Abstract
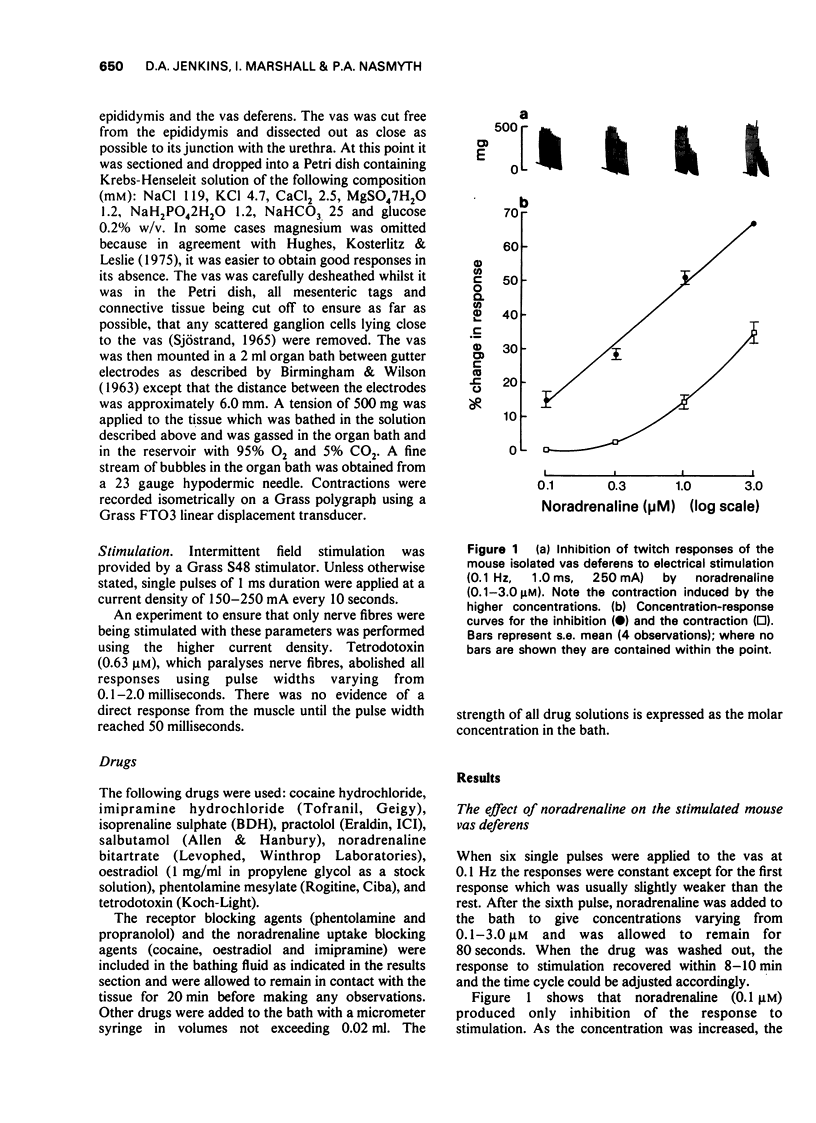
1 Noradrenaline (0.1-3.0 μM) inhibited the twitch responses to single pulse field stimulation of the isolated vas deferens of the mouse. The higher concentrations of noradrenaline (ca. 0.3-3.0 μM) were required to make the tissue contract.
2 Phentolamine (10 μM) abolished the contractor response to higher concentrations of noradrenaline and antagonized the inhibitory effect of lower concentrations on the twitch response.
3 Propranolol (10 μM) potentiated both the contractor and the inhibitory effect of noradrenaline on the twitch response.
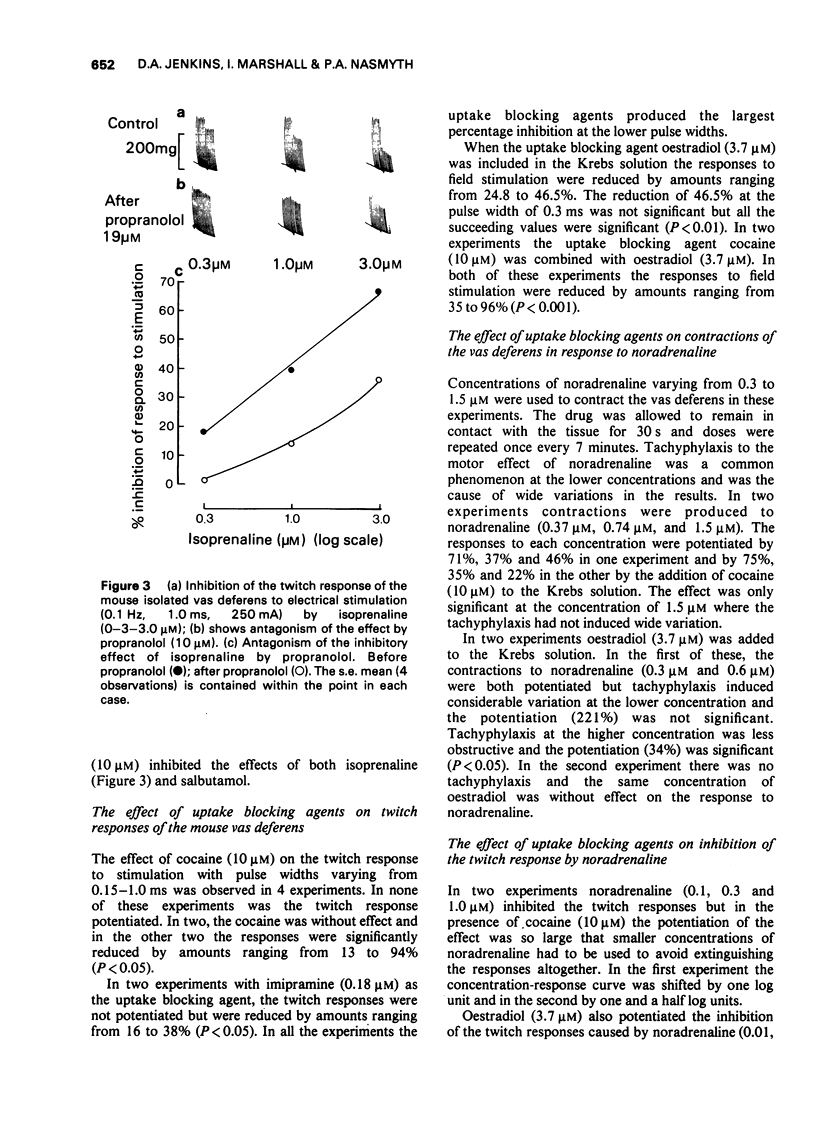
4 Isoprenaline (0.1-3.0 μM) and salbutamol (1.0-3.0 μM) both inhibited the twitch response. Their effects were antagonized by propranolol (10 μM), but not by practolol (10 μM).
5 The effects of uptake1 and uptake2 blocking agents were determined. Cocaine (10 μM) reduced the size of the twitch response in 2 out of 4 experiments. Imipramine (0.18 μM) also reduced the size of the twitch, as did oestradiol (3.7 μM) and a combination of cocaine and oestradiol.
6 Contractor responses to exogenous noradrenaline showed tachyphylaxis, but when this was not very marked, the response could be shown to be potentiated by uptake blocking agents.
7 The inhibitory effect of noradrenaline on the twitch response was greatly potentiated by cocaine (10 μM) and much less so by oestradiol (3.7 μM).
8 It is concluded that the transmitter responsible for the twitch response is either an unknown substance released from the sympathetic neurone, or noradrenaline acting upon a receptor with none of the characteristics of known α- or β-adrenoceptors. In either case, noradrenaline can inhibit the output, probably by stimulation of presynaptic α-adrenoceptors.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambache N., Dunk L. P., Verney J., Zar M. A. Inhibition of post-ganglionic motor transmission in vas deferens by indirectly acting sympathomimetic drugs. J Physiol. 1972 Dec;227(2):433–456. doi: 10.1113/jphysiol.1972.sp010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambache N., Zar M. A. Evidence against adrenergic motor transmission in the guinea-pig vas deferens. J Physiol. 1971 Jul;216(2):359–389. doi: 10.1113/jphysiol.1971.sp009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BHARGAVA K. P., KAR K., PARMAR S. S. INDEPENDENT CHOLINERGIC AND ADRENERGIC MECHANISMS IN THE GUINEA-PIG ISOLATED NERVE-VAS DEFERENS PREPARATION. Br J Pharmacol Chemother. 1965 Jun;24:641–650. doi: 10.1111/j.1476-5381.1965.tb01620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRMINGHAM A. T., WILSON A. B. PREGANGLIONIC AND POSTGANGLIONIC STIMULATION OF THE GUINEA-PIG ISOLATED VAS DEFERENS PREPARATION. Br J Pharmacol Chemother. 1963 Dec;21:569–580. doi: 10.1111/j.1476-5381.1963.tb02024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham A. T., Freeman M. A. The relation between stimulus frequency and the relative size of the components of the biphasic response of the vas deferens to electrical stimulation at different temperatures. J Physiol. 1976 Apr;256(3):747–759. doi: 10.1113/jphysiol.1976.sp011349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakeley A. G., Dearnaley D. P., Harrison V. The noradrenaline content of the vas deferens of the guinea-pig. Proc R Soc Lond B Biol Sci. 1970 Jan 20;174(1037):491–502. doi: 10.1098/rspb.1970.0007. [DOI] [PubMed] [Google Scholar]
- Enero M. A., Langer S. Z., Rothlin R. P., Stefano F. J. Role of the -adrenoceptor in regulating noradrenaline overflow by nerve stimulation. Br J Pharmacol. 1972 Apr;44(4):672–688. doi: 10.1111/j.1476-5381.1972.tb07306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnebo L. O., Hamberger B. Drug-induced changes in the release of ( 3 H)-noradrenaline from field stimulated rat iris. Br J Pharmacol. 1971 Sep;43(1):97–106. doi: 10.1111/j.1476-5381.1971.tb07160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness J. B., Campbell G. R., Gillard S. M., Malmfors T., Cobb J. L., Burnstock G. Cellular studies of sympathetic denervation produced by 6-hydroxydopamine in the vas deferens. J Pharmacol Exp Ther. 1970 Jul;174(1):111–122. [PubMed] [Google Scholar]
- Henderson G., Hughes J., Kosterlitz H. W. A new example of a morphine-sensitive neuro-effector junction: adrenergic transmission in the mouse vas deferens. Br J Pharmacol. 1972 Dec;46(4):764–766. doi: 10.1111/j.1476-5381.1972.tb06901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins D. A., Marshall I., Nasmyth P. A. Proceedings: Is morphine inhibition to the twitch response of the mouse vas deferens mediated via noradrenaline? Br J Pharmacol. 1975 Oct;55(2):267P–268P. [PMC free article] [PubMed] [Google Scholar]
- Jones M. E., Spriggs T. L. Noradrenaline and motor transmission in the vas deferens of the mouse. Br J Pharmacol. 1975 Mar;53(3):323–331. [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Puig M. Effect of flow-stop on noradrenaline release from normal spleens and spleens treated with cocaine, phentolamine or phenoxybenzamine. Br J Pharmacol. 1971 Oct;43(2):359–369. [PMC free article] [PubMed] [Google Scholar]
- SJOSTRAND N. O. Effect of reserpine and hypogastric denervation on the noradrenaline content of the vas deferens and the seminal vesicle of the guinea-pig. Acta Physiol Scand. 1962 Nov-Dec;56:376–380. doi: 10.1111/j.1748-1716.1962.tb02513.x. [DOI] [PubMed] [Google Scholar]
- Spriggs T. L., Lever J. D., Rees P. M., Graham J. D. Controlled formaldehyde-catecholamine condensation in cryostat sections to show adrenergic nerves by fluorescence. Stain Technol. 1966 Nov;41(6):323–327. doi: 10.3109/10520296609116333. [DOI] [PubMed] [Google Scholar]
- Swedin G. Biphasic mechanical response of the isolated vas deferens to nerve stimulation. Acta Physiol Scand. 1971 Apr;81(4):574–576. doi: 10.1111/j.1748-1716.1971.tb04936.x. [DOI] [PubMed] [Google Scholar]
- von Euler U. S., Hedqvist P. Evidence for an alpha- and beta2-receptor mediated inhibition of the twitch response in the guinea pig vas deferens by noradrenaline. Acta Physiol Scand. 1975 Apr;93(4):572–573. doi: 10.1111/j.1748-1716.1975.tb05853.x. [DOI] [PubMed] [Google Scholar]


