Abstract
Vibrio fischeri induces both anaerobic respiration and bioluminescence during symbiotic infection. In many bacteria, the oxygen-sensitive regulator FNR activates anaerobic respiration, and a preliminary study using the light-generating lux genes from V. fischeri MJ1 cloned in Escherichia coli suggested that FNR stimulates bioluminescence. To test for FNR-mediated regulation of bioluminescence and anaerobic respiration in V. fischeri, we generated fnr mutants of V. fischeri strains MJ1 and ES114. In both strains, FNR was required for normal fumarate- and nitrate-dependent respiration. However, contrary to the report in transgenic E. coli, FNR mediated repression of lux. ArcA represses bioluminescence, and ParcA-lacZ reporters showed reduced expression in fnr mutants, suggesting a possible indirect effect of FNR on bioluminescence via arcA. Finally, the fnr mutant of ES114 was not impaired in colonization of its host squid, Euprymna scolopes. This study extends characterization of FNR to the Vibrionaceae and underscores the importance of studying lux regulation in its native background.
Keywords: Photobacterium, Aliivibrio, luciferase, autoinduction, symbiosis
Introduction
Vibrio fischeri is a model for investigations of bioluminescence and mutualistic symbioses, two fields connected by the importance of oxygen. O2 is a substrate for the luminescence-producing enzyme luciferase, and luciferase may benefit V. fischeri by generating a more reduced environment in or near cells (Visick, et al., 2000, Timmins, et al., 2001). Reduction of O2 could be especially advantageous for this facultative anaerobe when it is colonizing animal tissue and may minimize the host’s ability to generate reactive oxygen species (Visick, et al., 2000). Luminescence emanating from bacteria colonizing the symbiotic light organ of the host indicates that O2 is present; however, evidence suggests that luciferase is O2-limited in this environment (Boettcher, et al., 1996) despite its high affinity (Km ~35 nM) for O2 (Bourgois, et al., 2001). Moreover, anaerobic respiration is apparently induced in symbiotic V. fischeri (Proctor & Gunsalus, 2000), consistent with the idea that [O2] is low in the light organ.
One regulator that might control anaerobic respiration and luminescence in response to [O2] is FNR. FNR regulates genes during the switch between aerobic and anaerobic growth in Escherichia coli and other bacteria, and it often activates genes responsible for anaerobic respiration (Browning, et al., 2002, Reents, et al., 2006, Fink, et al., 2007). Although FNR is expressed during both aerobic and anaerobic growth, it is only functional under microaerobic or anaerobic conditions due to its dependence on an oxygen-labile 4Fe-4S center (Khoroshilova, et al., 1995, Lazazzera, et al., 1996, Khoroshilova, et al., 1997, Kiley & Beinert, 1998). Under anaerobic conditions, [4Fe–4S]-FNR forms a functional dimer that binds DNA at a 5’-TTGAT(N4)ATCAA-3’ FNR-box sequence (Eiglmeier, et al., 1989), and it activates or represses transcription depending on the location of binding relative to the promoter (Wing, et al., 1995, Meng, et al., 1997, Marshall, et al., 2001).
FNR was reported to activate bioluminescence in transgenic E. coli carrying the V. fischeri MJ1 luxR-luxICDABEG region, which encodes the autoinducer-dependent lux activator LuxR, the autoinducer synthase LuxI, and the Lux proteins that produce bioluminescence (Muller-Breikreutz & Winkler, 1993). Although FNR-mediated regulation of luminescence is cited frequently (Meighen, 1994, Spiro, 1994, Sitnikov, et al., 1995, Ulitzur & Dunlap, 1995, Stevens & Greenberg, 1999), these data were only presented in preliminary form in a symposium report (Muller-Breikreutz & Winkler, 1993).
We have examined fnr in two V. fischeri strains: ES114 and MJ1. ES114’s genome is sequenced, and its symbiosis with the squid E. scolopes can be reconstituted in the laboratory (Ruby, et al., 2005, Stabb, 2006); however, like most isolates from these animals ES114 is not visibly luminescent in culture (Boettcher & Ruby, 1990). In contrast, MJ1 has bright luminescence typical of isolates from the pinecone fish Monocentris japonica, but this symbiosis is not yet experimentally tractable. The genes required for luminescence and autoinduction are similar in the two strains, with the luxICDABEG operon adjacent to and divergently transcribed from luxR (Gray & Greenberg, 1992). However, there are differences in the luxR-luxI intergenic region, and notably there is a putative FNR box upstream of luxR in MJ1 that is absent in ES114. Our goals were to examine V. fischeri to assess FNR’s regulation of luminescence and anaerobic respiration, and to determine whether FNR contributes to symbiotic competence.
Materials and Methods
Bacteria and media
Bacterial strains used in this study are described in Table 1. E. coli was grown in LB (Miller, 1992) or in M9 (Sambrook, et al., 1989) supplemented with 1 mg ml−1 casamino acids, 40 mM glycerol and 40 mM of either sodium nitrate or sodium fumarate. V. fischeri was grown in LBS (Stabb, et al., 2001), SWT (Boettcher & Ruby, 1990) wherein seawater was replaced with Instant Ocean (Aquarium Systems, Mentor, OH), SWTO medium (Bose, et al., 2007), or in a defined salts medium (Adin, et al., 2009) with 40 mM glycerol as a carbon source, 1 mg ml−1 casamino acids, and 40 mM of sodium nitrate or sodium fumarate. 15 mg ml−1 agar was added to solidify media for plating. Anaerobic growth on plates was assessed using the GasPak EZ Anaerobic Container System from Becton, Dickinson and Company (Sparks, MD). Antibiotics were added as previously described for selection (Stabb & Ruby, 2002), and N-3-oxo-hexanoyl homoserine lactone (3-oxo-C6-HSL) autoinducer was added to media at 140 nM.
Table 1.
Select bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Reference |
|---|---|---|
| Bacterial strains | ||
| E. coli | ||
| CC118λpir | Δ(ara-leu) araD ΔlacX74 galE galK phoA20 thi-1 rpsE rpoB argE(Am) recA1, lysogenized with λpir |
(Herrero, et al., 1990) |
| DH5α | F- F80dlacZΔM15 Δ(lacZYA-argF)U169 deoR supE44 hsdR17 recA1 endA1 gyrA96 thi-1 relA1 |
(Hanahan, 1983) |
| DH5αλpir | DH5α lysogenized with λpir | (Dunn, et al.,2005) |
| MC4100 | F- araD139 Δ(argF lac) U169 rpsL150 relA1 flbB5301 deoC1 ptsF25 rbsR |
(Silhavy, 1984) |
| PC2 | MC4100 Δfnr | (Cotter & Gunsalus, 1992) |
| V. fischeri | ||
| AMJ2 | ES114 ΔarcA | (Bose, et al., 2007) |
| ANS23 | ES114 ΔarcA∷lacZ (allele exchanged from pAS31 into ES114) |
this study |
| ANS24 | ES114 fnr∷tmpR ΔarcA∷lacZ (allele exchanged from pAS31 into JB1) |
this study |
| ANS25 | ES114 fnr∷tmpR lacIq PA1/34luxCDABEG (allele exchanged from pJLB5 into JB22) |
this study |
| ES114 | wild-type isolate from E. scolopes | (Boettcher & Ruby, 1990) |
| EVS102 | ES114 ΔluxCDABEG | (Bose, et al., 2008) |
| EVS601 | MJ1 Δfnr∷tmpR (allele exchanged from pCDW5 into MJ1) | this study |
| JB1 | ES114 Δfnr∷tmpR (allele exchanged from pJLB5 into ES114) |
this study |
| JB2 |
fnr restored in JB1 (wild-type allele exchanged from pEVS136 into JB1) |
this study |
| JB8 | ES114 fnr∷tmpR ΔarcA (allele exchanged from pJLB70 into AMJ2) |
this study |
| JB11 | MJ1 ΔarcA (allele exchanged from pJLB76 into MJ1) | (Bose, et al., 2007) |
| JB12 | MJ1 fnr∷tmpR ΔarcA (allele exchanged from pJLB76 into EVS601) |
this study |
| JB22 | ES114 lacIq PA1/34luxCDABEG | (Bose, et al., 2008) |
| JB28 | MJ1 ΔarcA∷lacZ (allele exchanged from pJLB139 into MJ1) |
this study |
| JB29 | MJ1 fnr∷tmpR ΔarcA∷lacZ (allele exchanged from pJLB139 into EVS601) |
this study |
| JB27 |
fnr restored in EVS601 (wild-type allele exchanged from pJLB69 into EVS601) |
this study |
| MJ1 | wild-type isolate from Monocentris japonica | (Ruby & Nealson, 1976) |
| VCW2G7 | ES114 luxI (frameshift mutation) | (Lupp, et al., 2003) |
| Select plasmidsb | ||
| pAS31 | R6Kγ, ColE1, chmR, ampR, ES114 ΔarcA∷lacZ allele | this study |
| pCDW5 | R6Kγ, ColE1, chmR kanR, MJ1 Δfnr∷tmpR allele | this study |
| pDMA5 | p15A oriV oriTRP4 lacZα chmR | (Dunn, et al., 2005) |
| pEVS136 | R6Kγ, ermR, ES114 fnr | this study |
| pJLB5 | R6Kγ, ermR, ES114 Δfnr∷tmpR allele | this study |
| pJLB6 | p15A, chmR, ES114 fnr | this study |
| pJLB69 | R6Kγ, ColE1, chmR kanR, MJ1 fnr | this study |
| pJLB70 | R6Kγ, ColE1, ermR kanR, ES114 Δfnr∷tmpR allele | this study |
| pJLB76 | R6Kγ, ColE1, chmR, ampR, MJ1 ΔarcA | (Bose, et al., 2007) |
| pJLB139 | R6Kγ, ColE1, chmR, ampR, MJ1 ΔarcA∷lacZ allele | this study |
| Oligonucleotidesc | ||
| AS1310RTF2 | TAT TGG TTA AAG AGC GCC CAT GG | this study |
| AS1310RTR2 | CAC TTC AGC GAA ATA GAT GGC | this study |
| EVS97 | CCG GGT ACC ATG GTT GGT GAT GGA ATA AAT GAT GC |
this study |
| EVS98 | CCG GGT ACC TTT TGA AGC TTA TTG AAA TTG TAT TG |
this study |
| JBLACZ1 | CTG ACT CTG GGT AAC ACT ACT TCT TCT GTG |
this study |
| JBLACZ2 | TTA TTT TTG ACA CCA GAC CAA CTG GTA ATG G |
this study |
Drug resistance abbreviations: ampR, ampicillin resistance (bla); chmR, chloramphenicol resistance (cat); ermR, erythromycin resistance; kanR, kanamycin resistance (aph); and tmpR trimethoprim resistance (dfr).
All plasmids listed contain the RP4 origin of transfer. Replication origin(s) are denoted as p15A, R6Kγ, and/or ColE1.
Oligonucleotide sequences are provided in the 5’-3’ orientation.
Genetic manipulations
Cloning was performed using standard procedures, with plasmids transformed in E. coli strain DH5α or DH5αλpir, as previously described (Bose, et al., 2008). Cloned PCR products were sequenced to ensure that unintended alterations were not incorporated. Sequencing was conducted at the University of Michigan DNA Sequencing Core Facility or at the University of Georgia Molecular Genetics Instrumentation Facility. Plasmids were mobilized into V. fischeri from E. coli by triparental mating using strain CC118λpir with pEVS104 as a helper (Stabb & Ruby, 2002), and mutations were placed on the chromosome by allelic exchange. Parent strains and plasmids used for allelic exchange are listed in Table 1.
Key plasmids and oligonucleotides are described in Table 1, and an overview of allele construction follows. To mutate fnr, a ~3.3 kb region of the V. fischeri genome centered on fnr was PCR amplified with primers EVS97 and EVS98 using ES114 or MJ1 genomic DNA as template, and the fragments were ultimately subcloned into pEVS136 and pJLB69, respectively (Table 1). We generated Δfnr∷tmpR alleles by replacing the ClaI to AvrII fragment of fnr with the trimethoprim-resistance gene (tmpR) from pJLB1 (Dunn, et al., 2005) on a BstBI to AvrII fragment, resulting in tmpR replacing an internal 255-bp fragment beginning in the middle of fnr, with tmpR in the same orientation as fnr. The ES114-derived Δfnr∷tmpR allele was placed in pJLB5 and pJLB70, and the MJ1-derived Δfnr∷tmpR allele was used in pCDW5. For complementation of E. coli with ES114 fnr, we ligated the fnr-containing BsrBI-PstI fragment from pEVS136 into SmaI- and PstI-digested pDMA5 generating pJLB6. To place lacZ under control of the arcA promoter, we PCR amplified a ~3.1-kb fragment containing an engineered lacZ (Tomich, et al., 1988) using pVSV3 (Dunn, et al., 2006) as template and primers JBLACZ1 and JBLACZ2 (Table 1). We cloned this product into SmaI-digested pAJ4 and pJLB55 (Bose, et al., 2007), which carry regions flanking arcA from ES114 and MJ1, respectively, with the sequence between the start and stop codons of arcA replaced by a 6-bp SmaI recognition site. The ParcA-lacZ alleles contain the arcA start codon, followed by a 5’-CCC-3’ proline codon, and then the lacZ reporter (Tomich, et al., 1988) from its second codon onward. These ES114- and MJ1-derived alleles were subcloned into pAS31 and pJLB139, respectively.
Growth and luminescence
Overnight cultures in LBS were diluted 1:1000 into SWTO and incubated at 24°C with shaking (200 rpm). Aerobic cultures contained 50 ml of SWTO in 250-ml flasks. For anaerobic cultures, aerobically-grown overnight cultures were diluted 1:10 in LBS before inoculation of 0.2 ml into 20 ml SWTO in 165-ml sealed bottles with a headspace containing 5% CO2, 10% H2, and 85% N2. 500-µl samples were removed periodically and culture optical density (OD595) was determined using a BioPhotometer (Brinkman Instruments, Westbury, NY) or a SmartSpec 3000 (BioRad Laboratories). After measuring OD595, cuvettes were covered with parafilm and shaken vigorously for ~10 sec to aerate the sample, followed by determination of luminescence using a GLOMAX 20/20 luminometer (Promega, Madison, WI).
Quantitative RT-PCR
Triplicate aerobic cultures of ES114 and JB1 were grown in LBS to an OD595 ~2.1. 1-ml samples were removed, added to microcentrifuge tubes containing 1/5 volume 5% (v/v) phenol pH 4.3 with 95% (v/v) ethanol, and placed on ice for 30 min. Samples were centrifuged and the pellets stored at −80°C overnight. Pellets were thawed, and RNA was isolated using Absolutely RNA Minipreps (Stratagene, La Jolla, CA). RNA was treated using the Turbo DNA-free kit (Applied Biosystems, Foster City, CA) and RNA quantity and purity were assessed using a Biotek Synergy 2 plate reader with Take3 Multi-Volume Plate and software (Winooski, VT). RNA was then stored at −80°C. cDNA was synthesized with the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen, Carlsbad, CA), and reactions were cleaned with a DNA Clean & Concentrator-5 kit (Zymo Research, Orange, CA). cDNA was quantified using the Synergy 2 plate reader. Real-time PCR was performed using the MyIQ Single-Color Real-Time PCR Detection System (BioRad, Hercules, CA), and reactions were set up using the BioRad IQ SYBR Green Supermix. Primers AS1310RTF2 and AS1310RTR2 were used to determine the level of VF1310 cDNA. ES114 genomic DNA was used to generate a standard curve. Real time PCR data were analyzed using BioRad IQ™5 software.
lacZ reporter expression
To determine ParcA-lacZ reporter expression, strains were grown overnight in LBS and diluted 1:1000 in 20 ml SWTO in 250-ml baffled flasks and grown at 24°C with shaking to an OD of ~0.1. 400 µl were removed to inoculate 20 ml SWTO in anaerobic bottles. These were incubated at 24°C with shaking until peak luminescence was reached. Strains were also grown aerobically in 20 ml SWTO in 250-ml baffled flasks and incubated 24°C with shaking until peak luminescence was reached. Culture samples were taken, cells were pelleted, the supernatant was discarded, and the pellet frozen at −20°C. The next day the pellet was thawed and resuspended in Z-buffer for determination of β-galactosidase activity expressed as Miller units as previously described (Miller, 1992).
Symbiotic Colonization Assays
Inoculant strains were grown unshaken in 5 ml of SWT in 50-ml conical tubes at 28°C to an OD595 of 0.3–1.0, and cultures were diluted in Instant Ocean to a density no higher than 1700 CFU ml−1. In each experiment the inoculant density of wild-type and mutants strains was equivalent, and this was checked by plating the inocula on LBS. Hatchling squid were placed in these inocula for up to 14 h before being rinsed in V. fischeri-free Instant Ocean. To study infection kinetics, the squid were placed in 5 ml of inoculant in scintillation vials, and the onset of luminescence was monitored using a LS6500 scintillation counter (Beckman Coulter, Fullerton, CA). For mixed-strain competitions, hatchlings were exposed to an inoculum containing a ~1:1 ratio of wild type and mutant. At 48 h post-inoculation, individual squid were homogenized and dilution plated on LBS. Resulting colonies were patched onto LBS with added trimethoprim to determine the ratio of strains in each animal. Inocula were similarly plated and patched to determine the starting ratio. The relative competitiveness index (RCI) was determined by dividing the mutant to wild type ratio in each animal by the ratio of these strains in the inoculum. Mean RCI was calculated from log-transformed data.
Results
Identification of V. fischeri fnr
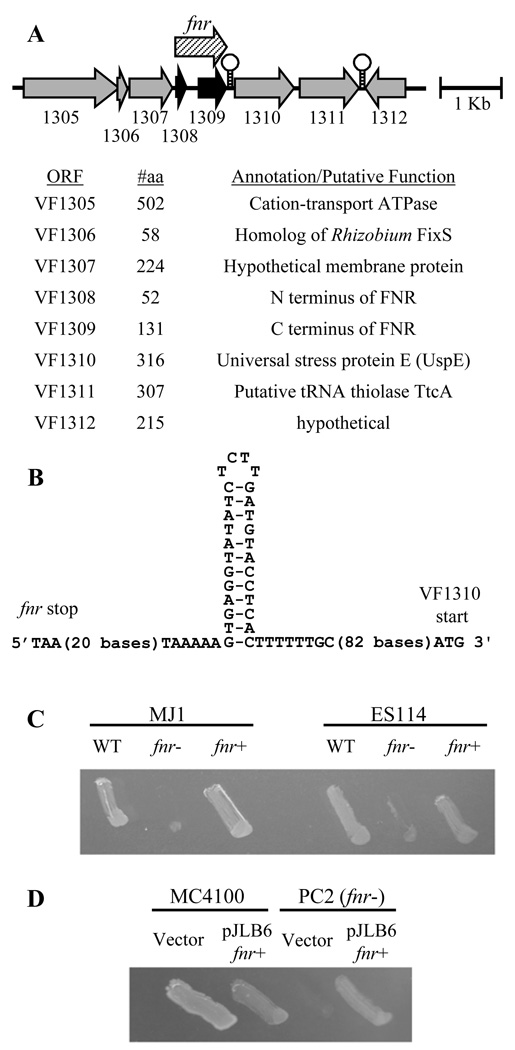
BLAST searches (Altschul, et al., 1990) of the V. fischeri ES114 genome revealed similarity of ORF’s VF1308 and VF1309 to the N and C termini of E. coli FNR, respectively (Fig. 1A). We suspected that a sequencing error had led to the misannotation of fnr as two genes, and we therefore cloned and sequenced the region spanning VF1308 and VF1309. We found five errors in the genome database, leading to an erroneously predicted truncation of VF1308, which we corrected in Genbank (Mandel, et al., 2008). In the revised sequence, VF1308 encodes a protein that is the same length as, and shares 84% identity with, E. coli FNR. This ES114 FNR is identical to the previously deposited V. fischeri MJ1 FNR (accession CAE47558). Importantly, the residues necessary for interactions with RNA polymerase (Williams, et al., 1997, Lonetto, et al., 1998, Blake, et al., 2002, Lamberg, et al., 2002), 4Fe–4S center assembly (Spiro & Guest, 1988, Kiley & Beinert, 1998), and DNA recognition (Spiro, et al., 1990) in E. coli are conserved in V. fischeri FNR. Using TransTermHP (Kingsford, et al., 2007) we also found a likely Rho-independent transcriptional terminator downstream of fnr (Fig. 1A and B). Given the 142-bp spacing and strong putative terminator between fnr and VF1310 (Fig. 1B), it seems likely that these are expressed on separate transcripts. Using quantitative RT-PCR we found that the fnr∷tmpR allele in mutants described below did not affect transcript levels for ORF1310.
FIG. 1.
Genomic context and function of fnr in V. fischeri. (A) Gene arrangement around fnr in V. fischeri ES114. Numbers represent the corresponding VF#### ORF designation. Stem-loop icons indicate the positions of Rho-independent transcriptional terminators predicted using TransTermHP (Kingsford, et al., 2007), with a confidence score of 100 in each case. “#aa” indicates the number of amino acids encoded by each ORF. VF1308 and VF1309 (black arrows) indicate ORFs with similarity to the N and C termini of E. coli FNR, respectively. The striped arrow shows the complete fnr based on our sequence revision. (B) The predicted Rho-independent transcriptional terminator between fnr and VF1310. (C) Growth of V. fischeri MJ1, fnr mutant EVS601, and restored fnr+ strain JB27 along with ES114, fnr mutant JB1, and restored fnr+ strain JB2 on defined medium with glycerol and fumarate, incubated in anaerobic jars at 28°C. (D) E. coli MC4100 and fnr mutant PC2 with vector pDMA5 or pJLB6, which contains the V. fischeri ES114 fnr, grown on M9 medium with glycerol and nitrate in anaerobic jars at 37°C.
We next made mutants disrupted in the putative fnr in V. fischeri ES114 and MJ1. We did not observe any attenuation of these strains under aerobic growth conditions, consistent with the role of FNR in other bacteria. E. coli fnr mutants do not grow anaerobically with nitrate or fumarate as an electron acceptor (Lambden & Guest, 1976), and we found that V. fischeri fnr mutants were similarly attenuated. Specifically, in minimal medium under anaerobic conditions, ES114 and MJ1 displayed nitrate- or fumarate-dependent growth on a non-fermentable carbon source (glycerol) that was lacking in the fnr mutants (e.g., Fig. 1C). Restoring fnr by replacing the fnr∷tmpR allele with the wild-type allele by a crossover exchange back into these mutants recovered the ability to respire anaerobically. We restored the wild-type fnr allele on the chromosome in this way (replacing fnr∷tmpR) rather than providing it in trans due to concerns that fnr provided in multicopy can show uncharacteristic effects such as gene activation under aerobic conditions (Reyes-Ramirez & Sawers, 2006) and a narrowing of the difference between better and poorer FNR activation sites (Scott, et al., 2003). However, because our V. fischeri-derived allele-replacement constructs were not appropriate (homologous) for exchange into E. coli, we provided the putative fnr of V. fischeri ES114 to E. coli in trans on plasmid pJLB6, which restored anaerobic respiration of E. coli fnr mutant PC2 on nitrate (Fig. 1D). Taken together our results indicate that the putative V. fischeri FNR is similar in both sequence and function to E. coli FNR.
Repression of luminescence by FNR
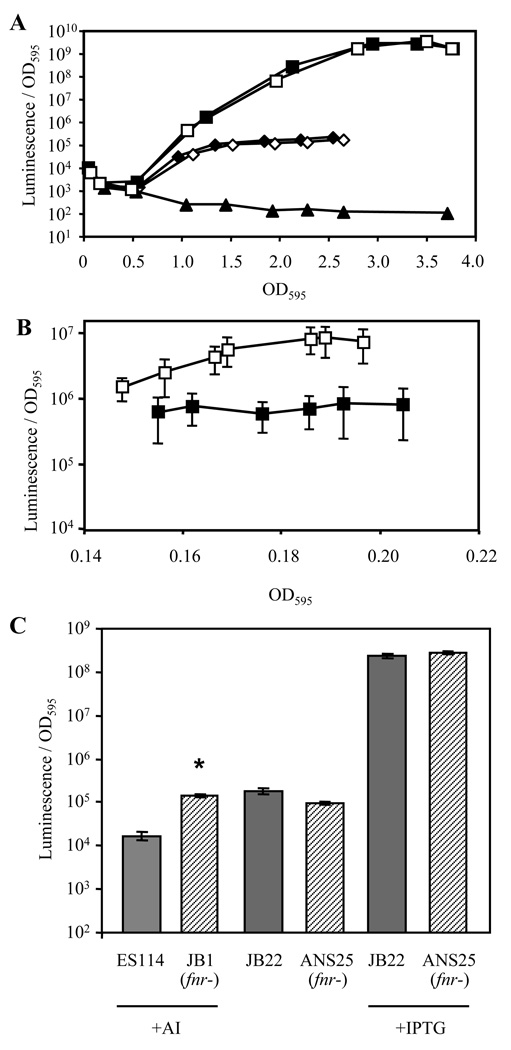
We tested whether FNR regulates lux expression by monitoring the luminescence of strains grown aerobically or anaerobically (Fig. 2A and B). The luminescence of the fnr mutants was similar to that of their parent strains under aerobic conditions (Fig. 2A). FNR is inactivated by oxygen, and we therefore also assessed lux expression anaerobically. Luciferase uses oxygen as a substrate, so anaerobic cultures do not luminesce; however, as with all luminescence measurements, samples removed from anaerobic bottles were shaken for ~10 sec to saturate luciferase with oxygen prior to measuring luminescence. When grown anaerobically, luminescence was higher in fnr mutant EVS601 than in MJ1 (Fig. 2B). The magnitude of this difference varied between 1.5- to 20-fold, and averaged 8-fold, in five experiments. The luminescence of ES114 and fnr mutant JB1 was below background, appearing the same as a dark ΔluxCDABEG strain (data not shown), which raised the possibility that FNR regulates lux in ES114 but that overall luminescence is below detection. To test this possibility, we added the luminescence-stimulating autoinducer 3-oxo-C6-HSL to anaerobic cultures of ES114 and its fnr mutant JB1. 3-oxo-C6-HSL stimulated luminescence of ES114 and JB1, and under these conditions JB1 was brighter than ES114 (Fig. 2C).
FIG. 2.
Luminescence per OD595 of fnr mutants. In Panels A and B, specific luminescence is shown at different culture densities for V. fischeri ES114 (solid diamonds), ES114 fnr mutant JB1 (empty diamonds), MJ1 (solid squares), MJ1 fnr mutant EVS601 (empty squares), and dark ΔluxCDABEG mutant EVS102 (solid triangles) grown in batch cultures that were; (A) aerobic (50 ml medium in 250-ml flask) or (B) anaerobic (20 ml medium in 165-ml bottles with anaerobic headspace) at 24°C with shaking (200 rpm). ES114, JB1, and EVS102 were omitted from panel B, because luminescence was not detected above background for these strains under these conditions. Bars in panel B indicate standard deviation (n=5). Error bars were omitted in panel A, because they were generally smaller than (and never extended above) the data symbols. For Panel C, ES114 (wild type), JB22 (lacIq PA1/34-lux), and their respective fnr mutants (represented by hatched bars) JB1 and ANS25, respectively (Table 1), were grown under anaerobic conditions. “AI” indicates supplementation with 140 nM 3-oxo-C6-HSL autoinducer, and “IPTG” indicates isopropyl-β-D-thiogalactoside was added to 2 mM to induce luxCDABEG expression in strains containing lacIq PA1/34-lux. Data is the average peak luminescence per OD595 with standard deviation (n=2). Asterisks indicate that the fnr mutant was significantly (p<0.01) brighter than the corresponding isogenic fnr-positive strain. Other comparisons were not significant (p>0.05).
We considered the possibility that increased luminescence in V. fischeri fnr mutants could result from increased availability of luciferase’s substrates due to physiological effects of this global regulator. To test this possibility, we disrupted fnr in a background where the luxCDABEG genes are under control of LacIq and a non-native promoter. In this background, FNR had no significant effect (p>0.05) on luminescence (Fig. 2C). Thus, the repressive effect of FNR on luminescence is dependent on the native lux promoter.
The luxICDABEG operon can be subject to positive feedback regulation, because the autoinducer synthase LuxI generates 3-oxo-C6-HSL, which in combination with LuxR stimulates luxICDABEG transcription. Given the amount of 3-oxo-C6-HSL added exogenously to the cultures (Fig. 2C), we predicted that endogenously produced autoinducer would have no further stimulatory effect, and therefore the effect of FNR on luminescence in this experiment would not have a significant LuxI-mediated positive-feedback component. We examined luxI point mutant VCW2G7 and found that, as predicted, it achieved the same luminescence as wild type under anaerobic conditions with added 3-oxo-C6-HSL (data not shown).
Analysis of FNR boxes
It was suggested that a putative FNR box upstream of luxR might underpin FNR-mediated regulation of luminescence in MJ1 (Muller-Breikreutz & Winkler, 1993); however, attempts to define a footprint using FNR*, an E. coli FNR derivative that is active aerobically (Kiley & Reznikoff, 1991), failed to show binding to this site (Ann Stevens, personal communication). To further explore how FNR might affect luminescence, we conducted a “Virtual Footprint” analysis with the PRODORIC database (Munch, et al., 2005), searching the V. fischeri genome for FNR boxes using a weighted consensus matrix based on data from E. coli. As expected, high PWM scores (≥7.0) were skewed toward intergenic regions. Such putative FNR boxes numbered in the hundreds, consistent with FNR’s global role in E. coli, and these included intergenic regions upstream of genes involved in anaerobic metabolism (e.g., upstream of nitrate and nitrite reductases). However, the best FNR box matches in the lux intergenic region of MJ1 and ES114 returned scores of 6.73 and only 5.88, respectively. To put this in perspective, >25,000 sites with no skew toward intergenic regions returned scores ≥5.9. Although we cannot rule out the possibility that FNR directly binds to the lux intergenic region, we believe this model is unlikely, especially in strain ES114.
FNR-mediated repression of arcA
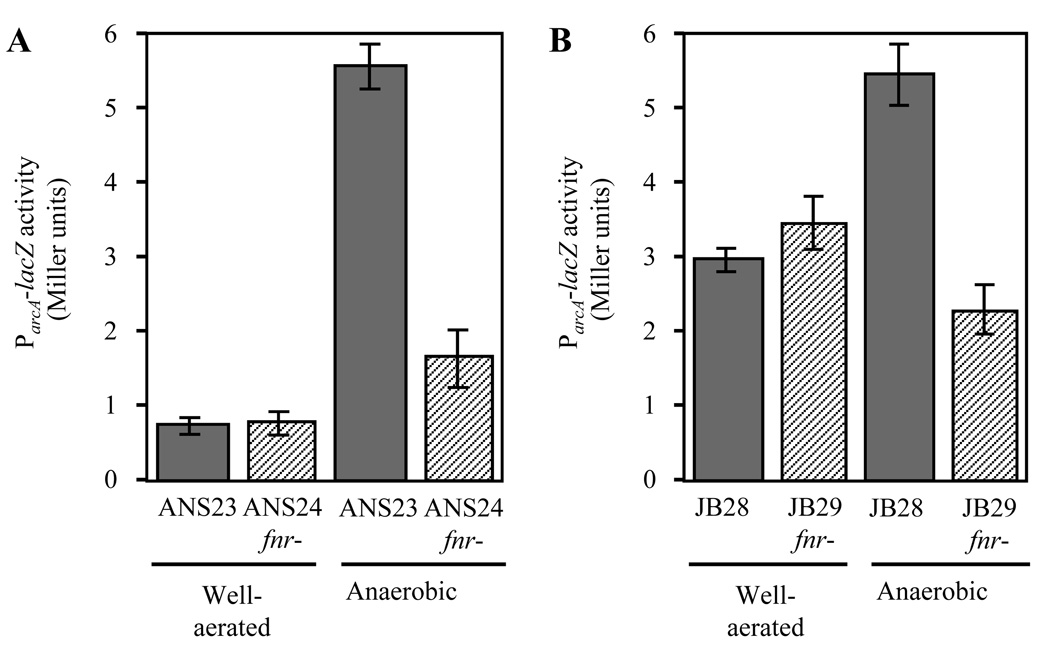
Virtual Footprinting did suggest a possible indirect effect of FNR on luminescence. The highest PWM score returned in this analysis (7.67) was found in six intergenic regions, one of which was upstream of arcA. In E. coli, FNR activates arcA (Compan & Touati, 1994), and in ES114 ArcA strongly represesses the lux operon (Bose, et al., 2007). If FNR activates arcA in V. fischeri, this might explain FNR’s repressive effect on luminescence. Using ParcA-lacZ transcriptional reporters, we found that fnr was responsible for a ~2-fold to 4-fold activation of the arcA promoter(s) anaerobically in ES114 and MJ1 backgrounds (Fig. 3).
FIG. 3.
FNR-mediated regulation of arcA promoter-lacZ reporters. LacZ reporter activity expressed in Miller units for (Panel A) ES114 derivatives ANS23 (ΔarcA∷lacZ) and ANS24 (ΔarcA∷lacZ Δfnr∷tmpR), or (Panel B) the MJ1 derivatives JB28 (ΔarcA∷lacZ) and JB29 (ΔarcA∷lacZ Δfnr∷tmpR). Culture conditions (aerobic or anaerobic) are as described in the Figure 2 legend. Averages with standard deviation are indicated (n=3). The LacZ reporter activity shown is approximately 100-fold above the background determined using strains ES114 and JB1, which lack the ΔarcA∷lacZ allele.
FNR is not necessary for host colonization
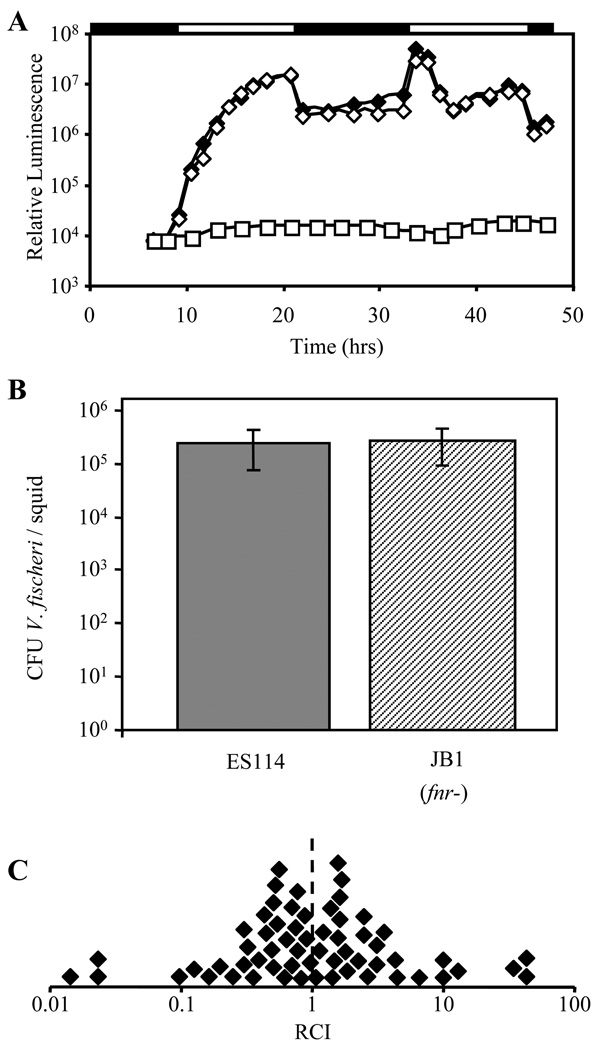
We tested whether FNR was important for symbiotic colonization by ES114 using established measures of symbiotic competence (Adin, et al., 2009). The onset of symbiotic luminescence (Fig. 4A), colonization levels (Fig. 4B), and colonization competitiveness (Fig. 4C) were similar for ES114 and fnr mutant JB1 during the first two days of infection. The fnr mutant was also equally competitive up to 90 hrs after inoculation (data not shown). Furthermore, the fnr mutation did not appear to affect the symbiosis in a ΔarcA mutant background (data not shown). We conclude that FNR is not necessary for colonization during the first days of a symbiotic infection.
FIG. 4.
Colonization of E. scolopes by fnr mutant and wild type. (A) Average symbiotic luminescence in E. scolopes hatchlings inoculated with ES114 (solid diamonds), or the fnr mutant JB1 (empty diamonds), (n=14). Control squid receiving no V. fischeri inoculum (empty squares) did not yield any bioluminescence (n=4). Bars above graph indicate periods of ambient light (empty bar) and darkness (solid bar). (B) Average colonization levels in CFU V. fischeri per squid 36 h after inoculation with ES114 (solid bar) or JB1 (hatched bar). Treatments are not significantly different (p=0.7). Bars indicate standard deviation (n=14 for ES114 and 13 for JB1). (C) Competitiveness of JB1 when presented in a mixed (~1:1) inoculum with wild type and recovered from squid after 48 h. Each symbol represents the RCI determined from one squid, defined as the ratio of JB1:ES114 in the squid divided by the ratio in the inoculum. Combined data from three experiments is presented. The dashed line represents equal competitiveness and in this case is also the mean RCI (n=60).
Discussion
In this study we investigated the oxygen-sensitive regulator FNR in V. fischeri. V. fischeri fnr complemented an E. coli fnr mutant, and like fnr in E. coli it is required for fumarate- and nitrate-dependent anaerobic respiration. Moreover, our data and another recent bioinformatic analysis (Ravcheev, et al., 2007) suggest that the FNR-box recognition site is conserved in V. fischeri. For example, we observed fnr-mediated regulation of reporters for arcA (Fig. 3), dmsA (Dunn & Stabb, 2008), torE (Dunn & Stabb, 2008), and yfiD (data not show), which have predicted FNR boxes upstream. Taken together, FNR’s function in V. fischeri appears similar to that in its fellow γ-proteobacterium E. coli. As the first experimental examination of FNR in the Vibrionaceae, this study should underpin future efforts to understand FNR-mediated regulation in this important bacterial family.
We initiated this study largely because FNR is cited as an activator of luminescence in V. fischeri (examples include (Meighen, 1994, Spiro, 1994, Sitnikov, et al., 1995, Ulitzur & Dunlap, 1995, Stevens & Greenberg, 1999)). However, that paradigm was based on a preliminary study that used the MJ1 lux genes cloned in E. coli (Muller-Breikreutz & Winkler, 1993). Our results appear to contradict that report, showing instead that FNR mediates repression of the luminescence-generating lux system in V. fischeri under anaerobic conditions (Fig. 2). It is perhaps unsurprising that lux regulation should be different in transgenic E. coli than in V. fischeri. For example, LitR, which activates luxR transcription, is absent in E. coli (Fidopiastis, et al., 2002). It is also possible that FNR does activate luminescence in V. fischeri under different conditions than those tested here, and that the discrepancy between our study and previous work simply reflects methodological differences.
Repression of the lux genes anaerobically may minimize production of luciferase when its O2 substrate is unavailable. This is consistent with the finding that luminescence is repressed by the ArcAB two-component regulatory system, which is more active under relatively reduced conditions (Bose, et al., 2007). The observation that arcA∷lacZ reporters showed lower expression in the absence of fnr (Fig. 3) suggests that the effect of FNR on bioluminescence may at least in part be indirect and mediated by FNR’s stimulation of arcA. Consistent with this idea, fnr did not exert much influence on luminescence in arcA mutant backgrounds, although arcA fnr double mutants were noticeably attenuated in anaerobic growth (data not shown). We speculate that FNR may amplify the repressive effect of ArcA on luminescence under reduced conditions. Athough we cannot rule out the possibility that FNR exerts a direct effect by binding the lux region, as described above we believe this model is unlikely. In either case, FNR apparently contributes to regulation that effectively turns off expression of the lux genes in ES114 under anaerobic conditions, which is easily rationalized given that luciferase requires O2 to generate light.
Given the suggestion that anaerobic respiration is important for symbiotic V. fischeri (Proctor & Gunsalus, 2000), and the fact that FNR can contribute to virulence factor production and/or colonization by pathogens (Baltes, et al., 2005, Bartolini, et al., 2006, Fink, et al., 2007, Zigha, et al., 2007), we hypothesized that fnr would play a role in the symbiotic light organ. However, the fnr mutant had no discernable attenuation in colonizing E. scolopes during the first 90 hrs of infection. V. fischeri, like other members of the Vibrionaceae family, is a cosmopolitan member of marine communities that is found in fish gut tracts and sediments where [O2] is low. Future studies may show ecological relevance of FNR for V. fischeri in such environments outside E. scolopes.
Acknowledgements
We thank Chandra Carpenter and Noreen Lyell for technical assistance. Genomic sequencing of Vibrio fischeri was supported by the W. M. Keck Foundation. ANS was supported by a University of Georgia Graduate Research Fellowship and a National Defense Science and Engineering Graduate Fellowship. This study was supported by grants from the National Science Foundation (CAREER MCB-0347317), the National Institutes of Health (RO1 A150661 to Margaret McFall-Ngai), and the Army Research Office (49549LSII).
References
- Adin DM, Engle JT, Goldman WE, McFall-Ngai MJ, Stabb EV. Mutations in ampG and lytic transglycosylase genes affect the net release of peptidoglycan monomers from Vibrio fischeri. J. Bacteriol. 2009;191:2012–2022. doi: 10.1128/JB.01547-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Baltes N, N'Diaye M, Jacobsen ID, Maas A, Buettner FF, Gerlach GF. Deletion of the anaerobic regulator HlyX causes reduced colonization and persistence of Actinobacillus pleuropneumoniae in the porcine respiratory tract. Infect. Immun. 2005;73:4614–4619. doi: 10.1128/IAI.73.8.4614-4619.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartolini E, Frigimelica E, Giovinazzi S, et al. Role of FNR and FNR-regulated, sugar fermentation genes in Neisseria meningitidis infection. Mol. Microbiol. 2006;60:963–972. doi: 10.1111/j.1365-2958.2006.05163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake T, Barnard A, Busby SJ, Green J. Transcription activation by FNR: evidence for a functional activating region 2. J. Bacteriol. 2002;184:5855–5861. doi: 10.1128/JB.184.21.5855-5861.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher KJ, Ruby EG. Depressed light emission by symbiotic Vibrio fischeri of the sepiolid squid Euprymna scolopes. J. Bacteriol. 1990;172:3701–3706. doi: 10.1128/jb.172.7.3701-3706.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher KJ, Ruby EG, McFall-Ngai MJ. Bioluminescence in the symbiotic squid Euprymna scolopes is controlled by a daily biological rhythm. J. Comp. Physiol. 1996;179:65–73. [Google Scholar]
- Bose JL, Rosenberg CS, Stabb EV. Effects of luxCDABEG induction in Vibrio fischeri: enhancement of symbiotic colonization and conditional attenuation of growth in culture. Arch. Microbiol. 2008;190:169–183. doi: 10.1007/s00203-008-0387-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose JL, Kim U, Bartkowski W, et al. Bioluminescence in Vibrio fischeri is controlled by the redox-responsive regulator ArcA. Mol. Microbiol. 2007;65:538–553. doi: 10.1111/j.1365-2958.2007.05809.x. [DOI] [PubMed] [Google Scholar]
- Bourgois J-J, Sluse FE, Baguet F, Mallefet J. Kinetics of light emission and oxygen consumption by bioluminescent bacteria. J. Bioengerg. Biomembr. 2001;33:353–363. doi: 10.1023/a:1010615508916. [DOI] [PubMed] [Google Scholar]
- Browning D, Lee D, Green J, Busby S. Secrets of bacterial transcription initiation taught by the Escherichia coli FNR protein. In: Hodgson DA, Thomas CM, editors. Signals, switches, regulons, and cascades : control of bacterial gene expression. Cambridge, United Kingdom: Cambridge University Press; 2002. pp. 127–142. [Google Scholar]
- Compan I, Touati D. Anaerobic activation of arcA transcription in Escherichia coli: roles of Fnr and ArcA. Mol. Microbiol. 1994;11:955–964. doi: 10.1111/j.1365-2958.1994.tb00374.x. [DOI] [PubMed] [Google Scholar]
- Cotter PA, Gunsalus RP. Contribution of the fnr and arcA gene products in coordinate regulation of the cytochrome o (cyoABCDE) and d (cydAB) oxidase genes in Escherichia coli. FEMS Microbiol. Lett. 1992;91:31–36. doi: 10.1016/0378-1097(92)90558-6. [DOI] [PubMed] [Google Scholar]
- Dunn AK, Stabb EV. Genetic analysis of trimethylamine N-oxide reductases in the light organ symbiont Vibrio fischeri ES114. J. Bacteriol. 2008;190:5814–5823. doi: 10.1128/JB.00227-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn AK, Martin MO, Stabb EV. Characterization of pES213, a small mobilizable plasmid from Vibrio fischeri. Plasmid. 2005;54:114–134. doi: 10.1016/j.plasmid.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Dunn AK, Millikan DS, Adin DM, Bose JL, Stabb EV. New rfp- and pES213-derived tools for analyzing symbiotic Vibrio fischeri reveal patterns of infection and lux expression in situ. Appl. Environ. Microbiol. 2006;72:802–810. doi: 10.1128/AEM.72.1.802-810.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiglmeier K, Honore N, Iuchi S, Lin EC, Cole ST. Molecular genetic analysis of FNR-dependent promoters. Mol. Microbiol. 1989;3:869–878. doi: 10.1111/j.1365-2958.1989.tb00236.x. [DOI] [PubMed] [Google Scholar]
- Fidopiastis PM, Miyamoto C, Jobling MG, Meighen EA, Ruby EG. LitR, a new transcriptional activator in Vibrio fischeri, regulates luminescence and symbiotic light organ colonization. Mol. Microbiol. 2002;45:131–143. doi: 10.1046/j.1365-2958.2002.02996.x. [DOI] [PubMed] [Google Scholar]
- Fink RC, Evans MR, Porwollik S, et al. FNR is a global regulator of virulence and anaerobic metabolism in Salmonella enterica serovar Typhimurium (ATCC 14028s) J. Bacteriol. 2007;189:2262–2273. doi: 10.1128/JB.00726-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray KM, Greenberg EP. Physical and functional maps of the luminescence gene cluster in an autoinducer-deficient Vibrio fischeri strain isolated from a squid light organ. J. Bacteriol. 1992;174:4384–4390. doi: 10.1128/jb.174.13.4384-4390.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Herrero M, De Lorenzo V, Timmis KN. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in Gram-negative bacteria. J. Bacteriol. 1990;172:6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoroshilova N, Beinert H, Kiley PJ. Association of a polynuclear iron-sulfur center with a mutant FNR protein enhances DNA binding. Proc. Natl. Acad. Sci. USA. 1995;92:2499–2503. doi: 10.1073/pnas.92.7.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoroshilova N, Popescu C, Munck E, Beinert H, Kiley PJ. Iron-sulfur cluster disassembly in the FNR protein of Escherichia coli by O2: [4Fe–4S] to [2Fe–2S] conversion with loss of biological activity. Proc. Natl. Acad. Sci. USA. 1997;94:6087–6092. doi: 10.1073/pnas.94.12.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiley PJ, Reznikoff WS. Fnr mutants that activate gene expression in the presence of oxygen. J. Bacteriol. 1991;173:16–22. doi: 10.1128/jb.173.1.16-22.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiley PJ, Beinert H. Oxygen sensing by the global regulator, FNR: The role of the iron-sulfur cluster. FEMS Microbiol. Rev. 1998;22:341–352. doi: 10.1111/j.1574-6976.1998.tb00375.x. [DOI] [PubMed] [Google Scholar]
- Kingsford CL, Ayanbule K, Salzberg SL. Rapid, accurate, computational discovery of Rho-independent transcription terminators illuminates their relationship to DNA uptake. Genome Biol. 2007;8:R22. doi: 10.1186/gb-2007-8-2-r22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambden PR, Guest JR. Mutants of Escherichia coli K12 unable to use fumarate as an anaerobic electron acceptor. J. Gen. Microbiol. 1976;97:145–160. doi: 10.1099/00221287-97-2-145. [DOI] [PubMed] [Google Scholar]
- Lamberg KE, Luther C, Weber KD, Kiley PJ. Characterization of activating region 3 from Escherichia coli FNR. J. Mol. Biol. 2002;315:275–283. doi: 10.1006/jmbi.2001.5241. [DOI] [PubMed] [Google Scholar]
- Lazazzera BA, Beinert H, Khoroshilova N, Kennedy MC, Kiley PJ. DNA binding and dimerization of the Fe-S-containing FNR protein from Escherichia coli are regulated by oxygen. J. Biol. Chem. 1996;271:2762–2768. doi: 10.1074/jbc.271.5.2762. [DOI] [PubMed] [Google Scholar]
- Lonetto MA, Rhodius V, Lamberg K, Kiley P, Busby S, Gross C. Identification of a contact site for different transcription activators in region 4 of the Escherichia coli RNA polymerase σ70 subunit. J. Mol. Biol. 1998;284:1353–1365. doi: 10.1006/jmbi.1998.2268. [DOI] [PubMed] [Google Scholar]
- Lupp C, Urbanowski M, Greenberg EP, Ruby EG. The Vibrio fischeri quorum-sensing systems ain and lux sequentially induce luminescence gene expression and are important for persistence in the squid host. Mol. Microbiol. 2003;50:319–331. doi: 10.1046/j.1365-2958.2003.t01-1-03585.x. [DOI] [PubMed] [Google Scholar]
- Mandel MJ, Stabb EV, Ruby EG. Comparative genomics-based investigation of resequencing targets in Vibrio fischeri: focus on point miscalls and artefactual expansions. BMC Genomics. 2008;9:138. doi: 10.1186/1471-2164-9-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall FA, Messenger SL, Wyborn NR, Guest JR, Wing H, Busby SJ, Green J. A novel promoter architecture for microaerobic activation by the anaerobic transcription factor FNR. Mol. Microbiol. 2001;39:747–753. doi: 10.1046/j.1365-2958.2001.02262.x. [DOI] [PubMed] [Google Scholar]
- Meighen EA. Genetics of bacterial bioluminescence. Annu. Rev. Genet. 1994;28:117–139. doi: 10.1146/annurev.ge.28.120194.001001. [DOI] [PubMed] [Google Scholar]
- Meng W, Green J, Guest JR. FNR-dependent repression of the ndh gene expression requires two upstream FNR-binding sites. Microbiology. 1997;143:1521–1532. doi: 10.1099/00221287-143-5-1521. [DOI] [PubMed] [Google Scholar]
- Miller JH. A short course in bacterial genetics. New York: Cold Spring Harbor Laboratory Press; 1992. [Google Scholar]
- Muller-Breikreutz K, Winkler UK. Anaerobic expression of the Vibrio fisheri lux regulon in E. coli is FNR-Dependent. In: Szalay AA, Kricka LJ, Stanley P, editors. Bioluminescence and Chemiluminescence. New York: Wiley; 1993. pp. 142–146. [Google Scholar]
- Munch R, Hiller K, Grote A, Scheer M, Klein J, Schobert M, Jahn D. Virtual Footprint and PRODORIC: an integrative framework for regulon prediction in prokaryotes. Bioinformatics. 2005;21:4187–4189. doi: 10.1093/bioinformatics/bti635. [DOI] [PubMed] [Google Scholar]
- Proctor LM, Gunsalus RP. Anaerobic respiratory growth of Vibrio harveyi, Vibrio fischeri, and Photobacterium leiognathi with trimethylamine N-oxide, nitrate, and fumarate: ecological implications. Environ. Microbiol. 2000;2:399–406. doi: 10.1046/j.1462-2920.2000.00121.x. [DOI] [PubMed] [Google Scholar]
- Ravcheev DA, Gerasimova AV, Mironov AA, Gelfand MS. Comparative genomic analysis of regulation of anaerobic respiration in ten genomes from three families of gamma-proteobacteria (Enterobacteriaceae, Pasteurellaceae, Vibrionaceae) BMC Genomics. 2007;8:54. doi: 10.1186/1471-2164-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reents H, Munch R, Dammeyer T, Jahn D, Hartig E. The Fnr regulon of Bacillus subtilis. J. Bacteriol. 2006;188:1103–1112. doi: 10.1128/JB.188.3.1103-1112.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Ramirez F, Sawers RG. Aerobic activation of transcription of the anaerobically inducible Escherichia coli focA-pfl operon by fumarate nitrate regulator. FEMS Microbiol. Lett. 2006;255:262–267. doi: 10.1111/j.1574-6968.2005.00077.x. [DOI] [PubMed] [Google Scholar]
- Ruby EG, Nealson KH. Symbiotic association of Photobacterium fischeri with the marine luminous fish Monocentris japonica: A model of symbiosis based on bacterial studies. Biol. Bull. 1976;151:574–586. doi: 10.2307/1540507. [DOI] [PubMed] [Google Scholar]
- Ruby EG, Urbanowski M, Campbell J, et al. Complete genome sequence of Vibrio fischeri: a symbiotic bacterium with pathogenic congeners. Proc. Natl. Acad. Sci. USA. 2005;102:3004–3009. doi: 10.1073/pnas.0409900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: a laboratory manual. New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Scott C, Partridge JD, Stephenson JR, Green J. DNA target sequence and FNR-dependent gene expression. FEBS letters. 2003;541:97–101. doi: 10.1016/s0014-5793(03)00312-0. [DOI] [PubMed] [Google Scholar]
- Silhavy TJ, Berman Michael L, Enquist Lynn W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1984. [Google Scholar]
- Sitnikov DM, Schineller JB, Baldwin TO. Transcriptional regulation of bioluminesence genes from Vibrio fischeri. Mol. Microbiol. 1995;17:801–812. doi: 10.1111/j.1365-2958.1995.mmi_17050801.x. [DOI] [PubMed] [Google Scholar]
- Spiro S. The FNR family of transcriptional regulators. Antonie van Leeuwenhoek. 1994;66:23–36. doi: 10.1007/BF00871630. [DOI] [PubMed] [Google Scholar]
- Spiro S, Guest JR. Inactivation of the FNR protein of Escherichia coli by targeted mutagenesis in the N-terminal region. Mol. Microbiol. 1988;2:701–707. doi: 10.1111/j.1365-2958.1988.tb00080.x. [DOI] [PubMed] [Google Scholar]
- Spiro S, Gaston KL, Bell AI, Roberts RE, Busby SJ, Guest JR. Interconversion of the DNA-binding specificities of two related transcription regulators, CRP and FNR. Mol. Microbiol. 1990;4:1831–1838. doi: 10.1111/j.1365-2958.1990.tb02031.x. [DOI] [PubMed] [Google Scholar]
- Stabb EV. The Vibrio fischeri-Euprymna scolopes light organ symbiosis. In: Thompson FL, Austin B, Swings J, editors. The Biology of Vibrios. Washington, D.C: ASM Press; 2006. pp. 204–218. [Google Scholar]
- Stabb EV, Ruby EG. RP4-based plasmids for conjugation between Escherichia coli and members of the Vibrionaceae. Methods Enzymol. 2002;358:413–426. doi: 10.1016/s0076-6879(02)58106-4. [DOI] [PubMed] [Google Scholar]
- Stabb EV, Reich KA, Ruby EG. Vibrio fischeri genes hvnA and hvnB encode secreted NAD+-glycohydrolases. J. Bacteriol. 2001;183:309–317. doi: 10.1128/JB.183.1.309-317.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens AM, Greenberg EP. Transcriptional activation by LuxR. In: Dunny GM, Winans SC, editors. Cell-cell signaling in bacteria. Washington, D.C: ASM Press; 1999. pp. 231–242. [Google Scholar]
- Timmins GS, Jackson SK, Swartz HM. The evolution of bioluminescent oxygen consumption as an ancient oxygen detoxification mechanism. J. Molec. Evol. 2001;52:321–332. doi: 10.1007/s002390010162. [DOI] [PubMed] [Google Scholar]
- Tomich CS, Kaytes PS, Olsen MK, Patel H. Use of lacZ expression to monitor transcription. Plasmid. 1988;20:167–170. doi: 10.1016/0147-619x(88)90022-4. [DOI] [PubMed] [Google Scholar]
- Ulitzur S, Dunlap PV. Regulatory circuitry controlling luminescence autoinduction in Vibrio fischeri. Photochem. Photobiol. 1995;62:625–632. [Google Scholar]
- Visick KL, Foster J, Doino J, McFall-Ngai M, Ruby EG. Vibrio fischeri lux genes play an important role in colonization and development of the host light organ. J. Bacteriol. 2000;182:4578–4586. doi: 10.1128/jb.182.16.4578-4586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SM, Savery NJ, Busby SJ, Wing HJ. Transcription activation at class I FNR-dependent promoters: identification of the activating surface of FNR and the corresponding contact site in the C-terminal domain of the RNA polymerase alpha subunit. Nucleic Acids Res. 1997;25:4028–4034. doi: 10.1093/nar/25.20.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing HJ, Williams SM, Busby SJ. Spacing requirements for transcription activation by Escherichia coli FNR protein. J. Bacteriol. 1995;177:6704–6710. doi: 10.1128/jb.177.23.6704-6710.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigha A, Rosenfeld E, Schmitt P, Duport C. The redox regulator Fnr is required for fermentative growth and enterotoxin synthesis in Bacillus cereus F4430/73. J. Bacteriol. 2007;189:2813–2824. doi: 10.1128/JB.01701-06. [DOI] [PMC free article] [PubMed] [Google Scholar]