Abstract
In the filamentous, heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120, expression of the nitrate assimilation nirA operon takes place in the absence of ammonium and the presence of nitrate or nitrite. Several positive-action proteins that are required for expression of the nirA operon have been identified. Whereas NtcA and NtcB exert their action by direct binding to the nirA operon promoter, CnaT acts by an as yet unknown mechanism. In the genome of this cyanobacterium, open reading frame (ORF) all0605 (the nirB gene) is found between the nirA (encoding nitrite reductase) and ntcB genes. A nirB mutant was able to grow at the expense of nitrate as a nitrogen source and showed abnormally high levels of nirA operon mRNA both in the presence and in the absence of nitrate. This mutant showed increased nitrate reductase activity but decreased nitrite reductase activity, an imbalance that resulted in excretion of nitrite, which accumulated in the extracellular medium, when the nirB mutant was grown in the presence of nitrate. A nirA in-frame deletion mutant also showed a phenotype of increased expression of the nirA operon in the absence of ammonium, independent of the presence of nitrate in the medium. Both NirB and NirA are therefore needed to keep low levels of expression of the nirA operon in the absence of an inducer. Because NirB is also needed to attain high levels of nitrite reductase activity, NirA appears to be a negative element in the nitrate regulation of expression of the nirA operon in Anabaena sp. strain PCC 7120.
Assimilatory nitrate reduction is carried out by many plants, algae, fungi, and bacteria. It involves the uptake of nitrate into the cell and its two-step reduction via nitrite to ammonium, which is incorporated into carbon skeletons. In bacteria, uptake is carried out by ABC-type or MFS transporters, and reduction involves the direct transfer of electrons to nitrate and nitrite, via nitrate reductase and nitrite reductase, respectively, from iron-sulfur or flavin-containing donor proteins (28). Expression of the nitrate assimilation system is frequently subjected to dual regulation, with repression by ammonium and induction by nitrate (or nitrite). Whereas repression is exerted by the general nitrogen control system of the bacterium, a variety of different mechanisms appear to exist to mediate induction (28).
Cyanobacteria are photoautotrophs that carry out oxygenic photosynthesis. Nitrate and ammonium are excellent sources of nitrogen for cyanobacteria in general, and many strains are able to use urea or to fix atmospheric nitrogen (15). In cyanobacteria, reduction of nitrate to ammonium is catalyzed by two ferredoxin-dependent enzymes, nitrate reductase and nitrite reductase. Genes encoding nitrite reductase (nirA), an ABC-type nitrate/nitrite uptake transporter (nrtABCD), and nitrate reductase (narB) are clustered together, constituting the nirA operon (nirA-nrtABCD-narB), in the genomes of Synechococcus elongatus strain PCC 7942 (hereafter referred to as S. elongatus) and Anabaena sp. strain PCC 7120 (13). Several genes involved in the biosynthesis of the nitrate reductase molybdenum cofactor (molybdopterin guanine dinucleotide) and two additional genes, narM and nirB, that affect nitrate reductase and nitrite reductase activity levels, respectively, have also been identified in S. elongatus (13). The nirB gene has been shown to be required for attaining maximum levels of nitrite reductase, and its inactivation provokes an imbalance between nitrate and nitrite reduction, resulting in release of nitrite into the external medium (36).
Nitrate reductase and nitrite reductase activities are lower in ammonium-grown than in nitrate-grown cyanobacterial cells (13, 15). Expression of these enzyme activities takes place at appreciable levels in the absence of nitrate or nitrite in some cyanobacteria, such as S. elongatus, but not in the heterocyst-forming, N2-fixing cyanobacteria, such as Anabaena sp. strain PCC 7120. Thus, in the non-N2-fixing cyanobacteria, the nitrate assimilation system is subjected mainly to ammonium-promoted repression, whereas in the N2-fixing cyanobacteria, in addition to the repression by ammonium, induction by nitrate or nitrite is required to attain high levels of expression, giving rise to a “nitrate effect” in this type of cyanobacteria (13, 15).
Expression of the nirA operon upon ammonium withdrawal is promoted by the NtcA protein, a CAP family transcription factor that is widespread among cyanobacteria (25). NtcA activity is enhanced by 2-oxoglutarate, a putative signal of C-to-N balance in the cyanobacterial cell (17, 30) that can act on NtcA both directly (37-39) and indirectly, via the signal transduction protein PII (3, 33). In addition to NtcA, a route-specific, LysR-type transcriptional regulator, NtcB, is involved in the regulation of nirA operon expression (1, 2, 18, 27). In contrast to NtcA, which is strictly necessary for expression of the nirA operon in all investigated cyanobacterial strains, NtcB is involved in regulation with different stringency levels depending on the cyanobacterial strain. In the case of Anabaena sp. strain PCC 7120, the NtcB protein is strictly required for expression of the nirA operon and for growth at the expense of nitrate, and expression of ntcB itself takes place from an NtcA-dependent promoter (18). A third positive regulatory element of nirA operon expression in Anabaena sp. strain PCC 7120 is the CnaT protein (20), which shows overall sequence similarity to glycosyltransferases. An Anabaena cnaT insertional mutant is unable to use nitrate as a nitrogen source due to a defect in activation of transcription of the nirA operon. However, CnaT does not appear to be a DNA-binding protein, and consequently, the effect of CnaT on nirA operon expression could be indirect.
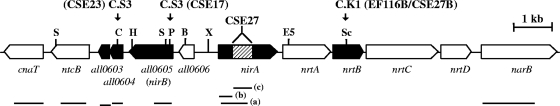
Four open reading frames (ORFs) (all0603, all0604, all0605, and all0606) are located between the nirA and ntcB genes in the genome of Anabaena sp. strain PCC 7120, with the same orientation as ntcB (Fig. 1). all0603 encodes a 101-amino-acid transcriptional regulator of the XRE family. all0604 encodes a 119-amino-acid polypeptide that shows no homology with any protein of known function. all0605 (previously designated orf398) (19) encodes a protein with sequence similarity to several proteins previously characterized in S. elongatus: the phycocyanobilin lyase alpha subunit CpcE (26% identity in a 163-amino-acid overlap), the NblB polypeptide involved in phycobilisome degradation (22% identity in an N-terminal 180-amino-acid overlap plus 23% identity in a C-terminal 148-amino-acid overlap), and the NirB protein (23% identity in a 331-amino-acid overlap). all0606 (previously designated orf136) (19) encodes a protein similar to the cytochrome b6f complex iron-sulfur protein PetC. Besides all0606, three other Anabaena sp. strain PCC 7120 ORFs, all2453, all4511, and all1512, show overall homology to the cytochrome b6f complex iron-sulfur protein PetC (26).
FIG. 1.
Genomic region of Anabaena sp. strain PCC 7120 bearing the nitrate assimilation gene cluster. Genes and ORFs are indicated by thick arrows, which also show the direction of transcription. Black arrows correspond to the ORFs investigated in this work. The locations of the restriction sites into which gene cassettes (C.S3 for strains CSE17 and CSE23 and C.K1 for strains EF116B and CSE27B) were inserted are indicated. The region deleted from nirA in strain CSE27 is indicated with a hatched bar. Abbreviations for some restriction endonuclease sites: B, BglI; C, ClaI; E5, EcoRV; H, HindIII; P, PvuII; S, SpeI; Sc, ScaI; and X, XbaI. Horizontal lines below the genes denote probes used for Northern analyses.
In this study, we show that in addition to the positive elements described above, the expression of the Anabaena nirA operon is subject to the action of two negative elements, the products of ORF all0605 (the Anabaena nirB gene) and nirA, which repress the expression of the nirA operon when nitrate is absent from the culture medium, giving rise to the aforementioned nitrate effect in Anabaena sp. strain PCC 7120.
MATERIALS AND METHODS
Strains and growth conditions.
Anabaena sp. (also known as Nostoc sp.) strain PCC 7120 was routinely grown photoautotrophically at 30°C under white light (about 25 μE s−1 m−2), with shaking for liquid cultures. Media used for growth were BG11 (NaNO3 as the nitrogen source) (34), BG110 (BG11 without nitrate), and BG110NH4+ [BG110 supplemented with 2 mM NH4Cl and 4 mM N-tris(hydroxymethyl)methyl-2-aminoethane sulfonic acid (TES)-NaOH buffer, pH 7.5]. For growth on plates, medium solidified with separately autoclaved 1% agar (Difco) was used. When appropriate, antibiotics were added to plates at the following final concentrations: streptomycin (Sm), 5 μg/ml; spectinomycin (Sp), 5 μg/ml; and neomycin (Nm), 30 μg/ml. In liquid cultures, antibiotic concentrations used were as follows: Sm, 2 μg/ml; Sp, 2 μg/ml; and Nm, 5 μg/ml. Strains CSE17 (19), CSE23, CSE271, and CSE272 were routinely grown in BG110NH4+ medium supplemented with Sm and Sp. Strains EF116B and CSE27B were routinely grown in BG110NH4+ medium supplemented with Nm. CSE27 and CSE172 were routinely grown in BG110NH4+ medium.
For derepression experiments, cells grown in BG110NH4+ (BG110 supplemented with 4 mM NH4Cl and 8 mM TES-NaOH buffer, pH 7.5) medium bubbled with a mixture of air and CO2 (1% [vol/vol] CO2) at 30°C in the light (75 to 100 μE s−1 m−2) were harvested by filtration and washed with BG110 medium, resuspended in the medium indicated in each experiment, and incubated under the same conditions. All media used for derepression experiments were supplemented with 12 mM NaHCO3.
Escherichia coli DH5α, HB101, XL1-Blue, and ED8654 were grown in Luria-Bertani medium as described previously (4).
Generation of mutant strains.
The method of sacB-mediated positive selection for double recombinants of Anabaena sp. (7) was used to generate mutant strains EF116B (nrtB::C.K1), CSE27 (ΔnirA), CSE27B (ΔnirA nrtB::C.K1), and CSE23 (all0604::C.S3) and to complement mutant strain CSE17 (all0605::C.S3). Plasmids pCSE111B (for CSE23), pCSE129 (for CSE172), pCSE142B (for CSE27), pCSE149 (for CSE271 and CSE272), and pCSE152B (for EF116B and CSE27B) were transferred to the cyanobacterial parental strain by conjugation (11). See Table 1 for a description of the strains and plasmids used in this study. Plasmid pRL623 was used as a helper plasmid in conjugations, except for the generation of strain CSE23, for which plasmids pRL528 and pRL591-W45 were used as helper plasmids. In all cases, plasmid pRL443 was used as a conjugative plasmid. For generation of strains CSE27 and CSE172, some sucrose-sensitive exconjugants (Smr Spr for CSE27 and Smr Spr Nmr for CSE172) were grown in BG110NH4+ liquid medium without antibiotics. These cultures were sonicated in a cleaning bath and plated on BG110NH4+ solid medium containing 5% sucrose. Double recombinants were identified by their sucrose-resistant, antibiotic-sensitive phenotype. In CSE27, a 666-bp internal fragment of nirA, corresponding to nucleotides 598 to 1263 of the 1,611-nucleotide coding region, was deleted from the genome, and as a consequence of the in-frame deletion of nucleotides, the modified nirA gene should encode a protein of 304 amino acid residues. In all cases, the genomic structure of the resultant Anabaena mutant strain was checked by Southern analysis or PCR analysis to confirm the absence of parental chromosomes in the resultant Anabaena strains.
TABLE 1.
Cyanobacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Reference |
|---|---|---|
| Strains | ||
| PCC 7120 | Wild-type Anabaena strain | 34 |
| EF116 | Derivative of Anabaena sp. strain PCC 7120 unable to fix nitrogen under aerobic conditions | 41 |
| EF116B | Nmr derivative of strain EF116; nrtB::C.K1 | This work |
| CSE17 | Smr Spr derivative of strain PCC 7120; all0605::C.S3 | 19 |
| CSE172 | Sms Sps derivative of strain CSE17; all0605::C.S3 replaced by a wild-type version of all0605 | This work |
| CSE23 | Smr Spr derivative of strain PCC 7120; all0604::C.S3 | This work |
| CSE27 | Derivative of strain EF116; ΔnirA | This work |
| CSE27B | Nmr derivative of strain CSE27; ΔnirA nrtB::C.K1 | This work |
| CSE271 | Smr Spr derivative of strain CSE27; pCSE149 plasmid integrated into nucA region of the alpha megaplasmid | This work |
| CSE272 | Smr Spr derivative of strain CSE27; pCSE149 plasmid integrated into nirA region of the Anabaena chromosome | This work |
| Plasmids | ||
| pCSE111B | 2.79-kb SpeI fragment from pCSE73 (19), bearing AccI-ended C.S3 gene cassette (12) inserted into the ClaI site of all0604, cloned into pRL278; used to generate mutant strain CSE23 | This work |
| pCSE129 | 1.94-kb XbaI/HindIII fragment from pCSE26 (19), which contains part of all0605 and all of all0606, cloned into pRL278; used to complement mutant strain CSE17 | This work |
| pCSE142 | 1,166-bp product of PCR with primers nir-7120-23, nir-7120-25, nir-7120-26, and nrtA-7120-3, with pCSE26 as the template, cloned in pGEM-T Easy (Promega); presents a deletion of a 666-bp internal segment of nirA corresponding to nucleotides 598 to 1263 of the 1,611-nucleotide coding region | This work |
| pCSE142B | 1.3-kb PvuII/SalI fragment from pCSE142 between the NruI/XhoI sites of pRL277; used to generate mutant strain CSE27 | This work |
| pCSE152B | 2.9-kb ClaI/XbaI fragment from pCSE2 (19), bearing HincII-ended C.K1 gene cassette (12) inserted into the ScaI site of nrtB (in the same direction as nrtB), cloned into pRL277; used to generate mutant strains EF116B and CSE27B | This work |
| pCSE149 | 5.3-kb EcoRV/PvuII fragment from pCSE78B (19) inserted between the EcoRV/NruI sites of pCSEL24 (31); used to complement mutant strain CSE27 | This work |
| pRL277 | Smr Spr; sacB-carrying, mobilizable vector | 5 |
| pRL278 | Nmr; sacB-carrying, mobilizable vector | 5 |
| pRL443 | Kms derivative of conjugative plasmid RP4 | 11 |
| pRL528 | Mobilization helper; encodes M.AvaI and M.Eco47II | 11 |
| pRL591-W45 | Mobilization helper; encodes M.EcoT22I | 10 |
| pRL623 | Mobilization helper; encodes M.AvaI, M.Eco47II, and M.EcoT22I | 10 |
DNA isolation and Southern blot analysis.
Isolation of DNA from Anabaena sp. was performed as previously described (7). For Southern blots, restriction endonuclease-digested DNA was subjected to electrophoresis in agarose gels and transferred to Hybond-N+ membranes following the instructions of the manufacturer. Labeling of probes with 32P and hybridization were performed as described previously (8, 21).
RNA isolation and analysis.
RNA from Anabaena sp. was prepared as described previously (29). The resulting RNA preparations were treated with RNase-free DNase I to eliminate contaminating DNA. For Northern blot analysis, RNA (approximately 20 to 25 μg) was subjected to electrophoresis in denaturing formaldehyde gels, transferred to Hybond-N+ membranes, and subjected to hybridization at 65°C as described previously (8). DNA probes used in Northern experiments were as follows: nirA probe, PCR-generated DNA fragment obtained using primers nir-7120-15 and nir-7120-16 (probe a), nir-7120-23 and nir-7120-25 (probe b), or nir-7120-30 and nir-7120-31 (probe c); narB probe, PCR-generated DNA fragment obtained using primers N-narB-7120 and C-narB-7120; ntcA probe, NcoI/SalI DNA fragment from pCSAM61 (29); ntcB probe, PCR-generated DNA fragment obtained using primers Nc-ntcB and ntcB-3 (18); cnaT probe, HincII/BstXI DNA fragment from pCSE118 (20), bearing most of the Anabaena cnaT gene; all0603 probe, PCR-generated DNA fragment obtained using primers all0603-1 and all0603-2; all0604 probe, PCR-generated DNA fragment obtained using primers all0604-1 and all0604-2; and all0605 (nirB) probe, PCR-generated DNA fragment obtained using primers orf398-2 and nir-7120-10. The sequences of primers not described previously are shown in Table 2. For PCR-generated probes, Anabaena chromosomal DNA, plasmid pCSE21 (19), or plasmid pCSE95 (18) was used as a template. Primer extension experiments were performed as described elsewhere (4), using 20 to 25 μg of RNA and primer nir-1 (19). Results were visualized and quantified with a Cyclone storage phosphorimaging system and OptiQuant image analysis software (Packard).
TABLE 2.
Oligodeoxynucleotide primers used in this work
| Primer | Sequence (5′-3′)a |
|---|---|
| nir-7120-6 | GGT GTT GGT CGT GGG TAC |
| nir-7120-10 | GCA AGC GAT CGC ACT GCC |
| nir-7120-15 | GCA ACA GAC CGA GAT CAT CG |
| nir-7120-16 | CCC CAT TCA TCA ATT AGC C |
| nir-7120-23 | CTA CCC CCA AAG CCA GCC TC |
| nir-7120-25 | GAG GGC GAA CGC ATG AAC TGA ATT ATC CC |
| nir-7120-26 | ACG TTC ATG CGT TCG CCC TCA TCG AAA CC |
| nir-7120-27 | CGG GAT AAT TCA GTT CAT GC |
| nir-7120-28 | TTC CGG CAC GGG CGC ACA ATT TGG CAA CTT CG |
| nir-7120-30 | GGA AAT CAA CGA TTT AGC CTT TGT TCC |
| nir-7120-31 | GTT GCA AAA TTG TGC GCC CGT G |
| orf398-2 | CAT AGT GCA GAT GAT TTG TC |
| orf398-4 | TCA GTG CAG CGA TCG CAT GG |
| nrtA-7120-2 | TCC AAT CTT GCC GCA TAC |
| nrtA-7120-3 | TCT AGA GGA AGT ACA GCC ATG TAC C |
| N-narB-7120 | GGA GCG AAG CGA CGT GAC |
| C-narB-7120 | GGT CAG TTG GGT AAA CTC |
| all0603-1 | CGA AGC CAT TTG ATG AAC |
| all0603-2 | CAA TCG AAC TGA GAA ATC AC |
| all0604-1 | AAT GAC CTG GGA GGT AGA G |
| all0604-2 | TCA TAG GAA CCC CTC TG |
| nui-7120-4 | ATG AGT GAG TCT GAA TAC CC |
Introduced restriction enzyme cutting sites are shown in bold.
Nitrate uptake.
Nitrate uptake assays were performed as previously described (14). Ammonium-grown cells (4 to 5 μg of chlorophyll a/ml) were derepressed by incubation for 4 h in BG11 medium. Cells were then harvested by filtration, washed with 10 mM Tricine-NaOH buffer (pH 8.1), resuspended in the same buffer to 10 μg of chlorophyll a/ml, and incubated under culture conditions. Uptake assays were started by the addition of NaNO3 (100 to 110 μM [final concentration]). Nitrate disappearance was determined by estimating the concentration of nitrate in the medium in aliquots of the cell suspensions after removal of the cells by filtration through Millipore HA 0.45-mm-pore-size filters. The nitrate concentration was determined by high-performance liquid chromatography (HPLC), using a Partisil 10 SAX WCS analytical column (4.6 mm × 250 mm; 10 μm) from Whatman International Ltd. (England).
Enzyme activities.
Nitrate reductase (23) and nitrite reductase (24) activities were measured with dithionite-reduced methyl viologen as the reductant in cells made permeable with mixed alkyltrimethylammonium bromide. The samples of cells added to enzymatic assays of nitrate reductase and nitrite reductase activities contained 5 and 25 μg of chlorophyll a, respectively. Activity units correspond to μmol of nitrite produced (nitrate reductase) or removed (nitrite reductase) per minute.
RESULTS AND DISCUSSION
Verification of strain CSE17.
Construction of mutant strain CSE17 (all0605::C.S3 or nirB::C.S3) has been described previously (19). When ammonium-grown cells of this mutant were incubated in the absence of ammonium in cultures bubbled with CO2-enriched air, they showed abnormally low levels of nitrite reductase activity in nitrate-containing medium and abnormally high levels of nitrate reductase activity in medium containing no combined nitrogen (see below). These data confirm previously reported data (19).
To verify that the phenotype shown by strain CSE17 results from inactivation of all0605 (nirB) and not from any additional mutation elsewhere in the genome, the mutated version of nirB in CSE17 was replaced by a wild-type version of the gene (see Materials and Methods for details). In strain CSE172, the genomic structure in the nirB region is identical to that of the wild-type strain PCC 7120 (not shown). Nitrate and nitrite reductase levels in strain CSE172 were similar to those of the wild type rather than to those of the parental strain, CSE17, showing that the phenotype of strain CSE17 results from the inactivation of nirB.
Two small ORFs, all0604 and all0603, whose coding sequences overlap by 3 nucleotides, are located downstream of all0605 (Fig. 1). The gene cassette inserted into all0605 in strain CSE17 (C.S3) (12) contains transcriptional terminators that are effective in Anabaena sp. strain PCC 7120 (e.g., see reference 19). To verify that the phenotype of strain CSE17 results from inactivation of all0605 and not from a polar effect on a downstream gene, the expression of all0604-all0603 was investigated and a mutant of all0604 was constructed with the same cassette, C.S3 (see Fig. 1 and Materials and Methods for details). Mutant strain CSE23 (all0604::C.S3) was able to grow in medium containing ammonium, nitrate, or no combined nitrogen and exhibited nitrate reductase and nitrite reductase activity levels that were similar (within 10%) to those of the wild-type strain. Additionally, the expression of nirA and narB in mutant strain CSE23, investigated by Northern analysis, showed no appreciable difference from that observed in the wild-type strain PCC 7120 (not shown).
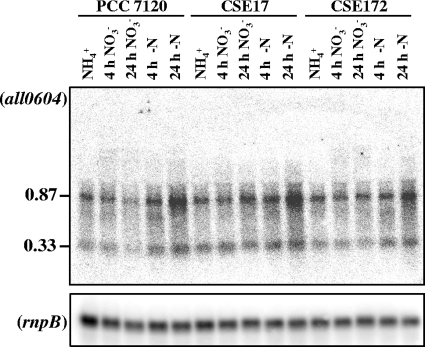
To investigate the expression of all0604 and all0603, Northern analysis was performed with probes for each of these ORFs and with RNAs isolated from strains PCC 7120, CSE17, and CSE172. The all0604 probe hybridized to bands of about 0.33 kb (likely corresponding to an all0604 transcript) and 0.87 kb (likely corresponding to a transcript covering both all0604 and all0603) (Fig. 2). Only the latter band was obtained using an all0603 probe, and no expression of all0603 was observed in mutant strain CSE23 (all0604::C.S3) (not shown). Thus, a bicistronic transcript is produced, but a monocistronic transcript was also observed that may have resulted from premature transcription termination downstream of all0604. The 0.87-kb bicistronic transcript accumulated at an increased level after 24 h of incubation in the absence of combined nitrogen. No significant differences in the expression profiles of all0603 and all0604 were observed between strains PCC 7120, CSE17, and CSE172 (Fig. 2). The results of analysis of the nirB mutant (all0605::C.S3) and of expression of all0604-all0603 both indicate that the phenotype of CSE17 cannot be ascribed to a polar effect on the expression of downstream genes.
FIG. 2.
Northern analysis of the expression of all0604 in strains PCC 7120, CSE17, and CSE172. Hybridization assays were carried out using RNAs isolated from cells grown with ammonium (NH4+) or grown with ammonium and incubated for 4 or 24 h in medium containing nitrate (NO3−) or no combined nitrogen (−N). Hybridization to rnpB (40) served as a loading and transfer control (lower panel). The positions and sizes (in kb) of the detected transcripts are shown on the left.
Effect of inactivation of nirB on the expression of nitrate assimilation enzyme activities and genes.
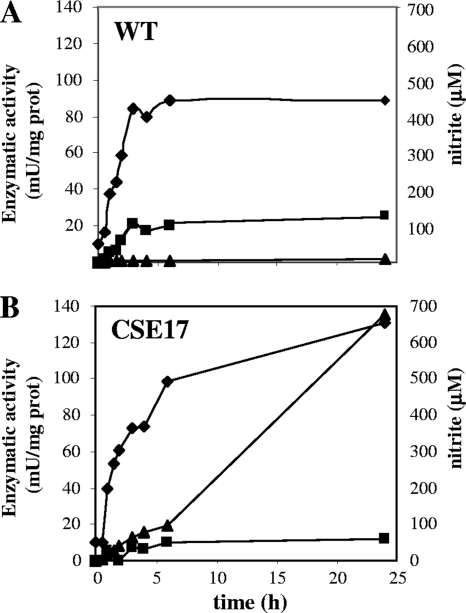
The development of nitrate and nitrite reductase activities was analyzed in cultures of wild-type strain PCC 7120 and mutant strain CSE17 during a 24-h induction experiment in nitrate-containing media. In the wild type (Fig. 3A), both enzymatic activities reached a plateau after 6 h of incubation, with activities of about 89 mU (mg of protein)−1 for nitrate reductase and about 23 mU (mg of protein)−1 for nitrite reductase. In the mutant (Fig. 3B), the nitrite reductase activity also reached a plateau at 6 h, showing an activity of about 11 mU (mg of protein)−1, whereas the nitrate reductase activity increased during the course of the experiment, to reach an activity of about 131 mU (mg of protein)−1 at 24 h. An accumulation of nitrite in the culture medium was observed specifically for strain CSE17, indicating that its nitrite reductase is unable to reduce all the nitrite produced by nitrate reductase, with the excess nitrite being excreted.
FIG. 3.
Changes in levels of nitrate and nitrite reductase activities and nitrite accumulation in the culture medium after transfer of ammonium-grown cells to nitrate-containing medium. (A) Strain PCC 7120 (WT); (B) strain CSE17. Diamonds, nitrate reductase activity; squares, nitrite reductase activity; triangles, nitrite concentration in the medium.
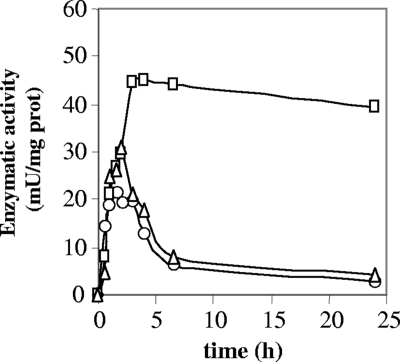
The development of nitrate reductase in strains PCC 7120, CSE17, and CSE172 was also studied in medium lacking combined nitrogen (Fig. 4). Expression was observed, and after reaching a peak shortly after the beginning of the incubation, the activity decayed in the wild type, but much less so in strain CSE17. Strain CSE172 behaved like the wild type. The level of nitrate reductase activity after 24 h in the absence of nitrate was about 15-fold higher in mutant CSE17 than in the wild type. These results show that inactivation of nirB has profound effects on the enzyme activities in the nitrate assimilation system, resulting in low levels of nitrite reductase and, especially when the cells are incubated in the absence of nitrate, abnormally high levels of nitrate reductase.
FIG. 4.
Changes in levels of nitrate reductase activity after transfer of ammonium-grown cells to a medium containing no combined nitrogen. Circles, strain PCC 7120; squares, strain CSE17; triangles, strain CSE172.
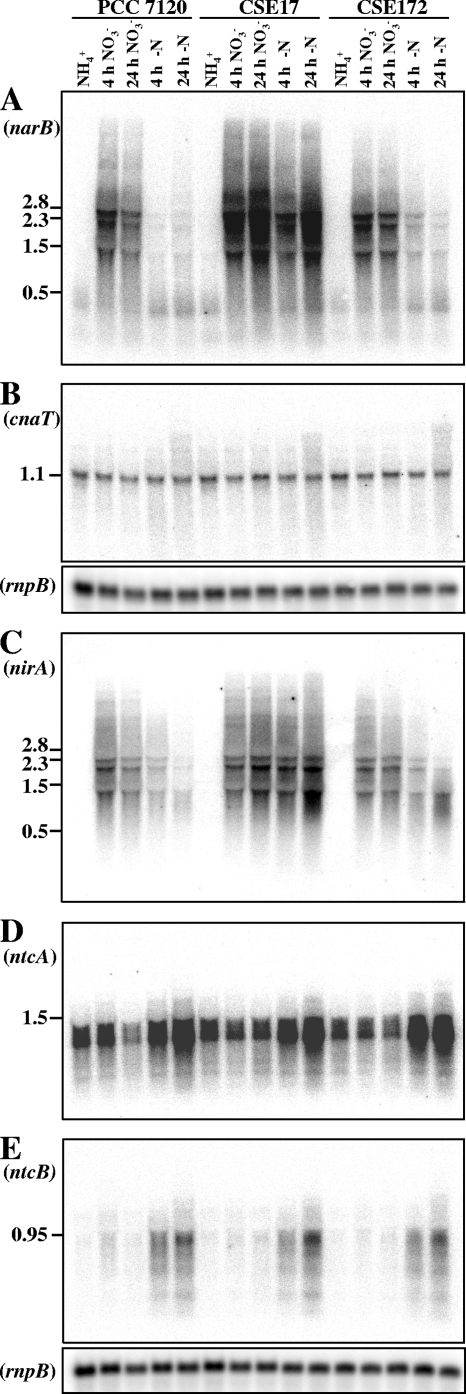
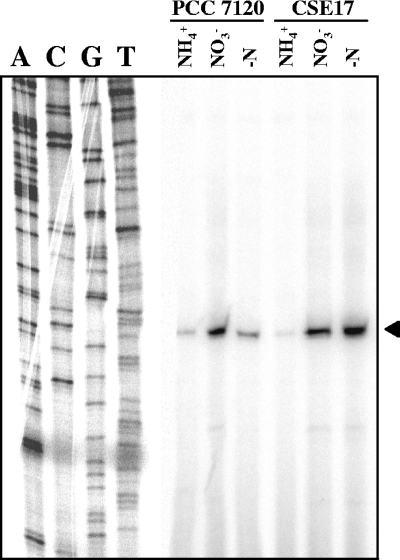
To test whether the abnormal levels of nitrate and nitrite reductase activities shown by strain CSE17 resulted from an altered expression of the nirA operon, Northern analyses were carried out with probes for the nirA and narB genes. Hybridizations were performed with total RNAs isolated from cells of strains PCC 7120, CSE17, and CSE172 incubated under different nitrogen regimens. As previously reported for the nirA operon (19), which produces a long transcript of close to 10 kb that is unstable, only a smear of degraded RNA products could be detected with both the nirA and narB probes (Fig. 5). In strains PCC 7120 and CSE172, a high level of expression of narB took place only in medium without ammonium in the presence of nitrate. In the nirB mutant (strain CSE17), however, a high level of expression was observed in the absence of ammonium both in the presence and in the absence of nitrate (Fig. 5A). A similar expression profile was obtained with the nirA probe (Fig. 5C). Levels of the 5′ region of the nirA operon transcript in strain CSE17 were also analyzed by primer extension analysis. The obtained data (Fig. 6) corroborated those obtained by Northern experiments, suggesting that the increased nirA operon transcript levels observed in strain CSE17 in the absence of combined nitrogen corresponded to more utilization of the nirA operon promoter in this strain.
FIG. 5.
Northern analysis of expression of the nirA operon and of some regulatory genes in strains PCC 7120, CSE17, and CSE172. Hybridization assays were carried out using RNAs isolated from cells grown with ammonium (NH4+) or grown with ammonium and incubated for 4 or 24 h in medium containing nitrate (NO3−) or no combined nitrogen (−N). The hybridization probes used (see Materials and Methods for details) corresponded to narB (A), cnaT (B), nirA (C; fragment a in Fig. 1), ntcA (D), and ntcB (E). Hybridization to rnpB (40) served as a loading and transfer control for each of the two filters used. The positions of some size markers (A and C) and identified transcripts (B, D, and E) are shown (in kb) on the left.
FIG. 6.
Primer extension analysis of expression of the nirA gene in strains PCC 7120 and CSE17. Primer extension assays were carried out using oligonucleotide nir-1 as a primer and RNAs isolated from cells grown with ammonium (NH4+) or grown with ammonium and incubated for 4 h in medium containing nitrate (NO3−) or no combined nitrogen (−N). The arrowhead points to the extension product identifying the Anabaena nirA operon tsp. The sequencing ladders presented were generated with the same primer used in the primer extension reactions, with plasmid pCSE26 as template.
To test whether the expression of the positive regulatory elements NtcA, NtcB, and CnaT was affected in mutant strain CSE17, Northern analysis was performed. The expression patterns observed for the ntcA, ntcB, and cnaT genes under the different nitrogen regimens tested were similar in strains PCC 7120, CSE17, and CSE172 (Fig. 5) and similar to those previously reported (18, 20, 29). These results indicate that NirB is not involved in the regulation of transcription of these genes and, additionally, that the altered levels of nirA operon expression observed in strain CSE17 do not result from altered expression of the positive regulatory elements NtcA, NtcB, and CnaT. Therefore, NirB appears to be a factor inhibiting the expression of the nirA operon, especially in the absence of nitrate, in Anabaena sp. strain PCC 7120.
The accumulation of nirA operon transcripts in strain CSE17, observed with probes for nirA and narB, explains the higher nitrate reductase activity observed in this mutant in both the presence and absence of nitrate; however, it does not explain the low levels of nitrite reductase activity observed in the presence of nitrate. A similar phenotype of expression of low levels of nitrite reductase activity has been observed for a nirB mutant of S. elongatus, although no effect of the corresponding mutation on the nirA operon transcript level has been reported (35). It has been proposed that NirB is required as a chaperone or scaffold for expression of maximum nitrite reductase activity (35). The similarity of NirB to CpcE and NblB is based on the presence in these proteins of a number of HEAT-like repeats, which consist of tandemly repeated amino acid modules that appear to function in protein-protein interactions and may have a scaffolding role (22). Anabaena NirB appears to have six such HEAT repeats. A role based on the presence of these structural motifs would explain why the NirB proteins from S. elongatus and Anabaena sp. strain PCC 7120, two homologous proteins that appear to have similar functions in nitrate reduction and are therefore orthologues, show such a low degree of identity (23%). If NirB works through protein-protein interactions, it is conceivable that NirB interacts with the nitrite reductase, allowing it to reach a maximally active conformation for the development of its enzymatic activity. In turn, this raises the possibility of regulation of the nirA operon by nitrite reductase itself rather than by NirB.
Study of a nirA mutant.
Different mutant strains with changes in the Anabaena nirA operon generated by lux-tagged Tn5 transposon mutagenesis have been described (6). One of those mutants, strain TLN10 (nirA::Tn5), shows a phenotype similar to that of the nirB mutant. When cells are transferred from ammonium-containing medium to medium lacking combined nitrogen, a luciferase activity develops in strain TLN10 that is very high compared to that shown by the mutant strains TLN12 (nrtC::Tn5) and TLN21 (with Tn5 inserted in the nrtD-narB intergenic region) (6). Given that in both cases, i.e., the nirB mutant and strain TLN10, the nitrite reductase activity is affected (we assume that the Tn5 insertion inactivates nitrite reductase), we decided to mutate the nirA gene in order to check whether the nitrite reductase is involved in the regulation of expression of the nirA operon.
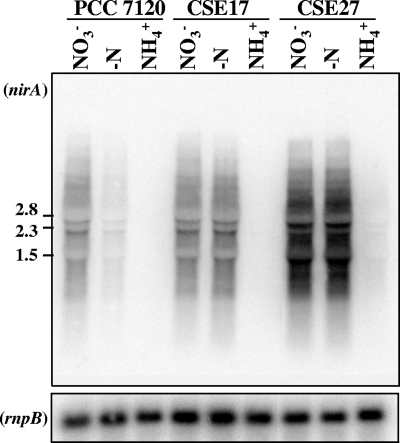
A nirA mutant, strain CSE27, was constructed by deleting in frame a 666-bp internal fragment of nirA, corresponding to nucleotides 598 to 1263 of the 1,611-nucleotide coding region (see Fig. 1 and Materials and Methods for details). When nitrate was used as a nitrogen source, strain CSE27 was unable to grow and nitrite accumulated in the culture medium (not shown). The expression of nirA in mutant strain CSE27 was investigated by Northern analysis, using RNAs isolated from strains PCC 7120, CSE17, and CSE27 (Fig. 7). As is the case for strain CSE17, high expression of the nirA operon in the absence of ammonium in both the presence and absence of nitrate was observed in strain CSE27, and the observed levels of expression were even higher than those in strain CSE17.
FIG. 7.
Northern analysis of expression of nirA in strains PCC 7120, CSE17, and CSE27. Hybridization assays were carried out using RNAs isolated from cells grown with ammonium (NH4+) or grown with ammonium and incubated for 4 h in medium containing nitrate (NO3−) or no combined nitrogen (−N). Fragment b in Fig. 1 was used as a nirA probe (see Materials and Methods for details). Hybridization to rnpB (40) served as a loading and transfer control (lower panel). The positions and sizes (in kb) of some size standards are shown on the left. The small change in the pattern of hybridization bands for strain CSE27 compared to that for strains CSE17 and PCC 7120 resulted from the deletion of 666 bp from nirA in CSE27.
To verify that the phenotype shown by strain CSE27 resulted from inactivation of nirA, a wild-type version of the nirA gene bearing the complete nirA operon promoter was introduced into strain CSE27. Plasmid pCSE149 (Table 1), a derivative of pCSEL24 (31), can recombine with the nirA region in the chromosome and with the nucA region in the Anabaena alpha megaplasmid. In the exconjugant strains CSE271 and CSE272, pCSE149 was integrated into the alpha megaplasmid (Fig. 8A) and into the chromosome (Fig. 8B), respectively, in such a way that in both cases one copy of the nirA operon was maintained as in CSE27 and an intact nirA gene, together with its promoter, was additionally present. The genomic structure of these strains was confirmed by PCR analysis using different primer pairs (nui-7120-4 with nir-7120-28, nir-7120-27 with nrtA-7120-2, nir-7120-6 with orf398-4, ntcs3 [19] with nrtA-7120-2, and ntcs3 with nrtA-7120-3) (Table 2). Both strains were able to grow using nitrate as a nitrogen source. The expression of narB was investigated by Northern analysis, using total RNAs isolated from strains CSE271 and CSE272 and, as controls, from strains PCC 7120 and CSE27 (Fig. 8C). In contrast to strain CSE27, strains CSE271 and CSE272 presented an expression of narB similar to that shown by strain PCC 7120. These results indicate that the introduction of a wild-type copy of nirA into strain CSE27 restored a wild-type phenotype of nirA operon expression at the mRNA level. Using the DNA fragment deleted in CSE27 as a nirA probe, we were also able to corroborate an ammonium- and nitrate-regulated expression of the wild-type copy of nirA introduced in strains CSE271 and CSE272 (Fig. 8D). Interestingly, however, the nirA copy in the alpha megaplasmid (strain CSE271) was expressed at higher levels than that present in the chromosome (strain CSE272).
FIG. 8.
Genomic structure of nirA complementing constructs in strains CSE271 and CSE272 and Northern analysis of the expression of narB and nirA in strains PCC 7120, CSE27, CSE271, and CSE272. The complementing constructs included ORF all0606, the nirA operon promoter region, and the nirA gene incorporated into the nucA-nuiA region of the alpha megaplasmid (A) or into the chromosomal nirA region (B). Primers (A and B): 1, orf398-4; 2, nir-7120-6; 3, nir-7120-27; 4, nrtA-7120-3; 5, nrtA-7120-2; 6, nui-7120-4; 7, nir-7120-28; and 8, ntcs3 (18). Hybridization assays were carried out using RNAs isolated from cells of the indicated strains grown with ammonium (NH4+) or grown with ammonium and incubated for 4 h in medium containing nitrate (NO3−) or no combined nitrogen (−N). The hybridization probes used (see Materials and Methods) corresponded to narB (C) and nirA (D; fragment c in Fig. 1). Hybridization to rnpB (40) served as a loading and transfer control. The positions and sizes (in kb) of some size standards are shown on the left.
As described in the introduction, a high level of expression of the nirA operon in Anabaena sp. strain PCC 7120 requires both the absence of ammonium and the presence of nitrate or nitrite, which acts as an inducer, in the culture medium. The results described above suggest that nitrite reductase, in addition to its role in nitrite reduction to ammonium during nitrate assimilation, has a negative role in the expression of the nirA operon when this cyanobacterium is incubated in the absence of both ammonium and nitrate in the culture medium. A simple interpretation of our results would be that in the nirB and nirA mutants, which have low levels of and lack nitrite reductase, respectively, nitrite accumulates in the cells up to levels that strongly induce the expression of the nirA operon. However, whereas this interpretation would be evident for those cultures supplemented with nitrate, we observed similarly increased nirA operon mRNA levels in the nirB and nirA mutants incubated in the absence as well as in the presence of added nitrate. The possibility remains, nevertheless, that traces of nitrate or nitrite present in the BG110 medium were concentrated within the cells to act as inducers.
Mutants of the NrtABCD transporter.
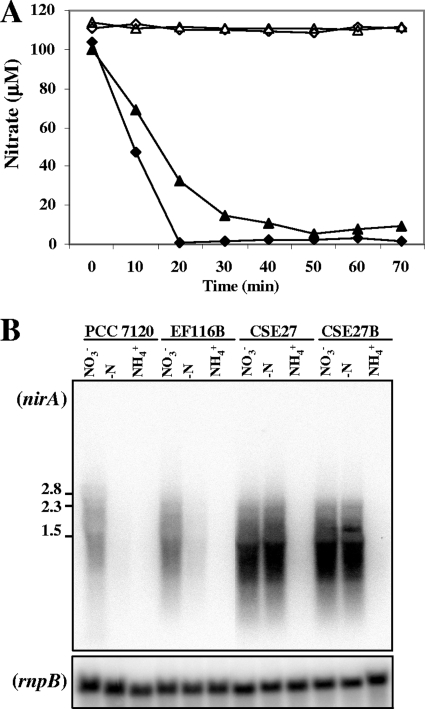
An Anabaena ABC-type nitrate transporter, NrtABCD, has been identified previously (19). To check whether nitrate concentrated within the cells from traces present in the medium could act as an inducer, the nrtB gene, which encodes the transmembrane component of the nitrate transporter, was mutated in strain CSE27 and in its parental strain, EF116 (see Table 1 for details). For this, the C.K1 cassette, which confers resistance to neomycin and does not bear transcriptional terminators, was introduced into the ScaI restriction site of nrtB in the same orientation as this gene (see Fig. 1 and Materials and Methods for details). Two mutants, strains EF116B (nrtB::C.K1) and CSE27B (ΔnirA nrtB::C.K1), were obtained that were defective in the active transport of nitrate (Fig. 9A). The expression of the nirA gene was studied by Northern analysis, and both mutants presented expression profiles similar to those shown by their respective parental strains (Fig. 9B). The fact that similar expression levels were observed in the presence and absence of added nitrate in spite of the absence of an active transporter makes it unlikely that traces of nitrate play a role in regulation.
FIG. 9.
Nitrate uptake and Northern analysis of expression of nirA in strains PCC 7120, EF116B, CSE27, and CSE27B. (A) Ammonium-grown cells of strains PCC7120 (closed diamonds), EF116B (open diamonds), CSE27 (closed triangles), and CSE27B (open triangles) were washed, resuspended in BG11 medium (17.6 mM NaNO3), and incubated for 4 h as indicated in Materials and Methods for derepression of the nitrate assimilation system. Cells were then harvested and used for nitrate uptake assays (see Materials and Methods). At the indicated times, nitrate was measured by HPLC, using aliquots withdrawn from the assay mixture. (B) Hybridization assays were carried out using RNAs isolated from cells of the indicated strains grown with ammonium (NH4+) or grown with ammonium and incubated for 4 h in medium containing nitrate (NO3−) or no combined nitrogen (−N). Fragment b in Fig. 1 was used as the nirA probe (see Materials and Methods). Hybridization to rnpB (40) served as a loading and transfer control (lower panel). The positions and sizes (in kb) of some size standards are shown on the left.
The Anabaena NrtABCD transporter also mediates the uptake of nitrite into cells (32; our unpublished results), but nitrite can also enter into cyanobacterial cells by means of diffusion of nitrous acid (16). Diffusion is not a mechanism that could concentrate nitrite within the cells, however, and direct determination of nitrite levels in cell extracts from filaments incubated without added nitrate under the conditions described in this work showed negligible levels of nitrite (not shown). Our results argue against accumulation of an inducer (nitrate or nitrite) being responsible for the increased levels of expression of the nirA operon observed in nirB and nirA mutants. They suggest instead that NirA (the nitrite reductase) and NirB (a possible nitrite reductase chaperone) somehow inhibit the expression of the nirA operon when wild-type Anabaena filaments are incubated in the absence of both ammonium and nitrate.
Concluding remarks.
As mentioned above, both Anabaena nirA and nirB mutants have lost the nitrate effect on expression of the nirA operon (Fig. 7). We do not know whether the observed phenotype of the nirB mutant results just from the lack of NirB protein or is related to the possible role of NirB as a scaffolding protein in the maturation of the nitrite reductase. If the latter were the case, then the nitrite reductase would be the key element in the negative regulation of the nirA operon in the absence of ammonium. How nitrite reductase could exert this negative role remains to be elucidated. A plausible hypothesis is that the nitrite reductase acts as a modulator of a transcription factor. Because NtcA is a general N control transcription factor and NtcB is a nitrate assimilation pathway-specific transcriptional activator, NtcB could be favored as a candidate target for nitrite reductase. Indeed, a role of NtcB in mediating a nitrite effect on the expression of the nirA operon in S. elongatus has been suggested (1). The nitrite reductase could switch from being an inhibitor of the NtcB transcriptional activator in the absence of nitrate or nitrite to acting as an enzyme in the presence of these nitrogen sources. It was recently found that some enzymes, termed trigger enzymes, can control gene expression in response to the availability of their substrates through different mechanisms, by binding to either DNA or RNA or by modulating the activity of transcription factors through either covalent modification or protein-protein interactions (9). Our results suggest that nitrite reductase from Anabaena sp. strain PCC 7120 is a trigger enzyme that, in addition to its role in nitrite reduction to ammonium, exerts a regulatory role on the nitrate assimilation system.
Acknowledgments
We thank A. M. Muro-Pastor and A. Valladares for their help during this work and A. Herrero for discussion. The use of DNA sequences from the DOE-Joint Genome Institute and the Kazusa DNA Research Institute (Japan) databases is acknowledged.
This work was supported by grant numbers BFU2005-07672 and BFU2008-03811 from the Ministerio de Ciencia y Tecnología (Spain).
Footnotes
Published ahead of print on 26 March 2010.
REFERENCES
- 1.Aichi, M., and T. Omata. 1997. Involvement of NtcB, a LysR family transcription factor, in nitrite activation of the nitrate assimilation operon in the cyanobacterium Synechococcus sp. strain PCC 7942. J. Bacteriol. 179:4671-4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aichi, M., N. Takatani, and T. Omata. 2001. Role of NtcB in activation of nitrate assimilation genes in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 183:5840-5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldehni, M. F., J. Sauer, C. Spielhaupter, R. Schmid, and K. Forchhammer. 2003. Signal transduction protein P(II) is required for NtcA-regulated gene expression during nitrogen deprivation in the cyanobacterium Synechococcus elongatus strain PCC 7942. J. Bacteriol. 185:2582-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ausubel, F. M., R. Brent, R. E. Kingston, D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 2009. Current protocols in molecular biology. Greene Publishing and Wiley-Interscience, New York, NY.
- 5.Black, T. A., Y. Cai, and C. P. Wolk. 1993. Spatial expression and autoregulation of hetR, a gene involved in the control of heterocyst development in Anabaena. Mol. Microbiol. 9:77-84. [DOI] [PubMed] [Google Scholar]
- 6.Cai, Y., and C. P. Wolk. 1997. Nitrogen deprivation of Anabaena sp. strain PCC 7120 elicits rapid activation of a gene cluster that is essential for uptake and utilization of nitrate. J. Bacteriol. 179:258-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai, Y. P., and C. P. Wolk. 1990. Use of a conditionally lethal gene in Anabaena sp. strain PCC 7120 to select for double recombinants and to entrap insertion sequences. J. Bacteriol. 172:3138-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Church, G. M., and W. Gilbert. 1984. Genomic sequencing. Proc. Natl. Acad. Sci. U. S. A. 81:1991-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Commichau, F. M., and J. Stülke. 2008. Trigger enzymes: bifunctional proteins active in metabolism and in controlling gene expression. Mol. Microbiol. 67:692-702. [DOI] [PubMed] [Google Scholar]
- 10.Elhai, J., A. Vepritskiy, A. M. Muro-Pastor, E. Flores, and C. P. Wolk. 1997. Reduction of conjugal transfer efficiency by three restriction activities of Anabaena sp. strain PCC 7120. J. Bacteriol. 179:1998-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elhai, J., and C. P. Wolk. 1988. Conjugal transfer of DNA to cyanobacteria. Methods Enzymol. 167:747-754. [DOI] [PubMed] [Google Scholar]
- 12.Elhai, J., and C. P. Wolk. 1988. A versatile class of positive-selection vectors based on the nonviability of palindrome-containing plasmids that allows cloning into long polylinkers. Gene 68:119-138. [DOI] [PubMed] [Google Scholar]
- 13.Flores, E., J. E. Frías, L. M. Rubio, and A. Herrero. 2005. Photosynthetic nitrate assimilation in cyanobacteria. Photosynth. Res. 83:117-133. [DOI] [PubMed] [Google Scholar]
- 14.Flores, E., M. G. Guerrero, and M. Losada. 1983. Photosynthetic nature of nitrate uptake and reduction in the cyanobacterium Anacystis nidulans. Biochim. Biophys. Acta 722:408-416. [Google Scholar]
- 15.Flores, E., and A. Herrero. 1994. Assimilatory nitrogen metabolism and its regulation, p. 487-517. In D. A. Bryant (ed.), The molecular biology of cyanobacteria. Kluwer Academic Publishers, Dordrecht, Netherlands.
- 16.Flores, E., A. Herrero, and M. G. Guerrero. 1987. Nitrite uptake and its regulation in the cyanobacterium Anacystis nidulans. Biochim. Biophys. Acta 896:103-108. [Google Scholar]
- 17.Forchhammer, K. 1999. The PII protein in Synechococcus PCC 7942 senses and signals 2-oxoglutarate under ATP-replete conditions, p. 549-553. In G. A. Peschek, W. Löffelhardt, and G. Schmetterer (ed.), The phototrophic prokaryotes. Kluwer Academic/Plenum Publishers, New York, NY.
- 18.Frías, J. E., E. Flores, and A. Herrero. 2000. Activation of the Anabaena nir operon promoter requires both NtcA (CAP family) and NtcB (LysR family) transcription factors. Mol. Microbiol. 38:613-625. [DOI] [PubMed] [Google Scholar]
- 19.Frías, J. E., E. Flores, and A. Herrero. 1997. Nitrate assimilation gene cluster from the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 179:477-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frías, J. E., A. Herrero, and E. Flores. 2003. Open reading frame all0601 from Anabaena sp. strain PCC 7120 represents a novel gene, cnaT, required for expression of the nitrate assimilation nir operon. J. Bacteriol. 185:5037-5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frías, J. E., A. Mérida, A. Herrero, J. Martín-Nieto, and E. Flores. 1993. General distribution of the nitrogen control gene ntcA in cyanobacteria. J. Bacteriol. 175:5710-5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Groves, M. R., and D. Barford. 1999. Topological characteristics of helical repeat proteins. Curr. Opin. Struct. Biol. 9:383-389. [DOI] [PubMed] [Google Scholar]
- 23.Herrero, A., E. Flores, and M. G. Guerrero. 1985. Regulation of nitrate reductase cellular levels in the cyanobacteria Anabaena variabilis and Synechocystis sp. FEMS Microbiol. Lett. 26:21-25. [Google Scholar]
- 24.Herrero, A., and M. G. Guerrero. 1986. Regulation of nitrite reductase in the cyanobacterium Anacystis nidulans. J. Gen. Microbiol. 132:2463-2468. [Google Scholar]
- 25.Herrero, A., A. M. Muro-Pastor, and E. Flores. 2001. Nitrogen control in cyanobacteria. J. Bacteriol. 183:411-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaneko, T., Y. Nakamura, C. P. Wolk, T. Kuritz, S. Sasamoto, A. Watanabe, M. Iriguchi, A. Ishikawa, K. Kawashima, T. Kimura, Y. Kishida, M. Kohara, M. Matsumoto, A. Matsuno, A. Muraki, N. Nakazaki, S. Shimpo, M. Sugimoto, M. Takazawa, M. Yamada, M. Yasuda, and S. Tabata. 2001. Complete genomic sequence of the filamentous nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. DNA Res. 8:205-213. [DOI] [PubMed] [Google Scholar]
- 27.Maeda, S., M. Okamura, M. Kobayashi, and T. Omata. 1998. Nitrite-specific active transport system of the cyanobacterium Synechococcus sp. strain PCC 7942. J. Bacteriol. 180:6761-6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moreno-Vivián, C., and E. Flores. 2007. Nitrate assimilation in bacteria, p. 263-282. In H. Bothe, S. J. Ferguson, and W. E. Newton (ed.), Biology of the nitrogen cycle. Elsevier B.V., Amsterdam, Netherlands.
- 29.Muro-Pastor, A. M., A. Valladares, E. Flores, and A. Herrero. 2002. Mutual dependence of the expression of the cell differentiation regulatory protein HetR and the global nitrogen regulator NtcA during heterocyst development. Mol. Microbiol. 44:1377-1385. [DOI] [PubMed] [Google Scholar]
- 30.Muro-Pastor, M. I., J. C. Reyes, and F. J. Florencio. 2001. Cyanobacteria perceive nitrogen status by sensing intracellular 2-oxoglutarate levels. J. Biol. Chem. 276:38320-38328. [DOI] [PubMed] [Google Scholar]
- 31.Olmedo-Verd, E., A. M. Muro-Pastor, E. Flores, and A. Herrero. 2006. Localized induction of the ntcA regulatory gene in developing heterocysts of Anabaena sp. strain PCC 7120. J. Bacteriol. 188:6694-6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paz-Yepes, J., E. Flores, and A. Herrero. 2009. Expression and mutational analysis of the glnB genomic region in the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 191:2353-2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paz-Yepes, J., E. Flores, and A. Herrero. 2003. Transcriptional effects of the signal transduction protein P(II) (glnB gene product) on NtcA-dependent genes in Synechococcus sp. PCC 7942. FEBS Lett. 543:42-46. [DOI] [PubMed] [Google Scholar]
- 34.Rippka, R., J. Deruelles, J. B. Waterbury, M. Herdman, and R. Y. Stanier. 1979. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 111:1-61. [Google Scholar]
- 35.Suzuki, I., N. Horie, T. Sugiyama, and T. Omata. 1995. Identification and characterization of two nitrogen-regulated genes of the cyanobacterium Synechococcus sp. strain PCC7942 required for maximum efficiency of nitrogen assimilation. J. Bacteriol. 177:290-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Suzuki, I., H. Kikuchi, S. Nakanishi, Y. Fujita, T. Sugiyama, and T. Omata. 1995. A novel nitrite reductase gene from the cyanobacterium Plectonema boryanum. J. Bacteriol. 177:6137-6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanigawa, R., M. Shirokane, S. Maeda Si, T. Omata, K. Tanaka, and H. Takahashi. 2002. Transcriptional activation of NtcA-dependent promoters of Synechococcus sp. PCC 7942 by 2-oxoglutarate in vitro. Proc. Natl. Acad. Sci. U. S. A. 99:4251-4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valladares, A., E. Flores, and A. Herrero. 2008. Transcription activation by NtcA and 2-oxoglutarate of three genes involved in heterocyst differentiation in the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 190:6126-6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vázquez-Bermúdez, M. F., A. Herrero, and E. Flores. 2002. 2-Oxoglutarate increases the binding affinity of the NtcA (nitrogen control) transcription factor for the Synechococcus glnA promoter. FEBS Lett. 512:71-74. [DOI] [PubMed] [Google Scholar]
- 40.Vioque, A. 1997. The RNase P RNA from cyanobacteria: short tandemly repeated repetitive (STRR) sequences are present within the RNase P RNA gene in heterocyst-forming cyanobacteria. Nucleic Acids Res. 25:3471-3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wolk, C. P., Y. Cai, L. Cardemil, E. Flores, B. Hohn, M. Murry, G. Schmetterer, B. Schrautemeier, and R. Wilson. 1988. Isolation and complementation of mutants of Anabaena sp. strain PCC 7120 unable to grow aerobically on dinitrogen. J. Bacteriol. 170:1239-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]