Abstract
We assessed the ability of a gamma-secretase inhibitor to promote the in vitro differentiation of induced embryonic pancreatic precursor cell aggregates into functional islet-like clusters when encapsulated within a three-dimensional hydrogel. Undifferentiated pancreatic precursor cells were isolated from E.15 rat embryos, dissociated into single cells, and aggregated in suspension-rotation culture. Aggregates were photoencapsulated into poly(ethylene glycol) hydrogels with entrapped collagen type 1 and cultured for 14 days with or without a gamma-secretase inhibitor. Gene expression, proinsulin content, and C-peptide release were measured to determine differentiation and maturation of encapsulated precursor cell aggregates. In the control medium, scattered breakthrough beta cell differentiation was observed; however, cells remained largely insulin negative. Upon addition of a gamma-secretase inhibitor the majority of cells in clusters became insulin positive, and insulin per DNA and glucose-stimulated insulin release measurements for these cultures were comparable with those for adult rat islets. Cluster counts after culture day 14 were 88% of those initially encapsulated, demonstrating excellent cluster survival in hydrogel culture. These results indicate that concerted differentiation of pancreatic precursor cell aggregates into functionally mature islet-like clusters can be achieved in poly(ethylene glycol)-based hydrogel cultures by blocking cell contact-mediated Notch signaling with a gamma-secretase inhibitor.
Introduction
Donor tissue shortage is a significant challenge facing islet cell transplantation therapy.1,2 Any alternative cell source to cadaveric islets must have not only a robust proliferative capacity but also the ability to selectively and efficiently differentiate into insulin-producing beta cells. Embryonic pancreatic precursor cells are proliferative cells not yet terminally differentiated; therefore, by deliberately modifying the cells' environment one could theoretically direct their differentiation fate. In vivo studies involving human, porcine, and rat fetal pancreatic tissue have successfully corrected hyperglycemia in animal models of diabetes.3–7 However, mechanisms to promote the in vitro differentiation of pancreatic precursor cells into a uniform population of functional beta cells have not been achieved. Additional research has focused on developing a Pdx-1+ cell population from embryonic stem cells, with the belief that properly forming and functioning islets must be generated through a cell type indistinguishable from those found in the developing pancreatic bud.8–10 Therefore, determining how to selectively differentiate embryonic pancreatic precursors into functioning islets may provide a blueprint for the in vitro differentiation of embryonic stem cells that have been encouraged toward a pancreatic precursor cell-like fate. In vitro differentiation of embryo-derived cells is an important step to ensure their safe transplantation, free from the risk of unwanted ectopic tissue or tumor formation when implanted in vivo.11,12
Poly(ethylene glycol) (PEG)-based hydrogels are a mechanically supportive three-dimensional culture platform providing minimal cell–material interaction. This cell–material neutrality provides a blank slate in which to study the effects of specific signaling molecules on an encapsulated cell population and subsequently use this information to enhance viability or encourage differentiation of desirable cell types. Importantly, PEG hydrogel encapsulation can also protect islets from immune-mediated destruction.13
We have previously shown that dissociated pancreatic precursor cells encapsulated as single cells in PEG hydrogels selectively differentiate into beta cells; however, cell numbers significantly decrease over 7 days of culture and the cells remain immature and unresponsive to glucose.14 These findings indicate that beta cell differentiation may be this cell population's default pathway and also suggest that cell aggregation, as demonstrated for adult islets, may be important for differentiating pancreatic precursor cell viability and function.15–17 The preference of adult islets for cell–cell contact is in direct conflict with the creation of a uniform population of functional islets from pancreatic precursors because cell–cell interactions prevent the concerted differentiation of multipotent precursor cells in the developing pancreas. Differentiation suppression is mediated by the Notch signaling pathway, which is activated by ligand–receptor interactions and propagated by cleavage and subsequent nuclear translocation of Notch intracellular domain via gamma-secretase.18 In the nucleus, Notch intracellular domain activates the transcription of target genes, such as Hes1, that results in precursor cell maintenance in the developing pancreas.19 Notch signaling pathway deficiencies lead to precocious and premature differentiation of pancreatic progenitors into endocrine cells,19,20 suggesting that blocking Notch signaling could be used to promote uniform in vitro differentiation.
In this study we utilized a PEG-based hydrogel as a culture platform to determine (1) whether precursor cell aggregation benefits cell survival in hydrogel culture and (2) whether enhanced in vitro differentiation of pancreatic precursor cell aggregates could be achieved through the interruption of the Notch signaling pathway. We first determined the appropriate cell aggregating conditions to increase the number of precursor cell clusters formed before hydrogel encapsulation. Next, we demonstrated cell–cell contact-mediated suppression of differentiation in PEG hydrogel culture. Finally, we utilized a gamma-secretase inhibitor to block cell–cell contact-mediated Notch signaling and promote differentiation of precursor cell aggregates into mature glucose-responsive islet-like clusters.
Materials and Methods
Pancreatic precursor cell isolation, encapsulation, and culture
Pregnant Sprague-Dawley rats were purchased from Charles River Laboratories. Animals were treated and euthanized in accordance with the National Institutes of Health Office of Laboratory Animal Welfare Principles of Laboratory Animal Care and Institutional Animal Care and Use Committee (IACUC) guidelines.
PEG macromers were synthesized as previously described.14,21 Dorsal pancreatic rudiments were removed from E.15 rat embryos and dissociated into single-cell suspensions.14 This initial cell population has been previously characterized and contains ∼65% Pdx1+/insulin− pancreatic precursor cells, ∼25% vimentin+ mesenchymal cells, and <1% of differentiated insulin or glucagon+ cells.22 For clustering experiments, cells were placed in nonadherent tissue culture wells on an orbital shaker (80 rpm) for 30, 60, or 120 min. Dissociated or clustered cells were photoencapsulated into 7.5wt% PEG hydrogels with 0.5 mg/mL entrapped collagen (PEGCol) at a cell concentration of 3 × 106 cells/mL.22 Hydrogel discs (3.6 mm in diameter and 1.5 mm thick) were photopolymerized using 0.025wt% photoinitiator (Irgacure 2959) for 10 min at 365 nm ultraviolet light (∼4 mW/cm2).14 PEGCol hydrogels are superior to unmodified PEG hydrogels or pure collagen gels at maintaining viability of encapsulated, spontaneously formed pancreatic precursor cell clusters by providing both mechanical stability and a source of β1-integrin signalling.22–24 A 50:50 mixture of hydrolytically degradable and nondegradable PEG macromers were used to preserve stability over long-term in vitro culture, a modification that would also facilitate maintenance of hydrogel structural integrity for in vivo implantation.25 PEGCol cultures were maintained at 37°C/5% CO2 in RPMI-1640 medium supplemented with 10% fetal bovine serum, 1% penicillin–streptomycin, and 0.1% N2 (all vol./vol.), 0.5 μg/mL Fungizone, and 2 mM L-glutamine. All medium components were purchased from Invitrogen. Gamma-secretase inhibitor IX (N-[N-(3,5-Difluorophenacetyl-L-alanyl)]-S-phenyl-glycine t-Butyl Ester) (DAPT) was used at 0.25 μM (Calbiochem). The medium was changed every 1–2 days. PEGCol hydrogel mesh size was measured at ∼60Å immediately after polymerization, indicating that the small molecule gamma-secretase inhibitor (432.5 Da), along with molecules such as insulin (5808 Da, ∼14.7Å) and glucose (180 Da, ∼4Å), could freely diffuse into and out of the cultures.
Cluster counts, size, and viability
Immediately after encapsulation, and on culture days 7 and 14, hydrogel culture discs were observed using Live/Dead fluorescent probe membrane integrity assay (viable and dead cells are labeled green and red, respectively) (Molecular Probes). Each image represents a series of 45–50 optical sections, 30 μm apart. Clusters size was indicated by measured diameter and clusters >25 μm were counted; for nonspherical clusters, the average of three diameter measurements was utilized.
Quantitative real-time polymerase chain reaction (RT-PCR)
On culture days 1, 3, 7, and 14, hydrogel cultures were removed from the medium, placed in TriReagent, and stored at −20°C. RNA isolation, cDNA synthesis, and polymerase chain reaction (PCR) amplification and quantification were carried out as previously described.14 Primer sequences used for insulin (Ins1 and 2), glucose transporter type 2 (Glut2), pancreatic and duodenal homeobox 1 (Pdx1), Mafa, glucagon (Gcg), vimentin (Vim), and amylase (Amy) have been previously published.14 For hairy and enhancer of split-1 (Hes1) and neurogenin 3 (Ngn3), the following primer sequences were used: Hes1 5′oligo, CCC,ACC,TCT,CTC,TTC,TGA,CG and 3′oligo, AGG,CGC,AAT,CCA,ATA,TGA,AC; Ngn3 5′oligo, CCA,CGA,AGT,GCT,CAG,TTC,CAA and 3′oligo, GCG,GAG,TTA,AGG,TTG,TGC,ATG.
Immunohistochemistry
On days 7 and 14, hydrogel cultures were fixed in 4% paraformaldehyde and dehydrated in 15% sucrose (wt/vol.). About 40 μm frozen sections were stained using standard immunohistochemical techniques with the following antibodies: mouse anti-insulin (1/750; Sigma), mouse anti-glucagon (1/1000; Sigma), mouse anti-amylase (1/300; Sigma), and rabbit anti-Ki67 (1/500; Abcam). Fluorophore-conjugated secondary antibodies were used (1/300; Invitrogen).
DNA and insulin content
On culture days 7 and 14, PEGCol hydrogel culture discs were transferred to cell lysis buffer, homogenized via pellet crusher, and sonicated for 15 s (4W). Supernatant DNA, insulin, and proinsulin content was measured using a Quant-iT PicoGreen dsDNA assay (Invitrogen), and rat insulin and proinsulin ELISA kits (Mercodia), respectively, per manufacture's instructions.
Glucose challenge test
On days 7 and 14, hydrogel discs were washed in the low-glucose serum-free medium and incubated in either low (1.1 mM) or high (16.7 mM) glucose medium for 2 h.14 These glucose concentrations have been utilized to test the glucose responsiveness of PEG hydrogel-encapsulated pancreatic precursor cells and MIN6 beta cells in previous studies.14,22,26 After incubation, supernatant C-peptide concentrations were measured using rat C-peptide ELISA kits (Mercodia).
Statistical analysis
Between group differences were analyzed using a two-tailed, unpaired Student's t-test when only two groups were being compared. Specifically, for gene expression, insulin and DNA content, and insulin release data through day 7 (i.e., when there were only two conditions), a Student's t-test was used to measure between group differences and not the effect of time. When three or more groups were being compared, a one-way analysis of variance was utilized; significant main effects were then followed with Newman-Keul's post-hoc analyses. Significance was set at p < 0.05 and n ≥ 3 separate experiments for all results shown. Data presented as mean ± standard error of the mean unless otherwise noted.
Results
Cluster formation before encapsulation
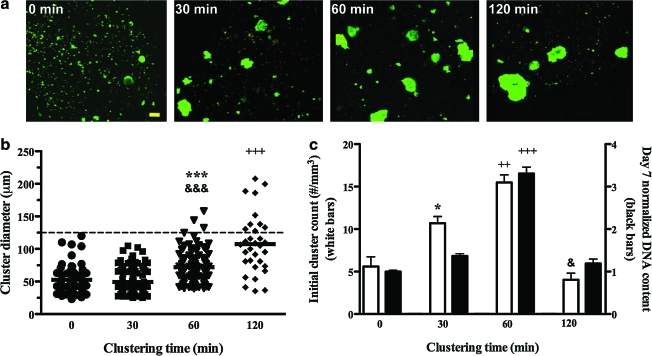
Pancreatic precursor cells were induced to form clusters using suspension rotation incubation for 0, 30, 60, and 120 min on an orbital shaker. Cadherin-mediated interactions were a likely mediator of the cell–cell adhesion that facilitated aggregation during this incubation, as these cell adhesion proteins, specifically E-cadherin, are found on the surface of all epithelial cells of the developing and adult pancreas, including pancreatic precursor cells.27 Clusters resulting from the orbital shaker incubation were subsequently encapsulated in PEGCol hydrogels and observed using a Live/Dead stain (Fig. 1a). The sizes of individual cell clusters along with mean cluster size were measured from these images (Fig. 1b). DNA content measured after 7 days of PEGCol hydrogel culture was highest for the 60-min cluster condition (Fig. 1c; black bars), demonstrating a survival benefit over dissociated cells (Fig. 1a; 0 min) and fewer, but larger cell clusters (Fig. 1a; 120 min). These results mimic those for initial cluster counts (Fig. 1c; white bars), suggesting that an increase in cluster number benefits encapsulated precursor cell survival. On the basis of cluster number and DNA content data, a clustering time of 60 min was utilized for all subsequent experiments.
FIG. 1.
Characterization of pancreatic precursor cell clusters after 0, 30, 60, and 120 min of orbital shaker incubation. (a) Images of clusters formed after orbital shaker incubation for the indicated time after Live/Dead stain application. Scale bar, 50 μm. Initial cluster size (b) and number (c, left axis, white bars) were determined from Live/Dead images and are given for each condition along with day 7 DNA contents (c, right axis, black bars). (b) Cluster size was determined by measuring the diameter of encapsulated clusters and is presented as a scatter plot, with one data point present for each cluster. Horizontal bars represent the mean, and dotted line indicates a cluster size of 125 μm. (c) Initial cluster counts (white bars) are shown as clusters per mm3 of hydrogel and culture day 7 DNA contents are shown normalized to the 0-min condition. DNA concentration comparisons (black bars) show enhanced cell survival in the 60-min conditions compared to all other conditions. The term “survival” is used to describe the DNA data because the DNA content decreases from day 0 in all conditions. For example, DNA content on day 7 is ∼20% and 60% of day 0 values for the 0- and 60-min clustering conditions, respectively. Both cluster counts and DNA contents are presented as the mean ± standard error of the mean (SEM). *p < 0.05 and ***p < 0.001 compared to 0-min condition. &p < 0.05 and &&&p < 0.001 compared to 30-min condition. ++p < 0.01 and +++p < 0.001 compared to all other conditions. Color images available online at www.liebertonline.com/ten.
Differentiation of precursor cell clusters encapsulated in PEGCol hydrogels
Clusters were encapsulated in PEGCol hydrogels and cultured in the medium with or without a gamma-secretase inhibitor. RT-PCR, immunohistochemistry, and proinsulin concentrations were used to compare the differentiation of encapsulated clusters during 2 weeks of culture.
Gene expression
RT-PCR was used to measure relative fold-differences in Hes1, Ngn3, Ins, and Pdx1 expression for clustered pancreatic precursor cells encapsulated in PEGCol hydrogels and cultured in the control medium or medium supplemented with a gamma-secretase inhibitor (Fig. 2). At all time points, Hes1 expression was significantly decreased in the presence of gamma-secretase inhibitor, verifying interrupted Notch signaling. Conversely, Ngn3 expression, a marker of endocrine specification, was significantly increased with gamma-secretase inhibitor exposure by culture days 3 and 7. Additionally, Ins expression was ∼6.4-fold higher on day 7 in the presence of gamma-secretase inhibitor, a pattern mimicked by a similar increase (∼5-fold) in Pdx1 expression.
FIG. 2.
Expression of genes important in pancreatic precursor cell differentiation during 7 days of culture with and without γ-secretase inhibitor. Cell clusters were induced via 60-min orbital shaker incubation before encapsulation (day 0) and then cultured in PEGCol hydrogels for 7 days. (a–d) Quantitative real-time polymerase chain reaction (RT-PCR) obtained gene expression results for Hes1 (a), Ngn3 (b), Ins (c), and Pdx1 (d) in PEGCol hydrogel cultures both without (control; white bars) and with (black bars) γ-secretase inhibitor added to the medium. All values are normalized to time-matched control condition and shown as mean ± SEM. *p < 0.05 and ***p < 0.001 compared to time-matched control. PEGCol, poly(ethylene glycol) hydrogels with entrapped collagen.
After in vivo implantation, Notch inhibition would not be maintained; therefore, a transient dose of gamma-secretase inhibitor was used to determine if differentiated cells could be weaned from Notch inhibition without detrimental effects to function and maturation. Relative fold-differences in Hes1, Ins, Pdx1, Glut2, Mafa, and Gcg gene expression were measured in hydrogel cultures from three experimental conditions: (1) cultures exposed to no gamma-secretase inhibitor (control), (2) cultures exposed to 7 days of gamma-secretase inhibitor, and then switched to the control medium for 7 days, and (3) cultures exposed to gamma-secretase inhibitor for 14 days of incubation (Fig. 3). When gamma-secretase inhibitor was withdrawn from hydrogel cultures after 7 days, Hes1 gene expression rebounded, indicating continued competence of Notch signaling (Fig. 3a); however, there was not a significant difference in the differentiation of these encapsulated pancreatic precursor cell clusters compared to cultures exposed to gamma-secretase inhibitor for 14 days. Cultures exposed to gamma-secretase inhibitor for 7 or 14 days demonstrated significantly more beta cell differentiation (Ins, Pdx1, Mafa, and Glut2) and alpha cell differentiation (Gcg) than controls. Although values are shown normalized to the control condition, expression of genetic markers indicative of beta cell differentiation significantly increased for both control and gamma-secretase inhibitor conditions overtime. For example, Ins gene expression increased ∼12,000-fold from day 1 to 14 in the control condition, whereas it increased ∼55,000-fold in gamma-secretase-exposed cultures over this same time period.
FIG. 3.
Expression of genes important in endocrine cell differentiation after 14 days of culture without γ-secretase inhibitor or exposed to γ-secretase inhibitor for either 7 or 14 days. (a–f) Cell clusters were induced via a 60-min orbital shaker incubation before encapsulation and then cultured in PEGCol hydrogels for 14 days. RT-PCR obtained gene expression results for Hes1 (a), Ins (b), Pdx1 (c), Glut2 (d), MafA (e), and Gcg (f) are shown for the control condition (white bars), with 7 days of γ-secretase inhibitor (checked bars), and with 14 days of γ-secretase inhibitor (black bars). All values are normalized to control condition and shown as mean ± SEM. *p < 0.05, **p < 0.01, and ***p < 0.001 compared with control.
The gene expression levels of vimentin and amylase were measured as markers of mesenchyme and exocrine tissue, respectively. Consistent with our previously published work, vimentin gene expression was undetectable by day 7, indicating that contaminating mesenchymal cells present upon dissection/dissociation of the pancreatic bud were unable to survive in PEGCol hydrogel culture (data not shown).22 Additionally, amylase gene expression, a marker for exocrine differentiation, did not change significantly from the low level of gene expression measured on day 0 for any culture time or condition tested (data not shown).14 These results are likely coupled, as mesenchymal influences are thought to be required for exocrine development.28
Proinsulin content
Proinsulin content, which selectively measures endogenous insulin production and not uptake from the serum-containing culture medium, was measured to determine if enhanced insulin gene expression translates into enhanced insulin synthesis and storage. Hydrogel encapsulated precursor cell clusters exposed for any duration to gamma-secretase inhibitor had significantly greater proinsulin:DNA ratios (Fig. 4a, b). Corroborating gene expression results, there were not significant differences in proinsulin contents between cultures exposed to 7 versus 14 days of gamma-secretase inhibitor. Initially upon encapsulation, both proinsulin and insulin levels in hydrogel cultures were undetectable (Table 1), consistent with previously published work22 and the embryonic stage of development at which the precursor cells were harvested. Proinsulin:insulin ratios for clustered cells in PEGCol hydrogel cultures ranged between 1:26 and 1:32, comparable to previously published values for adult rat islets.29
FIG. 4.
Proinsulin-to-DNA ratios and glucose-stimulated insulin release (GSIR) values after 7 and 14 days of culture in all medium conditions. (a, b) Proinsulin and DNA measurements were made on the supernatant of homogenized PEGCol hydrogel cultures after 7 (a) and 14 (b) days of culture. Values were obtained as a ratio of proinsulin to DNA (ng insulin/ng DNA) and are shown normalized to the time-matched no γ-secretase inhibitor control condition. (c, d) Medium C-peptide concentrations were measured after a 2-h incubation of day 7 (c) or day 14 (d) PEGCol hydrogel culture discs in either low (1.1 mM glucose) or high (16.7 mM glucose) glucose medium. GSIR values were calculated by normalizing the amount of C-peptide released in the high-glucose medium to the amount of C-peptide released in the low-glucose medium. A GSIR value of 1 would suggest a culture that does not differentially release C-peptide/insulin in response to a higher glucose concentration and is indicated by the horizontal line on each graph. All values are shown as mean ± SEM. *p < 0.05 and ***p < 0.001 compared to time-matched control condition. #p < 0.05, ##p < 0.01, ###p < 0.001 compared to 1.1 mM glucose medium C-peptide concentration (values not shown).
Table 1.
Measured Proinsulin and Insulin Values (ng Insulin/ng DNA) Along with C-Peptide Concentrations (nM) in the High-Glucose Medium Initially (day 0) and For All Day 7 and 14 Culture Conditions
| |
|
Day 7 |
Day 14 |
|||
|---|---|---|---|---|---|---|
| Medium condition | Day 1 | No γ-secretase inhibitor | + γ-secretase inhibitor | No γ-secretase inhibitor | γ-secretase inhibitor (7 days) | γ-secretase inhibitor (14 days) |
| Proinsulin content (ng proinsulin/ng DNA) | 0 | 0.013 ± 0.003 | 0.06 ± 0.01a | 0.04 ± 0.01 | 0.15 ± 0.01b | 0.16 ± 0.01b |
| Insulin content (ng insulin/ng DNA) | 0 | 0.38 ± 0.07 | 1.6 ± 0.2b | 1.3 ± 0.3 | 4.6 ± 0.5b | 4.1 ± 0.4a |
| High-glucose medium C-peptide concentrations (pM) | 0 | 270 ± 50 | 2800 ± 170 | 1800 ± 300 | 7400 ± 900b | 7600 ± 500b |
Values shown are mean ± standard error of the mean.
p < 0.01 and bp < 0.001 compared to time-matched no γ-secretase inhibitor control.
Functional differentiation
Released C-peptide concentrations were measured in low- and high-glucose media and glucose-stimulated insulin release (GSIR) values were calculated. On day 7, both control cultures and cultures exposed to gamma-secretase inhibitor had GSIR values >1, indicating glucose responsiveness. Importantly, precursor cluster-loaded PEGCol hydrogels cultured with gamma-secretase inhibitor were significantly more glucose responsive than controls (Fig. 4c). On day 14, encapsulated clusters exposed to 7 or 14 days of gamma-secretase inhibitor, but not cultures in the control medium, remained glucose responsive (Fig. 4d). C-peptide concentrations in the high-glucose medium are listed in Table 1. Although control cultures were glucose responsive on day 7, ∼10-fold more insulin was released in the high-glucose medium when cultures were exposed to gamma-secretase inhibitor. This is a further indication of the significantly enhanced beta cell differentiation in the gamma-secretase inhibitor–supplemented medium.
Immunohistochemistry
In the absence of gamma-secretase inhibitor, precursor cell clusters showed scattered insulin+ cells after 7 days of culture while differentiation of neighboring cells was notably absent (Fig. 5b, d). In contrast, groups of insulin+ cells were present within clusters exposed to gamma-secretase inhibitor on day 7 (Fig. 5c, e). By day 14, insulin+ cells remained the minority in the control condition (Fig. 6a), whereas insulin+ cells comprised the majority of all remaining clusters, regardless of cluster distance from the hydrogel surface, in both gamma-secretase inhibitor–exposed conditions (Fig. 6b, c). Additionally, Ki67+ proliferative cells were present in both control and gamma-secretase inhibitor–exposed cultures on day 7 (Fig. 5f, g). By day 14, proliferative capacity had diminished significantly, with only a few scattered Ki67+ cells remaining in any culture condition (Fig. 6g–i). Exocrine differentiation was not observed as there were no amylase+ cells on day 7 or 14 (data not shown), corroborating gene expression results.
FIG. 5.
Immunostaining of initial cell population and after 7 days of culture. (a; day 0) Pancreatic buds were dissociated into single cells and immediately fixed and stained with an antibody directed against insulin. Scattered and weakly insulin+ cells were seen (arrow head); however, they made up <1% of the total cell population. Scale bar, 20 μm. (b–g; day 7) Hydrogel sections from cultures without added γ-secretase inhibitor (b, d) and from those with γ-secretase inhibitor added to the culture medium (c, e) were incubated with an antibody directed against insulin. Upper images are taken at a lower magnification (b, c). In the control medium, scattered single cells within pancreatic precursor cell aggregates have differentiated into insulin+ cells; however, the majority of the cells in the cluster remain insulin− and there is notable absence of grouped insulin+ cells (b, d). When γ-secretase inhibitor was added to the culture medium, neighboring groups of cells are insulin+ within each cluster (c, e). Day 7 hydrogel sections were also costained for insulin and the proliferation marker Ki67 (f, g). Proliferating cells were present in both control (f) and γ-secretase inhibitor–exposed (g) culture conditions. Scale bar for (b, c), 50 μm. Scale bar for (d–g), 20 μm. Color images available online at www.liebertonline.com/ten.
FIG. 6.
Immunostaining of day 14 cultures. PEGCol hydrogel sections were incubated with antibodies directed against insulin (a–c), glucagon (d–f), and Ki67 (g–i), and representative images are shown from control cultures (a, d, g) and cultures exposed to 7 (b, e, h) or 14 (c, f, i) days of γ-secretase inhibitor. In the control medium, insulin+ cells remain the minority population in cell clusters (a). Scattered glucagon+ cells are also present in control cultures (d). In conditions where γ-secretase inhibitor was added to the medium for either 7 or 14 days, insulin+ cells are the predominant cell type found in all remaining encapsulated clusters (b, c). Glucagon+ cells are also apparent within each cell cluster (e, f). Only a few scattered Ki67+ proliferative cells remained on day 14 in any condition (g–i), suggesting either a decrease in proliferative capacity over time or, in the γ-secretase inhibitor–exposed conditions, a decrease in proliferative capacity with increasing differentiation. Scale bar, 20 μm. Color images available online at www.liebertonline.com/ten.
Cell cluster size and viability over 14 days in PEGCol culture
On days 7 and 14, DNA concentrations were significantly higher in gamma-secretase inhibitor exposed cultures (Fig. 7a, b). For gamma-secretase inhibitor conditions, cluster number and diameter were assessed and compared to initial (day 0) values (Fig. 7c). Cluster counts decreased from 13 ± 1 to 11.6 ± 1 clusters/mm3 of hydrogel between days 0 and 14, indicating that 88% of clusters remained after 2-weeks of hydrogel culture. It is possible that clusters present on day 14 are generated from single proliferating cells and are not the same clusters encapsulated on day 0; however, we have previously published results using singly dissociated precursor cell clusters and observed no cell clusters after 7 or 10 days of culture.14 Additionally, if single cells were able to proliferate to form clusters, we would have expected more clusters on days 7 and 14 than on day 0. Cluster size increased through the first 7 days of culture; however, cluster size decreased between days 7 and 14, such that the day 14 cluster size was not different from initial measurements. Cluster size range also narrowed by day 14 such that all remaining clusters were below 125 μm in diameter (Fig. 7c, dotted line).
FIG. 7.
DNA content, cluster size, and cluster viability through 14 days of culture. (a, b) DNA measurements were made on the supernatant of homogenized PEGCol hydrogel cultures from all conditions after 7 (a) and 14 (b) days of culture. Values shown are as mean ± SEM and are normalized to the control condition. ***p < 0.001 compared to time-matched control. (c) Median cluster size (solid horizontal lines), interquartile range bounded by the upper and lower quartiles (gray bars), and cluster size range (whiskers) are shown on days 0, 7, and 14 for cultures exposed to γ-secretase inhibitor. Dotted line denotes a cluster size of 125 μm. Day 14 values shown in (c) include measurements from cultures exposed to both 7 and 14 days of γ-secretase inhibitor, as no cluster size differences were observed between these two conditions. +p < 0.001 and p < 0.05 for day 7 mean cluster size compared to day 0 and 14, respectively. (d, e) Live/Dead images of cultures exposed to γ-secretase inhibitor for 7 (d) or 14 (e) days. Larger clusters are apparent on day 7 compared with day 14. Scale bar, 50 μm. Color images available online at www.liebertonline.com/ten.
Discussion
Here we describe the in vitro culture of pancreatic precursor cell clusters in mechanically supportive PEGCol hydrogels as a platform for testing the utility of a gamma-secretase inhibitor to promote selective endocrine differentiation. In the control medium, breakthrough insulin+ cell differentiation was observed; however, the majority of cells remained negative for exocrine or endocrine markers. These results are consistent with (1) an in vivo study in which ectopic Notch activation before and during the secondary transition promoted maintenance of undifferentiated epithelium30 and (2) an in vitro mesenchyme-free whole bud culture study in which differentiation also failed to progress.28 If the pancreatic precursor cells utilized for this study had been left in vivo for a comparable 7 days of differentiation (i.e., E.15 through birth), substantial exocrine and endocrine development would have occurred; however, repression of differentiation remains dominant in our cultures. We hypothesize that the predominant signal in our in vitro cultures is cell–cell signaling, including Notch receptor–ligand interactions that lead to stunted differentiation; therefore, our cultures are directed by Notch signaling to a greater degree than would occur within the complex milieu of signals that comprises the in vivo environment.
Although active Notch signaling has been linked to cell proliferation and interrupted Notch signaling to organ hypoplasia,19,31 our cultures demonstrated proliferative capacity with and without gamma-secretase inhibitor. Further, DNA concentrations on both days 7 and 14 were higher in cultures supplemented with gamma-secretase inhibitor, suggesting that differentiated cells have greater long-term viability in our PEGCol hydrogels. These data support the hypothesis that Notch signaling specifically effects cell differentiation without a direct role in cell proliferation, such that cells maintain a proliferative capacity appropriate to the stage of differentiation that is independent of Notch signaling. The disconnection between Notch signaling and proliferation observed in our cultures is similar to in vivo data, where ectopic Notch activation results in a smaller pancreatic cell mass compared to wild-type controls.30
Precursor cell aggregates undergo islet-like differentiation over 14 days in culture when encapsulated in PEGCol hydrogels and exposed to gamma-secretase inhibitor. Although other signaling pathways may be interrupted, decreased Hes1 expression in our cultures, coupled with prior in vivo studies,20 supports our hypothesis that enhanced islet-like differentiation is due to the gamma-secretase inhibitor's ability to block Notch signaling. Ngn3 is transiently expressed during pancreatic development by differentiating endocrine cells,32 and cultures exposed to gamma-secretase inhibitor transition through a period of elevated Ngn3 expression compared to controls, thus indicating a period of enhanced endocrine specification similar to that observed in Hes1−/− mice in vivo.19 By day 14, when cultures are composed predominantly of differentiated endocrine cells, Ngn3 expression was undetectable in gamma-secretase inhibitor–treated cultures, consistent with the fact that Ngn3 is not expressed in hormone+ endocrine cells.33,34 Additionally, exposure to gamma-secretase inhibitor significantly enhanced beta cell differentiation, as evidenced by elevated Ins and Pdx1 expression and increased numbers of insulin+ cells on culture days 7 and 14. Pdx1 is present in low levels in all pancreatic precursor cells, but restricted to and highly expressed in differentiated beta cells as development progresses.35,36 MafA expression and Glt2 expression are also indicative of beta cell differentiation and likewise were elevated in gamma-secretase inhibitor–exposed cultures on day 14. Taken together, these results indicate that the addition of gamma-secretase inhibitor successfully interrupted the Notch signaling pathway and enhanced beta cell differentiation and maturation of encapsulated precursor cell clusters over the 2-week culture period. The abundance of beta cells in day 14 cultures could also be explained by beta cell proliferation; however, the diminished Ki67+ staining on day 14 when the majority of cell in clusters are insulin+ and the lack of insulin+ cell clusters in the control cultures makes this explanation unlikely.
Pancreatic precursor cells isolated just before the secondary transition have a default differentiation tendency toward an endocrine, and specifically a beta cell, fate.14,28 Therefore, we propose that upon removal of the repressive Notch signaling, clusters of precursor cells in our hydrogel culture platform are allowed to proceed down their default developmental pathway, resulting in the differentiation of precursor cell aggregates into islet-like clusters. The results from our study appear to conflict with recent data showing that culturing an intact pancreatic bud with a gamma-secretase inhibitor for 5 days increases the number of differentiating Ngn3+ endocrine progenitor cells but does not facilitate insulin+ beta cell differentiation.37 The authors hypothesized that this phenomenon was due to signals from remaining mesenchyme acting downstream of Ngn3 expression to prevent beta cell differentiation. We have shown previously that mesenchymal cells do not survive culture in PEGCol hydrogels,22 both explaining this discrepancy and highlighting a benefit of culturing within a synthetic PEG hydrogel platform.
Interestingly, transient (7 day) and continuous (14 day) exposure to gamma-secretase inhibitor resulted in equivalent beta cell differentiation, as measured by gene expression, total insulin content, and GSIR values. Hes1 gene expression rebounded in medium-switched cultures, indicating continued competence of the Notch signaling pathway; however, the ultimate composition of the cultures was unaffected by this Notch signaling reactivation. Pancreatic precursor cells and committed Ngn3+ endocrine precursors are sensitive to Notch signaling; however, fully differentiated beta cells are not.30 In vivo ectopic Notch signaling activation arrests pancreatic precursor cell differentiation and, when coupled to Ngn3 expression, halts the differentiation of endocrine progenitors into fully differentiated cells.19,30 The gamma-secretase inhibitor used in this study (DAPT) has been shown to persist in cell culture 3–4 days after application,38 which would allow for the continued gamma-secretase inhibitor–mediated differentiation of endocrine-committed Ngn3-expressing cells into fully differentiated Notch-insensitive cells even after the day 7 medium switch.
Differentiated islet-like clusters within our PEGCol hydrogel cultures contain insulin at levels comparable to mature rodent islets. Insulin per DNA values (ng insulin/ng DNA) for cultured rat islets are ∼239 and for freshly isolated rodent islets are between 3.5 and 4.40 Our cultures compare favorably to the freshly isolated islets, as day 14 insulin per DNA values were 4.6 and 4.1 when exposed to 7 or 14 days of gamma-secretase inhibitor, respectively. These results were confirmed by proinsulin measurements consistent with known proinsulin to insulin ratios,29 making it highly unlikely that these measurements were a result of insulin uptake from the medium. Additionally, pancreatic precursor cell clusters encapsulated in a PEGCol hydrogel culture platform and exposed to gamma-secretase inhibitor were glucose responsive by day 7 (GSIR ∼3.3), and maintained their glucose responsiveness for an additional 7 days in culture (GSIR ∼3). Adult rat islets cultured in the same medium used for our study (RPMI; 11 mM glucose) have GSIR values of ∼3.4 and ∼2.8 after 4 and 7 days of culture, respectively,39,41 indicating that our differentiated islet-like clusters had comparable GSIR values to those reported for mature islets. In contrast to functional differentiation of precursor cell clusters, we have previously demonstrated that encapsulated and differentiated single cells do not differentially release insulin in response to changes in medium glucose concentrations,14 a result consistent with single adult beta cells.17
After significantly increasing in size from day 0 to 7, differentiated islet-like clusters on day 14 are all below 125 μm in diameter, a cutoff above which mature isolated rat islets have lower in vitro viability and stimulated insulin release, as well as a diminished in vivo efficacy.42 Among necessary nutrients for cell growth, oxygen availability is typically limiting due to its low solubility in the absence of a carrier protein such as hemoglobin,43 and studies indicate that the maximum size of islets that maintain sufficient oxygen diffusion ranges from 100 to 150 μm.44,45 Our data demonstrate that encapsulated cell clusters transition through a period of proliferation and increasing cluster size; however, once differentiation commences, differentiating islet-like clusters self-regulate to a size conducive for sufficient oxygen diffusion throughout the aggregate.
In summary, we report in this study the in vitro differentiation of induced clusters of undifferentiated embryonic pancreatic precursor cells into islet-like structures within a synthetic hydrogel platform. We have shown that inhibiting Notch signaling between neighboring precursor cells within clusters permits islet-like differentiation; conversely, when Notch signaling is not interrupted, only scattered insulin+ cells are observed. After 2 weeks in culture, clusters of precursor cells encapsulated within a PEGCol hydrogel and exposed to gamma-secretase inhibitor have similar insulin contents to those of freshly isolated adult rodent islets. Additionally, glucose responsiveness is comparable to mature islets cultured in similar base media. Cluster counts on day 14 are 88% of those on day 0, indicating excellent maintenance of cluster viability. Importantly, robust viability and islet-like differentiation were achieved in a transplantable scaffold known to protect encapsulated islets from immune infiltration.13 This study highlights the utility of the mechanically supportive and bioinert hydrogel culture platform to investigate specific effects of individual signaling molecules and pathways on pancreatic precursor cell development. Further, understanding how to convert Pdx1+ precursor cells into mature glucose-responsive beta cells in vitro is an important step toward creating a cell source that can replace or augment cadaveric islet donors. Progress has been made toward the meaningful differentiation of human embryonic stem cells into functional beta cells8–10; however, successful differentiation has so far only been demonstrated after in vivo implantation.11 If heterogeneous and undifferentiated embryonic-derived tissue could be terminally differentiated in vitro before implantation, a significant reduction in the risk associated with these potentially unlimited cell sources could be achieved. Our study suggests that a combination of precursor cell clustering, hydrogel encapsulation, and exposure to gamma-secretase inhibitor may provide an in vitro platform to facilitate final stages of differentiation from precursor cells into mature islet-like clusters.
Acknowledgments
This project was supported by Award No. VUMC35144 from the Beta Cell Biology Consortium and also by Award No. F30DK081278 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health.
Disclosure Statement
No competing financial interests exist.
References
- 1.Serup P. Madsen O.D. Mandrup-Poulsen T. Islet and stem cell transplantation for treating diabetes. BMJ. 2001;322:29. doi: 10.1136/bmj.322.7277.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liao Y.H. Verchere C.B. Warnock G.L. Adult stem or progenitor cells in treatment for type 1 diabetes: current progress. Can J Surg. 2007;50:137. [PMC free article] [PubMed] [Google Scholar]
- 3.Rogers S.A. Liapis H. Hammerman M.R. Intraperitoneal transplantation of pancreatic anlagen. Asaio J. 2003;49:527. doi: 10.1097/01.mat.0000084174.33319.7f. [DOI] [PubMed] [Google Scholar]
- 4.Rogers S.A. Liapis H. Hammerman M.R. Normalization of glucose post-transplantation of pig pancreatic anlagen into non-immunosuppressed diabetic rats depends on obtaining anlagen prior to embryonic day 35. Transpl Immunol. 2005;14:67. doi: 10.1016/j.trim.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Wu F. Jagir M. Powell J.S. Long-term correction of hyperglycemia in diabetic mice after implantation of cultured human cells derived from fetal pancreas. Pancreas. 2004;29:e23. doi: 10.1097/00006676-200407000-00064. [DOI] [PubMed] [Google Scholar]
- 6.Lukinius A. Korsgren O. The transplanted fetal endocrine pancreas undergoes an inherent sequential differentiation similar to that in the native pancreas. An ultrastructural study in the pig-to-mouse model. Diabetes. 2001;50:962. doi: 10.2337/diabetes.50.5.962. [DOI] [PubMed] [Google Scholar]
- 7.Beattie G.M. Otonkoski T. Lopez A.D. Hayek A. Functional beta-cell mass after transplantation of human fetal pancreatic cells: differentiation or proliferation? Diabetes. 1997;46:244. doi: 10.2337/diab.46.2.244. [DOI] [PubMed] [Google Scholar]
- 8.D'Amour K.A. Bang A.G. Eliazer S. Kelly O.G. Agulnick A.D. Smart N.G. Moorman M.A. Kroon E. Carpenter M.K. Baetge E.E. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 9.Kahan B.W. Jacobson L.M. Hullett D.A. Ochoada J.M. Oberley T.D. Lang K.M. Odorico J.S. Pancreatic precursors and differentiated islet cell types from murine embryonic stem cells: an in vitro model to study islet differentiation. Diabetes. 2003;52:2016. doi: 10.2337/diabetes.52.8.2016. [DOI] [PubMed] [Google Scholar]
- 10.Shiraki N. Yoshida T. Araki K. Umezawa A. Higuchi Y. Goto H. Kume K. Kume S. Guided differentiation of embryonic stem cells into Pdx1-expressing regional-specific definitive endoderm. Stem Cells. 2008;26:874. doi: 10.1634/stemcells.2007-0608. [DOI] [PubMed] [Google Scholar]
- 11.Kroon E. Martinson L.A. Kadoya K. Bang A.G. Kelly O.G. Eliazer S. Young H. Richordson M. Smart N.G. Cunningham J. Agulnick A.D. D'Amour K.A. Carpenter M.K. Baetge E.E. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 12.Shi Y. Hou L. Tang F. Jiang W. Wang P. Ding M. Deng H. Inducing embryonic stem cells to differentiate into pancreatic beta cells by a novel three-step approach with activin A and all-trans retinoic acid. Stem Cells. 2005;23:656. doi: 10.1634/stemcells.2004-0241. [DOI] [PubMed] [Google Scholar]
- 13.Lee D.Y. Nam J.H. Byun Y. Effect of polyethylene glycol grafted onto islet capsules on prevention of splenocyte and cytokine attacks. J Biomater Sci Polym Ed. 2004;15:753. doi: 10.1163/156856204774196144. [DOI] [PubMed] [Google Scholar]
- 14.Mason M.N. Mahoney M.J. Selective beta-cell differentiation of dissociated embryonic pancreatic precursor cells cultured in synthetic polyethylene glycol hydrogels. Tissue Eng Part A. 2009;15:1343. doi: 10.1089/ten.tea.2008.0290. [DOI] [PubMed] [Google Scholar]
- 15.Lucas-Clerc C. Massart C. Campion J.P. Launois B. Nicol M. Long-term culture of human pancreatic islets in an extracellular matrix: morphological and metabolic effects. Mol Cell Endocrinol. 1993;94:9. doi: 10.1016/0303-7207(93)90046-m. [DOI] [PubMed] [Google Scholar]
- 16.Montesano R. Mouron P. Amherdt M. Orci L. Collagen matrix promotes reorganization of pancreatic endocrine cell monolayers into islet-like organoids. J Cell Biol. 1983;97:935. doi: 10.1083/jcb.97.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wojtusciszyn A. Armanet M. Morel P. Berney T. Bosco D. Insulin secretion from human beta cells is heterogeneous and dependent on cell-to-cell contacts. Diabetologia. 2008;51:1843. doi: 10.1007/s00125-008-1103-z. [DOI] [PubMed] [Google Scholar]
- 18.Kadesch T. Notch signaling: the demise of elegant simplicity. Curr Opin Genet Dev. 2004;14:506. doi: 10.1016/j.gde.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Jensen J. Pedersen E.E. Galante P. Hald J. Heller R.S. Ishibashi M. Kageyama R. Guillement F. Serup P. Madsen O.D. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- 20.Apelqvist A. Li H. Sommer L. Beatus P. Anderson D. Honjo T. de Angelis M. Lendahl U. Edlund H. Notch signalling controls pancreatic cell differentiation. Nature. 1999;400:877. doi: 10.1038/23716. [DOI] [PubMed] [Google Scholar]
- 21.Sawhney A. Pathak C. Hubbell J. Bioerodible hydrogels based on photopolymerized poly(ethylene glycol)-co-poly(alpha-hydroxy acid) diacrylate macromers. Macromolecules. 1993;26:581. [Google Scholar]
- 22.Mason M.N. Arnold C.A. Mahoney M.J. Entrapped collagen type 1 promotes differentiation of embryonic pancreatic precursor cells into glucose-responsive beta-cells when cultured in three-dimensional PEG hydrogels. Tissue Eng Part A. 2009;15:3799. doi: 10.1089/ten.tea.2009.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yashpal N.K. Li J. Wheeler M.B. Wang R. Expression of {beta}1 integrin receptors during rat pancreas development—sites and dynamics. Endocrinology. 2005;146:1798. doi: 10.1210/en.2004-1292. [DOI] [PubMed] [Google Scholar]
- 24.Wang R.N. Rosenberg L. Maintenance of beta-cell function and survival following islet isolation requires re-establishment of the islet-matrix relationship. J Endocrinol. 1999;163:181. doi: 10.1677/joe.0.1630181. [DOI] [PubMed] [Google Scholar]
- 25.Bryant S.J. Anseth K.S. Controlling the spatial distribution of ECM components in degradable PEG hydrogels for tissue engineering cartilage. J Biomed Mater Res A. 2003;64:70. doi: 10.1002/jbm.a.10319. [DOI] [PubMed] [Google Scholar]
- 26.Weber L.M. Hayda B.S. Anseth K.A. Cell-matrix interactions improve beta cell survival and insulin secretion in three-dimensional culture. Tissue Eng Part A. 2008;14:1959. doi: 10.1089/ten.tea.2007.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esni F. Stoffers D.A. Takeuchi T. Leach S.D. Origin of exocrine pancreatic cells from nestin-positive precursors in developing mouse pancreas. Mech Dev. 2004;121:15. doi: 10.1016/j.mod.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Gittes G.K. Galante P.E. Hanahan D. Rutter W.J. Debase H.T. Lineage-specific morphogenesis in the developing pancreas: role of mesenchymal factors. Development. 1996;122:439. doi: 10.1242/dev.122.2.439. [DOI] [PubMed] [Google Scholar]
- 29.Borjesson A. Carlsson C. Altered proinsulin conversion in rat pancreatic islets exposed long-term to various glucose concentrations or interleukin-1beta. J Endocrinol. 2007;192:381. doi: 10.1677/joe.1.06676. [DOI] [PubMed] [Google Scholar]
- 30.Murtaugh L.C. Stanger B.Z. Kwan K.M. Melton D.A. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci USA. 2003;100:14920. doi: 10.1073/pnas.2436557100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Artavanis-Tsakonas S. Matsuno K. Fortini M.E. Notch signaling. Science. 1995;268:225. doi: 10.1126/science.7716513. [DOI] [PubMed] [Google Scholar]
- 32.Schwitzgebel V.M. Scheel D.W. Conners J.R. Kalamaras J. Lee J.E. Anderson D.J. Sussel L. Johnson J.D. German M.S. Expression of neurogenin3 reveals an islet cell precursor population in the pancreas. Development. 2000;127:3533. doi: 10.1242/dev.127.16.3533. [DOI] [PubMed] [Google Scholar]
- 33.Gradwohl G. Dierich A. LeMeur M. Guillemot F. Neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci USA. 2000;97:1607. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jensen J. Heller R.S. Funder-Nielsen T. Pedersen E.E. Lindsell C. Weinmaster G. Madsen O.D. Serup P. Independent development of pancreatic alpha- and beta-cells from neurogenin3-expressing precursors: a role for the notch pathway in repression of premature differentiation. Diabetes. 2000;49:163. doi: 10.2337/diabetes.49.2.163. [DOI] [PubMed] [Google Scholar]
- 35.Le Lay J. Stein R. Involvement of PDX-1 in activation of human insulin gene transcription. J Endocrinol. 2006;188:287. doi: 10.1677/joe.1.06510. [DOI] [PubMed] [Google Scholar]
- 36.Edlund H. Developmental biology of the pancreas. Diabetes. 2001;50(Suppl 1):S5. doi: 10.2337/diabetes.50.2007.s5. [DOI] [PubMed] [Google Scholar]
- 37.Duvillie B. Attali M. Bounacer A. Ravassard P. Basmaciogullari A. Scharfmann R. The mesenchyme controls the timing of pancreatic beta-cell differentiation. Diabetes. 2006;55:582. doi: 10.2337/diabetes.55.03.06.db05-0839. [DOI] [PubMed] [Google Scholar]
- 38.Olivier A. Lauret E. Gonin P. Galy A. The Notch ligand delta-1 is a hematopoietic development cofactor for plasmacytoid dendritic cells. Blood. 2006;107:2694. doi: 10.1182/blood-2005-03-0970. [DOI] [PubMed] [Google Scholar]
- 39.Tian Y. Laychock S.G. Prolactin regulates adenylyl cyclase and insulin secretion in rat pancreatic islets. Mol Cell Endocrinol. 2003;204:75. doi: 10.1016/s0303-7207(03)00126-6. [DOI] [PubMed] [Google Scholar]
- 40.Rulifson I. Karnik S. Heiser P. Ten Berge D. Chen H. Gu X. Taketo M. Nusse R. Hebrok M. Kim S.K. Wnt signaling regulates pancreatic beta cell proliferation. Proc Natl Acad Sci USA. 2007;104:6247. doi: 10.1073/pnas.0701509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holmes M.A. Clayton H.A. Chadwick D.R. Bell P.R. London N.J. James R.F. Functional studies of rat, porcine, and human pancreatic islets cultured in ten commercially available media. Transplantation. 1995;60:854. [PubMed] [Google Scholar]
- 42.MacGregor R.R. Williams S.J. Tong P.Y. Kover K. Moore W.V. Stehno-Bittel L. Small rat islets are superior to large islets in in vitro function and in transplantation outcomes. Am J Physiol Endocrinol Metab. 2006;290:E771. doi: 10.1152/ajpendo.00097.2005. [DOI] [PubMed] [Google Scholar]
- 43.Tannock I.F. Oxygen diffusion and the distribution of cellular radiosensitivity in tumours. Br J Radiol. 1972;45:515. doi: 10.1259/0007-1285-45-535-515. [DOI] [PubMed] [Google Scholar]
- 44.Lehmann R. Zuellig R.A. Kugelmeier P. Baenninger P.B. Moritz W. Perren A. Clavien P.A. Webber M. Spinas G.A. Superiority of small islets in human islet transplantation. Diabetes. 2007;56:594. doi: 10.2337/db06-0779. [DOI] [PubMed] [Google Scholar]
- 45.Buchwald P. FEM-based oxygen consumption and cell viability models for avascular pancreatic islets. Theor Biol Med Model. 2009;6:5. doi: 10.1186/1742-4682-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]