Abstract
Human natural killer (NK) cells are lymphocytes that destroy tumor and virally infected cells. Previous studies have shown that exposures of NK cells to tributyltin (TBT) greatly diminish their ability to destroy tumor cells (lytic function) while activating mitogen-activated protein kinases (MAPK) (p44/42, p38, and JNK) in the NK cells. The signaling pathway that regulates NK lytic function appears to include activation of protein kinase C (PKC) as well as MAPK activity. The TBT-induced activation of MAPKs would trigger a portion of the NK lytic signaling pathway, which would then leave the NK cell unable to trigger this pathway in response to a subsequent encounter with a target cell. In the present study we evaluated the involvement of PKC in the inhibition of NK lysis of tumor cells and activation of MAPKs caused by TBT exposures. TBT caused a 2–3 fold activation of PKC at concentrations ranging from 50–300 nM (16–98 ng/mL), indicating that activation of PKC occurs in response to TBT exposures. This would then leave the NK cell unable to respond to targets. Treatment with the PKC inhibitor, bisindolylmaleimide I, caused an 85% decrease in the ability of NK cells to lyse tumor cells validating the involvement of PKC in the lytic signaling pathway. The role of PKC in the activation of MAPKs by TBT was also investigated using bisindolylmaleimide I. The results indicated that in NK cells where PKC activation was blocked there was no activation of the MAPK, p44/42 in response to TBT. However, TBT-induced activation of the MAPKs, p38 and JNK did not require PKC activation. These results indicate the pivotal role of PKC in the TBT-induced loss of NK lytic function including the activation of p44/42 by TBT in NK cells.
INTRODUCTION
Organotin compounds are chemicals that are used widely for industrial and consumer purposes (Kimbrough, 1976; Roper, 1992; Kannan et al., 1998; Karpiak et al., 2001). Tributyltin (TBT) is an example of a butyltin (BT). BTs have been widely used in industrial, agricultural and domestic settings and are known significant environmental contaminants (Laughlin and Linden, 1985; Tanabe et al., 1998; Loganathan et al., 2000). TBT is the most toxic of BTs and was mainly in use in wood preservation, marine antifouling paints, disinfection of circulating industrial cooling waters, and slime control in paper mills (Kimbrough, 1976; Roper, 1992; Yamada et al., 1993). TBT is found in human food, such as fish (Kannan et al., 1995a,b,c; Kannan and Falandyz, 1997). It is also found in various household products such as siliconized-paper baking parchments and shower curtains (Yamada et al., 1993). In animals, TBT causes irritation of the eye and the skin, together with inflammation of the respiratory tract (Snoeij et al., 1987; Kupper, 1989; WHO, 1990; Corsini et al., 1996). Studies using human intestinal Caco-2 cells have shown that exposure to TBT may disorder the intestinal barrier functions (Tsukazaki et al., 2004). In humans, TBT residue has been detected in blood (Kannan et al., 1999; Whalen et al., 1999). Food ingestion may act as a route of entry into the human body. Additional routes of entry may include absorption through the skin (Baaijens, 1987) and possibly inhalation for those who are occupationally exposed (WHO/FAO, 1984).
Natural killer (NK) cells are a subset of lymphocytes that are known to have the ability to kill tumor cells, virally infected cells and antibody-coated cells (Cooper et al., 2001; Wu and Lanier, 2003; Vivier et al., 2004) without the need for in vitro or in vivo activation (Moretta et al., 2002). NK cells are the earliest and possibly predominant defense against tumor cells (Wu and Lanier, 2003; Vivier et al., 2004). NK cells also appear to have a central role in immune defense against viral infection as evidenced by a greatly increased incidence of viral infection in individuals where the NK subset of lymphocytes is completely absent (Fleisher et al., 1982; Biron et al., 1989). NK cells are defined by the absence of the T-cell receptor/CD3 complex and the presence of CD56 and/or CD16 on their surface (Wu and Lanier, 2003; Vivier et al., 2004). Target cells become susceptible to lysis by NK cells when they lose or down-regulate major histocompatibility complex class I expression, which protects target cells in which it is expressed (Tajima et al., 2004).
TBT in blood could suppress immune cells’ function, including NK cells. An interference with NK cell function predisposes animals, including humans, to viral infection and tumors. Individuals who lack the NK cell phenotype exhibit increased rates of viral infection (Fleischer et al., 1982; Biron et al., 1989). TBT has been detected in human blood in our laboratory and elsewhere (Kannan et al., 1999; Whalen et al., 1999), and further studies have found that TBT causes a decrease in the ability of human NK cells to destroy their target cells (Whalen et al., 1999; Dudimah et al, 2007).
Protein kinase C (PKC) catalyzes the phosphorylation of hydroxyl groups of serine and threonine amino acid residues in a variety of proteins and thus plays an important role in signal transduction and the regulation of various cellular functions (Nishizuka 1995; Rosse et al., 2010). There are several isoforms of PKC (Rosse et al., 2010). The binding of tumor cells to NK cells stimulate protein-tyrosine kinase (PTK) activation and PTK causes activation of a phospholipase Cγ (PLCγ) which produces diacylglycerol (DAG) and inositol trisphosphate (IP3) (Steele and Brahmi, 1988a; Ting et al., 1992; Whalen et al., 1993; Jevremovic et al., 1999) leading to activation of a protein kinase C (Graves et al., 1986; Nishizuka 1988; Steele and Brahmi, 1988b; Procopio et al., 1989). PTK activation also leads to activation of guanine nucleotide exchange factors (GEFs) which activate various small GTP-binding (G) proteins (Jiang et al., 2000; Perussia, 2000; Jevremovic et al., 2001; Vivier et al., 2004). Activation of small G-proteins (Ras or Rac) stimulate activation of mitogen-activated protein kinase (MAPK) kinase kinases (MAP3Ks) leading to activation of MAPK kinases (MAP2Ks) which activate MAPKs (Derijard et al., 1995; Han et al., 1996; Jiang et al., 2000; Wei et al., 2000; Chuang et al., 2003). PKC appears to activate p44/42 via activation of Ras (Pearson et al., 2001; Trakul et al., 2005) and/or direct activation of the MAP3K for p44/42, Raf-1 (Ueffing et al., 1997; Xuan et al., 2005).
Previous studies have found that activation of mitogen-activated protein kinases (MAPKs) occurs very rapidly in response to TBT exposure (Aluoch and Whalen., 2005; Aluoch, et al., 2006; Aluoch et al., 2007). Recent studies have indicated that TBT activates p44/42 via activation of its MAP2K, MEK, and that activation of p44/42 can cause a loss of lytic function in NK cells (Abraha and Whalen, 2009; Dudimah et al., 2010). As PKC is able to regulate the activation of p44/42, PKC activation may be a consequence of TBT exposures that causes the loss of lytic function in human NK cells. Thus, in the present study we examined whether TBT can activate PKC and whether PKC is a necessary upstream activator for TBT-induced MAP kinase phosphorylation and loss of lytic function in NK cells.
MATERIALS AND METHODS
Preparation of human NK cells
Peripheral blood from healthy adult (male and female) volunteer donors was used for this study (Red Cross, Portland, OR, or Key Biologics, Memphis, TN). Highly purified NK cells were obtained using a rosetting procedure. Buffy coats were mixed with 0.6–0.8 ml of RosetteSep human NK cell enrichment antibody cocktail (StemCell Technologies, Vancouver, BC, Canada) per 45 ml of buffy coat. The mixture was incubated for 20–25 min at room temperature (25° C). Following the incubation, 5–8 ml of the mixture was layered onto 4 ml of Ficoll-Hypaque (1.077 g/ml) (Sigma) and centrifuged at 1200 g for 30–40 min. The cell layer was collected and washed twice with phosphate buffered saline (PBS), pH 7.2 and stored in complete media (RPMI-1640 supplemented with 10% heat-activated BCS, 2 mM L-glutamine and 50 μg penicillin G with 50 μg streptomycin/ml) at 1 million cells/ml. The resulting cell preparation was >95% CD16+, 0% CD3+ by fluorescence microscopy and flow cytotometry (Whalen et al., 2002a).
Chromium Release Assay
NK cytotoxicity was measured using a 51Cr release assay (Whalen et al., 1999). The target cell in all cytotoxicity assays were the NK-susceptible K562 (Human Chronic Myelogenous Leukemia) cell line (ATCC, Manassas, VA). K562 cells (3 million) were incubated with 50 μCi 51Cr in 0.3–0.5 ml of BCS for 1.5 h at 37 °C in 19:1 air/CO2. Following this incubation, the target cells were washed twice with media. Following the various exposures, NK (effector) cells (1.2×105/100 μL for 12:1 ratio with target cells) were added to the wells of round-bottom microwell plates. The effectors were diluted to 6:1 ratio (0.6×105/100 μL) and 3:1 ratio (0.3×105/100 μL); each ratio was tested in triplicate. Targets were added (1 × 104/100 μl) to each well, and the plate was centrifuged at 300 × g for 3 min and incubated for 2 h at 37 °C (air/CO2, 19:1). After incubation, a 0.1-ml aliquot of the supernatant was collected and counted for radioactivity for 60 s in a Packard COBRA gamma counter (Packard Instrument Co., Meriden, CT). Specific lysis inhibition was calculated as follows: [1- [100 × [(test c.p.m. − spontaneous c.p.m.)/(maximum c.p.m. − spontaneous c.p.m.)]]. Maximum release was produced by adding 100 μl of 10% Triton X-100.
Chemical preparation
Tributyltin was purchased from Aldrich Chemical Co. (Milwaukee, WI). TBT was a neat standard (liquid), which was homogenously suspended in double de-ionized H2O to give a 1 mM solution. This TBT solution was diluted with media to achieve final concentrations. The range of concentrations of TBT used in treating the cells (25 to 300 nM) was based on previous studies (Whalen et al., 1999 and Whalen et al., 2002a). The concentration of TBT used in the experiments is not far greater than the highest concentration that was detected in human blood (as high as 260 nM) (Kannan et al., 1999; Whalen et al., 1999).
PKC inhibitor, Bisindolylmaleimide I, was purchased from Calbiochem, Inc. (San Diego, CA). This PKC inhibitor was dissolved initially in DMSO to make a 5 mM stock. These stock solutions were diluted in media to achieve final concentrations of 50 μM and 25 μM.
Cell viability
Cell viability was determined by trypan blue exclusion prior to and following each exposure period. Cell numbers and their viability did not vary among experimental conditions. Cell viability was normally greater than 90% for both control and TBT-treated cells.
Cell lysates
To examine whether TBT exposures caused activation of PKC in NK cells, cell lysates were prepared by exposing NK cells to 25–300 nM TBT or control for 10 min. Following these treatments, the cells were centrifuged and washed with 1 ml of PBS and centrifuged for 2 min again and the cell pellets were then lysed with 500 μl/10 million cells of lysis buffer. The cell lysates were stored frozen at −80 °C up to the point when they were run on sodium dodecylsulfate polyacrylamide gel electrophoresis (SDS-PAGE). Control and TBT-exposed cells for a given experimental setup were from an individual donor.
To examine if PKC activation was involved in TBT-induced activation of MAPKs, cells lysates were made using: (1) NK cells exposed to media (control); (2) NK cells exposed to 25 μM Bisindolylmaleimide I (PKC inhibitor) for 1hr, followed by media for 10 min; (3) NK cells were exposed to media for 1 h followed by exposure to 200 nM TBT for 10 min; (4) NK cells were exposed to media for 1 h followed by exposure to 100 nM TBT for 10 min; (5) NK cells were exposed to 25 μM Bisindolylmaleimide I for 1hr followed by exposure to 200 nM TBT for 10 min (6) NK cells were exposed to 25 μM Bisindolylmaleimide I for 1hr followed by exposure to 100 nM TBT for 10 min. to determine the involvement of PKC in activation of MAPKs. Following the above treatments, the cells were centrifuged and the cell pellets were lysed using 500 μl of lysis buffer (Active motif, Carlsbad, CA) per 10 million cells. The cell lysates were stored frozen at −80 °C up to the point when they were run on SDS-PAGE. Control and treated cells for a given experimental setup (described above) were from an individual donor. Each of the experimental setups (1–6) was repeated a minimum of three times using cells from different donors.
Western blot
Cell lysates were run on 10% SDS-PAGE and transferred to PVDF (polyvinylidene Difluoride) membrane. The PVDF was immunoblotted with anti-phospho-p44/42 (Thr202/Tyr204), anti-p44/42 anti-phospho-p38 (Thr180/Tyr182), anti-p38, anti-phospho-SAPK/JNK (Thr183/Tyr185), anti-SAPK/JNK and anti-β-actin antibodies (Cell Signaling Technologies, Beverly, MA). Antibodies were visualized using ECL chemiluminescent detection system (Amersham, Piscataway, NJ) and Kodak Image Station (Kodak, Rochester, NY). The density of each protein band was determined by densitometric analysis using the Kodak Image Station analysis software. The settings on the image station were optimized to detect the largest possible signal range and to prevent saturation of the system. Samples from all the experimental setups (listed in the cell lysates section) were run on a separate gel/blot. A given experimental setup (as described in the cell lysate section) always had its own internal control. Thus, differences and changes in protein expression are determined relative to the internal control. This determination provides relative quantitation by evaluating whether a given treatment changed expression of phospho-p44/42, or p44/42 or phospho-p38, or p38 or phospho-SAPK/JNK, or SAPK/JNK relative to untreated cells. β-Actin levels were determined for each condition to verify that equal amounts of protein were loaded. In addition, the density of each protein band was normalized to β-actin to correct for small differences in protein loading among the lanes.
Statistical analysis
Analysis of variance (ANOVA) followed by pair-wise comparison of data was carried out for all studies. A significant ANOVA was followed by pair wise comparisons of control versus TBT-exposed data using a t-test. A minimum of three separate experiments were carried out for measurement of lytic function (n ≥ 9). A minimum of three separate determinations were carried out for each Western blot experimental set-up (n ≥ 3) and statistical significance was noted at p < 0.05.
RESULTS
Effect of 10 min exposures to 25–300 nM TBT on protein kinase C (PKC) activation state
Figure 1 shows the effect of a 10 min exposure to 25–300 nM TBT on the activating phosphorylation of PKC. The 10 min exposure to 50–300 nM TBT produced an increase in phosphorylation of PKC; there was a significant 2–3 fold increase in phosphorylation of PKC when NK cells were exposed to 50–300 nM TBT (Figure 1A). Exposure to 25 nM TBT did not cause any significant activating phosphorylation of PKC and thus is not shown on the bar graph (Figure 1A). Figure 1B shows the results from a representative experiment.
Figure 1.
Effects of exposures of human NK cells to TBT for 10 minutes on the phosphorylation state of PKC. A) Fold increase in phospho-PKC as compared to control cells following 10 min exposures to 300 nM to 50 nM TBT. Values are mean ±S.D. from at least three separate experiments using different donors (triplicate determinations for each experiment, n ≥ 9). An asterisk indicates a significant increase as compared to control (p<0.05). B) Representative western blot of the effect of 10 min exposures of NK cells to 25 nM to 300 nM TBT on the phosphorylation state of PKC. Upper bands: (1) control cells, (2) NK cells exposed to 300 nM TBT, (3) NK cells exposed to 200 nM TBT, (4) NK cells exposed to 100 nM TBT, (5) NK cells exposed to 50 nM TBT, (6) NK cells exposed to 25 nM TBT. lower bands: β-actin for the corresponding lanes.
Effects of inhibition of protein kinase C (PKC) on lytic function NK cells
Figure 2 shows inhibition of NK lysis by the PKC inhibitor, Bisindolylmaleimide I. NK cells were pretreated with a range of concentrations of Bisindolylmaleimide I inhibitor for 1 h before incubation with 51Cr-labeled K562 tumor cells to test for lysis. NK cells exposed to Bisindolylmaleimide I showed a statistically significant decrease in their lytic function. Decrease in lytic function of 92.6 and 88.6 % were seen with 50 and 25 μM Bisindolylmaleimide I, respectively (p<0.01) (Figure 2).
Figure 2.
Effect of Bisindolylmaleimide I on the lytic function of NK cells. Starting from the left Bar, NK cells were treated with the indicated concentrations of Bisindolylmaleimide I (Bis I) for 1 h. Lysis of the target cells was measured by using a 51Cr release assay. X-axis represents the indicated concentrations of PKC inhibitor. Y-axis represents the % control lysis activity. *indicates significant difference compared to the control (p<0.05)
Effects of protein kinase C (PKC) inhibition on TBT-induced decreases in NK lytic function
In an effort to determine if TBT-activation of PKC was responsible for the TBT-induced loss of cytotoxicity, we investigated the effect of Bisindolylmaleimide I on the decrease in NK lytic function seen with TBT exposures. To address this, NK cells were incubated with bisindolylmaleimide I prior to a 1 h exposure to 200 or 100 nM TBT (lower concentrations were not tested). Additionally, the effects of 1 h exposures to bisindolylmaleimide I alone, and 200 or 100 nM TBT were also examined in the same cells. Following the incubation period, the cells were washed twice with the media and incubated for 24 h period before assaying for cytotoxic function. The rationale was that if bisindolylmaleimide I was inhibiting PKC during TBT exposures, then TBT would be unable to activate PKC and thus unable to block NK function. There was a significant decrease in lytic function when NK cells were exposed to 200 or 100 nM TBT alone followed by 24 h in TBT-free media causing a 85 and 42 % decrease in lytic function as compared to control (Figure 3). Decrease in lytic function of 78, 93 and 85 % were seen with 25 μM Bisindolylmaleimide I alone, 25 μM bisindolylmaleimide I followed by 200 or 100 nM TBT respectively (Figure 3) (p<0.05). Thus, the bisindolylmaleimide I inhibition of NK lytic function was not reversible, so it was not possible to determine if it had blocked the effects of TBT.
Figure 3.
Effect of PKC inhibition on TBT-induced decreases in NK lytic function in 24 h period following a 1 h exposure TBT. Starting from the left Bar 1 = NK cells were treated with 25 μM Bisindolylmaleimide I (Bis I) followed by control media for 1 h, Bar 2 = NK cells were treated with control media for 1 h followed by exposure to 200 nM TBT for 1 h, Bar 3 = NK cells were treated with control media for 1 h followed by exposure to 100 nM TBT for 1 h, Bar 4 = NK cells were treated with 25 μM Bisindolylmaleimide I (Bis I) for 1 h followed by exposure to 200 nM TBT for 1 h and Bar 5 = NK cells were treated with 25 μM Bisindolylmaleimide I (Bis I)for 1 h followed by exposure to 100 nM TBT for 1 h., washed twice with gel media and were incubated for 24 hrs. The lysis of the target cells were measured the same way as fig. 6. X-axis represents treatment conditions. Y-axis represents the % control lysis. *indicates significant difference compared to the control (p<0.05)
Effect of protein kinase C (PKC) inhibition on TBT-induced phosphorylation of the mitogen- activated protein kinase (MAPK), p44/42
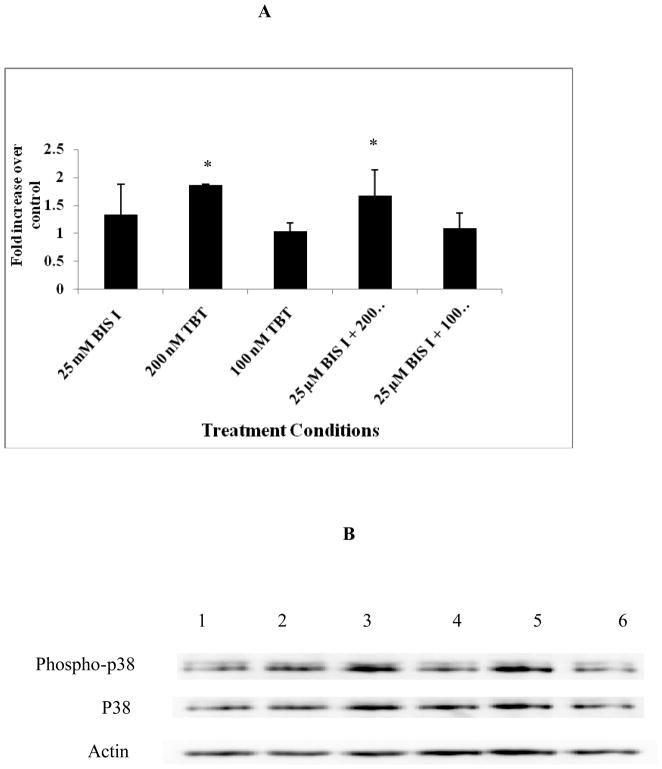
Figure 4A shows the changes in activating phosphorylation of p44/42 when NK cells were exposed to TBT in the presence and absence of the PKC inhibitor, bisindolylmaleimide I (results combined from at least 3 separate experiments). Bisindolylmaleimide I alone had no effect on the level of activation of p44/42. Exposure of NK cells to 200 nM for 10 min caused an average increase in the activating phosphorylation of p44/42 of 5.5 fold compared to controls (Figure 4A) (p> 0.05). This activating phosphorylation was completely blocked when the NK cells were exposed to the PKC inhibitor prior to exposure to TBT (Figure 4A) There was no change in the overall p44/42 levels with any of the treatments (p>0.05). A representative experiment of the effects of Bisindolylmaleimide I on TBT-induced phosphorylation of p44/42 is shown in Figure 4B.
Figure 4.
Effect of the PKC inhibitor, Bisindolylmaleimide I, on the TBT-induced phosphorylation of p44/42. A.) Fold increase in the levels of phospho-p44/42 compared to control. Values are mean ± S.D. from at least three separate experiments using cells from different donors *indicates significant difference compared to the control (p<0.05). B.) Representative experiment of Phospho-p44/42 (Upper bands), total p44/42 (Middle bands) and β-actin (Lower Bands): (1) control cells treated with TBT free media for 1h, (2) NK cells exposed to 25 μM bisindolylmaleimide I for 1 h followed by 10 min exposure to control media (3) NK cells exposed to control for 1 h followed by 200 μM TBT for 10 min (4) NK cells exposed to control for 1 h followed by 100 μM TBT for 10 min, (5) NK cells exposed to 25 μM bisindolylmaleimide I for 1 h followed by 200 μM TBT for 10 min, (6) NK cells exposed to 25 μM bisindolylmaleimide I for 1 h followed by 100 μM TBT for 10 min
Effect of protein kinase C (PKC) inhibition on TBT-induced phosphorylation of the mitogen-activated protein kinase (MAPK), p38
The role of PKC in the TBT-induced phosphorylation of p38 using bisindolylmaleimide I was also examined. Changes in activating phosphorylation of p38 combined from at least 3 separate experiments are shown in Figure 5A. Again, bisindolylmaleimide I did not have any effect on the phosphorylation state of p38. Exposure of NK cells to 200 nM TBT for 10 min caused an approximately 2 fold increase in phospho-p38 compared to control (Figure 5A) (p< 0.05). In NK cells where PKC activation had been blocked by 25 μM bisindolylmaleimide I prior to the 10 min exposure to TBT, the activating phosphorylation of p38 was still seen (Figure 5A and B). There was no significant difference in the overall p38 levels with any of the treatments (p>0.05). Figure 8B show a representative experiment of the effects of PKC inhibition by bisindolylmaleimide I on TBT-induced phosphorylation of p38.
Figure 5.
Effect of the PKC inhibitor, Bisindolylmaleimide I, on the TBT-induced phosphorylation of p38. A.) Fold increase in the levels of phospho-p38 compared to control. Values are mean ± S.D. from at least three separate experiments using cells from different donors *indicates significant difference compared to the control (p<0.05). B.) Representative experiment of Phospho-p38 (Upper bands), total p38 (Middle bands) and β-actin (Lower Bands): Lanes are defined as in the legend for Figure 4.
Effect of protein kinase C (PKC) inhibition on the TBT-induced phosphorylation of the mitogen-activated protein kinase (MAPK), JNK
We also examined the role of PKC in the TBT-induced activation of JNK in freshly isolated NK cells using bisindolylmaleimide I Again, 25 μM bisindolylmaleimide I alone did not cause an activating phosphorylation of JNK. Ten minute exposures of NK cells to 100 nM TBT caused activating phosphorylations of JNK ranging from 1.2 −2 fold. However, due to considerable variations in the dataset, the average value was not statistically significant (p>0.05). A representative experiment showing the effects of PKC inhibition on activating phosphorylation of JNK is shown in Figure 6. In this experiment the increase in phospho-JNK in response to 100 nM TBT was 2 fold and this was unchanged when the PKC activity of the NK cells was inhibited prior to exposure to TBT. This same pattern was seen in three replicate experiments.
Figure 6.
Effect of the PKC inhibitor, Bisindolylmaleimide I, on the TBT-induced phosphorylation of JNK. Representative experiment of Phospho-JNK (Upper bands), total JNK (Middle bands) and β-actin (Lower Bands): (1) control cells treated with TBT free media for 1h, (2) NK cells exposed to 25 μM bisindolylmaleimide I for 1 h followed by 10 min exposure to control media (3) NK cells exposed to control for 1 h followed by 200 μM TBT for 10 min (4) NK cells exposed to control for 1 h followed by 100 μM TBT for 10 min, (5) NK cells exposed to 25 μM bisindolylmaleimide I for 1 h followed by 200 μM TBT for 10 min, (6) NK cells exposed to 25 μM bisindolylmaleimide I for 1 h followed by 100 μM TBT for 10 min. lower bands: β-actin for the corresponding lanes.
DISCUSSION
Previous studies have shown that TBT has the ability to decrease human NK cell lytic function (Whalen et al., 1999; Whalen et al., 2002a,b; Dudimah et al., 2007). Our laboratory has previously also shown that TBT exposure can activate the mitogen-activated protein kinases (MAPKs) including, p44/42, p38, and JNK in freshly isolated human NK cells (Aluoch et al., 2006; Aluoch et al., 2007). Due to the fact that activation of MAPKs is also a part of the signaling pathway activated by the lytic process in NK cells (Trotta et al., 1998;2000); this TBT-induced activation would leave the NK cell unable to respond to a subsequent encounter with a tumor cell. Moreover, we have recently shown that inhibition of the immediate upstream activator of p44/42, the mitogen-activated protein kinase kinase (MAP2K), MEK1/2, can completely block the activation of p44/42 by TBT (Abraha and Whalen, 2009). This indicated that TBT was activating p44/42 via activation of the MAPK signaling pathway that is also stimulated by activation of the lytic process in NK cells (Abraha and Whalen, 2009). We have previously shown that the earliest signaling enzyme in the lytic pathway, protein tyrosine kinase (PTK), was not activated by TBT exposures (Aluoch et al., 2006). However, as was described above activation of protein kinase C (PKC) can lead to phosphorylation and activation of MAPKs (Pearson et al., 2001; Trakul et al., 2005; Ueffing et al., 1997; Xuan et al., 2005). The goal of the present study was to further extend our knowledge of the signal transduction pathways utilized by TBT to activate MAPKs and ultimately decreases the lytic function of NK cells. Thus, in this study we have examined the involvement of protein kinase C in TBT-induced loss of lytic function as well as in the TBT-induced phosphorylation of MAPKs.
In the present study, we examined the effect of TBT exposures on phosphorylation of protein kinase C (PKC). Our results indicated that there was an increase in phosphorylation of PKC when NK cells were exposed to 50–300 nM TBT. Previously, we have shown that the most upstream signal in the lytic pathway, protein tyrosine kinase activity, was not affected by TBT exposures (Aluoch et al., 2006). Thus, the current results indicate that PKC is the most upstream component of the NK lytic signaling pathway to be affected by TBT exposure.
Next, we employed a selective inhibitor of protein kinase C (PKC) to determine whether PKC activation is a requisite step in the process by which NK cells lyse tumor target cells. These studies showed that there was a decrease in lytic function (89%) when PKC was inhibited, with Bisindolylmaleimide I, verifying that PKC activation is a required step in the process by which NK cells lyse tumor target cells C (Graves et al., 1986; Steele and Brahmi, 1988b; Procopio et al., 1989).
Previously we showed that activation of the mitogen-activated protein kinase (MAPK), p44/42 by a selective p44/42 activator phorbol 12-myristate 13-acetate (PMA) was able to cause decreases in NK lytic function in a manner similar to TBT (Dudimah et al. 2010). PMA is likely activating p44/42 via its capacity to activate protein kinase C (PKC), which as mentioned above is an upstream activator of p44/42 (Ueffing et al., 1997; Pearson et al., 2001; Trakul et al., 2005; Xuan et al., 2005). In a separate study we also showed that inhibition of p44/42 activation (by an inhibitor of its immediate upstream activator MEK1/2) could not entirely prevent the negative effects of TBT on NK lytic function (Abraha and Whalen, 2009). The fact that activation of p44/42 by PMA which also activate PKC leads to decreases in NK function similar to those seen with TBT indicated that a likely reason why blocking MAPK activation alone did not prevent TBT-induced decrease in lytic function was that there was a role for PKC in this effect as well. Here we examined if blocking PKC prior to exposures to TBT could block the negative effects of TBT. However, although bisindolylmaleimide I, is reported to be a reversible inhibitor of PKC, it is not so in NK cells. Thus, it was not possible to determine if preventing TBT-induced activation of PKC could prevent the loss of lytic function. However, the role of PKC activation in TBT-induced activation of MAPKs was investigated. The results indicated that while PKC activation was required for TBT-induced activation of p44/42 it was not required for activation of p38 and JNK by TBT. These results indicate that in NK cells, TBT-induced phosphorylation of p44/42 but not p38 and JNK can be a consequence of PKC activation. This is consistent with a PKC activation of the mitogen activated protein kinase kinase kinase (MAP3K) for p44/42, Raf-1, which is not shared with p38 and JNK activation pathways (Ueffing et al., 1997; Pearson et al., 2001; Trakul et al., 2005; Xuan et al., 2005).
This study has widened our understanding of how a range of TBT concentrations can cause inhibition of cytotoxicity function of NK cells. The results from this study indicate that (1) TBT exposure activates protein kinase C (PKC) (2) activation of NK-cell PKC by TBT would leave the NK cell unable to respond to a subsequent encounter with a target cell, resulting in the observed loss of lytic function. (3) TBT-induced activation of the MAPK, p44/42, is due to activation of PKC. (4) TBT-induced activation of the MAPKs p38 and JNK is not a consequence of PKC activation.
Acknowledgments
This research was supported by Grant 2S06GM-08092-34 from the National Institutes of Health.
References
- Abraha AB, Whalen MM. The role of p44/42 activation in tributyltin- induced inhibition of human natural killer cells: effects of MEK inhibitors. Journal of Applied Toxicology. 2009;29:165–173. doi: 10.1002/jat.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aluoch AO, Odman-Ghazi SO, Whalen MM. Alteration of an essential NK cell signaling pathway by low doses of tributyltin in human natural killer cells. Toxicology. 2006;224:229–237. doi: 10.1016/j.tox.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Aluoch AO, Whalen MM. Tributyltin-induced effects on MAP kinases p38 and p44/42 in human natural killer cells. Toxicology. 2005;209:263–277. doi: 10.1016/j.tox.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Aluoch AO, Odman-Ghazi SO, Whalen MM. Pattern of MAP kinases p44/42 and JNK activation by non-lethal doses of tributyltin in human natural killer cells. Arch Toxicology. 2007;81:271–277. doi: 10.1007/s00204-006-0155-4. [DOI] [PubMed] [Google Scholar]
- Baaijens PA. Health effect screening and biological monitoring for workers in organotin industries. Toxicology analytics of the tributyltins: the present F status, Proceedings of the ORTEPA Workshop; Berlin. 15–16 May; Vlissingen-Oost, The Netherlands: ORTEP-Association; 1986. pp. 191–208. [Google Scholar]
- Biron CA, Byron KS, Sullivan JL. Severe herpes virus in an adolescent without natural killer cells. New Engl J Med. 1989;320:1731–1735. doi: 10.1056/NEJM198906293202605. [DOI] [PubMed] [Google Scholar]
- Chuang SS, Lee JK, Mathew PA. Protein kinase C is involved in 2B4 (CD244)-mediated cytotoxicity and AP-1 activation in natural killer cells. Immunology. 2003;109:432–439. doi: 10.1046/j.1365-2567.2003.01662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- Corsini E, Bruccoleri A, Marinovich M, Galli CL. Endogenous Interleukin-1 alpha associated with skin irritation induced by tributyltin. Toxicol Appl Pharmacol. 1996;138:268–274. doi: 10.1006/taap.1996.0125. [DOI] [PubMed] [Google Scholar]
- Derijard B, Raingeaud J, Barrett T, Wu IH, Han J, Ulevitch RJ, Davis RJ. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- Dudimah FD, Odman-Ghazi SO, Hatcher F, Whalen MM. Effect of tributyltin (TBT) on ATP levels in human natural killer (NK) cells: Relationship to TBT-induced decrease in NK function. J Appl Toxicol. 2007;27:86–94. doi: 10.1002/jat.1202. [DOI] [PubMed] [Google Scholar]
- Dudimah FD, Griffey D, Wang X, Whalen MM. Activation of p44/42 MAPK plays a role in the TBT-induced loss of human natural killer (NK) cell function. Cell Biol Toxicol. 2010 doi: 10.1007/s10565-010-9154-6. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher G, Koven N, Kamiya H, Henle W. A non-X-linked syndrome with susceptibility to severe Epstein-Bar virus infections. J Pediatr. 1982;100:727–730. doi: 10.1016/s0022-3476(82)80572-6. [DOI] [PubMed] [Google Scholar]
- Graves SS, Bramhall J, Bonavida B. Studies on the lethal hit stage of natural killer cll0mediated cytotoxcity. I. both phorbol ester and ionophore are required for release of natural killer cytotoxic factors (NKCF), suggesting a role for protein kinase C activity. J Immunol. 1986;137:1977–1984. [PubMed] [Google Scholar]
- Han J, Lee JD, Jiang Y, Li Z, Feng L, Ulevitch RJ. Characterization of the structure and function of a novel MAP kinase kinase (MKK6) J Biol Chem. 1996;271:2886–2891. doi: 10.1074/jbc.271.6.2886. [DOI] [PubMed] [Google Scholar]
- Jevremovic D, Billadeau DD, Schoon RA, Dick CJ, Leibson PJ. Regulation of NK cell-mediated cytotoxicity by the adaptor protein 3BP2. J Immunol. 2001;166:7219–7228. doi: 10.4049/jimmunol.166.12.7219. [DOI] [PubMed] [Google Scholar]
- Jiang K, Zhong B, Gilvary DL, Corliss BC, Hong-Geller E, Wei S, Djeu JY. Pivotal role of phoshoinositide-3 kinase in regulation of cytotoxicity in natural killer cells. Nat Immunol. 2000;1:419–425. doi: 10.1038/80859. [DOI] [PubMed] [Google Scholar]
- Kannan K, Tanabe S, Tatsukawa R. Occurrence of butyltin residues in certain foodstuffs. Bull Environ Contam Toxicol. 1995a;55:510–516. doi: 10.1007/BF00196029. [DOI] [PubMed] [Google Scholar]
- Kannan K, Tanabe S, Tatsukawa R, Williams RJ. Butyltin residues in fish from Australia, Papua New Guinea and the Solomon Islands. Int J Environ Anal Chem. 1995b;61:263–273. [Google Scholar]
- Kannan K, Tanabe S, Iwata H, Atsukawa R. Butyltins in muscle and liver of fish collected from certain Asian and Oceanian countries. Environ Pollut. 1995c;90:279–290. doi: 10.1016/0269-7491(95)00028-p. [DOI] [PubMed] [Google Scholar]
- Kannan K, Falandyz J. Butyltin residues in sediment, fish, fish-eating birds, harbour porpoise and human tissues from the Polish coast of the Baltic Sea. Mar Pollut Bull. 1997;34:203–207. [Google Scholar]
- Kannan K, Senthilkumar K, Giesy JP. Occurrence of butyltin compounds in human blood. Environ Sci Technol. 1999;33:1776–1779. [Google Scholar]
- Kannan K, Villeneuve DL, Blankenship AL, Giesy JP. Interaction of tributyltin with 3,3′,4,4′,5-pentachlorobiphenyl-induced ethoxyresorufin O-deethylase activity in rat hepatoma cells. J Toxicol Enrivon Health A. 1998;55:373–384. doi: 10.1080/009841098158412. [DOI] [PubMed] [Google Scholar]
- Karpiak VC, Bridges RJ, Eyer CL. Organotins disrupt components of glutamate homeostasis in rat astrocyte cultures. J Toxicol Environ Health A. 2001;63:273–287. doi: 10.1080/15287390151143668. [DOI] [PubMed] [Google Scholar]
- Kimbrough RD. Toxicity and health effects of selected organotins compounds: a review. Environ Health Perspect. 1976;14:51–56. doi: 10.1289/ehp.761451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupper TS. Mechanism of cutaneous inflammation: interaction between epidermal cytokines, adhesion molecules and leukocytes. Arch Dermatol. 1989;125:1406–1412. doi: 10.1001/archderm.125.10.1406. [DOI] [PubMed] [Google Scholar]
- Laughlin RB, Linden O. Fate and effects of organotin compounds. Ambio. 1985;14:88–94. [Google Scholar]
- Loganathan BG, Kannan K, Owen DA, Sajwan KS. Butyltin compounds in freshwater ecosystems. In: Lipnick RL, Hermens J, Jones K, Muir D, editors. Persistent, Bioaccumulative, and Toxic Chemicals. I Fate and Exposure, Am Chem Soc Pub. Oxford Univ. Press; London: 2000. [Google Scholar]
- Moretta L, Biassoni R, Bottino C, Mingari MC, Moretta A. Natural killer cells: a mystery no more. Scand J Immunol. 2002;55:229–232. doi: 10.1046/j.1365-3083.2002.01055.x. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988;334:661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocrine Rev. 2001;22:153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- Perussia B. Signaling for cytotoxicity. Nat Immunol. 2000;1:372–374. doi: 10.1038/80808. [DOI] [PubMed] [Google Scholar]
- Procopio ADG, Paolini R, Gismondi A, Picolli M, Adamo S, Cavallo G, Frati L, Santoni A. Effects of protein kinase C (PK-C) activators and inhibitors on human large granular lymphocytes (LGL): Role of PK-C on natural killer (NK) activity. Cell Immunol. 1989;118:470–481. doi: 10.1016/0008-8749(89)90394-8. [DOI] [PubMed] [Google Scholar]
- Roper WL. Toxicological profile for tin. U.S. Department of Health and Human Services. Agency for Toxic Substances and Disease Registry; US: 1992. [Google Scholar]
- Rosse C, Linch M, Kermogant S, Cameron AJM, Boeckeler K, Parker PJ. PKC and the control of localized signal dynamics. Nat Rev Mol Cell Biol. 2010;11:103–112. doi: 10.1038/nrm2847. [DOI] [PubMed] [Google Scholar]
- Snoeij NJ, Penninks AH, Seinen H. Biological activity of organotin compounds: an overview. Environ Res. 1987;44:335–353. doi: 10.1016/s0013-9351(87)80242-6. [DOI] [PubMed] [Google Scholar]
- Steele TA, Brahmi Z. Phosphatidylinositol metabolism accompanies early activation events in tumor target cell-stimulated human natural killer cells. Cell Immunol. 1988a;112:402–413. doi: 10.1016/0008-8749(88)90309-7. [DOI] [PubMed] [Google Scholar]
- Steele TA, Brahmi Z. Inhibition of human natural killer cell activity by the protein kinase C inhibitor 1-(5-isoquinolinesulfonyl)-2-methylpiperazine is an early but post-binding event. J Immunol. 1988b;141:3164–3169. [PubMed] [Google Scholar]
- Tajima K, Matsumoto N, Ohmori K, Wada H, Ito M, Suzuki K, Yamamoto K. Augmentation of NK cell-mediated cytotoxicity to tumor cells by inhibitory NK cell receptor blockers. Int Immunol. 2004;16:385–393. doi: 10.1093/intimm/dxh021. [DOI] [PubMed] [Google Scholar]
- Tanabe S, Prudente M, Mizuno T, Hasegawa J, Iwata H, Miyazaki N. Butyltin contamination in marine mammals from north Pacific and Asian coastal waters. Environ Sci Technol. 1998;32:193–198. [Google Scholar]
- Ting AT, Karnitz LM, Schoon RA, Abraham R, Leibson PJ. Fc gamma receptor activation induces the tyrosine phosphorylation of both phospholipase C (PLC)-gamma 1 and PLC-gamma 2 in natural killer cells. J Exp Med. 1992;176:1751–1755. doi: 10.1084/jem.176.6.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting AT, Schoon RA, Abraham RT, Leibson PJ. Interaction between protein kinase C-dependent and G protein dependent pathways in the regulation of natural killer cell granule exocytosis. J Biol Chem. 1992;267:3957. [PubMed] [Google Scholar]
- Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, Duhamel L, Charon D, Kirilovsky J. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- Trakul N, Rosner MR. Modulation of the MAP kinase signaling cascade by Raf kinase inhibitory protein. Cell Res. 2005;15:19–23. doi: 10.1038/sj.cr.7290258. [DOI] [PubMed] [Google Scholar]
- Trotta R, Puorro KA, Paroli M, Azzoni L, Abebe B, Eisenlohr LC, Perussia B. Dependence of both spontaneous and antibody-dependent, granule exocytosis-mediated NK cell cytotoxicity on extracellular signal-related kinases. J Immunol. 1998;161:6648–6656. [PubMed] [Google Scholar]
- Trotta R, Fettucciari K, Azzoni L, Abebe B, Puorro KA, Eisenlohr LC, Perussia B. Differential role of p38 and c-Jun N-terminal kinase 1 mitogen-activated protein kinases in NK cell cytotoxicity. J Immunol. 2000;165:1782–1789. doi: 10.4049/jimmunol.165.4.1782. [DOI] [PubMed] [Google Scholar]
- Tsukazaki M, Satsu H, Mori A, Sugita-Konishi Y, Shimizu M. Effects of tributyltin on barrier functions in human intestinal Caco-2 cells. Biochem Biophys Res Commun. 2004;315:991–997. doi: 10.1016/j.bbrc.2004.01.147. [DOI] [PubMed] [Google Scholar]
- Ueffing M, Lovric J, Philipp A, Mischak H, Kolch W. Protein kinase C-epsilon associates with the Raf-1 kinase and induces the production of growth factors that stimulate Raf-1 activity. Oncogene. 1997;15:2921–2927. doi: 10.1038/sj.onc.1201477. [DOI] [PubMed] [Google Scholar]
- Vivier E, Nunès JA, Vely F. Natural Killer Cell Signaling Pathways. Science. 2004;306:1517–1519. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- Wei S, Gilvery DL, Corliss BC, Sebti S, Sun J, Straus DB, Liebson PJ, Trapani JA, Hamilton AD, Weber MJ, Djeu JY. Direct tumor lysis by NK cells uses a Ras-Independent Mitogen-Activated Protein Kinase Signal Pathway. J Immunol. 2000;165:3811–3819. doi: 10.4049/jimmunol.165.7.3811. [DOI] [PubMed] [Google Scholar]
- Whalen MM, Doshi RN, Homma Y, Bankhurst AD. Phospholipase C Activation in the Cytotoxic Response of Human Natural Killer Cells Requires Protein-tyrosine Kinase Activity. Immunology. 1993;79:542–547. [PMC free article] [PubMed] [Google Scholar]
- Whalen MM, Loganathan BG, Kannan K. Immunotoxicity of environmentally relevant concentrations of butyltins on human natural killer cells in vitro. Environ Res. 1999;81:108–116. doi: 10.1006/enrs.1999.3968. [DOI] [PubMed] [Google Scholar]
- Whalen MM, William TB, Green SA, Loganathan BG. Interleukins 2 and 12 produce recovery of cytotoxic function in tributyltin-exposed human natural killer cells. Environ Res. 2002a;88:199–209. doi: 10.1006/enrs.2002.4332. [DOI] [PubMed] [Google Scholar]
- Whalen MM, Green SA, Loganathan BG. Brief Butyltin Exposure Induces Irreversible Inhibition of the Cytotoxic Function on Human Natural Killer Cells, In vitro. Environ Res. 2002b;88:19–29. doi: 10.1006/enrs.2001.4318. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) Environmental Health Criteria 116. WHO; Geneva: 1990. Tributyltin compounds. [Google Scholar]
- World Health Organization (WHO)/Food and Agriculture Organization of the United Nations (FAO) Data sheet on pesticides No. 65: Bis(tributyltin) oxide. World Health Organization; Geneva: 1984. (VBC/PDS/DS/85.65) [Google Scholar]
- Wu J, Lanier LL. Natural killer cells and cancer. Adv Cancer Res. 2003;90:127–156. doi: 10.1016/s0065-230x(03)90004-2. [DOI] [PubMed] [Google Scholar]
- Xuan YT, Guo Y, Zhu Y, Wang OL, Rokosh G, Messing RO, Bolli R. Role of the protein kinase C-epsilon-Raf-1-MEK-1/2-p44/42 MAPK signaling cascade in the activation of signal transducers and activators of transcription 1 and 3 and induction of cyclooxygenase-2 after ischemic preconditioning. Circulation. 2005;112:1971–1978. doi: 10.1161/CIRCULATIONAHA.105.561522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Fuji Y, Mikami E, Kawamura N, Hayakawa J. Small-scale survey of organotin compounds in household commodities. J AOAC Int. 1993;76:436–441. [Google Scholar]