Nonalcoholic fatty liver disease (NAFLD) refers to an increasingly diagnosed condition involving triglyceride accumulation in hepatocytes, resulting in a broad spectrum of liver injury. This injury ranges from simple hepatic steatosis, steatohepatitis, and chronic fibrosis to cirrhosis, and pathologically resembles alcoholic hepatitis in individuals without extensive alcohol use.1 This disease process has become progressively more prevalent and it is important that clinicians are fully aware of its pathophysiology and clinical implications.
The term nonalcoholic fatty liver disease was introduced by Schaffner and Thaler2 in 1986 and is the current terminology used to describe a progressive spectrum of liver disease beginning with simple steatosis and leading to non-alcoholic steatohepatitis (NASH), fibrosis, and ultimately, cirrhosis (Fig. 1).
Figure 1.
Histologic progression of nonalcoholic fatty liver disease from simple steatosis to cirrhosis.
The term nonalcoholic steatohepatitis was coined by Ludwig and colleagues3 in 1980 to describe a cohort of 20 patients with no history of alcohol abuse but with liver biopsies demonstrating fatty change, lobular inflammation, focal necrosis, and Mallory bodies consistent with alcoholic hepatitis. These patients were predominantly female and moderately obese. Nonalcoholic steatohepatitis is the term currently used to describe patients in whom simple steatosis has progressed to histologic evidence of inflammation, including hepatocyte ballooning, mixed acute and chronic lobular inflammation, and zone 3 perisinusoidal fibrosis.4,5 Progression of NAFLD, a relatively benign condition, to NASH is characterized by hepatic infiltration of inflammatory cells and subsequent hepatocellular injury. This inflammatory phase distinguishes NASH from simple NAFLD.6,7
Recent clinical observations have shown a clear link between development of NASH and presence of the metabolic syndrome. The metabolic syndrome is defined as insulin resistance, hypertension, dyslipidemia, and visceral obesity,8–11 and some have suggested that NAFLD be considered an additional feature of the metabolic syndrome.12 There is evidence that inflammatory changes seen in the metabolic syndrome might be an important feature of the progression of NAFLD to NASH. Obesity and insulin resistance have been found to be associated with a chronic state of systemic inflammation involving abnormal cytokine release, secondary mediators, and signaling pathways.13–15
The pathophysiology of the progression of NAFLD to NASH has yet to be fully elucidated. The first insult is the production and accumulation of triglycerides in the liver. This is primarily due to the presence of insulin resistance and hyperinsulinemia resulting in dysregulated lipogenesis and lipolysis peripherally and increased hepatic fatty acid synthesis.16 This results in simple steatosis. Progression to NASH is thought to evolve by way of a “2-hit” phenomenon17 (Fig. 2). The primary insult is lipid accumulation leading to development of steatosis, and the second is oxidative stress and lipid peroxidation resulting in cytokine-mediated recruitment and retention of inflammatory cells. Multiple cytokines, chemokines, and adipokines have been implicated in the development of NASH, including leptin,18–21 adiponectin,21,22 tumor necrosis factor–α,23,24 resistin,25,26 interluekin-6,27 and others.
Figure 2.
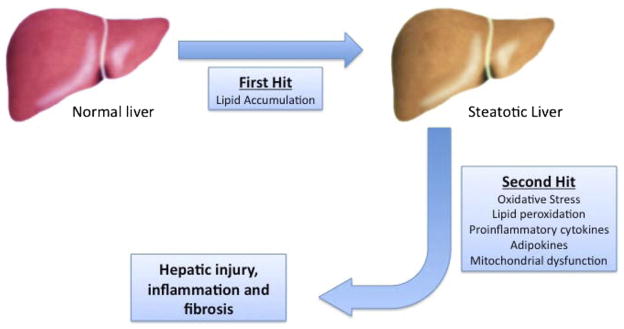
Progression of nonalcoholic fatty liver disease demonstrating the 2-hit hypothesis. The first hit consists of lipid accumulation resulting in simple benign steatosis. This primes the liver for a second hit, which can be multifactorial. This 2-hit process results in hepatic injury, inflammation, and ultimately fibrosis.
Epidemiology
NAFLD remains the most common liver abnormality in the world and the most common liver abnormality in children 2 to 19 years of age.28 The prevalence of NAFLD and NASH varies widely in the general population. The discrepancies for this stem largely from variation in invasive and noninvasive diagnostic modalities. Hospital series and population-based studies using noninvasive diagnostic techniques reveal the prevalence of NAFLD to be between 10% and 30%.29–34 Autopsy studies performed to further probe the epidemiology of NAFLD show that its prevalence ranges between 16% and 64%.35–39 These and other studies have also demonstrated that the incidence of NAFLD is increased substantially in obese patients.35,37,39,40 Interestingly, in a review of liver biopsies from asymptomatic patients with elevated liver function tests of unknown etiology, no correlation was found between histologic finding of NAFLD or NASH and obesity, hyperlipidemia, or diabetes.38 Prevalence of NAFLD in the general population remains alarmingly high, and diagnosis of this disease will likely increase as the noninvasive modalities for diagnosis improve in sensitivity and specificity.
Progression of relatively benign hepatic steatosis to NASH remains an important clinical finding, as it is predictive of progression of disease. The incidence of NASH determined from autopsy and liver biopsies has also been found to range widely between 1% and 32%.39–43 Prevalence of NASH in the general population remains difficult to determine because it requires histologic examination to make the diagnosis.
Diagnosis
Diagnosis of NAFLD and distinction of this relatively benign entity from NASH remain challenging, as noninvasive modalities are still not routinely used to differentiate the two entities. The only definitive method to diagnosis NAFLD and distinguish it from NASH and fibrosis remains liver biopsy with histologic examination.44 To standardize pathologic diagnosis, the Pathology Committee of the NASH Clinical Research Network designed and validated a scoring system of 14 histologic features examining liver biopsy findings detailing steatosis, fibrosis, inflammation, and liver cell injury.45 An NAFLD activity score >5 was universally associated with NASH and an NAFLD activity score >3 was considered not consistent with NASH (Tables 1 and 2).45
Table 1.
Nonalcoholic Fatty Liver Disease Activity Score
| Finding | Extent | Score |
|---|---|---|
| Steatosis | <5% | 0 |
| 5–33% | 1 | |
| >33–66% | 2 | |
| >66% | 3 | |
| Lobular inflammation | No foci | 0 |
| <2 foci/200× | 1 | |
| 2–4 foci/200× | 2 | |
| >4 foci/200× | 3 | |
| Hepatocyte ballooning | Few balloon cells | 1 |
| Many cells | 2 |
Table 2.
Nonalcoholic Fatty Liver Disease Activity Score Criteria
| Score | Findings |
|---|---|
| ≥5 | Diagnostic of NASH |
| 3–4 | Nondiagnostic of NASH |
| ≤2 | Excludes NASH |
NASH, nonalcoholic steatohepatitis.
Natural history
The natural history of NAFLD has been examined in several long-term studies. The difficulty with the reported literature is their short follow-up periods and selection biases in those studies using liver biopsy. In <4% of patients, simple steatosis progresses to cirrhosis46–50 (Fig. 3). Patients undergoing serial biopsies showed fibrosis stage progressing in 33% of patients and regressing in 20% to 30%.49,51 The rate of fibrotic change was found to be slow (0.02 stages/year) and diabetes and elevated body mass index (BMI) were found to be risk factors for progression of fibrosis.
Figure 3.
Histology of progression from steatosis to nonalcoholic steatohepatitis (NASH) cirrhosis. (A) Normal liver. Hematoxylin and eosin staining 200×. (B) Diffuse macrocytic steatosis with inflammatory changes. Hematoxylin and eosin staining 200×. (C) Macrocytic steatosis with neutrophil accumulation inflammation (NASH). Hematoxylin and eosin staining 400×. (D) NASH with pericellular fibrosis. Trichrome straining 400×. (E) NASH cirrhosis with bridging fibrosis. Trichrome staining 400×.
The largest of these studies reported on 420 patients with NAFLD, NASH, or cryptogenic cirrhosis during a 20-year period, with a mean follow-up of 7.6 years.50 Mortality in this group was approximately 13%, considerably worse than matched general population. Liver-related deaths occurred in <2% and liver disease was the third leading cause of death after malignancy and heart disease. Cirrhosis was found to be an independent risk factor for death and was diagnosed in 5% of patients. Cirrhosis-related complications developed in approximately 3% of patients and hepatocellular carcinoma developed in <1% of patients.
When NAFLD patients are classified according to histological grades, substantial differences in survival are observed.48 Reviewing outcomes of 132 NAFLD patients with biopsy-diagnosed and staged disease, those with early disease (fatty liver alone and steatohepatitis) showed considerably less cirrhosis and fewer liver-related deaths in comparison with those with advanced disease (steatonecrosis and steatonecrosis plus fibrosis).
The incidence of NASH progressing to end-stage liver disease requiring liver transplantation remains more difficult to extrapolate. NAFLD diagnosed by liver biopsy in 109 Danish patients revealed 1% developed cirrhosis and that life expectancy was similar to that in the normal population.47 Charlton and colleagues52 reviewed 1,207 patients evaluated for liver transplantation at the Mayo Clinic and found NASH as the primary cause of liver disease in less than 3%.
A clear association exists between obesity and hepatocellular carcinoma and multiple case series have shown that the natural progression of NASH cirrhosis is to hepatocellular carcinoma.53–56 This is similar to other causes of cirrhosis, but what remains a concern is the increased incidence of hepatocellular carcinoma in patients with NAFLD without cirrhotic change, which has been now reported in small series.55,57,58 This implicates obesity itself in the development of hepatic cancer and warrants multicenter studies to identify risk factors.
Current clinical data clearly demonstrate that simple hepatic steatosis is a relatively benign condition, with few patients progressing to NASH. What clinical and animal models have been unable to conclusively determine are the specific factors or markers that predict progression to a more ominous pathologic condition that can result in end-stage liver disease. This remains an area of intensive clinical and basic science research.
Future methods of noninvasive diagnosis
As progression from simple steatosis to NASH remains very important in predicting patient outcomes from this disease, the diagnosis of NASH with noninvasive modalities remains an area of intense research.
Noninvasive blood tests and biomarkers measuring a broad spectrum of patient and serum components including hypertension, age, gender, BMI, aspartate transaminase, alanine transaminase, insulin resistance, and hyaluronic acid have yet to demonstrate consistently acceptable sensitivity and specificity.9,59–61
Ultrasonography is an inexpensive test that has shown some promise in screening for NAFLD and has been found to have a positive predictive value of 95.4% in obese patients.62 The shortcomings of ultrasound center around operator variability, difficulty in assessment of steatosis <30%, and inability to distinguish benign fatty liver disease from NASH and fibrosis.63 Computed tomography and magnetic resonance imaging have shown promise in identifying patients with steatosis. Localized proton magnetic resonance spectroscopy accurately measures hepatic triglyceride content, providing a sensitive, quantitative, noninvasive method of measuring hepatic steatosis.64 However, this modality is also unable to differentiate between simple steatosis, NASH, and fibrosis.
A promising new modality based on ultrasound technology that measures tissue elasticity, termed transient elastography, has been found to be a noninvasive technique that can assess hepatic fibrosis.65 Transient elastography has yet to show success in long-term monitoring of fibrosis and the best noninvasive test might combine imaging and biomarkers. Much effort and progress have been made toward developing a successful noninvasive method of evaluating the progression of NAFLD from steatosis to fibrosis, liver biopsy with histological examination remains the only proven and reliable method.
Hepatic surgery and steatosis
Outcomes of patients undergoing major hepatic resection have improved substantially in recent years because of advances in perioperative patient care, resectional technique, instrumentation, and intensive care unit management. The modern mortality rate for hepatic resection is <6.0%.66–73
The effects of steatosis on postoperative complications and perioperative mortality in hepatic surgery have been less thoroughly evaluated. Studies that have selected hepatic resection patients with steatosis have demonstrated mortality ranging widely from 0 to 14%.68,73–77 Morbidity was found to be considerably higher in patients with moderate to severe steatosis compared with those with mild steatosis.74,76
The first large single-center hepatic surgery review that specifically addressed the effects of steatosis on postoperative recovery was done by Behrns and colleagues74 in 1998. This retrospective review of 135 patients looked at the histology of intraoperative specimens and classified patients into 3 groups: no steatosis, mild steatosis (<30%), and moderate to severe steatosis (>30%). Perioperative mortality was found to be 3%, 7%, and 14%, respectively. Longer operative times and increased rate of blood transfusion were also associated with steatosis severity. In addition, postoperative hepatic dysfunction was demonstrated with substantially elevated bilirubin and aspartate amino-transferase in the moderate to severe steatosis patients compared with those with mild to no steatotic change.
The largest single-center review of perioperative hepatic surgery outcomes was from Jarnagin and colleagues68 of the Memorial Sloan-Kettering Cancer Center, who looked at 1,803 hepatic resections. In a majority of patients, histologic review of specimens revealed no evidence of parenchymal abnormalities. Steatosis was identified in 325 patients and, in contrast with other reviews, did not affect perioperative outcomes. The authors attributed this to the relatively small number of steatotic patients, in comparison with those with normal hepatic pathology. In a subset analysis of these 325 patients, Kooby and colleagues76 separated patients into mild (<30%) and marked (>30%) subgroups and compared them with 160 matched nonsteatotic patients. Steatosis was found to be an independent predictor of complications, but again did not demonstrate a significant trend toward increased 60-day mortality.
An important matched case-control study of 58 patients undergoing major hepatectomy demonstrated postoperatively higher transaminases and bilirubin levels, increased blood loss, transfusions, and complications in patients with steatosis.78 This study confirmed other reports of increased postoperative morbidity associated with major hepatic resection in the steatotic patient and also showed that patients with pure macrosteatosis versus microsteatosis had increased mortality and morbidity.
The specific complications seen with hepatobiliary surgery in the steatotic patient involve wound, hepatobiliary-related, and gastrointestinal adverse events in the postoperative period. Wound complications include infections, seromas, and hernias. Hepatobiliary adverse events include cholangitis, biliary leak and obstruction, liver failure, hepatic artery infusion pump failure, ascites, perihepatic fluid collection, and perihepatic abscess. Finally, commonly seen gastrointestinal complications include hemorrhage, bowel obstruction, paralytic ileus, infectious diarrhea. and pancreatitis. No difference has been seen in the incidence of hemorrhagic, thrombotic, renal, cardiovascular, and pulmonary complications.74,76
Patients with hepatic steatosis >30% can undergo major hepatic resection with caution in the hands of an experienced hepatobiliary surgeon with the anticipation of higher rates of postoperative complications and mortality rates similar to those in patients with no steatosis. The short-term and long-term effects of hepatic resection in NASH have yet to be fully elucidated.
NAFLD in obesity surgery
Central to the pathogenesis of NAFLD is obesity and insulin resistance. As the prevalence of NAFLD has been shown to be growing steadily in industrialized Western nations with the continual growth of the obese population, the effect of weight loss on NAFLD and NASH has been intensely studied. Patients with NAFLD who are unable to achieve or maintain weight loss despite dietary and physical activity modifications can undergo Roux-en-Y gastric bypass, gastroplasty, or adjustable gastric banding to achieve an ideal body weight.
Roux-en-Y gastric bypass has been demonstrated to show substantial improvement in steatosis, lobular inflammation, and portal and lobular fibrosis in patients with biopsy-proven NASH before bypass.79,80 In a study with short-term follow-up after Roux-en-Y gastric bypass, the histopathologic criteria for NASH were no longer found post-weight loss in 89% of patients.79 Weight loss after adjustable gastric band placement has also been shown to have substantial effects on NASH. In a recent study by Dixon and colleagues,81 36 patients who underwent gastric band placement had liver biopsies after 2 years showing considerable improvement in NASH liver histopathology. A recent study of 87 patients undergoing gastroplasty for morbid obesity showed improvement of liver histopathology after weight loss.82
Although the results of obesity surgery on the histopathologic findings of NASH in patients with successful weight reduction are impressive, the long-term effects have yet to be elucidated and careful consideration must be given to the morbidity and expense of obesity surgery as a treatment modality for NAFLD and NASH.
NAFLD in liver transplantation
There are multiple implications of NAFLD in liver transplantation. First, the frequency and degree of NAFLD in the worldwide cadaveric donor liver pool and the risks of short-term and long-term hepatic graft function deserve particular consideration as the demand for organs continues to progressively outstrip availability. Second, the natural progression of NAFLD to NASH to end-stage liver disease requiring liver transplantation is relevant in defining the current frequency, outcomes, and expected increase in those requiring liver transplantation. As described here, the incidence of hepatic steatosis ranges from 16% to 64% in asymptomatic patients, who make up the potential deceased donor population. Use of steatotic grafts for liver transplantation has long been associated with poor short-term and long-term outcomes.83 However, there are varying reports of early and late graft dysfunction and primary nonfunction based on the degree of fatty infiltration.
Estimation of hepatic steatosis is defined as the percentage of hepatocytes seen on histology to contain fat droplets in the cell cytoplasm. The steatosis can be further differentiated into micro- and macrovesicular. Microvesicular refers to a collection of multiple inclusions of fat that does not displace the hepatocyte nucleus, and macrovesicular describes a single fat inclusion causing displacement of the cell nucleus84,85 (Fig. 4). Although many studies in liver transplantation using steatotic grafts combine microvesicular and macrovesicular steatosis into a single total steatosis percentage, the extent of ischemia reperfusion injury has been shown to differ, based on the type of steatosis in animal and human studies. Severe macrovesicular steatosis has been shown to have a clear impact on graft function and incidence of primary nonfunction.85 On the other hand, use of grafts with severe microvesicular steatosis has not been shown to have significant effects on primary nonfunction and graft and patient survival.86,87
Figure 4.
Histology of macrocytic and microcytic hepatic steatosis. (A) Macrocytic steatosis. Hematoxylin and eosin staining 400×. Fat vacuoles cause displacement of the hepatocyte nuclei. (B) Microcytic steatosis. Hematoxylin and eosin staining 400×. Small fat vacuoles clustered within hepatocyte with no displacement of nuclei.
Often the assessment of hepatic steatosis is made by visual inspection and palpation of the liver during organ donation. The ability of the transplantation surgeon to reliably assess steatosis by visual inspection at the time of the donor operation has been found to have a positive predictive value of <20% for mild steatosis and approximately 70% for severe steatotic change of the liver.88 Biopsy has proven to be a much more reliable indicator of macrovesicular steatotic change of the liver graft. Many centers rely on this modality to determine the appropriateness of a particular allograft for clinical usage. Even in the same liver biopsy specimen, there can be an underestimation of hepatic steatosis based on the type of staining method.89
BMI has been shown to be an inconsistent predictor of hepatic steatosis. In a review of patients undergoing evaluation as live hepatic donors, those with BMI <25 were found to have no evidence of steatosis on biopsy, and 76% of those with BMI >28 were found to have some degree of steatosis on biopsy.90 However, in another review of 100 consecutive live donor evaluation hepatic biopsies, 73% of those with BMI > 25 were found to have little to no hepatic steatosis, and approximately 10% of patients with BMI <25 were found to have some degree of NAFLD.63 Despite these contradictory results, most live donor liver transplantation programs use liver biopsy to evaluate for donor steatosis if the donor BMI is >25.
A strong correlation remains between donor liver steatosis and primary nonfunction and primary delayed function of the transplanted allograft. As early as 1991, Adam and colleagues published a review of the outcomes of 390 liver transplant recipients with frozen-section biopsy of the donor graft.88 The primary nonfunction rate was found to be 13% in grafts with biopsy-proven steatosis of >30% versus a primary nonfunction rate of <3% in those with no steatotic change in the graft. Other single-center reviews have also demonstrated increased primary nonfunction rates in severely steatotic donor grafts.91,92
Modern single-center reviews have demonstrated a much lower rate of primary nonfunction with use of the steatotic liver. In recent reviews, primary nonfunction rates of <5.0% have been noted from the use of hepatic donors organs having graft steatosis of <30%.77,83,92–94 In contrast to earlier reports that demonstrated very high rates of primary nonfunction from transplantations performed using donor livers with severe steatosis,88,91,92 more recent review have shown that moderate to severe steatotic donor organs can be used with primary nonfunction rates ranging from 2% to 5% (Table 3). These new reports of successful transplantation of severely steatotic livers with low primary nonfunction counter the conventional tenets of graft acceptability in the United States and will require additional evaluation.77 It is interesting to note that despite severe steatosis of the donor graft, simple steatosis will resolve 7 to 10 days after transplantation, implying an important role of the host systemic milieu in NAFLD. However, the price paid for successful use of severely steatotic liver allografts is markedly increased rates of primary delayed function.77,86,93–95
Table 3.
Outcomes of Liver Transplantation with Steatotic Grafts
| First author | Year | Donors | Grade | PNF, % | PDF, % | 1-y Patient survival, % | 3-y Patient survival, % | 1-y Graft survival, % | 3-y Graft survival, % |
|---|---|---|---|---|---|---|---|---|---|
| McCormack77 | 2007 | 20 | Severe | 5 | 30 | 95 | 84.4 | 95 | 84.4 |
| Yoo93 (UNOS data) | 2003 | 1,363 193 47 |
<19 20 to 35 >35 |
3.3 2.6 4.3 |
|||||
| Verran95 | 2003 | 72 48 |
<30 >30 |
0 2 |
17 35 |
84.7 79.2 |
76.5 62.5 |
||
| Soejima114 | 2003 | 23 6 |
<20 20 to 50 |
0 0 |
90.9 80.0 |
80.7 80.0 |
|||
| Angele94 | 2008 | 175 50 |
<30 >30 |
2 4 |
87* 77* |
||||
| Fishbein86 | 1997 | 40 | >30 | 5 | 10 | 80.0 | 72.5 | ||
| Marsman83 | 1996 | 59 | <30 | 5.1 | 5.1 | 80* | 76* | ||
| Ploeg92 | 1993 | 29 10 5 |
<30 30 to 60 >60 |
5 0 80 |
16 30 20 |
||||
| D’Alessandro91 | 1991 | 26 8 |
<60 >60 |
3.8 87.5 |
4-month survival.
PDF, primary delayed function; PNF, primary nonfunction; UNOS, United Network for Organ Sharing.
Primary delayed function as defined by peak aspartate aminotransferase or alanine aminotransferase >2000 U/L,96 is a clinical entity in which the newly implanted hepatic allograft works to submaximal capacity for a varying time period after transplantation. During this time, the patient can be prone to infection, renal dysfunction, and considerable coagulopathy, making management quite challenging. Such issues can drive up the resources used, in addition to overall cost of liver replacement, considerably.
The mechanisms behind the increased incidence of primary nonfunction in steatotic grafts have not been fully elucidated, but microcirculatory changes in the preserved steatotic liver have been shown to have a key role. In vivo microscopy of steatotic liver grafts has demonstrated narrow and irregular sinusoids, blood cell adhesion to the sinusoidal walls, and enhanced Kupffer cell phagocytic activity as culprits in the dysfunction of these grafts.97 Other studies have demonstrated a direct correlation between the status of the hepatic microcirculation and the degree of hepatic steatosis.98,99 Doppler flow studies in murine and human liver transplant grafts have also shown reduced blood flow in steatotic as compared with nonsteatotic livers.100,101
The final determination of the use of steatotic cadaveric liver grafts for liver transplantation should be made after histologic quantification of macrocytic steatotic change. Most centers avoid the use of donor grafts with histologic evidence of >30% macrosteatosis. However, in the continuing era of increasing demand and cadaveric organ shortage, caution should be used in employing generalized guidelines. The final determination of the use of >30% steatotic grafts should be made by an experienced liver transplantation surgeon with a thorough understanding of the recipient’s pathophysiology, Model for End-Stage Liver Disease (MELD) score, and center outcomes and experience with steatotic grafts. The ability to choose the particular recipient for a particular fatty donor allograft is key to its successful use. Some recipients, depending on their acuity of illness and other specific factors, will do well with fatty livers up to 40%, and other potential recipients certainly will not. One of the other variables of which transplantation surgeons have control is cold ischemic time, which is the period of time in which the donor liver is left without being perfused. Many studies have shown that with increased cold ischemic time, all donor organs, and especially fatty ones, do poorly. Grafts with NASH should be not be transplanted.
Living donor liver transplantation
In order to minimize poor recipient and donor results, nearly all liver transplantation centers will not accept live donors with histologic liver steatosis of >30%.102 In comparison with cadaveric donors with mild steatosis (<5%), live donor grafts with between 5% and 30% steatosis have been shown to have slightly decreased regeneration at 3 months, with the same amount of regeneration observed in both groups at 1 year.103
In a disturbing single-institutional report of 3 live donor grafts with NASH that were procured and transplanted, 2 of 3 donors had postoperative courses without complication, but there was 1 donor death.104 Although all recipients showed improvement of steatosis, the donor death highlights the importance of minimizing donor risk with exclusion of NASH donors.
The effort to maximize donor and recipient outcomes has led to frequent biopsy of donor livers, as noninvasive techniques have limitations in the detection of steatosis. This has led to a wealth of information about the incidence of NAFLD in the healthy donor and outcomes with mild steatosis in live donor liver transplantation. In a retrospective analysis of 589 donors during 13 months, the incidence of 5% steatosis was 51.4%, >30% steatosis was 10.4%, and NASH was 2.2%.105 Older age, obesity, and hypertriglyceridemia have been shown to be risk factors for steatosis.
There remains some controversy in predicting degree of steatosis without biopsy. Some have shown that liver biopsy can be safely avoided in those with normal BMI and no risk factors,90 and others have found only a weak correlation between steatosis and BMI, emphasizing the need for biopsy to determine the suitability of live donor candidates.63 The answer might lie in performing biopsies in those with risk factors, to minimize biopsy risk and maximize donor steatosis detection.
Liver transplantation for NASH
When patients are evaluated for liver transplantation as treatment for NASH, thorough evaluation and optimization of risk factors are critically important to minimize perioperative complications.
There is a considerable increase in long-term mortality for obese patients with liver transplantation, mostly as a result of cardiovascular events. Patients with morbid obesity have increased immediate, 1-, and 2-year mortality and primary nonfunction.106 In addition, this group of patients has a higher incidence of diabetes mellitus, which has been shown to have a substantial negative impact on survival after liver transplantation.107 With these risk factors in mind, it is important to optimize risk factors in this group of patients.
The natural history of NASH after liver transplantation has not yet been fully defined. Several centers have reported NASH recurrence in the transplanted liver.108–112 Of these studies, only cumulative steroid exposure was shown to correlate with development of fatty liver in transplanted graft.108 Most other clinical outcomes have shown outcomes in recipients with NASH to be similar to outcomes in other groups undergoing liver transplantation.113
The incidence of obesity in children and adults in the United States has increased profoundly during the past decade. It is estimated that 17.1% of children and adolescents and 32.2% of adults are overweight.114 With such rapid increases in prevalence of obesity and diagnosis of NAFLD and NASH, the need for hepatic resectional therapy, liver transplantation, or use of steatotic grafts will increase. It becomes very important that hepatobiliary and liver transplantation surgeons fully understand the disease pathology and implications of surgical intervention in this rapidly growing group of patients.
It is important to realize that simple hepatic steatosis is a relatively benign condition that poses little risk for progression to NASH and cirrhosis. Hepatic resectional therapy can be performed safely in patients with mild simple hepatic steatosis (<30%). Those with more severe steatosis can proceed with indicated resectional therapy with the expectation of increased morbidity, but mortality similar to those with no steatotic change. Clinical studies have yet to fully elucidate the ability to metric functional synthetic capacity and complications associated with hepatic resection in those with NASH.
The use of steatotic grafts in liver transplantation can be safe in those with biopsy-proven macrocytic steatosis of <30%. Although previous studies have shown substantially increased rates of primary nonfunction and patient mortality in those transplanted with moderate to severely steatotic grafts, more modern single-center studies have shown acceptable outcomes.
Moderate to severely steatotic grafts should only be used in select patients at centers with experience with high-risk transplantation. The effects of recurrence of baseline hepatic disease with use of steatotic grafts, if any, have yet to be fully established.
Because the diagnosis of NASH is relatively new, conclusive multicenter outcomes data for liver transplantation for end-stage liver disease secondary to this diagnosis have yet to be reported. It is important that as hepatic surgeons continue to offer invasive treatment to patients with NAFLD and NASH, and perform liver transplantation with steatotic organs, they remain aware of the operative risks in these patients and proceed with understanding and caution.
Abbreviations and Acronyms
- BMI
body mass index
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
Footnotes
Disclosure Information: Nothing to disclose.
Author Contributions
Study conception and design: Tevar
Acquisition of data: Tevar, Clarke, Wang, Rudich, Woodle, Lentsch, Edwards
Analysis and interpretation of data: Tevar, Clarke, Wang, Rudich, Woodle, Lentsch, Edwards
Drafting of manuscript: Tevar, Clarke, Wang, Rudich, Woodle, Lentsch, Edwards
Critical revision: Tevar, Clarke, Wang, Rudich, Woodle, Lentsch, Edwards
References
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Schaffner F, Thaler H. Nonalcoholic fatty liver disease [review] Prog Liver Dis. 1986;8:283–298. [PubMed] [Google Scholar]
- 3.Ludwig J, Viggiano TR, McGill DB, et al. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease [see comment] Mayo Clin Proc. 1980;55:434–438. [PubMed] [Google Scholar]
- 4.Diehl AM, Goodman Z, Ishak KG. Alcohollike liver disease in nonalcoholics. A clinical and histologic comparison with alcohol-induced liver injury. Gastroenterology. 1988;95:1056–1062. [PubMed] [Google Scholar]
- 5.Brunt EM, Janney CG, Di Bisceglie AM, et al. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 6.Diehl AM, Li ZP, Lin HZ, et al. Cytokines and the pathogenesis of non-alcoholic steatohepatitis. Gut. 2005;54:303–306. doi: 10.1136/gut.2003.024935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med. 2000;343:1467–1476. doi: 10.1056/NEJM200011163432007. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Monzon C, Martin-Perez E, Iacono OL, et al. Characterization of pathogenic and prognostic factors of nonalcoholic steatohepatitis associated with obesity. J Hepatol. 2000;33:716–724. doi: 10.1016/s0168-8278(00)80301-3. [DOI] [PubMed] [Google Scholar]
- 9.Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- 10.Chitturi S, Abeygunasekera S, Farrell GC, et al. NASH and insulin resistance: Insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology. 2002;35:373–379. doi: 10.1053/jhep.2002.30692. [DOI] [PubMed] [Google Scholar]
- 11.Diehl AM, Clarke J, Brancati F. Insulin resistance syndrome and nonalcoholic fatty liver disease. Endocr Pract. 2003;9(Suppl 2):93–96. doi: 10.4158/EP.9.S2.93. [DOI] [PubMed] [Google Scholar]
- 12.Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 13.Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterology. 2007;132:2169–2180. doi: 10.1053/j.gastro.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 14.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. [review] Hepatology. 2006;43(Suppl 1):S99–S112. doi: 10.1002/hep.20973. [DOI] [PubMed] [Google Scholar]
- 17.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 18.Shen J, Sakaida I, Uchida K, et al. Leptin enhances TNF-alpha production via p38 and JNK MAPK in LPS-stimulated Kupffer cells. Life Sci. 2005;77:1502–1515. doi: 10.1016/j.lfs.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Sakaida I, Jinhua S, Uchida K, et al. Leptin receptor-deficient Zucker (fa/fa) rat retards the development of pig serum-induced liver fibrosis with Kupffer cell dysfunction. Life Sci. 2003;73:2491–2501. doi: 10.1016/s0024-3205(03)00653-2. [DOI] [PubMed] [Google Scholar]
- 20.Kitade M, Yoshiji H, Kojima H, et al. Leptin-mediated neo-vascularization is a prerequisite for progression of nonalcoholic steatohepatitis in rats. Hepatology. 2006;44:983–991. doi: 10.1002/hep.21338. [DOI] [PubMed] [Google Scholar]
- 21.Kaser S, Moschen A, Cayon A, et al. Adiponectin and its receptors in non-alcoholic steatohepatitis. Gut. 2005;54:117–121. doi: 10.1136/gut.2003.037010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kowdley KV, Pratt DS. Adiponectin—tipping the scales from NAFLD to NASH? Gastroenterology. 2005;128:511–513. doi: 10.1053/j.gastro.2004.10.047. author reply 513. [DOI] [PubMed] [Google Scholar]
- 23.Tomita K, Tamiya G, Ando S, et al. Tumour necrosis factor alpha signalling through activation of Kupffer cells plays an essential role in liver fibrosis of non-alcoholic steatohepatitis in mice. Gut. 2006;55:415–424. doi: 10.1136/gut.2005.071118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Copaci I, Micu L, Voiculescu M. The role of cytokines in non-alcoholic steatohepatitis. A review. J Gastrointestin Liver Dis. 2006;15:363–373. [PubMed] [Google Scholar]
- 25.Bertolani C, Sancho-Bru P, Failli P, et al. Resistin as an intra-hepatic cytokine: overexpression during chronic injury and induction of proinflammatory actions in hepatic stellate cells. Am J Pathol. 2006;169:2042–2053. doi: 10.2353/ajpath.2006.060081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarrar MH, Baranova A, Collantes R, et al. Adipokines and cytokines in non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2008;27:412–421. doi: 10.1111/j.1365-2036.2007.03586.x. [DOI] [PubMed] [Google Scholar]
- 27.Hong F, Radaeva S, Pan HN, et al. Interleukin 6 alleviates hepatic steatosis and ischemia/reperfusion injury in mice with fatty liver disease. Hepatology. 2004;40:933–941. doi: 10.1002/hep.20400. [DOI] [PubMed] [Google Scholar]
- 28.Schwimmer JB, Deutsch R, Kahen T, et al. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 29.Lonardo A, Bellini M, Tartoni P, et al. The bright liver syndrome. Prevalence and determinants of a “bright” liver echopattern. Ital J Gastroenterol Hepatol. 1997;29:351–356. [PubMed] [Google Scholar]
- 30.Bellentani S, Saccoccio G, Masutti F, et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112–117. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- 31.Nomura H, Kashiwagi S, Hayashi J, et al. Prevalence of fatty liver in a general population of Okinawa, Japan. Jpn J Med. 1988;27:142–149. doi: 10.2169/internalmedicine1962.27.142. [DOI] [PubMed] [Google Scholar]
- 32.El-Hassan AY, Ibrahim EM, al-Mulhim FA, et al. Fatty infiltration of the liver: analysis of prevalence, radiological and clinical features and influence on patient management. Br J Radiol. 1992;65:774–778. doi: 10.1259/0007-1285-65-777-774. [DOI] [PubMed] [Google Scholar]
- 33.Bedogni G, Miglioli L, Masutti F, et al. Incidence and natural course of fatty liver in the general population: the Dionysos study. Hepatology. 2007;46:1387–1391. doi: 10.1002/hep.21827. [DOI] [PubMed] [Google Scholar]
- 34.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 35.Hilden M, Christoffersen P, Juhl E, et al. Liver histology in a ‘normal’ population—examinations of 503 consecutive fatal traffic casualties. Scand J Gastroenterol. 1977;12:593–597. doi: 10.3109/00365527709181339. [DOI] [PubMed] [Google Scholar]
- 36.Hultcrantz R, Glaumann H, Lindberg G, et al. Liver investigation in 149 asymptomatic patients with moderately elevated activities of serum aminotransferases. Scand J Gastroenterol. 1986;21:109–113. doi: 10.3109/00365528609034632. [DOI] [PubMed] [Google Scholar]
- 37.Ground KE. Prevalence of fatty liver in healthy male adults accidentally killed. Aviat Space Environ Med. 1984;55:59–61. [PubMed] [Google Scholar]
- 38.Daniel S, Ben-Menachem T, Vasudevan G, et al. Prospective evaluation of unexplained chronic liver transaminase abnormalities in asymptomatic and symptomatic patients [see comment] Am J Gastroenterol. 1999;94:3010–3014. doi: 10.1111/j.1572-0241.1999.01451.x. [DOI] [PubMed] [Google Scholar]
- 39.Wanless I, Lentz J. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–1110. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- 40.Byron D, Minuk GY. Clinical hepatology: profile of an urban, hospital-based practice. Hepatology. 1996;24:813–815. doi: 10.1002/hep.510240410. [DOI] [PubMed] [Google Scholar]
- 41.Berasain C, Betes M, Panizo A, et al. Pathological and virological findings in patients with persistent hypertransaminasaemia of unknown aetiology. Gut. 2000;47:429–435. doi: 10.1136/gut.47.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee RG. Nonalcoholic steatohepatitis: a study of 49 patients. Hum Pathol. 1989;20:594–598. doi: 10.1016/0046-8177(89)90249-9. [DOI] [PubMed] [Google Scholar]
- 43.Nonomura A, Mizukami Y, Unoura M, et al. Clinicopathologic study of alcohol-like liver disease in non-alcoholics; non-alcoholic steatohepatitis and fibrosis. Gastroenterol Jpn. 1992;27:521–528. doi: 10.1007/BF02777789. [DOI] [PubMed] [Google Scholar]
- 44.Wieckowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and future [review] Hepatology. 2007;46:582–589. doi: 10.1002/hep.21768. [DOI] [PubMed] [Google Scholar]
- 45.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 46.Teli MR, James OF, Burt AD, et al. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995;22:1714–1719. [PubMed] [Google Scholar]
- 47.Dam-Larsen S, Franzmann M, Andersen IB, et al. Long term prognosis of fatty liver: risk of chronic liver disease and death. Gut. 2004;53:750–755. doi: 10.1136/gut.2003.019984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 49.Harrison SA, Torgerson S, Hayashi PH. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study [see comment] Am J Gastroenterol. 2003;98:2042–2047. doi: 10.1111/j.1572-0241.2003.07659.x. [DOI] [PubMed] [Google Scholar]
- 50.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study [see comment] Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 51.Adams LA, Sanderson S, Lindor KD, et al. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies [see comment] J Hepatol. 2005;42:132–138. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 52.Charlton M, Kasparova P, Weston S, et al. Frequency of non-alcoholic steatohepatitis as a cause of advanced liver disease. Liver Transpl. 2001;7:608–614. doi: 10.1053/jlts.2001.25453. [DOI] [PubMed] [Google Scholar]
- 53.Regimbeau JM, Colombat M, Mognol P, et al. Obesity and diabetes as a risk factor for hepatocellular carcinoma. Liver Transplant. 2004;10(Suppl):S69–S73. doi: 10.1002/lt.20033. [DOI] [PubMed] [Google Scholar]
- 54.Cotrim HP, Parana R, Braga E, et al. Nonalcoholic steatohepatitis and hepatocellular carcinoma: natural history? Am J Gastroenterol. 2000;95:3018–3019. doi: 10.1111/j.1572-0241.2000.03241.x. [DOI] [PubMed] [Google Scholar]
- 55.Zen Y, Katayanagi K, Tsuneyama K, et al. Hepatocellular carcinoma arising in non-alcoholic steatohepatitis. Pathol Int. 2001;51:127–131. doi: 10.1046/j.1440-1827.2001.01174.x. [DOI] [PubMed] [Google Scholar]
- 56.Marrero JA. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36:1349–1354. doi: 10.1053/jhep.2002.36939. [DOI] [PubMed] [Google Scholar]
- 57.Bullock RE, Zaitoun AM, Aithal GP, et al. Association of non-alcoholic steatohepatitis without significant fibrosis with hepatocellular carcinoma. J Hepatol. 2004;41:685–686. doi: 10.1016/j.jhep.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 58.Kawada N, Imanaka K, Kawaguchi T, et al. Hepatocellular carcinoma arising from non-cirrhotic nonalcoholic steatohepatitis. J Gastroenterol. 2009;44:1190–1194. doi: 10.1007/s00535-009-0112-0. [DOI] [PubMed] [Google Scholar]
- 59.Palekar NA, Naus R, Larson SP, et al. Clinical model for distinguishing nonalcoholic steatohepatitis from simple steatosis in patients with nonalcoholic fatty liver disease. Liver Int. 2006;26:151–156. doi: 10.1111/j.1478-3231.2005.01209.x. [DOI] [PubMed] [Google Scholar]
- 60.Poynard T, Ratziu V, Charlotte F, et al. Diagnostic value of biochemical markers (NashTest) for the prediction of non alcoholo steato hepatitis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2006;6:34. doi: 10.1186/1471-230X-6-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gholam PM, Flancbaum L, Machan JT, et al. Nonalcoholic fatty liver disease in severely obese subjects. Am J Gastroenterol. 2007;102:399–408. doi: 10.1111/j.1572-0241.2006.01041.x. [DOI] [PubMed] [Google Scholar]
- 62.Mottin CC, Moretto M, Padoin AV, et al. The role of ultrasound in the diagnosis of hepatic steatosis in morbidly obese patients. Obes Surg. 2004;14:635–637. doi: 10.1381/096089204323093408. [DOI] [PubMed] [Google Scholar]
- 63.Ryan CK, Johnson LA, Germin BI, et al. One hundred consecutive hepatic biopsies in the workup of living donors for right lobe liver transplantation. Liver Transpl. 2002;8:1114–1122. doi: 10.1053/jlts.2002.36740. [DOI] [PubMed] [Google Scholar]
- 64.Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 65.Sporea I, Sirli R, Deleanu A, et al. Liver stiffness measurement by transient elastography in clinical practice. J Gastrointestin Liver Dis. 2008;17:395–399. [PubMed] [Google Scholar]
- 66.Kobayashi A, Kawasaki S, Miyagawa S, et al. Results of 404 hepatic resections including 80 repeat hepatectomies for hepatocellular carcinoma. Hepatogastroenterology. 2006;53(71):736–741. [PubMed] [Google Scholar]
- 67.Asiyanbola B, Chang D, Gleisner AL, et al. Operative mortality after hepatic resection: are literature-based rates broadly applicable? J Gastrointest Surg. 2008;12:842–851. doi: 10.1007/s11605-008-0494-y. [DOI] [PubMed] [Google Scholar]
- 68.Jarnagin WR, Gonen M, Fong Y, et al. Improvement in peri-operative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397–406. doi: 10.1097/01.SLA.0000029003.66466.B3. discussion 406–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iwatsuki S, Starzl TE. Personal experience with 411 hepatic resections. Ann Surg. 1988;208:421–434. doi: 10.1097/00000658-198810000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Imamura H, Seyama Y, Kokudo N, et al. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198–1206. doi: 10.1001/archsurg.138.11.1198. discussion 1206. [DOI] [PubMed] [Google Scholar]
- 71.Hemming AW, Reed AI, Fujita S, et al. Role for extending hepatic resection using an aggressive approach to liver surgery. J Am Coll Surg. 2008;206:870–875. doi: 10.1016/j.jamcollsurg.2007.12.036. discussion 875–878. [DOI] [PubMed] [Google Scholar]
- 72.Finch MD, Crosbie JL, Currie E, et al. An 8-year experience of hepatic resection: indications and outcome. Br J Surg. 1998;85:315–319. doi: 10.1046/j.1365-2168.1998.00585.x. [DOI] [PubMed] [Google Scholar]
- 73.Belghiti J, Hiramatsu K, Benoist S, et al. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. doi: 10.1016/s1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- 74.Behrns KE, Tsiotos GG, DeSouza NF, et al. Hepatic steatosis as a potential risk factor for major hepatic resection. J Gastroin-test Surg. 1998;2:292–298. doi: 10.1016/s1091-255x(98)80025-5. [DOI] [PubMed] [Google Scholar]
- 75.Little SA, Jarnagin WR, DeMatteo RP, et al. Diabetes is associated with increased perioperative mortality but equivalent long-term outcome after hepatic resection for colorectal cancer. J Gastrointest Surg. 2002;6:88–94. doi: 10.1016/s1091-255x(01)00019-1. [DOI] [PubMed] [Google Scholar]
- 76.Kooby DA, Fong Y, Suriawinata A, et al. Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg. 2003;7:1034–1044. doi: 10.1016/j.gassur.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 77.McCormack L, Petrowsky H, Jochum W, et al. Use of severely steatotic grafts in liver transplantation: a matched case-control study. Ann Surg. 2007;246:940–946. doi: 10.1097/SLA.0b013e31815c2a3f. discussion 946–948. [DOI] [PubMed] [Google Scholar]
- 78.McCormack L, Petrowsky H, Jochum W, et al. Hepatic steatosis is a risk factor for postoperative complications after major hepatectomy: a matched case-control study. Ann Surg. 2007;245:923–930. doi: 10.1097/01.sla.0000251747.80025.b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barker KB, Palekar NA, Bowers SP, et al. Non-alcoholic steato-hepatitis: effect of Roux-en-Y gastric bypass surgery. Am J Gastroenterol. 2006;101:368–373. doi: 10.1111/j.1572-0241.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 80.Liu X, Lazenby AJ, Clements RH, et al. Resolution of nonalcoholic steatohepatits after gastric bypass surgery. Obes Surg. 2007;17:486–492. doi: 10.1007/s11695-007-9086-2. [DOI] [PubMed] [Google Scholar]
- 81.Dixon JB, Bhathal PS, Hughes NR, et al. Nonalcoholic fatty liver disease: improvement in liver histological analysis with weight loss. Hepatology. 2004;39:1647–1654. doi: 10.1002/hep.20251. [DOI] [PubMed] [Google Scholar]
- 82.Jaskiewicz K, Raczynska S, Rzepko R, et al. Nonalcoholic fatty liver disease treated by gastroplasty. Dig Dis Sci. 2006;51:21–26. doi: 10.1007/s10620-006-3077-3. [DOI] [PubMed] [Google Scholar]
- 83.Marsman WA, Wiesner RH, Rodriguez L, et al. Use of fatty donor liver is associated with diminished early patient and graft survival. Transplantation. 1996;62:1246–1251. doi: 10.1097/00007890-199611150-00011. [DOI] [PubMed] [Google Scholar]
- 84.Selzner M, Clavien PA. Fatty liver in liver transplantation and surgery. Semin Liver Dis. 2001;21:105–113. doi: 10.1055/s-2001-12933. [DOI] [PubMed] [Google Scholar]
- 85.Selzner N, Selzner M, Jochum W, et al. Mouse livers with macrosteatosis are more susceptible to normothermic ischemic injury than those with microsteatosis. J Hepatol. 2006;44:694–701. doi: 10.1016/j.jhep.2005.07.032. [DOI] [PubMed] [Google Scholar]
- 86.Fishbein TM, Fiel MI, Emre S, et al. Use of livers with microvesicular fat safely expands the donor pool. Transplantation. 1997;64:248–251. doi: 10.1097/00007890-199707270-00012. [DOI] [PubMed] [Google Scholar]
- 87.Urena MA, Ruiz-Delgado FC, Gonzalez EM, et al. Assessing risk of the use of livers with macro and microsteatosis in a liver transplant program. Transplant Proc. 1998;30:3288–3291. doi: 10.1016/s0041-1345(98)01033-1. [DOI] [PubMed] [Google Scholar]
- 88.Adam R, Reynes M, Johann M, et al. The outcome of steatotic grafts in liver transplantation. Transplant Proc. 1991;23(1 Pt 2):1538–1540. [PubMed] [Google Scholar]
- 89.Urena GMA, Colina Ruiz-Delgado F, Moreno Gonzalez E, et al. Hepatic steatosis in liver transplant donors: common feature of donor population? World J Surg. 1998;22:837–844. doi: 10.1007/s002689900479. [DOI] [PubMed] [Google Scholar]
- 90.Rinella ME, Alonso E, Rao S, et al. Body mass index as a predictor of hepatic steatosis in living liver donors. Liver Transpl. 2001;7:409–414. doi: 10.1053/jlts.2001.23787. [DOI] [PubMed] [Google Scholar]
- 91.D’Alessandro AM, Kalayoglu M, Sollinger HW, et al. The predictive value of donor liver biopsies for the development of primary nonfunction after orthotopic liver transplantation. Transplantation. 1991;51:157–163. doi: 10.1097/00007890-199101000-00024. [DOI] [PubMed] [Google Scholar]
- 92.Ploeg RJ, D’Alessandro AM, Knechtle SJ, et al. Risk factors for primary dysfunction after liver transplantation—a multivariate analysis. Transplantation. 1993;55:807–813. doi: 10.1097/00007890-199304000-00024. [DOI] [PubMed] [Google Scholar]
- 93.Yoo HY, Molmenti E, Thuluvath PJ. The effect of donor body mass index on primary graft nonfunction, retransplantation rate, and early graft and patient survival after liver transplantation. Liver Transpl. 2003;9:72–78. doi: 10.1053/jlts.2003.50006. [DOI] [PubMed] [Google Scholar]
- 94.Angele MK, Rentsch M, Hartl WH, et al. Effect of graft steatosis on liver function and organ survival after liver transplantation. Am J Surg. 2008;195:214–220. doi: 10.1016/j.amjsurg.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 95.Verran D, Kusyk T, Painter D, et al. Clinical experience gained from the use of 120 steatotic donor livers for orthotopic liver transplantation. Liver Transpl. 2003;9:500–505. doi: 10.1053/jlts.2003.50099. [DOI] [PubMed] [Google Scholar]
- 96.Mor E, Klintmalm GB, Gonwa TA, et al. The use of marginal donors for liver transplantation. A retrospective study of 365 liver donors. Transplantation. 1992;53:383–386. doi: 10.1097/00007890-199202010-00022. [DOI] [PubMed] [Google Scholar]
- 97.Teramoto K, Bowers JL, Kruskal JB, et al. Hepatic micro-circulatory changes after reperfusion in fatty and normal liver transplantation in the rat. Transplantation. 1993;56:1076–1082. doi: 10.1097/00007890-199311000-00005. [DOI] [PubMed] [Google Scholar]
- 98.Seifalian AM, Piasecki C, Agarwal A, et al. The effect of graded steatosis on flow in the hepatic parenchymal microcirculation. Transplantation. 1999;68:780–784. doi: 10.1097/00007890-199909270-00009. [DOI] [PubMed] [Google Scholar]
- 99.Hayashi M, Tokunaga Y, Fujita T, et al. The effects of cold preservation on steatotic graft viability in rat liver transplantation. Transplantation. 1993;56:282–287. doi: 10.1097/00007890-199308000-00005. [DOI] [PubMed] [Google Scholar]
- 100.Seifalian AM, Chidambaram V, Rolles K, et al. In vivo demonstration of impaired microcirculation in steatotic human liver grafts. Liver Transpl Surg. 1998;4:71–77. doi: 10.1002/lt.500040110. [DOI] [PubMed] [Google Scholar]
- 101.Brandhagen D, Fidler J, Rosen C. Evaluation of the donor liver for living donor liver transplantation. Liver Transpl. 2003;9(Suppl 2):S16–S28. doi: 10.1053/jlts.2003.50222. [DOI] [PubMed] [Google Scholar]
- 102.Cho JY, Suh KS, Kwon CH, et al. Mild hepatic steatosis is not a major risk factor for hepatectomy and regenerative power is not impaired. Surgery. 2006;139:508–515. doi: 10.1016/j.surg.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 103.Yamamoto K, Takada Y, Fujimoto Y, et al. Nonalcoholic steatohepatitis in donors for living donor liver transplantation. Transplantation. 2007;83:257–262. doi: 10.1097/01.tp.0000250671.06456.3f. [DOI] [PubMed] [Google Scholar]
- 104.Lee JY, Kim KM, Lee SG, et al. Prevalence and risk factors of non-alcoholic fatty liver disease in potential living liver donors in Korea: a review of 589 consecutive liver biopsies in a single center. J Hepatol. 2007;47:239–244. doi: 10.1016/j.jhep.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 105.Nair S, Verma S, Thuluvath PJ. Obesity and its effect on survival in patients undergoing orthotopic liver transplantation in the United States. Hepatology. 2002;35:105–109. doi: 10.1053/jhep.2002.30318. [DOI] [PubMed] [Google Scholar]
- 106.Shields PL, Tang H, Neuberger JM, et al. Poor outcome in patients with diabetes mellitus undergoing liver transplantation. Transplantation. 1999;68:530–535. doi: 10.1097/00007890-199908270-00015. [DOI] [PubMed] [Google Scholar]
- 107.Carson K, Washington MK, Treem WR, et al. Recurrence of nonalcoholic steatohepatitis in a liver transplant recipient. Liver Transpl Surg. 1997;3:174–176. doi: 10.1002/lt.500030211. [DOI] [PubMed] [Google Scholar]
- 108.Contos MJ, Cales W, Sterling RK, et al. Development of nonalcoholic fatty liver disease after orthotopic liver transplantation for cryptogenic cirrhosis. Liver Transpl. 2001;7:363–373. doi: 10.1053/jlts.2001.23011. [DOI] [PubMed] [Google Scholar]
- 109.Czaja AJ. Recurrence of nonalcoholic steatohepatitis after liver transplantation. Liver Transpl Surg. 1997;3:185–186. doi: 10.1002/lt.500030215. [DOI] [PubMed] [Google Scholar]
- 110.Kim WR, Poterucha JJ, Porayko MK, et al. Recurrence of nonalcoholic steatohepatitis following liver transplantation. Transplantation. 1996;62:1802–1805. doi: 10.1097/00007890-199612270-00021. [DOI] [PubMed] [Google Scholar]
- 111.Molloy RM, Komorowski R, Varma RR. Recurrent nonalcoholic steatohepatitis and cirrhosis after liver transplantation. Liver Transpl Surg. 1997;3:177–178. doi: 10.1002/lt.500030212. [DOI] [PubMed] [Google Scholar]
- 112.Malik SM, deVera ME, Fontes P, et al. Outcome after liver transplantation for NASH cirrhosis. Am J Transplant. 2009;9:782–793. doi: 10.1111/j.1600-6143.2009.02590.x. [DOI] [PubMed] [Google Scholar]
- 113.Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 114.Soejima Y, Shimada M, Suehiro T, et al. Use of steatotic graft in living-donor liver transplantation. Transplantation. 2003;76:344–348. doi: 10.1097/01.TP.0000071205.52835.A4. [DOI] [PubMed] [Google Scholar]




