Abstract
Objective
Identification of the neural mechanisms underlying medication overuse headache resulting from triptans.
Methods
Triptans were administered systemically to rats by repeated intermittent injections or by continuous infusion over 6 days. Periorbital and hind paw sensory thresholds were measured to detect cutaneous allodynia. Immunofluorescent histochemistry was employed to detect changes in peptidic neurotransmitter expression in identified dural afferents. Enzyme-linked immunoabsorbent assay was used to measure calcitonin gene-related peptide (CGRP) levels in blood.
Results
Sustained or repeated administration of triptans to rats elicited time-dependent and reversible cutaneous tactile allodynia that was maintained throughout and transiently after drug delivery. Triptan administration increased labeling for CGRP in identified trigeminal dural afferents that persisted long after discontinuation of triptan exposure. Two weeks after triptan exposure, when sensory thresholds returned to baseline levels, rats showed enhanced cutaneous allodynia and increased CGRP in the blood following challenge with a nitric oxide donor. Triptan treatment thus induces a state of latent sensitization characterized by persistent pronociceptive neural adaptations in dural afferents and enhanced responses to an established trigger of migraine headache in humans.
Interpretation
Triptans represent the treatment of choice for moderate and severe migraine headaches. However, triptan overuse can lead to an increased frequency of migraine headache. Overuse of these medications could induce neural adaptations that result in a state of latent sensitization, which might increase sensitivity to migraine triggers. The latent sensitization could provide a mechanistic basis for the transformation of migraine to medication overuse headache.
Migraine is a debilitating disorder characterized as an episodic, unilateral throbbing cephalic pain that may occur with or without aura.1 Although multiple mechanistic hypotheses of migraine have been proposed, including cortical spreading depression, vasodilation, plasma protein extravasation and sensitization of nociceptive dural afferents, the underlying pathophysiology of this disorder remains unclear.2–5 Sensitization of primary afferent neurons may lead to sensitization of second- and third-order neurons in the trigeminal nucleus caudalis and thalamus, resulting in cephalic and extracephalic cutaneous allodynia.6 – 8
Considerable evidence implicates calcitonin gene-related peptide (CGRP) in migraine headache. Blood levels of CGRP are elevated during migraine attacks,9 and intravenous (iv) CGRP10 or administration of a nitric oxide (NO) donor11 produces migraine headache in migraineurs, but not in controls.12 Additionally, NO donor challenge to migraineurs results in elevated jugular blood levels of CGRP that occurs with headache pain.11,13,14 Triptans are suggested to inhibit the release of pronociceptive transmitters, including CGRP, through actions on 5-hydroxytryptamine (5-HT)1B/1D presynaptic receptors on trigeminal afferents, thus preventing the development of central sensitization if administered early in the course of an attack.15–19 Recently, CGRP receptor antagonists have been shown to have clinical efficacy in the treatment of migraine.20,21
The frequent use of triptans to treat migraine headache can lead to medication overuse headache (MOH) and has been demonstrated to be a major risk for the transformation of episodic to chronic migraine, representing an important unmet medical need.22–25 MOH is defined by >15 headaches per month, during regular overuse (>15 days per month) of acute analgesics or symptomatic drugs for >3 months. The mechanisms underlying MOH are unknown.24,26 We reasoned that the induction of MOH, and perhaps the transformation of episodic to chronic migraine by triptans, might result as a consequence of triptan-induced neural adaptations within the trigeminal afferents that could lower the thresholds to stimuli that trigger migraine headache.
Here, we report that the exposure of uninjured rats to triptans over several days leads to cephalic and extracephalic cutaneous allodynia accompanied by neural adaptations in dural primary afferent fibers. After termination of triptan exposure, sensory thresholds, but not neuronal adaptations, return to baseline. Such adaptations appear to underlie behavioral and neurochemical hypersensitivity to a stimulus known to trigger migraine in humans despite normal sensory thresholds. Persistent triptan-induced pronociceptive neural adaptations may provide a basis for MOH resulting in altered thresholds to migraine triggers.
Materials and Methods
Animals
Adult male Sprague Dawley rats (175–250g; n = 6–12) were maintained in a climate-controlled room on a 12-hour light/dark cycle with food and water ad libitum. Testing conformed to the specifications of the International Association for study of Pain and the National Institutes of Health guidelines for the handling and use of laboratory animals, and were approved by the Institutional Animal Care and Use Committee of the University of Arizona.
Drug Administration
Continuous drug infusion was performed with Alzet osmotic minipumps (Cupertino, CA; model 2001; 1μl/hr/7 days subcutaneously); the day of pump implant was considered day 0. Infused drugs were sumatriptan (0.6mg/kg/day; GlaxoSmithKline), naratriptan hydrochloride (200μg/kg/day; US Pharmacopeia reference standard), and the 5-HT1B/1D antagonist GR127935 (1mg/kg/day; Tocris Bioscience, Ellisville, MO). The infusion dose of sumatriptan was determined from our previous studies,27 whereas that of naratriptan was derived from literature values indicating a 2- to 3-fold greater potency for this compound.28 The dose of GR127935 was chosen to maintain selectivity for 5-HT1B/D receptors. Bolus doses of this compound have been shown to have only moderate activity at the 5-HT2 receptor and none at 5-HT3, 5-HT7, or the remaining 5-HT1 receptor sub-types.29 Naproxen (100mg/kg subcutaneously [sc]; Sigma, St. Louis, MO), α-CGRP8–37 (0.45mg/kg iv; Bachem Biochemicals, King of Prussia, PA), L-732,138 (NK-1 antagonist, 10mg/kg sc; Tocris), and sodium nitroprusside (SNP) (3mg/kg intraperitoneally [ip]; Sigma-Aldrich) were given as bolus injections. The dose of naproxen employed is consistent with doses employed in rats to elicit antinociception.27,30,31 The doses of the remaining antagonists were based on previous studies.27
Evaluation of Tactile Sensitivity
Withdrawal thresholds to von Frey filaments applied to the peri-orbital region and the plantar surface of the hind paw were determined by acclimating the rats in clear cages for 45 minutes. The von Frey filaments were applied perpendicularly to the peri-orbital region of the facial region or to the plantar surface of the hind paw until it buckled slightly, and held for 3 to 6 seconds or until withdrawal occurred (positive response); thresholds were determined by the “up-down” method.32 Maximum filament strengths were 8g and 15g for the periorbital region and hind paw, respectively. For the evaluation of tactile sensitivity in response to exposure to a NO donor, rats were injected with SNP (3mg/kg ip), and mechanical thresholds were measured at 1-hour intervals for 6 hours.
Tracer Injection
Four days before perfusion, animals were anesthetized with a combination of ketamine and xylazine (Sigma-Aldrich, St. Louis, MO; 80mg/kg and 12mg/kg). Two holes were made in the skull without tearing the dura, and Fluoro-Gold (Fluorochrome, Denver, CO; 10μl; 4% in saline) was injected onto the dura. Holes were covered with bone wax to prevent tracer spreading. Animals were sacrificed for tissue extraction, undamaged dura at the injection sites was evaluated, and only animals with intact dura were used for immunofluorescent labeling of trigeminal neuronal profiles. To label the maxillary nerve branch of the trigeminal ganglion, the extreme opening of the infraorbital canal was palpated and exposed, and Fluoro-Gold (5μl) was injected.
Immunohistochemistry
Rats were transcardially perfused with 0.1M phosphate-buffered saline (PBS) followed by 4% formaldehyde/12.5% picric acid solution in 0.1M PBS. Trigeminal ganglia were removed and cryoprotected (20% sucrose), and 10μm frozen sections were cut. They were incubated with primary antibody against CGRP (rabbit or guinea pig; Peninsula Laboratories, San Carlos, CA), substance P (rabbit or guinea pig; Peninsula Laboratories), or NF200 (mouse; Sigma-Aldrich) for 24 hours. Secondary antibodies were goat antirabbit/guinea pig immunoglobulin G (IgG) conjugated with either Alex fluor-488 or Alex fluor-694 (Molecular Probes, Eugene, OR) and goat antimouse IgG conjugated with Alex fluor-488 for 2 hours. Analysis of colocalization of 2 neuromarkers was performed using dual-labeling immunofluorescence. Because the primary antibodies for the neuromarkers were raised from different species, selective secondary antibodies were employed. For each group, 2 sections of the trigeminal ganglion were taken, and the number of neuronal profiles expressing each marker was counted at the appropriate wavelength for each marker. The sections were then incubated in ethidium bromide (5μg/ml) or DAPI for 30 seconds to visualize and count all neuronal profiles in each section of trigeminal ganglion.
CGRP Plasma Levels
Rats were anesthetized with isoflurane, and direct cardiac puncture was performed to remove 1.5ml of blood into a heparinized syringe 0, 1.5, 3.5, or 5.5 hours after injection of the NO donor. Only 1 blood draw was performed on each rat. The blood was centrifuged (3,000rpm for 15 minutes), and 200μl of plasma was withdrawn for CGRP detection with an enzyme-linked immunoabsorbent assay kit (SPlbio, Cayman Chemicals, Ann Arbor, MI); samples were run in duplicate.
Statistical Analyses
Behavioral studies among groups and across time were analyzed by 2-factor analysis of variance (ANOVA). One-factor ANOVA and Fisher least significant difference test were used with behavioral changes from baseline values. Allodynia precipitated by NO donor or stress was converted to percentage allodynia as 100 × response of treated/control group and compared by Student t test. Counts of trigeminal profiles were collected per section, and transformed to the percentage of that seen in vehicle-treated control groups. Data among groups was analyzed by ANOVA followed by Fisher least significant difference test.
Results
Persistent Triptan Administration Produces Generalized Tactile Allodynia
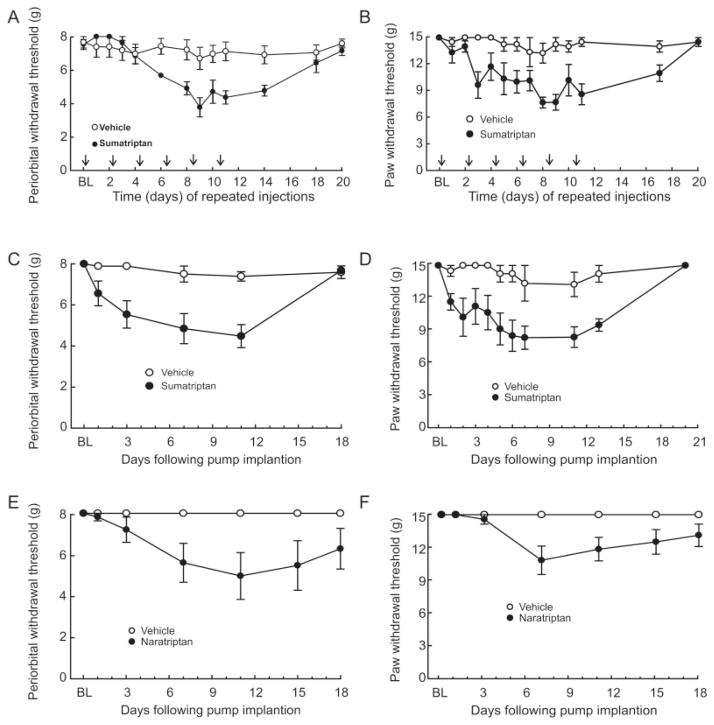
Rats received repeated injections of sumatriptan (0.6mg/kg sc) 48 hours apart, and behavioral responses to tactile stimuli applied to the periorbital region and to the hind paw were determined in the morning before injection. Six repeated injections of sumatriptan, given every second day, produced time-dependent, significant ( p < 0.05) reductions in periorbital and hind paw withdrawal thresholds relative to the saline-treated group (Fig 1A, B). Thresholds remained lower for several days following termination of triptan delivery. Recovery of sensory thresholds to preinjection baseline levels occurred within 20 days after the first injection (see Fig 1A, B).
FIGURE 1.
Sustained exposure to triptans reduces sensory thresholds to light tactile stimuli applied to the periorbital region and the hind paws of rats. Repeated injections of sumatriptan (0.6mg/kg subcutaneously [sc]) decrease the withdrawal thresholds to light tactile stimuli applied to (A) the periorbital region or (B) the hind paws of rats. Sumatriptan or saline was injected 6 times, at 48-hour intervals, and sensory thresholds were evaluated every 24 hours. Withdrawal responses to von Frey filaments are significantly (p < 0.05) reduced in a time-dependent manner. Continuous infusion of sumatriptan (0.6mg/kg/day sc) decreased withdrawal thresholds to light tactile stimuli applied to (C) the periorbital region or (D) the hind paws of rats. Sumatriptan or saline was continuously administered through an osmotic minipump for 6 days, after which the minipumps were removed. Withdrawal responses to von Frey filaments were significantly (p < 0.05) reduced in a time-dependent manner. Continuous infusion of naratriptan (0.6mg/kg/day sc) decreased withdrawal thresholds to light tactile stimuli applied to (E) the periorbital region or (F) the hind paws of rats. Naratriptan or saline was continuously administered through an osmotic minipump for 6 days, after which the minipumps were removed. Withdrawal responses to von Frey filaments were significantly (p < 0.05) reduced in a time-dependent manner. BL = baseline.
In separate experiments, sumatriptan (0.6mg/kg/day) or saline was infused by osmotic minipump for 6 days, and the pumps were removed. The withdrawal thresholds were significantly reduced within 1 day of minipump implantation (see Fig 1C, D, p < 0.05). Removal of the minipump on day 6 resulted in a gradual return of withdrawal thresholds to preimplantation baseline values by day 18. Likewise, infusion of naratriptan (0.2 mg/kg/day) produced significant reductions in peri-orbital and hind paw withdrawal thresholds that resolved after removal of the pumps (see Fig 1E, F), indicating that triptan-induced allodynia is not specific to a single molecule and may be a property of this class of medications.33
Sumatriptan-Induced Allodynia Is Receptor Mediated
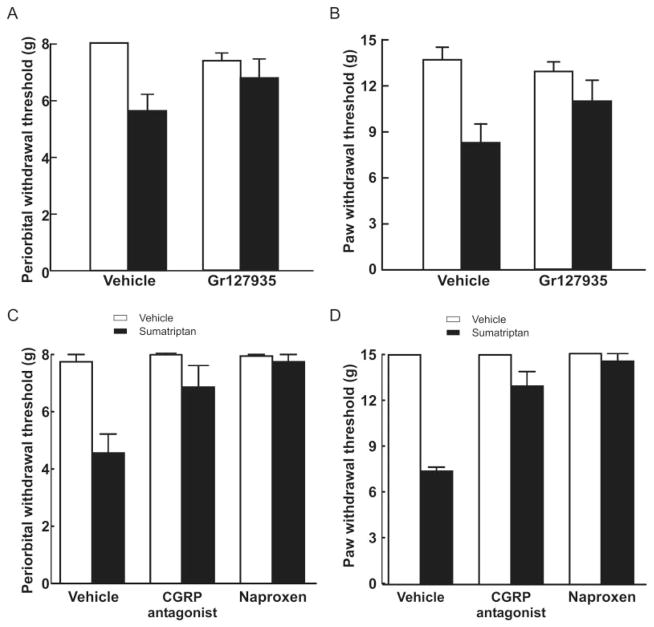
GR127935 (1mg/kg/day), a nonselective antagonist at 5-HT1B/1D receptors, was coinfused with sumatriptan in separate minipumps. Sumatriptan coinfused with saline produced significant ( p < 0.05) reductions in periorbital and hind paw withdrawal thresholds, whereas the coinfusion of sumatriptan with GR127935 did not produce allodynia (see Fig 1A, B). Withdrawal thresholds were significantly ( p < 0.05) elevated relative to those obtained with sumatriptan/vehicle on day 4 after initiation of the infusion (Fig 2A, B). Coinfusion of GR127935 with saline did not alter either periorbital or hind paw withdrawal thresholds.
FIGURE 2.
Effects of sumatriptan exposure are mediated by serotoninergic receptors. Coinfusion of sumatriptan (0.6mg/kg/day) with a nonselective antagonist at 5-hydroxytryptamine1B/1D receptors, GR127935 (1 mg/kg/day), significantly (p < 0.05) prevented the development of both (A) periorbital and (B) hind paw allodynia. There was no significant difference (p > 0.05) between the responses of animals receiving GR127935 with saline and vehicle/saline. Rats received vehicle or sumatriptan through an osmotic minipump for 6 days. On day 6 after evaluation of the cutaneous allodynia in sumatriptan-exposed animals, rats received a single injection of the calcitonin gene-related peptide (CGRP) antagonist α-CGRP(8–37) (0.45mg/kg intravenously), and (C) periorbital and (D) hind paw sensory thresholds were evaluated. The response thresholds of rats receiving α-CGRP(8–37) were significantly (p < 0.05) greater than those of sumatriptan-treated rats receiving vehicle infusion 30 minutes after the injection. Naproxen sodium (100mg/kg subcutaneously) produced a similar significant (p < 0.05) blockade of sumatriptan-induced (C) periorbital and (D) hind paw allodynia within 1 hour of the injection. Neither α-CGRP(8–37) nor naproxen sodium altered the sensory thresholds of saline-treated animals.
Reversal of Sumatriptan-Induced Allodynia
Clinical studies show that naproxen34 and CGRP antagonists35 are effective against migraine pain, whereas NK-1 receptor antagonists are not.36–39 Accordingly, these substances were tested against sumatriptan-induced allodynia. Once periorbital and hind paw tactile allodynia were established on day 6 of sumatriptan infusion, rats received a single injection of naproxen sodium (100mg/kg sc) or vehicle. Naproxen produced a significant increase in periorbital and hind paw withdrawal thresholds, indicating reversal of allodynia (see Fig 2C, D, p < 0.05). Withdrawal thresholds were similar to presumatriptan baseline values 1 hour after the naproxen injection and gradually returned to the allodynic state after a further 5 hours (not shown). Similarly, α-CGRP8–37 (0.45mg/kg iv) produced reversal of periorbital and hind paw sensitivity to light tactile stimuli. Response thresholds increased to the saline/vehicle values within 30 minutes of the injection and returned to allodynic sensory thresholds within 1 hour (data not shown). The NK-1 antagonist L-732,138 (10mg/kg sc), given at a dose effective against substance P-induced hypersensitivity, did not elevate either periorbital (F4,20 = 0.9479, p = 0.457) or hind paw (F4,25 = 0.938, p = 0.458) sensory thresholds (data not shown).
Increased CGRP-Positive Dural Afferent Neurons after Sumatriptan Treatment
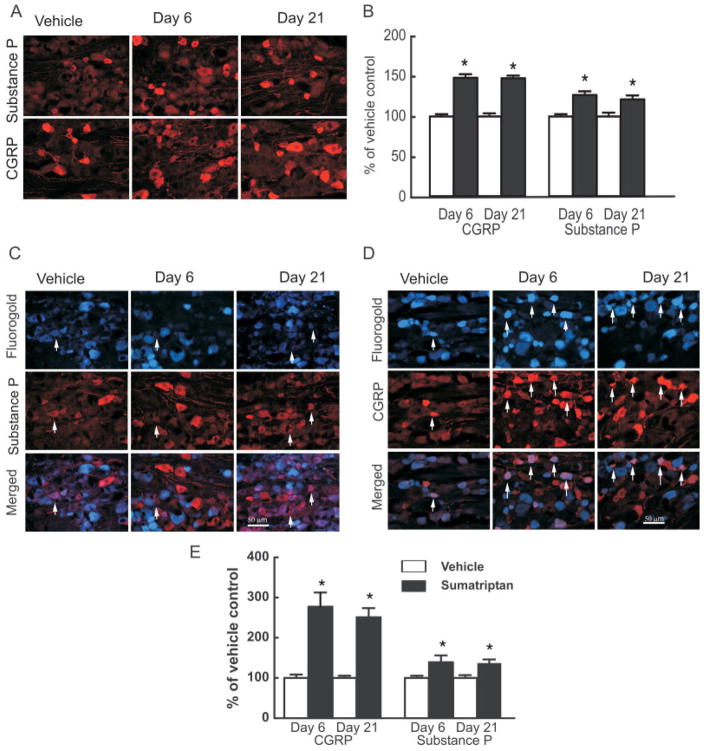
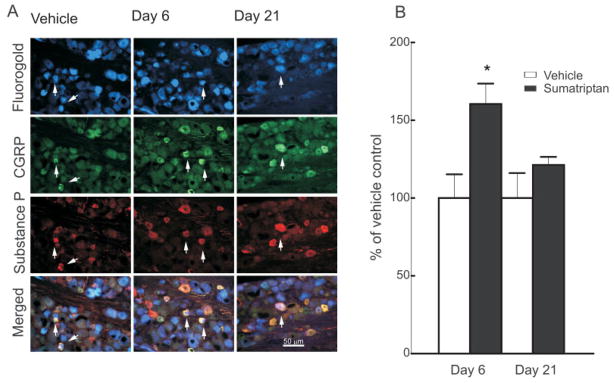
Sustained administration of sumatriptan for 6 days produced significant ( p < 0.05) and prolonged increases in trigeminal neuronal profiles expressing CGRP and substance P (Fig 3A). In the vehicle-treated group at day 6, approximately 29% of the counted trigeminal neuronal profiles were positively labeled for CGRP, and 19% were positive for substance P. Trigeminal neuronal profiles labeled for CGRP from sumatriptan-treated rats were increased to 149 ± 4.5% and 148 ± 3.3% of the saline control group on day 6 of sumatriptan treatment and 15 days following its termination (ie, day 21), respectively (see Fig 3B). Trigeminal neuronal profiles labeled for substance P in sections obtained from sumatriptan-treated rats were increased to a smaller but nevertheless significant ( p < 0.05) degree to 126 ± 6.8% and 121 ± 4.9% of the saline control group on days 6 and 21, respectively.
FIGURE 3.
Sustained infusion of sumatriptan (0.6mg/kg/day) for 6 days promoted increased and persistent expression of the neuropeptides calcitonin gene-related peptide (CGRP) and substance P in the trigeminal ganglia of rats. (A) Immunofluorescence labeling for CGRP or substance P in the trigeminal ganglion is shown for sections obtained 6 and 21 days after implantation of a minipump delivering vehicle (saline) or sumatriptan. (B) Sumatriptan exposure resulted in a significant (p < 0.05) increase in CGRP- and substance P-labeled profiles in the trigeminal ganglia, relative to vehicle-infused animals, at both days 6 and 21. Additionally, dural afferents were identified by application of Fluoro-Gold to the dura, and profiles expressing labeling for (C) substance P and (D) CGRP were evaluated in the trigeminal ganglia 6 and 21 days after sumatriptan exposure. (E) The relative proportion of profiles obtained from sumatriptan-treated animals and expressing both the retrograde label and label for CGRP or substance P in the trigeminal ganglia showed a significant and persistent (day 6 and day 21) increase in CGRP and substance P labeling relative to vehicle-infused animals. Notably, the increases in numbers of neuronal profiles labeled for CGRP were significantly (p < 0.05) greater than those expressing substance P. These observations suggest that sumatriptan exposure results in a substantial increase in dural afferents labeled for CGRP, with only a small increase in substance P expression in these cells.
Following retrograde labeling of dural afferents with Fluoro-Gold, no differences ( p = 0.502; t59 = 0.6756) were observed in the proportion of trigeminal profiles between the vehicle-treated (20.3 ± 0.35%) and sumatriptan-treated (20.8 ± 0.55%) groups. Triptan exposure produced a small but significant ( p < 0.05) increase in labeling for substance P, and a substantially greater and significant increase for CGRP, in the dural afferent subpopulation of trigeminal neurons (see Fig 3C–E). Sumatriptan exposure produced a significant ( p < 0.05) increase in retrogradely labeled neuronal profiles expressing CGRP to 277 ± 35% and 251 ± 23% relative to vehicle-infused animals on days 6 and 21, respectively, and substance P-positive profiles increased significantly to 139 ± 17% and 137 ± 9.2% ( p < 0.05) of saline-treated control groups on days 6 and 21, respectively (see Fig 3E).
Sumatriptan Treatment Increased CGRP-Positive Maxillary Afferent Neurons
Possible changes in labeling for peptidic transmitters in maxillary afferents were examined to determine if triptan-induced effects were specific to dural afferents. Sumatriptan treatment also resulted in a significant ( p < 0.05) increase in the proportion of retrogradely labeled maxillary trigeminal profiles expressing CGRP to 159 ± 10% and 157 ± 9% of the saline-treated group on days 6 and 21, respectively. Approximately 25% of the labeled profiles from the saline-treated control group expressed CGRP. The sumatriptan-induced changes in maxillary profiles expressing CGRP were similar to those of the trigeminal nucleus overall, but significantly ( p < 0.05) less than the increases observed in dural afferents on days 6 and 21. The proportions of retrogradely labeled trigeminal profiles expressing substance P on days 6 and 21 were not significantly different ( p > 0.05) from the vehicle-treated group, which represented 34% of all of the counted retrogradely labeled profiles. The proportion of trigeminal profiles labeled with Fluoro-Gold between the vehicle control and the sumatriptan-treated groups did not differ significantly (61.1 ± 0.93% and 61.0 ± 0.80%, respectively).
Increased CGRP-Positive Peptide-Poor and Myelinated Dural Afferent Neurons after Sumatriptan Treatment
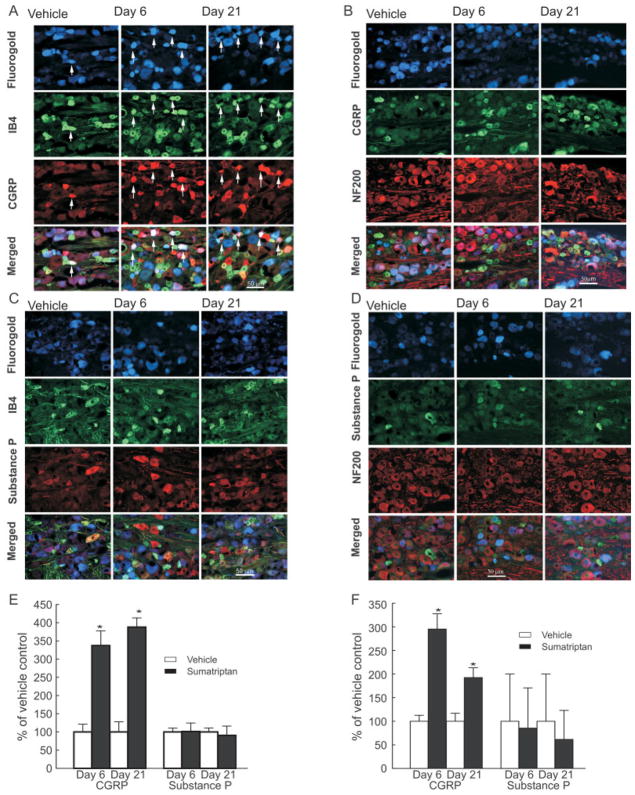
The marked sumatriptan-induced upregulation of CGRP, relative to substance P, was maintained in dural afferents labeled for IB4 (ie, peptide-poor) or NF200 (myelinated). In tissue from the control group, only 25 of 79 counted IB4-labeled dural profiles also expressed CGRP, and only 10 of 81 profiles also expressed substance P in the control group. Expression of CGRP was increased 3-fold in sumatriptan-exposed, retrogradely labeled trigeminal profiles that were colabeled for IB4 on both days 6 and 21 (Fig 4A, C, E). In contrast, retrogradely labeled trigeminal profiles also labeled with IB4 showed no significant change in expression of substance P (see Fig 4B, E).
FIGURE 4.
Sustained infusion of sumatriptan leads to an increased expression of calcitonin gene-related peptide (CGRP) in normally peptide-poor unmyelinated fibers and in myelinated fibers. Dural afferents were identified by administration of Fluoro-Gold to the dura, 4 days before collecting trigeminal tissue for immunofluorescent imaging. Trigeminal ganglion sections were obtained from rats 6 and 21 days after initiation of sumatriptan infusion (0.6mg/kg/day) and labeled for (A, B) CGRP or (C, D) substance P. The sections were also labeled for reactivity to (A, C) IB4 or (B, D) NF200. (E, F) The proportion of retrogradely labeled profiles also showing label for CGRP and for either IB4 or NF200 or for substance P and for either IB4 or NF200 were determined relative to that shown by sections obtained from saline-treated rats. Sumatriptan infusion resulted in a significant (*p < 0.05) increase in retrogradely labeled trigeminal profiles labeled for CGRP and either (E) IB4 or (F) NF200 6 and 21 days after initiation of infusion. In contrast, levels of substance P were not significantly elevated (p > 0.05) in any of the sections studies. Notably, substance P expression in profiles labeled for NF200 was very low, representing <1% of all profiles counted. (F) These low counts resulted in the large standard errors shown. These results indicate that sumatriptan exposure produces a preferential increase in CGRP expression in dural afferents that do not normally express this peptide, or express it at a low level, or in myelinated dural afferents.
Similarly, sumatriptan exposure led to a marked 3-fold increase in expression of CGRP in retrogradely labeled myelinated (ie, labeled with NF200) trigeminal profiles on days 6 and 21 (see Fig 4B, D, F). On day 6, of the 57 identified dural afferents labeled for NF200, 14 also expressed label for CGRP (saline control on day 6). Only 1 of 80 myelinated dural afferent expressed label for substance P after either saline or sumatriptan treatment (see Fig 4D, F). Moreover, there was little change in substance P expression 6 and 21 days after sumatriptan infusion in CGRP-labeled profiles (Fig 5).
FIGURE 5.
Sumatriptan treatment increased the coexpression of substance P with calcitonin gene-related peptide (CGRP) in dural afferents. (A) After retrolabeling the dural afferents of the trigeminal ganglia, sections were prepared for fluorescent staining to visualize CGRP and substance P at day 6 and 21 after sumatriptan exposure. (B) The numbers of profiles expressing substance P and CGRP were counted. Sumatriptan exposure induced a small but significant (*p < 0.05) increase in coexpression of CGRP and substance P, relative to vehicle-infused animals, observed at day 6 but not at day 21 after sumatriptan pump implantation.
Triptan Treatment Results in Increased Sensitivity to an NO Donor Challenge
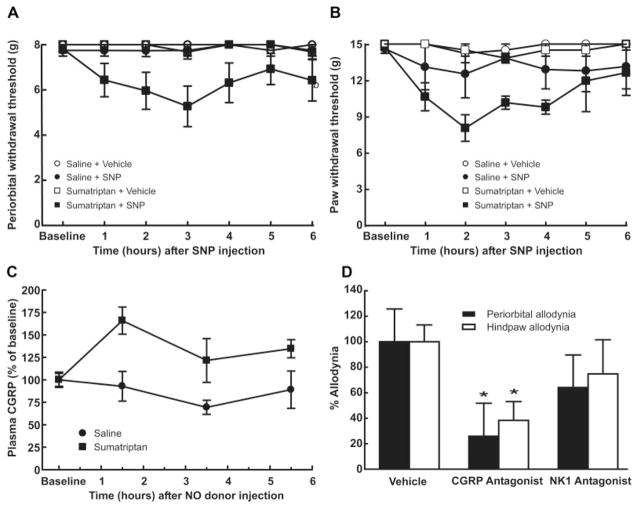
Once periorbital and hind paw tactile allodynia were established on day 6 of sumatriptan infusion, rats were allowed to recover for an additional 14 days and were then challenged with (3mg/kg ip); SNP triggered behavioral signs of periorbital and hind paw tactile allodynia in sumatriptan, but not saline, pre-exposed rats (Fig 6A, B). Periorbital withdrawal thresholds were reduced from 8g (baseline) to 5.27 ± 0.90g, and hind paw thresholds were reduced from 15g to 8.05 ± 1.1g within 2 to 3 hours (see Fig 6A, B). Similar results were seen with naratriptan exposure (data not shown).
FIGURE 6.
Sumatriptan-induced latent sensitization. On day 20 after pump implantation, an acute injection of the nitric oxide (NO) donor sodium nitroprusside (SNP) (3 mg/kg intraperitoneally [ip]) significantly (p < 0.05) reduced both the (A) periorbital and (B) hind paw withdrawal thresholds to probing with von Fey filaments. SNP injection in saline-exposed rats did not change the withdrawal thresholds. (C) Rats were pre-exposed to sumatriptan infusion (0.6mg/kg/day for 6 days) and challenged with SNP (3mg/kg ip) after 20 days. Blood samples (1ml) were withdrawn 1.5, 3.5, and 5.5 hours after the injection. Sumatriptan exposure resulted in a significant (p < 0.05) and time-dependent increase in calcitonin gene-related peptide (CGRP) levels. No changes in CGRP blood levels were observed in saline-exposed rats. (D) Twenty days after implantation of minipumps, rats received SNP (3mg/kg ip) followed after 30 minutes by α-CGRP(8–37) (0.45mg/kg intravenously). Systemic administration of α-CGRP(8–37) significantly (*p < 0.05) reduced behavioral signs of periorbital and hind paw tactile allodynia within 1 hour of the injection. In a second set of studies, the NK-1 antagonist L-732,138 (10mg/kg subcutaneously) was administered 30 minutes after SNP. Treatment with L-732,138 failed to abolish the development of tactile allodynia of the face or hind paws. Behavioral responses were not altered by vehicle, α-CGRP(8–37), or L-732,138 in saline exposed rats (data not shown).
The effects of α-CGRP(8–37) (0.45mg/kg iv) or of L-732,138 (10mg/kg sc) on SNP-induced cutaneous allodynia in rats previously treated with sumatriptan were tested. The CGRP antagonist significantly ( p < 0.05) decreased SNP-induced periorbital and hind paw allodynia to 25.8 ± 26% and 38.3 ± 14.7% of that seen in vehicle-treated animals, respectively, whereas the NK-1 receptor antagonist was without effect (see Fig 6D). Neither vehicle nor NK-1 antagonist injections given to saline-treated rats produced any changes in withdrawal thresholds (data not shown).
CGRP LEVELS IN BLOOD
Elevated blood levels of CGRP have been associated with evoked and spontaneous migraine in clinical experiments.11 Rats previously exposed to sumatriptan or saline received SNP (3mg/kg ip) on day 20. Although basal levels of CGRP were similar in either saline or sumatriptan pre-exposed rats, the SNP challenge resulted in a time-dependent, significant ( p < 0.05) increase of CGRP in the blood of the sumatriptan pre-exposed group, which reached a peak of 161.3 ± 25.2% of the saline control group at 90 minutes (see Fig 6C). No significant ( p > 0.05) changes in CGRP blood levels from baseline values were observed after NO donor injection to saline-exposed rats.
Discussion
The preclinical study of primary headache is particularly challenging, because such pain occurs in the absence of tissue injury (ie, dysfunctional pain).40 Although the triptans represent a highly selective therapeutic treatment against migraine, repeated overuse of these drugs can lead to increased frequency of migraine.41,42 We reasoned that triptan-induced increases in migraine frequency may result from neural adaptations that result in lowering of response thresholds to stimuli that trigger headache. This approach might therefore allow for the study of headache pain in the uninjured state.
In the present study, intermittent or sustained administration of sumatriptan or naratriptan elicited time-dependent allodynia that was generalized to cephalic and extracephalic regions of the body and sustained during the period of triptan exposure. Allodynia was reversed by systemic naproxen and the CGRP receptor antagonist, α-CGRP8–37, consistent with the clinical efficacy of these agents37,38,43–45 against migraine headache. In contrast, sumatriptan-induced allodynia was not reversed by L732,718 at a dose that blocked substance P-induced nociceptive behaviors,27 consistent with the lack of clinical efficacy of NK-1 antagonists in migraine.46
The iv administration of CGRP to volunteers elicits migraine headache in susceptible individuals but not in nonmigraineurs,10,47 and migraine headaches appear to be associated with elevated blood levels of CGRP.9 Moreover, the administration of NO or an NO donor elicits migraine headache along with release of CGRP in subjects with migraine.11,48 Here, once triptan-induced cutaneous allodynia resolved, there remained a persistent upregulation of trigeminal cells positively labeled for CGRP, most prominently observed in afferents innervating the dura. Additionally, animals previously exposed to triptans, but not controls, showed an enhanced behavioral sensitivity to challenge with an NO donor and increased levels of CGRP in the blood. The increased availability of CGRP may form the basis for changes in thresholds at which migraine attacks can be triggered and could result in the development of more frequent migraine headaches.
Although 5-HT1B/D receptors are broadly distributed throughout the nervous system, triptan activity appears specific for headache pain. Sumatriptan given systemically or into the rostral ventromedial medulla reversed periorbital and hind paw allodynia as well as the activation of pain facilitation cells within the right ventricular mass (RVM) produced by an inflammatory cocktail applied to the dura of the rat,27 suggesting that sustained sumatriptan may ultimately engage descending pain facilitation mechanisms from the RVM to elicit generalized allodynia. Further investigations will be needed to determine the specific role that the RVM may play in mediating triptan-induced cephalic and extracephalic allodynia. Additionally, broad distribution of triptan receptors in the trigeminal and dorsal root ganglia49 may contribute to the observed generalized allodynia.
Sumatriptan exposure produced a significant and long-lasting increase in the number of trigeminal profiles positively labeled for CGRP, and a more modest but significant increase in those labeled for substance P. Remarkably, these changes were more pronounced in identified dural afferents than in the whole trigeminal ganglion. Moreover, identified maxillary afferents showed an up-regulation of CGRP, but not of substance P, that was similar in magnitude to that seen in the whole trigeminal ganglion, but less than that seen in identified dural afferents. It seems possible that dural afferents might respond differently than other afferents to sustained triptan exposure, a finding that may be functionally relevant. The differential changes observed with substance P and CGRP may imply differential expression of receptors for triptans on CGRP-positive, substance P-negative cells and, as previously suggested, such fibers may be of increased relevance to migraine pain.50 Since Fluoro-Gold molecules diffuse passively across neuronal membranes,51 it is unlikely that triptan exposure altered its uptake, especially because no differences were observed in the proportion of Fluoro-Gold–labeled trigeminal profiles between the vehicle or sumatriptan-treated groups.
Upregulation of CGRP was prominent in IB4-labeled, normally peptide-deficient profiles, suggesting that triptan exposure might induce a phenotypic switch, contributing to increased excitability and responsivity to stimuli that trigger migraine. The preferential upregulation of CGRP in myelinated trigeminal afferents, including Aδ fibers, also suggests a novel mechanism that may contribute to neuronal sensitization.52–54 This is consistent with observations that neuronal plasticity has been consistently observed following administration of analgesic drugs. Upregulation of excitatory transmitters, along with hyperalgesia, has been noted preclinically following opiate administration,55–58 and hyperalgesia has also been observed in clinical studies.59 Behavioral and neurochemical changes suggestive of central sensitization have been reported preclinically with persistent exposure to cannabinoids60 and α2-adrenergic agonists.61 These findings suggest that the triptan-induced plasticity is consistent with the profile of inhibitory agonists at G-protein–coupled receptors on afferent fibers.
The increased numbers of CGRP-labeled dural profiles persisted after the cutaneous allodynia resolved. Even 14 days after terminating sumatriptan exposure, rats showed enhanced behavioral responses to an NO donor, which may be related to the persistent increase in CGRP. The NO-induced behavioral signs of cutaneous allodynia were delayed in onset and persisted for several hours, consistent with the clinical observations of NO-triggered migraine.62 Such enhanced sensitivity to a triggering event may reflect the presence of sensitization of primary afferent fibers, central sensitization, or both.
The mechanisms by which prolonged activation of the 5-HT1B/1D receptors by triptans could lead to neuro-plastic changes or transcriptional events within the neurons possessing these receptors, which manifest as upregulation of CGRP, and to a much lesser extent, substance P, remain to be determined. The increased numbers of neuronal profiles positively labeled for CGRP or substance P might reflect either increased peptide expression or reduced release or degradation. However, increased levels of CGRP after NO challenge were found only in blood from the triptan pre-exposed rats. Potential translational significance is suggested, because patients with spontaneous migraine or cluster headache show increased blood CGRP that correlated with pain score.9,63,64 Moreover, nitroglycerin evokes migraine headache and elevated blood levels of CGRP that correspond temporally with the headache, whereas administration of nitroglycerin to volunteers who did not develop a migraine headache did not provoke elevated levels of CGRP.11 Although not all studies have confirmed this clinical result,65 the present result may allow for the preclinical measurement of a potential biomarker of headache pain, offering opportunities for determination of efficacy of possible novel therapies.
Conclusions
These studies reveal persistent pronociceptive adaptations after exposure to triptan drugs that result in enhanced sensitivity to a stimulus that has been demonstrated to trigger migraine in humans. These findings are likely to be relevant to the adaptive changes that underlie MOH and may also offer insights into the possible pathology of chronic migraine. Interestingly, animals with triptan exposure show many characteristics in common with clinical migraine including (1) lack of tissue injury; (2) normal sensory thresholds between periods of pain (ie, allodynia); (3) enhanced sensitivity to NO donor challenge; (4) the presence of delayed and generalized allodynia, which is reversed by CGRP receptor, but not NK-1, antagonists; and (5) increased levels of CGRP in the blood after an NO donor challenge. These observations suggest that both repeated intermittent or sustained, continuous triptan treatment can induce a state of latent sensitization, resulting in neural adaptations that may decrease thresholds to triggers of migraine. Insights into the mechanisms by which triptan-induced latent sensitization occurs may offer novel approaches to the development of treatments that do not induce MOH, as might insights into mechanisms that may underlie chronic migraine.
References
- 1.Society HCCotIH. The International Classification of Headache Disorders (second edition) Cephalalgia. 2004;24:1–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 2.Launer LJ, Terwindt GM, Ferrari MD. The prevalence and characteristics of migraine in a population-based cohort: the GEM study. Neurology. 1999;53:537–542. doi: 10.1212/wnl.53.3.537. [DOI] [PubMed] [Google Scholar]
- 3.Bolay H, Moskowitz MA. The neurobiology of migraine and transformation of headache therapy. In: Waxman S, editor. From neuroscience to neurology: neuroscience, molecular medicine, and the therapeutic transformation of neurology. San Diego, CA: Elsevier; 2004. pp. 107–123. [Google Scholar]
- 4.Bolay H, Moskowitz MA. The emerging importance of cortical spreading depression in migraine headache. Rev Neurol (Paris) 2005;161:655–657. doi: 10.1016/s0035-3787(05)85108-2. [DOI] [PubMed] [Google Scholar]
- 5.Buzzi MG, Bonamini M, Moskowitz MA. Neurogenic model of migraine. Cephalalgia. 1995;15:277–280. doi: 10.1046/j.1468-2982.1995.1504277.x. [DOI] [PubMed] [Google Scholar]
- 6.Burstein R, Collins B, Jakubowski M. Defeating migraine pain with triptans: a race against the development of cutaneous allodynia. Ann Neurol. 2004;55:19–26. doi: 10.1002/ana.10786. [DOI] [PubMed] [Google Scholar]
- 7.Burstein R, Jakubowski M. Analgesic triptan action in an animal model of intracranial pain: a race against the development of central sensitization. Ann Neurol. 2004;55:27–36. doi: 10.1002/ana.10785. [DOI] [PubMed] [Google Scholar]
- 8.Landy S, Rice K, Lobo B. Central sensitisation and cutaneous allodynia in migraine: implications for treatment. CNS Drugs. 2004;18:337–342. doi: 10.2165/00023210-200418060-00001. [DOI] [PubMed] [Google Scholar]
- 9.Goadsby PJ, Edvinsson L, Ekman R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann Neurol. 1990;28:183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- 10.Lassen LH, Haderslev PA, Jacobsen VB, et al. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54– 61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- 11.Juhasz G, Zsombok T, Modos EA, et al. NO-induced migraine attack: strong increase in plasma calcitonin gene-related peptide (CGRP) concentration and negative correlation with platelet serotonin release. Pain. 2003;106:461–470. doi: 10.1016/j.pain.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Olesen J, Iversen HK, Thomsen LL. Nitric oxide supersensitivity: a possible molecular mechanism of migraine pain. Neuroreport. 1993;4:1027–1030. doi: 10.1097/00001756-199308000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Fanciullacci M, Alessandri M, Figini M, et al. Increase in plasma calcitonin gene-related peptide from the extracerebral circulation during nitroglycerin-induced cluster headache attack. Pain. 1995;60:119–123. doi: 10.1016/0304-3959(94)00097-X. [DOI] [PubMed] [Google Scholar]
- 14.Vanmolkot F, Van der Schueren B, de Hoon J. Sumatriptan causes parallel decrease in plasma CGRP concentration and migraine headache during nitroglycerin-induced migraine attack. Cephalalgia. 2006;26:1037–1038. doi: 10.1111/j.1468-2982.2006.01133_1.x. author reply 1038–1039. [DOI] [PubMed] [Google Scholar]
- 15.Dodick D, Silberstein S. Central sensitization theory of migraine: clinical implications. Headache. 2006;46(suppl 4):S182–S191. doi: 10.1111/j.1526-4610.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- 16.Burstein R. Deconstructing migraine headache into peripheral and central sensitization. Pain. 2001;89:107–110. doi: 10.1016/s0304-3959(00)00478-4. [DOI] [PubMed] [Google Scholar]
- 17.Burstein R, Yarnitsky D, Goor-Aryeh I, et al. An association between migraine and cutaneous allodynia. Ann Neurol. 2000;47:614– 624. [PubMed] [Google Scholar]
- 18.Suwattanasophon C, Phansuwan-Pujito P, Srikiatkhachorn A. 5-HT(1B/1D) serotonin receptor agonist attenuates nitroglycerin-evoked nitric oxide synthase expression in trigeminal pathway. Cephalalgia. 2003;23:825–832. doi: 10.1046/j.1468-2982.2003.00583.x. [DOI] [PubMed] [Google Scholar]
- 19.Ho TW, Ferrari MD, Dodick DW, et al. Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial. Lancet. 2008;372:2115–2123. doi: 10.1016/S0140-6736(08)61626-8. [DOI] [PubMed] [Google Scholar]
- 20.Olesen J, Diener HC, Husstedt IW, et al. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N Engl J Med. 2004;350:1104–1110. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- 21.Ho TW, Mannix LK, Fan X, et al. Randomized controlled trial of an oral CGRP receptor antagonist, MK-0974, in acute treatment of migraine. Neurology. 2008;70:1304–1312. doi: 10.1212/01.WNL.0000286940.29755.61. [DOI] [PubMed] [Google Scholar]
- 22.D’Amico D, Grazzi L, Usai S, et al. Disability pattern in chronic migraine with medication overuse: a comparison with migraine without aura. Headache. 2005;45:553–560. doi: 10.1111/j.1526-4610.2005.05109.x. [DOI] [PubMed] [Google Scholar]
- 23.Diener HC, Limmroth V. Medication-overuse headache: a worldwide problem. Lancet Neurol. 2004;3:475–483. doi: 10.1016/S1474-4422(04)00824-5. [DOI] [PubMed] [Google Scholar]
- 24.Olesen J, Bousser MG, Diener HC, et al. New appendix criteria open for a broader concept of chronic migraine. Cephalalgia. 2006;26:742–746. doi: 10.1111/j.1468-2982.2006.01172.x. [DOI] [PubMed] [Google Scholar]
- 25.Bigal ME, Serrano D, Buse D, et al. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008;48:1157–1168. doi: 10.1111/j.1526-4610.2008.01217.x. [DOI] [PubMed] [Google Scholar]
- 26.Limmroth V, Katsarava Z, Fritsche G, et al. Features of medication overuse headache following overuse of different acute headache drugs. Neurology. 2002;59:1011–1014. doi: 10.1212/wnl.59.7.1011. [DOI] [PubMed] [Google Scholar]
- 27.Edelmayer RM, Vanderah TW, Majuta L, et al. Medullary pain facilitating neurons mediate allodynia in headache-related pain. Ann Neurol. 2009;65:184–193. doi: 10.1002/ana.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Connor HE, Feniuk W, Beattie DT, et al. Naratriptan: biological profile in animal models relevant to migraine. Cephalalgia. 1997;17:145–152. doi: 10.1046/j.1468-2982.1997.1703145.x. [DOI] [PubMed] [Google Scholar]
- 29.De Vries P, Apaydin S, Villalon CM, et al. Interactions of GR127935, a 5-HT(1B/D) receptor ligand, with functional 5-HT receptors. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:423–430. doi: 10.1007/pl00004964. [DOI] [PubMed] [Google Scholar]
- 30.Clarke GD, MacPherson IS, Petrone G, Spangler RS. Antinociceptive effects of non-steroidal anti-inflammatory drugs in a rat model of unilateral hindpaw inflammation. Eur J Pharmacol. 1994;257:103–108. doi: 10.1016/0014-2999(94)90700-5. [DOI] [PubMed] [Google Scholar]
- 31.Karim F, Kanui TI, Mbugua S. Effects of codeine, naproxen and dexamethasone on formalin-induced pain in the naked mole-rat. Neuroreport. 1993;4:25–28. doi: 10.1097/00001756-199301000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 33.Dowson AJ, Dodick DW, Limmroth V. Medication overuse headache in patients with primary headache disorders: epidemiology, management and pathogenesis. CNS Drugs. 2005;19:483–497. doi: 10.2165/00023210-200519060-00002. [DOI] [PubMed] [Google Scholar]
- 34.Nestvold K, Kloster R, Partinen M, Sulkava R. Treatment of acute migraine attack: naproxen and placebo compared. Cephalalgia. 1985;5:115–119. doi: 10.1046/j.1468-2982.1985.0502115.x. [DOI] [PubMed] [Google Scholar]
- 35.Lipton RB, Dodick DW. CGRP antagonists in the acute treatment of migraine. Lancet Neurol. 2004;3:332. doi: 10.1016/S1474-4422(04)00764-1. [DOI] [PubMed] [Google Scholar]
- 36.Goldstein DJ, Wang O, Saper JR, et al. Ineffectiveness of neurokinin-1 antagonist in acute migraine: a crossover study. Cephalalgia. 1997;17:785–790. doi: 10.1046/j.1468-2982.1997.1707785.x. [DOI] [PubMed] [Google Scholar]
- 37.Burgey CS, Paone DV, Shaw AW, et al. Synthesis of the (3R,6S)-3-amino-6-(2,3-difluorophenyl)azepan-2-one of telcagepant (MK-0974), a calcitonin gene-related peptide receptor antagonist for the treatment of migraine headache. Org Lett. 2008;10:3235–3238. doi: 10.1021/ol8011524. [DOI] [PubMed] [Google Scholar]
- 38.Doods H, Arndt K, Rudolf K, Just S. CGRP antagonists: unravelling the role of CGRP in migraine. Trends Pharmacol Sci. 2007;28:580–587. doi: 10.1016/j.tips.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 39.May A, Goadsby PJ. Substance P receptor antagonists in the therapy of migraine. Expert Opin Investig Drugs. 2001;10:673–678. doi: 10.1517/13543784.10.4.673. [DOI] [PubMed] [Google Scholar]
- 40.Scholz J, Woolf CJ. The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci. 2007;10:1361–1368. doi: 10.1038/nn1992. [DOI] [PubMed] [Google Scholar]
- 41.Bigal ME, Ferrari M, Silberstein SD, et al. Migraine in the triptan era: lessons from epidemiology, pathophysiology, and clinical science. Headache. 2009;49(suppl 1):S21–S33. doi: 10.1111/j.1526-4610.2008.01336.x. [DOI] [PubMed] [Google Scholar]
- 42.Bigal ME, Lipton RB. Excessive acute migraine medication use and migraine progression. Neurology. 2008;71:1821–1828. doi: 10.1212/01.wnl.0000335946.53860.1d. [DOI] [PubMed] [Google Scholar]
- 43.Dodick D, Freitag F. Evidence-based understanding of medication-overuse headache: clinical implications. Headache. 2006;46(suppl 4):S202–S211. doi: 10.1111/j.1526-4610.2006.00604.x. [DOI] [PubMed] [Google Scholar]
- 44.Jakubowski M, Levy D, Goor-Aryeh I, et al. Terminating migraine with allodynia and ongoing central sensitization using parenteral administration of COX1/COX2 inhibitors. Headache. 2005;45:850– 861. doi: 10.1111/j.1526-4610.2005.05153.x. [DOI] [PubMed] [Google Scholar]
- 45.Jakubowski M, Levy D, Kainz V, et al. Sensitization of central trigeminovascular neurons: blockade by intravenous naproxen infusion. Neuroscience. 2007;148:573–583. doi: 10.1016/j.neuroscience.2007.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rost K, Fleischer F, Nieber K. Neurokinin 1 receptor antagonists-– between hope and disappointment [in German] Med Monatsschr Pharm. 2006;29:200–205. [PubMed] [Google Scholar]
- 47.Arulmani U, Maassenvandenbrink A, Villalon CM, Saxena PR. Calcitonin gene-related peptide and its role in migraine pathophysiology. Eur J Pharmacol. 2004;500:315–330. doi: 10.1016/j.ejphar.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 48.Edvinsson ML, Edvinsson L. Comparison of CGRP and NO responses in the human peripheral microcirculation of migraine and control subjects. Cephalalgia. 2008;28:563–566. doi: 10.1111/j.1468-2982.2008.01558.x. [DOI] [PubMed] [Google Scholar]
- 49.Ahn AH, Basbaum AI. Tissue injury regulates serotonin 1D receptor expression: implications for the control of migraine and inflammatory pain. J Neurosci. 2006;26:8332–8338. doi: 10.1523/JNEUROSCI.1989-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ma QP, Hill R, Sirinathsinghji D. Colocalization of CGRP with 5-HT1B/1D receptors and substance P in trigeminal ganglion neurons in rats. Eur J Neurosci. 2001;13:2099–2104. doi: 10.1046/j.0953-816x.2001.01586.x. [DOI] [PubMed] [Google Scholar]
- 51.Wessendorf MW. Fluoro-Gold: composition, and mechanism of uptake. Brain Res. 1991;553:135–148. doi: 10.1016/0006-8993(91)90241-m. [DOI] [PubMed] [Google Scholar]
- 52.Vetter G, Geisslinger G, Tegeder I. Release of glutamate, nitric oxide and prostaglandin E2 and metabolic activity in the spinal cord of rats following peripheral nociceptive stimulation. Pain. 2001;92:213–218. doi: 10.1016/s0304-3959(01)00258-5. [DOI] [PubMed] [Google Scholar]
- 53.Meller ST, Gebhart GF. Nitric oxide (NO) and nociceptive processing in the spinal cord. Pain. 1993;52:127–136. doi: 10.1016/0304-3959(93)90124-8. [DOI] [PubMed] [Google Scholar]
- 54.Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- 55.Gardell LR, King T, Ossipov MH, et al. Opioid receptor-mediated hyperalgesia and antinociceptive tolerance induced by sustained opiate delivery. Neurosci Lett. 2006;396:44– 49. doi: 10.1016/j.neulet.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 56.Ossipov MH, Lai J, King T, et al. Antinociceptive and nociceptive actions of opioids. J Neurobiol. 2004;61:126–148. doi: 10.1002/neu.20091. [DOI] [PubMed] [Google Scholar]
- 57.Ossipov MH, Lai J, Vanderah TW, Porreca F. Induction of pain facilitation by sustained opioid exposure: relationship to opioid antinociceptive tolerance. Life Sci. 2003;73:783–800. doi: 10.1016/s0024-3205(03)00410-7. [DOI] [PubMed] [Google Scholar]
- 58.Rivat C, Laboureyras E, Laulin JP, et al. Non-nociceptive environmental stress induces hyperalgesia, not analgesia, in pain and opioid-experienced rats. Neuropsychopharmacology. 2007;32:2217–2228. doi: 10.1038/sj.npp.1301340. [DOI] [PubMed] [Google Scholar]
- 59.Young WB. Medication overuse headache. Curr Treat Options Neurol. 2001;3:181–188. doi: 10.1007/s11940-001-0053-2. [DOI] [PubMed] [Google Scholar]
- 60.Gardell LR, Burgess SE, Dogrul A, et al. Pronociceptive effects of spinal dynorphin promote cannabinoid-induced pain and antinociceptive tolerance. Pain. 2002;98:79– 88. doi: 10.1016/s0304-3959(01)00475-4. [DOI] [PubMed] [Google Scholar]
- 61.Quartilho A, Mata HP, Ibrahim MM, et al. Production of paradoxical sensory hypersensitivity by alpha 2-adrenoreceptor agonists. Anesthesiology. 2004;100:1538–1544. doi: 10.1097/00000542-200406000-00029. [DOI] [PubMed] [Google Scholar]
- 62.Neeb L, Reuter U. Nitric oxide in migraine. CNS Neurol Disord Drug Targets. 2007;6:258–264. doi: 10.2174/187152707781387233. [DOI] [PubMed] [Google Scholar]
- 63.Edvinsson L, Goadsby PJ. Neuropeptides in the cerebral circulation: relevance to headache. Cephalalgia. 1995;15:272–276. doi: 10.1046/j.1468-2982.1995.1504272.x. [DOI] [PubMed] [Google Scholar]
- 64.Goadsby PJ, Edvinsson L. Human in vivo evidence for trigemi-novascular activation in cluster headache. Neuropeptide changes and effects of acute attacks therapies. Brain. 1994;117(pt 3):427–434. doi: 10.1093/brain/117.3.427. [DOI] [PubMed] [Google Scholar]
- 65.Sarchielli P, Alberti A, Russo S, et al. Nitric oxide pathway, Ca2+, and serotonin content in platelets from patients suffering from chronic daily headache. Cephalalgia. 1999;19:810– 816. doi: 10.1046/j.1468-2982.1999.1909810.x. [DOI] [PubMed] [Google Scholar]