Abstract
Whereas TGF-β is essential for the development of peripherally induced Foxp3+ regulatory T cells (iTreg cells) and Th17 cells, the intracellular signaling mechanism by which TGF-β regulates development of both cell subsets is less understood. In this study, we report that neither Smad2 nor Smad3 gene deficiency abrogates TGF-β–dependent iTreg induction by a deacetylase inhibitor trichostatin A in vivo, although the loss of the Smad2 or Smad3 gene partially reduces iTreg induction in vitro. Similarly, SMAD2 and SMAD3 have a redundant role in development of Th17 in vitro and in experimental autoimmune encephalomyelitis. In addition, ERK and/or JNK pathways were shown to be involved in regulating iTreg cells, whereas the p38 pathway predominately modulated Th17 and experimental autoimmune encephalomyelitis induction. Therefore, selective targeting of these intracellular TGF-β signaling pathways during iTreg and Th17 cell development might lead to the development of therapies in treating autoimmune and other chronic inflammatory diseases.
Transforming growth factor-β plays a crucial role in the differentiation of Foxp3+ regulatory T cells (Treg cells) and IL-17–producing (Th17) cells. TGF-β enables anti-CD3 or Ag-stimulated naive CD4+ cells to become Foxp3+ Treg cells in the presence of IL-2 (1, 2) or Th17 in the presence of IL-6 or IL-21 (3–6). The development of Foxp3+ Treg cells and Th17 cells is reciprocal (4).
Although most studies focus on the role of proinflammatory cytokines, such as IL-6 or IL-21, signaling pathways in the induction of Th17 cells, few studies have investigated the role of TGF-β signaling pathways in Th17 cell generation. Similarly, the critical role of TGF-β in inducing Foxp3+ Treg cells (iTreg cells) is well established (7–10), it is less clear, however, which downstream pathways of TGF-β signaling are involved in the development of Foxp3+ iTreg cells.
The cellular response to TGF-β varies by cell type and the context of the stimulus. In lymphocytes, TGF-β binds to its cognate receptor complex composed of type I (ALK5) and type II receptors. TGF-β type I receptor (TβRI) and type II receptor (TβRII) associate as interdependent components of a heteromeric complex. TβRII is required to activate TβRI in the ligand–receptor complex, and activated TβRI Ser/Thu kinases phosphorylate downstream specific SMAD2 and SMAD3. Lack of either TβRI or TβRII will terminate the cellular response to TGF-β (11). Upon phosphorylation, these two SMADS bind to their common partner, SMAD4, to form SMAD2–SMAD4 and SMAD3–SMAD4 complexes. These complexes then translocate to the nucleus and modulate target gene expression (12, 13). Mice with homozygous targeted disruption of the Smad2 or Smad4 gene are early embryonic lethal at day 9.5 and days 6.5–8.5, respectively (14, 15). Thus, these Smads play critical, nonredundant roles in early embryonic development. In addition, the role of Smad2 and Smad4 as tumor suppressor genes is now well established in humans, suggesting that either Smad2 or Smad4 plays an important function in cell growth regulation (16). Unlike Smad2 and Smad4 null mice, Smad3 null mice are viable and survive to adulthood (17). Accumulating evidence has revealed that Smad3 is essential for the suppressive effect of TGF-β on IL-2 production and T cell proliferation (18). Smad3 is also required for the suppressive effects of TGF-β on Th2 type cytokine productions and Th2 type disease in the skin (19).
In addition to classic SMAD signaling pathways, TGF-β can activate SMAD-independent pathways, such as MAPKs, in T cells (20). For example, TGF-β inhibition of IFN-γ–induced signaling and Th1 gene expression in CD4+ T cells is Smad3 independent but MAPKs dependent (21). These studies further revealed that the inhibition of the MEK/ERK pathway completely eliminates the inhibitory effects of TGF-β on IFN-γ responses in T cells.
Several studies have recently begun to explore the role of SMAD molecules of TGF-β downstream in the development of Foxp3+ cells induced by TGF-β. Tone et al. (22) observed that SMAD3 is essential for the induction of Foxp3 by TGF-β–primed CD4+ cells using an antagonist of SMAD3. Xiao et al. (23) also observed that all-trans retinoic acid (atRA) promotes iTreg cell differentiation via enhancing SMAD3 expression and phosphorylation. Using Smad3 knockout (KO) mice, Jana et al. (24) reported that the ability of TGF-β to induce Foxp3 in TCR-stimulated CD4+ cells was significantly diminished in Smad3 KO mice compared with wild type (WT) mice, although they believed that TGF-β SMAD-independent pathways also play an important role. Deficiency of Smad4 resulted in a 50% reduction of Foxp3 expression by TGF-β and did not affect Th17 cell development by IL-6 and TGF-β (25). It has been known that the proinflammatory cytokine IL-6 promotes Th17 cells and inhibits Foxp3 induction by TGF-β. In addition, IL-6 trans-signaling augmented the expression of the TGF-β signaling inhibitor SMAD7. Consequently, SMAD7 overexpression in T cells rendered CD4+CD25− T cells resistant to the induction of Foxp3 (26). Nonetheless, how TGF-β SMAD-dependent or -independent pathways affect the iTregs and Th17 cell development, especially in vivo, remains largely unknown. Because the balance between Treg and Th17 cells affects the pathogenesis and development of many autoimmune diseases, it is clear that understanding the molecular basis of TGF-β signaling pathways in the development of iTreg and Th17 cells may therefore provide insight into clinical immune pathologies and lead to strategies for intervention.
In this study, we examine the role of SMAD and non-SMAD pathways in the development of iTreg cells and Th17 cells by in vitro and in vivo experimental models using Smad2, Smad3, JNK2, and ERK1 KO mice. We crossed Smad2fx/fx and hCD2-Cre mice to generate lymphocyte-specific Smad2 conditional knock out (CKO) mice since conventional Smad2 KO mice are embryonic lethal (12). We found that neither Smad2 nor Smad3 alone is sufficient for the differentiation of Th17 cells and Th17 cell-mediated experimental autoimmune encephalomyelitis (EAE). Whereas both Smad2 and Smad3 play a partial role in the development of Foxp3+ iTreg cells induced by TGF-β in vitro, either Smad2 or Smad3 is redundant for the induction of deacetylase inhibitor (Trichostatin A, TsA)-initiated iTreg cells in vivo, although TGF-β signaling plays a crucial role in the increase of Foxp3+ Treg cells after TsA treatment. Whereas p38 MAPK mainly regulates Th17 cell differentiation and EAE development, ERK MAPK plays a central role in the development of Foxp3+ iTreg cells. The data in this study show that unique TGF-β signaling pathways can differentially control the development of Foxp3+ Treg cells and Th17 cells.
Materials and Methods
Mice
C57BL/6 and JNK2 KO mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Floxed TβRII mice were provided by Dr. Harold Moses at Vanderbilt University (Nashville, TN). ERK1 KO mice were provided by Dr. Gary Landreth at Case Western Reserve University (Cleveland, OH). Smad2fx/fx and Smad3 KO mice were provided by Dr. Xiao-Fan Wang at Duke University (Durham, NC) and Dr. Michael Winstein at Ohio State University (Columbus, OH). hCD2-Cre mice were provided by Dr. Dimitris Kioussis at the National Institute for Medical Research (London, U.K.). Foxp3 GFP knock-in mice were a gift from Dr. A. Y. Rudensky (University of Washington, Seattle, WA). Lymphocyte-specific Smad2 CKO (Smad2fx/fx/hCD2-Cre) mice were generated by crossing Smad2fx/fx mice with Smad2fx/+/hCD2-Cre mice. Mice with a Smad2fx/fx genotype were used as a normal control. Similarly, lymphocyte-specific TβRII CKO mice were generated by crossing the homozygous floxed-TβRII(TβRIIfx/fx) mice with heterozygous TβRIIfx/+/hCD2-Cre mice. Inducible TβRII whole body KO adult mice were generated from the mice with genotypes of TβRIIfx/−/Rosa26-rtTA/TetO-Cre by feeding them with doxycycline containing food (625 mg/kg; TestDiet, Richmond, IN) and drinking water (0.5 mg/ml; Sigma-Aldrich, St. Louis, MO) at the age of 1-mo-old to avoid embryonic lethality. After 1 mo doxycycline induction, TβRII KO was verified in a variety of tissues by RT-PCR and Western blot (data not included). These doxycycline-induced TβRII KO adult mice were then used for experiments at 3 mo old. All experiments using mice were performed in accordance with protocols approved by the Institutional Animal Care and Use Committee of University of Southern California and Children’s Hospital Los Angeles Research Institute.
Flow cytometry and Abs
Cell suspensions were stained for FACS analysis using the following: PE-, FITC-, or CyChrome-conjugated anti-CD4 (RM4-5), CD8 (53-6.7), CD11b (M1/70), B220 (RA36B2), CD62L (MEL-14), and respective matched control IgG Abs (BD Pharmingen, San Diego, CA). The PE-conjugated anti-mouse Foxp3 staining kit (FJK-16s), PE–anti-17A (17B7), and FITC-anti–IFN-γ (XMG1.2). PE-rat IgG2aκ and FITC-rat IgG1κ were purchased from eBioscience (San Diego, CA). Samples were analyzed on an LSRII or sorted using a FACSAria (BD Biosciences, San Diego, CA).
Cell differentiation and functional assay
T cells were prepared from spleen cells by collecting nylon wool column nonadherent cells as described previously (27). CD4+ T cells were isolated by negative selection. T cells were labeled with PE-conjugated anti-CD8, anti-CD11b, and anti-B220 mAbs, incubated with anti-PE magnetic beads, and loaded onto MACS separation columns (Miltenyi Biotec, Auburn, CA). The CD4+ cells were further labeled with FITC-conjugated anti-CD25 mAb, and CD4+CD25− and CD4+CD25+ cells were obtained by cell sorting (purity > 99%). To prepare naive CD4+CD25− cells, CD4+CD25− cells were labeled with PE-conjugated anti-CD62L and positively selected by anti-PE magnetic beads (CD4+CD62L+CD25− cells). Naive CD4+CD25− cells were stimulated with anti-CD3/CD28 coated beads (Invitrogen, Carlsbad, CA) at a bead-to T cell ratio of 1:5 in the presence of IL-2 (20 U/ml; R&D Systems, Minne-apolis, MN) and TGF-β (2 ng/ml; R&D Systems) for 4 d for Foxp3+ iTreg cells (CD4TGF-β) or without TGF-β for control cells (CD4Med). These cells were stimulated with soluble anti-CD3 (1 μg/ml) and anti-CD28 (10 μg/ml) in the presence of IL-6 (10 ng/ml) and TGF-β (2 ng/ml), anti–IL-4 (10 μg/ml), anti–IFN-γ (10 μg/ml) for 3 d for Th17 cell differentiation; 10 μM of SB203580 (p38 inhibitor) or SP600125 (JNK inhibitor) or 50 μM of PD98059 (ERK inhibitor) (Calbiochem/Merck Biosciences) was added to the cultures every 24 h. Three to 10 μM SIS3 (SMAD3 inhibitor; Calbiochem) or 5 μM LY-364947 (ALK5 inhibitor; Sigma-Aldrich) was added to naive CD4+ cells 1 h before TCR stimulation. An equivalent volume of DMSO was added to cultures only as a vehicle control. To assess the suppressive activities, T cells labeled with CFSE (Invitrogen) were stimulated with soluble anti-CD3 (0.025 μg/ml) with irradiated non-T cells as APCs (1:1). CD4+ conditioned (CD4Med and CD4TGF-β) cells generated from different mice were added at a ratio of conditioned cells to T responder cells (Tcon/Tresp) of 1:4, and suppression of cycling CFSE-labeled T cells was assessed on the CD4+ cell gate of T responder cells as described previously (1). AIM-V serum-free medium (Invitrogen Life Technologies, Carlsbad, CA) supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 10 mM HEPES (Invitrogen Life Technologies) was used for the generation of CD4+ iTreg or control cells. RPMI 1640 medium supplemented as described with 10% heat-inactivated FCS (HyClone Laboratories, Logan, UT) was used for all other cultures.
Quantitative real-time PCR
Total RNA was extracted with the RNeasy Mini Kit (Qiagen, Valencia, CA). cDNA was generated using an Omniscript RT kit (Qiagen). Foxp3 mRNA expression was quantified with ABsolute SYBR Green ROX mix (Thermo, Waltham, MA). The samples were run in triplicate, and the relative expression of Foxp3 was determined by normalizing the expression of each target to hypoxanthine guanine phosphoribosyl transferase (HPRT). Primer sequences were as follows: HPRT 5′-TGA AGA GCT ACT GTA ATG ATC AGT CAA C-3′ and 5′-AGC AAG CTT GCA ACC TTA ACC A-3′; Foxp3 primers: 5′-CCC AGG AAA GAC AGC AAC CTT-3′ and 5′-TTC TCA CAA CCA GGC CAC TTG-3′.
ELISA
IL-17 levels in the supernatants were performed according to the manufacturer’s instructions with Quantikine M kits from R&D Systems.
Cytokine analysis
T cells were isolated from spleens, lymph nodes, and blood in EAE mice at day 18 after immunization, then they were stimulated in vitro with PMA (50 ng/ml) and ionomycin (100 ng/ml) for 5 h, with brefeldin A (5 μg/ml) added after 1 h of incubation. In other experiments, T cells were stimulated in vitro with MOG35–55 peptide (50 μg/ml) for 3 d, and intracellular IL-17 and IFN-γ expression was stained and analyzed by FACS.
Western blot analysis
A total of 5 × 106 CD4+ cells with various treatments were lysed in RIPA buffer. Specific proteins were detected by immunoblotting after the method published previously (28). Equal amounts (10 μg) of total cell lysate proteins were separated in NuPAGE 4–12% gradient SDS-PAGE gels using a MOP buffering system (Invitrogen). After protein was transferred into polyvinylidene difluoride membrane, proteins of interest were detected by specific Abs. All chemicals were purchased from Sigma-Aldrich. All Abs were purchased from Cell Signaling (Danvers, MA).
TsA and p38 inhibitor administration
TsA was purchased from Sigma-Aldrich, dissolved in DMSO, and administered i.p. for 7 d at 1 mg/kg/d unless otherwise specified. The p38 inhibitor (SB203580) was obtained from Alexis Biochemicals (San Diego, CA) and dissolved in 2% DMSO. SB203580 (0.5 mg/kg) were injected i.p. into WT mice 2 d after immunization with MOG35–55 peptide and injection was repeated every other day for 7 d. Mice receiving 2% DMSO solution only were used as controls.
Induction of EAE
EAE was induced by s.c. immunization of mice into the flanks with 100 μl of an emulsion of 100 μg of MOG35–55 peptide and 250 μg of Myco-bacterium tuberculosis H37RA (BD Diagnostic Systems, Sparks, MD) in complete Freund’s adjuvant. In addition, the animals received pertussis toxin (150 ng/mouse; Sigma-Aldrich) i.p. on days 0 and 2 (29). Clinical signs of EAE were assigned scores according to the following: 0, no symptoms; 1, loss of muscle tone in tail; 2, hindlimb weakness; 3, hind-limb paralysis of one (3.0) or both (3.5); 4, hindlimb and forelimb paralysis; 5, loss of temperature control or moribund. Scores are shown as mean daily clinical scores for all mice per group.
Statistical analysis
Results are presented as mean ± SEM. Student t test was used to assess statistical significance between two groups, and one-way ANOVA was used to assess statistical significance between EAE scores.
Results
SMAD2 or SMAD3 plays partial roles in the induction of Foxp3+ iTreg cells in vitro
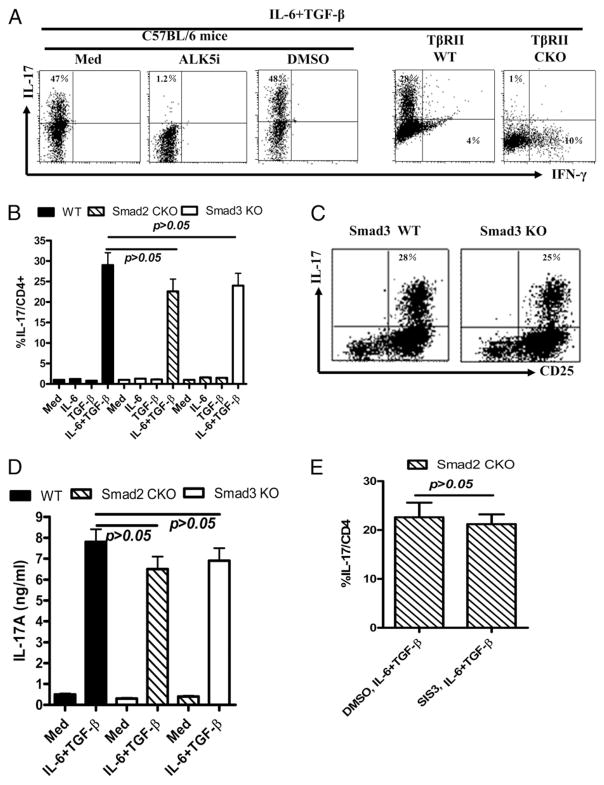
The role for TGF-β-SMAD or SMAD-independent MAPK pathways in Foxp3+ iTreg and Th17 cells has not yet been well defined, especially in vivo. In this study, we demonstrated that whereas TGF-β plays an important role in the induction of Foxp3+ iTreg cells as published (7–10), the TGF-β type I receptor (TβRI or ALK5) and/or TβRII-mediated TGF-β signaling in lymphocytes is essential for the induction of Foxp3+ iTreg cells, because either inhibition of ALK5 (TβRI) or genetic blockade of TβRII almost completely abrogates the induction of Foxp3 expression and the related suppressive activities in TGF-β–primed CD4+ cells (Fig. 1A, 1B). ALK5 inhibitor (LY-364947) is a selective, ATP-competitive inhibitor of TβRI (ALK5) kinase. It is much less potent at related kinases for TβRII and for MLK-7, a kinase in the MAP kinase signal pathway that is closely related to TGF-β RII (30). Others have reported that injection of a similar ALK5 inhibitor resulted in the specific blockade of TβRI kinase activation and subsequent repression of TGF-β–mediated growth inhibition in vivo (31). Because ALK5 is not directly soluble in aqueous solutions, we used DMSO to make a stock solution prior to diluting in aqueous solutions. As a control, we used DMSO alone in the cultures to exclude the possibility that DMSO-dissolved ALK5 inhibitor abolished Foxp3 induction on CD4+ cells by TGF-β is due to its nonspecific toxicity (Fig. 1A, 1B).
FIGURE 1.
Distinct role of TGF-β and SMAD signals in the induction of Foxp3+ regulatory T cells in vitro. Naive CD4+ cells isolated from WT, TβR-II CKO, Smad2 CKO and Smad3 KO mice were cultured with anti-CD3/CD28–coated beads (1 bead to 5 cells) and IL-2 (20 U/ml) in the absence (CD4Med) or presence of 2 ng/ml TGF-β (CD4TGF-β) for 4 d. ALK5 inhibitor (5 μM) or DMSO was added to some cultures. Foxp3 expression and suppressive activities of these cells were analyzed by FACS. A, Percentages of Foxp3 expression by CD4+ cells from wide type mice treated with ALK5 inhibitor or control DMSO or from TβR-II CKO mice. Values are mean ±SEM of three independent experiments (left panel) and a representative of dot plot flow data (right panel). B, The suppressive activity of CD4Med and CD4TGF-β cells generated as in A. These conditioned cells with a ratio of 1:4 (one conditioned cell to four responder cells, Tcon/Tresp = 1:4) were added to CFSE-labeled T responder cells isolated from WT mice and cocultured with anti-CD3 (0.025 μg/ml) in the presence of APCs from WT mice for 3 d. Histogram data are gated on CD4+ cells of T responder cells. Data are the representative of three similar experiments. C, Expression of Foxp3 protein (left panel) and mRNA (right panel) by TGF-β–primed or control CD4+CD25+ cells in WT, Smad2 CKO, and Smad3 KO mice. Experiments were conducted similarly as in A, and the percentages of cells expressing Foxp3 among CD4+CD25+ cells were analyzed by FACS (left panel). Values are mean ± SEM of three independent experiments. Foxp3 mRNA between various groups of CD4+CD25+ cells was determined by quantitative RT-PCR (right panel). Values are mean ± SEM of triplicate samples of one experiment. Similar results were obtained in a second experiment. D and E, The percentage of suppressive activities of CD4 conditioned (CD4Med and CD4TGF-β) cells generated from WT, Smad2 CKO, and Smad3 KO mice was calculated as (A − B)/A × 100%, where A is the number of responder CD4+ T cells cycling at baseline, and B is the number of responder CD4+ T cells cycling at cocultures with CD4+ conditioned cells (Tcon/Tresp = 1:4). Experiments were conducted similarly as in B. F, Naive CD4+ cells were pretreated with or without SIS3 (3–10 μM) for 1 h and then stimulated with or without TGF-β (2 ng/ml) for 24 h. SMAD3 and p-SMAD3 expression was analyzed by Weston blotting. G, Naive CD4+ cells isolated from Smad2 CKO were stimulated as in A with or without SIS3 (10 μM) for 4 d. Foxp3 expression of by TGF-β–primed CD4+ cells was analyzed by FACS. The differences were analyzed using Student t test; p < 0.05 was considered significant.
Given the crucial role of TGF-β signal pathways in the differentiation of Foxp3+ iTreg cells and critical role of SMAD2 or SMAD3 in mediating many effects of TGF-β signaling in T cells, we next dissected the downstream SMAD2- or SMAD3-mediated TGF-β pathways in regulating the development of Foxp3+ iTreg cells in vitro using Smad3 KO mice and lymphocyte-specific Smad2 CKO mice. Because conventional Smad2 KO mice are early embryonic lethal, we generated lymphocyte-targeted Smad2 CKO mice by crossbreeding floxed Smad2 and hCD2-Cre mice (32). We compared Foxp3 expression between the related KO mice and the controls at the protein level by Foxp3 immunostaining and FACS analysis (Fig. 1C, left panel, 1D), and at the mRNA level by quantitative RT-PCR (Fig. 1C, right panel). We observed that TGF-β–primed CD4+ CD25+ cells still expressed substantial amounts of Foxp3 mRNA and drove the expression of Foxp3 protein in a substantial percentage of CD4+ cells in the Smad2 or Smad3 KO cells. However, Foxp3+ cell numbers and Foxp3 mean fluorescence intensity in the TGF-β–primed CD4+CD25+ cells from Smad2 CKO or Smad3 KO mice was slightly (~20–30%) and significantly lower than those from WT mice (Fig. 1C, left panel, 1D). We also compared the suppressive activities of these cells. As shown in Fig. 1E, the suppressive activity of TGF-β–induced CD4+ cells from Smad2 CKO and Smad3 KO mice were significantly decreased compared with those from WT mice, suggesting that either SMAD2 or SMAD3 plays a partial (~20–30%) role in the induction of Foxp3+ iTregs in vitro. In addition, these data also suggest that Foxp3 protein, but not its mRNA level in CD4+CD25+ cells, more accurately reflects the suppressive function that is consistent with previous reports (33).
To determine whether SMAD2 and SMAD3 compensate each other in Foxp3 induction, because a lack of either Smad2 or Smad3 did not completely abrogate the Foxp3 induction on CD4+ cells by TGF-β, naive CD4+ cells isolated from Smad2 CKO mice were then treated with SIS3, an SMAD3 antagonist. After 1 h pretreatment, TGF-β–stimulated SMAD3 phosporylation in these cells was blocked (Fig. 1F). Nonetheless, induced Foxp3 expression by simultaneous TCR stimulation and TGF-β activation was comparable to that in the cells from Smad2 CKO mice without SMAD3 inhibition (Fig. 1G). The suppressive activities on T cell proliferation were not changed (data not shown). These results indicate that Foxp3 induction on TGF-β–primed CD4+ cells from Smad2 CKO is not due to the compensation of SMAD3, further demonstrating that SMAD2 and SMAD3 play a partial but incomplete role in the development of Foxp3+ iTreg cells in vitro.
SMAD2 or SMAD3 is redundant for TsA-induced increase of Foxp3+ Treg cells in vivo
We further examined the role of SMAD2 and SMAD3 in contributing to TGF-β–induced Foxp3+ regulatory T cell differentiation in vivo. Because TGF-β has a short t1/2, it is impractical to induce Foxp3+ iTreg cells in vivo using exogenous TGF-β. However, recent observations showed that administration of the deacetylase inhibitor TsA promoted Foxp3+ cell development and function, and mechanistic studies revealed that TsA converted and induced iTreg cells in the periphery and possibly increased thymic output of natural Treg cells (nTreg cells) in vivo (34). It has to be noted that TsA is able to directly act on histone/protein deacetylases of the Foxp3 gene, which then regulates Foxp3 chromatin remodeling, gene expression, and function (34). To exclude the possibility that TsA bypasses the TGF-β signaling pathway to upregulate Foxp3 frequency and function in vivo, we have generated mice with genotype of TβRIIfx/−/Rosa26-rtTA/TetO-Cre in which the floxed-TβRII allele can be deleted with doxycycline-induced Cre expression in the whole body, including the immune system in adults, to avoid a developmental lethal phenotype of conventional TβRII knockout. As reported before (34), daily treatment with TsA for 7 d markedly increased the percentages (Fig. 2A) and total number (Fig. 2B) of CD4+CD25+Foxp3+ cells in spleen cells in WT or Foxp3 GFP knock-in mice. TsA treatment also increased Foxp3+ cell frequency in lymph nodes and peripheral blood and did not alter the Foxp3+ cell frequency in the thymus (data not shown), suggesting that TsA increases but does not redistribute Foxp3+ cells. However, similar TsA treatment was unable to increase Foxp3+ cells in the spleen (Fig. 2A) and other organs (data not shown) in doxycycline-induced TβRII KO mice.
FIGURE 2.
The role of SMAD signals in the induction of Foxp3+ regulatory T cells in vivo. A, Doxycycline-induced TβRII KO, as described in Materials and Methods, and WT mice were given DMSO or TsA (1 mg/kg/d, i.p., for 7 d) and CD4+Foxp3+ cell frequency in spleens was analyzed by FACS. B, Foxp3 GFP knock-in mice were treated with DMSO or TsA as in A with or without i.p. treatment with ALK5 inhibitor (1 mg/kg/d, every other day). Foxp3+ (GFP+) cell numbers in spleens were analyzed by FACS at 7 d after treatment. Values are mean ± SEM of three mice each group. Experiments were repeated with similar results. C, TsA but not DMSO treatment induces CD103 expression in Foxp3+ cells. D, WT, Smad2 CKO, and Smad3 KO mice were treated with DMSO or TsA as in A, and CD4+Foxp3+ cell frequency in spleens in each group was analyzed by FACS. Data indicate mean ± SEM of five mice each group (D) and representative of these experiments with dot plot flow data (E). The differences were analyzed by Student t test; p < 0.05 was considered significantly different.
We also confirmed that TsA promoted iTreg cells through the TGF-β signaling pathway using other approaches. We found that injection of an ALK5 inhibitor also significantly abolished the enhancement of CD4+Foxp3+ cells in vivo by TsA administration (Fig. 2B). Treatment with TsA induced the expression of CD103, a TGF-β–inducible surface integrin (35), on Foxp3 (GFP)-positive cell populations (Fig. 2C), further suggesting that TGF-β signaling pathway involves in the Foxp3+ cell conversion in vivo in this setting. These data demonstrate that the induction of Treg by TsA in vivo was dependent on the TGF-β signal.
We next determined the role of SMAD2 or SMAD3 in the induction of Foxp3+ cells in vivo after TsA treatment. As shown in Fig. 2D and 2E, TsA treatment significantly increased the frequency of cells expressing Foxp3 in WT, Smad2 CKO and Smad3 KO mice. Although the increase of Foxp3+ cell frequency was slightly lower in Smad2 CKO and Smad3 KO mice than in WT mice, these differences were not statistically different (p = 0.06). We also had evidence that the suppressive activities of splenic CD4+CD25+ cells sorted from WT, Smad2 CKO, and Smad3 KO mice after treatment of TsA were comparable (data not shown). Our results showed that, although TsA increased Foxp3 induction in WT mice, abrogation of all TGF-β signaling by deleting the TβRII gene blocked this TsA-induced Foxp3 increase. Given that TsA is also able to induce Foxp3+ cells in Smad2 CKO or Smad3 KO mice, our current data suggest that TsA increases Foxp3 induction in vivo through a Smad2/3 independent TGF-β pathway.
TGF-β but not SMAD2 or SMAD3 is essential for the induction of Th17 cells
Recent studies in mice revealed that a combination of TGF-β and IL-6 or IL-21 initiated IL-17–producing T cell development (3–6). As in iTreg cell development, we now show that TGF-β/TβR signaling pathways are essential for Th17 cell differentiation. As in previous reports (3–6), when stimulated with anti-CD3 and anti-CD28 Abs in the presence of IL-6, TGF-β, anti–IFN-γ, and anti–IL-4 for 3 d, >40% of naive CD4+ cells isolated from normal C57BL/6 mice can be differentiated into IL-17–producing cells. The combination of both IL-6 and TGF-β seems to be crucial, because either single cytokine cannot induce Th17 cell development (data not shown). We now demonstrate that the TGF-β signaling pathway is essential for the Th17 cell development induced by both IL-6 and TGF-β cytokines. As shown in Fig. 3A, addition of an ALK5 (TβRI) inhibitor completely prevented Th17 cell differentiation in CD4+ cells. The DMSO control did not interfere with IL-6/TGF-β–driven Th17 cell differentiation, excluding the possibility that the vehicle had cell toxicity that nonspecifically suppressed Th17 cell differentiation. In addition, TβRII deficiency also prohibited induction of Th17 development, but instead drove Th1 cell development when naive CD4+ cells were cultured with a combination of TGF-β and IL-6 (Fig. 3A), suggesting that the TGF-β/TβR signaling pathway is essential for Th17 cell development and Th1 cell suppression. These results also emphasize the crucial importance of either TβRI or TβRII in TGF-β signaling events.
FIGURE 3.
TGF-β but not SMAD2 or SMAD3 plays a crucial role in the induction of Th17 cells invitro. Naive CD4+ T cells isolated from WT, TβRII CKO, Smad2 CKO, and Smad3 KO mice were stimulated with soluble anti-CD3 (1 μg/ml) and anti-CD28 (10 μg/ml) in the presence of TGF-β (2 ng/ml), IL-6 (10 ng/ml), anti–IL-4 (10 μg/ml), and anti–IFN-γ (10 μg/ml) for 3 d. ALK5 inhibitor (5 μM) was added to some cultures. Supernatants were harvested for measuring soluble IL-17. A, Naive CD4+ cells isolated from WT and TβRII CKO mice were stimulated with anti-CD3 and anti-CD28 in the presence of TGF-β and IL-6 as above for 3 d. ALK5 inhibitor or DMSO vehicle control was added to CD4+ cells from WT mice. Intracellular IL-17 and IFN-γ expression by CD4+ cells was analyzed by FACS. Data are representative of three separate experiments. Th17 cell differentiation in WT, Smad2 CKO, or Smad3 KO mice values indicate mean ± SEM of three separate experiments (B) and the representative of Th17 cell differentiation in WT and Smad3 KO mice from these experiments (C). D, IL-17 levels were assayed with an ELISA using supernatants from stimulated naive CD4+ cells from various mice as indicated in B. E, Naive CD4+ cells isolated from Smad2 CKO mice were pretreated with SIS3 (10 μM) for 1 h and then stimulated under Th17 conditions as described above. Intracellular IL-17 was analyzed by FACS. Values indicate mean ± SEM of three separate experiments. The differences were analyzed by Student t test; p < 0.05 was considered significantly different.
We also determined the role of SMAD2 or SMAD3 in Th17 cell differentiation. As shown in Fig. 3B–D, a deficiency of Smad2 or Smad3 gene in T cells did not significantly decrease intracellular IL-17 production (Fig. 3B, 3C) or soluble IL-17 secretion by CD4+ cells that have been treated with TGF-β and IL-6 simultaneously in vitro (Fig. 3D). TGF-β was also able to induce IL-17 in Smad2 CKO T cells treated with SMAD3 inhibitor in the presence of IL-6 (Fig. 3E). These results suggest that the TGF-β/TβR signaling pathway is essential, but SMAD2 and SMAD3 are redundant for Th17 cell development in vitro.
To assess the role of SMAD3 in the regulation of Th17 cells in vivo, we used a Th17 cell-mediated inflammatory disease model, EAE in either WT or Smad3 KO mice. Chronic EAE was induced in mice with MOG35–55/complete Freund’s adjuvant and pertussis toxin as previously reported (29). As shown in Fig. 4A, both WT and Smad3 KO mice developed similar diseases at day 7–24 after immunization. Immunization of both WT and Smad3 KO mice increased the frequency of CD4+ Th17 cells in the draining lymph nodes, spleens, and blood of both groups over nonimmunized mice, but there were no significant differences in the frequencies of Th17 cells between the two immunized groups, although Th17 cell development was slightly lower in Smad3 KO mice than in WT mice (Fig. 4B, 4C). These results demonstrate that the SMAD3 pathway plays no essential role in Th17 cell differentiation in vivo or in Th17-mediated disease development.
FIGURE 4.
The absence of Smad3 gene does not alter IL-17 production in vivo, nor does it affect the development of Th17-mediated disease. A, WT and Smad3 KO mice were immunized with MOG35–55 to induce EAE. Disease severities were scored as described in Materials and Methods. (EAE scores are similar in two groups.) The figure is representative of two similar experiments (n = 4 mice per group). B, IL-17 and IFN-γ production by CD4+ cells in draining lymph nodes, spleens, and blood in WT and Smad3 KO mice at day 18 after immunization with MOG35–55. Values indicate mean ± SEM of four mice (B). The differences were analyzed by Student t test; p < 0.05 was considered significantly different. C, Representative cytokine expression data from B.
atRA mediates iTreg and Th17 cells via a SMAD3 independent pathway
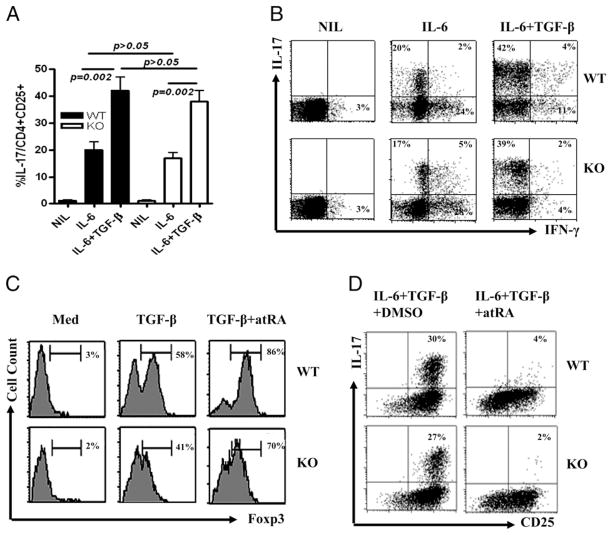
Recent studies have demonstrated that IL-6 is capable of enabling nTreg cells to convert to Th17 cells and that the TGF-β produced by nTreg cells is crucial for this conversion (36, 37). These studies have revealed that only purified Foxp3+CD25+ cells, but not Foxp3−CD25+ cells, can convert to Th17 cells when stimulated with IL-6 (36, 37). To assess the role of SMAD3 in this Th17 conversion, we have isolated thymic nTreg cells from WT and Smad3 KO mice using FACS sorting on the CD4 and CD25 gates (these cells expressed >99% of CD25). Foxp3 expression by sorting CD4+CD25+ cells in both WT and Smad3 KO mice was similarly high (>85%). nTreg subsets from different mice were stimulated with anti-CD3/CD28 Abs in the presence of IL-6 or IL-6 plus TGF-β with anti–IFN-γ and anti–IL-4 for 3 d. As shown in Fig. 5A and 5B, the addition of IL-6 enabled >20% nTreg cells from both WT and Smad3 KO mice to convert to Th17 and Th1 cells, respectively. The addition of exogenous TGF-β to IL-6 further enhanced the efficiency of conversion from nTreg to Th-17 and also suppressed Th1 conversion (Fig. 5B). These effects were all independent of SMAD3. The effect of TGF-β on suppressing the conversion of nTregs to Th1 cells via SMAD3 independent pathway is consistent with a previously reported study that TGF-β suppresses Th1 cell differentiation via an SMAD3-independent pathway (21).
FIGURE 5.
The SMAD3-independent effect of atRA on promoting Foxp3+ Treg cells and suppressing Th17 cells. A, Splenic CD4+CD25+CD62L+ cells sorted from WT and Smad3 KO mice (purity of both cell populations expressed >99% of CD25 and CD62L) were stimulated with immobilized anti-CD3 and soluble anti-CD28 for 3 d with anti–IL-4 and anti–IFN-γ with or without IL-6 and/or TGF-β. IL-17+ cell conversion from nTreg cells was determined by FACS. Values indicate mean ± SEM of three separate experiments. The differences were analyzed by Student t test; p < 0.05 was considered significantly different. B, Representative intracellular cytokine data from A. Naive CD4+ cells from WT and Smad3 KO mice were cultured under a condition polarizing toward iTreg cells (C) or (D) Th17 cells. Where indicated, atRA was used at 10 nM. Foxp3 expression is gated on CD4+ cells in C, and IL-17 expression is gated on activated CD4+ cells in D. FACS analysis was performed after 4 d of culture. C and D are representative of five separate experiments.
atRA, one of the vitamin A derivatives, has been highlighted for its role in immune regulation. This agent promotes Foxp3+ iTreg cell generation by TGF-β and restrains Th17 cell development by TGF-β and IL-6 (38). We have evidence that atRA promotes Foxp3+ iTreg development and restrains Th17 development through TGF-β signaling, because atRA alone was unable to promote iTreg cells in the absence of exogenous TGF-β (data not shown). Xiao et al. (23) have made similar observations; they also found that atRA enhanced TGF-β signaling by increasing the expression and phosphorylation of SMAD3. However, we demonstrate that SMAD3 plays a partial role in promoting Foxp3+ iTreg cell development, the addition of atRA to TGF-β–primed CD4+ cells from Smad3 KO mice still significantly increased Foxp3 expression (Fig. 5C) and promoted suppressive activities in vitro (data not shown), suggesting that other signaling pathways of TGF-β downstream play an important role in the promoting Foxp3+ cell differentiation in vitro. Similarly, the absence of Smad3 did not affect the suppressive effect of atRA on TGF-β and IL-6–induced Th17 cell differentiation in CD4+ cells (Fig. 5D). These studies provide further evidence that, although SMAD2 or SMAD3 contribute only partially to Foxp3+ iTreg cell induction in vitro, neither of these SMADs are required for Th17 cell differentiation in vitro and that SMAD3 is redundant for Th17 cell development in vivo.
MAPK pathways play a dominant role in the differentiation of iTreg and Th17 cells
Given that SMAD signaling is not essential for iTreg or Th17 cell differentiation, we next investigated whether non-SMAD pathways of TGF-β signaling are needed for the development of these cells. As described previously, MAPKs including ERK, JNK, and p38 constitute major non-SMAD signaling pathways that play a supplemental role in mediating the intracellular responses to TGF-β (20). We observed that CD4+ cells stimulated with TGF-β significantly increased activation of ERK and JNK (Fig. 6A) but not p38 (data not shown). The addition of either a JNK or ERK inhibitor, particularly an ERK inhibitor, markedly attenuated Foxp3 expression in TGF-β–primed CD4+ cells (Fig. 6B). Conversely, the addition of a p38 inhibitor did not alter Foxp3 expression in TGF-β–primed CD4+ cells. Using CD4+ cells from ERK1 and JNK2 KO mice further strengthened this conclusion (Fig. 6C). We recently reported that BMP-2 and BMP-4, members of the TGF-β superfamily, promoted the CD4+Foxp3+ cell development induced by TGF-β through ERK and JNK pathways (39). Moreover, the addition of JNK and ERK inhibitors further suppressed Foxp3 expression in TGF-β–primed CD4+ cells from Smad3 KO mice (Fig. 6B), suggesting that the JNK and ERK MAPK pathways in iTreg cell differentiation are also independent of SMAD3. The important role of the ERK pathway in iTreg cell induction might help to explain why ERK1 KO mice exhibit increased susceptibility to experimental autoimmune encephalomyelitis (40).
FIGURE 6.
Role of MAPKs in the differentiation of Foxp3+ Treg and Th17 cells. A, Splenic CD4+ CD62L+ cells isolated from C57BL/6 mice were activated with anti-CD3/CD28 Abs with or without TGF-β for 24 h. ERK and JNK activation was measured using Western blotting. Experiments were repeated three times with similar results. B, iTreg cells were induced from WT and Smad3 KO mice as in Fig. 1A. MAPK inhibitors (P38i and JNKi, 10 μM; ERKi, 50 μM) were added to cultures, and Foxp3 expression was determined by FACS. Values indicate mean ± SEM of four independent experiments. C, Foxp3 induction in CD4+ cells from WT, ERK1 KO, and JNK2 KO mice. Results were representative of three similar experiments. D–F, Th17 cell differentiation in CD4+ cells from WT, Smad3 KO, ERK1 KO, and JNK2 KO mice. MAPK inhibitors were added to cultures every 24 h for 3 d in some cultures. D, Percentages of CD4+CD25+ cells expressing IL-17. Values indicate mean ± SEM of five independent experiments. E, Flow cytometric data representative of the five separate experiments depicted as histograms in D. F, Th17 cell differentiation in CD4+ cells from WT, ERK1 KO and JNK2 KO mice. Values are mean ± SEM of three independent experiments. G, The addition of MAPK inhibitors does not affect CD4+ cell proliferation; 2 × 105 naive CD4+ cells were cultured under a condition polarizing toward Th17 cells with DMSO or MAPK inhibitors with similar concentrations as in B for 3 d. [3H]Thymidine incorporation was measured by liquid scintillation. All differences were analyzed by Student t test; p < 0.05 was considered significantly different.
Unlike Foxp3+ iTreg cell differentiation, Th17 cell development induced by TGF-β and IL-6 requires the JNK and p38 MAPKs pathways. As shown in Fig. 6D and 6E, addition of the p38 inhibitor, especially the JNK inhibitor, markedly inhibited Th17 cell development. Conversely, the addition of an ERK inhibitor did not attenuate Th17 cell production in this setting (Fig. 6D, 6E). In addition, IL-6 and TGF-β had a decreased ability to induce CD4+ cells from JNK2 KO mice to become Th17 cells, although Th17 cell differentiation by CD4+ cells from ERK1 KO mice was intact (Fig. 6F). Similarly, the addition of JNK and p38 inhibitors equivalently suppressed Th17 cell production in vitro in CD4+ cells from both WT and Smad3 KO mice (Fig. 6E), suggesting a SMAD3-independent role for the JNK and p38 MAPK pathways in Th17 cell induction. The addition of JNK, ERK, and p38 inhibitors did not alter the T cell activation, because >90% of these cells expressed CD25 (Fig. 6E) and total viable cell numbers (data not shown). In addition, these MAPK inhibitors did not affect the anti-CD3/CD28–stimulated CD4+ cell proliferation (Fig. 6G). Thus, Th17 suppression by JNK and p38 inhibitors is not due to global T cells suppression. In fact, others have previously reported that Smad4 is not essential for Th17 development, although it significantly contributes to TGF-β–primed Foxp3+ cell induction (25). Moreover, injection of a p38 inhibitor significantly suppressed EAE development (Fig. 7A) and Th17 cell production in regional lymph nodes (Fig. 7B, 7C). This helps to explain why injection of a p38 inhibitor can prevent the onset and progression of collagen-induced arthritis, another Th17 cell-mediated disease (41). A schematic model for the role of TGF-β signaling pathways in iTreg and Th17 cell development has been suggested (Fig. 8).
FIGURE 7.
Injection of p38 inhibitor markedly alleviates EAE and downregulates Th17 cell production. A, WT mice were immunized with MOG35–55 (100 μg per mouse), followed by the administration of pertussis toxin (150 ng per mouse; Sigma-Aldrich) on days 0 and 2. The p38 inhibitor (SB203580) was injected i.p. into WT mice 2 d after immunization with MOG35–55 peptide, and injection was repeated every other day for 7 d. Mice that received 2% DMSO solution only were used as controls. Disease severities were scored with a standard as described in Materials and Methods. The differences between two groups in time points indicated were statistically analyzed using GraphPad Prism 4 (GraphPad, La Jolla, CA. *p < 0.05; **p < 0.01; ***p < 0.001. The figure shows one representative of two similar experiments (n = 5 mice per group). B, IL-17 production in CD4+ cells in draining lymph nodes, spleens, and blood in two groups of mice at day 18 after immunization with MOG35–55. Values indicate mean ± SEM of five mice in each group. The differences were analyzed by the Student t test; p < 0.05 was considered as significantly different. C, Representative cytometric data of IL-17 and IFN-γ production by CD4+ cells in lymph nodes from EAE mice (n = 5) treated with or without p38 inhibitor.
FIGURE 8.
Schematic model for the role of TGF-β signaling pathways in iTreg and Th17 cell development. TGF-β binding induces heteromeric complex formation of TβR-II and TβR-I. This complex activates SMAD2 or SMAD3, as well as the ERK and JNK MAPKs pathways, which induces Foxp3 expression and drives the development of induced regulatory T cells when IL-2 is present. Conversely, this complex activates STAT3, JNK, and p38 MAPKs, promoting RORγt-expressing CD4+ cells to differentiate into Th17 cells when IL-6 is present. Foxp3 expression can suppress RORγt+ cells from conversion to Th17 cells, but IL-6 signaling pathway molecules, such as STAT3 and SMAD7, also have a negative feedback on Foxp3+ induced regulation differentiation.
Discussion
A subset of CD4+ cells that express the IL-2 receptor α-chain (CD25) called Treg cells play a vital role in the maintenance of central and/or peripheral tolerance, the frequency alteration and/or dysfunction of these cells has been linked to many autoimmune diseases (42–44). These cells are characterized by expression of a unique transcription factor known as Foxp3. Foxp3 is essential for the development and suppressive activity of Treg cells, and Foxp3 deficiency leads to multiorgan autoimmune disease, as found in scurfy mice and in human patients with immune dysregulation, polyendocrinopathy, enteropathy, and X–linked syndrome (45, 46). There are two distinct subsets of Treg cells in peripheral lymphoid organs: nTregs cells that develop in the thymus after recognition of high-affinity self-Ag, and iTreg cells that develop from conventional T cells as a consequence of peripheral exposure to Ag and specific cytokines, such as TGF-β or IL-10 (47). Both Treg cell types compose the Treg cell network and may have a synergistic action or have different targets that maintain immune homeostasis, although they have possibly different developmental mechanisms (48). For example, whereas TGF-β is not needed for the development of nTreg cells, this cytokine plays an important role in the development of iTreg cells (48, 49). We now provide the evidence that TGF-β/TβR signaling is essential for Foxp3+ iTreg cell induction. Although we blocked ALK5 (TβRI) or knocked out TβRII, naive CD4+ cells stimulated with anti-CD3/CD28 Abs lost their responses to TGF-β to become Foxp3+ suppressor cells, indicating that both TβRI and TβRII are crucial in the TGF-β signaling pathway. Previous studies have demonstrated that both TβRI and TβRII work as an integrated complex to phosphorylate downstream specific SMAD2 and SMAD3 (11). Using Foxp3+ iTreg or Th17 cell (below) induction, we further confirm that both TβRI and TFβRII signaling pathways are equivalently required for TGF-β response in CD4+ T cells.
Given that iTreg cells share similar phenotypic and functional characteristics with nTreg cells, it is likely that the manipulation of iTreg cells can treat many autoimmune diseases. Therefore, it is important to understand the mechanisms by which iTreg cells are induced. The importance of TGF-β signaling pathways has been recognized in the induction of CD4+ iTreg cells; however, few studies have investigated the role of downstream molecules of the TGF-β signaling pathways in the development of iTreg cells. SMAD3 plays an important role in many effects of TGF-β on T cells. For example, SMAD3 is essential for the suppressive effect of TGF-β on IL-2 production and T cell proliferation (18). In addition, SMAD3 is also required for the suppressive effects of TGF-β on Th2-type cytokine productions and Th2-type disease in the skin (19). Tone et al. (22) recently identified a Foxp3 enhancer and observed that the transcription factors Smad3 and NFAT are required for activity of this Foxp3 enhancer, implicating that SMAD3 is possibly important for Foxp3 induction. Xiao et al. (23) also observed that atRA promotes iTreg cell differentiation by enhancing SMAD3 expression and phosphorylation. Jana et al. (24) also reported that lack of Smad3 gene significantly decreased TGF-β–induced Foxp3 expression in vitro. However, the role of SMAD3 in Foxp3+ iTreg cell induction is partial, because a lack of Smad3 did not completely abolish the Foxp3+ cell induction, which is different from the blockade of TβRI and lack of TβRII.
We have systematically investigated the role of SMAD2 or SMAD3 in the TGF-β–induced Foxp3+ iTreg cell induction both in vitro and in vivo. Our findings revealed that either SMAD2 or SMAD3 plays a partial (~20–30%) role in the differentiation of Foxp3+ iTreg cells induced by TGF-β in vitro using Smad3 KO and Smad2 CKO mice. Whereas others reported Foxp3 expression in only 12% of TGF-β–induced CD4+CD25+ cells from Smad3 KO mice (24), our protocol demonstrated that >47% of these cells expressed Foxp3 when obtained from either Smad2 CKO or Smad3 KO mice. We believe the different precursors that we and others used might account for this Foxp3 induction discrepancy. Although we have used naive CD4+CD25− cells as precursors, others have used total CD4+CD25− cells. We and others have previously reported that TGF-β sufficiently induces Foxp3+ iTregs from naive CD4+ rather than memory CD4+ precursors (1, 2). Recently, Hill et al. (50) reported that cytokines produced by memory CD4+ cells interfered with Foxp3 expression induced by TGF-β. We also excluded the possibility that SMAD2 or SMAD3 mutually compensates each other, because we found that the combination of the deficiency of Smad2 and a specific inhibitor of SMAD3 (i.e., SIS3) did not significantly alter the Foxp3 expression and suppressive activity in vitro, further suggesting the partial but nonessential roles of SMAD2 and SMAD3 in Foxp3+ iTreg cell induction.
It is known that atRA can increase Foxp3 expression and function by TGF-β–primed CD4+ cells, and activated SMAD3 could contribute to this promotion. However, using Smad3 KO mice, we observed that atRA significantly increased Foxp3 expression in TGF-β–primed CD4+ cells, although the levels and MIF of Foxp3 is slightly lower than in WT mice. These results further suggest that other SMAD3-independent TGF-β–downstream signaling pathways are involved in the differentiation and promotion of TGF-β–induced Foxp3+ cells.
Although a partial role for SMAD2 or SMAD3 in TGF-β–induced Foxp3+ iTreg cell development in vitro has been identified, it is less clear whether these SMAD molecules play any role in the Foxp3+ iTreg cell induction in vivo. Using the deacetylase inhibitor TsA, we confirmed previous reports demonstrating that TsA promotes Foxp3+ iTreg cells in vivo (34). Interestingly, we also found that the TGF-β signaling pathway is crucial for this effect, because TsA no longer increased Foxp3+ iTreg cells in CD4+ cells lacking the TβRII. TsA also increased the percentages of cells expressing Foxp3 in either Smad2 CKO or Smad3 KO mice, suggesting that TsA enhances Foxp3+ iTreg cell induction in vivo through a SMAD2/3-independent TGF-β pathway. We are currently developing Smad2 and Smad3 double KO mice to exclude the possibility that SMAD2 or SMAD3 mutually compensates for one another in vivo. Thus, non-SMAD pathways might play a more important role than do SMAD pathways in the development of TGF-β-iTreg cells.
Recent studies found that TGF-β enables CD4+ cells to become Th17 cells when combined with proinflammatory cytokines, such as IL-6 or IL-21 (3–6). IL-23 promotes the survival and population expansion of Th17 cells (3, 4). Th17 cells play an important role in host defense; however, IL-17 produced by Th17 cells is also crucial for chronic inflammation in many disease models, such as EAE and colitis (51, 52). Proinflammatory cytokines and related cytokine signaling molecules play a critical role in Th17 cell development. For example, IL-6, IL-21, and IL-23 each can activate STAT3 protein, and this activation is essential for the expression of transcription factor RORγt and thus Th17 cell induction (53, 54). In addition to STAT3 activation, IFN regulatory factor 4 is also a decisive factor during the IL-21–mediated stage of Th17 cell development (53).
Notably, the TGF-β signaling pathway is also essential for Th17 cell development, because deficiency of the TGF-β receptor results in the inhibition of Th17 cell differentiation (Fig. 3A). As with Foxp3+ iTreg cell induction, blockading TβRI or knocking out TβRII almost completely prevented Th17 cell differentiation induced by a combination of IL-6 and TGF-β. Few studies have explored the roles of TGF-β downstream signaling pathways in the differentiation of these cells. Whereas Yang et al. (25) reported that SMAD4 is redundant for Th17 cell differentiation, we show neither SMAD2 nor SMAD3 is required for Th17 cell differentiation and Th17 cell-mediated disease. Combination of both Smad2 deficiency and SMAD3 inhibitor does not significantly alter Th17 cell differentiation, excluding the possibility that SMAD2 or SMAD3 can compensate each other for this cell lineage differentiation. Moreover, atRA equivalently suppresses Th17 cell differentiation induced by IL-6 and TGF-β between WT and Smad3 KO mice, further indicating a redundant role of SMAD3 in Th17 cell differentiation and implicating that other TGF-β downstream signaling pathways may be required for Th17 cell development. This finding helps to exclude the possibility that increased IFN-γ suppresses Th17 cell differentiation owing to IFN-γ–mediated SMAD suppression (54).
MAPKs constitute mostly regulators for the non-SMAD pathway of TGF-β signaling (20). In this study, we have shown that MAPKs predominately affect the Foxp3+ iTreg and Th17 cell differentiation, which is similar with Th1 cell differentiation but not Th2 cell differentiation (19, 21). However, we have also identified the different requirements of MAPKs in the development of both iTreg and Th17 cell lineages. Although ERK activation is crucial for iTreg cell differentiation, its activation or deficiency does not affect Th17 cell differentiation. Conversely, JNK and a p-38 inhibitor markedly suppressed Th17 cell differentiation and Th17 cell-mediated EAE, but did not alter iTreg cell induction. Using cell activation and proliferation experiments, we show that these MAPK inhibitors specifically suppressed Foxp3+ iTreg or Th17 cell differentiation rather than nonspecific global T cell suppression. It is still unclear as to why JNK deficiency affects the development of both lineages. It is possible that JNK plays a similar role in both iTreg and Th17 cell differentiation depending on the cytokine milieu.
We summarize our finding in a paradigm shown in Fig. 8. TGF-β signaling has a profound regulatory effect on lymphocyte differentiation and conversion, depending on the subset of lymphocytes and costimulated cytokines. Simultaneous stimulation in CD4+ cells by TGF-β and IL-2 activates both SMAD-independent and SMAD-dependent pathways to promote iTreg cell generation, whereas combined TGF-β and IL-6 signaling activates cell conversion from CD4+ and even nTreg cells to Th17 cells through SMAD-independent pathways. Foxp3 expression can suppress RORγt+ cells from conversion to Th17 cells, but IL-6 signaling pathway molecules, such as STAT3 and SMAD7, also have a negative feedback on Foxp3+ induced regulation differentiation.
We therefore identify novel signaling pathways for the TGF-β/TβR complex in the differential development of Foxp3+ iTreg and Th17 cells. The identification of these signaling pathways sheds light on the mechanisms by which SMAD and non-SMAD pathways differently induce specific cellular phenotypes. Knowledge of TGF-β signaling pathways that mediate specific cellular responses will allow for the development of therapeutics that can target specific TGF-β responses in autoimmune and inflammatory diseases.
Acknowledgments
We thank H. Moses, G. Landreth, X.F. Wang, M. Winstein, and D. Kioussis for providing floxed TβR-II, ERK1 KO, Smad2fx/fx, Smad3 KO, and hCD2-Cre mice; and W. Gilmore and L. Pei for help in establishing the EAE model.
This work was supported by National Institutes of Health Grant R01 HL068597, American College of Rheumatology Research and Education Foundation’s Within Our Reach, the Arthritis Foundation, the Outstanding Youth Scientist Investigator Award from National Natural Science Foundation of China (30728007), and the Foundation of Liver Surgery Center of Jiangsu Province in China.
Abbreviations used in this paper
- atRA
all-trans retinoic acid
- CKO
conditional knockout
- EAE
experimental autoimmune encephalomyelitis
- HPRT
hypoxanthine guanine phosphoribosyl transferase
- iTreg cell
induced Foxp3+ regulatory T cell
- KO
knockout
- nTreg cell
natural regulatory T cell
- TβRI
TGF-β type I receptor
- TβRII
TGF-β type II receptor
- Treg cell
regulatory T cell
- TsA
trichostatin A
- WT
wild type
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-beta to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J Immunol. 2007;178:2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 2.Davidson TS, DiPaolo RJ, Andersson J, Shevach EM. Cutting Edge: IL-2 is essential for TGF-beta-mediated induction of Foxp3+ T regulatory cells. J Immunol. 2007;178:4022–4026. doi: 10.4049/jimmunol.178.7.4022. [DOI] [PubMed] [Google Scholar]
- 3.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 5.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 6.Korn T, Bettelli E, Gao W, Awasthi A, Jäger A, Strom TB, Oukka M, Kuchroo VK. IL-21 initiates an alternative pathway to induce proinflammatory T(H)17 cells. Nature. 2007;448:484–487. doi: 10.1038/nature05970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng SG, Gray JD, Ohtsuka K, Yamagiwa S, Horwitz DA. Generation ex vivo of TGF-beta-producing regulatory T cells from CD4+CD25− precursors. J Immunol. 2002;169:4183–4189. doi: 10.4049/jimmunol.169.8.4183. [DOI] [PubMed] [Google Scholar]
- 8.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25− naive T cells to CD4+ CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25− cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol. 2004;172:5213–5221. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 10.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. Cutting edge: TGF-beta induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–5153. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 11.Wrana JL, Attisano L, Cárcamo J, Zentella A, Doody J, Laiho M, Wang XF, Massagué J. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992;71:1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- 12.Lagna G, Hata A, Hemmati-Brivanlou A, Massagué J. Partnership between DPC4 and SMAD proteins in TGF-beta signalling pathways. Nature. 1996;383:832–836. doi: 10.1038/383832a0. [DOI] [PubMed] [Google Scholar]
- 13.Rubtsov YP, Rudensky AY. TGFbeta signalling in control of T-cell-mediated self-reactivity. Nat Rev Immunol. 2007;7:443–453. doi: 10.1038/nri2095. [DOI] [PubMed] [Google Scholar]
- 14.Nomura M, Li E. Smad2 role in mesoderm formation, left-right patterning and craniofacial development. Nature. 1998;393:786–790. doi: 10.1038/31693. [DOI] [PubMed] [Google Scholar]
- 15.Yang X, Li C, Xu X, Deng C. The tumor suppressor SMAD4/DPC4 is essential for epiblast proliferation and mesoderm induction in mice. Proc Natl Acad Sci USA. 1998;95:3667–3672. doi: 10.1073/pnas.95.7.3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hata A, Lo RS, Wotton D, Lagna G, Massagué J. Mutations increasing autoinhibition inactivate tumour suppressors Smad2 and Smad4. Nature. 1997;388:82–87. doi: 10.1038/40424. [DOI] [PubMed] [Google Scholar]
- 17.Datto MB, Frederick JP, Pan L, Borton AJ, Zhuang Y, Wang XF. Targeted disruption of Smad3 reveals an essential role in transforming growth factor beta-mediated signal transduction. Mol Cell Biol. 1999;19:2495–2504. doi: 10.1128/mcb.19.4.2495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKarns SC, Schwartz RH, Kaminski NE. Smad3 is essential for TGF-beta 1 to suppress IL-2 production and TCR-induced proliferation, but not IL-2-induced proliferation. J Immunol. 2004;172:4275–4284. doi: 10.4049/jimmunol.172.7.4275. [DOI] [PubMed] [Google Scholar]
- 19.Anthoni M, Fyhrquist-Vanni N, Wolff H, Alenius H, Lauerma A. Transforming growth factor-beta/Smad3 signalling regulates inflammatory responses in a murine model of contact hypersensitivity. Br J Dermatol. 2008;159:546–554. doi: 10.1111/j.1365-2133.2008.08696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park IK, Letterio JJ, Gorham JD. TGF-beta 1 inhibition of IFN-gamma-induced signaling and Th1 gene expression in CD4+ T cells is Smad3 independent but MAP kinase dependent. Mol Immunol. 2007;44:3283–3290. doi: 10.1016/j.molimm.2007.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tone Y, Furuuchi K, Kojima Y, Tykocinski ML, Greene MI, Tone M. Smad3 and NFAT cooperate to induce Foxp3 expression through its enhancer. Nat Immunol. 2008;9:194–202. doi: 10.1038/ni1549. [DOI] [PubMed] [Google Scholar]
- 23.Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, Kuchroo VK. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol. 2008;181:2277–2284. doi: 10.4049/jimmunol.181.4.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jana S, Jailwala P, Haribhai D, Waukau J, Glisic S, Grossman W, Mishra M, Wen R, Wang D, Williams CB, Ghosh S. The role of NF-kappaB and Smad3 in TGF-beta-mediated Foxp3 expression. Eur J Immunol. 2009;39:2571–2583. doi: 10.1002/eji.200939201. [DOI] [PubMed] [Google Scholar]
- 25.Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dominitzki S, Fantini MC, Neufert C, Nikolaev A, Galle PR, Scheller J, Monteleone G, Rose-John S, Neurath MF, Becker C. Cutting edge: trans-signaling via the soluble IL-6R abrogates the induction of FoxP3 in naive CD4+CD25 T cells. J Immunol. 2007;179:2041–2045. doi: 10.4049/jimmunol.179.4.2041. [DOI] [PubMed] [Google Scholar]
- 27.Zheng SG, Wang JH, Koss MN, Quismorio F, Jr, Gray JD, Horwitz DA. CD4+ and CD8+ regulatory T cells generated ex vivo with IL-2 and TGF-beta suppress a stimulatory graft-versus-host disease with a lupus-like syndrome. J Immunol. 2004;172:1531–1539. doi: 10.4049/jimmunol.172.3.1531. [DOI] [PubMed] [Google Scholar]
- 28.Chen H, Zhuang F, Liu YH, Xu B, Del Moral P, Deng W, Chai Y, Kolb M, Gauldie J, Warburton D, et al. TGF-beta receptor II in epithelia versus mesenchyme plays distinct roles in the developing lung. Eur Respir J. 2008;32:285–295. doi: 10.1183/09031936.00165407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stromnes IM, Goverman JM. Active induction of experimental allergic encephalomyelitis. Nat Protoc. 2006;1:1810–1819. doi: 10.1038/nprot.2006.285. [DOI] [PubMed] [Google Scholar]
- 30.Li HY, Wang Y, Heap CR, King CH, Mundla SR, Voss M, Clawson DK, Yan L, Campbell RM, Anderson BD, et al. Dihydropyrrolopyrazole transforming growth factor-beta type I receptor kinase domain inhibitors: a novel benzimidazole series with selectivity versus transforming growth factor-beta type II receptor kinase and mixed lineage kinase-7. J Med Chem. 2006;49:2138–2142. doi: 10.1021/jm058209g. [DOI] [PubMed] [Google Scholar]
- 31.Kano MR, Bae Y, Iwata C, Morishita Y, Yashiro M, Oka M, Fujii T, Komuro A, Kiyono K, Kaminishi M, et al. Improvement of cancer-targeting therapy, using nanocarriers for intractable solid tumors by inhibition of TGF-beta signaling. Proc Natl Acad Sci USA. 2007;104:3460–3465. doi: 10.1073/pnas.0611660104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Boer J, Williams A, Skavdis G, Harker N, Coles M, Tolaini M, Norton T, Williams K, Roderick K, Potocnik AJ, Kioussis D. Transgenic mice with hematopoietic and lymphoid specific expression of Cre. Eur J Immunol. 2003;33:314–325. doi: 10.1002/immu.200310005. [DOI] [PubMed] [Google Scholar]
- 33.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 34.Tao R, de Zoeten EF, Ozkaynak E, Chen C, Wang L, Porrett PM, Li B, Turka LA, Olson EN, Greene MI, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–1307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 35.Nakamura K, Kitani A, Fuss I, Pedersen A, Harada N, Nawata H, Strober W. TGF-beta 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J Immunol. 2004;172:834–842. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 36.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25−Foxp3− T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 37.Zheng SG, Wang J, Horwitz DA. Cutting edge: Foxp3+CD4+CD25 + regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J Immunol. 2008;180:7112–7116. doi: 10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]
- 38.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 39.Lu L, Ma J, Wang X, Wang J, Zhang F, Yu J, He G, Xu B, Brand DD, Horwitz DA, et al. Synergistic effect of TGF-beta superfamily members on the induction of Foxp3+ Treg. Eur J Immunol. 2010;40:142–152. doi: 10.1002/eji.200939618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agrawal A, Dillon S, Denning TL, Pulendran B. ERK1−/− mice exhibit Th1 cell polarization and increased susceptibility to experimental autoimmune encephalomyelitis. J Immunol. 2006;176:5788–5796. doi: 10.4049/jimmunol.176.10.5788. [DOI] [PubMed] [Google Scholar]
- 41.Nishikawa M, Myoui A, Tomita T, Takahi K, Nampei A, Yoshikawa H. Prevention of the onset and progression of collagen-induced arthritis in rats by the potent p38 mitogen-activated protein kinase inhibitor FR167653. Arthritis Rheum. 2003;48:2670–2681. doi: 10.1002/art.11227. [DOI] [PubMed] [Google Scholar]
- 42.Valencia X, Lipsky PE. CD4+CD25+FoxP3+ regulatory T cells in autoimmune diseases. Nat Clin Pract Rheumatol. 2007;3:619–626. doi: 10.1038/ncprheum0624. [DOI] [PubMed] [Google Scholar]
- 43.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 44.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 45.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 46.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 47.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martínez-Llordella M, Ashby M, Nakayama M, Rosenthal W, Bluestone JA. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Horwitz DA, Zheng SG, Gray JD. Natural and TGF-beta-induced Foxp3(+)CD4(+) CD25(+) regulatory T cells are not mirror images of each other. Trends Immunol. 2008;29:429–435. doi: 10.1016/j.it.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 49.Piccirillo CA, Letterio JJ, Thornton AM, McHugh RS, Mamura M, Mizuhara H, Shevach EM. CD4(+)CD25(+) regulatory T cells can mediate suppressor function in the absence of transforming growth factor beta1 production and responsiveness. J Exp Med. 2002;196:237–246. doi: 10.1084/jem.20020590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hill JA, Hall JA, Sun CM, Cai Q, Ghyselinck N, Chambon P, Belkaid Y, Mathis D, Benoist C. Retinoic acid enhances Foxp3 induction indirectly by relieving inhibition from CD4+CD44hi Cells. Immunity. 2008;29:758–770. doi: 10.1016/j.immuni.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 52.Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, et al. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nat Immunol. 2007;8:247–256. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 53.Wei L, Laurence A, Elias KM, O’Shea JJ. IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 2007;282:34605–34610. doi: 10.1074/jbc.M705100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanaka K, Ichiyama K, Hashimoto M, Yoshida H, Takimoto T, Takaesu G, Torisu T, Hanada T, Yasukawa H, Fukuyama S, et al. Loss of suppressor of cytokine signaling 1 in helper T cells leads to defective Th17 differentiation by enhancing antagonistic effects of IFN-gamma on STAT3 and Smads. J Immunol. 2008;180:3746–3756. doi: 10.4049/jimmunol.180.6.3746. [DOI] [PubMed] [Google Scholar]