohistochemical study of SIBLING proteins in reactionary dentin of rat molars at different ages. Eur J Oral Sci. 2006;114:216–222. doi: 10.1111/j.1600-0722.2006.00353.x. [DOI] [PubMed] [Google Scholar]
- Murayama T, Iwatsubo R, Akiyama S, Amano A, Morisaki I. Familial hypophosphatemic vitamin D-resistant rickets: dental findings and histologic study of dentin. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:310–316. doi: 10.1067/moe.2000.107522. [DOI] [PubMed] [Google Scholar]
- Pereira CM, de Andrade CR, Vargas PA, Coletta RD, de Almeida OP, Lopes MA. Dental alterations associated with X-linked hypophosphatemic rickets. J Endod. 2004;30:241–245. doi: 10.1097/00004770-200404000-00015. [DOI] [PubMed] [Google Scholar]
- Qin C, Brunn JC, Jones J, et al. A comparative study of sialic acid-rich proteins in rat bone and dentin. Eur J Oral Sci. 2001;109:133–141. doi: 10.1034/j.1600-0722.2001.00001.x. [DOI] [PubMed] [Google Scholar]
- Qin C, Brunn JC, Cadena E, Ridall A, Tsujigiwa H, Nagatsuka H. The expression of dentin sialoprotein gene in bone. J Dent Res. 2002;81:392–394. doi: 10.1177/154405910208100607. [DOI] [PubMed] [Google Scholar]
- Qin C, Brunn JC, Baba O, Wygant JN, McIntyre BW, Butler WT. Dentin sialoprotein isoforms: detection and characterization of a high molecular weight dentin sialoprotein. Eur J Oral Sci. 2003a;111:235–242. doi: 10.1034/j.1600-0722.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- Qin C, Brunn JC, Cook RG, et al. Evidence for the proteolytic processing of dentin matrix protein 1. Identification and characterization of processed fragments and cleavage sites. J Biol Chem. 2003b;278:34700–34708. doi: 10.1074/jbc.M305315200. [DOI] [PubMed] [Google Scholar]
- Qin C, Baba O, Butler WT. Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis. Crit Rev Oral Biol Med. 2004;15:126–136. doi: 10.1177/154411130401500302. [DOI] [PubMed] [Google Scholar]
- Qin C, Huang B, Wygant JN, et al. A chondroitin sulfate chain attached to the bone dentin matrix protein 1 NH2-terminal fragment. J Biol Chem. 2006;281:8034–8040. doi: 10.1074/jbc.M512964200. [DOI] [PubMed] [Google Scholar]
- Qin C, D’Souza R, Feng JQ. Dentin matrix protein 1 (DMP1): new and important roles for biomineralization and phosphate homeostasis. J Dent Res. 2007;86:1134–1141. doi: 10.1177/154405910708601202. [DOI] [PubMed] [Google Scholar]
- Ritchie HH, Li X. A novel rat dentin mRNA coding only for dentin sialoprotein. Eur J Oral Sci. 2001;109:342–347. doi: 10.1034/j.1600-0722.2001.00093.x. [DOI] [PubMed] [Google Scholar]
- Rowe PS, Oudet CL, Francis F, et al. Distribution of mutations in the PEX gene in families with X-linked hypophosphataemic rickets (HYP) Hum Mol Genet. 1997;6:539–549. doi: 10.1093/hmg/6.4.539. [DOI] [PubMed] [Google Scholar]
- Ruchon AF, Marcinkiewicz M, Siegfried G, et al. Pex mRNA is localized in developing mouse osteoblasts and odontoblasts. J Histochem Cytochem. 1998;46:459–468. doi: 10.1177/002215549804600405. [DOI] [PubMed] [Google Scholar]
- Sabbagh Y, Boileau G, Campos M, Carmona AK, Tenenhouse HS. Structure and function of disease-causing missense mutations in the PHEX gene. J Clin Endocrinol Metab. 2003;88:2213–2222. doi: 10.1210/jc.2002-021809. [DOI] [PubMed] [Google Scholar]
- Sodek J, Ganss B, Mckee MD. Osteopontin. Crit Rev Oral Biol Med. 2000;11:279–303. doi: 10.1177/10454411000110030101. [DOI] [PubMed] [Google Scholar]
- Sreenath T, Thyagarajan T, Hall B, et al. Dentin sialophosphoprotein knockout mouse dentin display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J Biol Chem. 2003;278:24874–24880. doi: 10.1074/jbc.M303908200. [DOI] [PubMed] [Google Scholar]
- Steiglitz BM, Ayala M, Narayanan K, George A, Greenspan DS. Bone morphogenetic protein-1/Tolloid-like proteinases process dentin matrix protein-1. J Biol Chem. 2004;279:980–986. doi: 10.1074/jbc.M310179200. [DOI] [PubMed] [Google Scholar]
- Sugars RV, Olsson ML, Waddington R, Wendel M. Substitution of bovine dentine sialoprotein with chondroitin sulfate glycosaminoglycan chains. Eur J Oral Sci. 2006;114:89–92. doi: 10.1111/j.1600-0722.2006.00271.x. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Sreenath T, Haruyama N, et al. Dentin sialoprotein and dentin phosphoprotein have distinct roles in dentin mineralization. Matrix Biol. 2009;28:221–229. doi: 10.1016/j.matbio.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaix PH, Doulaverakis M, George A, et al. In vitro effects of dentin matrix protein-1 on hydroxyapatite formation provide insights into in vivo functions. J Biol Chem. 2004;279:18115–18120. doi: 10.1074/jbc.M314114200. [DOI] [PubMed] [Google Scholar]
- Terasawa M, Shimokawa R, Terashima T, Ohya K, Takagi Y, Shimokawa H. Expression of dentin matrix protein 1 (DMP1) in nonmineralized tissues. J Bone Miner Metab. 2004;22:430–438. doi: 10.1007/s00774-004-0504-4. [DOI] [PubMed] [Google Scholar]
- Thompson DL, Sabbagh Y, Tenenhouse HS, et al. Ontogeny of Phex/PHEX protein expression in mouse embryo and subcellular localization in osteoblasts. J Bone Miner Res. 2002;17:311–320. doi: 10.1359/jbmr.2002.17.2.311. [DOI] [PubMed] [Google Scholar]
- Verdelis K, Ling Y, Sreenath T, et al. DSPP effects on in vivo bone mineralization. Bone. 2008;43:983–990. doi: 10.1016/j.bone.2008.08.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao S, Yu C, Chou X, et al. Dentinogenesis imperfecta 1 with or without progressive hearing loss is associated with distinct mutations in DSPP. Nat Genet. 2001;27:201–204. doi: 10.1038/84848. [DOI] [PubMed] [Google Scholar]
- Yamakoshi Y, Hu JC, Fukae M, et al. Porcine dentin sialoprotein is a proteoglycan with glycosaminoglycan chains containing chondroitin 6-sulfate. J Biol Chem. 2005;280:1552–1560. doi: 10.1074/jbc.M409606200. [DOI] [PubMed] [Google Scholar]
- Ye L, Macdougall M, Zhang S, et al. Deletion of dentin matrix protein-1 leads to a partial failure of maturation of predentin into dentin, hypomineralization, and expanded cavities of pulp and root canal during postnatal dentin development. J Biol Chem. 2004;279:19141–19148. doi: 10.1074/jbc.M400490200. [DOI] [PubMed] [Google Scholar]
- Ye L, Mishina Y, Chen D, et al. Dmp1-deficient mice display severe defects in cartilage formation responsible for a chondrodysplasia-like phenotype. J Biol Chem. 2005;280:197–203. doi: 10.1074/jbc.M412911200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhao J, Li C, et al. DSPP mutation in dentinogenesis imperfecta Shields type II. Nat Genet. 2001;27:151–152. doi: 10.1038/84765. [DOI] [PubMed] [Google Scholar]