Abstract
PURPOSE
To test the hypothesis that increased pelvic bone marrow (BM) irradiation is associated with increased hematologic toxicity (HT) in cervical cancer patients undergoing chemoradiotherapy (CRT), and to develop a normal tissue complication probability (NTCP) model for HT.
METHODS AND MATERIALS
We tested associations between hematologic nadirs during CRT and the volume of BM receiving ≥ 10 and 20 Gy (V10 and V20) using a previously developed linear regression model. The validation cohort consisted of 44 cervical cancer patients treated with concurrent cisplatin and pelvic radiotherapy. Subsequently, these data were pooled with 37 identically treated patients from a prior study, forming a cohort of 81 patients for NTCP analysis. Generalized linear modeling was used to test associations between hematologic nadirs and dosimetric parameters, adjusting for body mass index. Receiver operating characteristic curves were used to derive optimal dosimetric planning constraints.
RESULTS
In the validation cohort, significant negative correlations were observed between white blood cell count (WBC) nadir and V10 (regression coefficient (β)=−0.060, p=0.009) and V20 (β=−0.044, p=0.010). In the combined cohort, the (adjusted) β estimates for log(WBC) vs. V10 and V20 were: −0.022 (p=0.025) and −0.021 (p=0.002), respectively. Patients with V10 ≥ 95% were more likely to experience grade ≥ 3 leukopenia (68.8% vs. 24.6%, p<0.001) as were patients with V20 > 76% (57.7% vs. 21.8%, p=0.001).
CONCLUSIONS
These findings support the hypothesis that HT increases with increasing pelvic BM volume irradiated. Efforts to maintain V10 < 95% and V20 < 76% may reduce HT.
Keywords: cervical cancer, hematologic toxicity, bone marrow, normal tissue complication probability
INTRODUCTION
Concurrent chemoradiotherapy (CRT) is standard treatment for locally advanced cervical cancer(1-6). Concomitant chemotherapy, however, increases hematologic toxicity (HT), particularly leukopenia and neutropenia(1,2,6-8), predisposing patients to infection, hospitalization, and requirements for transfusions and growth factors. Importantly, HT can also lead to delayed or missed chemotherapy cycles and treatment breaks(2,6-9), potentially compromising disease control(6,10-12). Reduction of HT is, therefore, an important goal.
It is known that both radiation and chemotherapy are myelosuppressive, but the extent to which pelvic radiation contributes to HT in patients undergoing CRT is not well understood. Approximately 50% of the body's hematopoietically active bone marrow (BM) is located in the pelvis and lower spine(13,14) and is contained within conventional radiation therapy (RT) ports. BM stem cells are radiosensitive(15) and damage to these cells is a principal cause of acute HT(14). In patients receiving pelvic RT alone, HT is rarely a problem, due to compensatory increased hematopoiesis in unirradiated BM. When chemotherapy is given concurrently, however, additional BM injury and myelosuppression predispose patients to HT, making effects of pelvic BM irradiation a greater concern.
Previous studies have identified relationships between HT and the volume of pelvic BM receiving ≥ 10 and 20 Gy (V10 and V20) in patients undergoing concurrent chemotherapy and pelvic RT(16-19), suggesting that techniques designed to reduce BM irradiation, such as intensity modulated RT (IMRT), could reduce HT(20). However, identifying BM as a planning constraint for IMRT could increase the radiation dose to other pelvic organs. As such, the optimal clinical implementation of BM-sparing techniques is limited by the lack of accurate normal tissue complication probability (NTCP) models quantifying the relationship between HT and BM radiation. The present study aimed to validate previously observed associations between HT and BM dosimetric parameters and to develop a more robust NTCP model, providing evidence for specific BM dose-volume planning constraints for pelvic RT.
METHODS AND MATERIALS
Patients and Study Design
This study was approved by the Institutional Review Board at each institution. To validate previously identified associations between HT and V10 and V20, we aimed to test these associations in a “validation” cohort. The validation cohort comprised cervical cancer patients treated with concurrent weekly cisplatin and pelvic RT at the University of California San Diego (UCSD) and University of Illinois Chicago (UIC) between January 2005 and December 2008. Eligible patients had biopsy-proven clinical stage I-IVA or recurrent cervical carcinoma, and no prior history of chemotherapy or pelvic irradiation. Patients treated with extended field (para-aortic) RT (EFRT) were excluded. Fifty-four patients were screened for eligibility; 8 patients treated with EFRT were excluded and 2 patients were excluded due to prior history of chemotherapy, leaving 44 patients eligible. Thirty patients were from UCSD and 14 from UIC.
To generate evidence-based dosimetric guidelines for RT planning, as well as robust estimates of the effect of dosimetric parameters and covariates on HT, the validation cohort was combined with 37 patients from a previously described “training” cohort(16), forming an 81-patient “combined” cohort for analysis. The training cohort comprised cervical cancer patients treated with pelvic IMRT and concurrent weekly cisplatin at the University of Chicago and affiliated hospitals between January 2000 and December 2004. Inclusion and exclusion criteria were identical to those used in the validation cohort.
Chemotherapy Delivery, Transfusions and Growth Factors
Chemotherapy consisted of weekly cisplatin(40 mg/m2;maximum:80 mg) delivered concurrently with external beam RT. Cisplatin was held under the following conditions: white blood cell count (WBC)<2.0×109/L, absolute neutrophil count (ANC)<1.0×109/L, platelet count<50×109/L or creatinine clearance<50 mL/min.
In the validation cohort, five patients were transfused with packed red blood cell transfusions during treatment. Ten patients received Epoetin alfa 40,000 units weekly for low serum hemoglobin, at the discretion of the treating physician. No patients received platelet transfusions or granulocyte-monocyte colony stimulating factors.
Radiation Simulation, Planning and Delivery, Bone Marrow Delineation
All patients were simulated in the supine position with customized immobilization. At UCSD, patients were simulated using a GE Lightspeed (Surrey,UK) computed tomography (CT) simulator with 2.5 mm slice thickness. At UIC, patients were simulated using a Philips Picker PQ 5000(Cleveland,OH) CT simulator with 3.0 mm slice thickness. Intravenous, rectal, and/or bladder contrast were given at the discretion of the treating physician. Patients were not given specific instructions regarding bladder or rectal filling prior to simulation.
The clinical target volume (CTV) consisted of the uterus, cervix, and gross tumor, upper half of the vagina, parametria, and regional lymph nodes. The lymph nodes were defined by encompassing the contrast-enhanced vasculature with a 7 mm margin. The planning target volume (PTV) was defined by a uniform 3-D expansion around the CTV, using 7-10 mm margins around the lymph nodes, 10-15 mm around the vagina, parametria, and uterus, and 15-20 mm around the cervix and gross disease. The bladder, rectum, and small bowel were contoured as avoidance structures for all patients. Bone marrow was not routinely used as an avoidance structure.
The majority of patients (41 of 44, 94%) received pelvic IMRT. Plans were generated using 6, 15, and/or 18 MV photons (Eclipse, Varian Medical Systems, Palo Alto, CA). For IMRT, co-planar 7-9 field plans were generated. Pelvic radiation dose was 39.6-50.4 Gy in 1.8 Gy daily fractions. Patients treated definitively received either five high-dose-rate (HDR) intracavitary brachytherapy insertions twice weekly or 1-2 low-dose-rate (LDR) insertions following external beam RT. The prescribed dose for HDR brachytherapy was 5.5-6.0 Gy per fraction to point A. The prescribed dose for LDR brachytherapy was 30-40 Gy to point A. The dose to pelvic BM from brachytherapy was considered negligible.
For each patient, the external contour of all bones within the pelvis was delineated on the planning CT using bone windows as previously described(16), and BM volumes receiving ≥10, 20, 30, and 40 Gy (V10, V20, etc.) were quantified.
Hematologic Toxicity
All patients had complete blood counts (CBC) with differentials weekly during CRT. Endpoints of interest were white blood cell count (WBC), absolute neutrophil count (ANC), and hemoglobin (HGB) nadirs occurring during or within two weeks of the end of CRT. Hematologic toxicity was graded according to the Radiation Therapy Oncology Group acute radiation toxicity scoring criteria(21).
Statistical analysis
For analysis on the validation cohort, we tested the hypothesis that hematologic nadirs were associated with V10 and V20 using least squares regression. We also conducted univariate analyses on potential confounders: body mass index (BMI), age(median centered), race, comorbidity, and clinical stage. BMI was defined as weight in kilograms divided by height in meters squared and was log(base e) transformed to eliminate skew. Log(BMI) and age were treated as continuous variables; all other covariates were treated as dichotomous. Race was classified as black versus other. Any chronic medical condition was considered a comorbidity. Clinical stage was divided into early (stage I-IIA), and advanced (IIB-IIIA). We planned to conduct multiple regression analyses including significant dosimetric parameters and statistically significant covariates, if any were found. We also conducted secondary analyses on the association of hematologic nadirs and other potentially predictive dosimetric parameters including V30, V40, and mean BM dose. Three patients from the training cohort had missing data for mean BM dose and the mean value from the training cohort was imputed. Dosimetric parameters were treated as continuous variables.
Generalized linear modeling was used to test for correlations between dosimetric parameters and hematologic endpoints in the combined cohort. Covariates found to be significant on univariate analysis were included in the multivariate model. Tests for normality were performed with the Shapiro-Wilk statistic. We used a log transform to eliminate skew in the dependent variables. We used logistic regression analysis to test whether the probability of holding chemotherapy was significantly associated with BM dosimetric parameters (controlling for effects of covariates). We also tested this association using a log transform of the number of cycles held (log(x)+1) as a dependent variable in generalized linear models. R2 and adjusted R2 were used to evaluate goodness of model fit. Receiver operating characteristic (ROC) curves were used to evaluate the value of specific dose-volume parameters for predicting toxicity. Differences in means and proportions were compared with the t-test and chi square test, respectively. Statistical programming was performed using SAS v9.1.3 (SAS Institute, Cary,NC).
RESULTS
Patients characteristics
The median age and BMI were similar in the training and validation cohorts (Table 1). More patients were Hispanic and fewer were white in the validation cohort compared to the training cohort (p<0.01). Fewer patients in the validation cohort had stage I-IIA disease (32% vs. 59%, p=0.01, respectively).
Table 1.
Patient and tumor characteristics
| Characteristic | Training Cohort | Validation Cohort | p | Combined Cohort |
|---|---|---|---|---|
| Patients, n | 37 | 44 | 81 | |
| Mean age, years (s.d.) | 51.5 (15.1) | 51.1 (14.6) | 0.9 | 51.3 (14.7) |
| Race, n (%) | <0.01* | |||
| Black | 23 (62.2) | 12 (27.3) | 35 (43.2) | |
| Hispanic | 1 (2.7) | 19 (43.2) | 20 (24.7) | |
| White | 13 (35.1) | 9 (20.5) | 22 (27.2) | |
| Other | 0 (0) | 4 (9.1) | 4 (4.9) | |
| Mean body mass index, kg/m2 (s.d.) | 27.8 (6.1) | 27.5 (7.1) | 0.8 | 27.6 (6.6) |
| Comorbidity (%) | 23 (62.2) | 26 (59.1) | 0.9 | 49 (60.5) |
| Clinical Stage, n (%) | 0.01*† | |||
| IA2 | 0 (0) | 1 (2.3) | 1 (1.2) | |
| IB | 2 (5.4) | 1 (2.3) | 3 (3.7) | |
| IB1 | 2 (5.4) | 4 (9.1) | 6 (7.4) | |
| IB2 | 14 (37.8) | 5 (11.4) | 19 (23.4) | |
| IIA | 4 (10.8) | 3 (6.8) | 7 (8.6) | |
| IIB | 10 (27.0) | 21 (47.7) | 31 (38.3) | |
| IIIA | 1 (2.7) | 0 (0) | 1 (1.2) | |
| IIIB | 3 (8.1) | 8 (18.2) | 11 (13.6) | |
| Recurrent | 1 (2.7) | 1 (2.3) | 2 (2.4) |
s.d., standard deviation
statistically significant
Compares early (stage I-IIA) vs. advanced (stage IIB-IIIB)
Radiation Delivery
The median pelvic dose in the validation cohort was 45 Gy (range:39.6-50.4 Gy). Thirty-six patients underwent intracavitary brachytherapy. Of these, 24 (67%) received HDR and 12 (33%) received LDR. The median point A dose was 75 Gy for patients receiving HDR (range:69-75) and 85 Gy for patients receiving LDR (range:75-85). Descriptive statistics of external beam RT dose-volume parameters are presented in Table 2. Mean BM volume was 1058.3 cm3 (standard deviation:169.9; range:714.5–1462.7).
Table 2.
Descriptive statistics of bone marrow dosimetric parameters
| Training Cohort mean (s.d.) | Validation Cohort mean (s.d.) | p | Combined Cohort mean (s.d.) | |
|---|---|---|---|---|
| V10 | 91.3 (3.6) | 89.4 (5.6) | 0.09 | 90.3 (4.9) |
| V20 | 74.5 (6.1) | 73.4 (7.6) | 0.5 | 73.9 (6.9) |
| V30 | 53.4 (7.5) | 47.8 (8.8) | <0.01* | 50.3 (8.6) |
| V40 | 27.7 (10.3) | 21.5 (7.9) | <0.01* | 24.3 (9.5) |
| Mean Pelvic Bone Marrow Dose | 29.8 (2.7) | 28.3 (3.0) | 0.03* | 29.0 (2.9) |
V10, V20, V30,V40: bone marrow volume receiving ≥ 10, 20, 30, 40 Gy; s.d., standard deviation
statistically significant
Chemotherapy Delivery
The median cisplatin dose delivered (per cycle) in the validation cohort was 69 mg. All patients were scheduled to receive either five (30%) or six (70%) cycles of chemotherapy. Of 30 patients scheduled to receive six cycles, 11 received all six cycles, 6 received five cycles, and 13 had ≥ 2 cycles held. Of 14 patients scheduled to receive five cycles, 9 received all five cycles, 2 received four cycles, and 2 received one cycle. Most patients received four (20%), five (34%), or six (25%) cycles of chemotherapy. At least one cycle of chemotherapy was held in 55% of patients.
Hematologic Toxicity
The median baseline WBC, ANC, HGB, and platelet counts in the validation cohort were 7.9 k/μL (range 3.8-15.4), 4.8 k/μL (range 1.4-11.5), 12.0 g/dL (range 7-15.3), and 296 k/μL (range 148-529), respectively. Acute hematologic toxicities for the validation and combined cohorts appear in Table 3. The median WBC, ANC, HGB, and platelet count nadirs were 2.0 k/μL (range:0.6-4.6), 1.3 k/μL (range:0.3-3.6), 9.9 g/dL (range:7.2-13.2), and 137 k/μL (range:54-306), respectively.
Table 3.
Acute hematologic toxicity
| Training Cohort n (%) | Validation Cohort n (%) | p | Combined Cohort n (%) | |
|---|---|---|---|---|
| Leukopenia | 0.02* | |||
| Grade 0 | 9 (24) | 2 (5) | 11 (14) | |
| Grade 1 | 7 (19) | 4 (9) | 11 (14) | |
| Grade 2 | 13 (35) | 19 (43) | 32 (40) | |
| Grade 3 | 8 (22) | 16 (36) | 24 (30) | |
| Grade 4 | 0 (0) | 3 (7) | 3 (4) | |
| Neutropenia | 0.20 | |||
| Grade 0 | 19 (51) | 12 (27) | 31 (38) | |
| Grade 1 | 4 (11) | 7 (16) | 11 (14) | |
| Grade 2 | 10 (27) | 16 (36) | 26 (32) | |
| Grade 3 | 4 (11) | 7 (16) | 11 (14) | |
| Grade 4 | 0 (0) | 2 (5) | 2 (2) | |
| Anemia | 0.18 | |||
| Grade 0 | 9 (24) | 11 (25) | 20 (25) | |
| Grade 1 | 20 (54) | 16 (36) | 36 (44) | |
| Grade 2 | 6 (16) | 16 (36) | 22 (27) | |
| Grade 3 | 2 (5) | 1 (2) | 3 (4) | |
| Grade 4 | 0 (0) | 0 (0) | 0 (0) | |
| Thrombocytopenia | 0.48 | |||
| Grade 0 | 33 (89) | 36 (82) | 69 (85) | |
| Grade 1 | 3 (8) | 4 (9) | 7 (9) | |
| Grade 2 | 1 (3) | 4 (9) | 5 (6) | |
| Grade 3 | 0 (0) | 0 (0) | 0 (0) | |
| Grade 4 | 0 (0) | 0 (0) | 0 (0) |
statistically significant
Validation Study
Univariate analysis identified statistically significant relationships between decreasing WBC nadir and increasing V10, V20, V30, V40, and mean BM dose (Table 4). The regression coefficient (β) indicates the change in WBC nadir for each unit change in the dosimetric parameter. We observed a trend towards an association between log(BMI) and WBC nadir (p=0.068). No other covariate was significantly associated with WBC nadir. In the subgroup of patients treated with IMRT, univariate β estimates for WBC nadir as a function of V10, V20, V30, V40, and mean BM dose were −0.055 (p=0.019), −0.055 (p=0.012), −0.029 (p=0.10), −0.025 (p=0.20), and −0.11 (p=0.032), respectively.
Table 4.
Univariate analysis of factors associated with WBC nadir in the validation cohort
| WBC Nadir (k/μL) |
|||
|---|---|---|---|
| β | 95% CI | p | |
| V10 | −0.060 | −0.10, −0.02 | 0.009* |
| V20 | −0.044 | −0.08, −0.01 | 0.010* |
| V30 | −0.035 | −0.06, −0.01 | 0.020* |
| V40 | −0.036 | −0.07, −0.004 | 0.029* |
| Mean Pelvic Bone Marrow Dose | −0.131 | −0.21, −0.05 | 0.002* |
| log(BMI) | 0.917 | −0.07, 1.90 | 0.068 |
| Age | 0.002 | −0.02, 0.02 | 0.798 |
| Black Race | −0.347 | −0.94, 0.24 | 0.244 |
| Stage | −0.06 | −0.63, 0.52 | 0.836 |
| Comorbidity | 0.051 | −0.49, 0.60 | 0.852 |
CI: confidence interval; WBC: white blood cell count; β: regression coefficient (e.g., 1% increase in V20 corresponds to a reduction in WBC nadir of 0.044 k/μL); BMI: body mass index.
statistically significant
Generalized Linear Modeling on Combined Cohort
In the combined cohort, increasing V10, V20, V30, and mean BM dose were associated with significantly decreased log(WBC nadir) on univariate analysis (Table 5). Of the covariates tested, only log(BMI) was significantly associated with decreasing log(WBC nadir). There was a non-significant association between advanced stage and decreased log(WBC nadir) (p=0.056).
Table 5.
Univariate analysis of factors associated with log(WBC nadir) in the combined cohort
| log(WBC Nadir) (k/μL) |
|||
|---|---|---|---|
| β | 95% CI | p | |
| V10 | −0.028 | −0.05, −0.008 | 0.006* |
| V20 | −0.023 | −0.04, −0.01 | <0.001* |
| V30 | −0.012 | −0.02, −0.0004 | 0.043* |
| V40 | −0.010 | −0.02, 0.001 | 0.067 |
| Mean Pelvic Bone Marrow Dose | −0.037 | −0.07, −0.004 | 0.028* |
| log(BMI) | 0.555 | 0.17, 0.94 | 0.005* |
| Age | 0.002 | −0.004, 0.01 | 0.494 |
| Black Race | −0.019 | −0.22, 0.18 | 0.853 |
| Stage | −0.19 | −0.38, 0.005 | 0.056 |
| Comorbidity | −0.037 | −0.24, 0.16 | 0.715 |
CI: confidence interval; WBC: white blood cell count; β: regression coefficient (e.g., 1% increase in V20 corresponds to a reduction in log(WBC nadir) of 0.023 k/μL); BMI: body mass index.
statistically significant
Multiple regression analysis adjusting for log(BMI) was performed on the combined cohort. Increasing V10, V20, V30, and mean BM dose were associated with significantly decreased log(WBC nadir) and log(ANC nadir) (Table 6). We observed a non-significant association between V40 and ANC nadir (p=0.069). There was an association between increasing V20 and a decreasing HGB nadir (β=−0.053,95% CI:−0.10,−0.01, p=0.016). The dependent variable was not normally distributed and so was changed to the log scale in the combined cohort model. We did not apply this transformation to the validation cohort because we were seeking to replicate findings of the previous analysis.
Table 6.
Generalized linear model of hematologic nadirs as a function of dosimetric parameters in the combined cohort
| log (WBC Nadir) (k/μL) |
log (ANC Nadir) (k/μL) |
HGB Nadir (g/dL) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | p | β | 95% CI | p | β | 95% CI | p | |
| V10 | −0.022 | −0.04, −0.003 | 0.025* | −0.029 | −0.05, −0.005 | 0.019* | −0.054 | −0.12, 0.01 | 0.094 |
| V20 | −0.021 | −0.03, −0.01 | 0.002* | −0.027 | −0.04, −0.01 | 0.001* | −0.053 | −0.10, −0.01 | 0.016* |
| V30 | −0.011 | −0.02, −0.0001 | 0.048* | −0.014 | −0.03, 0.001 | 0.034* | −0.020 | −0.06, 0.02 | 0.256 |
| V40 | −0.008 | −0.02, 0.003 | 0.143 | −0.011 | −0.02, 0.001 | 0.069 | −0.007 | −0.04, 0.03 | 0.689 |
| Mean Dose | −0.350 | −0.07, −003 | 0.033* | −0.043 | −0.08, −0.004 | 0.030* | −0.069 | −0.17, 0.03 | 0.189 |
CI: confidence interval; WBC: white blood cell count; ANC: absolute neutrophil count; HGB: hemoglobin; β: regression coefficient. Note: coefficients adjusted for effect of log(body mass index).
statistically significant
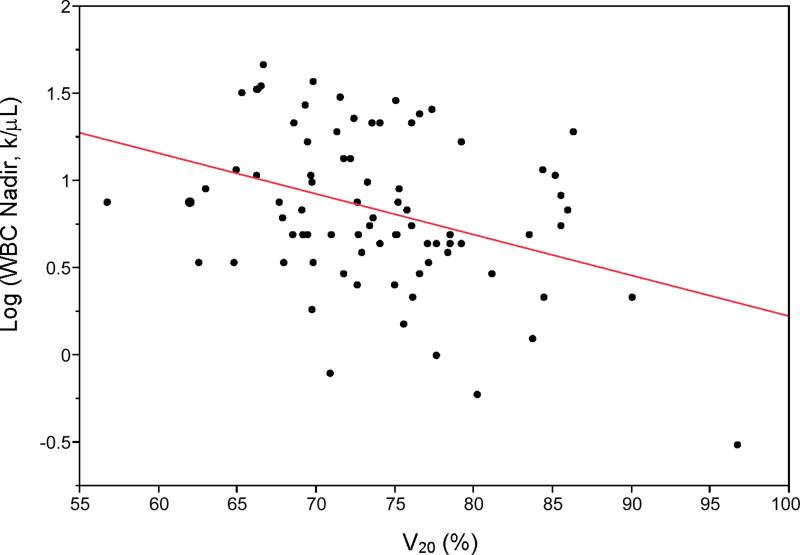
Figure 1 plots log(WBC nadir) versus V20 in the combined cohort, with the trend line superimposed. The R2 and adjusted R2 values of the multivariate model are 0.21 and 0.18, respectively, indicating a fair amount of unexplained variation in the log-linear model. In most regression models, log(BMI) was significantly correlated with log(hematologic nadir); for example, in the regression of WBC nadir versus V20, the coefficient estimate for log(BMI) was 0.47 (95% CI:0.11,0.84,p=0.012). The best prediction equation for WBC nadir from our data is:
| (1) |
Figure 1.
Univariate plot of white blood cell count log(WBC Nadir) vs. bone marrow volume receiving 20 Gy or more (V20) in the combined cohort. Regression coefficient (β) = −0.021 k/μL/%, p=0.002.
*Log transformed.
In the subgroup of patients treated with IMRT, multivariate β estimates for log(WBC nadir) as a function of V10, V20, V30, V40, and mean BM dose were −0.018 (p=0.067), −0.018 (p=0.017), −0.007 (p=0.21), −0.003 (p=0.52), and −0.017 (p=0.31), respectively.
Figure 2 shows a plot of observed versus predicted values of the WBC nadir from the regression model, along with 80% prediction intervals. The width of the prediction intervals indicates the degree of uncertainty around individual predictions.
Figure 2.
Plot of observed white blood cell count (WBC) nadir (k/μL) vs. predicted WBC nadir in the combined cohort.
To identify optimal thresholds for dosimetric planning, we analyzed the ROC curves for grade ≥ 3 HT versus V10 and V20 (Figure 3). The optimal V10 cutoff, indicated by the upper-left-most point of the ROC curve, was 95%. Patients with V10 ≥ 95% were more likely to experience grade ≥ 3 toxicity (68.8% vs. 24.6%,p<0.001). The sensitivity and specificity for this threshold were 44.4% and 90.7%, respectively. The positive and negative predictive value for V10 ≥ 95% were 68.8% (95% CI:58.6%,79.0%) and 75.4% (95% CI:66.0%,84.8%), respectively, with a relative risk of 2.79 (95% CI:1.63,4.79). The optimal V20 cutoff was 76%. Patients with V20 ≥ 76% were more likely to experience grade ≥ 3 toxicity (57.7% vs. 21.8%, p=0.001). The sensitivity and specificity for this threshold were 55.6% and 79.6%, respectively. The positive and negative predictive value of V20 ≥ 76% were 57.7% (95% CI:46.9%,68.5%) and 78.2% (95% CI:69.2%,87.2%), respectively, with a relative risk of 2.64 (95% CI:1.45,4.81). The cutoffs V10 ≥ 90% and V20 ≥ 80% were added to Figure 3 for reference.
Figure 3.
Receiver Operating Characteristic curves for grade ≥ 3 toxicity as a function of V10 (a) and V20 (b). The sensitivity and specificity corresponding to the optimal choice of cutoff is indicated by the intersection of the left-most diagonal line and the ROC curve.
Associations between Dosimetric Parameters and Chemotherapy Delivery
We did not find significant associations between BM dosimetric parameters and the probability of having ≥ 1 cycle of chemotherapy held. The number of cycles of chemotherapy held, however, was significantly associated with V20 on univariate (β=0.018; 95% CI:0.005,0.036, p=.047) but not multivariate (β=0.017; 95%CI:−0.001,0.035, p=0.066) analysis.
DISCUSSION
A goal of this study was to validate previous studies identifying an association(16,17) between HT and the volume of BM irradiated in patients undergoing concurrent chemotherapy and pelvic RT. The results of this study strongly support the hypothesis that the V10 and V20 of pelvic BM volume are predictors of acute HT in such patients. Our results suggest that efforts to maintain V10<95% and V20<76% could reduce the probability of grade ≥ 3 leukopenia by more than 50% in cervical cancer patients receiving CRT.
The hypothesized dependence of HT on the volume of BM irradiated is based on several lines of evidence. Many pre-clinical studies D have demonstrated the high radiosensitivity of hematopoietic stem cells(15). Damage to these cells is a primary contributor to myelosuppression(14). Radiation is known to cause acute and chronic pathologic and radiographic changes to the BM(14,22,23). Furthermore, RT induced BM injury is dependent on both dose and volume(24-26).
The reduction of CRT-associated HT could improve tolerance to more aggressive chemotherapy, potentially enhancing disease control in patients with pelvic malignancies. Though reported rates of acute HT vary in the literature, grade ≥ 3 HT occurs in approximately 27% of women undergoing CRT (Table 7). Peters et al. found that progression-free and overall survival were increased in patients receiving more chemotherapy cycles(6). Recently, Parker et al. found that distant metastasis was more common in cervical cancer patients receiving fewer chemotherapy cycles, and HT was the primary cause of missed chemotherapy(10). We found weak evidence supporting an association between increasing pelvic BM V20 and poorer chemotherapy delivery. Analysis in a larger data set may be required to establish the validity of these observations. Nonetheless, outcomes for patients with advanced disease are still sub-optimal, suggesting that treatment could be intensified for some patients. Preliminary clinical studies of combination chemotherapy given concurrently with RT have demonstrated promising disease control; however, the high rate of HT is a limiting factor(27-30). Finally, limiting CRT-associated BM injury incurred during definitive therapy may improve tolerance to subsequent chemotherapy given in the adjuvant or recurrent/metastatic setting. Phase III trials of either topotecan or paclitaxel in combination with cisplatin have shown improved response rate(31,32), disease-free survival(31,32), and overall survival(31). Grade ≥ 3 HT, however, occurred in over 65% of patients, limiting chemotherapy delivery(31,32).
Table 7.
Acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy
| Study | Chemotherapy | External Beam Radiation Dose (Gy) | N | Grade ≥ 3 Hematologic Toxicity (%) |
|---|---|---|---|---|
| Whitney (1999)5 | HU | 40-60 | 191 | 41% |
| 5FU, CDDP | 40-60 | 177 | 7% | |
| Peters (2000)6 | CDDP | 49 | 127 | 38% |
| None | 49 | 116 | 3% | |
| Rose (1999)2 | CDDP | 40.8-51.0 | 176 | 23% |
| CDDP, 5FU, HU | 40.8-51.0 | 173 | 48% | |
| HU | 40.8-51.0 | 177 | 23% | |
| Pearcey (2002)42 | CDDP | 45 | 127 | 5% |
| None | 45 | 126 | 0% | |
| Keys (1999)1 | CDDP | 45 | 183 | 21% |
| None | 45 | 186 | 2% | |
| Torres (2008)8 | CDDP, 5FU | 45 | 76 | 24% |
| CDDP, 5FU | 45 | 115 | 41% | |
| CDDP | 45 | 111 | 23% |
5FU, 5-Fluorouracil; MMC, mitomycin-C; HU, hydroxyurea; CDDP, cisplatin
The application of highly conformal techniques, such as IMRT, is one potential strategy to reduce BM irradiation and, consequently, HT. An initial study by Brixey et al. found that both BM dose and HT were reduced in patients treated with concurrent chemotherapy and IMRT, compared to patients treated with conventional 4-field box techniques(33). Subsequent studies have demonstrated that IMRT plans can be further optimized to reduce BM irradiation compared to conventional techniques in patients with pelvic malignancies(20,34,35).
The development of clinically useful BM sparing techniques is presently limited by a lack of reliable NTCP models to quantify the impact of increasing BM radiation on HT(16,17). Such models are necessary to predict the degree of BM sparing required to effect significant reductions in HT. This study examined generalized linear NTCP modeling in a larger cohort than has previously been studied. On the whole, the models explain a moderate proportion of the variability in observed toxicity rates; however, as shown in Figures 1 and 2, there is a considerable degree of unexplained variation. Consequently, these models have only modest predictive value. One possibility is that the bony contour was used as a surrogate for BM, potentially weakening the observed associations. Another likely explanation is that the primary explanatory variables are derived from dose-volume histograms, which fail to account completely for variations in the 3-D BM dose distribution. Emerging approaches using high-dimensional data analysis for NTCP modeling (36) may provide a more powerful tool for analyzing these relationships.
A second consideration is that BM is not a homogeneously functioning organ, but rather comprises subregions of hematopoietically active “red” BM and inactive “yellow” BM. These subregions cannot be distinguished on computed tomography(37-39), however, studies using functional imaging such as magnetic resonance imaging, positron emission tomography (PET), and single photon emission computed tomography (SPECT) have revealed that active BM tends to be concentrated in specific subregions in the pelvis, namely the iliac crests, medial ilium, sacrum and lumbar spine(39-41). Previous studies have found that HT increases with increasing volume of lumbar/sacral BM irradiated(16,17). Understanding the effects of radiation in active BM subregions, and refining efforts to selectively spare active BM could help optimize BM-sparing radiation techniques.
This study has several limitations. The data in this study were derived from retrospective cohorts, and further study in a prospective cohort would be desirable to affirm these findings. All patients in this study had cervical cancer, so it is uncertain whether our findings are specific to this disease. The use of IMRT in cervical cancer, particularly in patients with an intact uterus, is controversial and not yet widely accepted. Further studies will be necessary to optimize and test BM-sparing techniques in order to demonstrate a clinically significant impact.
This study also has several strengths. The study was hypothesis-driven, seeking to validate previously observed associations in an independent test cohort. Most of the patients in the validation cohort were treated at a different institution, supporting the generalizability of our findings. Finally, the use of IMRT and differences in treatment planning, physician contouring, and patient anatomy provided a good range and variation of BM doses, permitting us to evaluate the extent to which this variation was correlated with HT.
In summary, this study lends strong support to the hypothesis that V10 and V20 of pelvic BM are important predictors of HT in cervical cancer patients undergoing CRT. Efforts to maintain V10 < 95% and V20 < 76% could significantly reduce HT, but further research is needed to optimize and determine the clinical significance of BM-sparing techniques.
Acknowledgments
Supported by grants from the American Society of Clinical Oncology and NIH T32 RR023254
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interests:None
Presented at the 51st meeting of the American Society of Radiation Oncology, Chicago, IL, 2009
REFERENCES
- 1.Keys HM, Bundy BN, Stehman FB, et al. Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med. 1999;340:1154–1161. doi: 10.1056/NEJM199904153401503. [DOI] [PubMed] [Google Scholar]
- 2.Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340:1144–1153. doi: 10.1056/NEJM199904153401502. [DOI] [PubMed] [Google Scholar]
- 3.Morris M, Eifel PJ, Lu J, et al. Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med. 1999;340:1137–1143. doi: 10.1056/NEJM199904153401501. [DOI] [PubMed] [Google Scholar]
- 4.Eifel PJ, Winter K, Morris M, et al. Pelvic irradiation with concurrent chemotherapy versus pelvic and para-aortic irradiation for high-risk cervical cancer: an update of radiation therapy oncology group trial 90-01. J Clin Oncol. 2004;22:872–880. doi: 10.1200/JCO.2004.07.197. [DOI] [PubMed] [Google Scholar]
- 5.Whitney CW, Sause W, Bundy BN, et al. Randomized comparison of fluorouracil plus cisplatin versus hydroxyurea as an adjunct to RT in stage IIB-IVA carcinoma of the cervix. J Clin Oncol. 1999;17:1339–1348. doi: 10.1200/JCO.1999.17.5.1339. [DOI] [PubMed] [Google Scholar]
- 6.Peters WA, Liu PY, Barrett RJ, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000;18:1606–1613. doi: 10.1200/JCO.2000.18.8.1606. [DOI] [PubMed] [Google Scholar]
- 7.Green JA, Kirwan JM, Tierney JF, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix. Lancet. 2001;358:781–786. doi: 10.1016/S0140-6736(01)05965-7. [DOI] [PubMed] [Google Scholar]
- 8.Torres MA, Jhingran A, Thames HD, et al. Comparison of treatment tolerance and outcomes in patients with cervical cancer treated with concurrent chemoradiotherapy in a prospective randomized trial or with standard treatment. Int J Radiat Oncol Biol Phys. 2008;70:118–125. doi: 10.1016/j.ijrobp.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 9.Abu-Rustum NR, Lee S, Correa A, et al. Compliance with and acute hematologic toxic effects of chemoradiation in indigent women with cervical cancer. Gynecol Oncol. 2001;81:88–91. doi: 10.1006/gyno.2000.6109. [DOI] [PubMed] [Google Scholar]
- 10.Parker K, Gallop-Evans E, Hanna L, et al. Five years’ experience treating locally advanced cervical cancer with concurrent chemoradiotherapy and high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys. 2009;74:140–146. doi: 10.1016/j.ijrobp.2008.06.1920. [DOI] [PubMed] [Google Scholar]
- 11.Lanciano RM, Pajak TF, Martz K, et al. The influence of treatment time on outcome for squamous cell cancer of the uterine cervix treated with radiation. Int J Radiat Oncol Biol Phys. 1993;25:391–397. doi: 10.1016/0360-3016(93)90058-4. [DOI] [PubMed] [Google Scholar]
- 12.Perez CA, Grigsby PW, Castro-Vita H, et al. Carcinoma of the uterine cervix. Impact of prolongation of overall treatment time and timing of brachytherapy on outcome of RT. Int J Radiat Oncol Biol Phys. 1995;32:1275–1288. doi: 10.1016/0360-3016(95)00220-S. [DOI] [PubMed] [Google Scholar]
- 13.Ellis RE. The distribution of active bone marrow in the adult. Phys Med Biol. 1961;5:255–258. doi: 10.1088/0031-9155/5/3/302. [DOI] [PubMed] [Google Scholar]
- 14.Mauch P, Constine L, Greenberger J, et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys. 1995;31:1319–1339. doi: 10.1016/0360-3016(94)00430-S. [DOI] [PubMed] [Google Scholar]
- 15.Hall E, Giaccia A. Clinical response of normal tissues. In: H EJ, AJ G, editors. Radiobiology for the Radiologist. 6 ed. Lippincott Williams & Wilkins; Philadelphia: 2006. pp. 333–337.pp. 461–332. [Google Scholar]
- 16.Mell LK, Kochanski JD, Roeske JC, et al. Dosimetric predictors of acute hematologic toxicity in cervical cancer patients treated with concurrent cisplatin and intensity-modulated pelvic radiotherapy. Int J Radiat Oncol Biol Phys. 2006;66:1356–1365. doi: 10.1016/j.ijrobp.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 17.Mell LK, Schomas DA, Salama JK, et al. Association between bone marrow dosimetric parameters and acute hematologic toxicity in anal cancer patients treated with concurrent chemotherapy and IMRT. Int J Radiat Oncol Biol Phys. 2008;70:1431–1437. doi: 10.1016/j.ijrobp.2007.08.074. [DOI] [PubMed] [Google Scholar]
- 18.Mutyala S, Thawani N, Vainshtein JM, et al. Dose constraint recommendations and a predictive nomogram of incidence of hematological toxicity for cervix cancer patients treated with concurrent cisplatin and IMRT (abstr.) Int J Radiat Oncol Biol Phys. 2008;72:S359–S360. [Google Scholar]
- 19.Giangreco DT, Albuquerque K, Norton J, et al. Predictors of hematologic toxicity and implications for bone-marrow sparing pelvic IMRT for cervical cancer (abstr.) Int J Radiat Oncol Biol Phys. 2007;69:S399. doi: 10.1016/j.ijrobp.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 20.Mell LK, Tiryaki H, Ahn KH, et al. Dosimetric comparison of bone marrow-sparing IMRT versus conventional techniques for treatment of cervical cancer. Int J Radiat Oncol Biol Phys. 2008;71:1504–1510. doi: 10.1016/j.ijrobp.2008.04.046. [DOI] [PubMed] [Google Scholar]
- 21.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group and the European Organization for Research and Treatment of Cancer. Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 22.Blomlie V, Rofstad EK, Skjonsberg A, et al. Female pelvic bone marrow: serial MR imaging before, during, and after RT. Radiology. 1995;194:537–543. doi: 10.1148/radiology.194.2.7824737. [DOI] [PubMed] [Google Scholar]
- 23.Fajardo LF, Berthrong M, Anderson RE. Hematopoietic Tissue. In: Fajardo LF, Berthrong M, Anderson RE, editors. Radiation Pathology. Oxford University Press; Oxford: 2001. pp. 379–388. [Google Scholar]
- 24.Rubin P, Landman S, Mayer E, et al. Bone marrow regeneration and extension after extended field irradiation in Hodgkin's disease. Cancer. 1973;32:699–711. doi: 10.1002/1097-0142(197309)32:3<699::aid-cncr2820320324>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 25.Sacks EL, Goris ML, Glatstein E, et al. Bone marrow regeneration following large field radiation: influence of volume, age, dose, and time. Cancer. 1978;42:1057–1065. doi: 10.1002/1097-0142(197809)42:3<1057::aid-cncr2820420304>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 26.Scarantino CW, Rubin P, Constine LS. The paradoxes in patterns and mechanism of bone marrow regeneration after irradiation. Radiother Oncol. 1984;2:215–225. doi: 10.1016/s0167-8140(84)80062-6. [DOI] [PubMed] [Google Scholar]
- 27.Gatcliffe TA, Tewari KS, Shah A, et al. A feasibility study of topotecan with standard-dose cisplatin and concurrent primary RT in locally advanced cervical cancer. Gynecol Oncol. 2009;112:85–89. doi: 10.1016/j.ygyno.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 28.Dueñas-Gonzalez A, Cetina-Perez L, Lopez-Graniel C, et al. Pathologic response and toxicity assessment of chemoradiotherapy with cisplatin versus cisplatin plus gemcitabine in cervical cancer. Int J Radiat Oncol Biol Phys. 2005;61:817–823. doi: 10.1016/j.ijrobp.2004.07.676. [DOI] [PubMed] [Google Scholar]
- 29.Mundt AJ, Rotmensch J, Waggoner SE, et al. Phase I trial of concomitant vinorelbine, cisplatin, and pelvic irradiation in cervical carcinoma and other advanced pelvic malignancies. Gynecol Oncol. 2004;92:801–805. doi: 10.1016/j.ygyno.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 30.Petera J, Odrazka K, Frgala T, et al. External beam radiotherapy and high-dose brachytherapy combined with cisplatin and paclitaxel in patients with advanced cervical carcinoma. Gynecol Oncol. 2005;99:334–338. doi: 10.1016/j.ygyno.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Long HJ, Bundy BN, Grendys EC, et al. Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix. J Clin Oncol. 2005;23:4626–4633. doi: 10.1200/JCO.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 32.Moore DH, Blessing JA, McQuellon RP, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix. J Clin Oncol. 2004;22:3113–3119. doi: 10.1200/JCO.2004.04.170. [DOI] [PubMed] [Google Scholar]
- 33.Brixey CJ, Roeske JC, Lujan AE, et al. Impact of IMRT on acute hematologic toxicity in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2002;54:1388–1396. doi: 10.1016/s0360-3016(02)03801-4. [DOI] [PubMed] [Google Scholar]
- 34.Mundt AJ, Roeske JC. Can IMRT replace brachytherapy in the management of cervical cancer? Counterpoint. Brachytherapy. 2002;1:192–194. doi: 10.1016/S1538-4721(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 35.Milano MT, Jani AB, Farrey KJ, et al. Intensity-modulated radiation therapy in the treatment of anal cancer: toxicity and clinical outcome. Int J Radiat Oncol Biol Phys. 2005;63:354–361. doi: 10.1016/j.ijrobp.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 36.Liang Y, Rose BS, Lewis J, et al. Investigating effects of pelvic bone marrow radiation dose on acute hematologic toxicity using high dimensional data analysis (abstr.) Medical Physics. 2009;36:2781. [Google Scholar]
- 37.Hartsock RJ, Smith EB, Petty CS. Normal variations with aging of the amount of hematopoietic tissue in bone marrow from the anterior iliac crest. Am J Clin Pathol. 1965;43:326–331. doi: 10.1093/ajcp/43.4.326. [DOI] [PubMed] [Google Scholar]
- 38.Vogler JB, Murphy WA. Bone marrow imaging. Radiology. 1988;168:679–693. doi: 10.1148/radiology.168.3.3043546. [DOI] [PubMed] [Google Scholar]
- 39.Basu S, Houseni M, Bural G, et al. Magnetic resonance imaging based bone marrow segmentation for quantitative calculation of pure red marrow metabolism using 2-deoxy-2-[F-18]fluoro-D-glucose-PET. Mol Imaging Biol. 2007;9:361–365. doi: 10.1007/s11307-007-0112-5. [DOI] [PubMed] [Google Scholar]
- 40.Roeske JC, Mundt AJ. Incorporation of MRI into intensity modulated whole-pelvic radiation treatment to reduce the volume of pelvic bone marrow irradiated. Int Congress Series. 2004:307–312. [Google Scholar]
- 41.Roeske JC, Lujan A, Reba RC, et al. Incorporation of SPECT bone marrow imaging into intensity modulated whole-pelvic RT treatment planning for gynecologic malignancies. Radiother Oncol. 2005;77:11–17. doi: 10.1016/j.radonc.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 42.Pearcey R, Brundage M, Drouin P, et al. Phase III trial comparing radical radiotherapy with and without cisplatin chemotherapy in patients with advanced squamous cell cancer of the cervix. J Clin Oncol. 2002;20:966–72. doi: 10.1200/JCO.2002.20.4.966. [DOI] [PubMed] [Google Scholar]