Abstract
WAVE1 (the Wiskott-Aldrich syndrome protein (WASP)-family verprolin homologous protein 1) is a key regulator of Arp (actin-related protein) 2/3 complex-mediated actin polymerization. We have established previously that the state of phosphorylation of WAVE1 at three distinct residues controls its ability to regulate actin polymerization and spine morphology. Cyclin-dependent kinase 5 (Cdk5) phosphorylates WAVE1 at Ser310, Ser397 and Ser441 to a high basal stoichiometry, resulting in inhibition of WAVE1 activity. Our previous and current studies show that WAVE1 can be dephosphorylated at all three sites and thereby activated upon stimulation of the D1 subclass of dopamine receptors and of the NMDA subclass of glutamate receptors, acting through cAMP and Ca2+ signaling pathways, respectively. Specifically we have identified PP-2A and PP-2B as the effectors for these second messengers. These phosphatases act on different sites to mediate receptor-induced signaling pathways, which would lead to activation of WAVE1.
Keywords: WAVE1, Cdk5, PP-2A, PP-2B, dopamine, NMDA
INTRODUCTION
Actin plays critical roles in various membrane-cytoskeleton-coupled processes such as cell movement, vesicular trafficking events including endocytosis and exocytosis, cytokinesis and intracellular movement of pathogens (Rottner et al. 2004; Lanzetti 2007; Heasman and Ridley 2008). In neurons, reorganization of the actin cytoskeleton is required for neurite extension, axonal guidance, cycling of neurotransmitter vesicles, dendritic spine formation and synaptic plasticity (Luo 2002; Dillon and Goda 2005). Abnormal regulation of the actin cytoskeleton is associated with mental retardation (Newey et al. 2005) and cognitive deficits (Frangiskakis et al. 1996) as well as with neurodegenerative diseases (Minamide et al. 2000; Zhao et al. 2006).
Reorganization of the actin cytoskeleton is tightly controlled by various regulatory proteins that govern uncapping, severing and filament formation (Pollard et al. 2000). The WASP family proteins use their C-terminal VCA domain to stimulate the Arp2/3 complex to nucleate the de novo synthesis and branching of actin filaments (Takenawa and Suetsugu 2007). WAVE1, a member of the WASP family, is abundant in brain, where its highest levels are found in cerebral cortex, hippocampus, amygdala and striatum (Dahl et al. 2003; Soderling et al. 2003). WAVE1 is critical for the development and function of the central nervous system (CNS). WAVE1-null mice show CNS-related problems such as limb weakness, neuroanatomical malformations and behavioral abnormalities including reduced anxiety, sensorimotor retardation, and deficits in hippocampal-dependent learning and memory (Dahl et al. 2003; Soderling et al. 2003). Homozygote WAVE1 knockout mice also exhibit reduced body size and reduced viability.
In neurons, WAVE1 is localized to dendrites and dendritic spines (Pilpel and Segal 2005; Kim et al. 2006b; Soderling et al. 2007; Sung et al. 2008). WAVE1 is also localized to axonal growth cones (Nozumi et al. 2003; Soderling et al. 2007) as well as to mitochondria (Cheng et al. 2007; Sung et al. 2008). As a result, WAVE1 plays critical roles in growth cone dynamics, neurite outgrowth, dendritic spine morphogenesis and synaptic plasticity (Kim et al. 2006b; Soderling et al. 2007). WAVE1 also mediates neuronal activity-induced mitochondrial trafficking to dendritic spines and spine morphogenesis (Sung et al. 2008). Furthermore WAVE1 is localized to oligodendrocytes and plays a role in CNS myelination (Kim et al. 2006a).
Previously, we have identified WAVE1 as a novel target of p35/Cdk5 (Kim et al. 2006b). WAVE1 is phosphorylated at multiple sites by Cdk5 in vitro and in intact mouse neurons. Phosphorylation of WAVE1 by Cdk5 inhibits its ability to regulate Arp2/3 complex-dependent actin polymerization. In brain, WAVE1 is basally phosphorylated with high stoichiometry but the level of phosphorylation is reduced by stimulation of striatal slices with a dopamine D1 agonist or with forskolin, a stimulator of adenylyl cyclase, both of which elevate cAMP levels. Thus WAVE1 is largely in an inactive form under basal conditions, but can be activated by neurotransmitters such as dopamine, that increase the levels of cAMP. Previously we also observed a critical role for NMDA receptor-dependent signaling in WAVE1 dephosphorylation following repetitive depolarization of primary cortical neurons (Sung et al. 2008). Thus, phosphorylation and dephosphorylation of WAVE1 are both likely to be important mechanisms involved in the regulation of actin polymerization and, in turn, of neuronal function.
The molecular mechanisms that mediate neuronal stimulation-induced WAVE1 dephosphorylation have not been investigated. The cAMP-mediated reduction in phosphorylation of WAVE1 could be caused by inhibition of kinases or stimulation of phosphatases. However, activation of the cAMP pathway had no effect on Cdk5 activity (Kim et al. 2006b) suggesting the involvement of protein phosphatases in cAMP-mediated WAVE1 dephosphorylation. In the present study, we have investigated the effect of specific inhibitors of protein phosphatases on cAMP or NMDA receptor-induced WAVE1 dephosphorylation. We have also analyzed the phosphorylation of WAVE1 in DARPP-32 (Dopamine and adenosine 3',5'-monophosphate-regulated phosphoprotein, 32 kilodaltons) knockout mice (Fienberg et al. 1998) and in RCS (regulator of calmodulin signaling ) knockout mice (Rakhilin et al. 2004), to evaluate the role of protein phosphatase 1 (PP-1) and PP-2B, respectively, in the dephosphorylation of WAVE1. The results obtained indicate that both PP-2A and PP-2B are major WAVE1 phosphatases, which differentially mediate receptor-mediated dephosphorylation of the various sites in WAVE1.
EXPERIMENTAL PROCEDURES
Preparation of striatal slices
Male C57BL/6 mouse (6–8 weeks old) brains were quickly removed following rapid decapitation and placed in ice-cold, oxygenated Krebs-HCO3− buffer (124 mM NaCl, 4 mM KCl, 26 mM NaHCO3, 1.5 mM CaCl2, 1.25 mM KH2PO4, 1.5 mM MgSO4 and 10 mM d-glucose, pH 7.4). Coronal slices (350 µm) were prepared using a vibrating blade microtome, VT1000S, (Leica Microsystems, Nussloch, Germany). Striata were dissected from the slices in ice-cold Krebs-HCO3− buffer. Each slice was placed in a polypropylene incubation tube with 2 mL of fresh Krebs-HCO3− buffer. The slices were pre-incubated at 30°C under constant oxygenation with 95% O2/5% CO2 for 60 min. The buffer was replaced with fresh Krebs-HCO3− buffer after 30 min of pre-incubation. Slices were treated with either forskolin (1 µM) for 30 min or NMDA (100 µM) for 10 min in the absence or presence of various drugs as specified in each experiment. DMSO (2 µL/2 mL) was used as a vehicle for forskolin, okadaic acid, cyclosporin A and tautomycetin. After drug treatment, slices were transferred to micro centrifuge tubes, frozen on dry ice, and stored at −80°C until assayed.
Chemicals and reagents
Drugs were obtained from the following sources: okadaic acid (OKA) and NMDA from Sigma (St Louis, MO, USA); cyclosporin A (CyA) from Tocris (Ellisville, MO, USA); forskolin from Alexis Biochemicals (Lausen, Switzerland), and tautomycetin from Calbiochem (San Diego, CA, USA).
Immunoblotting
Frozen tissue samples were sonicated in boiling 1% SDS and boiled for an additional 10 min. Small aliquots of the homogenate were retained for protein determination by the BCA protein assay method (Pierce, Rockford, IL) using bovine serum albumin as a standard. Equal amounts of protein (10 µg) were separated by SDS-PAGE (4–20% polyacrylamide gels) and transferred to nitrocellulose membranes. The membranes were immunoblotted using anti-Cdk5 (C-8, 1:2,000, Santa Cruz), anti-p35 (C-19, 1:2,000, Santa Cruz), anti-WAVE1 (C-terminus, 1:10,000) antibodies and phosphorylation state-specific antibodies for phospho-Ser310 (1:10,000), -Ser397 (1:5,000) or -Ser441 (1:1,000) (Kim et al. 2006b). Antibody binding was detected using the enhanced chemiluminescence (ECL) immunoblotting detection system or the Odyssey infrared imaging system (LI-COR, Lincoln, NE, USA). For the ECL immunoblotting detection system, membranes were incubated with horseradish peroxidase-linked goat anti-rabbit IgG antibody (1:10000) (Pierce, Rockford, IL, USA). Chemiluminescence was detected by autoradiography using Kodak autoradiography film, and phospho-WAVE1 bands were quantified by densitometry, using NIH Image 1.63 software. For the Odyssey infrared imaging system, membranes were incubated with an IRDYE 800CW-conjugated goat anti-rabbit IgG antibody (1:7500) (LI-COR, Lincoln, NE, USA). Fluorescence at infrared wavelengths was detected by the Odyssey Imager, and quantified using Odyssey software (LI-COR).
Data Analysis
The data are expressed as means ± standard error (SE) of the means and were analyzed using statistical methods as described in the figure legends.
RESULTS
Forskolin-induced WAVE1 dephosphorylation
Our previous studies showed that, under basal conditions, WAVE1 in mouse striatal slices was highly phosphorylated at Ser310, Ser397 and Ser441 by Cdk5 (Kim et al. 2006b). Treatment of slices with a dopamine D1 receptor agonist or with forskolin for 30 min induced dephosphorylation of WAVE1, indicating a role for cAMP signaling in the dephosphorylation of WAVE1 (Kim et al. 2006b). Stimulation of striatal slices with a dopamine D2 receptor agonist did not induce dephosphorylation (not shown). In contrast, there was a trend for a slight increase in phosphorylation at Ser310 and Ser397 (-fold change after the treatment with 1 µM quinpirole for 30 min; Ser310, 1.19±0.12; Ser397, 1.29±0.27; Ser441, 1.07±0.18; n=5, P>0.05 for all sites compared to control). These results indicate the specific role of dopamine D1 receptor signaling in the dephosphorylation of WAVE1.
We examined the time-course of the effect of forskolin on WAVE1 dephosphorylation in mouse striatal slices. Stimulation of slices with forskolin (1 µM) significantly decreased WAVE1 phosphorylation at Ser310 and Ser397 after 15 min of incubation and this dephosphorylation was sustained for at least 30 min (Fig 1D,E). Forskolin treatment also resulted in dephosphorylation at Ser441 but this was not observed until 30 min of incubation (Fig.1F). No difference in total levels of WAVE1 was found (Fig.1C). Moreover, the levels of p35 and CdK5 were not altered by forskolin stimulation (Fig. 1A,B), which is consistent with our previous observation that Cdk5 activity was unaffected by treatment with forskolin (Kim et al. 2006b). These results suggest that the decrease in WAVE1 phosphorylation does not stem from a down-regulation of Cdk5/p35. Instead, protein phosphatases are likely to mediate the cAMP-dependent dephosphorylation of WAVE1. This hypothesis is supported by the relatively slower onset of the effect of forskolin on WAVE1 dephosphorylation compared to those on cAMP-dependent phosphorylation of various substrates, such as Thr34 of DARPP-32 in striatal slices. Therefore, we investigated the possible involvement of three major serine/threonine protein phosphatases in brain (PP-1, PP-2A and PP-2B) in the cAMP-induced dephosphorylation of WAVE1.
Figure 1. Time course of forskolin-induced WAVE1 dephosphorylation.
Mouse striatal slices were incubated without (DMSO vehicle) or with forskolin (Forsk, 1 µM) for the indicated times. The levels of total p35 (A), Cdk5 (B), and WAVE1 (C) and the phosphorylation of WAVE1 at Ser310 (D), Ser397 (E), and Ser441 (F) were measured by immunoblotting (upper) and the results were quantified by densitometry (lower). The data were normalized to the values obtained from control (DMSO) treated slices at each time point. Data represent means ± SEM for four experiments. *, P< 0.05, **, P< 0.01; ***, P< 0.001 versus the value at 0 min; one-way ANOVA with Tukey’s multiple comparison test.
Role of PP-2A in forskolin-induced WAVE1 dephosphorylation
Okadaic acid (OKA) is a preferential inhibitor of PP-2A but can inhibit PP-1 at higher concentrations. Our previous studies in striatal slices indicated that 1 µM OKA potently inhibited the activity of PP-2A, while this concentration of OKA reduced PP-1 activity only by ~33% (Nishi et al. 1999). In order to determine the appropriate concentration of okadaic acid for experiments designed to evaluate the role of PP-2A in forskolin-induced WAVE1 dephosphorylation, mouse striatal slices were incubated with various concentrations of OKA for 60 min, and the levels of phosphorylation of the different sites in WAVE1 were measured (Fig. 2A). OKA increased the levels of WAVE1 phosphorylation at Ser310 and Ser441 in a dose-dependent manner with a maximal effect at 0.5–1 µM but had no effect on phosphorylation at Ser397 (Fig. 2A). Our previous studies showed that the basal stoichiometry of Ser397 in striatum is relatively high (~0.85) compared to the stoichiometries of Ser310 (~0.58) and Ser441 (~0.27) (Kim et al. 2006b). It seems likely that we did not see any increase in the phosphorylation at Ser397 in response to incubation with OKA because phosphorylation of this site was close to maximal under basal conditions.
Figure 2. The role of PP-2A in forskolin-induced dephosphorylation of WAVE1.
A, Striatal slices were incubated in the absence (DMSO vehicle) or presence of the indicated concentrations of okadaic acid (OKA) for 1 hr. The levels of phospho-WAVE1 (upper) and total WAVE1 (not shown) were measured by immunoblotting. Treatment of the slices with different concentration of OKA did not significantly alter the level of total WAVE1. The results were quantified by densitometry, and the phospho-site data normalized to total WAVE1 (lower). Data were also normalized to the values obtained with DMSO (1 hr) treated slices. Data represent means ± SEM for four experiments. *, P< 0.05, ***, P< 0.001 versus the value at 0 (DMSO only); one-way ANOVA with Tukey’s multiple comparison test. B–D, Striatal slices were pretreated with DMSO or okadaic acid (OKA, 1 µM) for 90 min, during the last 30 min of which forskolin (Forsk, 1 µM) was added, as indicated. The levels of the phosphorylation of WAVE1 (upper) at Ser310 (B), Ser397 (C) and Ser441 (D), and total WAVE1 (not shown) were measured by immunoblotting. The representative immunoblot images correspond to the four groups of the treatment as indicated. The indicated treatments did not significantly alter the level of total WAVE1. Results were quantified by densitometry and the phospho-site data normalized to total WAVE1 (lower). Data, normalized to control (Cont) values, represent means ± SEM for at least six experiments. **, P< 0.01; ***, P< 0.001 versus control; †, P< 0.05 versus forskolin alone; ‡, P< 0.05 versus OKA alone; one-way ANOVA with Tukey’s multiple comparison test.
We next examined the role of PP-2A in mediating the effect of forskolin on WAVE1 dephosphorylation. Pretreatment with OKA (1 µM) elevated the basal level of phosphorylation at Ser310 and Ser441 (1.7 ± 0.2 and 1.6 ± 0.29 fold compared to control, respectively), but not at Ser397 (Fig. 2B–D). Pretreatment with OKA completely blocked forskolin-induced WAVE1 dephosphorylation at Ser310 and Ser397. However, forskolin treatment still resulted in a significant reduction in phosphorylation of Ser441 when compared to treatment with OKA alone. Thus, PP-2A likely mediates cAMP-induced dephosphorylation at Ser310 and Ser397. Although PP-2A regulates the basal level of phosphorylation at Ser441, its contribution to cAMP-induced dephosphorylation at this site appears negligible.
Role of PP-1 in forskolin-induced WAVE1 dephosphorylation
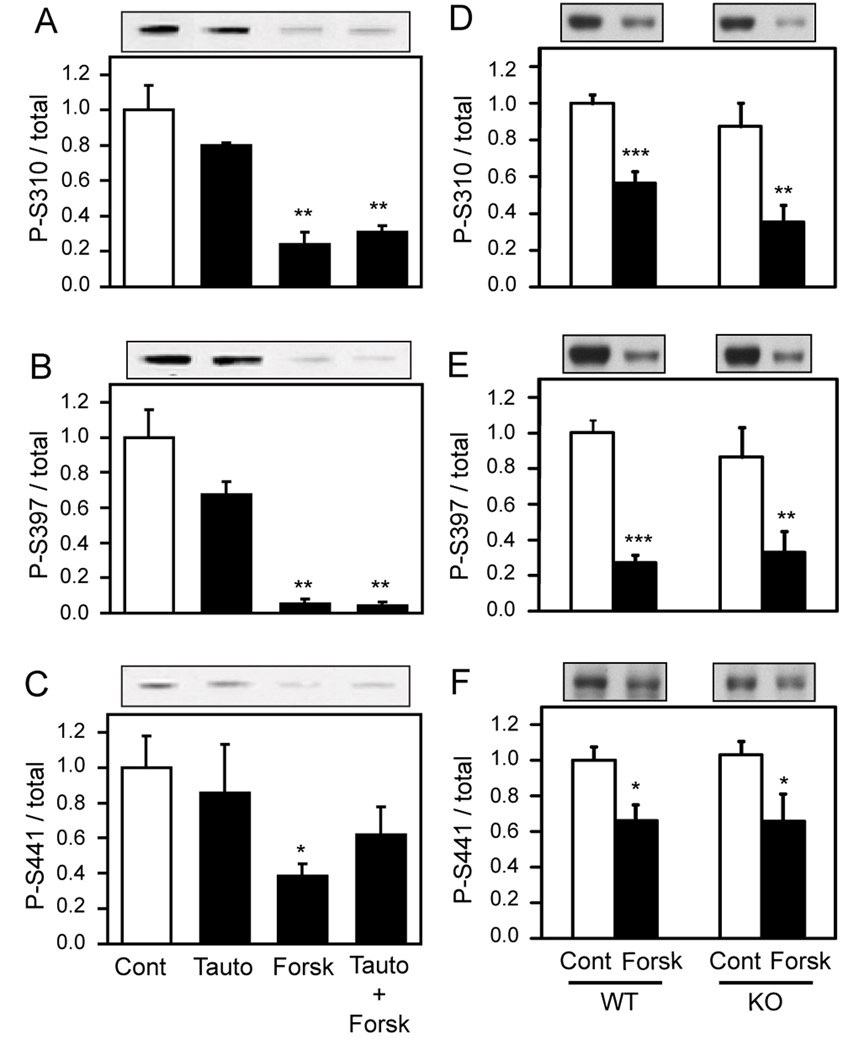
To investigate whether PP-1 is at all involved in WAVE1 dephosphorylation, striatal slices were incubated with tautomycetin, which inhibits PP-1 more potently than PP-2A (Mitsuhashi et al. 2001; Mitsuhashi et al. 2003). Treatment of slices with tautomycetin (5 µM) alone for 60 min did not affect the basal level of WAVE1 phosphorylation. Tautomycetin did not affect forskolin-induced WAVE1 dephosphorylation either at Ser310 or Ser397 (Fig. 3A,B). With respect to Ser441, tautomycetin appeared to have a small inhibitory effect on forskolin-induced dephosphorylation at this site but this did not reach significance (Fig. 3C). We also studied WAVE1 phosphorylation in slices from mice in which DARPP-32, a well-characterized inhibitor of PP-1 that is enriched in striatum, was knocked out (Hemmings et al. 1984; Greengard et al. 1999). Previously the level of phosphorylation of PP-1 substrates was shown to decrease in DARPP-32 knockout mice, due to reduced inhibition of PP-1 (Fienberg et al. 1998; Snyder et al. 1998). Forskolin-induced dephosphorylation was not altered at any of the three sites in slices from DARPP-32 knockout mice compared to their wild-type controls (Fig. 3D–F). Together, these results suggest that PP-1 does not play a major role in WAVE1 dephosphorylation.
Figure 3. The role of PP-1 in forskolin-induced dephosphorylation of WAVE1.
A–C, Striatal slices were pretreated with DMSO or tautomycetin (Tauto, 1 µM) for 90 min, during the last 30 min of which forskolin (Forsk, 1 µM) was added, as indicated. The levels of phospho-Ser310 (A), phospho-Ser397 (B) or phospho-Ser441 (C) of WAVE1 (upper) and total WAVE1 (not shown) were measured by immunoblotting. The representative immunoblot images correspond to the four groups of the treatment as indicated. The indicated treatments did not significantly alter the level of total WAVE1. The data were analyzed using the Odyssey infrared imaging system and the phospho-site data was normalized to total WAVE1 (lower). Data, normalized to control (Cont) values, represent means ± SEM for four experiments; one-way ANOVA with Tukey’s multiple comparison test. D–F, Striatal slices prepared from wild-type (WT) or DARPP-32 knockout mice (KO) were incubated in the presence of DMSO (Cont) or forskolin (Forsk, 1 µM) for 30 min. The representative immunoblot images for WT or DARPP-32 KO slices are shown in upper panels and quantification is shown in the lower panels. Data, normalized to control values for WT, represent means ± SEM for six experiments. *, P< 0.05; **, P< 0.01; ***, P< 0.001 versus control, Student’s t test.
Role of PP-2B in forskolin-induced WAVE1 dephosphorylation
To examine the role of PP-2B in forskolin-induced WAVE1 dephosphorylation, slices were incubated with a specific inhibitor of PP-2B, cyclosporin A (CyA, 10 µM) for 60 min in the absence or presence of forskolin. Pretreatment with cyclosporin A did not affect the basal level of WAVE1 phosphorylation at any of the three sites but was able to block forskolin-induced dephosphorylation at Ser441 (Fig. 4C). Forskolin-induced dephosphorylation at Ser310 and Ser397 was not affected by the pretreatment with CyA (Fig. 4A,B). We also examined the phosphorylation of WAVE1 in RCS (regulator of calcium/calmodulin-dependent signaling) knockout mice to further address the role of PP-2B. Like DARPP-32, RCS is enriched in striatum where G protein-coupled receptor (GPCR)-dependent activation of protein kinase A (PKA) leads to phosphorylation of RCS at Ser55 and increases its binding to calmodulin (CaM) (Rakhilin et al. 2004). Phospho-RCS can then act as a competitive inhibitor of CaM-dependent enzymes, including PP2B. Increasing RCS phosphorylation was found to block GPCR- and PP2B-mediated suppression of L-type Ca2+ currents in striatal neurons (Rakhilin et al. 2004). Conversely, genetic deletion of RCS significantly increased this modulation. We found no difference in forskolin-induced WAVE1 dephosphorylation at Ser310 in striatal slices from wild-type and RCS knockout mice (Fig. 4D). We observed a small, but not statistically significant, increase in forskolin-induced dephosphorylation at Ser397 and Ser441 in slices from RCS knockout mice compared to their wild-type controls (Fig. 4E,F).
Figure 4. The role of PP-2B in forskolin-induced dephosphorylation of WAVE1.
A–C, Striatal slices were pretreated with DMSO or cyclosporin A (CyA, 10 µM) for 90 min, during the last 30 min of which forskolin (Forsk, 1 µM) was added, as indicated.. The levels of phospho-WAVE1 (upper) and total WAVE1 (not shown) were measured by immunoblotting. The representative immunoblot images correspond to the four groups of the treatment as indicated. The indicated treatments did not significantly alter the level of total WAVE1. The data were quantified by densitometry and the phospho-site data were normalized to total WAVE1. Data, normalized to control values, represent means ± SEM for eight experiments. *, P< 0.05; ***, P< 0.001 versus control, one-way ANOVA with Tukey’s multiple comparison test. D–F, Striatal slices prepared from WT or RCS KO mice were incubated in the presence of DMSO (Cont) or forskolin (Forsk, 1 µM) for 30 min. The representative immunoblot images for WT or RCS KO slices are shown in upper panels and quantification is shown in the lower panels. Data, normalized to control values, represent means ± SEM for nine experiments. *, P< 0.05; ***, P< 0.001 versus control, Student’s t test.
Role of PP-2A and PP-2B in NMDA-induced WAVE1 dephosphorylation
We next examined the role of striatal NMDA receptor signaling on WAVE1 dephosphorylation in mouse striatal slices. Stimulation with NMDA (100 µM) significantly decreased the level of phosphorylation at all three WAVE1 sites within 5 min, and the dephosphorylation was sustained for at least 30 min (Fig. 5D–F). NMDA treatment in striatal slices did not result in the down-regulation of p35 or CdK5 (Fig. 5A,B) and the level of total WAVE1 remained unaltered (Fig. 5C). These results indicate that protein phosphatases are likely to be involved in NMDA-induced dephosphorylation of WAVE1, as in the case with cAMP-mediated WAVE1 dephosphorylation.
Figure 5. Time course of NMDA-induced WAVE1 dephosphorylation.
Striatal slices were incubated with NMDA (100 µM) for the indicated times. Levels of total p35 (A), Cdk5 (B), and WAVE1 (C) and the phosphorylation of WAVE1 at Ser310 (D), Ser397 (E), and Ser441 (F) were measured by immunoblotting (upper) and results quantified by the Odyssey infrared imaging system (lower). Quantitative results were normalized to the values obtained from slices at 0 min. Data represent means ± SEM for experiments. **, P< 0.01; ***, P< 0.001 compared with 0 min, one-way ANOVA with Tukey’s multiple comparison test.
To examine the mechanism by which NMDA reduced the level of WAVE1 phosphorylation, NMDA-induced WAVE1 dephosphorylation was measured in the presence or absence of OKA or CyA (Fig. 6). Pretreatment of striatal slices with OKA (1 µM) for 60 min blocked the effect of NMDA on WAVE1 dephosphorylation at Ser310 and Ser441, but not at Ser397 (Fig. 6A–C). Pretreatment of slices with CyA (10 µM) for 60 min significantly blocked the effect of NMDA on WAVE1 dephosphorylation only at Ser397 (Fig. 6D–F). Tautomycetin did not affect NMDA-induced WAVE1 dephosphorylation at any of the three sites (data not shown). These results suggest that NMDA-induced WAVE1 dephosphorylation at Ser310 and Ser441 is mediated by PP-2A, whereas WAVE1 dephosphorylation at Ser397 is mediated by PP-2B.
Figure 6. The role of PP-2A and PP-2B in NMDA-induced dephosphorylation of WAVE1.
Striatal slices were preincubated either with (A–C) okadaic acid (OKA, 1 µM) or (D–F) cyclosporin A (CyA, 10 µM) for 40 min and then incubated with NMDA (100 µM) for an additional 10 min. Control slices were pretreated with DMSO for 40 min and then incubated in the presence of vehicle (water) for 10 min (Cont).The levels of phospho-WAVE1 (upper) and total WAVE1 (not shown) were measured by immunoblotting. The representative immunoblot images correspond to the four groups of the treatment as indicated. The indicated treatments did not significantly alter the level of total WAVE1. The data were analyzed using the Odyssey infrared imaging system, and phospho-site data were normalized to total WAVE1 (lower). Data, normalized to control values, represent means ± SEM for five to six experiments. **, P< 0.01, ***, P< 0.001 versus control; one-way ANOVA with Tukey’s multiple comparison test.
DISCUSSION
The function of WAVE1 in actin polymerization and dendritic spine formation is largely suppressed by phosphorylation (Kim et al. 2006b). Under basal conditions, WAVE1 is phosphorylated by Cdk5 to a high stoichiometry (~60 to 90% depending on the site of phosphorylation). Our current studies have revealed a complex pattern of regulation of WAVE1 in response to activation of PP-2A and PP-2B acting at the three Cdk5 sites of WAVE1 (Fig. 7). We find that protein dephosphorylation is mediated by both PP-2A and PP-2B acting at the three Cdk5 sites of WAVE1, which would lead to WAVE1 activation. In brain, neurotransmitters trigger the rearrangement of actin filaments presumably through receptor-mediated signal transduction mechanisms involving WASP/WAVE family proteins. This actin rearrangement plays pivotal roles in axonal and dendritic development, synapse formation and plasticity. The current studies have thus identified novel neurotransmitter-mediated signaling pathways that are linked to activation of WAVE1.
Figure 7. The role of PP-2A and PP-2B in neurotransmitter-induced dephosphorylation of WAVE1.
Cdk5 phosphorylates WAVE1 at Ser310, Ser397 and Ser441 with high stoichiometry under basal conditions (blue arrows). Stimulation of dopamine D1 receptors induces cAMP/PKA signaling. PP-2A mediates dephosphorylation at Ser310 and Ser397, while PP-2B mediates dephosphorylation at Ser441. Stimulation of NMDA receptors increases intracellular calcium levels. Calcium-dependent PP-2A mediates dephosphorylation at Ser310 and Ser441, while PP-2B mediates dephosphorylation at Ser397.
In striatal slices, dopamine acting upon D1 receptors increases cAMP level through activation of receptor-coupled Gsα and adenylyl cyclase. In the current studies we utilized forskolin to directly activate adenylyl cyclase to increase cAMP signaling. Forskolin led to the dephosphorylation of Ser310, Ser397 and Ser441, with PP-2A being involved in dephosphorylation of Ser310 and Ser397, while PP-2B is involved in dephosphorylation of only Ser441 (Fig. 7). PP-2A is composed of catalytic C subunit, scaffolding A subunit and regulatory B subunit (Virshup and Shenolikar 2009). There are at least 15 different B subunit isoforms that are generated from four B subunit gene families, and there are many multiple splicing variants. The B subunits are critical for controlling localization, substrate specificity and catalytic activity of the C subunit. Our previous studies have shown that one particular B subunit, B56δ, was enriched in striatum, could be phosphorylated and activated by PKA, and that the PP-2A heterotrimeric complex containing B56δ was responsible for dopamine-mediated dephosphorylation of DARPP-32 at Thr75 (Ahn et al. 2007a). Given the similar features of phosphorylation and dephosphorylation between WAVE1 (Kim et al. 2006b) and DARPP-32 at Thr75 (Bibb et al. 1999; Nishi et al. 2000), it seems likely that PP2A containing the B56δ subunit may mediate cAMP-induced WAVE1 dephosphorylation. The discovery that cAMP could lead to increased dephosphorylation of DARPP-32 was somewhat unexpected, but the results from the present study support the idea that cAMP-mediated dephosphorylation may be a common process that affects multiple proteins in neurons.
cAMP signaling also leads to activation of PP-2B, a calcium-dependent protein phosphatase, and this enzyme is involved in the dephosphorylation of WAVE1 at Ser441. The precise mechanism involved in regulation of PP-2B is not known. However, previous studies have found in various cell types that L-type calcium channels can be activated via phosphorylation by PKA. Moreover, our previous studies have shown that dopamine can activate L-type calcium channels through D1 receptor-mediated regulation of PKA (Surmeier et al. 1995). Thus an increase in intracellular calcium through L-type calcium channels may be the mechanism involved in the PP-2B-mediated dephosphorylation at Ser441.
We have also found that activation of NMDA receptors leads to dephosphorylation of WAVE1, that this also involves PP-2A and PP-2B, but that the pattern of dephosphorylation is distinct from that of cAMP-mediated dephosphorylation (Fig. 7). NMDA receptor-induced increases in intracellular calcium would be expected to lead to activation of PP-2B. Notably, however, only phospho-Ser397 was dephosphorylated by PP-2B, while NMDA-induced dephosphorylation of Ser310 and Ser441 was mediated by PP-2A. In another previous study, we have also found that the B”/PR72 PP2A subunit is enriched in striatum (Ahn et al. 2007b). B”/PR72 has EF-hand motifs, binds to calcium, and was shown to be involved in NMDA/AMPA-induced dephosphorylation of Thr75 of DARPP-32 (Ahn et al. 2007b). Thus, PP-2A containing B”/PR72 may mediate NMDA-induced dephosphorylation of WAVE1 at Ser310 and Ser441.
Our previous studies have found in primary cultured cortical and hippocampal neurons that repetitive depolarization leads to dephosphorylation of WAVE1 at Cdk5 sites (Sung et al. 2008). In these studies in cultured neurons, the dephosphorylation of WAVE1 was associated with down-regulation of p35, the regulatory subunit of Cdk5. Co-incubation with the NMDA receptor antagonist 2-amino-5-phosphonovaleric acid (APV) blocked p35 down-regulation and the decrease in WAVE1 phosphorylation, but co-incubation with an AMPA/kainate receptor antagonist, 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) had no effect. The reason for the differences observed in the mechanisms of reduced phosphorylation of WAVE1 in cultured neurons compared to striatal slices is not known. However, differential expression of PP-2A heterotrimers containing the B”/PR72 subunit may be responsible in part.
Synaptic vesicle endocytosis involves the dephosphin family of phosphoproteins, which includes amphiphysin I and dynamin I. These proteins are constitutively phosphorylated by Cdk5 and are dephosphorylated by the calcium-dependent activation of PP-2B in response to depolarization (Cousin and Robinson 2001; Tan et al. 2003; Tomizawa et al. 2003). Post-synaptically, WAVE1 is phosphorylated by Cdk5 and dephosphorylated in response to stimulation of calcium-dependent activation of PP-2A and PP-2B. Given the similarity in the regulatory mechanisms found for presynatic dephosphins and WAVE1, WAVE1 might be considered as a postsynatic dephosphin, which couples neurotransmitter-mediated signaling pathways to morphological changes in dendritic spines.
One of the interesting features of the current study is the convergence of different second messenger-regulated protein phosphatases on different sites in WAVE1. For example, Ser397 is a good substrate for cAMP-stimulated PP-2A but not for calcium-stimulated PP-2A, while Ser441 can be dephosphorylated by PP-2B when slices are stimulated with forskolin but not when stimulated with NMDA. The specificity of different PP-2A activities towards specific sites is presumably a reflection of the ability of different B subunits to influence the selective dephosphorylation of different sites. However, it is not clear what the basis is for the selective activation of PP-2B towards different sites. It is possible that there is a hierarchy in terms of the relationship of PP-2A and PP-2B: for example, if a specific B subunit of PP-2A is active toward a given site, then the sites might not be available for PP-2B, perhaps because of some structural occlusion. However, if PP-2A is not targeted to a specific site then this could make it available for another phosphatase such as PP-2B (Fig. 7).
In summary, our studies indicate that neurotransmitters such as dopamine and glutamate activate WAVE1 by reducing the level of serine phosphorylation through the involvement of PP-2A as well as PP-2B. Future studies will be required to investigate how the phosphorylation at serine residues of WAVE1 influences the other features of the regulation of WAVE1, including intermolecular interactions of the WAVE1 complex (Eden et al. 2002; Ismail et al. 2009), interaction of the WAVE1 complex with diverse upstream activators such as phosphoinositides, Rac and SH3-domain-containing proteins (Takenawa and Miki, 2001), dimerization/oligomerization of WAVE1 (Padrick et al. 2008) and phosphorylation at tyrosine residues (Ardern et al. 2006). Dopaminergic and glutamatergic transmission in striatum and morphological changes in dendritic spines in striatal neurons have been implicated in many psychiatric and neurological disorders including drug addiction (Lee et al. 2006; Kalivas 2009; Kim et al. 2009) and Parkinson’s disease (Day et al. 2006; Deutch et al. 2007). The signal transduction mechanisms we have found in this study may therefore play crucial regulatory roles in striatal synaptic plasticity, and alterations in these pathways may be associated with these psychiatric and neurological disorders. Future studies of regulation of WAVE1 in mouse models of psychiatric and neurological disorders will hopefully give new insight into the synaptic pathology of these disorders.
Acknowledgements
This work was supported by funding from the National Institute on Drug Abuse - DA10044 (Y.K., A.C.N. and P.G).
Abbreviations
- WAVE1
Wiskott-Aldrich syndrome protein (WASP)-family verprolin homologous protein 1
- Arp
actin-related protein
- Cdk5
cyclin-dependent kinase 5
- PP-2A
protein phosphatase 2A
- PP-2B
protein phosphatase 2B
- CNS
central nervous system
- DARPP-32
Dopamine and adenosine 3',5'-monophosphate-regulated phosphoprotein (32 kilodaltons)
- RCS
regulator of calmodulin signaling
- PP-1
protein phosphatase 1
- ECL
enhanced chemiluminescence
- SE
standard error
- OKA
okadaic acid
- CyA
cyclosporin A
References
- Ahn JH, McAvoy T, Rakhilin SV, Nishi A, Greengard P, Nairn AC. Protein kinase A activates protein phosphatase 2A by phosphorylation of the B56delta subunit. Proc. Natl Acad. Sci. USA. 2007a;104:2979–2984. doi: 10.1073/pnas.0611532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn JH, Sung JY, McAvoy T, Nishi A, Janssens V, Goris J, Greengard P, Nairn AC. The B"/PR72 subunit mediates Ca2+-dependent dephosphorylation of DARPP-32 by protein phosphatase 2A. Proc. Natl Acad. Sci. USA. 2007b;104:9876–9881. doi: 10.1073/pnas.0703589104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardern H, Sandilands E, Machesky LM, Timpson P, Frame MC, Brunton VG. Src-dependent phosphorylation of Scar1 promotes its association with the Arp2/3 complex. Cell Motil. Cytoskeleton. 2006;63:6–13. doi: 10.1002/cm.20101. [DOI] [PubMed] [Google Scholar]
- Bibb JA, Snyder GL, Nishi A, Yan Z, Meijer L, Fienberg AA, Tsai LH, Kwon YT, Girault JA, Czernik AJ, Huganir RL, Hemmings HC, Jr, Nairn AC, Greengard P. Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature. 1999;402:669–671. doi: 10.1038/45251. [DOI] [PubMed] [Google Scholar]
- Cheng A, Arumugam TV, Liu D, Khatri RG, Mustafa K, Kwak S, Ling HP, Gonzales C, Xin O, Jo DG, Guo Z, Mark RJ, Mattson MP. Pancortin-2 interacts with WAVE1 and Bcl-xL in a mitochondria-associated protein complex that mediates ischemic neuronal death. J. Neurosci. 2007;27:1519–1528. doi: 10.1523/JNEUROSCI.5154-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin MA, Robinson PJ. The dephosphins: dephosphorylation by calcineurin triggers synaptic vesicle endocytosis. Trends Neurosci. 2001;24:659–665. doi: 10.1016/s0166-2236(00)01930-5. [DOI] [PubMed] [Google Scholar]
- Dahl JP, Wang-Dunlop J, Gonzales C, Goad ME, Mark RJ, Kwak SP. Characterization of the WAVE1 knock-out mouse: implications for CNS development. J. Neurosci. 2003;23:3343–3352. doi: 10.1523/JNEUROSCI.23-08-03343.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, Mugnaini E, Deutch AY, Sesack SR, Arbuthnott GW, Surmeier DJ. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat. Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Colbran RJ, Winder DJ. Striatal plasticity and medium spiny neuron dendritic remodeling in parkinsonism. Parkinsonism Relat. Disord. 2007;13 Suppl 3:S251–S258. doi: 10.1016/S1353-8020(08)70012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillon C, Goda Y. The actin cytoskeleton: integrating form and function at the synapse. Annu. Rev. Neurosci. 2005;28:25–55. doi: 10.1146/annurev.neuro.28.061604.135757. [DOI] [PubMed] [Google Scholar]
- Eden S, Rohatgi R, Podtelejnikov AV, Mann M, Kirschner MW. Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature. 2002;418:790–793. doi: 10.1038/nature00859. [DOI] [PubMed] [Google Scholar]
- Fienberg AA, Hiroi N, Mermelstein PG, Song W, Snyder GL, Nishi A, Cheramy A, O'Callaghan JP, Miller DB, Cole DG, Corbett R, Haile CN, Cooper DC, Onn SP, Grace AA, Ouimet CC, White FJ, Hyman SE, Surmeier DJ, Girault J, Nestler EJ, Greengard P. DARPP-32: regulator of the efficacy of dopaminergic neurotransmission. Science. 1998;281:838–842. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- Frangiskakis JM, Ewart AK, Morris CA, Mervis CB, Bertrand J, Robinson BF, Klein BP, Ensing GJ, Everett LA, Green ED, Proschel C, Gutowski NJ, Noble M, Atkinson DL, Odelberg SJ, Keating MT. LIM-kinase1 hemizygosity implicated in impaired visuospatial constructive cognition. Cell. 1996;86:59–69. doi: 10.1016/s0092-8674(00)80077-x. [DOI] [PubMed] [Google Scholar]
- Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat. Rev. Mol. Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- Hemmings HC, Jr, Greengard P, Tung HY, Cohen P. DARPP-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phosphatase-1. Nature. 1984;310:503–505. doi: 10.1038/310503a0. [DOI] [PubMed] [Google Scholar]
- Ismail AM, Padrick SB, Chen B, Umetani J, Rosen MK. The WAVE regulatory complex is inhibited. Nat. Struct. Mol. Biol. 2009;16:561–563. doi: 10.1038/nsmb.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kim HJ, DiBernardo AB, Sloane JA, Rasband MN, Solomon D, Kosaras B, Kwak SP, Vartanian TK. WAVE1 is required for oligodendrocyte morphogenesis and normal CNS myelination. J. Neurosci. 2006a;26:5849–5859. doi: 10.1523/JNEUROSCI.4921-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Teylan MA, Baron M, Sands A, Nairn AC, Greengard P. Methylphenidate-induced dendritic spine formation and DeltaFosB expression in nucleus accumbens. Proc. Natl Acad. Sci. USA. 2009;106:2915–2920. doi: 10.1073/pnas.0813179106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Sung JY, Ceglia I, Lee KW, Ahn JH, Halford JM, Kim AM, Kwak SP, Park JB, Ryu SH, Schenck A, Bardoni B, Scott JD, Nairn AC, Greengard P. Phosphorylation of WAVE1 regulates actin polymerization and dendritic spine morphology. Nature. 2006b;442:814–817. doi: 10.1038/nature04976. [DOI] [PubMed] [Google Scholar]
- Lanzetti L. Actin in membrane trafficking. Curr. Opin. Cell Biol. 2007;19:453–458. doi: 10.1016/j.ceb.2007.04.017. [DOI] [PubMed] [Google Scholar]
- Lee KW, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc. Natl Acad. Sci. USA. 2006;103:3399–3404. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L. Actin cytoskeleton regulation in neuronal morphogenesis and structural plasticity. Annu. Rev. Cell Dev. Biol. 2002;18:601–635. doi: 10.1146/annurev.cellbio.18.031802.150501. [DOI] [PubMed] [Google Scholar]
- Minamide LS, Striegl AM, Boyle JA, Meberg PJ, Bamburg JR. Neurodegenerative stimuli induce persistent ADF/cofilin-actin rods that disrupt distal neurite function. Nat. Cell Biol. 2000;2:628–636. doi: 10.1038/35023579. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi S, Matsuura N, Ubukata M, Oikawa H, Shima H, Kikuchi K. Tautomycetin is a novel and specific inhibitor of serine/threonine protein phosphatase type 1, PP1. Biochem. Biophys. Res. Commun. 2001;287:328–331. doi: 10.1006/bbrc.2001.5596. [DOI] [PubMed] [Google Scholar]
- Mitsuhashi S, Shima H, Tanuma N, Matsuura N, Takekawa M, Urano T, Kataoka T, Ubukata M, Kikuchi K. Usage of tautomycetin, a novel inhibitor of protein phosphatase 1 (PP1), reveals that PP1 is a positive regulator of Raf-1 in vivo. J. Biol. Chem. 2003;278:82–88. doi: 10.1074/jbc.M208888200. [DOI] [PubMed] [Google Scholar]
- Newey SE, Velamoor V, Govek EE, Van Aelst L. Rho GTPases, dendritic structure, and mental retardation. J. Neurobiol. 2005;64:58–74. doi: 10.1002/neu.20153. [DOI] [PubMed] [Google Scholar]
- Nishi A, Snyder GL, Nairn AC, Greengard P. Role of calcineurin and protein phosphatase-2A in the regulation of DARPP-32 dephosphorylation in neostriatal neurons. J. Neurochem. 1999;72:2015–2021. doi: 10.1046/j.1471-4159.1999.0722015.x. [DOI] [PubMed] [Google Scholar]
- Nishi A, Bibb JA, Snyder GL, Higashi H, Nairn AC, Greengard P. Amplification of dopaminergic signaling by a positive feedback loop. Proc. Natl Acad. Sci. USA. 2000;97:12840–12845. doi: 10.1073/pnas.220410397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozumi M, Nakagawa H, Miki H, Takenawa T, Miyamoto S. Differential localization of WAVE isoforms in filopodia and lamellipodia of the neuronal growth cone. J. Cell Sci. 2003;116:239–246. doi: 10.1242/jcs.00233. [DOI] [PubMed] [Google Scholar]
- Padrick SB, Cheng HC, Ismail AM, Panchal SC, Doolittle LK, Kim S, Skehan BM, Umetani J, Brautigam CA, Leong JM, Rosen MK. Hierarchical regulation of WASP/WAVE proteins. Mol. Cell. 2008;32:426–438. doi: 10.1016/j.molcel.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilpel Y, Segal M. Rapid WAVE dynamics in dendritic spines of cultured hippocampal neurons is mediated by actin polymerization. J. Neurochem. 2005;95:1401–1410. doi: 10.1111/j.1471-4159.2005.03467.x. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Blanchoin L, Mullins RD. Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu. Rev. Biophys. Biomol. Struct. 2000;29:545–576. doi: 10.1146/annurev.biophys.29.1.545. [DOI] [PubMed] [Google Scholar]
- Rakhilin SV, Olson PA, Nishi A, Starkova NN, Fienberg AA, Nairn AC, Surmeier DJ, Greengard P. A network of control mediated by regulator of calcium/calmodulin-dependent signaling. Science. 2004;306:698–701. doi: 10.1126/science.1099961. [DOI] [PubMed] [Google Scholar]
- Rottner K, Lommel S, Wehland J, Stradal TE. Pathogen-induced actin filament rearrangement in infectious diseases. J. Pathol. 2004;204:396–406. doi: 10.1002/path.1638. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Fienberg AA, Huganir RL, Greengard P. A dopamine/D1 receptor/protein kinase A/dopamine- and cAMP-regulated phosphoprotein (Mr 32 kDa)/protein phosphatase-1 pathway regulates dephosphorylation of the NMDA receptor. J. Neurosci. 1998;18:10297–10303. doi: 10.1523/JNEUROSCI.18-24-10297.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderling SH, Langeberg LK, Soderling JA, Davee SM, Simerly R, Raber J, Scott JD. Loss of WAVE-1 causes sensorimotor retardation and reduced learning and memory in mice. Proc. Natl Acad. Sci. USA. 2003;100:1723–1728. doi: 10.1073/pnas.0438033100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderling SH, Guire ES, Kaech S, White J, Zhang F, Schutz K, Langeberg LK, Banker G, Raber J, Scott JD. A WAVE-1 and WRP signaling complex regulates spine density, synaptic plasticity, and memory. J. Neurosci. 2007;27:355–365. doi: 10.1523/JNEUROSCI.3209-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung JY, Engmann O, Teylan MA, Nairn AC, Greengard P, Kim Y. WAVE1 controls neuronal activity-induced mitochondrial distribution in dendritic spines. Proc. Natl Acad. Sci. USA. 2008;105:3112–3116. doi: 10.1073/pnas.0712180105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Bargas J, Hemmings HC, Jr, Nairn AC, Greengard P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron. 1995;14:385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- Takenawa T, Miki H. WASP and WAVE family proteins: key molecules for rapid rearrangement of cortical actin filaments and cell movement. J. Cell Sci. 2001;114:1801–1809. doi: 10.1242/jcs.114.10.1801. [DOI] [PubMed] [Google Scholar]
- Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat. Rev. Mol. Cell Biol. 2007;8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- Tan TC, Valova VA, Malladi CS, Graham ME, Berven LA, Jupp OJ, Hansra G, McClure SJ, Sarcevic B, Boadle RA, Larsen MR, Cousin MA, Robinson PJ. Cdk5 is essential for synaptic vesicle endocytosis. Nat. Cell Biol. 2003;5:701–710. doi: 10.1038/ncb1020. [DOI] [PubMed] [Google Scholar]
- Tomizawa K, Sunada S, Lu YF, Oda Y, Kinuta M, Ohshima T, Saito T, Wei FY, Matsushita M, Li ST, Tsutsui K, Hisanaga S, Mikoshiba K, Takei K, Matsui H. Cophosphorylation of amphiphysin I and dynamin I by Cdk5 regulates clathrin-mediated endocytosis of synaptic vesicles. J Cell Biol. 2003;163:813–824. doi: 10.1083/jcb.200308110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virshup DM, Shenolikar S. From promiscuity to precision: protein phosphatases get a makeover. Mol Cell. 2009;33:537–545. doi: 10.1016/j.molcel.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Zhao L, Ma QL, Calon F, Harris-White ME, Yang F, Lim GP, Morihara T, Ubeda OJ, Ambegaokar S, Hansen JE, Weisbart RH, Teter B, Frautschy SA, Cole GM. Role of p21-activated kinase pathway defects in the cognitive deficits of Alzheimer disease. Nat Neurosci. 2006;9:234–242. doi: 10.1038/nn1630. [DOI] [PubMed] [Google Scholar]