Abstract
N-Methyl-d-aspartate (NMDA) receptors, the main mediators of excitatory synaptic transmission, are heterotetrameric receptors. Typically, glycine binding NR1 subunits co-assemble with glutamate binding NR2 subunits to form a functional receptor. Here we have used luminescence resonance energy transfer (LRET) investigations to establish the specific configuration in which these subunits assemble to form the functional tetramer and show that the dimer of dimers structure is formed by the NR1 subunits assembling diagonally to each other. The distances measured by LRET are consistent with the NMDA structure predicted based on cross-linking investigations and on the structure of the full-length α-amino-5-methyl-3-hydroxy-4-isoxazole propionic acid (AMPA) receptor structure (1). Additionally, the LRET distances between the NR1 and NR2A subunits within a dimer measured in the desensitized state of the receptor are longer than the distances in the previously published crystal structure of the isolated ligand binding domain of NR1-NR2A. Because the dimer interface in the isolated ligand binding domain crystallizes in the open channel structure, the longer LRET distances would be consistent with the decoupling of the dimer interface in the desensitized state. This is similar to what has been previously observed for the AMPA subtype of the ionotropic glutamate receptors, suggesting a similar mechanism for desensitization in the two subtypes of the glutamate receptor.
Keywords: Fluorescence Resonance Energy Transfer (FRET); Glutamate Receptors NMDA; Glutamate Receptors Ionotropic (AMPA, NMDA); Ion Channels; Ligand-binding Protein
Introduction
N-Methyl-d-aspartate (NMDA)3 receptors are members of the ionotropic glutamate receptor channel family, which mediate most of the fast excitatory synaptic transmissions in the central nervous system. The glutamate receptor family consists of three subtypes: kainate receptors, α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors, and NMDA receptors (2–4). Although the overall topology is similar among the three subtypes of the glutamate receptors, the NMDA receptors differ in many ways from the AMPA and kainate receptor subtypes. Functionally, the NMDA receptors are highly permeable to calcium ions and exhibit voltage-dependent block by physiological concentrations of Mg2+ and act as a coincidence detector, permitting ion flow only upon depolarization of the receiving cell. Structurally, unlike the AMPA and kainate receptors that can form homomeric receptors, the NMDA receptors are obligate heteromeric receptors composed of homologous NR1, NR2, and/or NR3 subunits.
A large fraction of the neuronal NMDA receptors are composed of glycine binding NR1 and glutamate binding NR2 subunits (5–8). The structure of the isolated ligand binding domain of the NMDA receptor suggests that the tetrameric receptor has a dimer of dimer arrangement, with each dimer having one glycine binding subunit and one glutamate binding subunit (9). The recent full-length structure of the AMPA receptor published while this manuscript was in review along with cross-linking data suggest that the dimers are arranged such that the NR1 subunits are diagonal to each other, as are the NR2 subunits (1) (see Fig. 1A). Here we have obtained LRET-based distances between the subunits and show that the distances match the arrangement predicted based on the full-length AMPA receptor structure.
FIGURE 1.
Possible arrangements of NR1-NR2A receptor agonist binding domains 1 and 2 based on full-length AMPA receptor crystal structure. The position of the NR1 subunit on the AMPA receptor structure is colored in red for domain 1 and in pink for domain 2. The position of NR2A subunit on the AMPA receptor structure is colored in green for domain 1 and in pale green for domain 2. Homologous sites tagged in the modified ΔNR1* and ΔNR2A* subunit for LRET measurements are highlighted.
Apart from the subunit arrangements, due to the limited number of structures available for the NMDA receptors, most of the insight into the mechanism of activation and desensitization has been based on the extensive structure-function investigations of the AMPA subtype. The mechanism based on these structures is that agonist binding induces cleft closure in the ligand binding domain, leading to channel opening, which causes stress on the transmembrane segments (9–22). To relieve this stress, it is thought that the dimer interface decouples, which in turn results in channel closure (desensitization). The mechanism for desensitization in the AMPA receptors is based on the observation that stabilizing the dimer interface using either mutations or modulators such as cyclothiazide results in a stabilization of the open channel form (12). There is no direct structural evidence for the same because the structural studies of the isolated ligand binding domain lack the transmembrane segments and hence do not have the strain that drives the decoupling. A “desensitized-like” structure was obtained by artificially decoupling the dimer interface by introducing a cysteine at position 729 or 725, which formed a disulfide bond formation deep in the interface (18). In the case of the NMDA receptors, stabilization of the dimer interface through disulfide bonds slows the rate of desensitization; however, it does not block desensitization, as seen in the AMPA receptors. There is currently one structure of the NR1-NR2A dimer, and because it lacks the transmembrane domain, this is expected to be the structure of the open channel form of the receptor. Hence there is no direct structural insight into the desensitized state of the NMDA receptor, and it is still not known whether dimer decoupling is the mechanism of desensitization in this subtype of the glutamate receptors.
EXPERIMENTAL PROCEDURES
Modifications Introduced in the NMDA Receptor
For studying the subunit arrangement of the NMDA receptor, we used modified NR1 and NR2A subunits of the NMDA receptor lacking the N-terminal domain. For NR1, residues 5–357 were deleted, and for NR2A, residues 1–385 were deleted (ΔNR1/ΔNR2A). For NR2A, a modified influenza hemagglutinin served as the signal peptide sequence (23). The ΔNR1 and ΔNR2A subunits were further modified to eliminate the single non-disulfide bonded accessible cysteine, Cys-459, in NR1 and Cys-399 and Cys-460 in NR2A by mutating them to serine. These modified subunits ΔNR1* and ΔNR2A* were used for LRET investigations by introducing cysteines and or histidine tags to serve as donor- and acceptor-tagging sites. Mutations in the plasmids were introduced using the Stratagene QuikChange site-directed mutagenesis kit (Stratagene), and the integrity of the plasmid after cloning was verified by sequencing. The sites tagged for LRET measurements are displayed in the full-length AMPA structure (see Fig. 1) and NR1-NR2A crystal structure (see Fig. 3).
FIGURE 3.
Sites that were tagged in the modified ΔNR1* and ΔNR2A* subunit for LRET measurements within the dimer are highlighted in the crystal structure of NR1-NR2A dimer.
Receptor Expression and Tagging
Oocytes were isolated and maintained as described previously in Mg2+-free storage solution (22). The ΔNR1*:ΔNR2A* mutant NMDA receptors were expressed in oocytes by injecting the RNA (mMessage mMachine T7 kit, Ambion) in a 1:2 ratio, respectively. Injection, blocking, and membrane preparations were performed as described previously (22). To measure background LRET, 1 unit of thrombin (Calbiochem) was added to the membrane preparation for 2–3 h at 4 °C. After digestion, LRET lifetimes were recorded and subtracted from the non-digested signal to determine the lifetime from the modified NMDA receptor protein.
Fluorophores
ATTO 465 maleimide and NiSO4 were purchased from Sigma-Aldrich, maleimide derivative of terbium chelate was purchased from Invitrogen, and fluorescein maleimide was purchased from Biotium. (Ni-NTA)2-Cy3 chelate was prepared as described previously (19).
Fluorescence Spectroscopy
The fluorescence measurements were performed using a cuvette-based fluorescence lifetime spectrometer QuantaMaster model QM3-SS from Photon Technology International. A high power pulsed xenon lamp served as the excitation source, whereas emitted light was collected and passed through a monochromator on to a detector. The sample was held at 15 °C with a Peltier TE temperature controller. Data were collected using Fluorescan software (Photon Technology International) and analyzed using Origin 4.0 software (OriginLab Corp.). Three to four sets of data were averaged and fitted to obtain the lifetimes shown in Table 1. Each individual data set was examined to ensure that similar trends were maintained. LRET lifetimes were obtained by studying the sensitized emission at 510 nm for ATTO 465, 515 nm for fluorescein, and 575 nm for (Ni-NTA)2-Cy3 chelate, and the donor-only lifetimes were collected at 545 nm. The fluorescence decay was fitted to a function that is a sum of discrete exponentials, and the goodness of the exponential fit was determined from the random residual distribution with a chi-square value being close to unity. R0 values were determined for each donor:acceptor pair using Equations 1 and 2.
TABLE 1.
Fluorescence lifetimes and distances for ΔNR1*:ΔNR2A* receptors
| Protein | Donor:Acceptor fluorophore pair | Donor lifetime | Sensitized emission lifetime (desensitized) | Distance desensitized state |
|---|---|---|---|---|
| μs | μs | Å | ||
| ΔNR1*Thr-396Histag/ΔNR1*T396C-Th:ΔNR2A* | Terbium chelate:(Ni-NTA)2Cy3 | 1900 ± 1 | No LRET | >100 |
| ΔNR1*:ΔNR2A*Asn-404Histag/ΔNR2A*N404C-Th | Terbium chelate:(Ni-NTA)2Cy3 | 1750 ± 21 | 672 ± 12 | 60.1 ± 0.3 |
| ΔNR1*Thr-396Histag:ΔNR2A* N404C-Th | Terbium chelate:(Ni-NTA)2Cy3 | 1904 ± 5 | 1254 ± 36 | 72.5 ± 0.8 |
| 318 ± 24 | 49.7 ± 0.6 | |||
| ΔNR1*Th-Q525C-Th:ΔNR2A* | Terbium chelate:fluorescein | 1606 ± 8 | 1089 ± 12 | 51 ± 0.3 |
| ΔNR1*525tetrahistag:ΔNR2A*L777C | Terbium chelate:Ni2+ | 1754 ± 5 | 1155 ± 42 | 13.3 ± 0.2 |
| ΔNR1*775tetrahistag:ΔNR2A*S519C | Terbium chelate:Ni2+ | 1681 ± 10 | 1330 ± 50 | 14.9 ± 0.4 |
| ΔNR1*T396C-Th, A715C:ΔNR2A* | Terbium chelate:ATTO 465 | 1704 ± 18 | 790 ± 16 | 35.1 ± 0.2 |
 |
 |
 |
In Equation 1, J is the overlap integral, FD is the fluorescence spectrum of donor, λ is the wavelength, and ϵA is the absorption spectra of the acceptor tagged to the protein. The J value is used in Equation 2 to determine R0 of the fluorophore pair. In Equation 2, k is the orientation factor, ϕD is the quantum yield of donor, and n is the refractive index. The R0 value measured for the following donor:acceptor pairs was: for terbium chelate, (Ni-NTA)2-Cy3 chelate, 65 Å; for terbium chelate:fluorescein, 45 Å; for terbium chelate:ATTO 465, 36 Å; and for terbium chelate:Ni2+, 12 Å. The time constants of donor fluorescence decay in the absence of acceptor and either donor fluorescence or sensitized acceptor fluorescence in the presence of the acceptor, along with the R0 values, were used in the Förster's equation (Equation 3) to determine the distance between the donor and acceptor.
Two-electrode Voltage Clamp
The functionality of all the constructs used in the LRET measurements was established using two-electrode voltage clamp measurements. Two-electrode voltage clamp recordings were performed as detailed previously (22). The dose-response curves for the mutants used in LRET measurements are shown in supplemental Fig. 1.
RESULTS AND DISCUSSION
Subunit Arrangement of the NMDA Receptor
LRET lifetimes were used to determine distances between the N terminus of the agonist binding domains of NR1 (residue 396) and NR2A subunits (residue 404) (Fig. 1). To determine the LRET specific to the NMDA receptors, we have introduced thrombin site(s) in the ΔNR1* and ΔNR2A* subunits that allowed for selectively cleaving the donor or acceptor fluorophore tagged to these subunits. The LRET lifetime probed after thrombin cleavage allows for the quantification of the nonspecific LRET, which is then subtracted from the LRET signal to provide the signal from the modified NMDA receptors. The LRET lifetimes for all the constructs shown are the final difference lifetime.
The ΔNR1*T396C-Th/ΔNR1*396Histag:ΔNR2A* tagged with terbium chelate:(Ni-NTA)2-Cy3 chelate donor:acceptor pair was used to measure the distance between residue 396 on one NR1 subunit with respect to the same residue on the second NR1 subunit (Fig. 2A). This construct did not show any significant LRET, suggesting that the distance between the residue 396 on one NR1 subunit with respect to the same residues on the second NR1 subunit is longer than that measurable by the terbium chelate:(Ni-NTA)2-Cy3 chelate donor:acceptor pair. Given that the R0 for this pair is 65 Å, for distances >100 Å, the efficiency of transfer decreases to around 6% (supplemental Fig. 2). Thus it can be concluded that the distance between residues 396 on the two NR1 subunits is greater than 100 Å. Similarly, to measure the distance between 404 on one NR2A subunit and the same residue on the second NR2A subunit, the ΔNR1*:ΔNR2A*Asn-404Histag/ΔNR2A*N404C-Th construct was used and tagged with terbium chelate:(Ni-NTA)2-Cy3 chelate donor:acceptor pair. The LRET lifetime for this construct (Fig. 2B) could be well represented by a single exponential decay, and the distance obtained from this lifetime was 60 Å. The LRET lifetime for the ΔNR1*396Histag:ΔNR2A*N404C-Th receptor tagged with terbium chelate:(Ni-NTA)2-Cy3, used to measure the distance between residue 396 on NR1 and residue 404 on NR2A, required a two-exponential fit (Fig. 2C). The two lifetimes indicate two distances between the NR1 and NR2A subunits. The LRET-based distances at the N terminus of the agonist binding domain are compared with the distances for equivalent residues on the full-length AMPA receptor structure for the three possible configurations in Table 2. The longer distance between the agonist binding domain N terminus in the NR1 subunits and the shorter distance between the agonist binding domain of the NR2 subunit is consistent with the configuration shown in Fig. 1A. To further confirm that the NMDA receptors are assembled in the configuration shown in Fig. 1A, we also measured the distance between residue 525 on one NR1 subunit with respect to the same residue on the second NR1 subunit. This distance changes significantly between the three possible configurations (Table 2) and hence can be differentiated in the LRET investigations. For measuring this distance, the ΔNR1*Th-Q525C-Th:ΔNR2A* construct was used and labeled with a 1:1 ratio of terbium chelate and fluorescein. The LRET lifetime could be well represented by a single exponential decay, suggesting primarily a single configuration for the NMDA receptor (Fig. 2D). Additionally, the LRET-based distance of 51 Å is closest to the distance of 49 Å seen in the configuration shown in Fig. 1A, thus revealing that the NMDA receptors are primarily in the configuration shown in Fig. 1A.
FIGURE 2.
A, LRET lifetimes for ΔNR1*Thr-396Histag/ΔNR1*T396C-Th:ΔNR2A* labeled with terbium chelate:(Ni-NTA)2Cy3 as measured by the sensitized emission of acceptor at 575 nm in the presence of saturating concentrations of agonists. No LRET signal was observed. B, LRET lifetimes for ΔNR1*:ΔNR2A*Asn-404Histag/ΔNR2A*N404C-Th labeled with terbium chelate:(Ni-NTA)2Cy3 as measured by the sensitized emission of acceptor at 575 nm with saturating concentrations of agonists. C, LRET lifetimes measured at 575 nm for ΔNR1*Thr-396Histag:ΔNR2A* N404C-Th labeled with terbium chelate:(Ni-NTA)2Cy3 under saturating concentrations of agonists. D, LRET lifetimes measured at 515 nm for ΔNR1*Th-525C-Th:ΔNR2A* labeled with terbium chelate:fluorescein at saturating concentrations of agonists. For all mutants, the sensitized lifetime shown is the difference between the lifetimes obtained before and after thrombin digestion. The residuals for the lifetime fits are shown below for each measurement (the y axis is in linear scale).
TABLE 2.
Comparison of distances between desensitized state LRET measurements to antagonist-bound crystal structure of AMPA receptor and the other possible configurations
| NMDA/homologous AMPA receptor | Distance based on LRET | AMPA receptor (PDB-3KG2) |
||
|---|---|---|---|---|
| Distance based on configuration A (Fig. 1A) | Distance based on configuration B (Fig. 1B) | Distance based on configuration C (Fig. 1C) | ||
| Å | Å | Å | Å | |
| NR1–396 to NR2A-404/AMPA-393 to AMPA-393 | 72.5 | 67 | 67 | 56,102 |
| NR1–396 to NR1–396/AMPA-393 to AMPA-393 | >100 | 101 | 56 | 72 |
| NR2A-404 to NR2A-404/AMPA-393 to AMPA-393 | 60 | 56 | 102 | 67 |
| NR1–525 to NR1–525/AMPA-487 to AMPA-487 | 51 | 49 | 82 | 65 |
The configuration established by the LRET-based distances is consistent with the structure that was predicted based on the AMPA receptor full-length structure and the cross-linking experiments where it has been shown that residue Glu-699 on NR1 subunits cross-links with the corresponding residue Glu-699 on the second NR1 subunit. Additionally, no cross-linking was observed between Lys-641 or Arg-645 and Asn-675/Glu-699 (in NR1) and Lys-641 or Arg-645 (in NR2), as would be expected for configurations in Fig. 1, B and C (1). These cross-linking investigations, however, focused on the interactions of the subunits in the domain 2 of the agonist binding segments. The LRET investigations and hence the distances are obtained on domain 1 of the agonist binding domain. Hence the distances, in addition to confirming the arrangement, also validate the twist seen in the structure wherein the domains 2 of the agonist binding domain of the NR1 subunits are closer than the domains 2 of the NR2 subunits, whereas the domains 1 of the agonist binding domains of NR2 subunits are in closer proximity relative to domains 1 of the NR1 subunits (Fig. 1A). This twist ultimately results in the crossover in the dimer interactions seen in the N-terminal domains of the AMPA receptors (1).
Distances within the Dimer Interface
In addition to determining the distances between the dimer, we have also used LRET to determine the distance between NR1 and NR2A subunits within the dimer. Two sets of residues expected to be in close proximity in the dimer interface 525 on NR1 and 777 on NR2, as well as 775 on NR1 and 519 on NR2, were used to study the changes within the dimer (Fig. 3). To selectively measure the distance within the dimer and not across the dimer, a cysteine was introduced on one subunit and a tetrahistidine tag was introduced on the second, and were tagged with a maleimide derivative of terbium chelate to serve as the donor and Ni2+ ion (R0 is 12 Å) to serve as the acceptor. The short R0 ensured that there was no transfer from the residues across the dimer interface. Additionally, the short R0 also ensured that the nonspecific labeling of cysteine residues far from the histidine tag does not contribute toward the LRET signal. Hence in the case where short distances were measured, it was not necessary to introduce a thrombin cleavage site. The LRET lifetimes for ΔNR1*525tetrahistag:ΔNR2A*L777C and ΔNR1*775tetrahistag:ΔNR2A*S519C (Fig. 4) could be well represented by a single exponential decay, and the corresponding distances are shown in Table 1. The distances within the dimer obtained with the LRET investigations are compared with the crystal structure distances for the NR1-NR2A dimer in Table 3. The LRET distances for all the constructs were obtained in the presence of saturating concentrations of glutamate and glycine; hence the distances represent primarily the desensitized form of the receptor. The crystal structures of the isolated ligand binding domain of the NR1-NR2A dimer, on the other hand, lack the transmembrane domains that drive the formation of the desensitized state and hence most likely represent the structure of the dimer in the open channel state of the protein and not the desensitized state. The longer distances in the desensitized state of the NMDA receptor relative to the distances expected for the open channel state of the receptor suggest that there is a decoupling of the dimer interface in the desensitized state. To further establish that such a comparison can be made between the LRET investigations and the x-ray structures, we have measured the distance within a given subunit using the ΔNR1*T396C-Th, A715C:ΔNR2A* tagged with terbium chelate, and ATTO 465 as donor and acceptor. This construct allows us to determine the distance between residue 396 and 715 in the NR1 subunit. The LRET lifetime is well represented with a single exponential with the distance corresponding to 35.1 Å, which is similar to the distance of 34.3 Å observed in the crystal structure (Fig. 5). It should be noted that the efficiency of transfer from the second NR1 subunit is expected to be 0.2% for transfer between 396 and 396 and 3.3% for transfer between 715 and 715 for the terbium chelate and ATTO 465 as donor and acceptor, and hence these do not contribute to the LRET signal. The desensitized LRET-based distances are also compared with the changes in distances observed for homologous sites in the desensitized-like crystal structure of S729C AMPA and the cyclothiazide-bound open channel crystal structure of the AMPA receptor (Table 3). The changes in distances between the desensitized and open channel state across the dimer for the NMDA receptors are similar to that observed for the AMPA receptors. This similarity further confirms that the dimer interface is decoupled in the NMDA receptors and also provides the evidence for a similar mechanism for desensitization in the NMDA receptors as seen in the AMPA receptors.
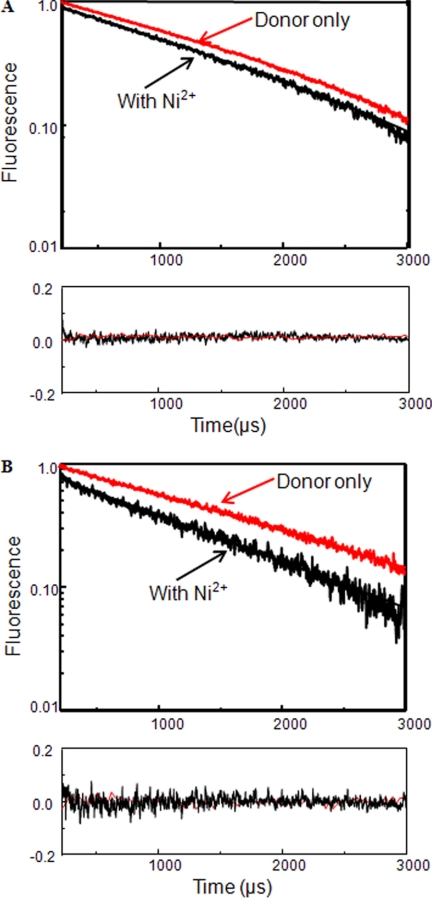
FIGURE 4.
A, LRET lifetimes for ΔNR1*775tetrahistag:ΔNR2A*S519C labeled with terbium chelate before (Donor only) and after the addition of Ni2+. B, LRET lifetimes for ΔNR1*525tetrahistag:ΔNR2A*L777C labeled with terbium chelate before (Donor only) and after the addition of Ni2+. The residuals for the above lifetime fits are shown below each measurement, and the y axis is in linear scale.
TABLE 3.
Comparison of changes in distances between desensitized state and open channel state of NMDA and AMPA receptors
| Protein | NMDA receptor |
Protein | AMPA receptor |
||||
|---|---|---|---|---|---|---|---|
| Distance based on LRET desensitized | Distance in X-ray structure PDB 2A5T; open (9) | Difference between LRET and X-ray structure (PDB 2A5T) (9) | Distance in X-ray structure (PDB 2I3W); desensitized (18) | Distance in X-ray structure (Å) (PDB 1LBC); open (12) | Difference between X-ray structures PDB 2I3W (18) and PDB1LBC (12) | ||
| Å | Å | Å | Å | Å | Å | ||
| NR1–396 to NR2A-404 | 49.7 | 41.5 | 8.2 | GluR2–393 to GluR2–393 | 54 | 46 | 8 |
| NR1–525 to NR2A-777 | 13.3 | 7 | 6.3 | GluR2–487 to GluR2–748 | 17 | 9 | 8 |
| NR1–775 to NR2A-519 | 14.9 | 7 | 7.9 | GluR2–484 to GluR2–748 | 17 | 8 | 9 |
| NR1–396 to NR1–715 | 35.1 | 34.3 | 0.8 | GluR2–393 to GluR2–687 | 35.2 | 34.5 | 0.70 |
FIGURE 5.
LRET lifetimes for ΔNR1*T396C-Th, A715C:ΔNR2A* labeled with terbium chelate:ATTO 465 as measured by the sensitized emission of acceptor at 510 nm under saturating concentrations of agonists. The donor:acceptor lifetime shown here is the difference between the lifetimes obtained before and after thrombin digestion. The residuals are shown below the lifetime measurement, and the y axis is in linear scale.
This work was supported, in whole or in part, by National Institutes of Health Grant R01GM073102 (to V. J.). This work was also supported by American Heart Association Grant 0855081F.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
- NMDA
- N-methyl-d-aspartic acid
- NR1
- N-methyl-d-aspartic acid receptor subunit 1
- NR2A
- N-methyl-d-aspartic acid receptor subunit 2A
- AMPA
- α-amino-5-methyl-3-hydroxy-4-isoxazole propionic acid
- LRET
- luminescence resonance energy transfer
- (Ni-NTA)2-Cy3
- biscyanine derivative of nickel-nitrilotriacetic acid
- Th
- thrombin.
REFERENCES
- 1.Sobolevsky A. I., Rosconi M. P., Gouaux E. (2009) Nature 462, 745–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hollmann M., O'Shea-Greenfield A., Rogers S. W., Heinemann S. (1989) Nature 342, 643–648 [DOI] [PubMed] [Google Scholar]
- 3.Keinänen K., Wisden W., Sommer B., Werner P., Herb A., Verdoorn T. A., Sakmann B., Seeburg P. H. (1990) Science 249, 556–560 [DOI] [PubMed] [Google Scholar]
- 4.Moriyoshi K., Masu M., Ishii T., Shigemoto R., Mizuno N., Nakanishi S. (1991) Nature 354, 31–37 [DOI] [PubMed] [Google Scholar]
- 5.Johnson J. W., Ascher P. (1987) Nature 325, 529–531 [DOI] [PubMed] [Google Scholar]
- 6.Watanabe M., Inoue Y., Sakimura K., Mishina M. (1992) Neuroreport 3, 1138–1140 [DOI] [PubMed] [Google Scholar]
- 7.Cull-Candy S., Brickley S., Farrant M. (2001) Curr. Opin. Neurobiol. 11, 327–335 [DOI] [PubMed] [Google Scholar]
- 8.Waxman E. A., Lynch D. R. (2005) Neuroscientist 11, 37–49 [DOI] [PubMed] [Google Scholar]
- 9.Furukawa H., Singh S. K., Mancusso R., Gouaux E. (2005) Nature 438, 185–192 [DOI] [PubMed] [Google Scholar]
- 10.Armstrong N., Sun Y., Chen G. Q., Gouaux E. (1998) Nature 395, 913–917 [DOI] [PubMed] [Google Scholar]
- 11.Armstrong N., Gouaux E. (2000) Neuron 28, 165–181 [DOI] [PubMed] [Google Scholar]
- 12.Sun Y., Olson R., Horning M., Armstrong N., Mayer M., Gouaux E. (2002) Nature 417, 245–253 [DOI] [PubMed] [Google Scholar]
- 13.Jin R., Banke T. G., Mayer M. L., Traynelis S. F., Gouaux E. (2003) Nat. Neurosci. 6, 803–810 [DOI] [PubMed] [Google Scholar]
- 14.Gouaux E. (2004) J. Physiol. 554, 249–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayer M. L. (2005) Curr. Opin. Neurobiol. 15, 282–288 [DOI] [PubMed] [Google Scholar]
- 16.Du M., Reid S. A., Jayaraman V. (2005) J. Biol. Chem. 280, 8633–8636 [DOI] [PubMed] [Google Scholar]
- 17.Mankiewicz K. A., Rambhadran A., Du M., Ramanoudjame G., Jayaraman V. (2007) Biochemistry 46, 1343–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong N., Jasti J., Beich-Frandsen M., Gouaux E. (2006) Cell 127, 85–97 [DOI] [PubMed] [Google Scholar]
- 19.Ramanoudjame G., Du M., Mankiewicz K. A., Jayaraman V. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 10473–10478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oswald R. E., Ahmed A., Fenwick M. K., Loh A. P. (2007) Curr. Drug Targets 8, 573–582 [DOI] [PubMed] [Google Scholar]
- 21.Mankiewicz K. A., Rambhadran A., Wathen L., Jayaraman V. (2008) Biochemistry 47, 398–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez J., Rambhadran A., Du M., Jayaraman V. (2008) Biochemistry 47, 10027–10032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rachline J., Perin-Dureau F., Le Goff A., Neyton J., Paoletti P. (2005) J. Neurosci. 25, 308–317 [DOI] [PMC free article] [PubMed] [Google Scholar]