Abstract
Pilot data showed that adding intratumoral (IT) injection of dendritic cells (DCs) prolongs survival of patients affected by glioblastoma multiforme (GBM) treated by subcutaneous (SC) delivery of DCs. Using a murine model resembling GBM, we investigated the immunological mechanisms underlying this effect. C57BL6/N mice received brain injections of GL261 glioma cells. Seven days later, mice were treated by 3 SC injections of DCs with or without 1 IT injection of DCs. DC maturation, induced by pulsing with GL261 lysates, was necessary to develop effective immune responses. IT injection of pulsed (pDC), but not unpulsed DCs (uDC), increased significantly the survival, either per se or in combination with SC-pDC (P < .001 vs controls). Mice treated by IT-pDC plus SC-pDC survived longer than mice treated by SC-pDC only (P = .03). Injected pDC were detectable in tumor parenchyma, but not in cervical lymph nodes. In gliomas injected with IT-pDC, CD8+ cells were significantly more abundant and Foxp3+ cells were significantly less abundant than in other groups. Using real-time polymerase chain reaction, we also found enhanced expression of IFN-γ and TNF-α and decreased expression of transforming growth factor-beta (TGF-β) and Foxp3 in mice treated with SC-pDC and IT-pDC. In vitro, pDC produced more TNF-α than uDC: addition of TNF-α to the medium decreased the proliferation of glioma cells. Overall, the results suggest that IT-pDC potentiates the anti-tumor immune response elicited by SC-pDC by pro-immune modulation of cytokines in the tumor microenvironment, decrease of Treg cells, and direct inhibition of tumor proliferation by TNF-α.
Keywords: dendritic cells, glioma, intratumoral vaccination, tumor microenvironment
Strategies for cancer immunotherapy critically rely on the use of dendritic cells (DCs) for antigen presentation in peripheral lymph nodes, where CD8+ T lymphocytes are instructed to initiate a cytolitic anti-tumor response.1 Such a response, however, has to develop in a tumor microenvironment that is strongly characterized by the presence of diverse immunosuppressive factors, allowing the tumor to escape immune surveillance:2 brain tumors provide an excellent example of this scenario.3 Several clinical experiences have been reported for DC immunotherapy of malignant gliomas and specifically of glioblastoma multiforme (GBM), the most frequent and aggressive of primary brain tumors.4 Results in terms of safety have been satisfactory but clinical efficacy, especially when treating relapses of GBM, can certainly be increased.5 The production of different immune suppressive cytokines, particularly transforming growth factor-beta (TGF-β), and the presence of CD4 + CD25 + Foxp3+ T cells (T-regulatory cells, Treg) has been correlated to evidence of low or decreased efficacy of DC immunotherapy in preclinical models and in patients.6–9 Thus, combined strategies linking peripheral immune responses to a modification of the tumor microenvironment are desirable in order to improve the clinical potential of DC immunotherapy.
Direct intratumoral (IT) injection of unpulsed DCs (uDC) has been attempted in different tumors, including GBM:10–17 the results may encourage further investigations on the molecular and cellular modifications that the local presence of DCs implies for cancer biology. We have done this in a murine model of malignant glioma obtained by brain injection of GL261 cells,18 studying the effects of IT injection of pulsed DC (pDC) with tumor lysate in established GL261 tumors. Results suggest that IT-DCs do not migrate to cervical lymph nodes and increase survival significantly in mice when combined with peripheral vaccination.
This enhanced efficacy, therefore, may be due to direct, significant changes in the tumor microenvironment, which are modulated by IT-pDC combined with the increased recruitment of specific T cells induced by SC-pDC. These data may have clinical implications for future clinical trials in patients affected by GBM and malignant gliomas.
Material and Methods
Cell Cultures
GL261 cells were grown in DMEM (EuroClone), 20% fetal bovine serum, l-glutamine, and penicillin/streptomycin. Immature DCs were prepared from the bone marrow (BM) of C57BL6/N mice and from C57BL/6-TgN(ACTbEGFP) transgenic mice, both syngeneic to GL261 cells. Briefly, mice were sacrificed and BM was flushed from the femur and tibia. Cells were cultured in 6-well plates for 5 days in Iscove's medium (Sigma Aldrich, St. Louis, MO) in the presence of 5 ng/mL rmGM-CSF and 5 ng/mL rmIL-4 (Li StarFISH, Milan, Italy). After 3 days, 70% of the medium was replaced with fresh medium containing GM-CSF and IL-4. For cytokine assays, culture supernatants were collected on day 5 (immature DC) or on day 6 after pulsing with tumor lysate (mature DC). Concentrations of IL-6 (R&D Systems), TNF-α, and IFN-γ (T1/Th2 Kit, CBA system, Becton Dickinson, San Diego, CA) were defined according to manufacturer's instructions.
DC phenotype was characterized using the anti-mouse CD11c, CD80, antibodies CD86, MHC class I (H2b), class II (Ia), and CD40 (Pharmingen, San Diego).
T-cell Monitoring
The spleen and lymph nodes were analyzed for T-cell subsets using anti-mouse CD3-FITC, CD4-PE-Cy5, CD8-PE, and CD25-FITC, all from Pharmingen and FoxP3-PE, from eBioscience (San Jose, CA). FACS analyses were conducted on an FACSCalibur flow cytometer (Becton Dickinson).
Ipsilateral or contralateral cervical or axillary lymph nodes constituted four separate groups. Two or three lymph nodes from each group were removed from each mouse (n = 4 for each group) at each time point (ie, day 15, 24, and 31 after GL261 cells injection). Cell suspensions were prepared from pooled lymph nodes from each group by gentle mechanical disaggregation and counted. The number of viable cells per group of lymph nodes was determined using trypan blue exclusion and ranged from 3 to 6 million cells. The total number of lymphocytes in the different groups of lymph nodes obtained from treated mice was comparable with that of control mice.
Cell staining for phenotypic analysis was carried out as described by the eBioscience protocol.
Briefly, 1 million cells were surface stained with anti-mouse CD4-PE-Cy5 and anti-mouse CD25-FITC and subsequently with anti-mouse Foxp3-PE (FJK-16s) or isotype control. About 500 000 cells were stained for CD3/CD8 (co-staining).
DC Labeling and Loading
DCs from C57BL6/N were labeled by superparamagnetic iron oxide nanoparticles (Endorem; Guebert, Genova) before pulsing with GL261 lysate. On day 5, Endorem (15 µg/mL) was added to the culture medium and incubated for 24 h at 37°C, 5% CO2. Higher concentrations of Endorem induced nonspecific maturation of DCs (data not shown). On day 6 after labeling, DCs were loaded using tumor lysate obtained by sonication of 1 × 107 GL261 cells. DCs were pulsed as described by Ashley et al.19 Cell surface markers such as MHC class I and II, CD80, CD86, CD40 (Pharmingen) were evaluated on labeled DCs before (uDC + Endorem) and after pulsing (pDC+ Endorem) and compared with unlabeled DCs.
DC Vaccinations
A total of 100 C57BL6/N mice received brain injections of 1 × 105 GL261 cells (coordinates with respect to the bregma: 0.7 mm posterior, 3 mm left lateral, 3.5 mm deep, into the nucleus caudatum). On day 7 after tumor implantation, mice were treated by subcutaneous (SC) injections of 1 × 106 pDC (3 injections, each spaced 1 week) apart with or without one IT injection of 2 × 105 pDC or uDC, respectively (IT injection was performed once, at the same time of the first SC injection). DCs were injected into the tumor using the same stereotactic coordinates. For the survival studies, DCs injected were GFP positive and experimental groups were as follows: PBS only as control (n = 15); IT-uDC (n = 10); IT-pDC (n = 10); SC-pDC (n = 31); IT-uDC plus SC-pDC (n = 10); IT-pDC plus SC-pDC (n = 14). For monitoring studies, DCs injected IT or SC alone were GFP or Endorem positive to track the potential migration toward peripheral lymph nodes, while DCs SC injected for the other combination of treatments were obtained from naïve mice to allow the identification of potential IT-DC migration toward the lymph nodes.
Real-Time Polymerase Chain Reaction
RNA for real-time polymerase chain reaction (RT-RCR) from paraffin-embedded material was extracted using reagents included in the Absolutely RNA FFPE kit (Stratagene, San Diego, CA). cDNA was synthesized from the RNA using oligo(dT) and M-MLV Reverse Transcriptase (Life Technologies). PCR was performed using a GeneAmp 5700 Sequencer Detector (Applied Biosystems, CA). Gene-specific oligonucleotide probes were included in the Assay on Demand Kit (Applied Biosystems). Amplification values obtained for each gene used were normalized to the endogenous control β-2-microglobulin.
Histology and Immunohistochemistry
A representative number of brain and lymph nodes from each group were embedded in OCT, snap frozen in cold isopenthane, and stored in liquid nitrogen or post-fixed in 4% paraformaldehyde for paraffin embedding. Frozen sections were analyzed using anti-CD8 antibodies (Novocastra Laboratories), whereas paraffin-embedded sections were analyzed with rat monoclonal IgG1 anti-CD3, anti-CD4 (1:20, Novocastra Laboratories); and anti-Foxp3 (eBioscience) antibodies.
Quantification of Tumor Infiltrating Lymphocytes by Immunohistological Analysis
The absolute cell number of CD8 + , CD4 + , and Foxp3 + total infiltrating lymphocytes (TILs) were calculated in 5 independent high power fields corresponding to 0.2 mm2 for a total of 1 mm2 of tumor area for each samples. Positive cells were counted only within the tumor area (TILs) with the exclusion of peritumoral lymphocytes. Two representative mice for each treatment group at both time points have been compared: IT-pSC plus SC-pDC, SC-pDC, and control on day 15 and 24 after tumor implantation. Results have been expressed as mean number of positive cells ± SD for each group. The positive rates were counted thrice manually from the photographs by two observer (M.R. and P.L.P.). Student's t-test was performed for evaluating the significance of data. Statistical significance was determined at the ≤.05 level.
Statistical Analysis
Survival estimates and median survivals were determined using the Kaplan–Meier method. Student's t-test was used for calculating the significance of data. Statistical significance was determined at the <.05 level and investigated using MedCalc, version 9.3.
Results
IT Injection of pDC Increases Survival of Mice Bearing GL261 Glioma
The anti-tumor efficacy of IT injection of DCs was evaluated per se or in combination with SC injection of DCs in immune-competent C57BL6/N mice with established GL261 tumors. Kaplan–Meier analysis of survival is shown in Fig. 1A and B and in Supplementary Material, Table S1. All controls (tumor-bearing mice treated with PBS) died by day 35. Treated mice were monitored for 200 days. SC-uDC injection had no therapeutic effect and mice survived as long as controls. IT-uDC injection had a minor effect on survival, as only 10% of mice survived the tumor challenge (P < .02). IT injection of pDC, however, extended survival to 30% of the mice (P < .001 vs control).
Fig. 1.
IT injection of pDC enhances anti-tumor efficacy of peripheral administration of DC. Kaplan–Meier survival curves represent the percentage of surviving animals over time (in days). Mice received 1 × 105 GL261-glioma cells IT on day 0 and were treated with three SC injections of 1 × 106 pDC spaced 1 week apart and/or one IT injection of 2 × 105 GL261 Lysate-LPDC or UDC on day 7 after tumor implantation. The experimental conditions were: control mice received PBS (n = 15, reported in [A] and [B]); (A) IT-pDC (n = 10; P = .001 vs PBS); IT-uDC (n = 10; P = .02 vs PBS); IT-uDC plus SC-pDC (n = 10; P < .001 vs PBS); SC-uDC (n = 10, P = .2); (B) SC-pDC (n = 31, P < .0001 vs PBS); IT-pDC plus SC-pDC (n = 14; P < .0001 vs PBS). We divided the survival curves for clarity. Control curves are indeed the same. In Supplementary Material, Table S1, all P-values for treatment groups were reported vs control and vs each other group.
Treatment with SC-pDC led to survival of 50% of the mice (P < .001 vs control): combining IT-uDC left unchanged the anti-tumor efficacy of SC-pDC. In contrast, combination with IT-pDC increased survival to 78% of the mice. The survival rate of IT-pDC plus SC-pDC mice was significantly higher than that of control mice (P < .0001) and, interestingly, of SC-pDC mice (P = .03).
These data demonstrate that DC pulsing with tumor lysate is necessary to develop an immune-based, effective anti-tumor response in the GL261 model of glioma and that IT injection of pDC is effective per se and significantly increases the efficacy of SC-pDC.
After Injection into Brain Gliomas, DCs Remain Viable and Active
As a first step in the study of the effects of IT DCs, we investigated their fate by monitoring the distribution of Endorem-loaded or GFP-positive DCs injected into the tumor mass. DC labeling by Endorem (see Materials and Methods) did not change significantly the expression of relevant maturation markers, as evaluated by flow cytometry, in comparison with GFP or naïve DC (Supplementary Material, Fig. S1A, C, and D). Histological staining with anti-GFP antibody revealed the presence of DC-GFP in the tumor a mass of vaccinated IT-pDC plus SC-pDC mice and of direct interaction between DC-GFP and tumor cells (Fig. 2A and B).
Fig. 2.
Intra-tumoral detection of pDC labeled by GFP or Endorem. DC-GFP can interact with tumor cells (A) and contact them with long spiky arms (B). Histological evaluations using Prussian Blue staining revealed iron in DC loaded with Endorem ex vivo 1 week after IT injection (C). Loaded DC can interact with tumor infiltrating, CD3+ lymphocytes (D).
Prussian Blue staining showed Endorem-loaded DCs in the tumor bulk and evidence of an interaction with T cells (Fig. 2C and D). These studies also suggested that transplanted DCs were viable for 1 week after IT injection. Analysis of cervical lymph nodes in 8 mice 1 week after DC injection, however, failed to detect Endorem-positive (n = 4 mice) or GFP-positive DCs (n = 4 mice). The persistence of pDC into the tumor, their interaction with tumor infiltrating CD3+ lymphocytes (Fig. 2D), and the absence of DC in regional lymph nodes all suggest that DCs did not migrate a long distance from their injection site into the brain, implying that their biological activity was mostly mediated in situ.
Increase of CD8+ Cytotoxic Cells and Decrease of Treg in Gliomas of IT-pDC Vaccinated Mice
Tumor infiltrating lymphocytes were characterized by immunohistochemistry performed 15 and 24 days after implantation of GL261 cells. Quantitative evaluation of the data is summarized in Table 1: the statistical analysis comparing treated groups and controls showed significant differences.
Table 1.
Quantitative analysis of TILs by immunohistochemistry
| Treatment | Tumor size (mm)a | Tumor infiltrating lymphocytesb |
|||
|---|---|---|---|---|---|
| CD3 | CD4 | CD8 | Foxp3 | ||
| IT-pDC + SC-pDC (15 days) | 2 | 34.5 ± 3.7**** | 19.1 ± 2.6** | 30.6 ± 4.1**** | 7.0 ± 1.4* |
| SC-pDC (15 days) | 2 | 19.7 ± 3.0*** | 21.5 ± 1.9*** | 15 ± 3.1*** | 8.9 ± 1.6 |
| Control (15 days) | 3 | 13 ± 3.2 | 14.3 ± 2.9 | 9.1 ± 2.1 | 8.5 ± 1.5 |
| IT-pDC + SC-pDC (24 days) | 3 | 29.5 ± 3.9*** | 16.4 ± 2.4** | 23.9 ± 2.3**** | 8.3 ± 2.1* |
| SC-pDC (24 days) | 5 | 19.9 ± 2.9** | 17.8 ± 2.7** | 12.2 ± 1.9*** | 15.4 ± 2.1* |
| Control (24 days) | 6 | 15.3 ± 3.3 | 12.7 ± 1.9 | 7.9 ± 2.0 | 13.3 ± 2.5 |
aMajor tumor diameter from a representative mouse for each group.
bAverage ± standard deviation of positive cells in 5 areas/mouse per group (10 areas/group). From each group two representative mice were analyzed (number of positive cells/5 different 40× HPF).
****P < 10−12; ***P < 10−8; **P < 10−4; *P < .05.
At both time points we found a significant increase of CD8+ T cells in tumors treated with IT-pDC plus SC-pDC compared with SC-pDC only (P < 10−8) or controls (P < 10−11). On day 15 a significant decrease of CD4+ cells in tumor was present in IT-pDC plus SC-pDC compared with SC-pDC (P < .01) or with control (P < 10−4). The differences in TIL composition were more evident on day 24 after tumor implantation. In particular, controls showed very few CD3+ and CD8+ T cells compared with treated tumors. Foxp3+ cells appeared significantly less in tumors from mice treated with IT-pDC plus SC-pDC than SC-pDC only (P = .01 on day 15; P < 10−6 on day 24 ) or controls (P = .05) Interestingly, no significant difference was observed in the Foxp3 expression among SC-pDC or control groups. Examples of histological evaluation are shown in Figs. S2 and S3 (Supplementary Material).
The distribution of TILs was characteristic on day 24 after GL261 cell injection (Supplementary Material, Fig. S3). Interestingly, while control and SC-pDC mice showed several CD4 + Foxp3+ cells preferentially dispersed in the tumor parenchyma, IT-pDC plus SC-pDC mice showed a peculiar perivascular distribution of the CD4 + Foxp3+ cells.
Tumor diameters were measured at both time points in all mice investigated (4 for each group). Mice injected IT with pDC exhibited delayed tumor growth, compared with control and SC-pDC mice, in agreement with survival data (Table 1).
IT DCs Modulate the Glioma Microenvironment by Decreasing TGF-β and Increasing TNF-α and IFN-γ Expression
To characterize the effect of IT injection of pDC on the tumor microenvironment, we investigated the expression of key molecules for immune suppression (TGF-β, Foxp3) or activation (IFN-γ and TNF-α) by RT-PCR on paraffin-embedded tumors from treated and control mice. These mice were the same also investigated by immunohistochemistry (Supplementary Material, Figs. S2 and S3). We found that in mice treated by SC-pDC only compared with those treated by IT-pDC plus SC-pDC TGF-β expression was 2.2 (P = .009, day 15) and 1.6-fold higher (P = .01 day 24), respectively (Fig. 3A), and Foxp3 expression was 2.6 (P = .007 day 15) and 1.2-fold (P = .03 day 24) higher (Fig. 3B). IFN-γ expression, on the contrary, was 2-fold higher in IT-pDC plus SC-pDC than in SC-pDC only (P = .03 on day 15; P = .002 on day 24) (Fig. 3C) and TNF-α was 3.8-fold higher on day 15 (P = .03) and 1.5-fold on day 24 (P = .07) (Fig. 3D).
Fig. 3.
Intra-tumoral levels of TGF-β, Foxp3, IFN-γ, and TNF-α. Tumors from control and treated mice were studied for the expression of TGF-β, Foxp3, IFN-γ, and TNF-α by RT–PCR at two different time points. Histograms show the evaluation of tumor environment 15 and 24 days after tumor implantation. (A) and (B) IT-pDC plus SC-pDC–treated mice TGF-β and Foxp3 expression is significantly lower than in SC-pDC–treated mice (TGF-β P = .009 and P = .01; Foxp3 P = .007 and P = .03, on day 15 and 24, respectively). (C) and (D) The same mice with decreased expression of TGF-β corresponded to higher expression of IFN-γ in IT-pDC plus SC-pDC–treated mice compared with SC-pDC–treated mice, particularly 24 days after tumor implantation (P = .03, 15 days; P = .002, 24 days). In contrast, on day 15, TNF-α expression was significantly higher in IT-pDC plus SC-pDC–treated mice than in SC-pDC–treated mice (P = .005, 15 days; P = .07, 24 days). Relative expression of cytokines in vaccinated mice was compared with controls.
The cytokine concentration was also evaluated by RT-PCR on frozen tumors from the same groups of mice on day 15 and 24: results confirmed the expression patterns described above (data not shown).
TNF-α Released by Mature DCs Decreases Proliferation of GL261 Glioma Cells
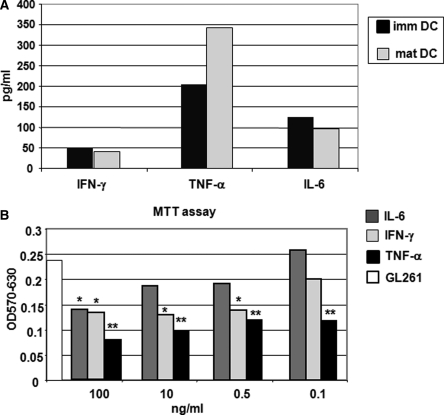
The to gain hints on the direct contribution of IT-pDC to tumor microenvironment, we studied cytokine production of freshly isolated BM-derived DC-GFP. For this, we used conditioned media from immature DCs (ie, DC cultured for 5 days in the presence of GM-CSF and IL-4) and mature DCs (ie, 24 hours after pulsing with tumor lysate). Maturation was defined according to phenotypic analysis by flow cytometry, considering significantly increased expression of MHC class I and II, co-stimulatory molecules, and CD40 (Supplementary Material, Fig. S1A). Three key cytokines produced by mature DCs were considered: TNF-α, IFN-γ, and IL-6.20–23 pDCs produced more TNF-α, but not IFN-γ or IL-6, than uDCs (Fig. 4A). The same evaluation was performed on immature and mature naïve DCs loaded with Endorem: the concentration of secreted cytokines was similar (data not shown). To define the effect of TNF-α, IFN-γ, and IL-6 on tumor proliferation, we cultured GL261cells for 24 hours in the presence of “physiological” (100 or 500 pg/mL) or “supra-physiological” (10 or 100 ng/mL) concentrations of each of these cytokines. The MTT assay showed a significant reduction of GL261 proliferation only at “supra physiological” concentrations of IL-6 (100 ng/mL; P = .001) and IFN-γ (P < .001), but not in the presence of 100 pg/mL. TNF-α–treated cells, however, showed a significant reduction in GL261 proliferation also at “physiological” concentrations, suggesting that TNF-α is very effective at inhibiting GL261 growth in vitro and, possibly, in vivo (Fig. 4B).
Fig. 4.
Mature DCs produce “therapeutic” levels of TNF-α. For quantitative determination of cytokines produced by DCs, we tested the secretion of IL-6, TNF-α, and IFN-γ. The histograms in (A) show cytokine secretion by immature DCs (before pulsing) and mature DCs (24 hours after pulsing). Mature DCs (24 hours after pulsing) produce a higher level of TNF-α (mDC 343 pg/mL vs immDC 204 pg/mL); levels of IL-6 and IFN-γ were similar to immature DCs. (B) The histogram shows a significant decrease of cell proliferation observed after exposure to each concentration of TNF-α (black histogram, P < .0003). The exposure to IFN-γ decreased GL261 proliferation at higher concentrations (light grey histogram, P < .001) but not at 0.1 ng/ml (P = .2). The dark grey histograms show that GL261 were sensitive only to the highest concentration of IL-6 (100 ng/ml; P = .001).
SC-pDCs Induce the Generation of GL261-specific Effector Lymphocytes
To characterize the direct effects of treatment on T-cell function, the spleen and draining lymph nodes were harvested, counted for total number of cells, and monitored by flow cytometry 15 and 24 days after tumor implantation.
Splenocytes for in vitro cytotoxicity studies were monitored before and after prestimulation with GL261 in controls (n = 2 on day 15, n = 2 on day 24), IT-pDC plus SC-pDC (n = 4 on day 15, n = 3 on day 24), SC-pDC only (n = 4 on day 15, n = 3 on day 24), and one healthy mouse (Table 2).
Table 2.
Flow cytometry monitoring of GL261-specific T cells from splenocytes of controls and vaccinated mice before and after GL261 prestimulation on day 15 and 24 after tumor injection
| Time points | Treatment | % CD3 +/CD8 + |
% CD4 + CD25- |
% CD4 +/CD25 + |
JAM-test | |||
|---|---|---|---|---|---|---|---|---|
| Splenocytes | Prestimulated splenocytes | Splenocytes before prestimulation | Splenocytes after prestimulation | Splenocytes | Splenocytes after | E/T 100/1 % lysis | ||
| 15 days after tumor injection | IT-pDC + SC-pDC1 | 5.8 | 28.7 | 15.2 | 30.5 | 8 | 5 | 40.1 |
| IT-pDC + SC-pDC2 | 10.3 | 34.7 | 16 | 31 | 10 | 6 | 40.6 | |
| IT-pDC + SC-pDC3 | 11.8 | 47 | 18 | 36 | 10 | 8 | 27.3 | |
| IT-pDC + SC-pDC4 | 25 | 49 | 18 | 42 | 11 | 5 | 17.6 | |
| SC-pDC1 | 13.3 | 33 | 12.2 | 28.7 | 12.8 | 7 | 30.4 | |
| SC-pDC2 | 8.6 | 47 | 12.4 | 23.8 | 12 | 8 | 31.7 | |
| SC-pDC3 | 10.2 | 30.6 | 22.6 | 27 | 12 | 11 | 25.6 | |
| SC-pDC4 | 14.8 | 29.8 | 21.6 | 31.1 | 12 | 8 | 20.1 | |
| CTRL1 | 8.9 | 20.1 | 18.7 | 26.6 | 16.5 | 8 | 10 | |
| CTRL2 | 15.5 | 8.2 | 15.3 | 19.4 | 12 | 12.2 | 11.2 | |
| 24 days after tumor injection | IT-pDC + SC-pDC5 | 11 | 44 | 18 | 49 | 13 | 6.8 | 81 |
| IT-pDC + SC-pDC6 | 9 | 34 | 18 | 32.1 | 11 | 6.7 | 84 | |
| IT-pDC + SC-pDC8 | 12.6 | 37.3 | 15 | 35 | 11 | 3 | 50 | |
| SC-pDC5 | 12 | 41 | 14 | 38.2 | 13 | 7.6 | 87 | |
| SC-pDC6 | 11.5 | 47 | 15 | 40.6 | 11 | 7.8 | 85 | |
| SC-pDC8 | 10.4 |
47 | 14 | 37 | 12 | 5 | 68 | |
| CTRL3 | 11.5 | 25 | 8 | 18.2 | 11.6 | 9.9 | 27.4 | |
| CTRL4 | 8 | 13 | 12 | 18 | 13 | 11.2 | 12.3 | |
| % CD3 + /CD8 + | % CD4 + CD25- | % CD4 + /CD25 + | JAM-test | |||||
| Splenocytes | Prestimulated splenocytes | Splenocytes | Prestimulated splenocytes | Splenocytes | Prestimulated splenocytes | E/T 100/1 % lysis | ||
| Healthy mouse | 11.9 | 21.5 | 22.9 | 28.6 | 8.4 | 2.4 | 12 | |
This stimulation resulted in an expansion of CD3 + /CD8+ and CD4 + /CD25− T cells in vaccinated mice compared with control mice. In IT-pDC plus SC-pDC, CD8+ T cells increased from 13.2 ± 8.2 to 39.9 ± 9.8 after prestimulation (P = .005) on day 15 and from 10.9 ± 1.8 to 38.4 ± 5.1 (P = .001) on day 24. In SC-pDC only, the CD8+ T cells increase from 11.7 ± 2.8 to 35.1 ± 8.1 (P = .001) on day 15 and from 11.3 ± 0.8 to 45 ± 3.5 (P = .001) on day 24. Control mice showed a slight and not significant increase of CD8+ T cells at both time points. Thus both treatment conditions implied a similar, significant increase of “peripheral” CD8+ T cells after stimulation (when compared with controls).
To evaluate if GL261-specific effector lymphocytes were generated in response to systemic vaccination, prestimulated splenocytes from the same mice were investigated for in vitro cytotoxicity 15 and 24 days after injection of tumor cells. Splenocytes from mice treated by either IT-pDC plus SC-pDC or SC-pDC only showed similar strong lytic activity against GL261 cells compared with controls (Table 2 and Supplementary Material, Fig. S4), confirming that increased survival of mice treated with IT-pSC plus SC-pDC vs SC-pDC only is not dependent on the generation of more effective immune responses at the periphery.
Monitoring of lymphocytes was performed on cervical and axillary lymph nodes obtained from the same mice and from mice on day 31 after tumor implantation. On day 15 and 24, in the cervical and axillary lymph nodes of both groups of treated mice, T cells were at similar levels. In fact, we observed a significant increase of CD3 + /CD8+ and CD4 + /CD25− T cells, and a significant decrease of CD4 + /CD25+ when compared with controls (Supplementary Material, Tables S2 and S3).
Overall, we can conclude that IT-pDC are not effective to potentiate the peripheral immune reaction.
Time-Dependent Increase of CD4 + CD25 + Foxp3+ T Cells in the Spleen and Draining Lymph Nodes of Tumor-Bearing Mice
To study the peripheral subpopulation of Treg, we evaluated by flow cytometry and RT-PCR the expression of Foxp3 on CD4 + CD25+ T cells isolated from the spleen, cervical, and axillary lymph nodes ipsilateral and contralateral to the tumor of control, IT-pDC plus SC-pDC and SC-pDC mice at different time points.
In Table 2, we reported the characterization of CD4 + /CD25+ splenocytes from treated and control mice, monitored before and after prestimulation. A significant decrease of CD4 + CD25+ T cells was evident at different time points in IT-pDC plus SC-pDC mice: from 9.8 ± 1.6 to 6.0 ± 1.4 (P = .007) on day 15 and from 11.6 ± 1.2 to 5.5 ± 2.1 (P = .01) on day 24. Splenocytes from SC-pDC only-treated mice had a similar decrease: from 12.2 ± 0.4 to 8.5 ± 1.7 (P = .005) on day 15 and from 12 ± 1 to 6.8 ± 1.6 (P = .008) on day 24.
Control mice showed a significant increase of CD4 + CD25+ T cells when compared with treated mice (P = .007 or P = .05 IT-pDC plus SC-pDC vs control; P = .05 or P = .01 SC-pDC vs controls on day 15 or 24, respectively). Differences between the two groups of treated mice were not significant. Similarly, ipsilateral, but not contralateral, lymph nodes of control mice showed a time-dependent increase of CD4 + CD25+ T cells (Supplementary Material, Tables S2 and S3, and data not shown).
The Treg fraction was monitored in healthy mice, controls, IT-pDC plus SC-pDC- and SC-pDC- vaccinated mice (n = 4 for each group and time point, including those reported in Table 2 and Supplementary Material, Table S2) by flow cytometry. Foxp3 expression in CD4+ CD25+ cells isolated on day 15, 24, and 31 from cervical lymph nodes and from spleens, and on day 31 from axillary lymph nodes. Tumor cells were implanted in the left hemisphere; however, Treg cells were evaluated in both ipsilateral and contralateral cervical and axillary lymph nodes. No significant differences were observed between right and left lymph nodes of treated (SC-pDC or IT-pDC plus SC-pDC) and healthy mice, whereas in control mice Treg percentage was significantly higher in ipsilateral cervical lymph nodes (not shown).
As shown in Fig. 5, a time-dependent, significant increase in the percentage of Foxp3+ cells was detectable in cervical lymph nodes after tumor implantation. In treated mice, the percentage of Treg decreased significantly when compared with controls. The P-values for each time point of treated vs control or healthy mice are reported in Supplementary Material, Table S4. A similar trend was confirmed by evaluating Foxp3 expression by RT-PCR in CD4 + CD25+ immunoselected T cells from the spleen and lymph nodes on day 24 and 31 (Supplementary Material, Fig. S5B and C).
Fig. 5.
Flow cytometric characterization of Treg from cervical lymph nodes. Foxp3+ cells from cervical lymph nodes show a time-related increase in control mice compared with vaccinated mice (from 10.0 ± 1.7% at 15 days to 19.5 ± 1.2% at 24 days, P = .001), reaching a maximum of 26.5 ± 4.9% at 31 days (P = .003 vs day 14; P = .03 vs day 24). In treated mice, the percentage of Treg decreases significantly when compared with controls (IT-pDC plus SC-pDC 6.3 ± 1.8, P = .0005; 9.4 ± 2.9, P = .04; 9.8 ± 1.3, P = .0004; SC-pDC 7.4 ± 0.6, P = .003; 10.6 ± 0.5, P = .01; 11.4 ± 1.2, P = .0007 on day 15, 24 and 31 respectively). In vaccinated mice, the percentage remains similar to healthy mice (9.4 ± 0.2%), but decreases slightly at 15 days. In Supplementary Material, Table S4, all P values for treated groups are reported vs controls and vs healthy mice.
The Treg fraction monitored by flow cytometry Foxp3 expression on CD4+ CD25+ cells isolated from axillary lymph nodes and from spleens of healthy mice, controls, and IT-pDC plus SC-pDC vaccinated mice was reported in Supplementary Material, Fig. S5A.
Discussion
A number of studies have shown that DC immunotherapy of cancer, and specifically of gliomas, has an interesting therapeutic potential but that important limitations are created locally by cytokines produced by the tumor that may induce T-cell anergy and amplification of the Treg fraction.24 Thus, together with the identification of relevant neoplastic targets for DC “vaccination”, the reversal of tumor-mediated immune suppression appears to be of paramount importance for the success of DC-based immunotherapy of cancer.25 Different strategies have been explored to locally modify tumor immune suppression: eg, the use of antisense oligonucleotides to quench expression of one of the most important of immunosuppressive cytokines, TGF-β.6,26,27 Yamanaka et al. in particular, have found that patients with both intratumoral and intradermal administration of DC (n = 7) had a longer survival time than the patients with intradermal administration only (n = 11; P = .043).16 Our results support the idea that IT delivery of DC may have increased anti-tumor efficacy by creating an IT environment more favorable to the development of T-cell–mediated immune responses.
This is demonstrated by the prolonged survival of mice with GL261 malignant gliomas treated by IT-pDC only or in combination with SC-pDC. Histological, quantitative analysis shows that in these mice the number of CD8+ T cells infiltrating the tumor is significantly higher and the number of immunosuppressive Treg cells is significantly lower. At the molecular level, in agreement with this, the amount of TGF-β and Foxp3 decreases and that of IFN-γ increases.
Several evidences support the concept that Foxp3 is a necessary and sufficient marker for mouse Treg expansion and function. Ectopic Foxp3 expression has been shown to drive Treg function, lack of Foxp3 has been correlated with a lack of Treg cells.28–31 Thus, the decrease of Foxp3 in our model, as evaluated by quantitative histological evaluation and by RT-PCR within tumors, indicates a decreased number of functionally active Tregs.
We believe that these results are mostly mediated by the DCs acting locally and surviving for several days after injection, as we could not find any evidence of DCs migrating from intracranial tumors to cervical or axillary lymph nodes. On the contrary, using similar experimental conditions (ie, DC manufacturing, number of cells injected, animal strain), we previously found that SC injection in the flanks was followed, 1 week later, by evidence of migrating, labeled DCs to axillary draining lymph nodes.32 Previous data have proposed that, after intracranial injection, DC may migrate to cervical lymph nodes.33,34 One interesting observation, possibly explaining this discrepancy with our data, suggests that only DCs that are injected into the cerebrospinal fluid, but not into brain parenchyma, are actually able to reach lymph nodes in the vicinity.35 Thus, our stereotaxic coordinates for DC injection may not favor their migration to the lymph nodes, implying that their biological activity is mostly mediated in situ.
For this reason, we do not find an increased efficacy at the periphery of IT-pDC plus SC-pDC over SC-pDC only, when monitoring T cells in the spleens and draining lymph nodes and their cytotoxic effects in vitro. The SC injection of DCs induces a systemic anti-tumor effect and is effective in eliciting T-cell responses and creating a convergence of immune response elements to the tumor site. IT-pDC can modulate the tumor microenvironment and potentiate the anti-tumor response, significantly increasing survival in combination with SC-pDC and exerting different functions on tumor biology.
Together with temporary release of pro-inflammatory cytokines by DCs, it is also conceivable that DCs initiate some dialogue with surrounding microglia, a possibility not explored by our experiments but suggested by previous evidence showing that under inflammatory conditions a fraction of CD11b+ microglial cells may express the DCs marker CD11c.36 Other data also showed that DCs may amplify T-cell–mediated immune responses in the CNS.37 The role of DCs in the CNS, however, has also been reported as an immunosuppressive one, with DCs inhibiting activation of T cells.38 Interestingly, however, such an inhibitory role was played by DCs showing a “phenotype similar to immature BM-derived DC”.38 This draws attention to the central role played by DC maturation in raising effective T-cell responses against gliomas in the experiments we have reported. In particular, the use of IT-uDC only led to survival of 10% of the mice and the addition of IT-uDC to SC-pDC was ineffective. This is a relevant issue, as several reports have described the effects of IT injection of DC in glioma models in rodents.11,12,17 In these studies, however, DC were not loaded with tumor homogenates or induced to maturation by in vitro exposure to appropriate cytokines, rather they were co-injected with irradiated glioma cells11,12 or transduced to overexpress IL-23.17 IT injection of DCs in other tumors has also been performed but had to be supplemented by other treatments aimed at enhancing antigen presentation, like apoptosis of tumor cells in situ obtained by tumor irradiation,13 hyperthermia,39–41 or photodynamic therapy.42 Thus, we propose that DC maturation, which in our system is obtained by loading DCs ex vivo with a tumor lysate, is a critical requisite for developing effective anti-tumor responses by IT injection of DCs. Observations showing that infiltration of malignant melanomas by mature DCs, defined by expression of the maturation marker LAMP, is significantly correlated to prolonged survival, providing indirect confirmation of our claim.43
Our results support the idea that IT-pDC may contrast tumor immune suppression favoring T-cell activation, as demonstrated by the significant presence of CD8+ TILs and by significant increased levels of IFN-γ on day 24 after tumor injection, ie, 10 days after IT “vaccination”. TNF-α, on the contrary, was highly expressed on day 15 (8 days after IT “vaccination”) and less on day 24, which is consistent with the limited life span of DCs injected IT.
High levels of TNF-α produced by pDC were confirmed in vitro and, most importantly, we showed that those levels of TNF-α could decrease the in vitro proliferation of GL261 GBM cells. As a consequence, we also propose that pDC may have a TNF-mediated anti-tumor effect that may amplify that of CD8+ cells that have been primed peripherally by SC-pDC “vaccination”. Interestingly, combined expression of IFN-γ and TNF-α may stimulate expression by local astrocytes of the CXCR3 cytokine fractalkine:44 fractalkine may amplify anti-tumor immune responses by involving not only CD8+ T cells but also NK cells.45 Although not investigated in our studies, this defines an interesting scenario, as the crosstalk between DC and NK cells has an important role in the coordination of innate and adaptive immune responses.46
These findings may have translational relevance for clinical applications of DC immunotherapy of gliomas and possibly other solid tumors. IT injection of immature DC has already been performed, for instance, in metastatic cancers10 and malignant gliomas16 with intriguing results. Furthermore, an inverse relationship has been demonstrated between the degree of local immune suppression in GBM, measured as the amount of TGF-β expressed locally, and the success of DC immunotherapy.47 Thus, our findings encourage the clinical use of IT-pDC, to be delivered (stereotaxically or through the use of Rickam catheters) in parallel to peripheral treatment by SC-pDC, as a promising tool to enhance the efficacy of DC immunotherapy in malignant gliomas and other solid tumors.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
This work has been supported by grants from the Associazione Italiana per la Ricerca sul Cancro, the Minister of Health, Il Fondo di Gio, and the Associazione per le Ricerche in Neurologia.
Supplementary Material
Acknowledgment
We thank Sara Nava, Silvia Musio, and Fulvio Baggi for help with the JAM assay and the CBA system analysis.
Conflict of interest statement. None declared.
References
- 1.Palucka AK, Ueno H, Fay JW, Banchereau J. Taming cancer by inducing immunity via dendritic cells. Immunol Rev. 2007;220:129–150. doi: 10.1111/j.1600-065X.2007.00575.x. [DOI] [PubMed] [Google Scholar]
- 2.Gajewski TF, Meng Y, Blank C, et al. Immune resistance orchestrated by the tumor microenvironment. Immunol Rev. 2006;213(1):131–145. doi: 10.1111/j.1600-065X.2006.00442.x. [DOI] [PubMed] [Google Scholar]
- 3.Gomez GG, Kruse CA. Mechanisms of malignant glioma immune resistance and sources of immunosuppression. Gene Ther Mol Biol. 2006;10(a):133–146. [PMC free article] [PubMed] [Google Scholar]
- 4.de Vleeschouwer S, Rapp M, Sorg RV, et al. Dendritic cell vaccination in patients with malignant gliomas: current status and future directions. Neurosurgery. 2006;59(5):988–999. doi: 10.1227/01.NEU.0000245595.38957.3E. discussion 999–1000. [DOI] [PubMed] [Google Scholar]
- 5.Hussain SF, Heimberger AB. Immunotherapy for human glioma: innovative approaches and recent results. Expert Rev Anticancer Ther. 2005;5(5):777–790. doi: 10.1586/14737140.5.5.777. [DOI] [PubMed] [Google Scholar]
- 6.Platten M, Wick W, Weller M. Malignant glioma biology: role for TGF-beta in growth, motility, angiogenesis, and immune escape. Microsc Res Tech. 2001;52(4):401–410. doi: 10.1002/1097-0029(20010215)52:4<401::AID-JEMT1025>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 7.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-{beta}1 maintains suppressor function and Foxp3 expression in CD4 + CD25+ regulatory T cells. J Exp Med. 2005;201(7):1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang HY, Wang R-F. Regulatory T cells and cancer. Curr Opin Immunol. 2007;19(2):217. doi: 10.1016/j.coi.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Grauer OM, Nierkens S, Bennink E, et al. CD4 + FoxP3+ regulatory T cells gradually accumulate in gliomas during tumor growth and efficiently suppress antiglioma immune responses in vivo. Int J Cancer. 2007;121(1):95–105. doi: 10.1002/ijc.22607. [DOI] [PubMed] [Google Scholar]
- 10.Triozzi PL, Khurram R, Aldrich WA, Walker MJ, Kim JA, Jaynes S. Intratumoral injection of dendritic cells derived in vitro in patients with metastatic cancer. Cancer. 2000;89(12):2646–2654. doi: 10.1002/1097-0142(20001215)89:12<2646::aid-cncr18>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 11.Kikuchi T, Akasaki Y, Abe T, Ohno T. Intratumoral injection of dendritic and irradiated glioma cells induces anti-tumor effects in a mouse brain tumor model. Cancer Immunol Immunother. 2002;51(8):424–430. doi: 10.1007/s00262-002-0297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehtesham M, Kabos P, Gutierrez MA, Samoto K, Black KL, Yu JS. Intratumoral dendritic cell vaccination elicits potent tumoricidal immunity against malignant glioma in rats. J Immunother. 2003;26(2):107–116. doi: 10.1097/00002371-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Teitz-Tennenbaum S, Li Q, Rynkiewicz S, et al. Radiotherapy potentiates the therapeutic efficacy of intratumoral dendritic cell administration. Cancer Res. 2003;63(23):8466–8475. [PubMed] [Google Scholar]
- 14.Yamanaka R, Tsuchiya N, Yajima N, et al. Induction of an antitumor immunological response by an intratumoral injection of dendritic cells pulsed with genetically engineered Semliki Forest virus to produce interleukin-18 combined with the systemic administration of interleukin-12. J Neurosurg. 2003;99(4):746–753. doi: 10.3171/jns.2003.99.4.0746. [DOI] [PubMed] [Google Scholar]
- 15.Kuwashima N, Nishimura F, Eguchi J, et al. Delivery of dendritic cells engineered to secrete IFN-alpha into central nervous system tumors enhances the efficacy of peripheral tumor cell vaccines: dependence on apoptotic pathways. J Immunol. 2005;175(4):2730–2740. doi: 10.4049/jimmunol.175.4.2730. [DOI] [PubMed] [Google Scholar]
- 16.Yamanaka R, Homma J, Yajima N, et al. Clinical evaluation of dendritic cell vaccination for patients with recurrent glioma: results of a clinical phase I/II trial. Clin Cancer Res. 2005;11(11):4160–4167. doi: 10.1158/1078-0432.CCR-05-0120. [DOI] [PubMed] [Google Scholar]
- 17.Hu J, Yuan X, Belladonna ML, et al. Induction of potent antitumor immunity by intratumoral injection of interleukin 23-transduced dendritic cells. Cancer Res. 2006;66(17):8887–8896. doi: 10.1158/0008-5472.CAN-05-3448. [DOI] [PubMed] [Google Scholar]
- 18.Szatmari T, Lumniczky K, Desaknai S, et al. Detailed characterization of the mouse glioma 261 tumor model for experimental glioblastoma therapy. Cancer Sci. 2006;97(6):546–553. doi: 10.1111/j.1349-7006.2006.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashley DM, Faiola B, Nair S, Hale LP, Bigner DD, Gilboa E. Bone marrow-generated dendritic cells pulsed with tumor extracts or tumor RNA induce antitumor immunity against central nervous system tumors. J Exp Med. 1997;186(7):1177–1182. doi: 10.1084/jem.186.7.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4 + CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299(5609):1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 21.Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65(8):3437–3446. doi: 10.1158/0008-5472.CAN-04-4262. [DOI] [PubMed] [Google Scholar]
- 22.Chatzidakis I, Fousteri G, Tsoukatou D, Kollias G, Mamalaki C. An essential role for TNF in modulating thresholds for survival, activation, and tolerance of CD8+ T cells. J Immunol. 2007;178(11):6735–6745. doi: 10.4049/jimmunol.178.11.6735. [DOI] [PubMed] [Google Scholar]
- 23.Villeneuve J, Tremblay P, Vallieres L. Tumor necrosis factor reduces brain tumor growth by enhancing macrophage recruitment and microcyst formation. Cancer Res. 2005;65(9):3928–3936. doi: 10.1158/0008-5472.CAN-04-3612. [DOI] [PubMed] [Google Scholar]
- 24.Rescigno M, Avogadri F, Curigliano G. Challenges and prospects of immunotherapy as cancer treatment. Biochim Biophys Acta. 2007;1776(1):108–123. doi: 10.1016/j.bbcan.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Liao YP, Schaue D, McBride WH. Modification of the tumor microenvironment to enhance immunity. Front Biosci. 2007;12:3576–3600. doi: 10.2741/2336. [DOI] [PubMed] [Google Scholar]
- 26.Fakhrai H, Dorigo O, Shawler DL, et al. Eradication of established intracranial rat gliomas by transforming growth factor beta antisense gene therapy. Proc Natl Acad Sci USA. 1996;93(7):2909–2914. doi: 10.1073/pnas.93.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fakhrai H, Mantil JC, Liu L, et al. Phase I clinical trial of a TGF-beta antisense-modified tumor cell vaccine in patients with advanced glioma. Cancer Gene Ther. 2006;13(12):1052–1060. doi: 10.1038/sj.cgt.7700975. [DOI] [PubMed] [Google Scholar]
- 28.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–226. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 29.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22(3):329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 30.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4 + CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 31.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4 + CD25+ T regulatory cells. Nat Immunol. 2003;4(4):337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 32.Pellegatta S, Poliani PL, Corno D, et al. Neurospheres enriched in cancer stem-like cells are highly effective in eliciting a dendritic cell-mediated immune response against malignant gliomas. Cancer Res. 2006;66(21):10247–10252. doi: 10.1158/0008-5472.CAN-06-2048. [DOI] [PubMed] [Google Scholar]
- 33.Tsugawa T, Kuwashima N, Sato H, et al. Sequential delivery of interferon-alpha gene and DCs to intracranial gliomas promotes an effective antitumor response. Gene Ther. 2004;11(21):1551–1558. doi: 10.1038/sj.gt.3302300. [DOI] [PubMed] [Google Scholar]
- 34.Karman J, Ling C, Sandor M, Fabry Z. Initiation of immune responses in brain is promoted by local dendritic cells. J Immunol. 2004;173(4):2353–2361. doi: 10.4049/jimmunol.173.4.2353. [DOI] [PubMed] [Google Scholar]
- 35.Hatterer E, Davoust N, Didier-Bazes M, et al. How to drain without lymphatics? Dendritic cells migrate from the cerebrospinal fluid to the B-cell follicles of cervical lymph nodes. Blood. 2006;107(2):806–812. doi: 10.1182/blood-2005-01-0154. [DOI] [PubMed] [Google Scholar]
- 36.Fischer H-G, Reichmann G. Brain dendritic cells and macrophages/microglia in central nervous system inflammation. J Immunol. 2001;166(4):2717–2726. doi: 10.4049/jimmunol.166.4.2717. [DOI] [PubMed] [Google Scholar]
- 37.Karman J, Chu HH, Co DO, Seroogy CM, Sandor M, Fabry Z. Dendritic cells amplify T cell-mediated immune responses in the central nervous system. J Immunol. 2006;177(11):7750–7760. doi: 10.4049/jimmunol.177.11.7750. [DOI] [PubMed] [Google Scholar]
- 38.Suter T, Biollaz G, Gatto D, et al. The brain as an immune privileged site: dendritic cells of the central nervous system inhibit T cell activation. Eur J Immunol. 2003;33(11):2998–3006. doi: 10.1002/eji.200323611. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka K, Ito A, Kobayashi T, et al. Intratumoral injection of immature dendritic cells enhances antitumor effect of hyperthermia using magnetic nanoparticles. Int J Cancer. 2005;116(4):624–633. doi: 10.1002/ijc.21061. [DOI] [PubMed] [Google Scholar]
- 40.Guo J, Zhu J, Sheng X, et al. Intratumoral injection of dendritic cells in combination with local hyperthermia induces systemic antitumor effect in patients with advanced melanoma. Int J Cancer. 2007;120(11):2418–2425. doi: 10.1002/ijc.22551. [DOI] [PubMed] [Google Scholar]
- 41.Mukhopadhaya A, Mendecki J, Dong X, et al. Localized hyperthermia combined with intratumoral dendritic cells induces systemic antitumor immunity. Cancer Res. 2007;67(16):7798–7806. doi: 10.1158/0008-5472.CAN-07-0203. [DOI] [PubMed] [Google Scholar]
- 42.Saji H, Song W, Furumoto K, Kato H, Engleman EG. Systemic antitumor effect of intratumoral injection of dendritic cells in combination with local photodynamic therapy. Clin Cancer Res. 2006;12(8):2568–2574. doi: 10.1158/1078-0432.CCR-05-1986. [DOI] [PubMed] [Google Scholar]
- 43.Ladanyi A, Kiss J, Somlai B, et al. Density of DC-LAMP(+) mature dendritic cells in combination with activated T lymphocytes infiltrating primary cutaneous melanoma is a strong independent prognostic factor. Cancer Immunol Immunother. 2007;56(9):1459–1469. doi: 10.1007/s00262-007-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshida H, Imaizumi T, Fujimoto K, et al. Synergistic stimulation, by tumor necrosis factor-alpha and interferon-gamma, of fractalkine expression in human astrocytes. Neurosci Lett. 2001;303(2):132–136. doi: 10.1016/s0304-3940(01)01699-8. [DOI] [PubMed] [Google Scholar]
- 45.Xin H, Kikuchi T, Andarini S, et al. Antitumor immune response by CX3CL1 fractalkine gene transfer depends on both NK and T cells. Eur J Immunol. 2005;35(5):1371–1380. doi: 10.1002/eji.200526042. [DOI] [PubMed] [Google Scholar]
- 46.Walzer T, Dalod M, Robbins SH, Zitvogel L, Vivier E. Natural-killer cells and dendritic cells: “l'union fait la force”. Blood. 2005;106(7):2252–2258. doi: 10.1182/blood-2005-03-1154. [DOI] [PubMed] [Google Scholar]
- 47.Liau LM, Prins RM, Kiertscher SM, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11(15):5515–5525. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.