Abstract
Despite palliative treatments, tumor-induced bone disease (TIBD) remains highly debilitating for many cancer patients and progression typically results in death within two years. Therefore, more effective therapies with enhanced antiresorptive and cytotoxic characteristics are needed. We developed bisphosphonate-chemotherapeutic conjugates designed to bind bone and hydrolyze, releasing both compounds, thereby targeting both osteoclasts and tumor cells. This study examined the effects of our lead compound, MBC-11 (the anhydride formed between arabinocytidine (AraC)-5’-phosphate and etidronate), on bone tumor burden, bone volume, femur bone mineral density (BMD), and overall survival using two distinct mouse models of TIBD, the 4T1/luc breast cancer and the KAS-6/1-MIP1α multiple myeloma models. In mice orthotopically inoculated with 4T1/luc mouse mammary cells, MBC-11 (0.04 µg/day; s.c.) reduced the incidence of bone metastases to 40% (4/10), compared to 90% (9/10; p=0.057) and 100% (5/5; p=0.04) of PBS- or similarly-dosed, zoledronate-treated mice, respectively. MBC-11 also significantly decreased bone tumor burden compared to PBS- or zoledronate-treated mice (p=0.021, p=0.017, respectively). MBC-11 and zoledronate (0.04 µg/day) significantly increased bone volume by two- and four-fold, respectively, compared to PBS-treated mice (p=0.005, p<0.001, respectively). In mice systemically injected with human multiple myeloma KAS-6/1-MIP1α cells, 0.04 and 4.0 µg/day MBC-11 improved femur BMD by 13% and 16%, respectively, compared to PBS (p=0.025, p=0.017, respectively) at 10 weeks post-tumor cell injection and increased mean survival to 95 days compared to 77 days in mice treated with PBS (p=0.047). Similar doses of zoledronate also improved femur BMD (p≤0.01 vs PBS) and increased mean survival to 86 days, but this was not significantly different than in PBS-treated mice (p=0.53). These results demonstrate that MBC-11 decreases bone tumor burden, maintains bone structure, and may increase overall survival, warranting further investigation as a treatment for TIBD.
Keywords: bone cancer, bisphosphonates, chemotherapeutics, drug conjugation and targeting
INTRODUCTION
Tumor-induced bone disease (TIBD) is one of the major causes of morbidity and mortality in cancer patients. It is found in 65%–95% of patients with multiple myeloma and advanced breast and prostate cancers [1]. Because TIBD is associated with considerable morbidity and a median survival of less than two years [1], the development of more effective therapies is warranted [2].
Bisphosphonates, particularly zoledronate (Fig. 1A), are bone-specific palliative treatments that reduce tumor-induced skeletal complications [3–5]. Unfortunately, TIBD still progresses in cancer patients necessitating the development of analogs with enhanced anti-resorptive and cytotoxic characteristics to improve the treatment of patients with TIBD [6]. As part of our tumor targeting approach, we exploited the bone-seeking properties of bisphosphonates for targeted delivery of chemotherapeutics. Bisphosphonates and cytotoxic agents can be covalently linked, allowing the intact conjugate to leave the circulation and release both drugs in the bone microenvironment [7,8]. We hypothesize that such conjugates will combine antiresorptive and antitumor activities while localizing at the site of tumor cell-induced bone destruction.
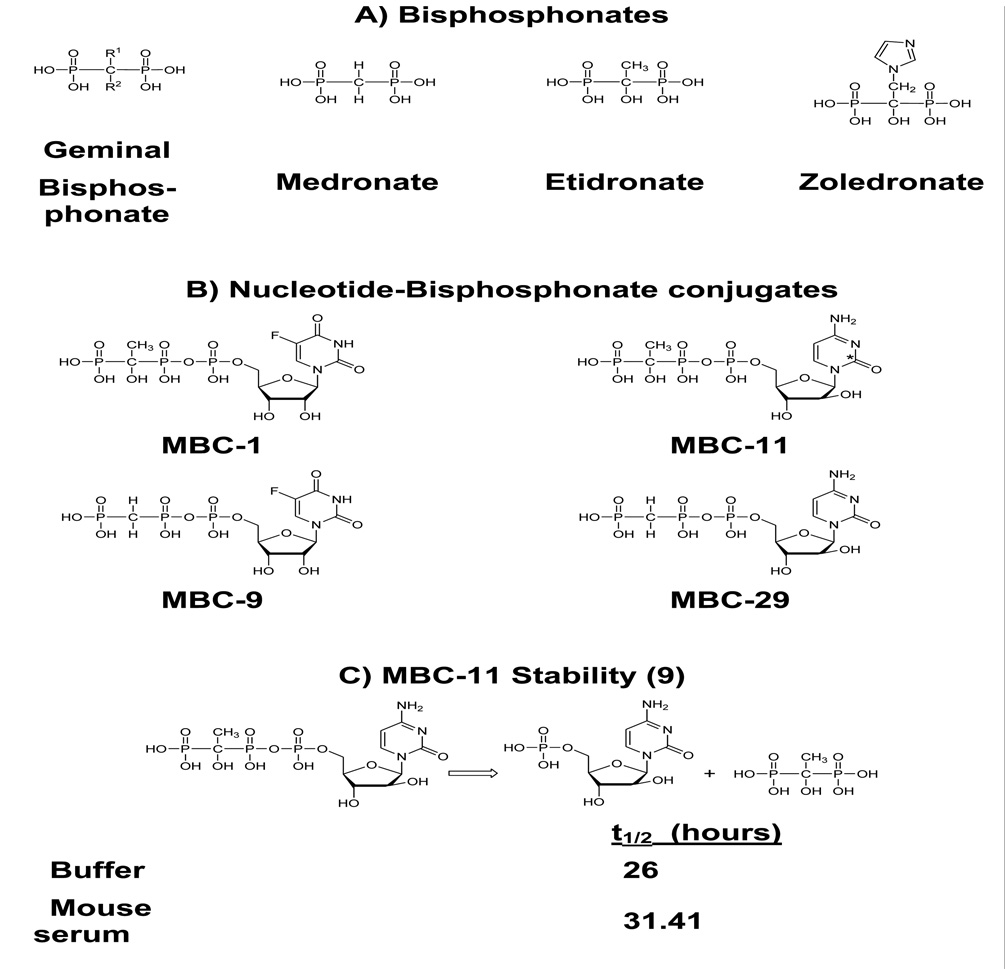
Figure 1. Chemical structures of bisphosphonates and associated conjugates and stability of MBC-11.
A. Chemical structures of bisphosphonates. B. Chemical structures of nucleotide-bisphosphonate conjugates, shown as free acids. * Indicates position of [14-C] label C. Chemical hydrolysis and ex vivo stability of MBC-11 in mouse and human sera [9].
Chemically, these unique compounds are nucleoside-5’-triphosphate analogs in which the β– and γ–phosphorus atoms are linked via a carbon instead of an oxygen atom. Figure 1B illustrates the structures of select conjugates with the cytotoxic analogs, 5-fluorouridine (FUR) or arabinocytosine (AraC), linked to the bisphosphonates, medronate (to give the conjugates MBC-9 and MBC-29) or etidronate (to give the conjugates MBC-1 and MBC-11) . MBC-11 hydrolyzes with the release of etidronate and AraC-5’-phosphate in buffer solutions at physiological pH, temperature and magnesium concentration with a t1/2 ~ 26 hours [9] (Fig 1C). The same hydrolysis pattern was observed in mouse sera with t1/2 of 33 hours [9] (Fig 1C). The conjugates’ hydroxyapatite affinity, mechanism and products of hydrolysis, and stability in mouse and human serum are consistent with the design principles of targeted bone delivery and release of chemotherapeutic on a meaningful timescale [9] . Indeed, our unpublished radiolabeled distribution data in rats demonstrated that the amount of radioactivity in bone associated with conjugated AraC was approximately twice that of AraC alone. Furthermore, the radioactivity decreased over the five hour time course of the experiment in a manner consistent with the measured hydrolysis (Supplemental Table S1).
In addition to anti-resorptive properties, experimental data suggest that bisphosphophonates have both indirect and direct effects on tumor cells [10–15]. However, the in vitro anti-proliferative effects against tumor cells typically require micromolar concentrations (with potentially greater systemic toxicity) [14,16–19] compared to the nanomolar concentrations of the newer nitrogen-containing bisphosphonates needed to inhibit bone resorption [13,14,20]. Our anti-neoplastic bisphosphonate conjugates may be promising drugs that increase local concentrations of cytotoxic agents improving efficacy, without increasing systemic toxicity. Our previous in vitro results demonstrated that MBC-11 was at least 100 times more potent than zoledronate at inhibiting breast cancer cell proliferation as well as inducing bone cell mineralization [18]. MBC-11 also had increased anti-tumor activity compared to AraC alone, and the compound as a whole was needed for maximum efficacy [18] . Thus, drugs that inhibit cancer cell growth and induce bone cell mineralization could revolutionize therapies for TIBD.
In this proof-of-concept study, we describe the tolerability of our lead compound, MBC-11 (the tolerability and efficacy of MBC-29, the medronate analog of MBC-11, are also described; the tolerability and efficacy results of MBC-1 and -9 are shown in the Supplemental Information) and its effects on bone tumor burden, bone structure, bone mineral density and animal survival using two distinct mouse models of TIBD, the 4T1/luciferase (4T1/luc) breast cancer and the KAS-6/1-MIP1α multiple myeloma models.
MATERIALS and METHODS
Materials
MBC-1, 9, 11, and 29 were synthesized at AM Chemicals, Oceanside, CA. The 4T1/luc mouse mammary cancer cells were obtained from Dr. Toshiyuki Yoneda, and the KAS-6/1-MIP1α multiple myeloma cells were obtained from Dr. David Dingli. The KAS-6/1, DP-6, and KP-6 multiple myeloma cells were obtained from Dr. Diane Jelinek. Ethanol, 10% neutral buffered formalin (NBF), sterile phosphate buffered saline (PBS) without calcium and magnesium, EDTA, AraC, FUR, and fluorouracil (FU) were obtained from Sigma (St. Louis, MO). Etidronate was obtained from MGI Pharm Inc (Minnetonka, MN), and zoledronate was obtained from the Mayo Clinic Pharmacy, Rochester, MN (manufacturer: Novartis Pharma Stein AG, Stein, Switzerland). Tritiated thymidine was obtained from Amersham Biosciences (Sweden). Dulbecco’s Modified Eagle’s Medium (DMEM) was obtained from Invitrogen (Carlsbad, CA). The Luciferase Assay System Kit and Reporter Lysis Buffer were obtained from Promega Corp. (Madison, WI), and the Protein DC Assay Kit was obtained from BioRad (Hercules, CA).
Methods
Tolerability of the conjugates
The tolerability and efficacy animal experiments were performed in accordance with National and Institutional guidelines and approved by the Mayo Clinic’s Institutional Animal Care and Use Committee. Female Balb/c and SCID mice (four-six weeks old) were obtained from Harlan Sprague Dawley (Houston, TX) and injected (subcutaneous; s.c.) daily with 500, 100, 1, or 0.01 µg/100 µl of MBC-1, -9, 11, or -29 or PBS (vehicle) for 24 (Balb/c) or 49 (SCID) days (n=5–6 per dose group). The mice were checked daily and their general health and weight (grams; g) were recorded twice weekly. Animals were euthanized (CO2 asphyxiation) when they developed signs of toxicity (e.g., persistent weight loss of > 20% body weight, lethargy, lack of water and food intake and grooming, loss of righting reflex) or after 24 or 49 days of drug administration. Blood was collected from the majority of the mice at the time of sacrifice and renal function was assessed by measurement of plasma creatinine and blood urea nitrogen (BUN) using a Creatinine Analyzer2 and a BUN Analyzer 2, respectively (Beckman Instruments, Fullerton, CA, USA) as previously described [21]. At the time of sacrifice, gross pathology of the organs was examined, individual organs were harvested, weighed, fixed in 10% formalin overnight, and stored in 70% ethanol for future histological analyses.
4T1/luc Orthotopic Breast Cancer Model
We used the well-established spontaneous bone metastasis animal model of breast cancer, which has been extensively utilized to examine the efficacy of many bisphosphonate compounds for the treatment of breast cancer bone metastases [22–25]. This model produces bone metastases in nearly 100% of animals. Histological examination reveals the occurrence of profound osteoclastic bone resorption and luciferase activity assays confirm tumor burden [24,25].
The 4T1/luc mouse mammary cancer cells were cultured in Dulbecco’s Modified Eagle’s Medium supplemented with 5% fetal calf serum in humidified atmosphere enriched with 5% CO2. Subconfluent 4T1/luc cells were re-fed with fresh medium 24 hours before injection. Washed cells (500,000) were suspended in 0.1 ml of sterile PBS and injected (similar to s.c. injection) into mammary fatpads of four-five week old female Balb/c mice at day 0. Primary breast tumors form approximately one week after cell inoculation, metastases to lung and liver develop within two weeks post-inoculation, while metastases to bone, adrenals, kidneys, spleen, and heart occur by three weeks post-inoculation. The mice are typically dead by four weeks after tumor cell injection [25]. In our study, mice were sacrificed on day 21/22 or at endstage (by day 28). Although day 28 data is not typically reported in this model due to the advanced stage of the disease, no significant differences in the incidence or severity of detectable bone luciferase activity were observed between any treatment groups at day 28 (Supplemental Table S2).
Breast Cancer Efficacy Study
Approximately four-week old female Balb/c mice were housed under a 12 hour light/dark cycle, with ad libitum access to food and water. They were inoculated (s.c. injection into their mammary fatpads) with 500,000 4T1/luc cells at day 0. Daily s.c. administration of compounds resuspended in 100 µl of sterile PBS was started seven days after tumor cell inoculation, at the time of primary breast tumor formation. Mice were given 0.04, 0.4, or 4.0 µg/day of MBC-11, -9, and -1, 0.04 or 4.0 µg/day of MBC-29, etidronate, AraC, AraC+etidronate, FUR, FU, and zoledronate. PBS was included as the vehicle control. As the other control compounds (e.g., AraC, FUR, FU, etidronate, AraC+etidronate, zoledronate) were not examined at the mid-dose level, the effects of the 0.40 µg/day dose are presented in Supplemental Table S3. The animals were dosed at mass equivalents (e.g. 4 ug/mouse/day). It should be noted that due to the differences in molecular weights, the molar exposure of mice to control compounds was approximately two-fold higher than the (mass-equivalent) test compound, e.g. 4ug/day of MBC-11 =391 nmol/day; etidronate = 971 nmol/day, AraC = 822 nmol/day; and zoledronate = 689.
At the time of sacrifice (CO2 asphyxiation), breast tumors, hearts, lungs, adrenals, kidneys, spleens, and livers were harvested and stored frozen at −80°C until luciferase activity assays were performed. Both hind-limbs were also excised, and one hind-limb (tibia and femur) was immediately frozen in liquid nitrogen and stored at −80°C for future luciferase activity assays. The other hind-limb was immersed in 10% formalin overnight and stored in 70% ethanol until decalcification (4.13% EDTA for two weeks) and histomorphometric examination (see below). The metastatic bone tumor burden was determined directly by measuring the luciferase content in one hind-limb and indirectly by measuring percent bone volume in the other hind-limb using bone histomorphometry analyses of stained hematoxylin and eosin (H&E) tissue sections using the OsteoMeasure System (OsteoMetrics, Decatur, GA) as described below.
Detection of Luciferase-Expressing Tumor Cells in Bone Lysates
At the time of luciferase assays, frozen bones (tibia and femur) were crushed and homogenized in 0.250 ml Reporter Lysis Buffer. Luciferase activity was measured in the supernatant of freeze-thawed whole homogenates using a TD20/20 Luminometer (Turner Designs, Sunnyvale, CA) and the Luciferase Assay System Kit according to the manufacturer’s instructions. The luciferase activity was normalized to total tissue protein using the Protein DC Assay. As the measurement of luciferase in the bones provides a more global and less subjective assessment of tumor burden, this assay was used to to compare the intramedullary tumor burden.
Bone Histomorphometry
The percent bone volume in the distal femur of one hind-limb (from each mouse) was determined by bone histomorphometry analyses. Tissue sections (5 µm) were made following conventional methods and stained with H&E. Representative sections from the center part of each femur were used to determine the percent of bone volume under a microscope at 10× magnification. Bone volume of the distal femur from endstage mice was measured in longitudinal H&E stained sections using the OsteoMeasure System. Bone volume was measured 350 µm from the growth plate in two 700 µm2 fields of the same tissue section and results are expressed as percent bone volume per total area measured.
In Vitro Multiple Myeloma Cell Proliferation Assays
The myeloma cells were obtained from three different myeloma patients and cell lines were generated and designated as KAS-6/1, DP-6, and KP-6 [26]. In vitro cell proliferation was measured by tritiated thymidine cell incorporation assays as previously described [27].
KAS-6/1-MIP1α Mouse Model
We utilized an animal model of multiple myeloma in which systemic administration of KAS-6/1-MIP1α myeloma cells into immunocompromised female mice produces progressive diffuse bone loss measured by BMD (Supplemental Fig. S1). In this animal model, initiation of bone loss occurs within two weeks after tumor cell injection, hind-limb paralyses occur within seven to eight weeks post-injection, and death typically occurs within eight to nine weeks post-injection. Human KAS-6/1 myeloma cells were genetically engineered to carry the osteoclast activating factor, MIP1α [28], which induces the observed bone density loss in these mice. No BMD loss was observed in SCID mice injected with parent KAS-6/1 cells (1×107 cells in 100 µl PBS i.v.; n=10; Supplemental Fig. S1) and the observed BMD increase was similar to animals not injected with any tumor cells (data not shown). In contrast, a progressive decrease in BMD was observed in the femurs and lumbar spines of the animals that were injected with the KAS-6/1-MIP1α cells starting within two weeks after cell injection (Supplemental Fig. S1). This model does not lead to localized bone destruction (lytic lesions) but diffuse osteoporosis due to diffuse marrow infiltration by the cells, similar to what is often observed in multiple myeloma.
Multiple Myeloma Efficacy Study
Approximately 4 week old female CB17 SCID mice were obtained from Harlan-Sprague Dawley Industries (Houston, TX) and housed in a barrier facility, under a 12 hour light/dark cycle, with ad libitum access to food and water. Approximately twenty-four hours before tumor cell injection, un-anesthetized mice were irradiated with 250 cG Cesium using a Mark 1–25 gamma irradiator (J. L. Shepherd and Associates, San Francisco, CA). KAS-6/1-MIP1α myeloma cells (1 × 107) were injected into the tail vein of properly restrained mice at day 0. The compounds and dosages were based on the results from our tolerability and breast cancer efficacy studies and included 0.04, 0.4, or 4.0 µg/day of MBC-29, -11, and -1, 0.04 or 4.0 µg/day of etidronate, AraC, AraC+etidronate, FUR, FU, and zoledronate. PBS was included as the vehicle control. As the control compounds were not examined at the mid-dose level, the effects of the 0.40 µg/day dose are presented in Supplemental Table S3. Daily s.c. administration of the compounds resuspended in sterile PBS (100 µl) was started two weeks after cell injection at the time of initial bone loss until the necessary sacrifice of the animals. Mice were sacrificed (CO2 asphyxiation) when they could no longer obtain food and water on their own, lost their righting reflex, lost excessive weight (≥ 10% of body weight), or if persistent paralysis occurred.
Bone density scans were routinely performed on anesthetized mice every two weeks starting at the time of cell injection until the necessary sacrifice date using dual-energy x-ray absorptiometry (DEXA) measured by a Lunar-PIXImus instrument (Lunar Corp., Madison, WI). At the time of sacrifice, the animals were euthanized and the gross pathology of individual organs (e.g., liver, spleen, kidneys, lungs, and heart) was examined for any type of metastatic tumor burden. Upon macroscopic evaluation, we did not observe any tumors in the examined organs.
Statistical Analysis
Parametric tests were performed when Normality and Equal Variance tests were passed, and non-parametric tests were performed when normality tests failed. Parametric multiple group comparisons were performed using One Way ANOVA with Tukey post-hoc tests and non-parametric multiple group comparisons were performed using Kruskal-Wallis One Way ANOVA on Ranks with Dunn’s post-hoc tests. Parametric and non-parametric two group comparisons were performed using Student’s T-test and Wilcoxon Rank Sum tests, respectively. Likelihood Ratio Chi square (χ2) analyses were used to test for significant differences in incidence of bone metastases and BMD loss among multiple groups. Fisher's Exact tests were used to test for significant differences in the incidence of bone metastases and BMD loss between two groups. Two-way ANOVA analyses were used to test for differences between the effects of the five tested concentrations and of the compounds on the in vitro cell proliferation of multiple myeloma cell lines. Survival was plotted using Kaplan-Meier curves, and Log-Rank tests were used to assess significance of the grouping factor. Multiple comparison tests’ p-values of ≤0.05 were used to warrant further investigation into differences between two groups. As a trend (p = 0.078) for differences in BMD change at 10 weeks post-tumor cell injection was observed among the 0.04µg/day treatment groups, pairwise comparisons were performed to avoid missing any trends for significant effects. Pairwise comparisons were all two-sided and p-values of ≤0.05 were considered statistically significant. For the breast cancer efficacy studies, four to 10 animals per treatment dose group were included for day 21 and for endstage data analyses. For the multiple myeloma efficacy experiments, three to 10 animals per treatment dose group were included for 10 weeks and for endstage data analyses. The number of mice used in analyses is indicated in the tables and figures. The results for MBC-11 are compiled from three separate experiments.
RESULTS
Tolerability of MBC-11 in Balb/c and SCID mice
BALB/c mice treated with up to 500 µg/day of MBC-11 had an average weight gain of 1–2 g over the 24 day dosing period (Table 1). SCID mice treated with up to 500 µg/day of either MBC-11 or MBC-29 had an average weight gain of 2–3g over the 49 day dosing period (Table 1). These weight gains were similar to the weight gains of mice treated with PBS. Renal function (measured by BUN and creatinine levels) was typically within the normal range (BUN: 15–24 mg/dl; creatinine: 0.17–0.24 mg/dl) for mice treated with MBC-11 or MBC-29. Supplemental Tables S4 and S5 show that mice treated with at least 50 µg/day of MBC-1 and -9 had significantly decreased body weight and abnormal BUN and creatinine levels compared to mice treated with PBS.
Table 1. General Health of Balb/c and SCID Mice Treated with MBC-11 or MBC-29.
Weight change (grams; g) is the difference between weight of animal at time of sacrifice and the weight of animal at start of compound administration. Blood urea nitrogen (BUN) and creatinine levels were measured at the time of sacrifice. Values represent the average ± standard deviation (ave. ± s.d.) and the number in parentheses represents the number of animals analyzed. Normal ranges of BUN: 15–24 mg/dl; creatinine: 0.17–0.24 mg/dl.
| Compound | Dose (µg/day) |
Body Weight Change (g) ave. ± s.d |
BUN (mg/dl) ave. ± s.d |
Creatinine (mg/dl) ave. ± s.d |
Dosing Duration (Days Alive) |
|---|---|---|---|---|---|
| Balb/c mice | |||||
| MBC-11 | 500 | + 1.5 ± 0.5 (5) | 20.5 ± 2.6 (6) | 0.19 ± 0.06 (5) | 24 |
| MBC-11 | 100 | + 2.1 ± 1.0 (5) | 20.2 ± 2.2 (5) | 0.19 ± 0.06 (5) | 24 |
| MBC-11 | 1 | + 1.1 ± 0.7 (5) | 21.2 ± 2.4 (5) | 0.13 ± 0.05 (5) | 24 |
| MBC-11 | 0.01 | + 1.2 ± 0.8 (5) | 23.6 ± 2.5 (5) | 0.15 ± 0.09 (5) | 24 |
| PBS | + 1.4 ± 0.9 (5) | 15.4 ± 4.2 (5) 24.2 ± 6.0 (5) |
0.24 ± 0.04 (5) 0.17 ± 0.06 (5) |
7 24 |
|
| SCID mice | |||||
| MBC-11 | 500 | + 2.0 ± 1.0 (6) | 16.0 ± 4.3 (6) | 0.19 ± 0.03 (6) | 49 |
| MBC-11 | 100 | + 3.1 ± 0.7 (5) | 23.5 ± 10.5 (5) | 0.22 ± 0.10 (4) | 49 |
| MBC-11 | 1 | + 2.7 ± 0.9 (5) | 26.4 ± 5.4 (5) | 0.17 ± 0.04 (5) | 49 |
| MBC-11 | 0.01 | + 2.6 ± 0.5 (5) | 17.40 ± 3.1 (5) | 0.21 ± 0.14 (5) | 49 |
| MBC-29 | 500 | + 2.1 ± 1.0 (6) | 22.00 ± 4.7 (6) | 0.23 ± 0.04 (5) | 49 |
| MBC-29 | 100 | + 2.4 ± 0.6 (5) | 21.80 ± 3.5 (5) | 0.19 ± 0.06 (5) | 49 |
| MBC-29 | 1 | + 2.9 ± 0.5 (5) | 31.40 ± 3.2 (5) | 0.24 ± 0.13 (5) | 49 |
| MBC-29 | 0.01 | + 2.9 ± 0.6 (5) | 21.00 ± 4.7 (5) | 0.28 ± 0.17 (5) | 49 |
| PBS | + 2.9 ± 0.5 (6) | 22.6 ± 4.2 (5) | 0.27 ± 0.09 (5) | 49 | |
The effects of MBC-11 on bone tumor burden in mice orthotopically inoculated with mouse mammary 4T1/luc cells and sacrificed at day 21
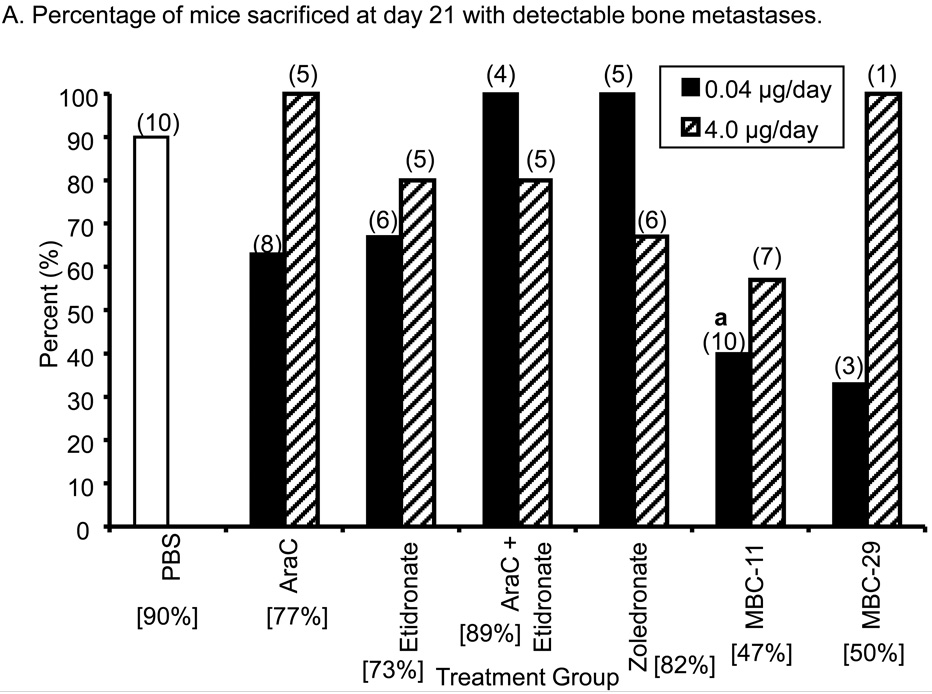
Figure 2A shows that 63% to 100% of mice treated with PBS (9/10), AraC (10/13), etidronate (8/11), AraC+etidronate (8/9), and zoledronate (9/11) had detectable bone metastases at day 21. Fifty percent of four mice treated with MBC-29 and 47% of 17 mice treated with MBC-11 had detectable bone metastases.at day 21 Significant differences in the incidence of bone metastases were observed between the PBS and 0.04 µg/day treatment groups (χ2 p=0.042). Mice treated with 0.04 µg/day MBC-11 appeared to have a lower incidence of bone metastases of 40% compared to those treated with PBS (90%; p=0.057) or 0.04 µg/day zoledronate (100%; p=0.044). No significant differences in bone metastases incidence were observed between the PBS and 4.0 µg/day treatment groups (χ2 p=0.44).
Figure 2. The effects of MBC-11, MBC-29, and control treatments on bone tumor burden in 4T1/luc-inoculated mice.
A. Percentage of mice sacrificed at day 21 with detectable bone luciferase activity. Detectable bone luciferase activity was defined as > 1 unit of luciferase activity (U)/mg total bone tissue protein. Parenthetic values indicate group size. Bracketed values indicate the overall percentage of mice displaying detectable bone luciferase activity from each treatment group. Likelihood Ratio χ2 analysis of PBS and 0.04 µg/day dose groups: p=0.024; two-tail Fisher's Exact test: a: MBC-11 vs. PBS p=0.057; MBC-11 vs. zoledronate p=0.044.
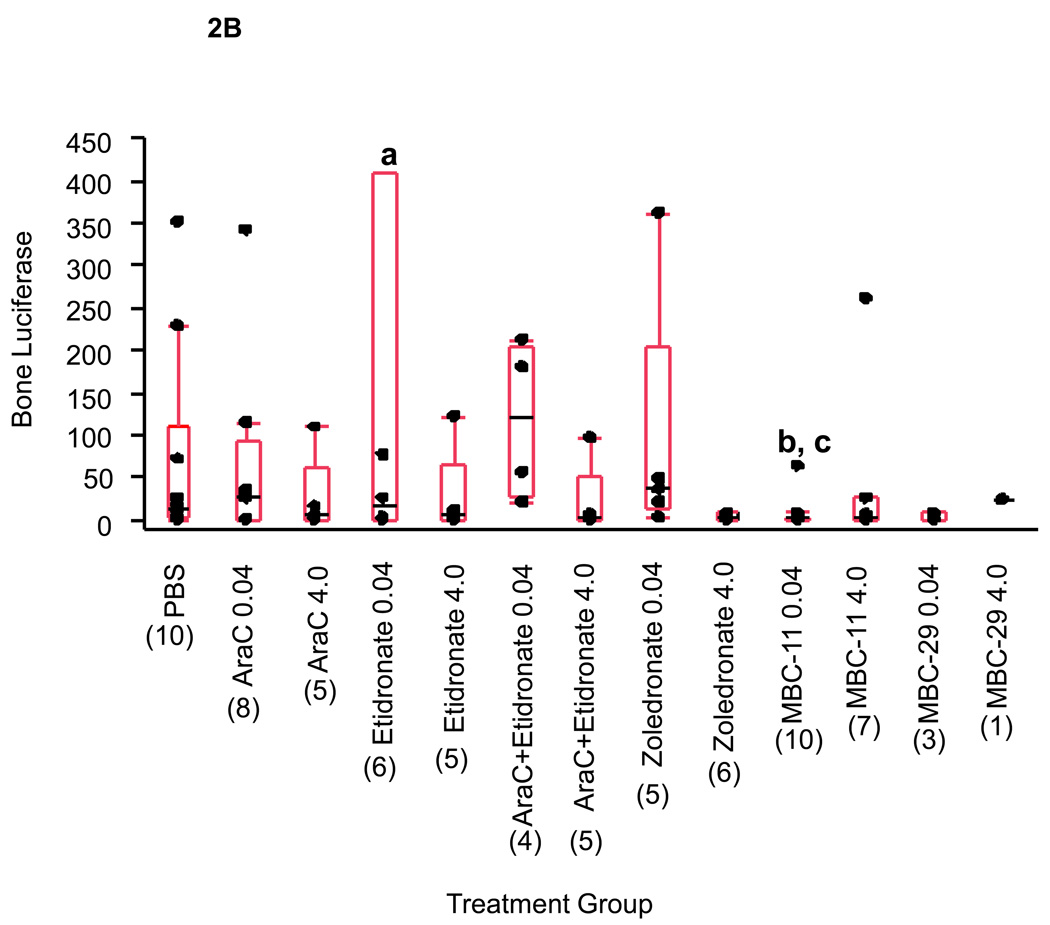
B. Luciferase activity in bone tissue from mice sacrificed at day 21. Luciferase activity was normalized to total bone tissue protein (U/mg protein). Box plot values represent the 25%, median, and 75% quartiles luciferase activity, and parenthetic values indicate group size. a: For graphing purposes, a luciferase activity value of 1410 U/mg for the etidronate 0.04 µg/day treatment group was not shown. b: Kruskal-Wallis ANOVA on Ranks analysis of PBS and 0.04 µg/day dose groups: p=0.042. c: Wilcoxon Rank Sum analyses: p=0.021, p=0.013, and p=0.017 for MBC-11 vs PBS, AraC+etidronate, and zoledronate, respectively. The median luciferase activity in bone tissue of mice sacrificed at day 21 for MBC-1 and -9, FU, and FUR is presented in Supplemental Figure S2.
Figure 2B shows that the amount of bone luciferase activity at day 21 was significantly different between the PBS and 0.04 µg/day treatment groups (Kruskal-Wallis ANOVA on Ranks: p=0.042). Wilcoxon Rank Sum tests demonstrated that: (1) bone luciferase content was not significantly different between mice treated with PBS or 0.04 µg/day of AraC, etidronate, AraC+etidronate, or zoledronate (p=1.0, 0.96, 0.30, and 0.36, respectively); and (2) the bone luciferase content was significantly lower in mice treated with 0.04 µg/day MBC-11 than in PBS, AraC+etidronate, and zoledronate-treated mice (p=0.021, 0.013, and 0.017, respectively). No significant differences were observed between the PBS and 4.0 µg/day treatment groups (Kruskal-Wallis ANOVA p=0.54).
The effects of MBC-11 on bone volume and architecture in mice sacrificed at day 28, endstage (Figure 3)
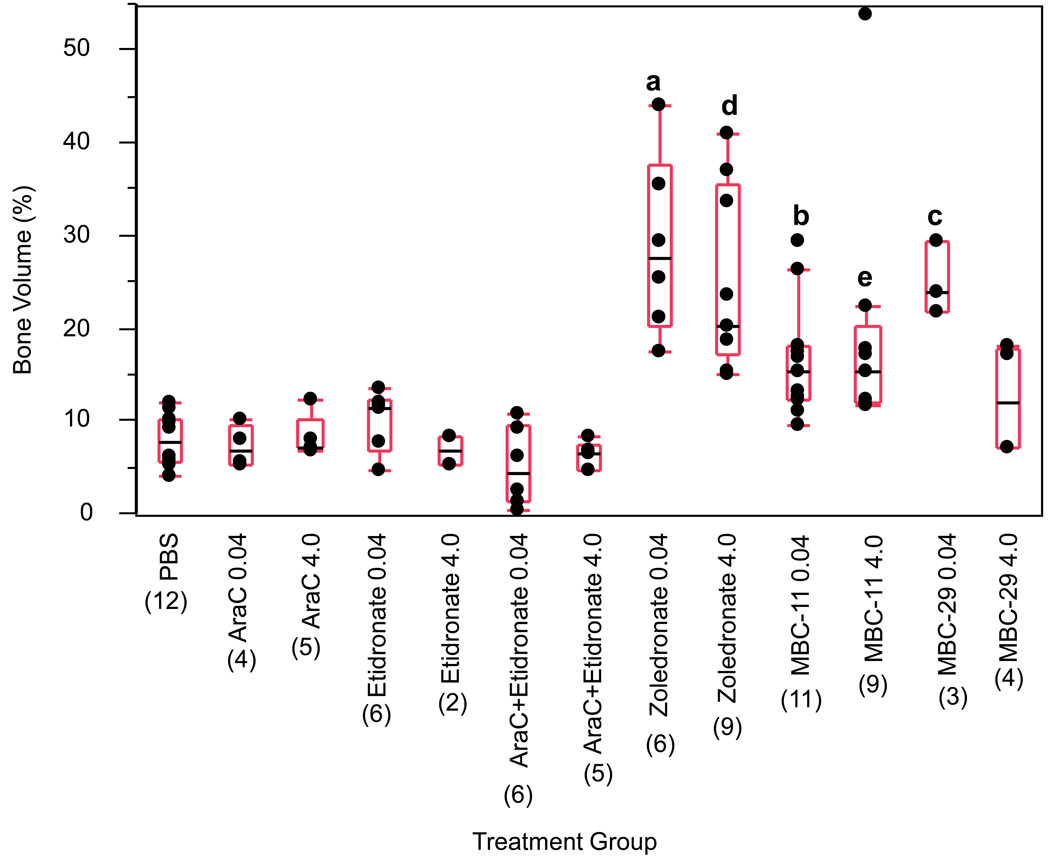
Figure 3. The effects of MBC-11, MBC-29, and control treatments on bone volume of femurs from 4T1/luc-inoculated mice sacrified at endstage (day 28).
Box plot values represent the 25%, median, and 75% quartiles of percent bone volume and parenthetic values indicate group size. ANOVA analyses for each dose group: p<0.001. ANOVA/Tukey analyses of PBS and 0.04µg/day dose groups: a : p<0.001 for zoledronate vs each treatment except MBC-29 (p=0.947); b: p=0.005, p=0.058, p=0.22, and p=0.002 for MBC-11 vs PBS, AraC, etidronate, and AraC+etidronate, respectively; c: p<0.001, p=0.001, p=0.004, p<0.001, and p=0.20 for MBC-29 vs PBS, AraC, etidronate, AraC+etidronate, and MBC-11, respectively. Kruskal-Wallis ANOVA/Dunn’s analyses of PBS and 4.0 µg/day dose groups: d : p<0.05 for zoledronate vs AraC+etidronate; e: p<0.05 for MBC-11 vs AraC+etidronate. The median bone volume in mice treated with MBC-1 and -9, FU, and FUR and sacrificed at endstage is shown in Supplemental Table S6.
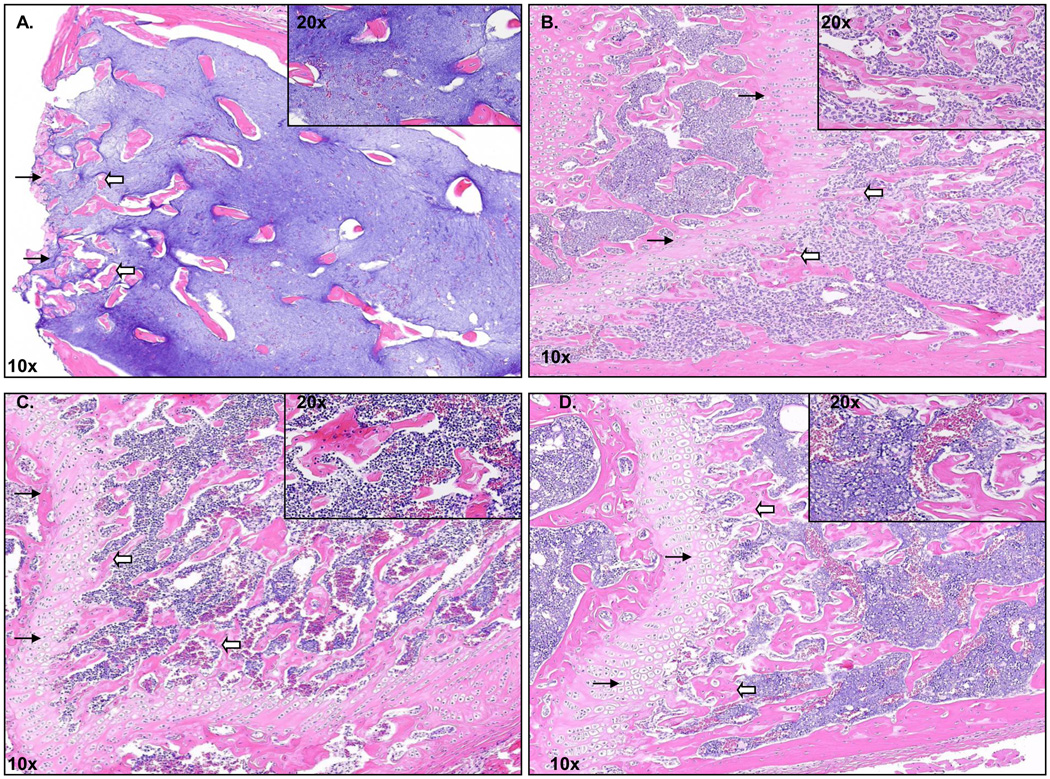
Bone volume in 15 mice treated with zoledronate (mean ± s.e.m. bone volume of combined doses of zoledronate: 26.5 ± 2.47) was used as a positive control and was comparable to that observed in five healthy mice with no tumor cells injected (29.2 ± 4.82). Bone volume was significantly different between the PBS and 0.04 µg/day or 4.0 µg/day treatment groups (ANOVA p<0.001 for each dose). Bone volume was significantly higher in mice treated with 0.04 µg/day zoledronate than in mice treated with 0.04 µg/day of any other compound except for MBC-29 (ANOVA/Tukey p<0.001). Bone volume in mice treated with 0.04 µg/day MBC-11 was higher than in mice treated with PBS (ANOVA/Tukey, p=0.005), 0.04µg/day AraC+etidronate (ANOVA/Tukey, p=0.002), and possibly 0.04 µg/day AraC (ANOVA/Tukey, p=0.058). Bone volume was higher in mice treated with 4.0 µg/day zoledronate or MBC-11 than in mice treated with 4.0 µg/day AraC+etidronate (ANOVA/Dunn’s, p<0.05). Figure 4 illustrates representative H&E staining of femur bone histology. The loss of the growth plate and trabeculae was extensive, and distinct cell membrane boundaries and nuclei could not be completely discerned in the bone marrow of mice treated with PBS (Fig. 4A). In contrast, the growth plate and trabeculae remained intact in mice treated with 0.04 µg/day of MBC-11 (Fig. 4B) or zoledronate (Fig. 4C), similar to that observed in healthy mice (Fig. 4D).
Figure 4. Bone histology of distal femur from Balb/c mice.
A. Representative femur bone histology from PBS-treated endstage 4T1/luc mice. B. Representative femur bone histology from 0.04 µg/day zoledronate-treated endstage 4T1/luc mice. C. Representative femur bone histology from 0.04 µg/day MBC-11-treated endstage 4t1/luc mice. D. Representative femur bone histology of a non-tumor cell injected mouse. Black arrows represent the growth plate and white arrow blocks represent the trabeculae. Magnification for all pictures is 10× (inset is 20×).
The effects of MBC-11 on human multiple myeloma cell proliferation in vitro
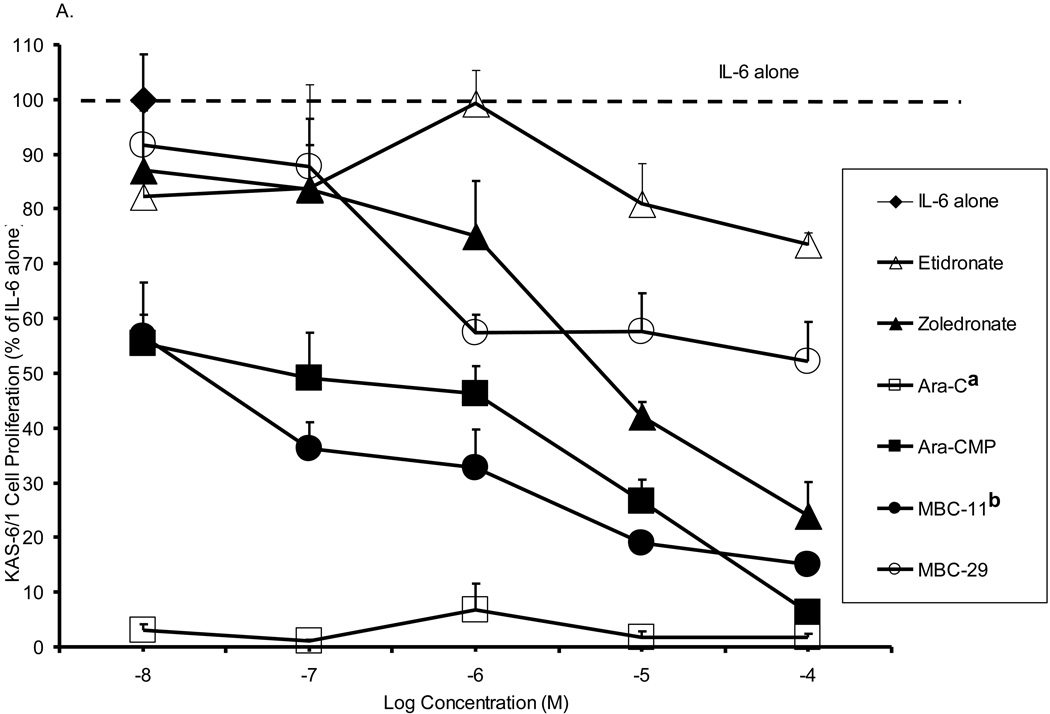
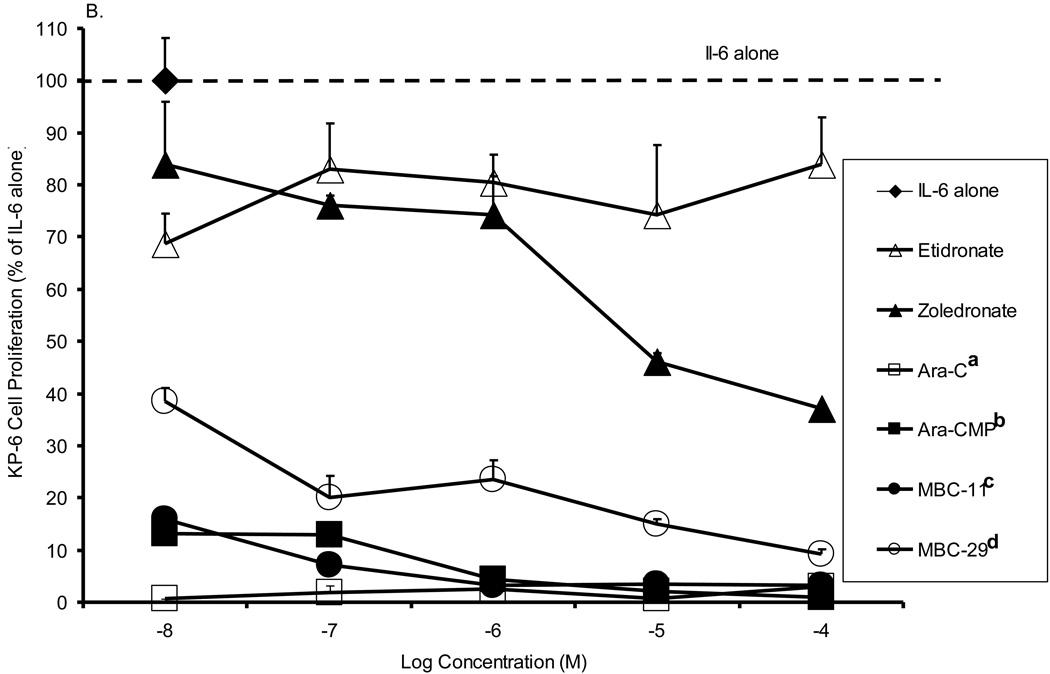
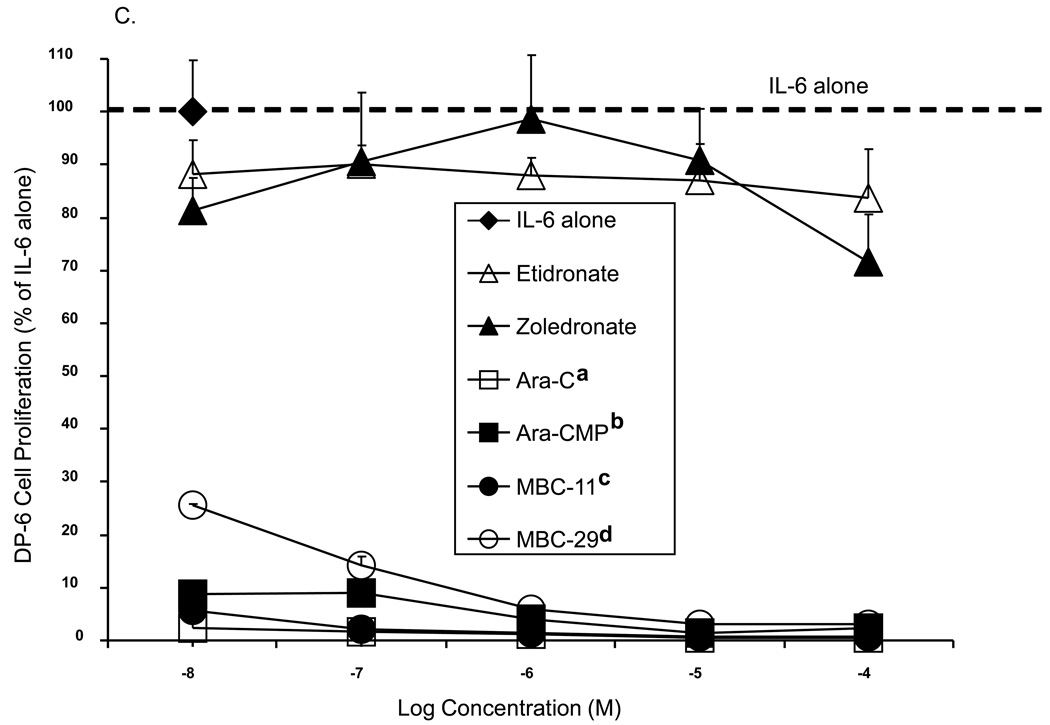
Figure 5 shows the effects of MBC-11 and -29 and the respective control treatments on cell proliferation of three multiple myeloma cell lines. For each cell line, significant differences in the inhibition of multiple myeloma cell proliferation were observed between the compounds (p<0.001). When comparing cell proliferation to the positive assay control (IL-6 alone), most compounds significantly inhibited multiple myeloma cell proliferation of each cell line at the majority of the tested concentrations. Etidronate was the weakest of the compounds at inhibiting multiple myeloma cell growth, and the inhibition was not significantly different than that observed by zoledronate across the concetration range for any of the cell lines examined . Yet, zoledronate decreased KAS-6/1 (Fig. 5A) and KP-6 (Fig. 5B) cell proliferation to ~ 45% at 10−5 M (p<0.0001 vs IL-6), whereas etidronate decreased KAS6/1 cell proliferation to only 78–82% (p<0.01 vs IL-6). MBC-29 decreased DP-6 (Fig. 5C) and KP-6 cell growth to <40% between 10−8 and 10−4 M (p<0.0001 vs IL-6 at each concentration), which was significantly different than the inhibition produced by etidronate (p<0.001) or zoledronate (p<0.001) for either cell line. MBC-29 decreased KAS-6/1 cell growth to 60% at 10−6 M where the effect appeared to plateau (p<0.0001). This inhibition was not significantly different than that observed by etidronate or zoledronate. In contrast, the cytotoxic agent, AraC, nearly abolished the growth of all three cell lines between 10−8 and 10−4 M (p<0.0001). This inhibition was significantly different than the inhibition produced by etidronate (p<0.001 all cell lines), zoledronate (p<0.001 all cell lines), or MBC-29 (p<0.001 KAS-6/1), but not by MBC-11. Further, Ara-CMP and MBC-11 showed similar activity profiles and significantly inhibited growth of all three cell lines between 10−8 and 10−4 M (p<0.0001 vs IL-6 at each concentration). Ara-CMP and MBC-11 decreased KAS-6/1 cell growth from approximately 56% at 10−8 M to 15% and 6%, respectively at 10−5 M (Fig. 5A). Both compounds nearly abolished KP-6 (Fig. 5B) and DP-6 (Fig. 5C) cell proliferation at all tested concentrations. This inhibition was significantly greater than that produced by etidronate or zoledronate (p<0.001 for all), but not by AraC.
Figure 5. The effects of MBC-11, MBC-29, and control treatments on multiple myeloma cell proliferation in vitro.
A. KAS-6/1 cell proliferation. Two-way ANOVA analyses: compound effect p<0.001; dose effect p=0.328. a: Tukey p<0.001 AraC vs etidronate, zoledronate, and MBC-29, p=0.053 AraC vs Ara-CMP. b: Tukey p=0.03 MBC-11 vs MBC-29. B. KP-6 cell proliferation. Two-way ANOVA analyses: compound effect p<0.001; dose effect p=0.068. a: Tukey p<0.001 AraC vs etidronate and zoledronate; b: Tukey p<0.001 Ara-CMP vs etidronate and zoledronate, p=0.041 Ara-CMP vs MBC-29; c: Tukey p<0.001 MBC-11 vs etidronate and zoledronate; d: Tukey p<0.001 MBC-29 vs etidronate and zoledronate. C. DP-6 cell proliferation. Two-way ANOVA analyses: compound effect p<0.001; dose effect p=0.098. a: Tukey p<0.001 AraC vs etidronate and zoledronate; b: Tukey p<0.001 Ara-CMP vs etidronate and zoledronate; c: Tukey p<0.001 MBC-11 vs etidronate and zoledronate; d: Tukey p<0.001 MBC-29 vs etidronate and zoledronate. Data are presented as percent of cell proliferation by IL-6 alone (positive control) and values represent the mean of duplicate or triplicate analyses ± the standard deviation in the presence of IL-6. Cell proliferation was also measured in the absence of IL-6, and these values were similar to background levels (data not shown).
The effects of MBC-11 on BMD in mice injected with human KAS-6/1-MIP1α multiple myeloma cells (Figure 6)
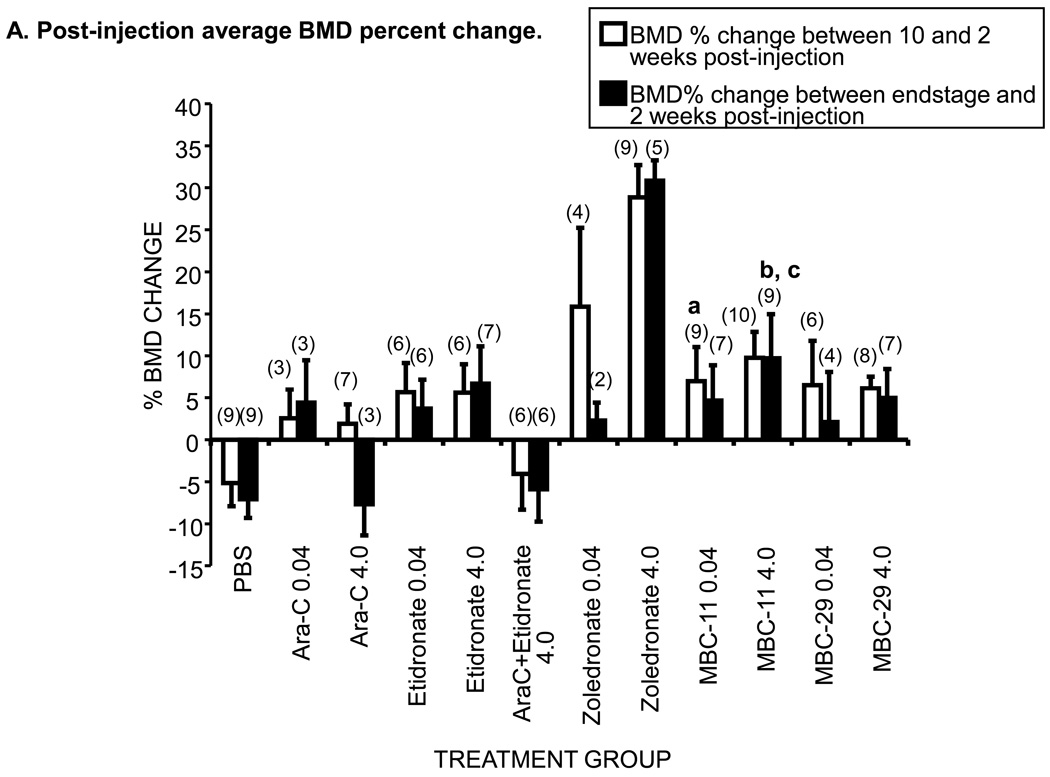
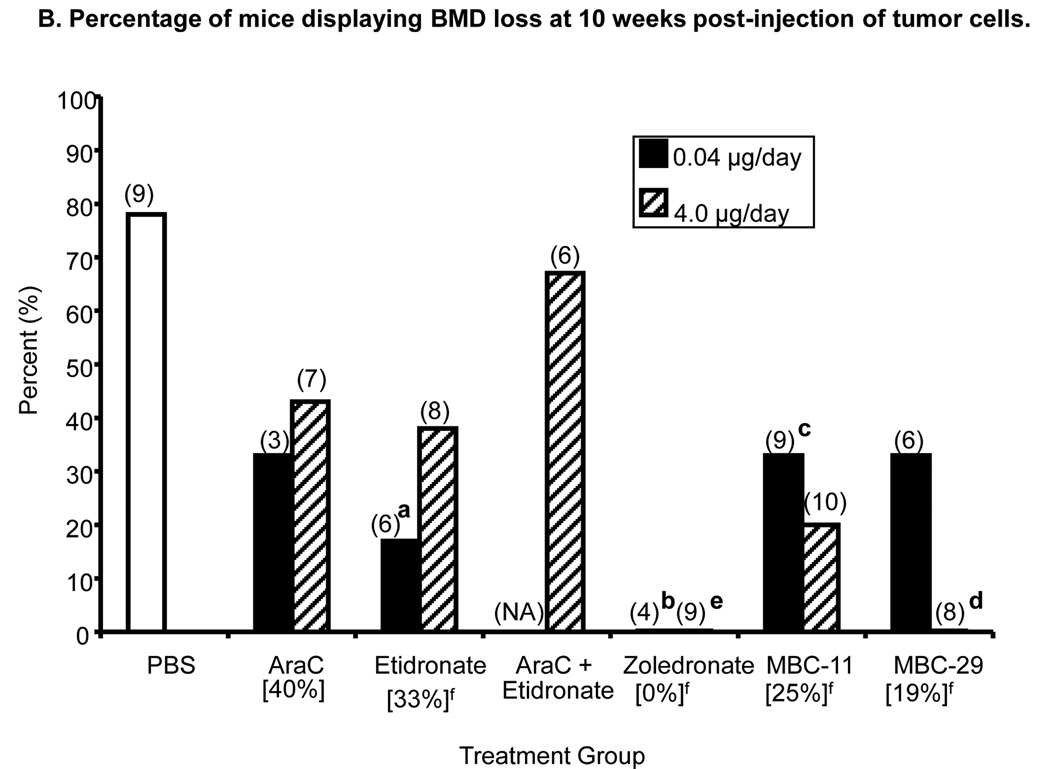
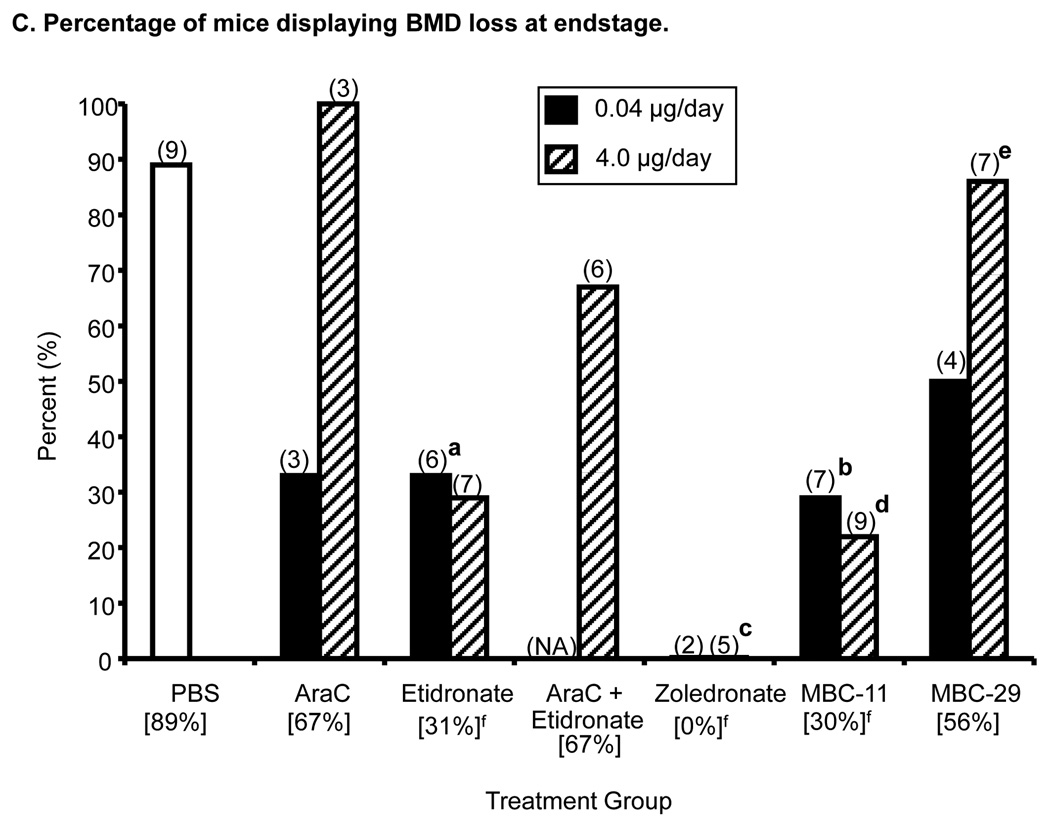
Figure 6. The effects of MBC-11, MBC-29, and control treaments on femur bone mineral density (BMD) of KAS-6/1-MIP1α-injected mice.
A. Post-injection average percent BMD changes between two and 10 weeks and two weeks and endstage in affected mice. Error bars represent standard error of the mean. Parenthetic values indicate group size. Week 10 analysis included mice that were sacrificed by or still alive at 10 weeks post-tumor cell injection. Endstage analysis included animals that were sacrificed or censored (overdosed, OD) and not the mice that were alive at study end. The average percent BMD loss between two weeks post-tumor cell injection and pre-injection typically ranged between 5–7% for all treatment groups except average initial losses of < 3.5% were observed for 0.04µg/day AraC, 0.04µg/day etidronate, and 0.04 and 4.0 µg/day zoledronate treatment groups (Supplemental Table S7). a: Week10 ANOVA of PBS and 0.04µg/day dose groups: p=0.078. t-tests: p=0.025 MBC-11 vs PBS; p=0.052 MBC-29 vs PBS; p=0.015 zoledronate vs PBS. b: Week 10 ANOVA of PBS and 4.0µg/day dose groups: p<0.001; ANOVA/Tukey: p=0.011 MBC-11 vs PBS; p<0.001 zoledronate vs PBS. c:Endstage ANOVA of PBS and 4.0µg/day dose groups: p<0.001; ANOVA/Tukey, p= 0.027 MBC-11 vs PBS; p<0.001 zoledronate vs PBS.
B. Percentage of mice displaying BMD loss at 10 weeks post-tumor cell injection. Parenthetic values indicate group size. Bracketed values represent the overall percentage of mice displaying BMD loss from each treatment group (when dose groups are pooled). Likelihood Ratio χ2 analyses of PBS and 0.04 µg/day dose groups, p=0.011; two-tailed Fisher’s Exact tests: a: etidronate vs PBS, p=0.041; b: zoledronate vs PBS, p=0.021. Likelihood Ratio χ2 analyses of PBS and 4.0 µg/day dose groups, p=0.0003; two-tailed Fisher’s Exact tests: c: MBC-11 vs PBS, p=0.023; d: MBC-29 vs PBS, p=0.002; e: zoledronate vs PBS, p=0.002. Secondary analyses included f: Likelihood Ratio χ2 analyses across PBS and the two dose levels pooled for each compound: p=0.0007; Two-tailed Fisher’s Exact tests: PBS versus etidronate, zoledronate, MBC-11, and MBC-29: p=0.036, 0.0002, 0.023, and 0.007, respectively. NA: not applicable.
C. The percentage of mice displaying BMD loss at endstage. Parenthetic values indicate group size. Bracketed values represent the overall percentage of mice displaying BMD loss from each treatment group (when dose groups are pooled). Likelihood Ratio χ2 analyses of PBS and 0.04 µg/day dose groups, p=0.046; Two-tailed Fisher’s Exact tests: a: etidronate vs PBS, p=0.08; b: MBC-11 vs PBS, p=0.035; Likelihood Ratio χ2 analyses of PBS and 4.0 µg/day dose groups, p=0.001; Two-tailed Fisher’s Exact tests: c: zoledronate vs PBS, p=0.02 d: MBC-11 vs PBS, p=0.015; e: MBC-29 vs PBS, p=0.09. Secondary analyses included f: Likelihood Ratio χ2 analyses across PBS and the two dose levels pooled for each compound: p=0.001; Two-tailed Fisher’s Exact tests: PBS vs etidronate, zoledronate and MBC-11: p=0.09, 0.003 and 0.017, respectively. NA: not applicable. The effects of MBC-1 and FUR on BMD change are shown in Supplemental Figure S3.
Nine mice without tumor cells displayed an average BMD gain of 18.1 ± 2.5% at 10 weeks post-injection. Again, zoledronate served as a positive control, and all mice treated with zoledronate displayed a BMD gain at 10 weeks post-tumor cell injection and at endstage (Fig. 6A). At 10 weeks post-injection, differences in BMD change appear to exist among the 0.04 µg/day treatment groups (ANOVA p=0.078) and were significant among the 4.0 µg/day treatment groups (p<0.001). At endstage, no differences in BMD change were observed among the 0.04 µg/day treatment groups (ANOVA p=0.139) and significant differences in BMD change were observed among the 4.0 µg/day treatment groups (ANOVA p<0.001). Figure 6A demonstrates that mice treated with 0.04 µg/day or 4.0 µg/day AraC displayed minimal BMD gains at 10 weeks (n=10) and had a 1.63% average BMD loss at endstage (n=6). Mice treated with 0.04 µg/day or 4.0 µg/day etidronate showed BMD gains of ~6% at 10 weeks and 3–7% at endstage. Mice treated with 4.0 µg/day AraC+etidronate displayed an average 5–7% BMD loss at 10 weeks and at endstage. At 10 weeks post-injection, mice treated with 4.0 µg/day MBC-11 (n=10) displayed an average 9.8% BMD gain, which was significantly higher than the average 5.1% BMD loss observed in the mice treated with PBS (ANOVA/Tukey: p=0.011). At endstage, mice treated with 4.0 µg/day MBC-11 (n=9) continued to have BMD gain at endstage (9.7%; ANOVA/Tukey: p=0.027 vs PBS).
Figure 6B shows that the incidence of BMD loss was significantly different between the PBS and 0.04 and 4.0 µg/day treatment groups at 10 weeks post- tumor cell injection (χ2: p=0.011 and p=0.0003, respectively). As expected, 0% incidence of BMD loss was observed in mice treated with either 0.04 or 4.0 µg/day zoledronate, which was significantly lower than the 78% (7/9) incidence observed in mice treated with PBS (p=0.021 and 0.002, respectively). The 20% and 0% incidences of BMD loss observed in mice treated with 4.0 µg/day MBC-11 and MBC-29, respectively, were significantly lower than in PBS-treated mice (p=0.023 and p=0.002, respectively). Secondary analyses showed that the incidence of BMD loss was significantly different between PBS and the two dose levels pooled for each compound (χ2: p=0.0007). Thirty percent (3/10), 67% (4/6), 29% (4/14), 0% (0/13), 26% (5/19), and 14% (2/14) of mice treated with AraC, AraC+etidronate, etidronate, zoledronate, MBC-11, and MBC-29, respectively, displayed BMD loss at 10 weeks post-tumor cell injection. The incidences in mice treated with etidronate, zoledronate, MBC-11, and MBC-29 were significantly lower than the 78% incidence of BMD loss observed in mice treated, with PBS (p=0.036, 0.0002, 0.023, and 0.007, respectively).
Figure 6C shows that the incidence of BMD loss was significantly different between the PBS and 0.04 and 4.0 µg/day treatment groups at endstage (χ2: p=0.046 and p=0.001, respectively). Again, 0% incidence of BMD loss was observed in mice treated with either 0.04 or 4.0 µg/day zoledronate. The incidence in the high dose group of zoledronate was significantly lower than the 89% (8/9) incidence observed in mice treated with PBS (p=0.021). The 29% and 22% incidences of BMD loss in mice treated with either 0.04 or 4.0 µg/day MBC-11 were also significantly lower than in mice treated with PBS (p=0.035 and 0.015, respectively). Secondary analyses showed that the incidence of BMD loss was significantly different between PBS and the two dose levels pooled for each compound (χ2: p=0.001). Sixty-seven percent (4/6), 67% (4/6), 30% (4/13), 0% (0/7), 25% (4/16), and 55% (6/11) of mice treated with AraC, AraC+etidronate, etidronate, zoledronate, MBC-11, and MBC-29, respectively, displayed BMD loss at endstage. The incidences in mice treated with zoledronate and MBC-11 were significantly lower than the 89% incidence of BMD loss observed in mice treated with PBS (p=0.003 and 0.017, respectively).
The effects of MBC-11 on survival of mice inoculated with KAS-6/1-MIP1α multiple myeloma cells
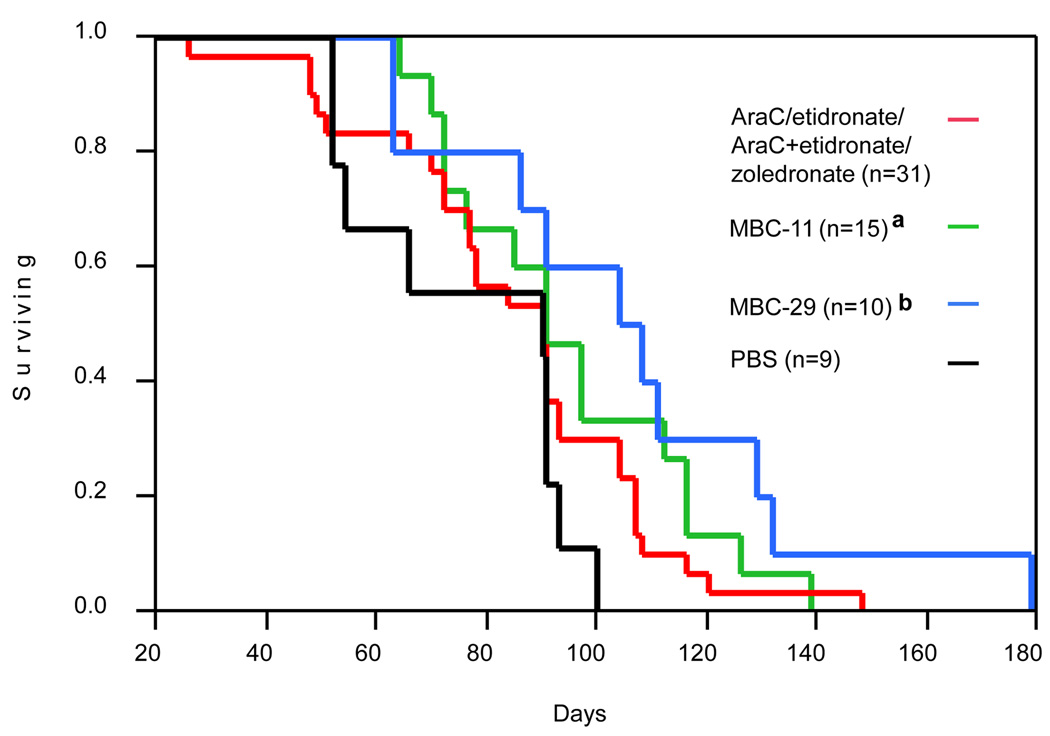
Kaplan-Meier survival curves of mice injected with KAS-6/1-MIP1α cells and treated with PBS, 0.04/4.0 µg/day MBC-11, 0.04/4.0 µg/day MBC-29, or 0.04/4.0 µg/day AraC, etidronate, AraC+etidronate, and zoledronate are illustrated in Figure 7. Table 2 indicates the mean, minimum, and maximum survival times of mice treated with individual doses of each compound. No difference in survival was observed between mice treated with PBS and any of the control treatments. The mean survival was increased by approximately 18 and 30 days in mice treated with MBC-11 [n=15; lower and upper 95 % confidence intervals (95% CI): 72–97; p=0.047] and MBC-29 (n=10; 95% CI: 63–111; p=0.011), respectively, compared to mice treated with PBS (n=9; 95% CI: 52–91).
Figure 7. Kaplan-Meier survival curves of KAS-6/1-MIP1α-injected mice.
Kaplan-Meier Survival Curves of animals treated with PBS, 0.04/4.0µg/day MBC-11, 0.04/4.0µg/day MBC-29, and the data pooled from the different control treatments (0.04/4.0µg/day AraC, etidronate, AraC+etidronate, and zoledronate). n: the number of mice analyzed for the respective group. Survival was significantly different across these treatment groups (Log-Rank: p=0.048). a: Log-Rank PBS vs MBC-11, p=0.047; b: Log-Rank PBS vs MBC-29, p=0.012. Kaplan-Meier survival curves for all treatments are presented in Supplemental Figure S4.
Table 2. Mean and range of survival of KAS-6/1-MIP1α-injected mice.
Mean, minimum, and maximum survival times are indicated in days. Data include those animals that had to be sacrificed by week 22 (154 days) and four animals that were sacrificed at either 179 (MBC-29 4.0µg/day) days. Censored data included nine animals that were overdosed and are not included in analyses (etidronate n = 2; zoledronate n= 1; MBC-29 n= 1; MBC-11 n= 3; MBC-1 n = 2). Kaplan-Meier Survival Curves of animals from all treatment groups are presented in online Supplemental Figure S4.
| Compound | Number of animals |
Mean Survival (days) |
Minimum survival (days) |
Maximum Survival (days) |
|---|---|---|---|---|
| PBS | 9 | 76.5 | 52 | 100 |
| AraC 4.0 µg/day | 5 | 95 | 66 | 148 |
| AraC 0.04 µg/day | 3 | 69 | 48 | 90 |
| Etidronate 4.0 µg/day | 7 | 72 | 26 | 116 |
| Etidronate 0.04 µg/day | 4 | 64 | 37 | 104 |
| AraC+Etidronate 4.0 µg/day | 6 | 85 | 49 | 107 |
| Zoledronate 4.0 µg/day | 5 | 87 | 51 | 120 |
| Zoledronate 0.04 µg/day | 1 | 84 | 84 | 84 |
| MBC-11 4.0 µg/day | 9 | 96 | 64 | 139 |
| MBC-11 0.40 µg/day | 2 | 123 | 76 | 170 |
| MBC-11 0.04 µg/day | 6 | 94 | 72 | 126 |
| MBC-29 4.0 µg/day | 6 | 115 | 86 | 179 |
| MBC-29 0.40 µg/day | 5 | 113 | 45 | 157 |
| MBC-29 0.04 µg/day | 4 | 92 | 63 | 129 |
DISCUSSION
Our unique conjugates are designed to combine the bone trafficking property of a bisphosphonate with phosphate ester hydrolysis to release a chemotherapeutic payload in the bone compartment. In this way, after local hydrolysis within the skeleton, the bisphosphonate will inhibit osteoclasts while the chemotherapeutic agent reaches high enough local concentrations to effectively kill tumor cells. Thus, by targeting the two primary cell types responsible for the vicious cycle of bone metastases [29,30], we hope to eliminate cancer cells in bone while maintaining bone structure. A key component for the design is the stability of the phospho-ester bond that must be maintained until the compound localizes to bone such that both compounds reach pharmacologically useful concentrations at the bone tumor site.
Our kinetic data support the idea that bisphosphonates can be used successfully as bone-seeking vehicles for cytotoxic drugs [31,32] and suggest that our conjugation design appears to be hydrolyzed on an effective time scale. It was previously shown that phospho-ester bond hydrolysis of MBC-11 in mouse sera had a t1/2 of ~33 hours [9] while the peak concentration in the skeleton was reached within 30 minutes after i.v. administration. These observations are consistent with the pharmacokinetics and tissue distribution properties of several bisphosphonates [33,34] and indicate that MBC-11 can be delivered to the bone mainly intact, where both compounds (AraC and etidronate) are presumably released [35]. Furthermore, our unpublished observations demonstrated that MBC-11 accumulated in rat bone at concentrations more than two-fold greater than free AraC. Since the radiolabel was present on the C2 pyrimidine ring position of the nucleoside, these observations are consistent with rapid binding of MBC-11 to bone followed by release of AraC on the necessary timescale desired for improved drug delivery to the bone target.
Bisphosphonates have been utilized as effective bone-specific drug delivery systems[32], in which radiochemical conjugates have shown potential clinical utility as treatment modalities for painful bone metastases [36]. In addition, several groups previously synthesized and tested various bisphosphonate-anti-neoplastic drug conjugates with the cytotoxic agents, methotrexate, melphalan, doxorubicin, or cis-platinum covalently attached via an amide bond using the terminal amino group of pamidronate [37–40]. The in vivo activity of these compounds was modest and no data were presented on stability or accumulation kinetics in bone. The covalent bond between the components of these conjugates was perhaps too chemically and/or enzymatically stable to provide the desired concentrations of a cytotoxic compound in bone. Most likely, intact, negatively charged conjugates were unable to effectively inhibit their respective targets to show significant anti-resorptive and/or antitumor activity. The current results suggest that our approach, using the unique chemical bridge of which the hydrolytic stability can be altered through chemical modifications may be the first suitable method for specific delivery of anti-neoplastic agents to bone tumor sites.
MBC-11 was very well tolerated by both immunocompetent and immunodeficient mice. We observed that mice treated daily for 49 days with up to 500 µg/day (~20 mg/kg) of MBC-11 did not show weight loss or elevated BUN and creatinine levels. In addition, hemoglobin, white blood cell count, platelet count, reticulocyte count did not change in dogs given five daily i.v. doses of up to75 mg/kg/day of MBC-11 (unpublished data).
MBC-11 was effective at decreasing bone tumor burden and increasing bone volume in mice with breast cancer-induced bone disease. No significant differences were observed between the high and low doses, suggesting the concentrations of MBC-11 used in this study may be beyond the linear range of its dose-response curve. The lack of a dose response could be due to the low sample size in some groups and the relatively high inter-animal variability of luciferase content for select treatment groups. We also observed that MBC-11 had no inhibitory effect on lung metastasis formation (unpublished data), consistent with the compound’s design to release drug in the bone compartment and specifically target the skeletal tumor burden.
Our in vivo and in vitro results suggest that zoledronate and etidronate at high levels (mM-µM in our study) have associated anti-tumor activity and support previous findings that these high concentrations are necessary for their cytotoxic activity [13–15]. Similar to previous investigators [41,42], we observed that high levels of zoledronate (>10 µM) inhibited the growth of different types of multiple myeloma cells in vitro. We observed that much lower MBC-11 levels (10−8M) dramatically inhibited multiple myeloma cell proliferation indicating that MBC-11 was ~100–1000 times more potent than zoledronate or etidronate at inhibiting multiple myeloma cell proliferation. These results are consistent with published results that demonstrated MBC-11 was 100 times more potent than zoledronate at inhibiting breast cancer cell growth [18].
In contrast to previous findings that demonstrated positive effects of both i.v. and s.c. administration of zoledronate on bone and lung metastases as well as survival [11,23,43], we did not observe any significant benefit of zoledronate on bone and lung metastases or survival in vivo (unpublished data). Other investigations demonstrated that intravenous zoledronate decreased bone and lung metastases at day 22 and modestly but significantly prolonged survival by ~5 days in 4T1-inoculated mice [23]. Clinical doses of zoledronate inhibited skeletal tumor growth in the B02/GFP.2 mouse model [11], but a high dose of zoledronate (5 mg/kg, 1×/week) did not affect tumor progression into extramedullary spaces [44]. Zoledronate also prolonged survival by 12 days in the 5T2MM myeloma model [43]. The lack of a significant benefit by zoledronate on survival in our studies is likely due to differences in the cumulative dose, routes of administration, and types of animal models used in each study.
Moreover, we observed that MBC-11 improved BMD and prolonged survival of mice injected with multiple myeloma cells. Although we did not observe any effect on survival of mice with breast cancer-induced TIBD, we did observe that MBC-11 prolonged survival of vehicle-treated mice with multiple myeloma-induced TIBD by ~18 days. This is most likely due to the differences in the disease progression timeline of the two models. The breast cancer model has a much more aggressive disease course with a shorter survival time compared to the multiple myeloma model. Our data support the idea that adjuvant MBC-11 therapy may improve the quality of life and survival in patients with TIBD. In clinical studies, the impact of bisphosphonates on metastatic tumor burden and survival is controversial [45–48]. A recent updated analysis of a trial of clodronate as adjuvant therapy showed this agent to significantly reduce bone metastasis and was associated with improved survival [47]. These findings are different from those of Saarto and coworkers, which showed a potential increase in visceral metastases [46]. Recent preliminary data demonstrated that adjuvant zoledronate when added to endocrine therapy, significantly reduced the risk of breast cancer recurrance or death by 36% beyond clinical benefits achieved with hormone therapy alone [49,50]. Adjuvant bisphosphonates are now emerging strategies in bone health management for early-stage cancer patients [3,10].
The mechanism of action of aminobisphosphonates on tumor cells is not well understood and so our current work with MBC-11 is not able to discern if the therapeutic effect was due to direct inactivation of osteoclasts, tumor cells or both. In the multiple myeloma model, MBC-11 reversed the BMD loss suggesting direct effects on the osteoclasts. Furthermore, the survival of these mice treated with MBC-11 was significantly improved compared to untreated mice and appeared (but not statistically significant) to be improved beyond that of zoledronate-treated mice. If zolendronate is effective solely through osteoclast inhibition, then this may suggest that MBC-11 is additionally targeting tumor cells. Future studies will address the effects of MBC-11 on the different target cell populations.
Regarding the conjugate design and the relationship between effective dose levels of MBC-11 and its parental moities, it is worth noting that: 1) AraC is not used for the treatment of solid tumors, such as breast cancer; 2) the clinically used dose levels of AraC for haematologic malignancies is 100 mg/m2/day or ~ 2.63 mg/kg/day, over 1000-fold greater than effective levels of MBC-11 in our experiments; 3) zoledronate alone demonstrated delayed onset of skeletal complications with no effect on survival in clinical trials; and 4) zoledronate’s antiresorptive activity is 10,000-fold more potent than etidronate, the bisphosphonate in MBC-11. Given this context with MBC-11 demonstrating improvement in AraC trafficking to bone, and high tolerability, we plan to examine higher dose levels and i.v. administration (as used with clinical AraC and zoledronate infusion) in anticipation of observing greater effects on existing bone lesions and/or the prevention of the onset of bone metastasis. In addition, we are examining the feasibility of conjugating the newer generation, more potent aminobisphosphonates (e.g., zoledronate, pamidronate) with anti-neoplastics to produce conjugates with enhanced anti-resorptive and potentially cytotoxic properties for improved treatment of TIBD.
Overall, successful development of our unique drug-design concept would result in production of new cytotoxic-bisphosphonates with low systemic toxicity and increased local efficacy to treat patients with TIBD. As such, these conjugates have the potential to improve the control of disease-related symptoms, minimize treatment-related toxicity, and limit the intrusion of therapy on activities of daily life. This would result in improvement in the patients’ quality of life and perhaps prolong patient survival, two of the main considerations important in selecting a treatment program [51] . In summary, our results demonstrating that MBC-11 can be delivered to bone, is well tolerated, exhibits anti-tumor activity, and may prolong survival suggest that MBC-11 is a promising therapy for TIBD.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by United States National Institutes of Health grants, 1R41CA097577 and 1R41CA105583, MBC Pharma research grants RRF31J and 3X1127, and MBC Pharma Inc.. We thank Drs. Nelly Paduykova, Sergei Mikhailov (Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, Moscow, Russia), and Dr. Andrei Guzaev (AM Chemicals, Oceanside CA) for the synthesis of the conjugates. We thank Mr. Darren Riehle for making digital images of the bone histology and Dr. Kara Lukasiewicz for critical review of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Dedicated to the memories of Professor Marat Karpeisky and Dr. Hal Dixon, co-inventors of MBC conjugates and founders of MBC Pharma, Inc.
Conflicts of Interest: Dr. Monica Reinholz received research funding for supplies and animals from MBC Pharma to supplement a portion of the in vivo efficacy studies that was not supported by the NIH grants. Dr. Reinholz, her spouse, or any of her dependents have no personal financial interest (such as company stocks or shares or equity) in MBC Pharma. Drs. Zinnen, Sebesta, and Karpeisky were employees of MBC Pharma at the time the work was performed. All other co-authors have no personal financial interest in MBC Pharma or conflicts of interest.
Contributor Information
Monica M. Reinholz, Email: reinholz.monica@mayo.edu.
Shawn P. Zinnen, Email: szinnen@mbcpharma.com.
Amylou C. Dueck, Email: dueck.amylou@mayo.edu.
David Dingli, Email: dingli.david@mayo.edu.
Gregory G. Reinholz, Email: reinholz.gregory@mayo.edu.
Leslie A. Jonart, Email: lesliejonart@yahoo.com.
Kathleen A. Kitzmann, Email: kitzmann.kathleen@mayo.edu.
Amy K. Bruzek, Email: amybruzek@gmail.com.
Vivian Negron, Email: negron.vivian@mayo.edu.
Abdalla K. Abdalla, Email: abdalla.sen@gmail.com.
Bonnie K. Arendt, Email: arendt.bonnie@mayo.edu.
Anthony J. Croatt, Email: croatt.anthony@mayo.edu.
Luis Sanchez-Perez, Email: luis.sanchez-perez@duke.edu.
David P. Sebesta, Email: David.sebesta@gmail.com.
Harri Lönnberg, Email: Harlon@utu.fi.
Toshiyuki Yoneda, Email: yoneda@uthscsa.edu.
Karl A. Nath, Email: nath.karl@mayo.edu.
Diane F. Jelinek, Email: jelinek.diane@mayo.edu.
Stephen J. Russell, Email: russell.stephen@mayo.edu.
James N. Ingle, Email: ingle.james@mayo.edu.
Thomas C. Spelsberg, Email: spelsberg.thomas@mayo.edu.
Alexander Karpeisky, Email: akarpeisky@mbcpharma.com.
Wilma L. Lingle, Email: lingle.wilma@mayo.edu.
REFERENCES
- 1.Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 Pt 2):6243s–6249s. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- 2.Lipton A, Berenson JR, Body JJ, Boyce BF, Bruland OS, Carducci MA, Cleeland CS, Clohisy DR, Coleman RE, Cook RJ, Guise TA, Pearse RN, Powles TJ, Rogers MJ, Roodman GD, Smith MR, Suva LJ, Vessella RL, Weilbaecher KN, King L. Advances in treating metastatic bone cancer: summary statement for the First Cambridge Conference. Clin Cancer Res. 2006;12(20 Pt 2):6209s–6212s. doi: 10.1158/1078-0432.CCR-06-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aapro M, Abrahamsson PA, Body JJ, Coleman RE, Colomer R, Costa L, Crino L, Dirix L, Gnant M, Gralow J, Hadji P, Hortobagyi GN, Jonat W, Lipton A, Monnier A, Paterson AH, Rizzoli R, Saad F, Thurlimann B. Guidance on the use of bisphosphonates in solid tumours: recommendations of an international expert panel. Ann Oncol. 2008;19(3):420–432. doi: 10.1093/annonc/mdm442. [DOI] [PubMed] [Google Scholar]
- 4.Body J-J. Bisphosphonates for malignancy-related bone disease: current status, future developments. Supportive Care in Cancer. 2006;V14(5):408–418. doi: 10.1007/s00520-005-0913-5. [DOI] [PubMed] [Google Scholar]
- 5.Costa L. Bisphosphonates: reducing the risk of skeletal complications from bone metastasis. Breast. 2007;16 Suppl 3:S16–S20. doi: 10.1016/j.breast.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Hortobagyi GN. Unmet needs in metastatic bone disease and its complications: is progress possible? Semin Oncol. 2001;28(2) Suppl 6:1–3. [PubMed] [Google Scholar]
- 7.Karpeisky MY, Padyukova NS, Mikhailov SN, Dixon HBF, Tzeitline G. Bisphosphonate conjugates and methods of making and using the same. 6,214,812 B1. Boulder, CO, US: MBC Research, Inc; United States patent US. 2001
- 8.Karpeisky MY, Padyukova NS, Mikhailov SN, Dixon HBF, Tzeitline G. Bisphosphonate conjugates and methods of making and using the same. 6,750,340 B2. Boulder, CO, US: MBC Research, Inc; United States patent US. 2004
- 9.Ora M, Lonnberg T, Florea-Wang D, Zinnen S, Karpeisky A, Lonnberg H. Bisphosphonate derivatives of nucleoside antimetabolites: hydrolytic stability and hydroxyapatite adsorption of 5'-beta,gamma-methylene and 5'-beta,gamma-(1-hydroxyethylidene) triphosphates of 5-fluorouridine and ara-cytidine. J Org Chem. 2008;73(11):4123–4130. doi: 10.1021/jo800317e. [DOI] [PubMed] [Google Scholar]
- 10.Coleman RE. Emerging strategies in bone health management for the adjuvant patient. Semin Oncol. 2007;34(6) Suppl 4:S11–S16. doi: 10.1053/j.seminoncol.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Daubine F, Le Gall C, Gasser J, Green J, Clezardin P. Antitumor effects of clinical dosing regimens of bisphosphonates in experimental breast cancer bone metastasis. J Natl Cancer Inst. 2007;99(4):322–330. doi: 10.1093/jnci/djk054. [DOI] [PubMed] [Google Scholar]
- 12.Zheng Y, Zhou H, Brennan K, Blair JM, Modzelewski JR, Seibel MJ, Dunstan CR. Inhibition of bone resorption, rather than direct cytotoxicity, mediates the anti-tumour actions of ibandronate and osteoprotegerin in a murine model of breast cancer bone metastasis. Bone. 2007;40(2):471–478. doi: 10.1016/j.bone.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Clezardin P, Ebetino FH, Fournier PG. Bisphosphonates and cancer-induced bone disease: beyond their antiresorptive activity. Cancer Res. 2005;65(12):4971–4974. doi: 10.1158/0008-5472.CAN-05-0264. [DOI] [PubMed] [Google Scholar]
- 14.Green JR. Bisphosphonates: preclinical review. Oncologist. 2004;9 Suppl 4:3–13. doi: 10.1634/theoncologist.9-90004-3. [DOI] [PubMed] [Google Scholar]
- 15.Senaratne SG, Colston KW. Direct effects of bisphosphonates on breast cancer cells. Breast Cancer Res. 2002;4(1):18–23. doi: 10.1186/bcr412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Senaratne SG, Pirianov G, Mansi JL, Arnett TR, Colston KW. Bisphosphonates induce apoptosis in human breast cancer cell lines. Br J Cancer. 2000;82(8):1459–1468. doi: 10.1054/bjoc.1999.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clezardin P, Fournier P, Boissier S, Peyruchaud O. In vitro and in vivo antitumor effects of bisphosphonates. Curr Med Chem. 2003;10(2):173–180. doi: 10.2174/0929867033368529. [DOI] [PubMed] [Google Scholar]
- 18.Reinholz GG, Getz B, Sanders ES, Karpeisky MY, Padyukova N, Mikhailov SN, Ingle JN, Spelsberg TC. Distinct mechanisms of bisphosphonate action between osteoblasts and breast cancer cells: identity of a potent new bisphosphonate analogue. Breast Cancer Res Treat. 2002;71(3):257–268. doi: 10.1023/a:1014418017382. [DOI] [PubMed] [Google Scholar]
- 19.Clezardin P. The antitumor potential of bisphosphonates. Semin Oncol. 2002;29(6) Suppl 21:33–42. doi: 10.1053/sonc.2002.37420. [DOI] [PubMed] [Google Scholar]
- 20.Green JR, Muller K, Jaeggi KA. Preclinical pharmacology of CGP 42'446, a new, potent, heterocyclic bisphosphonate compound. J Bone Miner Res. 1994;9(5):745–751. doi: 10.1002/jbmr.5650090521. [DOI] [PubMed] [Google Scholar]
- 21.Pittock ST, Norby SM, Grande JP, Croatt AJ, Bren GD, Badley AD, Caplice NM, Griffin MD, Nath KA. MCP-1 is up-regulated in unstressed and stressed HO-1 knockout mice: Pathophysiologic correlates. Kidney Int. 2005;68(2):611–622. doi: 10.1111/j.1523-1755.2005.00439.x. [DOI] [PubMed] [Google Scholar]
- 22.Lelekakis M, Moseley JM, Martin TJ, Hards D, Williams E, Ho P, Lowen D, Javni J, Miller FR, Slavin J, Anderson RL. A novel orthotopic model of breast cancer metastasis to bone. Clin Exp Metastasis. 1999;17(2):163–170. doi: 10.1023/a:1006689719505. [DOI] [PubMed] [Google Scholar]
- 23.Hiraga T, Williams PJ, Ueda A, Tamura D, Yoneda T. Zoledronic acid inhibits visceral metastases in the 4T1/luc mouse breast cancer model. Clin Cancer Res. 2004;10(13):4559–4567. doi: 10.1158/1078-0432.CCR-03-0325. [DOI] [PubMed] [Google Scholar]
- 24.Mundy G. Preclinical models of bone metastases. Semin Oncol. 2001;28(4) Suppl 11:2–8. doi: 10.1016/s0093-7754(01)90225-8. [DOI] [PubMed] [Google Scholar]
- 25.Yoneda T, Michigami T, Yi B, Williams PJ, Niewolna M, Hiraga T. Actions of bisphosphonate on bone metastasis in animal models of breast carcinoma. Cancer. 2000;88(12 Suppl):2979–2988. doi: 10.1002/1097-0142(20000615)88:12+<2979::aid-cncr13>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 26.Westendorf JJ, Ahmann GJ, Greipp PR, Witzig TE, Lust JA, Jelinek DF. Establishment and characterization of three myeloma cell lines that demonstrate variable cytokine responses and abilities to produce autocrine interleukin-6. Leukemia. 1996;10(5):866–876. [PubMed] [Google Scholar]
- 27.Jelinek DF, Witzig TE, Arendt BK. A role for insulin-like growth factor in the regulation of IL-6-responsive human myeloma cell line growth. J Immunol. 1997;159(1):487–496. [PubMed] [Google Scholar]
- 28.Abe M, Hiura K, Wilde J, Moriyama K, Hashimoto T, Ozaki S, Wakatsuki S, Kosaka M, Kido S, Inoue D. Matsumoto T. Role for macrophage inflammatory protein (MIP)-1alpha and MIP-1beta in the development of osteolytic lesions in multiple myeloma. Blood. 2002;100(6):2195–2202. [PubMed] [Google Scholar]
- 29.Brown SA, Clines GA, Guise TA. Local effects of malignancy on bone. Curr Opin Endocrinol Diabetes Obes. 2007;14(6):436–441. doi: 10.1097/MED.0b013e3282f15419. [DOI] [PubMed] [Google Scholar]
- 30.Yoneda T, Hiraga T. Crosstalk between cancer cells and bone microenvironment in bone metastasis. Biochem Biophys Res Commun. 2005;328(3):679–687. doi: 10.1016/j.bbrc.2004.11.070. [DOI] [PubMed] [Google Scholar]
- 31.Uludag H. Bisphosphonates as a foundation of drug delivery to bone. Curr Pharm Des. 2002;8(21):1929–1944. doi: 10.2174/1381612023393585. [DOI] [PubMed] [Google Scholar]
- 32.Zhang S, Gangal G, Uludag H. 'Magic bullets' for bone diseases: progress in rational design of bone-seeking medicinal agents. Chem Soc Rev. 2007;36(3):507–531. doi: 10.1039/b512310k. [DOI] [PubMed] [Google Scholar]
- 33.Fleisch H. Bisphosphonates in Bone Disease. 4 ed ed. San Diego, CA: Academic Press; 2000. [Google Scholar]
- 34.Ezra A, Golomb G. Administration routes and delivery systems of bisphosphonates for the treatment of bone resorption. Adv Drug Deliv Rev. 2000;42(3):175–195. doi: 10.1016/s0169-409x(00)00061-2. [DOI] [PubMed] [Google Scholar]
- 35.Stepensky D, Kleinberg L, Hoffman A. Bone as an effect compartment: models for uptake and release of drugs. Clin Pharmacokinet. 2003;42(10):863–881. doi: 10.2165/00003088-200342100-00001. [DOI] [PubMed] [Google Scholar]
- 36.Hirabayashi H, Fujisaki J. Bone-specific drug delivery systems: approaches via chemical modification of bone-seeking agents. Clin Pharmacokinet. 2003;42(15):1319–1330. doi: 10.2165/00003088-200342150-00002. [DOI] [PubMed] [Google Scholar]
- 37.Fabulet O, Sturtz GA. Synthesis of gem-bisphosphonic doxorubicinconjugates. Phosphorus, Sulfer Silicon Relat. Elem. 1995;101(1–4):225–234. [Google Scholar]
- 38.Hosain F, Spencer RP, Couthon HM, Sturtz GL. Targeted delivery of antineoplastic agent to bone: biodistribution studies of technetium-99m-labeled gem-bisphosphonate conjugate of methotrexate. J Nucl Med. 1996;37(1):105–107. [PubMed] [Google Scholar]
- 39.Sturtz GL, Couthon HM, Fabulet O, Mian MA, Rosini S. Synthesis of gem-bisphosphonic methotrexate conjugates and their biological response towards Walker's osteosarcoma. Eur J Med Chem. 1993;28(11):899–903. [Google Scholar]
- 40.Wingen F, Sterz H, Blum H, Moller H, Pittermann W, Pool BL, Sinn HJ, Spring H, Schmahl D. Synthesis, antitumor activity, distribution and toxicity of 4-[4-[bis(2-chloroethyl)amino]phenyl]-1-hydroxybutane-1 1-bisphosphonic acid (BAD), a new lost derivative with increased accumulation in rat osteosarcoma. J Cancer Res Clin Oncol. 1986;111(3):209–219. doi: 10.1007/BF00389236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shipman CM, Rogers MJ, Apperley JF, Graham R, Russell G, Croucher PI. Anti-tumour activity of bisphosphonates in human myeloma cells. Leuk Lymphoma. 1998;32(1–2):129–138. doi: 10.3109/10428199809059253. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi R, Shimazaki C, Inaba T, Okano A, Hatsuse M, Okamoto A, Hirai H, Ashihara E, Nakagawa M. A newly developed bisphosphonate, YM529, is a potent apoptosis inducer of human myeloma cells. Leuk Res. 2001;25(1):77–83. doi: 10.1016/s0145-2126(00)00087-4. [DOI] [PubMed] [Google Scholar]
- 43.Croucher PI, De Raeve H, Perry M, Hijzen A, Shipman CM, J L, J G, Van Marck E, Van Camp B, Vanderkerken K. Zoledronic acid treatment of 5T2MM-bearing mice inhibits the development of myeloma bone disease: evidence for decreased osteolysis, tumor burden and angiogenesis, and increased survival. J Bone Miner Res. 2003;18(3):0482–0492. doi: 10.1359/jbmr.2003.18.3.482. [DOI] [PubMed] [Google Scholar]
- 44.Buijs JT, Que I, Lowik CW, Papapoulos SE, van der Pluijm G. Inhibition of bone resorption and growth of breast cancer in the bone microenvironment. Bone. 2008 doi: 10.1016/j.bone.2008.10.047. [DOI] [PubMed] [Google Scholar]
- 45.Hillner BE, Ingle JN, Chlebowski RT, Gralow J, Yee GC, Janjan NA, Cauley JA, Blumenstein BA, Albain KS, Lipton A, Brown S. American Society of Clinical Oncology 2003 update on the role of bisphosphonates and bone health issues in women with breast cancer. J Clin Oncol. 2003;21(21):4042–4057. doi: 10.1200/JCO.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 46.Saarto T, Vehmanen L, Virkkunen P, Blomqvist C. Ten-year follow-up of a randomized controlled trial of adjuvant clodronate treatment in node-positive breast cancer patients. Acta Oncol. 2004;43(7):650–656. doi: 10.1080/02841860410032885. [DOI] [PubMed] [Google Scholar]
- 47.Powles T, Paterson A, McCloskey E, Schein P, Scheffler B, Tidy A, Ashley S, Smith I, Ottestad L, Kanis J. Reduction in bone relapse and improved survival with oral clodronate for adjuvant treatment of operable breast cancer. Breast Cancer Res. 2006;8(2):R13. doi: 10.1186/bcr1384. [ISRCTN83688026]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petrut B, Trinkaus M, Simmons C, Clemons M. A primer of bone metastases management in breast cancer patients. Curr Oncol. 2008;15 Supplement 1:S50–S57. doi: 10.3747/co.2008.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gnant M, Mlineritsch B, Luschin-Ebengreuth G, Kainberger F, Kassmann H, Piswanger-Solkner JC, Seifert M, Ploner F, Menzel C, Dubsky P, Fitzal F, Bjelic-Radisic V, Steger G, Greil R, Marth C, Kubista E, Samonigg H, Wohlmuth P, Mittlbock M, Jakesz R. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 5-year follow-up of the ABCSG-12 bone-mineral density substudy. Lancet Oncol. 2008;9(9):840–849. doi: 10.1016/S1470-2045(08)70204-3. [DOI] [PubMed] [Google Scholar]
- 50.Lyseng-Williamson KA. Zoledronic Acid: a review of its use in breast cancer. Drugs. 2008;68(18):2661–2682. doi: 10.2165/0003495-200868180-00010. [DOI] [PubMed] [Google Scholar]
- 51.Ali SM, Harvey HA, Lipton A. Metastatic breast cancer: overview of treatment. Clin Orthop Relat Res. 2003;(415 Suppl):S132–S137. doi: 10.1097/01.blo.0000092981.12414.7b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.