Abstract
Importance of the field
Acute Myeloid Leukemia (AML) and myelodysplastic syndrome (MDS) incidence in the United States increase with age. Given the progressive aging of the general population, incidence of these diseses is likely to continue to rise in the future. There is an earnest need for therapeutic developments because of the poor prognosis of these diseases. Since the knowledge of molecular genetics in AML and MDS has expanded recently, targeted therapeutics should offer an exciting new frontier for advancement. Of all the targeted inhibitors developed, tipifarnib represents one of the few compounds with some activity as a single agent.
Areas to be covered in this review
Described in this review are the molecular targets of tipifarnib, safety and tolerability of the drug, chemistry, and clinical efficacy in AML.
What the reader will gain
The reader will gain a thorough understanding of tipifarnib as it relates to the current and future use of the drug in AML.
Take home message
The future of tipifarnib, along with other molecularly-targeted drugs, lies in achieving a better understanding of leukemia biology and harnessing the activity of this agent using predictive biomarkers for improved patient selection.
Keywords: Acute myeloid leukemia, myelodysplastic syndrome, Ras, farneslytransferase, treatment, lipid
2.5 Introduction
2.5.1 Disease Incidence and Prevalence
Acute Myeloid Leukemia (AML) incidence in the United States was assessed from 1973–1998 by the Surveillance, Epidemiology, and End Results (SEER) program(Xie, Davies et al. 2003). Results of incidence and 5-year survival were based on nine reporting areas and the specific rates for age (birth-19, 20–44, 45–64, and 65 + years), gender, and race (black, white) were examined. Each year, a total of 18,717 cases of AML were identified with the highest incidence in individuals over 65 years of age (incidence rate of 150 per million based on 1970 US standards). The incidence of AML in children (birth to 19 years of age) was 1,187 cases per million. The 5 and 10 year survival rates in this study, among the various age groups, were calculated for patients with all types of leukemia including AML, acute lymphoblastic leukemia (ALL), chronic myeloid leukemia (CML), and chronic lymphoblastic leukemia (CLL). Patients with AML had the worst prognosis among all types of leukemia with 5-year survival rates of 22% (birth-19 years), 13.83% (22–44 years), 6.52% (45–64 years), and 2.49% (over 65 years old) (Xie, Davies et al. 2003). About half of older AML patients have adverse prognostic features at diagnosis, i.e. unfavorable cytogenetics and/or AML arising after MDS. These patients are less responsive and less tolerant to conventional chemotherapy and are potential candidates for experimental approaches as first line therapy. An active oral therapy, such as tipifarnib, could offer therapeutic opportunity to frail patients.
Myelodysplastic syndromes (MDS) are characterized by incompetent hematopoiesis that leads to single or multi-lineage peripheral cytopenias with the development of AML in approximately 30–40% of cases(Greenberg, Young et al. 2002). The etiology of MDS is unknown and the factors leading to AML progression are not well characterized. Registration of MDS to population-based cancer registries in the United States was initiated in 2001 and results were recently reported (Ma, Does et al. 2007; Rollison, Howlader et al. 2008). Data from the North American Association of Central Cancer Registries (NAACCR) and SEER programs, encompassing 82% of the US population estimated that the average case numbers for the entire United States, based on more than 40,000 observations, was 3.3 per 100,000 annually for 2001 through 2003(Rollison, Howlader et al. 2008). Incidence rates increased with age (P < .05) and were highest among whites and non-Hispanics.(Rollison, Howlader et al. 2008). The incidence of both AML and MDS is expected to further increase in the years to come, given the progressive aging of the general population.
2.5.2 Unmet medical needs
There is a serious unmet medical need for the discovery of new pharmaceutical or biological therapies with disease remitting activity that target the molecular and cellular abnormalities that drive clonal expansion and survival of leukemic cells. Available data suggest that pharmacological modulators of signal transduction pathways hold promise and may ultimately lead to improved outcome for AML patients. None of these agents as single therapy, however, has shown significant activity possibly due to the complexity of signaling events and disease heterogeneity. Signaling molecules that have been targets in AML include FLT3(Mattison, Ostler et al. 2007; Pinheiro, de Sa Moreira et al. 2008), Ras/Raf/MAPK(Padua, Carter et al. 1988; Ehmann, Horn et al. 2006), mTOR(Recher, Dos Santos et al. 2005), KIT(Faderl, Pal et al. 2009), and VEGF(Dickson and Shami 2001) Of all the targeted inhibitors tested, tipifarnib represents one of the few compounds with single agent activity in individuals with both AML and MDS(Lancet and Karp 2003). In this review, information will be provided about the molecular targets of tipifarnib and the future of this agent for the treatment of AML.
2.6 Body of the Review
2.6.1 Farnesyltransferase inhibitors as molecular bullets
Mutations of RAS genes, or activation of RAS in the absence of mutations, occurs in high frequency in MDS and AML suggesting that targeted disruption of this important signaling molecule may be used therapeutically(Saglio, Serra et al. 1989; Auewarakul, Lauhakirti et al. 2006; Bacher, Haferlach et al. 2006; Ehmann, Horn et al. 2006; Niimi, Harada et al. 2006). Leukemic cells from roughly 10–40% of MDS cases and 15–25% of AML cases have been reported to have RAS mutations(Padua, Carter et al. 1988; Paquette, Landaw et al. 1993; Neubauer, Greenberg et al. 1994; Padua and West 2000; Reuter, Morgan et al. 2000).
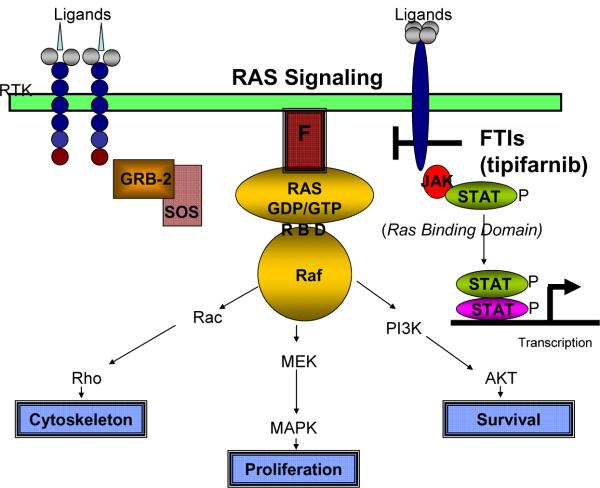
RAS is a small farneslyated, GTPase that is critical for many receptor-mediated pathways leading to MEK/ERK activation.(Li, Batzer et al. 1993) Inactive Ras binds GDP but when activated, GDP is exchanged for GTP enabling Ras to bind Raf-1 and signal to downstream intermediates.(de Rooij and Bos 1997) The mechanism of this activation is dependent on the NH2-terminal domain of Raf-1 known as the Ras-binding domain (RBD) that reversibly interacts with Ras in the plasma membrane (pathway shown diagrammatically in Figure 1).(de Rooij and Bos 1997)
Figure 1. Summary of Ras signaling pathway.
RAS is activated by receptor tyrosine kinase (RTK) activation with several ligands that invoke these intermediates. Activation of the receptor leads to the activation of Ras nucleotide exchange factors such as GRB-2 and SOS that mediate the exchange of GDP for GTP and the recruitment of Raf and the activation of downstream factors such as Rac, Rho that mediate changes in the cytoskeleton, MEK and MAPK that mediate proliferation and PI3K and AKT that are necessary for survival. Other factors that mediate transcription such as the Janus Activating Kinase (Jak) lead to the Signal Transducer and Activator of Transcription (STAT) phosphorylation and transcription. This pathway has also been linked to tipifarnib response but the mechanism is unknown.
The farnesyl group on Ras is necessary for movement from the cytoplasm to the plasma membrane and for membrane anchorage; a process that is essential for activation.(de Rooij and Bos 1997) Farnesyltransferase is the enzyme that catalyses the transfer of a 15-carbon isoprenyl farnesyl moiety to the carboxy terminus of RAS and other proteins containing the peptide target sequence “CAAX, often referred to as a CAAX-box”. A related enzyme, geranylgeranyl transferase I (GGT-1) catalyzes the addition of a 20-carbon geranylgeranyl group to CAAX-containing proteins. The “X” residue determines the specificity of these two enzymes.(Basso, Kirschmeier et al. 2006) Under physiologic conditions, the specificities of these enzymes are relatively well maintained. Targeted disruption of FTase activity leads to the inactivation of RAS function.(Agrawal and Somani 2009).
2.6.2 Introduction to the compound
Farnesyltransferase inhibitors (FTIs) represent an effective method to disrupt RAS function by blocking its ability to anchor to the plasma membrane, causing a GDP-locked, inactive state.(Lerner, Qian et al. 1995; Lerner, Zhang et al. 1997; Bolick, Landowski et al. 2003; Santucci, Mackley et al. 2003) Different FTIs have been synthesized and shown to have both in vitro and in vivo activity in cell lines or animal models, respectively(Tamanoi 1993; Sebti and Hamilton 1996). Several FTIs have entered clinical trials, beginning with dose and schedule optimization and identification of toxicities. Tipifarnib (initially R115777) is a very potent and highly selective inhibitor of farnesyltransferase (FTase) that was first developed in April 1997.(Norman 2002) Although the drug is highly selective for FTase, a plethora of activities result from the numerous cellular proteins that require farnesylation for activity. Tipifarnib inhibited proliferation of several cell lines with both mutated and non-mutated RAS.(Mesa, Tefferi et al. 2003) In most cell types, repressed proliferation was not accompanied by the induction of cell death suggesting that the drug has cytostatic rather than cytotoxic activity. Tipifarnib-induced apoptosis was observed, however, in both multiple myeloma cell lines and in primary cultures from patients with multiple myeloma suggesting that the drug is capable of inducing apoptosis in some neoplastic cell lineages other than AML.(Le Gouill, Pellat-Deceunynck et al. 2002; Cortes, Albitar et al. 2003; Ochiai, Uchida et al. 2003; Alsina, Fonseca et al. 2004) Evidence clearly shows that the mechanism of action is cell-type dependent and includes inhibition of angiogenesis, induction of apoptosis, and antiproliferative effects.
Prenylation catalyzed by geranylgeranyltransferase type 1 is not sensitive to suppression by tipifarnib. GGTase plays an important role in resistance to tipifarnib and other FTIs since selective inhibition of farneslyation often leads to a compensatory increase in geranylgeranylation that restores membrane anchor-associated activation and cellular function (Mor et al. 2007).
There have been no data that clearly link suppression of Ras with clinical response to tipifarnib. This may be due to the pleotrophic effects of the drug against other farnesylated cellular targets(Basso, Kirschmeier et al. 2006). Many targets of FTIs have been described and some are likely to be undefined. A list of the known farnesylated proteins inhibited by tipifarnib is provided in Table 1. A better understanding of leukemia biology and the molecular targets of tipifarnib is needed to fully understand the mechanism of action of this drug and more effectively apply it to select patients with AML.
Table 1.
Known farnesylated proteins that may be molecular targets for tipifarnib.
| Protein Target* | Prenylation Sequence | Function |
|---|---|---|
| H-Ras | CSVL | GTPase |
| Rheb | CSVM | GTPase |
| Rheb2 | CHLM | GTPase |
| HDJ2 | CQTS | Unknown |
| K-Ras | CVIM | GTPase |
| N-Ras | CVVM | GTPase |
| CENP E | CKTQ | Kinesin motor protein |
| CENP E | CKVQ | Chromosome passenger protein |
| Prelamin A | CSIM | Nuclear envelope |
| Rho B | CKVL | GTPase |
| PRL1,2,3 | CCIQ, CCVQ, CCVM | Tyrosine phosphatase |
| Rho D | CCVT | GTPase |
| Rho 6 | CSIM | GTPase |
| Rho 7 | CNLM | GTPase |
| Tc10 | CLIT | GTPase |
Adaptation from Basso, AD et al. J Lipid Res 2006; 47:15-31.24
2.6.3 Chemistry
Tipifarnib is a methyl-quinolinone derivative (molecular formula C27H22Cl2N4O) that acts as a potent and selective nonpeptidomimetic inhibitor of farnesyl tranferase protein (FTP) both in vitro and in vivo(Norman 2002). Tipifarnib is supplied as 100 mg (white, in boxes of 126 tablets each) circular film-coated tablets. Tablets should be protected from moisture and stored at room temperature (15–25°C, 59–77°F) with stability at least 36 months. Tablet bioavailability increases with a meal so the drug should be administer with food.
2.6.4 Pharmacodynamics
Competitive inhibition of farnesyltransferase enzyme activity, using K-Ras peptide as a substrate, occurred with an IC50 of 7.9 nM.(End et al. 2001) Forty of 53 (75%) human tumor cell lines were found to be sensitive to tipifarnib in vitro and most of these sensitive cell lines carried H-RAS or N-RAS mutations. In contrast, fifty percent of tumor cell lines displaying mutant K-ras genes were resistant or required higher concentrations for growth inhibition. Oral administration of tipifarnib in xenograft models carrying mutant H-RAS and K-RAS revealed that twice daily doses with 6.25–100 mg/kg inhibited tumor growth in vivo(End et al, 2001)). The ratio of unfarnesylated and farnesylated HDJ2, a farnesylated chaperone protein with unknown biological function, in peripheral blood mononuclear cells is accepted as the best marker of pharmacological efficacy and pharmacodynamics(Karp, Lancet et al. 2001).
2.6.5 Pharmacokinetics and metabolism
Pharmacokinetic studies were performed with tipifarnib and revealed a linear relationship between dose and maximum plasma concentration(Karp, Lancet et al. 2001; Patnaik et al. 2003). There was also a linear relationship between the dose and area under the curve over 12 hours at all dosage levels(Karp, Lancet et al. 2001). In individuals with AML, weekly marrow samples demonstrated a dose-dependent accumulation of tipifarnib in bone marrow with large increases in concentration occurring at 600 mg twice daily. Tipifarnib is currently available as a tablet and the bioavailability of tablets was compared to a capsule formulation, in patients with solid tumors but not in AML(Crul, de Klerk et al. 2002). The investigators used tipifarnib administered once daily in doses of 300 or 400 mg. Blood samples were drawn up to 24 hours after drug administration, and the plasma levels were measured using high performance liquid chromatography (HPLC)(Crul, de Klerk et al. 2002). The pharmacokinetic parameters examined included time to maximal plasma concentration (Tmax), half-life (t 1/2), maximal plasma concentration (Cmax) and area under the curve at twenty-four hours (AUC24h). The point estimates of the log-normalized Cmax and AUC24h were 0.94 and 0.92, respectively (0.81–1.09 and 0.83–1.03 90%CI)(Crul, de Klerk et al. 2002).
2.6.6 Clinical efficacy
2.6.6.1 Phase I studies
One of the earliest phase 1 trials of poor-risk acute leukemias revealed that tipifarnib was associated with a clinical response in 10 (29%) of the 34 evaluable patients, including 2 complete remissions(Karp, Lancet et al. 2001). This promising response rate, as a single agent in poor risk leukemias, produced excitement over the potential of tipifarnib for the treatment of AML(Karp, Lancet et al. 2001). Cohorts of patients received tipifarnib at doses ranging from 100 mg twice daily (bid) up to 1200 mg bid for up to 21 days. Dose-limiting toxicity was observed at 1200 mg bid(Karp, Lancet et al. 2001). Central neurotoxicity, including ataxia, confusion, and dysarthria, proved to be the dose-limiting toxicity. Non-dose-limiting toxicities included myelosuppression, nausea, paresthesias, renal insufficiency, polydipsia(Cortes, Albitar et al. 2003) In vivo, 300 mg bid actively suppressed farnesylation of lamin A and HDJ-2. Genetic analyses of mutation status failed to reveal a linkage between N-RAS mutations and clinical response. Comparison of peripheral blood samples obtained before and after treatment showed that phosphorylated ERK, an effector molecule that is downstream of Ras-mediated signaling, was reduced in roughly 50% of patients after one cycle of treatment. Tipifarnib clinical response failed correlate with the presence of mutant RAS and other biomarkers examined, which has been an enigma that has vexed the scientific community throughout the clinical development as single agent therapy for AML in Phase II and Phase III studies.
Due to the modest activity as a single agent, the efficacy of tipifarnb combinations was examined in preclinical and a phase I studies (Karp, Flatten et al. 2009). Synergistic activity was observed when an AML cell line (HL-60) and primary cells from AML patients were treated with tipifarnib in combination with the topoisomerase II inhibitor etoposide. Tipifarnib increased the amount of apoptosis induced by etoposide and reduced the number of colonies present in colony formation assays after 10–14 days. The mechanism for the synergistic activity was explored and found to be through inhibition of the ribosomal S6 protein phosphorylation and p70 S6 kinase 1, which is consistent with a combined effect on mTOR signaling. Results of these preclinical studies led to a phase 1 clinical trial to examine the tolerability and efficacy of tipifarnib and etoposide combination therapy in AML patients, A total of 101 patients were evaluated for eligibility and 84 were enrolled and treated with a 14- or 21-day course of tipifarnib at a dose of either 600 or 400 mg. The dose of etoposide was either 100 or 200 mg. In vivo, the investigators explored whether tipifarnib plus etoposide decreased ribosomal S6 phosphorylation. Of the 84 patients enrolled, 21 patients (84%) achieved a complete response. Using a poynomia model to estimate the relationship between the therapeutic dose of the drug combination and response, the response tended to be higher in patients who received a higher dose of both drugs (tipifarnib 600mg twice daily for 14-days plus etoposide dose 150 mg daily on days 1–3 and 8–10 or 400 mg twice daily tipifarnib for 14-days in combination with 200 mg daily etoposide on days 1–3 and 8–10).
An additional phase I study investigated the use of tipifarnib in combination with standard dose and schedule of daurorubine/cytarabine induction therapy (Brandwein et al, 2009) of AML in patients age 60 years and over. Tipifarnib was dose evaluated over four levels (200, 300, 400, and 600 mg po bid) in days six to 15. In this study, there were no dose-limiting toxicolities due to delayed hematologic recovery. The regimen was well tolerated and the maximum tolerated dose was not reached. The investigators recommended that the 600 mg bid dose of tipifarnib be considered for further study in this combination regimen. Despite these promising trials, failure to identify the precise mechanism of action of tipifarnib for leukemia therapy has been problematic but has engendered further investigations to uncover the basic mechanisms of leukemogenesis.
2.6.6.2 Phase II studies
Successful enzyme target inhibition, low toxicity, and promising response rates, prompted further investigation of tipifarnib in phase II trials. These trials focused on clarifying the response rate and identifying the downstream signal transduction targets that may be modified by these agents in order to understand a precise molecule linkage to response. It was anticipated that this information would generate optimal use of FTIs in patients with AML and lead to the development of more successful combination. A summary of Phase I, II and III trials is shown in Table 2. (Harousseau, Lancet et al. 2007; Karp, Lancet et al, 2001; Harousseau, Martinelli et al. 2009; Kirschbaum et al, 2007; Karp, Flatten et al, 2009; Brandwein et al, 2009; Lancet et al, 2007; Erba et al, 2007; Karp, Smith et al, 2008)
Table 2.
Therapy of AML with single agent tipifarnib.
| Author | Response | Dose-Schedule | Patients | |
|---|---|---|---|---|
| Phase I | ||||
| Karp, Lancet (2001) | 2/34 (8%) CR | 100–1200,21 of 28 days | relapse/ref ractory | |
| Kirschbaum (2007) | 3/9 (33%) CR | 400–1400, alternate week | relapse/ref ractory | |
| Karp, Flatten (2009) | Response based on dose (N=84) | Combination therapy tipifarnib and etoposide | Newly diagnosed Age> 70 | |
| Brandwein (2009) | 10/22 (45%) CR | Combination therapy with conventional induction and consolidation therapy | Newly diagnosed Age> 70 | |
| Phase II | ||||
| Karp, Smith (2008) | 15/48(31%), continuous CR for median 28+ months | 400 mg bid 14 of 21 days 48 weeks | poor-risk AML | |
| Harousseau (2007) | 11/169 (7%) months | 600, 21 of 28 days | relapse/ref ractory | |
| Lancet (2007) | 22/158 (14%) months | 600, 21 of 28 for 63 days | Poor risk age≥65 | |
| Phase III | ||||
| Harousseau (2009) | 18/228 (8%) compared to hydroxyurea | 600, 21 of 28 days | Newly diagnosed, Age≥70 |
After completion of several Phase II trials, it was obvious that tipifarnib failed to demonstrated significant responses in unselected AML patients despite obvious activity in the initial trials. Therefore, the attention then turned to better understanding the molecular mechanism of the drug starting with investigations to identify baseline predictive markers associated with response. Recent gene expression profiles from the bone marrow of patients from a phase 2 study of the FTI tipifarnib in older adults with AML revealed that the ratio of RASGRP1/APTX gene expression prior to treatment displayed high fidelity and excellent accuracy as a predictive marker of response(Raponi, Lancet et al. 2008). RASGRP1 is a guanine nucleotide exchange factor involved in the activation of RAS, and the APTX (aprataxin) gene product is involved in DNA excision repair(Gueven, Becherel et al. 2004). In addition to predicting response, the classifier also proved to be an independent predictor of improved overall survival (154 vs 56 days; P < .001). For use of tipifarnib in combination and as a single agent in AML, this molecular signature warrants further validation in a prospective study.
2.6.6.3 Phase III trial
Due to the tolerability, efficacy of tipifarnib, and the unmet need for therapy in elderly AML, tipifarnib was investigated for the treatment of older patients not eligible for transplantation. This phase 3 trial was conducted as first-line therapy in patients ≥ 70 years old with newly diagnosed, de novo, or secondary AML.(Harousseau, Martinelli et al. 2009) In this open-label study, the efficacy and safety of tipifarnib was compared with best supportive care (BSC), including hydroxyurea. Tipifarnib was administered at a dose of 600 mg orally twice daily for 21 out of a 28-day cycle. The primary endpoint was overall survival. A total of 457 patients enrolled, with 24% of the patients being 80 years of age or older. The median survival was 107 days for the tipifarnib arm and 109 days for the BSC arm. The hazard ratio (tipifarnib vs BSC) for overall survival was 1.02 (P value by stratified log-rank test, .843). The complete response rate for tipifarnib was lower in this study (8%) compared to previous Phase II studies. Cytopenias were the most frequently observed grade 3 or 4 adverse events. Therefore, the conclusion from this randomized study was that tipifarnib treatment, in this patient population, was not better than standard of care.
2.6.7 Safety and tolerability
2.6.7.1 Animal Studies
Cataracts were noted in preclinical toxicology studies only. Cataracts were noted in Wistar rats treated by oral gavage in repeated dose toxicity studies for 3 months at doses of 40 and 60 mg/kg (product literature). Rats treated for 7 weeks by oral gavage at 60 mg/kg also apparently developed cataracts. Cataracts were not noted in Wistar rats treated by continuous intravenous infusion for two weeks at daily doses of 12, 24 and 48 mg/kg. Cataracts were not noted in extensive preclinical toxicology studies performed in beagle dogs and rabbits.
2.6.7.2 Reported Adverse Events and Potential Risks in Humans
Safety observations from single agent clinical studies have been combined (n=726). These consist of Phase 1, Phase 2, and Phase 3 studies, with various dosing regimens in both adults and children with various types of cancers.(Schellens et al, 2001; Punt et al, 2000; Horak et al 2000; Richards, Thibault et al, 2003; Van de Velde et al, 2003; Richards, De Porre et al, 2002 OK; Howes et al, 2003;Palmer, Kerstens et al, 2003). The most frequently reported adverse events are general symptoms (body as a whole including fatigue, fever, asthenia, pain, back pain, peripheral edema, chest pain, edema, rigors, and leg pain) or gastrointestinal symptoms. The overall incidence of these adverse events is similar between participants receiving tipifarnib compared to placebo.(Howes et al, 2003)
The most frequently reported drug related adverse events are associated with myelosuppression: granulocytopenia (23%), thrombocytopenia (23%), anemia (21%), and leukopenia (12%). Drug-related adverse events were considered by the investigator to be possibly, probably, or very likely associated with the administration of tipifarnib.(Palmer, Thibault et al, 2003) The most common signs of myelosuppression, reported as adverse events in ≥10% of treated participants, were anemia (39%), thrombocytopenia (32%), granulocytopenia (29%), and leukopenia (12%). Almost all of these events occurred at doses of 300 mg bid and above, and the majority of these events were grade 3 and 4 (NCI-CTC version 2.0).
Other major adverse events reported included fatigue, asthenia, lethargy, and malaise. Less frequently reported adverse events included photosensitivity, rash/desquamation, anorexia, diarrhea, nausea, vomiting, and elevated lipase. An adverse event more frequently occurring in AML included febrile neutropenia, more specifically fever of unknown origin without clinically or microbiologically documented infection (ANC<1.0 × 109/L, fever ≥ 38.5°C) and infection (documented clinically or microbiologically) with Grade 3 or 4 neutrophils (ANC<1.0 × 109/L).
Electrolyte abnormalities occurred rarely and included hyopkalemia, which was reported as an adverse event in 5% of participants receiving tipifarnib at 300 mg bid and 38% of participants receiving doses above 300mg bid. Investigators considered hypokalemia to be drug-related in 0.8% of cases at 300 mg bid (no Grade 3 or 4) and 10.5% of cases at higher doses (4.2% Grade 3 or 4). The majority of the latter occurred in participants with AML receiving 600 mg bid, a large proportion of whom were also receiving concomitant diuretics or amphotericin B.
The most serious non-hematolgoical toxicity reported in early studies was mood alteration, motor neuropathy, and sensory neuropathy. Neurotoxicity was observed predominantly in participants receiving continuous daily dosing of tipifarnib at 300 mg bid. or above. Neurotoxicity has been operationally defined as any incidence of paresthesia, peripheral neuropathy, hypoesthesia, and muscle weakness. This adverse event was first observed in tipifarnib-GBR-1.(Howes et al, 2003) In the first cohort, 15 of 41 participants (37%) treated continuously developed neurotoxicity, of which several were severe and long lasting. In contrast, 1 of 35 participants (2.9%) in the second cohort treated cyclically experienced neurotoxicity. In the large Phase 3 colorectal cancer study(Palmer, Thibault et al 2003), neuropathy was reported by 16.3% of participants treated with tipifarnib (1.7% were Grade 3 or 4) and 12% of participants receiving placebo (all Grade 1 or 2).
Other AEs reported on tipifarnib trials but with an unknown relationship to tipifarnib were tachycardia, palpitation, hypotension, peripheral edema, fever, rigors, weight decrease, erythematous rash, alopecia, hot flashes, dehydration, dyspepsia, constipation, flatulence, stomatitis, pharyngitis, taste perversion, dry mouth, intestinal obstruction, ileus, hyperbilirubinemia, increased SGOT (aspartate aminotransferase), sepsis, muscle weakness, hypomagnesemia, hyperglycemia, hypercalcemia, increased lactic dehydrogenase, elevated amylase, elevated lipase, paresthesia, dizziness, tremor, extrapyramidal reaction, involuntary movements, dysphonia, depression, seizures (2 cases), abnormal vision, chest pain, arthralgia, myalgia, headache, abdominal pain, pneumonia, pneumonitis, pulmonary infiltrates, dyspnea, cough, hiccoughs, increased creatnine, dysuria, urinary tract infections. Because of preclinical toxicity studies (see above), initial human trials of tipifarnib included extensive ophthalmologic evaluation for development of cataracts. None were observed.
Most of the deaths occurred at doses above 300 mg bid and were in subjects with leukemia. The majority of fatal adverse events (58/88, 66%) were related to infection (sepsis, pneumonia, infection, infection fungal and infection bacterial). Hemorrhage (intracranial, pulmonary, GI or vascular hemorrhage) accounted for 15/88 fatal events (17%). Myelosuppression (granulocytopenia, leucopenia, pancytopenia, anemia and thrombocytopenia) accounted for 12/88 fatal events (14%).
2.7 Conclusion
Tipifarnib as single agent appeared to have promising activity in AML in early phase clinical trials. Tipifarnib was granted “Fast Track” status by the FDA in 2004 and was also granted Orphan Drug status. However, in 2005 FDA did not grant approval for tipifarnib, a request based on Phase II data. Subsequently, a Phase III study did not confirm activity as a single agent for the treatment of older AML patients.
Other uses of tipifarnib are being investigated in trials supported by CTEP, including the two phase I trials discussed previously investigating the addition of tipifarnib to induction AML therapy (Karp, Hatten et al 2009; Brandwein et al, 2009). There is also interest in examining the potential benefit of tipifarnib maintenance therapy in poor-risk AML. In a phase II trial, maintenance tipifarnib at a dose of 400 mg bid for 14 or 21 doses was given for a maximum of 16 cycles (48 weeks) in poor-risk AML patients achieving first remission (Karp, Smith, et al, 2008). Compared to historically similar patients, the authors observed disease free prolongation in patients with secondary AML and adverse cytogenetics. These results suggested that tipifarnib maintenance might be beneficial for subsets of patients with poor-risk AML. Based on these results, ECOG 2902 is conducting a phase III trial of maintenance tipifarnib versus observation alone in poor-risk AML patients in remission. This study has met its accrual goal of 139 patients. Follow-up and data analyses are ongoing and results of this study are eagerly awaited.
2.8 Expert Opinion
The future of tipifarnib is uncertain at this time. It is the authors' understanding that Johnson & Johnson Pharmaceutical is no longer supporting tipifarnib as a therapeutic for AML because of the negative Phase III trial utilizing tipifarnib as a single agent. Therefore, further development of tipifarnib for MDS/ALM therapeutics appears questionable. However, utilization of tipifarnib in other settings such as maintenance therapy or in combination with induction therapy may prove to be useful. Combinations of FTI and inhibitors of cholesterol synthesis simvastatin have apoptosis promoting effects in combination against human CD34+ AML cell lines in vitro(van der Weide, et al., 2009). Although not yet tested in the clinical setting, these results suggest that the combination of tipifarnib and statins may be better than single agent in some AML patients. The future of tipifarnib lies in achieving a better understanding of the mechanism of clinical response and the use of predictive biomarkers for patient selection. The predictive power of the ratio of RASGRP1/APTX gene expression not only provides a potentially important biomarker of response, it also suggests that responsive patients have underlying pathology related to abnormalities in the DNA excision repair pathway(Raponi, Lancet et al. 2008). Validation of this predictive marker is critical for future therapeutic approaches and may generate important information that will restore interest in tipifarnib as an active agent in AML and MDS.
2.9 Drug summary box.
Drug summary box
| Drug name | tipifarnib |
| Phase | Phase 1, 11 and 111 |
| Indication | Acute Myeloid Leukemia (investigational) |
| Pharmacology description/mechanism of action | methyl-quinolinone derivative that acts as a potent and selective nonpeptidomimetic inhibitor of farnesyl tranferase protein (FTP) |
| Route of administration | oral |
| Chemical structure |

|
| Pivotal trials | See table 2 |
2.10 Annotated bibliography
- Agrawal AG, Somani RR. Farnesyltransferase inhibitor as anticancer agent. Mini Rev Med Chem. 2009;9(6):638–52. doi: 10.2174/138955709788452702. [DOI] [PubMed] [Google Scholar]; Review article that describes the development of FTIs for the treatment of cancer.
- Alsina M, Fonseca R, et al. Farnesyltransferase inhibitor tipifarnib is well tolerated, induces stabilization of disease, and inhibits farnesylation and oncogenic/tumor survival pathways in patients with advanced multiple myeloma. Blood. 2004;103(9):3271–7. doi: 10.1182/blood-2003-08-2764. [DOI] [PubMed] [Google Scholar]; Manuscript describes a phase II trial of tipifarnib for the treatment of patients with multiple myeloma (MM).
- Auewarakul CU, Lauhakirti D, et al. Frequency of RAS gene mutation and its cooperative genetic events in Southeast Asian adult acute myeloid leukemia. Eur J Haematol. 2006;77(1):51–6. doi: 10.1111/j.1600-0609.2006.00663.x. [DOI] [PubMed] [Google Scholar]; Report that describes the frequency of Ras mutations in 239 Thai de novo adult AML patients using polymerase chain reaction-single-strand conformational polymorphism analysis.
- Bacher U, Haferlach T, et al. Implications of NRAS mutations in AML: a study of 2502 patients. Blood. 2006;107(10):3847–53. doi: 10.1182/blood-2005-08-3522. [DOI] [PubMed] [Google Scholar]; In this manuscript, the authors analyzed 2502 patients with acute myeloid leukemia at diagnosis for NRAS mutations around the hot spots at codons 12, 13, and 61 and correlated the results to cytomorphology, cytogenetics, other molecular markers, and prognostic relevance of these mutations.
- Basso AD, Kirschmeier P, et al. Lipid posttranslational modifications. Farnesyl transferase inhibitors. J Lipid Res. 2006;47(1):15–31. doi: 10.1194/jlr.R500012-JLR200. [DOI] [PubMed] [Google Scholar]; Article describing the role and proteins that undergo prenylation and the role of this modication on the transforming activity of Ras.
- Bolick SC, Landowski TH, et al. The farnesyl transferase inhibitor, FTI-277, inhibits growth and induces apoptosis in drug-resistant myeloma tumor cells. Leukemia. 2003;17(2):451–7. doi: 10.1038/sj.leu.2402832. [DOI] [PubMed] [Google Scholar]; Results describe the preclinical development of FTIs for the treatment of multiple myeloma and the combination of these agents with a geranylgeranyltransferase I inhibitor (GGTI)-2166 and the topoisomerase II inhibitors.
- Brandwein JM, Leber BG, Howson-Jan K, et al. A phase 1 study of tipifarnib combined with conventional induction and consolidation therapy for previously untreated patients with Acute Myeloid Leukemia aged 60 years and over. Leukemia. 2009;23 doi: 10.1038/leu.2008.341. [DOI] [PubMed] [Google Scholar]; Rationale and scientific support for tipifarnib combination therapy in AML.
- Caraglia M, Marra M, et al. The farnesyltransferase inhibitor R115777 (ZARNESTRA) enhances the pro-apoptotic activity of interferon-alpha through the inhibition of multiple survival pathways. Int J Cancer. 2007;121(10):2317–30. doi: 10.1002/ijc.22964. [DOI] [PubMed] [Google Scholar]; Interferon alpha (IFNalpha) induces an EGF-Ras-->Raf-1-->Erk dependent survival pathway counteracting apoptosis induced by the cytokine. In this paper, the authors have evaluated the effects of the combination between farnesyl-transferase inhibitor (FTI) R115777 and IFNalpha on the growth inhibition and apoptosis of cancer cells.
- Cortes J, Albitar M, et al. Efficacy of the farnesyl transferase inhibitor R115777 in chronic myeloid leukemia and other hematologic malignancies. Blood. 2003;101(5):1692–7. doi: 10.1182/blood-2002-07-1973. [DOI] [PubMed] [Google Scholar]; The clinical activity of the farnesyl transferase inhibitor R115777 was investigated in 22 patients with chronic myelogenous leukemia (CML) in chronic, accelerated, or blastic phase and in 8 patients with myelofibrosis (MF) and 10 patients with multiple myeloma (MM).
- Crul M, de Klerk GJ, et al. Phase I clinical and pharmacologic study of chronic oral administration of the farnesyl protein transferase inhibitor R115777 in advanced cancer. J Clin Oncol. 2002;20(11):2726–35. doi: 10.1200/JCO.2002.09.116. [DOI] [PubMed] [Google Scholar]; The purpose of this study was to determine the maximum-tolerated dose, toxicities, and pharmacokinetics of R115777, a farnesyl transferase inhibitor, when administered continuously via the oral route.
- de Rooij J, Bos JL. Minimal Ras-binding domain of Raf1 can be used as an activation-specific probe for Ras. Oncogene. 1997;14(5):623–5. doi: 10.1038/sj.onc.1201005. [DOI] [PubMed] [Google Scholar]; In this manuscript, the authors show that the Gst-RBD fusion protein precipitates transfected RasL61 (RasGTP) but not RasN17 (RasGDP) from cell lysates. In addition, they demonstrate for two different cell lines that the increase in RasGTP is reflected by an increase in Ras bound to Gst-RBD. From these results we conclude that the minimal Ras-binding domain of Raf1 is an excellent activation specific-probe for Ras.
- Dickson DJ, Shami PJ. Angiogenesis in acute and chronic leukemias. Leuk Lymphoma. 2001;42(5):847–53. doi: 10.3109/10428190109097703. [DOI] [PubMed] [Google Scholar]; Describes the role of angiogenesis in the pathophysiology of leukemias.demonstrates the potential role for VEGF as an autocrine growth factor in AML has been suggested.
- Ehmann F, Horn S, et al. Detection of N-RAS and K-RAS in their active GTP-bound form in acute myeloid leukemia without activating RAS mutations. Leuk Lymphoma. 2006;47(7):1387–91. doi: 10.1080/10428190600565925. [DOI] [PubMed] [Google Scholar]; In the present study, RAS proteins were shown to be present in an activated, GTP-bound form, in AML patients (n = 10).
- End DW, Smets G, et al. Characterization of the antitumor effects of the selective farnesyl protein transferase inhibitor R115777 in vivo and in vitro. Cancer Res. 2001;61(1) [PubMed] [Google Scholar]; This work was important to establish the specificity and sensitivity of tipifarnib in vitro and in vivo in a nude mouse. This also shows the dosage range needed to inhibit different Ras family proteins.
- Erba HP, Kopecky KJ, Kirschbaum MH, et al. Phase II studies of different schedules and doses of the farnesyl transferase inhibitor tipifarnib (R115777, Zarnestra, NSC-702818) for patients of age 70 or older with previously untreated acute myeloid leukemia (AML): A north american intergroup study (SO432) Ash Annual Meeting Abstracts. 2007;110:440. [Google Scholar]; Published abstract about the results of a phase II study of tipifarnib in patients with AML.
- Faderl S, Pal A, et al. Kit inhibitor APcK110 induces apoptosis and inhibits proliferation of acute myeloid leukemia cells. Cancer Res. 2009;69(9):3910–7. doi: 10.1158/0008-5472.CAN-08-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]; Describes preclinical studies with a new inhibitor targeting Kit in AML.
- Greenberg PL, Young NS, et al. Myelodysplastic syndromes. Hematology (Am Soc Hematol Educ Program) 2002:136–61. doi: 10.1182/asheducation-2002.1.136. [DOI] [PubMed] [Google Scholar]; This article reviews the developing understanding of biologic and molecular lesions in MDS and recently available biospecific drugs that are potentially capable of abrogating these abnormalities.
- Gueven N, Becherel OJ, et al. Aprataxin, a novel protein that protects against genotoxic stress. Hum Mol Genet. 2004;13(10):1081–93. doi: 10.1093/hmg/ddh122. [DOI] [PubMed] [Google Scholar]; Ataxia-oculomotor apraxia (AOA1) is a neurological disorder with symptoms that overlap those of ataxia-telangiectasia, a syndrome characterized by abnormal responses to double-strand DNA breaks and genome instability. The gene mutated in AOA1, APTX, is predicted to code for a protein called aprataxin that contains domains of homology with proteins involved in DNA damage signalling and repair. These results demonstrate that aprataxin influences the cellular response to genotoxic stress very likely by its capacity to interact with a number of proteins involved in DNA repair.
- Harousseau JL, Lancet JE, et al. A phase 2 study of the oral farnesyltransferase inhibitor tipifarnib in patients with refractory or relapsed acute myeloid leukemia. Blood. 2007;109(12):5151–6. doi: 10.1182/blood-2006-09-046144. [DOI] [PubMed] [Google Scholar]; Description of results of a this phase 2 study evaluated the efficacy and safety of the oral farnesyltransferase inhibitor tipifarnib in adults with refractory or relapsed acute myeloid leukemia (AML).
- Harousseau JL, Martinelli G, et al. A randomized phase 3 study of tipifarnib compared with best supportive care, including hydroxyurea, in the treatment of newly diagnosed acute myeloid leukemia in patients 70 years or older. Blood. 2009;114(6):1166–73. doi: 10.1182/blood-2009-01-198093. [DOI] [PubMed] [Google Scholar]; Results of the only phase 3, multicenter, open-label study evaluated the efficacy and safety of tipifarnib compared with best supportive care (BSC), including hydroxyurea, as first-line therapy in elderly patients (>or=70 years) with newly diagnosed, de novo, or secondary acute myeloid leukemia. This trial was registered at www.clinicaltrials.gov as #NCT00093990.
- Horak I, Bowden C, Palmer P, et al. Phase I trial to determine the safety and pharmacokinetics of R115777, a farnesyltransferase inhibitor. JRF Clinical Research Report R115777-USA-1, October 2000. EDMS-USTI-2338340. 2000 [Google Scholar]; This is a pharmaceutical report describing the safety and pharmacokinetics of tipifarnib.
- Howes A, Michiels B, Zannikos P, et al. An open study of the efficacy, safety and pharmacodynamics of the farnesyl protein inhibitor, R115777, in advanced breast cancer. JRF Clinical Research Report R115777-GBR-1, September 2003, EDMS-PSDB-1675498 [Google Scholar]; This is a pharmaceutical report describing the safety and pharmacodynamics of tipifarnib.
- Karp JE, Flatten K, et al. Active oral regimen for elderly adults with newly diagnosed acute myelogenous leukemia: a preclinical and phase 1 trial of the farnesyltransferase inhibitor tipifarnib (R115777, Zarnestra) combined with etoposide. Blood. 2009;113(20):4841–52. doi: 10.1182/blood-2008-08-172726. [DOI] [PMC free article] [PubMed] [Google Scholar]; Results of preclinical and clinical efficacy of tipifarnib plus etoposide in elderly adult AML patients who are not candidates for conventional induction chemotherapy. These clinical studies are registered at www.clinicaltrials.gov as #NCT00112853.
- Karp JE, Lancet JE, et al. Clinical and biologic activity of the farnesyltransferase inhibitor R115777 in adults with refractory and relapsed acute leukemias: a phase 1 clinical-laboratory correlative trial. Blood. 2001;97(11):3361–9. doi: 10.1182/blood.v97.11.3361. [DOI] [PubMed] [Google Scholar]; Results of a phase 1 trial of orally administered R115777 in 35 adults with poor-risk acute leukemias.
- Karp JE, Smith BD, et al. Phase II trial of tipifarnib as maintenance therapy in first complete remission in adults with acute myelogenous leukemia and poor-risk features. Clin Cancer Res. 2008;14(10):3077–82. doi: 10.1158/1078-0432.CCR-07-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]; Results of a phase II trial of maintenance tipifarnib monotherapy for 48 adults with poor-risk AML in first CR.
- Kirschbaum MH, Stein AS, et al. A phase I study of farnesyltransferase inhibitor tipifarnib in a week-on week-off dose schedule in acute myelogenous leukemia. ASH Annual Meeting Abstracts. 2007;110:891. [Google Scholar]; Results of a phase I study in AML.
- Lancet JE, Karp JE. Farnesyl transferase inhibitors in myeloid malignancies. Blood Rev. 2003;17(3):123–9. doi: 10.1016/s0268-960x(03)00008-0. [DOI] [PubMed] [Google Scholar]; Review article of the use of FTIs in myeloid malignancies.
- Le Gouill S, Pellat-Deceunynck C, et al. Farnesyl transferase inhibitor R115777 induces apoptosis of human myeloma cells. Leukemia. 2002;16(9):1664–7. doi: 10.1038/sj.leu.2402629. [DOI] [PubMed] [Google Scholar]; Preclinical results of tipifarnib as an interesting therapeutical approach in multiple myeloma.
- Lerner EC, Qian Y, et al. Ras CAAX peptidomimetic FTI-277 selectively blocks oncogenic Ras signaling by inducing cytoplasmic accumulation of inactive Ras-Raf complexes. J Biol Chem. 1995;270(45):26802–6. doi: 10.1074/jbc.270.45.26802. [DOI] [PubMed] [Google Scholar]; Article describes the molecular mechanism of tipifarnib.
- Lerner EC, Zhang TT, et al. Inhibition of the prenylation of K-Ras, but not H- or N-Ras, is highly resistant to CAAX peptidomimetics and requires both a farnesyltransferase and a geranylgeranyltransferase I inhibitor in human tumor cell lines. Oncogene. 1997;15(11):1283–8. doi: 10.1038/sj.onc.1201296. [DOI] [PubMed] [Google Scholar]; Article describes the molecular mechanism of tipifarnib.
- Li N, Batzer A, et al. Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signalling. Nature. 1993;363(6424):85–8. doi: 10.1038/363085a0. [DOI] [PubMed] [Google Scholar]; Results indicate that the Grb2/hSos1 complex couples activated EGF receptor to Ras signalling.
- Ma X, Does M, et al. Myelodysplastic syndromes: incidence and survival in the United States. Cancer. 2007;109(8):1536–42. doi: 10.1002/cncr.22570. [DOI] [PubMed] [Google Scholar]; Manuscript that describes the incidence of MDS in the United States.
- Mattison RJ, Ostler KR, et al. Implications of FLT3 mutations in the therapy of acute myeloid leukemia. Rev Recent Clin Trials. 2007;2(2):135–41. doi: 10.2174/157488707780599320. [DOI] [PubMed] [Google Scholar]; Describes the role of the FMS-like tyrosine kinase 3 (FLT3), which is a type III receptor tyrosine kinase that is expressed on the surface of hematopoietic stem cells and plays an important role in normal hematopoiesis. FLT3 is mutated in approximately one-third of cases of acute myeloid leukemia (AML) with normal karyotype.
- Mesa RA, Tefferi A, et al. In vitro antiproliferative activity of the farnesyltransferase inhibitor R115777 in hematopoietic progenitors from patients with myelofibrosis with myeloid metaplasia. Leukemia. 2003;17(5):849–55. doi: 10.1038/sj.leu.2402901. [DOI] [PubMed] [Google Scholar]; Results show that multiple myeloma progenitors are sensitive to clinically achievable R115777 concentrations in vitro and provide a potential explanation for the thrombocytopenia observed with R115777 during the treatment of other hematologic malignancies.
- Mor A, Dustin ML, et al. Small GTPases and LFA-1 reciprocally modulate adhesion and signaling. Immunol Rev. 2007;218:114–25. doi: 10.1111/j.1600-065X.2007.00538.x. [DOI] [PubMed] [Google Scholar]; Review of the role of small guanosine triphosphatases (GTPases) in LFA-1 signaling.
- Neubauer A, Greenberg P, et al. Mutations in the ras proto-oncogenes in patients with myelodysplastic syndromes. Leukemia. 1994;8(4):638–41. [PubMed] [Google Scholar]; Patients with MDS were analyzed for the presence of mutations in codons 12, 13, and 61 of the N- and K-ras proto-oncogenes.
- Niimi H, Harada H, et al. Hyperactivation of the RAS signaling pathway in myelodysplastic syndrome with AML1/RUNX1 point mutations. Leukemia. 2006;20(4):635–44. doi: 10.1038/sj.leu.2404136. [DOI] [PubMed] [Google Scholar]; AML1/RUNX1 mutations have been reported frequently in myelodysplastic syndrome (MDS) patients, especially those diagnosed with refractory anemia with excess blast (RAEB), RAEB in transformation (RAEBt), or AML following MDS (these categories are defined as MDS/AML). Although AML1 mutations are suspected to play a pivotal role in the development of MDS/AML, acquisition of additional genetic alterations is also necessary. We analyzed gene alterations in MDS/AML patients with AML1 mutations, comparing them to alterations in those without an AML1 mutation. AML1 mutations were significantly associated with -7/7q-, whereas MDS/AML patients without AML1 mutations showed a high frequency of -5/5q- and a complex karyotype.
- Norman P. Tipifarnib (Janssen Pharmaceutica) Curr Opin Investig Drugs. 2002;3(2):313–9. [PubMed] [Google Scholar]; Summary of research activity published by Janssen for the development of tipifarnib (formerly known as R-1 15777), an inhibitor of RAS farnesylation, for the potential treatment of neoplasia [287030], [289610].
- Ochiai N, Uchida R, et al. Effect of farnesyl transferase inhibitor R115777 on the growth of fresh and cloned myeloma cells in vitro. Blood. 2003;102(9):3349–53. doi: 10.1182/blood-2003-03-0851. [DOI] [PubMed] [Google Scholar]; The effect of R115777 (tipifarnib) on the growth of fresh and cloned myeloma cells in vitro.
- Padua RA, Carter G, et al. RAS mutations in myelodysplasia detected by amplification, oligonucleotide hybridization, and transformation. Leukemia. 1988;2(8):503–10. [PubMed] [Google Scholar]; The authors assessed the mutational activation of H, K, and NRAS in myelodysplasia (MDS) by polymerase chain reaction and hybridization with synthetic oligonucleotide probes.
- Padua RA, West RR. Oncogene mutation and prognosis in the myelodysplastic syndromes. Br J Haematol. 2000;111(3):873–4. [PubMed] [Google Scholar]; In a series of myelodysplastic syndrome patients, mutational status (particularly RAS mutation) was found to be prognostic for survival, independently of four previously reported scoring systems (Bournemouth, Sanz, Lille, International).
- Palmer P, Thibault A, Zannikos P, et al. A randomized, double-blind, placebo-controlled Phase 3 study of chronic oral adminstration of farnesyltransferase inhibitor R11577 plus supportive care versus supportive care alone in subjects with advanced colorectal cancer, after chemotherapy failure. J&JPRD Clinical Study report R115777-INT-9 EDMS-PSDB-1814193. 2003 [Google Scholar]; This is a pharmaceutical report that describes the clinical outcome of tipifarnib treatment and adverse events in advanced colorectal cancer.
- Palmer P, Kerstens R, Zannikos P, et al. A phase 2 trial of R115777, an oral farnesyltransferase inhibitor (FTI), in subjects with advanced urothelail tract transitional cell carcinoma. J&JPRD Clinical Study Report R115777-INT-10, EDMS-PSDB-2125081. 2003 [Google Scholar]; This is a pharmaceutical report that describes the results and toxicity of tipifarnib in a phase 2 trial.
- Paquette RL, Landaw EM, et al. N-ras mutations are associated with poor prognosis and increased risk of leukemia in myelodysplastic syndrome. Blood. 1993;82(2):590–9. [PubMed] [Google Scholar]; Manuscript described the clinical significance of N-ras mutations in the myelodysplastic syndrome (MDS) archival bone marrow samples from 252 patients were studied for the presence of N-ras exon I mutations using polymerase chain reaction amplification and differential oligonucleotide hybridization.
- Patnaik A, Eckhardt SG, et al. A phase I, pharmacokinetic, and biological study of the farnesyltransferase inhibitor tipifarnib in combination with gemcitabine in patients with advanced malignancies. Clin Cancer Res. 2003;9(13):4761–71. [PubMed] [Google Scholar]; Study to assess the feasibility of administering tipifarnib, an oral nonpeptidomimetic competitive inhibitor of farnesyltransferase, in combination with gemcitabine and recommend doses for disease-directed clinical trials. The study also sought to identify drug-drug pharmacokinetic interactions, evaluate effects on protein farnesylation, and seek preliminary evidence for clinical activity.
- Pinheiro RF, de Sa Moreira E, et al. FLT3 internal tandem duplication during myelodysplastic syndrome follow-up: a marker of transformation to acute myeloid leukemia. Cancer Genet Cytogenet. 2008;183(2):89–93. doi: 10.1016/j.cancergencyto.2008.02.006. [DOI] [PubMed] [Google Scholar]; Authors studied FLT3 ITD, prospectively, in 50 MDS patients at diagnosis, at 6 and 12 months follow-up, and at any other time-point if AML transformation was detected.
- Punt C, Palmer P, Seifert W, et al. A Phase-I study to determine the safety and maximum tolerated dose of 28 days oral administration of farnesyltransferase inhibitor R115777 in subjects with advanced cancer. JRF Clinical Research Report Synopsis R115777-BEL-7 (terminated study), October 2000 EDMS-BEBE-1834493. 2000 [Google Scholar]; This is a pharmaceutical report that describes the efficacy and toxicity of tipifarnib in subjects with advanced cancer.
- Raponi M, Lancet JE, et al. A 2-gene classifier for predicting response to the farnesyltransferase inhibitor tipifarnib in acute myeloid leukemia. Blood. 2008;111(5):2589–96. doi: 10.1182/blood-2007-09-112730. [DOI] [PubMed] [Google Scholar]; Development of a method to predict response to farnesyltransferase inhibitors (FTIs) in older adults with previously untreated acute myeloid leukemia (AML). The authors made a novel observation that a 2 gene classifier of RASGRP1/APTX gene expression ratio predicted response to tipifarnib
- Recher C, Dos Santos C, et al. mTOR, a new therapeutic target in acute myeloid leukemia. Cell Cycle. 2005;4(11):1540–9. doi: 10.4161/cc.4.11.2159. [DOI] [PubMed] [Google Scholar]; The mTOR (mammalian target of rapamycin) serine threonine kinase is involved in the regulation of the cell cycle, apoptosis and angiogenesis. mTOR inhibitors (rapamycin, or analogues such as CCI-779, RAD001, AP23573), which have been shown to have a potent anti-neoplastic effect in many solid tumor models, are now being used in clinical trials. In this review, the authors discussed the possible mechanisms of mTOR activation, the mechanisms involved in the inhibition of cell proliferation by rapamycin, the possible resistance mechanisms and ways of improving rapamycin efficacy in the context of AML.
- Reuter CW, Morgan MA, et al. Targeting the Ras signaling pathway: a rational, mechanism-based treatment for hematologic malignancies? Blood. 2000;96(5):1655–69. [PubMed] [Google Scholar]; This review presents an overview on some inhibitors of the Ras signaling pathway, including their specificity and effectiveness in vivo. Because Ras signaling plays a crucial role in the pathogenesis of some hematologic malignancies, the potential therapeutic usefulness of these inhibitors is discussed.
- Richards H, De Porre P, Thibault A, et al. A phase 2 trial to determine the antitumor activity of farnesyltransferase inhibitor R115777 in subjects with relapsed small cell lung cancer. JRF Clinical Research Report R115777-USA-8, EDMS-PSDB-1508187. 2002 [Google Scholar]; This is a pharmaceutical report that describes the efficacy and toxicity of tipifarnib in patients with relapsed small cell lung cancer.
- Richards H, Thibault A, Jia X, et al. A Phase I trial to determine the safety and pharmacokinetics of 21-day dosing of a farnseyltransferase inhibitor, R115777 (ZARNESTRA™) JRF Clinical Research Report R115777-USA-3, July 2001. EDMS-USTI-2474164. 2001 [Google Scholar]; This is a pharmaceutical report that describes the safety and pharmacokinetics of tipifarnib using a 21-day dosing schedule.
- Rollison DE, Howlader N, et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001–2004, using data from the NAACCR and SEER programs. Blood. 2008;112(1):45–52. doi: 10.1182/blood-2008-01-134858. [DOI] [PubMed] [Google Scholar]; Manuscript describing the MDS incidence rates from 2001 through 2004, in the United States.
- Saglio G, Serra A, et al. N-ras mutations in myeloid leukemias. Tumori. 1989;75(4):337–40. doi: 10.1177/030089168907500407. [DOI] [PubMed] [Google Scholar]; Mutations were found in five out of twenty (25%) untreated AML cases at onset. No mutations were detected in the complete remission samples, two of them with N-ras mutations during the leukemic phase. Two out of the four leukemia relapses were positive for the same N-ras mutation shown at presentation, whereas no new mutations were found in the other two initially negative cases. These data suggest that a partial overlapping between initiation and progression factors could exist in naturally occurring tumors.
- Santucci R, Mackley PA, et al. Farnesyltransferase inhibitors and their role in the treatment of multiple myeloma. Cancer Control. 2003;10(5):384–7. doi: 10.1177/107327480301000505. [DOI] [PubMed] [Google Scholar]; Review of therapies targeting Ras farnesylation, which may be a valuable approach to treatment of Multiple myeloma.
- Schellens J, Palmer P, Selfert W, et al. A phase 1 study to determine the safety and MTD of chronic oral administation of farnesyltransferase inhibitor R115777 in patients with advanced cancer. JRF Clinical REsearch Report R115777-BEL-,EDMS-BEBE-2341152. 2001 [Google Scholar]; This is a pharmaceutical report that describes the safety and maximum tolerated dose of tipifarnib in patients with advanced cancer..
- Sebti SM, Hamilton AD. Rational design of Ras prenyltransferase inhibitors as potential anticancer drugs. Biochem Soc Trans. 1996;24(3):692–9. doi: 10.1042/bst0240692. [DOI] [PubMed] [Google Scholar]; Article highlighting the chemistry and rationale design of farnesyltransferase inhibitors.
- Tamanoi F. Inhibitors of Ras farnesyltransferases. Trends Biochem Sci. 1993;18(9):349–53. doi: 10.1016/0968-0004(93)90072-u. [DOI] [PubMed] [Google Scholar]; FTase inhibitors may be useful in blocking the action of Ras proteins, in further characterizing protein prenyltransferases, and in elucidating the regulation of cholesterol metabolism.
- Van de Velde H, Thibault A, Hoffman K, et al. A pilot phase 2 study of farnesyltransferase inhibitor R115777 in subjects with superficial bladder cancer. JRF Clinical Research rEport R115777-USA-7. EDMS-PSDB-1500663. 2003 [Google Scholar]; This is a pharmaceutical report that describes the safety and efficacy of tipifarnib in a phase 2 study in bladder cancer.
- Van der Weide K, Jonge-Peeters S, Kuipers K, et al. Combining Simvastiatin with the farnesyltransferase inhibitor tipifarnib results in an enhanced cytotoxic effect in a subset of primary CD34+ acute myeloid leukemia samples. Clin Cancer Res. 2009;15(9):3078–83. doi: 10.1158/1078-0432.CCR-08-3004. [DOI] [PubMed] [Google Scholar]; In this manuscript, the authors show that the inhibitory effects of the cholesterol synthesis inhibitor simvastatin promotes the anti-leukemia activity of farnesyltransferase inhibitor tipifarnib in human CD34+ acute myeloid leukemia (AML) cells.
- Xie Y, Davies SM, et al. Trends in leukemia incidence and survival in the United States (1973–1998) Cancer. 2003;97(9):2229–35. doi: 10.1002/cncr.11316. [DOI] [PubMed] [Google Scholar]; Leukemia incidence (including acute lymphoblastic leukemia [ALL], acute myeloid leukemia [AML], chronic myeloid leukemia [CML], and chronic lymphoblastic leukemia [CLL]) and 5-year survival rates were obtained from the Surveillance, Epidemiology, and End Results (SEER) program.