Many bacterial species contain protein shell-bound polyhedral inclusions referred to as bacterial microcompartments (BMCs). The earliest of these to be described and analyzed in detail was the carboxysome (1), an organelle that is essential for efficient CO2 fixation in cyanobacteria and many chemoautotrophs. By cosequestering ribulose 1,5-bisphosphate carboxylase/oxygenase (RubisCO) and a carbonic anhydrase, the carboxysome provides a compartment with elevated CO2 levels that overcome some of the kinetic deficiencies of RubisCO (2). Reverse genetic studies of isolated carboxysome proteins revealed that homologues of the major shell protein genes occur in the genomes of heterotrophic bacteria within operons for metabolic pathways that are completely unrelated to autotrophic CO2 fixation (3). Recent comparative genomics analysis suggested that these bacterial organelles play a much broader role than originally surmised, because the genetic potential for BMC formation is widespread among the bacterial lineages (4). Salmonella enterica uses two different BMCs (Pdu and Eut) for the degradation of propanediol and ethanolamine, respectively. Sequestering as many as four sequential enzymes from the respective pathways in polyhedral protein shells is thought to prevent diffusion and consequent loss of volatile aldehyde intermediates that might damage cellular structures. In a recent issue of PNAS, a report by Fan et al. (5) identifies a short peptide signal, present on the N terminus of some enzymes contained within the Pdu BMC of S. enterica, as directing the placement of these enzymes inside the organelle. Their study constitutes a major step toward elucidating the self-assembly processes that bring about BMC formation within the bacterial cytoplasm.
Despite their function as organelles and the apparent permselectivity of their shells, BMCs are not surrounded by a lipid bilayer-based membrane like their eukaryotic counterparts but are instead bordered by a thin protein shell. The shells of BMCs that have been purified and studied directly were shown to be composed of several thousand copies of orthologous polypeptides with conserved domains (Pfam 00936); recent crystallographic studies revealed that individual monomers of the predominant BMC shell proteins are arranged into thin hexamers that strongly interact in an “edge-on” fashion to form the flat facets of the polyhedral compartments (6–8). Another, albeit minor, shell constituent (Pfam 03319) that appears to occur in all BMCs forms the pentamers thought to occupy the vertices of the polyhedral structures (9). One of the great mysteries surrounding BMCs is the mechanism by which their shells, all of which apparently are built from proteins that belong to the same two structural families, function in widely diverse metabolic pathways and package a large variety of different and seemingly structurally unrelated enzymes. Because BMCs lack a bounding lipid bilayer-based membrane, the mechanisms that target polypeptides to their interior are likely different from those that guide newly synthesized proteins to the plasma membrane. Related to the targeting question is that of the mechanism by which the proper stoichiometric ratio of the encapsulated enzymes, which is likely crucial to BMC function, is ensured during assembly of the organelle.
Two pathways were previously put forth for the biogenesis of BMCs that are based on electron micrographs of apparent carboxysome assembly intermediates. One of these proposes that a preassembled RubisCO core guides assembly of the polyhedral shell (10). The other model posits the existence of a preassembled (partial) shell that directs the packaging of RubisCO into the BMC interior (11). Clearly, carboxysome shell formation is not dependent on the presence of RubisCO, as was shown for a Halothiobacillus neapolitanus RubisCO deletion mutant that forms empty BMC shells of otherwise normal shape and composition (12). However, that same study provided strong evidence for specific interactions between payload and shell proteins by identifying the large subunit of the enzyme as the factor that determines whether a particular RubisCO species can be packaged into the carboxysome. Contrary to the N-terminal peptide that is identified by Fan et al. (5) on some enzymes of the Pdu BMC, sequence comparisons between carboxysomal and noncarboxysomal large subunits of RubisCO from a variety of autotrophic bacteria did not reveal an obvious packaging signal (12).
As Fan et al. (5) point out, a variety of short peptide sequences have been reported to mediate assembly of larger protein complexes. Perhaps most relevant to BMC packaging is the landmark study by Sutter et al. (13) that identified the short C-terminal extension on those DyP and Flp homologues that are packaged into the polyhedral encapsulin nanocompartment as necessary and sufficient for targeting. Previous reports had also noted that two subunits (PduD and PduE) of the Pdu BMC-associated diol dehydratase contain N-terminal segments that are not required for enzymatic activity but were speculated to play a role in BMC assembly (14). Fan et al. (5) have now taken a closer look at the sequences of several enzymes that are packaged into the Pdu BMC of S. enterica. Alignment of CoA-dependent propionaldehyde dehydrogenase (PduP) sequences from a number of organisms revealed that a 20-aa N-terminal extension is present in most of the homologues suspected to be associated with a BMC. Recombinant versions of Salmonella PduP lacking this N-terminal extension, when expressed in a Salmonella pduP deletion mutant, yield BMCs with greatly reduced PduP activity.
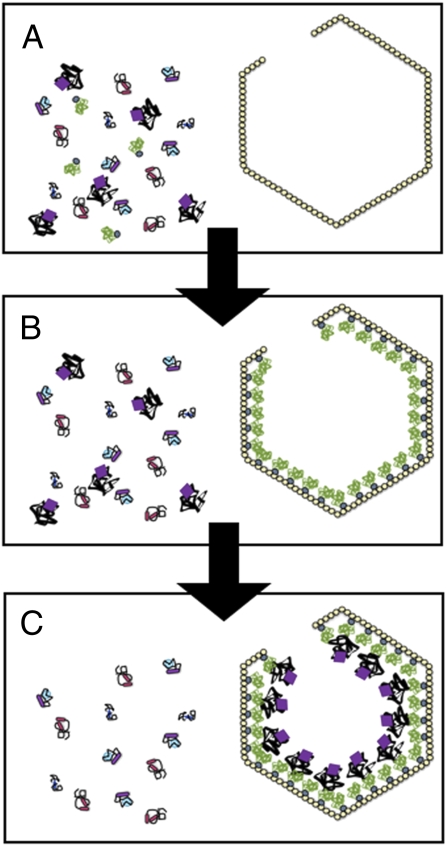
The N-terminal extension was also found to target three heterologous reporter proteins into the BMC of the pduP deletion mutant. Through a series of cleverly designed experiments with GFP fused to N-terminal extensions of various lengths, the group convincingly demonstrates that the N-terminal signal sequence is necessary and sufficient to direct GFP to the interior of the Pdu BMC (Fig. 1 A and B). The specificity of the packaging signal was tested with a construct featuring the first 18 residues of the N-terminal extension. By controlling the levels of the fusion protein in a WT S. enterica background, Fan et al. (5) show that increasing amounts of GFP are packaged into the Pdu BMC in competition with endogenous PduP as expression levels of the fusion protein increase.
Fig. 1.
Targeting of sequestered proteins to the interior of BMCs. As described in PNAS (5), a short extension of ≈20 amino acids found on the N terminus of PduP directs the enzyme to the lumen of the Pdu BMC. (A and B) Fan et al. (5) propose that the N-terminal segment binds specific sites on the interior surface of shell protein assemblies. (C) Additional protein interactions are likely to occur between shell-anchored proteins and those found deeper in the BMC interior and would account for the solid appearance of BMC interiors observed with various imaging methods.
Fan et al. (5) suggest that the N-terminal sequences of PduP proteins and similar extensions of five other BMC proteins may contribute to a general mechanism for BMC sequestration that depends on specific interactions of the different packaging signals with individual
The genetic potential for BMC formation is widespread among the bacterial lineages.
shell components. The idea put forth by these investigators that different encapsulation signals on other BMC proteins may bring about pairing with specific shell protein partners is intriguing because it suggests a mechanism for orienting and organizing BMC enzyme contents so that diffusion of intermediates between them is optimized. However, because not all BMC proteins contain an N-terminal extension, the nature of any additional signal(s) that ensure inclusion of these proteins in the BMC is not clear.
We would like to propose that in addition to the interactions between N-terminal packaging sequences of lumen enzymes and shell constituents, specific interactions likely exist between these shell-anchored proteins and others that are located deeper inside the BMC lumen (Fig. 1C), similar to previously characterized subcarboxysomal protein assemblies (15, 16). Such contacts would explain the regular arrangement of carboxysomal RubisCO molecules in concentrical layers and the fact that the interior of the great majority of carboxysomes appears to be solidly filled with proteins (17). Specific interactions between BMC components that are not in direct contact with the BMC shell could also explain why traces of mutant PduP lacking the N-terminal peptide were found to be present in isolated BMCs by Fan et al. (5).
Questions remaining about the processes that govern the assembly of the BMC shell relate to the mechanism of protein encapsulation. The extent to which other cellular components are involved in guiding the proper assembly of the BMC and closing of its shell is not yet known. Transmission electron microscopy and electron cryotomography revealed that carboxysomes are associated with a variety of filamentous structures in cyanobacteria and chemoautotrophs (17, 18). Whether these structures play a role in enzyme targeting and BMC assembly is not known. However, a recent report by Silver and coworkers (19) implicated the two bacterial cytoskeleton proteins ParA and MreB in mediating arrangement of carboxysomes in the cell and in their segregation during cell division in the cyanobacterium Synechococcus PCC 7942, thus lending credence to the idea that carboxysomes, and likely other BMCs, interact with cellular structures that are not part of the BMC itself.
The discovery of a signal sequence that is capable of directing enzyme encapsulation into a BMC is a landmark in our understanding of BMC assembly. The report by Fan et al. (5) leads the way for searches of additional packaging signals in other types of BMCs and for the development of these bacterial organelles toward biotechnological applications.
Acknowledgments
We thank Scott Walper for help with the figure. Carboxysome research in our laboratories is supported by National Science Foundation Grants MCB 0851094 and MCB 0818680.
Footnotes
The authors declare no conflict of interest.
See companion article on page 7509 in issue 16 of volume 107.
References
- 1.Shively JM, Ball F, Brown DH, Saunders RE. Functional organelles in prokaryotes: Polyhedral inclusions (carboxysomes) of Thiobacillus neapolitanus. Science. 1973;182:584–586. doi: 10.1126/science.182.4112.584. [DOI] [PubMed] [Google Scholar]
- 2.Badger MR, Price GD. The CO2 concentrating mechanism in cyanobacteria and microalgae. Physiol Plant. 1992;90:529–536. [Google Scholar]
- 3.Shively JM, et al. Sequence homologs of the carboxysomal polypeptide CsoS1 of the thiobacilli are present in cyanobacteria and enteric bacteria that form carboxysomes-polyhedral bodies. Can J Bot. 1998;76:906–916. [Google Scholar]
- 4.Bobik TA. Polyhedral organelles compartmenting bacterial metabolic processes. Appl Microbiol Biotechnol. 2006;70:517–525. doi: 10.1007/s00253-005-0295-0. [DOI] [PubMed] [Google Scholar]
- 5.Fan C, et al. Short N-terminal sequences package proteins into bacterial microcompartments. Proc Natl Acad Sci USA. 2010;107:7509–7514. doi: 10.1073/pnas.0913199107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerfeld CA, et al. Protein structures forming the shell of primitive bacterial organelles. Science. 2005;309:936–938. doi: 10.1126/science.1113397. [DOI] [PubMed] [Google Scholar]
- 7.Tanaka S, Sawaya MR, Yeates TO. Structure and mechanisms of a protein-based organelle in Escherichia coli. Science. 2010;327:81–84. doi: 10.1126/science.1179513. [DOI] [PubMed] [Google Scholar]
- 8.Tsai Y, et al. Structural analysis of CsoS1A and the protein shell of the Halothiobacillus neapolitanus carboxysome. PLoS Biol. 2007;5:e144. doi: 10.1371/journal.pbio.0050144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka S, et al. Atomic-level models of the bacterial carboxysome shell. Science. 2008;319:1083–1086. doi: 10.1126/science.1151458. [DOI] [PubMed] [Google Scholar]
- 10.Orus MI, Rodriguez ML, Martinez F, Marco E. Biogenesis and ultrastructure of carboxysomes from wild type and mutants of Synechococcus sp. strain PCC 7942. Plant Physiol. 1995;107:1159–1166. doi: 10.1104/pp.107.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Price GD, Badger MR. Evidence for the role of carboxysomes in the cyanobacterial CO2-concentrating mechanism. Can J Bot. 1991;69:963–973. [Google Scholar]
- 12.Menon BB, Dou Z, Heinhorst S, Shively JM, Cannon GC. Halothiobacillus neapolitanus carboxysomes sequester heterologous and chimeric RubisCO species. PLoS One. 2008;3:e3570. doi: 10.1371/journal.pone.0003570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sutter M, et al. Structural basis of enzyme encapsulation into a bacterial nanocompartment. Nat Struct Mol Biol. 2008;15:939–947. doi: 10.1038/nsmb.1473. [DOI] [PubMed] [Google Scholar]
- 14.Tobimatsu T, Kawata M, Toraya T. The N-terminal regions of beta and gamma subunits lower the solubility of adenosylcobalamin-dependent diol dehydratase. Biosci Biotechnol Biochem. 2005;69:455–462. doi: 10.1271/bbb.69.455. [DOI] [PubMed] [Google Scholar]
- 15.Cot SS-W, So AK-C, Espie GS. A multiprotein bicarbonate dehydration complex essential to carboxysome function in cyanobacteria. J Bacteriol. 2008;190:936–945. doi: 10.1128/JB.01283-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Long BM, Badger MR, Whitney SM, Price GD. Analysis of carboxysomes from Synechococcus PCC7942 reveals multiple Rubisco complexes with carboxysomal proteins CcmM and CcaA. J Biol Chem. 2007;282:29323–29335. doi: 10.1074/jbc.M703896200. [DOI] [PubMed] [Google Scholar]
- 17.Iancu CV, et al. Organization, structure, and assembly of alpha-carboxysomes determined by electron cryotomography of intact cells. J Mol Biol. 2010;396:105–117. doi: 10.1016/j.jmb.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen TE. Cyanobacterial cell inclusions of irregular occurrence: Systematic and evolutionary implications. Cytobios. 1984;39:35–62. [Google Scholar]
- 19.Savage DF, Afonso B, Chen AH, Silver PA. Spatially ordered dynamics of the bacterial carbon fixation machinery. Science. 2010;327:1258–1261. doi: 10.1126/science.1186090. [DOI] [PubMed] [Google Scholar]