Abstract
Previous studies demonstrated that the nef gene is a critical determinant of the pathogenicity of simian immunodeficiency virus (SIV) in macaques. In the present study, we evaluated the effect of a spontaneous frameshift mutation in the C-terminus of the nef gene of the minimally pathogenic SIVsmH4i clone. This clone exhibited a single nucleotide deletion in the nef gene relative to pathogenic SIV clones that resulted in a frameshift and addition of 46 amino acids to the C-terminus of Nef. We generated a corrected version of this clone, SIVsmH4i Nef+ that restored Nef protein expression. Inoculation of macaques with SIVsmH4i resulted in delayed and low levels of peak viremia. This contrasted with improved kinetics and robust peak viremia in macaques inoculated with the corrected version. Despite the restoration of in vivo replication ability, neither clone resulted in memory CD4+ T cell loss or disease in a period of two years.
Keywords: SIV, Macaques, Rhesus, Pathogenesis, Nef
Introduction
Simian immunodeficiency virus (SIV) infection of macaques has become a valuable surrogate model for human AIDS (Daniel et al., 1985) for vaccine and pathogenesis studies. SIVs originating from sooty mangabey monkeys (both SIVsm and SIVmac) are the most widely used for vaccine studies. However, there is a relative paucity of pathogenic SIV clones from the sooty mangabey lineage for evaluation of vaccine efficacy. In addition, many of these viruses such as SIVmac239 and SIVsmE543-3 are difficult to neutralize; therefore they are more reflective of viruses at the most resistant end of the spectrum of primary HIV isolates. The development of alternative SIV strains with greater sensitivity to neutralization, but retaining pathogenicity, would be useful for the evaluation of vaccine strategies intended to induce neutralizing antibody responses.
Our lab has focused on a lineage of SIV originally derived from a naturally SIVsm-infected sooty mangabey, E038 that was subsequently passaged in a rhesus macaque, F236 (Baskin et al., 1988). Virus isolated in H9 cells from PBMC at the time that F236 developed AIDS was used to generate the first molecular clone of SIVsm (Hirsch et al., 1989b) and in subsequent animal passages to generate more pathogenic virus strains. SIVsmH4 has been the basis for immunogens in a number of vaccine studies (Hirsch et al., 1996; Moss et al., 1996; Ourmanov et al., 2000a,b; Sharpe et al., 2001). The original proviral clone had a spontaneous frameshift mutation in the integrase domain of pol that impaired virus replication in vitro but was corrected by mutagenesis to generate SIVsmH4i. Despite robust replication in vitro, SIVsmH4i was minimally pathogenic in macaques (Hirsch et al., 1989a) resulting in extremely low primary and often undetectable post-acute viremia. This contrasted with the robust replication and pathogenicity of the closely related SIVsmE543-3 clone (Hirsch et al., 1997; Kuwata et al., 2007) that was derived from passage of SIVsmF236 in a rhesus macaque, E543. These clones also differ in a number of biologic properties that are related to differences in their envelope genes. Thus SIVsmH4i does not infect macaque monocyte derived macrophages (MDM) in vitro and is extremely sensitive to neutralizing antibody whereas SIVsmE543-3 replicates in MDM and is much more difficult to neutralize (Hirsch et al., 1997). Comparative analysis of nef gene sequences relative to other SIVsm/mac clones revealed a single nucleotide deletion at position 761 of SIVsmH4i nef gene, which results in a frameshift and the addition of 46 amino acids to the C-terminus of Nef (see Fig. 1).
Fig. 1.
Comparison of nucleotide (A) and amino acid (B) sequences of the C-terminus of Nef of the original SIVsmH4i, the corrected version and sequences found at various time points post-infection in macaques H729. The nucleotide sequence of the original clone at the top is aligned with nucleotide substitutions below and identical nucleotides indicated by a dash (-). Gaps are indicated by a dot (.). The amino acid sequence of the C-terminus of Nef is shown in single amino acid code using the same symbols.
Previous studies have demonstrated that HIV or SIV Nef is one of the major determinants of virulence (Kestler et al., 1991; Kirchhoff et al., 1995, 2008). Nef is a small myristoylated protein devoid of enzymatic activity. It is mainly localized in the paranuclear region with reduced expression at the plasma membrane and serves as an adaptor protein to divert host cell proteins to aberrant functions that amplify viral replication (Arold and Baur, 2001; Geyer et al., 2001). Nef regulates multiple host factors in order to optimize the cellular environment for virus replication (Foster and Garcia, 2008). Four in vitro functions common to both HIV and SIV Nefs are: 1) downregulation of cell surface levels of CD4 (Garcia and Miller, 1991; Lundquist et al., 2002); 2) down-regulation of surface levels of major histocompatibility class I (MHC-I) molecules (Atkins et al., 2008; Schwartz et al., 1996); 3) mediation of cellular signaling and activation (Wei et al., 2005); and 4) enhancement of viral particle infectivity by CD4 independent mechanisms (Campbell et al., 2004; Pizzato et al., 2007). However, for many years considered as an amplifier of HIV replication, the functions of Nef are far more complex. In T cells, Nef could be viewed as a T cell receptor-associated adaptor protein exerting a number of specific signaling functions through the assembly of multi-protein complexes (Arien and Verhasselt, 2008). By targeting the T cell receptor (TCR), Nef may not only prime viral replication but, more importantly, ensure viral survival through distinct mechanisms of immune evasion and anti-apoptosis (Arrode et al., 2008). Recent studies have suggested that SIV Nef may have Vpu-like activity in overcoming the inhibitory activity of tetherin (Jia et al., 2009).
The majority of SIV Nef mutations that have been studied have been the result of premature stop codons or internal deletions, thus resulting in a truncated Nef protein. In contrast, this particular mutation was unique in that the majority of the nef reading frame was intact but was fused to an irrelevant sequence at the C-terminus. This particular mutation was in a region that did not overlap envelope and thus did not affect any other open reading frames. To assess the role of this particular mutation in the attenuated phenotype of SIVsmH4i, we corrected the mutation to generate SIVsmH4i Nef+ and investigated its replication and pathogenicity in rhesus macaques.
Results
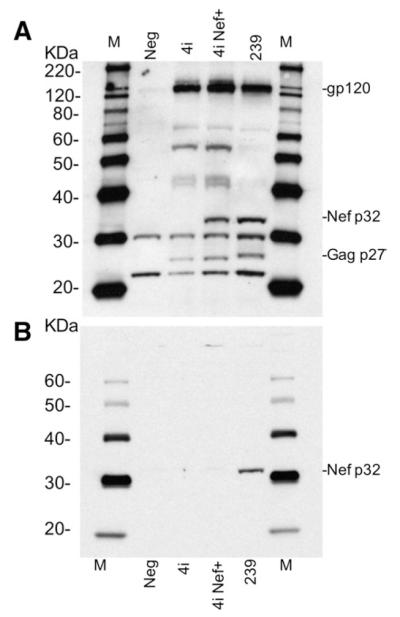
To evaluate the impact of this unique nef mutation in SIV pathogenesis, we inserted an “A” at position 761 in nef of SIVsmH4i by PCR mutagenesis (Fig. 1A) to exactly replicate the sequence found in both SIVmac239 and SIVsmE543-3. The resulting clone designated SIVsmH4i Nef+ was isogenic to the parental strain in all other genes. Following transfection of 293T cells, both viruses generated similar levels of reverse transcriptase activity (data not shown). As shown in Fig. 2, similar levels of viral proteins were observed by western blot analysis of transfected cells using plasma from a SIV-infected rhesus macaque E544 for detection. The major difference between the protein profiles was the absence of a viral protein of approximately 32 kDa in the SIVsmH4i-transfected cells relative to SIVsmH4i Nef+. This protein co-migrated with a similar protein in SIVmac239-transfected cells in the predicted size range of the SIV Nef protein. Since there are no anti-sera specific for SIVsm Nef protein, we used antiserum to SIVmac251 Nef to identify this protein as Nef in SIVmac239-transfected cells (Fig. 2B). As expected, the 32 kDa protein of SIVmac239 reacted with the Nef antibody. Due to significant differences in the sequence of SIVsmH4i Nef, this antiserum did not react with Nef in SIVsmH4i Nef+-transfected cells. The size of Nef in SIVsmH4i would be predicted to be at least 5 kDa larger than prototypic Nef. However, this larger molecular weight species was not identified in SIVsmH4i-transfected cells (Fig. 2A). We presume that the absence of Nef expression is the result of instability resulting from the additional amino acids on the C-terminus.
Fig. 2.
Western blot analysis of the Nef protein expression in SIVsmH4i and SIVsmH4I Nef+-transfected 293T cells. A. Western blotting with RhE544 anti-SIV serum, demonstrates a 32 kDa protein consistent with the size of Nef in both SIVsmH4i Nef+ and SIVmac239 infected cells expressing Nef protein. A comparable but larger molecular weight species cannot be identified in SIVsmH4i-transfected cells. B. SIVsmH4i, SIVsmH4in, and SIVmac239 infected 293T cell lysate western blotting with anti-SIVmac251 Nef monoclonal antibody, which only recognizes SIVmac239 Nef protein, confirms that the 32 kDa protein expressed in panel A is indeed Nef.
Correction of the Nef mutation restores Nef protein function
The relative replication ability of SIVsmH4i Nef+ and SIVsmH4i viruses, normalized for reverse transcriptase (RT) activity of input virus, was compared in CEMx174 cells and macaque PBMCs in vitro (Fig. 3). Both viruses replicated relatively efficiently with similar kinetics. To evaluate whether these viruses differed in terms of any of the known biologic properties of Nef, we compared the effect of infection with SIVsmH4i and SIVsmH4i Nef+ on the expression of CD4 and MHC Class I on a genetically engineered clone of CEMx174 cells – 5.25.EGFP.Luc.M7, that express multiple entry receptors and co-receptors for SIV. The M7-Luc cells also express the enhanced green fluorescent protein gene under a Tat-responsive promoter allowing us to gate on infected cells to evaluate whether they down-regulated these cell surface proteins. As shown by flow cytometric analysis of M7-Luc cells infection in Fig. 4, SIVsmH4i Nef+ clearly down-regulated MHC-I molecules (Fig. 4, left panels) whereas the original SIVsmH4i had no effect on MHC-I expression levels. The extent of down-regulation was similar to that observed for the pathogenic clone, SIVsmE543-3. The picture was more complex with CD4 expression since envelope expression alone can induce CD4 down-regulation. Thus both viruses induced down-regulation of CD4, however, CD4 down-regulation was more profound in cells infected with SIVsmH4i Nef+, similar in extent to that observed with SIVsmE543-3 (Fig. 4, right panels).
Fig. 3.
Comparison of replication of SIVsmH4i and SIVsmH4i Nef+ in CEMx174 cells and PBMCs in vitro. A. SIVsmH4i Nef+ virus replicated more efficiently than the parental SIVsmH4i virus in CEMx174 cells (P<0.05). B. SIVsmH4i Nef+ virus replicated more efficiently than the parental SIVsmH4i virus in rhesus PBMC cells (P<0.05).
Fig. 4.
Comparison of the ability of SIVsmH4i and SIVsmH4iNef+ to down-regulate MHC-I and CD4 on cell surface following infection of M7-Luc cells. M7-Luc cells were infected with SIVsmH4i, SIVsmH4iNef+ or SIVsmE543-3 in triplicate and tested at 68 h post-infection. To measure the level of MHC-I and CD4, cells were stained with anti-human CD4-APC, 7-AAD and HLA-A2-PE. Approximately 30,000 live infected cells (as determined by EGFP positive and 7-AAD negative gating) were counted by flow cytometry and FACS histograms of cell distribution for representative samples are shown in the upper panels. The mock-infected M7-Luc cells were used to set the MHC-I and CD4 positive gates and infected M7-Luc cells stained with isotype control antibodies were used to set the negative gates. The lower panels show the percentage of cells which down-regulated MHC-I or CD4 molecules as indicated by HLA-A2 low or CD4 low expression. The brackets on the graphs represent 95% CI and numbers represent the P value for the unpaired t-test comparing means of indicated triplicates of cells infected with different SIVs.
Correction of the Nef mutation restores in vivo virus replication
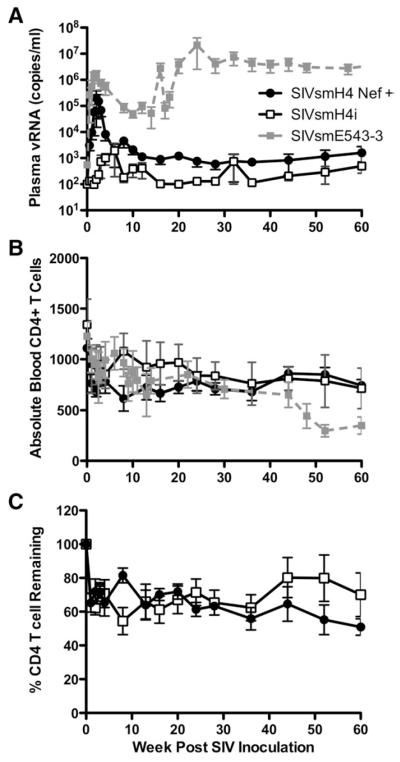
Finally, to evaluate viral replication and pathogenicity in vivo, two groups of four rhesus macaques each were inoculated intravenously with 5000 TCID50 of SIVsmH4i or SIVsmH4i Nef+. Macaques were monitored sequentially for viral replication by plasma viral RNA (Fig. 5A) and effects on peripheral CD4+ T cells by flow cytometry (Figs. 5B and C). Macaques inoculated with SIVsmH4i Nef+ demonstrated peak plasma viral RNA levels of 104 to 106 copies per ml between 14 and 21 days post-inoculation followed by persistent but low levels of plasma viremia (102 to 104) for the remaining course of the infection (Fig. 5A). This contrasted with a delayed peak of viremia from between 4 and 12 weeks p.i. in macaques inoculated with the parental SIVsmH4i. Due to the delay and decreased magnitude of viremia, plasma viral RNA levels were significantly lower in macaques infected with SIVsmH4i at 2 and 3 weeks (Mann Whitney U, P=0.028). Peak plasma viral RNA levels were also considerably lower in these animals, ranging from 103 to 104, with one exception, macaque, H729. Two macaques had undetectable viremia (<100 copies/ml) during the chronic phase of infection (H724 and H710). Indeed, one of these macaques only exhibited detectable plasma viremia at one time point (12 weeks p.i). There was a trend to lower viral load in the group inoculated with SIVsmH4i throughout the chronic phase of infection (Mann Whitney U, P=0.057).
Fig. 5.
Comparison of plasma viremia and CD4 T cell counts in peripheral blood of SIVsmH4i (left panels) and SIVsmH4i Nef+ (right panels). A. Plasma viral RNA levels are shown sequentially throughout infection of each of the macaques. B. Absolute CD4+ T cell numbers in peripheral blood are shown sequentially for all macaques. C. The percent of preinoculation CD4+ T cells remaining at various time points after infection is shown.
Spontaneous correction of the Nef mutation in one macaque
A single nucleotide change in the original SIVsmH4i could result in correction of the nef frameshift. This was of particular interest in macaque H729 from the SIVsmH4i group that exhibited higher viral load. We therefore evaluated a portion of nef amplified by RT-PCR from plasma viral RNA at various time points during infection in each of the animals with the exception of the animal with undetectable viremia, H724. Direct sequencing of the products revealed the appropriate virus sequence in each of the animals at early time points. When compared with the SIVsmH4i inoculum, the correct sequence was detected in plasma of H729 at 3 weeks but was completely replaced with a variant with a single “T” insertion by 6 weeks p.i. (Fig. 1A). This mutation was associated with increasing virus load (arrow in Fig. 5A). The virus in this animal was not identical to SIVsmH4i Nef+ due to an additional substitution at position 762, resulting in a Leu to Tyr substitution at amino acid 252 of Nef (Fig. 1B). Thus, the increase in plasma viremia corresponded to the appearance of a reversion of the nef frameshift mutation. The original mutation was stable in the other three macaques in the SIVsmH4i group until at least 40 weeks p.i., the last time point evaluated. If rhesus macaque H729 is excluded from comparison of viral load, a significant and sustained increase in plasma viremia at both peak and setpoint was observed in macaques inoculated with the SIVsmH4i Nef+ virus (Fig. 6; Mann Whitney, P<0.0001).
Fig. 6.
Sequential comparison of mean plasma viremia (A), absolute CD4+ T cell counts (B) and percent CD4 cell remaining (C) in peripheral blood of SIVsmH4i (unfilled squares) and SIVsmH4i Nef+ (black circles) inoculated macaques. Mean plasma viremia (A) and CD4+ T cell counts (B) are also compared to a cohort of six SIVsmE543-3-infected rhesus macaques (grey symbols), a fully pathogenic infection.
Infection with SIVsmH4i Nef+ results in slow CD4+ T cell depletion
Whole blood samples from the eight rhesus macaques were analyzed sequentially for CD4+ T cell levels by flow cytometry. As shown in Fig. 5B, a transient decline in CD4+ T cell levels was observed during primary viremia. Subsequently, the CD4+ T cell levels remained relatively stable in both groups of macaques. Analysis of memory CD4+ T cell subsets in the blood did not reveal significant changes in this subset and a transient decline in CD4+ T cells in bronchoalveolar lavage (BAL) was only observed in the macaque with highest peak viremia (H727) (data not shown). Since the absolute pre-inoculation CD4+ T cell counts varied widely between individual macaques, we also evaluated the percent of CD4+ T cell remaining as compared to pre-inoculation values (Fig. 5C). The SIVsmH4i group maintained higher percentage of CD4+ T cells than SIVsmH4iNef+ group (Fig. 6, Mann Whitney U, P=0.04), consistent with lower mean viral RNA load observed in these animals. Data analyses that excluded the one SIVsmH4i macaque with reversion of the Nef mutation gave very similar results (data not shown). The study was terminated at 60 and 84 weeks post-inoculation and at that time point, CD4+ T cell numbers remained above AIDS-defining levels and there was no evidence of clinical immunodeficiency in any of the macaques and no significant findings were observed on pathologic examination. Despite improved in vivo replication, SIVsmH4in was still less robust than the closely related pathogenic, neutralization-resistant SIVsmE543-3 (Goldstein et al., 2000; Hirsch et al., 1997) as evidenced by lower chronic phase viremia (Fig. 6A) and CD4+ T cell decline (Fig. 6B).
SIVsmH4i Nef+ induces more robust antibody responses
The kinetics of antibody responses was compared between the two groups by western blot analysis. Overall, the antibody responses in the SIVsmH4i group were weaker than observed in animals infected with SIVsmH4i Nef+ (data not shown). We therefore evaluated the neutralizing antibody responses in the two groups. Serum samples collected at 20 weeks post-infection from both groups of macaques neutralized not only homologous SIVsmH4i viruses, but also cross-neutralized the closely related viruses, SIVsmE660 and SIVsmE543. These two viruses represent slightly heterologous strains that are relatively sensitive and resistant to neutralization respectively. Titers were consistently higher in the Nef− corrected SIVsmH4i Nef+ group (P<0.05, see Table 1), presumably due to more robust virus replication.
Table 1.
Magnitude and cross-reactivity of neutralizing antibodies detected 20 weeks post-infection.
| Nab titer to SIVa |
SIVsmH4i groupb |
SIVsmH4i Nef+ groupb |
||||||
|---|---|---|---|---|---|---|---|---|
| H724 | H728 | H710 | H729 | H726 | H727 | H715 | H732 | |
| SIVsmH4i | 450 | 488 | 388 | 437 | 2441 | 2729 | 2638 | 2483 |
| SIVsmE660 | 433 | 459 | 391 | 428 | 2338 | 2655 | 2573 | 2429 |
| SIVsmE543 | 57 | 64 | 51 | 62 | 326 | 349 | 337 | 329 |
Values are the dilution or concentration at which RLU were reduced 50% compared to those in virus control wells.
Serum samples from each animals were obtained 20 weeks post-infection.
Discussion
This study confirms the importance of the Nef protein in SIV pathogenesis. Correction of a unique nef frameshift mutation in the attenuated SIVsmH4i, restored virus replication in vitro and in vivo. Although the SIVsmH4i Nef was predicted to be significantly larger in molecular weight, analysis of cell lysates of cells transfected with this virus found no evidence for either the wild type Nef protein or a larger species. However, correction of the frameshift in SIVsmH4i Nef+ resulted in expression of a Nef protein that co-migrated with the Nef protein of SIVmac239. We assume therefore, that addition of nonsense amino acids on the C-terminus of Nef results in instability or aberrant trafficking and degradation of the protein. Consistent with that hypothesis, SIVsmH4i did not induce down-regulation of MHC-I antigens in infected cells and had a reduced ability to down-regulate CD4 expression, both properties that were intact for the corrected SIVsmH4i Nef+. These two properties map to different regions of the Nef protein, confirming that the entire protein is apparently not expressed in SIVsmH4i.
Most importantly, macaques inoculated with SIVsmH4i Nef+ exhibited higher peak viral load with normal kinetics and persistence of level viremia during chronic infection. In contrast, macaques infected with SIVsmH4i showed lower peak viral loads and delayed kinetics of virus replication consistent with a Nef defect. There was not a clear distinction in the pathogenicity of the two viruses. However, macaques inoculated with the original SIVsmH4i maintained higher percentage of their CD4+ T cells than those inoculated with SIVsmH4i Nef+, consistent with lower mean viral RNA load. Consistent with higher viral replication, macaques inoculated with SIVsmH4i Nef+ group had higher Nab titers than the macaques inoculated with SIVsmH4i. The neutralizing phenotype of the two viruses did not differ from one another. Therefore, SIVsmH4i Nef+ clearly replicates more efficiently which could be useful for SIV pathogenesis and vaccine research.
SIVsmH4i Nef+ did not result in AIDS after 1 to 1.5 year of follow-up. Based on the slowly declining CD4+ T cell counts, persistence of plasma viremia and previous studies with the original SIVsmH4i clone in macaques (Johnson et al., 1991), it is probable that this virus would eventually result in AIDS after longer follow-up. The underlying reason for the reduced virulence of this virus is not clear. The original SIVsmH4 lambda clone was derived from a long term passage of SIVsmF2236 in H9 cells that apparently contributed to the two attenuating mutations; these were the result of deletion of a single nucleotide in integrase and nef. Although all other genes appear intact, this clone may have additional attenuating mutations in other genes. Alternatively we have speculated that the neutralization phenotype may result in a virus that is more readily controlled by neutralizing antibodies. Studies are underway to investigate the pathogenicity of a SIVsmH4i Nef+ chimera expressing the E543-3 envelope to investigate this latter possibility.
Despite the lack of a clearly pathogenic phenotype, correction of the Nef mutation restored primary virus replication to levels that would allow its use in testing prophylactic strategies aimed at preventing infection. In addition, infection with SIVsmH4i Nef+ clearly resulted in robust SIV-specific homologous and heterologous neutralizing antibody titers. As antibodies act as a first line of defense against HIV infection, the elicitation of humoral immunity should be a component of an effective HIV vaccine. The ability of antibodies to confer protection against HIV has been demonstrated by several studies using the passive transfer of neutralizing antibodies in the non-human primate challenge model (Baba et al., 2000; Eda et al., 2006; Nishimura et al., 2003; Veazey et al., 2003). Efforts have been made to induce a similarly protective humoral immune response by vaccination with antigens derived from HIV. Thus far, the results have been disappointing. Humoral immune responses elicited by vaccination display activities that are generally much less potent and broad as compared to those induced during natural infection (Burke and Barnett, 2007). Therefore, more work is required to improve protective antibody responses in non-human primate models. Our study provides a useful reagent for vaccine research since virus from SIVsmH4in clone has a more readily neutralizable envelope protein than many other more pathogenic SIV strains.
Methods
QuickChange site-directed mutagenesis PCR
A 5 kb fragment of pSIVsmH4i digested with Hind III and Asp I retaining nef was religated after a Klenow fill-in reaction to generate a subclone for PCR mutagenesis. The QuickChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) was used according to the manufacturer’s instructions (Kunkel, 1985) to introduce an additional “A” into the nef gene sequence using primers, 5′ GGC TAA CCG CAA GAG GCC TTT ATA AAA TGG CTG ACA AGA AGG 3′ and 5′ CCT TCT TGT CAG CCA TTT TAT AAA GGC CTC TTG CGG TTA GCC 3′ to replicate the nucleotide sequence found in SIVmac239 and SIVsmE543-3. The resulting clone was sequenced to confirm the mutation and a 1 kb Nde I/Sal I fragment containing the mutation was used to replace corresponding fragment into the full length pSIVsmH4i clone to create pSIVsmH4iNef+.
Viruses, cells, and replication assay
The pSIVsmH4i and pSIVsmH4i Nef+ clones were transfected into 293T cells to generate cell free viruses using FuGENE transfection reagent (Roche, Indianapolis, IN) according to the manufacturer’s instructions. Cell free supernatants from 293T transfected cells were normalized for p27 antigen content and used to infect CEMx174 T cells or macaque PBMC. Cells were incubated with virus for 2-3 h, then pelleted and resuspended in fresh culture medium. Virus replication was detected by 32P reverse transcriptase (RT) assay as previously reported (Sears et al., 1999). PBMC were separated from peripheral blood of healthy uninfected macaques by density centrifugation (LSM, ICN Biomedicals, Inc., Aurora, OH) according to the manufacturer’s instructions. PBMC were suspended in RPMI 1640, supplemented with 10% human AB serum, penicillin (100 μg/ml), streptomycin (100 μg/ml) and 10% IL-2 at density of 106 cells/ml and cultured with twice weekly media changes.
Western blot detection of Nef protein expression
Western blotting was performed to detect viral Nef protein expression in 293T cells transfected with SIVsmH4i, SIVsmH4i Nef+ or SIVmac239. The cells were harvested at 72 h post transfection, lysed with RIPA buffer (Upstate, Temecula, CA). Probes were heated to 100 °C for 5 min in NuPAGE lithium dodecyl sulphate sample buffer supplemented with NuPAGE reducing agent and subjected to electrophoresis on a NuPAGE 4-12% Bis-Tris gel (Invitrogen, Carlsbad, CA). MagicMark™ XP Western Protein Standard (Invitrogen, Carlsbad, CA) was used as molecular weight marker. The resolved samples were transferred to 0.2 μm nitrocellulose membrane using iBlot transfer unit (Invitrogen, Carlsbad, CA) as suggested by the manufacturer. Serum from a SIVsmF236-infected rhesus macaque, E544 was used as a primary antibody for detection of viral proteins. This antiserum reacts with the Env, Gag, Pol and Nef proteins of SIV. To block non-specific binding the membrane was immersed in 5% non-fat dried milk, 0.1% Tween 20 (Sigma, Saint Louis, MO) in Tris buffered saline, pH7.6 (TBS-T) for 1 h at room temperature on an orbital shaker. After blocking, the membrane was incubated with a 1:1000 dilution of the primary antibody the overnight, then briefly rinsed with two changes of wash buffer and washed 3 times for 10 min in TBS-T. After incubation with a 1:10,000 dilution of a sheep anti-human Ig conjugated with horseradish peroxidase (Amersham, Piscataway, NJ) for 1 h and intensive washing, proteins on the membrane were detected by enhanced chemiluminescence with ECL Plus Western Blotting Detection Reagent (Amersham, Piscataway, NJ) A 1:500 dilution of SIVmac251 Nef specific monoclonal antibody (17.2) as primary antibody and a sheep anti-mouse Ig horseradish peroxidase conjugate (Amersham, Piscataway, NJ) as a secondary antibody was used to detect SIVmac239 Nef. This monoclonal antibody was obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: SIVmac251 Nef monoclonal antibody (17.2) from Dr. Kai Krohn and Dr. Vladimir Ovod.
Animals and viruses
A total of eight juvenile, colony-bred, Indian-origin rhesus macaques (Macaca mulatta) were randomly separated into two groups and inoculated with either SIVsmH4i or SIVsmH4i Nef+ virus. Animals were maintained in accordance with the guidelines of the Committee on the Care and Use of Laboratory Animals under a NIAID-approved animal study protocol and were housed in a Biosafety Level 2 facility using BSL3 practices. Virus stocks generated in 293T cells were normalized by tissue culture infectious dose 50% (TCID50) on TZM-bl cells in vitro. The animals were then inoculated intravenously with 5000 TCID50 of the SIVsmH4i or SIVsmH4i Nef+ virus. EDTA-coagulated blood samples were collected from macaques and monitored sequentially for plasma viral RNA levels, CD4+ T cell subsets, and SIV-specific neutralizing antibody titers. Bronchoalveolar lavages (BAL) were collected sequentially for evaluating mucosal CD4+ T cell depletion.
Plasma viral load
Viral RNA was isolated from cryopreserved plasma samples using the QIAamp Viral RNA Mini Kit (Qiagen, Hilden, Germany). Reverse transcription was carried out using TaqMan reverse transcription reagents (Applied Biosystems, Foster City, CA) and random RETRO-script decamers (Ambion) for priming. Viral RNA levels in plasma were then determined by quantitative real-time PCR using a Prism 7700 sequence detector (Applied Biosystems, Foster City, CA) as detailed previously (Seth et al., 2000). A serial 5-fold dilution series of the standard RNA template was assayed in duplicate to generate a standard curve for each assay. RT-PCR for each plasma samples were performed in triplicate including one control reaction processed without addition of reverse transcriptase for potential DNA contamination. Results of assay were normalized to the volume of plasma extracted and expressed as SIV RNA copy equivalents per ml of plasma, as described for HIV-1 (Piatak et al., 1993a,b). Interassay variation was less than 25% (c.v.).
Sequence evaluation of the nef gene in infected macaques
To assess the stability of the frameshift mutation in macaques infected with the original SIVsmH4i, nef sequences were amplified by RT-PCR from plasma samples at different times post-infection. The sequences of primers for nef gene RT-PCR used for amplification were: forward: 5′ ATG AAT ACC CCC TGG AGG AAC C 3′ and reverse: 5′ CTT GTG GAA AGT CCC TGC TGT C 3′. Viral RNA was extracted from plasma using QIAamp Viral RNA Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions and SuperScript III One-Step RT-PCR System with Platinum Taq DNA Polymerase (Invitrogen, Carlsbad, CA) was used for RT-PCR. The products of RT-PCR were sequenced directly after purification and sequence results were analyzed by Vector NTI software.
Flow cytometric analysis
Blood, and mononuclear cells isolated from bronchoalevolar lavage (BAL), and lymph node (LN) biopsies were stained with combinations of the following fluorochrome-conjugated monoclonal antibodies: CD3 (fluorescein isothiocyanate [FITC] or peridinin chlorophyll protein-Cy5.5), CD4 (phycoerythrin [PE], peridinin chlorophyll protein-Cy5.5, or allophycocyanin [APC]), CD28 (FITC), CD95 (APC), CCR5 (PE), CD8 (FITC or APC), CD8 beta (PE), CD20 (FITC or PE), and mouse immunoglobulin G1 and G2 isotype-matched controls. Percentages of memory/naïve cells in CD4+ T cells were determined using CD28 and CD95 as makers, as described previously (Nishimura et al., 2004).
The ability of Nef to down-regulate MHC-I and CD4 on infected cells was assessed by infecting a genetically engineered clone of CEMx174 cells, 5.25.EGFP.Luc.M7 (M7-Luc), that expresses multiple entry receptors and co-receptors for SIV (Montefiori, 2004). The M7-Luc cells also express Tat-responsive reporter genes for firefly luciferase and enhanced green fluorescent protein (EGFP) that allowing to gate on infected cells. M7-Luc cells were grown in RPMI-1640 medium containing 2 mM l-glutamine, 25 mM HEPES, 10% heat-inactivated FBS and 50 μg/ml gentamycin. For routine maintenance, cells were grown in medium supplemented with 0.2 mg/ml hygromycin B, 0.3 mg/ml genetecin (G418) and 0.5 μg/ml puromycin. Supplemented drugs were removed before cell infection. For fluorocytometric assay 15 million cells were infected at 37 °C with 2000 TCID50 of SIVsmH4i, SIVsmH4iNef+ or SIVsmE543-3 in 8 ml of growth medium supplemented with 15 μg/ml of DEAE-dextran. After a 6 hour incubation, the cell suspension was diluted with 12 ml of growth medium and incubated at 37 °C in parallel with mock-infected M7-Luc cells as a control. All infections were done in parallel in triplicate. Approximately two million cells from each sample were tested at 68 h post-infection. Cells were pelleted by low speed centrifugation, washed with PBS, resuspended in 200 μl of Stain Buffer (BD Pharmigen) and stained with anti-human CD4-APC, antihuman HLA-A2-PE and 7-amino-actinomycin D (7-AAD) at 4 °C for 30 min. After staining cells were washed once with 2 ml of Stain Buffer, once with 2 ml of PBS containing 5 μl/ml actinomycin D (Sigma, Saint Louis, MO), resuspended in 100 μl of this solution and fixed by adding 20 μl of 2% formaldehyde in PBS. Live infected cells were gated as EGFP positive and 7-AAD negative, and the percentage of infected cells population in the MHC-I low or CD4 low gates were measured to compare with MHC-1 high and CD4 high mock-infected M7-Luc cells collected on the same time.
All antibodies were obtained from BD Biosciences except CD8 beta (Beckman Coulter, Fullerton, CA) and stained cells were analyzed by four-color flow cytometry using a FACSCalibur (BD Biosciences). Data acquisition and analysis were performed using CellQuest (BD Biosciences) and Flowjo (TreeStar, San Carlos, CA) software.
Detection of SIV-specific antibody responses
Serology for antibodies to SIV was performed by Western blot analysis, as previously described (Hirsch et al., 1995). Neutralizing antibodies (Nabs) were measured as reductions in Luc reporter gene expression in TZM-bl cells after infection with various SIVsm viruses pre-incubated with macaque serum samples as described previously (Montefiori, 2004). This assay is a modified version of the assay used by Wei et al. (Wei et al., 2003). Briefly, 50 TCID50 of virus was incubated with various dilutions of test samples (starting with 1 to 20 with eight threefold stepwise dilutions) in triplicate for 1 h at 37 °C in a total volume of 100 μl growth medium in 96-well flat-bottom culture plates (Corning-Costar). Freshly trypsinized cells (10,000 cells in 100 μl of growth medium) were added to each well. One set of six control wells received cells plus virus (virus control), and another set of six wells received cells only (background control). Approximately 36 h after incubation, a culture medium was removed from each well and 50 μl of Cell Lysing Buffer (Promega) was added to the cells. After a 15 min incubation at room temperature to allow cell lysis, 30 μl of cell lysate was transferred to 96-well black solid OptiPlates-96F plates (PerkinElmer) for measurements of luminescence using a Mithras LB940 luminometer (Bethold Technologies)) that inject luciferase assay substrate (Promega) into each well. The 50% inhibitory dose (ID50) was defined as the serum dilution that caused a 50% reduction in luciferase activity compared to virus control wells after subtraction of background. To calculate the dilution of serum that neutralized 50% of infectious virus (ID50), the inhibitory dose-response curve was fit with a nonlinear function (a four-parameter dose-response curve equation) using GraphPad Prism 5 software (GraphPad Software, Inc.).
Acknowledgments
We thank Simoy Goldstein, Que Dang and Takeo Kuwata for technical assistance, and Bioqual Inc. (Rockville, MD) for conducting the animal studies. This work was supported by the intramural program of NIAID, NIH.
References
- Arien KK, Verhasselt B. HIV Nef: role in pathogenesis and viral fitness. Curr. HIV Res. 2008;6(3):200–208. doi: 10.2174/157016208784325001. [DOI] [PubMed] [Google Scholar]
- Arold ST, Baur AS. Dynamic Nef and Nef dynamics: how structure could explain the complex activities of this small HIV protein. Trends Biochem. Sci. 2001;26(6):356–363. doi: 10.1016/s0968-0004(01)01846-1. [DOI] [PubMed] [Google Scholar]
- Arrode G, Hegde R, Jin Y, Singh DK, Narayan O, Chebloune Y. Nef modulates the immunogenicity of Gag encoded in a non-infectious HIV DNA vaccine. Vaccine. 2008;26(31):3795–3804. doi: 10.1016/j.vaccine.2008.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkins KM, Thomas L, Youker RT, Harriff MJ, Pissani F, You H, Thomas G. HIV-1 Nef binds PACS-2 to assemble a multikinase cascade that triggers major histocompatibility complex class I (MHC-I) down-regulation: analysis using short interfering RNA and knock-out mice. J. Biol. Chem. 2008;283(17):11772–11784. doi: 10.1074/jbc.M707572200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini LA, Posner MR, Katinger H, Stiegler G, Bernacky BJ, Rizvi TA, Schmidt R, Hill LR, Keeling ME, Lu Y, Wright JE, Chou TC, Ruprecht RM. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 2000;6(2):200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- Baskin GB, Murphey-Corb M, Watson EA, Martin LN. Necropsy findings in rhesus monkeys experimentally infected with cultured simian immunodeficiency virus (SIV)/delta. Vet. Pathol. 1988;25(6):456–467. doi: 10.1177/030098588802500609. [DOI] [PubMed] [Google Scholar]
- Burke B, Barnett SW. Broadening our view of protective antibody responses against HIV. Curr. HIV Res. 2007;5(6):625–641. doi: 10.2174/157016207782418533. [DOI] [PubMed] [Google Scholar]
- Campbell EM, Nunez R, Hope TJ. Disruption of the actin cytoskeleton can complement the ability of Nef to enhance human immunodeficiency virus type 1 infectivity. J. Virol. 2004;78(11):5745–5755. doi: 10.1128/JVI.78.11.5745-5755.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel MD, Letvin NL, King NW, Kannagi M, Sehgal PK, Hunt RD, Kanki PJ, Essex M, Desrosiers RC. Isolation of T-cell tropic HTLV-III-like retrovirus from macaques. Science. 1985;228(4704):1201–1204. doi: 10.1126/science.3159089. [DOI] [PubMed] [Google Scholar]
- Eda Y, Murakami T, Ami Y, Nakasone T, Takizawa M, Someya K, Kaizu M, Izumi Y, Yoshino N, Matsushita S, Higuchi H, Matsui H, Shinohara K, Takeuchi H, Koyanagi Y, Yamamoto N, Honda M. Anti-V3 humanized antibody KD-247 effectively suppresses ex vivo generation of human immunodeficiency virus type 1 and affords sterile protection of monkeys against a heterologous simian/human immunodeficiency virus infection. J. Virol. 2006;80(11):5563–5570. doi: 10.1128/JVI.02095-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JL, Garcia JV. HIV-1 Nef: at the crossroads. Retrovirology. 2008;5:84. doi: 10.1186/1742-4690-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia JV, Miller AD. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature. 1991;350(6318):508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- Geyer M, Fackler OT, Peterlin BM. Structure-function relationships in HIV-1 Nef. EMBO Rep. 2001;2(7):580–585. doi: 10.1093/embo-reports/kve141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S, Brown CR, Dehghani H, Lifson JD, Hirsch VM. Intrinsic susceptibility of rhesus macaque peripheral CD4(+) T cells to simian immunodeficiency virus in vitro is predictive of in vivo viral replication. J. Virol. 2000;74(20):9388–9395. doi: 10.1128/jvi.74.20.9388-9395.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch VM, Dapolito G, McGann C, Olmsted RA, Purcell RH, Johnson PR. Molecular cloning of SIV from sooty mangabey monkeys. J. Med. Primatol. 1989a;18(3-4):279–285. [PubMed] [Google Scholar]
- Hirsch VM, Olmsted RA, Murphey-Corb M, Purcell RH, Johnson PR. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature. 1989b;339(6223):389–392. doi: 10.1038/339389a0. [DOI] [PubMed] [Google Scholar]
- Hirsch VM, Dapolito G, Johnson PR, Elkins WR, London WT, Montali RJ, Goldstein S, Brown C. Induction of AIDS by simian immunodeficiency virus from an African green monkey: species-specific variation in pathogenicity correlates with the extent of in vivo replication. J. Virol. 1995;69(2):955–967. doi: 10.1128/jvi.69.2.955-967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch VM, Fuerst TR, Sutter G, Carroll MW, Yang LC, Goldstein S, Piatak M, Jr., Elkins WR, Alvord WG, Montefiori DC, Moss B, Lifson JD. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J. Virol. 1996;70(6):3741–3752. doi: 10.1128/jvi.70.6.3741-3752.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch V, Adger-Johnson D, Campbell B, Goldstein S, Brown C, Elkins WR, Montefiori DC. A molecularly cloned, pathogenic, neutralization-resistant simian immunodeficiency virus, SIVsmE543-3. J. Virol. 1997;71(2):1608–1620. doi: 10.1128/jvi.71.2.1608-1620.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia B, Serra-Moreno R, Neidermyer W, Rahmberg A, Mackey J, Fofana IB, Johnson WE, Westmoreland S, Evans DT. Species-specific activity of SIV Nef and HIV-1 Vpu in overcoming restriction by tetherin/BST2. PLoS Pathog. 2009;5(5):e1000429. doi: 10.1371/journal.ppat.1000429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PR, Hamm TE, Goldstein S, Kitov S, Hirsch VM. The genetic fate of molecularly cloned simian immunodeficiency virus in experimentally infected macaques. Virology. 1991;185(1):217–228. doi: 10.1016/0042-6822(91)90769-8. [DOI] [PubMed] [Google Scholar]
- Kestler HW, III, Ringler DJ, Mori K, Panicali DL, Sehgal PK, Daniel MD, Desrosiers RC. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65(4):651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- Kirchhoff F, Greenough TC, Brettler DB, Sullivan JL, Desrosiers RC. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N. Engl. J. Med. 1995;332(4):228–232. doi: 10.1056/NEJM199501263320405. [DOI] [PubMed] [Google Scholar]
- Kirchhoff F, Schindler M, Specht A, Arhel N, Munch J. Role of Nef in primate lentiviral immunopathogenesis. Cell. Mol. Life Sci. 2008;65(17):2621–2636. doi: 10.1007/s00018-008-8094-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel TA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc. Natl. Acad. Sci. U. S. A. 1985;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwata T, Byrum R, Whitted S, Goeken R, Buckler-White A, Plishka R, Iyengar R, Hirsch VM. A rapid progressor-specific variant clone of simian immunodeficiency virus replicates efficiently in vivo only in the absence of immune responses. J. Virol. 2007;81(17):8891–8904. doi: 10.1128/JVI.00614-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist CA, Tobiume M, Zhou J, Unutmaz D, Aiken C. Nef-mediated downregulation of CD4 enhances human immunodeficiency virus type 1 replication in primary T lymphocytes. J. Virol. 2002;76(9):4625–4633. doi: 10.1128/JVI.76.9.4625-4633.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montefiori DC. Evaluating neutralizing antibodies against HIV, SIV and SHIV in luciferase reporter gene assays. In: Coligan AMKJE, Margulies DH, Shevach EM, Strober W, Coico R, editors. Current Protocols in Immunology. John Wiley & Sons; New York, N.Y: 2004. pp. 12.11.1–12.11.15. [DOI] [PubMed] [Google Scholar]
- Moss B, Carroll MW, Wyatt LS, Bennink JR, Hirsch VM, Goldstein S, Elkins WR, Fuerst TR, Lifson JD, Piatak M, Restifo NP, Overwijk W, Chamberlain R, Rosenberg SA, Sutter G. Host range restricted, non-replicating vaccinia virus vectors as vaccine candidates. Adv. Exp. Med. Biol. 1996;397:7–13. doi: 10.1007/978-1-4899-1382-1_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y, Igarashi T, Haigwood NL, Sadjadpour R, Donau OK, Buckler C, Plishka RJ, Buckler-White A, Martin MA. Transfer of neutralizing IgG to macaques 6 h but not 24 h after SHIV infection confers sterilizing protection: implications for HIV-1 vaccine development. Proc. Natl. Acad. Sci. U. S. A. 2003;100(25):15131–15136. doi: 10.1073/pnas.2436476100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y, Igarashi T, Donau OK, Buckler-White A, Buckler C, Lafont BA, Goeken RM, Goldstein S, Hirsch VM, Martin MA. Highly pathogenic SHIVs and SIVs target different CD4+ T cell subsets in rhesus monkeys, explaining their divergent clinical courses. Proc. Natl. Acad. Sci. U. S. A. 2004;101(33):12324–12329. doi: 10.1073/pnas.0404620101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ourmanov I, Bilska M, Hirsch VM, Montefiori DC. Recombinant modified vaccinia virus ankara expressing the surface gp120 of simian immunodeficiency virus (SIV) primes for a rapid neutralizing antibody response to SIV infection in macaques. J. Virol. 2000a;74(6):2960–2965. doi: 10.1128/jvi.74.6.2960-2965.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ourmanov I, Brown CR, Moss B, Carroll M, Wyatt L, Pletneva L, Goldstein S, Venzon D, Hirsch VM. Comparative efficacy of recombinant modified vaccinia virus Ankara expressing simian immunodeficiency virus (SIV) Gag-Pol and/or Env in macaques challenged with pathogenic SIV. J. Virol. 2000b;74(6):2740–2751. doi: 10.1128/jvi.74.6.2740-2751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatak M, Jr., Luk KC, Williams B, Lifson JD. Quantitative competitive polymerase chain reaction for accurate quantitation of HIV DNA and RNA species. Biotechniques. 1993a;14(1):70–81. [PubMed] [Google Scholar]
- Piatak M, Jr., Saag MS, Yang LC, Clark SJ, Kappes JC, Luk KC, Hahn BH, Shaw GM, Lifson JD. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science. 1993b;259(5102):1749–1754. doi: 10.1126/science.8096089. [DOI] [PubMed] [Google Scholar]
- Pizzato M, Helander A, Popova E, Calistri A, Zamborlini A, Palu G, Gottlinger HG. Dynamin 2 is required for the enhancement of HIV-1 infectivity by Nef. Proc. Natl. Acad. Sci. U. S. A. 2007;104(16):6812–6817. doi: 10.1073/pnas.0607622104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz O, Marechal V, Le Gall S, Lemonnier F, Heard JM. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 1996;2(3):338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- Sears JF, Repaske R, Khan AS. Improved Mg2+-based reverse transcriptase assay for detection of primate retroviruses. J. Clin. Microbiol. 1999;37(6):1704–1708. doi: 10.1128/jcm.37.6.1704-1708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A, Ourmanov I, Schmitz JE, Kuroda MJ, Lifton MA, Nickerson CE, Wyatt L, Carroll M, Moss B, Venzon D, Letvin NL, Hirsch VM. Immunization with a modified vaccinia virus expressing simian immunodeficiency virus (SIV) Gag-Pol primes for an anamnestic Gag-specific cytotoxic T-lymphocyte response and is associated with reduction of viremia after SIV challenge. J. Virol. 2000;74(6):2502–2509. doi: 10.1128/jvi.74.6.2502-2509.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe S, Polyanskaya N, Dennis M, Sutter G, Hanke T, Erfle V, Hirsch V, Cranage M. Induction of simian immunodeficiency virus (SIV)-specific CTL in rhesus macaques by vaccination with modified vaccinia virus Ankara expressing SIV transgenes: influence of pre-existing anti-vector immunity. J. Gen. Virol. 2001;82(Pt 9):2215–2223. doi: 10.1099/0022-1317-82-9-2215. [DOI] [PubMed] [Google Scholar]
- Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, Hu Q, Ketas T, Marx PA, Klasse PJ, Burton DR, Moore JP. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat. Med. 2003;9(3):343–346. doi: 10.1038/nm833. [DOI] [PubMed] [Google Scholar]
- Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. Antibody neutralization and escape by HIV-1. Nature. 2003;422(6929):307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- Wei BL, Arora VK, Raney A, Kuo LS, Xiao GH, O’Neill E, Testa JR, Foster JL, Garcia JV. Activation of p21-activated kinase 2 by human immunodeficiency virus type 1 Nef induces merlin phosphorylation. J. Virol. 2005;79(23):14976–14980. doi: 10.1128/JVI.79.23.14976-14980.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]