Abstract
While a number of chronic pain conditions are much more prevalent in women than men, the role of estrogen in regulating nociception remains unclear. Estrogen receptors (ER) are known to be expressed in various parts of the nociceptive pathway, including in the small-sized primary sensory neurons of the dorsal root ganglion (DRG). This study evaluated the effects of long-term estrogen replacement on pain sensitivity and neuropeptide expression in the DRG of female Sprague Dawley rats. The goal was to evaluate whether estrogen modulates nociceptive neuropeptides in the DRG in a manner consistent with its effects on pain sensitivity. Our results show that long term (28 days) ovariectomy (ovx) of adult rats induces a profound thermal and mechanical hyperalgesia of the hindpaw and tail compared to ovariectomized animals that were continuously estrogen-treated (ovx+E). Significant changes in the expression of two neuropeptides, substance P (SP) and calcitonin gene related peptide (CGRP), were observed using immunocytochemistry and in situ hybridization (ISH) in the small lumbar DRG neurons which contain ER. CGRP and SP were differentially regulated by estrogen, with SP showing a significant downregulation at both the peptide and mRNA level while CGRP and its mRNA were increased in the DRG of estrogen-treated animals. We also evaluated the development of mechanical allodynia after partial sciatic nerve injury and found that both ovx and ovx+E animals developed significant allodynia within a week of the partial nerve injury, which continued for at least one month. The estrogen treated animals showed a partial amelioration of the extent of the allodynia at 2 weeks post injury. Overall, the results suggest that estrogen has significant anti-nociceptive actions that can be directly correlated with changes in expression of two peptides in the small nociceptive ERα expressing neurons of the DRG.
Keywords: Gonadal steroids, estrogen, steroid receptors, neuropeptides, peripheral neurons, sensory neurons, DRG cells
INTRODUCTION
While it is clear that primary sensory neurons in the dorsal root ganglion (DRG) express estrogen receptors (ER), the role of these receptors in the functioning of the nociceptive system is not completely understood. Studies have shown that ERα is expressed only in the small, nociceptive neurons in the rat DRG while ERβ mRNA is expressed in all DRG neurons (Taleghany et al. 1999). Many of the small DRG neurons are also known to contain neuropeptides involved in nociception, such as substance P (SP) and calcitonin gene-related peptide (CGRP). While SP is expressed mainly in small neurons of the DRG, CGRP has a wider distribution and is found in some intermediate-sized neurons as well as in small cells (Lee et al. 1985). In other neural systems, estrogen has been shown to have a negative effect on SP expression. For example, estrogen treatment has been shown to decrease SP levels in the anterior pituitary (Brown et al. 1990; O'Halloran et al. 1990; Ma et al. 1997a). In neurons of the bed nucleus of the stria terminalis, as well as those in the ventromedial nucleus of cycling female rats, SP immunoreactivity is lowest during proestrus, when estrogen levels are high (Micevych et al. 1988). In infundibular neurons of the hypothalamus, the mRNA levels of beta preprotachykinin (β-PPT) from which SP is generated were shown to be lower in young women and higher in postmenopausal women (Rance and Young 1991). A study has shown that long term treatment of rats with the hormone replacement therapy drug, Premarin, significantly downregulates the level of β-PPT mRNA in rat DRG neurons (Liuzzi et al. 1999a). The downregulation of SP by estrogen suggests that estrogen may have antinociceptive effects in the DRG system.
In contrast to its effects on SP expression, estrogen is known to positively enhance the expression of CGRP, which plays a neuromodulatory role in the pain/temperature system. Estrogen treatment has been shown to increase CGRP expression in the rat anterior pituitary (Gon et al. 1990) and in the preoptic area of the brain (Yuri and Kawata 1993; 1994a; 1994b). In postmenopausal women, hormone replacement therapy has been shown to elevate plasma CGRP levels (Spinetti et al. 1997). While some studies have determined an increase in CGRP in the DRG by estrogen (Gangula, et al. 2000; 2009) it has also been reported that estrogen may reduce CGRP in the rat DRG (Yang et al. 1998). The prediction that this would have for estrogen’s effect on pain sensitivity is thus complicated and in need of additional study.
The literature indicates that the sign or direction of estrogen’s modulation on pain in experimental models is not always uniform, with reports pointing to estrogen as both pro-nociceptive (Coyle et al. 1996) or anti-nociceptive (Multon et al. 2005; Craft et al. 2008; Sanoja and Cervero 2008). The extent to which estrogen effects are related to changes in neuropeptide expression in primary sensory neurons has not been systematically evaluated. Thus, our present study examined the effects of estrogen replacement in ovariectomized rats on SP and CGRP expression at the peptide and mRNA level in DRG neurons and compared those with changes in baseline pain sensitivity as well as to neuropathic pain parameters related to the hindpaw. We undertook a systematic examination of several features of pain sensitivity in ovariectomized and estrogen-replaced rats: Baseline thermal and mechanical pain thresholds, and the development of tactile allodynia in the hindpaw after partial sciatic nerve injury.
MATERIALS AND METHODS
Animals and hormone conditions
Female Sprague-Dawley rats (Harlan Sprague Dawley, Indianapolis, IN) weighing 200–250 gm were used for the experiments. All animals were acquired, cared for, and surgically handled in accordance with guidelines specified in the NIH Guide for the Care and Use of Laboratory Animals under a Rosalind Franklin University IACUC approved protocol. Animals were fed standard Purina rat chow and had water available continuously; the light/dark cycle was 12:12. All of the animals were surgically ovariectomized under Halothane anesthesia using aseptic procedures. They were then either treated continuously with estrogen using implanted 17β-estradiol capsules (ovx+E group) or implanted with blank capsules (ovx control). The estrogen treatment consisted of a silastic capsule filled with 100% crystalline 17β-estradiol (Sigma) that was implanted subcutaneously in the lower back immediately after the ovariectomy; capsules were left in place until animals were euthanized. The estrogen packed capsules consisted of 1 cm long pieces of silastic tubing (0.62 mm inner diameter, 0.95 mm outer diameter) that were sealed at both ends with wooden plugs; capsules were soaked in phosphate buffered saline (PBS) overnight prior to implantation (Ahmed et al. 2006, Lauber et al. 1991). In the control group (ovx), a blank silastic capsule was implanted subcutaneously in the small of the back just after the ovariectomy. In our past experiments (see Taleghany et al 1999) using identically constructed implants, supraphysiological levels of estrogen (190 pg/ml) were obtained at 21–28 days post-implantation. For immunocytochemistry and in situ hybridization experiments the animals were killed 28 days after the ovariectomy using sodium pentobarbital overdose and decapitation. Lumbar dorsal root ganglia were removed from the animals after death and uterine horns were inspected at necropsy to confirm the treatment conditions.
Partial Sciatic Nerve Ligation
Rats were anesthetized with sodium pentobarbital (60 mg/kg, i.p.) and partial sciatic nerve ligations were done in the right hindleg as described previously by Coyle (Coyle et al. 1995). The outer muscle layer was separated until the nerve was visualized over the obturator muscle. The ligature was placed distal to where the posterior biceps semitendinosus nerve branches off from the common sciatic nerve. A 6 mm taper needle and 6-0 silk suture were placed through approximately 1/3-1/2 of the common sciatic nerve’s thickness. The suture was then tightly knotted, ligating a portion of the sciatic nerve. The overlying muscle was closed with suture and the skin was stapled. The foot of the animal was painted with a dilute picric acid solution to discourage self-mutilation; staples were removed 7–8 days after the nerve injury surgery. Nerve injury surgeries were done 28 days after ovariectomies in ovx and ovx+E groups. Pain sensitivity testing was done 7, 13, 20 and 27 days after the nerve injury surgery. At the conclusion of the testing, animals were euthanized by pentobarbital overdose and decapitation. The uterine horns and the capsules were inspected during necropsy to validate the hormone condition.
Pain sensitivity testing
In unlesioned animals, baseline sensitivity was evaluated 28 days post ovariectomy in both ovx and ovx+E animals. In the partial nerve injury experiments, animals were evaluated just prior to the partial nerve injury (time 0) then again at 7, 13, 20 and 27 days after injury. To test thermal sensitivity, we determined the latency for an animal to remove its paw, or flick its tail, away from a radiant heat source generated by a commercial analgesia meter (Model 33 Tail Flick analgesia meter, IITC Inc., Woodland Hills, CA). The distal centimeter of the tail was painted black with a Sharpie pen and the animals were placed into a Plexiglas box divided into 4 chambers (8 × 8 × 8 inches) and allowed to acclimate for about 15 minutes. Then, the radiant heat source of the analgesia meter was focused onto the distal blackened area of the tail and the time until the tail was flicked or withdrawn was recorded. Each animal was tested twice with a 2 min interval between tests, and an average withdrawal time was calculated. The analgesia meter was then used to evaluate the sensitivity of the hind paw in the following manner. The animals were given about a 5 min rest and then the radiant heat source of the analgesia meter was manually focused onto the plantar surface of the hindpaw and activated. The time until the animal withdrew the paw (lifted and shook or licked) was measured in seconds. The paw was tested twice with a 2 min interval between trials and the times were averaged for each animal.
Mechanical sensitivity thresholds were evaluated using graded Von Frey monofilaments as outlined previously (Coyle et al. 1996). The day before the first data collection session, animals were conditioned during two 90 min sessions. The animals were placed into a Plexiglas box divided into 4 chambers (8 × 8 × 8 inches) over a wire mesh floor and allowed to acclimate for 30 min. They were then stimulated with an 8.51 gm force monofilament (the hindpaw was poked several times) every 10 minutes for an hour. After 30 min the conditioning pokes were reinitiated every 10 minutes for an hour and then the animals were returned to their home cages. For the actual testing, the animals were placed in the small Plexiglas cubicles over a wire mesh floor and Von Frey monofilaments ranging from 0.17–75.86 gm were applied to the plantar surface of the hindpaw starting with the lowest pressure filament. The minimum gram force (filament size) needed to induce paw withdrawal was recorded. Testing started with the weakest filament and a single trial consisted of applying each filament over a 3–5 second interval 6–8 times (poking) to the hindpaw plantar surface. Once an animal responded by withdrawing the paw, the next three higher filaments were also tested. The minimum gram force that induced paw withdrawal was only recorded if the animal also responded to the next three higher filaments. Care was taken to not stimulate the same spot twice in succession to avoid sensitization. Three trials were done with a 15 min break in between each trial. The minimum gram force needed for paw withdrawal was averaged from the three trials. Behavioral data were evaluated by ANOVA followed by Tukey’s tests with p < .05 considered significant.
Immunocytochemistry
Upon euthanasia by sodium pentobarbital overdose and decapitation, lumbar DRGs were removed from the animals, fixed by overnight immersion in 4% paraformaldehyde, rinsed in PBS, dehydrated through alcohols and xylene, and infiltrated with paraffin. The blocks were cut at 10 µm thickness on a microtome and sections were mounted onto Super Frost slides (Fisher Scientific). The slides were stored at room temperature until used for immunocytochemistry. Two antibodies were used for immunostaining. The CGRP polyclonal antibody (Amersham Life Science, Arlington Heights, IL) was used at a final dilution of 1:5000. The SP polyclonal antibody (Incstar, Stillwater, MN) was used at a final dilution of 1:4000. For SP, but not CGRP, it was necessary to use an "unmasking" procedure to obtain good immunostaining from the paraffin sections. We used a microwave unmasking method as previously described (Shi et al. 1991). After the unmasking, (or for the CGRP experiments, after routine deparaffinization and rehydration), sections were incubated in 4% normal goal serum (NGS) in PBS for 1 hr. They were then reacted with primary antibody (diluted in 1% NGS in PBS) overnight at room temperature. The sections were washed with PBS and the antigens visualized using the ABC Vectastain (peroxidase) Elite Kit (Vector Laboratories, Burlingame, CA). Sections were washed in H2O, dehydrated through alcohols, cleared in xylene, and cover slips were applied using Permount.
For quantitative assessment of the immunocytochemically-processed material, cell counts were done. For this, we randomly selected cellular areas of DRGs from 4–8 animals in blinded slides and photographed those at low magnification (10 × objective). The photographs were printed, overlaid with a standardized grid, and cell counts were manually compiled. Care was taken to exclude regions of the DRG that were largely axonal and to count only cells with clear margins and a clear immunological signal. The counts were normalized to the unit pixel area of the grid evaluated. Cell counts from ovx and ovx+E groups were then compared using the Student’s t-test at p < 0.05.
In situ hybridization (ISH)
The SP probe was a 567 bp riboprobe encoding rat β-preprotachykinin cDNA (Carter and Krause 1990). The probe for rat α-CGRP was a 400 bp fragment from the calcitonin/α-CGRP gene, including 160 bp of the 3’ non-coding sequence for α-CGRP mRNA (Amara et al. 1985.) Antisense and control (sense) riboprobes were both labeled with 33 P-UTP (New England Nuclear) by linearizing the plasmids and then transcribing with the appropriate polymerase according to the protocol of a commercial in vitro transcription kit (Riboprobe Combinations System, Promega Inc.). The ISH conditions were described previously (Taleghany et al. 1999). For autoradiography, slides were dipped in Kodak NTB2 emulsion (diluted 1:1 in 600 mM ammonium acetate), air-dried, and incubated in dark boxes containing dessicant at 4°C. After appropriate exposure times (7–8 days) the sections were developed in Kodak D-19 developer and fixer, lightly stained with toludine blue, and cover slips were applied using Permount.
Emulsion autoradiograms of DRG sections after ISH were quantified using computer assisted image analysis. The density of silver grains overlying the small-sized DRG neurons (cells having a cross sectional area < 600 µm2) was determined. All slides were blind coded with a number to avoid possible bias during counting. For grain counting, edges of sections were routinely avoided; only cells with clear cytoplasmic borders, a visible nucleus, and positive tagging above background were included. Background grain counts were defined by measuring grain densities over several axonal regions in each section, and these were subtracted from neuron grain density measurements. Sections were viewed at 100× under oil and neurons were randomly selected from 3–4 histological sections of each DRG. We routinely counted 30–80 cells per animal. Mean grain density values of ovx and ovx+E groups were compared using an unpaired Student’s t-test with p < 0.05 considered significant.
RESULTS
Estrogenic modulation of CGRP and SP expression in DRG neurons
Immunocytochemistry was performed on paraffin sections using polyclonal antibodies to CGRP and SP in two different groups (ovx and ovx+E). Results showed robust CGRP-ir in many small-sized DRG neurons, as well as in some medium-sized neurons (Figure 1). In contrast, SP-ir was largely restricted to the small neurons in ovx and ovx+E groups (Figure 1). Qualitative observations indicated that CGRP-ir in the DRG was enhanced in the ovx+E group relative to the ovx controls. In contrast, SP-ir appeared markedly reduced in the ovx+E group compared to the ovx controls. Quantitative examination by cell counting of the immunostained material confirmed that estrogen treatment differentially affected the expression of SP (negatively) and CGRP (positively) in the DRG. The peptides did not exhibit a perceptible shift in the cell type in which expression occurred. SP was almost exclusively found in small sized neurons (<600 µm2) in both ovx and ovx+E while CGRP was present in small and medium-sized (600–1000 µm2) neurons in both hormone conditions. Large neurons (>1000 µm2) of the DRG were typically devoid of substance P peptide expression in our experiments and CGRP was only rarely observed in the large cells. For substance P, cell counting analysis revealed a mean of 9.1 (+/− 2.7) cells per unit area of the DRG in the ovx condition and 4.3 (+/−1.2) cells per unit area in the estrogen treated condition (data not shown). This reduction in number of cells expressing substance P was statistically significant (p < .05, n= 4). For CGRP, cell counting analysis revealed a mean of 8.7 (+/− 2.6) cells per unit area of the DRG in the ovx condition and 12.5 (+/− 2.9) cells per unit area in the ovx+E condition (data not shown). This increase was statistically significant at p <. 05, n =8).
Figure 1.
Immunocytochemical evidence for the estrogenic modulation of SP and CGRP expression in the DRG. Sections of rat DRGs were reacted with polyclonal antibodies specific to SP and CGRP and visualized using the Vectastain ABC peroxidase kit. Representative examples of staining in the DRG of ovx and ovx+E animals are presented. Note reduced level of SP immunostaining in the +E condition compared to the enhanced level of CGRP immunostaining in the +E condition. Bar=70µm.
ISH of histological sections of DRG neurons with radiolabeled probes was performed to study the changes in mRNAs encoding CGRP and SP in ovx and ovx+E animals. Autoradiography of tissue hybridizations with 33P-labeled riboprobes specific for CGRP and SP mRNAs was done and quantified by grain counting. Figure 2 shows representative autoradiograms of SP mRNA localization in small-sized DRG neurons of ovx (A) and ovx+E (B) animals. To enable statistical verification of changes resulting from estrogen treatment, the silver grain densities overlying DRG cells were determined from the autoradiograms. The grain density (number of grains/unit area) over individual small-sized (<600 µm2) cells was determined using an image analysis system. The mean grain density was determined by averaging the grain densities of 30–80 individual, randomly selected neurons that were counted for each animal (n = 8). Figure 2 (bottom) shows that grain counts from DRG sections hybridized with a 33P-labeled CGRP riboprobe were significantly higher in the ovx+E group (0.53 grains/µm2) as compared to the ovx group (0.32 grains/µm2). In contrast, DRG sections hybridized with a 33P-labeled SP riboprobe revealed a significant decrease in the mean grain density of the ovx+E group (0.46 grains/µm2) as compared to the ovx group (1.09 grains/µm2). Thus, the ISH experiments showed that the mRNA changes were similar in sign to the changes observed by examining the peptide levels in the DRG with immunocytochemistry.
Figure 2.
In situ hybridization evidence for the estrogenic modulation of SP and CGRP mRNA expression in the DRG. Sections of rat DRGs were hybridized with specific 33P-labeled riboprobes and autoradiograms were prepared. Top panels show examples of SP mRNA localization in ovx (A) and estrogen-treated (B) groups in bright field photomicrographs of emulsion coated sections. Quantitative data grain counting analyses are shown in the lower graph. Average grain densities (number of grains/µm2) and SEM for small-sized DRG neurons are plotted for the ovx and +E groups with both riboprobes as indicated. The asterisks* indicate significant differences (p<0.05, n= 8) between the two groups for each mRNA probe.
Estrogenic modulation of pain sensitivity
We next evaluated baseline pain sensitivity to both thermal and mechanical stimuli in the chronically ovx animals compared to the ovx+E treated group of animals. The initial tests evaluated the latency of tail flick away from a radiant heat point generated by a commercial analgesia meter. The mean latency to tail flick was 1.86 sec in the ovx animals and 2.82 sec in the ovx+E group (Figure 3, left). This difference was significant (p < .05) and indicated that estrogen had an anti-nociceptive effect. We also examined the sensitivity of the hind paw to a thermal stimulus. Rats were tested with a radiant heat source aimed at the mid-hindpaw region and the latency to withdraw the paw was measured. The mean latency of withdrawal for ovx animals was 5.73 sec while that of the ovx+E group was 11.2 sec (Fig. 3, right). This difference was significant (p < .05) and indicated an anti-nociceptive action of estrogen on this baseline parameter of thermal sensitivity.
Figure 3.
Baseline thermal sensitivity levels of hindpaw and tail are influenced by estrogen treatment. The graphs show the mean time (sec) to withdrawal of the paw or tail as indicated from a thermal point source emitted from an analgesia meter. Assessments were made one month after ovariectomy (ovx) without or with (+E) estrogen replacement done by capsule implants. The mean latencies +/− SEM are plotted; significant differences between the groups are (p<. 05, n = 8) are indicated by asterisks.
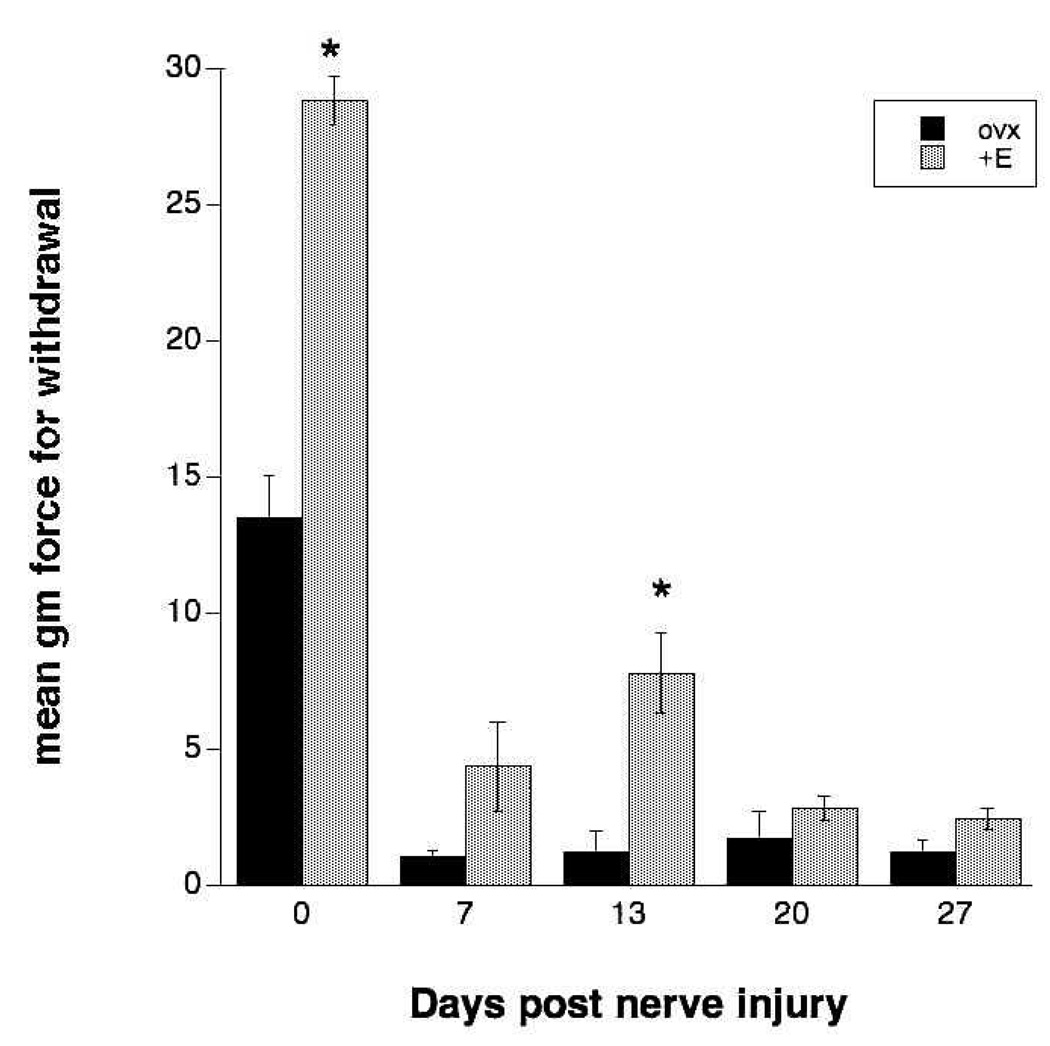
Mechanical sensitivity of the hindpaw was assessed using graded von Frey monofilaments. The animals in both ovx and ovx+E groups were tested before partial sciatic nerve lesion and then at various times after the lesion (7,13,20, 27 days) to assess the development of allodynia. Figure 4A shows that the presurgical scores (time 0) for ovx and ovx+E groups were significantly different, with the ovx animals showing a lower threshold filament strength needed for paw withdrawal than the ovx+E animals. Between 7 – 27 days after partial sciatic nerve injury, animals in both the ovx and ovx+E groups showed a marked increase in sensitivity to the mechanical stimuli compared to the comparison values obtained before surgery (Figure 4A). Estrogen treatment did not prevent the development of hypersensitivity but did have a significant ameliorative effect on the extent of allodynia that developed at 13 days, but not other times, post lesion. Data were next normalized to each group’s prelesion control and expressed as a percentage (Fig. 4B). Both groups showed significant hypersensitivity to non-noxious mechanical stimuli but estrogen had a significant ameliorative, albeit partial, effect at day 13 (askerisk indicates significance in post-hoc testing at p < .05; n = 8). At 13 days, the animals in the ovx+E group showed a sensitivity to mechanical probes that was 27% of the average filament gm force needed to elicit a withdrawal response in the pre-lesion control. The ovx controls were considerably more hypersensitive at the same time interval (just 9% of the prelesion control filament gm force could elicit a response). We concluded that the estrogen treatment did not completely reverse the development of allodynia after partial sciatic nerve lesion but that it had a small but significant effect in ameliorating the extent of hypersensitivity at 2 weeks post injury.
Figure 4.
Mechanical allodynia of the hindpaw develops after partial sciatic nerve injury and is influenced by estrogen treatment. A. Sensitivity of the hindpaw to von Frey filaments was evaluated and the mean gm force of the monofilament needed to elicit paw withdrawal was determined in groups of animals that were ovariectomized (ovx) for 28 days prior to the nerve injury vs. those ovariectomized and treated with estrogen capsule implants. Both groups developed significant allodynia by day 7 post lesion; estrogen-treated animals were less sensitive than ovx counterparts at day 13 (asterisk indicates significance in post-hoc testing at p < .05; n = 8). B. Data were normalized to each group’s prelesion control and expressed as a percentage. Both groups showed significant hypersensitivity to non-noxious mechanical stimuli but estrogen had a significant ameliorative, albeit partial, effect at day 13 (askerisk indicates significance in post-hoc testing at p < .05; n = 8).
DISCUSSION
The goal of these experiments was to evaluate whether estrogen modulates nociceptive neuropeptides in the dorsal root ganglion in a manner that is consistent with its effects on pain sensitivity. Our results show that long term ovariectomy of adult rats induces a profound thermal and mechanical hyperalgesia of the hindpaw and tail compared to ovx animals that are continuously estrogen-treated. We also detected substantial changes in the expression of two neuropeptides, SP and CGRP, in the small lumbar DRG neurons that we previously showed contain ERα (Taleghany et al. 1999) during this same time frame. Interestingly, CGRP and SP were differentially regulated by estrogen, with SP showing a robust downregulation at both the peptide and mRNA level while CGRP and its mRNA were increased in the DRG of estrogen-treated animals. We evaluated the development of mechanical allodynia after partial sciatic nerve injury and found that both ovx and estrogen-treated animals developed significant allodynia within a week of the nerve injury which continued for at least one month. The animals with continuous estrogen replacement showed a partial amelioration of the extent of the hypersensitivity at 2 weeks post injury. Overall, the results suggest that estrogen has significant anti-nociceptive actions that can be correlated with changes in expression of two peptides in the small nociceptive ERα expressing neurons in the DRG.
The existing literature from both human and animal studies as to whether estrogen is pro- or anti-nociceptive is inconsistent. Clearly some chronic pain syndromes, such as migraine and temporomandibular joint pain, are more prevalent in women than men, suggesting that estrogen is involved (Rasmussen and Breslau 1993; Marcus 1995; Berkley 1997; Craft 2007; Gupta et al. 2007). Some model systems have revealed that acute estrogen administration after ovariectomy enhances pain responses and sensitivity (Fillingem and Ness 2000; Flake et al. 2005; Yan et al. 2007). However, when estrogen levels are constantly elevated as in pregnancy, pain sensitivity is known to decrease (Ginzler 1980). The absence of estrogen has often been shown to result in an increase in pain sensitivity. Long term ovariectomy results in hyperalgesia in adult mice on both mechanical and thermal tests (Sanoja and Cervero 2008) and increased pain responses to formalin injection (Gaumond et al. 2002; Multon et al. 2005; Mannino et al. 2007). Response time in tail flick is increased by estrogen in ovariectomized animals (Martinez-Gomez et al. 1994) Our present studies support an anti-nociceptive role for estrogen in pain sensitivity, both at a baseline level for hindpaw and tail, as well as in a model of mechanical allodynia of the hindpaw.
We postulate that the changes in sensitivity of the DRG system to thermal and mechanical nociceptive stimuli are intimately related to changes in the expression of neuropeptides, and SP in particular. We observed a very robust downregulation in SP and its mRNA by estrogen but curiously found an increase in CGRP expression in the estrogen-treated animals. It was surprising to find that CGRP, which we predicted would decrease when sensitivity was decreased, showed the opposite effect. Thus, in our experiments, substance P was the peptide that best correlated with pain sensitivity trends. Estrogen treatment in other experimental paradigms has also been reported to differentially regulate SP and CGRP. For example, in the anterior pituitary and hypothalamus, estrogen treatment downregulates SP expression but upregulates CGRP expression (Brown et al. 1990; Gon et al. 1990; O'Halloran et al. 1990; Herbison and Theodosis 1992; Ma et al. 1997a). While some studies have shown an increase in CGRP in the DRG by estrogen treatment (Gangula et al. 2009) it has also been reported that estrogen can reduce CGRP (Yang et al. 1998). Our findings provide additional support for the conclusion that estrogen is a positive regulator of CGRP and a negative regulator of SP. The focus was on peripheral changes and we did not evaluate any possible CNS changes that may be present. It would be of interest to compare the central projections of SP and CGRP neurons in this paradigm in the future.
It is not known if the effects of estrogen on CGRP or SP are direct or indirect. Since the human α-CGRP gene contains a sequence with only one base difference to the estrogen response element (ERE) it is likely that CGRP expression could be directly influenced by estrogen in a classical receptor-mediated transcriptional manner. However, it has also been postulated that neurotrophic factors such as NGF may be involved (Gangula et al. 2000). Their study of cultured DRG neurons from adult rats showed that estrogen upregulated CGRP levels only when NGF was also present, suggesting a more indirect mechanism. Our studies do not speak directly to this issue but it is known that the small nociceptive DRG neurons we analyzed contain not only estrogen receptors (Taleghany et al. 1999) but also trkA, the high affinity NGF receptor (Toran-Allerand et al. 1992). Long term (90 d) estrogen treatment has been reported to decrease trkA as well as SP mRNA levels in the rat DRG (Liuzzi et al. 1999). Interestingly, short term (7 d) estrogen replacement had opposite effects on trkA expression in the DRG (Liuzzi, et al. 1999b). Our study used a 28 day ovx and ovx+E time frame, which is considered a long-term condition that models menopause. The loss of estrogen, such as that associated with menopause, may have different effects on the DRG system than short-term cyclic variations.
The partial sciatic nerve injury model that we used in this study faithfully produced many of the symptoms of causalgia in humans but without an inflammatory component (Selzer et al. 1990; Shir and Selzer 1990). In the model, only a portion of the rat sciatic nerve is tightly ligated with silk suture to axotomize ~50% of the axons in the nerve while leaving other axons intact. This model was used by the Coyle laboratory to discover gender differences in neuropathic pain symptoms (Coyle et al. 1995). There is complexity in this model since some neurons will be separated from their targets (which may supply trophic molecules like NGF and others) while other uninjured neurons may not experience withdrawal from trophic factors, and may in fact see increased levels of trophic factors from the Schwann cells and other components present in an injured sciatic nerve. For example, studies have shown an increased presence of p75, the low affinity NGF receptor, in Schwann cells of the injured sciatic nerve (Taniuchi et al. 1988). Our present study did not evaluate how partial nerve injury influences neuropeptide expression in injured vs. uninjured DRG neurons. However, it is known that complete sciatic nerve injury downregulates SP levels in the DRG, and that this can be rescued by treatment with exogenous NGF (Fitzgerald et al. 1985; Wong and Oblinger 1991). In the case of CGRP, which coexists with SP in many of the small DRG neurons, axotomy is also known to result in downregulation of expression (Noguchi et al . 1990). Studies have shown that the downregulation of CGRP after axotomy can be rescued by treatment with NGF (Verge et al. 1995). In future studies it will be important to retrogradely tag intact and injured DRG neurons after partial sciatic nerve lesion and to compare the changes in injured vs. uninjured neurons. Such assays will help delineate the changes that coincide with and may be integral to the development of allodynia in this model system. In addition, it would also be of interest to retrogradely tag neurons innervating different tissues to provide more information about whether the changes are significant for cutaneous, muscular or visceral regions. The effects of estrogen are robust in the DRG, suggesting that a fuller understanding of the role of estrogen in the primary nociceptive neuron will be useful in developing strategies for pain syndromes that have a clear prevalence in women.
ACKNOWLEDGMENTS
This work was supported by NIH grant AG-13338 to MMO. The authors would like to thank Dr. James Krause for providing the β-preprotachykinin clone and Dr. Susan Amara for providing the α CGRP clone.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahmed Y, Lin DL, et al. Effect of estrogen on urethral function and nerve regeneration following pudendal nerve crush in the female rat. J. Urol. 2006;175:1948–1952. doi: 10.1016/S0022-5347(05)00894-3. [DOI] [PubMed] [Google Scholar]
- Amara SG, Arriza JL, et al. Expression in brain of a messenger RNA encoding a novel neuropeptide homologous to calcitonin gene-related peptide. Science. 1985;209:1094–1097. doi: 10.1126/science.2994212. [DOI] [PubMed] [Google Scholar]
- Berkley K. Sex differences in pain. Behav Brain Sci. 1997;20:371–380. doi: 10.1017/s0140525x97221485. [DOI] [PubMed] [Google Scholar]
- Brown ER, Harlan RE, et al. Gonadal steroid regulation of substance P (SP)-encoding messenger ribonucleic acids in the rat anterior pituitary and hypothalamus. Endocrin. 1990;126:330–340. doi: 10.1210/endo-126-1-330. [DOI] [PubMed] [Google Scholar]
- Carter MS, Krause JE. Structure, expression and some regulatory mechanisms of the rat preprotachykin in gene encoding Substance P, Neurokinin A, Neuropeptide K and Neuropeptide gamma. J. Neurosci. 1990;10:2203–2214. doi: 10.1523/JNEUROSCI.10-07-02203.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle DE, Sehlhorst CS, et al. Intact female rats are more susceptible to the development of tactile allodynia than ovariectomized female rats following partial sciatic nerve ligation (PSNL) Neurosci. Lett. 1996;203:37–40. doi: 10.1016/0304-3940(95)12259-1. [DOI] [PubMed] [Google Scholar]
- Coyle DE, Sehlhorst CS, et al. Female rats are more susceptible to the development of neuropathic pain using the partial sciatic nerve ligation (PSNL) model. Neurosci. Lett. 1995;186:135–138. doi: 10.1016/0304-3940(95)11304-f. [DOI] [PubMed] [Google Scholar]
- Craft R. Modulation of pain by estrogens. Pain. 2007;132 Supplement 1:S3–S12. doi: 10.1016/j.pain.2007.09.028. [DOI] [PubMed] [Google Scholar]
- Craft R, Ulibarri C, et al. Dose -and time-dependent estradiol modulation of morphine antinociception in adult female rats. Eur J Pain. 2008;12(4):472–479. doi: 10.1016/j.ejpain.2007.07.014. [DOI] [PubMed] [Google Scholar]
- Fillingem R, Ness T. Sex-related hormonal influences on pain and analgesic responses. Neurosci Biobehav Rev. 2000;24:485–501. doi: 10.1016/s0149-7634(00)00017-8. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M, Wall PD, et al. Nerve growth factor counteracts the neuro physiological and neuro chemical effects of chronic sciatic nerve section. Brain Res. 1985;332:131–141. doi: 10.1016/0006-8993(85)90396-8. [DOI] [PubMed] [Google Scholar]
- Flake N, Bonebreak D, et al. Estrogen and inflammation increase the excitability of rat temporomandibular joint afferent neurons. J Neurophysiol. 2005;93:1585–1597. doi: 10.1152/jn.00269.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangula P, Chauhan M, et al. Age-related changes in dorsal root ganglia, circulating and vascular calciton in gene-related peptide (CGRP) concentrations in female rats: Effect of female sex steroid hormones. Neurosci Lett. 2009;454:118–123. doi: 10.1016/j.neulet.2009.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangula P, Lanlua P, et al. Regulation of calcitonin gene-related peptide expression in dorsal root ganglia of rats by female sex steroid hormones. Biology Reprod. 2000;62:1033–1039. doi: 10.1095/biolreprod62.4.1033. [DOI] [PubMed] [Google Scholar]
- Gaumond I, Arsenault P, et al. The role of sex hormones on formal in -induced nociceptive responses. Brain Res. 2002;958:139–145. doi: 10.1016/s0006-8993(02)03661-2. [DOI] [PubMed] [Google Scholar]
- Ginzler A. Endorphin-mediated increases in pain threshold during pregnancy. Science. 1980;210:193–195. doi: 10.1126/science.7414330. [DOI] [PubMed] [Google Scholar]
- Gon G, Giaid A, et al. Localization of immunoreactivity for calcitonin gene-related peptide in the rat anterior pituitary during ontogeny and gonadal steroid manipulations and detection of its messenger ribonucleic acid. Endocrinol. 1990;127:2618–2629. doi: 10.1210/endo-127-6-2618. [DOI] [PubMed] [Google Scholar]
- Gupta S, Mehrotra S, et al. Potential role of female sex hormones in the patho physiology of migraine. Pharmacol Ther. 2007;113(2):321–340. doi: 10.1016/j.pharmthera.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Herbison AE, Theodosis DT. Immunocytochemical identification of oestrogen receptors in preoptic neurons containing calcitonin gene-related peptide in the male and female rat. Neuroendocrin. 1992;56:761–764. doi: 10.1159/000126304. [DOI] [PubMed] [Google Scholar]
- Lauber AH, Mobbs CV, et al. Estrogen receptor mRNA expression in rat hypothalamus as a function of genetic sex and estrogen dose. Endocrinol. 1991;129:3180–3186. doi: 10.1210/endo-129-6-3180. [DOI] [PubMed] [Google Scholar]
- Lee Y, Takami K, et al. Distribution of calcitonin gene-related peptide in the rat peripheral nervous system with reference to its coexistence with substance P. Neuroscience. 1985;15:1227–1237. doi: 10.1016/0306-4522(85)90265-9. [DOI] [PubMed] [Google Scholar]
- Liuzzi FJ, Scoville SA, et al. Long-termestrogen replacement coordinately decreases trkA and B-PPT mRNA levels in dorsal root ganglion neurons. Exp. Neurol. 1999;155:260–267. doi: 10.1006/exnr.1998.6999. [DOI] [PubMed] [Google Scholar]
- Liuzzi FJ, Scoville SA, et al. Long-termestrogen replacement coordinately decreases trkA and B-PPT mRNA levels in dorsal root ganglion neurons. Exp. Neurol. 1999a;155:260–267. doi: 10.1006/exnr.1998.6999. [DOI] [PubMed] [Google Scholar]
- Liuzzi FJ, Scoville SA, et al. Effects of short-term estrogen replacement on trkA mRNA levels in axotomized dorsal root ganglion neurons. Exp. Neurol. 1999b doi: 10.1006/exnr.1999.7169. in press. [DOI] [PubMed] [Google Scholar]
- Ma D, Zhao C, et al. Response of substance P-immunoreactive nerve fibres in the anterior pituitary to plasma oestrogen levels in the rat. J Neuroendocrinol. 1997a;9:735–740. doi: 10.1046/j.1365-2826.1997.00636.x. [DOI] [PubMed] [Google Scholar]
- Mannino C, South S, et al. Estradiol replacement in ovariectomized rats is antihyperalgesic in the formal in test. J Pain. 2007;8:334–342. doi: 10.1016/j.jpain.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Marcus D. Inter relationships of neurochemicals, estrogen and recurring headache. Pain. 1995;62:129–139. doi: 10.1016/0304-3959(95)00052-T. [DOI] [PubMed] [Google Scholar]
- Martinez-Gomez M, Cruz Y, et al. Assessing pain threshold in the rat: changes with estrus and time of day. Physiol Behav. 1994;55(4):651–657. doi: 10.1016/0031-9384(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Micevych PE, Matt DW, et al. Concentrations of cholecystokinin, substance P, and bombesin in discrete regions of male and female rat brain:sex differences and estrogen effects. Exp Neurol. 1988;100:416–425. doi: 10.1016/0014-4886(88)90119-7. [DOI] [PubMed] [Google Scholar]
- Multon S, Pardutz A, et al. Lack of estrogen increases pain in the trigeminal formal in model:a behavioral and immunocytochemical study of transgenic ARKO mice. Pain. 2005;114(1):257–265. doi: 10.1016/j.pain.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Senba E, et al. alpha-CGRP and beta-CGRP mRNAs are differentially regulated in the rat spinal cord and dorsal root ganglion. Mol. Brain Res. 1990;7:299–304. doi: 10.1016/0169-328x(90)90080-w. [DOI] [PubMed] [Google Scholar]
- O'Halloran DJ, Jones PM, et al. The regulation of neuropeptide expression in rat anterior pituitary following chronic manipulation of estrogen status:a comparison between substance P, neuropeptide Y, neurotensin, and vasoactive intestinal peptide. Endocrinol. 1990;127:1463–1469. doi: 10.1210/endo-127-3-1463. [DOI] [PubMed] [Google Scholar]
- Rance NE, Young WSI. Hypertrophy and increased gene expression of neurons containing neurokin in-B and substance P messenger ribonucleic acids in the hypothalami of post menopausal women. Endocrin. 1991;128:2239–2247. doi: 10.1210/endo-128-5-2239. [DOI] [PubMed] [Google Scholar]
- Rasmussen B, Breslau N. Migraine: Epidemiology. In: Olsen J, Tfelt-Hansen P, Welch K, editors. The Headaches. New York, NY: Raven Press; 1993. pp. 169–173. [Google Scholar]
- Sanoja R, Cervero F. Estrogen modulation of ovariectomy-induced hyperalgesia in adult mice. Eur J Pain. 2008;12(5):573–581. doi: 10.1016/j.ejpain.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Selzer Z, Dubner R, et al. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- Shi S-R, Key ME, et al. Antigen retrieval in formalin-fixed, paraffin-embedded tissues:an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991;39 doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- Shir Y, Selzer Z. A-fibers mediate mechanical hyperesthesia and allodynia and C-fibers mediate thermal hyperalgesia in a new model of causaliform pain disorders in rats. Neurosci. Lett. 1990;115:62–67. doi: 10.1016/0304-3940(90)90518-e. [DOI] [PubMed] [Google Scholar]
- Spinetti A, Margutti A, et al. Hormonal replacement therapy affects calcitonin gene-related peptide and atrial natriuretic peptide secretion in post menopausal women. Eur J Endocrinol. 1997;137:664–669. doi: 10.1530/eje.0.1370664. [DOI] [PubMed] [Google Scholar]
- Taleghany N, Sarajari S, et al. Differential expression of estrogen receptor alpha and beta in rat dorsal root ganglion neurons. J Neurosci Res. 1999;57(5):603–615. [PubMed] [Google Scholar]
- Taniuchi M, Clark HB, et al. Expression of nerve growth factor receptors by Schwann cells of axotomized peripheral nerves:ultrastructural location, suppression by axonal contact, and binding properties. J. Neurosci. 1988;8:664–681. doi: 10.1523/JNEUROSCI.08-02-00664.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toran-Allerand CD, Miranda RC, et al. Estrogen receptors colocalize with low-affinity nerve growth factor receptors in cholinergic neurons of the basal for ebrain. PNAS. 1992;89:4668–4672. doi: 10.1073/pnas.89.10.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verge VMK, Richardson PM, et al. Differential influence of nerve growth factor on neuropeptide expression in vivo: A novel role in peptide suppression in adult sensory neurons. J. Neurosci. 1995;15:2081–2096. doi: 10.1523/JNEUROSCI.15-03-02081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J, Oblinger MM. NGF rescues substance P expression but not neuro filament or tubul in gene expression in axotomized sensory neurons. J. Neurosci. 1991;11:543–552. doi: 10.1523/JNEUROSCI.11-02-00543.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T, Liu B, et al. Estrogen amplifies pain responses to uterine cervical distension in rats by altering transient receptor potential-1 function. Anesth Analg. 2007;104:1246–1250. doi: 10.1213/01.ane.0000263270.39480.a2. [DOI] [PubMed] [Google Scholar]
- Yang Y, Ozawa H, et al. Immunocytochemical analysis of sex differences in calcitonin gene-related peptide in the rat dorsal root ganglion, with special reference to estrogen and its receptor. Brain Res. 1998;791:35–42. doi: 10.1016/s0006-8993(98)00021-3. [DOI] [PubMed] [Google Scholar]
- Yuri K, Kawata M. Time-course analysis of changes in calcitonin gene-related peptide and methionine-enkephal in-immunoreactivity in the female rat preoptic area after estrogen treatment. Neuroscience. 1993;55:1067–1074. doi: 10.1016/0306-4522(93)90320-f. [DOI] [PubMed] [Google Scholar]
- Yuri K, Kawata M. Estrogen affects calcitonin gene-related peptide-and methionine-enkephal in-immunoreactive neuron in the female rat preoptic area. Neurosci. Lett. 1994a;169:5–8. doi: 10.1016/0304-3940(94)90343-3. [DOI] [PubMed] [Google Scholar]
- Yuri K, Kawata M. Estrogen receptor -immunoreactive neurons contain calcitonin gene-related peptide, methionine-enkephal in or tyrosine hydroxylase in the female rat preoptic area. Neurosci Res. 1994b;21:135–141. doi: 10.1016/0168-0102(94)90155-4. [DOI] [PubMed] [Google Scholar]