Abstract
Spermatogenesis is a highly regulated process of germ cell proliferation and differentiation, starting from spermatogonia to spermatocytes and giving rise to spermatids, the future spermatozoa. In addition to endocrine regulation, testicular cell–cell interactions are essential for spermatogenesis. This precise control is mediated through paracrine/autocrine pathways, direct intercellular contacts and through intercellular communication channels, consisting of gap junctions and their constitutive proteins, the connexins. Gap junctions are localized between adjacent Leydig cells, between Sertoli cells and between Sertoli cells and specific germ cells. This review focuses on the distribution of connexins within the seminiferous epithelium, their participation in gap junction channel formation, the control of their expression and the physiological relevance of these junctions in both the Sertoli–Sertoli cell functional synchronization and the Sertoli–germ cell dialogue. In this review, we also discuss the potential implication of disrupted connexin in testis cancer, since impaired expression of connexin has been described as a typical feature of tumoral proliferation.
Keywords: connexin, spermatogenesis, cell proliferation, fertility, cancer
1. Introduction
Testis exerts two major functions, an endocrine function, characterized by synthesis of androgens, and an exocrine function via spermatogenesis. Spermatogenesis, which takes place within seminiferous tubules, is a highly controlled process that may be divided into three principal phases: spermatogonia proliferation, spermatocyte meiosis and spermatid differentiation (Clermont 1972). In addition, developing germ cells must progressively migrate from the basal to the adluminal compartment of seminiferous tubules so that fully differentiated spermatids can be released into the lumen at spermiation. Within the seminiferous epithelium, spermatogenesis is supported by Sertoli cells that provide structural and nutritional supports for the developing germinal cells (Griswold 1993). Spermatogenesis requires a functional hypothalamo-pituitary system, involving luteinizing hormone (LH) and follicle stimulating hormone (FSH), and a local control via paracrine signals. FSH stimulates the proliferation of Sertoli cells during the neonatal period. LH indirectly controls spermatogenesis through androgens produced by Leydig cells located in the interstitial compartment between the seminiferous tubules.
Local cell–cell interactions, including direct contact-dependent junctional pathways, are also essential in the regulation of mammalian spermatogenesis and in the maintenance of the male phenotype (Mruk & Cheng 2004). These junctions are composed of specialized proteins implicated in cell adhesion (cadherin, catenin, nectin, integrin, etc.) and in the regulation of paracellular diffusion, the establishement and maintenance of cell polarity, and in cell attachment (occludin, claudin, Zonula occludens, etc.) (reviewed in Cheng & Mruk 2002). A third type of junction present in the testis is gap junctions and their constitutive proteins, connexins (Cxs). The present review seeks to update the recent developments on Cx identification and function in the control of spermatogenesis and their alteration in testicular diseases such as cancer of the testis.
2. Gap junction channels and connexins
Gap junctions are intercellular plasma membrane channels that create electric and metabolic coupling from cell to cell in a wide variety of tissues. Gap junctions are formed by the docking of two hemi-channels, termed connexons, present at opposing plasma membranes of adjacent cells, resulting from the oligomerization of six protein subunits named connexins. Connexons, which are delivered to the cell surface, assemble into gap junction plaques that coalesce within the plane of the cell membrane (figure 1). Today, 20 members of this multigene family, mapped on different chromosomes, have been cloned in the mouse genome (mCx23, mCx26, mCx29, mCx30, mCx30.2, mCx30.3, mCx31, mCx31.1, mCx32, mCx33, mCx36, mCx37, mCx39, mCx40, mCx43, mCx45, mCx46, mCx47, mCx50 and mCx57), while 21 connexin genes (hCx23, hCx25, hCx26, hCx30, hCx30.2, hCx30.3, hCx31, hCx31.1, hCx31.9, hCx32, hCx36, hCx37, hCx40, hCx40.1, hCx43, hCx45, hCx46, hCx47, hCx50, hCx59 and hCx62) have been identified in human (Willecke et al. 2002; Sohl & Willecke 2003). The current nomenclature is based on their molecular weights. Cxs assemble into homomeric or heteromeric hemi-channels, which can dock with hemichannels from adjacent cells to form homotypic or heterotypic gap junction channels when the same Cxs or different Cxs associate. Depending on the Cx type, the pore diameter can vary from 6.5 to 15 Å and allow the passage of molecules less than 1.5 kDa such as amino acids or short peptides, ions (Cl−, Na+, K+ and Ca2+) and second messengers such as cyclic adenosine monophosphate (cAMP) and inositol 1,4,5-trisphosphate (IP3; Bruzzone et al. 1996). Since heterotypic channels exhibit different unitary conductance and permeability to signalling molecules that pass through these channels, it is probable that the different Cxs support functional specialization in different cell types (reviewed in Cottrell & Burt 2005).
Figure 1.
Schematic representation of gap junction plaque, gap junction channel and connexins. Gap junctions are aqueous channels formed by the docking of two hemichannels (connexons) between two adjacent cells. Each hemi-channel is composed of six transmembrane proteins (connexins). Each connexin monomer is composed of four transmembrane domains with two extracellular loops, one intracellular loop and intracellular carboxyl and amino ends.
This process of molecular signal exchange through gap junctions, called gap junction intercellular communication (GJIC), appears to be involved in several cellular processes including homeostasis, electrical coupling, intercellular synchronization, metabolic support, embryogenesis, hormone responsiveness and control of cell growth (reviewed in Hervé et al. 2007). GJIC can be regulated at different levels including, Cx, connexon docking and gating of the channel. The co-expression of at least two different Cxs that form a heteromeric connexon within the same cell is another mechanism by which the channel function can be controlled (Martin & Evans 2004).
The regulation of Cx expression depends on a fine-tuned balance involving various processes, such as gene transcription, mRNA processing, protein synthesis, post-translational modifications (phosphorylation), assembly in the Golgi apparatus, transport to the cell surface, anchoring to the cytoskeleton, regulation of internalization and degradation of the protein (Saez et al. 2003). In pathological situations, disruption of GJIC, which could be caused by several mechanisms such as mutation of Cx, altered expression of the Cx genes (methylation) or impaired trafficking of the protein to the plasma membrane, can lead to a loss of balanced control between cell proliferation, differentiation and apoptosis (Chipman et al. 2003). An interesting feature is that impaired GJIC and Cx expression have been reported in numerous human tumours, almost all malignant cell lines, and after exposure to tumour promoters and oncogene expression (Trosko & Ruch 1998; Mesnil et al. 2005; Pointis et al. 2007). The notion that Cx are tumour-suppressor genes came from the observation that Cx transfection into transformed cells restores normal cell growth, leading to the proposal that pharmacological Cx up-regulation may be of therapeutic interest in the treatment of cancer (Trosko & Ruch 2002; Salameh & Dhein 2005). In addition to the classical role of gap junction, recent studies reported that undocked channels also exert physiological roles. Indeed, hemi-channels could be involved in numerous cellular processes such as the release of ATP, NAD+, prostaglandins and glutamate, intercellular Ca2+ wave propagation, cell-volume control and the passage of survival signals (reviewed in Spray et al. 2006). Cxs themselves may function as scaffold protein for the attachment of cytoplasmic elements and potentially play a role other than that classically described for intercellular communication (Huang et al. 1998).
More recently, a second family of three gene-encoding proteins named pannexins (Panx1, Panx2 and Panx3) were cloned in vertebrates and these proteins were found homologous to the invertebrate gap junction proteins innexins (Bruzzone et al. 2003; Baranova et al. 2004). However, in contrast to connexins, which constitute the intercellular gap junction channels, it has been postulated that pannexins play an important biological paracrine role as single membrane channels that allow the release of ATP and, thus, the modulation of the intercellular Ca2+ wave transmission between cells (Scemes et al. 2007).
3. Connexin distribution in the testis
The testis is a well-organized tissue that needs efficient and fast intercellular communication involved in germ cell proliferation, migration, differentiation and apoptosis. The cross-talk between testicular cells may be mediated through several mechanisms including paracrine/autocrine pathways, direct intercellular contact (via adhesion molecules), as well as gap junctional pathways (reviewed in Mruk & Cheng 2004; Pointis & Segretain 2005).
In rodents, transcripts for Cx26, Cx30.3, Cx31, Cx31.1, Cx37, Cx40, Cx45 and Cx46 were detected in the foetal testis (Juneja 2003) and for Cx26, 30.2, 31, 31.1, 32, 33, 37, 40, 43, 46 and 50 in the adult testis (Risley 2000). However, the precise localization of the corresponding proteins has not been clearly demonstrated (table 1). Cx43, the most predominant Cx, is located in both interstitial and seminiferous tubule compartments. Cx43 is the only Cx expressed in Leydig cells (Risley et al. 1992; Pérez-Armendariz et al. 1994; Varanda & de Carvalho 1994; Batias et al. 1999). It has been suggested that Cx43 gap junctions in Leydig cells may coordinate the androgenic secretory activities of these cells associated with specific stages of the seminiferous epithelium. Cx43 was detected within the seminiferous tubules from birth to all postnatal stages (Bravo-Moreno et al. 2001; Perez-Armendariz et al. 2001). Cx43 immunostaining was first localized in the adluminal region of the growing tubules. At the onset of the blood–testis barrier formation, the Cx43 signal progressively moves towards the basal region of the tubules following the seminiferous epithelium compartmental reorganization (Tan et al. 1996; Batias et al. 2000). The Cx43 signal shift from the luminal to the basal region of the seminiferous tubules could be due to the progressive reorganization of palissadic epithelial-like Sertoli cells to the final typical tree-like aspect of the adult Sertoli cells and to the large germ cell proliferation, which invade most of the spaces within the tubules and participate in the Sertoli cell remodelling. In the mature testis Cx43 is mainly found at the level of the blood–testis barrier between Sertoli cells and probably allows the synchronization of Sertoli cell function metabolism during the first wave of spermatogenesis and during specific stages of the adult seminiferous epithelium (Risley et al. 1992). Cx43 present between Sertoli and germ cells could promote Sertoli cell to germ cell metabolic coupling and cell signalling (Batias et al. 1999, 2000; Gilleron et al. 2009).
Table 1.
Distribution of connexin (Cx) mRNA and protein in whole testis and in isolated populations of testicular cells. SC: Sertoli cells; LC: Leydig cells; GC: total germ cells; MC: myoid cells; EC: endothelial cells; SMC: vascular smooth muscle cells; sp: spermatogonia; st: spermatocytes; sd: spermatids. ‘X’ indicates presence of the mRNA/protein when investigated.
| testicular cell types |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| connexin | SC | LC | GC | MC | EC | SMC | whole testis | mRNA/protein | references |
| Cx26 | — | — | — | — | — | — | X | mRNA | Zhang & Nicholson (1989) |
| X | — | — | — | — | — | — | protein | Risley et al. (1992) | |
| X | — | st, sd | X | — | — | X | mRNA | Risley (2000) | |
| — | — | — | — | — | — | X | protein | Brehm et al. (2002) | |
| Cx30.2 | — | X | — | — | — | X | X | mRNA, protein | Nielsen & Kumar (2003) |
| Cx31 | — | — | st, sd | — | — | — | X | mRNA, protein | Mok et al. (1999) |
| X | — | st, sd | X | — | — | X | mRNA | Risley (2000) | |
| — | — | — | — | — | — | X | mRNA | Luk et al. (2003) | |
| Cx31.1 | — | — | — | — | — | — | X | mRNA | Hennemann et al. (1992) |
| — | — | — | — | — | — | X | mRNA | Risley (2000) | |
| — | — | — | — | — | X | — | mRNA | Nielsen et al. (2002) | |
| Cx31.9 | — | — | — | — | — | — | X | mRNA | White et al. (2002) |
| Cx32 | — | — | — | — | — | — | X | mRNA | Zhang & Nicholson (1989) |
| X | — | — | — | — | — | — | protein | Risley et al. (1992) | |
| X | — | st, sd | — | — | — | X | mRNA | Risley (2000) | |
| X | — | — | — | — | — | X | protein | Gilleron et al. (2009, in press) | |
| Cx33 | — | — | — | — | — | — | X | mRNA | Haefliger et al. (1992) |
| X | — | — | — | — | — | — | protein | Tan et al. (1996) | |
| — | — | X | — | — | — | X | mRNA | Chung et al. (1999) | |
| X | — | st, sd | — | — | — | X | mRNA | Risley (2000) | |
| X | — | — | — | — | — | X | protein | Fiorini et al. (2004a,b) | |
| X | — | st | — | — | — | X | mRNA, protein | Fisher et al. (2005) | |
| Cx37 | — | — | — | — | — | — | X | mRNA | Haefliger et al. (1992) |
| — | — | — | — | X | — | — | protein | Tan et al. (1996) | |
| X | — | st, sd | X | — | — | X | mRNA | Risley (2000) | |
| Cx40 | X | — | st, sd | X | — | — | X | mRNA | Risley (2000) |
| Cx43 | X | X | st, sg | X | X | — | X | protein | Risley et al. (1992) |
| — | X | — | — | — | — | — | protein | Varanda & de Carvalho (1994) | |
| — | X | — | — | — | — | — | protein | Pérez-Armendariz et al. (1994) | |
| X | X | st, sg | — | — | — | — | mRNA, protein | Batias et al. (1999, 2000) | |
| X | — | st, sd | X | — | — | X | mRNA | Risley (2000) | |
| X | X | X | — | — | — | X | protein | Bravo-Moreno et al. (2001) | |
| X | — | sg | — | — | — | — | protein | Gilleron et al. (2009) | |
| X | — | X | X | — | — | — | protein | Gilleron et al. (in press) | |
| X | — | st | — | — | — | — | protein | Godet et al. (2008) | |
| — | — | — | — | — | — | X | protein | Brehm et al. (2002) | |
| Cx45 | X | — | st, sd | X | — | — | X | mRNA | Risley (2000) |
| Cx46 | X | — | — | — | — | — | X | mRNA | Risley (2000) |
| Cx50 | — | — | st, sd | — | — | — | X | mRNA | Risley (2000) |
| Cx57 | — | — | — | — | — | — | X | mRNA | Manthey et al. (1999) |
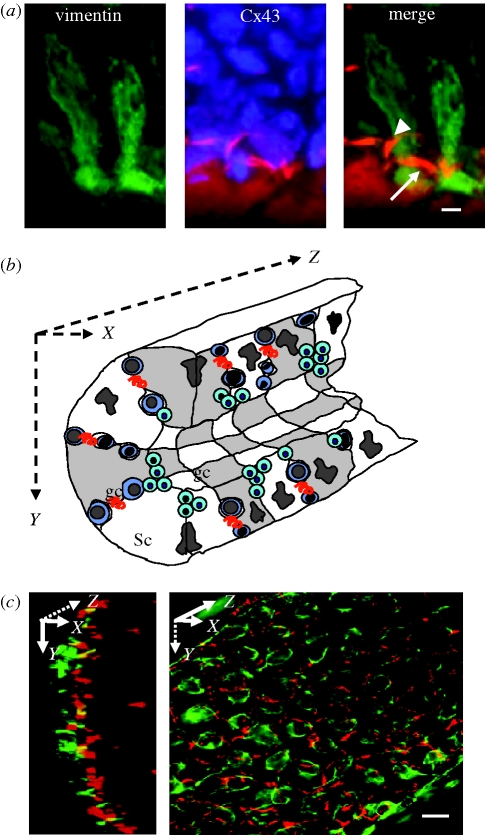
Cx43 mRNA are specifically localized in the basal seminiferous compartment and equally distributed in the somatic cells, Sertoli cells, and in spermatogonia and spermatocytes of rat (Batias et al. 2000) and human (Defamie et al. 2003), suggesting that Cx43 gap junction channels could allow communication between these two cell types. Other studies suggested that the multiple gap junction communication pathways that occur within the seminiferous epithelium cells could offer different permeability properties and different regulatory properties (Risley et al. 2002). By developing a sophisticated assay that allows the correlation of three parameters (function, cell identification and Cx43 expression), we demonstrated that Cx43 gap junction coupling occurs between adjacent Sertoli cells, and between Sertoli cells and spermatogonia and early and late spermatocytes, but not between Sertoli cells and spermatids (figure 2a; Decrouy et al. 2004). A similar conclusion was also drawn from observations in the catfish Pseudoplatystoma fasciatum, demonstrating that Cx32, the homologue of Cx43 in this species, was localized in Sertoli cells and inside germinal cysts present at the base of the seminiferous tubules and containing secondary spermatogonia and primary spermatocytes (Batlouni et al. 2005).
Figure 2.
Connexin 43 expression within the rat seminiferous epithelium. (a) Cx43 immunolabelling (red fluorescence) in Sertoli cells immunopositive for vimentin (green fluorescence), a specific marker of Sertoli cell within the seminiferous epithelium. Nuclei of the two adjacent Sertoli cells and germ cells are identified by DAPI staining (blue fluorescence). Merge indicates that Cx43 immunosignal is detected between Sertoli cells (arrow) and between Sertoli and germ cells (arrowhead). (b) Schematic three-dimensional representation of a seminiferous tubule with Sertoli cells (Sc) and germ cells (gc). The red curved lines represent gap junctions. (c) Isolated seminiferous tubules are observed in two different angles as described in the schematic representation. Side view (left panel, XY axis) and face view (right panel, XZ axis) show the basal organization of Sertoli cells determined by vimentin immunolabeling (green fluorescence) and the localization of Cx43 (red fluorescence). Note that Cx43 is present between Sertoli cells distributed along the XY axis and the XZ axis. Scale bars, (a) 5 µm; (c) 15 µm.
Cx26 and Cx32 are present in the apical regions of the seminiferous epithelium (Risley et al. 1992). We have recently demonstrated that Cx32 was present in Sertoli cells, but undetectable in germ cells (Gilleron et al. 2009), in contrast to Cx31, which was only found in germ cells, such as spermatogonia and early spermatocytes (leptotene, zygotene; Mok et al. 1999).
The Cx33 gene has been cloned in the rodent testis (Haefliger et al. 1992) and Cx33 mRNAs have been detected in this organ (Chang et al. 1996). The product of Cx33 gene appears specifically restricted to Sertoli cells (Tan et al. 1996; Fiorini et al. 2004a,b; Fisher et al. 2005; Lee et al. 2006), although others detected the presence of Cx33 mRNA in whole germ cells and in enriched preparations of pachytene spermatocytes and round spermatids (Chung et al. 1999; Risley 2000). Cx33 has been described as the only wild-type Cx able to exert an inhibitory effect on gap junction channel function by altering the junctional conductance when coexpressed with other Cxs (Chang et al. 1996, 1999). The mechanism(s) by which Cx33 blocks the gap junction coupling has been extensively studied by our group. In the testis, Cx33 is able to specifically associate with Cx43 and not with Cx26 and Cx32, and to sequester Cx43 within early endosomes when overexpressed in a Sertoli cell line (Fiorini et al. 2004a,b). By using Cx33 and Cx43 transfected Sertoli cells, we recently demonstrated that the two Cxs can be assembled into the same connexons (heteroconnexons) that trafficked from the Golgi apparatus to the plasma membrane with the same velocity as the Cx43 homoconnexons. However, in contrast to Cx43 connexons, these heterocomplex structures were not stable at the plasma membrane and were rapidly internalized (Carette et al. 2009), explaining both the uncou09pling of Cx33 transfected Sertoli cells and the intracytoplasmic accumulation of the Cx33–Cx43 complex previously described (Fiorini et al. 2004a,b).
4. Control of connexin 43 in the testis
There is now evidence that testicular Cx expression has a multi-component control system dependent on the hormonal environment, the cell types and the developmental and spermatogenic stages. However, neither the hormonal effectors nor the mechanisms that control Cx cell communication in the testis have been clearly characterized.
In Leydig cells, Cx43 mRNA is present in 20-day-old rat, reaches a plateau at day 40 and remains at high levels in 65- and 80-day-old rats, suggesting that the expression of Cx43 is dependent on the stage of development (You et al. 2000). These authors also reported that human chorionic gonadotropin diminished Cx43 mRNA and protein in rat Leydig cells, in vitro and in vivo, and caused a redistribution of Cx43 to the Leydig cell plasma membrane. Studies on the TM3 Leydig cell line demonstrated that increased testosterone secretion in response to LH was associated with reduced intercellular communication, suggesting that gap junction mediated coupling could be the modulator of hormone secretion in Leydig cells and consequently of spermatogenesis (Goldenberg et al. 2003).
Sertoli cells proliferate during foetal and prepubertal life in mammals and are influenced by multiple endocrine and paracrine factors (for review, see Sharpe et al. 2003). The key hormones involved in this process are FSH and thyroid hormones. Since Cx43 is an FSH-responsive gene, the possibility that FSH controls neonatal Sertoli cell proliferation and differentiation, partly through Cx activation, has been suggested. This hypothesis is in agreement with data showing that in the ovary FSH increases the levels of Cx43 mRNA, reduces the cell proliferation and enhances the differentiation of granulosa cells, the homologue of testicular Sertoli cells (Sommersberg et al. 2000). However, such a process has not been clearly established for Sertoli cells. Other studies demonstrated that FSH and cAMP control the electrical coupling (Grassi et al. 1986), the functional coupling (Pluciennik et al. 1994) and the Cx43 intracellular redistribution in Sertoli cells (Lablack et al. 1998), suggesting that FSH could also exert short-term effects on Cx43. Recently, we demonstrated in both in vitro and ex vivo models that FSH and its second messenger cAMP could alter gap junction plaque structures within 1 h (Gilleron et al. in press). Another possibility is that cAMP increases gap junction plaque formation through accretion of Cx43-channels due to PKA activation, as evidenced in cardiac myocytes (Somekawa et al. 2005). Altogether, these data suggest that in addition to potential genomic effects on Cx43, gonadotropin also exerts a non-genomic action.
Thyroid hormones, specifically through triiodothyronine (T3), can also control Cx43 expression in Sertoli cells and participate in the regulation of Sertoli cell proliferation during neonatal development as recently demonstrated in vivo and in vitro (St-Pierre et al. 2003; Gilleron et al. 2006). The latter study also demonstrates that T3 may control Cx function at both genomic and non-genomic levels. The genomic effect, which leads to increased Cx43 levels in Sertoli cells, is associated with a reduced rate of cell proliferation. The non-genomic effect of T3 may result in an increase of gap junction plaque number and size resulting from activated Cx43 trafficking and dependent on the actin network (Gilleron et al. 2006). This non-classical and rapid effect could participate in the coordination of Sertoli cell metabolism and also ensure metabolic and signalling coupling to germ cells.
Steroids have also been reported to regulate Cx43-dependent gap junctional communication between Sertoli cells. Previous studies demonstrated that testosterone and 17β-oestradiol (17β-E2) downregulated the electrical and diffusional couplings between Sertoli cells in primary culture (Hervé et al. 1996; Pluciennik et al. 1996). The identification of putative oestrogen response sites at the promoter region of Cx43 gene suggests that 17β-E2 increases Cx43 through a genomic pathway mediated by the nuclear oestrogen receptor (Lefebvre et al. 1995). However, no alteration of Cx43 protein levels was reported in the TM4 Sertoli cell line exposed to 17β-E2 for 24 h (Aravindakshan & Cyr 2005). We recently demonstrated that 17β-E2 as well as 5α-dihydrotestosterone affected cell–cell coupling within 1 h, by disrupting gap junction plaque at the plasma membrane and promoting gap junction plaque internalization, suggesting that 17β-E2 can control Sertoli cell gap junctions through both genomic and non-genomic pathways (Gilleron et al. in press). However, the specific mechanism(s) by which 17β-E2 rapidly altered gap junction plaque is unknown. The possibility that this effect could be mediated through membraneous oestrogen receptor and protein kinase activation involved in Cx trafficking could be hypothesized. Indeed, it has been reported that 17β-E2 stimulates a Src-mediated translocation of oestrogen receptors to the plasma membrane in Sertoli cells (Lucas et al. 2008). In addition, there is evidence that the proto-oncogene leads to Cx43 channel closure (reviewed in Lau 2005) and also to gap junction plaque internalization, as we recently described (Gilleron et al. 2008). A role of oestrogens in Cx43 regulation is also supported by toxicological studies, which show that compounds with oestrogen-like activity altered Sertoli cell Cx43 gap junctions (Kang et al. 2002; Fiorini et al. 2004a,b; Aravindakshan & Cyr 2005; de Montgolfier et al. 2008; Fiorini et al. 2008).
Another interesting feature is that retinoids, which participate in the control of spermatogenesis by controlling cell cycle regulation and cell–cell interactions (Wolgemuth & Chung 2007), are also physiological regulators of Cx43 expression in Sertoli cells. This is supported by the observation that testicular Cx43 transcripts and protein are reduced in a mutant mouse deficient in the retinoid X receptor β (Batias et al. 2000) and the findings that Cx43 mRNA levels are undetectable in the seminiferous epithelium of vitamin A-deficient rat but are restored after vitamin A administration (Luk et al. 2003).
Whether junctional proteins, such as adhesion and tight junction molecules, which are intermingled with Cx at the blood–testis barrier, control Cx43 gap junction at this level, could be also hypothesized. Indeed, it has been reported that E-cadherin influences the intracellular trafficking and function of Cx26 and Cx43 (Hernandez-Blazquez et al. 2001). ZO-1 silencing in Sertoli cells by siRNA resulted in significant impairment of Cx43 (D. Carette, J. Gilleron, D. Segretain & G. Pointis 2008, unpublished data).
Other data reported that Cx43 in Sertoli cells varies with the stages of spermatogenesis in several species such as mouse (Batias et al. 1999, 2000; Yu et al. 2003; Fiorini et al. 2006), rat (Risley et al. 1992; Tan et al. 1996), human (Steger et al. 1999; Mauro et al. 2008) and fish (de Montgolfier et al. 2007). These observations led to suggest that a group of germ cells or specific germ cells could control Cx expression in the somatic cells. This hypothesis has also been supported in mutant mice with specific arrest of spermatogenesis (Batias et al. 1999). Interestingly in the rainbow trout testis, specific Cx are localized to different testicular cell types, suggesting that the messages exchanged between these cells are different and that they change as a function of the stage of spermatogenesis (de Montgolfier et al. 2007). In guinea pig and mink, the distribution of Cx43 within the seminiferous tubules changed with germ cell differentiation in a stage-dependent manner and with the dynamic change of the blood–testis barrier accompanying the translocation of spermatocytes into the adluminal compartment (Pelletier 1995).
5. Physiological relevance of connexins and gap junction channels in spermatogenesis
There is now strong evidence that Cx43 plays several essential roles during testis development and later in the control of spermatogenesis. From immunohistochemical studies, it has been proposed that Cx gap junctions form a highly controlled transverse and longitudinal intercellular communication network within the seminiferous epithelium (figure 2b,c) (Gilleron et al. in press). In addition, the functional diversity of gap junctions, related to the nature of the Cx, may allow controlled communication among the many interacting Sertoli cells and germ cell stages to ensure the local synchronization of germ cell proliferation and differentiation (Risley et al. 2002).
The direct evidence of the involvement of Cx43 in testicular development comes from observations in transgenic mice, in which the Cx genes were invalidated (table 2). While knock-out animals for Cx31, Cx32, Cx40, Cx46 and Cx50 show normal fertility (Willecke et al. 1999), Cx43 deficient mice exhibit 50 per cent depletion in primordial germ cells (PGCs) in foetal testes (Juneja et al. 1999). More recent findings showed that Cx43 is essential for PGC motility in 8.5- and 11.5-day-old embryos and cell survival in older embryos, with abnormal p53 activation playing a crucial role in the apoptotic loss of PGCs at the later stages in the Cx43 knock-out mouse embryos (Francis & Lo 2006).
Table 2.
Effect of Cx gene invalidation and protein inhibition by mimetic inhibitory peptides on mouse and rat spermatogenesis. Seminiferous tubules showing Sertoli-cell-only syndrome are totally devoid of germ cells. In mice Cx43KI32 and Cx43KI40 the coding sequence of the Cx43 gene is substituted by the coding sequences of Cx32 or Cx40. The pan-Cx mimetic peptide virtually recognized all the identified Cxs found in the testis.
| connexin (Cx) | effect of Cx gene invalidation or protein inhibition on spermatogenesis | references |
|---|---|---|
| KO Cx26 | unknown since embryonic lethality | Gabriel et al. (1998) |
| KO Cx31 | no effect on spermatogenesis | Plum et al. (2001) |
| KO Cx32 | no effect on spermatogenesis | Nelles et al. (1996) |
| KO Cx33 | unknown since embryonic lethality | |
| KO Cx37 | no alteration of spermatogenesis but females are sterile | Simon et al. (1997) |
| KO Cx40 | no alteration of spermatogenesis | Simon et al. (1998), Kirchhoff et al. (1998) and van Rijen et al. (2001) |
| KO Cx43 | germ cell deficiency in foetal testis and in postnatally grafted testis primordial germ cell apoptosis | Reaume et al. (1995), Juneja et al. (1999) and Francis & Lo (2006) |
| replacement of Cx43 by Cx26 | presence of spermatogonia and some primary spermatocytes | Winterhager et al. (2007) |
| KO Cx46 | no alteration of spermatogenesis | Gong et al. (1997) |
| KO Cx47 | no alteration of spermatogenesis | Odermatt et al. (2003) |
| KO Cx50 | no alteration of spermatogenesis | White et al. (1998) |
| Cx43 KI 32 Cx43 KI 40 | alteration of spermatogenesis (Sertoli-cell-only syndrome) | Plum et al. (2000) |
| double KO Cx37/Cx40 | impairment of spermatogenesis due to vascular anomalies | Simon & McWhorter (2002) |
| conditional Sertoli cell Cx43 KO | inhibition of Sertoli cell maturation, alteration of spermatogenesis | Brehm et al. (2007) and Sridharan et al. (2007) |
| KO innexin 4 | reduced number of spermatogonia in Drosophila | Tazuke et al. (2002) |
| Pan-Cx inhibitory mimetic peptide | germ cell apoptosis after intratesticular injection | Lee et al. (2006) |
| Cx43 inhibitory mimetic peptides | increased Sertoli cell proliferation and spermatogonia survival in vitro | Gilleron et al. (2009, in press) |
During the neonatal period, Cx43 appears to be essential in the control of Sertoli cell proliferation since testes from treated neonatal rats with 6-N-propyl-2-thiouracil (PTU), a reversible goitrogen, show an increased Sertoli cell proliferation within the seminiferous epithelium associated with reduced Cx43 levels (Gilleron et al. 2006) and an altered Cx43 localization in the neonatal testis (St-Pierre et al. 2003). By using an in vitro proliferation model of germ and Sertoli cells associated with the use of Cx mimetic inhibitory peptides, we recently demonstrated that Cx43 gap junctions between Sertoli cells participate in the control of Sertoli cell proliferation and that Cx43 gap junctions between Sertoli cells and spermatogonia are indirectly involved in germ cell number by controlling germ cell survival rather than germ cell proliferation (Gilleron et al. 2009).
To assess the potential role of Cx43 in spermatogenesis, testes from the null mutant foetuses were grafted under the kidney capsules of adult males and postnatal testis development was analysed since such mice die shortly after birth (Reaume et al. 1995). As reported above for foetuses, germ cell deficiency persists postnatally, giving rise to a ‘Sertoli cell only’ SCO phenotype (Roscoe et al. 2001). The substitution of the Cx43 gene by the coding sequences of Cx32 and Cx40 in knock-in mice (Cx43KI32 and Cx43KI40) results in mature animals with severe impairments of spermatogenesis with SCO seminiferous tubules (Plum et al. 2001). A direct relationship between Cx43 and germ cell maturation has also been reported in non-mammalian testes. Reduced spermatogonia number has been described in Drosophila males carrying a null mutation in the zero population growth (zpg) locus that encodes the germ line-specific gap junction protein, innexin 4 (Tazuke et al. 2002). In frog testis, Cx43 transcript and protein show a parallel temporal and spatial pattern of expression throughout the reproductive annual cycle, with higher levels when spermatogenesis is at a maximum level (Izzo et al. 2006).
These observations on the role of Cx43 gap junctions in germ cell proliferation during adulthood have been supported by indirect evidence. During stages IX and X of spermatogenesis, we reported that Cx43 was poorly expressed within seminiferous tubules whereas the Cx33 was strongly expressed (Fiorini et al. 2006). At about these stages of spermatogenesis, residual bodies formed from the excess of germ cell cytoplasm are phagocytosed by Sertoli cells and this process is known to induce the secretion of IL-1α, an activator of spermatogonial DNA synthesis (Amjad et al. 2006). Thus, we hypothesized that Sertoli cell phagocytosis of residual bodies could control germ cell proliferation by stimulating IL-1α and controlling Cx33 and Cx43 gene expression. In these conditions, the induction of the negative Cx33 could overcome the control exerted by Sertoli cells through Cx43-based gap junctions on germ cell proliferation (Pointis et al. 2009). IL-1α has also been reported to restructure the blood–testis barrier during stages VIII–XI of spermatogenesis and to alter the distribution of tight and adherens junction molecules (Sarkar et al. 2008). Thus, it is possible that the cytokine affects all similar connexins since gap junctions are intermingled with tight and adherens junctions at this level.
Recently, two laboratories have independently developed Sertoli cell conditional Cx43 knock-out mice using Cre-loxP methodology for a better analysis of the role of Cx43 in spermatogenesis (Brehm et al. 2007; Sridharan et al. 2007). From these studies, it appears that Cx43 is essential for spermatogenesis, probably through controlling the cessation of proliferation and the differentiation of Sertoli cells. In one of these studies a marked reduction of the spermatogonia number per tubule was measured in Sertoli cell Cx43KO−/− mouse testes, suggesting that Sertoli cell Cx43 plays a major role in supporting germ cell proliferation and/or survival (Brehm et al. 2007). From these studies, it has also been suggested that Sertoli cell Cx43 must exhibit unique regulatory or physiological properties since its loss in Sertoli cell Cx43 knock-out mice cannot be compensated by other Cxs known to be expressed in Sertoli cells and spermatogonia. This hypothesis was also supported by the findings that Cx26 and Cx32, two other Cxs expressed in Sertoli cells, cannot functionally replace Cx43 (Plum et al. 2001; Winterhager et al. 2007). However, taken together, these data in Cx43 KO mice do not prove the existence of a close relationship between the alteration of the Sertoli–germ cell Cx43 gap junctions and the reduced germ cell proliferation. Although in some cell types, the loss of function of a Cx can be compensated by other Cxs (Minkoff et al. 1999), this is unlikely in the testis since the Cx43 gene has conserved an individual and specific role to maintain proliferation and survival of germ cells by both regulating intercellular communications between germ cells and adjacent supporting cells during spermatogenesis.
Another interesting question is whether Cx43 gap junctions between Sertoli cells and germ cells are essential during the whole spermatogenic process or only during specific phases. In Sertoli cell conditional Cx43 knock-out mice, descriptions have recorded a reduced number of unidentified early stage germ cells or an arrest of spermatogenesis at the level of spermatogonia (Brehm et al. 2007; Sridharan et al. 2007). One day after intratesticular injection of Cx43 mimetic peptide, mostly spermatocytes and elongated spermatids were found in the seminiferous epithelium, whereas they contained only primary spermatocytes, and spermatogonia on day 15 post-treatment (Lee et al. 2006). More recently, it has been demonstrated that a close association between Sertoli cells and pachytene spermatocytes, propably via Cx43 gap junctions, is essential for meiotic progression of rat spermatocytes (Godet et al. 2008). These findings are in agreement with the previous immunohistochemical studies indicating that the gap junction protein is found in Sertoli cells and in specific germ cells (see §3). They suggest that Cx43 between Sertoli and germ cells are involved in at least two processes: the proliferation of spermatogonia and the late maturation of spermatocytes. This hypothesis is reinforced by the fact that these two germ cell types express Cx43 mRNA (Batias et al. 2000) and by the functionality of the Cx43 gap junction channel formed between Sertoli cells and these germ cells (Decrouy et al. 2004).
The mechanism(s) by which Cx43 gap junctions control germ cell proliferation is (are) poorly understood. In general, gap junctions may promote cell survival by controlling the flux of necrotic or apoptotic signal effectors or propagating diffusion of vital signals, thereby contributing to cell survival (Krysko et al. 2005). Intratesticular injection of Cx inhibitory mimetic peptides for pan-Cx and Cx43 but not for Cx31 and Cx33 led to a total dysregulation of spermatogenesis and subsequently triggered germ cell apoptosis via the caspase-3 and the NF-kappaB pathway (Lee et al. 2006). In Cx43 knock-out mice, primordial germ cell deficiency was associated with abnormal p53 activation (Francis & Lo 2006). By using an in vitro model of neonatal germ cell proliferation, we recently demonstrated that Cx43 and Cx32 participate in the control of Sertoli cell proliferation and that only Cx43 is involved in germ cell growth by controlling spermatogonia survival rather than proliferation (Gilleron et al. 2009). These data strongly support the possibility that testicular Cx43 maintains the number of germ cells by regulating Cx43 gap junctions between germ cells and adjacent supporting cells. In addition, it is probable that the physiological significance of Cx on spermatogenesis is the result of fine tuned-balance involving multiple elements such as the nature of Cx involvedash, their association to form functional channels, the communicating cell types (Sertoli–Sertoli or Sertoli–germ cells), the stage of the testicular development, the spermatogenic stages and the hormonal control. In view of recent studies demonstrating a role of hemi-channels (see §1), we cannot exclude that Sertoli hemi-channels act as a paracrine conduit to spread factors that modulate the fate of germ cells.
Another possibility is that Cxs and/or gap junctions may control the germ cell proliferation process through indirect regulation of tight and adherens junctions. Indeed, this putative regulation has been recently discussed in a review paper for several cell types, such as hepatocytes, mouse hepatic cells and in human airway epithelial Calu-3 cells (Kojima et al. 2007). At the level of the blood–testis barrier adherens junctions, tight junctions and gap junctions are closely intermingled with each other and the possibility that Cx-based gap junctions exert such an effect has been postulated (Lee et al. 2007). This hypothesis is also supported by previous studies in continual (guinea pig) and seasonal breeder (mink) testes, suggesting that Cx43 between Sertoli cells participates in the formation and regulation of the blood–testis barrier (Pelletier 1995). It has recently been reported that the blockage of Cx functions by using pan-connexin peptide, which recognizes all the characterized Cx within the testis, concomitantly leads to a diminished occludin expression and upregulated N-cadherin expression (Lee et al. 2006). Since Cx and occludin are present at the membrane within a protein complex, it has been postulated by these authors that the dysregulation of ZO-1, a common adaptor shared by occludin- and connexin-associated protein complexes (Lee & Cheng 2004), indirectly hampers the stability and integrity of the occludin associated protein complex.
Further analyses of junctional tight and adherens protein expression in Sertoli cell conditional Cx43 knock-out mice compared with their wild-type and heterozygous littermates could provide new information on a possible relationship between germ cell deficiency in the transgenic mice and the altered blood–testis barrier.
6. Gap junction and connexin 43 in pathological testes
To date, although altered testicular Cx43 expression has been reported in mutant rodents with testicular defects and in hypofertile men (Pointis & Segretain 2005; Matsuo et al. 2007; Pointis et al. 2009; table 3), a direct relationship between altered Cx and spermatogenesis arrest has not been clearly established. In Sertoli cells of patients with impaired spermatogenesis, alteration of Cx43 expression has been suggested to be a marker of undifferentiated Sertoli cell functionality (Defamie et al. 2003; Altay et al. 2008); however, no testicular alteration was reported in men with Cx gene mutations (Lai-Cheong et al. 2007). Thus, it has been postulated that altered Cx expression is a consequence of impaired testicular function rather than the cause of the testicular pathology. However, these data do not exclude the possibility that in response to environmental toxicants altered Cx expression may represent one mechanism responsible for the dysfunction of spermatogenesis, as suggested by in vitro findings (Defamie et al. 2001; Mograbi et al. 2003; Fiorini et al. 2004a,b, 2008; Zhou et al. 2008) and studies in rat and trout in vivo exposed to endocrine disrupting chemicals (Aravindakshan et al. 2004; de Montgolfier et al. 2008).
Table 3.
Testicular diseases in which connexin expression was impaired.
| diseases | species | connexin | references | |
|---|---|---|---|---|
| genetic infertility | ebo/ebo, jun-d−/− mutants | rat, mouse | Cx43 | Batias et al. (1999) |
| RXR−/− mutants | mouse | Cx43 | Batias et al. (2000) | |
| mosaic mutation and a partial deletion in the long arm of the Y chromosome | mouse | Cx43 | Kotula-Balak et al. (2007) | |
| Klinefelter's syndrome (47XXY) | human | Cx43 | Kotula-Balak et al. (2007) | |
| idiopathic azoospermia | hypospermatogenesis | human | Cx43 | Steger et al. (1999) |
| Sertoli cell only syndrome | human | Cx43 | Defamie et al. (2003) | |
| cryptorchidy | rat | Cx43 | Defamie et al. (2003) | |
| azoospermia and oligospermia | human | Cx43 | Matsuo et al. (2007) | |
| cryptorchidy | horse | Cx43 | Hejmej & Bilińska (2008) | |
| testis cancer | carcinoma in situ, seminoma | human | Cx26, Cx43 | Brehm et al. (2002) |
| seminoma | human | Cx40, Cx43 | Okada et al. (2003) | |
| carcinoma in situ, seminoma | human | Cx43 | Brehm et al. (2006) | |
| seminoma and seminoma cell line | human | Cx43 | Roger et al. (2004) | |
| Donner et al. (2004) | ||||
| Mauro et al. (2008) | ||||
| seminoma and neoplastic Sertoli cells | dog | Cx43 | Rüttinger et al. (2008) | |
| Leydig cell tumour | mouse | Cx43 | Segretain et al. (2003) |
As generally described for neoplastic cells, Cx gene expression is abnormally downregulated in most testicular tumoral tissue and cell lines. In human testis infiltrated with carcinoma-in-situ (CIS) or seminoma, Cx43 was undetectable (Brehm et al. 2002) and reduced Cx43 mRNA levels were measured in the tumoral testis (Okada et al. 2003; Brehm et al. 2006). It is interesting to note that the reduced intratubular Cx43 gene expression during the testicular tumoral progression from CIS to seminoma was concomitantly associated with an up-regulation of another Cx, Cx26, within Sertoli cells and its accumulation within the cytoplasm (Brehm et al. 2002). Cx26 overexpression and cytoplasmic accumulation has also been reported in numerous carcinomas (pancreas, head and neck, breast, colon, prostate), in keratinocyte-derived skin tumours and in human papillary thyroid and follicular thyroid cancers (reviewed in Cronier et al. 2009). Altogether these lines of evidence appear to be inconsistent with the conventional described role of Cxs as tumour suppressors. In addition, it has been recently demonstrated that cytoplasmic accumulation of Cx proteins may exert a favourable effect for tumour progression (Omori et al. 2007). Whether such a situation occurs in the course of tumoral testicular progression is at present questionable. This hypothesis is also supported by the observation that Cx26 is the only known Cx that cannot be controlled by phosphorylation of its C-terminal tail. Although overexpression of cytoplasmic Cx, different from Cx43, may play a role in tumour progression processes, such as invasion and metastasis (Li et al. 2007), there is evidence that the first stages of testicular neoplasia are associated with impaired Cx43 expression. Indeed, by using neoplastic cells originating from the JKT-1 seminoma cell line, we reported that overexpression of Cx43 by transfection of a Cx43-GFP vector not only restored gap junctional intercellular communication but also blocked abnormal proliferation of these cells (Roger et al. 2004). In this study, we also demonstrated that Cx43 protein was aberrantly trafficked, with accumulation of the protein within the cytoplasm of neoplasic cells. In pure human testicular seminoma, aberrant cytoplasmic Cx43 accumulation has also been demonstrated (Mauro et al. 2008). In agreement with these findings, the Cx43 signal was mainly sequestered within early endosomes at the earlier stage of testicular tumours confined to Leydig cells and in the BLT1 Leydig cell line originating from the tumour (Segretain et al. 2003). Altered trafficking of Cx43 is also observed in Sertoli cells exposed to carcinogens (Defamie et al. 2001; Mograbi et al. 2003; Gilleron et al. 2008). Impaired gap junction coupling and Cx dysfunction have been associated with the action of carcinogens, being a feature of cancer per se. From these collected studies, we suggested that impaired Cx43 trafficking is an early event associated with uncontrolled cell proliferation that could serve as a neoplastic marker during tumour germ cell progression. Interestingly, in human testicular cancer, it has been suggested that the tumour progression may be correlated with an impaired function of Sertoli cells resulting from an undifferentiated status of this somatic cell (reviewed in Sharpe et al. 2003). Since we have previously demonstrated that coupling via gap junctions occured efficiently between adjacent Sertoli cells but also unidirectionally from Sertoli cells to germ cells (Decrouy et al. 2004), it could be hypothesized that the loss of proliferation-suppressing signals from Sertoli cells to adjacent CIS cells through Cx43 gap junction channels, due to altered Sertoli cell Cx43 expression, may lead to uncontrolled CIS cell proliferation, which subsequently increases their invasive potentiality.
7. Conclusions and future perspectives
The pivotal role played by gap junction intercellular communication and Cx43, the predominant Cx within the testis, in testicular physiology is now established. The indispensability of Cx43 for normal testicular development and spermatogenesis has been clearly demonstrated in knock-out and transgenic mice. Furthermore, Cx43 appears to be a good marker of Sertoli cell function and is implicated in the control of neonatal Sertoli cell proliferation and differentiation, and germ cell proliferation in adulthood.
Several areas of gap junction and Cx functions in the testis could be examinated in the future. The specific functions for the other Cxs expressed in the testis such as Cx26, Cx31, Cx32 and the rodent-specific inhibitory member of the gap junction protein family, Cx33, remain to be defined. In addition, one can wonder whether Cx33-like sequences, which have been found on the X chromosome in other species such as humans, chimpanzee and cow, can encode functional proteins comparable to rodent Cx33 and thus exert a similar function (Cruciani & Mikalsen 2006).
Another interesting point would be to know whether the role of Cx in the testis is mediated only through the formation of gap junctions, through hemi-channels, or as signalling molecules, as recently reported (Kardami et al. 2007).
The potential role of pannexins, gap junction proteins homologous to invertebrate innexins and recently identified in mammals, also remains to be investigated in the testis. Indeed, mRNAs coding for PANX1, one isoform of the human pannexin family, have been detected in the human testis (Baranova et al. 2004) but not in the mouse testis (Bruzzone et al. 2003).
Research of new testicular Cx partners and analysis of their potential roles in regard to the complexity of spermatogenesis is also an important challenge for the future. Their specific involvement in the trafficking of the different Cxs present in Sertoli cells, the stability and degradation of the homotypic or heterotypic gap junction channels between Sertoli cells and between Sertoli and germ cells will be interesting to examine.
In addition to their roles in normal Sertoli and germ cell proliferation, dysfunction of gap junction proteins could be related to testicular pathologies associated with abnormal germ cell proliferation such as hypospermatogenesis and human testicular seminoma (figure 3). A better knowledge of Cx control in response to testicular toxic agents through analysis of epigenetic inactivation of Cx genes (Vinken et al. 2009) and of impaired trafficking and interaction of Cx with other protein partners (Pointis et al. 2007; Gilleron et al. 2008) will contribute to our understanding of the aetiology and progression of testicular cancer so that better prevention, diagnosis and therapy can be achieved.
Figure 3.
Schematic illustration of events and effectors that control spermatogenesis. In the physiological situation, hormones, specific Cx association between compatible Cxs, and Cx interacting proteins control gap junction communication between Sertoli cells and between Sertoli and germ cells. In physiopathological conditions a rupture of this equilibrium (genetic, epigenetic, environmental influence) may be conducive either to hypofertility or abnormal tumor cell proliferation.
Acknowledgements
The preparation of this review and our studies reported herein were supported by the Institut National de la Santé et de la Recherche Médicale (INSERM) and grants from the French Ministry of Research and Technology, ANR (05-PCOD-006-02), ARC and Novexel. The authors would like to thank Dr Kirsty Grant for reading the manuscript, Jeannine Colombani for secretarial assistance and to acknowledge the friendship and support of Dr François Spezia.
Footnotes
One contribution of 17 to a Theme Issue ‘The biology and regulation of spermatogenesis’.
References
- Altay B., Turna B., Oktem G., Aktuğ H., Semerci B., Bilir A.2008Immunohistochemical expression of connexin 43 and occludin in the rat testis after epididymal and vasal ligation. Fertil. Steril. 90, 141–147 (doi:10.1016/j.fertnstert.2007.05.065) [DOI] [PubMed] [Google Scholar]
- Amjad A. I., Söder O., Sultana T.2006Role of testicular interleukin-1alpha IL-1alpha in testicular physiology and disease. J. Coll. Physicians Surg. Pak. 16, 55–60 [PubMed] [Google Scholar]
- Aravindakshan J., Cyr D. G.2005Nonylphenol alters connexin43 levels and connexin43 phosphorylation via an inhibition of the p38-mitogen-activated protein kinase pathway. Biol. Reprod. 72, 1232–1240 (doi:10.1095/biolreprod.104.038596) [DOI] [PubMed] [Google Scholar]
- Aravindakshan J. M., Gregory M., Marcogliese D. J., Fournier M., Cyr D. G.2004Consumption of xenoestrogen-contaminated fish during lactation alters adult male reproductive function. Toxicol. Sci. 81, 179–189 (doi:10.1093/toxsci/kfh174) [DOI] [PubMed] [Google Scholar]
- Baranova A., et al. 2004The mammalian pannexin family is homologous to the invertebrate innexin gap junction proteins. Genomics 83, 706–716 (doi:10.1016/j.ygeno.2003.09.025) [DOI] [PubMed] [Google Scholar]
- Batias C., Defamie N., Lablack A., Thepot D., Fenichel P., Segretain D., Pointis G.1999Modified expression of testicular gap-junction connexin 43 during normal spermatogenic cycle and in altered spermatogenesis. Cell. Tissue Res. 298, 113–121 (doi:121.10.1007/s004419900076) [DOI] [PubMed] [Google Scholar]
- Batias C., Siffroi J. P., Fenichel P., Pointis G., Segretain D.2000Connexin43 gene expression and regulation in the rodent seminiferous epithelium. J. Histochem. Cytochem. 48, 793–805 [DOI] [PubMed] [Google Scholar]
- Batlouni S. R., Carreño F. R., Romagosa E., Borella M. I.2005Cell junctions in the germinal epithelium may play an important role in spermatogenesis of the catfish P. fasciatum (Pisces, Siluriformes). J. Mol. Histol. 36, 97–110 (doi:10.1007/s10735-004-4115-0) [DOI] [PubMed] [Google Scholar]
- Bravo-Moreno J. F., Diaz-Sanchez V., Montoya-Flores J. G., Lamoyi E., Saez J. C., Perez-Armendariz E. M.2001Expression of connexin43 in mouse Leydig, Sertoli, and germinal cells at different stages of postnatal development. Anat. Rec. 264, 13–24 (doi:10.1002/ar.1100) [DOI] [PubMed] [Google Scholar]
- Brehm R., Marks A., Rey R., Kliesch S., Bergmann M., Steger K.2002Altered expression of connexins 26 and 43 in Sertoli cells in seminiferous tubules infiltrated with carcinoma-in-situ or seminoma. J. Pathol. 197, 647–653 (doi:10.1002/path.1140) [DOI] [PubMed] [Google Scholar]
- Brehm R., Rüttinger C., Fischer P., Gashaw I., Winterhager E., Kliesch S., Bohle R. M., Steger K., Bergmann M.2006Transition from preinvasive carcinoma in situ to seminoma is accompanied by a reduction of connexin 43 expression in Sertoli cells and germ cells. Neoplasia 8, 499–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brehm R., et al. 2007A Sertoli cell-specific knockout of connexin43 prevents initiation of spermatogenesis. Am. J. Pathol. 171, 19–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone R., White T. W., Goodenough D. A.1996The cellular Internet: on-line with connexins. Bioessays 18, 709–718 [DOI] [PubMed] [Google Scholar]
- Bruzzone R., Hormuzdi S. G., Barbe M. T., Herb A., Monyer H.2003Pannexins, a family of gap junction proteins expressed in brain. Proc. Natl Acad. Sci. USA. 100, 13 644–13 649 (doi:10.1073/pnas.2233464100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carette D., Gilleron J., Decrouy X., Fiorini C., Diry M., Segretain D., Pointis G.2009Connexin 33 impairs functionality of gap junction by accelerating connexin 43 gap junction plaque endocytosis. Traffic 10, 1272–1285 (doi:10.1111/j.1600-0854.2009.00949) [DOI] [PubMed] [Google Scholar]
- Chang M., Werner R., Dahl G.1996A role for an inhibitory connexin in testis? Dev. Biol. 175, 50–56 (doi:10.1006/dbio.1996.0094) [DOI] [PubMed] [Google Scholar]
- Cheng C. Y., Mruk D. D.2002Cell junction dynamics in the testis: Sertoli-germ cell interactions and male contraceptive development. Physiol. Rev. 82, 825–874 (doi:10.1152/physrev.00009.20020031-9333/02) [DOI] [PubMed] [Google Scholar]
- Chipman J. K., Mally A., Edwards G. O.2003Disruption of gap junctions in toxicity and carcinogenicity. Toxicol. Sci. 71, 146–153 [DOI] [PubMed] [Google Scholar]
- Chung S. S., Lee W. M., Cheng C. Y.1999Study on the formation of specialized inter-Sertoli cell junctions in vitro. J. Cell. Physiol. 181, 258–272 [DOI] [PubMed] [Google Scholar]
- Clermont Y.1972Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol. Rev. 52, 198–236 [DOI] [PubMed] [Google Scholar]
- Cottrell G. T., Burt J. M.2005Functional consequences of heterogeneous gap junction channel formation and its influence in health and disease. Biochim. Biophys. Acta 1711, 126–141 (doi:10.1016/j.bbamem.2004.11.013) [DOI] [PubMed] [Google Scholar]
- Cronier L., Crespin S., Strale P. O., Defamie N., Mesnil M.2009Gap junctions and cancer: new functions for an old story. Antioxid. Redox. Signal. 2, 323–338 (doi:10.1089/ars.2008.2153) [DOI] [PubMed] [Google Scholar]
- Cruciani V., Mikalsen S. O.2006The vertebrate connexin family. Cell. Mol. Life Sci. 63, 1125–1140 (doi:10.1007/s00018-005-5571-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Montgolfier B., Dufresne J., Letourneau M., Nagler J. J., Fournier A., Audet C., Cyr D. G.2007The expression of multiple connexins throughout spermatogenesis in the rainbow trout testis suggests a role for complex intercellular communication. Biol. Reprod. 76, 2–8 (doi:10.1095/biolreprod.106.054288) [DOI] [PubMed] [Google Scholar]
- de Montgolfier B., Fournier M., Audet C., Marcogliese D. J., Cyr D. G.2008Influence of municipal effluents on the expression of connexins in the brook trout (Salvelinus fontinalis) testis. Aquat. Toxicol. 86, 38–48 (doi:10.1016/j.aquatox.2007.09.013) [DOI] [PubMed] [Google Scholar]
- Decrouy X., Gasc J. M., Pointis G., Segretain D.2004Functional characterization of Cx43 based gap junctions during spermatogenesis. J. Cell. Physiol. 200, 146–154 (doi:10.1002/JCP.10473) [DOI] [PubMed] [Google Scholar]
- Defamie N., Mograbi B., Roger C., Cronier L., Malassine A., Brucker-Davis F., Fenichel P., Segretain D., Pointis G.2001Disruption of gap junctional intercellular communication by lindane is associated with aberrant localization of connexin43 and zonula occludens-1 in 42GPA9 Sertoli cells. Carcinogenesis 22, 1537–1542 [DOI] [PubMed] [Google Scholar]
- Defamie N., Berthaut I., Mograbi B., Chevallier D., Dadoune J. P., Fenichel P., Segretain D., Pointis G.2003Impaired gap junction connexin43 in Sertoli cells of patients with secretory azoospermia: a marker of undifferentiated Sertoli cells. Lab. Invest. 83, 449–456 [DOI] [PubMed] [Google Scholar]
- Donner J., Kliesch S., Brehm R., Bergmann M.2004From carcinoma in situ to testicular germ cell tumour. APMIS 112, 79–88 (doi:10.1111/j.1600-0463.2004.apm1120201.x) [DOI] [PubMed] [Google Scholar]
- Fiorini C., et al. 2004aDominant negative effect of connexin33 on gap junctional communication is mediated by connexin43 sequestration. J. Cell Sci. 117, 4665–4672 (doi:10.1242/jcs.01335) [DOI] [PubMed] [Google Scholar]
- Fiorini C., Tilloy-Ellul A., Chevalier S., Charuel C., Pointis G.2004bSertoli cell junctional proteins as early targets for different classes of reproductive toxicants. Reprod. Toxicol. 18, 413–421 (doi:10.1016/j.reprotox.2004.01.002) [DOI] [PubMed] [Google Scholar]
- Fiorini C., Decrouy X., Defamie N., Segretain D., Pointis G.2006Opposite regulation of connexin 33 and connexin 43 by LPS and IL-1alpha in spermatogenesis. Am. J. Physiol. Cell. Physiol. 290, C733–C740 (doi:10.1152/ajpcell.00106.20050363-6143/06) [DOI] [PubMed] [Google Scholar]
- Fiorini C., Gilleron J., Carette D., Valette A., Tilloy A., Chevalier S., Segretain D., Pointis G.2008Accelerated internalization of junctional membrane proteins (connexin 43, N-cadherin and ZO-1) within endocytic vacuoles: an early event of DDT carcinogenicity. Biochim. Biophys. Acta 1778, 56–67 (doi:10.1016/j.bbamem.2007.08.032) [DOI] [PubMed] [Google Scholar]
- Fischer P., Brehm R., Konrad L., Hartmann S., Kliesch S., Bohle R. M., Bergmann M.2005Connexin 33: a rodent-specific member of the gap junction protein family? J. Androl. 26, 75–84 [PubMed] [Google Scholar]
- Francis R. J., Lo C. W.2006Primordial germ cell deficiency in the connexin 43 knockout mouse arises from apoptosis associated with abnormal p53 activation. Development 133, 3451–3460 (doi:10.1242/dev.02506) [DOI] [PubMed] [Google Scholar]
- Gabriel H. D., Jung D., Bützler C., Temme A., Traub O., Winterhager E., Willecke K.1998Transplacental uptake of glucose is decreased in embryonic lethal connexin26-deficient mice. J. Cell Biol. 140, 1453–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleron J., Nebout M., Scarabelli L., Senegas-Balas F., Palmero S., Segretain D., Pointis G.2006A potential novel mechanism involving connexin 43 gap junction for control of Sertoli cell proliferation by thyroid hormones. J. Cell Physiol. 209, 153–161 [DOI] [PubMed] [Google Scholar]
- Gilleron J., Fiorini C., Carette D., Avondet C., Falk M., Segretain D., Pointis G.2008Molecular reorganization of the connexin 43, Zonula occludens-1 and c-Src complexes during gap junction plaque endocytosis in response to a non genomic carcinogen. J. Cell. Sci. 121, 4069–4078 (doi:10.1242/jcs.033373) [DOI] [PubMed] [Google Scholar]
- Gilleron J., Carette D., Durand P., Pointis G., Segretain D.2009Connexin 43 a potential regulator of cell proliferation and apoptosis within the seminiferous epithelium. Int. J. Biochem. Cell Biol. 41, 1381–1390 (doi:10.1016/j.biocel.2008.12.008) [DOI] [PubMed] [Google Scholar]
- Gilleron J., Carette D., Segretain D., Pointis G.In press Three dimensional analysis of connexin43 gap junction in the ex-vivo rat seminiferous tubules: short-term effects of spermatogenic regulators. Micro. Res. Techn. (doi:10.1002/jemt.20731) [DOI] [PubMed] [Google Scholar]
- Godet M., Sabido O., Gilleron J., Durand P.2008Meiotic progression of rat spermatocytes requires mitogen-activated protein kinases of Sertoli cells and close contacts between the germ cells and the Sertoli cells. Dev. Biol. 315, 173–188 (doi:10.1016/j.ydbio.2007.12.019) [DOI] [PubMed] [Google Scholar]
- Goldenberg R. C., Fortes F. S., Cristancho J. M., Morales M. M., Franci C. R., Varanda W. A., Campos de Carvalho A. C.2003Modulation of gap junction mediated intercellular communication in TM3 Leydig cells. J. Endocrinol. 177, 327–335 [DOI] [PubMed] [Google Scholar]
- Gong X., Li E., Klier G., Huang Q., Wu Y., Lei H., Kumar N. M., Horwitz J., Gilula N. B.1997Disruption of alpha3 connexin gene leads to proteolysis and cataractogenesis in mice. Cell 91, 833–843 (doi:10.1016/S0092-8674(00)80471-7) [DOI] [PubMed] [Google Scholar]
- Grassi F., Monaco L., Fratamico G., Dolci S., Iannini E., Conti M., Eusebi F., Stefanini M.1986Putative second messengers affect cell coupling in the seminiferous tubules. Cell. Biol. Int. Rep. 10, 631–639 [DOI] [PubMed] [Google Scholar]
- Griswold M. D.1993Protein secretion by Sertoli cells: general considerations. In The Sertoli cell (eds Russell L. D., Griswold M. D.), pp. 195–200 Clearwater, FL: Cache River Press [Google Scholar]
- Haefliger J. A., Bruzzone R., Jenkins N. A., Gilbert D. J., Copeland N. G., Paul D. L.1992Four novel members of the connexin family of gap junction proteins. Molecular cloning, expression, and chromosome mapping. J. Biol. Chem. 267, 2057–2064 [PubMed] [Google Scholar]
- Hejmej A., Bilińska B.2008The effects of cryptorchidism on the regulation of steroidogenesis and gap junctional communication in equine testes. Endokrynol. Pol. 59, 112–118 [PubMed] [Google Scholar]
- Hennemann H., Dahl E., White J. B., Schwarz H. J., Lalley P. A., Chang S., Nicholson B. J., Willecke K.1992Two gap junction genes, connexin 31.1 and 30.3, are closely linked on mouse chromosome 4 and preferentially expressed in skin. J. Biol. Chem. 267, 17 225–17 233 [PubMed] [Google Scholar]
- Hernandez-Blazquez F. J., Joazeiro P. P., Omori Y., Yamasaki H.2001Control of intracellular movement of connexins by E-cadherin in murine skin papilloma cells. Exp. Cell Res. 270, 235–247 (doi:10.1006/excr.20015342) [DOI] [PubMed] [Google Scholar]
- Hervé J. C., Pluciennik F., Verrecchia F., Bastide B., Delage B., Joffre M., Deleze J.1996Influence of the molecular structure of steroids on their ability to interrupt gap junctional communication. J. Membr. Biol. 149, 179–187 (doi:10.1007/s002329900018) [DOI] [PubMed] [Google Scholar]
- Hervé J. C., Bourmeyster N., Sarrouilhe D., Duffy H. S.2007Gap junctional complexes: from partners to functions. Prog. Biophys. Mol. Biol. 94, 29–65 (doi:10.1016/j.pbiomolbio.2007.03.010) [DOI] [PubMed] [Google Scholar]
- Huang R. P., Fan Y., Hossain M. Z., Peng A., Zeng Z. L., Boynton A. L.1998Reversion of the neoplastic phenotype of human glioblastoma cells by connexin 43 (Cx43). Cancer Res. 58, 5089–5096 [PubMed] [Google Scholar]
- Izzo G., d'Istria M., Ferrara D., Serino I., Aniello F., Minucci S.2006Connexin 43 expression in the testis of the frog Rana esculenta. Zygote 14, 349–357 (doi:10.1017/S096719940600390X) [DOI] [PubMed] [Google Scholar]
- Juneja S. C.2003mRNA expression pattern of multiple members of connexin gene family in normal and abnormal fetal gonads in mouse. Indian J. Physiol. Pharmacol. 47, 147–156 [PubMed] [Google Scholar]
- Juneja S. C., Barr K. J., Enders G. C., Kidder G. M.1999Defects in the germ line and gonads of mice lacking connexin43. Biol. Reprod. 60, 1263–1270 [DOI] [PubMed] [Google Scholar]
- Kang K. S., Lee Y. S., Kim H. S., Kim S. H.2002Di-(2-ethylhexyl) phthalate-induced cell proliferation is involved in the inhibition of gap junctional intercellular communication and blockage of apoptosis in mouse Sertoli cells. J. Toxicol. Environ. Health 65, 447–459 [DOI] [PubMed] [Google Scholar]
- Kardami E., Dang X., Iacobas D. A., Nickel B. E., Jeyaraman M., Srisakuldee W., Makazan J., Tanguy S., Spray D. C.2007The role of connexins in controlling cell growth and gene expression. Prog. Biophys. Mol. Biol. 94, 245–264 (doi:10.1016/j.pbiomolbio.2007.03.009) [DOI] [PubMed] [Google Scholar]
- Kirchhoff S., Nelles E., Hagendorff A., Krüger O., Traub O., Willecke K.1998Reduced cardiac conduction velocity and predisposition to arrhythmias in connexin40-deficient mice. Curr. Biol. 8, 299–302 (doi:10.1016/S0960-9822(98)70114-9) [DOI] [PubMed] [Google Scholar]
- Kojima T., Murata M., Go M., Spray D. C., Sawada N.2007Connexins induce and maintain tight junctions in epithelial cells. J. Membr. Biol. 217, 13–19 (doi:10.1007/s00232-007-9021-4) [DOI] [PubMed] [Google Scholar]
- Kotula-Balak M., Hejmej A., Sadowska J., Bilinska B.2007Connexin 43 expression in human and mouse testes with impaired spermatogenesis. Eur. J. Histochem. 51, 261–268 [PubMed] [Google Scholar]
- Krysko D. V., Leybaert L., Vandenabeele P., D'Herde K.2005Gap junctions and the propagation of cell survival and cell death signals. Apoptosis 10, 459–469 [DOI] [PubMed] [Google Scholar]
- Lablack A., Bourdon V., Defamie N., Batias C., Mesnil M., Fenichel P., Pointis G., Segretain D.1998Ultrastructural and biochemical evidence for gap junction and connexin 43 expression in a clonal Sertoli cell line: a potential model in the study of junctional complex formation. Cell Tissue Res. 294, 279–287 (doi:10.1007/s004410051178) [DOI] [PubMed] [Google Scholar]
- Lai-Cheong J. E., Arita K., McGrath J. A.2007Genetic diseases of junctions. J. Invest. Dermatol. 127, 2713–2725 (doi:10.1038/sj.jid.5700727) [DOI] [PubMed] [Google Scholar]
- Lau A. F.2005c-Src: bridging the gap between phosphorylation- and acidification-induced gap junction channel closure. Sci STKE. 291, e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N. P. Y., Cheng C. Y.2004Adaptors, junction dynamics, and spermatogenesis. Biol. Reprod. 71, 392–404 (doi:10.1095/biolreprod.104.027268) [DOI] [PubMed] [Google Scholar]
- Lee N. P., Leung K. W., Wo J. Y., Tam P. C., Yeung W. S., Luk J. M.2006Blockage of testicular connexins induced apoptosis in rat seminiferous epithelium. Apoptosis 11, 1215–1229 [DOI] [PubMed] [Google Scholar]
- Lee N. P., Yeung W. S., Luk J. M.2007Junction interaction in the seminiferous epithelium: regulatory roles of connexin-based gap junction. Front. Biosci. 12, 1552–1562 (doi:10.1007/s10495-006-6981-2) [DOI] [PubMed] [Google Scholar]
- Lefebvre D. L., Piersanti M., Bai X. H., Chen Z. Q., Lye S. J.1995Myometrial transcriptional regulation of the gap junction gene, connexin-43. Reprod. Fertil. Dev. 7, 603–611 [DOI] [PubMed] [Google Scholar]
- Li Q., Omori Y., Nishikawa Y., Yoshioka T., Yamamoto Y., Enomoto K.2007Cytoplasmic accumulation of connexin32 protein enhances motility and metastatic ability of human hepatoma cells in vitro and in vivo. Int. J. Cancer 121, 536–546 [DOI] [PubMed] [Google Scholar]
- Lucas T. F., Siu E. R., Esteves C. A., Monteiro H. P., Oliveira C. A., Porto C. S., Lazari M. F.200817beta-Estradiol induces the translocation of the estrogen receptors ESR1 and ESR2 to the cell membrane, MAPK3/1 phosphorylation and proliferation of cultured immature rat Sertoli cells. Biol. Reprod. 78, 101–114 (doi:10.1095/biolreprod.107.063909) [DOI] [PubMed] [Google Scholar]
- Luk J. M., et al. 2003Identification of novel genes expressed during spermatogenesis in stage-synchronized rat testes by differential display. Biochem. Biophys. Res. Commun. 307, 782–790 (doi:10.1016/S0006-291X(03)01250-6) [DOI] [PubMed] [Google Scholar]
- Manthey D., Bukauskas F., Lee C. G., Kozak C. A., Willecke K.1999Molecular cloning and functional expression of the mouse gap junction gene connexin-57 in human HeLa cells. J. Biol. Chem. 274, 14 716–14 723 [DOI] [PubMed] [Google Scholar]
- Martin P. E., Evans W. H.2004Incorporation of connexins into plasma membranes and gap junctions. Cardiovasc. Res. 62, 378–387 (doi:10.1016/j.cardiores.2004.01.016) [DOI] [PubMed] [Google Scholar]
- Matsuo Y., Nomata K., Eguchi J., Aoki D., Hayashi T., Hishikawa Y., Kanetake H., Shibata Y., Koji T.2007Immunohistochemical analysis of connexin43 expression in infertile human testes. Acta Histochem. Cytochem. 40, 69–75 (doi:10.1267/ahc.07001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro V., Chevallier D., Gilleron J., Carette D., Defamie N., Gasc J. M., Senegas-Balas F., Segretain D., Pointis G.2008Aberrant cytoplasmic accumulation of connexin 43 in human testicular seminoma. Open Biomarkers J. 1, 20–27 (doi:10.2174/1875318300801010020) [Google Scholar]
- Mesnil M., Crespin S., Avanzo J. L., Zaidan-Dagli M. L.2005Defective gap junctional intercellular communication in the carcinogenic process. Biochim. Biophys. Acta 1719, 125–145 (doi:10.1016/j.bbamem.2005.11.004) [DOI] [PubMed] [Google Scholar]
- Minkoff R., Bales E. S., Kerr C. A., Struss W. E.1999Antisense oligonucleotide blockade of connexin expression during embryonic bone formation: evidence of functional compensation within a multigene family. Dev. Genet. 24, 43–56 [DOI] [PubMed] [Google Scholar]
- Mograbi B., Corcelle E., Defamie N., Samson M., Nebout M., Segretain D., Fenichel P., Pointis G.2003Aberrant connexin 43 endocytosis by the carcinogen lindane involves activation of the ERK/mitogen-activated protein kinase pathway. Carcinogenesis 24, 1415–1423 (doi:10.1093/carcin/bgg093) [DOI] [PubMed] [Google Scholar]
- Mok B. W., Yeung W. S., Luk J. M.1999Differential expression of gap-junction gene connexin 31 in seminiferous epithelium of rat testes. FEBS Lett. 453, 243–248 (doi:10.1016/S0014-5793(99)00714-0) [DOI] [PubMed] [Google Scholar]
- Mruk D. D., Cheng C. Y.2004Sertoli–Sertoli and Sertoli–germ cell interactions and their significance in germ cell movement in the seminiferous epithelium during spermatogenesis. Endocr. Rev. 25, 747–806 (doi:10.1210/er2003-0022) [DOI] [PubMed] [Google Scholar]
- Nelles E., et al. 1996Defective propagation of signals generated by sympathetic nerve stimulation in the liver of connexin32-deficient mice. Proc. Natl Acad. Sci. USA 93, 9565–9570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen P. A., Kumar N. M.2003Differences in expression patterns between mouse connexin-30.2 (Cx30.2) and its putative human orthologue, connexin-31.9. FEBS Lett. 540, 151–156 (doi:10.1016/S0014-5793(03)00252-7) [DOI] [PubMed] [Google Scholar]
- Nielsen P. A., Beahm D. L., Giepmans B. N., Baruch A., Hall J. E., Kumar N. M.2002Molecular cloning, functional expression, and tissue distribution of a novel human gap junction-forming protein, connexin-31.9. Interaction with Zonula occludens protein-1. J. Biol. Chem. 277, 38 272–38 283 (doi:10.1074/jbc.M205348200) [DOI] [PubMed] [Google Scholar]
- Odermatt B., et al. 2003Connexin 47 (Cx47)-deficient mice with enhanced green fluorescent protein reporter gene reveal predominant oligodendrocytic expression of Cx47 and display vacuolized myelin in the CNS. J. Neurosci. 23, 4549–4559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K., et al. 2003Analysis of gene-expression profiles in testicular seminomas using a genome-wide cDNA microarray. Int. J. Oncol. 23, 1615–1635 [PubMed] [Google Scholar]
- Omori Y., Li Q., Nishikawa Y., Yoshioka T., Yoshida M., Nishimura T., Enomoto K.2007Pathological significance of intracytoplasmic connexin proteins: implication in tumor progression. J. Membr. Biol. 218, 73–77 (doi:10.1007/s00232-007-9048-6) [DOI] [PubMed] [Google Scholar]
- Pelletier R. M.1995The distribution of connexin 43 is associated with the germ cell differentiation and with the modulation of the Sertoli cell junctional barrier in continual (guinea pig) and seasonal breeders' (mink) testes. J. Androl. 16, 400–409 [PubMed] [Google Scholar]
- Perez-Armendariz E. M., Romano M. C., Luna J., Miranda C., Bennett M. V., Moreno A. P.1994Characterization of gap junctions between pairs of Leydig cells from mouse testis. Am. J. Physiol. 267, C570–C580 (doi:409.0363-6143/94) [DOI] [PubMed] [Google Scholar]
- Perez-Armendariz E. M., Lamoyi E., Mason J. I., Cisneros-Armas D., Luu-The V., Bravo Moreno J. F.2001Developmental regulation of connexin 43 expression in fetal mouse testicular cells. Anat. Rec. 264, 237–246 [DOI] [PubMed] [Google Scholar]
- Pluciennik F., Joffre M., Deleze J.1994Follicle-stimulating hormone increases gap junction communication in Sertoli cells from immature rat testis in primary culture. J. Membr. Biol. 139, 81–96 [DOI] [PubMed] [Google Scholar]
- Pluciennik F., Verrecchia F., Bastide B., Hervé J. C., Joffre M., Deleze J.1996Reversible interruption of gap junctional communication by testosterone propionate in cultured Sertoli cells and cardiac myocytes. J. Membr. Biol. 149, 169–177 (doi:10.1007/s002329900017) [DOI] [PubMed] [Google Scholar]
- Plum A., et al. 2000Unique and shared functions of different connexins in mice. Curr. Biol. 10, 1083–1091 (doi:10.1016/S0960-9822(00)00690-4) [DOI] [PubMed] [Google Scholar]
- Plum A., Winterhager E., Pesch J., Lautermann J., Hallas G., Rosentreter B., Traub O., Herberhold C., Willecke K.2001Connexin31-deficiency in mice causes transient placental dysmorphogenesis but does not impair hearing and skin differentiation. Dev. Biol. 231, 334–347 (doi:10.1006/dbio.2000.0148) [DOI] [PubMed] [Google Scholar]
- Pointis G., Segretain D.2005Role of connexin-based gap junction channels in testis. Trends Endocrinol. Metab. 16, 300–306 (doi:10.1016/j.tem.2005.07.001) [DOI] [PubMed] [Google Scholar]
- Pointis G., Fiorini C., Gilleron J., Carette D., Segretain D.2007Connexins as precocious markers and molecular targets for chemical and pharmacological agents in carcinogenesis. Curr. Med. Chem. 14, 2288–2303 [DOI] [PubMed] [Google Scholar]
- Pointis G., Fiorini C., Gilleron J., Carette D., Segretain D.2009Connexins in the male reproductive system. In Connexins: a guide (eds Harris A., Loke D.), pp. 449–510 New York, NY: Humana Press [Google Scholar]
- Reaume A. G., de Sousa P. A., Kulkarni S., Langille B. L., Zhu D., Davies T. C., Juneja S. C., Kidder G. M., Rossant J.1995Cardiac malformation in neonatal mice lacking connexin43. Science 267, 1831–1834 (doi:10.1126/science.7892609) [DOI] [PubMed] [Google Scholar]
- Risley M. S.2000Connexin gene expression in seminiferous tubules of the Sprague-Dawley rat. Biol. Reprod. 62, 748–754 [DOI] [PubMed] [Google Scholar]
- Risley M. S., Tan I. P., Roy C., Saez J. C.1992Cell-, age- and stage-dependent distribution of connexin43 gap junctions in testes. J. Cell Sci. 103, 81–96 [DOI] [PubMed] [Google Scholar]
- Risley M. S., Tan I. P., Farrell J.2002Gap junctions with varied permeability properties establish cell-type specific communication pathways in the rat seminiferous epithelium. Biol. Reprod. 67, 945–952 [DOI] [PubMed] [Google Scholar]
- Roger C., Mograbi B., Chevallier D., Michiels J. F., Tanaka H., Segretain D., Pointis G., Fenichel P.2004Disrupted traffic of connexin 43 in human testicular seminoma cells: overexpression of Cx43 induces membrane location and cell proliferation decrease. J. Pathol. 202, 241–246 (doi:10.1002/path.1509) [DOI] [PubMed] [Google Scholar]
- Roscoe W. A., Barr K. J., Mhawi A. A., Pomerantz D. K., Kidder G. M.2001Failure of spermatogenesis in mice lacking connexin43. Biol. Reprod. 65, 829–838 [DOI] [PubMed] [Google Scholar]
- Rüttinger C., Bergmann M., Fink L., Pesch S., Seitz K., Trautmann A., Steger K., Konrad L., Brehm R.2008Expression of connexin 43 in normal canine testes and canine testicular tumors. Histochem. Cell. Biol. 130, 537–548 (doi:10.1007/s00418-008-0432-9) [DOI] [PubMed] [Google Scholar]
- Saez J. C., Berthoud V. M., Branes M. C., Martinez A. D., Beyer E. C.2003Plasma membrane channels formed by connexins: their regulation and functions. Physiol. Rev. 83, 1359–1400 (doi:10.1152/physrev.00007.20030031-9333/03) [DOI] [PubMed] [Google Scholar]
- Salameh A., Dhein S.2005Pharmacology of gap junctions. New pharmacological targets for treatment of arrhythmia, seizure and cancer? Biochim. Biophys. Acta 1719, 36–58 (doi:10.1016/j.bbamem.2005.09.007) [DOI] [PubMed] [Google Scholar]
- Sarkar O., Mathur P. P., Cheng C. Y., Mruk D. D.2008Interleukin 1 alpha (IL1A) is a novel regulator of the blood-testis barrier in the rat. Biol. Reprod. 78, 445–454 (doi:10.1095/biolreprod.107.064501) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scemes E., Suadicani S. O., Dahl G., Spray D. C.2007Connexin and pannexin mediated cell–cell communication. Neuron. Glia Biol. 3, 199–208 (doi:10.1017/S1740925X08000069) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segretain D., et al. 2003Sequestration of connexin43 in the early endosomes: an early event of Leydig cell tumor progression. Mol. Carcinog. 38, 179–187 (doi:10.1002/mc.10160) [DOI] [PubMed] [Google Scholar]
- Sharpe R. M., McKinnell C., Kivlin C., Fisher J. S.2003Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 125, 769–784 [DOI] [PubMed] [Google Scholar]
- Simon A. M., McWhorter A. R.2002Vascular abnormalities in mice lacking the endothelial gap junction proteins connexin37 and connexin40. Dev. Biol. 251, 206–220 (doi:10.1006/dbio.2002.0826) [DOI] [PubMed] [Google Scholar]
- Simon A. M., Goodenough D. A., Li E., Paul D. L.1997Female infertility in mice lacking connexin 37. Nature 385, 525–529 (doi:10.1038/385525a0) [DOI] [PubMed] [Google Scholar]
- Simon A. M., Goodenough D. A., Paul D. L.1998Mice lacking connexin40 have cardiac conduction abnormalities characteristic of atrioventricular block and bundle branch block. Curr. Biol. 8, 295–298 (doi:10.1016/S0960-9822(98)70113-7) [DOI] [PubMed] [Google Scholar]
- Sohl G., Willecke K.2003An update on connexin genes and their nomenclature in mouse and man. Cell. Commun. Adhes. 10, 173–180 [DOI] [PubMed] [Google Scholar]
- Somekawa S., Fukuhara S., Nakaoka Y., Fujita H., Saito Y., Mochizuki N.2005Enhanced functional gap junction neoformation by protein kinase A-dependent and Epac-dependent signals downstream of cAMP in cardiac myocytes. Circ. Res. 97, 655–662 (doi:10.1161/01.RES.0000183880.49270.f9) [DOI] [PubMed] [Google Scholar]
- Sommersberg B., Bulling A., Salzer U., Fröhlich U., Garfield R. E., Amsterdam A., Mayerhofer A.2000Gap junction communication and connexin 43 gene expression in a rat granulosa cell line: regulation by follicle-stimulating hormone. Biol. Reprod. 63, 1661–1668 [DOI] [PubMed] [Google Scholar]
- Spray D. C., Ye Z. C., Ransom B. R.2006Functional connexin ‘hemichannels’: a critical appraisal. Glia 54, 758–773 (doi:10.1002/glia.20429) [DOI] [PubMed] [Google Scholar]
- Sridharan S., Simon L., Meling D. D., Cyr D. G., Gutstein D. E., Fishman G. I., Guillou F., Cooke P. S.2007Proliferation of adult Sertoli cells following conditional knockout of the Gap junctional protein GJA1 (connexin 43) in mice. Biol. Reprod. 76, 804–812 (doi:10.1095/biolreprod.106.059212) [DOI] [PubMed] [Google Scholar]
- Steger K., Tetens F., Bergmann M.1999Expression of connexin 43 in human testis. Histochem. Cell Biol. 112, 215–220 (doi:10.1007/s004180050409) [DOI] [PubMed] [Google Scholar]
- St-Pierre N., Dufresne J., Rooney A. A., Cyr D. G.2003Neonatal hypothyroidism alters the localization of gap junctional protein connexin 43 in the testis and messenger RNA levels in the epididymis of the rat. Biol. Reprod. 68, 1232–1240 (doi:10.1095/biolreprod.102.010504) [DOI] [PubMed] [Google Scholar]
- Tan I. P., Roy C., Saez J. C., Saez C. G., Paul D. L., Risley M. S.1996Regulated assembly of connexin33 and connexin43 into rat Sertoli cell gap junctions. Biol. Reprod. 54, 1300–1310 [DOI] [PubMed] [Google Scholar]
- Tazuke S. I., et al. 2002A germline-specific gap junction protein required for survival of differentiating early germ cells. Development 129, 2529–2539 [DOI] [PubMed] [Google Scholar]
- Trosko J. E., Ruch R. J.1998Cell–cell communication in carcinogenesis. Front. Biosci. 3, 208–236 [DOI] [PubMed] [Google Scholar]
- Trosko J. E., Ruch R. J.2002Gap junctions as targets for cancer chemoprevention and chemotherapy. Curr. Drug Targets 3, 465–482 [DOI] [PubMed] [Google Scholar]
- van Rijen H. V., et al. 2001Impaired conduction in the bundle branches of mouse hearts lacking the gap junction protein connexin40. Circulation 103, 1591–1598 [DOI] [PubMed] [Google Scholar]
- Varanda W. A., de Carvalho A. C.1994Intercellular communication between mouse Leydig cells. Am. J. Physiol. 267, C563–C569 [DOI] [PubMed] [Google Scholar]
- Vinken M., De Rop E., Decrock E., De Vuyst E., Leybaert L., Vanhaecke T., Rogiers V.2009Epigenetic regulation of gap junctional intercellular communication: more than a way to keep cells quiet? Biochim. Biophys. Acta 1795, 53–61 (doi:10.1016/j.bbcan.2008.08.002) [DOI] [PubMed] [Google Scholar]
- White T. W., Goodenough D. A., Paul D. L.1998Targeted ablation of connexin50 in mice results in microphthalmia and zonular pulverulent cataracts. J. Cell Biol. 143, 815–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T. W., Srinivas M., Ripps H., Trovato-Salinaro A., Condorelli D. F., Bruzzone R.2002Virtual cloning, functional expression, and gating analysis of human connexin31.9. Am. J. Physiol. Cell. Physiol. 283, C960–C970 (doi:10.1152/ajpcell.00163.20020363-6143/02) [DOI] [PubMed] [Google Scholar]
- Willecke K., Kirchhoff S., Plum A., Temme A., Thonnissen E., Ott T.1999Biological functions of connexin genes revealed by human genetic defects, dominant negative approaches and targeted deletions in the mouse. Novartis Found Symp. 219, 76–88 88–96 [DOI] [PubMed] [Google Scholar]
- Willecke K., Eiberger J., Degen J., Eckardt D., Romualdi A., Güldenagel M., Deutsch U., Söhl G.2002Structural and functional diversity of connexin genes in the mouse and human genome. Biol. Chem. 383, 725–737 (doi:10.1515/BC.2002.076,May2002) [DOI] [PubMed] [Google Scholar]
- Winterhager E., et al. 2007Replacement of connexin43 by connexin26 in transgenic mice leads to dysfunctional reproductive organs and slowed ventricular conduction in the heart. BMC Dev. Biol. 7, 26 (doi:10.1186/1471-213X-7-26) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolgemuth D. J., Chung S. S.2007Retinoid signaling during spermatogenesis as revealed by genetic and metabolic manipulations of retinoic acid receptor alpha. Soc. Reprod. Fertil. Suppl. 63, 11–23 [PMC free article] [PubMed] [Google Scholar]
- You S., Li W., Lin T.2000Expression and regulation of connexin43 in rat Leydig cells. J. Endocrinol. 166, 447–453 [DOI] [PubMed] [Google Scholar]
- Yu Z., Guo R., Ge Y., Ma J., Guan J., Li S., Sun X., Xue S., Han D.2003Gene expression profiles in different stages of mouse spermatogenic cells during spermatogenesis. Biol. Reprod. 69, 37–47 (doi:10.1095/biolreprod.102.012609) [DOI] [PubMed] [Google Scholar]
- Zhang J. T., Nicholson B. J.1989Sequence and tissue distribution of a second protein of hepatic gap junctions, Cx26, as deduced from its cDNA. J. Cell Biol. 109, 3391–3401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D. R., Zhou Y. C., Cui G. H., Guo X., Qin J., Gui Y. T., Cai Z. M.2008Gossypol repressed the gap junctional intercellular communication between Sertoli cells by decreasing the expression of Connexin43. Toxicol. In Vitro 22, 1719–1725 (doi:10.1016/j.tiv.2008.07.012) [DOI] [PubMed] [Google Scholar]