Abstract
To determine the potential consequences of plasmacytoid dendritic cell (pDC) accumulation in tissue sites observed in several autoimmune diseases, we measured type 1 interferon production from circulating human pDCs as a function of pDC concentration. The effects of interferon-alpha and blockade of the type 1 interferon receptor (IFNAR) on human pDC type 1 interferon and interferon-inducible transcription and protein production were measured. Human pDCs became far more efficient producers of interferon-alpha at concentrations beyond those normally present in blood, through an IFNAR-dependent mechanism. Extracellular interferon-alpha increased pDC production of type 1 interferons. The accumulation of pDCs in diseased tissue sites allows marked non-linear amplification of type 1 interferon production locally. The role of the IFNAR-dependent mechanism of interferon production by human pDCs is greater than previously suggested. IFNAR blockade has potential for diminishing type 1 interferon production by all human cells.
Keywords: Type 1 interferons, Systemic lupus erythematosus, Myositis, Plasmacytoid dendritic cells
Introduction
The type 1 interferons, which include interferon-alpha (IFNα) and interferon-beta (IFNβ), may play a significant role in several autoimmune diseases, particularly systemic lupus erythematosus (SLE; reviewed in [1; 2; 3]) and dermatomyositis (DM; reviewed in [4; 5]). Anti-IFNα neutralizing antibody therapy is currently being studied in clinical trials for both SLE [6; 7] and myositis [8], and various other approaches to modulating the type 1 interferon pathway have been considered.
Plasmacytoid dendritic cells (pDCs) are immune system cells capable of producing large amounts of type 1 interferons. pDCs have been observed to concentrate in diseased tissue sites, in DM muscle [9; 10] and skin [11; 12], and in SLE glomeruli [13] and skin [14; 15]. The effects of such concentration is unknown but it is notable that DM muscle shows marked enrichment of type 1 interferon-inducible transcripts in comparison to blood, even though both compartments are highly dominated by such transcripts (accounting for >85% of the most abundant 25 transcripts in both blood and muscle, of >18,000 measured). For example, ISG15 (interferon-stimulated gene 15) transcript was 570-fold increased in muscle but 9-fold increased in blood; Mx1 (myxovirus resistance protein 1) transcript was 281-fold increased in muscle but 6-fold increased in blood [16].
To further understand the consequences of human pDC accumulation in autoimmune disease tissue sites, we studied the effects of increasing pDC cell number on type 1 interferon production. We found that IFNα production by human pDCs proceeds exponentially, not linearly, within a physiological range of increasing cell numbers, an effect in part mediated by the type 1 interferon receptor (IFNAR). We demonstrate directly that type 1 interferons substantially augment their own production by pDCs, effects that have previously been demonstrated in non-human cells [17; 18; 19; 20; 21; 22], but only hypothesized for human pDCs [23; 24] based on indirect experiments and on mouse experiments [20; 21; 22; 25; 26; 27; 28].
Methods
pDC isolation, purification, stimulation, and viability
Normal donor peripheral blood mononuclear cells (PBMCs) were isolated over Ficoll-Paque (Amersham Biosciences). Plasmacytoid dendritic cells (pDCs) were purified using a BDCA-4 cell isolation kit (Miltenyi Biotec) with 2 steps of pDC enrichment on magnetic columns. The purity of isolated BDCA-2+CD123+ pDC was 92.02 % ± 1.02% by flow cytometry (n = 5).
For measurement of IFNα protein, varying number of pDCs as described below were stimulated in 200 µl assays in 96-well or 50 µl or 75 µl assays in 384-well plates with CpG oligodeoxynucleotide (ODN) type A, human toll-like receptor 9 (TLR9) ligand ODN2216 (InvivoGen) 5 µg/ml for 24 hours, and the supernatants removed and immediately assayed by ELISA. TLR9 agonists were used because TLR9 activating immunostimulatory DNA complexes are believed to be directly relevant to the mechanism of type 1 interferon production in SLE and DM [1; 4]. For measurement of priming and IFNAR blocking effects, these experiments were done in the absence and presence of IFNα-2a protein (PBL Biomedical, Product #11100-1) at varying doses as indicated and absence or presence of anti-IFNAR2 antibody (PBL Biomedical, Product #21385-1), with isotype control matched IgG2a antibody, both at 5 µg/ml (BD Pharmingen Product #554126).
For harvesting of RNA for transcript experiments, 10,000 pDCs in wells of 384-well microtiter plates were stimulated with ODN2216 at 5 µg/ml in the absence and presence of 25 pg/ml IFNα-2a for 1, 3, 6, 16, and 24 hours and then frozen at −80°C for subsequent RNA extraction. In separate experiments, PBMCs were compared with both the BDCA-4 positively selected fraction (pDCs) and the remaining BDCA4-depleted PBMC fraction. Triplicate wells of 10,000 PBMCs, 10,000 BDCA4-depleted PBMCs, and 10,000 BDCA4-positively selected cells (pDCs) from the same purification were stimulated with ODN2216 at 5 µg/ml for 6 hours. All cells were then frozen at −80°C for subsequent RNA extraction.
For harvesting of proteins for western blots, pDCs (BDCA4+; mean of 4 experiments 1.19 × 106 cells), PBMCs (mean of 2 experiments 8.40 × 105 cells), or BDCA4-depleted PBMCs (mean of 2 experiments 1.80 × 106 cells) in 15 ml tubes were stimulated with ODN2216 at 5 µg/ml for 24 hours. Supernatants and cells were separated and separately frozen.
Plasmacytoid dendritic cell viability before and after stimulation was measured by the method of trypan blue exclusion. Trypan blue was obtained from Sigma-Aldrich (cat# T8154) and cell counting performed as described at http://www.cascadebio.com/uploads/File/pdf/hemat.pdf.
Transcript measurements
Total RNA was extracted from pDCs using the Zymo Research Mini RNA Isolation I kit (Orange, CA). RNA purity and concentration were determined spectrophotometrically (260/280>1.9). RNA quality was assessed on an Agilent 2100 Bioanalyzer using the RNA 6000 Nano LabChip. The generation of single-stranded cDNA from 15 ng of total RNA was accomplished with the Invitrogen SuperScript First-Strand Synthesis SuperMix kit (Carlsbad, CA).
The Fluidigm Biomark system was used for high-throughput real-time PCR. A mixture of 48 TaqMan Gene Expression assays (Applied Biosystems) was prepared for use with the TaqMan PreAmp Master Mix Kit (Applied Biosystems). All samples were pre-amplified for 10-cycles of PCR according to the manufacturer’s directions.
For real-time PCR, all samples were run in triplicate against a set of 48 TaqMan Gene Expression Assays in BioMark 48.48 Dynamic Array chips (Fluidigm Corp). The untreated (0-hr.) controls were included on every array. Dynamic arrays were loaded using a NanoFlex 4-IFC Controller (Fluidigm Corp), and real-time reactions were performed and analyzed using BioMark Real-Time PCR System and Analysis software (Fluidigm Corp), respectively. Cycle thresholds (Cts) above 30 were excluded from the calculation. Delta-delta Cts (ΔΔCt) were calculated using the mean of the 2 reference genes (ACTB and UBC) and a calibrator sample and were converted to fold expression change by the following formula: 2−ΔΔCt.
Interferon-α ELISAs
Levels of IFNα protein were measured using a commercial multisubtype 96-well ELISA (#41105-1, PBL Biomedical, Piscataway, NJ; detecting IFNα subtypes as described at http://www.interferonsource.com/New_InterferonSource/ELISA/HumanELISA_Specificity.html), a limited subtype custom 384-well ELISA (detecting only IFNα-2a, -6, and -8), and a multisubtype custom 384-well ELISA otherwise identical to PBL #41105-1.
Western blots
For the preparation of intracellular P1S1 fractions, pDCs were washed with ice cold PBS, collected by centrifugation at 1,500 rpm for 10 minutes at 4° C, sonicated, placed on a rotator for 15 minutes, and incubated on ice for 45 minutes. Cells were then centrifuged at 13,000 rpm for 30 minutes at 4° C. Supernatants were collected (S1) and cell lysate was re-suspended in SDS (P1S1). Both S1 and P1S1 fractions were heated at 95° C for 5 minutes and stored at −80° C.
Blots were incubated with rabbit polyclonal anti-ISG15 antibody (# ab45285, Abcam, Cambridge, MA) at the dilution of 1:500 in 5%milk/PBST (0.1% Tween 20) for 18 hours at 4° C with shaking. After 5 washes of 5 minutes in PBST, the blots were incubated with secondary HRP-tagged goat anti-rabbit IgG antibody (# ab6721, Abcam, Cambridge, MA) at the dilution of 1:5000 in 5%milk/PBST for 1 hour at room temperature (RT), with shaking, and washed as above. SuperSignal West Pico Chemiluminescent Substrate (Pierce, Rockford IL) and Kodak films were used for visualization of the bands. The blots were stripped using Restore Western Blot Stripping Buffer (#21062, Pierce) for 45 minutes at 37°C, washed twice for 10 minutes with PBST and blocked for 1 hr in 5%milk/PBST. To determine reactivity against actin, immunoblotting was repeated as above using rabbit polyclonal anti-actin primary antibody (# sc1616, Santa Cruz Biotechnology, sc1616, Santa Cruz, CA) at the dilution of 1:10,000 for 1 hour at RT and secondary HRP-tagged goat anti-rabbit IgG antibody (# ab6721, Abcam) at the dilution of 1:10,000 for 1 hour at RT. To determine reactivity against MxA, immunoblotting was repeated as above using mouse anti-MxA primary antibody (courtesy of Professor Otto Haller, University of Freiburg, Freiburg, Germany) at the dilution of 1:500 for 1 hour at RT and secondary PowerVison Poly-HRP anti-mouse IgG (# DPVM-110HRP, ImmunoVision Technologies, Norwell MA) at the dilution of 1:2 for 1 hour at RT.
Results
pDCs become more efficient producers of IFNα in the company of other pDCs at concentrations above those present in blood
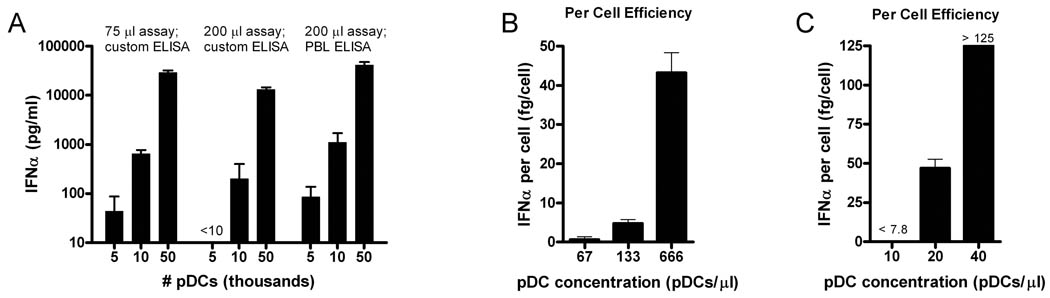
In studying the effects of various cell numbers and volumes on IFNα secretion by TLR9 stimulated human pDCs, we found that such secretion increased exponentially, not linearly, as a function of the number of pDCs stimulated (Figure 1A). This effect was seen in a variety of formats, using cells in both 200 µl volumes in 96-well stimulation plates and 50 or 75 µl volumes in 384-well stimulation plates, and both commercial and custom made ELISAs. When IFNα production was calculated on a per cell basis, TLR9-stimulated pDCs became far more efficient producers of IFNα as a function of increased cell density. For example, using an ELISA that detected only limited subtypes of IFNα, pDCs in a 5,000 cell suspension (66 pDCs/µl) produced 0.65 fg/cell, in a 10,000 cell suspension (133 pDCs/µl) 4.8 fg/cell, and in a 50,000 cell suspension (666 pDCs/µl) 43 fg/cell (Figure 1B). Unstimulated pDCs did not produce any detectable IFNα at any cell concentrations, as high as 666 pDCs/µl.
Figure 1. Non-linearity of pDC interferon-α (IFNα) production demonstrates concentration effects.
(A) ODN-stimulated pDC IFNα production increased greater than exponentially, not linearly, with increased cell numbers in 3 different assay formats, in either 75 µl or 200 µl assay volumes or as measured with a custom limited subtype or commercial multisubtype (PBL Biomedical) ELISA. IFNα production plotted with logarithmic scale. (B and C) IFNα production per cell depends on cell density, with marked increases in per cell output with increasing pDC cell concentration. In (B), custom limited subtype ELISA requires large cell numbers for IFNα detection. In (C), custom multisubtype ELISA allows the use of very small numbers of pDCs to study physiologically relevant numbers: 500 pDCs in a 50 µl assay (10 pDCs/µl) approximated the concentration of pDCs in whole blood (5–10 pDCs/µl). Increasing pDC concentration from this value (to 20 and 40 pDC/µl) shows non-linear marked increases in per cell efficiency.
Amplification effects were not due to changes in cell viability. Increasing concentrations of 80 and 160 pDCs/µl did not change trypan blue exclusion measured viability of 71% and 73% at 24 hours yet produced exponentially greater amounts of IFNα, 713 pg/ml and 10101 pg/ml, respectively (Supplementary Figure).
Previously published experiments with human pDCs have used large numbers greatly exceeding blood concentrations. In normal human blood, pDCs constitute approximately 0.1% of white blood cells, or 5–10 pDCs/µl. Previous studies have used 250 pDCs/µl [23; 24], 500 pDCs/µl [29; 30], 1000 pDCs/µl [31], or 1000–2000 pDCs/µl [32] in examining human pDC responses. Using a highly sensitive custom 384-well format ELISA detecting multiple subtypes of IFNα, we were able to examine the kinetics of pDC IFNα production at physiologically relevant cell numbers starting at the approximate concentration of pDCs in whole blood. We found that 10 pDCs/µl produced < 4 fg/cell while 20 pDCs/µl produced 47 fg/cell, and 40 pDCs/µl produced > 125 fg/cell (Figure 1C). Thus, at concentrations just starting to exceed those present normally in blood, a pDC concentration effect creates non-linear amplification of pDC IFNα production.
Extracellular IFNα increases TLR9-mediated pDC production of interferon-α protein
We studied the effect of added extracellular IFNα-2a to TLR9-stimulated pDCs. In independent experiments from separate pDC collections from 5 different donors, IFNα secretion at 24 hours increased a mean of 3.8-fold (range 1.8 – 10.0-fold) after exposure to 25 pg/ml of recombinant IFNα-2a (Figure 2A). The responsiveness to TLR9 agonist and extracellular IFNα-2a differed among donors. The increased IFNα detected in co-stimulated pDCs ranged from 215–634 pg/ml, which was not accounted for by the small amount (25 pg/ml) added during stimulation. The amplification with IFNα-2a was dose-dependent, maximal at 25 to 50 pg/ml (Figure 2B). The increased IFNα-2a mediated IFNα production was not due to changes in cell viability; 80 pDCs/µl had 24-hour viability of 71% and produced 713 pg/ml with ODN treatment alone while cells treated with ODN and IFNα had viability of 67% and produced 2516 pg/ml of IFNα (Supplementary Figure).
Figure 2. Direct demonstration of IFNα amplification effect on human pDCs.
(A) Five different donor pDC collections exposed to TLR9 agonist ODN2216 alone or with 25 pg/ml of IFNα-2a protein. (B) Dose-response effects of IFNα-2a on ODN2216 stimulated pDC production of IFNα. Control pDCs were stimulated with IFNα-2a alone, without ODN2216. 10,000 pDCs, 8 replicates, and custom limited subtype ELISA used for all IFNα measurements.
Kinetics of IFNα, IFNβ and IFNω transcription in human pDCs and enhanced transcription with extracellular IFNα
To understand if the autocrine amplification of IFNα protein on pDC production of IFNα occurred through increased transcription of IFNα we performed high-throughput RT-PCR on pDC RNA prepared from TLR9 stimulated pDCs without and with 25 pg/ml of added IFNα-2a. We measured transcripts for 10 IFNα subtypes and two other type 1 interferon family members β and ω prior to stimulation and at 1, 3, 6, 16, and 24 hours after stimulation. Type 1 interferon transcripts were detectably increased at 1 hour, increased substantially by 3–6 hours, and returned towards baseline at 16 and 24 hours (Figure 3). The greatest fold increases occurred for IFNα2 (96-fold at 6 hours) and IFNβ (297-fold at 6 hours) compared with other type 1 interferon subtypes. IFNα6 and IFNω transcript abundance peaked at 3 hours, while the other subtypes peaked at 6 hours.
Figure 3. Kinetics of TLR9 stimulated human pDC type 1 interferon transcription.
(A) 24-hour time-scale view of 10 IFNα subtypes, IFNβ, and IFNω transcript levels and their augmentation by the addition of IFNα-2a 25 pg/ml. Intensity = fold change from pre-ODN stimulation levels. (B) Fold-change for 10 subtypes of interferon-α, and interferon-β and –ω transcripts were measured in TLR9 stimulated pDCs in comparison to pDCs prior to stimulation at 1 and 6 hours. Note differing scales, as fold changes have increased substantially at 6 hours.
We found at all time points and for all type 1 interferon subtypes that addition of IFNα protein augmented type 1 interferon transcription. For example, with ODN stimulation alone after 6 hours, the mean increase for 10 IFNα subtypes was 64-fold, while stimulation with ODN together with IFNα-2a resulted in a 230-fold increase.
Interferon receptor blockade causes marked reduction in TLR9 mediated production of type 1 interferons and downstream type 1 interferon-inducible effects in pDCs
The receptor complex for type I IFN signaling is comprised of a heterologous transmembrane receptor complex comprised of the IFNAR2 and IFNAR1 chains. Blocking IFNAR interactions inhibits type I IFN signaling and subsequent IFN-mediated gene expression. We performed TLR9 stimulation experiments in the presence of a neutralizing antibody against IFNAR2. TLR9 stimulated pDCs produced 86–95% less IFNα protein than without receptor blockade (Figure 4; N=5 experiments, 3 without and 2 with IFNα priming). IgG2a antibody, an isotype matched control for the anti-IFNAR2 antibody, did not reduce IFNα production after ODN stimulation. The anti-IFNAR2 antibody mediated decrease in IFNα production was not accounted for by changes in cell viability (Supplementary Figure). pDCs appear to produce a small portion of IFNα independently of autocrine engagement of IFNAR but require IFNAR engagement for full IFNα production after TR9 stimulation. These findings are consistent with a model of TLR9 stimulated human pDCs secreting type 1 interferons that subsequently engage and activate the IFN receptor complex resulting in further amplification of IFNα production.
Figure 4. Effect of type 1 interferon receptor (IFNAR) blockade on IFNα production by human pDCs.
(A) Highly sensitive ELISA using low pDC cell numbers shows non-linear increase in IFNα production and that IFNAR blockade (anti-IFNAR2 antibody 5 µg/ml) markedly reduces IFNα production. (B) Lower sensitivity ELISA, 10,000 pDCs per condition. The effects of added IFNα-2a (25 pg/ml) increased IFNα production only in the absence of IFNAR blockade. (p<0.0001 for all comparisons).
The autocrine engagement of the IFNAR complex by pDC-secreted type 1 interferons suggested that pDCs might themselves also produce many type 1 interferon-inducible transcripts and proteins (interferon stimulated genes; ISGs). We found by real-time PCR that many such transcripts, such as Mx1, RSAD2, IFIT1, and ISG15 are abundantly produced by concentrated suspensions of pDCs (Figure 5A) but not by TLR9 stimulated mixed PBMCs or PBMCs depleted of pDCs (data not shown). pDC production of these was amplified in the presence of small amounts of added IFNα-2a protein. Production of Mx1 protein was similarly demonstrated in western blots prepared from intracellular fractions of concentrated pDC suspensions (Figure 5B). It is possible that type 1 interferon-inducible transcripts and proteins are being produced by non-pDCs contaminating these pDC purification experiments, a concern recently highlighted with regard to interpretations that pDCs produced IL-12 [29].
Figure 5. Concentrated pDC suspensions produce downstream type 1 interferon-inducible transcripts and proteins, augmented by addition of IFNα.
(A) Interferon-inducible transcripts produced by TLR9 stimulated >92% pure pDC suspensions measured by real-time quantitative PCR. Amplification of pDC production of type 1 interferon-inducible transcripts in the presence of small amounts of added IFNα-2a protein (25 pg/ml). 10,000 pDCs in 50 µl volumes used. (B) The interferon-inducible protein Mx1 is present in intracellular fractions and the ubiquitin-like modifier and cytokine protein ISG15 is secreted into cell media at 24 hours. (C) Further downstream effects of type 1 interferon signaling in which multiple intracellular proteins have been conjugated by ISG15 is evident. Unstimulated pDCs (No Tx) do not have or secrete detectable ISG15 or ISG15 conjugates. IFNAR blockade prevents ISG15 conjugation. Transcripts measured by quantitative real-time PCR.
To study the effect of IFNAR blockade on further downstream type 1 interferon signaling consequences, we examined pDC cell suspensions for the presence of ISG15 conjugated proteins. ISG15, a type 1 interferon-inducible protein, is a ubiquitin-like modifier and cytokine with poorly understood function. ISG15 conjugates to other proteins with the help of conjugating enzymes, including HERC5 and deconjugates via USP18. We found at 1, 3, and 6 hours after TLR9 stimulation of pDCs marked increases in the transcription of ISG15 pathway genes (Figure 5A; at 6 hours, ISG15 95-fold; HERC5 20-fold; and USP18 120-fold). TLR9 stimulated pDC mixtures secreted free ISG15 protein into their culture media and conjugated intracellularly ISG15 to multiple other proteins of unknown identity (Figure 5C; N=4 experiments). Unstimulated pDCs did not have detectable intracellular or secreted ISG15 protein at 24 hours. IFNAR2 blockade (N=2 experiments) abrogated production of ISG15 conjugates and secretion of ISG15. TLR9 stimulated mixed PBMCs or PBMCs depleted of pDCs did not produce ISG15 pathway transcripts or ISG15 conjugated proteins measurable by the methods we used (N=2 experiments).
Discussion
We show here that human pDCs become far more efficient producers of IFNα in the company of increasing numbers of other pDCs and that this effect is in part mediated by engagement of the type 1 interferon receptor (IFNAR). Within the circulating blood compartment, pDCs are not in close proximity to each other, with a concentration of approximately 5–10 pDCs/µl (0.1% of white blood cells). In our most sensitive ELISA studies, 10 pDCs/µl (500 pDCs in 50 µl volumes) produced < 78 pg/ml of IFNα. In contrast, concentrating pDCs further greatly enhances IFNα production: 20 pDCs/µl produced 939 pg/ml, and 40 pDCs/µl produced > 5000 pg/ml of IFNα. Because pDCs have been observed to concentrate in areas of diseased tissue (muscle in DM [9; 10]; and glomeruli [13] and skin [14] in SLE), the heightened per cell efficiency that activated pDCs confer upon each other might contribute to a transition from a protective to harmful phenotype for these cells.
We furthermore show directly that human pDCs exposed to extracellular IFNα substantially enhance their own production of type 1 interferons, an effect known as priming that was first observed in virally-stimulated chick chorioallantoic membranes in 1958 [17], chick embryo fibroblasts in 1966 [18], and mouse fibroblasts in 1971 [19]. Models that account for priming phenomena were developed in non-human non-pDC cells. Virally-induced maximal type 1 interferon production in mouse embryonic fibroblasts and splenocytes [20; 21; 22; 25; 26; 27; 28] involves an early stage of interferon regulatory factor 3 (IRF3)-[21; 25; 26] and IRF7-mediated [21; 26] interferon-β (IFNβ) and interferon-α-4 (IFNα4) production, autostimulation of the type 1 interferon receptor (IFNAR) by early secreted type 1 interferons (possibly predominantly IFNβ) [27], and then marked amplification of IRF7-mediated IFNα [21; 27] production. Priming of mouse embryo fibroblasts and splenocytes [22] with small amounts of type 1 interferons results in more rapid and larger responses to viral stimulation [20; 21], likely by increasing their pre-stimulation cytoplasmic IRF7 levels. Mouse pDCs differ from fibroblasts in that robust TLR9 and virus stimulated pDC type 1 interferon production is independent of IFNβ [33; 34] and critically dependent on IRF7 [35], which is constitutively present at high levels in mouse pDCs [33]. IFNAR autostimulation is not required for TLR7 [36] or viral [37] mediated robust mouse pDC production of IFNα, but is required for TLR9 [36; 38] mediated production.
In contrast to these mouse studies, autocrine effects in human pDCs have been less studied. Human pDCs also express high constitutive levels of IRF7 [23; 24]. An autocrine mechanism has been suspected for a second phase of human TLR9 stimulated (with CpG-A ODN) pDCs based on consequences of anti-IFNAR antibody exposure, which resulted in moderate 43% reduction in IFNα protein [23], 50–55% reductions in IFNα and IFNβ protein [24], and 40% reduction in IFNα transcript [32]. Demonstration that antibody binding to IFNAR reduces type 1 interferon production, however, is not sufficient for concluding that an autocrine feedback loop exists because antibodies against other pDC surface molecules that do not bind IFNα (BDCA2 [24; 39], ILT7 [40], and others) also inhibit pDC interferon production. Direct experiments are thus needed to conclude that human pDCs have an autocrine mechanism and we are aware of only one such published experiment in which a large dose of extracellular IFNβ, approximately 800 pg/ml, increased IFNα protein production only modestly by approximately 20% (1.2 fold) [32]. We found that a small dose of IFNα-2a protein (25 pg/ml) increased IFNα protein production by a mean of 280% (3.8-fold, range 1.8–10.0-fold, depending on the donor), as well as 6-hour transcript levels for IFNα 3.6-fold (mean of 10 IFNα subtypes), IFN-β 2.7-fold, and IFN-ω 1.4-fold. The biological factors responsible for the varying sensitivity of donor cells to added IFNα-2a were not studied here, however inheritable factors are known to play a role in downstream type 1 interferon-inducing properties of serum [41].
The inhibition in type 1 interferon production after IFNAR blockade is consistent with these direct experiments and with a model of human pDC early IFNAR-independent modest type 1 interferon production followed by a later phase of IFNAR-dependent production [23; 24]. Our results with IFNAR blockade, reducing IFNα protein production by 86–95%, differ from previous studies [23; 24] which found only moderate reductions (43–55%). However, these other studies used substantially less anti-IFNAR antibody per pDC. Whereas we used 10–100 pg/pDC of anti-IFNAR2, other studies used 0.5 pg/pDC [23] or 2 pg/pDC [24] of the same anti-IFNAR2 antibody we used. Doses of anti-IFNAR in these other studies were based on its effect on STAT1 phosphorylation [23] or on IFNα production by cells other than pDCs (γδ T cells [24]). The much greater degree of IFNα inhibition resulting from IFNAR blockade we observed suggests that in physiologically relevant circumstances in which pDCs accumulate in diseased tissue sites, the IFNAR-dependent phase dominates type 1 IFN production. Indeed we show that an event far downstream of TLR9 stimulation, the attachment of ISG15 protein to multiple other proteins, was inhibited by IFNAR blockade. Of note, such ISG15 conjugated proteins accumulate in DM muscle tissue [42].
The current observations predict several beneficial effects of IFNAR blockade for breaking cycles of type 1 interferon associated autoimmunity. Besides for its predicted ability to affect the downstream consequences of multiple type 1 interferons (such as IFNα, IFNβ, IFNκ, and IFNω) through blood and tissue reductions of type 1 interferon-inducible transcripts and proteins, these data predict that IFNAR blockade will inhibit production of the type 1 interferon proteins themselves (Figure 6). According to current models human cells other than pDCs are entirely dependent on IFNAR feedback for type 1 interferon production, so that IFNAR blockade is predicted to inhibit type 1 interferon production from all human cells. As such, this approach may be a highly potent means to interrupting a cycle of autoimmunity at three steps, the production of type 1 interferons from all cells, their downstream IFNAR mediated signaling on other immune cells, and the production of type 1 interferon inducible transcripts and proteins at tissue sites of disease activity.
Figure 6. Predicted effects of therapeutic targeting of interferon-alpha (IFNα) compared with targeting the type 1 interferon receptor (IFNAR).
Anti-IFNα neutralization might affect IFNα effects on immune system cells and tissue production of IFN-inducible proteins, but might not affect processes driven by IFNβ or other type 1 interferons. Anti-IFNAR blockade would inhibit (1) tissue production of IFN-inducible proteins regardless of the driving type 1 interferon responsible, (2) the effects of type 1 interferons on activation and maturation of other immune system cells, such as B cells and cytotoxic T cells, and (3) production of all type 1 interferons by pDCs and all other human cells.
Supplementary Material
Acknowledgements
Partial funding through NIH NINDS R21NS057225 and Muscular Dystrophy Association MDA3523 (to S.A.G), and by MedImmune, LLC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pascual V, Farkas L, Banchereau J. Systemic lupus erythematosus: all roads lead to type I interferons. Curr Opin Immunol. 2006;18:676–682. doi: 10.1016/j.coi.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 2.Crow MK. Type I interferon in systemic lupus erythematosus. Curr Top Microbiol Immunol. 2007;316:359–386. doi: 10.1007/978-3-540-71329-6_17. [DOI] [PubMed] [Google Scholar]
- 3.Ronnblom L, Alm GV, Eloranta ML. Type I interferon and lupus. Curr Opin Rheumatol. 2009;21:471–477. doi: 10.1097/BOR.0b013e32832e089e. [DOI] [PubMed] [Google Scholar]
- 4.Greenberg SA. Proposed immunologic models of the inflammatory myopathies and potential therapeutic implications. Neurology. 2007;69:2008–2019. doi: 10.1212/01.WNL.0000291619.17160.b8. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg SA. Inflammatory myopathies: disease mechanisms. Curr Opin Neurol. 2009;22:516–523. doi: 10.1097/WCO.0b013e3283311ddf. [DOI] [PubMed] [Google Scholar]
- 6.A phase 2A, multicenter, randomized, double-blind, placebo-controlled, parallel-dose study to evaluate the safety and tolerability of multiple subcutaneous doses of MEDI-545, a fully human anti-interferon-alpha monoclonal antibody, in subjects with systemic lupus erythematosus. http://clinicaltrials.gov/ct2/show/NCT00657189.
- 7.A phase I, randomized, placebo-controlled, double-blind, dose-escalation study of single and repeat doses of rhuMAb IFNalpha, administered through the SC or IV route, in adults with systemic lupus erythematosus. http://clinicaltrials.gov/ct2/show/NCT00541749.
- 8.A phase 1b, randomized, double-blind, placebo-controlled, multicenter study to evaluate safety of multiple-dose, intravenously administered MEDI-545, a fully human anti Interferon-alpha monoclonal antibody, in adult patients with dermatomyositis or polymyositis. http://clinicaltrials.gov/ct2/show/NCT00533091.
- 9.Greenberg SA, Pinkus JL, Pinkus GS, Burleson T, Sanoudou D, Tawil R, Barohn RJ, Saperstein DS, Briemberg HR, Ericsson M, Park P, Amato AA. Interferon-alpha/beta-mediated innate immune mechanisms in dermatomyositis. Ann Neurol. 2005;57:664–678. doi: 10.1002/ana.20464. [DOI] [PubMed] [Google Scholar]
- 10.Lopez de Padilla CM, Vallejo AN, McNallan KT, Vehe R, Smith SA, Dietz AB, Vuk-Pavlovic S, Reed AM. Plasmacytoid dendritic cells in inflamed muscle of patients with juvenile dermatomyositis. Arthritis Rheum. 2007;56:1658–1668. doi: 10.1002/art.22558. [DOI] [PubMed] [Google Scholar]
- 11.Wenzel J, Schmidt R, Proelss J, Zahn S, Bieber T, Tuting T. Type I interferon-associated skin recruitment of CXCR3+ lymphocytes in dermatomyositis. Clin Exp Dermatol. 2006;31:576–582. doi: 10.1111/j.1365-2230.2006.02150.x. [DOI] [PubMed] [Google Scholar]
- 12.McNiff JM, Kaplan DH. Plasmacytoid dendritic cells are present in cutaneous dermatomyositis lesions in a pattern distinct from lupus erythematosus. J Cutan Pathol. 2008;35:452–456. doi: 10.1111/j.1600-0560.2007.00848.x. [DOI] [PubMed] [Google Scholar]
- 13.Tucci M, Quatraro C, Lombardi L, Pellegrino C, Dammacco F, Silvestris F. Glomerular accumulation of plasmacytoid dendritic cells in active lupus nephritis: role of interleukin-18. Arthritis Rheum. 2008;58:251–262. doi: 10.1002/art.23186. [DOI] [PubMed] [Google Scholar]
- 14.Farkas L, Beiske K, Lund-Johansen F, Brandtzaeg P, Jahnsen FL. Plasmacytoid dendritic cells (natural interferon- alpha/beta-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am J Pathol. 2001;159:237–243. doi: 10.1016/s0002-9440(10)61689-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vermi W, Lonardi S, Morassi M, Rossini C, Tardanico R, Venturini M, Sala R, Tincani A, Poliani PL, Calzavara-Pinton PG, Cerroni L, Santoro A, Facchetti F. Cutaneous distribution of plasmacytoid dendritic cells in lupus erythematosus. Selective tropism at the site of epithelial apoptotic damage. Immunobiology. 2009;214:877–886. doi: 10.1016/j.imbio.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Walsh RJ, Kong SW, Yao Y, Jallal B, Kiener PA, Pinkus JL, Beggs AH, Amato AA, Greenberg SA. Type I interferon-inducible gene expression in blood is present and reflects disease activity in dermatomyositis and polymyositis. Arthritis Rheum. 2007;56:3784–3792. doi: 10.1002/art.22928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isaacs A, Burke DC. Mode of action of interferon. Nature. 1958;182:1073–1074. doi: 10.1038/1821073a0. [DOI] [PubMed] [Google Scholar]
- 18.Friedman RM. Effect of interferon treatment on interferon production. J Immunol. 1966;96:872–877. [PubMed] [Google Scholar]
- 19.Stewart WE, 2nd, Gosser LB, Lockart RZ., Jr Priming: a nonantiviral function of interferon. J Virol. 1971;7:792–801. doi: 10.1128/jvi.7.6.792-801.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harada H, Matsumoto M, Sato M, Kashiwazaki Y, Kimura T, Kitagawa M, Yokochi T, Tan RS, Takasugi T, Kadokawa Y, Schindler C, Schreiber RD, Noguchi S, Taniguchi T. Regulation of IFN-alpha/beta genes: evidence for a dual function of the transcription factor complex ISGF3 in the production and action of IFN-alpha/beta. Genes Cells. 1996;1:995–1005. doi: 10.1046/j.1365-2443.1996.870287.x. [DOI] [PubMed] [Google Scholar]
- 21.Sato M, Hata N, Asagiri M, Nakaya T, Taniguchi T, Tanaka N. Positive feedback regulation of type I IFN genes by the IFN-inducible transcription factor IRF-7. FEBS Lett. 1998;441:106–110. doi: 10.1016/s0014-5793(98)01514-2. [DOI] [PubMed] [Google Scholar]
- 22.Hata N, Sato M, Takaoka A, Asagiri M, Tanaka N, Taniguchi T. Constitutive IFN-alpha/beta signal for efficient IFN-alpha/beta gene induction by virus. Biochem Biophys Res Commun. 2001;285:518–525. doi: 10.1006/bbrc.2001.5159. [DOI] [PubMed] [Google Scholar]
- 23.Takauji R, Iho S, Takatsuka H, Yamamoto S, Takahashi T, Kitagawa H, Iwasaki H, Iida R, Yokochi T, Matsuki T. CpG-DNA-induced IFN-alpha production involves p38 MAPK-dependent STAT1 phosphorylation in human plasmacytoid dendritic cell precursors. J Leukoc Biol. 2002;72:1011–1019. [PubMed] [Google Scholar]
- 24.Kerkmann M, Rothenfusser S, Hornung V, Towarowski A, Wagner M, Sarris A, Giese T, Endres S, Hartmann G. Activation with CpG-A and CpG-B oligonucleotides reveals two distinct regulatory pathways of type I IFN synthesis in human plasmacytoid dendritic cells. J Immunol. 2003;170:4465–4474. doi: 10.4049/jimmunol.170.9.4465. [DOI] [PubMed] [Google Scholar]
- 25.Sato M, Tanaka N, Hata N, Oda E, Taniguchi T. Involvement of the IRF family transcription factor IRF-3 in virus-induced activation of the IFN-beta gene. FEBS Lett. 1998;425:112–116. doi: 10.1016/s0014-5793(98)00210-5. [DOI] [PubMed] [Google Scholar]
- 26.Sato M, Suemori H, Hata N, Asagiri M, Ogasawara K, Nakao K, Nakaya T, Katsuki M, Noguchi S, Tanaka N, Taniguchi T. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity. 2000;13:539–548. doi: 10.1016/s1074-7613(00)00053-4. [DOI] [PubMed] [Google Scholar]
- 27.Marie I, Durbin JE, Levy DE. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. Embo J. 1998;17:6660–6669. doi: 10.1093/emboj/17.22.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoneyama M, Suhara W, Fukuhara Y, Sato M, Ozato K, Fujita T. Autocrine amplification of type I interferon gene expression mediated by interferon stimulated gene factor 3 (ISGF3) J Biochem. 1996;120:160–169. doi: 10.1093/oxfordjournals.jbchem.a021379. [DOI] [PubMed] [Google Scholar]
- 29.Ito T, Kanzler H, Duramad O, Cao W, Liu YJ. Specialization, kinetics, and repertoire of type 1 interferon responses by human plasmacytoid predendritic cells. Blood. 2006;107:2423–2431. doi: 10.1182/blood-2005-07-2709. [DOI] [PubMed] [Google Scholar]
- 30.Krug A, Towarowski A, Britsch S, Rothenfusser S, Hornung V, Bals R, Giese T, Engelmann H, Endres S, Krieg AM, Hartmann G. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur J Immunol. 2001;31:3026–3037. doi: 10.1002/1521-4141(2001010)31:10<3026::aid-immu3026>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 31.Gibson SJ, Lindh JM, Riter TR, Gleason RM, Rogers LM, Fuller AE, Oesterich JL, Gorden KB, Qiu X, McKane SW, Noelle RJ, Miller RL, Kedl RM, Fitzgerald-Bocarsly P, Tomai MA, Vasilakos JP. Plasmacytoid dendritic cells produce cytokines and mature in response to the TLR7 agonists, imiquimod and resiquimod. Cell Immunol. 2002;218:74–86. doi: 10.1016/s0008-8749(02)00517-8. [DOI] [PubMed] [Google Scholar]
- 32.Osawa Y, Iho S, Takauji R, Takatsuka H, Yamamoto S, Takahashi T, Horiguchi S, Urasaki Y, Matsuki T, Fujieda S. Collaborative action of NF-kappaB and p38 MAPK is involved in CpG DNA-induced IFN-alpha and chemokine production in human plasmacytoid dendritic cells. J Immunol. 2006;177:4841–4852. doi: 10.4049/jimmunol.177.7.4841. [DOI] [PubMed] [Google Scholar]
- 33.Prakash A, Smith E, Lee CK, Levy DE. Tissue-specific positive feedback requirements for production of type I interferon following virus infection. J Biol Chem. 2005;280:18651–18657. doi: 10.1074/jbc.M501289200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erlandsson L, Blumenthal R, Eloranta ML, Engel H, Alm G, Weiss S, Leanderson T. Interferon-beta is required for interferon-alpha production in mouse fibroblasts. Curr Biol. 1998;8:223–226. doi: 10.1016/s0960-9822(98)70086-7. [DOI] [PubMed] [Google Scholar]
- 35.Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, Taniguchi T. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature. 2005;434:772–777. doi: 10.1038/nature03464. [DOI] [PubMed] [Google Scholar]
- 36.Asselin-Paturel C, Brizard G, Chemin K, Boonstra A, O'Garra A, Vicari A, Trinchieri G. Type I interferon dependence of plasmacytoid dendritic cell activation and migration. J Exp Med. 2005;201:1157–1167. doi: 10.1084/jem.20041930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barchet W, Cella M, Odermatt B, Asselin-Paturel C, Colonna M, Kalinke U. Virus-induced interferon alpha production by a dendritic cell subset in the absence of feedback signaling in vivo. J Exp Med. 2002;195:507–516. doi: 10.1084/jem.20011666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waibler Z, Anzaghe M, Ludwig H, Akira S, Weiss S, Sutter G, Kalinke U. Modified vaccinia virus Ankara induces Toll-like receptor-independent type I interferon responses. J Virol. 2007;81:12102–12110. doi: 10.1128/JVI.01190-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dzionek A, Sohma Y, Nagafune J, Cella M, Colonna M, Facchetti F, Gunther G, Johnston I, Lanzavecchia A, Nagasaka T, Okada T, Vermi W, Winkels G, Yamamoto T, Zysk M, Yamaguchi Y, Schmitz J. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J Exp Med. 2001;194:1823–1834. doi: 10.1084/jem.194.12.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao W, Rosen DB, Ito T, Bover L, Bao M, Watanabe G, Yao Z, Zhang L, Lanier LL, Liu YJ. Plasmacytoid dendritic cell-specific receptor ILT7-Fc epsilonRI gamma inhibits Toll-like receptor-induced interferon production. J Exp Med. 2006;203:1399–1405. doi: 10.1084/jem.20052454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niewold TB, Hua J, Lehman TJ, Harley JB, Crow MK. High serum IFN-alpha activity is a heritable risk factor for systemic lupus erythematosus. Genes Immun. 2007;8:492–502. doi: 10.1038/sj.gene.6364408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salajegheh M, Kong SK, Pinkus JL, Walsh RJ, Liao A, Nazareno R, Amato AA, Krastins B, Morehouse C, Higgs BW, Jallal B, Yao Y, Sarracino DA, Parker KC, Greenberg SA. Interferon-stimulated gene 15 (ISG15) conjugates proteins in dermatomyositis muscle with perifascicular atrophy. Ann Neurol. 2009 doi: 10.1002/ana.21805. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.