Abstract
Alcohol use disorders (abuse and dependence, AUD) are multifactorial phenomena, depending on the interplay of environmental and genetic variables. This review describes current developments in animal research that may help (a) develop gene therapies for the treatment of alcoholism, (b) understand the permissive role of stress on ethanol intake and (c) elucidate why exposure to ethanol early in life is associated with a greater risk of AUD. The polymorphisms found in liver alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) affect the elimination of ethanol and the susceptibility to ethanol intake. A highly-active ADH protects against alcoholism, an effect related to a pre-steady state burst in arterial acetaldehyde. Social stressors, such as repeated early maternal separation or social defeat, exert a permissive effect on ethanol intake, perhaps by altering the normal development of the hypothalamic–pituitary–adrenal axis. Ethanol exposure during gestation, infancy or adolescence increases the likelihood of AUD later in life. Early perception of ethanol’s positive and negative (anti-anxiety) reinforcing effects may play a role in this phenomenon. The review underscores the advantages of using pre-clinical animal models of AUD and highlights points of intersection between the topics to help design a more integrated approach for the study of alcohol-related problems.
Keywords: Ethanol consumption, gene therapy, stress, social stress, rat, mouse, ontogeny, behavioral sensitization, motivational learning, intake
INTRODUCTION
Alcohol abuse and dependence (AUD) are pervasive disorders. In a recent study conducted in the U.S. 42 percent of men and 19 percent of women reported having had alcohol-related problems at some point in their lives (Hassin et al., 2007). It is generally accepted that these disorders are determined by multiple genetic and environmental factors. This review focuses on several factors that contribute to modifying alcohol consumption, as revealed by recent pre-clinical animal research.
Specifically, the review will focus on a triad of factors encompassing drug-induced effects, genetic and environmental factors. We describe developments in animal research that may help (a) develop gene therapies for the treatment of alcoholism, (b) understand the permissive role of stress on ethanol intake and (c) elucidate why exposure to alcohol during adolescence significantly increases the likelihood of AUD later in life (DeWitt, 2000). While some of these topics have been extensively covered in the literature (see Pautassi et al., 2009; Rivera-Meza et al, 2009; Ducci and Goldman, 2008; Miczek et al., 2008; Spear and Varlinskaya, 2005), the one purpose of the review is to provide a brief account of recent findings presented at the RSA-ISBRA 2009 Conference and to highlight points of intersection between the topics, to help design a more integrated approach for the study of alcohol-related problems. Substantial effort is also made to explain the specific human phenomena that each animal model aims at reproducing and the translation between human and animal research.
Genetic determinants in alcohol metabolism and potential therapies for alcohol abuse disorders
Genetic factors have long been studied in the etiology of AUD. These factors have been unraveled through screening of family histories: those with first-, second- or third degree relatives with an AUD diagnosis are labeled as family history positive and face a significantly higher risk of developing alcohol dependence than unrelated individuals. Indeed, the level of heritability for alcohol dependence is of the order of 50% (Ducci & Goldman, 2008). The search for a specific, single gene that regulates the vulnerability to become an alcoholic has, however, generally proven elusive. AUD are likely polygenic disorders with more than one gene involved (Strat et al., 2008).
One way to narrow down the search for the genes that contribute to the development of AUD is to focus on bio-markers of alcohol problems. These so-called endophenotypes can be defined as indirect links between genes and the behavioral expressions of AUD (Dick & Agrawal, 2008). Among these markers, the P300 event-related potential (Singh and Basu, 2009) has been one of the most studied. Yet another research path involves conducting genome-wide scans of large cohorts of individuals who either met the diagnostic criteria for AUD or have a family history of AUD (Strat et al., 2008). These linkage studies, many conducted by the collaborative study on the genetics of alcoholism (COGA), led to the identification of several genes or genetic polymorphisms that appear associated with the vulnerability to develop alcoholism (Edenberg & Foroud, 2006).
Recently, a review by a number of prominent alcohol scientists (Crabbe et al 2006) addressed the polymorphisms that affect a wide range of alcohol responses (akin to endophenotypes in humans) in selected animal strains. These investigators, who analyzed 93 genes that affect alcohol responses, indicated: “despite the importance of inherited contributions, we know for certain that only two genes affect the alcoholism risk” (Crabbe et al 2006). These are the polymorphisms of genes coding for liver alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH). ADH and ALDH are the most important enzymes involved in alcohol metabolism. ADH oxidizes alcohol to acetaldehyde, which in turn is oxidized to acetic acid by ALDH2 (the high affinity liver mitochondrial ALDH). Both ADH and ALDH are polymorphic in humans. There are amino acid differences in these polymorphic enzymes that result from changes in single nucleotides, which change the activity of these enzymes (Edenberg, 2007). Some individuals carry a dominant negative ALDH2 allele (ALDH2*2). This polymorphism, highly prevalent in populations from East Asia, is associated with a less active ALDH2 and, hence, with high blood acetaldehyde levels upon ethanol intake. In these individuals, alcohol ingestion leads to a number of unpleasant effects, including hypotension, nausea, dizziness and a characteristic facial flushing response. This aversive reaction seems to act as a protective factor for alcohol abuse and alcoholism, reducing the likelihood of developing these disorders by 60–95% (Luczak et al., 2002; Tu & Israel, 1995). The discovery of this phenomenon is consistent with the use of pharmacological therapies, notably the use of disulfiram, a drug that blocks the metabolism of acetaldehyde after alcohol consumption (Kiefer and Mann, 2005). One in every ten physicians in the U.S. prescribes disulfiram (Mark et al., 2003). The use of disulfiram has, however, faced several obstacles, mainly the low drug-taking compliance of alcoholics and the inhibition of dopamine beta hydroxylase, which inhibits the synthesis of norepinephrine. When compliance is assured by a third person, disulfiram has a powerful inhibitory effect on alcohol consumption (Chick et al 1992).
There is also the belief that disulfiram has untoward hepatic effects, although this has been not clearly demonstrated, except for case reports of idiosyncratic hypersensitivity known to most drugs. The FDA, which critically follows untoward Phase IV reactions, has clearly not recalled disulfiram nor has pointed out to liver dysfunction contraindications (www.drugs.com/pro/disulfiram.html). Indeed, well conducted animal studies indicate that disulfiram protects from liver necrosis and inflammation induced by alcohol (Lindros et al 1999). Recently, Ivan Diamond and associates (Arolfo et al 2009), following nearly a decade of work on the search of analogs of the inhibitor of ALDH2 present in the Chinese herb kudzu, developed analogs that display dual effects; elevating acetaldehyde levels by inhibiting ALDH2, thus preventing ethanol volition, and also reducing relapse. Compliance for this short half-life analog will likely be met by slow release formulations.
Although at present there are no human gene therapies which modify the expression of the two genes that markedly affect the levels of acetaldehyde and thus volition, two recent pre-clinical studies provide a “proof of concept” for this possibility. In the first of these studies alcohol drinker rats (UChB), selectively bred for 70 generations for their high alcohol intake, were rendered alcohol dependent (and likely tolerant) by offering 10% ethanol and water for two months and subsequently alcohol was withdrawn for three days. When alcohol availability was reestablished in a limited access paradigm, these animals consumed 10-times more alcohol than naïve rats. Ocaranza and coworkers (2008) administered a single dose of an anti-Aldh2 antisense gene to these animals, which reduced liver ALDH2 activity by 80% and inhibited by 50% the voluntary ethanol intake. The inhibitory effect lasted for a full month, the duration of the study.
In this 2009 ISBRA-RSA-LASBRA symposium, Israel et al. concentrated primarily on the study of the mechanisms by which the high-activity of alcohol dehydrogenases (ADH1*B2) protects against alcoholism (Quintanilla et al., 2007; Rivera-Meza et al., 2009). This group demonstrated that an elevated liver ADH activity achieved by the administration of the rat analog of the high activity human ADH1B*2 cDNA to alcohol drinker rats led to a blood “acetaldehyde burst”, which inhibited alcohol intake by 50%. There were two important characteristics to this “burst” (i) it was short duration, disappearing 15 minutes after ethanol administration and (ii) it was only evidenced in arterial blood (blood that flows before perfusing most body tissues). The latter feature is explained by the fact that, unless the ALDH2 is inactivated chemically or genetically, virtually all body tissues have a high ALDH2 activity, which removes acetaldehyde from the blood; thus this metabolite of ethanol is not found in venous blood. Indeed, humans who are markedly protected against alcoholism by the ADH1B*2 allele show no venous acetaldehyde levels (Peng et al 2007). The short duration was explained by the initial contribution of endogenous blood and liver pyruvate, which reoxidizes NADH into NAD+, thus speeding the generation of acetaldehyde by ADH (Quintanilla et al 2007). Thus, the title of Israel’s talk was “It Takes Two to Tango”, namely proposing that both a high ADH and the high pyruvate levels that exist at the very start of ethanol metabolism are needed to generate an acetaldehyde burst, and an aversion to alcohol.
Overall, these studies suggest that increasing hepatic ADH activity or reducing liver ALDH2 activity by gene therapy may become valuable long-lasting adjuncts in the therapy of alcoholism. It is well known, however, that genetics can be modulated by environmental conditions, such as stress and precocious exposure to ethanol. The effects of stress and early exposure to ethanol on alcohol abuse or dependence are discussed below.
Environmental factors: permissive role of stress on alcohol intake
The Tension Reduction Hypothesis (Conger, 1956) depicts alcohol consumption as a self-medication behavior aimed at reducing an ongoing aversive state. In this framework ethanol intake would be mainly driven by the anxiolytic effects of alcohol. Indeed, a relationship between stress and alcohol consumption has been long claimed (for an early review, see Pohorecky, 1981). The exact nature of the association between stress events and ethanol consumption, though, is still unclear and many findings are contradictory. Exteroceptive nociceptive stimulation (i.e., footshock), prototypical common source of stress, has been observed to either increase, decrease or exert no effects on ethanol intake, as measured in pre-clinical animal models of ethanol intake. Factors such as intensity, frequency, and the interval between stress exposure and ethanol intake assessments are critical in determining the outcome of footshock on ethanol intake patterns (Caplan and Puglisi, 1986; Champagne and Kirouac, 1987; Volpicelli et al., 1990). For instance, rats given unavoidable and high-intense footshock exhibited heightened ethanol intake (Caplan and Puglisi, 1986). Yet in another rat study the same stressor failed to alter ethanol sensitivity when given twenty minutes before the intake assessment (Ponce et al., 2004). Similar controversies have been observed when employing alternative stress sources. For example, chronic swim and restraint stress have been observed to decrease and increase, respectively, ethanol intake in rodents (Boyce-Rustay et al., 2008; Overstreet et al., 2007).
It has been suggested that the validity of stress-related research can be enhanced by designing animal models that rely on biologically-significant stimuli and situations. Social stress models are among these ethologically relevant models. Social stress should be particularly relevant when the species of choice relies heavily on parental care and social organization. Among these models, the stress associated with social conflict and maternal separation exhibits good face and construct validity (see Miczek et al., 2008).
Social defeat and subsequent social subordination is a means to determine reduced access to food and resources, but can also be evoked in experimental conditions by introducing an intruder rat to an aggressive resident (Miczek et al., 2008). These conflicts, particularly when they are inescapable or unpredictable (Tornatzky & Miczek, 1993), lead to a number of short- and long-lasting hormonal, behavioral and neural changes and are also thought to play significant role in the maintenance of alcohol intake, facilitating the transition from a controlled to a more problematic pattern of drug self-administration (Tidey and Miczek, 1996). The evidence for the latter statement is substantial for psychostimulant drugs (e.g., cocaine, Tidey and Mickzek, 1997) but much less so for ethanol. Social defeat stress has a long-term increasing effect on ethanol intake in CRH1 mutant mice (Sillaber et al., 2002) and rats exposed to an odor cue previously paired with social defeat show significant reinstatement of ethanol self-administration (Funk et al. 2005). The latter finding suggests that social stress can endow previously neutral stimulus with the capability of inducing relapse into alcohol drinking. Other studies, however, indicate that chronic social defeat or subordination leads to a reduction in ethanol intake (Van Erp et al., 2001) or ethanol reinforcement (Van Erp & Miczek, 2001).
Besides differences in strain or species, the discrepancy in the effects of stress on ethanol intake might be explained by procedural differences. The heightened voluntary alcohol intake displayed by mutant mice (Sillaber et al., 2002) was observed after a relatively brief (3 consecutive days) schedule of repeated social defeat. On the other hand, the Van Erp et al., (2001) study employed three periods of five consecutive days of social stress. The postulates of the Yerkes-Dodson law (1908) may also help understand these differences. This law suggests that an inverted U-shaped curve is the best fit for the relationship between stress and drug taking. Brief, moderate and intermittent stress would yield behavioral activation, arousal and, if available, drug intake; whereas more intense stress events would lead to behavioral suppression and less engagement in drug taking (Miczek et al., 2008).
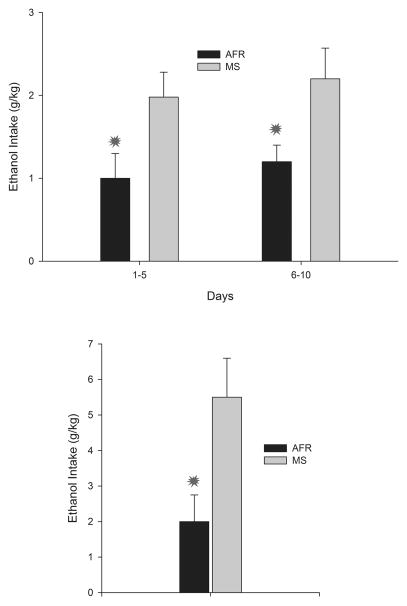
Maternal separation stress has been proposed as a model of early maltreatment. The disruption of the mother–infant bond, even for relatively brief periods of time, causes short (e.g., ultrasonic distress calls, Kraebel et al., 2002) and long-term alterations (e.g., differential functionality of the HPA axis, Plotsky and Meaney, 1993; Rosenfeld et al., 1992) in the developing organism. Animals subjected to relatively brief or prolonged episodes of social stress exhibit heightened anxiety when subsequently confronted with stressors and, perhaps more important for the purposes of this review, high vulnerability to initiate self-administration of several drugs of abuse, including ethanol (Huot et al., 2001). Perhaps one of the most impressive findings of the permissive role of social stress on ethanol intake has been provided by Huot and coworkers (2001). These researchers assessed the initial intake of ethanol and sucrose in adult animals that, on postnatal days 2–14, had been separated from their mother 180 minutes each day or reared under standard animal facility conditions. Early maternal separation engendered in adulthood a five-fold increase in ethanol intake and with a decrease in sucrose preference, the latter likely reflecting an anhedonic state (Huot et al., 2001). In a recent study, mice that underwent prolonged maternal separation (180 min per day, PDs 2–14; Cruz et al., 2008) not only drank more ethanol when assessed in a three-bottle procedure (see Figure 1, upper panel), but also exhibited higher operant ethanol-administration relative to those reared under standard conditions (particularly when offered a relatively high ethanol concentration, 10% v/v; see Figure 1, lower panel). Operant conditioning procedures provide more compelling evidence of ethanol preference, as they require animals execute a given behavior to gain access to ethanol.
Figure 1.
Upper panel Ethanol intake (gram per kilogram of body weight, g/kg) in adult mice that underwent maternal separation as infants (MS) or were reared under normal animal facility conditions (AFR). Ethanol intake was tested in a three-bottle choice procedure (6% ethanol, 10% ethanol or its vehicle, a 0.05% saccharin solution). To facilitate data visualization ethanol intake has been averaged over two 5-day blocks. Lower panel: Ethanol intake in the home cage (gram per kilogram of body weight, g/kg) of a 10% alcohol solution, by AFR and MS mice in 60-min sessions using an operant self-administration procedure. Asterisks indicate significant differences between a paired group and its corresponding unpaired control (p < 0.05). Vertical Bars indicate the standard error of the means (SEM). Adapted from: Cruz et al., (2008) Maternal separation stress in male mice: long-term increases in alcohol intake. Psychopharmacology 201, 459–468.
Some conflicting results on the effects of early maternal separation have been also reported. Employing procedures that closely resembled those of Huot et al., (2001), Jaworski an coworkers (2005) also observed greater ethanol consumption in rats with a history of prolonged maternal separation than in animals that had experienced no or minimal maternal separation. Yet the greatest level of ethanol consumption, among the conditions under analysis, was observed in non-handled animals.
It has been suggested (Cruz et al., 2008) that the effects of early maternal separation on ethanol intake are a by-product of the alterations induced by this treatment on the functioning of the hypothalamic–pituitary–adrenal (HPA) axis. Indeed, it is intriguing that maternal separation treatments usually occur during a developmental time point known as the stress hypo-responsive period (Walker et al., 1986). During approximately the first two weeks of life, rats have a blunted hormonal response to several stressors (Rosenfeld et al., 1992). Adequate maternal care during this period is critical for the integrity of HPA axis; even brief handling at this period is known to alter the functioning of the system (Stamatakis et al., 2008). In general terms, brief (15 min) and long (180 min) daily maternal separation result in hypo and hypersensitivity, respectively, of the HPA axis (Miczek et al., 2008). Intriguingly, some studies (e.g., Ploj et al., 2003) have suggested that brief maternal separation episodes may be linked with a decreased vulnerability to ethanol intake in adulthood. Early maternal separation as a mean to induce early stress shares many features with prenatal stress (PNS), a protocol in which dams are subjected to daily stress events, usually during the last section of the pregnancy (Campbell et al., 2009). The offspring of these dams exhibits alteration in the HPA axis function, enhanced sensitivity to stressors and greater operant responding for alcohol (Campbell et al., 2009).
There is also an extrahypothalamic corticotrophin releasing factor system; and this system has been implicated in the regulation of ethanol’s effects and ethanol dependence (Soomer and Saavedra, 2008). For instance, extrahypothalamic corticotropin-releasing factor receptors might be responsible for the expression of ethanol-induced behavioral sensitization, once already formed (Pastor et al., 2008), and also play a role in stress-induced reinstatement of alcohol seeking (Liu et al., 2002). Perhaps the changes on ethanol intake after early maternal separation relate to alterations in this extrahypothalamic system rather than on the HPA access. The important role of extrahypothalamic corticotrophin-releasing hormone receptors in the modulation of the alcohol-stress interaction has motivated the development of antagonists for this system. Among these, 3-(4-Chloro-2-morpholin-4-yl-thiazol-5-yl)-8-(1-ethylpropyl)-2,6-dimethylimidazo[1,2-b]pyridazine (MTIP) has proved effective in reducing alcohol consumption and blocking stress-induced relapse in alcohol dependent rats and in rats with genetic suceptibility to alcohol consumption (Gehlert et al., 2007).
Recent studies have provided an exciting new perspective as to how genetic factors interact with early social stress to produce phenotypes exhibiting heightened vulnerability to alcohol consumption. Barr et al. (2009) detected a single-nucleotide polymorphism (SNP) in the gen coding for the corticotropin-releasing factor (CRH) in rhesus macaques. These animals showed heightened release of adrenocorticotropic hormone and heightened sensitivity to the facilitating effects of early social stress upon alcohol consumption. These results suggest an interaction between genotype and early stress experiences. Analogous conclusions can be drawn from a human study that assessed if alterations in the CRH R1 gen would modulate the association between stressful life events and an early onset of alcohol consumption (Schmid et al., 2009). There was a negative correlation between numbers of stressful life events prior to drinking onset and age at first drink, but only for those subjects homozygotes for one of the SNP analyzed.
The facilitatory effects of stress on ethanol consumption or in ethanol effects are not restricted to the studies reviewed here. Stress can facilitate the expression of behavioral sensitization to ethanol (Roberts et al., 1995) and conditioned place preference induced by the drug (Matsuzawa et al., 1998; 2000). Also, it is important to remark that social stress leading to higher ethanol intake (Wolffgram, 1990) and increased sensitivity to ethanol’s motor activating effects (Araujo et al., 2005) can result from simply changing the standard housing conditions of rodents to create conditions of social isolation or social crowding.
In summary, stress successfully modulates initiation, maintenance and relapse of ethanol-self administration. The employment of species-specific stress models holds promise for understanding the exact mechanisms underlying these effects.
Environmental factors: early exposure to ethanol in early ontogeny and adolescence and later affinity for the drug
Epidemiological research (Baer et al., 2003) indicates that a prenatal history of ethanol exposure strongly predicts alcohol consumption in adolescence. The timing and magnitude of ethanol exposure during adolescence, in turn, relates to the likelihood of problematic drug use later in life (Grant and Dawson, 1997). In general terms, the earlier children start to drink, the higher the likelihood of alcohol abuse and dependence (“early debut effect”, Pedersen and Skrondal, 1998). The relationship, however, is not strictly linear, as there are developmental periods (e.g., 12–13 years; DeWitt, 2000) in which alcohol exposure exerts an even greater effect upon later affinity for the drug. Furthermore, early contact with ethanol is not restricted to the prenatal and adolescent stages of development. Cultural practices promote exposure to ethanol through milk during breastfeeding (Mennella and Beauchamp, 1993) as well as through routine medical procedures (Fildes, 1986; Flores-Huerta et al, 1992). For instance, in some cultures infants suffering from stomach spasms are treated with a heated cloth containing alcohol placed on the abdomen. The practice, which intends to alleviate the pain associated with the spasms, can yield high infantile blood ethanol concentrations (Croce, 1987). Pre-clinical research that employed an analogous high-dose exposure to ethanol during the preweanling period of the rat (PDs 6–12) found heightened ethanol consumption when tested at infancy (PD 15, Lopez and Molina, 1999) or adulthood (PD 120, Hayashi and Tadakoro, 1985).
There is an ongoing debate as to whether the relationship between age of first alcohol experience and problematic alcohol use reflects a causal relationship (Buchmann et al., 2009). On the one hand, exposure to alcohol early in life can alter the normal pattern of brain development or interfere with the accomplishment of several age-specific tasks. This possibility is particularly serious during adolescence, a time point in which the dopaminergic mesocorticolimbic system and the prefrontal cortex undergo profound changes and organisms are required to weaken their parental bonds and explore new social opportunities (Spear, 2000).
Several studies suggest that adolescence is a period of heightened vulnerability to the neurotoxic effects of alcohol (Medina et al., 2008). Alcohol drinking during adolescence is associated with a reduction in the volume of several brain structures, including the hippocampus and the prefrontal cortex (PFC) (Squeglia et al., 2009; Lubman et al., 2007). The ethanol-related damage to the PFC is usually greater in females than in males and seems to be driven by alterations in the volume of white matter (Medina et al., 2008). These findings are consistent with female drinkers having less frontal activation when tested in a spatial working memory test (Caldwell et al., 2005) and reduced blood flow into the brain when compared with age-matched light drinkers (Suzuki et al. 2002). Heavy-drinker adolescents also exhibited greater brain activation in several brain areas (including PFC) when exposed to alcohol-related advertisements than when exposed to neutral advertisements (Tapert et al., 2003).
Early onset of drinking and later alcohol abuse or dependence could also be the expression of a common genetic susceptibility. This hypothesis has received some support from twin studies (McGue et al., 2001; Prescott & Kendler, 1999). The mechanisms underlying these early exposure effects are not well understood and will be uncovered only by ontogenetic study. The use of animal models has proven to be a valuable tool for answering these questions (Spear & Varlinskaya, 2005).
The last years have seen an explosion in the study of ethanol’s effects during adolescence. Adolescent rats (i.e., animals 28–42 days old), when compared to adults (i.e., animals >70 days old) show a distinctive pattern of reactivity to drugs of abuse, including ethanol (Badanich et al, 2006; Camarini et al, 2008; Faria et al, 2008). Adolescents are less sensitive to a wide array of sedative (e.g., narcosis, motor coordination, Spear, 2004; White et al., 2002) and stimulant effects of ethanol (e.g. behavioral sensitization, Faria et al, 2008; Stevenson et al, 2008) but, conversely, show more cognitive impairment after treatment with the drug, particularly when assessed in tasks that require the integrity of the hippocampus (Markiwiese et al., 1998). It has been suggested that this differential pattern of reactivity to ethanol might put adolescents at risk for problematic drug use (Spear & Varlinskaya, 2005). The underlying rationale is that the drug’s sedative effects serve as biological cues to limit further drug intake, while heightened cognitive impairment may preclude adequate judgment about the size of the drinking bout or cause engagement in risky behavior, which in turn could lead to an escalation in drinking behavior. In agreement with these postulates, several pre-clinical studies reported heightened ethanol intake (2–3 times higher when measured as gram per kilogram of body weight) in adolescents than in adults, under a variety of testing circumstances (Brunell and Spear, 2005; Doremus et al., 2005; Truxell et al., 2007; Vetter et al., 2005; 2007).
Recent data also suggest that adolescent rats may be more sensitive to ethanol’s positive motivational reinforcing effects relative to their older counterparts. Adolescent, but not adult, rats expressed ethanol-mediated first (Philpot et al., 2003) and second-order conditioned preferences (Pautassi et al., 2008). By first-order conditioning preference we mean the capability of ethanol to transfer its appetitive motivational information to a distinctive conditional stimulus (CS1), which then is preferred at test. A second-order procedure comprises an additional set of pairings. In these pairings the CS1 signals a second stimulus (CS2). During this stage the CS1 transfers behavioral control to the CS2, which now can evoke conditioned preference (Molina et al., 2007). Furthermore, early work (Fernández Vidal et al., 2003) suggested that low-dose ethanol (0.5 g/kg) may induce conditioned taste preference. The study of ethanol-mediated conditioned preferences in adolescent animals, however, is still under development and more work is needed to achieve firm conclusions. Yet it is intriguing that adolescent rats exhibit conditioned preference by ethanol (Philpot et al., 2003; Pautassi et al., 2008). Several studies suggest that, at adulthood, rats and mice differ in their sensitivity to ethanol’s appetitive effects in the place conditioning paradigm, a first-order conditioning paradigm that measures conditioned preferences. Adult mice readily express ethanol-mediated place conditioning (dose: 0.5 – 4.0 g/kg; Cunningham et al., 2006). In contrast, the expression of ethanol-mediated place conditioning in adult rats has proven problematic. Unlike mice, rats tend to avoid contexts or surfaces that signal the drug (Cunningham et al., 1993). Some success has been observed when ethanol is co-administered with other drugs (Marglin, 1988) or with stressors such as footshock (Matsuzawa et al., 1998).
Interestingly, some studies with mice revealed a somehow different pattern, with adolescent mice requiring either a higher ethanol dose or more conditioning trials to express similar ethanol-mediated conditioned preference than adult mice (Dickinson et al., 2009). Also in mice, administration of 2.0 g/kg ethanol for 15 consecutive days in the ethanol-paired environment induced a typical pattern of behavioral sensitization in adult, but not in adolescent mice (Faria et al., 2008). The younger subjects showed much less ethanol-induced motor activity than adults and exhibited a progressive decrease in their locomotor frequency across trials, a finding that suggests tolerance to a low dose of ethanol. However, ethanol-treated adolescents, but not adults, exhibited context-independent behavioral sensitization and also a desensitized expression of immediate-early genes in the nucleus accumbens and prefrontal cortex (Faria et al., 2008). These results suggest that associative mechanisms involved in the context-dependent behavioral sensitization are not functionally mature in adolescent mice. Behavioral sensitization induced by ethanol and other drugs of abuse is thought to contribute to the sensitivity to the reinforcing effects of these psychotropics (Araujo et al., 2005).
Based on the previous studies, it could be postulated that the usual pattern of species-related differences in ethanol-mediated place conditioning reverses when testing takes places at adolescence rather than at adulthood. Adult mice and rats usually express preference and aversion, respectively, when trained with ethanol. Yet adolescent rats but not adolescent mice, apparently express place conditioning by ethanol. This is just a hypothesis, however, as these species have not been yet examined in a comparable manner during the adolescent stage and, therefore, the differences may relate to differences in housing conditions, apparatus, stimuli, or temporal parameters across the studies.
The mechanisms underlying the facilitative effects of early ethanol exposure on later drinking are, as mentioned, still poorly understood. A recent work, however, found that adolescent rats given chronic and intermittent ethanol (3.0 g/kg) exhibited heightened ethanol intake as adults and also exhibited alterations in the mesolimbic dopaminergic and glutamatergic systems (Pascual et al., 2009). Specifically, adolescent but not adults, given chronic ethanol had greater basal dopamine levels in the nucleus accumbens and a downregulated NMDA receptor expression in prefrontal cortex (Pascual et al., 2009). These results fit well with the hypothesis that ethanol exposure during adolescence may be interfering with the normal pattern of neural pruning and synaptic reorganization occurring at this developmental stage (Spear, 2000).
Yet another possibility is that early ethanol exposure may facilitate the effect of stress on alcohol drinking. As mentioned in the previous section, under certain conditions, stress exposure leads to greater affinity for alcohol. In an attempt to model the “early debut effect”, Siegmund et al. (2005) found that, among animals initiated with alcohol at either adolescence or adulthood, those initiated at adolescents drank more alcohol than adults if given foot-shock or swim stress. These results agree with a subsequent human study (Dawson et al., 2007) revealing that the number of stress episodes in the last year was associated with greater alcohol consumption in those subjects that began drinking at age < = 14 but not in those that delayed their first drink until age > = 18. Overall, these studies suggest that early ethanol initiation may interact with stress experiences, facilitating initiation or escalation in alcohol consumption.
Alcohol exposure can also occur before adolescence, in the womb, as a result of maternal consumption, and this exposure can increase the vulnerability to problematic drinking in adulthood. Several pre-clinical studies conducted with rats have indicated that maternal treatment with ethanol during late gestation (gestational days 17–20, dose 1–2 g/kg) is associated with heightened ethanol intake in infancy (Abate et al., 2000; 2004), adolescence (Chotro et al., 2003) or adulthood (Bond & Di Giusto, 1976). Teratogenic effects are well-known consequences of ethanol during early ontogeny, as can be observed in rats (Stanton & Goodlett, 1998), mice and in children diagnosed with fetal alcohol syndrome (FAS). Individuals with FAS have developmental delays expressed in low weight and height for their age group, a characteristic pattern of facial dismorphology and neurological alterations (i.e., agenesis of the corpus callosum) that translates into moderate to severe mental retardation. Yet the association between early exposure to ethanol and heightened ethanol affinity can be exhibited in the absence of obvious teratology (see Dominguez et al., 1996). Chotro et al. (2007) reviewed potential mechanisms underlying this association and proposed that heightened postnatal preference for ethanol can result from chemosensory learning about ethanol. Maternal ethanol intoxication results in the fetus being exposed to both the odor and taste of ethanol and to the pharmacological postabsorptive effects of the drug. According to these and other authors (Spear & Molina, 2005; Abate et al., 2008), fetuses may learn an association between the chemosensory effects of the drug and its positive, rewarding effects. This Pavlovian learning, in turn, could regulate later ethanol seeking and intake. Yet, it is also possible that prenatal alcohol serves as a stressor, hence producing a similar endophenotype as that resulting from prenatal stress (PNS) protocols. As mentioned in the previous section, PNS not only induces neuroendocrine and behavioral hyperresponsiveness to stressors but also heightened sensitivity to the reinforcing properties of substances of abuse and greater affinity for alcohol (Campbell et al., 2009).
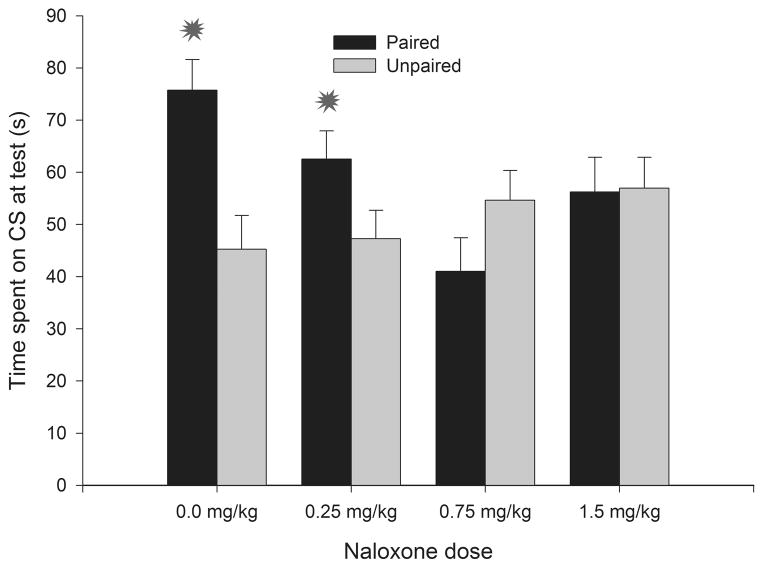
Altough reviewing the evidence for these proposals (Abate et al., 2008; Spear & Molina, 2005; Chotro et al., 2007) goes beyong the scope of this review, these theoretical accounts do highlight the need to assess ethanol’s reinforcing effects during the period of development corresponding to the third trimester in humans. The infant rat brain during the preweanling period (first three postnatal weeks, postnatal days 1 to 21, PDs 1–21) closely resembles the third trimester human (Goodlett and Johnson, 1997). The analysis of early sensitivity to ethanol in preweanling rats has indicated that infants are extremely sensititive to ethanol’s aversive effects, as indexed through conditioned aversion tests (Pautassi et al., 2002; 2005). But appetitive, positive rewarding effects are also apparent when employing first- and second-order place conditioning preparations (Pautassi et al., 2008; Nizhnikov et al., 2009; Molina et al., 2007). Similar to what has been found in adult rats, these reinforcing effects of ethanol seem to be facilitated by a prior history of non-reinforced exposure to the drug (Pautassi et al., 2008; Ponce et al., 2008) and can be blocked by opoid antagonism (Nizhnikov et al. 2009). Rat pups given pairings of a tactile CS (sandpaper) and 1.0 g/kg ethanol exhibit heightened preference for this CS than counterparts given unpaired exposure to this stimuli. This conditioned response, indicative of ethanol reinforcement, was completely inhibited in animals given naloxone (0.75 or 1.5 mg/kg) at time of conditioning. A lower dose of the antagonist (0.25 mg/kg) reduced but did not block, reinforcement (Nizhnikov et al. 2009). These results are depicted in Figure 2.
Figure 2.
Percent time spent on a conditioned stimulus (sandpaper, CS) as a function of conditioning procedures [sandpaper paired or unpaired with administration of 1.0 g/kg ethanol] and naloxone dose at time of conditioning (0.0, 0.25, 0.75 or 1.5 mg/kg). Ethanol was administered intragastrically, naloxone was given via the intraperitoneal route. Animals (14-day old rats) were tested in a two-way, 5-min preference test (sandpaper vs. cardboard). Asterisks indicate significant differences between a paired group and its corresponding unpaired control (p < 0.05). Vertical bars represent the standard error of the means (S.E.M.). Adapted from: Nizhnikov et al., (2009) Opioid antagonists block the acquisition of ethanol-mediated conditioned preference in infant rats. Alcohol 43, 347–358. Used with permission from publishers.
In summary, the study of ethanol reinforcement and intake in infant and adolescent rodents provides a window to analyze the effect of early onset of alcohol drinking upon later vulnerability to alcohol abuse and dependence. It also helps in the search for risk factors asssociated with heightened alcohol consumption during adolescence that favor the development of AUD.
DISCUSSION
Alcohol abuse and dependence (AUD) is a multifactorial phenomenon, requiring the interplay of environmental and genetic variables (Pautassi et al., 2008). It has been more difficult, however, to experimentally combine these different factors and assess the common and specific genetic and environmental contributions to AUD, although twin studies (e.g., Xian et al., 2008) and the use of rodents selectively bred for expressing differential sensitivity to ethanol’s effects have proven useful.
The present review describes recent pre-clinical advances on genetic and environmental factors that regulate ethanol seeking and intake. We first analyzed genetic polymorphisms of the enzymes associated with alcohol drinking. This natural pattern of genetic variability inspired the development of gene therapies aimed at interfering with the metabolism of a by-product of alcohol, acetaldehyde (Rivera-Meza et al., 2009). Exposure to maltreatment or aversive environments is known to strongly modulate alcohol consumption and relapse. We reviewed studies employing stress derived from social sources (i.e., social conflict and early maternal separation; Miczek et al., 2008) that, when contrasted with more traditional stress models, such as nociceptive exteroceptive stimulation (i.e., footshock), have the advantage of relying on age and species-specific aversive situations. We then changed the focus from adult rodents to the study of immature, preweanling (Pautassi et al., 2009) or adolescent (Faria, 2008) rats so as to provide information on the modulatory effect of early exposure to alcohol on later AUD development.
A common denominator across the experiments and lines of research reviewed is the aim of identifying sub-populations with differential susceptibility to develop AUD and, on the basis of that knowledge, to develop specific interventions for reducing problematic drinking. In other words, although pinpointing different mechanisms, each approach tries to understand why some individuals move quickly from alcohol use to abuse and dependence, while some subjects avoid engaging in problematic consumption. Another common denominator is that each model provides quite important information for understanding AUD, but, at the same time, fails to completely account for the total variance in the outcome under analysis. Indeed, discrepant findings across experiments seem to be the rule rather than the exception. Although alcoholism runs in families, the level of heritability of this disorder in the overall population rarely exceeds 50% (Ducci and Goldman, 2008). As mentioned, exposure to sources of stress often leads to heightened ethanol consumption (Overstreet et al., 2007), but sometimes is followed by a reduced propensity to engage in ethanol intake (Boyce-Rustay et al., 2008) or fails to alter this variable (Ponce et al., 2004). The effect of prior ethanol exposure is not immune to these considerations. Early exposure to ethanol is usually associated with heightened ethanol sensitivity (Abate et al., 2008) and apparently facilitates later learning comprising ethanol’s positive effects (Pautassi et al., 2008; Nizhnikov et al., 2009). However, some studies reported a lack of effect of age at drinking onset, in that initiation of exposure to ethanol during adolescence (via vapor inhalation, Slawecki & Betancourt, 2002; via oral forced access, Tolliver & Samson, 1991) did not affect ethanol consumption in adulthood.
One obvious weakness of the present studies, and a potential source of variability in their results, is the lack of interaction between the approaches. For instance, there is little to say about potential genetic modulation of the association between stress and ethanol intake. The work by Barr et al. (2009) with rhesus macaques helps understand these multiple phenomena. These researchers found an association between polymorphisms in the CRH gene and sensitivity to early-stress induced alcohol intake. As mentioned, early ethanol exposure can lead to heightened ethanol consumption later in life; the magnitude of this effect, though, markedly varies. Genetic differences in the response to ethanol may be important factors in the regulation of these differences. A fruitful path of research may involve analyzing early onset of drinking effects in lines of rats or mice selected for their high- or low sensitivity to ethanol’s effects. The present review also highlighted studies indicating that differential sensitivity to alcohol can result from adverse experiences early in life. Early stress in the form of maternal separation or prenatal stress affect alcohol affinity, apparently by altering the functioning of the HPA axis or the extrahypothalamic CRF system (Campbell et al., 2009; Cruz et al., 2008). The animal models described in the present review are certainly limited, but they should provide an avenue for analyzing the interactions between genetic polymorphism, early stress and early contact with the drug and how these influences affect initiation, maintenance and relapse into problematic alcohol intake.
For instance, epidemiological studies suggest that an early onset of ethanol consumption may exacerbate stress-mediated ethanol consumption (Dawson et al., 2007). To our knowledge, there have been little attempts to replicate this finding in a more controlled, experimental animal model. Yet it has been found that animals initiated with alcohol are more likely to engage in stress-mediated alcohol consumption than animals initiated at adulthood (Siegmund et al., 2005). This is a promising model for scrutinizing the interaction between stress-reactive drinking and alcohol consumption.
Novelty seeking and increased risk taking are considered hallmark traits of adolescence (Spear, 2000), that may represent “biological markers” or endophenotypes for drug dependence (Klebaur & Bardo, 1999). The analysis of ethanol reinforcement and sensitivity in genotypes or phenotypes exhibiting varying degrees of overlap between these traits has been, however, scarce. The work by Schramm-Sapyta (2008) represents progress towards this direction. Schramm-Sapyta et al. (2008) screened heterogeneous adolescent rats in terms of novelty-seeking, stress hormone levels and initial acceptance of ethanol. The aim was to use the normal range of individual differences in these variables to predict which individuals would later be vulnerable to high ethanol acceptance and relapse to drinking after an ethanol-deprivation period. Neither novelty-seeking nor the hormonal markers predicted later vulnerability to ethanol. Early ethanol intake, however, was significantly associated with later relapse-like behavior.
In summary, the present review focused on current developments in pre-clinical animal models with the aim of understanding different features associated with the initiation, maintenance or relapse into problematic ethanol consumption. Besides the scholarly purpose of describing their findings, the mini-review also underscored the need to promote interaction between these different approaches and translating animal research findings to epidemiological research.
Acknowledgments
This work was supported by by Fondeyt-Chile and the National Institute on Alcohol Abuse and Alcoholism (YI), the Agencia Nacional de Promocion Cientifica y Tecnologica (Argentina) and grant PRH-UNC (FONCyT-SPU) (Argentina) (RMP), and FAPESP (FUNDAÇÃO DE AMPARO À PESQUISA DO ESTADO DE SÃO PAULO) e CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico) (RC).
Footnotes
This review summarizes the proceedings of a symposium (“Determinants Of Ethanol Consumption: From Genetic To Environmental Influences”) organized and chaired by Rosana Camarini and presented at the joint ISBRA-RSA 2009 meeting. The symposium was sponsored by LASBRA (Latin American Society for Biomedical Research on Alcoholism).
References
- Abate P, Pepino M, Dominguez H, Spear N, Molina J. Fetal associative learning mediated through maternal alcohol intoxication. Alcohol Clin Exp Res. 2000;24:39–47. [PubMed] [Google Scholar]
- Abate P, Pepino M, Spear N, Molina J. Fetal learning with ethanol: correlations between maternal hypothermia during pregnancy and neonatal responsiveness to chemosensory cues of the drug. Alcohol Clin Exp Res. 2004;28:805–815. doi: 10.1097/01.alc.0000125354.15808.24. [DOI] [PubMed] [Google Scholar]
- Abate P, Pueta M, Spear NE, Molina JC. Fetal learning about ethanol and later ethanol responsiveness: evidence against “safe” amounts of prenatal exposure. Exp Biol Med (Maywood) 2008;233:139–154. doi: 10.3181/0703-MR-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo NP, Camarini R, Souza-Formigoni MLO, Carvalho RC, Ablio VC, Silva RH, Ricardo VP, Ribeiro RdA, Frussa-Filho R. The importance of housing conditions on behavioral sensitization and tolerance to ethanol. Pharmacol Biochem Behav. 2005;82:40–45. doi: 10.1016/j.pbb.2005.07.009. [DOI] [PubMed] [Google Scholar]
- Arolfo MP, Overstreet DH, Yao L, Fan P, Lawrence AJ, Tao G, Keung W-M, Vallee BL, Olive MF, Gass JT, Rubin E, Anni H, Hodge CW, Besheer J, Zablocki J, Leung K, Blackburn BK, Lange LG, Diamond I. Suppression of heavy drinking and alcohol seeking by a selective ALDH-2 inhibitor. Alcohol Clin Exp Res. 2009;33:1–10. doi: 10.1111/j.1530-0277.2009.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badanich KA, Adler KJ, Kirstein CL. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur J Pharmacol. 2006;21:95–106. doi: 10.1016/j.ejphar.2006.08.034. [DOI] [PubMed] [Google Scholar]
- Baer J, Sampson P, Barr H, Connor P, Streissguth A. A 21-year longitudinal analysis of the effects of prenatal alcohol exposure on young adult drinking. Arch Gen Psych. 2003;60:377–385. doi: 10.1001/archpsyc.60.4.377. [DOI] [PubMed] [Google Scholar]
- Barr CS, Dvoskin RL, Gupte M, Sommer W, Sun H, Schwandt ML, Lindell SG, Kasckow JW, Suomi SJ, Goldman D, Higley JD, Heilig M. Functional CRH variation increases stress-induced alcohol consumption in primates. Proc Natl Acad Sci US A. 2009;106:14593–14598. doi: 10.1073/pnas.0902863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond NW, Di Giusto EL. Effects of prenatal alcohol consumption in open-field behaviour and alcohol preference in rats. Psychopharmacologia. 1976;46:163–165. doi: 10.1007/BF00421386. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Janos AL, Holmes A. Effects of chronic swim stress on EtOH-related behaviors in C57BL/6J, DBA/2J and BALB/cByJ mice. Behav Brain Res. 2008;186:133–137. doi: 10.1016/j.bbr.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunell SC, Spear LP. Effect of stress on the voluntary intake of a sweetened ethanol solution in pair-housed adolescent and adult rats. Alcohol Clin Exp Res. 2005;29:1641–1653. doi: 10.1097/01.alc.0000179382.64752.13. [DOI] [PubMed] [Google Scholar]
- Buchmann AF, Schmid B, Blomeyer D, Becker K, Treutlein J, Zimmermann US, Jennen-Steinmetz C, Schmidt MH, Esser G, Banaschewski T, Rietschel M, Schumann G, Laucht M. Impact of age at first drink on vulnerability to alcohol-related problems: Testing the marker hypothesis in a prospective study of young adults. J Psychiatr Res. 2009 doi: 10.1016/j.jpsychires.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Caldwell LC, Schweinsburg AD, Nagel BJ, Barlett VC, Brown SA, Tapert SF. Gender and adolescent alcohol use disorders on bold (blood oxygen level dependent) response to spatial working memory. Alcohol Alcohol. 2005;40:194–200. doi: 10.1093/alcalc/agh134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camarini R, Griffin WC, 3rd, Yanke AB, Rosalina dos Santos B, Olive MF. Effects of adolescent exposure to cocaine on locomotor activity and extracellular dopamine and glutamate levels in nucleus accumbens of DBA/2J mice. Brain Res. 2008;1193:34–42. doi: 10.1016/j.brainres.2007.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JC, Szumlinski KK, Kippin TE. Contribution of early environmental stress to alcoholism vulnerability. Alcohol. 2009;43:547–554. doi: 10.1016/j.alcohol.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan MA, Puglisi K. Stress and conflict conditions leading to and maintaining voluntary alcohol consumption in rats. Pharmacol Biochem Behav. 1986;24:271–280. doi: 10.1016/0091-3057(86)90350-3. [DOI] [PubMed] [Google Scholar]
- Champagne F, Kirouac G. Effects of unavoidable electric shocks on voluntary alcohol consumption in the rat. Percept Mot Skills. 1987;64:335–338. doi: 10.2466/pms.1987.64.1.335. [DOI] [PubMed] [Google Scholar]
- Chick J, Gough K, Falkowski W, Kershaw, Mehta B, Ritson B, Ropner R, Torley D. Disulfiram treatment of alcoholism. Br J Psychiatry. 1992;161:84–89. doi: 10.1192/bjp.161.1.84. [DOI] [PubMed] [Google Scholar]
- Chotro M, Arias C. Prenatal exposure to ethanol increases ethanol consumption: a conditioned response? Alcohol. 2003;30:19–28. doi: 10.1016/s0741-8329(03)00037-5. [DOI] [PubMed] [Google Scholar]
- Chotro M, Arias C, Laviola G. Increased ethanol intake after prenatal ethanol exposure: Studies with animals. Neurosci Biobehav Rev. 2007;31:181–191. doi: 10.1016/j.neubiorev.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Conger JJ. Alcoholism: theory, problem and challenge. II. Reinforcement theory and the dynamics of alcoholism. Q J Stud Alcohol. 1956;17:296–305. [PubMed] [Google Scholar]
- Crabbe JC, Phillips TJ, Harris RA, Arends MA, Koob GF. Alcohol-related genes: contributions from studies with genetically engineered mice. Addiction Biology. 2006;11:195–269. doi: 10.1111/j.1369-1600.2006.00038.x. [DOI] [PubMed] [Google Scholar]
- Croce P. Secretaria de Medicina Sanitaria, editor. El alcohol y ninos, in Alcohol and Alcoholism. Sector Educacion para la Salud; Buenos Aires: 1987. [Google Scholar]
- Cruz FC, Quadros IM, Planeta CdS, Miczek KA. Maternal separation stress in male mice: long-term increases in alcohol intake. Psychopharmacology (Berl) 2008;201:459–468. doi: 10.1007/s00213-008-1307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Gremel C, Groblewski P. Drug-induced conditioned place preference and aversion in mice. Nat Protoc. 2006;4:1662–1670. doi: 10.1038/nprot.2006.279. [DOI] [PubMed] [Google Scholar]
- Dawson D, Grant B, Li T. Impact of age at first drink on stress-reactive drinking. Alcohol Clin Exp Res. 2007;31:69–77. doi: 10.1111/j.1530-0277.2006.00265.x. [DOI] [PubMed] [Google Scholar]
- DeWit DJ, Adlaf EM, Offord DR, Ogborne AC. Age at first alcohol use: a risk factor for the development of alcohol disorders. Am J Psychiatry. 2000;157:745–750. doi: 10.1176/appi.ajp.157.5.745. [DOI] [PubMed] [Google Scholar]
- Dick DM, Agrawal A. The Genetics of Alcohol and Other Drug Dependence. Alc Res Health. 2008;31:111–118. [PMC free article] [PubMed] [Google Scholar]
- Dickinson SD, Kashawny SK, Thiebes KP, Charles DY. Decreased sensitivity to ethanol reward in adolescent mice as measured by conditioned place preference. Alcohol Clin Exp Res. 2009;33:1246–1251. doi: 10.1111/j.1530-0277.2009.00950.x. [DOI] [PubMed] [Google Scholar]
- Dominguez H, Lopez M, Chotro M, Molina J. Perinatal responsiveness to alcohol’s chemosensory cues as a function of prenatal alcohol administration during gestational days 17–20 in the rat. Neurobiol Learn Mem. 1996;65:103–112. doi: 10.1006/nlme.1996.0012. [DOI] [PubMed] [Google Scholar]
- Doremus TL, Brunell SC, Rajendran P, Spear LP. Factors influencing elevated ethanol consumption in adolescent relative to adult rats. Alcohol Clin Exp Res. 2005;29:1796–1808. doi: 10.1097/01.alc.0000183007.65998.aa. [DOI] [PubMed] [Google Scholar]
- Ducci F, Goldman D. Genetic approaches to addiction: genes and alcohol. Addiction. 2008;103:1414–1428. doi: 10.1111/j.1360-0443.2008.02203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health. 2007;30:5–13. [PMC free article] [PubMed] [Google Scholar]
- Edenberg HJ, Foroud T. The genetics of alcoholism: identifying specific genes through family studies. Addict Biol. 2006;11:386–396. doi: 10.1111/j.1369-1600.2006.00035.x. [DOI] [PubMed] [Google Scholar]
- Faria RR, Lima Rueda AV, Sayuri C, Soares SL, Malta MB, Carrara-Nascimento PF, da Silva Alves A, Marcourakis T, Yonamine M, Scavone C, Giorgetti Britto LR, Camarini R. Environmental modulation of ethanol-induced locomotor activity: Correlation with neuronal activity in distinct brain regions of adolescent and adult Swiss mice. Brain Res. 2008;1239:127–140. doi: 10.1016/j.brainres.2008.08.056. [DOI] [PubMed] [Google Scholar]
- Fernandez-Vidal J, Spear N, Molina J. Adolescent rats discriminate a mild state of ethanol intoxication likely to act as an appetitive unconditioned stimulus. Alcohol. 2003;30:45–60. doi: 10.1016/s0741-8329(03)00093-4. [DOI] [PubMed] [Google Scholar]
- Fildes VA. Breasts, Bottles and Babies: A History of Infant Feeding. Edinburgh University Press; Edinburgh: 1986. [Google Scholar]
- Flores-Huerta S, Hernadez-Montes H, Argote RM, Villalpando S. Effects of ethanol consumption during pregnancy and lactation on the outcome and postnatal growth of the offspring. Ann Nutr Metab. 1992;36:121–128. doi: 10.1159/000177706. [DOI] [PubMed] [Google Scholar]
- Funk D, Harding S, Juzytsch W, Lê AD. Effects of unconditioned and conditioned social defeat on alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2005;183:341–349. doi: 10.1007/s00213-005-0194-1. [DOI] [PubMed] [Google Scholar]
- Garver E, Gc T, Cao QN, Aini M, Zhou F, Israel Y. Eliciting the low-activity aldehyde dehydrogenase Asian phenotype by an antisense mechanism results in an aversion to ethanol. J Exp Med. 2001;194:571–580. doi: 10.1084/jem.194.5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert DR, Cippitelli A, Thorsell A, Lê AD, Hipskind PA, Hamdouchi C, Lu J, Hembre EJ, Cramer J, Song M, McKinzie D, Morin M, Ciccocioppo R, Heilig M. 3-(4-Chloro-2-Morpholin-4-yl-Thiazol-5-yl)-8-(1-Ethylpropyl)-2,6-Dimethyl-Imidazo[1,2-b]Pyridazine: A Novel Brain-Penetrant, Orally Available Corticotropin-Releasing Factor Receptor 1 Antagonist with Efficacy in Animal Models of Alcoholism. J Neurosci. 2007;27:2718–2726. doi: 10.1523/JNEUROSCI.4985-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlett C, Johnson T. Neonatal binge ethanol exposure using intubation: timing and dose effects on place learning. Neurotoxicol Teratol. 1997;19:435–446. doi: 10.1016/s0892-0362(97)00062-7. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Hassin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Tadokoro S. Learning retardation and enhanced ethanol preference produced by postnatal pretreatments with ethanol in adult rats. Jpn J Pharmacol. 1985;37:269–276. doi: 10.1254/jjp.37.269. [DOI] [PubMed] [Google Scholar]
- Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology. 2001;158:366–373. doi: 10.1007/s002130100701. [DOI] [PubMed] [Google Scholar]
- Jaworski JN, Francis DD, Brommer CL, Morgan ET, Kuhar MJ. Effects of early maternal separation on ethanol intake, GABA receptors and metabolizing enzymes in adult rats. Psychopharmacology. 2005;181:8–15. doi: 10.1007/s00213-005-2232-4. [DOI] [PubMed] [Google Scholar]
- Kiefer S. Alcohol, Palatability, and Taste Reactivity. Neurosci Biobehav Rev. 1995;19:133–141. doi: 10.1016/0149-7634(94)00027-x. [DOI] [PubMed] [Google Scholar]
- Kleabaur JE, Bardo MT. Individual Differences in Novelty Seeking on the Playground Maze Predict Amphetamine Conditioned Place Preference. Pharm Biochem Behav. 1999;63:131–136. doi: 10.1016/s0091-3057(98)00258-5. [DOI] [PubMed] [Google Scholar]
- Kraebel KS, Brasser SM, Campbell JO, Spear LP, Spear NE. Developmental differences in temporal patterns and potentiation of isolation-induced ultrasonic vocalizations: influence of temperature variables. Dev Psychobiol. 2002;40:147–159. doi: 10.1002/dev.10022. [DOI] [PubMed] [Google Scholar]
- Lindros KO, Jokelainen K, Nanji AA. Acetaldehyde prevents nuclear factor-kappa B activation and hepatic inflammation in ethanol-fed rats. Lab Invest. 1999;79:799–806. [PubMed] [Google Scholar]
- Lopez MF, Molina JC. Chronic alcohol administration in the rat pup: effects upon later consumption of alcohol and other palatable solutions. Addict Biol. 1999;4:173–183. doi: 10.1080/13556219971678. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Yücel M, Hall WD. Substance use and the adolescent brain: a toxic combination? J Psychopharmacol. 2007;21:792–794. doi: 10.1177/0269881107078309. [DOI] [PubMed] [Google Scholar]
- Luczak SE, Elvine-Kreis B, Shea SH, Carr LG, Wall TL. Genetic risk for alcoholism relates to level of response to alcohol in Asian-American men and women. J Stud Alcohol. 2002;63:74–82. [PubMed] [Google Scholar]
- Mark TL, Kranzler HR, Song X, Bransberger P, Poole VH, Crossse S. Physicians’ opinions about medications to treat alcoholism. Addiction. 2003;98:617–626. doi: 10.1046/j.1360-0443.2003.00377.x. [DOI] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:416–421. [PubMed] [Google Scholar]
- Matsuzawa S, Suzuki T, Misawa M. Conditioned fear stress induces ethanol-associated place preference in rats. Euro J Pharmacol. 1998;341:127–130. doi: 10.1016/s0014-2999(97)01456-8. [DOI] [PubMed] [Google Scholar]
- Matsuzawa S, Suzuki T, Misawa M. Ethanol, but not the anxiolytic drugs buspirone and diazepam, produces a conditioned place preference in rats exposed to conditioned fear stress. Pharmacol Biochem Behavi. 2000;65:281–288. doi: 10.1016/s0091-3057(99)00224-5. [DOI] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Malone S, Elkins I. Origins and consequences of age at first drink. I. Associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcohol Clin Exp Res. 2001;25:1156–1165. [PubMed] [Google Scholar]
- Medina KL, McQueeny T, Nagel BJ, Hanson KL, Schweinsburg AD, Tapert SF. Prefrontal cortex volumes in adolescents with alcohol use disorders: unique gender effects. Alcohol Clin Exp Res. 2008;32:386–394. doi: 10.1111/j.1530-0277.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menella JA, Beauchamp GK. Beer, breast feeding, and folklore. Dev Psychobiol. 1993;26:459–466. doi: 10.1002/dev.420260804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Yap JJ, Covington HE. Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina JC, Pautassi RM, Truxell E, Spear N. Differential motivational properties of ethanol during early ontogeny as a function of dose and postadministration time. Alcohol. 2007;41:41–55. doi: 10.1016/j.alcohol.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. Li to Step Down as Director of the National Institute on Alcohol Abuse and Alcoholism. NIH News, National Institute on Alcohol Abuse and Alcoholism; Bethesda MD: 2008. [Google Scholar]
- Nizhnikov ME, Pautassi RM, Truxell E, Spear NE. Opioid antagonists block the acquisition of ethanol-mediated conditioned tactile preference in infant rats. Alcohol. 2009;43:347–358. doi: 10.1016/j.alcohol.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocaranza P, Quintanilla ME, Tampier L, Karahanian E, Sapag A, Israel Y. Gene therapy reduces ethanol intake in an animal model of alcohol dependence. Alcohol Clin Exp Res. 2008;32:52–57. doi: 10.1111/j.1530-0277.2007.00553.x. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Knapp DJ, Breese GR. Drug challenges reveal differences in mediation of stress facilitation of voluntary alcohol drinking and withdrawal-induced anxiety in alcohol-preferring P rats. Alcohol Clin Exp Res. 2007;31:1473–1481. doi: 10.1111/j.1530-0277.2007.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. J Neurochem. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Pastor R, McKinnon CS, Scibelli AC, Burkhart-Kasch S, Reed C, Ryabinin AE, Coste SC, Stenzel-Poore MP, Phillips TJ. Corticotropin-releasing factor-1 receptor involvement in behavioral neuroadaptation to ethanol: a urocortin1-independent mechanism. Proc Natl Acad Sci USA. 2008;105:9070–9075. doi: 10.1073/pnas.0710181105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Nizhnikov ME, Spear NE. Assessing appetitive, aversive, and negative ethanol-mediated reinforcement through an immature rat model. Neurosci Biobehav Rev. 2009;33:953–974. doi: 10.1016/j.neubiorev.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Myers M, Spear LP, Molina JC, Spear NE. Adolescent but not adult rats exhibit ethanol-mediated appetitive second-order conditioning. Alcohol Clin Exp Res. 2008;32:2016–2027. doi: 10.1111/j.1530-0277.2008.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Godoy JC, Spear NE, Molina JC. Early responsiveness to stimuli paired with different stages within the state of alcohol intoxication. Alcohol Clin Exp Res. 2002;26:644–654. [PubMed] [Google Scholar]
- Pautassi RM, Molina JC, Spear NE. Infant rats exhibit aversive learning mediated by ethanol’s orosensory effects but are positively reinforced by ethanol’s post-ingestive effects. Pharmacol Biochem Behav. 2008;88:393–402. doi: 10.1016/j.pbb.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pautassi RM, Ponce LF, Molina JC. Effects of early exposure to ethanol on subsequent learning mediated by the unconditional attributes of the drug. Latinamerican Journal of Psychology. 2005;37:149–166. [Google Scholar]
- Pedersen W, Skrondal A. Alcohol consumption debut: predictors and consequences. J Stud Alcohol. 1998;59:32–42. doi: 10.15288/jsa.1998.59.32. [DOI] [PubMed] [Google Scholar]
- Peng GS, Chen YC, Tsao TP, Wang MF, Yin SJ. Pharmacokinetic and pharmacodynamic basis for partial protection against alcoholism in Asians, heterozygous for the variant ALDH2*2 gene allele. Pharmacogenet Genomics. 2007;17:845–55. doi: 10.1097/FPC.0b013e3282609e67. [DOI] [PubMed] [Google Scholar]
- Philpot RM, Badanich KA, Kirstein CL. Place conditioning: age-related changes in the rewarding and aversive effects of alcohol. Alcohol Clin Exp Res. 2003;27:593–599. doi: 10.1097/01.ALC.0000060530.71596.D1. [DOI] [PubMed] [Google Scholar]
- Ploj K, Roman E, Nylander I. Long-term effects of maternal separation on ethanol intake and brain opioid and dopamine receptors in male Wistar rats. Neuroscience. 2003;121:787–799. doi: 10.1016/s0306-4522(03)00499-8. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Pohorecky L. The interaction of alcohol and stress. A review. Neurosci Biobehav Rev. 1981;5:209–229. doi: 10.1016/0149-7634(81)90003-8. [DOI] [PubMed] [Google Scholar]
- Ponce LF, Pautassi RM, Spear NE, Molina JC. Nursing from an ethanol-intoxicated dam induces short- and long-term disruptions in motor performance and enhances later self-administration of the drug. Alcohol Clin Exp Res. 2004;28:1039–1050. doi: 10.1097/01.alc.0000131298.32045.96. [DOI] [PubMed] [Google Scholar]
- Ponce LF, Pautassi RM, Spear NE, Molina JC. Ethanol-mediated operant learning in the infant rat leads to increased ethanol intake during adolescence. Pharmacol Biochem Behav. 2008;90:640–650. doi: 10.1016/j.pbb.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott CA, Kendler KS. Age at First Drink and Risk for Alcoholism: A Noncausal Association. Alcohol Clin Exp Res. 1999;23:101–107. [PubMed] [Google Scholar]
- Quintanilla ML, Tampier L, Sapag A, Gerdtzen Z, Israel Y. Sex differences, alcohol dehydrogenase, acetaldehyde burst and aversión to etanol: a systems perspective. Am J Physiol Endocrinol Metab. 2007;293:E531–E537. doi: 10.1152/ajpendo.00187.2007. [DOI] [PubMed] [Google Scholar]
- Quintanilla ME, Israel Y, Sapag A, Tampier L. The UChA and UChB rat lines: metabolic and genetic differences influencing ethanol intake. Addict Biol. 2006;11:310–323. doi: 10.1111/j.1369-1600.2006.00030.x. [DOI] [PubMed] [Google Scholar]
- Rivera-Meza M, Quintanilla ME, Tampier L, Mura CV, Sapag A, Israel Y. Mechanism of protection against alcoholism by an alcohol dehydrogenase polymorphism: development of an animal model. The FASEB J. 2009 doi: 10.1096/fj.09-132563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AJ, Lessov CN, Phillips TJ. Critical role for glucocorticoid receptors in stress- and ethanol-induced locomotor sensitization. J Pharmacol Exp Ther. 1995;275:790–797. [PubMed] [Google Scholar]
- Rosenfeld P, Wetmore JB, Levine S. Effects of repeated maternal separations on the adrenocortical response to stress of preweanling rats. Physiol Behav. 1992;52:787–791. doi: 10.1016/0031-9384(92)90415-x. [DOI] [PubMed] [Google Scholar]
- Schmid B, Blomeyer D, Treutlein J, Zimmermann US, Buchmann AF, Schmidt MH, Esser G, Rietschel M, Banaschewski T, Schumann G, Laucht M. Interacting effects of CRHR1 gene and stressful life events on drinking initiation and progression among 19-year-olds. Int J Neuropsychopharmacol. 2009:1–12. doi: 10.1017/S1461145709990290. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Kingsley MA, Rezvani AH, Propst K, Swartzwelder HS, Kuhn CM. Early ethanol consumption predicts relapse-like behavior in adolescent male rats. Alcohol Clin Exp Res. 2008;32:754–62. doi: 10.1111/j.1530-0277.2008.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund S, Vengeliene V, Singer MV, Spanagel R. Influence of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcohol Clin Exp Res. 2005;29:1139–1145. doi: 10.1097/01.alc.0000171928.40418.46. [DOI] [PubMed] [Google Scholar]
- Sillaber I, Rammes G, Zimmermann S, Mahal B, Zieglgänsberger W, Wurst W, Holsboer F, Spanagel R. Enhanced and delayed stress-induced alcohol drinking in mice lacking functional CRH1 receptors. Science. 2002;296:931–933. doi: 10.1126/science.1069836. [DOI] [PubMed] [Google Scholar]
- Singh SM, Basu D. The P300 event-related potential and its possible role as an endophenotype for studying substance use disorders: a review. Addict Biol. 2009;14:298–309. doi: 10.1111/j.1369-1600.2008.00124.x. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M. Effects of adolescent ethanol exposure on ethanol consumption in adult rats. Alcohol. 2002;26:23–30. doi: 10.1016/s0741-8329(01)00192-6. [DOI] [PubMed] [Google Scholar]
- Soomer WH, Saavedra JM. Targeting brain angiotensin and corticotrophin-releasing hormone systems interaction for the treatment of mood and alcohol use disorders. J Molec Med. 2008;86:723–728. doi: 10.1007/s00109-008-0333-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Varlinskaya EI. Adolescence. Alcohol sensitivity, tolerance, and intake. Recent Dev Alcohol. 2005;17:143–159. [PubMed] [Google Scholar]
- Spear LP. Adolescence and the Trajectory of Alcohol Use: Introduction to Part VI. Annals of the New York Academy of Sciences. 2004;1021:202–205. doi: 10.1196/annals.1308.025. Adolescent Brain Development: Vulnerabilities and Opportunities. [DOI] [PubMed] [Google Scholar]
- Spear NE, Molina JC. Fetal or infantile exposure to ethanol promotes ethanol ingestion in adolescence and adulthood: a theoretical review. Alcohol Clin Exp Res. 2005;29:909–929. doi: 10.1097/01.alc.0000171046.78556.66. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Tapert SF. The influence of substance use on adolescent brain development. Clin EEG Neurosci. 2009;40:31–38. doi: 10.1177/155005940904000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Oishi M, Mizutani T, Sato Y. Regional cerebral blood flow measured by the resting and vascular reserve (RVR) method in chronic alcoholics. Alcohol Clin Exp Res. 2002;26:95S–99S. doi: 10.1097/01.ALC.0000026984.37262.82. [DOI] [PubMed] [Google Scholar]
- Stamatakis A, Pondiki S, Kitraki E, Diamantopoulou A, Panagiotaropoulos T, Raftogianni A, Stylianopoulou F. Effect of neonatal handling on adult rat spatial learning and memory following acute stress. Stress. 2008;11:148–159. doi: 10.1080/10253890701653039. [DOI] [PubMed] [Google Scholar]
- Stanton M, Goodlett C. Neonatal ethanol exposure impairs eyeblink conditioning in weanling rats. Alcohol Clin Exp Res. 1998;22:270–275. [PubMed] [Google Scholar]
- Stevenson RA, Besheer J, Hodge CW. Comparison of ethanol locomotor sensitization in adolescent and adult DBA/2J mice. Psychopharmacology. 2008;197:361–370. doi: 10.1007/s00213-007-1038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strat YL, Ramoz N, Schumann G, Gorwood P. Molecular genetics of alcohol dependence and related endophenotypes. Curr Genomics. 2008;9:444–451. doi: 10.2174/138920208786241252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, Meloy MJ, Brown SA. Neural response to alcohol stimuli in adolescents with alcohol use disorder. Arch Gen Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Social defeat stress selectively alters mesocorticolimbic dopamine release: an in vivo microdialysis study. Brain Res. 1996;721:140–149. doi: 10.1016/0006-8993(96)00159-x. [DOI] [PubMed] [Google Scholar]
- Tidey JW, Miczek KA. Acquisition of cocaine self-administration after social stress: role of accumbens dopamine. Psychopharmacology. 1997;130:203–212. doi: 10.1007/s002130050230. [DOI] [PubMed] [Google Scholar]
- Tolliver G, Samson H. The influence of early postweaning ethanol exposure on oral self-administration behavior in the rat. Pharmacol Biochem Behav. 1991;38:575–580. doi: 10.1016/0091-3057(91)90016-u. [DOI] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol Behav. 1993;53:983–993. doi: 10.1016/0031-9384(93)90278-n. [DOI] [PubMed] [Google Scholar]
- Truxell E, Molina J, Spear N. Ethanol intake in the juvenile, adolescent, and adult rat: effects of age and prior exposure to ethanol. Alcohol Clin Exp Res. 2007;31:755–765. doi: 10.1111/j.1530-0277.2007.00358.x. [DOI] [PubMed] [Google Scholar]
- Tu GC, Israel Y. Alcohol consumption by orientals in North America is predicted largely by a single gene. Behav Genet. 1995;25:59–65. doi: 10.1007/BF02197242. [DOI] [PubMed] [Google Scholar]
- Van Erp AMM, Miczek KA. Persistent suppression of ethanol selfadministration by brief social stress in rats and increased startle response as index of withdrawal. Physiol Behav. 2001;73:301–311. doi: 10.1016/s0031-9384(01)00458-9. [DOI] [PubMed] [Google Scholar]
- Van Erp AMM, Tachi N, Miczek KA. Short or continuous social stress: Suppression of continuously available ethanol intake in subordinate rats. Behav Pharmacol. 2001;12:335–342. doi: 10.1097/00008877-200109000-00004. [DOI] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats under continuous, voluntary-access conditions. Alcohol Clin Exp Res. 2007;31:1159–1168. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter CS, Doremus-Fitzwater TL, Spear LP. Time course of elevated ethanol intake in adolescent relative to adult rats in a continuous-access situation. Alcohol Clin Exp Res. 2005;29:20A–20A. doi: 10.1111/j.1530-0277.2007.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli JR, Ulm RR, Hopson N. The bidirectional effects on alcohol preference in rats. Alcohol Clin Exp Res. 1990;14:913–916. doi: 10.1111/j.1530-0277.1990.tb01837.x. [DOI] [PubMed] [Google Scholar]
- Walker C, Perrin M, Vale W, Rivier C. Ontogeny of the stress response in the rat: role of the pituitary and the hypothalamus. Endocrinology. 1986;118:1445–1451. doi: 10.1210/endo-118-4-1445. [DOI] [PubMed] [Google Scholar]
- White A, Roberts D, Best P. Context-specific tolerance to the ataxic effects of alcohol. Pharmacol Biochem Behav. 2002;72:107–110. doi: 10.1016/s0091-3057(01)00731-6. [DOI] [PubMed] [Google Scholar]
- Wolffgramm J. Free choice ethanol intake of laboratory rats under different social conditions. Psychopharmacology (Berl) 1990;101:233–239. doi: 10.1007/BF02244132. [DOI] [PubMed] [Google Scholar]
- Yerkes RM, Dodson JD. The relation of strength of stimulus to rapidity of habit-formation. J Comp Neurology Psychol. 1908;18:459–482. [Google Scholar]
- Xian H, Scherrer JF, Grant JD, Eisen SA, True WR, Jacob T, Bucholz KK. Genetic and environmental contributions to nicotine, alcohol and cannabis dependence in male twins. Addiction. 2008;103:1391–1398. doi: 10.1111/j.1360-0443.2008.02243.x. [DOI] [PubMed] [Google Scholar]