Abstract
Background
Case-crossover is one of the most used designs for analyzing the health-related effects of air pollution. Nevertheless, no one has reviewed its application and methodology in this context.
Objective
We conducted a systematic review of case-crossover (CCO) designs used to study the relationship between air pollution and morbidity and mortality, from the standpoint of methodology and application.
Data sources and extraction
A search was made of the MEDLINE and EMBASE databases. Reports were classified as methodologic or applied. From the latter, the following information was extracted: author, study location, year, type of population (general or patients), dependent variable(s), independent variable(s), type of CCO design, and whether effect modification was analyzed for variables at the individual level.
Data synthesis
The review covered 105 reports that fulfilled the inclusion criteria. Of these, 24 addressed methodological aspects, and the remainder involved the design’s application. In the methodological reports, the designs that yielded the best results in simulation were symmetric bidirectional CCO and time-stratified CCO. Furthermore, we observed an increase across time in the use of certain CCO designs, mainly symmetric bidirectional and time-stratified CCO. The dependent variables most frequently analyzed were those relating to hospital morbidity; the pollutants most often studied were those linked to particulate matter. Among the CCO-application reports, 13.6% studied effect modification for variables at the individual level.
Conclusions
The use of CCO designs has undergone considerable growth; the most widely used designs were those that yielded better results in simulation studies: symmetric bidirectional and time-stratified CCO. However, the advantages of CCO as a method of analysis of variables at the individual level are put to little use.
Keywords: air pollution, crossover studies, epidemiologic methods, health, systematic review
The first epidemiologic studies on the impact of air pollution on health were undertaken as a consequence of the extreme pollution episodes that took place in the decades from 1930 to 1960. The association between air pollution and certain health variables was made clear by simple graphic representations or by comparisons of mortality rates for these time periods (Firket 1931; Logan 1953). Since that time, air pollution levels have fallen substantially, such that, to evaluate their effects on health, longer time series are required. To this end, epidemiologists began to use dynamic regression models in the 1970s that consisted of models in which the relationship between the dependent and explanatory variables were distributed over time, rather than being expected to occur simultaneously. Moreover, investigators were able to control for residual autocorrelation, with the error being specified by means of autoregressive integrated moving-average models (ARIMA). The problem with these types of models is that they assume that the dependent variable is distributed normally, which, in fact, is extremely rare in the daily outcome count variables of morbidity and mortality events (Saez et al. 1999).
The early 1990s saw the appearance of linear models based on Poisson regression, in which a parametric approach was used to control for trend and seasonality because the event counts more typically have a Poisson distribution. These models use the variable “time” and its transforms, quadratic and sinusoidal functions (sine or cosine) of different frequency and amplitude, to control for the effect on the dependent variable (mortality or morbidity) of unmeasured variables that may vary seasonally, such as in pollen concentration, meteorological variables, and influenza outbreaks, or that may have a trend, such as changes in a city’s population distribution, in order to ascertain the effect of such variables on the dependent variable (Saez et al. 1999). Insofar as changes in a city’s population pyramid are concerned, Poisson regression is particularly useful only when cases, rather than the entire population, can be enumerated, because this form of regression analysis does not require knowledge of the denominator as long as population flux is in steady state (Loomis et al 2005).
Nevertheless, Poisson regression poses the problem that, if any of these unmeasured variables follows a cyclical component of varying frequency and width (as might be the case of pollen concentration or influenza), the parametric functions of time or of its sinusoidal transforms cannot be easily “adapted” to such changes. These limitations led to the development of nonparametric Poisson regression with the application of generalized additive models (GAMs) that use nonparametric functions of the variable “time” (Kelsall et al. 1997), which adapt flexibly to the irregular cyclic components of unmeasured variables and allow for flexible fits for important variables, such as temperature, barometric pressure, and relative humidity, thus reducing any potential confounding due to these factors.
One difficulty with this method is that the number of degrees of freedom of the smoothed nonparametric function must be specified by the researcher, with discrepancies arising as to the most appropriate way to calculate this. Because inappropriate determination of the number of degrees of freedom can lead to bias in the estimates of nonparametric Poisson designs, epidmiologists focused on the case-crossover (CCO) design that purported to control time trends. The CCO design was proposed by Maclure (1991) to identify risk factors of acute events; it is characterized by the fact that each subject serves as his or her own control by assessing referent exposure at a point in time prior to the event. By virtue of its design, this type of study controls for the influence of confounding variables that remain constant in the subject at both dates, that of the event and that of the referent time, such as sex, smoking history, occupational history, and genetics. This design was initially used to assess the effect of exposures measured at an individual level (telephone calls and traffic accidents, physical or sexual activity, and acute myocardial infarction) and was not applicable to exposures with a time trend, such as air pollution. Thus, if an investigator selected exposure control dates before the effect, and there was a trend, prior exposures would be systematically higher or lower than at the date of the effect. To circumvent this bias, Navidi (1998) developed a variant of this design, bidirectional CCO, which is conceptually characterized by having control time periods before and after the event, something that made it possible to control for the effect of long-term trend and seasonality on the variable “exposure.” This design was already appropriate for ecologic-type exposures, such as air pollution, because the existence of registries means that the values of such exposure can be ascertained even after the event. In addition, pollution values are not affected by the presence of prior morbidity and mortality events. In the CCO design, the referent time periods represent the counterfactual exposure experience of the individual, had he or she not become sick; because in air pollution pre- and postevent exposure values are independent of the hazard-period exposure, those that are postevent referent can be appropriate. One advantage of CCO design over Poisson regression is its ability to assess potential effect modification (i.e., statistical interaction) at the individual level rather than at the group level (Figueiras et al. 2005). As an alternative analytic methodology to Poisson regression, the CCO approach allows for direct modeling of interaction terms, rather than depending on multiple subgroup analyses (Figueiras et al. 2005).
We conducted a systematic review of the CCO design used to study the relationship between air pollution and morbidity and mortality, from both a methodologic and an applied standpoint.
Materials and Methods
We conducted a bibliographic search in January 2009 using the MEDLINE (National Library of Medicine, Bethesda, MD, USA) and EMBASE (Elsevier, New York, NY, USA) databases and the key words case-crossover* and pollution*; the time frame was 1999 through 2008. From the total number of papers, we selected a series of reports based on the language used and the topic addressed in the title and/or abstract, thereby eliminating all that were not written in English or Spanish and that did not address the subject targeted for study. All the reports chosen in this way were reviewed, and additional reports were selected from among those cited in the respective references.
The reports retrieved were classified into two major groups: methodology reports in which new CCO designs were described or existing designs compared, generally by means of simulation studies, and application reports, in which some CCO design was applied for the purpose of analyzing the relationship between air pollution and health.
The methodology reports were in turn classified into those that conducted simulation studies to compare CCO designs with one another or with other designs, such as Poisson time-series, and those that described theoretical aspects pertaining to CCO design.
From the application reports, the following data were obtained for comparison: author, study location, year, dependent variable(s), independent variable(s), and type of CCO design (unidirectional, symmetric, semisymmetric, or time stratified). The modeling of interaction terms between pollutants and the individual characteristics of the subjects was also assessed, to record whether the reports had analyzed effect modification. For this purpose, only interactions with subjects’ individual variables were considered, with the following deemed ineligible: studies only reporting interactions between pollutants and pollen, meteorological variables, or other pollutants; and stratified analyses in which different models were constructed for each subgroup and no interaction term was included in a single model.
Results
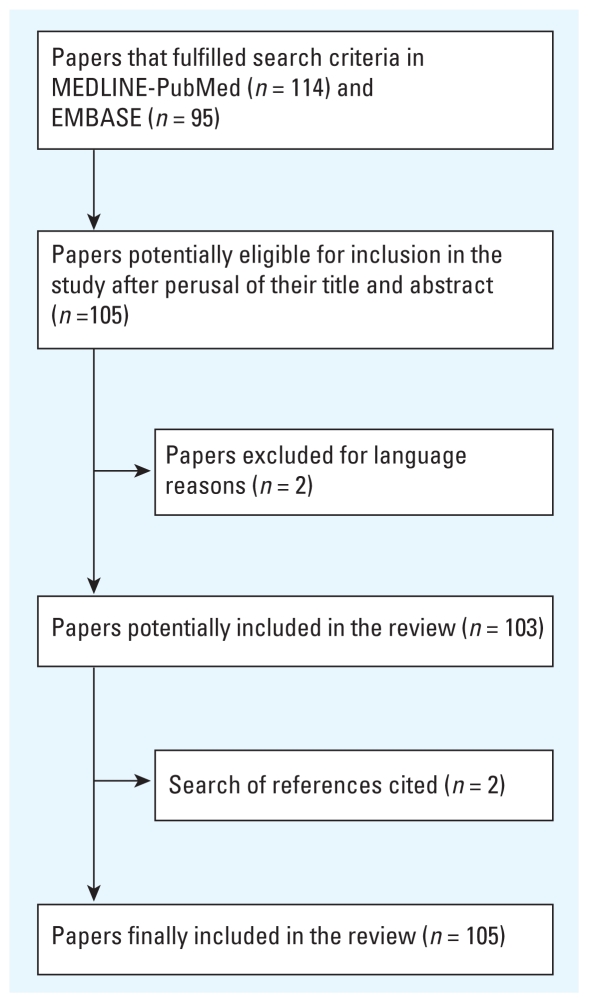
Figure 1 schematically depicts the results obtained in the bibliographic search. Of the total of 105 reports retrieved as a result of the bibliographic search, 24 addressed methodological aspects of CCO design (Bateson and Schwartz 1999, 2001; Figueiras et al. 2005; Fung et al. 2003; Hajat 2003; Jaakkola 2003; Janes et al. 2005a, 2005b; Kunzli and Schindler 2005a, 2005b; Lee et al. 2000; Levy et al. 2001a; Lu et al. 2008; Lu and Zeger 2007; Lumley and Levy 2000; Maclure 1991; Maclure and Mittleman 2008; Marshall and Jackson 1993; Mittleman 2005, Navidi 1998; Navidi et al. 1999; Navidi and Weinhandl 2002; Peters et al. 2006; Sheppard et al. 2001); the remaining studies applied CCO designs to study the relationship between different air pollutants and different outcome variables in terms of human health (Barnett et al. 2005, 2006; Bateson and Schwartz 2004; Boutin-Forzano et al. 2004; Carracedo-Martinez et al. 2008; Chang et al. 2005; Checkoway et al. 2000; Cheng et al. 2007; D’ippoliti et al. 2003; Filleul et al. 2004; Forastiere et al. 2005, 2007; Henrotin et al. 2007; Hinwood et al. 2006; Jalaludin et al. 2008; Johnston et al. 2007; Kan and Chen 2003; Karr et al. 2006; Kim et al. 2007; Kwon et al. 2001; Laurent et al. 2008; Lee and Schwartz 1999; Lee et al. 2007a, 2007b, 2008; Levy et al. 2001b; Lin et al. 2002, 2003, 2005; Ljungman et al. 2008; Luginaah et al. 2005; Maynard et al. 2007; Medina-Ramón et al. 2006; Neas et al. 1999; Peel et al. 2007; Perez et al. 2008; Peters et al. 2001, 2005; Pope et al. 2006, 2008; Rich et al. 2004, 2005, 2006a, 2006b; Romieu et al. 2004; Ruidavets et al. 2005; Schwartz 2004a, 2004b, 2005; Ségala et al. 2008; Son et al. 2008; Stafoggia et al. 2008; Sullivan et al. 2003, 2005; Sunyer and Basagana 2001; Sunyer et al. 2000, 2002; Symons et al. 2006; Tecer et al. 2008; Tsai et al. 2003a, 2003b, 2006a, 2006b; Villeneuve et al. 2006, 2007; Wellenius et al. 2005a, 2005b, 2006; Xu et al. 2008; Yamakazi et al. 2007; Yang 2008; Yang and Chen 2007; Yang et al. 2003, 2004a, 2004b, 2006b, 2007; Zanobetti and Schwartz 2005, 2006; Zeka et al. 2005, 2006).
Figure 1.
Identification of studies and inclusion criteria.
CCO design
Of the 24 reports that addressed CCO design, nine conducted simulation studies, one study compared the estimators obtained by different methods applied to real data, and the remaining 14 analyzed only theoretical aspects of CCO design, without performing simulations or comparisons.
Our review of methodological aspects revealed a trend in CCO bidirectional designs with regard to the choice of control periods (Table 1). The main bidirectional CCO designs, in chronological order of appearance, were as follows: a) full-stratum CCO, one of the designs initially proposed by Navidi (1998), in which all the days of the series except that of the event were taken as controls; b) random matched-pair CCO, which was also proposed by Navidi (1998) and consisted of taking any day of the series before or after the event, at random; c) symmetric CCO, proposed by Bateson and Schwartz (1999), which consisted of taking 2 days of the series as the controls, one before and one after the event, equidistant from the latter; d) time-stratified CCO, a design proposed by Lumley and Levy (2000), consisting of taking as control one or more days falling within the same time stratum as that in which the event occurred; for example, if “month” is established as the time stratum and the event occurs on, say, a Monday, then this is compared with all the Mondays in that same month; and e) semisymmetric CCO, proposed by Navidi and Weinhandl (2002), which consists of randomly choosing as control only one of the two controls used by symmetric CCO.
Table 1.
Comparison of different CCO designs.
| Reference | Type | Selection of controls | Advantages | Factors that can introduce bias | Selection of controls diagram |
|---|---|---|---|---|---|
| Maclure 1991 | CCO | One control point before theeffect | All possible confoundingfactors undergoing no changebetween control periods andeffect, automatically controlledfor by design | Long-term trends orseasonality |  |
| Navidi 1998 | Full-stratumbidirectional | For each case, all the days ofthe series other than that ofthe event taken as controls | Provides control for long-termtrends | Long-term trends (onlypartially controlledfor) or seasonality |  |
| Bateson andSchwartz 1999 | Symmetricbidirectional | Two at equal distance ofthe event | Provides adequate controlfor long-term trends andseasonality |  |
|
| Navidi andWeinhandl 2002 | Semisymmetricbidirectional | One chosen at random fromthe two used for symmetricbidirectional CCO | Provides adequate controlfor long-term trends andseasonality |  |
|
| Lumley and Levy2000 | Time stratified | One (or several) within thesame time stratum in whichthe event occurred | Provides adequate controlfor long-term trends andseasonality |  |
Arrows pointing up indicate case periods; horizontal arrows represent direction of time within 1 month; dashed lines indicate time periods of 1 day; vertical lines indicate control periods.
Simulation studies compare model predictions based on repeated samples drawn from a data set that represents the entire population of interest and for which true values are known because they were determined by the investigator when the data set was created in order to represent a scenario of interest. They compare the performance of different CCO designs (process or manner of functioning or operating) based on such indicators as efficiency (with relative increases in variance or standard error indicating less efficiency), bias (the difference between the model-estimated value and the true value of the parameter being estimated), and coverage (the proportion of replicate estimates that include the true value of the coefficient within their 95% confidence intervals). Simulation studies yielded the following results, in chronologic order (summarized in Table 2).
Table 2.
Characteristics of the scenarios of simulation studies on CCO designs applied to the relationship between air pollution and health.
| Reference | Long-term trend | Short-term trend (seasonality) | Pollutanta | Event variableb | Site of real data collection | Study period |
|---|---|---|---|---|---|---|
| Navidi 1998 | Yes | No | PM10 | S | 10 communities inSouthern California | 1 January 1994 to30 December 1994 |
| Bateson and Schwartz 1999 | Yesc | Yesc | PM10 | S | Seattle | 1988–1990 |
| Lumley and Levy 2000 | Yes | Yes | BS | S | King County(Washington) | 1989–1994 |
| Lee et al. 2000 | No | Yes | S | Mortality | Seoul | 1 October 1991 to30 September 1993 |
| Bateson and Schwartz 2001 | Yes | Yes | S | C | — | 3 years |
| Levy et al. 2001a | Yes | No | BS | S | King County(Washington) | 3 October 1988 to25 June 1994 |
| Navidi and Weinhandl 2002 | Yesc | Yesc | PM10 | S | Denver | 1989–1992 |
| Fung et al. 2003 | Yes | Yes | PM2.5 | S | Toronto | 1981–1993 |
| Figueiras et al. 2005 | Yesc | Yesc | PM10 | S | Barcelona | 1995–1997 |
—, simulation site only.
BS, black smoke; PM2.5, PM with aerodynamic diameter ≤ 2.5 μm; PM10, PM with aerodynamic diameter ≤ 10 μm; S, simulated.
S, simulated (variable generated mathematically on the basis of other variables that enter into the simulation); C, created (variable generated artificially, although not on the basis of other variables that enter into the simulation).
The simulations by Bateson and Schwartz (1999), Navidi and Weinhandl (2002), and Figueiras et al. (2005) share the same simulation scenario, in the sense that these authors use the same equation to generate trend and seasonality in the data series.
Navidi (1998), in a simulation scenario based on real data for particulate matter (PM) with aerodynamic diameter ≤ 10 μm (PM10) and an unmeasured confounding variable that generated a long-term trend, conducted a simulation in which unidirectional was compared with bidirectional full-stratum CCO design and observed that the bidirectional design resulted in less bias.
Bateson and Schwartz (1999), in a simulation scenario based on real PM10 data and an unmeasured confounding variable that generated long-term trend and seasonality (short-term trends), conducted a simulation to compare Poisson time-series regression design against different CCO designs, such as unidirectional, full-stratum, random matched pair, and symmetric, with control periods ranging from 1–4 weeks before and after the event. The results of this simulation showed that, whereas the symmetric CCO design performed best in terms of bias, it nevertheless displayed a lower efficiency (66%) than did the Poisson time-series designs.
Lumley and Levy (2000) compared symmetric with time-stratified CCO designs in a simulation scenario based on real black smoke data and an unmeasured confounding variable that generated long-term trend and seasonality; they observed better performance with the time-stratified CCO design, although both displayed a small degree of bias.
Lee et al. (2000), in a simulation scenario based on real mortality data and an unmeasured confounding variable that generated seasonality, compared unidirectional design with symmetric CCO and found that the latter performed better, although bias increased when the number of seasonality waves was incomplete.
Bateson and Schwartz (2001) set out to study the best distance at which to use control days in symmetric CCO design, in a scenario with trend and seasonality, in which all the variables were simulated. They studied control days ranging from 1–28 days before and after the event and observed that confounding was minimized when the spacing was equal to the period of exposure.
Levy et al. (2001a), in a simulation scenario based on real black smoke data and an unmeasured confounding variable that generated long-term trend but no seasonality, compared unidirectional with symmetric design, using different numbers of control periods and at different intervals from the event period, as well as the influence of autocorrelation (correlation of a temporal series variable with its own previous or posterior values) between control periods and overlapping (bias resulting from the use of incorrect referent periods), and concluded that the symmetric CCO design performed better, with less bias when the distance of the control periods from the event was 7 days and when autocorrelation and overlapping were avoided.
Navidi and Weinhandl (2002) conducted a simulation in a scenario based on real PM10 data and an unmeasured confounding variable that generated long-term trend and seasonality, in which they compared Poisson time-series design with the following CCO designs: symmetric with control periods separated by 7 days with respect to the case date, semisymmetric with the control period separated by 7 days with respect to the case date, random matched pair, and full-stratum. They concluded that the semisymmetric design performed best.
Fung et al. (2003) conducted a simulation in a simulation scenario based on real PM with aerodynamic diameter ≤ 2.5 μm (PM2.5) data and an unmeasured confounding variable that generated long-term trend and seasonality, in which they compared Poisson time-series design against unidirectional, symmetric, and semisymmetric CCO designs. They concluded that, although the symmetric design displayed a better performance in terms of bias than did the other designs studied, it was nonetheless similar to that of the Poisson time-series design, which showed a better coverage and statistical power thanks to its greater efficiency.
Figueiras et al. (2005), in a simulation study that used a simulation scenario based on real PM10 data and an unmeasured confounding variable that could generate long-term trend and seasonality, compared the Poisson time-series design with a number of CCO designs: symmetric, semisymmetric, time stratified, full symmetric (14 control periods before and after event) analyzed by longitudinal designs, and full semisymmetric (seven control periods before and after event) analyzed by longitudinal designs. They reported that the full semisymmetric design displayed the least bias together with the best coverage and statistical power but proved unstable when the beta value (strength of association between the pollutant and the event) varied with respect to the usual values. Although semisymmetric CCO displayed fewer biases than did symmetric or time-stratified CCO (both of which yielded similar results), it suffered from the drawback of having a lower statistical power.
It is particularly interesting to note that three of these simulation studies (Bateson and Schwartz 1999; Figueiras et al. 2005; Navidi and Weinhandl 2002) generated data for simulations using the same equations to determine trend and seasonality, before going on to use different real pollution data, such that comparable scenarios were investigated by each set of investigators.
In a separate study, Peters et al. (2006) analyzed a real database by means of a CCO and an alternative design (Poisson time-series design or Cox regression analysis) and then compared the results, observing that the time-stratified CCO design yielded results and conclusions similar to those of the Poisson time-series design and Cox regression analysis.
CCO studies of the relationship between pollution and health
CCO designs are increasingly being applied to the task of analyzing the relationship between air pollution and its short-term effects on health (Figure 2). Tables 3–5 provide a detailed description of the studies published to date.
Figure 2.
Trend in the use of different CCO methods for analyzing the short-term relationship between air pollution and health.
Table 3.
Studies of air pollution health effects using symmetric CCO.
| Reference | Countrya | Study populationb | Control periodc | Exposured | Outcome variablee |
|---|---|---|---|---|---|
| Neas et al. 1999 | US | GP | Days (± 7, 14, 21) | TSP | Nonaccidental M |
| Sunyer et al. 2000 | Sp | P with COPD > 35 years of age | Days (± 7) | BS | Nonaccidental M |
| Sunyer and Basagana 2001 | Sp | P with COPD > 35 years of age | Days (± 7) | PM10, CO, NO2, O3 | Nonaccidental M |
| Kwon et al. 2001 | SK | P with heart failure | Days (± 7, 14) | PM10, CO, NO2, SO2, O3 | Nonaccidental M |
| Yang et al. 2003 | Ca | GP < 14 and > 65 years of age | Days (± 7) | COH, CO, NO2, SO2, O3 | HA due to respiratory disease |
| Tsai et al. 2003a | Chi | GP | Days (± 7) | PM10, BS, CO, NO2, SO2, O3 | HA due to stroke |
| Tsai et al. 2003b | Chi | GP | Days (± 7) | PM10, BS, CO, NO2, SO2, O3 | Nonaccidental M |
| Lin et al. 2003 | Ca | GP > 6 and < 12 years of age | Days (± 14) | CO, NO2, SO2, O3 | HA due to asthma |
| Yang et al. 2004a | Chi | GP | Days (± 7) | PM10, CO, NO2, SO2, O3 | Nonaccidental M |
| Yang et al. 2004b | Chi | GP | Days (± 7) | PM10, CO, NO2, SO2, O3 | HA due to cardiovascular cause |
| Bateson and Schwartz 2004 | US | GP > 65 years of age | Days (± 6–14) | PM10 | HA due to cardiac or respiratory cause |
| Rich et al. 2004 | Ca | P with pacemaker | Days (± 7) | PM10, SO2, NO2, O3 | Cardiac arrhythmias |
| Chang et al. 2005 | Chi | GP | Days (± 7) | PM10, NO2, CO, O3 | HA due to cardiovascular cause |
| Luginaah et al. 2005 | Ca | GP | Days (± 14) | PM10, COH, NO2, SO2, CO, O3 | HA due to respiratory cause |
| Barnett et al. 2005 | Au, NZ | GP < 14 years of age | Days (± 2–14) | PM10, PM2.5, COH, NO2, SO2 | HA due to respiratory cause |
| Lin et al. 2005 | Ca | GP < 14 years of age | Days (± 14) | PM10, PM2.5, SO2, CO, NO2, O3 | HA due to respiratory infection |
| Yang et al. 2006 | Chi | GP | Days (± 7) | PM10, SO2, CO, O3, NO2 | Postneonatal M |
| Tsai et al. 2006b | Chi | GP | Days (± 7) | PM10, SO2, CO, O3, NO2 | HA due to asthma |
| Tsai et al. 2006a | Chi | GP | Days (± 7) | PM10, SO2, CO, O3, NO2 | Postneonatal M |
| Cheng et al. 2007 | Chi | GP | Days (± 7) | PM10, SO2, CO, O3, NO2 | HA due to pneumonia |
| Lee et al. 2007b | Chi | GP | Days (± 7) | PM10, SO2, CO, O3, NO2 | HA due to heart failure |
| Yang and Chen 2007 | Chi | GP | Days (± 7) | PM10, SO2, CO, O3, NO2 | HA due to COPD |
| Yang et al. 2007 | Chi | GP | Days (± 7) | PM10, SO2, CO, O3, NO2 | HA due to asthma |
| Lee et al. 2007a | Chi | GP | Days (± 7) | PM10, SO2, CO, O3, NO2 | HA due to COPD |
| Kim et al. 2007 | SK | GP | Days (± 7), days (± 7, 14) | PM10, SO2, CO, O3, NO2 | HE due to asthma |
| Henrotin et al. 2007 | Fr | GP | Days (± 7, 14, 21, 28) | PM10, SO2, CO, O3, NOx | Stroke |
| Ségala et al. 2008 | Fr | GP < 3 years of age | Days (± 7–8, 14–15) | PM10, BS, SO2, NO2 | HE due to bronchiolitis |
| Yang 2008 | Chi | GP | Days (± 7) | PM10, SO2, NO2, CO, O3 | HA heart failure |
| Tecer et al. 2008 | Tu | GP < 14 years of age | Days (± 7–14) | PM10, PM2.5 | HA respiratory diseases |
| Carracedo-Martinez et al.2008 | Sp | GP | Days (± 7) | BS, SO2 | ETC due to respiratory andcardiovascular causes |
| Son et al. 2008 | SK | GP | Days (± 7), days (± 7, 14), days (± 7, 14, 21) | PM10, SO2, NO2, CO, O3 | Postneonatal M |
Au, Australia; Ca, Canada; Chi, China; Fr, France; NZ, New Zealand; SK, South Korea; Sp, Spain; Tu, Turkey; US, United States of America.
COPD, chronic obstructive pulmonary disease; GP, general population; P, patients.
Interpretation of control periods: days (±7), 7th day before and after the case; days (±7, 14), days 7 and 14 before and after the case; days (± 7–14), days 7–14 before and after the case.
BS, black smoke; CO, carbon monoxide; COH, PM measured as haze coefficient; NO2, nitrogen dioxide; NOx, nitrogen oxide; O3, ozone; PM10, PM with aerodynamic diameter ≤ 10 μm; PM2.5, PM with aerodynamic diameter ≤ 2.5 μm; SO2, sulfur dioxide; TSP, total suspended PM.
ETC, emergency telephone calls; HA, hospital admission; HE, hospital emergency; M, mortality.
Table 5.
Studies of air pollution health effects using multiple CCO designs or those other than symmetric or time stratified.
| Reference | Countrya | Study populationb | Type of CCO designc | Exposured | Outcome variablee |
|---|---|---|---|---|---|
| Lee and Schwartz 1999 | SK | GP | U(–7d); U(–7, 14d); U(+7d); U(+7, 14d); SB(± 7d) | TSP, SO2, O3 | Nonaccidental M |
| Peters et al. 2001 | US | GP | U(–2, 3, 4d) | PM2.5 | Myocardial infarction |
| Lin et al. 2002 | Ca | GP 6–12 years of age | U(–14d); SB(± 14d) | PM10, PM2.5 | HA due to asthma |
| Kan and Chen 2003 | Chi | GP | U(–7, 14, 21d); SB(± 7, 14, 21d) | PM10, NO2, SO2 | Nonaccidental M |
| Filleul et al. 2004 | Fr | GP > 65 years of age | SSB(± 7d) | BS | Nonaccidental M and cardiovascular M |
| Boutin-Forzano et al. 2004 | Fr | GP > 3 and < 49 years of age | U(–7d) | SO2, NO2, O3 | HE due to asthma |
| Schwartz 2004a | US | GP | SB(± 7d); TS(m, d =T) | PM10 | Nonaccidental M |
| Ruidavets et al. 2005 | Fr | GP | U(–7, 14, 21, 28d); SB(± 7d) | O3, SO2, NO2 | HA due to myocardial infarction |
| Peters et al. 2005 | Ger | GP | U(–(1–3)d ); U(–(1–3)d, =h);SB(± 7, 14d); SB(± 7, 14d, =h); SB(± 7–14d); SB(±7–14d, =h); TS(m, =wd) ; TS(m, =wd, =h) | PM10, PM2.5, TSP, SO2, CO, NO, NO2, O3 | HA due to myocardial infarction |
| Villeneuve et al. 2007 | Ca | GP | SB(± 7, 14d); TS(m, =wd) | PM10, PM2.5, SO2, CO, O3, NO2 | HE due to asthma |
| Xu et al. 2008 | US | GP | SB(± 7, 14d); TS(m, =wd) | PM10, SO2 | HA respiratory and cardiovascular diseases |
Ca, Canada; Chi, China; Fr, France; Ger, Germany; SK, South Korea; US, United States of America.
GP, general population; P, patients.
SB, symmetric bidirectional CCO; SSB, semisymmetric bidirectional CCO; TS, time-stratified CCO; U, unidirectional CCO. Interpretation of control periods: (±7d), 7th day before and after the case; (±7; 14d), days 7 and 14 before and after the case; (±7–14d), days 7–14 before and after the case; (m, =wd), all the days of the same month as that of the case, which was the same day of the week; (m; =wd; =h), hours that coincide with those of the case, on days in the same month as the case, which were the same days of the week; (m; d =T), days in the same month as and having a temperature equal to that of the case date; U(–7d), one control day, 7 days before the case.
BS, black smoke; CO, carbon monoxide; NO2, nitrogen dioxide; O3, ozone; PM10, PM with aerodynamic diameter ≤ 10 μm; PM2.5, PM with aerodynamic diameter ≤ 2.5 μm; SO2, sulfur dioxide; TSP, total suspended PM.
HA, hospital admission; HE, hospital emergency; M, mortality.
The reports published by Lee and Schwartz (1999) and Neas et al. (1999) were the first studies to report the relationship between air pollution and mortality using a CCO design. These studies performed a reanalysis of the effects of air pollution and mortality in the cities of Philadelphia and Seoul, respectively, and obtained a relationship that proved statistically significant. These results are similar to those previously obtained with the Poisson time-series design and thus strengthen the relationship of causality, inasmuch as the same relationship was observed when different statistical methods were applied.
Analysis of which CCO designs were most commonly used in the published reports showed that 7.7% of these were unidirectional and the remainder bidirectional. The most frequently used bidirectional designs were symmetric (42.2% of studies) and time stratified (48.9% of studies). The semisymmetric bidirectional design was used in only one study. Figure 2 depicts the time trend in the use of the different CCO designs. Although unidirectional designs were used in the initial period, they were gradually discarded. Most of the published studies used a 1-day control period, but six studies used a 1-hr control period.
Most of the studies that employed symmetric CCO designs used day 7 before and after the event as the control days (n = 23), although a variety of other schemes were also used (Table 3). Studies that used time-stratified CCO typically selected a control day on the same day of the week during the same month as the event, although other schemes (e.g., selecting days during the same month with comparable temperature) were also used (Table 4). Studies that used unidirectional CCO designs used a variety of schemes to select control days (e.g., day 7 before the event) (Table 5).
Table 4.
Studies of air pollution health effects using time-stratified CCO.
| Reference | Countrya | Study populationb | Control periodc | Exposured | Outcome variablee |
|---|---|---|---|---|---|
| Checkoway et al. 2000 | US | GP | =month, =weekday | BS, PM10, SO2, CO | Cardiac arrest |
| Levy et al. 2001b | US | GP | =month, =weekday | PM2.5, PM10 | Cardiac arrest |
| Sunyer et al. 2002 | Sp | Asthmatic P > 14 yearsof age | =month, =weekday | PM10, BS, CO, NO2, SO2, O3 | M due to asthma |
| Sullivan et al. 2003 | US | GP | =month, =weekday | PM10, CO, SO2 | Cardiac arrest |
| D’Ippoliti et al. 2003 | Ita | GP | =month, =weekday | TSP, CO, NO2, SO2 | HA due to myocardial infarction |
| Schwartz 2004b | US | GP | =month, =weekday | PM10 | M accidental |
| Romieu et al. 2004 | Mex | GP >1 month and1 year of age | =month, =weekday | PM10 | M due to respiratory cause |
| Schwartz 2005 | US | GP | =month, days =temperature | O3 | Nonaccidental M |
| Sullivan et al. 2005 | US | GP | =month, =weekday | PM10, PM2.5, SO2, CO | Myocardial infarction |
| Wellenius et al. 2005a | US | GP > 65 years of age | =month, =weekday | PM10, CO, NO2, SO2, O3 | HA due to heart failure |
| Rich et al. 2005 | US | P with pacemaker | =month, =weekday, =hour | PM2.5 | Cardiac arrhythmias |
| Forastiere et al. 2005 | Ita | GP | =month, =weekday | PM10, CO, NO2, O3 | Out-of-hospital cardiovascular M |
| Zanobetti and Schwartz2005 | US | GP | =month, =weekday | PM10 | HA due to myocardial infarction |
| Zeka et al. 2005 | US | GP | =month, =weekday | PM10 | Nonaccidental M |
| Wellenius et al. 2005b | US | GP | =month, =weekday | PM10, SO2, CO, NO2 | HA due to stroke |
| Pope et al. 2006 | US | GP | =month, =weekday | PM2.5, PM10 | Ischemic coronary events |
| Villeneuve et al. 2006 | Ca | GP > 65 years of age | =month, =weekday | PM10, PM2.5, SO2, CO, O3,NO2 | HE due to ischemic stroke |
| Symons et al. 2006 | US | GP | =month, =weekday | PM2.5 | HA due to heart failure |
| Zeka et al. 2006 | US | GP | =month, =weekday | PM10 | Nonaccidental, cardiovascularand respiratory M |
| Medina-Ramon et al. 2006 | US | GP | =month, =weekday | PM10, O3 | HA due to pneumonia, COPD |
| Rich et al. 2006b | US | P with pacemaker | =month, =weekday, =hour | BS, PM2.5, SO2, CO, O3, NO2 | Paroxysmal auricular fibrillation Ep |
| Wellenius et al. 2006 | US | GP > 65 years of age | =month, =weekday | PM10 | HA heart failure |
| Karr et al. 2006 | US | GP < 1 year of age | =month, =weekday | PM2.5, CO, NO2 | HA due to bronchiolitis |
| Rich et al. 2006a | US | P with pacemaker | =month, =weekday, =hour | PM2.5, SO2, CO, O3, NO2 | Ventricular arrhythmia Ep |
| Zanobetti and Schwartz2006 | US | GP > 65 years of age | =month, days =temperature | PM2.5, BS, CO, O3, NO2 | HA due to myocardial infarction and pneumonia |
| Hinwood et al. 2006 | Au | GP | =month, =weekday | BS, PM10, PM2.5, CO, O3,NO2 | HA due to cardiovascular andrespiratory disease |
| Barnett et al. 2006 | Au, NZ | GP > 15 years of age | =month, all days but day ±1 | PM10, PM2.5, SO2, CO, O3, NO2 | HA due to cardiovascular causes |
| Forastiere et al. 2007 | Ita | GP | =month, =weekday | PM10 | Nonaccidental M |
| Peel et al. 2007 | Ca | GP | =month, =weekday | PM10, SO2, CO, O3, NO2 | HE due to cardiovascular causes. |
| Johnston et al. 2007 | Au | GP | =month, =weekday | PM10 | HA due to cardiovascular and respiratory causes |
| Maynard et al. 2007 | US | GP | =month, all days but 2 days between | BS, sulfate particles | Nonaccidental, cardiovascular and respiratory M |
| Yamakazi et al. 2007 | Jap | GP > 65 years of age | =month, =weekday, =hour | PM7, NO2, Ox | M due to stroke |
| Jalaludin et al. 2008 | Au | GP > 1 and < 14 years of age | =month, all days | PM10, PM2.5, SO2, NO2,O3, CO, | HE due to asthma |
| Perez et al. 2008 | Sp | GP | =month, =weekday | PM10, PM2.5 | Nonaccidental M |
| Laurent et al. 2008 | Fr | GP | =month, =weekday | PM10 SO2, NO2, O3 | ETC due to asthma |
| Lee et al. 2008 | Chi | GP | =month, =weekday | PM10, SO2, NO2, CO, O3 | HA heart failure |
| Pope et al. 2008 | US | GP | =month, =weekday | PM10, PM2.5 | HA heart failure |
| Ljungman et al. 2008 | Sw | P with pacemaker | =month, =weekday, =hour | PM10, NO2 | Ventricular arrhythmia Ep |
| Stafoggia et al. 2008 | Ita | GP > 35 years of age | =month, all days but 1 day between | PM10 | Nonaccidental M |
Au, Australia; Ca, Canada; Chi, China; Fr, France; Ita, Italy; Jap, Japan; Mex, Mexico; NZ, New Zealand; Sp, Spain; Sw, Sweden; US, United States of America.
GP, general population; P, patients.
Control periods: =month, =weekday, all the days of the same month as that of the case, which was the same day of the week; =month, =weekday, =hour, hours that coincide with those of the case, on days in the same month as the case, which were the same days of the week; =month, days =temperature, days in the same month as and having a temperature equal to that of the case date; =month, all days but 2 days between, all days in the same month as that of the case except 2 days between each control day.
BS, black smoke; CO, carbon monoxide; NO2, nitrogen dioxide; O3, ozone; PM10, PM with aerodynamic diameter ≤ 10 μm; PM2.5, PM with aerodynamic diameter ≤ 2.5 μm; SO2, sulfur dioxide; TSP, total suspended PM.
Ep, episode; ETC, emergency telephone calls; HA, hospital admission; HE, hospital emergency; M, mortality.
The dependent variables studied were mortality related in 25 cases and morbidity related in the remainder: hospital admissions in 35 studies, hospital emergencies in 7 studies, episodes of arrhythmias recorded in pacemakers in 5 studies, telephone calls to medical emergencies in 2 studies, and others based on disease-specific registers, such as stroke (1 study), cardiac arrest (3 studies), and ischemic heart disease (2 studies).
In 77 studies, the air pollutant analyzed was particulate level, mostly measured as PM10 (61 studies), followed by PM2.5 (22 studies), black smoke (11 studies), haze coefficient (3 studies), total suspended PM (4 studies), sulfate particles (1 study), and PM with aerodynamic diameter < 7mm (1 study). Insofar as gaseous air pollutants were concerned, sulfur dioxide was used on 47 studies, nitrogen dioxide on 48, ozone on 44, carbon monoxide on 43, and oxides of oxygen (Ox), oxides of nitrogen (NOx), and nitrogen oxide on 1 study each.
In most cases, the general population was studied. Patients were studied in only 9 studies: cardiac pacemaker carriers in 5, chronic obstructive pulmonary disease patients in 2, and asthma and heart failure patients in 1 study each.
Of all the studies that addressed application of CCO designs, 11 (13.6%) made use of analysis of effect modification of variables at the individual level.
Common steps and requirements for CCO study designs
The procedures followed in conducting a study into the relationship between air pollution and health, taking all reports on CCO design methodology and application into account, are outlined in the Appendix.
In brief, CCO studies begin by confirming that data meet a series of necessary requisites and end with a sensitivity analysis, after passing through a series of intermediate steps that include the transformation of the database into a matrix with CCO structure.
Discussion
This is the first systematic review to cover the application of CCO designs to the study of the health effects of air pollution. Use of CCO designs has risen steeply in recent years and from 2003 in particular, reaching a peak in 2006. Most of the new CCO designs that gradually appeared were based on simulation studies, which in many cases neither relied on the same scenarios nor assessed performance for variables with special characteristics, for example, discontinuous exposures. Most application studies have tended to study the effect of particulates on morbidity, yet few studies have taken advantage of the strength of CCO designs to assess potential effect modifications with individual variables.
CCO versus Poisson
The increase in the use of the CCO design appears to coincide with problems using Poisson regression models with GAM: as far back as 2002, Dominici et al. (2002) discovered that the most frequently used statistical packages gave rise to unstable estimators due to inadequate convergence criteria that could underestimate standard errors because of the presence of concurvity in the data (Ramsay et al. 2003). In part, the CCO design represents a solution to the problems posed by GAM methods, but before it can become generalized, a period of time is required. For instance, we observed no marked increase in the use of these designs until some years after the discovery of GAM-related problems; a peak in use occurred 2 years after the discovery of the problems of concurvity (analog to collinearity for nonlinear relationships). Currently, other (e.g., geographic) methods are also being used to analyze the link between air pollution and health (Zeger et al. 2008).
Different CCO designs and their evolution
We observed an ongoing effort to perfect the CCO design dating from the initial unidirectional design up to the bidirectional designs with their subtypes. Successive simulation studies have focused on studying the designs that yielded the best results in previous simulations. Symmetric bidirectional CCO and time-stratified CCO most often proved to be best in different simulations. In contrast, the semisymmetric design yielded contradictory results: in some simulation studies it proved better than the symmetric design, but other studies gave opposite results (Fung et al. 2003), which could be due to differences in the simulation scenario. One consistent finding, however, is that the statistical efficiency of semisymmetric CCO is low compared with that of the symmetric or time-stratified CCO methods.
The rapid adoption of symmetric and time-stratified CCO designs is noteworthy, in that these began to be applied in the very same year in which their methodology was first proposed in the scientific literature. In contrast, the semisymmetric CCO design was first proposed in 2002, yet the first report in which it was used to analyze the relationship between air pollution and health was published in 2004.
One possible explanation for the fact that different designs are used in practice is that they were discovered at different points in time: unidirectional were described before bidirectional methods, and within bidirectional methods, symmetric was described before time-stratified CCO. Unidirectional methods are being used less frequently because of important disadvantages, such as poor control of trends.
Of the three bidirectional methods, semisymmetric is used very little because of its negligible statistical power. Symmetric and time-stratified designs had a similar percentage of use, with a trend toward greater use of time-stratified designs, possibly because, from a theoretical point of view, they solve the “overlap bias” that symmetric designs otherwise display. However, simulation studies are not conclusive when it comes to comparing time-stratified with symmetric designs; for example, in their simulation study, Lumley and Levy (2000) reported that the time-stratified method was superior, but Figueiras et al. (2005) did not find this method to be better than the symmetric CCO.
The fact that the CCO designs most often used to analyze the relationship between air pollution and health are symmetric and time stratified, plus the rapid adoption of these same two models (they began to be used in the same year as they were proposed in the literature), together indicate that there is an interest in the correct application of this methodology. Control periods most frequently used for the symmetric design are 7 days before and after case, and for the time-stratified design, control periods are all the same days of the week as the case within the same month. Thus, these two approaches prevent problems of autocorrelation, and control for effect of day of the week.
Interpretation of application studies
In studies that use the CCO design to analyze the relationship between air pollution and health, the most frequently used exposure is that of hospital admissions. The greater use of hospital admissions than mortality as an outcome may be because, on the one hand, the hospital admission variable entails a greater number of events, thereby affording greater statistical power, and on the other hand, the time period from exposure until the event is shorter for hospital admissions than for mortality, thereby requiring a smaller number of lags, thus facilitating statistical analysis (American Thoracic Society 1985). The type of pollutant most frequently analyzed with CCO designs is airborne particulates, possibly because these have been widely studied and because exposure data are readily available. In terms of type of population, these studies seldom target diseased populations but focus instead on general populations, possibly because of the difficulty of obtaining records for a specific disease population (Filleul et al. 2004).
Lessons learned and new challenges
Although the application of nonparametric Poisson models amounted to a great advance over earlier designs, enabling more flexible control of unmeasured confounding variables that change over time, the problems detected, such as the difficulty in setting the number of degrees of freedom, seem to have heightened interest in other alternatives, such as CCO. These approaches make it possible to control for the influence of trend and seasonality by design. Initially, these designs resulted in certain biases in the estimators under very specific conditions, which were superseded by new control period sampling designs, although a decision must still be made as to precisely what is the most appropriate time interval between case and control periods.
In principle, CCO designs seem easier to model and involve fewer arbitrary decisions for the researcher than do Poisson time-series designs with GAM (type of smoother, number of degrees of freedom), yet CCO designs also entail arbitrariness in the selection of reference periods or sampling method.
There are no known study characteristics that would favor using one referent period over another, because the heterogeneity of the simulation studies in terms of their scenarios and results renders it impossible to draw any conclusion in this regard. Likewise, simulation studies have tended to concentrate on PM, and no simulation study assesses the latter’s behavior in discontinuous exposures (e.g., a high-ozone day). In this type of exposure where high proportions of cases and controls assume a value of zero, Poisson time series might, from a theoretical point of view, perform better than CCO methods, because the comparisons are made in the same person and, when the case and control periods have the same value, provide no statistical power when analyzed with conditional logistic regression. However, we are not aware of any simulation studies that have tested whether this assumption has any relevance in practice.
Theoretically, one of the great advantages of CCO designs is that individual data can be included to estimate effect modifications, but in practice most CCO-based studies on the relationship between air pollution and health do not analyze effect modification at the individual level. The scant use of this advantage might be due to the lack of availability of data at this level (Filleul et al. 2004).
Furthermore, thanks to the CCO design, we have more scientific evidence of the short-term association between air pollution and health, because at times reanalyses using CCO methodology have been run on data previously analyzed with Poisson methods, and similar results have been obtained (Lee and Schwartz 1999).
One possible challenge is the application of mixed models to the analysis of CCO designs, something that, on the one hand, could furnish greater statistical power and, on the other, could extend CCO designs to spatial-temporal models. Figueiras et al. (2005) attempted to apply longitudinal models to CCO designs but observed that, in the presence of autocorrelation, estimates might be biased. New approaches in this field could solve these problems.
From the standpoint of statistical analysis, Lu et al. (2008) have proposed that CCO models should be checked to see if assumptions for using CCO methodology were satisfied, via a series of diagnostic tools such as plotting the data. In practice, however, we have detected no CCO study on the relationship between air pollution and health that checked the models. Furthermore there are no formulas for calculating sample size (or statistical power) in CCO designs, and indeed, one study (Symons et al. 2006) applied a simulation to calculate the lower bound of detectable effects. A possible risk of CCO designs lies in “model shopping,” whereby multiple analyses are performed using different designs, and only the most interesting are then shown (Mittleman 2005). This problem can be solved, in part, by means of a sensitivity analysis, in which the authors show the results obtained with different CCO methods, and even compare the results against a generalized linear model with a Poisson response.
Limitations of our review
In assessing the reports that use effect modification with individual data, we encountered difficulties regarding use of different terminologies: some used the term “modification” to classify what is in reality “stratification into subgroups”; others referred to stratification but did not clarify whether different statistical models were used for each group of subjects of the variable “stratification,” or whether an interaction term was introduced into the model to assess effect modification. Furthermore, as with any systematic review, publication bias may be present.
Conclusions
The CCO design could be an attractive alternative to Poisson time-series analysis with GAM, but its advantages and drawbacks are still in the process of being understood. The use of CCO designs to study the relationship between air pollution and health has experienced a great upsurge, but with few exceptions, full advantage has not been taken in terms of effect modification or spatial-temporal analyses. Moreover, although a number of simulations have been conducted to study the performance of CCO designs, the performance of discontinuous exposures, such as ozone, remains to be studied. A further, very important challenge would be to undertake an in-depth longitudinal analysis of CCO designs, which would enhance their statistical power and enable them to be applied to spatial-temporal models.
Appendix. Applying CCO Designs to Study the Relationship between Air Pollution and Health
The steps to be followed to conduct a study into the relationship between air pollution and health, taking all reports on CCO design methodology and application into account, can be summarized as follows:
-
Confirm that the study variables meet the conditions for being able to study the association using a CCO:
Exposure variables must be transitory (prolonged exposures such as radon would not be valid).
Event variables must be acute (events such as cancer would not be valid).
Proportion of missing data must be small.
-
The databases obtained can be classified into one of the following types:
Contain only ecologic temporal cluster data.
Contain ecologic temporal and spatial cluster data.
Individual data available—this enables effect modification to be subsequently studied at the level of variables having characteristics pertaining to individuals.
For exposure variables, compute the individual (0, 1, 2, 3) or combined lags (0, 1, 2–3 . . .) depending on the nature of the dependent variable (longer lags are needed for mortality than for morbidity variables).
-
Transform the database into a matrix with a CCO structure, that is, with as many strata as there are events, and in each stratum there is a case period that would be formed by exposure at the time of the event (or the corresponding lag) and one (or more) control periods that would be formed by exposure in the periods selected as controls (e.g., in a symmetric CCO design, these could be day 7 before and after the event). For an ecologic database consisting solely of temporal cluster data, calculations are simplified because:
There are macros in S-Plus that transform an ecological matrix into a symmetric CCO, semisymmetric CCO, or time-stratified CCO (these may be requested from the corresponding author).
There is the possibility of conducting CCO studies using an ecologic matrix, with weighting for the daily number of events in the regression models. The advantages are that transformation into a CCO matrix is not necessary, the size of the database is smaller, and computing time is shorter.
-
To relate dependent and independent variables, perform the statistical analysis according to the following steps:
Construct a baseline model by introducing variables, such as temperature, ambient humidity, and atmospheric pressure. For these types of environmental variables, nonlinear risk exposure relationships might have to be checked. For the purpose, use can be made of different smoothers, such as natural splines, penalized splines, or smoothing splines. To decide whether a variable is retained in the model or the number of degrees of freedom of the smooth function, use the minimization criterion of the Akaike information criterion (Figueiras and Cadarso-Suarez 2001).
Construct the single-pollutant models by adding the pollutants to the baseline model.
Construct the multipollutant models by adding those pollutants to the baseline model that have obtained a given p-value in the single-pollutant model.
Analyze possible effect modification by reference to the statistical significance of the interaction term.
Analyze statistical power (Symons et al. 2006).
Check the models according to the method proposed by Lu et al. (2008).
Conduct a sensitivity analysis by analyzing the models using another type of CCO design or even a Poisson time series.
Report the results obtained.
This study was supported by grant CIBERESP-MET-007 from the Consortium for Biomedical Research in Epidemiology and Public Health [CIBER en Epidemiología y Salud Pública (CIBERESP)], Spain. A.T. was funded by project PI080354 [Fondo de Investigaciones Sanitarias (FIS)] of the Subdirectorate-General for Research Evaluation and Development and by project 200930I008 [Consejo Superior de Investigaciones Científicas (CSIC)].
We thank M. Benedict for his help with the English version of this article.
References
- American Thoracic Society. Guidelines as to what constitutes an adverse respiratory health effect, with special reference to epidemiologic studies of air pollution. Am Rev Respir Dis. 1985;13:666–668. [PubMed] [Google Scholar]
- Barnett AG, Williams GM, Schwartz J, Best TS, Neller AH, Petroeschevsky, et al. The effects of air pollution on hospitalizations for cardiovascular disease in elderly people in Australian and New Zealand cities. Environ Health Perspect. 2006;114:1018–1023. doi: 10.1289/ehp.8674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett AG, Williams GM, Schwartz J, Neller AH, Best TL, Petroeschevsky AL, et al. Air pollution and child respiratory health: a case-crossover study in Auckland New Zealand. Am J Respir Crit Care Med. 2005;171:1272–1278. doi: 10.1164/rccm.200411-1586OC. [DOI] [PubMed] [Google Scholar]
- Bateson TF, Schwartz J. Control for seasonal variation and time trend in case-crossover studies of acute effects of environmental exposures. Epidemiology. 1999;10:539–544. [PubMed] [Google Scholar]
- Bateson TF, Schwartz J. Selection bias and confounding in case-crossover analyses of environmental time-series data. Epidemiology. 2001;12:654–661. doi: 10.1097/00001648-200111000-00013. [DOI] [PubMed] [Google Scholar]
- Bateson TF, Schwartz J. Who is sensitive to the effects of particulate air pollution on mortality? A case-crossover analysis of effect modifiers. Epidemiology. 2004;15:143–149. doi: 10.1097/01.ede.0000112210.68754.fa. [DOI] [PubMed] [Google Scholar]
- Boutin-Forzano S, Adel N, Gratecos L, Jullian H, Garnier JM, Ramadour M, et al. Visits to the emergency room for asthma attacks and short-term variations in air pollution. A case-crossover study. Respiration. 2004;71:134–137. doi: 10.1159/000076673. [DOI] [PubMed] [Google Scholar]
- Carracedo-Martinez E, Sanchez C, Taracido M, Saez M, Jato V, Figueiras A. Effect of short-term exposure to air pollution and pollen on medical emergency calls: a case-crossover study in Spain. Allergy. 2008;63:347–353. doi: 10.1111/j.1398-9995.2007.01574.x. [DOI] [PubMed] [Google Scholar]
- Chang CC, Tsai SS, Ho SC, Yang CY. Air pollution and hospital admissions for cardiovascular disease in Taipei, Taiwan. Environ Res. 2005;98:114–119. doi: 10.1016/j.envres.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Checkoway H, Levy D, Sheppard L, Kaufman J, Koenig J, Siscovick D. A case-crossover analysis of fine particulate matter air pollution and out-of-hospital sudden cardiac arrest. Res Rep Health Eff Inst. 2000;(99):5–28. [PubMed] [Google Scholar]
- Cheng MF, Tsai SS, Wu TN, Chen PS, Yang CY. Air pollution and hospital admissions for pneumonia in a tropical city: Kaohsiung, Taiwan. J Toxicol Environ Health. 2007;70:2021–2026. doi: 10.1080/15287390701601020. [DOI] [PubMed] [Google Scholar]
- D’Ippoliti D, Forastiere F, Ancona C, Agabiti N, Fusco D, Michelozzi P, et al. Air pollution and myocardial infarction in Rome: a case-crossover analysis. Epidemiology. 2003;2003;14:528–535. doi: 10.1097/01.ede.0000082046.22919.72. [DOI] [PubMed] [Google Scholar]
- Dominici F, McDermott A, Zeger SL, Samet JM. On the use of generalized additive models in time-series studies of air pollution and health. Am J Epidemiol. 2002;156:193–203. doi: 10.1093/aje/kwf062. [DOI] [PubMed] [Google Scholar]
- Figueiras A, Cadarso-Suarez C. Application of nonparametric models for calculating odds ratios and their confidence intervals for continuous exposures. Am J Epidemiol. 2001;154:264–275. doi: 10.1093/aje/154.3.264. [DOI] [PubMed] [Google Scholar]
- Figueiras A, Carracedo-Martinez E, Saez M, Taracido M. Analysis of case-crossover designs using longitudinal approaches: a simulation study. Epidemiology. 2005;16:239–246. doi: 10.1097/01.ede.0000152915.58564.d3. [DOI] [PubMed] [Google Scholar]
- Filleul L, Rondeau V, Cantagrel A, Dartigues JF, Tessier JF. Do subject characteristics modify the effects of particulate air pollution on daily mortality among the elderly? J Occup Environ Med. 2004;46:1115–1122. doi: 10.1097/01.jom.0000144998.82543.9d. [DOI] [PubMed] [Google Scholar]
- Firket J. The cause of symptoms found in the Meuse Valley during the fog of December, 1930. Bull Acad R Med Belg. 1931;11:683–741. [Google Scholar]
- Forastiere F, Stafoggia M, Picciotto S, Bellander T, D’Ippoliti D, Lanki T, et al. A case-crossover analysis of out-of-hospital coronary deaths and air pollution in Rome, Italy. Am J Respir Crit Care Med. 2005;172:1549–1555. doi: 10.1164/rccm.200412-1726OC. [DOI] [PubMed] [Google Scholar]
- Forastiere F, Stafoggia M, Tasco C, Picciotto S, Agabiti N, Cesaroni G, et al. Socioeconomic status, particulate air pollution, and daily mortality: differential exposure or differential susceptibility. Am J Ind Med. 2007;50:208–216. doi: 10.1002/ajim.20368. [DOI] [PubMed] [Google Scholar]
- Fung KY, Krewski D, Chen Y, Burnett R, Cakmak S. Comparison of time series and case-crossover analyses of air pollution and hospital admission data. Int J Epidemiol. 2003;32:1064–1070. doi: 10.1093/ije/dyg246. [DOI] [PubMed] [Google Scholar]
- Hajat S. Commentary: comparison of time series and case-crossover analyses of air pollution and hospital admission data. [[accessed 1 July 2010]];Int J Epidemiol. 2003 32:1071. doi: 10.1093/ije/dyg300. Available: http://ije.oxfordjournals.org/cgi/content/full/32/6/1071. [DOI] [PubMed] [Google Scholar]
- Henrotin JB, Besancenot JP, Bejot Y, Giroud M. Short-term effects of ozone air pollution on ischaemic stroke occurrence: a case-crossover analysis from a 10-year population-based study in Dijon, France. Occup Environ Med. 2007;64:439–445. doi: 10.1136/oem.2006.029306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinwood AL, De Klerk N, Rodríguez C, Jacoby P, Runnion T, Rye P, et al. The relationship between changes in daily air pollution and hospitalizations in Perth, Australia 1992–1998: a case-crossover study. Int J Environ Health Res. 2006;16:27–46. doi: 10.1080/09603120500397680. [DOI] [PubMed] [Google Scholar]
- Jaakkola JJ. Case-crossover design in air pollution epidemiology. Eur Respir J Suppl. 2003;40:81s–85s. doi: 10.1183/09031936.03.00402703. [DOI] [PubMed] [Google Scholar]
- Jalaludin B, Khalaj B, Sheppeard V, Morgan G. Air pollution and ED visits for asthma in Australian children: a case-crossover analysis. Int Arch Occup Environ Health. 2008;81:967–974. doi: 10.1007/s00420-007-0290-0. [DOI] [PubMed] [Google Scholar]
- Janes H, Sheppard L, Lumley T. Case-crossover analyses of air pollution exposure data: referent selection strategies and their implications for bias. Epidemiology. 2005a;16:717–726. doi: 10.1097/01.ede.0000181315.18836.9d. [DOI] [PubMed] [Google Scholar]
- Janes H, Sheppard L, Lumley T. Overlap bias in the case-crossover design, with application to air pollution exposures. Stat Med. 2005b;24:285–300. doi: 10.1002/sim.1889. [DOI] [PubMed] [Google Scholar]
- Johnston FH, Bailie RS, Pilotto LS, Hanigan IC. Ambient biomass smoke and cardio-respiratory hospital admissions in Darwin, Australia. BMC Public Health. 2007;7:240. doi: 10.1186/1471-2458-7-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan H, Chen B. A case-crossover analysis of air pollution and daily mortality in Shanghai. J Occup Health. 2003;45:119–124. doi: 10.1539/joh.45.119. [DOI] [PubMed] [Google Scholar]
- Karr C, Lumley T, Shepherd K, Davis R, Larson T, Ritz B, et al. A case-crossover study of wintertime ambient air pollution and infant bronchiolitis. Environ Health Perspect. 2006;114:277–281. doi: 10.1289/ehp.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelsall JE, Samet JM, Zeger SL, Xu J. Air pollution and mortality in Philadelphia, 1974–1988. Am J Epidemiol. 1997;146:750–762. doi: 10.1093/oxfordjournals.aje.a009351. [DOI] [PubMed] [Google Scholar]
- Kim SY, O’Neill MS, Lee JT, Cho Y, Kim J, Kim H. Air pollution, socioeconomic position, and emergency hospital visits for asthma in Seoul, Korea. Int Arch Occup Environ Health. 2007;80:701–710. doi: 10.1007/s00420-007-0182-3. [DOI] [PubMed] [Google Scholar]
- Kunzli N, Schindler C. A call for reporting the relevant exposure term in air pollution case-crossover studies. J Epidemiol Community Health. 2005a;59:527–530. doi: 10.1136/jech.2004.027391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzli N, Schindler C. Case-crossover studies. Epidemiology. 2005b;16:592–593. doi: 10.1097/01.ede.0000165792.14924.f1. [DOI] [PubMed] [Google Scholar]
- Kwon HJ, Cho SH, Nyberg F, Pershagen G. Effects of ambient air pollution on daily mortality in a cohort of patients with congestive heart failure. Epidemiology. 2001;12:413–419. doi: 10.1097/00001648-200107000-00011. [DOI] [PubMed] [Google Scholar]
- Laurent O, Pedrono G, Segala C, Filleul L, Havard S, Deguen S, et al. Air pollution, asthma attacks, and socioeconomic deprivation: a small-area case-crossover study. Am J Epidemiol. 2008;168:58–65. doi: 10.1093/aje/kwn087. [DOI] [PubMed] [Google Scholar]
- Lee IM, Tsai SS, Chang CC, Ho CK, Yang CY. Air pollution and hospital admissions for chronic obstructive pulmonary disease in a tropical city: Kaohsiung, Taiwan. Inhal Toxicol. 2007a;19:393–398. doi: 10.1080/08958370601174818. [DOI] [PubMed] [Google Scholar]
- Lee IM, Tsai SS, Ho CK, Chiu HF, Wu TN, Yang CY. Air pollution and hospital admissions for congestive heart failure: are there potentially sensitive groups? Environ Res. 2008;108:348–353. doi: 10.1016/j.envres.2008.07.024. [DOI] [PubMed] [Google Scholar]
- Lee IM, Tsai SS, Ho CK, Chiu HF, Yang CY. Air pollution and hospital admissions for congestive heart faliure in a tropical city: Kaohsiung, Taiwan. Inhal Toxicol. 2007b;19:889–904. doi: 10.1080/08958370701479406. [DOI] [PubMed] [Google Scholar]
- Lee JT, Kim H, Schwartz J. Bidirectional case-crossover studies of air pollution: bias from skewed and incomplete waves. Environ Health Perspect. 2000;108:1107–1111. doi: 10.1289/ehp.001081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JT, Schwartz J. Reanalysis of the effects of air pollution on daily mortality in Seoul, Korea: a case-crossover design. Environ Health Perspect. 1999;107:633–636. doi: 10.1289/ehp.99107633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, Lumley T, Sheppard L, Kaufman J, Checkoway H. Referent selection in case-crossover analyses of acute health effects of air pollution. Epidemiology. 2001a;12:186–192. doi: 10.1097/00001648-200103000-00010. [DOI] [PubMed] [Google Scholar]
- Levy D, Sheppard L, Checkoway H, Kaufman J, Lumley T, Koenig J, et al. A case-crossover analysis of particulate matter air pollution and out-of-hospital primary cardiac arrest. Epidemiology. 2001b;12:193–199. [PubMed] [Google Scholar]
- Lin M, Chen Y, Burnett RT, Villeneuve PJ, Krewski D. The influence of ambient coarse particulate matter on asthma hospitalization in children: case-crossover and time-series analyses. Environ Health Perspect. 2002;110:575–581. doi: 10.1289/ehp.02110575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Chen Y, Burnett RT, Villeneuve PJ, Krewski D. Effect of short-term exposure to gaseous pollution on asthma hospitalisation in children: a bi-directional case-crossover analysis. J Epidemiol Community Health. 2003;57:50–55. doi: 10.1136/jech.57.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M, Stieb DM, Chen Y. Coarse particulate matter and hospitalization for respiratory infections in children younger than 15 years in Toronto: a case-crossover analysis. Pediatrics. 2005;116:e235–e240. doi: 10.1542/peds.2004-2012. [DOI] [PubMed] [Google Scholar]
- Ljungman PL, Berglind N, Holmgren C, Gadler F, Edvardsson N, Pershagen G, et al. Rapid effects of air pollution on ventricular arrhythmias. Eur Heart J. 2008;29:2894–2901. doi: 10.1093/eurheartj/ehn463. [DOI] [PubMed] [Google Scholar]
- Logan WPD. Mortality in the London fog incident, 1952. Lancet. 1953;1:336–338. doi: 10.1016/s0140-6736(53)91012-5. [DOI] [PubMed] [Google Scholar]
- Loomis D, Richardson DB, Elliott L. Poisson regression analysis of ungrouped data. Occup Environ Med. 2005;62:325–329. doi: 10.1136/oem.2004.017459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Symons JM, Geyh AS, Zeger SL. An approach to checking case-crossover analyses based on equivalence with time-series methods. Epidemiology. 2008;19:169–175. doi: 10.1097/EDE.0b013e3181632c24. [DOI] [PubMed] [Google Scholar]
- Lu Y, Zeger SL. On the equivalence of case-crossover and time series methods in environmental epidemiology. Biostatistics. 2007;8:337–344. doi: 10.1093/biostatistics/kxl013. [DOI] [PubMed] [Google Scholar]
- Luginaah IN, Fung KY, Gorey KM, Webster G, Wills C. Association of ambient air pollution with respiratory hospitalization in a government-designated “area of concern”: the case of Windsor, Ontario. Environ Health Perspect. 2005;113:290–296. doi: 10.1289/ehp.7300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley T, Levy D. Bias in the case-crossover design: implications for studies of air pollution. Environmetrics. 2000;11:689–704. [Google Scholar]
- Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133:144–153. doi: 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]
- Maclure M, Mittleman MA. Case-crossover designs compared with dynamic follow-up designs. Epidemiology. 2008;19:176–178. doi: 10.1097/EDE.0b013e318162afb9. [DOI] [PubMed] [Google Scholar]
- Marshall RJ, Jackson RT. Analysis of case-crossover designs. Stat Med. 1993;12:2333–2341. doi: 10.1002/sim.4780122409. [DOI] [PubMed] [Google Scholar]
- Maynard D, Coull BA, Gryparis A, Schwartz J. Mortality risk associated with short-term exposure to traffic particles and sulfates. Environ Health Perspect. 2007;115:751–755. doi: 10.1289/ehp.9537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina-Ramón M, Zanobetti A, Schwartz J. The effect of ozone and PM10 on hospital admissions for pneumonia and chronic obstructive pulmonary disease: a national multicity study. Am J Epidemiol. 2006;163:579–588. doi: 10.1093/aje/kwj078. [DOI] [PubMed] [Google Scholar]
- Mittleman MA. Optimal referent selection strategies in case-crossover studies: a settled issue. Epidemiology. 2005;16:715–716. doi: 10.1097/01.ede.0000183170.92955.25. [DOI] [PubMed] [Google Scholar]
- Navidi W. Bidirectional case-crossover designs for exposures with time trends. Biometrics. 1998;54:596–605. [PubMed] [Google Scholar]
- Navidi W, Thomas D, Langholz B, Stram D. Statistical methods for epidemiologic studies of the health effects of air pollution. Res Rep Health Eff Inst. 1999;(86):1–50. [PubMed] [Google Scholar]
- Navidi W, Weinhandl E. Risk set sampling for case-crossover designs. Epidemiology. 2002;13:100–105. doi: 10.1097/00001648-200201000-00016. [DOI] [PubMed] [Google Scholar]
- Neas LM, Schwartz J, Dockery D. A case-crossover analysis of air pollution and mortality in Philadelphia. Environ Health Perspect. 1999;107:629–631. doi: 10.1289/ehp.99107629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel JL, Metzger KB, Klein M, Flanders WD, Mulholland JA, Tolbert PE. Ambient air pollution and cardiovascular emergency department visits in potentially sensitive groups. Am J Epidemiol. 2007;165:625–633. doi: 10.1093/aje/kwk051. [DOI] [PubMed] [Google Scholar]
- Perez L, Tobias A, Querol X, Künzli N, Pey J, Alastuey A, et al. Coarse particles from Saharan dust and daily mortality. Epidemiology. 2008;19:800–807. doi: 10.1097/ede.0b013e31818131cf. [DOI] [PubMed] [Google Scholar]
- Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103:2810–2815. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- Peters A, von Klot S, Berglind N, Hörmann A, Löwel H, Nyberg F, et al. Comparison of different methods in analyzing short-term air pollution effects in a cohort study of susceptible individuals. [[accessed 1 July 2010]];Epidemiol Perspect Innov. 2006 3:10. doi: 10.1186/1742-5573-3-10. Available: http://www.epi-perspectives.com/content/3/1/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, von Klot S, Heier M, Trentinaglia I, Cyrys J, Hörmann A, et al. Particulate air pollution and nonfatal cardiac events. Part I. Air pollution, personal activities, and onset of myocardial infarction in a case-crossover study. Res Rep Health Eff Inst. 2005;(124):1–66. [PubMed] [Google Scholar]
- Pope CA, III, Muhlestein JB, May HT, Renlund DG, Anderson JL, Horne BD. Ischemic heart disease events triggered by short-term exposure to fine particulate air pollution. Circulation. 2006;114:2443–2448. doi: 10.1161/CIRCULATIONAHA.106.636977. [DOI] [PubMed] [Google Scholar]
- Pope CA, III, Renlund DG, Kfoury AG, May HT, Horne BD. Relation of heart failure hospitalization to exposure to fine particulate air pollution. Am J Cardiol. 2008;102:1230–1234. doi: 10.1016/j.amjcard.2008.06.044. [DOI] [PubMed] [Google Scholar]
- Ramsay TO, Burnett RT, Krewski D. The effect of concurvity in generalized additive models linking mortality to ambient particulate matter. Epidemiology. 2003;14:18–23. doi: 10.1097/00001648-200301000-00009. [DOI] [PubMed] [Google Scholar]
- Rich DQ, Kim MH, Turner JR, Mittleman MA, Schwartz J, Catalana PJ, et al. Association of ventricular arrhythmias detected by implantable cardioverter defibrillator and ambient air pollutants in the St Louis, Missouri metropolitan area. Occup Environ Med. 2006a;63:591–596. doi: 10.1136/oem.2005.023457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich DQ, Mittleman MA, Link MS, Schwartz J, Luttmann-Gibson H, Catalana PJ, et al. Increased risk of paroxysmal atrial fibrillation episodes associated with acute increases in ambient air pollution. Environ Health Perspect. 2006b;114:120–123. doi: 10.1289/ehp.8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich DQ, Schwartz J, Mittleman MA, Link M, Luttmann-Gibson H, Catalano PJ, et al. Association of short-term ambient air pollution concentrations and ventricular arrhythmias. Am J Epidemiol. 2005;161:1123–1132. doi: 10.1093/aje/kwi143. [DOI] [PubMed] [Google Scholar]
- Rich KE, Petkau J, Vedal S, Brauer M. A case-crossover analysis of particulate air pollution and cardiac arrhythmia in patients with implantable cardioverter defibrillators. Inhal Toxicol. 2004;16:363–372. doi: 10.1080/08958370490439515. [DOI] [PubMed] [Google Scholar]
- Romieu I, Ramirez-Aguilar M, Moreno-Macias H, Barraza-Villarreal A, Millar P, Hernández-Cadena L, et al. Infant mortality and air pollution: modifying effect by social class. J Occup Environ Med. 2004;46:1210–1216. [PubMed] [Google Scholar]
- Ruidavets JB, Cournot M, Cassadou S, Giroux M, Meybeck M, Ferrieres J. Ozone air pollution is associated with acute myocardial infarction. Circulation. 2005;111:563–569. doi: 10.1161/01.CIR.0000154546.32135.6E. [DOI] [PubMed] [Google Scholar]
- Saez M, Perez-Hoyos S, Tobias A, Saurina C, Barceló MA, Ballester F. Métodos de series temporales en los studies epidemiológicos sobre contaminación atmosférica [in Spanish] Rev Esp Salud Publica. 1999;73:133–143. [PubMed] [Google Scholar]
- Schwartz J. Is the association of airborne particles with daily deaths confounded by gaseous air pollutants? An approach to control by matching. Environ Health Perspect. 2004a;112:557–561. doi: 10.1289/ehp.6431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J. The effects of particulate air pollution on daily deaths: a multi-city case crossover analysis. Occup Environ Med. 2004b;61:956–961. doi: 10.1136/oem.2003.008250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J. How sensitive is the association between ozone and daily deaths to control for temperature? Am J Respir Crit Care Med. 2005;171:627–631. doi: 10.1164/rccm.200407-933OC. [DOI] [PubMed] [Google Scholar]
- Ségala C, Poizeau D, Mesbah M, Willems S, Maidenberg M. Winter air pollution and infant bronchiolitis in Paris. Environ Res. 2008;106:96–100. doi: 10.1016/j.envres.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Sheppard L, Levy D, Checkoway H. Correcting for the effects of location and atmospheric conditions on air pollution exposures in a case-crossover study. J Expo Anal Environ Epidemiol. 2001;11:86–96. doi: 10.1038/sj.jea.7500151. [DOI] [PubMed] [Google Scholar]
- Son JY, Cho YS, Lee JT. Effects of air pollution on postneonatal infant mortality among firstborn infants in Seoul, Korea: case-crossover and time-series analyses. Arch Environ Occup Health. 2008;63:108–113. doi: 10.3200/AEOH.63.3.108-113. [DOI] [PubMed] [Google Scholar]
- Stafoggia M, Schwartz J, Forastiere F, Perucci CA SISTI Group. Does temperature modify the association between air pollution and mortality? A multicity case-crossover analysis in Italy. Am J Epidemiol. 2008;167:1476–1485. doi: 10.1093/aje/kwn074. [DOI] [PubMed] [Google Scholar]
- Sullivan J, Ishikawa N, Sheppard L, Siscovick D, Checkoway H, Kaufman J. Exposure to ambient fine particulate matter and primary cardiac arrest among persons with and without clinically recognized heart disease. Am J Epidemiol. 2003;157:501–509. doi: 10.1093/aje/kwg015. [DOI] [PubMed] [Google Scholar]
- Sullivan J, Sheppard L, Schreuder A, Ishikawa N, Siscovick D, Kaufman J. Relation between short-term fine-particulate matter exposure and onset of myocardial infarction. Epidemiology. 2005;16:41–48. doi: 10.1097/01.ede.0000147116.34813.56. [DOI] [PubMed] [Google Scholar]
- Sunyer J, Basagana X. Particles, and not gases, are associated with the risk of death in patients with chronic obstructive pulmonary disease. Int J Epidemiol. 2001;30:1138–1140. doi: 10.1093/ije/30.5.1138. [DOI] [PubMed] [Google Scholar]
- Sunyer J, Basagaña X, Belmonte J, Antó JM. Effect of nitrogen dioxide and ozone on the risk of dying in patients with severe asthma. Thorax. 2002;57:687–693. doi: 10.1136/thorax.57.8.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunyer J, Schwartz J, Tobias A, Macfarlane D, Garcia J, Antó JM. Patients with chronic obstructive pulmonary disease are at increased risk of death associated with urban particle air pollution: a case-crossover analysis. Am J Epidemiol. 2000;151:50–56. doi: 10.1093/oxfordjournals.aje.a010121. [DOI] [PubMed] [Google Scholar]
- Symons JM, Wang L, Guallar E, Howell E, Dominici F, Schwab M, et al. A Case-crossover study of fine particulate matter air pollution and onset of congestive heart failure symptom exacerbation leading to hospitalization. Am J Epidemiol. 2006;164:421–433. doi: 10.1093/aje/kwj206. [DOI] [PubMed] [Google Scholar]
- Tecer LH, Alagha O, Karaca F, Tuncel G, Eldes N. Particulate matter [PM(2.5), PM(10–2.5), and PM(10)] and children’s hospital admissions for asthma and respiratory diseases: a bidirectional case-crossover study. J Toxicol Environ Health A. 2008;71:512–520. doi: 10.1080/15287390801907459. [DOI] [PubMed] [Google Scholar]
- Tsai SS, Chen CC, Hisieh HJ, Chang CC, Yang CY. Air pollution and postneonatal mortality in a tropical city: Kaohsiung, Taiwan. Inhal Toxicol. 2006a;18:185–189. doi: 10.1080/08958370500434214. [DOI] [PubMed] [Google Scholar]
- Tsai SS, Cheng M, Chiu H, Wu T, Yang CY. Air pollution and hospital admissions for asthma in a tropical city: Kaohsiung, Taiwan. Inhal Toxicol. 2006b;18:549–554. doi: 10.1080/08958370600686176. [DOI] [PubMed] [Google Scholar]
- Tsai SS, Goggins WB, Chiu HF, Yang CY. Evidence for an association between air pollution and daily stroke admissions in Kaohsiung, Taiwan. Stroke. 2003a;34:2612–2616. doi: 10.1161/01.STR.0000095564.33543.64. [DOI] [PubMed] [Google Scholar]
- Tsai SS, Huang CH, Goggins WB, Wu TN, Yang CY. Relationship between air pollution and daily mortality in a tropical city: Kaohsiung, Taiwan. J Toxicol Environ Health A. 2003b;66:1341–1349. doi: 10.1080/15287390306389. [DOI] [PubMed] [Google Scholar]
- Villeneuve PJ, Chen L, Rowe BH, Coates F. Outdoor air pollution and emergency department visits for asthma among children and adults: a case-crossover study in northern Alberta, Canada. [[accessed 2 July 2010]];Environ Health. 2007 6:40. doi: 10.1186/1476-069X-6-40. Available: http://www.ehjournal.net/content/6/1/40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve PJ, Chen L, Stieb D, Rowe BH. Associations between outdoor air pollution and emergency department visits for stroke in Edmonton, Canada. Eur J Epidemiol. 2006;21:689–700. doi: 10.1007/s10654-006-9050-9. [DOI] [PubMed] [Google Scholar]
- Wellenius GA, Bateson TF, Mittleman MA, Schwartz J. Particulate air pollution and the rate of hospitalization for congestive heart failure among Medicare beneficiaries in Pittsburgh, Pennsylvania. Am J Epidemiol. 2005a;161:1030–1036. doi: 10.1093/aje/kwi135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellenius GA, Schwartz J, Mittleman MA. Air pollution and hospital admissions for ischemic and hemorrhagic stroke among Medicare beneficiaries. Stroke. 2005b;36:2549–2553. doi: 10.1161/01.STR.0000189687.78760.47. [DOI] [PubMed] [Google Scholar]
- Wellenius GA, Schwartz J, Mittleman MA. Particulate air pollution and hospital admissions for congestive heart failure in seven United Status cities. Am J Cardiol. 2006;97:404–408. doi: 10.1016/j.amjcard.2005.08.061. [DOI] [PubMed] [Google Scholar]
- Xu X, Zborowski JV, Arena VC, Rager J, Talbott EO. Case-crossover analysis of air pollution and cardiorespiratory hospitalizations: using routinely collected health and environmental data for tracking: science and data. J Public Health Manag Pract. 2008;14:569–576. doi: 10.1097/01.PHH.0000338369.59080.9d. [DOI] [PubMed] [Google Scholar]
- Yamakazi S, Nitta H, Ono M, Green J, Fukuhara S. Intracerebral haemorrhage associated with hourly concentration of ambient particulate matter: case-crossover analysis. Occup Environ Med. 2007;64:17–24. doi: 10.1136/oem.2005.021097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CY. Air pollution and hospital admissions for congestive heart failure in a subtropical city: Taipei, Taiwan. J Toxicol Environ Health A. 2008;71:1085–1090. doi: 10.1080/15287390802114428. [DOI] [PubMed] [Google Scholar]
- Yang CY, Chang CC, Chuang HY, Tsai SS, Wu TN, Ho CK. Relationship between air pollution and daily mortality in a subtropical city: Taipei, Taiwan. Environ Int. 2004a;30:519–523. doi: 10.1016/j.envint.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Yang CY, Chen CC, Chen CY, Kuo HW. Air pollution and hospital admissions for asthma in a subtropical city: Taipei, Taiwan. J Toxicol Environ Health. 2007;70:111–117. doi: 10.1080/15287390600755059. [DOI] [PubMed] [Google Scholar]
- Yang CY, Chen CJ. Air pollution and hospital admissions for chronic obstructive pulmonary disease in a subtropical city: Taipei, Taiwan. J Toxicol Environ Health. 2007;70:1214–1219. doi: 10.1080/15287390701380880. [DOI] [PubMed] [Google Scholar]
- Yang CY, Chen YS, Yang CH, Ho SC. Relationship between ambient air pollution and hospital admissions for cardiovascular diseases in Kaohsiung, Taiwan. J Toxicol Environ Health A. 2004b;67:483–493. doi: 10.1080/15287390490276502. [DOI] [PubMed] [Google Scholar]
- Yang CY, Hsieh HJ, Tsai SS, Wu TN, Chiu HF. Correlation between air pollution and postneonatal mortality in a subtropical city: Taipei, Taiwan. J Toxicol Environ Health. 2006;69:2033–2040. doi: 10.1080/15287390600746181. [DOI] [PubMed] [Google Scholar]
- Yang Q, Chen Y, Shi Y, Burnett RT, McGrail KM, Krewski D. Association between ozone and respiratory admissions among children and the elderly in Vancouver, Canada. Inhal Toxicol. 2003;15:1297–1308. doi: 10.1080/08958370390241768. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J. The effect of particulate air pollution on emergency admissions for myocardial infarction: a multicity case-crossover analysis. Environ Health Perspect. 2005;113:978–982. doi: 10.1289/ehp.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanobetti A, Schwartz J. Air pollution and emergency admissions in Boston, MA. J Epidemiol Community Health. 2006;60:890–895. doi: 10.1136/jech.2005.039834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Dominici F, McDermott A, Samet JM. Mortality in the Medicare population and chronic exposure to fine particulate air pollution in urban centers (2000–2005) Environ Health Perspect. 2008;116:1614–1619. doi: 10.1289/ehp.11449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeka A, Zanobetti A, Schwartz J. Short term effects of particulate matter on cause specific mortality: effects of lags and modification by city characteristics. Occup Environ Med. 2005;62:718–725. doi: 10.1136/oem.2004.017012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeka A, Zanobetti A, Schwartz J. Individual-level modifiers of the effects of particulate matter on daily mortality. Am J Epidemiol. 2006;163:849–859. doi: 10.1093/aje/kwj116. [DOI] [PubMed] [Google Scholar]