Abstract
In most mammalian species aromatase is encoded by a single gene (Cyp19), which contains 18 exons, nine of them being translated. In man, the presence of a biologically active aromatase and oestrogen receptors (ERα and ERβ) has been reported in Leydig cells, and also in immature germ cells and ejaculated spermatozoa. Concerning aromatase, the amount of transcript and enzymatic activity are decreased in immotile compared with motile sperm. We have amplified aromatase mRNA by real-time polymerase chain reaction in spermatozoa from asthenospermic, teratospermic and asthenoteratospermic men and recorded, respectively, 44, 52 and 67 per cent decreases of the amount of transcripts compared with fertile donors. A high degree of correlation (r = −0.64) between the abnormal spermatozoa (especially microcephaly and acrosome malformations) and aromatase/GAPDH transcript ratio has been observed. Idiopathic infertility is a wide health problem and no treatment is currently available. In humans, even if the role of oestrogens in spermatogenesis is still a matter of debate, the observations of decreased sperm number and motility in men genetically deficient in aromatase, together with our data and those reported in the literature, may suggest a role for aromatase/oestrogens not only during the development and maintenance of spermatogenesis but also in the final maturation of spermatozoa.
Keywords: aromatase, oestrogens, oestrogen receptors, spermatozoa, fertility, man
1. Introduction
In mammals, oestrogens have for a long time been considered as a specific female hormone. However, since Zondek's work more than 70 years ago dealing with the presence of oestrogenic hormone in stallion urine (Zondek 1934), the relevance of oestrogens in the male gonad has been increasingly documented (see reviews by Carreau et al. 1999, 2008; Hess 2003; Akingbemi 2005). The androgen/oestrogen balance is essential for normal sexual development and reproduction in mammals. In the testis, the maintenance of this balance is fine tuned via endocrine and paracrine factors, but is also related to aromatase activity (see reviews by Saez 1994; Carreau et al. 2003). Aromatase that catalyses androgen conversion into oestrogens is a product of a single gene called Cyp19 (see reviews by Simpson et al. 1994; Conley & Hinshelwood 2001). Besides the negative effect exerted by oestrogens on the secretion of gonadotrophins, it has become apparent that oestrogens do play an important physiological role in men, especially after the discoveries of patients genetically deficient in aromatase (see reviews by Rochira et al. 2005 and Jones et al. 2007). In addition, decreased sperm counts and increased male reproductive tract disorders (cryptorchidism, hypospadia and testicular cancer) in men have been described and these pathologies have been observed mainly after exposure to endocrine disruptors with either oestrogenic or anti-androgenic actions (Toppari et al. 1996; Skakkebaek 2004). The localization of aromatase within mammalian testicular cells has been a subject of interest and controversies for a long time (Carreau 2007). Certainly the testis produces oestrogens, and the role of these hormones in male reproduction is being extensively studied in numerous mammals, including primates, taking into account the existence of specific receptors (ERα and ERβ) that are present throughout the genital tract (see reviews by Hess 2003; Carreau et al. 2006).
2. The aromatase complex
The aromatase complex is composed of two proteins: a ubiquitous NADPH-cytochrome P450 reductase and a specific cytochrome P450 aromatase (P450arom), which contains the haem and steroid binding pocket. P450arom is the product of a single gene located on chromosome 15q21.1 called Cyp19, which belongs to the cytochrome P450 gene family that contains more than 500 members. The Cyp19 gene is more than 120 kb in length of which 30 kb represents the coding region with nine exons and an additional larger region (nine untranslated exons I) starting at the 5′ end. Cyp19 gene expression is regulated by tissue-specific promoters producing alternate 5′-untranslated exons I that are then spliced onto a common 3′-splice acceptor site in exon II, upstream of the translation start site (see reviews by O'Donnell et al. 2001; Sebastian et al. 2002). Even though there is generation of Cyp19 variants with different 5′UTRs, the coding sequences are identical and give rise to a unique protein in humans.
3. Aromatase and oestrogen receptors in human testicular cells
(a). Aromatase expression
In humans, Leydig cells (Payne et al. 1976) and Sertoli cells produce oestrogens (Foucault et al. 1992; see Carreau 1996, 2007 for reviews) and spermatozoa can metabolize pregnenolone into oestrogens (Gunasegaram et al. 1995). Taking into account the presence of aromatase in rodent testis and the probable role of oestrogens in spermatogenesis (see Carreau et al. 1999 and Hess 2003 for reviews), we have checked the expression of aromatase in motile and immotile spermatozoa from healthy donors and studied the relevance of this protein to sperm quality (motility and survival). To rule out the possibility of any contamination by residual cells (germ cells and/or polynuclear cells), the liquefied semen samples were fractionated on a discontinuous Puresperm gradient; after centrifugation, high motile (greater than 90%) and low motile spermatozoa (less than 30%) were collected. Human granulosa cells (positive control for aromatase) were obtained from human follicular fluid at the in vitro fertilization (IVF) centre (CHRU Clemenceau-Caen). The quality of the sperm cell preparations was carefully analysed to eliminate any contamination of samples by leukocytes (by checking for the presence of CD45 transcript) and by Sertoli cells (by checking for the presence of Sertoli cell factor mRNA). All samples containing detectable levels of these transcripts were eliminated; conversely, the presence of c-kit transcript in round cells was used as a positive control for the presence of germ cells (Lambard et al. 2004a,b). In addition, since no information was available on the source(s) of oestrogens in immature human germ cells (mainly in spermatocytes and round spermatids), we used semen samples with more than 20 per cent of round cells (selected on the 47.5% layer of the Puresperm gradient) to examine by real-time polymerization chain reaction (RT-PCR) the putative expression of Cyp19.
In all samples studied individually, we have demonstrated the presence of aromatase mRNA (Lambard et al. 2003; see review by Galeraud-Denis et al. 2007), which is in agreement with other reports (Aquila et al. 2002; Rago et al. 2003). In immotile sperm, the amount of P450arom transcripts was 30 per cent lower than in motile samples; indeed, aromatase activity was higher in the motile fraction compared with immotile spermatozoa (Lambard et al. 2004b). In immature germ cells of these healthy donors, we also detected the presence aromatase transcripts (Lambard et al. 2004a). The sequence analyses of the polymerase chain reaction (PCR) products obtained were identical to each other and more than 98 per cent identical to the published sequence of human P450arom (Lambard et al. 2003). Using Western blots and a specific monoclonal antibody against a highly conserved region of aromatase, we have evidenced the presence of aromatase in both immature germ cells and ejaculated sperm cells, the protein being more abundant in spermatozoa containing cytoplasmic droplets. To better characterize the aromatase, we looked for the protein in microsomes obtained from crude germ cells and granulosa cells. Two bands were observed: a strong one at 53 kDa and a light one at 49 kDa, which were also present in spermatozoa; the predominant form was the 49 kDa band. The proteins obtained from granulosa cells and spermatozoa were deglycosylated; only the motility of aromatase in granulosa cells was slightly modified yielding a 49 kDa protein (Lambard & Carreau 2005). Our data are in agreement with those of Turner et al. (2002) who immunolocalized aromatase in cytoplasm surrounding elongated spermatids in man, and with those of Rago et al. (2003) demonstrating aromatase in the cytoplasmic droplets of ejaculated human spermatozoa.
More recently, we analysed aromatase expression by RT-PCR in spermatozoa of asthenospermic, teratospermic and asthenoteratospermic patients from Tunisia (Dr A. Saad, Hospital F Hached-Sousse). The amount of transcripts as compared with controls were decreased by 44, 52 and 67 per cent, respectively (Galeraud-Denis et al. 2007; Said et al. in press). It is noteworthy that a twofold decrease in the amount of aromatase transcripts has also been observed in a group of infertile men from Poland (Jedrzejczak et al. 2006).
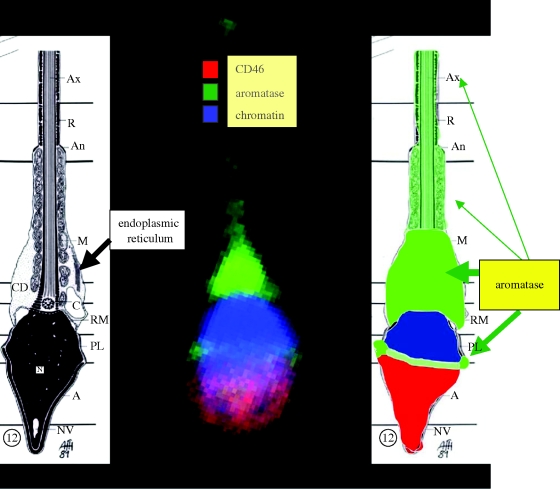
The most recent data collected were obtained using a confocal microscopy approach. Accordingly, two immunoreactive sites for aromatase have been demonstrated (Galeraud-Denis et al. 2007): one in the tail in which an intensive staining was observed as expected, and the second one corresponding to a tight but compact highly fluorescent zone of the head (equatorial region/upper postacrosomal region; figure 1). Our preliminary results in teratospermic patients showed a high degree of correlation (r = −0.64) between the aromatase/GAPDH mRNAs and the percentage of abnormal spermatozoa. More interesting may be the negative correlation (r = −0.56) observed between that ratio and head malformations (microcephaly or acrosome malformations) rather than with tail defects (r = −0.42). From studies of a small group of teratospermic men, it appears that annular aromatase (equatorial localization) is twofold less than in normospermic men.
Figure 1.
Localization of aromatase in ejaculated human spermatozoa using confocal microscopy. CD 46: specific marker of inner acrosomal membrane; chromatin is localized with DAPI and the aromatase is revealed by a polyclonal antibody. Specific antibodies targeted to specific markers of either the acrosome (CD46), or nucleus (DAPI) or mid-piece (Mitotracker Green) or tail (Tubulin) have been used.
Concerning the epididymal regions, Rago et al. (2003) and Carpino et al. (2007) have immunolocalized aromatase in the epithelial cells of human efferent ducts and in the proximal caput epididymis, suggesting an additional source of oestrogens in the male genital tract. Harada et al. (1993) have reported the existence of aromatase in prostate, which was recently confirmed by Ellem et al. (2004).
In the Rhesus monkey, it has been reported that testis and to a lesser extent epididymis contained two P450arom transcripts, of which one is truncated (Pereyra-Martinez et al. 2001). In addition, these authors reported that the aromatase activity was identical in testis and caput epididymis. Nevertheless, together these data show that epididymal regions are able to aromatize androgens into oestrogens.
(b). Oestrogen receptors
Oestrogens interact with oestrogen receptors (ERs), members of the nuclear receptor superfamily, which in turn modulate the expression of target genes (see Heldring et al. 2007 for review). The physiological role of oestrogens (via the classical genomic pathway) in male reproduction has been extensively reanalysed taking into account the presence of two ERs (ERα and ERβ) in most testicular cells and throughout the genital tract (see O'Donnell et al. 2001, Saunders et al. 2001 and Carreau et al. 2006 for reviews). In addition, a relevant role for oestrogens has recently been demonstrated via rapid membrane interactions leading to new signalling pathways (see Luconi et al. 2004, Watson et al. 2007 and Prossnitz et al. 2008 for reviews).
In immature germ cells and spermatozoa of healthy men, we have reported the presence of ERs, not only full length but also some variants (see Lambard & Carreau 2005 for review). In immature testicular cells several transcripts of ERα were observed, the wild type and a smaller one which corresponds to the ERα isoform lacking exon IV; in addition, we have detected another transcript corresponding to the exon I-deleted variant as described previously (Flouriot et al. 2000). Concerning ERβ mRNAs, the full-length and shorter isoforms were demonstrated in germ cells; the corresponding ER proteins were observed on Western blots using specific antibodies (Lambard et al. 2004a,b).
Regarding spermatozoa, even though the transcripts of ERα and ERβ were demonstrated, we were only able to show the presence of an ERα protein of 46 kDa corresponding to the isoform that lacks exon I; this variant could be located on the membrane and involved in rapid signalling as demonstrated in endothelial cells (Figtree et al. 2003). Aquila et al. (2004) and Solakidi et al. (2005) have reported the presence of ERα and ERβ proteins in human ejaculated spermatozoa, with some discrepancies on their respective localization. It is obvious that either the presence or the absence of these ER isoforms (table 1) in male primates is likely to be related to the various antibodies used as well as to the origin and quality of the tissues. In addition, the development of the male gonad could interfere with the cellular distribution of these ERs (review by Hess & Carnes 2004). Moreover, numerous spliced variants of ERβ have been detected in human testicular cells (Aschim et al. 2004). Therefore for human seminiferous tubules there are controversial data (table 1) indicating either the absence (Pelletier & El-Alfy 2000) or the presence of ERα and ERβ (Pentikaïnen et al. 2000; Makinen et al. 2001; Saunders et al. 2001, 2002). It is of note that Cheng et al. (1981) were the first to show a specific binding of oestradiol to human sperm; it was only in 1998 that Durkee et al. demonstrated the presence of ERα in human spermatozoa, data that were later confirmed by Luconi et al. (2006). It is also clear that ERs (mainly ERβ) are present in ductules, seminal vesicles (Saunders et al. 2001) as well as in the prostate (see reviews by Ellem et al. 2004; Ellem & Risbridger 2007). To summarize (table 1), it is obvious that ERs are widespread in the male genital tract, but still more data are necessary to clarify their roles, especially when taking into account either the presence or absence of ERα and ERβ in the various cell types.
Table 1.
Transcripts of aromatase and wild-type ERs in human testicular cells and genital tract. n.d., not determined; +, positive; −, negative.
4. Oestrogens and human reproduction
The presence of aromatase and ERs in most of the testicular cells has been demonstrated in several mammals, amongst them humans (see reviews of Carreau et al. 2008, 2009). Berensztein et al. (2006) have immunolocalized aromatase and ERβ in gonocytes and spermatogonia in newborn and infantile testes, but the role of oestrogens in that developing gonad remains to be clarified. In addition, the existence of ERβ isoforms in human testicular cells has been reported but their specific functions have not been fully elucidated even though a putative relationship between ERβ polymorphisms and infertility has been suggested (Aschim et al. 2005).
What is more interesting is the demonstration of a source and target of oestrogens in ejaculated spermatozoa (Carreau et al. 2007). Even though spermatozoa are considered to be biologically inert cells that transfer the paternal genome into the oocyte, the presence of mRNAs (see Naz 1998, Dadoune et al. 2005 and Dadoune 2009 for reviews) and their potential roles in spermatozoa are still controversial (see Martins & Krawetz 2005, Miller et al. 2005 and Galeraud-Denis et al. 2007 for review). But numerous data suggest that both transcriptional and translational activities could occur in the mitochondria of these haploid cells (Gur & Breibart 2006; Oliva 2006). Nevertheless, the role of oestrogens in man, especially in the reproductive function, is becoming more obvious after the report by Smith et al. (1994) concerning a man with a non-functional ERα and several reports relating to men deficient in aromatase (see review by Rochira et al. 2005). Aromatase deficiency is rather rare in men (seven cases published to date) and occurs following mutations in the Cyp19 gene; for example, in exons V and IX, which are critical for catalytic activity and androgen binding, respectively, with the resulting protein showing an absence of biological activity and thus very low (or nil) levels of oestrogens in the blood. One of the most dramatic effects recorded on aromatase mutation is on exon IV, which is critical for aromatase function and thus leads to the total absence of enzyme activity (Maffei et al. 2007). All together four exons (IV, V, IX and X) are required for the full biological activity of the protein, as is also observed in the rat aromatase gene (Levallet et al. 1998).
From a testicular biopsy of one of these aromatase-deficient adult men, it was reported by Maffei et al. (2007) that spermatogenesis varied from normal in some seminiferous tubules to highly reduced (hypospermatogenesis) in most of the others, therefore suggesting a decrease in germ cell number as observed in ArKO mice which lead to infertility at the age of 1 year (O'Donnell et al. 2001). The last (eighth case) patient described by Carani's group (Lanfranco et al. 2008) showed moderate alterations of sperm parameters (asthenoteratospermia) that did not return to normal after oestradiol treatment; a cryptorchidism history was recorded, as is frequently the case for the aromatase-deficient men described (Pura et al. 2003; Maffei et al. 2004; see Rochira et al. 2005 and Jones et al. 2007 for reviews).
But what is also very interesting is that these aromatase-deficient patients with variable degrees of fertility disorder provided additional information on the roles of oestrogens because they were studied in adulthood. Carani et al. (1997) were the first to report a tall stature with linear growth and the absence of epiphyseal closure, which is the main reason leading these patients to consult a specialist physician. It is of note that these men have normal or subnormal levels of gonadotrophins and testosterone (Rochira et al. 2005); in addition to these symptoms, most have problems concerning lipid and glucose metabolism without obvious cause (see Jones et al. 2006 and Zirilli et al. 2008 for reviews).
It is important to mention that the human spermatozoa produce oestrogens (Gunasegaram et al. 1995) and indeed they express a functional aromatase that is still active after ejaculation (Lambard & Carreau 2005); together with the presence of ERs (Aquila et al. 2004; Luconi et al. 2004; Solakidi et al. 2005), these data open new considerations about the role of oestrogens all along the male genital tract and likely also regarding sperm mobility and fertilization ability. Indeed, Fraser et al. (2006) have demonstrated that either oestradiol or a phytoestrogen (genistein) improves the capacitation and acrosome loss of human spermatozoa. It is also relevant to mention that more than 30 years ago it was reported that (i) there was a correlation between the amount of oestrogens in the seminal plasma and fertility and (ii) incubation of spermatozoa with oestradiol improves their motility (see review by Carreau et al. 1999). Besides the positive effects of oestrogens on human spermatogenesis, it has also been shown that aromatase inhibitors significantly improve the number and quality of spermatozoa of infertile men with a decreased blood testosterone/oestradiol ratio (Raman & Schlegel 2002). Concerning anti-oestrogen treatment of idiopathic male infertility, several studies have been realized with either tamoxifen or clomiphene with no significant effects on sperm parameters recorded (see Liu & Handelsman 2003 for review).
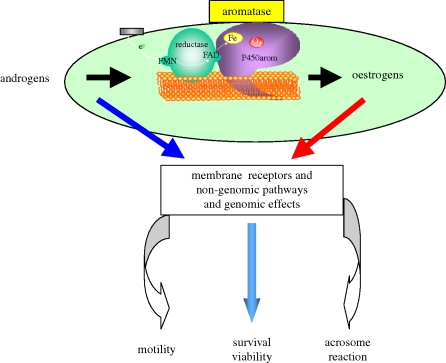
Therefore, the effects of oestrogens on human ejaculated spermatozoa are becoming increasingly obvious: besides the classical genomic effects, membrane ERs are connected with numerous signal transduction pathways involving rapid responses (see Luconi et al. 2004 for review), among them cAMP/PKA/AKAP (Muratori et al. 2008), nitric oxide (Herrero & Gagnon 2001), the MEK pathway, calcium channel and a calcium/calmodulin complex, all known to be concerned with sperm mobility and capacitation (see Revelli et al. 1998 for review). Aquila et al. (2004) have also shown a rapid membrane effect of oestrogens that in turn activate the PI3K/AKT pathway in the human ejaculated spermatozoa (figure 2).
Figure 2.
Putative role(s) of oestrogens in the male gamete.
5. Future developments
From knock out studies in mice and data reported concerning aromatase-deficient men, the oestrogen effects on spermatogenesis and spermiogenesis are clearly different (Grumbach & Auchus 1999); however, the role of oestrogens in male reproduction is becoming more obvious taking into account the existence of specific receptors all along the genital tract (see review of Jones et al. 2007). Indeed, it is absolutely clear that oestrogen therapy is really beneficial for these men deficient in aromatase (Carani et al. 1997; Maffei et al. 2007). In humans, aromatase is constitutively expressed not only in Leydig cells and Sertoli cells but also in germ cells, whatever the stage of development. Even though the requirement of oestrogens for spermatogenesis is not completely understood (not enough studies, more patients required, the complexity of oestrogen roles) beside its role in gonadotrophin control (Rochira et al. 2006), it is obvious that men with an aromatase deficiency or oestrogen resistance have altered (or abnormal) spermatogenesis.
The absence of semen analyses of course detracts from the final conclusion on sperm number and quality and thus on the putative fertility of these patients. Nevertheless, in these patients a decrease in sperm motility is recorded and we have a significant decrease of aromatase in immotile spermatozoa, which could suggest that aromatase/oestrogens are involved in the acquisition of sperm motility. Besides a relevant physiological role for these female hormones, it is also well known that excess oestrogens are very deleterious for testicular function. Indeed, Leydig cell tumours in which enhanced oestradiol production is recorded together with the presence of ERs lead to excess cell proliferation (Carpino et al. 2007). Seminoma cells express aromatase and a rapid membrane oestrogen effect as well as genomic ERs have been reported (Roger et al. 2005; Bouskine et al. 2008). In addition, it is well known that testicular germ cell tumours produce high amounts of oestrogens that induce a decrease in sperm motility and significant alterations in spermatogenesis (Nakazumi et al. 1996).
Whatever our knowledge of the role of oestrogens in male physiology, it is increasing, and at least as far as oestrogens are concerned in male gamete maturation, not only the existence of oestrogen sources but also the presence of ERs in immature germ cells and ejaculated sperm have been clearly demonstrated, therefore suggesting a potential role of oestrogens in the latter steps of sperm maturation (capacitation and/or acrosome reaction). Taking into account this new role of oestrogens (even if oestrogen-related genes are not yet elucidated), it will also be conceivable to develop some targeted pharmacological drugs for male contraception (figure 2). The presence of aromatase transcripts could be a marker of male gamete quality, and the existence of two sites of aromatase could be related to the implication of the oestrogens in motility and acrosome reaction. In addition, since aromatase, ERs and a recently described androgen receptor (Aquila et al. 2007) are simultaneously present in spermatozoa, a putative autocrine and/or intracrine role for oestradiol is suggested. At least oestrogens could play a role if we considered the presence of ERs in the mitochondria (Chen et al. 2007), a tremendously important organelle in the mid-piece of the spermatozoa. Finally, we would also draw attention to GPR30 (a transmembrane intracellular ER) that induces rapid mobilization of calcium in the cytoplasm (Prossnitz et al. 2008) and obviously would be of great interest for sperm activation before acrosome reaction.
Male fertility is decreasing in western countries and thus it is a society problem; consequently, extensive and comparative studies of mRNA maps in ejaculated spermatozoa from fertile and infertile men may contribute to a better understanding of the various aspects of male gamete maturation throughout the genital tract.
Acknowledgements
We are greatly indebted to our collaborators (Drs Lambard, de Vienne, Said, Saad and Chocat). All these works have been supported by funding from the French Ministry of Education and Research, a Polonium grant and the Région Basse-Normandie.
Footnotes
One contribution of 17 to a Theme Issue ‘The biology and regulation of spermatogenesis’.
References
- Akingbemi B. T.2005Estrogen regulation of testicular function. Reprod. Biol. Endocrinol. 3, 51 (doi:10.1186/1477-7827-3-51) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquila S., Sisci D., Gentile M., Middea E., Siciliano L., Ando S.2002Human ejaculated spermatozoa contain active P450 aromatase. J. Clin. Endocrinol. Metab. 87, 3385–3390 (doi:10.1210/jc.87.7.3385) [DOI] [PubMed] [Google Scholar]
- Aquila S., Sisci D., Gentile M., Middea E., Catalano S., Carpino A., Rago V., Ando S.2004Estrogen receptor (ER)α and ERβ are both expressed in human ejaculated spermatozoa: evidence for their direct interaction with phosphatidylinositol-3-OH Kinase/Akt pathway. J. Clin. Endocrinol. Metab. 89, 1443–1451 (doi:10.1210/jc.2003-031681) [DOI] [PubMed] [Google Scholar]
- Aquila S., et al. 2007Human sperm express a functional androgen receptor: effects on PI3K/AKT pathway. Hum. Reprod. 22, 2594–2605 (doi:10.1093/humrep/dem243) [DOI] [PubMed] [Google Scholar]
- Aschim E. L., Saether T., Wiger R., Grotmol T., Haugen T. B.2004Differential distribution of splice variants of estrogen receptor beta in human testicular cells suggests specific functions in spermatogenesis. J. Steroid Biochem. Mol. Biol. 92, 97–106 (doi:10.1016/j.jsbmb.2004.05.008) [DOI] [PubMed] [Google Scholar]
- Aschim E. L., Giwercman A., Ståhl O., Eberhard J., Cwikiel M., Nordenskjöld A., Haugen T. B., Grotmol T., Giwercman Y. L.2005The Rsal polymorphism in the estrogen receptor beta gene is associated with male infertility. J. Clin. Endocrinol. Metab. 90, 5343–5348 (doi:10.1210/jc.2005-0263) [DOI] [PubMed] [Google Scholar]
- Berensztein E. B., Baquedano M. S., Gonzalez C. R., Saraco N. I., Rodriguez J., Ponzio R., Rivarola M. A., Belgorosky A.2006Expression of aromatase, estrogen receptor α and β, androgen receptor, and cytochrome P-450 scc in the human early prepubertal testis. Pediatr. Res. 60, 740–744 (doi:10.1203/01.pdr.0000246072.04663.bb) [DOI] [PubMed] [Google Scholar]
- Bouskine A., Nebout M., Mograbi B., Brucker-Davis F., Roger C., Fenichel P.2008Estrogens promote human testicular germ cell cancer through a membrane mediated activation of extracellular regulated kinase and protein kinase A. Endocrinology 149, 565–573 (doi:10.1210/en.2007-1318) [DOI] [PubMed] [Google Scholar]
- Carani C., Qin K., Simoni M., Faustini-Fustini M., Serpente S., Boyd J., Korach K. S., Simpson E. R.1997Effect of testosterone and estradiol in a man with aromatase deficiency. N. Engl. J. Med. 337, 91–95 (doi:10.1056/NEJM199707103370204) [DOI] [PubMed] [Google Scholar]
- Carpino A., Rago V., Pezzi V., Carani C., Ando S.2007Detection of aromatase and estrogen receptors (ERα, ERβ1, ERβ2) in human Leydig cell tumor. Eur. J. Endocrinol. 157, 239–244 (doi:10.1530/EJE-07-0029) [DOI] [PubMed] [Google Scholar]
- Carreau S.1996Paracrine control of human Leydig cell and Sertoli cell functions. Folia Histochem. Cytobiol. 3, 111–119 [PubMed] [Google Scholar]
- Carreau S.2007Leydig cell aromatase: from gene to physiological role. In The Leydig cell in health and disease (eds Payne A. H., Hardy M. P.), pp. 189–195 Totowa, NJ: Humana Press [Google Scholar]
- Carreau S., Genissel C., Bilinska B., Levallet J.1999The oestrogen sources in the testis and the reproductive tract of the male. Int. J. Androl. 22, 211–223 (doi:10.1046/j.1365-2605.1999.00172.x) [DOI] [PubMed] [Google Scholar]
- Carreau S., Lambard S., Delalande C., Denis-Galeraud I., Bilinska B., Bourguiba S.2003Aromatase expression and role of estrogens in male gonad: a review. Reprod. Biol. Endocrinol. 1, 35 (doi:10.1186/1477-7827-1-35) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreau S., Delalande C., Silandre D., Bourguiba S., Lambard S.2006Aromatase and estrogen receptors in male reproduction. Mol. Cell. Endocrinol. 246, 65–68 (doi:10.1016/j.mce.2005.11.021) [DOI] [PubMed] [Google Scholar]
- Carreau S., Lambard S., Said L., Saad A., Galeraud-Denis I.2007RNA dynamics of fertile and infertile spermatozoa. Biochem. Soc. Trans. 35, 634–636 [DOI] [PubMed] [Google Scholar]
- Carreau S., Bourguiba S., Delalande C., Silandre D., Said L., Galeraud-Denis I., Lambard S.2008Estrogens and male reproduction. Curr. Med. Chem. Immunol. Endocrinol. Metab. Agents 8, 59–65 (doi:10.2174/187152208783790679) [Google Scholar]
- Carreau S., Delalande C., Denis-Galeraud I.2009Mammalian sperm quality and aromatase expression. Microsc. Res. Tech 72, 552–557 [DOI] [PubMed] [Google Scholar]
- Chen J. Q., et al. 2007Regulation of mitochondrial respiratory chain structure and function by estrogens/estrogen receptors and potential physiological/pathophysiological implications. Biochim. Biophys. Acta 1746, 1–17 [DOI] [PubMed] [Google Scholar]
- Cheng C. Y., Boettcher B., Rose R. J., Kay D. J., Tinneberg H. R.1981The binding of sex steroids to human spermatozoa. An autoradiographic study. Int. J. Androl. 4, 1–17 (doi:10.1111/j.1365-2605.1981.tb00685.x) [DOI] [PubMed] [Google Scholar]
- Conley A., Hinshelwood M.2001Mammalian aromatases. Reproduction 121, 685–695 (doi:10.1530/rep.0.1210685) [DOI] [PubMed] [Google Scholar]
- Dadoune J. P.2009Spermatozoal RNAs: what about their functions? Microsc. Res. Tech 72, 536–551 [DOI] [PubMed] [Google Scholar]
- Dadoune J. P., Pawlak A., Alfonsi M. F., Siffroi J. P.2005Identification of transcripts by macroarrays, RT-PCR and in situ hybridization in human ejaculate spermatozoa. Mol. Hum. Reprod. 11, 133–140 (doi:10.1093/molehr/gah137) [DOI] [PubMed] [Google Scholar]
- Durkee T. J., Mueller M., Zinaman M.1998Identification of estrogen receptor protein and messenger ribonucleic acid in human spermatozoa. Am. J. Obstet. Gynecol. 178, 1288–1295 (doi:10.1016/S0002-9378(98)70335-7) [DOI] [PubMed] [Google Scholar]
- Ellem S. J., Risbridger G. P.2007Treating prostate cancer: a rationale for targeting local oestrogens. Nat. Rev. Cancer 7, 621–627 (doi:10.1038/nrc2174) [DOI] [PubMed] [Google Scholar]
- Ellem S. J., Schmitt J. F., Pedersen J. S., Frydenberg M., Risbridger G. P.2004Local aromatase expression in human prostate is altered in malignancy. J. Clin. Endocrinol. Metab. 89, 2434–2441 (doi:10.1210/jc.2003-030933) [DOI] [PubMed] [Google Scholar]
- Figtree G. A., McDonald D., Watkins H., Channon K. M.2003Truncated estrogen receptor α 46 kDa isoform in human endothelial cells. Relationship to acute activation of nitric oxide synthase. Circulation 107, 120–126 (doi:10.1161/01.CIR.0000043805.11780.F5) [DOI] [PubMed] [Google Scholar]
- Flouriot G., Brand H., Denger S., Metivier R., Kos M., Reid G., Sonntag-Buck V., Gannon F.2000Identification of a new isoform of the human estrogen receptor alpha (hERα) that is encoded by distinct transcripts and that is able to repress hERα activation function-1. EMBO J. 19, 4688–4700 (doi:10.1093/emboj/19.17.4688) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foucault P., Carreau S., Kuczynski W., Guillaumin J. M., Bardos P., Drosdowsky M. A.1992The human Sertoli cells in vitro: lactate, estradiol-17ß and transferrin productions. J. Androl. 13, 361–367 [PubMed] [Google Scholar]
- Fraser L. R., Beyret E., Milligan S. R., Adeoya-Osiguwa S. A.2006Effects of estrogenic xenobiotics on human and mouse spermatozoa. Hum. Reprod. 21, 1184–1193 (doi:10.1093/humrep/dei486) [DOI] [PubMed] [Google Scholar]
- Galeraud-Denis I., Lambard S., Carreau S.2007Relationship between chromatin organization, mRNAs profile and human male gamete quality. Asian J. Androl. 9, 587–592 (doi:10.1111/j.1745-7262.2007.00310.x) [DOI] [PubMed] [Google Scholar]
- Grumbach M. M., Auchus R. J.1999Estrogen: consequences and implications of human mutations in synthesis and actions. J. Clin. Endocrinol. Metab. 84, 4677–4694 (doi:10.1210/jc.84.12.4677) [DOI] [PubMed] [Google Scholar]
- Gunasegaram R., Chew P. C. T., Loganath A., Peh K. L., Ratman S. S.1995A delta 4-3 keto pathway for testosterone synthesis in the human spermatozoa. Arch. Androl. 40, 49–57 (doi:10.3109/01485019808987927) [DOI] [PubMed] [Google Scholar]
- Gur Y., Breibart H.2006Mammalian sperm translate nuclear-encoded proteins by mitochondrial-type ribosomes. Gene Dev. 20, 411–416 (doi:10.1101/gad.367606) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada N., Utsumi T., Takagi Y.1993Tissue-specific expression of the human aromatase cytochrome P-450 gene by alternative use of multiple exons 1 and promoters, and switching of tissue-specific exons 1 in carcinogenesis. Proc. Natl Acad. Sci. USA 90, 11 312–11 316 (doi:10.1073/pnas.90.23.11312) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldring N., et al. 2007Estrogen receptors: how do they signal and what are their targets. Physiol. Rev. 87, 905–931 (doi:10.1152/physrev.00026.2006) [DOI] [PubMed] [Google Scholar]
- Herrero M. B., Gagnon C.2001Nitric oxide: a novel mediator of sperm function. J. Androl. 22, 349–356 [PubMed] [Google Scholar]
- Hess R. E.2003Estrogen in the adult male reproductive tract: a review. Reprod. Biol. Endocrinol. 9, 52 (doi:10.1186/1477-7827-1-52) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess R. A., Carnes K.2004The role of estrogen in the testis and the male reproductive tract: a review. Anim. Reprod. 1, 5–30 [Google Scholar]
- Jedrzejczak P., Januchowski R., Taszarek G., Laddach R., Pawelczyk L., Jagodzinski P. P.2006Quantitative analysis of CCR5 chemokine receptor and cytochrome P450 aromatase transcripts in swim-up spermatozoa isolated from fertile and infertile men. Arch. Androl. 52, 335–341 (doi:10.1080/01485010600692058) [DOI] [PubMed] [Google Scholar]
- Jones M. E., Boon W. C., Proietto J., Simpson E. R.2006Of mice and men: the evolving phenotype of aromatase deficiency. Trends Endocrinol. Metab. 17, 55–64 (doi:10.1016/j.tem.2006.01.004) [DOI] [PubMed] [Google Scholar]
- Jones M. E., McInnes K., Maffei L., Carani C., Simpson E. R.2007Recognizing rare disorders: aromatase deficiency. Nat. Clin. Pract. Endocrinol. Metabol. 3, 414–421 (doi:10.1038/ncpendmet0477) [DOI] [PubMed] [Google Scholar]
- Lambard S., Carreau S.2005Aromatase and estrogens in human male germ cells. Int. J. Androl. 28, 254–259 (doi:10.1111/j.1365-2605.2005.00546.x) [DOI] [PubMed] [Google Scholar]
- Lambard S., Galeraud-Denis I., Bouraïma H., Bourguiba S., Chocat A., Carreau S.2003Expression of aromatase in human ejaculated spermatozoa: a putative marker of motility. Mol. Hum. Reprod. 9, 117–124 (doi:10.1093/molehr/gag020) [DOI] [PubMed] [Google Scholar]
- Lambard S., Galeraud-Denis I., Saunders P. T. K., Carreau S.2004aHuman immature germ cells and ejaculated spermatozoa contain aromatase and oestrogen receptors. J. Mol. Endocrinol. 32, 279–289 (doi:10.1677/jme.0.0320279) [DOI] [PubMed] [Google Scholar]
- Lambard S., Galeraud-Denis I., Martin G., Levy R., Carreau S.2004bAnalysis and significance of mRNA in human ejaculated sperm from normozoospermic donors: relationship to sperm motility and capacitation. Mol. Hum. Reprod. 10, 535–541 (doi:10.1093/molehr/gah064) [DOI] [PubMed] [Google Scholar]
- Lanfranco F., Zirilli L., Baldi M., Pignatti E., Corneli G., Ghigo E., Aimaretti G., Carani C., Rochira V.2008A novel mutation in the human aromatase gene: insights on the relationship among serum estradiol, longitudinal growth and bone mineral density in an adult man under estrogen replacement treatment. Bone 43, 628–635 (doi:10.1016/j.bone.2008.05.011) [DOI] [PubMed] [Google Scholar]
- Levallet J., Delarue B., Mittre H., Carreau S.1998Alternative splicing events in the coding region of cytochrome P450 aromatase in adult male rat germ cells. J. Mol. Endocrinol. 20, 305–312 (doi:10.1677/jme.0.0200305) [DOI] [PubMed] [Google Scholar]
- Liu P. Y., Handelsman D. J.2003The present and future state of hormonal treatment for male infertility. Hum. Reprod. Update 9, 9–23 (doi:10.1093/humupd/dmg002) [DOI] [PubMed] [Google Scholar]
- Luconi M., Francavilla F., Porazzi I., Macerola B., Forti G., Baldi I.2004Human spermatozoa as a model for studying membrane receptors mediating rapid nongenomic effects of progesterone and estrogens. Steroids 69, 553–559 (doi:10.1016/j.steroids.2004.05.013) [DOI] [PubMed] [Google Scholar]
- Luconi M., Forti G., Baldi E.2006Pathophysiology of sperm mobility. Front. Biosci. 11, 1433–1447 (doi:10.2741/1894) [DOI] [PubMed] [Google Scholar]
- Maffei L., et al. 2004Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: effects of testosterone, alendronate and estradiol treatment. J. Clin. Endocrinol. Metab. 89, 61–70 (doi:10.1210/jc.2003-030313) [DOI] [PubMed] [Google Scholar]
- Maffei L., et al. 2007A novel compound heterozygous mutation of the aromatase gene in an adult man: reinforced evidence on the relationship between congenital oestrogen deficiency, adiposity and the metabolic syndrome. Clin. Endocrinol. 67, 218–224 (doi:10.1111/j.1365-2265.2007.02864.x) [DOI] [PubMed] [Google Scholar]
- Makinen S., Makela S., Zhang W. H., Warner M., Rosenlund B., Sami S., Hovatta O., Gustafsson J. A.2001Localization of oestrogen receptors alpha and beta in human testis. Mol. Hum. Reprod. 7, 497–503 (doi:10.1093/molehr/7.6.497) [DOI] [PubMed] [Google Scholar]
- Martins R. P., Krawetz S. A.2005RNA in human sperm. Asian J. Androl. 7, 1–4 [DOI] [PubMed] [Google Scholar]
- Miller D., Ostermeier G. C., Krawetz S. A.2005The controversy, potential and roles of spermatozoal RNA. Trends Mol. Med. 11, 156–163 (doi:10.1016/j.molmed.2005.02.006) [DOI] [PubMed] [Google Scholar]
- Muratori M., Luconi M., Marchiani S., Forti G., Baldi E.2008Molecular markers of human sperm functions. Int. J. Androl. 32, 25–45 (doi:10.1111/j.1365-2605.2008.00875.x) [DOI] [PubMed] [Google Scholar]
- Nakazumi H., Sasano H., Maehara I., Ozaki M., Tezuka F., Orikasa S.1996Estrogen metabolism and impaired spermatogenesis in germ cells tumors of the testis. J. Clin. Endocrinol. Metab. 81, 1289–1295 (doi:10.1210/jc.81.3.1289) [DOI] [PubMed] [Google Scholar]
- Naz R. K.1998Effect of actinomycine D and cycloheximide on human sperm function. Arch. Androl. 41, 135–142 (doi:10.3109/01485019808987955) [DOI] [PubMed] [Google Scholar]
- O'Donnell L., Robertson K. M., Jones M. E., Simpson E. R.2001Estrogen and spermatogenesis. Endocrinol. Rev. 22, 289–318 (doi:10.1210/er.22.3.289) [DOI] [PubMed] [Google Scholar]
- Oliva R.2006Protamines and male infertility. Hum. Reprod. Update 12, 417–435 (doi:10.1093/humupd/dml009) [DOI] [PubMed] [Google Scholar]
- Payne A. H., Kelch R. P., Musich S. S., Halpern M. E.1976Intratesticular site of aromatization in the human. J. Clin. Endocrinol. Metab. 42, 1081–1087 (doi:10.1210/jcem-42-6-1081) [DOI] [PubMed] [Google Scholar]
- Pelletier G., El-Alfy M.2000Immunocytochemical localization of estrogen receptors α and β in the human reproductive organs. J. Clin. Endocrinol. Metab. 85, 4835–4840 (doi:10.1210/jc.85.12.4835) [DOI] [PubMed] [Google Scholar]
- Pentikaïnen V., Erkkilä K., Suomalainen L., Otala M., Pentikaïnen M. O., Parvinen M., Dunkel L.2000Estradiol acts as a germ cell survival factor in the human testis in vitro. J. Clin. Endocrinol. Metab. 85, 2057–2067 (doi:10.1210/jc.85.5.2057) [DOI] [PubMed] [Google Scholar]
- Pereyra-Martinez A. C., Roselli C. E., Stadelman H. L., Resko J. A.2001Cytochrome P450 aromatase in testis and epididymis of male rhesus monkeys. Endocrine 16, 15–19 (doi:10.1385/ENDO:16:1:15) [DOI] [PubMed] [Google Scholar]
- Prossnitz E. R., Arterburn J. B., Smith H. O., Oprea T. I., Sklar L. A., Hathaway H. J.2008Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annu. Rev. Physiol. 70, 165–190 (doi:10.1146/annurev.physiol.70.113006.100518) [DOI] [PubMed] [Google Scholar]
- Pura M., Mittre H., Carreau S., Kottler M. L.2003Clinical findings in an adult man with a novel mutation of the aromatase gene. In Proc. Endocrine Society, vol. 467, p. 243 Philadelphia, PA: Endocrine Society [Google Scholar]
- Rago V., Bilinska B., Palma A., Ando S., Carpino A.2003Evidence of aromatase localization in cytoplasmic droplet of human immature ejaculated spermatozoa. Folia Histochem. Cytobiol. 41, 23–28 [PubMed] [Google Scholar]
- Raman J. D., Schlegel P. N.2002Aromatase inhibitors for male infertility. J. Urol. 167, 624–629 (doi:10.1016/S0022-5347(01)69099-2) [DOI] [PubMed] [Google Scholar]
- Revelli A., Massobrio M., Tesarik J.1998Nongenomic actions of steroid hormones in reproductive tissues. Endocrinol. Rev. 19, 3–17 (doi:10.1210/er.19.1.3) [DOI] [PubMed] [Google Scholar]
- Rochira V., Granata A. R. M., Madeo B., Zirilli L., Rossi G., Carani C.2005Estrogens in males: what have we learned in last 10 years? Asian J. Androl. 7, 3–20 (doi:10.1111/j.1745-7262.2005.00018.x) [DOI] [PubMed] [Google Scholar]
- Rochira V., et al. 2006Hypothalamic-pituitary gonadal axis in two men with aromatase deficiency: evidence that circulating estrogens are required at the hypothalamic level for the integrity of gonadotropin negative feedback. Eur. J. Endocrinol. 155, 513–522 (doi:10.1530/eje.1.02254) [DOI] [PubMed] [Google Scholar]
- Roger C., Lambard S., Bouskine A., Mograb B., Chevallier D., Nebout M., Pointis G., Carreau S., Fenichel P.2005Estrogen-induced growth inhibition of human seminoma cells expressing estrogen receptor β and aromatase. J. Mol. Endocrinol. 35, 191–199 (doi:10.1677/jme.1.01704) [DOI] [PubMed] [Google Scholar]
- Saez J. M.1994Leydig cells: endocrine, paracrine, and autocrine regulation. Endocr. Rev. 15, 574–626 [DOI] [PubMed] [Google Scholar]
- Said L., Galeraud-Denis I., Carreau S., Saad A.2009Relationship between semen quality and seminal plasma components: alpha-glucosidase, fructose, and citrate in infertile men compared with a normospermic population of Tunisian men. Andrologia 41, 150–156 [DOI] [PubMed] [Google Scholar]
- Saunders P. T. K., Sharpe R. M., Williams K., Macpherson S., Urquart H., Irvine D. S., Millar M. R.2001Differential expression of oestrogen receptor α and β proteins in the testes and male reproductive system of human and non-human primates. Mol. Hum. Reprod. 7, 227–236 (doi:10.1093/molehr/7.3.227) [DOI] [PubMed] [Google Scholar]
- Saunders P. T. K., Millar R., MacPherson S., Irvin D. S., Groome N. G., Evans L. R., Sharpe R. M., Scobie G. A.2002ERβ1 and the ERβ2 splice variant (ERβcx/β2) are expressed in distinct cell populations in the adult human testis. J. Clin. Endocrinol. Metab. 87, 2706–2715 (doi:10.1210/jc.87.6.2706) [DOI] [PubMed] [Google Scholar]
- Sebastian S., Takayama K., Shozu M., Bulun S. E.2002Cloning and characterization of a novel endothelial promoter of the human CYP19 (aromatase P450) gene that is up-regulated in breast cancer tissue. Mol. Endocrinol. 16, 2243–2254 (doi:10.1210/me.2002-0123) [DOI] [PubMed] [Google Scholar]
- Simpson E. R., et al. 1994Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocrinol. Rev. 15, 342–355 [DOI] [PubMed] [Google Scholar]
- Skakkebaek N. E.2004Testicular dysgenesis syndrome: new epidemiological evidence. Int. J. Androl. 27, 189–201 (doi:10.1111/j.1365-2605.2004.00488.x) [DOI] [PubMed] [Google Scholar]
- Smith E. P., Boyd J., Frank G. R., Takahashi H., Cohen R. M., Specker B., Williams T. C., Lubahn D. B., Korach K. S.1994Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N. Engl. J. Med. 331, 1056–1061 (doi:10.1056/NEJM199410203311604) [DOI] [PubMed] [Google Scholar]
- Solakidi S., Psarra A. M. G., Nikolaropoulos S., Sekeris C. E.2005Estrogen receptors alpha and beta (ERα and ERβ) and androgen receptor (AR) in human sperm: localization of ERβ and AR in mitochondria of the midpiece. Hum. Reprod. 20, 3481–3485 (doi:10.1093/humrep/dei267) [DOI] [PubMed] [Google Scholar]
- Toppari J., et al. 1996Male reproductive health and environmental xenoestrogens. Environ. Health Perspect. 104, 741–803 (doi:10.2307/3432709) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner K. J., et al. 2002Development and validation of a new monoclonal antibody to mammalian aromatase. J. Endocrinol. 172, 21–30 (doi:10.1677/joe.0.1720021) [DOI] [PubMed] [Google Scholar]
- Watson C. S., Alyea R. A., Jeng Y.-J., Kochukov M. Y.2007Nongenomic actions of low concentration estrogens and xenoestrogens on multiple tissues. Mol. Cell. Endocrinol. 274, 1–7 (doi:10.1016/j.mce.2007.05.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zirilli L., Rochira V., Diazzi C., Caffagni G., Carani C.2008Human models of aromatase deficiency. J. Steroid Biochem. Mol. Biol. 109, 212–218 (doi:10.1016/j.jsbmb.2008.03.026) [DOI] [PubMed] [Google Scholar]
- Zondek B.1934Mass excretion of oestrogenic hormone in the urine of the stallion. Nature (London) 193, 209–210 [Google Scholar]