Abstract
Bone repair depends on the coordinated action of numerous growth factors and cytokines to stimulate new skeletal tissue formation. Among all the growth factors involved in bone repair, Bone Morphogenetic Proteins (BMPs) are the only molecules now used therapeutically to enhance healing. Although BMPs are known as strong bone inducers, their role in initiating skeletal repair is not entirely elucidated. The aim of this study was to define the role of BMP2 during the early stages of bone regeneration and more specifically in regulating the fate of skeletal progenitors. During healing of non-stabilized fractures via endochondral ossification, exogenous BMP2 increased the deposition and resorption of cartilage and bone, which was correlated with a stimulation of osteoclastogenesis but not angiogenesis in the early phase of repair. During healing of stabilized fractures, which normally occurs via intramembranous ossification, exogenous BMP2 induced cartilage formation suggesting a role in regulating cell fate decisions. Specifically, the periosteum was found to be a target of exogenous BMP2 as shown by activation of the BMP pathway in this tissue. Using cell lineage analyses, we further show that BMP2 can direct cell differentiation towards the chondrogenic lineage within the periosteum but not the endosteum, indicating that skeletal progenitors within periosteum and endosteum respond differently to BMP signals. In conclusion, BMP2 plays an important role in the early stages of repair by recruiting local sources of skeletal progenitors within periosteum and endosteum and by determining their differentiation towards the chondrogenic and osteogenic lineages.
Keywords: Bone Morphogenetic Proteins, Fracture Repair, Endosteum, Skeletal Progenitor Cells, Cartilage and Bone formation
INTRODUCTION
During the early stages of bone regeneration, mesenchymal cells are recruited to the site of injury, where they proliferate and differentiate into chondrocytes and osteoblasts. Little is known about the cellular and molecular regulation of mesenchymal cell recruitment. Numerous secreted growth factors, among which bone morphogenetic proteins (BMP) have been shown to stimulate osteogenesis, however their impact on various sources of skeletal progenitors in vivo is not well understood. BMPs are secreted signaling molecules within the transforming growth factor-β family that regulate various biological processes during embryonic development and in adult life [1–2]. More specifically, BMPs play critical roles in cartilage and bone formation during skeletal development and repair [3–5]. Previous studies have reported that rhBMP2 increases torsion stiffness and strength of the fracture in a goat tibial fracture model and accelerates healing in a model of rabbit ulnar osteotomy. Similarly, rhBMP7 also accelerates healing of ulna segmental defects and increases the stiffness and strength of the bone regenerate in large animals [6–9]. Besides bone autografts and allografts, rhBMP2 and rhBMP7 are the only approved biological methods to stimulate bone repair in humans and are now clinically proven to be beneficial in the treatment of bone defects, fracture non-union and spinal fusion [10–11]. Yet further advances remain to be made to improve BMP treatments since their clinical and cost-effectiveness is not always evident [12]. It is clear that BMPs are indispensable for bone repair as there is a failure to initiate fracture repair in mice carrying a conditional mutation of the Bmp2 gene [13]. Whether BMPs act directly on proliferation and differentiation of skeletal progenitor cells and/or indirectly via regulating other cell types in the initial stages of repair is not entirely clear. Some studies indicate that BMPs may act via stimulating angiogenesis in synergy with VEGF, a key angiogenic factor [14–15]. Other studies showed direct effects of BMP2 on periosteal-derived cells and bone marrow-derived mesenchymal stem cells in vitro via induction of bone and cartilage markers, acceleration of osteogenic differentiation and mineralization [16–20]. Here, we analyzed the cellular responses to rhBMP2 during fracture healing with a particular focus on the early stages of repair. We used non-stabilized and stabilized mouse fracture models to compare the impact of rhBMP2 on cartilage and bone formation during healing via endochondral and intramembranous ossification. We evaluated how the effects on osteogenesis and chondrogenesis may be correlated with changes in angiogenesis, osteoclastogenesis and cell proliferation. Furthermore, we determined the role of rhBMP2 on skeletal cell fate and used lineage analyses to assess its direct effects on periosteum- and bone marrow/endosteum-derived cells.
MATERIALS AND METHODS
Non-stabilized and stabilized fractures
All procedures followed protocols approved by the UCSF Animal Care and Use Committee. Adult C57B6 wild type mice (males 3–4 month old) were anesthetized with an intraperitoneal injection of 50mg/ml Ketamine/0.5mg/ml Metedomidine (0.03ml/mouse). Closed, standardized non-stabilized fractures were produced via three point bending as previously described [21]. Briefly, the tibial was placed on the fracture jig and a 460g weight was dropped from 14 cm to create the fracture. These non-stabilized fractures heal through the formation of a cartilage intermediate [22]. To create stabilized fractures, which heal without a cartilage intermediate, an external fixator was placed prior to creating the fracture as previously described [22]. The external fixation frames consisted of two aluminum rings stabilized by three stainless-steel #0/56 threaded rods. The frames were secured to the tibia by 0.25mm insect pins (Anticorro: Fine Science Tools, Foster City, CA) and the pins were attached to the frame with #2/56 hexagonal nuts. The total weight of the apparatus was 7.2g. Following surgery, each animal was assessed via X-Ray to select for fractures that were minimally displaced in order to further minimize mechanical strength at the fracture site. In these conditions, healing occurs entirely via intramembranous ossification, as opposed to non-stabilized fractures, which heal primarily via endochondral ossification.
BMP2 treatment of fractures
For BMP2 treatment, 10μg of rhBMP2 (diluted in 7μl of PBS, Medtronic) was injected directly to the fracture site, or applied to an absorbable collagen sponge (ACS, Medtronic), implanted at the fracture site immediately after fracture. For controls, 7μl of PBS was applied with or without ACS. Mice were sacrificed by cervical dislocation following an intraperitoneal injection of 2% Avertin (0.5ml/mouse). Non-stabilized fractures were collected at 5, 7, 10, 14, and 21 days post-fracture (n=5/time point/group). Stabilized fractures were collected at 10 days post-fracture (n=5/time point/group). Samples were processed for histology and immunohistochemistry as described below.
Histological and histomorphometric analyses of cartilage and bone
Callus tissues were fixed overnight at 4°C in 4% paraformaldehyde, decalcified at 4°C in 19% EDTA (pH 7.4) for 10–14 days, then dehydrated in a graded ethanol series and embedded in paraffin. For both stabilized and non-stabilized fractures, 10μm thick sections were collected throughout the entire callus and analyzed by histomorphometry as previously described [21, 23–24]. To determine the amount of cartilage within each callus, every thirtieth section (300μm) was stained with Safranin-O/Fast Green. To determine the amount of bone, adjacent sections were stained with Milligan’s Trichrome. Images were captured from a Leica DM 5000 B light microscope (Leica Microsystems GmbH) that was equipped with a camera (Diagnostic). The area of callus, cartilage and bone on each section was determined using Adobe PhotoShop.
Immunohistochemistry
Sections were processed and prepared for immmunohistochemistry staining as previous described [25]. Briefly, after deparaffinization and hydration, antigen retrieval was performed by incubating with 0.05% trypsin and followed by 3% H2O2 in methanol for 15 min to inhibit endogenous peroxidase activity. Slides were treated with 1.5% donkey serum or 1.5% goat serum as indicated by manufacturer’s instruction (goat or rabbit ABC staining kit; Santa Cruz Biotechnology, Santa Cruz, CA). Sections were incubated with primary antibodies diluted (1:50) in the blocking serum in a humidified chamber at 4°C overnight. Primary antibodies were purchased from from Santa Cruz Biotechnology (Santa Cruz, CA): BMPRIA (sc-5676), BMPRIB (sc-5679), and BMPRII (sc-5683); and Chemicon (Millipore, Billerica, MA): affinity purified rabbit polyclonal antibody against phospho-Smad 1/5/8. Control sections were incubated with normal goat IgG or normal rabbit IgG serum. Detection of primary antibody binding was done using goat or rabbit ABC staining kit (Santa Cruz Biotechnology, Santa Cruz, CA). Sections were developed with DAB and counterstained with 1% Fast Green
Quantification of tissue vascularity in fracture calluses
To visualize and quantify blood vessels, serial tissues (10μm) that were 600μm apart were selected throughout the whole callus and immunohistochemistry using an anti-platelet endothelial cell adhesion molecules (PECAM) antibody was performed [21]. For each sample, six sections were analyzed. The length density (length of blood vessels per unit volume of the reference space) and the surface density (area of the outer surface of blood vessels per unit volume of the reference space) of the blood vessels within the fracture callus were estimated using an Olympus CAST system (Olympus) and Visiopharm software (Visioplarm) as previous described [26]. The whole callus was used as the reference space and its volume was estimated using Calvalieri’s Principle [27–28].
Quantification of osteoclasts in fracture calluses
Tartrate-resistant acid phosphatase (TRAP) staining was performed using a leukocyte acid phosphatase kit (Sigma) on serial sections (10μm) that were 300μm apart through the callus. Sections were counterstained with 1% Fast Green. The number of TRAP-positive cells per area of fracture callus was estimated using the Olympus CAST system and Visiopharm software.
Quantification of proliferating cells in facture calluses
To assess the effect of rhBMP2 on cell proliferation, bromodeoxyuridine (BrdU) staining was performed using BrdU staining kit (Zymed) on serial sections (10μm) that were 300μm apart through the callus. The number of proliferating cells per area of fracture callus was estimated using the Olympus CAST system and Visiopharm software.
Bone grafting and BMP2 treatment
Bone grafts were prepared and transplanted as previously described [29]. Briefly, grafts were isolated from Rosa 26 donors (males, 10 week old) that express the LacZ reporter gene ubiquitously in the C57B6 background. To follow cells derived from the periosteum, endosteum and bone marrow were removed from the graft using a scalpel. To follow cells derived from the endosteum, periosteum was removed from the graft. Positive controls were obtained by keeping intact both periosteum and endosteum from the graft and negative controls by removing both periosteum and endosteum. The graft was placed in a tibial cortical bone defect of host C57B6 wild type mice (males, 10 week old) with or without fracture. Unicortical defects and non-stabilized fractures were created as previously described [29]. PBS or rhBMP2 protein (3μg, Medtronic) was applied to ACS and placed adjacent to the periosteal or endosteal surface of the graft. When the graft orientation was switched, ACS with PBS or rhBMP2 was still placed adjacent to the periosteal surface of the graft (i.e. within the bone marrow cavity). Callus tissues were collected at 7, 10 and 14 days post-surgery and processed for histology and cell lineage analyses. Days 10 and 14 were the optimal time points to assess cartilage and bone as reported in Table 1 (n= 3–5 per time point) [29].
TABLE 1.
PERCENTAGE OF SAMPLES WITH CONTRIBUTION OF DONOR PERIOSTEUM (PO) AND ENDOSTEUM (EO) TO BONE AND CARTILAGE DURING BONE GRAFT HEALING
| Bone Graft Healing | ||||
|---|---|---|---|---|
| Contribution to Bone | Contribution to Cartilage | |||
| Donor PO | Donor EO | Donor PO | Donor EO | |
| Normal Graft Orientation | ||||
| PBS | 100 (n=4) | 100 (n=3) | 0a (n=4) | 0 (n=3) |
| BMP2 | 40 (n=5) | 100 (n=4) | 80a,b (n=5) | 0 (n=4) |
| Graft Orientation Switched | ||||
| PBS | 100 (n=5) | 100 (n=3) | 20 (n=5) | 0 (n=3) |
| BMP2 | 100 (n=3) | 100 (n=3) | 0b (n=3) | 0 (n=3) |
p-value < 0.01
p-value <0.05
Lineage analyses
Callus collected from host C57B6 transplanted with Rosa 26 bone grafts were fixed in 0.2 % glutaraldehyde, decalcified and cryo-embedded in OCT as described in [29]. Longitudinal 8μm thick sections were collected throughout the entire callus. Every thirtieth section was stained with X-gal and adjacent sections stained with SO and TC as described in [29]. As previously described, donor-derived chondrocytes and osteoblasts/osteocytes were positively stained with X-gal and were found adjacent to the graft [27]. Samples reported in Table 1 were selected as follows: Samples with more than 10 X-gal-positive chondrocytes or osteoblasts/osteocytes near the periosteum of the graft were selected as samples with contribution from the donor periosteum to cartilage or bone respectively. Similarly, samples with more than 10 X-gal-positive osteoblasts/osteocytes near the endosteum of the graft were selected as samples with contribution to bone from the donor endosteum.
Statistical analyses
The student t-test was used to compare experimental and control samples, and p-values of <0.05 were considered significant. In tables 1 and 2, all comparison groups did not show any statistical significance except the groups highlighted in superscript (a and b).
TABLE 2.
PERCENTAGE OF SAMPLES WITH CONTRIBUTION OF DONOR PERIOSTEUM (PO) AND ENDOSTEUM (EO) TO BONE AND CARTILAGE DURING NON-STABILIZED FRACTURE HEALING
| Non-Stabilized Fracture Healing | ||||
|---|---|---|---|---|
| Contribution to Bone | Contribution to Cartilage | |||
| Donor PO | Donor EO | Donor PO Donor EO | ||
| Normal Graft Orientation | ||||
| PBS | 75 (n=4) | 100 (n=3) | 25a (n=4) | 0 (n=3) |
| BMP2 | 60 (n=5) | 100 (n=3) | 100a, b (n=5) | 0 (n=3) |
| Graft Orientation Switched | ||||
| PBS | 100 (n=5) | 100 (n=4) | 20 (n=5) | 0 (n=4) |
| BMP2 | 100 (n=5) | 100 (n=4) | 40b (n=5) | 0 (n=4) |
p-value <0.05
RESULTS
rhBMP2 accelerates the formation and remodeling of bone and cartilage during non-stabilized fracture repair
In humans, rhBMP2 is usually applied to critical and non-critical size defects in combination with an absorbable collagen sponge (ACS). To assess the role of BMP2 in the early stages of bone repair, we started by comparing healing in mice that received rhBMP2 or PBS applied with ACS with mice that received direct injection of rhBMP2 or PBS into the fracture site. We first chose a model of non-stabilized fractures, which heal via endochondral ossification in order to examine both cartilage and bone. Histological analyses indicated that ACS was not completely resorbed within the callus by day 10 post-fractures (data not shown). Histomorphometric analyses revealed no significant difference in total callus volume between ACS/rhBMP2 and rhBMP2 or between ACS/PBS and PBS-treated samples by day 7 and day 10 post-fracture (Fig. 1A). However, there was a significantly reduced bone volume (Fig. 1B) and proportion of bone in the callus (data not shown) in ACS/rhBMP2 compared to rhBMP2-treated mice by day 7. In addition, there was a significantly greater volume of cartilage (Fig. 1C) and proportion of cartilage (data not shown) in the callus in ACS/rhBMP2 compared to rhBMP2 treated mice by day 10. Therefore, we carried on with direct injections of rhBMP2 or PBS into the fracture site to study the effects of rhBMP2 independent of ACS. rhBMP2-treated animals had a significantly bigger callus at days 7, 14 and 21 post-fracture compared with PBS controls (Fig. 1A). We observed more granulation tissue in rhBMP2-treated calluses compared to controls at day 7 post-fracture (data not shown). There was a significantly greater volume of bone (Fig. 1B) and of bone as a proportion of callus (data not shown) in rhBMP2-treated mice by days 10 and 14 post-fracture. Bone volume peaked by day 14 in rhBMP2-treated calluses and decreased at day 21, while the volume of bone remained high at day 21 in PBS-treated calluses compared to days 10 and 14. Thus rhBMP2 treatment accelerated not only bone deposition but also bone remodeling. The process of cartilage formation and removal was also accelerated in rhBMP2-treated samples. By day 7, there was a significantly greater volume of cartilage (Fig. 1C) and of cartilage as a proportion of callus (data not shown) in rhBMP2-treated samples (Fig. 1C and data not shown). The peak of cartilage volume was observed at day 10 in both rhBMP2 and PBS-treated mice and by day 14 there was a significantly reduced volume of cartilage (Fig. 1C) and of cartilage as a proportion of callus (data not shown) in rhBMP2-treated calluses compared to PBS-treated samples.
FIG. 1.
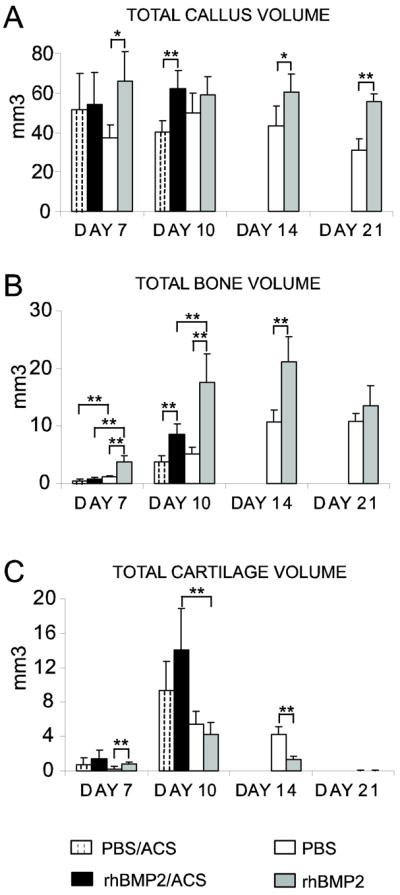
Effects of rhBMP2 treatment during the course of non-stabilized tibial fracture repair. Histomorphometric analyses of total callus volume (TV, A), total bone volume (BV, B), and total cartilage volume (CV, C in PBS/ACS, rhBMP2/ACS, PBS, and rhBMP2-treated mice at days 7, 10, 14 and 21 post-fracture (n=5 per group). *p< 0.05, **p<0.01. Bars represent mean ± s.d.
rhBMP2 affects osteoclastogenesis but not angiogenesis during early stages of fracture healing
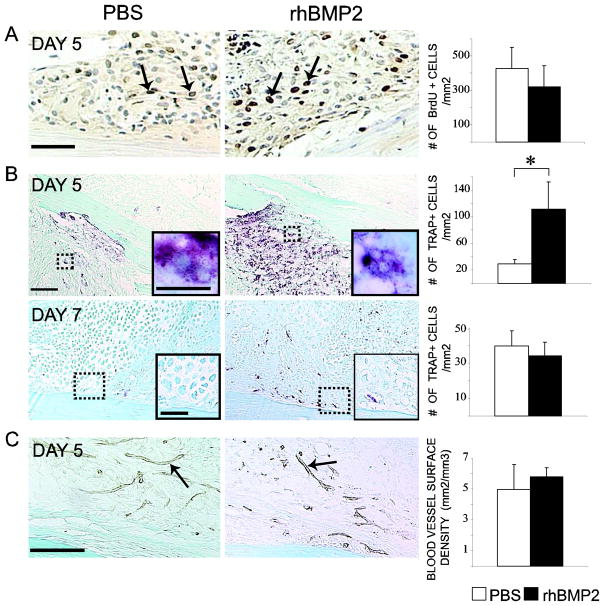
Changes in cell proliferation, osteoclastogenesis and angiogenesis may be associated with the stimulation of cartilage and bone during the early stages of repair [14, 30–32]. At day 5 post-fracture, BrdU positive proliferating cells were detected in both rhBMP2- and PBS-treated fracture calluses, however, there was no significant difference in the number of proliferating cells per area of callus between rhBMP2- and PBS-treated samples (Fig 2A). At day 5, TRAP-positive cells were mostly found within bone marrow, and to a lesser extent in granulation tissue near the fracture site. RhBMP2 treatment significantly increased the number of TRAP-positive cells per callus surface area (Fig 2B). By day 7, TRAP-positive cells were detected adjacent to hypertrophic cartilage and new bone in the periosteum of rhBMP2-treated samples, while they were still mainly detected within the bone marrow of control samples. Yet, the number of TRAP-positive cells per callus surface area did not differ between the two groups (Fig 2B). PECAM immunohistochemistry illustrated that rhBMP2 did not affect angiogenesis at 5 days post-fracture. No significant difference in length density and surface density of blood vessels was detected between rhBMP2- and PBS-treated samples (Fig 2C).
FIG. 2.
Effects of rhBMP2 on cell proliferation, osteoclastogenesis and angiogenesis during non-stabilized fracture repair. (A) BrdU immunohistochemistry staining (left, arrows) and stereological analyses of BrdU-positive cells per area of fracture callus (right, n=5 per group) in PBS- and rhBMP2-treated calluses at day 5 post-fracture. (B) TRAP staining (left) and stereology analyses of TRAP-positive cells per area of fracture callus (right, n=5 per group) in PBS- and rhBMP2-treated callus at days 5 and 7 post-fracture. There is a significantly increase in the number of TRAP-positive cells in rhBMP2-treated calluses compared to controls at day 5 (*p<0.05). Inserted boxes show high magnifications of multinucleated TRAP-positive cells in the bone marrow of rhBMP2- and PBS-treated calluses (B, top) and hypertrophic cartilage association with TRAP-positive cells in the periosteum of rhBMP2-treated samples (B, bottom). (C) PECAM immunohistochemistry (left, arrows) and stereology analyses of the surface density of blood vessel within the callus (right, n=5 per group) in PBS- and rhBMP2-treated calluses at day 5 post-fracture. Scale bars: (A) = 50μm; (B) = 100 μm; (B, inserted box, top) = 10μm; (B, inserted box, bottom) = 50μm; (C) = 100μm.
rhBMP2 induces cartilage formation during healing of stabilized fractures
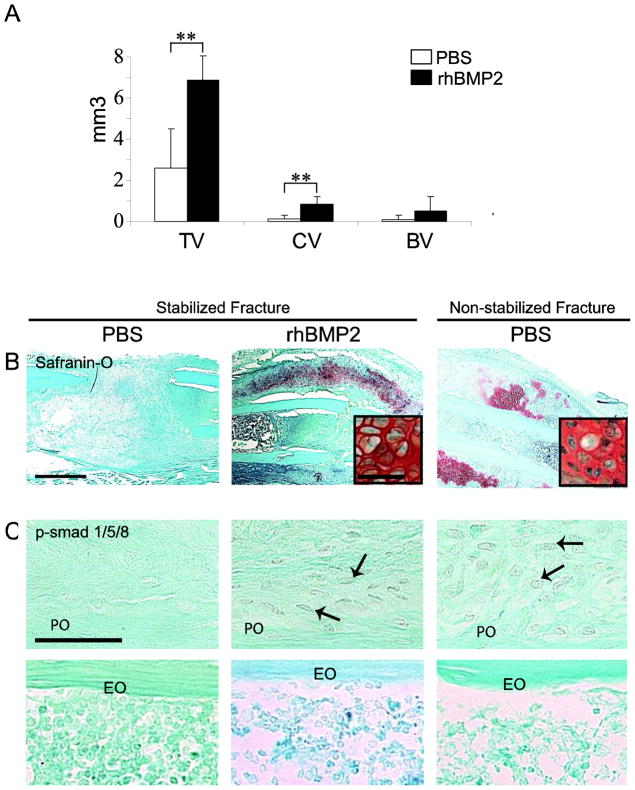
We next assessed the effects of direct rhBMP2 injections on healing of stabilized fractures, which normally occurs via intramembranous ossification [22, 29]. Histomorphometry indicated a significant greater volume of total callus and volume of cartilage in rhBMP2-treated than in PBS-treated calluses by day 10 post-fracture. However, the volume of bone was not significantly increased in rhBMP2-treated calluses (Fig 3A). Histological analyses indicated that new cartilage formed primarily at the periosteal surface of rhBMP2-treated injured bone but was negligible in PBS controls by day 10 of post-stabilized fracture (Fig. 3B). Concurrently, new bone formed both at the periosteal and endosteal surfaces in rhBMP2- and PBS-treated calluses by day 10 in stabilized fractures (data not shown). Immunohistochemical analyses showed that BMP receptors (IA, IB and II) and effectors (p-Smad 1/5/8) were not detected in periosteal cells of control stabilized fractures, but were detected in cells of the activated periosteum in rhBMP2-treated stabilized fractures and control non-stabilized fractures by day 5 (Fig 3C and data not shown, [25]). No immunoreactivity signal for BMP receptors and effectors was detected in the endosteum in both PBS and rhBMP2-treated fracture calluses (Fig 3C and data not shown).
FIG. 3.
rhBMP2 induces cartilage formation during stabilized fracture repair and activates BMP signaling pathway within periosteum. (A) Histomorphometric measurements of total callus (TV), total cartilage volume (CV) and total bone volume (BV) in PBS- and rhBMP2-treated calluses. There is a statistically significant increase in total callus volume and cartilage volume in rhBMP2 treated calluses compared with PBS-treated calluses (**p<0.01). (B) Safranin-O/Fast Green (SO) staining of sections through the callus of (left) PBS- and (middle) rhBMP2-treated stabilized fractures and (right) PBS-treated non-stabilized fractures at day 10 post-fracture. Inserted boxes show high magnification of chondrocytes. (C) Negative p-Smad 1/5/8 immunostaining in the periosteum (PO, top) of stabilized fractures treated with PBS (left) and positive staining in cells of the activated periosteum in rhBMP2- treated stabilized fractures (middle, arrows) and PBS-treated non-stabilized fractures (right, arrows) at day 5. Negative p-Smad 1/5/8 immunostaining in endosteum (EO, bottom). Scale bars (B) = 1mm; (B, inserted boxes) and (C) = 50 μm,
rhBMP2 stimulates chondrogenesis within periosteum
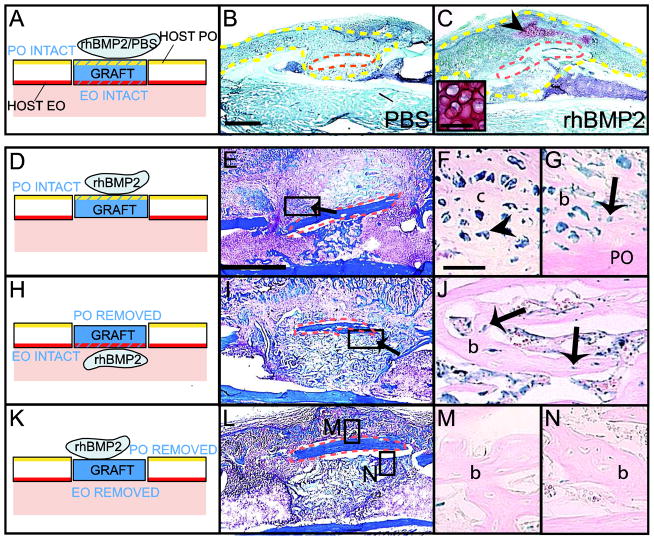
To directly address the role of rhBMP2 on cell recruitment and differentiation during the early stages of fracture repair, we used lineage analyses to distinguish the effects of rhBMP2 on periosteum and endosteum that contain skeletal progenitors [25]. For bone grafting, ACS was used as a carrier to deliver rhBMP2 to avoid diffusion of the solution at the open injury site. As observed during stabilized fracture repair, rhBMP2 promoted endochondral ossification during bone graft healing, which normally occurs via intramembranous ossification (Fig. 4A–C). In the rhBMP2-treated samples, new bone formed at the periosteal and endosteal surfaces of bone grafts (Fig. 4E and I, arrows) and cartilage was observed near the periosteal surface (Fig. 4C, arrowhead).
FIG. 4.
Distinct effects of rhBMP2 on periosteum- and endosteum-derived cells during bone repair. (A) Schematic representation of Rosa 26 bone grafts (blue) treated with PBS/ACS or rhBMP2/ACS and transplanted in the cortex of wild type host tibia. Safranin-O/Fast green (SO) staining at day 10 of (B) PBS/ACS and (C) rhBMP2/ACS treated samples. Cartilage (arrowhead and high magnification) is found at the periosteal surface of rhBMP2/ACS-treated samples (C). (D, H, K) (Left) Schematic representations of Rosa 26 bone grafts (blue) treated with rhBMP2/ACS transplanted in the cortex of wild type host tibia. (Middle) Longitudinal sections through the bone graft stained with Trichrome (TC) and (Right) adjacent sections stained with X-gal at 10 days following bone grafting. (F–G) X-gal-positive osteoblasts and osteocytes (arrow) and pre-hypertrophic chondrocytes (arrowhead) are found at the intact periosteal surface in rhBMP2-treated samples. (J) X-gal-positive osteoblasts and osteocytes (arrow) are found at the intact endosteal surface within bone marrow cavity but (M–N) not in negative controls. Dotted yellow lines delimit the callus. Dotted orange lines delimit the graft. c= cartilage; b=bone. Scale bars (B, C, E, I, L) = 1mm; (C, inserted box) = 50μm; (F, G, J, M and N) = 100μm.
To separate the effects of rhBMP2 on cell differentiation within periosteum and endosteum, Rosa26 bone grafts containing either periosteum or endosteum were transplanted to wild type hosts (Fig. 4D). In samples with periosteum intact, donor-derived chondrocytes were detected adjacent to the periosteum of the graft in 80% of the samples treated with rhBMP2, but not in PBS-treated samples (Fig. 4D–F and Table 1). Concurrently, donor-derived osteoblasts/osteocytes were detected adjacent to the periosteum of the graft in both rhBMP2-and PBS-treated samples (Fig. 4G and Table 1). In samples with endosteum intact, donor-derived osteoblasts/osteocytes were detected adjacent to the endosteum of the graft, and no donor-derived chondrocytes were detected at the endosteal surface and/or within bone marrow in both rhBMP2- and PBS-treated tissues (Fig. 4H–J and Table 1). In negative controls where both periosteum and endosteum were removed, no graft-derived osteoblasts, osteocytes or chondrocytes were detected (Fig 4K–N).
Effect of the tissue environment on the cellular response to rhBMP2
Since rhBMP2 treatment simulated chondrogenesis in the periosteum but not the endosteum in vivo, we wondered whether these differences were due to the tissue environments surrounding periosteum and endosteum or the intrinsic properties of these tissues (i.e. distinct populations of skeletal progenitors). First, we assessed the ability of rhBMP2 to modulate cell differentiation within periosteum and endosteum in an environment where fractures normally heal via endochondral ossification. To this end, we created a non-stabilized fracture adjacent to the graft at the time of bone grafting. In this environment, rhBMP2 increased the chondrogenic potential of periosteum (Table 2) but did not affect the osteogenic potential of periosteum (Table 2). Endosteum-derived cells exposed to rhBMP2 or PBS gave rise to osteoblasts and osteocytes but not chondrocytes next to the endosteum of the graft (Table 2).
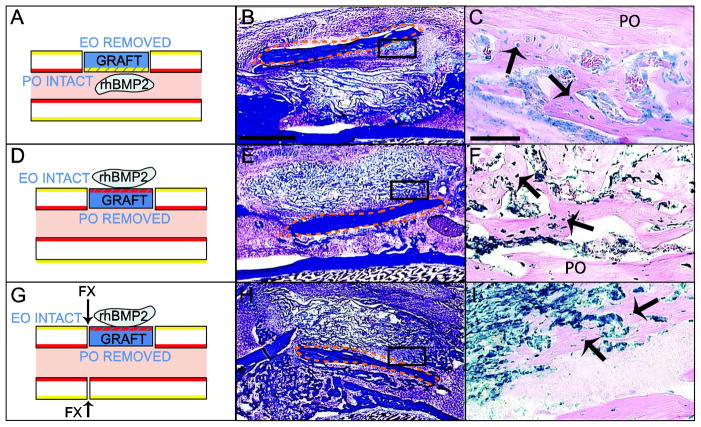
To further assess the impact of the tissue environment on the periosteal and endosteal response to rhBMP2, we switched the orientation of the grafts. During bone graft and fracture healing, donor periosteum-derived cells exposed to rhBMP2 or PBS differentiated into osteoblasts and osteocytes but not into chondrocytes within bone marrow (Figure 5A–C, Tables 1 and 2). The bone marrow environment reduced the chondrogenic potential of the periosteum in response to rhBMP2 (Tables 1 and 2). Placing the endosteum in the periosteal environment of the host did not affect the fate of endosteum-derived cells in response to rhBMP2 (Fig 5D–I and Table 1 and 2).
FIG. 5.
Effect of the tissue environment on periosteal and endosteal responses to rhBMP2 during bone repair. (Left) Schematic representation of Rosa26 bone grafts (blue) treated with rhBMP2/ACS. Intact periosteum (PO, yellow) was placed in the environment of bone marrow (A) and intact endosteum (EO, red) was placed in the environment of periosteum (D, G). Arrows indicate where the fracture is created (G). (Middle) Sections through the bone graft were stained with Trichrome (TC) and (Right) adjacent sections were stained with X-gal at 10 days following bone grafting. (A–C) During bone graft healing, X-gal-positive osteoblasts and osteocytes (arrows) derived from PO are found at the periosteal surface of the graft in the host bone marrow. (D–F) During bone graft and (G–I) non-stabilized fracture healing, X-gal-positive osteoblasts and osteocytes (arrows) derived from EO are located at the endosteal surface of the graft in the environment of the host PO. Dotted orange lines delimit the bone graft. Scale bars (B, E, H) = 1mm; (C, F, I) = 100μm.
DISCUSSION
BMP2 impacts both osteogenesis and chondrogenesis during bone repair
The current study shows that rhBMP2 accelerates the initial deposition of cartilage and bone in the fracture callus, increases the total amount of bone formation and induces an early onset of cartilage and bone remodeling, which results in accelerating the entire process of endochondral ossification. This is likely accompanied with the early enhancement of biomechanical properties of the callus as previously described [7–8, 11]. Numerous studies have illustrated the benefits of rhBMP2 treatment on bone repair by assessing its effects in late stages of repair, and our results further show that rhBMP2 acts as early as the inflammatory phase by acting both on osteoblast and chondrocyte differentiation [11, 33]. BMPs have long been described as stimulators of bone formation by supporting both osteogenic and chondrogenic differentiation [34–38]. In vitro, BMPs can also induce osteogenic and chondrogenic differentiation of mesenchymal stem cells [17, 39–41]. We further show that rhBMP2 induces repair via endochondral ossification regardless of the type of injury. BMP2 may act independently on both osteoblasts and chondrocytes, which express BMP receptors during endochondral ossification [25]. BMPs play therefore similar roles in repair and development, where BMP signaling has been shown to be essential for endochondral ossification in the appendicular skeleton [42–44] and to induce ectopic cartilage formation in the cranial skeleton [45–46]
Indirect effects of BMP2 on angiogenesis and osteoclastogenesis
The beneficial effects of BMPs on bone healing have often been associated with effects on blood vessels and osteoclasts during the hard callus phase of repair. Consistent with previous reports, we observed an increase in the amount of blood vessels during the hard callus phase of repair and this correlated with increased bone deposition in the callus [47–49]. The early onset of cartilage and bone formation in response to rhBMP2, however, was not initially associated with increased angiogenesis within the callus. Therefore, stimulation of angiogenesis by rhBMP2 may be a consequence of increased bone formation. In fact, activation of the BMP signaling pathway specifically in osteoblasts can induce skeletal angiogenesis [50]. Osteogenesis and angiogenesis are tightly coupled due to paracrine signals between osteoblasts and endothelial cells. BMP-induced angiogenesis has been shown to occur through osteoblast-derived vascular endothelial growth factor A [15]. Whether BMPs can stimulate angiogenesis via direct effects on endothelial cells is still unclear. We did observe activation of the BMP pathway in blood vessels adjacent to the new bone during the hard callus phase of repair but not during early stages of fracture healing [25]. This again supports the role of BMPs in stimulating angiogenesis specifically within bone and as a consequence of BMP-induced bone formation.
Bone formation is also closely coupled with bone remodeling. During the early stages of repair, we observed increased recruitment of TRAP-positive cells in fractures treated with rhBMP2. Our histological observations indicate that the early differentiation of osteoclasts was first apparent within the marrow cavity and may coincide with initial osteoblast differentiation, although no bone was detected yet in these areas. During the early soft callus phase, the total number of osteoclasts was similar in treated and untreated calluses, however accumulation of osteoclasts was linked with the early onset of cartilage hypertrophy and bone deposition at the periosteal surface within the callus. Matrix resorbing cells play an important role in the removal of bone debris after injury and in extracellular matrix remodeling as soon as new cartilage and bone are produced. During the hard callus phase, bone remodeling occurred faster in response to rhBMP2, suggesting again an increased in osteoclast activity and/or number. The apparent disparate effects of a single rhBMP2 injection on osteoclasts are presumably due to the dynamic process of bone repair, and are likely an indirect consequence of BMP-induced bone and cartilage formation. Previous reports have already showed that the stimulatory effect of BMPs on osteoclast formation is an indirect effect via osteoblasts or chondrocytes [35, 51–53]. Overall, these results indicate that the primary targets of rhBMP2 are not endothelial cells or osteoclasts at least during the initial phase of repair.
Direct effects of BMP2 on skeletal progenitors
The ability of BMPs alone to stimulate bone formation in vivo has long suggested that BMPs can recruit skeletal progenitors and directly induce their differentiation into osteoblasts and chondrocytes. Many in vitro studies have further supported these observations. However, the mechanisms of action of BMPs in the complex in vivo environment are still not clearly understood and the specific cell populations that are the target of BMPs are not characterized. There are several potential sources of skeletal progenitors within bone, soft tissues and blood vessels that potentially may be responsive to BMP signals after injury [54–57]. Although BMP could recruit skeletal progenitors from distant sites, our results point to an important role on local progenitors within bone [29]. Our previous studies showed that periosteum and endosteum are major local sources for skeletal progenitors with distinct osteogenic and chondrogenic potentials [25]. More specifically, the periosteum is the source of chondrocytes and osteoblasts while the endosteum only gives rise to osteoblasts in the callus. Given the ability of rhBMP2 to stimulate chondrogenesis in stabilized fractures, we suspected that the periosteum was a privileged target for rhBMP2. Our results show that rhBMP2 directly stimulates both chondrogenesis and osteogenesis within periosteum, but only osteogenesis within endosteum. These results were correlated with activation of the BMP pathway in activated periosteum but not endosteum during the early stages of repair. BMP2 can therefore regulate cell fate decisions by activating the BMP pathway specifically within periosteum that expresses BMP receptors. Although BMP receptors were undetectable via immunohistochemistry in this tissue prior to fracture, some level of expression must exist in order for periosteal cells to respond differentially to BMP signals. In addition, we show that the tissue environment can affect periosteal response to rhBMP2 as its chondrogenic potential was reduced within the bone marrow environment. We previously showed that the tissue environment can affect periosteal and endosteal cell fate, including the mechanical environment [21, 23, 29]. BMPs may act independent of the mechanical environment to control skeletal cell recruitment and differentiation. The BMP signaling pathway may also mediate the cellular response to mechanical signals at the fracture site to determine the program of bone repair. Indeed, the pathway is up-regulated in the periosteum of non-stabilized but not in stabilized fractures [25].
In sum, our work elucidates the role of BMP2 in regulating cartilage and bone formation in the initial stages of skeletal regeneration and identifies cellular targets of BMP2 in vivo. In particular, BMP2 acts specifically on the periosteum by modulating cell fate decision and differentiation. Further studies will be required to elucidate the regulation of skeletal cell fate at the molecular level and to better understand the functions and potential benefits of BMPs in vivo.
Acknowledgments
The authors thank R. Marcucio for helpful comments on the manuscript and Medtronic for providing rhBMP2. This work was supported by funds from NIH/NIDCR (DE016701), the Musculoskeletal Transplant Foundation, Osteosynthesis and Trauma Care (OTC) Foundation and the UCSF Research Evaluation and Allocation Committee to C.C.
Funding sources: NIH/NIDCR (DE016701), the Musculoskeletal Transplant Foundation, Osteosynthesis and Trauma Care (OTC) Foundation and the UCSF Research Evaluation and Allocation Committee
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yan Yiu Yu, Email: yuy@orthosurg.ucsf.edu.
Shirley Lieu, Email: lieus@orthosurg.ucsf.edu.
Chuanyong Lu, Email: chuanyong.lu@ucsf.edu.
Céline Colnot, Email: colnotc@orthosurg.ucsf.edu.
References
- 1.Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;a(13):1580–94. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- 2.Huang YH, et al. Bone morphogenetic proteins and osseointegration: current knowledge - future possibilities. Periodontol 2000. 2008;47:206–23. doi: 10.1111/j.1600-0757.2007.00240.x. [DOI] [PubMed] [Google Scholar]
- 3.Linkhart TA, Mohan S, Baylink DJ. Growth factors for bone growth and repair: IGF, TGF beta and BMP. Bone. 1996;19(1 Suppl):1S–12S. doi: 10.1016/s8756-3282(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 4.Kugimiya F, Kawaguchi H, Chung UI. BMP and bone formation. Clin Calcium. 2004;14(1):173–9. [PubMed] [Google Scholar]
- 5.Sellers RS, et al. Repair of articular cartilage defects one year after treatment with recombinant human bone morphogenetic protein-2 (rhBMP-2) J Bone Joint Surg Am. 2000;82(2):151–60. doi: 10.2106/00004623-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Blokhuis TJ, et al. Biomechanical and histological aspects of fracture healing, stimulated with osteogenic protein-1. Biomaterials. 2001;22(7):725–30. doi: 10.1016/s0142-9612(00)00238-6. [DOI] [PubMed] [Google Scholar]
- 7.Welch RD, et al. Effect of recombinant human bone morphogenetic protein-2 on fracture healing in a goat tibial fracture model. J Bone Miner Res. 1998;13(9):1483–90. doi: 10.1359/jbmr.1998.13.9.1483. [DOI] [PubMed] [Google Scholar]
- 8.Bouxsein ML, et al. Recombinant human bone morphogenetic protein-2 accelerates healing in a rabbit ulnar osteotomy model. J Bone Joint Surg Am. 2001;83-A(8):1219–30. doi: 10.2106/00004623-200108000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Cook SD, et al. Healing course of primate ulna segmental defects treated with osteogenic protein-1. J Invest Surg. 2002;15(2):69–79. doi: 10.1080/08941930290085822. [DOI] [PubMed] [Google Scholar]
- 10.White AP, et al. Clinical applications of BMP-7/OP-1 in fractures, nonunions and spinal fusion. Int Orthop. 2007;31(6):735–41. doi: 10.1007/s00264-007-0422-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Einhorn TA, et al. A single percutaneous injection of recombinant human bone morphogenetic protein-2 accelerates fracture repair. J Bone Joint Surg Am. 2003;85-A(8):1425–35. doi: 10.2106/00004623-200308000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Garrison KR, et al. Clinical effectiveness and cost-effectiveness of bone morphogenetic proteins in the non-healing of fractures and spinal fusion: a systematic review. Health Technol Assess. 2007;11(30):1–150. iii–iv. doi: 10.3310/hta11300. [DOI] [PubMed] [Google Scholar]
- 13.Tsuji K, et al. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38(12):1424–9. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- 14.Peng H, et al. VEGF improves, whereas sFlt1 inhibits, BMP2-induced bone formation and bone healing through modulation of angiogenesis. J Bone Miner Res. 2005;20(11):2017–27. doi: 10.1359/JBMR.050708. [DOI] [PubMed] [Google Scholar]
- 15.Deckers MM, et al. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology. 2002;143(4):1545–53. doi: 10.1210/endo.143.4.8719. [DOI] [PubMed] [Google Scholar]
- 16.Hanada K, Dennis JE, Caplan AI. Stimulatory effects of basic fibroblast growth factor and bone morphogenetic protein-2 on osteogenic differentiation of rat bone marrow-derived mesenchymal stem cells. J Bone Miner Res. 1997;12(10):1606–14. doi: 10.1359/jbmr.1997.12.10.1606. [DOI] [PubMed] [Google Scholar]
- 17.Zachos TA, Shields KM, Bertone AL. Gene-mediated osteogenic differentiation of stem cells by bone morphogenetic proteins-2 or -6. J Orthop Res. 2006;24(6):1279–91. doi: 10.1002/jor.20068. [DOI] [PubMed] [Google Scholar]
- 18.Thies RS, et al. Recombinant human bone morphogenetic protein-2 induces osteoblastic differentiation in W-20-17 stromal cells. Endocrinology. 1992;130(3):1318–24. doi: 10.1210/endo.130.3.1311236. [DOI] [PubMed] [Google Scholar]
- 19.Lou J, et al. Gene therapy: adenovirus-mediated human bone morphogenetic protein-2 gene transfer induces mesenchymal progenitor cell proliferation and differentiation in vitro and bone formation in vivo. J Orthop Res. 1999;17(1):43–50. doi: 10.1002/jor.1100170108. [DOI] [PubMed] [Google Scholar]
- 20.Hanada K, et al. BMP-2 induction and TGF-beta 1 modulation of rat periosteal cell chondrogenesis. J Cell Biochem. 2001;81(2):284–94. doi: 10.1002/1097-4644(20010501)81:2<284::aid-jcb1043>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 21.Colnot C, et al. Altered fracture repair in the absence of MMP9. Development. 2003;130(17):4123–33. doi: 10.1242/dev.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson Z, et al. A model for intramembranous ossification during fracture healing. J Orthop Res. 2002;20(5):1091–8. doi: 10.1016/S0736-0266(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 23.Behonick DJ, et al. Role of matrix metalloproteinase 13 in both endochondral and intramembranous ossification during skeletal regeneration. PLoS ONE. 2007;2(11):e1150. doi: 10.1371/journal.pone.0001150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu C, et al. Cellular basis for age-related changes in fracture repair. J Orthop Res. 2005;23(6):1300–7. doi: 10.1016/j.orthres.2005.04.003.1100230610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu YY, et al. Immunolocalization of BMPs, BMP antagonists, receptors, and effectors during fracture repair. Bone. 2009 doi: 10.1016/j.bone.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Lu C, et al. Effect of age on vascularization during fracture repair. J Orthop Res. 2008;26(10):1384–9. doi: 10.1002/jor.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gundersen HJ, et al. The efficiency of systematic sampling in stereology--reconsidered. J Microsc. 1999;193(Pt 3):199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- 28.Schionning JD, Larsen JO. A stereological study of dorsal root ganglion cells and nerve root fibers from rats treated with inorganic mercury. Acta Neuropathol. 1997;94(3):280–6. doi: 10.1007/s004010050704. [DOI] [PubMed] [Google Scholar]
- 29.Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res. 2009;24(2):274–82. doi: 10.1359/jbmr.081003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duvall CL, et al. Impaired angiogenesis, early callus formation, and late stage remodeling in fracture healing of osteopontin-deficient mice. J Bone Miner Res. 2007;22(2):286–97. doi: 10.1359/jbmr.061103. [DOI] [PubMed] [Google Scholar]
- 31.Pacicca DM, et al. Expression of angiogenic factors during distraction osteogenesis. Bone. 2003;33(6):889–98. doi: 10.1016/j.bone.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Parsons P, et al. Platelet-rich concentrate supports human mesenchymal stem cell proliferation, bone morphogenetic protein-2 messenger RNA expression, alkaline phosphatase activity, and bone formation in vitro: a mode of action to enhance bone repair. J Orthop Trauma. 2008;22(9):595–604. doi: 10.1097/BOT.0b013e318188dbb7. [DOI] [PubMed] [Google Scholar]
- 33.Edwards RB, 3rd, et al. Percutaneous injection of recombinant human bone morphogenetic protein-2 in a calcium phosphate paste accelerates healing of a canine tibial osteotomy. J Bone Joint Surg Am. 2004;86-A(7):1425–38. doi: 10.2106/00004623-200407000-00010. [DOI] [PubMed] [Google Scholar]
- 34.Zhu W, et al. Combined bone morphogenetic protein-2 and -7 gene transfer enhances osteoblastic differentiation and spine fusion in a rodent model. J Bone Miner Res. 2004;19(12):2021–32. doi: 10.1359/JBMR.040821. [DOI] [PubMed] [Google Scholar]
- 35.Paul S, Lee JC, Yeh LC. A comparative study on BMP-induced osteoclastogenesis and osteoblastogenesis in primary cultures of adult rat bone marrow cells. Growth Factors. 2009;27(2):121–31. doi: 10.1080/08977190802707324. [DOI] [PubMed] [Google Scholar]
- 36.Toh WS, et al. Effects of culture conditions and bone morphogenetic protein 2 on extent of chondrogenesis from human embryonic stem cells. Stem Cells. 2007;25(4):950–60. doi: 10.1634/stemcells.2006-0326. [DOI] [PubMed] [Google Scholar]
- 37.Schmitt B, et al. BMP2 initiates chondrogenic lineage development of adult human mesenchymal stem cells in high-density culture. Differentiation. 2003;71(9–10):567–77. doi: 10.1111/j.1432-0436.2003.07109003.x. [DOI] [PubMed] [Google Scholar]
- 38.Tang N, et al. BMP9-induced osteogenic differentiation of mesenchymal progenitors requires functional canonical Wnt/beta-catenin signaling. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luu HH, et al. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J Orthop Res. 2007;25(5):665–77. doi: 10.1002/jor.20359. [DOI] [PubMed] [Google Scholar]
- 40.Shen B, et al. BMP-2 Enhances TGF-beta3-Mediated Chondrogenic Differentiation of Human Bone Marrow Multipotent Mesenchymal Stromal Cells in Alginate Bead Culture. Tissue Eng Part A. 2009;15(6):1311–20. doi: 10.1089/ten.tea.2008.0132. [DOI] [PubMed] [Google Scholar]
- 41.Steinert AF, et al. Enhanced in vitro chondrogenesis of primary mesenchymal stem cells by combined gene transfer. Tissue Eng Part A. 2009;15(5):1127–39. doi: 10.1089/ten.tea.2007.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Retting KN, et al. BMP canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation. Development. 2009;136(7):1093–104. doi: 10.1242/dev.029926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoon BS, et al. Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. Proc Natl Acad Sci U S A. 2005;102(14):5062–7. doi: 10.1073/pnas.0500031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Minina E, et al. Expression of Fgf and Tgfbeta signaling related genes during embryonic endochondral ossification. Gene Expr Patterns. 2005;6(1):102–9. doi: 10.1016/j.modgep.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 45.Hu D, Colnot C, Marcucio RS. Effect of bone morphogenetic protein signaling on development of the jaw skeleton. Dev Dyn. 2008;237(12):3727–37. doi: 10.1002/dvdy.21781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barlow AJ, Francis-West PH. Ectopic application of recombinant BMP-2 and BMP-4 can change patterning of developing chick facial primordia. Development. 1997;124(2):391–8. doi: 10.1242/dev.124.2.391. [DOI] [PubMed] [Google Scholar]
- 47.Ripamonti U, et al. Complete regeneration of bone in the baboon by recombinant human osteogenic protein-1 (hOP-1, bone morphogenetic protein-7) Growth Factors. 1996;13(3–4):273–89. doi: 10.3109/08977199609003228. color plates III–VIII,pre bk. [DOI] [PubMed] [Google Scholar]
- 48.Suwa F, et al. SEM study on microvascular changes following implantation of bone morphogenetic protein combined with hydroxyapatite into experimental bone defects. J Osaka Dent Univ. 1998;32(1):27–34. [PubMed] [Google Scholar]
- 49.Zhang X, et al. Periosteal progenitor cell fate in segmental cortical bone graft transplantations: implications for functional tissue engineering. J Bone Miner Res. 2005;20(12):2124–37. doi: 10.1359/JBMR.050806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang F, et al. Sustained BMP signaling in osteoblasts stimulates bone formation by promoting angiogenesis and osteoblast differentiation. J Bone Miner Res. 2009;24(7):1224–33. doi: 10.1359/JBMR.090204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wutzl A, et al. Bone morphogenetic proteins 5 and 6 stimulate osteoclast generation. J Biomed Mater Res A. 2006;77(1):75–83. doi: 10.1002/jbm.a.30615. [DOI] [PubMed] [Google Scholar]
- 52.Usui M, et al. Murine and chicken chondrocytes regulate osteoclastogenesis by producing RANKL in response to BMP2. J Bone Miner Res. 2008;23(3):314–25. doi: 10.1359/JBMR.071025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Otsuka E, Notoya M, Hagiwara H. Treatment of myoblastic C2C12 cells with BMP-2 stimulates vitamin D-induced formation of osteoclasts. Calcif Tissue Int. 2003;73(1):72–7. doi: 10.1007/s00223-002-1071-0. [DOI] [PubMed] [Google Scholar]
- 54.Zuk PA, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7(2):211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 55.Lee JY, et al. Clonal isolation of muscle-derived cells capable of enhancing muscle regeneration and bone healing. J Cell Biol. 2000;150(5):1085–100. doi: 10.1083/jcb.150.5.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuznetsov SA, et al. Circulating skeletal stem cells. J Cell Biol. 2001;153(5):1133–40. doi: 10.1083/jcb.153.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Farrington-Rock C, et al. Chondrogenic and adipogenic potential of microvascular pericytes. Circulation. 2004;110(15):2226–32. doi: 10.1161/01.CIR.0000144457.55518.E5. [DOI] [PubMed] [Google Scholar]