Abstract
The present studies assessed the potential abuse liability and likely mechanism(s) of action of the wake-promoting agent modafinil.
Methods
Experiments assessed the locomotor sensitization (LS) and discriminative stimulus (DS) properties of modafinil in mouse and rat, respectively. Comparative data were generated with a range of psychostimulants and monoamine reuptake inhibitors.
Results
Repeated administration of d-amphetamine and cocaine, psychostimulants with high abuse liability, resulted in the induction and expression of LS in mice. Bupropion and caffeine, two psychostimulants not abused in humans, were not associated with LS. GBR12909 induced LS during repeated exposure, but there was no evidence of expression of LS after acute challenge following withdrawal. In contrast, repeated administration of modafinil resulted in the expression, but not induction, of LS. d-amphetamine, but not the μ-opioid agonist morphine or the nAChR agonist nicotine, fully substituted for the cocaine DS in rats. The selective dopamine transporter (DAT) inhibitor GBR12909 fully substituted, the preferential norepinephrine transporter (NET) inhibitor desipramine partially substituted, and the selective serotonin reuptake inhibitor citalopram failed to substitute for cocaine. Modafinil fully substituted for cocaine, similar to the mixed DAT/NET inhibitor bupropion.
Conclusions
Two preclinical assays indicated potential abuse liability of modafinil; drug discrimination studies suggest DAT blockade by modafinil is a likely mechanism of action in vivo.
Keywords: modafinil, locomotor sensitization, drug discrimination, cocaine, d-amphetamine, bupropion, citalopram, desipramine, GBR12909, caffeine, morphine, nicotine, rat, mouse
1. Introduction
Modafinil (2-[(Diphenylmethyl)sulfinyl]acetamide; Provigil®) is a wake-promoting agent currently approved for treatment of excessive daytime sleepiness, and is being investigated for use in the treatment of fatigue due to conditions such as cancer (Cooper et al. 2009) and amyotrophic lateral sclerosis (Rabkin et al. 2009). In addition, early clinical trials suggest that modafinil may be useful in treating cognitive disorders (Biederman and Pliszka 2008, Kahbazi et al. 2009) and deficits (Kohli et al. 2009). Preliminary clinical trials indicated that modafinil may be effective in treating cocaine dependence (Dackis et al. 2005; Hart et al. 2008), although a meta-analysis indicated that modafinil was not effective in reducing cocaine use (Castells et al. 2007). It is particularly important to consider the abuse liability of modafinil given the interest in using modafinil to treat both ADHD in children and adolescents (Biederman and Pliszka 2008) and amphetamine abuse in adults with ADHD (Mann and Bitsios 2009).
Despite reports that modafinil exhibits low abuse potential (Rush et al. 2002b; Deroche-Gamonet et al. 2002; for review, see Myrick et al. 2004), preclinical and human studies have warned that modafinil may posses significant abuse potential (Gold and Balster 1996; Stoops et al. 2005), at least in vulnerable populations, due to increased dopamine release in brain reward circuitry (Volkow et al. 2009). The present experiments aimed to add to the preclinical literature on the potential abuse liability of modafinil, using locomotor sensitization in drug-naïve mice and drug discrimination in rats trained to discriminate cocaine from saline.
Locomotor sensitization (LS) refers to the phenomenon in which repeated intermittent administration of a drug of abuse results in a progressive increase in the locomotor-stimulant effects of the drug during the repeated exposure phase and in response to acute drug challenge after a drug-free (‘withdrawal’) period (Short and Shuster 1976; Bartoletti et al. 1983; Reith 1986; Shoaib and Stolerman 1992). The induction and expression of LS is linked to multiple neuroadaptations in the mesocorticolimbic system, heavily implicated in reward-related behavior (Wolfe 1998; Vanderschuren and Kalivas 2000; Thomas et al. 2008). Due to the importance of LS in the development and persistence of addiction (Robinson and Berridge 1993), the assay may be useful in assessing the potential abuse liability of a novel compound by determining whether repeated intermittent exposure to that compound results in the emergence of a sensitized locomotor response. Based on modafinil-induced inhibition of the dopamine transporter and perhaps other monoamine transporters (Madras et al. 2006; Zolkowska et al. 2009), the present studies aimed to determine whether repeated exposure to modafinil would result in locomotor sensitization in mice. Mice were selected for locomotor sensitization studies based on the increasing popularity of mouse for LS studies, coupled with the potential use of genetically modified mice to identify neurobiological substrates of addiction.
Drug discrimination (DD) is an assay of operant behavior based on the interoceptive (‘discriminative stimulus’) properties of test compounds (Silverman and Ho 1976; Holzman 1985). Rats were selected for drug discrimination studies based on the long history of DD studies in rats. DD has been used to assess drug abuse liability, based on the idea that a test compound which substitutes for a drug of abuse shares the discriminative stimulus and pharmacological properties of that drug of abuse (for a critical review of the clinical translatability of DD and other abuse liability assessment procedures, see Carter and Griffiths 2009). Previously, modafinil has been shown to substitute for the cocaine discriminative stimulus (Gold and Balster 1996; Dopheide et al. 2007). DD studies also allow the exploration of pharmacological mechanisms of action. The pharmacological properties of modafinil are not yet entirely clear, but appear to include effects on norepinephrine, serotonin, glutamate, GABA, histamine and orexin signaling (for review, see Minzenberg and Carter 2008). Accumulating preclinical (Fuxe et al. 1992; Mignot et al. 1994; de Saint Hilaire et al. 2001; Wisor et al. 2001; Madras et al. 2006; Zolkowska et al. 2009) and clinical (Volkow et al. 2009) evidence points toward significant inhibition of the dopamine transporter as one of the main mechanisms of action, resurrecting concerns about the potential abuse liability of the compound. In the present studies, the discriminative stimulus properties of selective single (GBR12909, citalopram, desipramine) and dual (bupropion) monoamine reuptake inhibitors and modafinil were compared in cocaine-trained rats.
In summary, the present studies assessed the potential for abuse liability and likely mechanism(s) of action of the wake-promoting agent modafinil, via the locomotor sensitization and drug discrimination procedures in mice and rats, respectively.
2. Materials and Methods
2.1. Subjects
Male C57BL/6J mice were obtained from Jackson Laboratories (Bar Harbor, ME) and male Sprague-Dawley rats were obtained from Harlan Laboratories (Indianapolis, IN). After acclimation to the vivarium, animals were either ear-notched (mice) or tail-marked (rats). Mice were group-housed in OptiMICE ventilated cages and rats were single-housed in OptiRAT ventilated cages. All animals were maintained on a 12/12 hr light/dark cycle (lights on 0700 EST). The room temperature was maintained at 20–23°C with relative humidity at approximately 50%. For mice, food was available ad libitum for the duration of the study, except during testing. For rats, chow was restricted to maintain body weights at 85% of freefeeding age-matched controls. For all subjects, water was provided ad libitum for the duration of the study, except during testing. All testing was conducted during the light phase, 5 days per week. The behavioral tests were conducted according to established protocols approved by the PsychoGenics, Inc. IACUC committee in AALAC-accredited facilities, and in accordance with the Guide to Care and Use of Laboratory Animals (National Institutes of Health 1996).
2.2. Apparatus
Locomotor activity was measured in Plexiglas square chambers (27.3 × 27.3 × 20.3 cm; Med Associates Inc., St Albans, VT) surrounded by infrared photobeam sources and detectors. Mice were tested under ambient light and data were collected by Med Associates software.
All DD testing took place in operant conditioning chambers (30.5 cm × 24.1cm × 21.0 cm) located in sound-attenuating cubicles equipped with an exhaust fan (Med Associates, St Albans, VT). Each chamber contained two response levers situated on one wall of the chamber. A stimulus light was located above each lever and a house light was located at the top of the opposite wall. A pellet receptacle was situated between the two levers for delivery of food pellets (45 mg). Data were collected, and test session functions were controlled, by Med PC IV software (Med Associates, St Albans, VT).
2.3. Experimental Procedures
2.3.1. Locomotor Sensitization Studies
Locomotor sensitization was assessed over a 20 day testing period, and under four testing phases: baseline locomotor activity (3 consecutive days), drug-induced locomotion (5 consecutive days), wash-out (10 days), and challenge (2 days testing of vehicle and test compound according to a cross-over design: i.e., on day 1 half of subjects received drug and half vehicle; conditions were reversed on day 2). Test compounds were administered IP or PO (modafinil only, based on pilot data indicating locomotor activation occurred after only PO, not IP, modafinil administration) in a volume of 10 ml/kg, immediately prior to the mice being placed in the open field for a 30-minute test session, during the 5-day repeated exposure phase and the 2-day challenge phase.
2.3.2. Drug Discrimination Studies
Twelve rats were tested according to a double-alternation two-week schedule [Drug (D), Vehicle (V), D, D, V; V, D, V, V, D]. During training sessions, rats were administered either cocaine (10 mg/kg, IP) or vehicle and were immediately placed in the operant chambers. Five minutes later, the test session was initiated; this was signaled by illumination of the house-light and the stimulus lights. After cocaine administration, responding on one lever was reinforced by delivery of a 45 mg food pellet under a fixed-ratio 20 schedule; responses on the inappropriate lever reset the FR response requirement. After saline administration, responding on the opposite lever was reinforced. Lever assignation was counter-balanced across subjects. Delivery of each food pellet was immediately followed by a 20 s time-out period during which further lever-pressing had no consequence and the house and stimulus lights were extinguished. After consistent responding on the appropriate cocaine- or vehicle-associated lever for at least 80% of total lever presses across the whole session and during the first fixed-ratio 20 of the session, compound testing was initiated. During test sessions, responding on both levers was reinforced via food pellet delivery.
Test compounds were administered 15 (d-amphetamine, nicotine) or 30 (morphine, modafinil, GBR12909, desipramine, bupropion, citalopram) minutes prior to testing, either IP or SC (nicotine and morphine only) in a volume of 1 ml/kg. All test compounds were administered according to a randomized-order, counter-balanced, within-subjects design. Variable numbers of rats were exposed to each test compound, but all subjects were used to generate the cocaine dose-response function. Specific group sizes for each test compound are indicated in the Figure 3 legend.
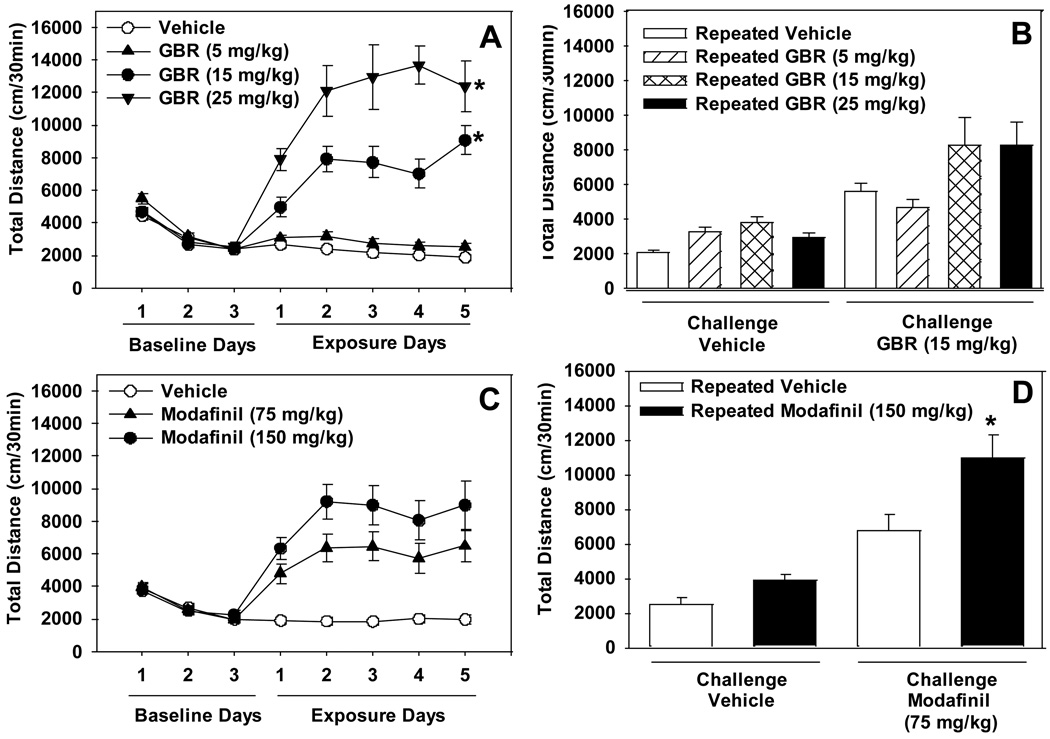
Figure 3. The effects of repeated administration of GBR12909 and modafinil on locomotor activity.
Panel A depicts total locomotor activity measured across 3 days of vehicle (baseline) and 5 days of GBR12909 (5, 15, or 25 mg/kg) or vehicle administration (n=10/group). Panel B shows locomotor activity measured after administration of a challenge dose of GBR12909 (15mg/kg) following the 10 day wash-out period. Panel C depicts total locomotor activity measured across 3 days of vehicle (baseline) and 5 days of modafinil (75 or 150 mg/kg) or vehicle treatment (n=13–14/group). Panel D shows total locomotor activity measured after administration of a challenge dose of modafinil (75 mg/kg) following the 10 day wash-out period. Asterisks (*p<0.05) indicate a significant difference compared to either Day 1 of drug exposure (Panels A and C) or the repeated vehicle-acute challenge groups (Panels B and D).
2.4. Drugs
Cocaine hydrochloride, nicotine bitartrate, morphine sulfate, d-amphetamine sulfate, modafinil, desipramine hydrochloride, citalopram, bupropion hydrochloride, GBR12909 and caffeine were purchased from Sigma-Aldrich (St. Louis, MO). Cocaine, nicotine, morphine, d-amphetamine, citalopram and caffeine were dissolved in saline; GBR12909, desipramine and bupropion were dissolved in water; modafinil was dissolved in 5% arabic gum solution. All doses are reported as salt, except nicotine (free base).
2.5. Statistical Analyses
Locomotor activity was measured as total distance traveled (cm), assessed via infrared beam breaks. Induction of LS (defined as a progressive increase in locomotor activity in drug-exposed subjects over the 5 consecutive days of repeated drug exposure) was assessed by analyzing the locomotor activity data obtained on the final baseline day and the five days of repeated drug/vehicle administration. Data were analyzed by repeated measures ANOVAs where Day (6 levels) was the repeated measures and Treatment (variable number of levels depending on number of doses tested) was defined as the between-subjects factor. Expression of LS (defined as increased locomotor activity in previously drug-exposed subjects compared to vehicle-exposed controls after acute challenge following the 10 day wash-out period) was assessed by analyzing the locomotor activity data obtained on the drug and vehicle challenge days, via a repeated measures ANOVA with Challenge (2 levels) as the repeated measure, and Exposure (variable levels depending on the number of doses tested) defined as the between-subjects factor.
Drug discrimination data were expressed as the percent of cocaine-appropriate lever responding, and as the rate of responding during the test session. Percent of cocaine-appropriate lever responding was calculated by dividing the number of responses on the drug-appropriate lever by the total responses on both levers. Response rates were calculated by dividing the total number of responses by the total duration of the session in seconds. If the response rate was less than 0.02 responses per second at a specific dose for a specific subject, cocaine-appropriate lever responding data for that subject at that dose were excluded. Linear regression analyses and ED50 values were performed using GraphPad Prism 5.0 software (La Jolla, CA). Full substitution was defined as more than 80% drug-appropriate responding; partial substitution was defined as between 20–80%, and lack of substitution was defined as less than 20% drug-appropriate responding. Data were analyzed by analyses of variance (ANOVA) followed by Fisher post-hoc comparisons where appropriate, using Statview software (SAS Institute, Inc., Cary, NC).
3. Results
3.1. The effects of repeated administration of cocaine, d-amphetamine, bupropion or caffeine on locomotor activity
3.1.1. Cocaine
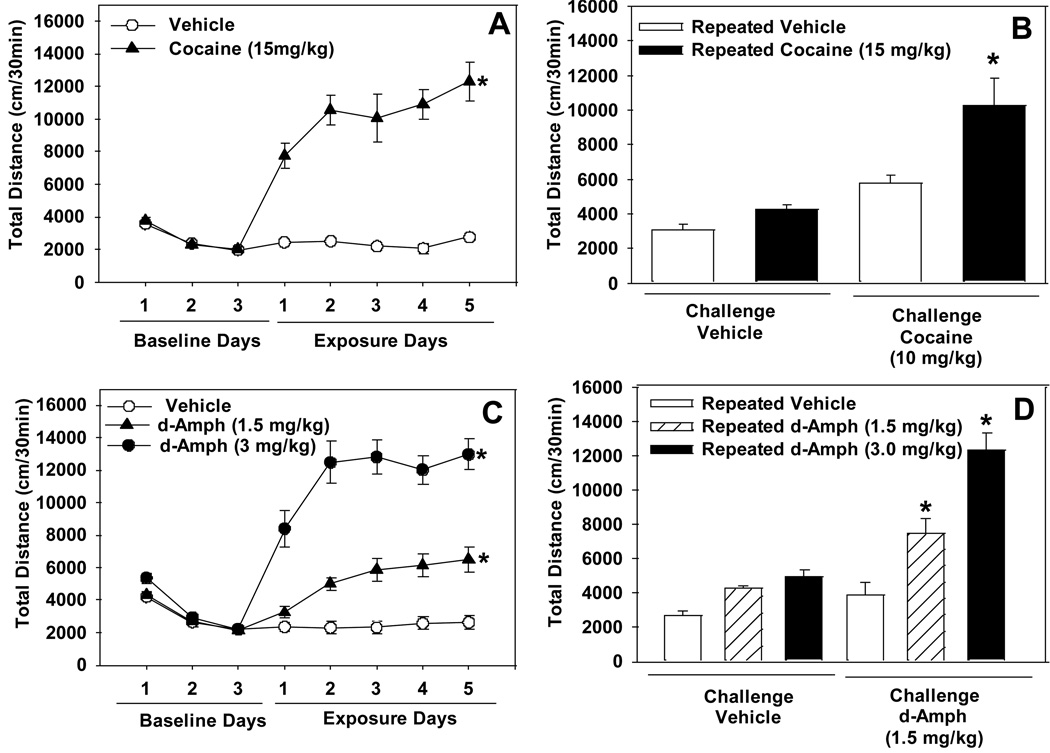
A repeated measures ANOVA on data obtained from all 5 days of cocaine/vehicle exposure did not reveal the induction of a sensitized locomotor response [very strong trend toward a significant Drug x Day interaction effect: F(4,72)=2.30, p=0.067; significant main effects of Day: F(4,72)=2.68, p<0.05 and Drug: F(1,18)=192.04, p<0.0001]. A repeated measures ANOVA comparing locomotor activity on Days 1 and 5 of cocaine/vehicle exposure revealed a significant Drug x Day interaction [F(1,18)=10.2, p<0.01]. Post-hoc tests showed that locomotor activity was significantly higher on Day 5 compared to Day 1 for subjects exposed to cocaine, but not vehicle (Figure 1A). A challenge dose of cocaine (10.0 mg/kg) following a 10 day wash-out period resulted in the expression of locomotor sensitization [significant Challenge X Exposure interaction effect: F(1,18)=5.39, p<0.05; significant main effects of Challenge: F(1,18)=37.05, p<0.0001]. Previous exposure to cocaine increased basal locomotor activity level [significant main effect of Exposure: F(1,18)=7.74, p<0.05]. Post-hoc tests showed that cocaine challenge elicited greater locomotor activation in mice that received repeated administration of cocaine versus vehicle (Figure 1B).
Figure 1. The effects of repeated administration of cocaine or d-amphetamine on locomotor activity.
Panel A depicts total locomotor activity measured across 3 days of vehicle (baseline) and 5 days of cocaine (15 mg/kg) or vehicle administration (n=10/group). Panel B shows total locomotor activity measured after administration of a challenge dose of cocaine (10 mg/kg) following the 10 day wash-out period. Panel C depicts total locomotor activity measured across 3 days of vehicle (baseline) and 5 days of d-amphetamine (1.5 or 3.0 mg/kg) or vehicle (n=10/group). Panel D shows total locomotor activity measured after administration of a challenge dose of d-amphetamine (1.5 mg/kg) following the 10 day wash-out period. Asterisks (*p<0.05) indicate a significant difference compared to either Day 1 of drug exposure (Panels A and C) or the repeated vehicle-acute challenge groups (Panels B and D).
3.1.2. Amphetamine
Repeated administration of amphetamine, but not vehicle, resulted in the induction of a sensitized locomotor response [significant Drug x Day interaction effect: (F(8,108)=10.54, p<0.0001; significant main effects of Day: F(4,108)=37.32, p<0.0001 and Drug: F(2,27)=47.39, p<0.0001]. Post-hoc tests showed that locomotor activity was significantly higher on days 2 through 5 compared to day 1 for subjects exposed to either 1.5 or 3.0 mg/kg amphetamine, but not vehicle (Figure 1C). A challenge dose of amphetamine (1.5 mg/kg) following a 10 day wash-out period resulted in the expression of locomotor sensitization [significant Challenge x Exposure interaction effect: F(2,27)=15.84, p<0.0001; significant main effect of Challenge: F(1,27)=73.09, p<0.0001; significant main effect of Exposure: F(2,27)=28.00, p<0.0001]. Post-hoc tests showed that a challenge dose of amphetamine elicited greater locomotor activation in mice that received repeated administration of amphetamine (1.5 or 3.0 mg/kg) versus vehicle (Figure 1D).
3.1.3. Bupropion
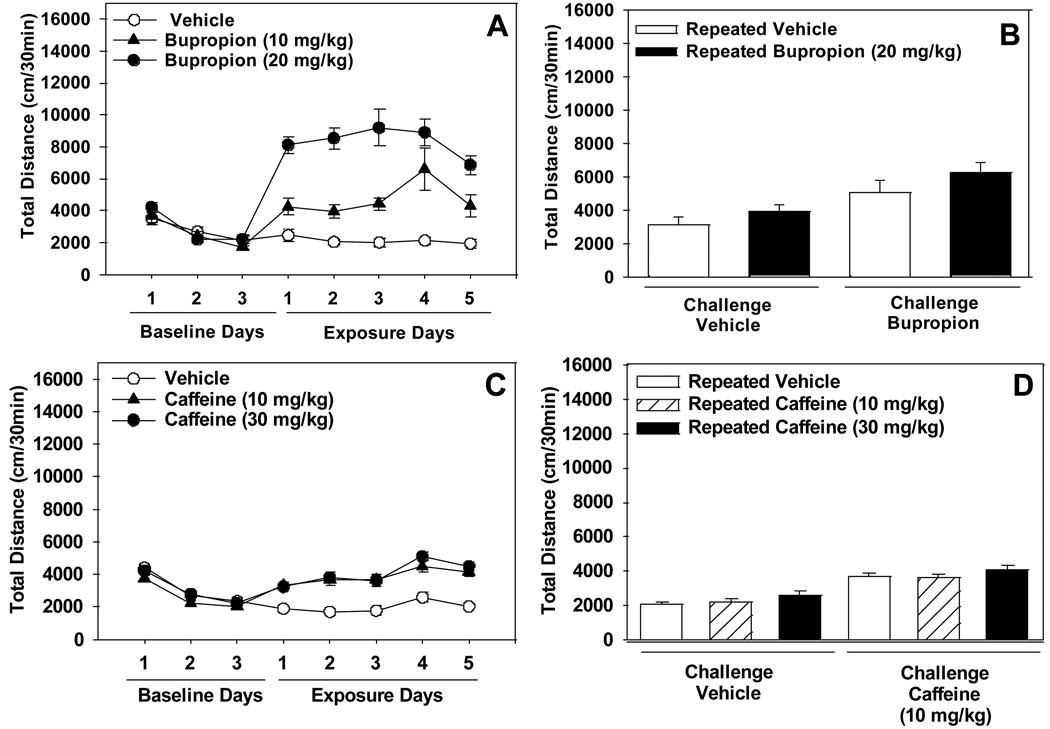
Repeated administration of bupropion (10.0 or 20.0 mg/kg) did not result in the induction of locomotor sensitization [no significant Drug x Day interaction effect: F(8,84)=1.74, n.s.], although acute bupropion increased locomotor activity compared to vehicle [significant main effect of Drug: F(2,21)=59.62, p<0.0001; significant main effect of Day: F(4,84)=2.88, p<0.05; Figure 2A]. A challenge dose of bupropion (10.0 mg/kg) following a 10 day wash-out period did not result in the expression of locomotor sensitization [no significant Challenge x Exposure interaction effect: F(1,10)=0.17, n.s.], although acute bupropion increased locomotor activity [significant main effect of Challenge: F(1,10)=30.99, p<0.001]. Previous exposure to bupropion or vehicle did not alter basal locomotor activity level [no significant main effect of Exposure: F(1,10)=0.96, n.s.; Figure 2B].
Figure 2. The effects of repeated administration of bupropion or caffeine on locomotor activity.
Panel A depicts total locomotor activity measured across 3 days of vehicle (baseline) and 5 days of bupropion (10 or 20 mg/kg) or vehicle administration (n=8/group). Panel B shows total locomotor activity measured after administration of a challenge dose of bupropion (10 mg/kg) following the 10 day wash-out period. Panel C depicts total locomotor activity measured across 3 days of vehicle (baseline) and 5 days of caffeine (10 or 30 mg/kg) or vehicle administration (n=10/group). Panel D shows total locomotor activity measured after administration of a challenge dose of caffeine (10 mg/kg) following the 10 day wash-out period. Asterisks (*p<0.05) indicate a significant difference compared to either Day 1 of drug exposure (Panels A and C) or the repeated vehicle-acute challenge groups (Panels B and D).
3.1.4. Caffeine
Repeated administration of caffeine (10.0 and 30.0 mg/kg) did not result in the induction of locomotor sensitization [no significant Drug x Day interaction effect: F(8,108)=1.61, n.s.], although caffeine increased locomotor activity [significant main effect of Drug: F(2,27)=30.10, p<0.0001; significant main effect of Day: F(4,108)=18.35, p<0.0001; Figure 2C). A challenge dose of caffeine (10.0 mg/kg) following a 10 day wash-out period did not result in the expression of locomotor sensitization, in subjects exposed to either 10.0 or 30.0 mg/kg [no significant Exposure x Challenge interaction effect: F(2,27)=0.16, n.s.], although acute caffeine increased locomotor activity [Challenge: F(1,27)=64.08, p<0.0001]. Previous exposure to caffeine or vehicle did not alter basal locomotor activity level [no significant main effect of Exposure: F(2,27)=1.94, n.s.; Figure 2D].
3.2. The effects of repeated administration of GBR12909 or modafinil on locomotor activity
3.2.1. GBR12909
Repeated administration of GBR12909 resulted in the induction of a sensitized locomotor response [significant Drug x Day interaction effect: F(12,144)=3.73, p<0.0001; significant main effect of Drug: F(3,36)=56.14, p<0.0001; significant main effect of Day: F(4,144)=5.28, p<0.001]. Post-hoc tests indicated that administration of either 15.0 or 25.0 mg/kg GBR12909, but not 5.0 mg/kg GBR12909 or vehicle, resulted in significantly higher locomotor activity on days 2 through 5 compared to day 1 (Figure 3A). A challenge dose of GBR12909 following a 10 day wash-out period did not result in the expression of locomotor sensitization [no significant Exposure x Challenge interaction effect: F(3,36)=2.17, n.s.] although acute administration of GBR 12909 increased locomotor activity [significant main effect of Challenge: F(1,36)=41.06, p<0.0001]. Previous exposure to GBR12909 versus vehicle increased basal locomotor activity [significant main effect of Exposure: F(3,36)=3.78, p<0.05; Figure 3B).
3.2.2. Modafinil
Repeated administration of modafinil did not result in the induction of locomotor sensitization [no significant Drug x Day interaction effect: F(8,148)=1.73, n.s.], although modafinil reliably increased locomotor activity [significant main effect of Drug:F(2,37)=21.65, p<0.0001]. There was some variation in the effect of modafinil across days [significant main effect of Day: F(4,148)=5.48, p<0.001; Figure 3C). A challenge dose of modafinil (75 mg/kg) following a 10 day wash-out period resulted in the expression of locomotor sensitization [significant Exposure x Challenge interaction effect: F(1,24)=5.18, p<0.05; significant main effect of Challenge: F(1,24)=86.12, p<0.0001; significant main effect of Exposure: F(1,24)=8.26, p<0.01]. Post-hoc tests showed that acute modafinil challenge resulted in a greater increase in locomotor activation in subjects previously exposed to modafinil versus vehicle (Figure 3D).
3.3. Assessment of the discriminative stimulus properties of d-amphetamine, morphine, nicotine, GBR12909, desipramine, citalopram, bupropion and modafinil in cocaine-trained rats
3.3.1. Drugs of abuse
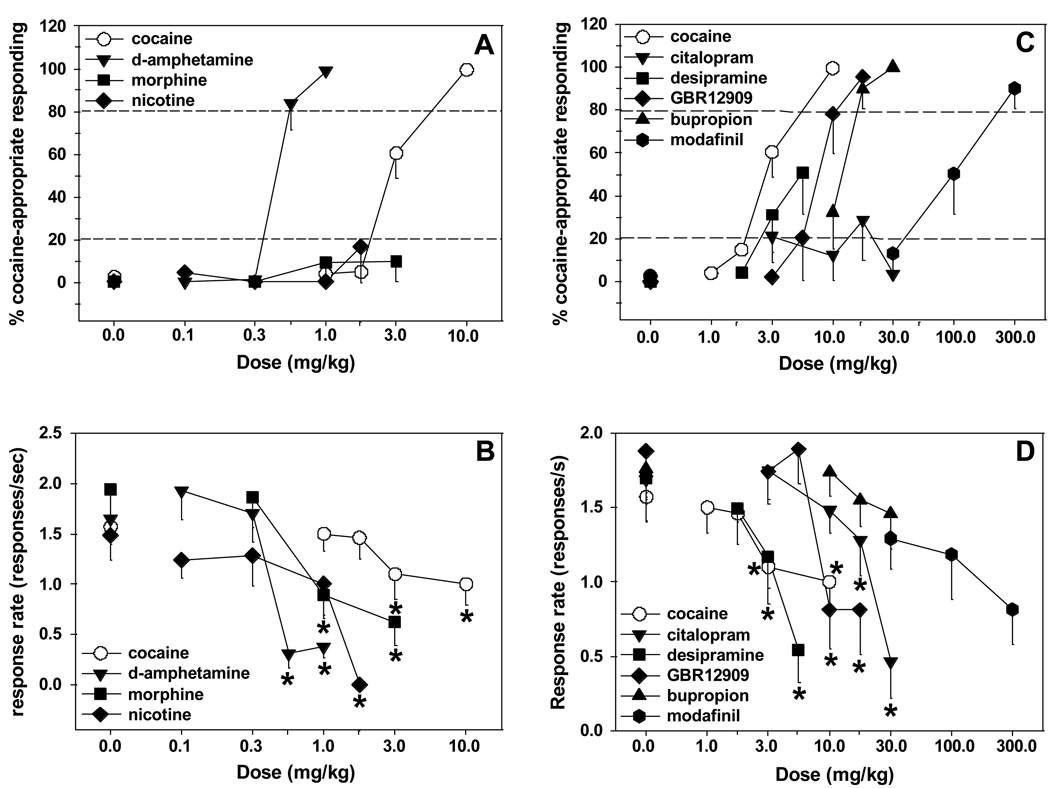
Administration of cocaine (0, 1.0, 1.7, 3.0 and 10.0 mg/kg IP) engendered a dose-dependent increase in percent cocaine-appropriate responding, with full substitution at 10 mg/kg, and an ED50 value of 2.94 (95% confidence limits 2.68–3.23) mg/kg (cocaine data are shown in all four panels of Figure 4 for reference purposes). Administration of d-amphetamine (0, 0.1, 0.3 and 0.56 mg/kg IP) engendered a dose-dependent increase in percent cocaine-appropriate responding, with full substitution at 0.56 (83.9%) and 1.0 (98.8%) mg/kg, and an ED50 value of 0.48 (95% confidence limits: 0.42–0.54) mg/kg. By contrast, administration of morphine (0, 0.3, 1.0 and 3.0 mg/kg SC) or nicotine (0, 0.1, 0.3, 1.0 mg/kg SC) did not substitute for cocaine at any dose tested (Figure 4A). Administration of cocaine [F(4,44)= 2.76, p<0.05], d-amphetamine [F(4,16)= 18.91, p<0.0001], morphine [F(3,15)= 5.85, p<0.01] and nicotine [F(4,20)= 6.3, p<0.01], resulted in decreased response rates. Post-hoc tests indicated that response rates were significantly decreased after administration of 3.0 and 10.0 mg/kg cocaine, 0.56 and 1.0 mg/kg d-amphetamine, 1.0 and 3.0 mg/kg morphine and 1.7 mg/kg nicotine (Figure 4B).
Figure 4. Discriminative stimulus properties of d-amphetamine, nicotine, morphine GBR12909, citalopram, desipramine, bupropion and modafinil in cocaine-trained rats.
The graphs depict the effects of d-amphetamine (n=5), nicotine (n=6) and morphine (n=6; Panels A and B), GBR12909 (n=7), citalopram (n=8), desipramine (n=8), bupropion (n=9) and modafinil (n=9; Panels C and D), on cocaine-appropriate responding and response rates in rats trained to discriminate cocaine (10 mg/kg IP) from saline. Panels A and C include the cocaine dose-response function obtained for all rats (n=12). The dashed lines represent the thresholds for full (80%) and partial (20%) substitution. Asterisks (*p<0.05, **p<0.01) indicate significant differences compared to vehicle.
3.3.2. Monoamine reuptake inhibitors and modafinil
Administration of GBR12909 (0, 3.0, 5.6, 10.0 and 17.0 mg/kg IP) engendered a dose-dependent increase in percent cocaine-appropriate responding, with full substitution (87.7%) at 17.0 mg/kg and an ED50 of 9.1 mg/kg (95% confidence limits 6.98–11.86) mg/kg. Administration of desipramine (0, 1.7, 3.0 and 5.6 mg/kg IP) exhibited partial substitution for cocaine at 3.0 (31.2%) and 5.6 (50.8%) mg/kg. It should be noted that only 7 and 6 out of 8 rats responded at 3.0 and 5.6 mg/kg doses, respectively. Administration of citalopram (0, 3.0, 10.0, 17.0 and 30.0 mg/kg IP) failed to substitute for cocaine at any dose tested, except at 17.0 mg/kg (28.5%), with only 7 and 3 out of 8 rats responding at the 17.0 and 30.0 mg/kg doses, respectively. Administration of bupropion (0, 10.0, 17.0 and 30.0 mg/kg) engendered a dose-dependent increase in percent cocaine-appropriate responding, with full substitution at 17.0 (91.2%) and 30.0 (99.94%) mg/kg IP, and an ED50 value of 11.78 (95% confidence limits 9.58–14.08) mg/kg. Administration of modafinil (0, 30, 100 and 300 mg/kg IP) exhibited full substitution for cocaine at 300 mg/kg (90.2%), with an ED50 value of 95.96 (95% confidence limits 42.93–214.5) mg/kg (Figure 4C). Administration of GBR12909 [F(4,24)= 14.15, p<0.0001], desipramine [F(3,21)= 11.24, p<0.0001] and citalopram [F(4,28)= 14.27, p<0.0001], but not bupropion [F(3,21)= 0.86, n.s.], resulted in decreased response rates. Post-hoc tests indicated that response rates were significantly decreased after administration of 10.0 and 17.0 mg/kg GBR12909, 3.0 and 5.6 mg/kg desipramine, and 17.0 and 30.0 mg/kg citalopram. There was a strong trend for modafinil to decrease response rates [F(3,21)= 2.99, p=0.054; Figure 4D).
4. Discussion
In the present studies, previous exposure to modafinil resulted in the expression of a sensitized locomotor response in mice and fully substituted for the cocaine discriminative stimulus in rats. The effects of modafinil in these two assays were shared with those of abused drugs such as cocaine and d-amphetamine. Conversely, non-abused compounds such as bupropion, desipramine, citalopram and caffeine either did not substitute for cocaine (desipramine and citalopram) or did not induce locomotor sensitization (bupropion and caffeine). Thus, the present data may indicate that modafinil exhibits potential abuse liability. Comparison of the effects of modafinil versus selective and dual monoamine reuptake inhibitors suggests that modafinil shares the same mechanism of action as dopamine (DA), but not serotonin (5-HT) reuptake inhibitors.
Locomotor sensitization occurs during, and after a period of enforced abstinence (‘withdrawal’) from, repeated administration of drugs of abuse such as cocaine (present studies; e.g., Reith 1986), d-amphetamine (present studies; e.g., Short and Shuster 1976), nicotine (e.g., Shoaib and Stolerman 1992) and morphine (e.g., Bartoletti et al. 1983). GBR12909 induced sensitization in the present studies, consistent with previous reports indicating cross-sensitization with cocaine (Baldo and Kelley 1991; Cornish and Kalivas 2001), although GBR12909-exposed subjects did not express locomotor sensitization after the withdrawal phase. In contrast with cocaine, d-amphetamine and GBR12909, repeated exposure to bupropion or caffeine did not result in the induction or expression of locomotor sensitization. Previously, bupropion induced locomotor sensitization in rats when given twice, but not once, daily (Nielsen et al. 1986; Nomikos et al. 1992). It should be noted, however, that in addition to potential species difference, which include differences between mouse and rat in metabolism of bupropion (Suckow et al. 1986), these studies (Nielsen et al. 1986; Nomikos et al. 1992) did not utilize a wash-out period, unlike the present studies. Previously, caffeine-induced sensitization was reported for mice (Hsu et al. 2009) and rats (Meliska et al. 1990). The differences between the Hsu et al. 2009 study and the present findings may be attributable to the duration of the washout period (3 versus 10 days, respectively) or the duration of caffeine exposure (14 days v 5 days, respectively). In the current LS protocol, repeated administration of modafinil (150 mg/kg) resulted in the expression, but not the induction, of locomotor sensitization. As far as these authors are aware, there are no previous reports on the locomotor sensitizing properties of modafinil.
In the DD assay, modafinil fully substituted for cocaine at 300 mg/kg, with an ED50 value of 95.96 mg/kg. Even at the dose of 300 mg/kg, there was no significant decrease in response rates. Consistent with the nature of DD as a pharmacologically specific screen for potential abuse liability (Balster and Bigelow 2003), the DA and NE releaser and reuptake inhibitor d-amphetamine fully substituted for the cocaine DS (ED50 0.48 mg/kg), as reported previously (D’Mello and Stolerman 1977; Huang and Wilson 1986). In contrast, morphine and nicotine did not substitute for cocaine at any dose tested, including behaviorally relevant doses as indicated by rate-depressant effects of the higher doses of morphine and nicotine. Although previously nicotine was reported to substitute for cocaine, the training dose of cocaine was considerably lower (5.6 mg/kg) compared to the present study (10.0 mg/kg; Cunningham et al. 2006; but see Desai et al. 1999). Morphine does not substitute for cocaine (Jarbe 1984; Wood and Emmet-Oglesby 1986; Negus et al. 1998).
Previous reports indicated that modafinil at 250 mg/kg substituted for cocaine (10.0 mg/kg IP) in rats (Gold and Balster 1996), and lower doses (64–128 mg/kg) partially substituted for 5.0 mg/kg cocaine (Dopheide et al. 2007). Self-administration is considered the gold standard for assessing the reinforcing properties of potential drugs of abuse (Balster and Bigelow 2003). Previous studies indicated that modafinil is not self-administered in drug-naïve rats from 0.28–1.7 mg/kg/infusion under a fixed-ratio 1 schedule of reinforcement and doses up to 256 mg/kg failed to induce a conditioned place preference in rats (Deroche-Gamonet et al. 2002). Modafinil was self-administered at 0.1 and 0.3 mg/kg/infusion in rhesus monkeys previously trained to self-administer cocaine (Gold and Balster 1996). The absence of self-administration of modafinil in rats versus monkeys may be due to the lack of prior psychostimulant exposure in the rat study (Gold and Balster 1996; Deroche-Gamonet et al. 2002). Supportive data include reinstatement of cocaine-seeking in rats after non-contingent modafinil at 64 mg/kg (Deroche-Gamonet et al. 2002). Nonetheless, modafinil failed to substitute for the cocaine DS in a human study and did not result in subjective ‘stimulant-like’ ratings (Rush et al. 2002a, b), although human subjects earned modafinil capsules under a progressive-ratio schedule when the self-administration session also required the performance of arithmetical, but not ‘relaxation’, tasks (Stoops et al. 2005). Overall, the preclinical data, including the present studies, present a mixed picture of the potential abuse liability of modafinil. An interesting addition to the preclinical data would be the operational assessment of psychological constructs previously associated with psychostimulant withdrawal, such as anhedonia (e.g., Paterson et al., 2000). Importantly, current clinical data suggest that abuse of modafinil is not highly prevalent (Myrick et al. 2004), perhaps due to the formulation properties of the available prescribed compound.
Locomotor sensitization and drug discrimination provide indirect measures of abuse-related properties of drugs. The present data highlight the possibility of identifying false positives in both assays. Bupropion provides an example of demonstrated abuse liability in multiple preclinical assays, yet does not appear to be abused in humans (present study; Nielsen et al. 1986; Nomikos et al. 1992; Lamb and Griffiths 1990; Tella et al. 1997). The formulation properties of prescribed preparations of bupropion most likely limit its utility as a drug of abuse. Thus, a crucial caveat for the interpretation of all preclinical abuse liability studies is the use of parenteral routes of administration of solubilized drug preparations that result in rapid onset of action. Finally, the use of food-restricted subjects in assays such as drug discrimination may enhance the rewarding properties of potential drugs of abuse (e.g., Carroll et al. 1981; Macenski and Meisch 1999). In summary, the identification of potential abuse liability of modafinil in the present studies must be considered in the context of the relative insolubility of prescribed modafinil preparations, which appear to successfully limit abuse in humans (Myrick et al. 2004). Regardless of modafinil’s abuse liability, the use of the DD assay in the present study allowed a preliminary exploration of the pharmacological mechanisms of action of modafinil in vivo.
The relative contribution of reuptake inhibition of dopamine, norepinephrine and serotonin to the cocaine DS were assessed. Specifically, the selective DAT inhibitor GBR12909 fully substituted for cocaine at 17.0 mg/kg IP (ED50=9.1 mg/kg), but the preferential NET inhibitor desipramine only partially substituted (50.8 ± 19.6%) for the cocaine DS at 5.6 mg/kg; selective serotonin reuptake inhibition via administration of citalopram only just exceeded the threshold for partial substitution (28.5 ± 18.4%) at the second-highest dose tested (17.0 mg/kg).The lack of dose-dependent effects for citalopram, coupled with the high variability in the data, suggest that serotonin reuptake did not substitute for the cocaine DS. Thus, the present data indicate that dopamine, but not norepinephrine or serotonin, reuptake inhibition is sufficient to fully mimic the cocaine DS, consistent with previous reports (Kleven et al. 1990; Baker et al. 1993; Spealman 1993; Filip and Papla 2001). Previously, a contributory role for NE involvement in the DS properties of low doses of cocaine (Terry et al. 1994; Spealman 1995) was identified; the present data indicated partial substitution with the preferential NET inhibitor desipramine. Interestingly, the mixed DA/NE reuptake inhibitor bupropion fully substituted for cocaine (ED50 value of 11.78 mg/kg), consistent with previous studies (Jones et al. 1980; Lamb and Griffiths 1990; Kleven et al. 1990; Baker et al. 1993), although Terry and Katz (1997) demonstrated that the DS properties of bupropion are mediated primarily via DAT inhibition. Thus, the present data considered in the context of previous studies suggest that modafinil substitutes for cocaine (10 mg/kg IP) via significant DAT inhibition in vivo. A contributory role for NET inhibition cannot be excluded based on the present data, but requires additional study.
The present studies indicated that modafinil may possess significant abuse liability due to the emergence of a sensitized locomotor response after repeated intermittent administration in mice and shared discriminative stimulus properties with cocaine in rats, suggesting significant DAT inhibitory effects. Nonetheless, in the context of the reported low abuse of modafinil in humans, the present studies underline the importance of crucial differences between clinical and preclinical settings, including drug solubility and rate of onset of drug action. Based on the current findings, future studies should compare the reinforcing properties of modafinil in psychostimulant-experienced and -naïve non-human primates, and further characterize the in vivo pharmacological mechanism(s) of action of modafinil via the use of selective DA and NE receptor antagonists in DD studies. Finally, studies should assess whether the cessation of chronic exposure to modafinil results in the emergence of anhedonia or dysphoria.
Acknowledgements
The authors gratefully acknowledge the technical contribution of Wenzhong Min, Michael Manzano, Christina Ruiz, Katie Cavino and Lucas Homa and the early contribution made by Dr. Su-Min Li to the drug discrimination studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baker LE, Riddle EE, Saunders RB, Appel JB. The role of monoamine uptake in the discriminative stimulus effects of cocaine and related compounds. Behav Pharmacol. 1993;4:69–79. [PubMed] [Google Scholar]
- Baldo BA, Kelley AE. Cross-sensitization between cocaine and GBR 12909, a dopamine uptake inhibitor. Brain Res Bull. 1991;27:105–108. doi: 10.1016/0361-9230(91)90289-v. [DOI] [PubMed] [Google Scholar]
- Balster RL, Bigelow GE. Guidelines and methodological reviews concerning drug abuse liability assessment. Drug Alcohol Depend. 2003;70:S13–S40. doi: 10.1016/s0376-8716(03)00097-8. [DOI] [PubMed] [Google Scholar]
- Bartoletti M, Gaiardi M, Gubellini G, Bacchi A, Babbini M. Long-term sensitization to the excitatory effects of morphine: a motility study in post-dependent rats. Neuropharmacology. 1983;22:1193–1196. doi: 10.1016/0028-3908(83)90080-1. [DOI] [PubMed] [Google Scholar]
- Biederman J, Pliszka SR. Modafinil improves symptoms of attention-deficit/hyperactivity disorder across subtypes in children and adolescents. J Pediatr. 2008;152:394–399. doi: 10.1016/j.jpeds.2007.07.052. [DOI] [PubMed] [Google Scholar]
- Carroll ME, France CP, Meisch RA. Intravenous self-administration of etonitazene, cocaine and phencyclidine in rats during food deprivation and satiation. J Pharmacol Exp Ther. 1981;217:241–247. [PubMed] [Google Scholar]
- Carter LP, Griffiths RR. Principles of laboratory assessment of drug abuse liability and implications for clinical development. Drug Alcohol Depend. 2009;105:S14–S25. doi: 10.1016/j.drugalcdep.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castells X, Casas M, Vidal X, Bosch R, Roncero C, Ramos-Quiroga JA, Capellà D. Efficacy of central nervous system stimulant treatment for cocaine dependence: a systematic review and meta-analysis of randomized controlled clinical trials. Addiction. 2007;102:1871–1887. doi: 10.1111/j.1360-0443.2007.01943.x. [DOI] [PubMed] [Google Scholar]
- Cooper MR, Bird HM, Steinberg M. Efficacy and safety of modafinil in the treatment of cancer-related fatigue. Ann Pharmacother. 2009;43:721–725. doi: 10.1345/aph.1L532. [DOI] [PubMed] [Google Scholar]
- Cornish JL, Kalivas PW. Cocaine sensitization and craving: differing roles for dopamine and glutamate in the nucleus accumbens. J Addict Dis. 2001;20:43–54. doi: 10.1300/J069v20n03_05. [DOI] [PubMed] [Google Scholar]
- Cunningham CS, Polston JE, Jany JR, Segert IL, Miller DK. Interaction of lobeline and nicotinic receptor ligands with the discriminative stimulus properties of cocaine and amphetamine. Drug Alcohol Depend. 2006;84:211–222. doi: 10.1016/j.drugalcdep.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O'Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30:205–211. doi: 10.1038/sj.npp.1300600. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Darnaudéry M, Bruins-Slot L, Piat F, Le Moal M, Piazza PV. Study of the addictive potential of modafinil in naive and cocaine-experienced rats. Psychopharmacology. 2002;161:387–395. doi: 10.1007/s00213-002-1080-8. [DOI] [PubMed] [Google Scholar]
- Desai RI, Barber DJ, Terry P. Asymmetric generalization between the discriminative stimulus effects of nicotine and cocaine. Behav Pharmacol. 1999;10:647–656. doi: 10.1097/00008877-199911000-00011. [DOI] [PubMed] [Google Scholar]
- D'Mello GD, Stolerman IP. Comparison of the discriminative stimulus properties of cocaine and amphetamine in rats. Br J Pharmacol. 1977;61:415–422. doi: 10.1111/j.1476-5381.1977.tb08434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dopheide MM, Morgan RE, Rodvelt KR, Schachtman TR, Miller DK. Modafinil evokes striatal [(3)H]dopamine release and alters the subjective properties of stimulants. Eur J Pharmacol. 2007;568:112–123. doi: 10.1016/j.ejphar.2007.03.044. [DOI] [PubMed] [Google Scholar]
- Filip M, Papla I. Does combined treatment with novel antidepressants and a dopamine D3 receptor agonist reproduce cocaine discrimination in rats? Pol J Pharmacol. 2001;53:577–585. [PubMed] [Google Scholar]
- Fuxe K, Janson AM, Rosén L, Finnman UB, Tanganelli S, Morari M, Goldstein M, Agnati LF. Evidence for a protective action of the vigilance promoting drug modafinil on the MPTP-induced degeneration of the nigrostriatal dopamine neurons in the black mouse: an immunocytochemical and biochemical analysis. Exp Brain Res. 1992;88:117–130. doi: 10.1007/BF02259133. [DOI] [PubMed] [Google Scholar]
- Gold LH, Balster RL. Evaluation of the cocaine-like discriminative stimulus effects and reinforcing effects of modafinil. Psychopharmacology. 1996;126:286–292. doi: 10.1007/BF02247379. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Rubin E, Foltin RW. Smoked cocaine self-administration is decreased by modafinil. Neuropsychopharmacology. 2008;33:761–768. doi: 10.1038/sj.npp.1301472. [DOI] [PubMed] [Google Scholar]
- Holtzman SG. Drug discrimination studies. Drug Alcohol Depend. 1985;14:263–282. doi: 10.1016/0376-8716(85)90061-4. [DOI] [PubMed] [Google Scholar]
- Hsu CW, Chen CY, Wang C-S, Chiu TH. Caffeine and a selective adenosine A2A receptor antagonist induce reward and sensitization behavior associated with increased phospho-Thr75 DARPP-32 in mice. Psychopharmacology. 2009;204:313–325. doi: 10.1007/s00213-009-1461-3. [DOI] [PubMed] [Google Scholar]
- Huang D, Wilson MC. Comparative discriminative stimulus properties of dl-cathinone, d-amphetamine, and cocaine in rats. Pharmacol Biochem Behav. 1986;24:205–210. doi: 10.1016/0091-3057(86)90339-4. [DOI] [PubMed] [Google Scholar]
- Järbe TU. Discriminative stimulus properties of cocaine. Effects of apomorphine, haloperidol, procaine and other drugs. Neuropharmacology. 1984;23:899–907. doi: 10.1016/0028-3908(84)90003-0. [DOI] [PubMed] [Google Scholar]
- Jones CN, Howard JL, McBennett ST. Stimulus properties of antidepressants in the rat. Psychopharmacology. 1980;67:111–118. doi: 10.1007/BF00431964. [DOI] [PubMed] [Google Scholar]
- Kahbazi M, Ghoreishi A, Rahiminejad F, Mohammadi MR, Kamalipour A, Akhondzadeh S. A randomized, double-blind and placebo-controlled trial of modafinil in children and adolescents with attention deficit and hyperactivity disorder. Psychiatry Res. 2009 doi: 10.1016/j.psychres.2008.06.024. doi:10.1016/j.psychres.2008.06.024. [DOI] [PubMed] [Google Scholar]
- Kohli S, Fisher SG, Tra Y, Adams MJ, Mapstone ME, Wesnes KA, Roscoe JA, Morrow GR. The effect of modafinil on cognitive function in breast cancer survivors. Cancer. 2009;115:2605–2616. doi: 10.1002/cncr.24287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleven MS, Anthony EW, Woolverton WL. Pharmacological characterization of the discriminative stimulus effects of cocaine in rhesus monkeys. J Pharmacol Exp Ther. 1990;254:312–317. [PubMed] [Google Scholar]
- Lamb RJ, Griffiths RR. Self-administration in baboons and the discriminative stimulus effects in rats of bupropion, nomifensine, diclofensine and imipramine. Psychopharmacology. 1990;102:183–190. doi: 10.1007/BF02245920. [DOI] [PubMed] [Google Scholar]
- Macenski MJ, Meisch RA. Cocaine self-administration under conditions of restricted and unrestricted food access. Exp Clin Psychopharmacol. 1999;7:324–337. doi: 10.1037//1064-1297.7.4.324. [DOI] [PubMed] [Google Scholar]
- Madras BK, Xie Z, Lin Z, Jassen A, Panas H, Lynch L, Johnson R, Livni E, Spencer TJ, Bonab AA, Miller GM, Fischman AJ. Modafinil occupies dopamine and norepinephrine transporters in vivo and modulates the transporters and trace amine activity in vitro. J Pharmacol Exp Ther. 2006;319:561–569. doi: 10.1124/jpet.106.106583. [DOI] [PubMed] [Google Scholar]
- Meliska CJ, Landrum RE, Landrum TA. Tolerance and sensitization to chronic and subchronic oral caffeine: effects on wheelrunning in rats. Pharmacol Biochem Behav. 1990;35:477–479. doi: 10.1016/0091-3057(90)90189-o. [DOI] [PubMed] [Google Scholar]
- Mann N, Bitsios P. Modafinil treatment of amphetamine abuse in adult ADHD. J Psychopharmacol. 2009;23:468–471. doi: 10.1177/0269881108091258. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Carter CS. Modafinil: a review of neurochemical actions and effects on cognition. Neuropsychopharmacology. 2008;33:1477–1502. doi: 10.1038/sj.npp.1301534. [DOI] [PubMed] [Google Scholar]
- Mignot E, Nishino S, Guilleminault C, Dement WC. Modafinil binds to the dopamine uptake carrier site with low affinity. Sleep. 1994;17:436–437. doi: 10.1093/sleep/17.5.436. [DOI] [PubMed] [Google Scholar]
- Myrick H, Malcolm R, Taylor B, LaRowe S. Modafinil: preclinical, clinical, and post-marketing surveillance--a review of abuse liability issues. Ann Clin Psychiatry. 2004;16:101–109. doi: 10.1080/10401230490453743. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Guide for the care and use of laboratory animals. Washington, D.C: National Academy Press; 1996. [Google Scholar]
- Negus SS, Gatch MB, Mello NK. Effects of mu opioid agonists alone and in combination with cocaine and D-amphetamine in rhesus monkeys trained to discriminate cocaine. Neuropsychopharmacology. 1998;18:325–338. doi: 10.1016/S0893-133X(97)00163-2. [DOI] [PubMed] [Google Scholar]
- Nielsen JA, Shannon NJ, Bero L, Moore KE. Effects of acute and chronic bupropion on locomotor activity and dopaminergic neurons. Pharmacol Biochem Behav. 1986;24:795–799. doi: 10.1016/0091-3057(86)90413-2. [DOI] [PubMed] [Google Scholar]
- Nomikos GG, Damsma G, Wenkstern D, Fibiger HC. Effects of chronic bupropion on interstitial concentrations of dopamine in rat nucleus accumbens and striatum. Neuropsychopharmacology. 1992;7:7–14. [PubMed] [Google Scholar]
- Paterson NE, Myers C, Markou A. Effects of repeated withdrawal from continuous amphetamine administration on brain reward function in rats. Psychopharmacology. 2000;152:440–446. doi: 10.1007/s002130000559. [DOI] [PubMed] [Google Scholar]
- Rabkin JG, Gordon PH, McElhiney M, Rabkin R, Chew S, Mitsumoto H. Modafinil treatment of fatigue in patients with ALS: a placebo-controlled study. Muscle Nerve. 2009;39:297–303. doi: 10.1002/mus.21245. [DOI] [PubMed] [Google Scholar]
- Reith ME. Effect of repeated administration of various doses of cocaine and WIN 35,065-2 on locomotor behavior of mice. Eur J Pharmacol. 1986;130:65–72. doi: 10.1016/0014-2999(86)90184-6. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Rush CR, Kelly TH, Hays LR, Baker RW, Wooten AF. Acute behavioral and physiological effects of modafinil in drug abusers. Behav Pharmacol. 2002;13:105–115. doi: 10.1097/00008877-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Rush CR, Kelly TH, Hays LR, Wooten AF. Discriminative-stimulus effects of modafinil in cocaine-trained humans. Drug Alcohol Depend. 2002;67:311–322. doi: 10.1016/s0376-8716(02)00082-0. [DOI] [PubMed] [Google Scholar]
- de Saint Hilaire Z, Orosco M, Rouch C, Blanc G, Nicolaidis S. Variations in extracellular monoamines in the prefrontal cortex and medial hypothalamus after modafinil administration: a microdialysis study in rats. Neuroreport. 2001;12:3533–3537. doi: 10.1097/00001756-200111160-00032. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Stolerman IP. MK801 attenuates behavioural adaptation to chronic nicotine administration in rats. Br J Pharmacol. 1992;105:514–515. doi: 10.1111/j.1476-5381.1992.tb09010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short PH, Shuster L. Changes in brain norepinephrine associated with sensitization to d-amphetamine. Psychopharmacology. 1976;48:59–67. doi: 10.1007/BF00423307. [DOI] [PubMed] [Google Scholar]
- Silverman PB, Ho BT. Discriminative response control by psychomotor stimulants. Psychopharmacol Commun. 1976;2:331–337. [PubMed] [Google Scholar]
- Spealman RD. Modification of behavioral effects of cocaine by selective serotonin and dopamine uptake inhibitors in squirrel monkeys. Psychopharmacology. 1993;112:93–99. doi: 10.1007/BF02247368. [DOI] [PubMed] [Google Scholar]
- Spealman RD. Noradrenergic involvement in the discriminative stimulus effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 1995;275:53–62. [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Fillmore MT, Glaser PE, Rush CR. Reinforcing effects of modafinil: influence of dose and behavioral demands following drug administration. Psychopharmacology. 2005;182:186–193. doi: 10.1007/s00213-005-0044-1. [DOI] [PubMed] [Google Scholar]
- Suckow RF, Smith TM, Perumal AS, Cooper TB. Pharmacokinetics of bupropion and metabolites in plasma and brain of rats, mice, and guinea pigs. Drug Metab Dispos. 1986;14:692–697. [PubMed] [Google Scholar]
- Tella SR, Ladenheim B, Cadet JL. Differential regulation of dopamine transporter after chronic self-administration of bupropion and nomifensine. J Pharmacol Exp Ther. 1997;281:508–513. [PubMed] [Google Scholar]
- Terry P, Katz JL. Dopaminergic mediation of the discriminative stimulus effects of bupropion in rats. Psychopharmacology. 1997;134:201–212. doi: 10.1007/s002130050443. [DOI] [PubMed] [Google Scholar]
- Terry P, Witkin JM, Katz JL. Pharmacological characterization of the novel discriminative stimulus effects of a low dose of cocaine. J Pharmacol Exp Ther. 1994;270:1041–1048. [PubMed] [Google Scholar]
- Thomas MJ, Kalivas PW, Shaham Y. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Logan J, Alexoff D, Zhu W, Telang F, Wang GJ, Jayne M, Hooker JM, Wong C, Hubbard B, Carter P, Warner D, King P, Shea C, Xu Y, Muench L, Apelskog-Torres K. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. J Am Med Assoc. 2009;301:1148–1154. doi: 10.1001/jama.2009.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisor JP, Nishino S, Sora I, Uhl GH, Mignot E, Edgar DM. Dopaminergic role in stimulant-induced wakefulness. J Neurosci. 2001;21:1787–1794. doi: 10.1523/JNEUROSCI.21-05-01787.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Wood DM, Emmett-Oglesby MW. Characteristics of tolerance, recovery from tolerance and cross-tolerance for cocaine used as a discriminative stimulus. J Pharmacol Exp Ther. 1986;237:120–125. [PubMed] [Google Scholar]
- Zolkowska D, Jain R, Rothman RB, Partilla JS, Roth BL, Setola V, Prisinzano TE, Baumann MH. Evidence for the involvement of dopamine transporters in behavioral stimulant effects of modafinil. J Pharmacol Exp Ther. 2009;329:738–746. doi: 10.1124/jpet.108.146142. [DOI] [PMC free article] [PubMed] [Google Scholar]