Abstract
Genes involved in the control of cell proliferation and survival (those genes most important to cancer pathogenesis) are often specifically regulated at the translational level, through RNA-protein interactions involving the 5’-untranslated region of the mRNA. IGF1R is a proto-oncogene strongly implicated in human breast cancer, promoting survival and proliferation of tumor cells, as well as metastasis and chemoresistance. Our lab has focused on the molecular mechanisms regulating IGF1R expression at the translational level. We previously discovered an internal ribosome entry site (IRES) within the 5’-untranslated region of the human IGF1R mRNA, and identified and functionally characterized two individual RNA-binding proteins, HuR and hnRNP C, which bind the IGF1R 5’-UTR and differentially regulate IRES activity. Here we have developed and implemented a high resolution northwestern profiling strategy to characterize, as a group, the full spectrum of sequence-specific RNA-binding proteins potentially regulating IGF1R translational efficiency through interaction with the 5’-untranslated sequence. The putative IGF1R IRES trans-activating factors (ITAFs) are a heterogeneous group of RNA-binding proteins including hnRNPs originating in the nucleus as well as factors tightly associated with ribosomes in the cytoplasm. The IGF1R ITAFs can be categorized into three distinct groups: (a) high molecular weight external ITAFs, which likely modulate the overall conformation of the 5’-untranslated region of the IGF1R mRNA and thereby the accessibility of the core functional IRES; (b) low molecular weight external ITAFs, which may function as general chaperones to unwind the RNA, and (c) internal ITAFs which may directly facilitate or inhibit the fundamental process of ribosome recruitment to the IRES. We observe dramatic changes in the northwestern profile of non-malignant breast cells downregulating IGF1R expression in association with acinar differentiation in 3-D culture. Most importantly, we are able to assess the RNA-binding activities of these putative translation-regulatory proteins in primary human breast surgical specimens, and begin to discern positive correlations between individual ITAFs and the malignant phenotype. Together with our previous findings, these new data provide further evidence that pathological dysregulation of IGF1R translational control may contribute to development and progression of human breast cancer, and breast metastasis in particular.
Keywords: Breast carcinoma, Sequence-specific RNA-binding proteins, Regulation of gene expression at the translational level
Introduction
Many genes involved in the control of cell proliferation and survival (i.e. those most important to cancer biology) are now known to be regulated specifically at the translational (RNA to protein) level {Gray, 1998 #32; Rajasekhar, 2003 #56}. The mRNAs associated with these genes contain very long 5’-untranslated regions, which function essentially as RNA “promoters”, with structural features and regulatory protein binding sites that provide mechanisms by which the translational efficiency of individual mRNAs can be specifically modulated. One of the most intriguing of these translation-regulatory features is the internal ribosomal entry site (IRES), which allows the 40S ribosomal subunit to bypass the highly structured regions of the 5’-UTR and arrive in the immediate vicinity of the initiation codon (Baird et al., 2006; Coleman and Miskimins, 2009; Prats and Prats, 2002). These gene-specific translation-regulatory mechanisms may be pathologically altered in malignant disease (Chappell et al., 2000; Shi et al., 2008), however this area, and in particular its relevance to breast cancer, remains vastly underexplored.
The type 1 insulin-like growth factor receptor (IGF1R) is a potent proto-oncogene strongly implicated in the development and progression of human breast cancer through its powerful mitogenic, anti-apoptotic, and potential transforming activities (Belfiore and Frasca, 2008; Jones et al., 2007; Kim et al., 2007; Law et al., 2008; LeRoith and Roberts, 2003; Romanelli et al., 2007; Yanochko and Eckhart, 2006; Zhang and Yee, 2000). IGF1R overexpression contributes significantly to the resistance of tumor cells to cytotoxic and targeted therapeutic agents (Gooch et al., 1999; Guix et al., 2008; Kurmasheva et al., 2009; Kurmasheva and Houghton, 2006; Miller et al., 2009; Resnicoff et al., 1995; Rexer et al., 2009; Scotlandi et al., 2002; Shi et al., 2005; Turner et al., 1997; Yuen et al., 2009; Zeng et al., 2009), as well as to the metastatic properties of the malignant cells (Lopez and Hanahan, 2002; Sachdev et al., 2004; Sachdev et al., 2009); chemoresistance and metastasis are two of the most significant clinical problems currently facing breast cancer treatment.
Our lab determined that translation of the human IGF1R mRNA is controlled by an IRES (Meng et al., 2005; Meng et al., 2008; Meng et al., 2010). We have positively identified and extensively characterized two of the sequence-specific RNA-binding proteins that interact specifically with the IGF1R 5’-UTR and differentially modulate IRES function. Our results established HuR as a potent IRES repressor (Meng et al., 2005), while hnRNP C appears to compete with HuR and activate the IGF1R IRES (Meng et al., 2008). However, we realized that there are multiple additional RNA-binding proteins interacting with the IGF1R 5’-untranslated sequence and potentially contributing to IGF1R translational regulation. The RNA recognition motif is one of the most common protein domains in the eukaryotic genome (Varani and Nagai, 1998), and approximately 8% of all human genes encode RNA-binding proteins, yet relatively few of these have been characterized in any detail (Pullmann et al., 2007). We set out to examine, as a group, the full spectrum of sequence-specific RNA-binding proteins which may be involved in regulating IGF1R translation, and the IRES in particular.
Here we have categorized the putative IGF1R translation-regulatory proteins according to intermolecular interactions within the cell, factors affecting affinity for the 5’-untranslated RNA, whether they bind within or outside of the core functional IRES, and relationship to IRES activation. We observe dramatic alterations in the pattern of protein binding to the IGF1R 5’-UTR / IRES accompanying differentiation of non-malignant breast epithelial cells in 3-D culture. Most importantly, northwestern profiles of primary human breast surgical specimens provide evidence for pathological dysregulation of IGF1R translational control in malignant breast epithelial cells, and particularly in breast metastases.
Materials and Methods
Recovery of sequence-specific RNA-binding proteins from cells
We tested multiple individual variables and two major protocols (A and B described below) for preparation of whole cell extracts. A series of hybrid (A/B) protocols were compared, and the most optimal of these (Protocol H) selected for use with primary breast surgical specimens.
Whole cell extract Protocol A: Cells were scraped from the surface of the flask and the cell pellet resuspended in 3X volumes of hypotonic lysis buffer (10 mM Tris, pH 7.8; 10 mM KCl, 3 mM MgCl2, 1 mM EDTA, 7 mM 2-mercaptoethanol, 0.25% NP-40, supplemented with AEBSF, leupeptin, aprotinin, and phosphatase inhibitor cocktail (Sigma)) and incubated on ice for 55 min with frequent gentle agitation. The suspension was brought to 2 mM CaCl2 and DNase I (60 u/ml) and micrococcal nuclease (500u/ml) were added and the incubation continued for 45 min at 4°C. Glycerol was added (to 10%) and NaCl added very slowly with stirring to a final concentration of 500 mM, and the incubation continued for an additional 35 min. Triton X-100 (1%), deoxycholate (0.5%), and SDS (0.1%) were added and incubated further for 35 min. Finally, 5 mM EDTA and 5 mM EGTA (to inactivate MNase) were added and incubated 15 min, then microfuged at top speed for 10 min and the supernatant recovered. Microdialysis was performed using Slide-a-lyzer units (Pierce) in 40 mM Tris pH 7.8; 50 mM KCl, 0.5 mM EDTA, 0.5 mM EGTA, 7 mM 2-mercaptoethanol, 0.1% NP-40. Glycerol (10%) was re-added and the samples frozen rapidly in dry ice/ ethanol and stored at -80°C.
Whole cell extract Protocol B: The cell pellet was resuspended in 5X volumes of cold RNP lysis buffer (10 mM Tris, pH 7.4; 100 mM NaCl, 2.5 mM MgCl2, 14 mM 2-mercaptoethanol, 0.5% Triton X-100, supplemented with protease and phosphatase inhibitors). The suspension was incubated on ice for 7 min, then sonicated (Branson) on ice for 5 seconds × 2 at a setting of 2. The sample was then microfuged at top speed for 10 min at 4°C, the supernatant recovered, rapidly frozen, and stored at -80°C.
Whole cell extract Protocol H (hybrid, human): Begin as in Protocol B. After sonication, glycerol was added to 10% and NaCl added slowly to 500 mM final concentration with stirring. The sample was microfuged, and microdialysis performed on the supernatant as described above in Protocol A. This hybrid protocol also effectively extracts IGF1R itself, enabling both northwestern and western analysis to be performed on the same sample (in fact the same blot).
High resolution Northwestern profiling of sequence-specific RNA-binding proteins
Extracts prepared from cultured cells or surgical specimens were separated by SDS/PAGE (10%) and transferred to nitrocellulose membrane using carbonate buffer. Proteins were renatured by sequential incubation of the blot first in isotonic detergent-free buffer (1X TBS, overnight), followed by a complex physiological buffer (Ren/Block solution: 40 mM Tris-Cl, pH 7.4, 60 mM KCl, 1 mM MgCl2, 10 mg/ml BSA, 6% glycerol, 0.25 mM spermidine, 50 μg/ml tRNA (as non-specific competitor), 75 μM ATP, 75 μM GTP, 0.01% NP-40, 250 mM beta-mercaptoethanol) for 2 hours at room temperature and then overnight at 4°C. The radiolabeled RNA probe (sense orientation) representing the human IGF1R 5’-untranslated sequence (including the IRES) was synthesized by in vitro transcription using T7 RNA polymerase (Promega) in the presence of 32P-UTP. The radiolabeled probe was added to the Ren/Block solution, MgCl2 concentration adjusted to 1 – 5 mM as indicated, and incubated with the blot for 2 hours at room temperature on a rotating platform. The blot was washed once in fresh Ren/Block solution plus 1 mg/ml heparin for 10 min, and then twice more in Ren/Block solution for 5 min each, then wrapped and exposed to autoradiographic film with intensifying screens.
Three-dimensional culture of breast cell lines
MCF-10A and T47D cells (obtained from ATCC) were placed in three-dimensional culture essentially as described (Debnath et al., 2002; Petersen et al., 1992). Briefly, 106 cells were resuspended in 1.2 ml Matrigel (B-D) and plated in 35 mm dishes with 2 ml media provided above the matrix. Development of acinar structures (MCF-10A) or cell clusters (T47D) were followed over time by phase contrast microscopy. Following 15 days incubation, embedded cells were harvested by depolymerization of the matrix using Cell Recovery Solution (B-D) according to the manufacturer’s instructions, and used for preparation of whole cell extracts (Protocol A) and northwestern profiling. Alternatively, 105 cells were seeded in 1 ml of 5% Matrigel on top of 0.5 ml 100% Matrigel. Northwestern results obtained from surface plated cells were identical to those embedded within the Matrigel. For confocal microscopic examination of 3-D cultures, cells were seeded on top of small quantities of Matrigel spread over the surface of 8-well chamber slides.
Acquisition of human breast surgical specimens
A series of primary invasive breast carcinoma specimens or metastases (snap frozen or embedded in OCT) was acquired through the UAB Comprehensive Cancer Center Tissue Procurement Facility under an IRB-approved protocol. Normal breast specimens from reduction mammoplasty were acquired for use as controls. All specimens were examined by a Board-certified pathologist with special expertise in breast cancer (A.F.) to assess the prevalence and distribution of normal or malignant breast epithelium. Gross microdissection was performed where appropriate to enrich for the epithelial component. From three to twelve consecutive sections were transferred directly from cryostat to cold lysis buffer with protease and phosphatase inhibitors and subjected to whole cell extraction (Protocol H) optimized for recovery of the IGF1R translation-regulatory proteins.
Results
IGF1R translational regulation
The human IGF1R mRNA contains an extraordinarily long 5’-untranslated region (5’-UTR) of 1,040 nucleotides (Fig. 1, contrast with an “average” 5’-UTR of ~75-250 nucleotides) (Cooke et al., 1991). The IGF1R 5’-UTR is projected to adopt a complex secondary structure with a high degree of internal base-pairing and an overall thermodynamic stability of greater than -500 kCal / mole (Meng et al, 2010). Indeed, we have found that this highly structured sequence represents a substantial impediment to ribosomal scanning and IGF1R translation initiation (Meng et al., 2008). In addition, an upstream open reading frame (uORF) within the 5’-UTR, encoding a 28 amino acid peptide with no relationship to the IGF1R protein, tends to derail the ribosome before it reaches the IGF1R coding sequence. An extremely G-C rich sequence immediately following the uORF termination codon decreases even further the efficiency of IGF1R translation initiation mediated by conventional ribosomal scanning.
Fig 1. IGF1R translational regulation: Architecture of the 5’-untranslated region of the human IGF1R mRNA.
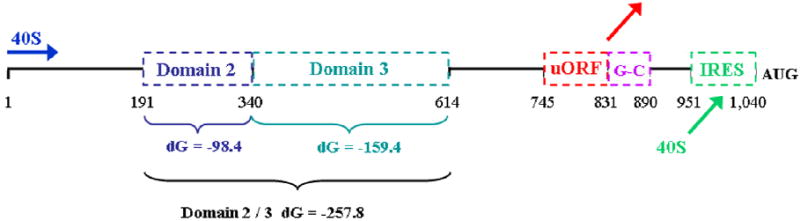
The structural and regulatory features contained within the 5’-untranslated region of the human IGF1R mRNA are indicated (drawn to scale, coordinates refer to distance in nucleotides). The conventional mechanism for translation initiation in eukaryotes involves a process of scanning by the 40S ribosomal subunit from the beginning of the mRNA (blue arrow) to the initiation codon. The complex IGF1R 5’-UTR presents multiple obstacles to ribosomal scanning. Domains 2 and 3 are highly stable secondary structures with individual free energies of -98.4 and -159.4 kCal/mol and a combined free energy of -257.8 kCal/mol. (For reference, a structure with dG > -60 kCal / mol is considered a significant obstacle to ribosomal scanning.) The upstream open reading frame (uORF) encodes a 28 amino acid peptide with no relationship to IGF1R. The uORF initiation codon is in good Kozak consensus, thus scanning ribosomes which reach this point will tend to initiate translation (upon joining of the 60S ribosomal subunit), and then dissociate from the mRNA upon reaching the uORF termination codon (red arrow). Ribosome dissociation is facilitated by the G-C rich element which immediately follows the uORF termination codon. We have referred to this G-C rich segment (56 out of 59 residues are G or C) as the “third obstacle”. Finally, however, there exists an internal ribosomal entry site (IRES, nucleotides 951 – 1040) positioned just upstream of the authentic IGF1R initiation codon. The IRES provides an alternative mechanism for IGF1R translation initiation, recruiting the 40S ribosomal subunit directly to the vicinity of the AUG (green arrow), allowing it to bypass the obstacles presented by the remainder of the 5’-UTR. Importantly, the IRES is not constitutively active, but rather is specifically regulated through competitive and cooperative interactions with multiple RNA-binding proteins recognizing specific sequence elements within or outside of the IGF1R IRES. The RNA-binding proteins regulating IGF1R translation are the subject of this investigation.
However, we have also discovered an internal ribosomal entry site (IRES) contained within the 5’-UTR which allows the ribosome to bypass the obstacles presented by the highly structured 5’-UTR, arriving in the immediate vicinity of the IGF1R initiation codon (Meng et al., 2005). Importantly, the IRES is not constitutively active (that would obviate the physiological function of the complex 5’-UTR), but rather is regulated through dynamic interactions with a series of sequence-specific RNA-binding proteins (Meng et al, 2005; Meng et al, 2008). In this manner, the IRES allows the translational efficiency of the IGF1R mRNA to be modulated independently of the global controls on the overall rate of protein synthesis in the cell.
Characterization of sequence-specific translation-regulatory proteins by the Northwestern assay: Recovery of functional RNA-binding proteins from cells
There have been two major factors impeding progress in the study of gene-specific translational regulation. First, to our knowledge, no systematic efforts had been made to establish an optimal protocol for extraction and recovery of sequence-specific RNA-binding proteins from cells. Second, the UV-crosslinking methods we have relied on to covalently capture sequence-specific RNA-binding proteins on their preferred substrates are effective only for U residues or uridine-derivatives, thus RNA-binding proteins with affinity for A-, C-, G-rich, or mixed sequences without neighboring U residues are very likely to be missed by these technologies. We set out to address each of these limitations.
We utilized the northwestern assay to characterize the RNA-binding proteins potentially regulating internal ribosomal entry and translational efficiency of the IGF1R mRNA. The northwestern is a solid phase functional assay which is complementary to UV crosslinking, capable of measuring the RNA-binding activities of individual proteins in a sequence-specific manner, but without constraints on target sequence composition. The northwestern is performed under highly stringent conditions, with inclusion of a large excess of tRNA as a non-specific competitor throughout the protocol, and addition of the polyanion heparin to the first wash, to eliminate weak or unstable RNA-protein interactions. While RNA-binding activity may reflect the abundance of the RNA-binding protein, it is well established that RNA-binding activity can be dynamically modulated e.g. through post-translational modifications to the RNA-binding protein, and there are documented instances in which RNA-binding activity does not correlate, and may even be inversely correlated, with abundance of the RNA-binding protein (Abdelmohsen et al., 2007; Jo et al., 2008; Meng et al., 2005). Having performed ~50 different northwestern experiments over the course of these studies, we came to recognize a very reproducible pattern of proteins interacting specifically with the IGF1R 5’-untranslated sequence. We have numbered the IGF1R northwestern bands from 1 through 10, beginning with highest molecular weight (Band 1 series ~125->200 kDa) to the lowest molecular weight (Band 10 ~25 kDa), using letter suffixes to designate additional bands in similar molecular weight range (i.e. Band 4b has a slightly lower apparent molecular weight than Band 4).
We evaluated three interventions to assist in biochemical extraction of these IGF1R translation-regulatory proteins from the nucleus: salt to disrupt ionic interactions, nuclease digestion to facilitate dissociation from high molecular weight nucleic acids, and detergents (particularly deoxycholate) to disintegrate microsomes associated with the rough ER, a proportion of which will tend to co-fractionate with the nucleus through attachment to the nuclear membrane. Incubation with 500 mM NaCl alone was sufficient for extraction of a significant number of the IGF1R 5’-UTR-binding proteins, however, a further increase in NaCl to 1M, digestion with DNase and micrococcal nuclease, or supplemental detergents were each effective in facilitating recovery of additional sequence-specific RNA-binding proteins from the nucleus (Fig. 2A).
Fig. 2. Characterization of Sequence-specific RNA-binding proteins by the Northwestern assay: Development of a protocol for optimal recovery of functional RNA-binding proteins from cells.
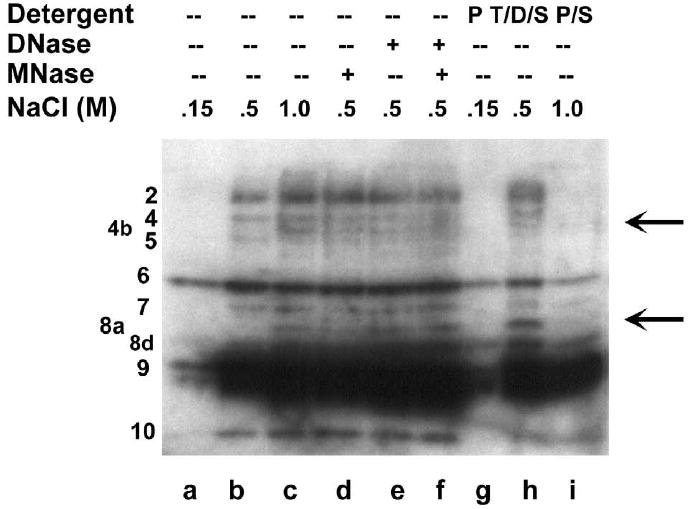
A: Northwestern assay evaluating recovery of sequence-specific RNA-binding proteins from the nucleus. Following hypotonic lysis of T47D breast tumor cells in the presence of 0.25% NP-40, pelleted nuclei were resuspended in an extraction buffer containing various concentrations of NaCl (lanes a through c). For lanes d through f, nuclei were incubated with DNase I and / or micrococcal nuclease prior to 500 mM NaCl. For lanes g through i, nuclear remnants following extraction with 150, 500, or 1000 mM NaCl respectively were resuspended and extracted with additional detergents: T-PER (Pierce), 1% Triton X-100/ 0.5% deoxycholate/ 0.1% SDS, or 50% T-PER/ 1% SDS respectively. Numerical designations for the proteins interacting specifically with the IGF1R 5’-untranslated RNA are indicated to the left. Note that the majority of bands detected by IGF1R northwestern required at minimum 500 mM NaCl for efficient extraction from the nucleus, while several individual RNA-binding proteins (particularly Bands 4b and 8a, see arrows) required additional NaCl, nuclease digestion, and/or supplemental detergent for efficient recovery. B: Cytoplasmic lysates obtained by hypotonic lysis of T47D cells were incubated with 500 mM NaCl and / or micrococcal nuclease, and then analyzed by northwestern. Note that the same manipulations which enhance recovery of RNA-binding proteins from the nucleus result in loss of RNA-binding activity in the cytoplasm. This phenomenon, which we refer to as “cytoplasmic susceptibility”, may be explained by targeted inactivation of RNA-binding translation-regulatory proteins once they become dissociated from their intended target sequences in the cytoplasm.
In general, cytoplasmic lysates yielded substantially fewer and less intense bands on northwestern than were recovered from the nucleus. Surprisingly, we found that the interventions which enhanced recovery of RNA-binding proteins from the nucleus were actually associated with loss of RNA-binding activity in the cytoplasm (Fig. 2B). Certain predominantly high molecular weight proteins were sensitive to micrococcal nuclease digestion, while a group of lower molecular weight proteins were sensitive to addition of high salt. To explain these findings, we have postulated that incubation with high salt or digestion with nuclease results in dissociation of cytoplasmic RNA-binding proteins from their native target mRNAs, and that a system apparently exists within the cytoplasm to rapidly inactivate (or degrade) any such free (unbound) translation-regulatory proteins (see Discussion).
Therefore, we began experimenting with preparation and analysis of whole cell extracts, utilizing the same interventions described above, but eliminating the differential centrifugation step that would separate the cytoplasmic and nuclear components. Indeed we found that there were a number of proteins which yielded relatively intense bands on the IGF1R northwestern when recovered from whole cell extracts, but were not detected at all, or with much lower intensity, in either the nucleus or the cytoplasm. This suggested that extraction of cytoplasmic proteins in the presence of nuclear components may protect these RNA-binding proteins from inactivation.
Sequence-specificity of RNA-binding proteins recognizing the IGF1R 5’-UTR: internal vs. external ITAFs
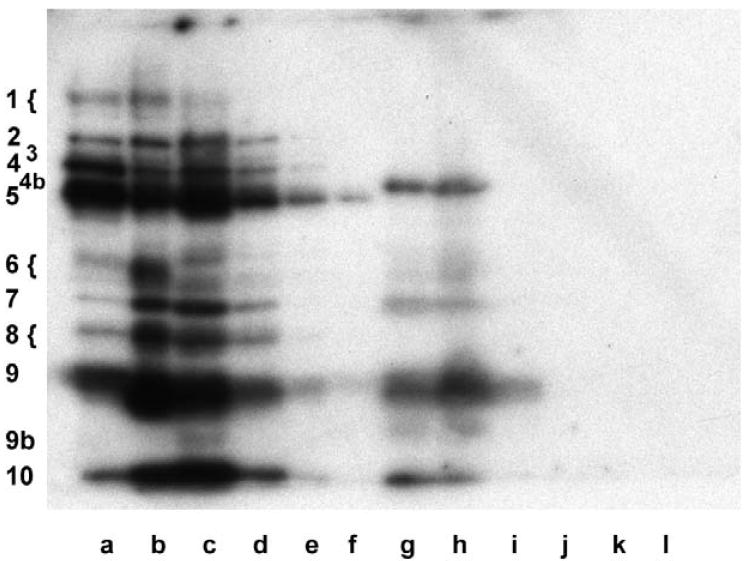
To assess the sequence-specificity of these RNA-protein interactions, we bisected the IGF1R 5’-UTR probe into two fragments. Several of the most intense bands detected by northwestern bound selectively to the smaller probe (90 nucleotides) representing the core functional IRES (Fig. 3). A distinct subset of bands was detected with the larger probe representing the ~950 nucleotides outside of the core IRES sequence. The term ITAF (IRES trans-acting factor) has been coined to describe RNA-binding proteins involved in regulation of IRES-mediated translation initiation (Pilipenko et al., 2000). As applied to IGF1R translational regulation, it appears there is a series of “external” ITAFs that bind the 5’-untranslated sequence outside of the IRES, as well as a group of “internal” ITAFs which bind within the core functional IRES.
Fig. 3. Sequence-specificity of RNA-binding proteins regulating IGF1R translation: internal and external ITAFs.
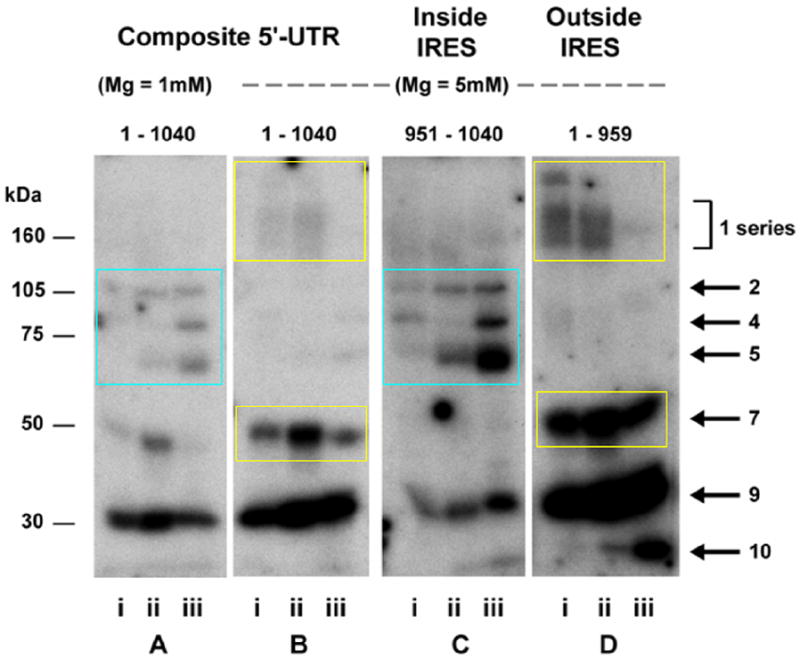
Northwestern assays were performed to assess the sequence-specificity of the RNA-protein interactions taking place on the 5’-untranslated region of the IGF1R mRNA. Three different RNA probes were synthesized: the full length 5’-UTR (nucleotides 1 – 1040, panels A and B), the isolated 90 nucleotide sequence (nucleotides 951-1040) to which the core functional IRES has been delimited (panel C), and the 5’-untranslated sequence from which the core functional IRES sequence has been deleted (nucleotides 1-959, panel D). Distinct patterns of protein binding are observed for the core IRES and the remainder of the 5’-UTR, while the pattern of protein binding to the full-length 5’-UTR (including the IRES) is a composite of those two series of bands. Pottential translation-regulatory proteins binding within the IGF1R core functional IRES are designated “internal” ITAFs (IRES trans-acting factors), while those binding 5’-untranslated sequence outside the core functional IRES are referred to as “external” ITAFs. The relative affinity or accessibility for the internal versus the external ITAFs can be modulated by varying the magnesium concentration (and thereby the level of secondary structure of the RNA). Molecular weight markers are shown on the left, and the positions of the IGF1R ITAFs are indicated on the right. Whole cell extracts: lanes i, MCF-10A, Protocol A; lanes ii, T47D, Protocol A; lanes iii, T47D, Protocol B.
Note that the pattern obtained using the full-length 5’-UTR (including the IRES) as probe is a composite of that obtained using the two individual probes, but that variation in magnesium concentration affects considerably the relative affinity of the external and internal ITAFs for the RNA. When magnesium concentration is low, the 5’-UTR is inherently less structured, and the core functional IRES becomes more accessible to the internal ITAFs (e.g. Bands 2, 4, and 5). Conversely, the higher degree of secondary structure that accompanies higher magnesium concentration favors binding of the external ITAFs (e.g. Bands 1, 7, and 9).
We have recently completed a systematic dissection of the sequence elements critical for IRES function, and found that point mutations or deletions of critical residues within the core functional IRES are associated with dramatic changes (positive or negative) in affinity of the internal ITAFs (but not the external ITAFs) for the IGF1R 5’-UTR (Meng et al, 2010), further confirming the sequence-specificity of these RNA-protein interactions.
Characterization of the IGF1R ITAFs by intermolecular association and subcellular fractionation
We felt it was important to further characterize the IGF1R 5’-UTR-binding proteins as to their relationship to other molecules and structures within the cell. Perhaps the best characterized group of RNA-binding proteins are the hnRNPs, which originate in the nucleus and appear to be heavily involved in processing of nascent mRNA (hnRNA). They are designated hnRNP A through U, roughly correlating with their molecular weights, and exist in a complex of well-defined stoichiometry (Dreyfuss et al., 1993). Dreyfuss and coworkers have determined that the entire complex of ~20 hnRNP proteins can be immunoprecipitated using monoclonal antibody 4F4 to hnRNP C, one of the core components of the complex and one of the most prevalent of the hnRNPs (Pinol-Roma et al., 1988). To determine whether some of the IGF1R ITAFs might belong to the hnRNP family, we used the 4F4 antibody to IP the hnRNP complex, and then separated those proteins by SDS / PAGE and performed northwestern analysis using the IGF1R 5’-UTR probe (Fig. 4A). We found that select bands amongst the northwestern profile were immunoprecipitated with the 4F4 antibody. One of these was Band 8d, which we have determined by western analysis performed sequentially on the same blot to be hnRNP C itself, and which we had previously identified using crosslinking technology as an IGF1R 5’-UTR-binding protein and potent IRES-activator (Meng et al, 2008). Bands 2 and 4b which co-immunoprecipitate with hnRNP C are also, by definition, members of the hnRNP family of RNA-binding proteins. However, none of the remainder of the IGF1R 5’-UTR-binding proteins detectable by northwestern appear to be hnRNPs.
Fig. 4. Characterization of IGF1R ITAFs by intermolecular association.
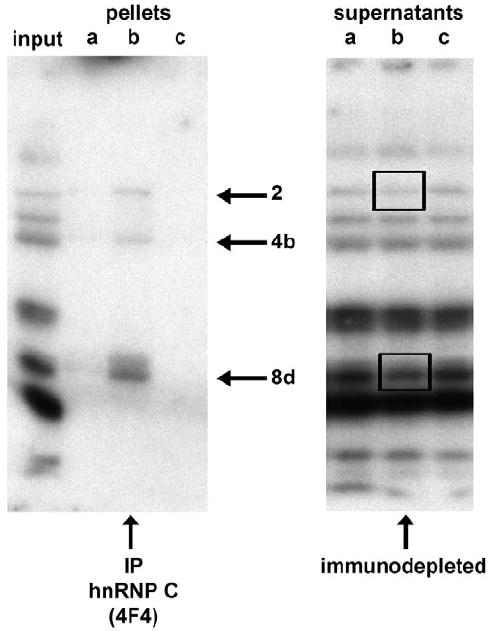
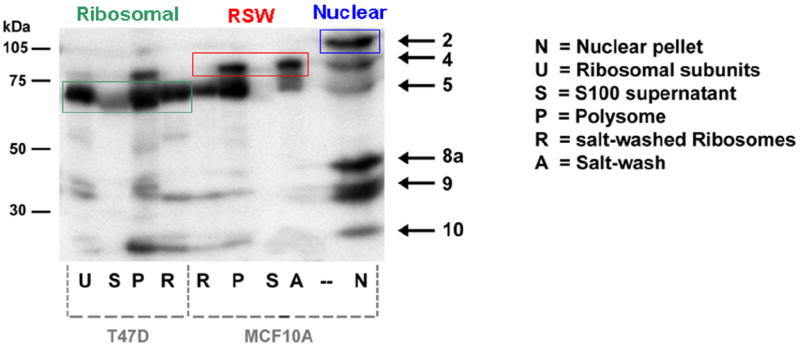
A: T47D whole cell extract (Protocol B) was subjected to immunoprecipitation using the 4F4 monoclonal antibody (mouse IgG1) to hnRNP C, which immunoprecipitates the hnRNP complex (composed of >20 proteins). Aliquots of the input, pellet, and supernatant were separated by SDS / PAGE and analyzed by northwestern using the full-length IGF1R 5’-UTR / IRES probe. Three of the IGF1R northwestern bands are selectively recovered by 4F4 immunoprecipitation (lane b): Band 8d, which is hnRNP C itself (as confirmed by western analysis performed sequentially on the same blot), as well as Bands 2 and 4b. Significant immunodepletion of hnRNP C (8d) and Band 2 is observed in the corresponding supernatant (lane b of right panel). Lanes a and c of each panel are negative control immunoprecipitations and supernatants (no immunodepletion) obtained using antibodies to two other RNA-binding proteins (3A2 mouse monoclonal IgG1 to HuR and goat polyclonal C-18 to TIAR). The experiment was repeated with identical results B: MCF-10A and T47D cells were subjected to conventional fractionation of the cytoplasm by differential ultracentrifugation to yield an S100 supernatant (lanes S) and polysomal pellet (lanes P). Aliquots of polysomal pellets were incubated in 500 mM KCl, and centrifuged again to yield a supernatant (ribosomal salt wash, lane A) and pellet (salt-washed ribosomes, lanes R). Finally, an aliquot of salt-washed ribosomes was incubated with puromycin and 500 mM KCl, and centrifuged again to yield free ribosomal subunits (lane U). Each of these fractions was analyzed by northwestern using the IGF1R IRES probe. Note that Bands 2 and 8a are exclusively nuclear, Band 5 is tightly associated with ribosomes, while Band 4 is quantitatively recovered in the ribosomal salt wash. A proportion of Bands 9 and 10 are also tightly associated with the ribosomes.
We were also very interested to determine whether some of the cytoplasmic IGF1R ITAFs detected by northwestern might be inherently associated with the translation machinery (Fig. 4B). We performed standard fractionation procedures to separate the 100,000 × g supernatant (S100) from the polysomal pellet, then salt washed the polysomes, and used puromycin with high salt to disassemble polysomes into individual ribosomal subunits. Each of these fractions was then separated on SDS/PAGE and analyzed by northwestern using the IGF1R IRES probe. Bands 2 and 8a are absolutely restricted to the nuclear fraction. However, Bands 4 and 5, two internal ITAFs which are among the most dominant features of the IGF1R northwestern profile, co-fractionate with polysomes. Curiously, Band 4 is quantitatively recovered in the ribosomal salt wash, while Band 5 remains closely associated with the ribosomes even after incubation with puromycin. It appears that a proportion of Bands 9 and 10 may also be tightly associated with ribosomes. Note that the IGF1R 5’-UTR-binding proteins are not generally present free in the S100 cytoplasmic supernatant.
There are now several known examples of canonical ribosomal proteins shown to be capable of sequence-specific interactions with non-ribosomal RNAs (including viral IRESs) and even extra-ribosomal regulatory functions (Fukushi et al, 2001; Chiocchetti et al, 2005; Wanzel et al, 2008). Therefore, we repeated the northwestern analysis on these same fractions using a 17.5% polyacrylamide gel to determine whether any of the canonical integral ribosomal proteins might function as ITAFs for IGF1R. Only one very low molecular weight (~15 kDa) protein was detected by northwestern, however, and it fractionated with the nucleus.
We also performed an IP-Northwestern analysis using an anti-phosphoserine antibody to determine which of the IGF1R ITAFs might be targets for serine phosphorylation. We observed that a subset of the high molecular weight external ITAFs (the Band 1 series) as well as Band 9 appear to contain phosphoserine residues (data not shown). A number of reports have demonstrated that the RNA-binding activity and/or subcellular localization of certain RNA-binding proteins can be influenced by signal transduction culminating with phosphorylation of serine residues (Abdelmohsen et al., 2007; Jo et al., 2008).
In conclusion, the putative IGF1R ITAFs detected by northwestern are a remarkably heterogeneous group of RNA-binding proteins, varying in their subcellular localization, intermolecular associations, and biochemical properties.
Distinctions in the northwestern profiles of T47D and MCF-10A cells: correlation with pathological activation of the IGF1R IRES and the malignant phenotype
We had previously demonstrated differences in the pattern of protein binding to the IGF1R 5’-UTR as detected by crosslinking, comparing the non-transformed human mammary epithelial cell line MCF-10A, in which IGF1R expression is low (normal) and IRES activity borders on the threshold of detection, to the breast carcinoma cell line T47D, in which IGF1R protein levels are elevated ~4 fold and IRES activity is substantially increased (Meng et al., 2008). We had observed differences in the relative RNA-binding activities of HuR (IRES repressor) and hnRNP C (IRES activator), along with two additional crosslinked proteins of ~60 and ~85 kDa which mirror HuR and hnRNP C respectively.
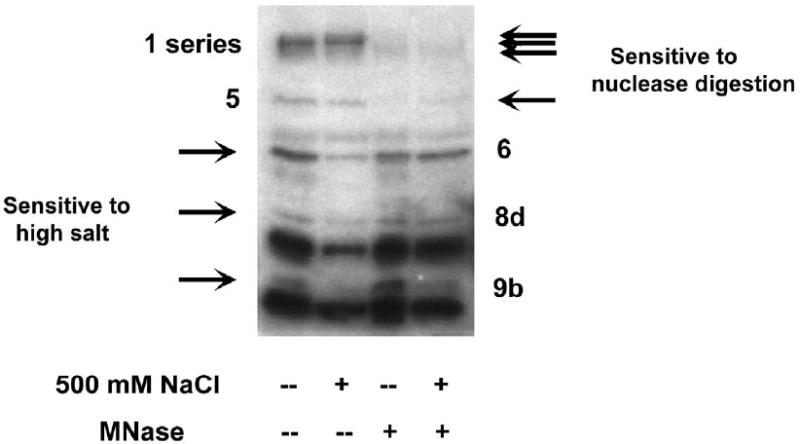
However, the northwestern assay reveals multiple RNA-binding proteins not detectable by crosslinking, particularly with use of the optimized whole cell extraction protocol, so we felt it was important to re-examine the status of the IGF1R translation-regulatory proteins in these two model cell lines using this technology. Many of the bands on the northwestern profiles of the T47D and MCF-10A cells exhibit very similar molecular weights and relative intensities, however, certain distinctions are observed (Fig. 5). The relative RNA-binding activities of Bands 2, 5, and 7 are considerably higher in the malignant T47D cell line than in the non-malignant MCF-10A cells, while just the reverse is true for Band 4, which is considerably more intense in MCF-10A. These results suggest that Bands 2, 5, and 7 may be positive ITAFs, involved in promoting IRES activity, while Band 4 may represent a negative ITAF, capable of inhibiting IRES function.
Fig. 5. Distinctions in the pattern of protein binding to the IGF1R 5’-UTR / IRES between non-malignant and malignant cultured breast cells.
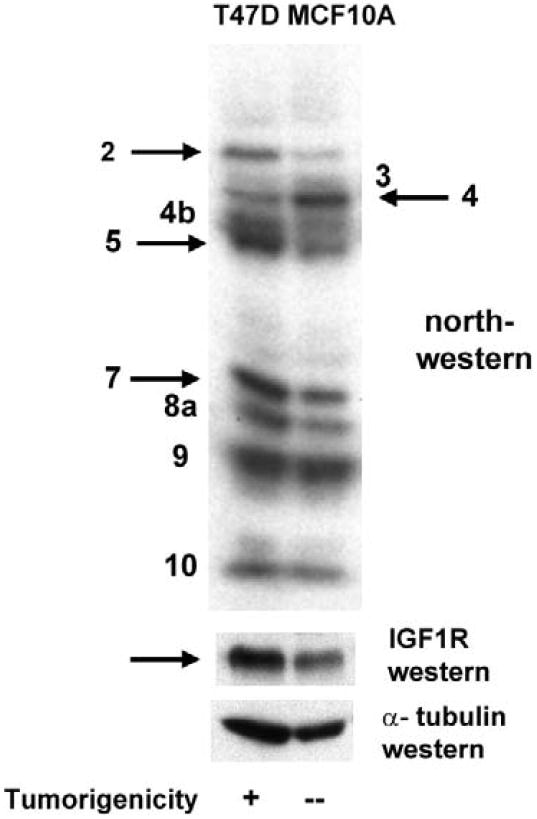
Whole cell extracts (Protocol B) of T47D (malignant) or MCF-10A (non-malignant) breast epithelial cells were prepared and subjected to northwestern analysis (4 × 105 cell equivalents per lane) using the IGF1R 5’-UTR / IRES probe. Though the patterns are generally quite similar, certain bands exhibit significant differences in relative intensity (RNA-binding activity). In particular, Bands 2, 5, and 7 are considerably more intense in T47D, correlating with the substantially higher IRES activity (Meng et al, 2005) and IGF1R protein levels in these cells. Band 4 is considerably more intense in MCF-10A cells, suggesting this may represent an IRES inhibitor.
Alterations to the northwestern profile of MCF-10A cells undergoing acinar differentiation in 3-D culture
The distinction between the normal and malignant phenotype is not evident in traditional monolayer tissue culture, where even normal breast epithelial cells fail to differentiate, having no contact with basement membrane material and no opportunity to become spatially organized. However, a number of investigators have now established the utility of a three-dimensional culture system to demonstrate the capacity of non-malignant breast epithelial cells to undergo a normal process of differentiation (Debnath et al., 2002; Petersen et al., 1992). We wished to determine whether IGF1R translational regulation might be altered when breast cells differentiate in 3-D culture. MCF-10A and T47D cells were seeded in Matrigel, and proliferation of the cells over time was assessed by both phase contrast (Fig. 6A,B) and confocal microscopy (Fig. 6C,D,E). As expected, the non-transformed MCF-10A breast epithelial cells formed relatively uniform small spherical clusters resembling acini of normal breast tissue, and polarization of cells on the circumference of these acinar structures was evident from the basal localization of the nuclei. Many of these acinar structures showed evidence of lumenal clearing via separation and apoptosis of centrally-located cells. In contrast, the malignant T47D breast tumor cells provided the same 3-D culture conditions formed large heterogeneous amorphous clusters, with no evidence of differentiation, polarity, apoptosis, or limitation of proliferation.
Fig. 6. Alterations in RNA-binding activities of IGF1R translation-regulatory proteins accompanying differentiation of non-malignant breast epithelial cells in 3-D culture.
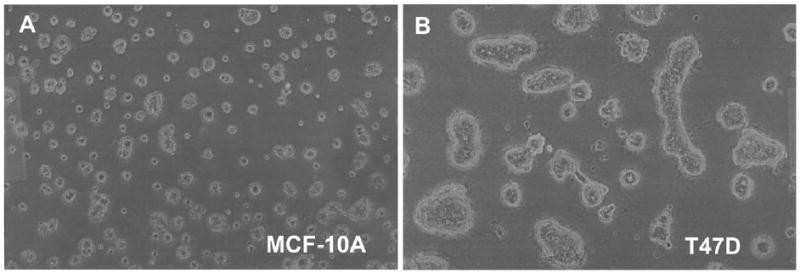
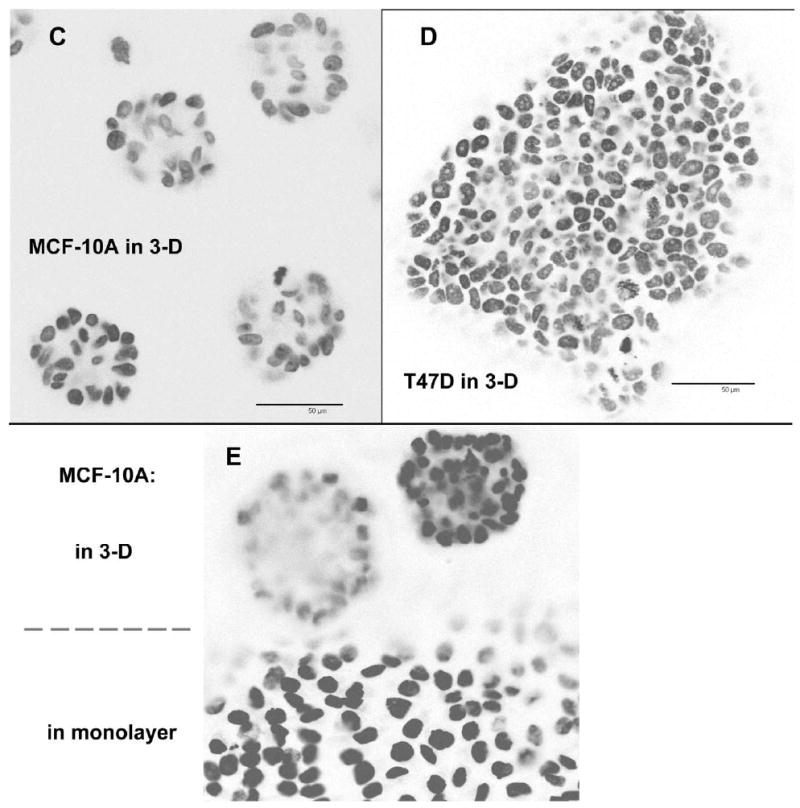
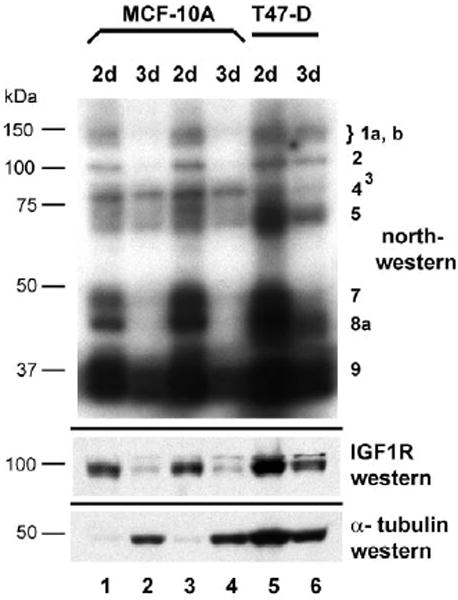
A, B: Single-cell suspensions of MCF-10A and T47D cells were embedded in Matrigel in 35 mm dishes and allowed to proliferate or differentiate for 15 days. Representative phase contrast micrographs reveal the distinction between the small, relatively uniform acinar structures formed by the non-transformed MCF-10A breast epithelial cells versus the large, amorphous clusters formed by the T47D breast tumor cells. C, D: MCF-10A and T47D cells were also plated on the surface of a thin layer of Matrigel on 8-well chamber slides to facilitate examination by confocal microscopy. Following fixation, nuclei were stained with DAPI, and a series of confocal images was captured demonstrating the organized morphology of the MCF-10A acinar structures versus the disorganized clusters of T47D cells. The immunofluorescent micrographs are shown in reverse image (nuclei are dark). Bar = 50 μm. E: MCF-10A cells were seeded on an 8-well chamber slide in which Matrigel had been intentionally spread so as to cover only a portion of the surface of the well, forming a “beach” with the inorganic growth surface. This allowed us to capture, in a single image, acinar structures on the surface of the Matrigel and a continuously proliferating monolayer on the adjacent uncoated portion of the slide. This illustrates the capacity of the non-transformed MCF-10A cells to differentiate and cease dividing when provided the appropriate environmental context, versus indefinite proliferation in standard monolayer culture. F: Northwestern analysis revealing changes in the pattern of protein binding to the IGF1R 5’-UTR / IRES accompanying differentiation of non-malignant MCF-10A breast epithelial cells in 3-D culture. MCF-10A and T47D cells were recovered from 3-D culture following dissolution of the matrix (see Materials and Methods) and whole cell extracts prepared (Protocol A). Extracts were also prepared in parallel from cells harvested from standard monolayer culture (2-D). Equal numbers of cell equivalents of these extracts were analyzed by northwestern using the IGF1R 5’-UTR / IRES probe. Results of western analyses for IGF1R (C-20 rabbit polyclonal, Santa Cruz) and alpha tubulin (B-5-1-2, Sigma) performed sequentially on the northwestern blot are shown below.
Cells cultured in 3-D were recovered by dissolution of the matrix and extracts were prepared in parallel from both standard monolayer and 3-D cultures of both cell lines. The changes in northwestern profile accompanying acinar differentiation of MCF-10A cells in 3-D were dramatic (Fig. 6F). The high molecular weight Band 1 series, as well as Bands 2, 7, and 8a were completely lost. Notably, Band 4, the internal ITAF which we had speculated might be an IRES inhibitor, was selectively retained in the northwestern profile of the MCF-10A cells grown in 3-D culture. At the same time, IGF1R protein levels, measured by western performed sequentially on the same blot, decreased precipitously. Alpha-tubulin levels increased substantially when MCF-10A cells were grown in 3-D, confirming that the decreases in IGF1R levels and northwestern band intensities were not the result of limited recovery of cells from 3D culture or underloading of protein on the gel. In contrast, changes in the northwestern profile of T47D cells grown in 3-D culture were limited, and IGF1R levels decreased only modestly, indicative of the failure of these breast tumor cells to differentiate appropriately in this environment. These results demonstrate the utility of the northwestern assay to discern changes in activities of sequence-specific RNA-binding proteins in association with phenotypic alterations, and a clear distinction in IGF1R translational regulation between the malignant and non-malignant breast epithelial cells.
Northwestern profiling of primary human breast surgical specimens: correlation with malignancy and metastasis
Our ultimate objective was to extend these studies to primary human breast specimens. First it was necessary to establish that functional RNA-binding proteins could be successfully extracted from surgical specimens, and that the northwestern technique would be sensitive enough to yield informative patterns from limited quantities of biological material. Recognizing the inherent cellular heterogeneity of both normal breast tissue and malignant breast tumor specimens, we attempted to use laser microdissection to isolate the epithelial component for extract preparation. We found that even minimal processing (i.e. hematoxylin staining) required for recognition of tissue architecture was incompatible with recovery of functional RNA-binding proteins. However, informative high resolution northwestern patterns were obtained from unstained specimens which had been grossly microdissected on the basis of distribution of normal or malignant epithelial cells as assessed by H&E staining of an adjacent cryosection. (Fig. 7A). We observed in the primary tumor specimen a discrete subset of the bands normally seen with cultured T47D cells. In particular, Band 4b, which is typically only a minor component of the northwestern profile of cultured breast tumor cells, was very intense in the primary tumor, along with Bands 7, 9, 9b, and 10, while a number of other bands usually prominent in the northwestern profile of cultured cells were completely absent in the primary tumor.
Fig. 7. Northwestern analysis of sequence-specific translation-regulatory proteins binding to the IGF1R 5’-UTR in primary human breast tumors, metastases, and normal tissues.
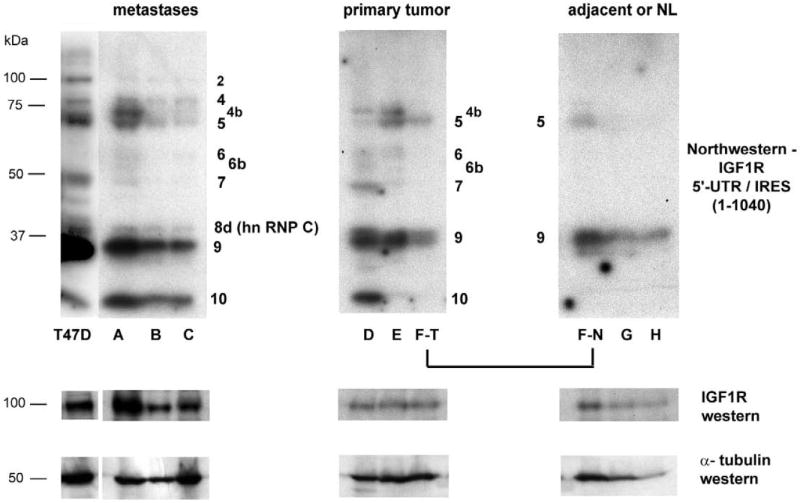
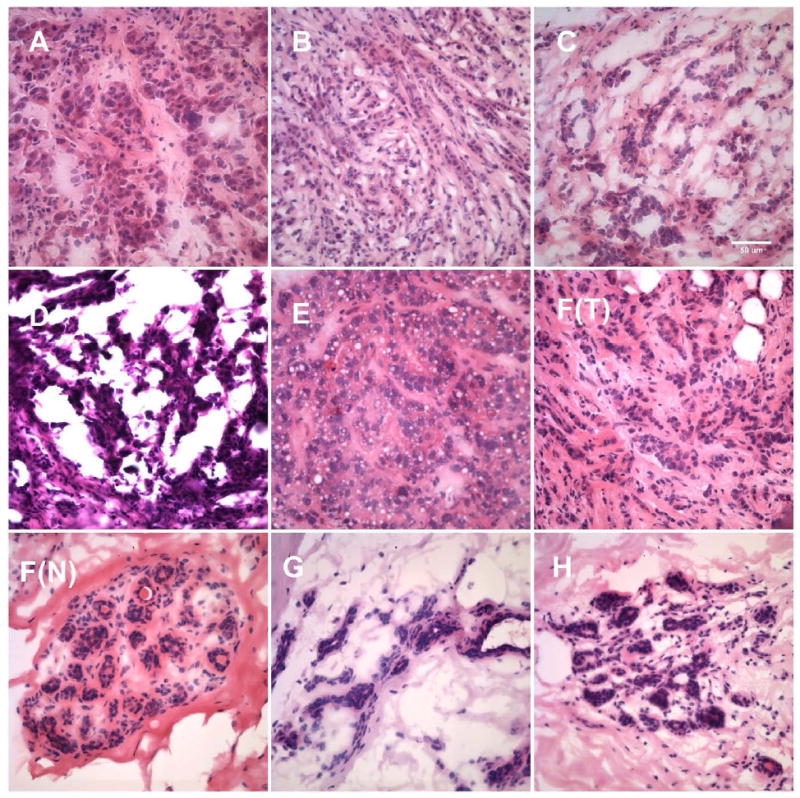
A: A primary human breast tumor specimen (poorly differentiated ductal carcinoma, specimen D, Table 1) was cryosectioned and subjected to varying degrees of processing to enable laser microdissection. Processed materials were then recovered from microdissection cap or slides and subjected to whole cell extraction (Protocol H) before performing northwestern analysis. Lane g: cryosections washed in water and immediately recovered from slides and transferred to lysis buffer (containing protease and phosphatase inhibitors). Lane h: cryosections (still in OCT) directly transferred to lysis buffer with no histological processing. Lane i: Sections stained with hematoxylin (standard protocol, but omitting final xylene rinse), then immediately recovered from slides and transferred to lysis buffer. Lane j: Sections stained with hematoxylin including final xylene rinse. Lane k: Sections stained with both hematoxylin and eosin. Lane l: Section stained with H&E and subjected to laser microdissection, with captured malignant epithelial cells selectively transferred to lysis buffer. Reference samples: Lane a: HeLa nuclear extract; Lane b: K-1 (T47D derivative) Protocol H whole cell extract; Lane c: T47D Protocol H whole cell extract (2.5 × 105 cell equivalents); Lane d: same as Lane c, one-fourth sample loaded; Lane e: same as Lane c, one-tenth of sample loaded; Lane f: same as Lane c, one-twenty-fifth of sample loaded. B: Human breast surgical specimens (snap frozen or embedded in OCT) were examined by H&E staining to assess distribution of epithelial cells and guide gross microdissection as appropriate. Relative proportion of malignant breast epithelial cells within each tumor sample ranged from ~85 to near 100%. Consecutive cryosections (unprocessed) were then transferred directly to lysis buffer and used for preparation of whole cell extract (Protocol H). Equivalent aliquots were subjected to northwestern analysis using the full-length IGF1R 5’-UTR / IRES probe. Specimens A through C are metastatic lesions of breast tumor origin. Specimens D through F(T) are primary invasive breast tumors. Specimen F(N) is adjacent histologically uninvolved tissue obtained from the same block as primary tumor F(T). Specimens G and H are normal breast tissue obtained from reduction mammoplasty. The results are representative of four replicate northwestern analyses performed on the same series of specimens. Molecular weight markers are indicated on the left, while the positions of IGF1R ITAFs are indicated adjacent to each panel. Results of western analyses for IGF1R (N-20 rabbit polyclonal, Santa Cruz) and alpha tubulin (B-5-1-2, Sigma) performed sequentially on the northwestern blot are shown below. C: Representative H&E stained sections of each of the human breast surgical specimens. Bar = 50 μm.
We extended this study to include a series of primary breast tumors, breast metastases, and non-malignant breast tissues (Fig. 7B). Band 4b was again one of the more dominant bands in 3 out of 6 of the malignant specimens (2 primary tumors and 1 metastasis), while Band 7 (an external ITAF) was highly active in two of the malignant specimens (1 primary tumor and 1 metastatic lesion), both of which had been described as poorly differentiated. Interestingly, Bands 2 and 8d (hnRNP C) were evident in each of the breast metastases, but none of the primary breast tumors or normal specimens. We have characterized hnRNP C as a potent activator of the IGF1R IRES. IGF1R protein levels were substantially increased in each of the metastatic lesions, approaching that observed in the T47D cells.
With the exception of Band 9, a low molecular weight external ITAF, the northwestern profiles of the two normal breast specimens (obtained from reduction mammoplasty) were negative. While the relatively low density of breast epithelial cells within the normal architecture of the breast tissue may be partly responsible, these results are also compatible with the relative inactivity of the IGF1R IRES expected in these normal tissues. However, the positivity of the northwestern profile obtained from the adjacent uninvolved tissue of specimen F, and its similarity (particularly Band 5) to that of the frankly malignant tissue is notable. This result is suggestive of a field effect (Heaphy et al., 2009), or perhaps a germ-line alteration predisposing this patient to dysregulation of IGF1R translation and the development of breast malignancy; this patient presented at age 47 with bilateral multifocal invasive carcinoma.
The clinical / pathological data associated with each of the human breast specimens are summarized in Table 1. Representative H&E images of each of the breast specimens are shown in Fig. 7C.
Table 1.
Clinical / pathological characteristics of human breast surgical specimens
| Age/Race/Gender | Diagnosis | ER PR Her2 |
Differentiation | Size | Lymph nodes | Notes |
|---|---|---|---|---|---|---|
| Metastatic lesions of breast tumor origin | ||||||
| A. 59WF | Ductal carcinoma Metastatic to Lung | ER-Neg PR-Neg Her2-Non-ampl |
Poor | previous history breast carcinoma (1 yr prior); tumor nodules in lung morphologically identical to original tumor (also ER,PR-Neg) | ||
| B. 60F | Lobular carcinoma Metastatic to Ovary | ER-Pos PR-Pos Her2-Non-ampl |
Moderate | previous history breast carcinoma (7 yr prior); involves both ovaries fallopian tube, omentum; ascites | ||
| C. 50F | Adenocarcinoma Metastatic to Ovary | ER-Pos PR-Pos |
Moderate | involves both ovaries alpha-lactalbumin-positive CA-125-negative | ||
| Primary invasive breast carcinoma | ||||||
| D. 52BF | Ductal carcinoma invasive | ER-Neg PR-Neg Her2-Non-ampl |
Poor | 8cm | 2/22-pos | |
| E. 50WF | Ductal carcinoma invasive | ER-Pos PR-Neg Her2-Amplified |
Poor | 7.5cm | 16/19-pos | angiolymphatic invasion |
| F. 47WF (T) | Bilateral, multifocal carcinoma | ER-Pos PR-Pos Her2-Non-ampl |
Moderate Moderate |
3.5cm (Left) 9.0cm (Right) |
4/28-pos 0/3 (i+) |
ductal and lobular features predominately lobular features |
| Adjacent uninvolved or Normal breast tissue | ||||||
| F. (N) | Adjacent uninvolved tissue from primary tumor F | histologically non-malignant | ||||
| G. 35WF | Normal breast (reduction) | benign parenchyma no mass lesions | ||||
| H. 26WF | Normal breast (reduction) | fibrosis no mass lesions | ||||
Discussion
RNA-binding translation-regulatory proteins: cytoplasmic susceptibility and nuclear protection
It would be dangerous to the phenotypic integrity of the cell for translation-regulatory proteins to retain their RNA-binding activities and have an opportunity to associate with other RNA molecules once freed from their original intended target mRNAs in the cytoplasm where translation is actively taking place. Such an occurrence could transform highly orchestrated translational regulation into chaotic protein synthesis. Thus it may benefit the cell to have in place a quality control system to rapidly and efficiently inactivate or destroy these RNA-binding proteins (through post-translational modification or targeted degradation) once they become free in the cytoplasm. Such a system could be responsible for the “cytoplasmic susceptibility” we have observed, wherein recovery of functional sequence-specific RNA-binding proteins from the cytoplasm is highly inefficient, particularly if interventions which favor dissociation of RNA-binding proteins from their native target sequences are utilized.
Within the nucleus, however, RNA-binding proteins are purposefully becoming associated with individual mRNA molecules for the first time in a controlled fashion. This cotranscriptional tagging of nascent mRNA with specific RNA-binding proteins likely serves to indicate the suitability of the mRNA for translation, the appropriate intracellular location for that mRNA to be translated, and the relative efficiency with which it should be translated. Thus it would be important for conditions within the nucleus to favor retention of RNA-binding activity, and indeed, recovery of active RNA-binding proteins from the nucleus is relatively efficient. In the preparation of whole cell extracts, where nuclear and cytoplasmic components are recovered together, we have observed that nuclear protection is generally dominant over cytoplasmic susceptibility, though this varies somewhat for individual proteins and between individual cell lines. In practical terms, this experience has taught us that cytoplasmic RNA-binding proteins are typically recovered much more efficiently in the context of a whole cell extract. However, if our interpretation is correct, the cellular systems responsible for nuclear protection and cytoplasmic susceptibility may carry far greater biological relevance.
A two-component model of IGF1R translational regulation: internal and external ITAFs
We have begun to characterize a series of sequence-specific RNA-binding proteins which may be involved in regulation of IGF1R expression at the translational level. On the basis of our northwestern results, we are able to divide the putative translation-regulatory proteins interacting with the IGF1R 5’-untranslated RNA sequence into two distinct categories: External ITAFs, which bind outside of the core functional IRES (nucleotides 1 – 959), and Internal ITAFs, which bind within the core IRES (nucleotides 951 – 1040).
The Band 1 series of proteins are high molecular weight external (~125->200 kDa) ITAFs which we have determined to be targets for serine phosphorylation. These proteins likely modulate the overall conformation of the 5’-untranslated region of the IGF1R mRNA, ultimately controlling the accessibility of the core functional IRES to the requisite internal ITAFs and the 40S ribosomal subunit. The large size of these cytoplasmic proteins and their sensitivity to micrococcal nuclease implies that they may contact a relatively large surface area of the RNA, perhaps bringing together disparate segments of the 5’-untranslated sequence, serving as a scaffold to coordinate intermolecular interactions involved in assembly of the translation-regulatory complex on the IGF1R 5’-UTR. Band 7 is a relatively low molecular weight external ITAF which disappears in association with downregulation of IGF1R expression when MCF-10A cells differentiate in 3-D, and its appearance in the northwestern profiles of human breast surgical specimens correlates with poor differentiation. The other low molecular weight external ITAFs (Bands 9 and 10), which are somewhat less sequence-specific, may serve as general RNA chaperones or helicases, facilitating IRES function by helping to untangle the complex 5’-UTR sequence.
The internal ITAFs, which generally occupy the intermediate molecular weight range of the northwestern profile, likely participate directly in the fundamental process of ribosome recruitment, either facilitating or interfering with IRES-mediated translation initiation. Band 5 is tightly associated with ribosomes, displays elevated RNA-binding activity in T47D cells (in which the IRES is pathologically upregulated (Meng et al, 2008)), and is detectable in the northwestern profiles of nearly all of the breast tumors and metastatic lesions, thus correlating with the malignant phenotype. Band 2 is a nuclear protein which displays greater RNA-binding activity in T47D cells, disappears completely when MCF-10A cells downregulate IGF1R and cease proliferation upon differentiation in 3-D culture, and is detectable in the northwestern profiles of each of the breast metastases analyzed in this study. Band 8d, an internal ITAF which we had previously characterized as the potent IRES activator hnRNP C, is also observed in the northwestern profiles of each of the metastatic lesions of breast tumor origin, in association with substantial increases in IGF1R protein levels.
In contrast, Band 4, which we have characterized as a component of the ribosomal salt wash, is unique amongst the internal ITAFs detected by northwestern in demonstrating an apparent negative correlation with IGF1R IRES activity. The RNA-binding activity of Band 4 is considerably higher in the non-malignant MCF-10A cells, which express low (normal) levels of IGF1R and borderline detectable IRES activity (Meng et al, 2008), than in malignant T47D cells. Furthermore, Band 4 selectively retains its RNA-binding activity upon terminal differentiation of MCF-10A cells in 3-D culture, when IGF1R protein levels drop precipitously and many of the other northwestern bands are disappearing. It is conceivable that these positive and negative IRES-regulatory proteins may initiate assembly of distinct ribonucleoprotein complexes on the IGF1R 5’-UTR, consistent with the model we originally proposed to explain how IGF1R translational regulation could be dynamically modulated (Meng et al., 2003). The properties of the IGF1R ITAFs are summarized in Table 2.
Table 2.
Properties of Sequence-specific RNA-binding proteins interacting with the IGF1R 5’-UTR / IRES
| Designation | MW | Relation to IRES | Intracellular Localization | Status in Breast cell lines | Change with 3-D culture MCF-10A | Status in Breast Specimens | Inter-molecular Association | Nuclear extraction/Cytoplasmic Susceptibility | Affect of Mg++ on Affinity |
|---|---|---|---|---|---|---|---|---|---|
| Band 1 series | ~125-250 kDa | External ITAFs | Cytoplasmic | Lost upon acinar differ. | serine phosph. | susceptible to MNase in cytoplasm | favored by ↑ Mg++ (↑RNA structure) | ||
| Band 2 | ~105 kDa | Internal ITAF | Nuclear | T47D>MCF-10A | Lost upon acinar differ. | positive 3 / 3 breast metastases | hnRNP | favored by ↓ Mg++ | |
| Band 3 | ~95 kDa | Internal ITAF | Nuclear | MCF-10A>T47D | Lost upon microdialysis | ||||
| Band 4 | ~85kDa | Internal ITAF | Cyto/Nucl | MCF-10A>T47D (inverse corr. IRES activity) | Selectively retained upon acinar differ. | positive 3 / 3 breast metastases | Ribosomal salt wash | favored by ↓ Mg++ | |
| Band 4b | ~75kDa | Internal ITAF | Nuclear | T47D>MCF-10A | highly active 1/3 metastases 2/3 1° tumors (corr. w/ poor diff) | hnRNP | recovery ↑ by 1M NaCl, DOC or nuclease | ||
| Band 5 | ~70 kDa | Internal ITAF | Cyt>Nuc | T47D>MCF-10A | active in 3/3 metastases 2/3 1° tumors (corr. malignant phenotype) | Tightly associated Ribosomes | susceptible to MNase in cytoplasm | favored by ↓ Mg++ | |
| Band 6 series | ~60 kDa | Internal ITAFs | Cyto / Nucl | active in 2/3 metastases 2/3 1° tumors | highly labile | susceptible to high salt in cytoplasm | |||
| Band 7 | ~ 50 kDa | External ITAF | Nuc>Cyt | T47D>MCF-10A | Lost upon acinar diff. | highly active 1/3 metastases 1/3 1° tumors (corr. w/ poor diff) | favored by ↑ Mg++ (↑ RNA structure) | ||
| Band 8a | ~45 kDa | Internal ITAF | Cyto / Nucl | Lost upon acinar diff. | recovery ↑ by 1M NaCl, DOC or nuclease | ||||
| Band 8d (hnRNP C) | 42 kDa | Internal ITAF | Nuc>Cyt | active in 3/3 metastases | hnRNP | susceptible to high salt in cytoplasm | |||
| Band 9 | ~37 kDa | External ITAF | Nuc>Cyt | Retained | readily detectable | serine phosph; asso Ribosomes | favored by ↑ Mg++ | ||
| Band 10 | ~25 kDa | External ITAF | Cyto / Nucl | readily detectable | asso Ribosomes | recovery ↑ by high salt |
Clinical / pathological implications: Dysregulation of IGF1R translational control in breast malignancy and metastasis
Our results suggest that cumulative changes in the activities of RNA-binding translation-regulatory proteins may occur during development and progression of human breast cancer, contributing to altered patterns of gene expression and the molecular pathogenesis of the disease. It is conceivable that such RNA-binding translation-regulatory proteins may have the capacity to function as oncogenes (or tumor suppressors) themselves, and that aberrant translational control of IGF1R may contribute to initiation and progression of breast malignancy. As little as 50% increase in IGF1R protein levels may allow cells to proliferate in an anchorage-independent manner (i.e. adopt a transformed phenotype) (Rubini et al., 1997). Furthermore, IGF1R is essentially required for establishment and maintenance of the malignant phenotype, as numerous oncogenes including SV40 large T antigen and ras fail to transform IGF1R -/- cells (Sell et al., 1993). Pathological activation of the IRES could represent a previously unrecognized mechanism for altered IGF1R expression in breast cancer, one which would not be evident from standard microarray analyses, and which may be even more important in metastatic lesions than in the primary tumor.
Acknowledgments
The primary human breast surgical specimens used in this study were acquired through the UAB Comprehensive Cancer Center Tissue Procurement Facility, under an IRB-approved protocol. The authors gratefully acknowledge the kind gift of 4F4 antibody to hnRNP C from Dr. Gideon Dreyfuss. This work was supported by a grant (CA108886) from the National Institutes of Health / National Cancer Institute.
Abbreviations
- IGF1R
type 1 insulin-like growth factor receptor
- IRES
internal ribosomal entry site
- ITAF
IRES trans-activating factor
- 5’-UTR
5’-untranslated region
- uORF
upstream open reading frame
Footnotes
Conflict of interest statement The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelmohsen K, Pullmann R, Jr, Lal A, Kim HH, Galban S, Yang X, Blethrow JD, Walker M, Shubert J, Gillespie DA, Furneaux H, Gorospe M. Phosphorylation of HuR by Chk2 regulates SIRT1 expression. Mol Cell. 2007;25(4):543–557. doi: 10.1016/j.molcel.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird SD, Turcotte M, Korneluk RG, Holcik M. Searching for IRES. RNA. 2006;12(10):1755–1785. doi: 10.1261/rna.157806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfiore A, Frasca F. IGF and insulin receptor signaling in breast cancer. J Mammary Gland Biol Neoplasia. 2008;13(4):381–406. doi: 10.1007/s10911-008-9099-z. [DOI] [PubMed] [Google Scholar]
- Chappell SA, LeQuesne JP, Paulin FE, deSchoolmeester ML, Stoneley M, Soutar RL, Ralston SH, Helfrich MH, Willis AE. A mutation in the c-myc-IRES leads to enhanced internal ribosome entry in multiple myeloma: a novel mechanism of oncogene de-regulation. Oncogene. 2000;19(38):4437–4440. doi: 10.1038/sj.onc.1203791. [DOI] [PubMed] [Google Scholar]
- Chiocchetti A, Gibello L, Carando A, Aspesi A, Secco P, Garelli E, Loreni F, Angelini M, Biava A, Dahl N, Dianzani U, Ramenghi U, Santoro C, Dianzani I. Interactions between RPS19, mutated in Diamond-Blackfan anemia, and the PIM-1 oncoprotein. Haematologica. 2005 Nov;90(11):1453–62. [PubMed] [Google Scholar]
- Coleman J, Miskimins WK. Structure and activity of the internal ribosome entry site within the human p27 Kip1 5’-untranslated region. RNA Biol. 2009;6(1):84–89. doi: 10.4161/rna.6.1.7572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke DW, Bankert LA, Roberts CT, Jr, LeRoith D, Casella SJ. Analysis of the human type I insulin-like growth factor receptor promoter region. Biochem Biophys Res Commun. 1991;177(3):1113–1120. doi: 10.1016/0006-291x(91)90654-p. [DOI] [PubMed] [Google Scholar]
- Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell. 2002;111(1):29–40. doi: 10.1016/s0092-8674(02)01001-2. [DOI] [PubMed] [Google Scholar]
- Dreyfuss G, Matunis MJ, Pinol-Roma S, Burd CG. hnRNP proteins and the biogenesis of mRNA. Annu Rev Biochem. 1993;62:289–321. doi: 10.1146/annurev.bi.62.070193.001445. [DOI] [PubMed] [Google Scholar]
- Fukushi S, Okada M, Stahl J, Kageyama T, Hoshino FB, Katayama K. Ribosomal protein S5 interacts with the internal ribosomal entry site of hepatitis C virus. J Biol Chem. 2001;276(24):20824–6. doi: 10.1074/jbc.C100206200. [DOI] [PubMed] [Google Scholar]
- Gooch JL, Van Den Berg CL, Yee D. Insulin-like growth factor (IGF)-I rescues breast cancer cells from chemotherapy-induced cell death--proliferative and anti-apoptotic effects. Breast Cancer Res Treat. 1999;56(1):1–10. doi: 10.1023/a:1006208721167. [DOI] [PubMed] [Google Scholar]
- Gray NK, Wickens M. Control of translation initiation in animals. Annu Rev Cell Dev Biol. 1998;14:399–458. doi: 10.1146/annurev.cellbio.14.1.399. [DOI] [PubMed] [Google Scholar]
- Guix M, Faber AC, Wang SE, Olivares MG, Song Y, Qu S, Rinehart C, Seidel B, Yee D, Arteaga CL, Engelman JA. Acquired resistance to EGFR tyrosine kinase inhibitors in cancer cells is mediated by loss of IGF-binding proteins. J Clin Invest. 2008;118(7):2609–2619. doi: 10.1172/JCI34588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaphy CM, Griffith JK, Bisoffi M. Mammary field cancerization: molecular evidence and clinical importance. Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-009-0504-0. [DOI] [PubMed] [Google Scholar]
- Jo OD, Martin J, Bernath A, Masri J, Lichtenstein A, Gera J. Heterogeneous nuclear ribonucleoprotein A1 regulates cyclin D1 and c-myc internal ribosome entry site function through Akt signaling. J Biol Chem. 2008;283(34):23274–23287. doi: 10.1074/jbc.M801185200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RA, Campbell CI, Gunther EJ, Chodosh LA, Petrik JJ, Khokha R, Moorehead RA. Transgenic overexpression of IGF-IR disrupts mammary ductal morphogenesis and induces tumor formation. Oncogene. 2007;26(11):1636–1644. doi: 10.1038/sj.onc.1209955. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Litzenburger BC, Cui X, Delgado DA, Grabiner BC, Lin X, Lewis MT, Gottardis MM, Wong TW, Attar RM, Carboni JM, Lee AV. Constitutively active type I insulin-like growth factor receptor causes transformation and xenograft growth of immortalized mammary epithelial cells and is accompanied by an epithelial-to-mesenchymal transition mediated by NF-kappaB and snail. Mol Cell Biol. 2007;27(8):3165–3175. doi: 10.1128/MCB.01315-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurmasheva RT, Dudkin L, Billups C, Debelenko LV, Morton CL, Houghton PJ. The insulin-like growth factor-1 receptor-targeting antibody, CP-751,871, suppresses tumor-derived VEGF and synergizes with rapamycin in models of childhood sarcoma. Cancer Res. 2009;69(19):7662–7671. doi: 10.1158/0008-5472.CAN-09-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurmasheva RT, Houghton PJ. IGF-I mediated survival pathways in normal and malignant cells. Biochim Biophys Acta. 2006;1766(1):1–22. doi: 10.1016/j.bbcan.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Law JH, Habibi G, Hu K, Masoudi H, Wang MY, Stratford AL, Park E, Gee JM, Finlay P, Jones HE, Nicholson RI, Carboni J, Gottardis M, Pollak M, Dunn SE. Phosphorylated insulin-like growth factor-i/insulin receptor is present in all breast cancer subtypes and is related to poor survival. Cancer Res. 2008;68(24):10238–10246. doi: 10.1158/0008-5472.CAN-08-2755. [DOI] [PubMed] [Google Scholar]
- LeRoith D, Roberts CT., Jr The insulin-like growth factor system and cancer. Cancer Lett. 2003;195(2):127–137. doi: 10.1016/s0304-3835(03)00159-9. [DOI] [PubMed] [Google Scholar]
- Lopez T, Hanahan D. Elevated levels of IGF-1 receptor convey invasive and metastatic capability in a mouse model of pancreatic islet tumorigenesis. Cancer Cell. 2002;1(4):339–353. doi: 10.1016/s1535-6108(02)00055-7. [DOI] [PubMed] [Google Scholar]
- Meng Z, Snyder RC, Shrestha K, Miller DM, Emanuel PD, Blume SW. Evidence for differential ribonucleoprotein complex assembly in vitro on the 5’-untranslated region of the human IGF-IR transcript. Mol Cell Endocrinol. 2003;200(1-2):127–140. doi: 10.1016/s0303-7207(02)00381-7. [DOI] [PubMed] [Google Scholar]
- Meng Z, King PH, Nabors LB, Jackson NL, Chen CY, Emanuel PD, Blume SW. The ELAV RNA-stability factor HuR binds the 5’-untranslated region of the human IGF-IR transcript and differentially represses cap-dependent and IRES-mediated translation. Nucleic Acids Res. 2005;33(9):2962–2979. doi: 10.1093/nar/gki603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Z, Jackson NL, Choi H, King PH, Emanuel PD, Blume SW. Alterations in RNA-binding activities of IRES-regulatory proteins as a mechanism for physiological variability and pathological dysregulation of IGF-IR translational control in human breast tumor cells. J Cell Physiol. 2008;217(1):172–183. doi: 10.1002/jcp.21486. [DOI] [PubMed] [Google Scholar]
- Meng Z, Jackson NL, Shcherbakov OD, Choi H, Blume SW. The human IGF1R IRES likely operates through a Shine-Dalgarno-like interaction with the G961 loop (E-site) of the 18S rRNA and is kinetically modulated by a naturally-polymorphic polyU loop. J Cell Biochem. 2010 doi: 10.1002/jcb.22569. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller TW, Perez-Torres M, Narasanna A, Guix M, Stal O, Perez-Tenorio G, Gonzalez-Angulo AM, Hennessy BT, Mills GB, Kennedy JP, Lindsley CW, Arteaga CL. Loss of Phosphatase and Tensin homologue deleted on chromosome 10 engages ErbB3 and insulin-like growth factor-I receptor signaling to promote antiestrogen resistance in breast cancer. Cancer Res. 2009;69(10):4192–4201. doi: 10.1158/0008-5472.CAN-09-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci U S A. 1992;89(19):9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilipenko EV, Pestova TV, Kolupaeva VG, Khitrina EV, Poperechnaya AN, Agol VI, Hellen CU. A cell cycle-dependent protein serves as a template-specific translation initiation factor. Genes Dev. 2000;14(16):2028–2045. [PMC free article] [PubMed] [Google Scholar]
- Pinol-Roma S, Choi YD, Matunis MJ, Dreyfuss G. Immunopurification of heterogeneous nuclear ribonucleoprotein particles reveals an assortment of RNA-binding proteins. Genes Dev. 1988;2(2):215–227. doi: 10.1101/gad.2.2.215. [DOI] [PubMed] [Google Scholar]
- Prats AC, Prats H. Translational control of gene expression: role of IRESs and consequences for cell transformation and angiogenesis. Prog Nucleic Acid Res Mol Biol. 2002;72:367–413. doi: 10.1016/s0079-6603(02)72075-8. [DOI] [PubMed] [Google Scholar]
- Pullmann R, Jr, Kim HH, Abdelmohsen K, Lal A, Martindale JL, Yang X, Gorospe M. Analysis of turnover and translation regulatory RNA-binding protein expression through binding to cognate mRNAs. Mol Cell Biol. 2007;27(18):6265–6278. doi: 10.1128/MCB.00500-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajasekhar VK, Viale A, Socci ND, Wiedmann M, Hu X, Holland EC. Oncogenic Ras and Akt signaling contribute to glioblastoma formation by differential recruitment of existing mRNAs to polysomes. Mol Cell. 2003;12(4):889–901. doi: 10.1016/s1097-2765(03)00395-2. [DOI] [PubMed] [Google Scholar]
- Resnicoff M, Abraham D, Yutanawiboonchai W, Rotman HL, Kajstura J, Rubin R, Zoltick P, Baserga R. The insulin-like growth factor I receptor protects tumor cells from apoptosis in vivo. Cancer Res. 1995;55(11):2463–2469. [PubMed] [Google Scholar]
- Rexer BN, Engelman JA, Arteaga CL. Overcoming resistance to tyrosine kinase inhibitors: lessons learned from cancer cells treated with EGFR antagonists. Cell Cycle. 2009;8(1):18–22. doi: 10.4161/cc.8.1.7324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanelli RJ, LeBeau AP, Fulmer CG, Lazzarino DA, Hochberg A, Wood TL. Insulin-like growth factor type-I receptor internalization and recycling mediate the sustained phosphorylation of Akt. J Biol Chem. 2007;282(31):22513–22524. doi: 10.1074/jbc.M704309200. [DOI] [PubMed] [Google Scholar]
- Rubini M, Hongo A, D’Ambrosio C, Baserga R. The IGF-I receptor in mitogenesis and transformation of mouse embryo cells: role of receptor number. Exp Cell Res. 1997;230(2):284–292. doi: 10.1006/excr.1996.3430. [DOI] [PubMed] [Google Scholar]
- Sachdev D, Hartell JS, Lee AV, Zhang X, Yee D. A dominant negative type I insulin-like growth factor receptor inhibits metastasis of human cancer cells. J Biol Chem. 2004;279(6):5017–5024. doi: 10.1074/jbc.M305403200. [DOI] [PubMed] [Google Scholar]
- Sachdev D, Zhang X, Matise I, Gaillard-Kelly M, Yee D. The type I insulin-like growth factor receptor regulates cancer metastasis independently of primary tumor growth by promoting invasion and survival. Oncogene. 2009 doi: 10.1038/onc.2009.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotlandi K, Avnet S, Benini S, Manara MC, Serra M, Cerisano V, Perdichizzi S, Lollini PL, De Giovanni C, Landuzzi L, Picci P. Expression of an IGF-I receptor dominant negative mutant induces apoptosis, inhibits tumorigenesis and enhances chemosensitivity in Ewing’s sarcoma cells. Int J Cancer. 2002;101(1):11–16. doi: 10.1002/ijc.10537. [DOI] [PubMed] [Google Scholar]
- Sell C, Rubini M, Rubin R, Liu JP, Efstratiadis A, Baserga R. Simian virus 40 large tumor antigen is unable to transform mouse embryonic fibroblasts lacking type 1 insulin-like growth factor receptor. Proc Natl Acad Sci U S A. 1993;90(23):11217–11221. doi: 10.1073/pnas.90.23.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Frost PJ, Hoang BQ, Benavides A, Sharma S, Gera JF, Lichtenstein AK. IL-6-induced stimulation of c-myc translation in multiple myeloma cells is mediated by myc internal ribosome entry site function and the RNA-binding protein, hnRNP A1. Cancer Res. 2008;68(24):10215–10222. doi: 10.1158/0008-5472.CAN-08-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Yan H, Frost P, Gera J, Lichtenstein A. Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther. 2005;4(10):1533–1540. doi: 10.1158/1535-7163.MCT-05-0068. [DOI] [PubMed] [Google Scholar]
- Turner BC, Haffty BG, Narayanan L, Yuan J, Havre PA, Gumbs AA, Kaplan L, Burgaud JL, Carter D, Baserga R, Glazer PM. Insulin-like growth factor-I receptor overexpression mediates cellular radioresistance and local breast cancer recurrence after lumpectomy and radiation. Cancer Res. 1997;57(15):3079–3083. [PubMed] [Google Scholar]
- Varani G, Nagai K. RNA recognition by RNP proteins during RNA processing. Annu Rev Biophys Biomol Struct. 1998;27:407–445. doi: 10.1146/annurev.biophys.27.1.407. [DOI] [PubMed] [Google Scholar]
- Wanzel M, Russ AC, Kleine-Kohlbrecher D, Colombo E, Pelicci PG, Eilers M. A ribosomal protein L23-nucleophosmin circuit coordinates Mizl function with cell growth. Nat Cell Biol. 2008;10(9):1051–61. doi: 10.1038/ncb1764. [DOI] [PubMed] [Google Scholar]
- Yanochko GM, Eckhart W. Type I insulin-like growth factor receptor over-expression induces proliferation and anti-apoptotic signaling in a three-dimensional culture model of breast epithelial cells. Breast Cancer Res. 2006;8(2):R18. doi: 10.1186/bcr1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen JS, Akkaya E, Wang Y, Takiguchi M, Peak S, Sullivan M, Protheroe AS, Macaulay VM. Validation of the type 1 insulin-like growth factor receptor as a therapeutic target in renal cancer. Mol Cancer Ther. 2009;8(6):1448–1459. doi: 10.1158/1535-7163.MCT-09-0101. [DOI] [PubMed] [Google Scholar]
- Zeng X, Sachdev D, Zhang H, Gaillard-Kelly M, Yee D. Sequencing of type I insulin-like growth factor receptor inhibition affects chemotherapy response in vitro and in vivo. Clin Cancer Res. 2009;15(8):2840–2849. doi: 10.1158/1078-0432.CCR-08-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Yee D. Tyrosine kinase signalling in breast cancer: insulin-like growth factors and their receptors in breast cancer. Breast Cancer Res. 2000;2(3):170–175. doi: 10.1186/bcr50. [DOI] [PMC free article] [PubMed] [Google Scholar]


