Abstract
The process of ovulation involves weakening of the follicular wall by proteolytic enzymes. The function of FURIN (also known as PCSK3) is to activate various proteolytic enzymes. In the present study, the expression, localization, and function of FURIN were investigated in the periovulatory rat ovary. Immature female rats were injected with equine chorionic gonadotropin followed by human chorionic gonadotropin (hCG) 48 h later to stimulate ovulation. Ovaries were collected at 0, 4, 8, 12, and 24 h after hCG injection. Administration of hCG increased Furin mRNA expression in both intact ovaries and cultured ovarian follicles to maximal levels at 8 and 12 h before decreasing at 24 h. In cultured granulosa cells, Furin mRNA levels were significantly induced at 12 h after hCG. In situ hybridization of Furin mRNA demonstrated expression in the granulosa cells, with predominant expression in the theca layer. Regulation studies demonstrated that Furin mRNA was induced in residual tissue by forskolin or amphiregulin. To examine the role of FURIN in protease activation and ovulation, rats were treated with a FURIN inhibitor and oocyte release was determined. There was a 38% decrease in the number of oocytes released in ovaries treated with the FURIN inhibitor. Likewise, the FURIN inhibitor decreased the activation of MMP2. The induction of Furin mRNA after treatment with hCG, along with the decrease in MMP2 activation and oocyte release after FURIN inhibition, supports the hypothesis that FURIN is upregulated during the preovulatory period, which results in activation of proteinases associated with the breakdown of the follicular wall during ovulation.
Keywords: follicle, ovary, ovulation, proteinase, theca cells
Furin mRNA is upregulated by hCG prior to ovulation and FURIN inhibition blocks MMP2 activation and oocyte release.
INTRODUCTION
In order for ovulation to take place, there must be an orchestrated breakdown of the apical wall of the preovulatory follicle. The midcycle surge of luteinizing hormone (LH) increases proteolytic activity that is postulated to play a role in this remodeling of the ovarian follicular extracellular matrix (ECM), thus leading to expulsion of the oocyte [1]. Among the proteases that increase after an LH stimulus are the matrix metalloproteinases (MMPs), which are a family of 25 distinct proteinases that degrade the ECM. It has previously been shown that LH and human chorionic gonadotropin (hCG), which mimics LH action, increase expression and activity of a variety of the MMPs such as the collagenases, gelatinases, MMP19, and the membrane-type MMPs in various species [2–4]. Although LH and hCG can stimulate the expression of the MMPs, these enzymes are synthesized in a latent form that must be activated. This activation can occur both intracellularly as well as extracellularly via a number of different mechanisms, including activation by other proteinases or by reduction-oxidation reactions [5–7].
One family of proteolytic enzymes that activates precursor proteins into biologically active proteins is the proprotein convertase subtilisin/kexin-like proteases (PCSKs). This family presently includes nine known proteinases: FURIN (PCSK3, PACE, FUR), PCSK1, PCSK2, PCSK4, PCSK5, PCSK6, PCSK7, MBTPS1, and PCSK9 [8]. Each of these PCSKs, alone or in combination with others, is able to activate a variety of proteinases, hormones, growth factors, and receptors via endoproteolytic cleavage [9]. The substrate specificity of the PCSKs is further determined by their subcellular, cellular, and tissue distribution [10]. The PCSKs have been linked to numerous disease states such as Alzheimer [11, 12], infection [8], and cancer [13–15]. In tumorigenesis, the ability of PCSKs to increase the growth and aggressiveness of cancers has been linked to their processing and activation of various molecules such as the MMPs [13–15].
Certain members of the MMP family, such as the membrane-type MMPs, contain a furin site (RRKR) that can be cleaved intracellularly by FURIN to convert the MMPs to their active form. When activated by FURIN, these membrane-type MMPs are inserted into the membrane in an active state. For example, the latent form of membrane type 1 (pro-MMP14) is cleaved by FURIN intracellularly and relocated to the plasma membrane, where it can activate latent MMP2 [16, 17]. As both MMP2 and MMP14 are stimulated during the periovulatory period [18], we hypothesized that LH could increase the expression of mRNA for Furin, which would activate the MMP system and impact ovulation.
Presently there is limited data on the role of FURIN in the ovary. In the teleost fish, Oryzias latipes, the highest expression of furin mRNA occurred in oocytes of small growing follicles, where it was postulated to activate MMPs [19]. Although little is known about FURIN in the ovary, there are a number of studies regarding other members of the PCSK family. For example, Pcsk6 mRNA was expressed by murine granulosa cells of small to medium preantral follicles, but PCSK6 was not induced in preovulatory follicles by hCG treatment; Diaz et al. [20] suggested that the action of PCSK6 may be on the processing of zona pellucida proteins or on TGFB ligands. A survey of several members of the PCSK family during development and after gonadotropin stimulation in the rat found Pcsk5 mRNA was expressed in the theca cells of growing follicles and upregulated by stimulation with eCG. Administration of hCG resulted in a further induction of Pcsk5 mRNA within 3 h in granulosa cells [21].
Exploration of an existing ovarian database [22] for potential PCSK5 substrates revealed a number of potential proteins that are associated with ovulation and are activated by PCSK5, such as the bone morphogenenic proteins, MMPs, and ADAMTS1 [21]. These previous findings with other members of the PCSK family would indicate that this family of proprotein convertases processes substrates associated with follicular growth and ovulatory events. In this study, we examine the regulation and localization of Furin during the periovulatory period and its involvement in the process of MMP activation and ovulation.
MATERIALS AND METHODS
Materials
Unless otherwise noted, all chemicals were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA). Molecular biological enzymes, primers, pCRII-TOPO Vector, culture media, and Trizol were purchased from Invitrogen Life Technologies, Inc. (Carlsbad, CA).
Animals
All animal procedures were approved by the University of Kentucky Animal Care and Use Committee. Immature female Sprague-Dawley rats were obtained from Harlan Laboratories, Inc. (Indianapolis, IN), provided with water and rat chow ad libitum, and maintained on a 12L:12D photoperiod. The rats were injected with 10 IU of equine chorionic gonadotropin (eCG) at 21–22 days of age to stimulate follicular development and 48 h later were injected with 10 IU of hCG to induce ovulation. In this model, ovulation occurs approximately 14–16 h after hCG administration [9]. Animals were killed at 0 (i.e., at the time of hCG administration), 4, 8, 12, and 24 h after hCG administration (three animals per time point). Ovaries were collected and stored for later extraction of total RNA or plasma membrane proteins, or processed for in situ hybridization.
Isolation and Culture of Ovarian Follicles, Granulosa Cells, and Residual Tissue
To isolate ovarian follicles, ovaries were collected from immature rats 48 h after eCG administration. Large preovulatory follicles were isolated by fine needle (33G) dissection. Follicles were cultured in 96-well plates with Eagle α-MEM supplemented with sodium bicarbonate, 1× penicillin/streptomycin, 1× ITS (insulin, transferin, and selenium), and 0.3% bovine serum albumin (BSA). Follicles were treated with or without hCG (1 IU/ml) and cultured for 0, 4, 8, 12, or 24 h. At the end of culture, follicles were placed in Trizol reagent and frozen at −70°C for RNA isolation to analyze Furin mRNA expression.
To isolate granulosa cells, ovaries collected from eCG-primed immature rats (48 h after eCG) were processed as described previously [23]. Briefly, granulosa cells were isolated by follicular puncture. Cells were pooled, filtered, pelleted by centrifugation at 200 × g for 5 min, and resuspended in defined medium consisting of Opti-MEM medium supplemented with sodium bicarbonate, 50 μg/ml of gentamycin, and 1× ITS. The cells were cultured in the absence or presence of hCG (1 IU/ml) for 0, 4, 8, 12, or 24 h. At the end of each culture period, cells were collected and snap-frozen for isolation of total RNA.
To isolate an enriched population of theca cells, ovaries were collected 48 h after eCG treatment, and granulosa cells were removed by follicular puncture. The remaining tissue (residual tissue) was cultured with or without phorbol 12-myristate 13-acetate (PMA; 20 nM) + forskolin (FSK; 10 μM). FSK and PMA have previously been used to mimic the effects of LH for the induction of Ptgs2, Pgr, and Snap25 mRNA in cultured rat granulosa cells [24–26] and were used in the present study because a more robust stimulation was observed with PMA + FSK than with hCG. Residual tissue was cultured in the absence or presence of various reagents for 0, 8, 12, or 24 h. At the end of each culture period, residual tissue was collected and snap-frozen for isolation of total RNA. For regulation studies, residual tissues were incubated for 12 h with either PMA (20 nM) to stimulate the protein kinase C (PKC) pathway, FSK (10 μM) to stimulate the cAMP pathway, PMA + FSK, amphiregulin (AREG; 250 ng/ml) to stimulate the epidermal growth factor receptor (EGFR) pathway, FSK + H89 (10 μM) to inhibit the protein kinase A (PRKA) pathway, or FSK + AG1478 (1 μM) to inhibit the EGFR pathway.
Generation of a Rat Furin Plasmid cDNA
A 597-bp cDNA fragment corresponding to rat Furin was generated by RT-PCR. Briefly, total RNA (1 μg) isolated from rat preovulatory ovaries (12 h after hCG) was reverse-transcribed at 42°C for 1 h using SuperScript II and Oligo dT primers. First-strand cDNA samples were amplified using oligonucleotide primer pairs (forward 5′-ATCTCAACGCTAACGATTGG-3′, reverse 5′-AGAAACCTTCCTCACACACC-3′) based on the previously reported sequence of rat Furin (GenBank accession no. NM 019331). The 597-bp PCR product was cloned into the pCRII-TOPO Vector. The DNA sequence of the cloned rat partial Furin cDNA was determined using a Standard ABI kit (Eurofins MWG/Operon, Huntsville, AL).
In Situ Hybridization of mRNA for Furin
Ovaries were collected at various times after hCG as outlined above and processed for in situ hybridization as described previously [27]. Briefly, the 597-bp segment of Furin mRNA was amplified using PCR and was used to generate sense and antisense RNA probes labeled with [α-35S]UTP (10 mCi/ml; PerkinElmer, Boston, MA) using appropriate RNA polymerases. Ovarian sections were hybridized overnight with 1 × 106 cpm 35S-labeled riboprobe per slide in a humidified chamber at 55°C. The next day, the slides were washed extensively and treated with RNase A (0.1 mg/ml). Tissue sections were washed again at high stringency, dried, dipped in Kodak NTB2 emulsion (Eastman Kodak, Rochester, NY), and exposed at 4°C for 21–42 days. To visualize the hybridized riboprobes, slides were developed with Kodak D19, counterstained with hematoxylin, and examined with an Eclipse E800 Nikon microscope (Nikon Corp., Melville, NY) under bright- and dark-field optics. A sense RNA probe was used as a control for nonspecific binding and was included for each ovary and each time point. A minimum of three slides with four sections per time were examined.
Osmotic Minipump Implantation
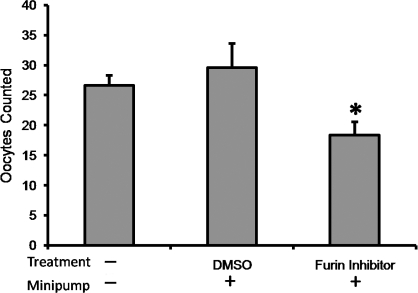
To determine the impact of FURIN on ovulation, a commercially available inhibitor, Furin Inhibitor I (Rec-Dec-RMVK; Calbiochem, San Diego, CA), was administered by i.p. infusion with Alzet osmotic minipumps (Durect Corp., Cupertino, CA). To accomplish this, animals were stimulated with eCG, 48 h later rats were anesthetized, and a single lateral dorsal incision was made directly above the fat pad of the right ovary. An osmotic minipump was inserted into the cavity and sutured to the muscle wall, keeping it in proximity to the right ovary. Animals received a minipump with either 50 μg (100 mM) of Furin Inhibitor I or 100 μl 0.1% dimethyl sulfoxide (DMSO) vehicle solvent (sham control group). The incision was closed, and rats were injected with 10 IU hCG (s.c.) 1 h after surgery. Rats were killed 24 h after surgery, and oocytes within the right oviduct were collected and counted. A group of animals without surgical manipulation served as the control group where the number of oocytes present in the right oviduct was analyzed.
Quantification of mRNA for Furin by Real-Time PCR
Total RNA was isolated from whole ovaries, cultured follicles, and cultured cells using Trizol reagent according to the manufacturer's protocol and was then quantified by spectrophotometry. Total RNA was treated with 0.2 U DNase I to eliminate possible contamination with genomic DNA. Synthesis of first-strand cDNA was performed by reverse transcription of 0.5 μg total RNA using superScript III with Oligo(dT)18 primer according to the manufacturer's protocol. Oligonucleotide primers corresponding to cDNA for rat Furin (forward 5′-ACATCTCCAGACTGGAACACG-3′, reverse 5′-AGGAATGAGTTGTCATGAAAGC-3′) and for rat Rpl32 (forward 5′-GAAGCCCAAGATCGTCAAAA-3′, reverse 5′-AGGATCTGGCCCTGGCCCTTGAATCT-3′) were designed using OMIGA software (v2.0, Oxford Molecular, Campbell, CA). The performance of each primer set was tested by sequencing the PCR product and by analyzing the dissociation curve in the MxPro real-time PCR analysis program (Stratagene, La Jolla, CA) after each real-time PCR reaction. The real-time PCR reactions contained 10% of the reverse transcription reaction product, 0.4 μM of forward and reverse primers, 0.3 μl of 1:10 diluted ROX reference dye (provided with SYBR Green ER qPCR SuperMix Universal kit; Invitrogen), and SYBR Green SuperMix. PCR reactions were performed on an Mx3000P QPCR System (Stratagene). The thermal cycling steps included 2 min at 50°C to permit optimal AmpErase uracil-N-glycosylase activity, 10 min at 95°C for initial denaturation, and then each cycle of 15 sec at 95°C, 30 sec at 58°C, and 45 sec at 72°C for 40 cycles, followed by 1 min at 95°C, 30 sec at 58°C, and then 30 sec at 95°C for ramp dissociation. The relative amount of Furin transcript was calculated using the 2−ΔΔCT method and normalized to the endogenous reference gene Rpl32.
Preparation of Plasma Membrane Fractions
Plasma membrane fractions were prepared according to a modification of a previously described method [28] as routinely performed in our laboratory [18]. Briefly, ovaries were homogenized in 40 mM Tris buffer (pH 7.4) containing 0.25 M sucrose using a Dounce homogenizer at 4°C. Homogenates were filtered through a nylon cell strainer (40 μM; BD Biosciences, Woburn, MA) and centrifuged at 700 × g for 20 min at 4°C. The supernatant was collected and centrifuged again at 10 000 × g for 1 h at 4°C to pellet the crude plasma membrane fractions. The pellet was resuspended in Tris buffer and stored at −70°C. The protein concentration of the plasma membrane fractions was determined using the Bradford method [29].
Gelatin Zymography and Morphometric Analysis
Plasma membrane fractions were subjected to gelatin zymography as previously described [18]. Briefly, plasma membrane fractions (30 μg protein per lane) were electrophoresed in 10% polyacrylamide gels containing 1 mg/ml of gelatin. After electrophoresis, the gel was washed and then incubated in Tris incubation buffer (50 mM Tris/HCl, 5 mM CaCl2; pH 7.4) at 37°C for at least 48 h. Subsequently, the gels were stained with Coomassie Brilliant Blue R250 dye. The density of the bands represents gelatinolytic activity, which was quantitated using MetaMorph Image analysis software (Universal Imaging Corp., West Chester, PA) as described previously [30]. To determine the effect of treatment on MMP2 activation, the ratio of active to latent MMP2 from each cell membrane sample was calculated.
Statistical Analyses
All data are presented as means ± SEM. An ANOVA was used to test differences in Furin mRNA expression at various times after hCG in whole ovaries, cultured follicles, residual tissue, or granulosa cells, and for regulation studies using residual tissue. ANOVA was used to test for differences in activation of MMP2 after treatment with the FURIN inhibitor. A paired Student t-test was used to test for differences between oocyte release in ovaries implanted with osmotic minipumps treated with or without the FURIN inhibitor. If ANOVA revealed significant effects of treatment, the means were compared by the Duncan test, with P < 0.05 considered significant.
RESULTS
Periovulatory Furin Expression in Ovarian Tissue
Administration of hCG increased Furin mRNA expression in intact ovaries to maximal levels at 8 and 12 h before decreasing to control (0 h) levels by 24 h (Fig. 1A). In cultured granulosa cells, expression of Furin mRNA did not change in control (i.e., vehicle treated) granulosa cells across the time of culture. In contrast, Furin mRNA expression increased at 12 h in the hCG-treated group and decreased to control (0 h) levels by 24 h (Fig. 1B). As expression of Furin mRNA increased in whole ovaries and this expression was not totally accountable by an induction in granulosa cells, the expression of Furin mRNA was also examined in ovarian follicles. This was accomplished by isolating follicles from ovaries of eCG-primed rats and culturing them with or without hCG. Furin mRNA expression in ovarian follicles increased at 12 h and decreased by 24 h in the hCG-treated group, when compared to control vehicle-treated follicles (Fig. 1C).
FIG. 1.
Stimulation of Furin mRNA expression by hCG in the rat ovary. Real-time PCR analysis shows the expression of Furin mRNA in whole ovary (A), granulosa cells (B), and ovarian follicles (C) after hCG administration. In A, rats were injected with eCG for 48 h and treated with hCG, and ovaries were collected at 0, 4, 8, 12, or 24 h after treatment. For B and C, rats were injected with eCG for 48 h, and granulosa cells (B) or follicles (C) were isolated and cultured in medium alone (Control) or with hCG (1 IU/ml) for 0, 4, 8, 12, or 24 h. Relative levels of mRNA for Furin were normalized to Rpl32 in each sample (mean ± SEM; n = 3 independent culture experiments). Bars with superscripts or asterisks are significantly different (P < 0.05) within each panel.
Furin Localization by In Situ Hybridization During the Periovulatory Period
Localization of Furin mRNA in intact ovaries collected at different times after hCG was analyzed by in situ hybridization. The cellular localization results demonstrated an hCG-dependent induction of Furin mRNA expression. Furin mRNA expression was low at 0 h (Fig. 2, A, B, I, and J) and 4 h (not shown) after hCG treatment. At 8 h after hCG, intense cellular expression of Furin mRNA was observed in the theca, interstitial, and surrounding stromal cells (Fig. 2, C, D, K, and L). At 12 h after hCG, expression was strongly localized in the theca-interstitial layer of preovulatory follicles (Fig. 2, E and F). By 24 h after hCG treatment, Furin mRNA expression was localized throughout the interstitium of the ovary (Fig. 2, G and H). Of interest was the finding that Furin mRNA was expressed in oocytes of follicles at different stages of development. A representative photomicrograph of an oocyte in a preantral follicle expressing Furin mRNA is presented (Fig. 2, I and J). Ovarian sections hybridized with a sense probe displayed only background levels of silver grain deposition (Fig. 2, M and N).
FIG. 2.
Cellular localization of Furin mRNA in periovulatory rat ovaries. Sections of rat ovaries obtained at 0 h (48 h after eCG) (A, B, I, J), 8 h (C, D, K, L), 12 h (E, F), or 24 h (G, H) after hCG injection were hybridized to the appropriate antisense Furin mRNA probe. Representative bright-field and corresponding dark-field photomicrographs are depicted. A–H illustrate low magnification localization of Furin mRNA throughout the ovary at different times after hCG. Higher magnification of an ovary obtained at 0 h (I, J) demonstrates the expression of Furin mRNA in the oocyte. Higher magnification of an ovary obtained at 8 h after hCG (K, L) demonstrates the expression of Furin mRNA in the theca layer (arrows) and the granulosa cell layer (arrowheads). Sections from an ovary collected at 8 h after hCG hybridized to a sense Furin mRNA probe exhibit only background deposition of silver grains (M, N). F, follicle. White bar = 250 μm (A–H), 20 μm (I and J), 200 μm (K and L), and 100 μm (M and N).
Regulation of Furin in Ovarian Residual Tissue
As Furin mRNA expression was primarily in the theca-interstitial cells, the regulation of Furin was analyzed in residual tissue, which represents an enriched theca-interstitial cell population. Furin mRNA was increased at 12 h after PMA + FSK treatment (Fig. 3A). To further explore Furin mRNA regulation, residual tissue was cultured with PMA or FSK alone to stimulate the PKC or protein kinase A pathways (PRKA), respectively, with PMA + FSK, or with amphiregulin to stimulate the EGFR pathway. At 12 h after treatment, Furin mRNA levels were stimulated by FSK or AREG, but not PMA alone (Fig. 3B). To determine regulation downstream of cAMP, residual tissue was cultured with FSK + H89 to inhibit the PRKA pathway or FSK + AG1478 to inhibit the EGFR pathway. The stimulatory effect of FSK was blocked by both AG1478 and H89 (Fig. 3C).
FIG. 3.
Regulation of Furin mRNA by activators or inhibitors of LH-induced intracellular pathways in cultured ovarian residual tissue. A) Real-time PCR analysis shows the expression of Furin mRNA in ovarian residual tissue after culture in medium alone (Control) or with Phorbol 12-myristate 13-acetate (PMA; 20 nM) + forskolin (FSK; 10 μM) for 0, 8, 12, or 24 h. B) Residual tissues were cultured for 12 h with PMA, FSK, or amphiregulin (Areg; 250 ng/ml). C) Residual tissues were also cultured for 12 h with FSK in the presence of the EGFR antagonist AG1478 (1 μM) or the protein kinase A pathway inhibitor H89 (10 μM). Relative levels of mRNA for Furin were normalized to Rpl32 in each sample (mean ± SEM; n = 3 independent culture experiments). Bars with no common superscripts are significantly different (P < 0.05).
Impact of a FURIN Inhibitor on Ovulation
To identify a possible function of FURIN in the ovary, rats were treated in vivo with a FURIN inhibitor prior to ovulation, and the number of oocytes released 24 h after hCG treatment was determined. Rats that did not undergo surgery (control) ovulated 26.6 ± 1.7 oocytes from the right ovary. Rats implanted with a DMSO-filled osmotic minipump ovulated 29.5 ± 4.1 oocytes from the treated (right) ovary, whereas animals implanted with minipumps containing the Furin inhibitor I showed a 38% decrease in the number of oocytes released from the treated ovary (18.3 ± 2.2 vs. 29.5 ± 4.1 oocytes; Fig. 4).
FIG. 4.
Inhibition of oocyte release by administration of Furin Inhibitor I in vivo. Rats were injected with eCG, and 48 h later an osmotic minipump (Alzet) was inserted into the peritoneal cavity in proximity with the right ovary. Animals received a minipump with either 50 μg of Furin Inhibitor I or an equivalent concentration of DMSO vehicle control (DMSO). Rats were injected with 10 IU of hCG 1 h after surgery. Twenty four hours after surgery, oocytes were counted in the right oviduct from the treated ovary (mean ± SEM; DMSO vehicle n = 10, FURIN inhibitor n = 9). A group of animals without surgical manipulation served as the control group (no treatment, no minipump; n = 6). Bars with asterisks are significantly different (P < 0.05).
Impact of a FURIN Inhibitor on MMP Activation
Isolated cell membranes from residual tissues cultured with the Furin Inhibitor I (50 μM) were analyzed by gelatin zymography to determine MMP2 activity. The dose of Furin Inhibitor I was based upon previous reports of attenuated processing of pro-MMP14, MMP2 activation, and impeded cell migration of cardiac fibroblast with the Furin Inhibitor I [31]. Residual tissues cultured with FSK, FSK + DMSO vehicle control, or FSK + Furin Inhibitor I displayed MMP2 activity in its proprotein form (72 kDa), intermediate form (66 kDa), and fully active form (62 kDa; Fig. 5A). The effect of each treatment on MMP2 activation was determined and expressed as a ratio of active MMP2 to latent MMP2. Treatment with the Furin Inhibitor I decreased the activation of MMP2 (Fig. 5B). Increasing the concentration of the Furin Inhibitor I (100 μM) had no further effect on the activation of MMP2 or the number of oocytes released.
FIG. 5.
Inhibition of MMP2 activation by Furin Inhibitor I. Rats were injected with eCG, and 48 h later residual tissue was collected. Residual tissues were cultured for 12 h with FSK (10 μM) to induce Furin expression, and then DMSO vehicle control or Furin Inhibitor I (50 μM) was added. Residual tissue was removed from culture at 24 h (i.e., 12 h after treatment with the Furin Inhibitor I), and cell membranes were isolated. Cell membrane proteins were subjected to gelatin zymography (A), and band densities were determined via MetaMorph Image Analysis software. Y-axis shows the ratio of active to inactive MMP2 (mean ± SEM; n = 3 independent culture experiments) (B). Bars with asterisks are significantly different (P < 0.05).
DISCUSSION
During the process of ovulation, there is a systematic breakdown of the wall of the preovulatory follicle to allow release of the oocyte. Associated with this follicular matrix remodeling is the induction of an array of proteinases including the MMPs, such as the gelatinases MMP2 and MMP9 [1]. Like other proteinases, MMPs are secreted in a nonactive, zymogen form that must be cleaved in order for the enzyme to be active [1]. One such family of activators is the PCSKs, which include FURIN or PCSK3 [8]. The present study demonstrates that hCG stimulates Furin mRNA expression and that this proprotein convertase plays a role in the ovulatory process. This is apparent from a number of lines of evidence, such as the temporal and spatial pattern of Furin mRNA induction as well as the decrease in MMP activity and oocyte release following administration of a FURIN inhibitor. For example, the stimulation of Furin mRNA in whole ovaries occurs at 8 to 12 h after hCG, which is prior to ovulation in this gonadotropin-induced model [9]. Experiments to elucidate the localization of Furin mRNA levels by in situ hybridization revealed intense expression in theca and adjacent stromal cells of preovulatory follicles after hCG administration. This pattern of cellular localization is similar to previous reports of high expression of the MMPs, such as mRNA for Mmp2, Mmp14, and Mmp19, in the theca and interstitial compartments [18, 32]. Thus, Furin mRNA is present in the same follicular compartment as MMP14, a proteinase that is known to be activated by FURIN [16, 17].
Presently, little is known regarding the expression and role of FURIN in the ovary. In fish, Furin mRNA was highly abundant in oocytes of small growing follicles [19]. The temporal expression pattern for furin in the fish was similar to that of matrix metallopeptidase 11b (stromelysin-3; human homolog MMP11) and matrix metallopeptidase 24 (human homolog MMP24), both of which contain furin sites, leading to the proposal that FURIN may activate these MMPs [19]. In the present study, Furin mRNA was also observed in oocytes and may play a role comparable to that in fish in activating other MMPs [19] or may be involved in the processing of zona pellucida proteins [20, 33].
Although there are limited reports of FURIN in the ovary, other members of the PCSK family have been described in the ovary. For example, in the mouse, Pcsk6 mRNA was found in granulosa cells of preantral follicles, but hCG treatment did not induce PCSK6 in preovulatory follicles. Diaz et al. [20] suggested that the action of PCSK6 may be on TGFB ligands and potentially GDF9 and AMH, which exert their biological effect during the same developmental period when PCSK6 is most highly expressed or alternatively by acting on zona pellucida proteins. Bae et al. [21] found that in the rat Pcsk5 mRNA was expressed in the theca cells of growing follicles and that eCG stimulated Pcsk5 mRNA expression during follicular growth. A further induction of Pcsk5 mRNA was observed in granulosa cells within 3 h of hCG administration. Exploration of an existing ovarian database [22] for potential PCSK5 substrates revealed a number of potential ovulatory proteins that are activated by PCSK5, such as the MMPs, ADAMTS1, and bone morphogenic proteins [21]. These previous findings with other members of the PCSK family suggest that this family of proprotein convertases is associated with follicular growth and ovulation.
To determine whether FURIN plays a role in the processing of MMPs and its impact on the ovulatory process, the present study explored the activation of MMP2 and oocyte release in the presence of a FURIN inhibitor. The rationale behind these experiments is that activation of ovarian MMP2 occurs at the cell surface and is dependent, in part, on an activated MMP14 [18]. MMP14 contains a furin activation site allowing FURIN to cleave the latent form of MMP14 intracelluarly, which is then inserted into the cell membrane [18]. Activated MMP14 subsequently activates MMP2, which is held to the cell surface by a trimolecular complex of MMP14/TIMP2/pro-MMP2 [34]. Thus, ovarian MMP2 activation is dependent upon active MMP14 present in the plasma membrane [18] and was used as an index of FURIN activity. The present finding that an inhibitor of FURIN reduced the activation of MMP2 provides indirect evidence that FURIN activates MMP14 in ovarian follicles, which in turn activates MMP2. It is, therefore, tempting to speculate that this decrease in active MMP following administration of the FURIN inhibitor diminishes the breakdown of the follicular ECM, thereby blocking oocyte release. Such speculation is supported by previous reports that inhibition of MMP activity blocks ovulation in the rat [35] and that antibodies against MMP2 block the ovulatory luteal transition in ewes [36]. Therefore, we investigated whether inhibition of FURIN would impact ovulation. Indeed, administration of a FURIN inhibitor reduced the number of oocytes released, which further supports the hypothesis that this proprotein convertase activates proteins associated with the ovulatory pathway. This may occur through the activation of MMP14 or, as shown in the present study, MMP2. However, furin cleavage sites are present in MMP11, MMP23, and other members of the membrane type MMPs, and FURIN has been demonstrated to activate MMP11 [5], indicating additional pathways of FURIN influence in the ovary. Also, the data must be interpreted with the understanding that the commercial inhibitor may block the actions of other members of the PCSK family. Irrespective of the specific substrate(s) cleaved by FURIN, the finding of a diminution of oocyte release following administration of a FURIN inhibitor suggests that FURIN facilitates the ovulatory process.
The administration of a FURIN inhibitor in the present study did not completely block oocyte release, suggesting that ovulation still occurs, albeit to a lesser degree, in inhibitor-treated animals. The ability of the animals to ovulate after treatment with the inhibitor is not totally unexpected, based upon previous reports, and may be related to a number of factors. First, the ability of the inhibitor to penetrate and be taken up by the ovary may affect the local concentration of the inhibitor at the follicular apex. Second, although the inhibitor decreases the activation of MMP2, there may be sufficient levels of the proteinase present to bring about rupture of some follicles. Finally, the breakdown of the follicle wall may entail an orchestrated process involving a number of proteinases, some of which have overlapping substrate specificities. For example, administration of collagenase inhibitors only reduces the number of oocytes released by 55% [37], whereas intrabursal injection of antibodies against plasminogen activators diminishes ovulation by 36%–44% [38], suggesting an incomplete blockade of oocyte release. Within this same context of multiple proteinases impacting ovulation, the commercial FURIN inhibitor may not block the actions of other members of the PCSK family, such as PCSK5, which potentially have a role in the ovulatory process. As noted previously, Pcsk5 mRNA was expressed in the theca cells and was increased in granulosa cells by hCG administration, leading to the suggestion of an involvement of this proprotein convertase in oocyte release [21].
Since the hCG-induced expression of PCSK appears to be important for ovulation, experiments were performed to determine the pathways responsible for the hCG induction of Furin mRNA. Our findings in cultured follicles and granulosa cells, in conjunction with the localization studies, suggested that Furin mRNA was primarily upregulated by hCG in theca-interstitial cells of the rat ovary. Therefore, residual tissue consisting of theca cells and interstitial tissue was used as a model to examine regulation of Furin expression. It is important to note, however, that residual tissue is a remnant of granulosa cell collection resulting in a heterogeneous tissue sample with variable amounts of granulosa cells remaining. This heterogenetity results in differences in the magnitude of responsiveness to hormonal stimuli. Regardless, the ability of PMA + FSK to upregulate Furin mRNA indicates that Furin mRNA could be regulated by either the cAMP or PKC pathway, or both. To dissect the regulatory pathway further, residual tissue was incubated with several downstream products of LH action. The EGF pathway was examined based upon previous reports that Furin mRNA levels are regulated by the EGFR pathway in gastric epithelial cells [39]. To determine if this pathway is also intact in the ovary, we cultured rat ovarian residual tissue with a ligand of EGFR, AREG. Expression of Furin mRNA after AREG treatment was equal to the stimulation after FSK, indicating regulation by both EGFR and cAMP pathways, both of which are necessary for successful ovulation [40, 41]. Recent findings have also shown that the EGFR and cAMP pathways can be interconnected. Panigone et al. [42] demonstrated that LH-induced EGFR phosphorylation is cAMP dependent, as the PRKA inhibitor, H89, blocked LH-induced EGFR phosphorylation. Since Furin mRNA seems to be regulated by both cAMP and EGFR, we hypothesized that EGFR induction of Furin mRNA is also cAMP dependent. FSK-induced Furin expression was surpressed by H89, confirming that Furin mRNA is regulated by cAMP through PRKA. In addition, inhibition of EGFR with AG1478 blocked the FSK-induced Furin expression, suggesting the FSK induction is working through EGFR. Whether this FSK induction occurs via stimulation of AREG in the theca or remaining granulosa cells remains to be determined. However, it is of interest that not only might the EGF pathway regulate Furin expression, but FURIN might positively regulate the EGF pathway. In human astrocytoma cells, FURIN is involved in shedding of heparin-binding EGF, which results in activation of the EGF receptor [43]. Thus, induction of Furin mRNA by the EGF pathway may result in a positive stimulatory feedback where EGF-like ligands induce Furin and FURIN then increases EGF receptor activation, which drives further activation of Furin mRNA expression.
In conclusion, Furin mRNA is upregulated by hCG prior to ovulation in vivo in rats and in vitro in cultured follicles. Furin mRNA is also upregulated in cultured residual tissue by FSK or AREG, suggesting regulation by cAMP and EGFR pathways. When treated intraperitoneally with a FURIN inhibitor, there was a decrease in ovulated oocytes, indicating a role for FURIN in ovulation. A potential mechanism for this diminution of ovulation is that inhibition of FURIN decreases active proteases available to breakdown the follicle wall. This idea was supported by our findings that cell membranes incubated with the FURIN inhibitor displayed a reduced amount of active MMP2. The ability of FURIN to activate not only MMPs, but many other proteins, suggests that this convertase may be a crucial player in ovulation.
Acknowledgments
The authors would like to acknowledge the assistance of Ms. Kathy Rosewell in the overall preparation of this manuscript.
Footnotes
1Supported by grants NCRR P20 RR15592 (T.E.C.), NIH HD057446 (T.E.C.), and a fellowship from the Endocrine Society (B.P.K.).
REFERENCES
- Curry TE, Jr, Osteen KG.The matrix metalloproteinase system: changes, regulation, and impact throughout the ovarian and uterine reproductive cycle. Endocr Rev 2003; 24: 428–465. [DOI] [PubMed] [Google Scholar]
- Curry TE, Jr, Dean DD, Woessner JF, Jr, LeMaire WJ.The extraction of a tissue collagenase associated with ovulation in the rat. Biol Reprod 1985; 33: 981–991. [DOI] [PubMed] [Google Scholar]
- Kaur C, Guraya SS.Effects of blocking ovulation with pentobarbitone on ovarian proteinases in rats. Curr Sci 1986; 55: 995–996. [Google Scholar]
- Reich R, Tsafriri A, Mechanic GL.The involvement of collagenolysis in ovulation in the rat. Endocrinology 1985; 116: 522–527. [DOI] [PubMed] [Google Scholar]
- Kleiner DE, Jr, Stetler-Stevenson WG.Structural biochemistry and activation of matrix metalloproteases. Curr Opin Cell Biol 1993; 5: 891–897. [DOI] [PubMed] [Google Scholar]
- Murphy G, Stanton H, Cowell S, Butler G, Knauper V, Atkinson S, Gavrilovic J.Mechanisms for pro matrix metalloproteinase activation. APMIS 1999; 107: 38–44. [DOI] [PubMed] [Google Scholar]
- Strongin AY, Collier IE, Bannikov G, Marmer BL, Grant GA, Goldberg GI.Mechanism of cell surface activation of 72-kDa type IV collagenase. Isolation of the activated form of the membrane metalloprotease. J Biol Chem 1995; 270: 5331–5338. [DOI] [PubMed] [Google Scholar]
- Scamuffa N, Calvo F, Chretien M, Seidah NG, Khatib AM.Proprotein convertases: lessons from knockouts. FASEB J 2006; 20: 1954–1963. [DOI] [PubMed] [Google Scholar]
- Sridaran R, Rodriguez-Sierra JF, Blake CA.Ovarian involvement in the timing mechanism that controls ovulation in rats. Biol Reprod 1979; 21: 505–509. [DOI] [PubMed] [Google Scholar]
- Stawowy P, Meyborg H, Stibenz D, Borges Pereira SN, Roser M, Thanabalasingam U, Veinot JP, Chretien M, Seidah NG, Fleck E, Graf K.Furin-like proprotein convertases are central regulators of the membrane type matrix metalloproteinase-pro-matrix metalloproteinase-2 proteolytic cascade in atherosclerosis. Circulation 2005; 111: 2820–2827. [DOI] [PubMed] [Google Scholar]
- Benjannet S, Elagoz A, Wickham L, Mamarbachi M, Munzer JS, Basak A, Lazure C, Cromlish JA, Sisodia S, Checler F, Chretien M, Seidah NG.Post-translational processing of beta-secretase (beta-amyloid-converting enzyme) and its ectodomain shedding. The pro- and transmembrane/cytosolic domains affect its cellular activity and amyloid-beta production. J Biol Chem 2001; 276: 10879–10887. [DOI] [PubMed] [Google Scholar]
- Creemers JW, Ines DD, Plets E, Serneels L, Taylor NA, Multhaup G, Craessaerts K, Annaert W, De SB.Processing of beta-secretase by furin and other members of the proprotein convertase family. J Biol Chem 2001; 276: 4211–4217. [DOI] [PubMed] [Google Scholar]
- Khatib AM, Siegfried G, Prat A, Luis J, Chretien M, Metrakos P, Seidah NG.Inhibition of proprotein convertases is associated with loss of growth and tumorigenicity of HT-29 human colon carcinoma cells: importance of insulin-like growth factor-1 (IGF-1) receptor processing in IGF-1-mediated functions. J Biol Chem 2001; 276: 30686–30693. [DOI] [PubMed] [Google Scholar]
- Khatib AM, Siegfried G, Chretien M, Metrakos P, Seidah NG.Proprotein convertases in tumor progression and malignancy: novel targets in cancer therapy. Am J Pathol 2002; 160: 1921–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried G, Basak A, Cromlish JA, Benjannet S, Marcinkiewicz J, Chretien M, Seidah NG, Khatib AM.The secretory proprotein convertases furin, PC5, and PC7 activate VEGF-C to induce tumorigenesis. J Clin Invest 2003; 111: 1723–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remacle AG, Rozanov DV, Fugere M, Day R, Strongin AY.Furin regulates the intracellular activation and the uptake rate of cell surface-associated MT1-MMP. Oncogene 2006; 25: 5648–5655. [DOI] [PubMed] [Google Scholar]
- Sato H, Kinoshita T, Takino T, Nakayama K, Seiki M.Activation of a recombinant membrane type 1-matrix metalloproteinase (MT1-MMP) by furin and its interaction with tissue inhibitor of metalloproteinases (TIMP)-2. FEBS Lett 1996; 393: 101–104. [DOI] [PubMed] [Google Scholar]
- Jo M, Thomas LE, Wheeler SE, Curry TE., JrMembrane-type 1 matrix metalloproteinase (MMP)-associated MMP-2 activation increases in the rat ovary in response to an ovulatory dose of human chorionic gonadotropin. Biol Reprod 2004; 70: 1024–1033. [DOI] [PubMed] [Google Scholar]
- Ogiwara K, Shinohara M, Takahashi T.Structure and expression of Furin mRNA in the ovary of the medaka, Oryzias latipes. J Exp Zoolog A Comp Exp Biol 2004; 301: 449–459. [DOI] [PubMed] [Google Scholar]
- Diaz FJ, Sugiura K, Eppig JJ.Regulation of Pcsk6 expression during the preantral to antral follicle transition in mice: opposing roles of FSH and oocytes. Biol Reprod 2008; 78: 176–183. [DOI] [PubMed] [Google Scholar]
- Bae JA, Park HJ, Seo YM, Roh J, Hsueh AJ, Chun SY.Hormonal regulation of proprotein convertase subtilisin/kexin type 5 expression during ovarian follicle development in the rat. Mol Cell Endocrinol 2008; 289: 29–37. [DOI] [PubMed] [Google Scholar]
- Leo CP, Vitt UA, Hsueh AJ.The Ovarian Kaleidoscope database: an online resource for the ovarian research community. Endocrinology 2000; 141: 3052–3054. [DOI] [PubMed] [Google Scholar]
- Mann JS, Kindy MS, Edwards DR, Curry TE., JrHormonal regulation of matrix metalloproteinase inhibitors in rat granulosa cells and ovaries. Endocrinology 1991; 128: 1825–1832. [DOI] [PubMed] [Google Scholar]
- Morris JK, Richards JS.Hormone induction of luteinization and prostaglandin endoperoxide synthase-2 involves multiple cellular signaling pathways. Endocrinology 1993; 133: 770–779. [DOI] [PubMed] [Google Scholar]
- Shimada M, Yanai Y, Okazaki T, Yamashita Y, Sriraman V, Wilson MC, Richards JS.Synaptosomal-associated protein 25 gene expression is hormonally regulated during ovulation and is involved in cytokine/chemokine exocytosis from granulosa cells. Mol Endocrinol 2007; 21: 2487–2502. [DOI] [PubMed] [Google Scholar]
- Sriraman V, Sharma SC, Richards JS.Transactivation of the progesterone receptor gene in granulosa cells: evidence that Sp1/Sp3 binding sites in the proximal promoter play a key role in luteinizing hormone inducibility. Mol Endocrinol 2003; 17: 436–449. [DOI] [PubMed] [Google Scholar]
- Simpson KS, Byers MJ, Curry TE., JrSpatiotemporal mRNA expression of the tissue inhibitors of metalloproteinases (TIMPs) in the ovary throughout the rat estrous cycle. Endocrinology 2000; 142: 2058–2069. [DOI] [PubMed] [Google Scholar]
- Luborsky JL, Slater WT, Behrman HR.Luteinizing hormone (LH) receptor aggregation: modification of ferritin-LH binding and aggregation by prostaglandin F2 alpha and ferritin-LH. Endocrinology 1984; 115: 2217–2226. [DOI] [PubMed] [Google Scholar]
- Bradford MM.A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72: 248–254. [DOI] [PubMed] [Google Scholar]
- Curry TE, Jr, Song L, Wheeler SE.Cellular localization of gelatinases and tissue inhibitors of metalloproteinases during follicular growth, ovulation and early luteal formation in the rat. Biol Reprod 2001; 65: 855–865. [DOI] [PubMed] [Google Scholar]
- Guo C, Piacentini L.Type I collagen-induced MMP-2 activation coincides with up-regulation of membrane type 1-matrix metalloproteinase and TIMP-2 in cardiac fibroblasts. J Biol Chem 2003; 278: 46699–46708. [DOI] [PubMed] [Google Scholar]
- Jo M, Curry TE., JrRegulation of matrix metalloproteinase-19 messenger RNA expression in the rat ovary. Biol Reprod 2004; 71: 1796–1806. [DOI] [PubMed] [Google Scholar]
- Kiefer SM, Saling P.Proteolytic processing of human zona pellucida proteins. Biol Reprod 2002; 66: 407–414. [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Sato H, Okada A, Ohuchi E, Imai K, Okada Y, Seiki M.TIMP-2 promotes activation of progelatinase A by membrane-type 1 matrix metalloproteinase immobilized on agarose beads. J Biol Chem 1998; 273: 16098–16103. [DOI] [PubMed] [Google Scholar]
- Brannstrom M, Woessner JF, Jr, Koos RD, Sear CHJ, LeMaire WJ.Inhibitors of mammalian tissue collagenase and metalloproteinases suppress ovulation in the perfused rat ovary. Endocrinology 1988; 122: 1715–1721. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Van Kirk EA, Murdoch WJ.Role of matrix metalloproteinase 2 in the ovulatory folliculo-luteal transition of ewes. Reproduction 2002; 124: 347–352. [DOI] [PubMed] [Google Scholar]
- Butler TA, Zhu C, Mueller RA, Fuller GC, LeMaire WJ, Woessner JF., JrInhibition of ovulation in the perfused rat ovary by the synthetic collagenase inhibitor SC 44463. Biol Reprod 1991; 44: 1183–1188. [DOI] [PubMed] [Google Scholar]
- Reich R, Miskin R, Tsafriri A.Follicular plasminogen activator: involvement in ovulation. Endocrinology 1985; 116: 516–521. [DOI] [PubMed] [Google Scholar]
- Kamimura H, Konda Y, Yokota H, Takenoshita S, Nagamachi Y, Kuwano H, Takeuchi T.Kex2 family endoprotease furin is expressed specifically in pit-region parietal cells of the rat gastric mucosa. Am J Physiol 1999; 277: G183–G190. [DOI] [PubMed] [Google Scholar]
- Holmes PV, Hedin L, Janson PO.The role of cyclic adenosine 3′,5′-monophosphate in the ovulatory process of the in vitro perfused rabbit ovary. Endocrinology 1986; 118: 2195–2202. [DOI] [PubMed] [Google Scholar]
- Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M.Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol 2007; 27: 1914–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panigone S, Hsieh M, Fu M, Persani L, Conti M.Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol Endocrinol 2008; 22: 924–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama K, Saito M, Sasaki M, Obara Y, Higashiyama S, Nakahata N.Thromboxane A2 receptor-mediated epidermal growth factor receptor transactivation: involvement of PKC-delta and PKC-epsilon in the shedding of epidermal growth factor receptor ligands. Eur J Pharm Sci 2009; 38: 504–511. [DOI] [PubMed] [Google Scholar]