Abstract
Severe injury and infection are associated with autonomic dysfunction. The realization that a dysregulation in autonomic function may predispose a host to excessive inflammatory processes has renewed interest in understanding the role of central nervous system (CNS) in modulating systemic inflammatory processes. Assessment of heart rate variability (HRV) has been used to evaluate systemic abnormalities and as a predictor of the severity of illness. Dissecting the relevance of neuroimmunomodulation in controlling inflammatory processes requires an understanding of the multiscale interplay between CNS and the immune response. A vital enabler in that respect is the development of a systems-based approach that integrates data across multiple scales, and models the emerging host response as the outcome of interactions of critical modules. Thus, a multiscale model of human endotoxemia, as a prototype model of systemic inflammation in humans, is proposed that integrates processes across the host from the cellular to the systemic host response level. At the cellular level interacting components are associated with elementary signaling pathways that propagate extracellular signals to the transcriptional response level. Further, essential modules associated with the neuroendocrine immune crosstalk are considered. Finally, at the systemic level, phenotypic expressions such as HRV are incorporated to assess systemic decomplexification indicative of the severity of the host response. Thus, the proposed work intends to associate acquired endocrine dysfunction with diminished HRV as a critical enabler for clarifying how cellular inflammatory processes and neural-based pathways mediate the links between patterns of autonomic control (HRV) and clinical outcomes.
Keywords: endotoxin, inflammation, modeling, heart rate variability
inflammation is a complex, multiscale physiological response of an organism to biological stressors that is required for immune surveillance and regeneration after injury (16). The major steps in an inflammatory cascade are initiation of the reaction, progression, and termination followed by resolution of inflammation. Under normal circumstances, the end point of inflammation is a favorable outcome. However, when anti-inflammatory processes fail, inflammation becomes prolonged and can lead to uncontrolled systemic inflammation, which, in turn, can eventuate in various disease conditions or aggravate an already existing disease process (43). It is, therefore, a dysregulation of the resolution of inflammation that, in many cases, causes detrimental effects for the host.
Physiological mechanisms regulating the inflammatory response involve not only the local release of anti-inflammatory cytokines but also hormonal influences (25). Recent studies indicate that the central nervous system (CNS) is a pivotal regulator of the immune response (12). A primary stress response pathway by which the CNS regulates the immune system is the hypothalamic-pituitary adrenal axis (HPA), through the production of glucocorticoids and other immunomodulatory signals. Furthermore, the autonomic nervous system communicates with the immune system through the release of neurotransmitters from sympathetic (SNS) and parasympathetic nerves (PNS) that monitor and regulate inflammation (70). These functions are integrated through a network of complex interactions between the immune, neuroendocrine, and autonomic systems. The integrity of this circuitry is essential for maintaining physiological homeostasis and therefore disruption of these functions may have untoward effects (65).
A characteristic feature of deterioration in the physiological status of the host is the evolved state of diminished signal variability among organ systems including the innate immune responsiveness and CNS (48). Clinical measures of heart rate variability (HRV) are noninvasive assessments that may reflect real-time alterations of physiological status (57). As a potential surrogate marker for systemic decomplexification, diminished HRV has received increasing attention in critical illness (79). It has been hypothesized that a reduction in HRV suggests an increased isolation of the heart from other organs (63). The hypothesis, originally introduced by Godin and Buchman (33), suggests that healthy organs behave like biological oscillators coupled to one another. Thus, reduced HRV reflects systemic-level loss of high level signal variability and is associated with a less “healthy” state not only in patients with cardiovascular diseases but also in other critically ill conditions that involve injury, severe infection, and sepsis (13, 54–57).
Diminished HRV is also induced by the acute systemic inflammatory condition mediated by endotoxin administration, a major component of the gram-negative bacteria outer membrane, to healthy subjects (2). In vivo human endotoxin challenge is an accepted surrogate model for studying the acute inflammatory response as it captures many of the clinically observed features of the initial systemic inflammatory phenotype (15, 28, 47, 69, 81). Endotoxin challenge evokes both significant dynamic transcriptional changes as well as hemodynamic and neuroendocrine responses that have been well described (22, 47).
Although considerable progress has been made in elucidating many of the components of inflammation and their regulation, most hypotheses related to the management and treatment of severe human inflammation have failed rigorous clinical testing (48). Even the improved capacity to acquire quantitative data in a clinical setting has generally failed to improve outcomes in acutely ill patients. It has been argued that this difficulty is related to the challenge of manipulating biocomplex internal regulatory systems when they are as ubiquitous as inflammation (19). There is an increasing recognition that progress in treating these processes requires a greater understanding of the multiscalar organization of biological systems and integrative initiatives are identified as valuable for the effective characterization of a complex system (75). It is believed that a systems-oriented mathematical modeling approach can yield significant insights into how the macroscopic response (phenotype) of a system emerges as the result of propagating information, in the form of disturbances, across an intricate web of interacting modules (3, 4, 32, 76).
In this study, a multiscale model of human endotoxemia, as a prototype model of systemic inflammation in humans, is developed that integrates regulatory processes across the host from the cellular to the systemic level and subsequently models the emerging host response as the outcome of interactions of critical modules. Based on our prior work, a cellular level physicochemical host response model is used as template for connecting extracellular signals and intracellular signaling cascades eventually leading to the emergent transcriptional dynamics (29–31). Driven by the premise that a characteristically enhanced endocrine hormone profile is elicited during the early-phase response to endotoxin injury, essential modules associated with the bidirectional communication between the immune response and the neuroendocrine axis (HPA, SNS) are considered. Accordingly, of particular relevance of this study are human data associated with plasma concentrations of neuroendocrine hormones including cortisol and catecholamines. Furthermore, clinical measurements of HRV are also incorporated to assess systemic abnormalities and decomplexification manifested by diminished physiological variability.
The proposed multilevel human inflammation model is characterized by the dynamic state of 24 coupled ordinary differential equations, and its validity is evaluated through a series of inflammatory relevant scenarios indicative of the complex dynamics of acute inflammatory responses and involve: 1) a self-limited inflammatory response elicited by low-dose endotoxin corresponding to successful inflammatory resolution within 24 h after the administration of the inflammatory stimulus (endotoxin, LPS), 2) an unconstrained inflammatory response that can be elicited under high concentrations of LPS characterized by a proinflammatory cytokine “storm” that contributes to derangements in neuroendocrine and autonomic activity, and finally 3) two scenarios associated with the implications of acute stress hormone infusion (cortisol, catecholamine excess) under conditions of either low or high infectious challenge. Under conditions of low-dose endotoxin, the acute pre-exposure of the host to either cortisol or catecholamines, e.g., 6 or 3 h before the main endotoxin challenge, respectively, modulates cytokine responses but does not change the overall host adaptability as quantified by HRV (2, 40). Furthermore, under conditions of severe injury, which is simulated as high concentration of the inflammatory stimulus (endotoxin), antecedent periods of stress hormone infusion attenuate aberrant proinflammatory responses that mitigate the subsequent amplified inflammatory response. Such attenuation suggests that the rates of the response may be well tuned in response to the anti-inflammatory influence of stress hormone background upon the systemic inflammatory manifestations of acute illnesses. Thus, a fundamental assumption of our model is the existence of two steady states that, depending on the anti-inflammatory “reservoir” of the host, can represent either “recovery/self-limited” or “uncontrolled/sustained inflammation” that might account for the transient clinical phenotype of severely stressed patients. Taken together, the appropriateness of the assumptions invoked in the development of this model is evaluated through its ability to enable such “predictions,” making it a crucial enabler for improving our understanding of how interacting inflammatory responses and neural-based mechanisms influence the ability of the host to regulate inflammation.
It is the goal of this study to demonstrate the feasibility of a relevant human inflammation model that bridges the initiating signal and phenotypic expressions (HRV) through semimechanistic-based host response models that include transcriptional dynamics, signaling cascades, and hormonal components that should not be viewed as distinct functional domains. Such a modeling approach could potentially provide invaluable insights into how disruption within these compartments contributes to morbidity and mortality in severely stressed patients.
MATERIALS AND METHODS
Human Endotoxin Model and Data Collection
Gene expression data used in this study were generated as part of the Inflammation and Host Response to Injury Large Scale Collaborative Project funded by the United States Public Health Service U54 GM-621119 (20). Human subjects were injected intravenously with endotoxin (CC-RE, lot 2) at a dose of 2 ng/kg body weight (endotoxin-treated subjects) or 0.9% sodium chloride (placebo-treated subjects). Following lysis of erythrocytes and isolation of total RNA from leukocyte pellets, (15), biotin-labeled cRNA was hybridized to the Hu133A and Hu133B arrays containing a total of 44,924 probes for measuring the expression level of genes that can be either activated or repressed in response to endotoxin. A set of 5,093 probe sets was characterized by significant variation (corresponding to 0.1% false discovery rate) across the time course of the experiment using the SAM software (67). The data are publicly available through the GEO Omnibus Database (http://www.ncbi.nlm.nih.gov/geo/) under the accession number GSE3284.
In addition to transcriptional profiling analysis, blood samples were also collected and analyzed to determine the plasma concentration of counterregulatory hormones including cortisol and epinephrine (2, 7). Specifically, cortisol levels were measured at 0, 0.5, 1, 1.5, 2, 3, 4, 6, and 24 h in relation to endotoxin administration (2), while the study period for epinephrine levels was 0, 2, 4 and 6 h after endotoxin administration (7). Furthermore, human volunteers were injected with the same amount of LPS while vital signs, including HRV indexes, were recorded (2). There are two basic approaches to quantifying HRV, namely time-domain methods and frequency-domain (spectral) analyses (10).
In time-domain analysis, the heart rate at any time is determined from the time interval between successive respiration peaks in the QRS complexes (resulting from sinus node depolarization) of ECG (RR intervals). From the distribution of RR intervals, statistical measures of variance such as standard deviation of the time interval between consecutive respiration peaks and the root-mean square of the difference between adjacent RR intervals are determined. The root mean square of the successive beat differences is the recommended estimate of short-term variability, while the standard deviation of normal interbeat intervals (SDNN) is for assessing longer-term variability (10). In addition to this, other time-domain measures that quantify the physiological complexity between organ systems include multiscale entropy (MSE) (23). However, SDNN and MSE both quantify the complexity of interactions between organ systems and generate equivalent results when predicting mortality in intensive care unit (58).
Analysis of HRV in the frequency domain requires more complex algorithms but provides additional information. Frequency-domain measurements using Fourier analysis calculate the power of selected frequencies within a given frequency range (e.g., parasympathetic frequency ranges) (79). Thus, spectral methods produce a decomposition of total variation of a data series into its frequency components, which reflect operation of a particular modulatory reflex. For instance, parasympathetic (vagal) function is, oftentimes, assessed using the high-frequency HRV, while low-frequency variability is a measure associated with both sympathetic and parasympathetic activation (38).
Although a wide variety of estimates of HRV have been employed including both global descriptive statistics and spectral methods in this study, the time-domain measure SDNN will be used to assess overall HRV, which will serve as a surrogate for systemic abnormalities. During the analysis of HRV, parameters and interbeat intervals were collected using ECG data at a rate of 256 samples/s where each QRS complex (which corresponds to the depolarization of the ventricles) was detected and the “normal-to-normal” (NN) intervals were tabulated. HRV measurements and plasma cortisol concentrations are employed from Ref. 2, while epinephrine concentrations are also assessed under the systemic inflammatory manifestations of human endotoxemia (7). The data have been appropriately deidentified, and appropriate IRB approval and informed, written consent were obtained from the volunteers.
Cellular Level Physicochemical Model of Systemic Inflammation in Humans
To establish quantifiable relationships among the various components of the endotoxin-induced inflammatory response, we have recently advocated a cellular, semimechanistic modeling approach (29–31) that explores three unique aspects. First, through the analysis of the leukocyte gene expression data we identify the essential responses characterizing the cellular (transcriptional) dynamics. Second, we explore the concepts of physicochemical modeling (1) to express the relations that connect extracellular signals and intracellular signaling cascades leading to the emergent transcriptional dynamics. Finally, we explore the pharmacodynamic concept of indirect response (IDR) (42) to establish implicit interactions among signaling molecules and emerging transcriptional responses. Our inability to precisely model the complex signaling events that characterize the host adaptation process to environmental changes makes IDR modeling appealing (26, 64).
In our endotoxin injury model, the elementary responses present the constitutive elements of the overall response and include a proinflammatory response (P) that consists of the early increased expression of cytokines and chemokines, an anti-inflammatory response (A) that serves as the immunoregulatory arm of the host defense system, and an energetic response (E) that involves the decreased expression of genes that participate in cellular bioenergetic processes. The inflammatory response is activated when endotoxin is recognized by microbial pattern recognition receptors (TLR4) (78) that ultimately trigger signaling modules for the activation of proinflammatory transcription factors. Although a large family of transcription factors is known to be involved in inflammation, we focus on a particular complex, NF-κB, as the archetypical signaling module for initiating and controlling the expression of proinflammatory genes (8, 37). We therefore assume that activation of NF-κB module serves as a proxy signal associated with the binding of LPS to its signaling receptor. Given that anti-inflammatory drugs, such as corticosteroids, play a pivotal role in modulating the progression of inflammation their contribution is further assessed in this model. By incorporating an appropriate pharmacokinetic/pharmacodynamic (PK/PD) model (41), we evaluate the possibility of a corticosteroid regulation of either the inhibitor of NF-κB or the anti-inflammatory component of the response. The mathematical representation of the cellular host response model is succinctly presented in Eqs. 1–6, while details of this model are discussed in the relevant publications (29–31).
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
In particular, Eqs. 1–4 simulate the propagation of LPS signaling on the cellular level, while the PK/PD action of exogenous corticosteroids is described by Eqs. 5 and 6. Note: cortisol is abbreviated F, after Kendall's substance F. However, one of the simplifying hypotheses made in this model is that the immunomodulatory role of hormonal influences, including endogenous cortisol, is not explicitly incorporated. It is now well established that the human response to endotoxin evokes both leukocyte transcriptional alterations and a neuroendocrine response characteristic of acute injury (46). Accordingly, a rise in circulating endocrine hormones is manifested 2–4 h following endotoxin administration (47). Moreover, diminished HRV, as a component of autonomic dysfunction, is induced by low-dose endotoxin to human subjects (2, 40). In the following section we will discuss modeling extensions associated with critical aspects of the neuroendocrine immune cross talk connecting the cellular response level with neural-based pathways where systemic abnormalities are assessed through HRV.
Modeling Neuroendocrine Immune System Interactions
The primary stress response pathway by which the CNS regulates the immune system is the HPA through the production of glucocorticoids (cortisol in primates; corticosterone in most rodents). The HPA, as one of the peripheral limbs of the stress system, responds to the proinflammatory cytokines produced by immune-mediated inflammatory reactions by releasing cortisol that inhibits proinflammatory cytokine expression (18, 77). To mathematically describe the dynamics of cortisol, a joint PK model (50) is employed as shown in the following equation (Eq. 7).
| (7a) |
| (7b) |
| (7c) |
Total plasma cortisol concentrations are defined as the additive effect between endogenous and exogenous cortisol. Upon inflammatory stimulation, the rate of change of total cortisol concentration, F, (Eq. 7a), is described by a zero-order production rate Kin,F stimulated by the proinflammatory response P, through the activation function HF,P, (Eq. 7b) and a first-order elimination rate constant Kout,F. Furthermore, the contribution of exogenous cortisol upon total cortisol concentrations is assessed through the stimulatory parameter Rin,F, which is active based on the binary variable wFex.
Given a quantification of the cortisol dynamics, Eqs. 5 and 6, the influence of cortisol on the host response to endotoxin can be simulated. However, it should be noted that these equations, as outlined in the original analysis (41), describe receptor/gene-mediated corticosteroid effects based on results from an in vivo adrenalectomized model, and therefore the baseline value of plasma cortisol is zero. However, in our model the baseline value of cortisol (F) equals to one (Note that it represents the concentration of cortisol relative to the measured response at t = 0 h), and Eq. 5 is modified as follows:
| (8) |
In the absence of exogenous cortisol (wFex = 0), the active steroid signal, FR(N), is normalized so that numerically it ranges between (0,1). Thus, any increase in the concentration of the active signal FR(N) will be relative to the trajectory that is elicited upon the systemic inflammatory manifestations of human endotoxemia. Regarding the immunosuppressive effects of glucocorticoids, antecedent periods of exogenously induced hypercortisolemia attenuate circulating levels of proinflammatory cytokines through an increase in plasma IL-10 concentrations during human endotoxemia (72). For purposes of this model, it is assumed that cortisol modulates the host response to endotoxin primarily via potentiation of IL-10 signaling (A). Such anti-inflammatory influence is quantified through the linear stimulatory function, HA,FRN, which is discussed below in Eq. 11.
Along the same lines, catecholamines such as epinephrine (EPI), also modulate a range of immune functions (59). Such hormones are secreted by the sympathetic nervous system pathway (SNS) and act via adrenergic receptors on immune cells (71). In addition, there is evidence indicating that the proinflammatory response (P) stimulates central components of the stress system through the afferent vagus nerve (27). We will therefore assume that the proinflammatory response (P) acts as the peripheral immune signal that stimulates not only the secretion of cortisol but also the secretion of EPI. The afferent transit mechanism that describes the propagation of the local proinflammatory signal (P) to the SNS is shown in Eq. 9.
| (9a) |
| (9b) |
| (9c) |
The dynamics of EPI, Eq. 9, are described in the same manner as in Eq. 7, where total EPI concentration is defined as the joint effect between endogenous and exogenous hormone. Upon the systemic inflammatory manifestations of human endotoxemia, the rate of change of total EPI concentration, Eq. 9a, is described by a zero-order production rate Kin,EPI stimulated by the proinflammatory response (P) through the linear function HEPI,P (Eq. 9b) and a first-order degradation rate (Kout,EPI). Furthermore, in the case of exogenous EPI, the stimulatory effect of such perturbation is simulated via the parameter Rin,EPI, which becomes activated in the presence of exogenous EPI controlled by the binary variable wEPIex.
Though proinflammatory cytokines stimulate the secretion of EPI, the latter attenuates the proinflammatory manifestations of human endotoxemia as supported by reduced TNF levels (73). The anti-inflammatory influence of EPI is shown to be mediated by β-adrenergic stimulation, resulting in an increase in cAMP intracellular levels followed by potentiation in the production rate of IL-10 signaling (A) (71, 73). To mathematically describe such postreceptor effect, a precursor-dependent indirect response model (51) is proposed where the precursor reflects the signaling receptor of epinephrine (REPI) as shown in Eq. 10.
| (10a) |
| (10b) |
| (10c) |
| (10d) |
The dynamic changes of REPI depend on an apparent zero-order production rate k0REPI. Furthermore, k1,REPI and k2,REPI represent first-order rate constants for the loss of the receptor (Eq. 10a). Since the response is triggered as a result of the formation of an activating complex associated with the binding of EPI to its receptor, EPIR represents the formed signaling complex that decays with a first-order rate k3,EPI (Eq. 10b). Furthermore, the stimulatory postadrenergic effect of sympathetic activity in favoring the production of cAMP signaling is described by the principles of a signal transduction model as outlined in (49, 68). In particular, the production and loss of the cAMP signaling depends on first-order rate constants that are equivalent to the reciprocal of the transit times (τ) consistent with the transit compartment model, while n is the shaping (scaling) factor (Eq. 10c) (51). Such a scaling factor is used to amplify the signal transduction cascade associated with the postadrenergic effect of EPI on the host. Regarding the immunosuppressive effect of epinephrine, a cAMP-dependent potentiation in IL-10 signaling (A) is quantified in Eq. 11 through the linear stimulatory function HA,cAMP. In addition to this, we previously mentioned that cortisol also increases IL-10 levels. Thus, such steroid-dependent immunomodulatory effect is quantified via the stimulatory function HA,FRN.
| (11) |
In addition to the neuroendocrine response evoked by endotoxin is the evolving concept of autonomic dysfunction as assessed by HRV indexes. Recent studies imply that disordered neuroendocrine functions are also associated with diminished HRV in stressed patients (48). To quantify systemic abnormalities, clinical measurements of HRV will be further incorporated.
Model for the Assessment of Reduced HRV in Human Endotoxemia
Clinical data associated with HRV measurements (47) establish that the host response to endotoxin causes a depression in both cardiac-vagal tone and in overall HRV and are consistent with prior studies (34, 61), indicating diminished physiological variability as a generalized response to human endotoxemia. Several studies have implied the use of HRV as a readily available vital sign (38) in the assessment of critically ill patients with the hope of earlier intervention for those patients deemed at higher risk (54, 55, 57, 80). Thus, the prognostic significance of HRV has made it a critical enabler to detect either physiological deterioration or response to therapy (79).
To quantify the effect of acute endotoxin injury on HRV a critical question that arises involves the relationship between proinflammatory markers and autonomic dysfunction. There is considerable human evidence indicating that systemic low-grade (pro)inflammatory activity is associated with reduced HRV (6, 52, 53). Assuming a linear relationship between proinflammation and HRV would imply that any modulations in the peripheral immune response will subsequently drive changes in hemodynamic parameters. However, it is important to realize that it cannot be taken for granted that factors modifying the magnitude of the immune response should affect all circulatory parameters equally (9).
Such nonlinearity in the sinus node transduction processes may arise from sensitivity of pacemaker discharge to the timing of pulsatile neural activity and from functional inhomogeneity within the sinus node tissue (14). In addition to this, the concept of nonlinearity in cardiovascular variability has been stressed in the hemodynamic parameters of endotoxin-induced systemic inflammation under conditions of prior endocrine stress hormone infusion (2, 40). One of the interesting observations was that the anti-inflammatory influence of endocrine hormones including cortisol and epinephrine does not extend to changes in HRV induced by a relatively low dose of the inflammatory stimulus (LPS). Thus, reduction in endotoxin-induced proinflammation does not influence autonomic dysfunction (HRV), at least in a context of self-limited systemic inflammatory disease that resolves within 12–24 h.
To quantify such nonlinear interactions, we will assume that the effect of peripheral proinflammation upon HRV response to endotoxin can be mathematically approximated by employing appropriate sigmoid activation functions as outlined in Eq. 11. Although the overall HRV is assessed, for instance, by evaluating the SDNN, the physiological background for such variation involves the activation of signal transduction mechanisms in the sinus node of the heart associated with the modulation of neuromediator concentrations (82). Thus, we introduce the signal Sf as a surrogate for the upregulation of such transduction processes in the heart and the relevant dynamics are described in Eq. 11.
| (12a) |
| (12b) |
| (12c) |
| (12d) |
The possible nonlinear modulatory effect of proinflammation (P) upon HRV is described by the dynamics of fP, (Eq. 12a), where the switch-like behavior is determined by the sigmoid function [tanh(P − w)] and w is a parameter greater than the proinflammatory response (P) elicited upon endotoxin-induced inflammation. This nonlinear gain modulatory function should be active under conditions of an inflammatory response and inactive when the system lies in its homeostasis. We therefore model such event based on the function, HP (Eq. 12d), where φ is an M-big number and HP takes values 0, when proinflammation (P) lies in its baseline (homeostasis), and 1, otherwise. The underlying rationale for this function is predicated upon a neurocomputational model (35) that aims at simulating the firing rate of neuronal activity. In our model, fP could therefore reflect the activation of efferent nerve activity on the heart eventually leading to the upregulation of intracellular mediators (Sf). The loss and production of such mediators, Eq. 12b, is thereby described by the principles of time-dependent transduction systems (51) depending on first-order rate constants, which are equivalent to the reciprocal of the transit times (τS), and nS is the shaping (scaling) factor (Eq. 12b). The dynamics of HRV (Eq. 12b) are described by a zero-order production rate (Kin,HRV) and a first-order degradation rate (Kout,HRV), which is stimulated by the effector biological signal (Sf). Taken together, the integrated module that describes critical aspects of the neuroendocrine immune system interactions is presented in Eq. 13.
 |
While the model of Eqs. 1–6 aims to describe the key determinants of the cellular response to endotoxin, the elements that constitute the module in Eq. 13 intend to connect the (cellular) inflammatory response with neural-based pathways (HPA, SNS), while systemic disruptions are assessed by physiological variables such as HRV.
RESULTS AND DISCUSSION
Elements of the Multiscale Host Response Model of Human Inflammation
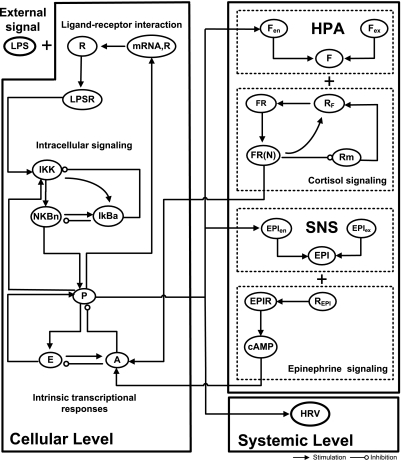
We have previously demonstrated that the transcriptional dynamics of human leukocytes exposed to bacterial endotoxin can be decomposed into to three elementary comprehensive responses (29). These elementary responses capture the functional dynamics and were shown to be related to proinflammatory (P), anti-inflammatory (A), and energetic (E) transcriptional events associated with the overall host response. The response is triggered by the activation of the NF-κB signaling module as a result of the formation of an activating signal associated with the binding of LPS to appropriate receptors (R). To introduce higher level biological information we further incorporate critical aspects of the neuroendocrine immune cross talk. A schematic illustration of the network architecture that constitutes the multilevel host response model is presented in Fig. 1. At the cellular level interacting components are associated with elementary signaling pathways that propagate extracellular signals to the emergent transcriptional response level. Essential modules associated with the release of endocrine stress hormones coupled with their immunosuppressive effects are also considered. Such hormones are integral parts of the bidirectional communication pathway between peripheral inflammation (cellular level) and the neuroendocrine axis (HPA, SNS) and interact with appropriate receptors potentiating the production rate of anti-inflammatory cytokines (A). Finally, clinical measurements, at the systemic level, of HRV are incorporated to assess systemic decomplexification manifested by deterioration in the physiological status of the host.
Fig. 1.
Basic topological interactions composing the multilevel model of endotoxin induced human inflammation. At the cellular level, interacting components involve the propagation of LPS signaling on the transcriptional response level (P, A, E) through the activation of endotoxin signaling receptor (R) and elementary signaling pathways (NF-κB signaling module). At the level of circulating hormones, essential modules are associated with the release of endocrine stress hormones from neuroendocrine axis (HPA, SNS) coupled with their anti-inflammatory influence on the host. The dynamics of cortisol and epinephrine (EPI) signaling involve components interacting at the cellular level. At the systemic level, physiological deterioration of the host is quantified by heart rate variability (HRV).
Estimation of Relevant Model Parameters
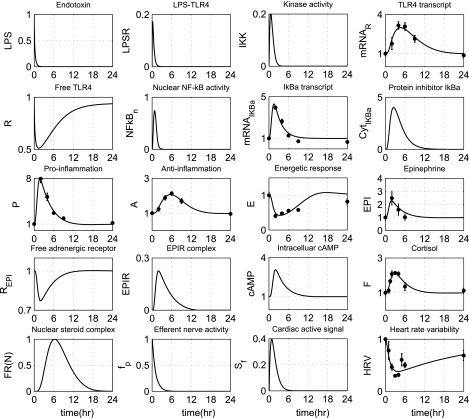
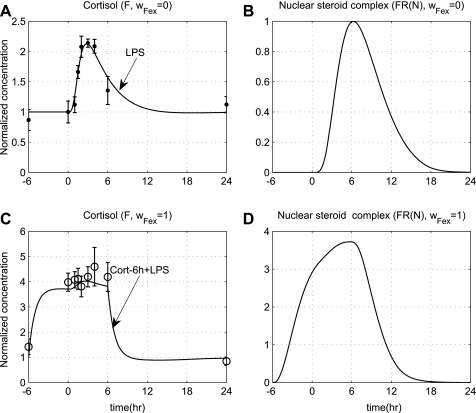
Standard parameter estimation techniques are applied to evaluate appropriate model parameters associated with the neuroendocrine immune system interactions (21). In particular, we estimate those model parameters that are involved in the dynamics of EPI, cortisol (F), anti-inflammation (A), and HRV. The relative experimental data are normalized by taking the ratio of the measured response at each time point of the study period with respect to the control time point (t = 0 h). Furthermore, parameter estimation is performed to estimate the parameter Rin,F under conditions of hydrocortisone infusion (exogenous cortisol), reproducing human plasma cortisol levels in subjects pre-exposed 6 h prior to LPS and continued for another 6 h after the endotoxin injection. All the other parameters associated with the propagation of LPS signaling on the transcriptional response level are maintained to agree with those presented in Ref. 30 and are shown in Table 1, while parameters relevant to the neuroendocrine immune system interactions are estimated and presented in Table 2. The performance of the multilevel human inflammation model is shown in Fig. 2. In our computational model the host restores homeostasis without any external perturbation. A self-limited inflammatory response to the endotoxin stimulus corresponds to resolved dynamic profiles for all the elements that constitute our model. In essence, a self-limited inflammatory response involves the successful elimination of the inflammatory stimulus within the first 2 h postendotoxin administration while followed by a subsequent resolution within 24 h. Although the kinetic parameters associated with the REPI interactions are not calibrated, the dynamic profile of β-adrenergic receptor (REPI) lies in qualitative agreement with the basis of receptor occupancy theory (49) in that the concentration of free adrenergic receptors decreases in the presence of the ligand (EPI). Regarding the gain modulatory effect of peripheral proinflammation in the heart (fP), such an exponential decrease would biologically reflect the decay rate of cardiac neuronal activity (44), which is eventually “translated” to the upregulation of neuromediator concentrations (Sf) in the heart. Furthermore, the reconstruction of plasma cortisol levels under conditions of either prior cortisol infusion or LPS only is shown in Fig. 3. While comparing the top and bottom panel of Fig. 3, both plasma cortisol levels (F) and the steroid active signal, FR(N) are expected to be greater under conditions of exogenously induced hypercortisolemia (bottom, wFex = 1) relative to the baseline cortisol profiles (top, wFex = 0).
Table 1.
Estimated values of the parameters involved in the propagation of LPS signaling on the transcriptional response level
| Parameter | Value | Parameter | Value | Parameter | Value |
|---|---|---|---|---|---|
| kLPS,1 | 4.500 | kNFκB,1 | 16.294 | Kout,A | 0.590 |
| kLPS,2 | 6.790 | kNFκB,2 | 1.1860 | Kin,E | 0.080 |
| ksyn | 0.020 | Kin,IκBa | 0.463 | Kout,E | 0.257 |
| k1 | 3.000 | kIκBa,1 | 13.273 | kmRNAR,P | 1.740 |
| k2 | 0.040 | kI,1 | 1.400 | kP,1 | 29.741 |
| k3 | 5.000 | kI,2 | 0.870 | kP,2 | 9.050 |
| k4 | 2.240 | Kin,P | 0.033 | kA,P | 0.01 |
| kin,mRNA,R | 0.090 | Kout,P | 0.332 | kA,E | 5.300 |
| kout,mRNA,R | 0.250 | Kin,A | 0.090 | kE,P | 2.216 |
Table 2.
Estimated values of parameters involved in the neuroendocrine immune axis
| Parameter | Value | Parameter | Value | Parameter | Value |
|---|---|---|---|---|---|
| Rin,F(wFex = 0) | 0 | k2,REPI | 5.465 | k0REPI | 11.011 |
| Kin,Fen | 0.842 | k3,REPI | 5.546 | Kout,A | 0.809 |
| kFen,P | 0.256 | τ | 0.053 | w | 10 |
| Kout,F | 1.058 | n | 5.509 | τS | 0.723 |
| Rin,F(wFex = 1) | 2.922 | Kin,A | 0.461 | nS | 1.185 |
| Kin,EPI | 5.921 | kA,cAMP | 0.145 | Kin,HRV | 0.038 |
| KEPI,P | 0.231 | kA,E | 0.534 | Kout,HRV | 0.038 |
| k1,REPI | 3.005 | kA,FRN | 0.401 | kHRV,S | 35.254 |
| kR,EPI | 0.845 | Kout,EPI | 7.286 | Kout,EPI | 7.286 |
Fig. 2.
Estimation of relevant model parameters intending to reproduce available experimental data associated with transcriptional signatures (A) and plasma counterregulatory hormones including EPI and cortisol (F) as well as clinical data (HRV). Solid lines (-) correspond to model predictions under conditions of low-dose endotoxin, while ● refers to experimental data expressed as means ± SE. The initial condition of endotoxin [LPS (t = 0 h) = 1] refers to LPS concentration relative to 2 ng/kg body weight.
Fig. 3.
A: plasma cortisol levels (F); B: steroid active signal, FR(N) under conditions of acute endotoxin injury (wFex = 0); C: simulated F; D: steroid active signals FR(N) under conditions of prior steroid infusion (wFex = 1), which is initiated at t = −6 h before LPS and continued for 6 h after LPS. Solid lines correspond to model predictions, while solid markers represent experimental data expressed as means ± SE.
Qualitative Assessment of the Model
Building a mathematical model that can predict relevant biological implications to the host response to endotoxin allows us to identify ways of both controlling and modulating such a complex phenomenon. In the following we will demonstrate the ability of our model to enable such predictions and provide further evidence of the appropriateness of the assumptions invoked in the development of the model. First, we explore the implications of increasing levels of initial insult (LPS), since this would probably constitute the most obvious disturbance. Then we explore possible reversibility in the dynamics of the host in response to an acute endocrine hormone stress infusion (cortisol, EPI excess). Finally, the implications of acute stress hormone infusion upon the systemic inflammatory manifestations of human endotoxemia will be evaluated. We opt therefore to validate the correctness of the proposed model by assessing the implications of anti-inflammatory treatment strategies that are active under conditions of either high or low infectious challenge.1
Implications of increased insult.
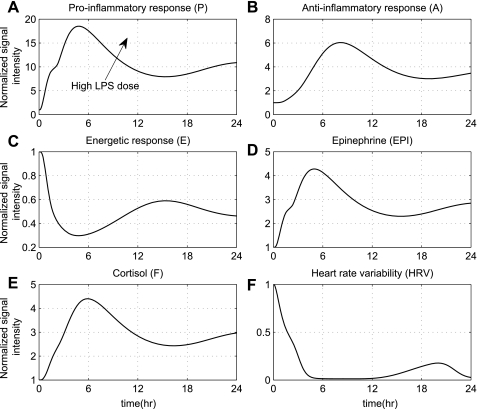
An increase in the dose of the inflammatory stimulus can be responsible for an overwhelming inflammatory response. Such situation in which the initial levels of endotoxin are increased is simulated in Fig. 4. This response can be equated with an exacerbated inflammatory state in the early phase of severe injury, which in our model is simulated as high concentration of the initial stimulus (LPS), e.g., four times greater than the nominal, which corresponds to 8 ng/kg [Note: maximum dose of LPS is administered safely to humans is 4 ng/kg (5)], which deregulates the host defense intrinsic dynamics toward a cytokine “burst”. Such a situation is further characterized by the uncontrolled secretion of endocrine hormones that are not adequate to balance (control) the overall immune response even after the circulating levels of LPS have been cleared. Phenotypically, such physiological deterioration is expressed as diminished HRV that does not return to baseline within 24 h (solid lines) as was seen in Fig. 2, where a lower dose of LPS was simulated compared with experimental data. Such computational results implicate the role of the host rather than the inflammatory stimulus itself, which is eventually cleared from the system, accounting for the progression of an unconstrained inflammatory response. Qualitatively, this amplification of the host immune response (trajectories of inflammatory relevant components do not return to baseline homeostasis) without present infection represents clinically stressed patients without documented infection (62).
Fig. 4.
Simulation of an unresolved inflammatory response due to high endotoxin concentration [LPS (t = 0 h) = 4]. Such high concentration of LPS (4 times greater than the nominal value) deregulates the NF-κB signaling module giving rise to an unconstrained immune response followed by abnormal hormonal responses that macroscopically are translated into diminished physiological variability.
Evaluation of stress hormone infusion in modulating the inflammatory response.
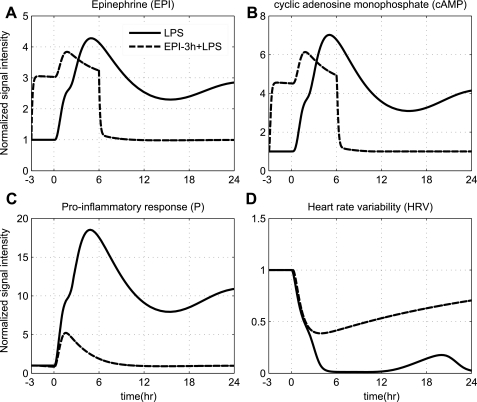
Since we have demonstrated the ability of our model to simulate the trajectory of an unconstrained inflammatory response, the potential of the proposed model is also demonstrated through its capability to respond to systematic perturbations that modulate the dynamics in favor of a balanced immune response coupled with a restoration in autonomic balance. Considerable attention has been given to the effectiveness of pharmacological agents such as ligands of adrenergic receptors in influencing the production rate of both pro- and anti-inflammatory cytokines (36, 39). In particular, significant modulations in the cytokine network was observed in human subjects exposed to EPI infusion (73), underscoring the role of neuroendocrine activity in dampening excessive proinflammatory effects. In particular, we opt to simulate the mode of an intervention strategy that mimics the activity of SNS pathway. Such intervention strategy results in potentiation of the total plasma concentration of EPI, which further increases the intracellular cAMP signaling (dashed lines, Fig. 5). Based on the anti-inflammatory effect of acute EPI infusion via cAMP-dependent mechanism, it is expected an increase in intracellular cAMP levels will attenuate the proinflammatory response (P) followed by a subsequent restoration in autonomic activity (HRV), which serves as a proxy indicator of improved survival (66). Thus, the acute pre-exposure of the host to EPI attenuates the proinflammatory response (P), which allows for recovery of HRV dynamics. Such improvement in autonomic activity underscores the role of epinephrine in improving cardiac index under severe conditions (i.e., low-output septic shock) as supported by Court et al. (24).
Fig. 5.
Simulating the effect of acute EPI infusion (wEPI,ex = 1), which is initiated 3 h prior to the main endotoxin challenge [LPS (t = 0 h) = 4] and continued for 6 h after LPS (Rin,EPI = 15), under conditions of severe inflammation. Dashed and solid lines represent the progression of a balanced (due to system's pre-exposure into EPI infusion) and unconstrained inflammatory response (due to high inflammatory challenge), [LPS (t = 0 h) = 4], respectively. Acute pre-exposure of the host to EPI attenuates the aberrant proinflammatory response (P) induced by high LPS concentration, which allows for recovery in HRV dynamics (restoration in autonomic balance).
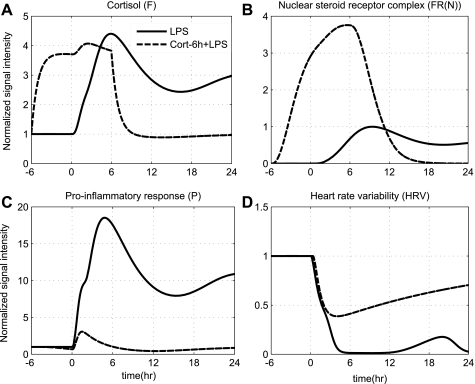
Furthermore, the CNS controls inflammation through the activation of HPA axis by releasing cortisol. Prior studies evaluating human responses within the context of antecedent stress hormone excess have shown that glucocorticoid excess, as produced by hydrocortisone injection (45) or 6 h infusion before LPS challenge, abrogates several features of human endotoxemia (7). In an effort to assess the impact of such hypercortisolemia as a potential endogenous in vivo anti-inflammatory mechanism, hydrocortisone infusion is initiated 6 h before the administration of high LPS concentration (Fig. 6). Note that in our model, the high LPS dose, which is simulated by simply varying the concentration of LPS at time zero, serves as one putative mode of dysregulation in the host defense intrinsic dynamics giving rise to unremitting inflammation. Prior mathematical models of inflammation also simulate severe inflammatory states by varying the initial conditions of the inflammatory insult (17). The acute pre-exposure of the host to cortisol, as represented by dashed lines in Fig. 6, attenuates proinflammatory responses that mitigate the subsequent amplified inflammatory response. Specifically, the initiation of such intervention strategy increases the total concentration of cortisol (F), which subsequently potentiates the active steroid signal [FR(N)]. Such an increase in total cortisol levels potentiates the anti-inflammatory arm of the system (A) immediately after the administration of LPS and thereby attenuates the proinflammatory response (P). Thus, the initiation of such an intervention strategy that indirectly attenuates the proinflammatory signaling (P) via potentiation of the humeral anti-inflammatory signaling (A) suffices to reverse the inflammatory dynamics and eventually restore autonomic balance. Collectively, such in silico predictions as illustrated in Figs. 5 and 6 annotate the impact of dynamic anti-inflammation on the host evoked by stress hormone background upon the systemic inflammatory manifestations of acute illnesses and suggest that the rates of this response may be well tuned to yield optimal outcomes. Thus, a fundamental assumption of our model is the existence of two steady states that, depending on the anti-inflammatory reservoir of the host, can represent either recovery/self-limited or uncontrolled/sustained HRV depression. Qualitatively, such equilibria might account for the transient clinical improvement (e.g., “survivors”) noted to critically ill patients that respond to a treatment. For example, in the study (55) among injured patients there exists a subset of severely stressed patients whose clinical condition improved upon treatment with exogenous steroid. However, we would like to point out that a direct comparison between our model predictions and clinical observations is beyond the scope of the present study. Instead, the overall goal of this study is to develop an in silico model of human endotoxemia that would allow us to evaluate antecedent stresses upon the systemic inflammatory manifestations of acute injury.
Fig. 6.
The effect of low-dose steroid administration initiated 6 h prior to endotoxin challenge (dashed lines) and continued for another 6 h after LPS (wFex = 1) under conditions of high LPS concentration (solid lines). Solid lines simulate the progression of a systemic inflammatory response syndrome (due to high LPS concentration), [LPS (t = 0 h) = 4], while dashed lines reflect the protective effect that can be exerted by hormonal(steroid) replacement therapy. The acute pre-exposure of the host to exogenous cortisol dampens the excessive proinflammatory effects induced by high LPS concentration, which allows for restoration in autonomic balance (HRV).
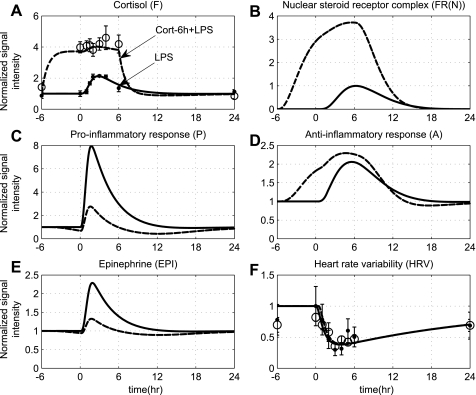
Although the immunosuppressive effects of corticosteroids upon the systemic inflammatory manifestations of human endotoxemia have been well described, the influence of this anti-inflammatory intervention on overall autonomic dysfunction is not well understood. Predicated upon this, the influence of steroid administration on a self-limited endotoxin-induced inflammatory response is simulated in Fig. 7. Although measurements of the transcript abundance of cytokines are not available under such conditions, soluble inflammatory markers (e.g., TNF-a, IL-8, Il-10) were measured and were significantly modulated by prior hydrocortisone treatment. Specifically, antecedent cortisol infusion blunts the proinflammatory cytokine response to LPS administration while enhancing some anti-inflammatory responses as reflected by increased plasma IL-10 concentrations (2, 72). Predicated upon the hypothesis that cytokine protein expression correlates well with gene expression (60), we seek to validate our model qualitatively by simulating an enhanced transcriptional anti-inflammatory response (A) followed by diminished proinflammation (P) under conditions of exogenously induced hypercortisolemia (dashed lines, Fig. 7). In addition to such attenuation in the proinflammatory response, antecedent cortisol infusion also induces hormonal changes and particularly reduction in plasma EPI concentrations. Such a decrease in endogenous EPI secretion under acute hypercortisolemia is simulated in Fig. 7E, thus validating the assumptions invoked in the development of the proposed integrated model. Remarkably, although acute hypercortisolemia significantly attenuated endotoxin-induced production of proinflammatory cytokines, such attenuation in a context of acute systemic inflammatory condition mediated by endotoxin administration (2) does not contribute to any alterations in HRV indexes. From a computational standpoint, such an effect is represented as superimposition of the solid and dashed lines in the HRV component as shown in Fig. 7F.
Fig. 7.
The effect of exogenously induced hypercortisolemia on autonomic dysfunction under the systemic inflammatory manifestations mediated by low-dose endotoxin. Solid lines simulate the progression of a self-limited endotoxin-induced inflammatory response, while dashed lines reflect the antecedent period of exogenously induced hypercortisolemia initiated 6 h prior to LPS administration and continued for 6 h after endotoxin (wFex = 1). Solid markers and ○ refer to experimental data (expressed as means ± SE) under conditions of acute endotoxin injury and prior hydrocortisone infusion, respectively, which do not vary across the 2 experimental conditions (LPS, Cort-6 h + LPS). Such prior cortisol infusion modulates cytokine responses (P, A) and hormonal responses (EPI), but there is no change in overall system adaptability as assessed by HRV (solid and dashed HRV lines overlap).
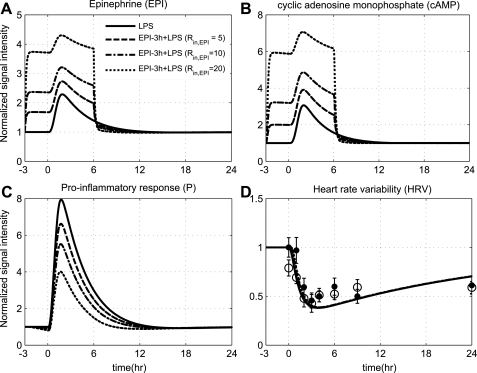
In addition to the influence of low-dose steroid on endotoxin-induced inflammation, recent data document that prior EPI exposure may attenuate the proinflammatory response, but such an anti-inflammatory influence does not extend to changes in overall system's adaptability (HRV) (40). Since increased catecholamine secretion accompanies modest infection and the propensity of a dose-dependent effect of EPI in inhibiting LPS-induced proinflammatory response has been documented in Ref. 74, we sought to simulate whether antecedent EPI infusion would modulate, in a dose-dependent manner, the cytokine responses to endotoxin (Fig. 8). In particular, increasing doses of acute sterile stress conditions modulate the innate immune system activation and particularly attenuate the proinflammatory response through potentiation of the anti-inflammatory effect of cAMP signaling. However, such attenuation in the progression of the proinflammatory response does not contribute to any changes in HRV response as experimentally observed (40) and is represented by the superimposition of predicted HRV dynamics in Fig. 8, which is consistent with the aforementioned results with steroid administration before LPS.
Fig. 8.
Dose-dependent modulation in the progression of the inflammatory reaction due to short-term EPI infusion (wEPI,ex = 1), initiated 3 h before LPS and continued for 6 h after LPS at increasing values of the parameter Rin,EPI = 5, 10, 20. Such intervention potentiates, in a dose-dependent manner (dashed and dotted lines), the secretion of EPI from SNS that through cAMP anti-inflammatory signaling can protect, in part, the host response attenuating the proinflammatory response (P). Such attenuation in the proinflammatory response relative to endotoxin administration (solid lines) does not extend to changes in autonomic balance (HRV) as represented by the superimposition of predicted HRV dynamics (solid and dashed lines overlap). Solid markers and ○ refer to relevant experimental data (expressed as means ± SE) under conditions of low-dose endotoxin administration and prior EPI infusion, respectively. These data have not been used as a training dataset but rather to validate the structure of the proposed model. Descriptive statistics in the original analysis (40) show that there was no significant variation between these experimental measurements (solid markers vs. open circles) from 0 h until 24 h after LPS exposure.
From a modeling standpoint, such responses are captured due to the possible nonlinear interaction between peripheral proinflammation and HRV. In particular, a fundamental assumption of the proposed study is that any reduction in the proinflammatory response relative to the constrained response evoked by low doses of endotoxin will not affect the magnitude of HRV relative to the naïve (LPS) injection. Such an assumption is primarily predicated upon evidence (2, 40) that indicates the existence of reduced differential proinflammatory responses within the context of antecedent stresses without altering endotoxin-induced HRV dynamics. On the other hand, under conditions of high inflammatory challenge, as illustrated in Fig. 4, an unconstrained proinflammatory response will account for a persistent diminished physiological variability (HRV) indicative of the severity of injury.
While the proposed model does not capture the sympathomimetic properties of EPI, we recognize that antecedent EPI infusion significantly reduced the parasympathetic tone, and there was a relative decrease in HRV in the initial hours after EPI administration (40). In future studies, we plan to describe dynamic changes in HRV as a result of cardiac autonomic imbalance, explicitly incorporating the interplay between efferent branches of the autonomic nervous system (sympathetic/parasympathetic outflow) that account for modulations in heart rate response. Such modeling extensions associated with the autonomic control of heart rate would allow us to simulate the vagolytic influence of EPI and therefore explore the possibility of developing more mechanistic-based and physiological relevant in silico disease progression models.
In summary, a multilevel human inflammation model is proposed that couples essential aspects of the complex bidirectional relationship between the neuroendocrine axis and the immune response. We addressed how to construct the topology and the dynamics of the underlying network linking processes across the host from the cellular to the systemic level. Essential modules associated with the secretion of endocrine stress hormones (cortisol, EPI) and their counterregulatory role are particularly taken into account, while phenotypic expressions such as HRV are further incorporated to assess systemic decomplexification. This work bridges the initiating signal and phenotypic expressions through semimechanistic-based host response models that include transcriptional dynamics, signaling cascades, and physiological (hormonal) components. Model parameters are appropriately evaluated to reproduce a self-limited inflammatory response that resolves within 24 h. The potential of the model is evaluated via computational tests performed to assess the implications of neuroendocrine activity across the host. Exploring the possible effects of systemic perturbations enables us to trace the dynamics of a systemic inflammatory response syndrome, improving our understanding of how interacting inflammatory responses and neural-based mechanisms influence the host's ability to regulate inflammation. Since both glucocorticoids and catecholamines are used clinically in the context of systemic inflammation, the proposed modeling has the potential for direct clinical relevance. Thus, such a modeling effort lays the foundation for a translational systems-based model of inflammation that could clarify the clinical contexts in which autonomic dysfunction contributes to morbidity and mortality in severely stressed patients.
GRANTS
The investigators acknowledge the contribution of NIGMS Inflammation and the Host Response to Injury Large-Scale Collaborative Project Award 2-U54-GM-062119. P. T. Foteinou and I. P. Androulakis acknowledge support from NIGMS Grant GM-082974, NSF 0519563, EPA GAD R 832721-010-RRA, and a Busch Biomedical Research Grant. S. E. Calvano and S. F. Lowry are supported, in part, by NIGMS Grant GM-34695.
DISCLAIMER
The Inflammation and the Host Response to Injury “Glue Grant” program is supported by the National Institute of General Medical Sciences (NIGMS). This manuscript was prepared using a dataset obtained from the Glue Grant program and does not necessarily reflect the opinions or views of the Inflammation and the Host Response to Injury Investigators or the NIGMS.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
Supplementary Material
ACKNOWLEDGMENTS
P. T. Foteinou and I. P. Androulakis acknowledge critical input and guidance from Prof. W. J. Jusko and R. R. Almon (SUNY Buffalo).
Footnotes
The online version contains an Appendix as supplemental material.
REFERENCES
- 1.Aldridge BB, Burke JM, Lauffenburger DA, Sorger PK. Physicochemical modelling of cell signalling pathways. Nat Cell Biol 8: 1195–1203, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Alvarez SM, Katsamanis Karavidas M, Coyle SM, Lu SE, Macor M, Oikawa LO, Lehrer PM, Calvano SE, Lowry SF. Low-dose steroid alters in vivo endotoxin-induced systemic inflammation but does not influence autonomic dysfunction. J Endotoxin Res 13: 358–368, 2007. [DOI] [PubMed] [Google Scholar]
- 3.An G. Introduction of an agent-based multi-scale modular architecture for dynamic knowledge representation of acute inflammation. Theor Biol Med Model 5: 11, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.An G, Faeder J, Vodovotz Y. Translational systems biology: introduction of an engineering approach to the pathophysiology of the burn patient. J Burn Care Res 29: 277–285, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andreasen AS, Krabbe KS, Krogh-Madsen R, Taudorf S, Pedersen BK, Moller K. Human endotoxemia as a model of systemic inflammation. Curr Med Chem 15: 1697–1705, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Aronson D, Mittleman MA, Burger AJ. Interleukin-6 levels are inversely correlated with heart rate variability in patients with decompensated heart failure. J Cardiovasc Electrophysiol 12: 294–300, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Barber AE, Coyle SM, Marano MA, Fischer E, Calvano SE, Fong Y, Moldawer LL, Lowry SF. Glucocorticoid therapy alters hormonal and cytokine responses to endotoxin in man. J Immunol 150: 1999–2006, 1993 [PubMed] [Google Scholar]
- 8.Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med 336: 1066–1071, 1997 [DOI] [PubMed] [Google Scholar]
- 9.Bendixen HH, Osgood PF, Hall KV, Laver MB. Dose-dependent differences in catecholamine action on heart and periphery. J Pharmacol Exp Ther 145: 299–306, 1964 [PubMed] [Google Scholar]
- 10.Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, Nagaraja HN, Porges SW, Saul JP, Stone PH, van der Molen MW. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology 34: 623–648, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Berntson GG, Cacioppo JT, Quigley KS. Autonomic determinism: the modes of autonomic control, the doctrine of autonomic space, and the laws of autonomic constraint. Psychol Rev 98: 459–487, 1991 [DOI] [PubMed] [Google Scholar]
- 12.Blalock JE. Harnessing a neural-immune circuit to control inflammation and shock. J Exp Med 195: F25–F28, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405: 458–462, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Brown GL, Eccles JC. The action of a single vagal volley on the rhythm of the heart beat. J Physiol 82: 211–241, 1934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, Chen RO, Brownstein BH, Cobb JP, Tschoeke SK, Miller-Graziano C, Moldawer LL, Mindrinos MN, Davis RW, Tompkins RG, Lowry SF, Inflamm and Host Response to Injury Large Scale Collab Res. Program A network-based analysis of systemic inflammation in humans. Nature 437: 1032–1037, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Cavaillon JM, Annane D. Compartmentalization of the inflammatory response in sepsis and SIRS. J Endotoxin Res 12: 151–170, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Chow CC, Clermont G, Kumar R, Lagoa C, Tawadrous Z, Gallo D, Betten B, Bartels J, Constantine G, Fink MP, Billiar TR, Vodovotz Y. The acute inflammatory response in diverse shock states. Shock 24: 74–84, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med 332: 1351–1362, 1995 [DOI] [PubMed] [Google Scholar]
- 19.Clermont G, Bartels J, Kumar R, Constantine G, Vodovotz Y, Chow C. In silico design of clinical trials: a method coming of age. Crit Care Med 32: 2061–2070, 2004 [DOI] [PubMed] [Google Scholar]
- 20.Cobb JP, Mindrinos MN, Miller-Graziano C, Calvano SE, Baker HV, Xiao W, et al. Inflammation and Host Response to Injury Large-Scale Collaborative Research Program Application of genome-wide expression analysis to human health and disease. Proc Natl Acad Sci USA 102: 4801–4806, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Contreras M, Ryan LM. Fitting nonlinear and constrained generalized estimating equations with optimization software. Biometrics 56: 1268–1271, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Copeland S, Warren HS, Lowry SF, Calvano SE, Remick D. Acute inflammatory response to endotoxin in mice and humans. Clin Diagn Lab Immunol 12: 60–67, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of complex physiologic time series. Phys Rev Lett 89: 068102, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Court O, Kumar A, Parrillo JE, Kumar A. Clinical review: myocardial depression in sepsis and septic shock. Crit Care 6: 500–508, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Czura CJ, Tracey KJ. Autonomic neural regulation of immunity. J Intern Med 257: 156–166, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Dayneka NL, Garg V, Jusko WJ. Comparison of four basic models of indirect pharmacodynamic responses. J Pharmacokinet Biopharm 21: 457–478, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elenkov IJ, Wilder RL, Chrousos GP, Vizi ES. The sympathetic nerve–an integrative interface between two supersystems: the brain and the immune system. Pharmacol Rev 52: 595–638, 2000 [PubMed] [Google Scholar]
- 28.Fannin RD, Auman JT, Bruno ME, Sieber SO, Ward SM, Tucker CJ, Merrick BA, Paules RS. Differential gene expression profiling in whole blood during acute systemic inflammation in lipopolysaccharide-treated rats. Physiol Genomics 21: 92–104, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Foteinou PT, Calvano SE, Lowry SF, Androulakis IP. An indirect response model of endotoxin-induced systemic inflammation. J Crit Care 22: 337–338, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foteinou PT, Calvano SE, Lowry SF, Androulakis IP. In silico simulation of corticosteroids effect on an NFκB-dependent physicochemical model of systemic inflammation. PLoS One 4: e4706, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foteinou PT, Calvano SE, Lowry SF, Androulakis IP. Modeling endotoxin-induced systemic inflammation using an indirect response approach. Math Biosci 217: 27–42, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Foteinou PT, Calvano SE, Lowry SF, Androulakis IP. Translational potential of systems-based models of inflammation. Clin Transl Sci 2: 85–89, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Godin PJ, Buchman TG. Uncoupling of biological oscillators: a complementary hypothesis concerning the pathogenesis of multiple organ dysfunction syndrome. Crit Care Med 24: 1107–1116, 1996 [DOI] [PubMed] [Google Scholar]
- 34.Godin PJ, Fleisher LA, Eidsath A, Vandivier RW, Preas HL, Banks SM, Buchman TG, Suffredini AF. Experimental human endotoxemia increases cardiac regularity: results from a prospective, randomized, crossover trial. Crit Care Med 24: 1117–1124, 1996 [DOI] [PubMed] [Google Scholar]
- 35.Gutkin BS, Dehaene S, Changeux JP. A neurocomputational hypothesis for nicotine addiction. Proc Natl Acad Sci USA 103: 1106–1111, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hasko G, Elenkov IJ, Kvetan V, Vizi ES. Differential effect of selective block of alpha 2-adrenoreceptors on plasma levels of tumour necrosis factor-alpha, interleukin-6 and corticosterone induced by bacterial lipopolysaccharide in mice. J Endocrinol 144: 457–462, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Hoffmann A, Levchenko A, Scott ML, Baltimore D. The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science 298: 1241–1245, 2002 [DOI] [PubMed] [Google Scholar]
- 38.Huikuri HV, Makikallio T, Airaksinen KE, Mitrani R, Castellanos A, Myerburg RJ. Measurement of heart rate variability: a clinical tool or a research toy? J Am Coll Cardiol 34: 1878–1883, 1999 [DOI] [PubMed] [Google Scholar]
- 39.Ignatowski TA, Spengler RN. Regulation of macrophage-derived tumor necrosis factor production by modification of adrenergic receptor sensitivity. J Neuroimmunol 61: 61–70, 1995 [DOI] [PubMed] [Google Scholar]
- 40.Jan BU, Coyle SM, Oikawa LO, Lu SE, Calvano SE, Lehrer PM, Lowry SF. Influence of acute epinephrine infusion on endotoxin-induced parameters of heart rate variability: a randomized controlled trial. Ann Surg 249: 750–756, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin JY, Almon RR, DuBois DC, Jusko WJ. Modeling of corticosteroid pharmacogenomics in rat liver using gene microarrays. J Pharmacol Exp Ther 307: 93–109, 2003 [DOI] [PubMed] [Google Scholar]
- 42.Jusko WJ, Ko HC. Physiologic indirect response models characterize diverse types of pharmacodynamic effects. Clin Pharmacol Ther 56: 406–419, 1994 [DOI] [PubMed] [Google Scholar]
- 43.Krishnamoorthy S, Honn KV. Inflammation and disease progression. Cancer Metastasis Rev 25: 481–491, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Lameris TW, de Zeeuw S, Duncker DJ, Tietge W, Alberts G, Boomsma F, Verdouw PD, van den Meiracker AH. Epinephrine in the heart: uptake and release, but no facilitation of norepinephrine release. Circulation 106: 860–865, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Lin E, Calvano SE, Coyle SM, Lowry SF. Physiologic hypercortisolemia in humans modulates CD95-signal transduction. Surg Forum 50: 288–290, 1999 [Google Scholar]
- 46.Lin E, Lowry SF. The human response to endotoxin. Sepsis 2: 255–262, 1998 [Google Scholar]
- 47.Lowry SF. Human endotoxemia: a model for mechanistic insight and therapeutic targeting. Shock 24, Suppl 1: 94–100, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Lowry SF, Calvano SE. Challenges for modeling and interpreting the complex biology of severe injury and inflammation. J Leukoc Biol 83: 553–557, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Mager DE, Jusko WJ. Pharmacodynamic modeling of time-dependent transduction systems. Clin Pharmacol Ther 70: 210–216, 2001 [DOI] [PubMed] [Google Scholar]
- 50.Mager DE, Lin SX, Blum RA, Lates CD, Jusko WJ. Dose equivalency evaluation of major corticosteroids: pharmacokinetics and cell trafficking and cortisol dynamics. J Clin Pharmacol 43: 1216–1227, 2003 [DOI] [PubMed] [Google Scholar]
- 51.Mager DE, Wyska E, Jusko WJ. Diversity of mechanism-based pharmacodynamic models. Drug Metab Dispos 31: 510–518, 2003 [DOI] [PubMed] [Google Scholar]
- 52.Malave HA, Taylor AA, NATTAMA J, Deswal A, Mann DL. Circulating levels of tumor necrosis factor correlate with indexes of depressed heart rate variability: a study in patients with mild-to-moderate heart failure. Chest 123: 716–724, 2003 [DOI] [PubMed] [Google Scholar]
- 53.Marsland AL, Gianaros PJ, Prather AA, Jennings JR, Neumann SA, Manuck SB. Stimulated production of proinflammatory cytokines covaries inversely with heart rate variability. Psychosom Med 69: 709–716, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Morris JA, Jr, Norris PR, Ozdas A, Waitman LR, Harrell FE, Jr, Williams AE, Cao H, Jenkins JM. Reduced heart rate variability: an indicator of cardiac uncoupling and diminished physiologic reserve in 1,425 trauma patients. J Trauma 60: 1165–1173, 2006 [DOI] [PubMed] [Google Scholar]
- 55.Morris JA, Jr, Norris PR, Waitman LR, Ozdas A, Guillamondegui OD, Jenkins JM. Adrenal insufficiency, heart rate variability, and complex biologic systems: a study of 1,871 critically ill trauma patients. J Am Coll Surg 204: 885–892, 2007 [DOI] [PubMed] [Google Scholar]
- 56.Norris PR, Morris JA, Jr, Ozdas A, Grogan EL, Williams AE. Heart rate variability predicts trauma patient outcome as early as 12 h: implications for military and civilian triage. J Surg Res 129: 122–128, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Norris PR, Ozdas A, Cao H, Williams AE, Harrell FE, Jenkins JM, Morris JA., Jr Cardiac uncoupling and heart rate variability stratify ICU patients by mortality: a study of 2088 trauma patients. Ann Surg 243: 804–812, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Norris PR, Stein PK, Morris JA., Jr Reduced heart rate multiscale entropy predicts death in critical illness: a study of physiologic complexity in 285 trauma patients. J Crit Care 23: 399–405, 2008 [DOI] [PubMed] [Google Scholar]
- 59.Padgett DA, Glaser R. How stress influences the immune response. Trends Immunol 24: 444–448, 2003 [DOI] [PubMed] [Google Scholar]
- 60.Prabhakar U, Conway TM, Murdock P, Mooney JL, Clark S, Hedge P, Bond BC, Jazwinska EC, Barnes MR, Tobin F, Damian-Iordachi V, Greller L, Hurle M, Stubbs AP, Li Z, Valoret EI, Erickson-Miller C, Cass L, Levitt B, Davis HM, Jorkasky DK, Williams WV. Correlation of protein and gene expression profiles of inflammatory proteins after endotoxin challenge in human subjects. DNA Cell Biol 24: 410–431, 2005 [DOI] [PubMed] [Google Scholar]
- 61.Rassias AJ, Holzberger PT, Givan AL, Fahrner SL, Yeager MP. Decreased physiologic variability as a generalized response to human endotoxemia. Crit Care Med 33: 512–519, 2005 [DOI] [PubMed] [Google Scholar]
- 62.Reyes WJ, Brimioulle S, Vincent JL. Septic shock without documented infection: an uncommon entity with a high mortality. Intensive Care Med 25: 1267–1270, 1999 [DOI] [PubMed] [Google Scholar]
- 63.Seely AJ, Christou NV. Multiple organ dysfunction syndrome: exploring the paradigm of complex nonlinear systems. Crit Care Med 28: 2193–2200, 2000 [DOI] [PubMed] [Google Scholar]
- 64.Sharma A, Jusko WJ. Characteristics of indirect pharmacodynamic models and applications to clinical drug responses. Br J Clin Pharmacol 45: 229–239, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sharshar T, Hopkinson NS, Orlikowski D, Annane D. Science review: The brain in sepsis–culprit and victim. Crit Care 9: 37–44, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stein PK, Kleiger RE. Insights from the study of heart rate variability. Annu Rev Med 50: 249–261, 1999 [DOI] [PubMed] [Google Scholar]
- 67.Storey JD, Xiao W, Leek JT, Tompkins RG, Davis RW. Significance analysis of time course microarray experiments. Proc Natl Acad Sci USA 102: 12837–12842, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun YN, Jusko WJ. Transit compartments versus gamma distribution function to model signal transduction processes in pharmacodynamics. J Pharm Sci 87: 732–737, 1998 [DOI] [PubMed] [Google Scholar]
- 69.Talwar S, Munson PJ, Barb J, Fiuza C, Cintron AP, Logun C, Tropea M, Khan S, Reda D, Shelhamer JH, Danner RL, Suffredini AF. Gene expression profiles of peripheral blood leukocytes after endotoxin challenge in humans. Physiol Genomics 25: 203–215, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tracey KJ. The inflammatory reflex. Nature 420: 853–859, 2002 [DOI] [PubMed] [Google Scholar]
- 71.Van der Poll T. Effects of catecholamines on the inflammatory response. Sepsis 4: 159–167, 2000 [Google Scholar]
- 72.Van der Poll T, Barber AE, Coyle SM, Lowry SF. Hypercortisolemia increases plasma interleukin-10 concentrations during human endotoxemia–a clinical research center study. J Clin Endocrinol Metab 81: 3604–3606, 1996 [DOI] [PubMed] [Google Scholar]
- 73.Van der Poll T, Coyle SM, Barbosa K, Braxton CC, Lowry SF. Epinephrine inhibits tumor necrosis factor-alpha and potentiates interleukin 10 production during human endotoxemia. J Clin Invest 97: 713–719, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Van der Poll T, Lowry SF. Epinephrine inhibits endotoxin-induced IL-1 beta production: roles of tumor necrosis factor-alpha and IL-10. Am J Physiol Regul Integr Comp Physiol 273: R1885–R1890, 1997. [DOI] [PubMed] [Google Scholar]
- 75.Vodovotz Y, Constantine G, Rubin J, Csete M, Voit EO, An G. Mechanistic simulations of inflammation: current state and future prospects. Math Biosci 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vodovotz Y, Csete M, Bartels J, Chang S, An G. Translational systems biology of inflammation. PLoS Comput Biol 4: e1000014, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Webster JI, Tonelli L, Sternberg EM. Neuroendocrine regulation of immunity. Annu Rev Immunol 20: 125–163, 2002 [DOI] [PubMed] [Google Scholar]
- 78.Wells CA, Ravasi T, Hume DA. Inflammation suppressor genes: please switch out all the lights. J Leukoc Biol 78: 9–13, 2005 [DOI] [PubMed] [Google Scholar]
- 79.Winchell RJ, Hoyt DB. Spectral analysis of heart rate variability in the ICU: a measure of autonomic function. J Surg Res 63: 11–16, 1996 [DOI] [PubMed] [Google Scholar]
- 80.Winchell RJ, Hoyt DB. Analysis of heart-rate variability: a noninvasive predictor of death and poor outcome in patients with severe head injury. J Trauma 43: 927–933, 1997 [DOI] [PubMed] [Google Scholar]
- 81.Wittebole X, Hahm S, Coyle SM, Kumar A, Calvano SE, Lowry SF. Nicotine exposure alters in vivo human responses to endotoxin. Clin Exp Immunol 147: 28–34, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zaza A, Lombardi F. Autonomic indexes based on the analysis of heart rate variability: a view from the sinus node. Cardiovasc Res 50: 434–442, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.