Abstract
Three cases of abnormalities of elastic fibers, two of them on the floor of the mouth and one on the lingual alveolar mucosa, close to the floor of the mouth, in a patient with history of homolateral squamous cell carcinoma of the floor of the mouth, are presented. Comparison with elastofibromatous changes and elastofibromas are made and their possible pathogenesis is discussed. It is suggested that increased awareness may facilitate recognition of such lesions as they can be easily overlooked, especially when they do not present as discrete tumors or they are associated with other “more significant” pathologic processes.
Keywords: Oral tumors, Elastic tissue, Elastofibroma, Hyperelastosis
Introduction
Acquired disorders of elastic fibers are broadly classified into disorders of increased elastic fibers, solar elastotic dermatoses, and disorders of decreased elastic fibers or elastolysis [1]. Although elastic fibers constitute a significant component of the connective tissue of the oral mucosa, intraoral acquired disorders of elastic tissue have hardly been described, save for the well-known actinic (solar) cheilitis. This may be due to either their true rarity or to being simply overlooked, in particular when they do not comprise the main component of a lesion. A review of the pertinent literature has disclosed only two cases of oral elastofibroma [2, 3], and a single case of penicillamine-induced elastosis of the lip mucosa in a patient with Wilson’s disease [4].
Herein, we present three cases of abnormalities of elastic fibers, two of them on the floor of the mouth and one on the lingual alveolar mucosa in a patient with history of homolateral squamous cell carcinoma of the floor of the mouth.
Methods and Materials
Three cases from the files of the Division of Oral and Maxillofacial Pathology, School of Dentistry, University of Minnesota were retrieved as part of a search looking for keywords “elastic fibers”, “elastofibroma” or “elastosis” among intraoral lesions in the period 1992–2009. Clinical data were obtained from pathology reports. Hematoxylin and eosin and Vierhoeff-van Gieson elastic stains were available in all cases.
Results
The Clinicopathologic Characteristics of the Three Cases are as Follows:
Case 1
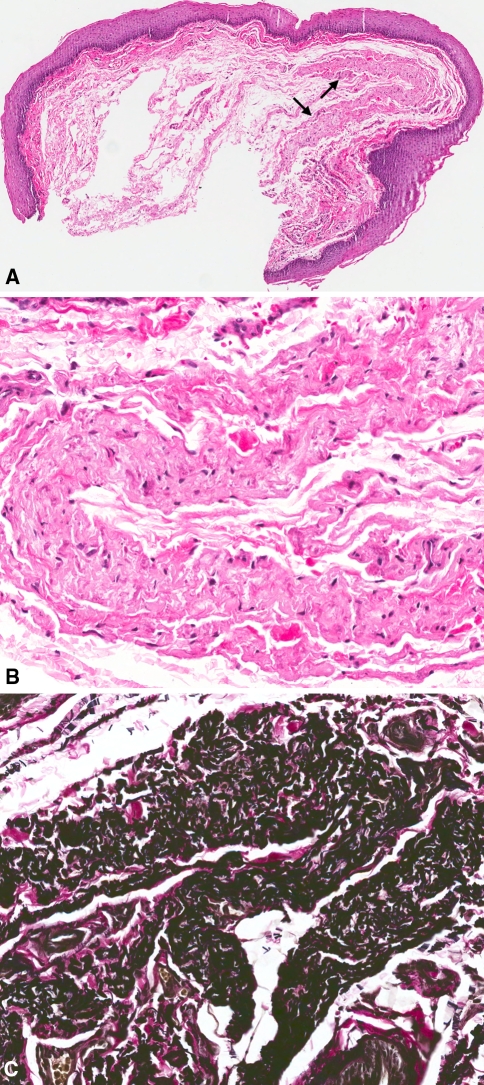
A 76-year-old Caucasian female presented with a “small, flat, white area” on the left anterior floor of the mouth. Microscopic evaluation revealed mucosal fragment surfaced by focally slightly hyperparakeratinizing stratified squamous epithelium (Fig. 1a). The connective tissue was loose and featured a well defined, pale eosinophilic to amphophilic, hypocellular and Congo red negative zone. Irregular fibrillar and globular deposits were present (Fig. 1b). Vierhoeff-van Gieson stain revealed thickened, occasionally wavy and fragmented elastic fibers (Fig. 1c). The diagnosis was focal hyperkeratosis and elastofibromatous changes suggestive of early elastofibroma.
Fig. 1.
a Mucosal fragment surfaced by hyperkeratinizing stratified squamous epithelium with a well defined, eosinophilic to amphophilic, and hypocellular area (arrows) in the connective tissue (H and E, original magnification ×40). b Irregular fibriliar and globular deposits (H and E, original magnification ×200). c Thickened, wavy, and fragmented elastic fibers (Vierhoeff-van Gieson elastic stain, original magnification ×200)
Case 2
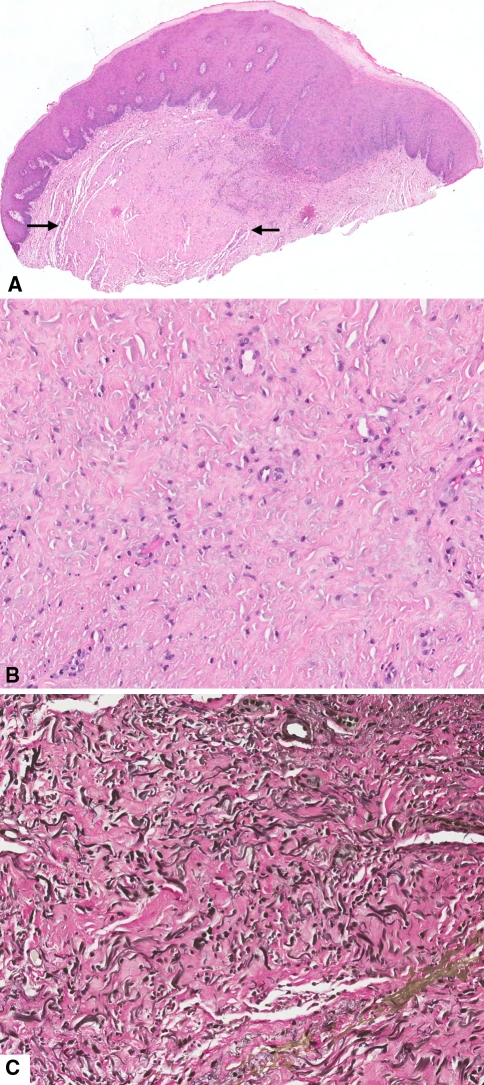
A 98-year-old Caucasian female presented clinically with “an area of leukoplakia” on the lingual aspect of the right alveolar mucosa close to the floor of the mouth. The patient had history of squamous cell carcinoma of the right floor of mouth. Microscopic evaluation revealed mucosal fragment surfaced by hyperkeratinizing stratified squamous epithelium featuring exfoliation of the superficial layer and acanthosis. Focally, cytologic variations of the basal cell layer were present. Subjacent to the epithelium there was fibrinous exudate and a neutrophilic infiltrate. Interestingly, and giving the lesion a partial nodular pattern, there was a mass of collagen fibers (Fig. 2a) where prominent bead-like, comma-shaped and crinkled, amphophilic deposits consistent with elastic fibers were present (Fig. 2b). Vierhoeff-van Gieson elastic stain revealed hyperplastic wavy, fragmented and less frequently globe-shaped elastic fibers intermingled with dense collagen (Fig. 2c). The diagnosis was hyperkeratosis with mild epithelial dysplasia, inflammation and elastofibromatous changes.
Fig. 2.
a Mucosal fragment surfaced by hyperkeratinizing stratified squamous epithelium with hypocellular area (arrows) in the connective tissue exhibiting dense collagen and ampophilic deposits consistent with elastic fibers. (H and E, original magnification ×20). b Amphophilic deposits consistent with elastic fibers (H and E, original magnification ×200). c Hyperplastic wavy, fragmented and globe-shaped elastic fibers intermingled with dense collagen (Vierhoeff-van Gieson elastic stain, original magnification ×200)
Case 3
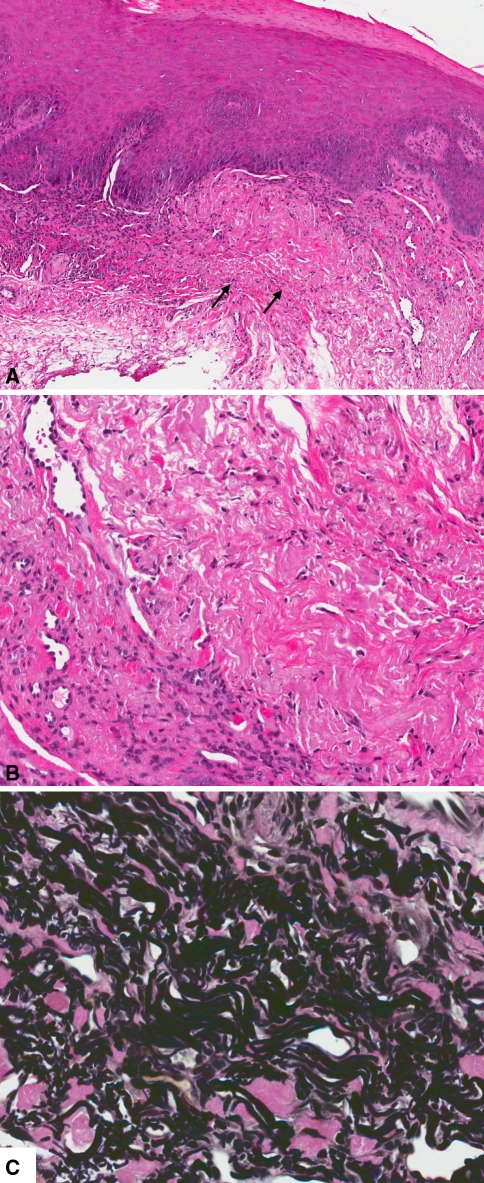
An 84-year-old female presented with a well-demarcated “ulcer” in the right floor of the mouth, just lingual to Wharton’s duct. The lesion was totally excised under the differential diagnosis of aphtha, traumatic lesion, or dysplasia. Microscopic examination revealed mucosal fragment covered by partially hyperparakeratinized and acanthotic stratified squamous epithelium, with focal basal cell hyperplasia and nuclear hyperchromatism of apparent reactive nature. The connective tissue exhibited chronic and subacute inflammation in association with increased vascularity and fibrosis (Fig. 3a). Predominantly in the area of fibrosis, and to a lesser extend in the inflamed region, the connective tissue exhibited amphophilic changes consistent with hyperplastic elastic tissue (Fig. 3b). Vierhoeff-van Gieson elastic stain revealed hyperplastic wavy and fragmented elastic fibers (Fig. 3c). There was no evidence of ulceration in the preparation examined. The diagnosis was “hyperkeratosis and inflammation, with hyperelastosis”.
Fig. 3.
a Mucosal fragment covered by partially hyperparakeratinized and acanthotic stratified squamous epithelium with increased vascularity and amphophilic/eosinophilic deposits (arrows) in the connective tissue (H and E, original magnification ×80). b Amphophilic changes consistent with hyperplastic elastic tissue (H and E, original magnification ×200). c Hyperplastic wavy and fragmented elastic fibers (Vierhoeff-van Gieson elastic stain, original magnification ×400)
Discussion
Elastic fibers constitute one of the main types of connective tissue fibers and are found around large elastic arteries, in the dermis, the lungs, ligaments, and auricular cartilage [5]. Mature elastic fibers are composed of a central cross-linked core of elastin protein, surrounded by a microfibriliar sheath mainly composed of the glycoprotein fibrillin. Elasticity and resilience of dynamic connective tissues are their most prominent functions, but they also regulate the activity of the TGB-β family of growth factors, and cell migration, survival and differentiation, through control of cell attachment. A wide range of pathologic conditions are related to elastic fibers and include lesions of increased elastic tissue (e.g. elastoma, linear focal elastotis, elastofibroma, elastoderma), solar elastotic syndromes (e.g. solar elastosis, elastotic nodules of the ear, erythema ab igne), decreased elastic tissue (e.g. anetoderma, cutis laxa, Williams’ syndrome) and variable or minor elastic tissue changes (e.g. wrinkles, leprechaunism, scar tissue) [6].
The first case in our series featured changes essentially similar to those described by Goldblum et al. [7] in a 58-year-old female with a 7-year history of multiple myeloma who was treated with chemotherapy and radiation to the arms and pelvis, in that there was no evidence of a nodular growth and no other associated pathologic process of any significance, save of focal subtle hyperparakeratosis in our case. These elastofibromatous changes may be an early stage in the development of elastofibroma. Similar lesions have been reported in other mucosal sites i.e. the small bowel and the trachea of an 88-year-old female and a 67 year-old male, respectively who died from unrelated causes [8]. It is not clear whether elastofibromatous changes represent a reaction to trauma [9] or an initial, subclinical stage in the development of an elastofibroma, as it has been proposed for the elastofibromatous changes commonly seen in routine autopsy cases in the thoracic fascia under the lower portion of the scapula [10]. Interestingly, elastofibromatous changes have been found to occur in 2.6% out of 426 of all routine dorsal spine biopsies in a study by Daum et al. [11] indicating the presence of a “degenerative process practically unknown to contemporary medicine” with specific characteristics and treatment.
Elastofibroma (elastofibroma dorsi), on the other hand, is a benign tumor situated almost exclusively in the subscapular area, between the lower portion of the scapula and the chest wall. It was first described by Järvi and Saxén in 1961 [12]. More than 300 cases have been reported up to now [1], 170 of them included in a study from Okinawa, Japan with more than 30% of patients found to have a family history [13]. Most patients are women, generally older than 55 years [14]. It typically manifests as a slowly growing, large, solid, ill-defined, and non-adherent mass of fibroelastic tissue that rarely causes tenderness, pain or functional disturbance [14]. Rare examples have been reported in other anatomic sites, including the, gastrointestinal tract [15, 16], greater omentum [17], eye [18, 19], and skin [20]. Bilateral and multiple tumors may be seen, especially in the subscapular area [13] or subcutaneously [20].
Microscopically, elastofibromas of the subscapular area consist of about equal proportions of fibrous and elastic tissue, variously sized islets of mature fat, and small amounts of interstitial mucoid material [13, 14]. The collagen fibers are swollen eosinophilic and intertwining, while the elastic fibers appear as homogenous, branched or unbranched fibrillar or globular structures, with a distinct linear arrangement [14], and may show a perivascular, pseudo-amyloid arrangement [21]. They react strongly with elastic stains, such as Weigert’s, Verhoeff’s or Gömöri’s methods, and present a central dense core and irregular edges [14]. Terms such as “beaded”, “test-tube brush”, “scrub-brush”, or “serrated” and when fragmented “flower-like”, “petaloid”, “moth-eaten”, or globular “chenille bodies”, have been used to describe their pattern variations. Histochemical evaluation has disclosed darkly stained central cord or cords surrounded by a weaker stained mantle substance of elastic tissue which contains in addition reticular fibers [22]. Ultrastructurally, the central cord does not differ in size from mature normal elastic tissue while the mantle features haphazard arrangement of electrolucent and electrodense fibrillar material. The reticular fibers are seen invading this mantle. Immunohistochemically, they are strongly positive for elastin [23–25] and vitronectin, show a patchy reaction for bone sialoprotein, and are negative for fibrillin-1, fibronectin and osteonectin [25]. Furthermore, they are digested by elastase or pepsin, and give a green fluorescence under ultraviolet light [14].
The origin of the spindle or stellate cells that are interspersed among the collagen and elastic fibers of elastofibroma is a matter of dispute. The cells are unanimously positive for vimentin [23–27], and most react for CD34 [23, 25–28] or bone sialoprotein [25]. Positive reaction for other antigens, such as promin-2, Cx43, Factor XIIIa, MEF-2 transcription factor [23, 27], and TGF-β [28] has been reported in some studies. They are considered as fibroblasts [28] or consistent with the CD34 + dendritic and fibroblast-like cells of the reticular dermis and fibro-collagenous soft tissues that are thought to act as potential mesenchymal stem cells [26]. It has also been proposed that they may be periosteal fibroblasts, as elastofibromas are usually, although not always, found in the proximity of the periosteum [29]. In contrast, they are not myofibroblasts, as is shown by negativity for myogenic markers, such as actins, desmin, calponin, or caldesmon [23–28], as well as their ultrastructural features [23]. Tryptase positive mast cells [26] and CD105 positive blood vessels [21] have been implicated in the pathogenesis of the lesion.
The pathogenesis of elastofibroma is debatable, but most evidence suggests a reactive lesion caused by a local mechanical stimulus that induces reactive abnormal elastogenesis by stromal cells [1, 21, 29] followed by degeneration, appearing first as segmentation of the hyperplastic fibers and then as breaking up of the fibers into globes that are gradually resorbed [22]. Elastotic degeneration of collagen fibers and degeneration of existing elastic fibers do not seem probable [29]. Interestingly, some investigators have suggested a neoplastic origin for elastofibroma based on the identification of clonal chromosomal abnormalities [30, 31] and the nonrandom inactivation pattern of X-chromosome shown in a few cases [23].
Two intraoral cases of elastofibroma have been documented [2]. Both tumors presented on the left side of the floor of the mouth and were asymptomatic, small white, smooth-surfaced, and well-circumscribed nodules. In one patient there was a history of accidental injury of the area, approximately 52 years before diagnosis of the elastofibroma [2], while in the other the tumor developed in site of a squamous cell carcinoma 2 years after resection and radiation therapy [3].
Two of our cases featuring hyperplastic elastic fibers, one with elastofibromatous-like appearance, were associated with other pathologic processes. One patient had history of homolateral squamous cell carcinoma, a similar setting as with the patient reported by Manchandu et al. [3]. In our case there was, however, mild epithelial dysplasia and focal acute inflammation. The other was in association with an “ulcer” and histologically featured reactive hyperparakeratosis, epithelial hyperplasia and chronic inflammation.
The predilection for the floor of the mouth and the frequency of such changes in the floor of the mouth and adjacent areas are unknown. However, one can hypothesize that the movement of the tongue and neck may necessitate the presence of a well developed elastic component in the connective tissue of the floor of the mouth. Elastic hyperplasia and elastofibromatous changes may be the result of trauma or aging. In our patients there was no history of penicillamine chelation treatment that may have caused a marked increase of elastic fibers. Be that as it may, as we stated in the introduction, such changes may be overlooked especially if they are early and when associated with other “more significant” pathologic processes. However, they should be recognized and, hopefully, studied in greater detail to elucidate their pathophysiologic significance.
In summary, we presented three oral lesions with elastic fiber abnormalities, two of them elastofibromatous, the third characterized by unusual hyperelastosis. Their predilection for the floor of the mouth and neighboring areas is of interest. The presence of such changes can be easily overlooked, especially when they do not present as discrete tumors or they are associated with other more significant pathologic processes. Increased awareness may enhance recognition of oral elastofibromatous and hyperelastotic changes and instigate studies to elucidate their pathogenesis and significance.
Acknowledgment
The authors are indebted to Mr. Jonathan Henriksen (University of Minnesota) for his superb assistance with the illustrations.
References
- 1.Lewis KG, Bercovitch L, Dill SW, Robinson-Bostom L. Acquired disorders of elastic tissue: part I. Increased elastic tissue and solar elastotic syndromes. J Am Acad Dermatol. 2004;51:1–21. doi: 10.1016/j.jaad.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Potter TJ, Summerlin DJ, Rodgers SF. Elastofibroma: the initial report in the oral mucosa. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;97:64–67. doi: 10.1016/j.tripleo.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 3.Manchandu R, Foote J, Alawi F. Elastofibroma presenting as an oral soft tissue mass. J Oral Pathol Med. 2008;37:125–126. doi: 10.1111/j.1600-0714.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- 4.Lewis BK, Chern PL, Stone MS. Penicillamine-induced elastosis of the mucosal lip. J Am Acad Dermatol. 2009;60:700–703. doi: 10.1016/j.jaad.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 5.Kielty CM. Elastic fibres in health and disease. Expert Rev Mol Med. 2006;8:1–23. doi: 10.1017/S146239940600007X. [DOI] [PubMed] [Google Scholar]
- 6.Weedon D. Skin Pathology. 2. Philadelphia: Churchill Livingstone; 2002. pp. 381–404. [Google Scholar]
- 7.Goldblum JR, Beals T, Weiss SW. Elastofibromatous change of the rectum. A lesion mimicking amyloidosis. Am J Surg Pathol. 1992;16:793–795. doi: 10.1097/00000478-199208000-00008. [DOI] [PubMed] [Google Scholar]
- 8.Schiffman R. Elastofibromatous lesion. Am J Surg Pathol. 1993;17:951. doi: 10.1097/00000478-199309000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Enjoji M, Sumiyoshi K, Sueyoshi K. Elastofibromatous lesion of the stomach in a patient with elastofibroma dorsi. Am J Surg Pathol. 1985;9:233–237. doi: 10.1097/00000478-198503000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Jarvi OH, Lansimies PH. Subclinical elastofibromas in the scapular region in an autopsy series. Acta Pathol Microbiol Scand A. 1975;83:87–108. doi: 10.1111/j.1699-0463.1975.tb01361.x. [DOI] [PubMed] [Google Scholar]
- 11.Daum O, Ferda J, Curik R, Choc M, Mukensnabl P, Michal M. Elastofibromatous changes in tissues from spinal biopsies. A degenerative process afflicting a small but important subset of patients operated for spinal canal compression: Report of 18 cases. Int J Surg Pathol. 2009. [DOI] [PubMed]
- 12.Jarvi O, Saxen E. Elastofibroma dorse. Acta Pathol Microbiol Scand Suppl. 1961;51(Suppl 144):83–84. [PubMed] [Google Scholar]
- 13.Nagamine N, Nohara Y, Ito E. Elastofibroma in Okinawa. A clinicopathologic study of 170 cases. Cancer. 1982;50:1794–1805. doi: 10.1002/1097-0142(19821101)50:9<1794::AID-CNCR2820500925>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 14.Enzinger FM, Weiss SW. Soft Tissue Tumors. 3. St.Louis, MO.: Mosbly; 1995. pp. 187–191. [Google Scholar]
- 15.Saint-Paul MC, Musso S, Cardot-Leccia N, Chevallier A, Myx A, Baldini E, et al. Elastofibroma of the stomach. Pathol Res Pract. 2003;199:637–639. doi: 10.1078/0344-0338-00474. [DOI] [PubMed] [Google Scholar]
- 16.Sakatani T, Shomori K, Adachi H, Hosoda A, Ito H. Elastofibroma of the sigmoid colon. Pathol Res Pract. 2000;196:205–207. doi: 10.1016/S0344-0338(00)80102-8. [DOI] [PubMed] [Google Scholar]
- 17.Tsutsumi A, Kawabata K, Taguchi K, Doi K. Elastofibroma of the greater omentum. Acta Pathol Jpn. 1985;35:233–241. doi: 10.1111/j.1440-1827.1985.tb02222.x. [DOI] [PubMed] [Google Scholar]
- 18.Austin P, Jakobiec FA, Iwamoto T, Hornblass A. Elastofibroma oculi. Arch Ophthalmol. 1983;101:1575–1579. doi: 10.1001/archopht.1983.01040020577016. [DOI] [PubMed] [Google Scholar]
- 19.Hsu JK, Cavanagh HD, Green WR. An unusual case of elastofibroma oculi. Cornea. 1997;16:112–119. doi: 10.1097/00003226-199701000-00019. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu S, Yasui C, Tateno M, Sato H, Homma S, Hirano E, et al. Multiple elastofibromas. J Am Acad Dermatol. 2004;50:126–129. doi: 10.1016/S0190-9622(03)00073-2. [DOI] [PubMed] [Google Scholar]
- 21.Kai K, Kusano K, Sakai M, Tabuchi M, Yunotani S, Miyazaki K, et al. Active neovascularization and possible vascular-centric development of gastric and periscapular elastofibromas. Virchows Arch. 2009;454:181–188. doi: 10.1007/s00428-008-0722-6. [DOI] [PubMed] [Google Scholar]
- 22.Järvi OH, Saxen AE, Hopsu-Havu VK, Wartiovaara JJ, Vaissalo VT. Elastofibroma––a degenerative pseudotumor. Cancer. 1969;23:42–63. doi: 10.1002/1097-0142(196901)23:1<42::AID-CNCR2820230105>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 23.Hisaoka M, Hashimoto H. Elastofibroma: clonal fibrous proliferation with predominant CD34-positive cells. Virchows Arch. 2006;448:195–199. doi: 10.1007/s00428-005-0053-9. [DOI] [PubMed] [Google Scholar]
- 24.Kayaselcuk F, Demirhan B, Kayaselcuk U, Ozerdem OR, Tuncer I. Vimentin, smooth muscle actin, desmin, S-100 protein, p53, and estrogen receptor expression in elastofibroma and nodular fasciitis. Ann Diagn Pathol. 2002;6:94–99. doi: 10.1053/adpa.2002.32377. [DOI] [PubMed] [Google Scholar]
- 25.Naouri M, Michenet P, Chassaing N, Martin L. Immunohistochemical characterization of elastofibroma and exclusion of ABCC6 as a predisposing gene. Br J Dermatol. 2007;156:755–758. doi: 10.1111/j.1365-2133.2006.07735.x. [DOI] [PubMed] [Google Scholar]
- 26.Gun BD, Bahadir B, Behzatoglu K, Gun MO, Ozdamar SO. Elastofibroma: a clinicopathologic and immunohistochemical study of seven cases and literature review. APMIS. 2007;115:115–119. doi: 10.1111/j.1600-0463.2007.apm_525.x. [DOI] [PubMed] [Google Scholar]
- 27.Yamazaki K. An ultrastructural and immunohistochemical study of elastofibroma: CD 34, MEF-2, prominin 2 (CD133), and factor XIIIa-positive proliferating fibroblastic stromal cells connected by Cx43-type gap junctions. Ultrastruct Pathol. 2007;31:209–219. doi: 10.1080/01913120701350365. [DOI] [PubMed] [Google Scholar]
- 28.Kuroda N, Hamaguchi N, Ohara M, Hirouchi T, Mizuno K, Hayashi Y, et al. Elastofibroma: a histochemical, immunohistochemical, and ultrastructural study of two patients. Med Mol Morphol. 2008;41:179–182. doi: 10.1007/s00795-007-0372-9. [DOI] [PubMed] [Google Scholar]
- 29.Kumaratilake JS, Krishnan R, Lomax-Smith J, Cleary EG. Elastofibroma: disturbed elastic fibrillogenesis by periosteal-derived cells? An immunoelectron microscopic and in situ hybridization study. Hum Pathol. 1991;22:1017–1029. doi: 10.1016/0046-8177(91)90010-M. [DOI] [PubMed] [Google Scholar]
- 30.McComb EN, Feely MG, Neff JR, Johansson SL, Nelson M, Bridge JA. Cytogenetic instability, predominantly involving chromosome 1, is characteristic of elastofibroma. Cancer Genet Cytogenet. 2001;126:68–72. doi: 10.1016/S0165-4608(00)00395-2. [DOI] [PubMed] [Google Scholar]
- 31.Batstone P, Forsyth L, Goodlad J. Clonal chromosome aberrations secondary to chromosome instability in an elastofibroma. Cancer Genet Cytogenet. 2001;128:46–47. doi: 10.1016/S0165-4608(01)00394-6. [DOI] [PubMed] [Google Scholar]