The feeding of human milk (milk from the infant’s own mother; excluding donor milk) during the NICU stay reduces the risk of short-and long-term morbidities in premature infants, including: enteral feed intolerance; nosocomial infection; necrotizing enterocolitis (NEC); chronic lung disease (CLD); retinopathy of prematurity (ROP); developmental and neurocognitive delay; and rehospitalization after NICU discharge.1–29 The mechanisms by which human milk provides this protection are varied and synergistic, and appear to change over the course of the NICU stay.30, 31 In brief, these mechanisms include specific human milk components that are not present in the milk of other mammals, such the type and amount of long-chain polyunsaturated fatty acids and digestible proteins, and the extraordinary number of oligosaccharides (approximately 130).32 Human milk also contains multiple lines of undifferentiated stem cells, with the potential to impact a variety of health outcomes through the lifespan.33 Other human milk mechanisms change over the course of lactation in a manner that complements the infant’s nutritional and protective needs. These mechanisms include immunological, anti-infective, anti-inflammatory, epigenetic, and mucosal membrane protecting properties.34–41 Thus, human milk from the infant’s mother cannot be replaced by commercial infant or donor human milk, and the feeding of human milk should be a NICU priority.
Recent evidence suggests that the impact of human milk on improving infant health outcomes and reducing the risk of prematurity-specific morbidities appears to be linked to specific critical exposure periods in the post-birth period during which the exclusive use of human milk and the avoidance of commercial formula may be most important.29–31, 42, 43 Similarly, there are other periods when high doses, but not necessarily exclusive use of human milk, may be important. This chapter will review the concept of “dose and exposure period” for human milk feeding in the NICU to precisely measure and benchmark the amount and timing of human milk use in the NICU. Similarly, the critical exposure periods when exclusive or high doses of human milk appear to have the greatest impact on specific morbidities will be reviewed. Finally, this chapter will summarize the current best practices for the use of human milk during and after the NICU stay for premature infants.
Dose and Exposure Period: Precise Measurement of Human Milk Use in the NICU
Research, practice, and quality improvement initiatives focused on the use of human milk in the NICU have been limited by the lack of a precise, quantitative measure of “human milk feeding” for premature infants.30 Whereas definitions for “breastfeeding” were standardized for term healthy infants in the early 1990s,44 these six categorical definitions do not capture the critical components of human milk feeding patterns for NICU infants.45 Additionally, the existing definitions for “human milk feeding” used in studies of premature infants are limited and inconsistent. For example, human milk feeding might vary from receiving “any” human milk to having received a specific volume threshold, such as 50 mL/kg/day. However, the measures usually do not specify when the infant received human milk and whether there were periods of exclusive or high doses of human milk feeding. Thus, quality improvement initiatives that focus only on increasing the percentage of NICU infants that are “human milk-fed” will be inadequate if specific amounts and time periods of human milk feeding are not specified.
A second category of quality improvement indicators focuses on the use of human milk at the time of NICU discharge. Examples of these indicators include “increasing the percentage of NICU infants that are exclusively breastfeeding or receiving exclusive human milk feedings” at the time of NICU discharge. Although this outcome is precise and easily-measured, it fails to capture the infant’s human milk feeding history throughout the NICU stay. Similarly, it is dichotomous with respect to the individual infant and mother. For example, a mother who had no desire to breastfeed may have provided her milk for her infant for a significant portion of the NICU stay so that her infant was fed exclusive human milk for a substantial period (e.g., 30 or 60 days). However, this mother-infant dyad would be classified as “not breastfeeding” at the time of discharge, even though the mother may have provided human milk throughout the most critical period of the infant’s development. Indeed, current evidence suggests there are relatively short, critical exposure periods post-birth when exclusive or high amounts of human milk are especially important in optimizing health outcomes for premature infants30 and reducing the risk of enteral feed intolerance, nosocomial infection, and inflammation-based morbidities such as NEC.7, 27, 28, 46, 47 From a cost outcomes perspective, these morbidities translate directly into higher costs of NICU care15, 48–50 and greater probability of long-term health problems.10–21 Thus, the infant who receives exclusive human milk feeding in the first month post birth may have better health outcomes than an infant who received low doses of human milk throughout the NICU stay.
Consistent with the emerging clinical and molecular evidence, quality improvement indicators should focus on measuring and benchmarking the “dose and exposure period” for human milk use in the NICU.31 The “dose” of human milk should be quantitatively measured for each infant, both as a percentage of total enteral feedings and in mL/kg/day for each day of the NICU stay. These simple calculations require only the total mLs of human milk and non-human milk fed to the infant each day. The “exposure period” refers to the specific days during the NICU stay during which the infant received any human milk feeding. For example, three quality indicators using dose and exposure period might be: “Increase to 75% the percentage of very low birth weight infants (VLBW; <1500 g) infants who receive a dose of human milk of at least 80% over the first month post-birth”; “Increase to 75% the percentage of extremely low birth weight infants (ELBW; <1000 g) infants who receive at least 50 mL/kg/d of human milk over the NICU stay”; and “Increase to 75% the percentage of ELBW infants who receive exclusive human milk feeding during the first 14 days post-birth.” These indicators are evidence-based, precise, objective, measurable and, as continuous variables, are easily related to health outcomes and cost of care in statistical and economic analyses.
Critical Exposure Periods for the Use of Human Milk
This section will review the clinical evidence for use of critical exposure periods to conceptualize and measure the dose of human milk feeding for premature infants in the NICU. Additionally, the underlying human milk mechanisms and their impact on the development of specific infant organs and systems during these critical exposure periods will be detailed. These four critical periods include: colostrum as the transition from intrauterine to extrauterine nutrition; the transition from colostrum to mature milk feedings during the first month post-birth; human milk feedings throughout the NICU stay; and human milk feedings after NICU discharge.
Colostrum: The Transition from Intrauterine to Extrauterine Nutrition in Mammals
The first critical exposure period for human milk feeding is the use of colostrum during the introduction and advancement of enteral feedings in the early post-birth period. Colostrum is secreted during the early days post-birth when the paracellular pathways between the mammary epithelium are open and permit the transfer of high molecular weight antibodies, anti-inflammatories, growth factors, and other protective components into the milk product. When colostrum is fed to the infant during the early post-birth period, the high molecular weight protective components of colostrum can pass through the open paracellular pathways in the infant gastrointestinal tract.51–55 Colostrum feedings are especially important for extremely immature infants because, during the last trimester in utero, the infants would have swallowed approximately 750 mL of amniotic fluid daily.54, 56 An array of growth factors in the swallowed amniotic fluid more than doubles the weight of the intestinal mucosa during this time.53, 54
Colostrum, with a profile of growth factors, anti-inflammatory and anti-infective components similar to amniotic fluid, is the transitional nutritive that facilitates the transition from intrauterine to extrauterine nutrition in mammals.53, 57 For extremely premature infants, the early administration of colostrum helps compensate for the shortened period of in-utero amniotic fluid swallowing. Initial colostrum feedings stimulate rapid growth in the intestinal mucosal surface area, facilitate the endocytosis of protein, and induce many digestive enzymes.53, 58, 59 In animal models, the intestinal tract does not mature comparably if colostrum is not the first feeding.53, 58, 59 This observation is true even when initial feedings consist of mature milk from the same mammalian species and are followed by colostrum.53, 55, 59 Furthermore, artificial feedings appear to exert a separate detrimental effect when they replace colostrum as initial postnatal nutrition in piglets, including atrophy of the gastrointestinal tract, higher concentrations of inducible nitric oxide synthase in the intestinal tissue, and elevated serum cortisol.53, 58 These structural and biochemical outcomes have been linked to necrotizing enterocolitis in laboratory animals.53, 60
Human colostrum is also quite different from mature milk, with higher concentrations of secretory IgA, growth factors, lactoferrin, anti-inflammatory cytokines, oligosaccharides, soluble CD14, antioxidants and other protective components.32, 61, 62, 62–64 Recent studies suggest an inverse relationship between the duration of pregnancy and the concentration of these agents in maternal colostrum, meaning that mothers of the least mature infants produce the most protective colostrum. Separate studies suggest that secretion of colostrum may be prolonged by several hours or days following extremely premature birth, and that the additional colostrum-type milk may be a specific protective mechanism for the compromised infant.36, 39 A recent study has also demonstrated the safety and feasibility of oropharyngeally administered colostrum prior to the introduction of trophic feedings in ELBW infants.65, 66 The mechanisms of protection with oropharyngeally administered colostrum, such as cytokine absorption via the oropharyngeal associated lymphoid tissues (OFALT) with subsequent systemic immunomodulation and the local interference with microbe attachment to the oral mucous membranes, may be additive to trophic feedings and may have a specific role in protection from ventilator-associated pneumonia.65, 66
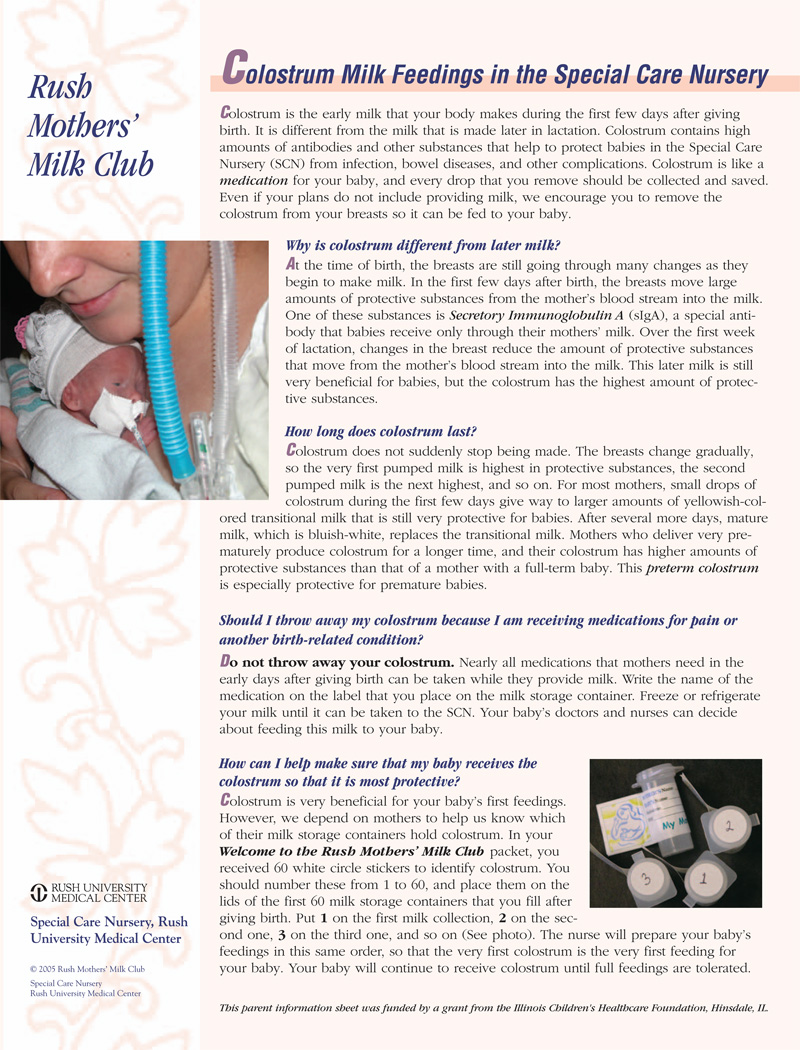
The evidence about the importance of colostrum as a first feeding has many implications in the NICU, especially for extremely premature infants who were not exposed to the growth factors in amniotic fluid during the last trimester. Box 1 summarizes clinical guidelines for colostrum feeding in the NICU, and Figure 1 is patient information handout that summarizes the importance of colostrum feeding for families of NICU infants.
Box 1.
Clinical Application for Colostrum Feedings in the NICU
Figure 1.
Parent Information Handout about the Importance of Collecting and Feeding Colostrum in the NICU
Early Enteral Feedings: Transition from Colostrum to Mature Milk During the First Month Post-Birth
A second critical period for high doses of human milk feedings is the first 14 to 28 days post-birth, when several studies have demonstrated a dose-response relationship between the amount of human milk received by VLBW and ELBW infants and specific clinical morbidities including enteral feed intolerance,28 nosocomial infection,7, 46 NEC,27, 67 CLD,47, 68 ROP,47 and total number of morbidities during the NICU stay.47 The mechanism by which the feeding of high doses of human milk impacts morbidities during this critical period is linked to structural and functional changes in the gastrointestinal tract that occur as enteral feedings are advanced. Human milk appears to program or stimulate many of these healthy processes, whereas formula appears to exert an independent detrimental effect.29, 69 Unfortunately, no previous study has examined the effect of donor human milk during this transition to full enteral feedings so, the impact of donor milk during this period is unknown.
During the first days of life, the gastrointestinal tract, sterile at birth, becomes colonized almost immediately with an array of commensal and potentially pathogenic bacteria. Many factors surrounding the birth of a premature or NICU infant, such as Cesarean birth, antibiotic use, and delayed enteral feedings predispose the intestine to a dysbiosis with respect to colonization and maturation.69–71 However, several independent studies indicate that human milk, which has both probiotic and prebiotic activity,32, 61, 62, 72, 73 results in a predominantly commensal gut microflora.74–76 In contrast, even small amounts of formula fed during this time appear to interrupt the protective colonization conferred by human milk.74–76 Related research indicates that soluble CD14, a pattern recognition molecule that functions as a co-receptor for toll-like receptors II and IV, is highly concentrated in human milk and is up to 20 times higher in milk than in the serum of lactating women.62, 63 In combination, the pre- and probiotics, and soluble CD14 provide the substrates for healthy bacterial-enterocyte crosstalk in the developing intestine.77
A second protective mechanism is that during the transition from colostrum to mature milk feedings during the first month post-birth is that these milks facilitate the closure of paracellular pathways by the formation of tight junctions between the enterocytes in the infant’s intestine. The closure of the paracellular pathways is positively associated with the volume human milk feeding.29 The resulting tight junctions inhibit the translocation of high molecular-weight bacteria and their toxins from the lumen of the gut to the bowel wall where they can up-regulate inflammatory processes through activation of the cytokine, interleukin 8 (IL-8).78–80 With little or no ability to mount a compensatory anti-inflammatory response, the extremely immature infant is susceptible to local inflammatory processes, such as NEC as well as the spread of inflammation to distal organs such as the lungs, eyes, and brain.79, 81 The specific human milk components linked with these functions include pre- and pro-biotics, oligosaccharides, soluble CD14, transforming growth factor-beta (TGF-B), epidermal growth factor (EGF),36, 37 interleukin-10 (IL-10)and lactoferrin (LF), all of which are concentrated most highly in the colostrum, the milk secreted prior to the closure of the paracellular pathways in the mammary epithelium.29, 32, 34–41, 61–63, 65, 66, 72–76, 78, 79, 81 Furthermore, during this critical exposure period, which coincides with the introduction and advancement of enteral feeds, formula appears to exert an independent, proinflammatory effect.29, 32, 34–41, 61–63, 65, 66, 72–76, 78, 79, 81
Dose of Human Milk During the NICU Stay
Five well-controlled studies of four cohorts of extremely premature and/or ELBW infants have linked the dose of human milk feedings (mL/kg/d) received throughout the NICU stay with specific health outcomes during or after the NICU stay.5, 6, 25, 26, 47 However, only one of these studies examined the effect of specific exposure periods within the NICU stay, and found that high doses of human milk during the first 14 days post-birth were most highly associated with the advantageous health outcomes that were noted throughout the NICU stay.47 In three of the four cohorts, premature infants who received the highest doses and/or exclusive feedings of fortified human milk had shorter hospital stays than formula-fed infants, despite the fact that the human milk fed infants either grew at a similar rate47 or more slowly5, 6 than the formula-fed infants. Although the remaining studies25, 26 did not find a feeding-related trend in hospital-based morbidities or length of the NICU stay, they established a dose-response relationship between the amount of human milk received during the NICU stay and better health outcomes during the first 18 and 30 months of age, corrected for prematurity.
In separate studies, Schanler compared health outcomes for extremely premature infants who received differing doses of human milk throughout the NICU stay.5, 6 In the first study,6 health outcomes were compared for infants who received ≥ 50 mL/kg/d of fortified human milk and those who received exclusive formula feedings. Infants who received human milk feedings had: fewer days of total parenteral nutrition (TPN); fewer episodes of enteral feed intolerance; a lower incidence of NEC, CLD, and ROP; and a dose-response relationship between the amount of human milk and the number of episodes of late onset sepsis. Even though the human milk-fed infants gained weight more slowly, they were discharged nearly 500 grams lighter and two weeks earlier than comparison formula-fed infants. The investigators speculated that the earlier discharge was a function of the lower incidence and severity of morbidities in the human milk-fed infants.
In a subsequent study5 Schanler studied 243 extremely premature infants whose mothers initiated lactation with the intent of providing human milk throughout the infants’ NICU stay. If the maternal milk supply was inadequate for all of the infants’ feedings, the infants were assigned randomly to receive either donor human milk or formula as a supplement to the mother’s own milk. Of the 243 infants, 29% received only their own mothers’ milk throughout the NICU stay, and the other 71% were distributed equally between the two randomized groups. Only minor differences were noticed between the two groups randomized to either supplementation with donor human milk or formula, with the donor milk supplemented group demonstrating a slightly lower incidence of CLD and slower weight gain. In contrast, the infants who received only their own mothers’ milk during the NICU stay had a lower incidence of all prematurity-specific morbidities, and were discharged a week sooner than the infants who required supplementation with either donor human milk or formula.
In a retrospective study of 277 ELBW infants, Patel et al., noted a similar trend toward earlier discharge in ELBW infants who received exclusive human milk feedings versus those who received exclusive formula feedings during the NICU stay.47 Although the human milk fed infants were one week less mature (25.2 vs 26.3 wks) at birth, and demonstrated the same growth velocity from regaining birthweight until NICU discharge (14.5 vs 14.6 g/kg/d), their length of stay in the NICU was 11.7 days shorter (90.2 days vs 101.9 days), and their postmenstrual age at NICU discharge was 2.8 weeks less (38.0 vs 40.8) than the formula fed infants. In this 5-year, retrospective cohort, Patel et al., also described a dose-response relationship between the dose of human milk (mL/kg/d) received during the first 14 days post-birth and number of morbidities during the NICU stay.
In a secondary analysis of the NICHD-funded glutamine trial of 1034 ELBW infants cared for in 19 NICUs in the United States,82 Vohr et al., found no differences in hospital-based morbidities or length of NICU stay among formula fed and human milk fed infants..26 However, the investigators reported a dose-response relationship between the amount of human milk received during the NICU stay and developmental outcomes at 18 months26 and 30 months of age25 in this cohort. At the 18-month evaluation26 there was no difference between formula and human milk fed groups in overall growth. However, the investigators reported that each 10 mL/kg/d of human milk received over the NICU stay was associated with a dose-response increase in scores on standardized neurocognitive and developmental tests, and with a reduced risk of rehospitalization during the first year of life. The most striking differences were observed between the exclusively formula fed group and the highest quintile of human milk dose received (110 mL/kg/d), with a 5-point IQ advantage for the high human milk group. The investigators concluded that this difference, when considered from a population perspective, translated into significant health care, educational, and societal cost savings over the lifespan for ELBW infants.
Vohr, et al.,25 followed this same cohort of infants, and reported outcome at 30 months of age, corrected for prematurity, for 773 of the original 1034 infants. The relationship between dose of human milk received during the NICU stay and neurocognitive and developmental outcome persisted through this second developmental time point, with each 10 mL/kg/d of human milk ingestion in the NICU adding to infants’ scores on standardized tests in a dose-response manner. The risk of rehospitalization remained lower as a function of human milk dose, especially for respiratory illnesses. Thus, it appears that human milk feedings during the NICU stay provide the foundation for better health outcomes during early childhood.
In summary, in three of the four cohorts of extremely premature and/or ELBW infants for whom dose of human milk during the NICU stay was measured, investigators reported a lower incidence and severity of morbidities, a shorter length of stay in the NICU, and hospital discharge at lower weights and/or postmenstrual age in infants who received exclusive or high doses of human milk.5, 6, 25, 26, 47 These data are especially compelling because in the three studies, the human milk-fed infants either grew similarly47 or more slowly5, 6 than comparable infants who received either low doses or no human milk.
In the single cohort for whom health outcomes were measured after the NICU stay, infants in all feeding groups grew similarly during the first 18 and 30 months of life, corrected for prematurity.25, 26 However, a dose-response relationship was described between the amount of human milk feedings received during the NICU stay and scores on tests of neurocognitive and developmental outcome and the risk of rehospitalization at both post-discharge time points. In all four cohorts, the most striking differences in outcome were between infants who had received high (or exclusive) doses of human milk and exclusive formula. The findings also suggest, though, that proportionately higher doses of human milk (but not necessarily exclusive or extremely high dose) received over the longer exposure period of the entire course of the NICU stay, impact the aforementioned outcomes. Thus, human milk feedings over the NICU stay do not have to be exclusive to confer benefit, but the greatest benefit appears linked to high doses or exclusive feedings of human milk.
The specific human milk mechanisms that impact these NICU and post-discharge outcomes are probably both protective and nutritive in nature. Several developmental outcome studies suggest that a lower incidence and severity of morbidities during the NICU stay translate into a shorter NICU stay, lower discharge weight and postmenstrual age, better neurocognitive and developmental outcome, and a lower risk of rehospitalization in premature infants.10, 11, 14–16, 18–21, 49, 83, 84 By providing primary protection from these morbidities during the early NICU stay, human milk may indirectly impact the associated longer-term outcomes.30
Additionally, many protective components in human milk may be equally, or even more important beyond the first 14–28 days post-birth, and probably affect these longer-term outcomes. Examples of these components include: antioxidant activity to counter the untoward effects of oxygen;41 the “customization” of antibodies via the enteromammary pathway, providing protection from specific pathogens in the NICU environment;85 oligosaccharides that inhibit the adhesion of pathogens to mucosal membranes in the mouth, throat, and gastrointestinal tract; the potential impact of oligosaccharides on neural development;9, 32, 86, 87 and other lesser-studied factors that impact tissue growth and metabolism, such as vascular endothelial growth factor, transforming growth factors, and leptin.88, 89 .
The Impact of Human Milk Feedings After the NICU Stay
Although it can be assumed that premature infants who continue to receive human milk after the NICU stay experience the same short- and long-term benefits as term infants,90 no well-controlled studies linking these outcomes with either dose or exposure period of human milk have been reported for premature infants after NICU discharge. In contrast, most research in this area has focused on comparing short-term growth velocity and other anthropometric measures for cohorts of premature infants who are discharged in one of three feeding categories: receiving exclusive human milk, either from the breast, bottle, or a combination of the two; receiving human milk feedings that are either supplemented with powdered formula or partially replaced with premature formulas, or receiving exclusive formula feedings. The findings from most of these studies suggest that premature infants grow more rapidly with exclusive formula feedings or when human milk feedings are either supplemented or partially replaced with formula. Although no studies have examined the impact of these practices on longer-term health outcomes, they are common NICU discharge instructions for human milk fed premature infants.91–94
However, a limitation in all of these post-discharge growth studies is the fact that human milk intake has seldom been measured precisely during breastfeeding, using accurate test-weighing procedures in the home.92, 93, 95–98 even though a series of well-controlled studies indicate that premature infants are vulnerable to under-consumption of milk during exclusive breastfeeding until they achieve term, corrected age.97, 99–102 Similarly, no study has included actual compositional measures of the human milk consumed by the infant, despite the extensive evidence indicating that the caloric content of human milk varies markedly throughout the day and within the same mother.103–107 Instead, the caloric content of human milk is assumed to be 20 calories per ounce for all feedings, a figure that is inconsistent with the research in this area.102 Thus, at the present time, it is unknown whether exclusively human milk-fed infants grow more slowly because they consume an inadequate volume of milk or because the milk that they consume is inadequate in calories or a specific nutrient such as protein. These are important distinctions, and are diagnosable and manageable for both research and practice, using human milk research technologies that enable an infant to continue receiving high doses of human milk.30, 42, 100, 102
In summary, nothing is known about the longer-term implications of feeding either exclusive human milk or human milk supplemented with commercial formula products. While short-term growth is important, replacement of human milk with commercial formula reduces the overall lifetime dose of human milk for premature infants. Studies with term, healthy infants suggest that many of the longer-term health benefits associated with breastfeeding such as higher scores on intelligence tests and protection from infections, eczema, and adult-onset morbidities, are conferred in a dose-response manner.90 Thus, replacement of human milk feedings with commercial products may accelerate short-term growth but has unknown implications for later-onset morbidities.
Best NICU Practices to Increase Dose and Exposure Period of Human Milk Feedings
This section will address the best practices for optimizing the dose and exposure period of human milk feeding for premature infants in the NICU. These practices are conceptualized into four aspects of care: encouraging the mother to provide her milk for her infant; providing cost-effective, expert lactation and human milk feeding support for families and staff in the NICU; prioritizing the initiation, establishment, and maintenance of maternal milk volume, and using lactation technologies to manage human milk feeding problems.
Encouraging the Mother to Provide her Milk for her Infant
Exclusive human milk feeding is uniformly recommended as the first food and as the only food during the first months of life by all of the major health organizations with an interest in infant health, including: the World Health Organization,108 the United States Breastfeeding Committee,109 and the American Academy of Pediatrics.110 The World Health Organization specifically addresses the importance of colostrum as the first feeding for infants in the immediate post-birth period,108 and the American Academy of Pediatrics specifically addresses the importance of human milk feeding for premature infants.110
Although the benefits of human milk feeding for premature infants are well-documented, many obstetricians, pediatricians, and nurses remain reluctant to encourage mothers to provide their milk and often simply accept the mother’s decision to formula feed without further discussion. These professionals often mistakenly assume that they do not have any influence over a mother’s feeding decision, or that they will increase the stress for a mother whose infant is in a critical care setting. Finally, some care providers think that it is unethical to encourage mothers to provide milk, and express concern that they are pressuring or coercing mothers at this sensitive time.
Recent research has dispelled many of these concerns and demonstrated that provider encouragement of human milk feeding for premature infants is effective regardless of the social and ethnic background of families, and that families depend upon health care providers to share this information with them.111–116 A recent review of the ethical issues related to promoting breastfeeding concluded that fully informing mothers of the health benefits of human milk was an ethical responsibility for health care professionals.112 Additionally, concerns that promotion of human milk feeding may make women feel guilty, coerced or forced into changing their decision were abated in a recent study of 21 mothers of VLBW infants who changed their feeding decision from formula to human milk.111 The study participants indicated that they changed their decision almost immediately after learning from a health care provider that their milk was a critical component in the overall management of their infants’ NICU plan of care.111 Indeed, one mother was so disturbed that she had not been told of the importance of her milk by professionals in the hospital where she gave birth, that she questioned the qualifications of the doctors and nurses who had cared for her and her baby in the referral hospital prior to her infant’s transport to the hospital where this research was conducted.
Although the efficacy and ethics of promoting breastfeeding are documented, the language used to promote the provision of human milk is important when speaking with women and their families. For example, many women do not wish to feed at the breast for a number of reasons, some of which are extremely sensitive, such as a history of sexual abuse. However, these women may be very amenable to using a breast pump to express their milk so it can be fed by bottle. Similarly, it is more appropriate to focus on providing milk for a limited period of time in order to “get the baby off to the best start,” than to engage in discussions about long-term milk expression or feeding at breast. All decisions about feeding at breast or long-term milk expression can be postponed until the infant’s condition is stable and the mother’s stress about the premature birth has begun to lessen. Then, these decisions can be made calmly and thoughtfully with the support of professionals, family members, and friends.
Although this initial discussion with the mother and family should be conducted in a non-directive and non-coercive manner, the benefits of human milk feeding, particularly of the colostrum and early post-birth feedings, should be clearly and scientifically communicated.30, 34, 42 Occasionally, the terms “non-coercive and non-directive” are misinterpreted to mean that feeding options are presented as if they were two equally safe and efficacious choices. Although the care provider must be supportive and caring in this discussion with families, the scientific evidence about human milk feedings should be shared just like any other NICU therapeutic option, which involves family decision-making. Examples of talking points that accurately translate scientific terminology about human milk into understandable parent information were summarized in a recent review paper.34 The health care provider who wants to provide encouragement and accurate information about human milk feedings, but who is not a lactation expert, will find this review paper useful in guiding these discussions with families of NICU infants.
In the Rush Mothers’ Milk Club lactation program, the perinatologists, neonatologists, nurses, and dietitians refer to human milk as a “medicine” that only the mother can provide. This explanation is accompanied by appropriate parent-focused information packets and handouts that translate the scientific principles about human milk and lactation into understandable words and concepts (Welcome to the Rush Mothers’ Milk Club).117 An additional resource is a recently completed parent-focused video about the importance of human milk feedings when an infant is born prematurely. This video, which features real families and infants from the Rush Mothers’ Milk Club program, is culturally sensitive, available in Spanish and can be used in a multitude of health care settings.117
Providing Cost-Effective, Expert Lactation and Human Milk Feeding Support for Families and Staff in the NICU
Whereas many maternal-infant health care providers recognize the importance of human milk for premature and NICU infants, individual institutions struggle with respect to implementing evidence-based models of lactation care for this population. Few neonatologists, NICU nurses, and dietitians are experts in the delivery of this care, and frequently turn to lactation consultants whose primary training and expertise is in the management of breastfeeding for term infants and their mothers. As a result, families are often “caught in the middle” with conflicting advice about the importance of human milk and the NICU-specific lactation problems they encounter, such as selecting and using an appropriate breast pump, collecting and storing their milk, and observing clinicians treat their infants’ slow weight gain with formula, even when they have an abundance of available milk. The conflicting advice that families receive about providing human milk in the NICU is well-documented, is a source of discouragement to mothers, and is a primary reason for lower doses and exposure periods of human milk feedings for recipient infants.118, 119
The best practice approach to solving inconsistencies in the management of human milk feedings in the NICU is no different from any other care issue: policies and procedures must be based on available scientific evidence rather than individual opinions and attitudes of staff members. This approach includes evidence-based education of personnel, the completion of human milk and lactation competencies, and the development of standardized policies and procedures to guide practice. Similarly, the notion that some staff members are “pro” or “con” human milk should be addressed by NICU administrators in a manner that is consistent with all other NICU therapies. Most NICUs would not tolerate professional staff members providing information to families based on whether they are “pro” or “con” ventilator management or medication regimens, and human milk feedings should be no different. Simply said, the evidence supports the use of human milk in the NICU, and personal attitudes or experiences of individual staff members to the contrary (“I didn’t breastfeed my babies and they turned out just fine…”) should not be a part of evidence-based practice in the NICU.
Numerous studies have demonstrated that health care professionals have very limited knowledge and skills related to assisting mothers with breastfeeding and providing milk for either healthy or NICU infants.120–123 However, several recent studies have demonstrated that educational interventions can improve provider knowledge, skills, and attitudes.124, 125 The United States Breastfeeding Committee has developed competencies for breastfeeding and lactation that are applicable to all care providers involved in the care of women and infants.126 These competencies include skills such as “know how and when to use technology and equipment to support breastfeeding” and “the ability to preserve breastfeeding under adverse conditions.”126 Likewise, the American Academy of Pediatrics Policy Statement on Breastfeeding details the expectations of pediatricians in promoting, supporting and protecting breastfeeding.110 Competencies specific to professionals who provide support to breast pump-dependent mothers and NICU infants have also been developed.117 Recent research demonstrates that a NICU-specific lactation education program was effective in changing NICU nurses’ knowledge and attitudes124 and that overall staff breastfeeding education in the NICU resulted in increased human milk feeding rates.125
The provision of clinical and educational support for NICU families and professionals is a specialty area that requires education and expertise both in complicated NICU situations and in the science of lactation and human milk. As such, NICU lactation programs should be under the direction of an advance-practice nurse, dietitian, or neonatologist. This professional needs expertise in the initiation, establishment, and maintenance of maternal milk volume in pump-dependent mothers who have numerous medical complications, and who may be taking multiple medications. Whereas lists of medications that either are or are not compatible with breastfeeding are useful in decision-making about term, healthy infants who will be breastfeeding exclusively,127 these decisions must be approached from an individualized risk-benefit perspective for the NICU infant (Figure 2). Similarly, the use of the highest possible dose and longest exposure period of human milk necessitates that this practitioner integrate technologies, such as the creamatocrit and test-weights, on a daily basis in order to prevent, diagnose and manage common NICU problems with human milk feedings.42, 102 The standardization of this model of practice requires interaction and education of NICU neonatologists, dieticians, nurses, subspecialists, and families.
Figure 2.
Parent Information Handout Explaining Decision-Making about Medications in Mothers’ Milk for Infants in the NICU
The optimal lactation team in the NICU can minimize its costs and increase its efficacy by incorporating the use of Breastfeeding Peer Counselors (BPCs). Although the role of the NICU-based BPC is new, research has demonstrated that BPCs in the NICU and in other settings improve human milk and lactation outcomes.128, 129 The Rush Mothers’ Milk Club has incorporated volunteer BPCs since 1997, and has employed BPCs as a part of the NICU lactation team since 2005, when this position was first funded with a foundation grant.130 These women (and one male counselor) complete a 5-day BPC training program in order to function as volunteers. Employed BPCs complete an additional 3-month orientation program117 so that they can acquire the necessary knowledge and skills to practice safely and effectively in the NICU environment. Two of the Rush Mothers’ Milk Club BPCs have also met the demanding clinical requirements for non-health care professionals without a college degree to become certified as International Board Certified Lactation Consultants (IBCLCs).
NICU-based BPCs can augment the work of the lactation specialists by performing many of the basic clinical services required in the NICU. For example, they can assume responsibility for teaching all mothers how to use the breast pump, how to clean the collection kit and how to safely collect, label, store, and transport their milk. However, equally important is that the BPC is a peer of the mother, and can help solve many of the cultural and ethnic lactation and human milk feeding problems that arise. This aspect is particularly important for African American mothers who are significantly more likely to experience preterm birth131 but less likely to breastfeed or provide their milk.132, 133 A recent study demonstrated that African American mothers experience issues with anxiety and trust when working with nurses and physicians.134
A recent study conducted with women in the Rush Mothers’ Milk Club demonstrated that the mothers preferred the BPCs to a health care professional when they sought help to address their personal barriers to providing milk for their infants.135 Additionally, the data from this study revealed that the BPC’s personal experience with an NICU infant and providing her milk had a profound impact on the new mother and influenced her decision to provide milk for her infant. The mothers who participated in this study also reported that the BPCs provided informational, instrumental, appraisal, and emotional support: The study also demonstrated that BPCs gave the mothers hope that their lives would “eventually return to normal,” and made them feel empowered with their decision to initiate and continue providing milk for their infants.
In addition to personnel, mothers need basic physical resources in the NICU in order to provide milk for their infants. These include: access to a hospital-grade dual electric breast pump and pump kit for adequate milk removal;42, 136 volume-based, rather than ration-based, allocation of containers for storing their expressed milk;42 refrigerator and freezer space for on-site milk storage of all milk to be fed to the infant during the NICU stay;42 and access to additional lactation equipment (e.g., nipple shields or infant scales to perform test weights) as needed to ensure that infants receive the highest dose of human milk.42, 100 A recent study demonstrated that the cost per 100mL of maternal human milk is less expensive than donor human milk and specialty formula for NICU infants. These and other health outcome data suggest that the NICU would realize a cost savings by promoting maternal human milk feeding over formula or donor human milk feedings.136 Another large clinical trial is underway to quantify the cost impact to the NICU for providing containers, volume-based refrigerator and freezer space, and additional support equipment.31
Prioritizing the Initiation, Establishment and Maintenance of Maternal Milk Volume
Prioritizing maternal milk volume is the single most important lactation-related responsibility for maternity and neonatal caregivers. An abundant milk volume ensures that the infant has access to exclusive human milk feedings and facilitates the transition to feeding at breast during and after the NICU stay, whereas maternal milk volume problems compromise these goals. Initiating, establishing and maintaining an adequate milk volume is, however, a demanding task for mothers of premature infants. These mothers are breast pump-dependent, meaning that they must rely on the breast pump to replace the sucking stimulation and milk removal functions of a healthy breastfeeding infant.137 As such, their needs are very different from those of a mother who is an occasional breast pump user, and can depend upon her infant to provide the necessary autocrine stimulus required for milk production.42
Several studies have demonstrated that breast pump-dependent women experience problems with delayed lactogenesis and inadequate milk volume,138–144 with one large study demonstrating that only 29% of mothers with extremely premature infants were able to provide exclusive human milk throughout the NICU stay.5 However, a recent randomized control trial137 comparing different breast pump suction patterns suggested that “running out of milk” is at least partially iatrogenic for mothers of VLBW infants, and that implementation of evidence-based best practices may reduce the number of women who do not produce an adequate volume of milk.42
The process of developing an adequate milk volume begins during pregnancy when the breast undergoes a number of anatomical and physiological changes in preparation for breastfeeding.145 Lactogenesis I occurs during the second trimester of pregnancy and is the phase of lactation wherein the mammary glands are sufficiently developed and differentiated to secrete a small amount of colostrum.52, 146 However, the milk secretion is suppressed throughout the remainder of pregnancy by high circulating levels of progesterone.147 After the delivery of the placenta, in the early post-birth period, circulating progesterone levels decline rapidly and, in response, lactogenesis II, the onset of copious milk secretion occurs and the mother senses the milk “coming in.”52, 147–149 Two recent studies137, 150 suggest that specific stimulatory interventions during the transition from lactogenesis I to lactogenesis II do not increase milk output during this time, but appear to have a programming effect on subsequent maternal milk volume. Whether these interventions hasten the decline in progesterone or exert some effect on the secretory mechanisms in the breast tissue is unknown.
Following the onset of lactogenesis II, milk synthesis and secretion are regulated by a combination of autocrine and endocrine processes that depend upon regular and effective milk removal via the feedback inhibitor of lactation (FIL) mechanism.151 For women who exclusively breastfeed a healthy infant, the transition from endocrine to autocrine mechanisms of control occurs seamlessly because the infant removes available milk, and the milk is replaced. Regular and effective milk removal by the infant serves to increase the mean maternal milk volume to approximately 600–625 mL/day by the end of the first week post-birth.147 The transition from lactogenesis II to a milk output that is sufficient for exclusive breastfeeding of the infant has been termed “coming to volume” by our research team.152 This short, but critical, transition is the time that most breast pump-dependent mothers experience milk volume problems that require rapid identification and resolution.42
In contrast to a term infant who regulates milk synthesis and secretion during this critical transition, breast pump-dependent mothers must undertake frequent and complete breast emptying with a breast pump. Numerous factors that are unique to these women, such as an ineffective breast pump, improperly fitting breast shields, infrequent pump use, or ending a pumping session before all of the available milk is removed, can compromise this transition. Similarly, the intense stress, fatigue, and pain in these early days can down-regulate prolactin via the dopaminergic prolactin inhibiting factor.52, 147 Best practices to prevent, diagnose, and manage milk volume problems in breast pump-dependent women have been summarized in a recent review manuscript.42 However, mothers need measurable milk volume targets and daily monitoring during the critical “coming to volume” transition. In our Rush Mothers’ Milk Club program, a BPC contacts each new mother on a daily basis during this period, either in the NICU or by telephone, and reviews with her each item in the brief checklist in Figure 3.
Figure 3.
“Coming to Volume” Checklist to be Completed Daily for Breast Pump-Dependent Mothers of NICU Infants until Daily Milk Volume is ≥ 350 mL for 5 Consecutive Days
A common problem experienced by breast pump-dependent mothers during this critical transition is that the administration of hormonal-based contraceptive methods in the early post-birth period appears to negatively affect the initiation of lactation and the “coming to volume” transition. Whereas estrogen-containing contraceptives have long been known to impact milk quality and quantity and should be avoided in the early port-birth period,153 progestin contraceptives have received limited study in the early post-birth period.154–158 However, the endocrine mechanism for lactogenesis II is the rapid decline in progesterone in the first days post-birth, and conditions that result in elevated progesterone levels, such as retained placental fragments are theca lutein cysts are known to compromise the initiation of lactation due to the continued progesterone secretion.159 After lactation has been fully established and the regulation of milk volume occurs via autocrine mechanisms, progestin-containing contraceptives are less likely to have a negative effect.127
Although the postponement of a subsequent pregnancy is an important aspect of post-birth care for mothers of premature infants,160 the selection of a contraceptive must consider the potential impact of the contraceptive on the initiation, establishment, and maintenance of maternal milk volume. Currently, the administration of progestin contraceptives such as depo medroxyprogesterone acetate in the immediate post-birth period is inconsistent with the guidelines of the United States Food and Drug Administration,161 and with recommendations of the World Health organization,153 and the American College of Obstetricians and Gynecologists.162 Since the obligation of maternal-child health care providers is to protect breastfeeding, progestins should be avoided in the early post-birth period for breast pump-dependent mothers of premature infants until research to support their use is available. However, these mothers should receive thorough contraceptive counseling and non-hormonal methods of contraception should be made available to them.
Using lactation technologies to manage human milk feeding problems
The prevention, identification and management of common human milk feeding problems in the NICU is a priority for NICU care providers and lactation specialists so that the infant can receive the highest possible dose of human milk, especially during critical exposure periods. Fortunately, many of the technologies that facilitate these processes have been thoroughly studied and by human milk and lactation scientists, and have been adapted for use in the clinical setting. These include: breast pump technology that is designed to meet the unique needs of breast pump-dependent women;42, 137, 163 the creamatocrit technique to accurately and quickly measure the lipid and caloric content in expressed human milk;102, 104, 164–167 the use of nipple shields to facilitate milk transfer during breastfeeding;168, 169 and test weights to accurately and precisely measure milk intake during breastfeeding.95–100 Use of these technologies is easy to learn and should be the standard of care in evidence-based NICU best practices for managing lactation and human milk feeding in the NICU.
Although there is a plethora of scientific literature detailing the scientific foundations and appropriate use of these technologies, they have not been universally integrated into routine NICU care. One reason is that many NICU personnel feel that it is just “too much work” to manage human milk feedings and lactation processes so scientifically. For example, many staff members want a simple visual scoring system to estimate milk intake during breastfeeding, or would prefer a single the use of a “default value” (e.g., 20 cal/ounce) for the caloric content in expressed human milk. The problem with these less objective mechanisms is that extensive research has demonstrated they are not accurate indicators of either milk intake during breastfeeding96–98 or caloric density in individual containers of mothers’ milk fed in the NICU.42, 102, 104, 165
The other primary reason that these effective technologies have not been integrated into NICU best practices is that many lactation proponents feel they are not necessary, and that the focus on “numbers” undermines mothers’ confidence. However, research with both test-weights.96, 97, 99 and creamatocrits164 has demonstrated that NICU mothers can easily learn both techniques and are reassured by knowing how much milk their infants consume and the caloric content of their milk. Another concern of lactation proponents is that these lactation technologies have been adapted from the research arena to the clinical setting by for-profit industries, so the industry’s profit motive versus the “need” for the products is questioned.170 However, nearly all other NICU products have evolved from industry and have been studied in industry-funded trials because federal dollars are more appropriately directed toward achieving the broader national health objectives for breastfeeding.171 Rather than focusing on the politics of infant feeding, lactation products should be selected based on the evidence that they are effective in ensuring that infants receive the highest dose of human milk, especially during critical exposure periods.
Use of Breast Pump Technology Designed for and Tested With Breast-Pump Dependent Mothers
Despite the fact that mothers of premature and NICU infants must remain breast pump dependent for weeks or months, very few studies have focused on the effectiveness, efficiency, comfort, and convenience of the hospital-grade electric breast pump that mothers use. In fact, many lactation proponents feel that it is unethical to recommend a specific type of breast pump, despite the fact that the literature supports the fact that certain breast pumps and breast pump features appear to be superior or more acceptable to pump-dependent mothers than are other pumps.137, 144, 150, 172, 173 In contrast, most of the research in this area has been focused on care practices which influence maternal milk volume such as skin-to-skin holding or pumping regimens (e.g., single versus double pumping). However, a breast pump is fundamental to a mother’s ability to produce milk, and it is critical that NICU mothers receive the most effective, efficient, comfortable, and effective breast pump available. Thus, NICU care givers should provide breast pump recommendations based on the scientific evidence available for the pump, which should include scientific, systematic evaluation of the pump characteristics by breast pump-dependent mothers. Mothers will need to use the pump until their infants consume all milk directly from the breast, which for most infants is when they achieve term, corrected age or slightly later.99
Use of the Creamatocrit Technology to Measure Lipid and Calories in Expressed Human Milk
The creamatocrit technique, which involves centrifuging a small specimen of human milk in a capillary tube and then calculating the percent of total milk volume equal to cream, has been the standard in the research arena since its first description for use with human milk in 1978.104, 166 The creamatocrit provides a quick, inexpensive, easy-to-perform, and accurate method of measuring the lipid and caloric content in expressed human milk102, 104, 164–167 Recently the laboratory equipment used in the research arena was adapted into a 2-pound, portable, user-friendly device (Creamatocrit Plus, Medela, Inc, McHenry, IL) that is ideal for use in the clinical setting.102, 104 Since it is well-established that the lipid and caloric content vary tremendously in individually collected milk samples104, 106, 165, 167, 174–176 and that NICU storage and feeding procedures further reduce baseline lipid and caloric content,102, 177, 178 this device should be an essential part of routine NICU care.
A complete review of best NICU practices for preventing, diagnosing, and managing slow weight gain in premature infants that are predominantly or exclusively human milk fed has been published.102 This review paper includes several NICU case studies that detail the use of the creamatocrit technique as a part of an overall plan for managing slow weight gain in the NICU setting without routine supplementation or “rescue” with commercial formulas.
Use of Nipple Shields During the Transition to Feeding at Breast for Premature Infants
Few premature infants are able to consume 100% of their feedings from the breast at the time of NICU discharge. A recent study of VLBW infants revealed that while 30.5% of VLBW infants received exclusive human milk at the time of discharge, less than 10% were feeding exclusively at the breast.101 Among the physiologic immaturities on the part of the premature infant is the fact that suction pressures, essential for creating and sustaining the nipple shape during breastfeeding, are not mature until approximately term, corrected age.100, 179 Although positioning techniques that include the mother’s hand supporting the infant’s head and scapulae can help compensate for the relative weight of the head and the immature suction pressures, many premature infants demonstrate greater milk transfer when feeding with an ultra-thin nipple shield.119, 168, 180
The modern nipple shield concentrates the infant’s suction pressure in the tunnel of the shield, stimulates the milk flow, and allows the infant to remove milk even with immature suction pressures. Although many lactation proponents think that nipple shields are unnecessary, overused, shorten duration of breastfeeding and compromise milk transfer to the premature infant, research clearly demonstrates that the nipple shield is advantageous in establishing and maintaining breastfeeding for many premature infants. The indications and correct usage of the nipple shield in preterm and late preterm infants have been summarized in a recent review paper.100
Use of Test-Weighing Technology to Measure Milk Intake During Breastfeeding
Test-weighing methods, whereby the infant is weighed pre- and post- breastfeeding under the identical conditions, have been the standard research technique for measuring milk intake during breastfeeding since the advent of electronic digital scales in the 1980s.181 In the past decade, a lightweight, portable infant scale for measuring test weights has been developed from the more cumbersome research scales of the 1980s.96 The adapted scale (BabyWeigh, Medela, Inc., McHenry IL), which features the ability to program in the “pre-feed” weight, and to calculate milk intake (1 mL = 1g) automatically after the infant is weighed post-feed, is ideal for use in the clinical setting or in the infant’s home.100, 119, 180
Numerous controlled, blinded clinical trials have demonstrated that test weighing is accurate95, 96, 181 and acceptable to mothers,96–99 and that breastfeeding effectiveness “tools” or scoring systems do not accurately estimate intake during breastfeeding.96, 97 A review paper that summarizes the indications and use of test-weights to manage the transition to exclusive feeding at the breast for preterm and late preterm infants has been published.100 This review paper features photographs and detailed clinical examples for integrating test-weights into an overall post-discharge management plan that includes nipple shield use and breast pump use for this population.
Summary
The evidence about human milk feedings for premature infants in the NICU indicates that there are critical exposure periods post-birth when exclusive or high doses of human milk provide the greatest protection from costly and handicapping morbidities in premature infants. These data should form the basis for research, practice, and quality outcome indicators in the NICU. Best practices to increase the dose and exposure period of human milk feedings in the NICU include: encouraging the mother to provide milk for her infant, providing cost-effective, expert lactation and human milk feeding support for families and staff; prioritizing the initiation, establishment and maintenance of maternal milk volume, and using lactation technologies to manage human milk feeding problems.
Clinical Application for Colostrum Feeding in the NICU.
Colostrum should be the first feeding received by the infant.
Colostrum may be used for trophic feedings and can also be administered safely via the oropharyngeal route with and/or prior to trophic feedings.
Colostrum should be fed in the order that it is produced, even if it has been previously frozen.
After the first 3–4 days of exclusive colostrum feedings, colostrum can be alternated with fresh mature milk (to protect infant from microorganisms in the NICU via the enteromammary pathway).
Colostrum should be stored in small, sterile, food-grade containers that are easily identifiable in the refrigerator/freezer by the nurse.
Colostrum containers should be numbered in the order they were collected, in a manner easily identifiable by the nurse.
Small expressed drops of colostrum can be diluted with 1–2 mL of sterile water to remove the drops from the pump collection kit and/or to achieve a desired feed volume. Dilution is not necessary for any other reason.
Colostrum should not be mixed with fortifier or commercial formula.
Removal of colostrum from the breast may be most effective with a combination of pumping and hand expression.
Formula should be avoided during the introduction and advancement of colostrum feedings, because formula may exert a separate detrimental effect on gastrointestinal integrity at this critical time.
Acknowledgement
Supported by NIH Grant NR010009
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lucas A, Cole TJ. Breast milk and neonatal necrotising enterocolitis. Lancet. 1990;336(8730 1990 Dec 22–29):1519–1523. doi: 10.1016/0140-6736(90)93304-8. [DOI] [PubMed] [Google Scholar]
- 2.El-Mohandes A, Picard M, Simmens S. Human milk utilization in the ICN decreases the incidence of bacterial sepsis [abstract] Pediatr Res. 1995;37:306A. [Google Scholar]
- 3.Hylander MA, Strobino DM, Dhanireddy R. Human milk feedings and infection among very low birth weight infants. Pediatrics. 1998;102(3):E38. doi: 10.1542/peds.102.3.e38. [DOI] [PubMed] [Google Scholar]
- 4.Hylander MA, Strobino DM, Pezzullo JC, Dhanireddy R. Association of human milk feedings with a reduction in retinopathy of prematurity among very low birthweight infants. Journal of Perinatology. 2001;21(6):356–362. doi: 10.1038/sj.jp.7210548. [DOI] [PubMed] [Google Scholar]
- 5.Schanler RJ, Lau C, Hurst NM, Smith EOB. Randomized trial of donor human milk versus preterm formula as substitutes for mothers' own milk in the feeding of extremely premature infants. Pediatrics. 2005;116(2):400–406. doi: 10.1542/peds.2004-1974. [DOI] [PubMed] [Google Scholar]
- 6.Schanler RJ, Shulman RJ, Lau C. Feeding strategies for premature infants: Beneficial outcomes of feeding fortified human milk versus preterm formula. Pediatrics. 1999;103(6 Pt 1):1150–1157. doi: 10.1542/peds.103.6.1150. [DOI] [PubMed] [Google Scholar]
- 7.Furman L, Taylor G, Minich N, Hack M. The effect of maternal milk on neonatal morbidity of very low-birth-weight infants. Arch Pediatr Adolesc Med. 2003;157(1):66–71. doi: 10.1001/archpedi.157.1.66. [DOI] [PubMed] [Google Scholar]
- 8.Furman L, Wilson-Costello D, Friedman H, Taylor HG, Minich N, Hack M. The effect of neonatal maternal milk feeding on the neurodevelopmental outcome of very low birth weight infants. J Dev Behav Pediatr. 2004;25(4):247–253. doi: 10.1097/00004703-200408000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Davidson B, Meinzen-Derr JK, Wagner CL, Newburg DS, Morrow AL. Fucosylated oligosaccharides in human milk in relation to gestational age and stage of lactation. Adv Exp Med Biol. 2004;554:427–430. doi: 10.1007/978-1-4757-4242-8_56. [DOI] [PubMed] [Google Scholar]
- 10.Hintz SR, Kendrick DE, Stoll BJ, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005;115(3):696–703. doi: 10.1542/peds.2004-0569. [DOI] [PubMed] [Google Scholar]
- 11.Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292(19):2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 12.Hack M. Young adult outcomes of very-low-birth-weight children. Semin Fetal Neonatal Med. 2006;11(2):127–137. doi: 10.1016/j.siny.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Hack M, Taylor HG, Drotar D, et al. Chronic conditions, functional limitations, and special health care needs of school-aged children born with extremely low-birth-weight in the 1990s. JAMA. 2005;294(3):318–325. doi: 10.1001/jama.294.3.318. [DOI] [PubMed] [Google Scholar]
- 14.Marlow N, Wolke D, Bracewell MA, Samara M, Group EPS. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352(1):9–19. doi: 10.1056/NEJMoa041367. [see comment] [DOI] [PubMed] [Google Scholar]
- 15.Payne NR, Carpenter JH, Badger GJ, Horbar JD, Rogowski J. Marginal increase in cost and excess length of stay associated with nosocomial bloodstream infections in surviving very low birth weight infants. Pediatrics. 2004;114(2):348–355. doi: 10.1542/peds.114.2.348. [DOI] [PubMed] [Google Scholar]
- 16.Perlman JM. Neurobehavioral deficits in premature graduates of intensive care--potential medical and neonatal environmental risk factors. Pediatrics. 2001;108(6):1339–1348. doi: 10.1542/peds.108.6.1339. [DOI] [PubMed] [Google Scholar]
- 17.Salhab WA, Perlman JM, Silver L, Sue Broyles R. Necrotizing enterocolitis and neurodevelopmental outcome in extremely low birth weight infants <1000 g. Journal of Perinatology. 2004;24(9):534–540. doi: 10.1038/sj.jp.7211165. [DOI] [PubMed] [Google Scholar]
- 18.Petrou S, Sach T, Davidson L. The long-term costs of preterm birth and low birth weight: Results of a systematic review. Child Care Health Dev. 2001;27(2):97–115. doi: 10.1046/j.1365-2214.2001.00203.x. [DOI] [PubMed] [Google Scholar]
- 19.Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117(4):1253–1261. doi: 10.1542/peds.2005-1368. [DOI] [PubMed] [Google Scholar]
- 20.Ehrenkranz RA, Younes N, Lemons JA, et al. Longitudinal growth of hospitalized very low birth weight infants. Pediatrics. 1999;104(2):280–289. doi: 10.1542/peds.104.2.280. [DOI] [PubMed] [Google Scholar]
- 21.Drotar D, Hack M, Taylor G, Schluchter M, Andreias L, Klein N. The impact of extremely low birth weight on the families of school-aged children. Pediatrics. 2006;117(6):2006–2013. doi: 10.1542/peds.2005-2118. [DOI] [PubMed] [Google Scholar]
- 22.Uraizee F, Gross S. Improved feeding tolerance and reduced incidence of sepsis in sick very low birthweight (VLBW) infants fed maternal milk [abstract] Pediatr Res. 1989;25:298A. [Google Scholar]
- 23.Simmer K, Metcalf R, Daniels L. The use of breastmilk in a neonatal unit and its relationship to protein and energy intake and growth. Journal of Paediatrics & Child Health. 1997;33(1):55–60. doi: 10.1111/j.1440-1754.1997.tb00992.x. [DOI] [PubMed] [Google Scholar]
- 24.Meinzen-Derr J, Poindexter B, Wrage L, Morrow AL, Stoll B, Donovan EF. Role of human milk in extremely low birth weight infants' risk of necrotizing enterocolitis or death. J Perinatol. 2009;29(1):57–62. doi: 10.1038/jp.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vohr BR, Poindexter BB, Dusick AM, et al. Persistent beneficial effects of breast milk ingested in the neonatal intensive care unit on outcomes of extremely low birth weight infants at 30 months of age. Pediatrics. 2007;120(4):e953–e959. doi: 10.1542/peds.2006-3227. [DOI] [PubMed] [Google Scholar]
- 26.Vohr BR, Poindexter BB, Dusick AM, et al. Beneficial effects of breast milk in the neonatal intensive care unit on the developmental outcome of extremely low birth weight infants at 18 months of age. Pediatrics. 2006;118(1):e115–e123. doi: 10.1542/peds.2005-2382. [DOI] [PubMed] [Google Scholar]
- 27.Sisk PM, Lovelady CA, Dillard RG, Gruber KJ, O'Shea TM. Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. [Accessed July 11, 2007];J Perinatol. 2007 27:428–433. doi: 10.1038/sj.jp.7211758. [DOI] [PubMed] [Google Scholar]
- 28.Sisk PM, Lovelady CA, Gruber KJ, Dillard RG, O’Shea TM. HM consumption and full enteral feeding among infants who weigh </= 1250 grams. Pediatrics. 2008;121(6):e1528–e1533. doi: 10.1542/peds.2007-2110. [DOI] [PubMed] [Google Scholar]
- 29.Taylor SN, Basile LA, Ebeling M, Wagner CL. Intestinal permeability in preterm infants by feeding type: Mother's milk versus formula. Breastfeed Med. 2009;4(1):11–15. doi: 10.1089/bfm.2008.0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel AL, Meier PP, Engstrom JL. The evidence for use of human milk in very low-birthweight preterm infants. [Accessed 4/15/2009];NeoReviews. 2007 8(11):e459. [Google Scholar]
- 31.Meier PP. Health benefits and cost of human milk for very low birthweight infants. 2007;1 R01-NR010009-01. [Google Scholar]
- 32.Miller J, McVeagh P. Human milk oligosaccharides: 130 reasons to breast-feed. Br J Nutr. 1999;82(55):333–335. doi: 10.1017/s0007114599001567. [DOI] [PubMed] [Google Scholar]
- 33.Cregan MD, Fan Y, Appelbee A, et al. Identification of nestin-positive putative mammary stem cells in human breastmilk. Cell Tissue Res. 2007;329(1):129–136. doi: 10.1007/s00441-007-0390-x. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez NA, Miracle DJ, Meier PP. Sharing the science on human milk feedings with mothers of very-low-birth-weight infants. J Obstet Gynecol Neonatal Nurs. 2005;34(1):109–119. doi: 10.1177/0884217504272807. [DOI] [PubMed] [Google Scholar]
- 35.Diaz-Gomez NM, Domenech E, Barroso F. Breast-feeding and growth factors in preterm newborn infants. Journal of Pediatric Gastroenterology & Nutrition. 1997;24(3):322–327. doi: 10.1097/00005176-199703000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Dvorak B, Fituch CC, Williams CS, Hurst NM, Schanler RJ. Increased epidermal growth factor levels in human milk of mothers with extremely premature infants. Pediatr Res. 2003;54(1):15–19. doi: 10.1203/01.PDR.0000065729.74325.71. [DOI] [PubMed] [Google Scholar]
- 37.Dvorak B, Fituch CC, Williams CS, Hurst NM, Schanler RJ. Concentrations of epidermal growth factor and transforming growth factor-alpha in preterm milk. Adv Exp Med Biol. 2004;554:407–409. doi: 10.1007/978-1-4757-4242-8_52. [DOI] [PubMed] [Google Scholar]
- 38.Goldman AS, Chheda S, Keeney SE, Schmalstieg FC, Schanler RJ. Immunologic protection of the premature newborn by human milk. Semin Perinatol. 1994;18(6):495–501. [PubMed] [Google Scholar]
- 39.Montagne P, Cuilliere ML, Mole C, Bene MC, Faure G. Immunological and nutritional composition of human milk in relation to prematurity and mother's parity during the first 2 weeks of lactation. J Pediatr Gastroenterol Nutr. 1999;29(1):75–80. doi: 10.1097/00005176-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 40.Ronayne de Ferrer PA, Baroni A, Sambucetti ME, Lopez NE, Ceriani Cernadas JM. Lactoferrin levels in term and preterm milk. J Am Coll Nutr. 2000;19(3):370–373. doi: 10.1080/07315724.2000.10718933. [DOI] [PubMed] [Google Scholar]
- 41.Shoji H, Shimizu T, Shinohara K, Oguchi S, Shiga S, Yamashiro Y. Suppressive effects of breast milk on oxidative DNA damage in very low birthweight infants. Archives of Disease in Childhood Fetal & Neonatal Edition. 2004;89(2):F136–F138. doi: 10.1136/adc.2002.018390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meier PP, Engstrom JL. Evidence-based practices to promote exclusive feeding of human milk in very low-birthweight infants. [Accessed 4/15/2009];NeoReviews. 2007 8(11):e467. [Google Scholar]
- 43.Claud EC, Walker WA. Bacterial colonization, probiotics, and necrotizing enterocolitis. J Clin Gastroenterol. 2008;42 Suppl 2:S46–S52. doi: 10.1097/MCG.0b013e31815a57a8. [DOI] [PubMed] [Google Scholar]
- 44.Labbok M, Krasovec K. Toward consistency in breastfeeding definitions. Stud Fam Plann. 1990;21(4):226–230. [PubMed] [Google Scholar]
- 45.Meier PP, Brown LP. Limitations of the labbok and krassovec breastfeeding classification for preterm infants (letter) J Nurs Midwif. 1997;42:1259–1260. [Google Scholar]
- 46.Meinzen-Derr J, Poindexter BB, Donovan EF, et al. Human milk and late-onset sepsis in infants 401–1000 grams: A secondary analysis; International Society for Research in Human Milk and Lactation: 12th International Conference; 2004. p. 44. [Google Scholar]
- 47.Patel AL, Engstrom JL, Goldman J, Fogg L, Meier P. Dose response benefits of human milk in extremely low birth weight premature infants. Pediatric Academic Societies. 2008 E-PAS2008:3777.1. [Google Scholar]
- 48.Petrou S, Mehta Z, Hockley C, Cook-Mozaffari P, Henderson J, Goldacre M. The impact of preterm birth on hospital inpatient admissions and costs during the first 5 years of life. Pediatrics. 2003;112(6 Pt 1):1290–1297. doi: 10.1542/peds.112.6.1290. [DOI] [PubMed] [Google Scholar]
- 49.Bisquera JA, Cooper TR, Berseth CL. Impact of necrotizing enterocolitis on length of stay and hospital charges in very low birth weight infants. Pediatrics. 2002;109(3):423–428. doi: 10.1542/peds.109.3.423. [DOI] [PubMed] [Google Scholar]
- 50.Weimer J. The economic benefits of breastfeeding: A review and analysis. USDA. 2001;13:1–14. [Google Scholar]
- 51.Neville MC. Anatomy and physiology of lactation. Pediatr Clin North Am. 2001;48(1):13–34. doi: 10.1016/s0031-3955(05)70283-2. [DOI] [PubMed] [Google Scholar]
- 52.Neville MC, Morton J, Umemura SLactogenesis. the transition from pregnancy to lactation. Pediatr Clin North Am. 2001;48(1):35–52. doi: 10.1016/s0031-3955(05)70284-4. [DOI] [PubMed] [Google Scholar]
- 53.Sangild PT, Siggers RH, Schmidt M, et al. Diet- and colonization-dependent intestinal dysfunction predisposes to necrotizing enterocolitis in preterm pigs. Gastroenterology. 2006;130(6):1776–1792. doi: 10.1053/j.gastro.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 54.Sangild PT, Schmidt M, Elnif J, Bjornvad CR, Westrom BR, Buddington RK. Prenatal development of gastrointestinal function in the pig and the effects of fetal esophageal obstruction. Pediatr Res. 2002;52(3):416–424. doi: 10.1203/00006450-200209000-00019. [DOI] [PubMed] [Google Scholar]
- 55.Sangild PT, Mei J, Fowden AL, Xu RJ. The prenatal porcine intestine has low transforming growth factor-beta ligand and receptor density and shows reduced trophic response to enteral diets. Am J Physiol Regul Integr Comp Physiol. 2009;296(4):R1053–R1062. doi: 10.1152/ajpregu.90790.2008. [DOI] [PubMed] [Google Scholar]
- 56.Sangild PT. Gut responses to enteral nutrition in preterm infants and animals. Exp Biol Med (Maywood) 2006;231(11):1695–1711. doi: 10.1177/153537020623101106. [DOI] [PubMed] [Google Scholar]
- 57.Underwood MA, Gilbert WM, Sherman MP. Amniotic fluid: Not just fetal urine anymore. J Perinatol. 2005;25(5):341–348. doi: 10.1038/sj.jp.7211290. [DOI] [PubMed] [Google Scholar]
- 58.Jensen AR, Elnif J, Burrin DG, Sangild PT. Development of intestinal immunoglobulin absorption and enzyme activities in neonatal pigs is diet dependent. J Nutr. 2001;131(12):3259–3265. doi: 10.1093/jn/131.12.3259. [DOI] [PubMed] [Google Scholar]
- 59.Mei J, Zhang Y, Wang T, Sangild PT, Xu RJ. Oral ingestion of colostrum alters intestinal transforming growth factor-beta receptor intensity in newborn pigs. Livestock Science. 2006;105:214–222. [Google Scholar]
- 60.Thymann T, Burrin DG, Tappenden KA, Bjornvad CR, Jensen SK, Sangild PT. Formula-feeding reduces lactose digestive capacity in neonatal pigs. Br J Nutr. 2006;95(6):1075–1081. doi: 10.1079/bjn20061743. [DOI] [PubMed] [Google Scholar]
- 61.Newburg DS, Walker WA. Protection of the neonate by the innate immune system of developing gut and of human milk. Pediatr Res. 2007;61(1):2–8. doi: 10.1203/01.pdr.0000250274.68571.18. [DOI] [PubMed] [Google Scholar]
- 62.Vidal K, Donnet-Hughes A. CD14: A soluble pattern recognition receptor in milk. In: Bosze Z, editor. Bioactive Components of Milk. New York: Springer; 2008. pp. 195–216. [DOI] [PubMed] [Google Scholar]
- 63.Labeta MO, Vidal K, Nores JE, et al. Innate recognition of bacteria in human milk is mediated by a milk-derived highly expressed pattern recognition receptor, soluble CD14. J Exp Med. 2000;191(10):1807–1812. doi: 10.1084/jem.191.10.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vidal K, Donnet-Hughes A. CD14: A soluble pattern recognition receptor in milk. Adv Exp Med Biol. 2008;606:195–216. doi: 10.1007/978-0-387-74087-4_7. [DOI] [PubMed] [Google Scholar]
- 65.Rodriguez NA, Meier PP, Groer MW, Zeller JM. Oropharyngeal administration of colostrum to extremely low birth weight infants: Theoretical perspectives. [Accessed 24 November 2009];Journal of Perinatology. 2009 29(1):1–7. doi: 10.1038/jp.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rodriguez NA, Meier PP, Groer MW, Zeller JM, Engstrom JL, Fogg L. A pilot study of the oropharyngeal administration of own mother’s colostrum to extremely low birth weight infants. Advances in Neonatal Care. 2010 doi: 10.1097/ANC.0b013e3181e94133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Meinzen-Derr J, Poindexter B, Wrage L, Morrow AL, Stoll B, Donovan EF. Role of human milk in extremely low birth weight infants' risk of necrotizing enterocolitis or death. J Perinatol. 2009;29(1):57–62. doi: 10.1038/jp.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patel AL, Engstrom JL, Meier PP, Kimura RE. Effect of human milk feedings on growth velocity and major morbidity in extremely low birth weight infants in the neonatal intensive care unit. Chicago, IL: 2006. [Google Scholar]
- 69.Chaud EC, Walker WA. Hypothesis: Inappropriate colonization of the premature intestine can cause necrotizing enterocolitis. FASEB Journal. 2001;15:1398–1403. doi: 10.1096/fj.00-0833hyp. [DOI] [PubMed] [Google Scholar]
- 70.Cotten CM, Taylor S, Stoll B, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123(1):58–66. doi: 10.1542/peds.2007-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Magne F, Suau A, Pochart P, Desjeux JF. Fecal microbial community in preterm infants. Journal of Pediatric Gastroenterology & Nutrition. 2005;41(4):386–392. doi: 10.1097/01.mpg.0000179855.38543.85. [DOI] [PubMed] [Google Scholar]
- 72.Martin R, Langa S, Reviriego C, et al. Human milk is a source of lactic acid bacteria for the infant gut. J Pediatr. 2003;143(6):754–758. doi: 10.1016/j.jpeds.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 73.Perez PF, Dore J, Leclerc M, et al. Bacterial imprinting of the neonatal immune system: Lessons from maternal cells? Pediatrics. 2007;119(3):e724–e732. doi: 10.1542/peds.2006-1649. [DOI] [PubMed] [Google Scholar]
- 74.Mackie RI, Sghir A, Gaskins HR. Developmental microbial ecology of the neonatal gastrointestinal tract. Am J Clin Nutr. 1999;69(5):1035S–1045S. doi: 10.1093/ajcn/69.5.1035s. [DOI] [PubMed] [Google Scholar]
- 75.Penders J, Thijs C, Vink C, et al. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118(2):511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 76.Harmsen HJ, Wildeboer-Veloo AC, Raangs GC, et al. Analysis of intestinal flora development in breast-fed and formula-fed infants by using molecular identification and detection methods. Journal of Pediatric Gastroenterology & Nutrition. 2000;30(1):61–67. doi: 10.1097/00005176-200001000-00019. [DOI] [PubMed] [Google Scholar]
- 77.Rautava S, Walker WA. Commensal bacteria and epithelial cross talk in the developing intestine. Curr Gastroenterol Rep. 2007;9(5):385–392. doi: 10.1007/s11894-007-0047-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Minekawa R, Takeda T, Sakata M, et al. Human breast milk suppresses the transcriptional regulation of IL-1beta-induced NF-kappaB signaling in human intestinal cells. American Journal of Physiology Cell Physiology. 2004;287(5):11. doi: 10.1152/ajpcell.00471.2003. [DOI] [PubMed] [Google Scholar]
- 79.Caicedo RA, Schanler RJ, Li N, Neu J. The developing intestinal ecosystem: Implications for the neonate. Pediatr Res. 2005;58(4):625–628. doi: 10.1203/01.PDR.0000180533.09295.84. [DOI] [PubMed] [Google Scholar]
- 80.Claud EC, Savidge T, Walker WA. Modulation of human intestinal epithelial cell IL-8 secretion by human milk factors. Pediatr Res. 2003;53(3):419–425. doi: 10.1203/01.PDR.0000050141.73528.AD. [DOI] [PubMed] [Google Scholar]
- 81.Schultz C, Temming P, Bucsky P, Gopel W, Strunk T, Hartel C. Immature anti-inflammatory response in neonates. Clinical & Experimental Immunology. 2004;135(1):130–136. doi: 10.1111/j.1365-2249.2004.02313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Poindexter BB, Ehrenkranz RA, Stoll BJ, et al. Parenteral glutamine supplementation does not reduce the risk of mortality or late-onset sepsis in extremely low birth weight infants. Pediatrics. 2004;113(5):1209–1215. doi: 10.1542/peds.113.5.1209. [DOI] [PubMed] [Google Scholar]
- 83.Hack M, Flannery DJ, Schluchter M, Cartar L, Borawski E, Klein N. Outcomes in young adulthood for very-low-birth-weight infants. N Engl J Med. 2002;346(3):149–157. doi: 10.1056/NEJMoa010856. [DOI] [PubMed] [Google Scholar]
- 84.Fanaroff AA, Korones SB, Wright LL, et al. Incidence, presenting features, risk factors and significance of late onset septicemia in very low birth weight infants. the national institute of child health and human development neonatal research network. Pediatr Infect Dis J. 1998;17(7):593–598. doi: 10.1097/00006454-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 85.Brandtzaeg P. The secretory immunoglobulin system: Regulation and biological significance: Focusing on human mammary glands. In: Davis MK, Isaacs CE, Hanson LA, Wright AL, editors. Integrating Population Outcomes, Biological Mechanisms and Research Methods in the Study of Human Milk and Lactation. New York: Plenum Press; 2002. pp. 1–16. [DOI] [PubMed] [Google Scholar]
- 86.Andersson B, Porras O, Hanson LA, Lagergard T, Svanborg-Eden C. Inhibition of attachment of streptococcus pneumoniae and haemophilus influenzae by human milk and receptor oligosaccharides. J Infect Dis. 1986;153(2):232–237. doi: 10.1093/infdis/153.2.232. [DOI] [PubMed] [Google Scholar]
- 87.Nakhla T, Fu D, Zopf D, Brodsky NL, Hurt H. Neutral oligosaccharide content of preterm human milk. Br J Nutr. 1999;82(5):361–367. doi: 10.1017/s0007114599001609. [DOI] [PubMed] [Google Scholar]
- 88.Siafakas CG, Anatolitou F, Fusunyan RD, Walker WA, Sanderson IR. Vascular endothelial growth factor (VEGF) is present in human breast milk and its receptor is present on intestinal epithelial cells. Pediatr Res. 1999;45(5 Pt 1):652–657. doi: 10.1203/00006450-199905010-00007. [DOI] [PubMed] [Google Scholar]
- 89.Resto M, O'Connor D, Leef K, Funanage V, Spear M, Locke R. Leptin levels in preterm human breast milk and infant formula. Pediatrics. 2001;108(1):E15. doi: 10.1542/peds.108.1.e15. [DOI] [PubMed] [Google Scholar]
- 90.Ip S, Chung M, Raman G, et al. Breastfeeding and maternal and infant health outcomes in developed countries. Agency for Health Care Research and Quality; 2007. Contract No. 290-02-0022. [PMC free article] [PubMed] [Google Scholar]
- 91.Schanler RJ. Post-discharge nutrition for the preterm infant. Acta Paediatr Suppl. 2005;94(449):68–73. doi: 10.1111/j.1651-2227.2005.tb02158.x. [DOI] [PubMed] [Google Scholar]
- 92.O'Connor DL, Jacobs J, Hall R, et al. Growth and development of premature infants fed predominantly human milk, predominantly premature infant formula, or a combination of human milk and premature formula. Journal of Pediatric Gastroenterology & Nutrition. 2003;37(4):437–446. doi: 10.1097/00005176-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 93.O'Connor DL, Khan S, Weishuhn K, et al. Growth and nutrient intakes of human milk-fed preterm infants provided with extra energy and nutrients after hospital discharge. Pediatrics. 2008;121(4):766–776. doi: 10.1542/peds.2007-0054. [DOI] [PubMed] [Google Scholar]
- 94.Griffin IJ. Postdischarge nutrition for high risk neonates. Clin Perinatol. 2002;29(2):327–344. doi: 10.1016/s0095-5108(02)00004-0. [DOI] [PubMed] [Google Scholar]
- 95.Meier PP, Lysakowski TY, Engstrom JL, Kavanaugh KL, Mangurten HH. The accuracy of test weighing for preterm infants. J Pediatr Gastroenterol Nutr. 1990;10(1):62–65. doi: 10.1097/00005176-199001000-00012. [DOI] [PubMed] [Google Scholar]
- 96.Meier PP, Engstrom JL, Crichton CL, Clark DR, Williams MM, Mangurten HH. A new scale for in-home test-weighing for mothers of preterm and high risk infants. Journal of Human Lactation. 1994;10(3):163–168. doi: 10.1177/089033449401000312. [see comment] [DOI] [PubMed] [Google Scholar]
- 97.Meier PP, Engstrom JL, Fleming BA, Streeter PL, Lawrence PB. Estimating milk intake of hospitalized preterm infants who breastfeed. J Hum Lact. 1996;12(1):21–26. doi: 10.1177/089033449601200106. [DOI] [PubMed] [Google Scholar]
- 98.Meier PP, Engstrom JL. Test weighing for term and premature infants is an accurate procedure. Arch Dis Child Fetal Neonatal Ed. 2007;92(2):F155–F156. doi: 10.1136/adc.2006.113480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hurst NM, Meier PP, Engstrom JL, Myatt A. Mothers performing in-home measurement of milk intake during breastfeeding of their preterm infants: Maternal reactions and feeding outcomes. J Hum Lact. 2004;20(2):178–187. doi: 10.1177/0890334404264168. [DOI] [PubMed] [Google Scholar]
- 100.Meier PP, Furman LM, Degenhardt M. Increased lactation risk for late preterm infants and mothers: Evidence and management strategies to protect breastfeeding. J Midwifery Womens Health. 2007;52(6):579–587. doi: 10.1016/j.jmwh.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 101.Davanzo R, Ronfani L, Brovedani P, Demarini S. Breastfeeding in Neonatal Intensive Care Unit Study Group. Breast feeding very-low-birthweight infants at discharge: A multicentre study using WHO definitions. Paediatr Perinat Epidemiol. 2009;23(6):591–596. doi: 10.1111/j.1365-3016.2009.01068.x. [DOI] [PubMed] [Google Scholar]
- 102.Meier PP, Engstrom JL. Preventing, diagnosing and managing slow weight gain in the human milk-fed very low birthweight infant. sulla nutrizione con latte materno. 2008 [Google Scholar]
- 103.Meier PP, Engstrom JL, Zuleger JL, et al. The creamatocrit plus: A new centrifuge for measuring creamatocrits with mother's milk. Cambridge, UK: 2004. [Google Scholar]
- 104.Meier PP, Engstrom JL, Zuleger JL, et al. Accuracy of a user-friendly centrifuge for measuring creamatocrits on mothers' milk in the clinical setting. Breastfeeding Medicine. 2006;1(2):79–87. doi: 10.1089/bfm.2006.1.79. [DOI] [PubMed] [Google Scholar]
- 105.Kent JC, Mitoulas LR, Cregan MD, Ramsay DT, Doherty DA, Hartmann PE. Volume and frequency of breastfeedings and fat content of breast milk throughout the day. Pediatrics. 2006;117(3):e387–e395. doi: 10.1542/peds.2005-1417. [DOI] [PubMed] [Google Scholar]
- 106.Spencer SA, Hull D. Fat content of expressed breast milk: A case for quality control. Br Med J (Clin Res Ed) 1981;282(6258):99–100. doi: 10.1136/bmj.282.6258.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tyson J, Burchfield J, Sentance F, Mize C, Uauy R, Eastburn J. Adaptation of feeding to a low fat yield in breast milk. Pediatrics. 1992;89(2):215–220. [PubMed] [Google Scholar]
- 108.World Health Organization. [Accessed November 28, 2009];Breastfeeding. Available at: http://www.who.int/topics/breastfeeding/en/.
- 109.United States Breastfeeding Committee. [Accessed November 28, 2009];USBC: A brief history. Available at: http://www.usbreastfeeding.org/AboutUs/History/tabid/62/Default.aspx. [Google Scholar]
- 110.AAP. Breastfeeding and the use of human milk. Pediatrics. 2005;115(2):496–506. doi: 10.1542/peds.2004-2491. [DOI] [PubMed] [Google Scholar]
- 111.Miracle DJ, Meier PP, Bennett PA. Mothers' decisions to change from formula to mothers' milk for very-low-birth-weight infants. J Obstet Gynecol Neonatal Nurs. 2004;33(6):692–703. doi: 10.1177/0884217504270665. [DOI] [PubMed] [Google Scholar]
- 112.Miracle DJ, Fredland V. Provider encouragement of breastfeeding: Efficacy and ethics. J Midwifery Womens Health. 2007;52(6):545–548. doi: 10.1016/j.jmwh.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 113.Lu MC, Lange L, Slusser W, Hamilton J, Halfon N. Provider encouragement of breast-feeding: Evidence from a national survey. Obstet Gynecol. 2001;97(2):290–295. doi: 10.1016/s0029-7844(00)01116-9. [DOI] [PubMed] [Google Scholar]
- 114.Pate B. A systematic review of the effectiveness of breastfeeding intervention delivery methods. J Obstet Gynecol Neonatal Nurs. 2009;38(6):642–653. doi: 10.1111/j.1552-6909.2009.01068.x. [DOI] [PubMed] [Google Scholar]
- 115.Sisk PM, Lovelady CA, Dillard RG, Gruber KJ. Lactation counseling for mothers of very low birth weight infants: Effect on maternal anxiety and infant intake of human milk. Pediatrics. 2006;117(1):e67–e75. doi: 10.1542/peds.2005-0267. [DOI] [PubMed] [Google Scholar]
- 116.Friedman S, Flidel-Rimon O, Lavie E, Shinwell ES. The effect of prenatal consultation with a neonatologist on human milk feeding in preterm infants. Acta Paediatr. 2004;93(6):775–778. doi: 10.1111/j.1651-2227.2004.tb03017.x. [DOI] [PubMed] [Google Scholar]
- 117.Rush Mothers' Milk Club. [Accessed November 28, 2009];Rush mothers' milk club. Available at: http://www.rushmothersmilkclub.com. [Google Scholar]
- 118.Meier PP, Engstrom JL, Mingolelli SS, Miracle DJ, Kiesling S. The rush mothers' milk club: Breastfeeding interventions for mothers with very-low-birth-weight infants. J Obstet Gynecol Neonatal Nurs. 2004;33(2):164–174. doi: 10.1177/0884217504263280. [DOI] [PubMed] [Google Scholar]
- 119.Meier PP. Supporting lactation in mothers with very low birth weight infants. Pediatr Ann. 2003;32(5):317–325. doi: 10.3928/0090-4481-20030501-08. [DOI] [PubMed] [Google Scholar]
- 120.Freed GL, Clark SJ, Cefalo RC, Sorenson JR. Breast-feeding education of obstetrics-gynecology residents and practitioners. Am J Obstet Gynecol. 1995;173(5):1607–1613. doi: 10.1016/0002-9378(95)90656-8. [DOI] [PubMed] [Google Scholar]
- 121.Hellings P, Howe C. Breastfeeding knowledge and practice of pediatric nurse practitioners. J Pediatr Health Care. 2004;18(1):8–14. doi: 10.1016/s0891-5245(03)00108-1. [DOI] [PubMed] [Google Scholar]
- 122.Register N, Eren M, Lowdermilk D, Hammond R, Tully MR. Knowledge and attitudes of pediatric office nursing staff about breastfeeding. J Hum Lact. 2000;16(3):210–215. doi: 10.1177/089033440001600305. [DOI] [PubMed] [Google Scholar]
- 123.Spear HJ. Baccalaureate nursing students' breastfeeding knowledge: A descriptive survey. Nurse Educ Today. 2006;26(4):332–337. doi: 10.1016/j.nedt.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 124.Bernaix LW, Schmidt CA, Arrizola M, Iovinelli D, Medina-Poelinez C. Success of a lactation education program on NICU nurses' knowledge and attitudes. J Obstet Gynecol Neonatal Nurs. 2008;37(4):436–445. doi: 10.1111/j.1552-6909.2008.00261.x. [DOI] [PubMed] [Google Scholar]
- 125.Merewood A, Philipp BL, Chawla N, Cimo S. The baby-friendly hospital initiative increases breastfeeding rates in a US neonatal intensive care unit. J Hum Lact. 2003;19(2):166–171. doi: 10.1177/0890334403252475. [DOI] [PubMed] [Google Scholar]
- 126.United States Breastfeeding Committee. [Accessed November 28, 2009];Core competencies in breastfeeding care for all health professionals. Available at: http://www.usbreastfeeding.org/LinkClick.aspx?link=Publications%2fCore-Competencies-2009-USBC.pdf&tabid=70&mid=388.
- 127.Hale TW. Medications and Mother's Milk. 13th ed. Amarillo, TX: Hale Publishing; 2008. [Google Scholar]
- 128.Rossman B. Breastfeeding peer counselors in the united states: Helping to build a culture and tradition of breastfeeding. J Midwifery Womens Health. 2007;52(6):631–637. doi: 10.1016/j.jmwh.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 129.Merewood A, Chamberlain LB, Cook JT, Philipp BL, Malone K, Bauchner H. The effect of peer counselors on breastfeeding rates in the neonatal intensive care unit: Results of a randomized controlled trial. Arch Pediatr Adolesc Med. 2006;160(7):681–685. doi: 10.1001/archpedi.160.7.681. [DOI] [PubMed] [Google Scholar]
- 130.Meier PP. Breastfeeding peer counselors in the NICU: Increasing access to care for very low birthweight infants. IL: 2005. (190,000) [Google Scholar]
- 131.Martin JA, Hamilton BE, Sutton PD, et al. Births: Final data for 2006. National Vital Statistics Reports. 2009;57(7):1–102. [PubMed] [Google Scholar]
- 132.Li R, Fridinger F, Grummer-Strawn L. Racial/ethnic disparities in public opinion about breastfeeding: The 1999--2000 healthstyles surveys in the united states. Advances in Experimental Medicine & Biology. 2004;554:287–291. doi: 10.1007/978-1-4757-4242-8_24. [DOI] [PubMed] [Google Scholar]
- 133.Li R, Darling N, Maurice E, Barker L, Grummer-Strawn LM. Breastfeeding rates in the united states by characteristics of the child, mother, or family: The 2002 national immunization survey. Pediatrics. 2005;115(1):e31–e37. doi: 10.1542/peds.2004-0481. [DOI] [PubMed] [Google Scholar]
- 134.Cricco-Lizza R. Black non-hispanic mothers’ perceptions about the promotion of infant-feeding methods by nurses and physicians. J Obstet Gynecol Neonatal Nurs. 2006;35(2):173–180. doi: 10.1111/j.1552-6909.2006.00033.x. [DOI] [PubMed] [Google Scholar]
- 135.Rossman B. Breastfeeding peer counselors in the neonatal intensive care unit: Maternal perspectives [Doctoral] Chicago, IL: University of Illinois-Chicago; 2009. [Google Scholar]
- 136.Jegier BJ, Meier PP, Engstrom JL, McBride TM. The initial maternal cost of providing 100 mL of human milk for very low birth weight infants in the neonatal intensive care unit. Breastfeeding Medicine. doi: 10.1089/bfm.2009.0063. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Meier PP, Engstrom JL, Hurst NM, et al. A comparison of the efficiency, efficacy, comfort, and convenience of two hospital-grade electric breast pumps for mothers of very low birthweight infants. Breastfeed Med. 2008;3(3):141–150. doi: 10.1089/bfm.2007.0021. [DOI] [PubMed] [Google Scholar]
- 138.Cregan MD, De Mello TR, Kershaw D, McDougall K, Hartmann PE. Initiation of lactation in women after preterm delivery. Acta Obstet Gynecol Scand. 2002;81(9):870–877. doi: 10.1034/j.1600-0412.2002.810913.x. [DOI] [PubMed] [Google Scholar]
- 139.Cregan MD, de Mello TR, Hartmann PE. Pre-term delivery and breast expression: Consequences for initiating lactation. Advances in Experimental Medicine & Biology. 2000;478:427–428. doi: 10.1007/0-306-46830-1_60. [DOI] [PubMed] [Google Scholar]
- 140.Hill PD, Aldag JC, Zinaman M, Chatterton RT. Predictors of preterm infant feeding methods and perceived insufficient milk supply at week 12 postpartum. J Hum Lact. 2007;23(1):32–38. doi: 10.1177/0890334406297277. [DOI] [PubMed] [Google Scholar]
- 141.Hill PD, Aldag JC, Chatterton RT, Zinaman M. Comparison of milk output between mothers of preterm and term infants: The first 6 weeks after birth. Journal of Human Lactation. 2005;21(1):22–30. doi: 10.1177/0890334404272407. [DOI] [PubMed] [Google Scholar]
- 142.Hill PD, Aldag JC, Chatterton RT, Zinaman M. Primary and secondary mediators' influence on milk output in lactating mothers of preterm and term infants. Journal of Human Lactation. 2005;21(2):138–150. doi: 10.1177/0890334405275403. [DOI] [PubMed] [Google Scholar]
- 143.Hill PD, Aldag JC, Chatterton RT. Effects of pumping style on milk production in mothers of non-nursing preterm infants. Journal of Human Lactation. 1999;15(3):209–216. doi: 10.1177/089033449901500310. [DOI] [PubMed] [Google Scholar]
- 144.Slusher T, Hampton R, Bode-Thomas F, Pam S, Akor F, Meier P. Promoting the exclusive feeding of own mother's milk through the use of hindmilk and increased maternal milk volume for hospitalized, low birth weight infants (< 1800 grams) in nigeria: A feasibility study. Journal of Human Lactation. 2003;19(2):191–198. doi: 10.1177/0890334403252490. [DOI] [PubMed] [Google Scholar]
- 145.Lawrence R, Lawrence R. Breastfeeding: A Guide for the Medical Profession. Sixth edition ed. Philadelphia: Mosby; 2005. [Google Scholar]
- 146.Kent JC. How breastfeeding works. J Midwifery Womens Health. 2007;52(6):564–570. doi: 10.1016/j.jmwh.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 147.Neville MC, Morton J. Physiology and endocrine changes underlying human lactogenesis II. J Nutr. 2001;131(11):3005S–3008S. doi: 10.1093/jn/131.11.3005S. [DOI] [PubMed] [Google Scholar]
- 148.Chapman D, Perez-Escamilla R. Maternal perception of the onset of lactation: A valid indicator of lactogenesis stage II? Adv Exp Med Biol. 2000;478:423–424. doi: 10.1007/0-306-46830-1_58. [DOI] [PubMed] [Google Scholar]
- 149.Perez-Escamilla R, Chapman D. Can women remember when their milk came in? Adv Exp Med Biol. 2001;501:567–572. [PubMed] [Google Scholar]
- 150.Morton J, Hall JY, Wong RJ, Thairu L, Benitz WE, Rhine WD. Combining hand techniques with electric pumping increases milk production in mothers of preterm infants. J Perinatol. 2009;29(11):757–764. doi: 10.1038/jp.2009.87. [DOI] [PubMed] [Google Scholar]
- 151.Knight CH, Peaker M, Wilde CJ. Local control of mammary development and function. Rev Reprod. 1998;3(2):104–112. doi: 10.1530/ror.0.0030104. [DOI] [PubMed] [Google Scholar]
- 152.Engstrom JL. Coming to volume. 2009 [Google Scholar]
- 153.World Health Organization. [Accessed November 28, 2009];Combined hormonal versus nonhormonal versus progestin-only contraception in lactation. Available at http://apps.who.int/rhl/fertility/contraception/dlcom/en/index.html. [Google Scholar]
- 154.Rodriguez MI, Kaunitz AM. An evidence-based approach to postpartum use of depot medroxyprogesterone acetate in breastfeeding women. Contraception. 2009;80(1):4–6. doi: 10.1016/j.contraception.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 155.King J. Contraception and lactation. J Midwifery Womens Health. 2007;52(6):614–620. doi: 10.1016/j.jmwh.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 156.Halderman LD, Nelson AL. Impact of early postpartum administration of progestin-only hormonal contraceptives compared with nonhormonal contraceptives on short-term breast-feeding patterns. Am J Obstet Gynecol. 2002;186(6):1250–1256. doi: 10.1067/mob.2002.123738. discussion 1256–8. [DOI] [PubMed] [Google Scholar]
- 157.Baheiraei A, Ardsetani N, Ghazizadeh S. Effects of progestogen-only contraceptives on breast-feeding and infant growth. Int J Gynaecol Obstet. 2001;74(2):203–205. doi: 10.1016/s0020-7292(01)00418-0. [DOI] [PubMed] [Google Scholar]
- 158.Truitt ST, Fraser AB, Grimes DA, Gallo MF, Schulz KF. Hormonal contraception during lactation. systematic review of randomized controlled trials. Contraception. 2003;68(4):233–238. doi: 10.1016/s0010-7824(03)00133-1. [DOI] [PubMed] [Google Scholar]
- 159.Hurst NM. Recognizing and treating delayed or failed lactogenesis II. J Midwifery Womens Health. 2007;52(6):588–594. doi: 10.1016/j.jmwh.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 160.Grisaru-Granovsky S, Gordon ES, Haklai Z, Samueloff A, Schimmel MM. Effect of interpregnancy interval on adverse perinatal outcomes--a national study. Contraception. 2009;80(6):512–518. doi: 10.1016/j.contraception.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 161.United State Food and Drug Administration. [Accessed November, 2009];Physician information for depo provera. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2004/20246s025lbl.pdf. [Google Scholar]
- 162.American College of Obstetricians and Gynecologists. Breastfeeding: Maternal and infant aspects. special report from ACOG. ACOG Clin Rev. 2007;12 suppl:1S–16S. Available from: http://www.acog.org/departments/underserved/clinicalReviewv12i1s.pdf. [Google Scholar]
- 163.Engstrom JL, Meier PP, Jegier BJ, Motykowski JE, Zuleger JL. Comparison of milk output from the right and left breasts during simultaneous pumping in mothers of very low birthweight infants. Breastfeeding Medicine. 2007 doi: 10.1089/bfm.2006.0019. in press. [DOI] [PubMed] [Google Scholar]
- 164.Griffin TL, Meier PP, Bradford LP, Bigger HR, Engstrom JL. Mothers' performing creamatocrit measures in the NICU: Accuracy, reactions, and cost. J Obstet Gynecol Neonatal Nurs. 2000;29(3):249–257. doi: 10.1111/j.1552-6909.2000.tb02046.x. [DOI] [PubMed] [Google Scholar]
- 165.Meier PP, Engstrom JL, Murtaugh MA, Vasan U, Meier WA, Schanler RJ. Mothers' milk feedings in the neonatal intensive care unit: Accuracy of the creamatocrit technique. J Perinatol. 2002;22(8):646–649. doi: 10.1038/sj.jp.7210825. [DOI] [PubMed] [Google Scholar]
- 166.Lucas A, Gibbs JA, Lyster RL, Baum JD. Creamatocrit: Simple clinical technique for estimating fat concentration and energy value of human milk. Br Med J. 1978;1(6119):1018–1020. doi: 10.1136/bmj.1.6119.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wang CD, Chu PS, Mellen BG, Shenai JP. Creamatocrit and the nutrient composition of human milk. J Perinatol. 1999;19(5):343–346. doi: 10.1038/sj.jp.7200204. [DOI] [PubMed] [Google Scholar]
- 168.Meier PP, Brown LP, Hurst NM, et al. Nipple shields for preterm infants: Effect on milk transfer and duration of breastfeeding. J Hum Lact. 2000;16(2):106–114. doi: 10.1177/089033440001600205. quiz 129–31. [DOI] [PubMed] [Google Scholar]
- 169.Chertok IR, Schneider J, Blackburn S. A pilot study of maternal and term infant outcomes associated with ultrathin nipple shield use. J Obstet Gynecol Neonatal Nurs. 2006;35(2):265–272. doi: 10.1111/j.1552-6909.2006.00028.x. [DOI] [PubMed] [Google Scholar]
- 170.Meier P. Concerns regarding industry-funded trials. J Hum Lact. 2005;21(2):121–123. [PubMed] [Google Scholar]
- 171.Brown LP, Bair AH, Meier PP. Does federal funding for breastfeeding research target our national health objectives? Pediatrics. 2003;111(4 Pt 1):e360–e364. doi: 10.1542/peds.111.4.e360. [DOI] [PubMed] [Google Scholar]
- 172.Slusher T, Slusher IL, Biomdo M, Bode-Thomas F, Curtis BA, Meier P. Electric breast pump use increases maternal milk volume in african nurseries. J Trop Pediatr. 2007;53(2):125–130. doi: 10.1093/tropej/fml066. [DOI] [PubMed] [Google Scholar]
- 173.Engstrom JL. Comfort and effectiveness of new pumping patterns for the initiation and maintenance of lactation in breast pump-dependent mothers of premature infants. IL: 2006. (12000) [Google Scholar]
- 174.Hytten FE. Clinical and chemical studies in human lactation. Br Med J. 1954;1(4855):175–182. doi: 10.1136/bmj.1.4855.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Daly SE, Di Rosso A, Owens RA, Hartmann PE. Degree of breast emptying explains changes in the fat content, but not fatty acid composition, of human milk. Exp Physiol. 1993;78(6):741–755. doi: 10.1113/expphysiol.1993.sp003722. [DOI] [PubMed] [Google Scholar]
- 176.Weber A, Loui A, Jochum F, Buhrer C, Obladen M. Breast milk from mothers of very low birthweight infants: Variability in fat and protein content. Acta Paediatr. 2001;90(7):772–775. [PubMed] [Google Scholar]
- 177.Brennan-Behm M, Carlson GE, Meier P, Engstrom J. Caloric loss from expressed mother's milk during continuous gavage infusion. Neonatal Network Journal of Neonatal Nursing. 1994;13(2):27–32. [PubMed] [Google Scholar]
- 178.Greer FR, McCormick A, Loker J. Changes in fat concentration of human milk during delivery by intermittent bolus and continuous mechanical pump infusion. J Pediatr. 1984;105(5):745–749. doi: 10.1016/s0022-3476(84)80294-2. [DOI] [PubMed] [Google Scholar]
- 179.Lau C, Alagugurusamy R, Schanler RJ, Smith EO, Shulman RJ. Characterization of the developmental stages of sucking in preterm infants during bottle feeding. Acta Paediatr. 2000;89(7):846–852. [PubMed] [Google Scholar]
- 180.Meier PP. Breastfeeding in the special care nursery. prematures and infants with medical problems. Pediatr Clin North Am. 2001;48(2):425–442. doi: 10.1016/s0031-3955(08)70035-x. [DOI] [PubMed] [Google Scholar]
- 181.Woolridge MW, Butte N, Dewey KG, Ferris AM, Garza C, Keller RP. Methods for the measurement of milk volume intake of the breastfed infant. In: Jensen RG, Neville MC, editors. Human Lactation: Milk Components and Methodologies. New York: Plenum Press; 1985. pp. 5–20. [Google Scholar]