Abstract
The aim of this clinical trial was to investigate safety and efficacy when combining cetuximab with bevacizumab and irinotecan in patients with recurrent primary glioblastoma multiforme (GBM). Patients were included with recurrent primary GBM and progression within 6 months of ending standard treatment (radiotherapy and temozolomide). Bevacizumab and irinotecan were administered IV every 2 weeks. The first 10 patients received bevacizumab 5 mg/kg, but this was increased to 10 mg/kg after interim safety analysis. Irinotecan dose was based on whether patients were taking enzyme-inducing antiepileptic drugs or not: 340 and 125 mg/m2, respectively. Cetuximab 400 mg/m2 as loading dose followed by 250 mg/m2 weekly was administered IV. Forty-three patients were enrolled in the trial, of which 32 were available for response. Radiographic responses were noted in 34%, of which 2 patients had complete responses and 9 patients had partial responses. The 6-month progression-free survival probability was 30% and median overall survival was 29 weeks (95% CI: 23–37 weeks). One patient had lacunar infarction, 1 patient had multiple pulmonary embolisms, and 3 patients had grade 3 skin toxicity, for which 1 patient needed plastic surgery. One patient was excluded due to suspicion of interstitial lung disease. Three patients had deep-vein thrombosis; all continued on study after adequate treatment. Cetuximab in combination with bevacizumab and irinotecan in recurrent GBM is well tolerated except for skin toxicity, with an encouraging response rate. However, the efficacy data do not seem to be superior compared with results with bevacizumab and irinotecan alone.
Keywords: bevacizumab, cetuximab, EGFR, glioblastoma multiforme, irinotecan
Glioblastoma multiforme (GBM) continues to be a devastating disease with a median survival for newly diagnosed GBM of only 15 months.1 The prognosis for recurrent GBM is even worse with a median survival of 3–9 months when using traditional chemotherapeutic agents.2,3 However, several recent publications have demonstrated a significant improvement in the treatment of recurrent GBM using the angiogenesis inhibitor bevacizumab plus the topoisomerase 1 inhibitor irinotecan.4–6
Primary GBM arises de novo, whereas secondary GBM develops from pre-existing low-grade astrocytomas.7 Primary and secondary GBM are clinically indistinguishable. However, genotypically, there are differences, which could be used in the search for improved treatment.8,9 One target could be the epidermal growth factor receptor (EGFR), which is known to be overexpressed and/or amplified in 35%–45% of primary GBM tumors and has been shown to correlate with poor prognosis.9–12 The EGFR tyrosine kinase inhibitors (TKIs), gefitinib and erlotinib, have been used in phase I and II trials for treatment of recurrent GBM, either alone or in combination with conventional chemotherapy.13–15 Results from these studies are not uniform, although several indicate modest efficacy for TKIs in GBM.16,17 Cetuximab is a chimeric monoclonal antibody that binds to EGFR with high affinity, competes for ligand binding, and down-regulates cell-surface receptor expression.18,19 In vitro and in vivo studies using glioma cells that overexpress and/or amplify EGFR have shown reduction in cell viability with cetuximab.20–22
GBM is one of the most highly vascularized tumors with extremely elevated levels of proangiogenic factors, including vascular endothelial growth factor (VEGF) that induces tumor angiogenesis.23 VEGF promotes endothelial cell proliferation and migration in human gliomas and has been associated with poor prognosis in high-grade glioma.24 Bevacizumab is a humanized immunoglobulin G1 that binds to and inhibits the activity of the human VEGF ligand (VEGF-A) and has been used in combination with cytotoxic chemotherapy in colorectal, lung, and breast cancers.25 At the initiation of the study, there were several reports of promising effect when combining bevacizumab with irinotecan in high-grade glioma, and the results from these clinical trials have subsequently been published, confirming these observations.4,5,26 Irinotecan is able to cross the blood-brain barrier (BBB) but demonstrates only limited effect against high-grade glioma as a single-agent therapy, with response rates between 0% and 15%.27–30
Phase I studies have shown that erlotinib and gefitinib cannot be combined with irinotecan31,32 and, at the time of initiation of this study, the BOND-2 data showed that the combination of cetuximab, bevacizumab, and irinotecan is feasible,33 and clinical activity of cetuximab in GBM has been reported.34 We therefore combined bevacizumab and irinotecan with cetuximab in patients with high-grade glioma. With this combination, the aim was to target both angiogenesis through VEGF inhibition and tumors likely to overexpress EGFR, which, accordingly, were expected to benefit the most from EGFR inhibition. In addition, in vitro and in vivo results have shown that EGFR inhibition leads to reduced angiogenesis, which indicates a possible synergistic effect of cetuximab and bevacizumab on angiogenesis.35–37
Patients and Methods
Patient Selection
Adult patients (age ≥18 years) with histologically proven primary GBM (WHO classification)38 and MRI-verified recurrent or progressive disease (PD) were eligible for inclusion. Moreover, patients had to have progression within 6 months of finishing standard treatment with concomitant radiotherapy and temozolomide followed by adjuvant temozolomide.1 Reintroduction of temozolomide was not allowed. Debulking surgery was performed, if possible, before entering the study but no other tumor reductive treatments were accepted. Basic clinical and laboratory evaluations were performed within 2 weeks and MRI scan within 4 weeks of starting study treatment. Eligibility criteria were: WHO performance status 0–2; ≥4 weeks from prior surgery and/or chemotherapy; life expectancy >3 months; neutrophils ≥1500/mm3; platelets ≥125 000/mm3; hemoglobin ≥6.2 mmol/L; ASAT and/or ALT <3× upper limit of normal (ULN); bilirubin ≤1.5× ULN; cholesterol ≤7 mmol/L; normal creatinine clearance; and activated partial thromboplastin time (APTT) ≤35 seconds and/or international normalized ratio (INR) from 0.8 to 1.2. Fertile women had to use contraception. Exclusion criteria were: prior EGFR- or VEGFR-based therapy; any medical, social, or physiological condition which could prevent adequate follow-up; any other active malignancy or previous malignancies within the previous 5 years, except adequately treated basal or squamous cell carcinoma of the skin or carcinoma in situ; any significant cardiac disease (New York Heart Association Class II or greater), arrhythmia, congestive heart failure, acute myocardial infarction within 6 months, or unstable angina pectoris; any serious on-going infection, illness, or medical condition; requirement of therapeutic anticoagulation, aspirin, nonsteroidal anti-inflammatory drugs, or clopidogrel; BP >150/100 mm Hg; proteinuria WHO grade 2 or greater; and pregnant or breast-feeding women.
The study was financed by the Danish National Board of Health and conducted in accordance with the Declaration of Helsinki and ICH Guideline for Good Clinical Practice.39 Approval was obtained from the Ethics Committee and Danish Medicines Agency. Each patient signed written informed consent prior to enrollment.
Treatment
Bevacizumab and irinotecan were administered every 2 weeks (days 1 and 15) and each cycle of treatment was defined as 2 treatment administrations (4 weeks). The first 10 patients included in the study received bevacizumab 5 mg/kg without significant side effects and with no dose-limiting toxicities observed defined as grade 4 hematological toxicity or grade 3 nonhematological toxicity (according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0 [CTCAE 3.0]) except for headache, fatigue, nausea, vomiting, and alopecia if not sufficiently medically palliated. Consequently, after a planned safety analysis when these patients had received at least 2 cycles, bevacizumab was increased to 10 mg/kg in these and subsequent patients. Bevacizumab was administered by slow IV infusion over 90, 60, and 30 minutes for the first, second, and subsequent doses, respectively. IV irinotecan 340 mg/m2 for patients receiving enzyme-inducing antiepileptic drugs (EIAEDs) and 125 mg/m2 for patients not receiving EIAEDs was administered 60 minutes prior to bevacizumab. Atropine 1 mg SC was given 10 minutes prior to irinotecan to prevent cholinergic syndrome. Cetuximab was administered by slow IV infusion on days 1, 8, 15, and 22, with 400 mg/m2 as the loading dose on day 1 followed by 250 mg/m2 weekly. Appropriate antiemetics and/or antidiarrheal agents were permitted. Patients on corticosteroids were required to have a stable dose for at least 7 days before baseline MRI scan.
Cetuximab could be reduced once to 200 mg/m2 for grade 3 or 4 skin toxicity and was discontinued for grade 3 or 4 hypersensitivity reactions. These patients were allowed to continue on-study without cetuximab. Reduction in bevacizumab dose was not permitted. For unmanageable bevacizumab-related side effects (grade 3 or 4 hypertension, pulmonary embolism, severe hemorrhage, arterial thromboembolic event, grade 3 or 4 proteinuria, and GI perforation), study treatment was discontinued. Irinotecan was reduced to 80% of starting dose for grade 4 neutropenia or febrile neutropenia or ≥ grade 3 toxicity (except alopecia) in the following cycles. For grade 4 neutropenia or febrile neutropenia after dose reduction, irinotecan was reduced to 60%. No further dose reduction was allowed. Treatment was discontinued in the case of tumor progression, unmanageable grade 4 toxicity, or at the request of the patient. The physician could terminate study treatment if continuation was deemed unsafe. Patients went off-study if treatment had to be postponed for more than 2 weeks.
Patient Evaluation
Evaluation was performed within 14 days of initiating therapy and included full medical history, physical and neurological examinations, performance status examination, complete blood count with differential and platelet counts, APTT or INR, serum chemistry profile, creatinine clearance, and urinary protein dipstick analysis. T1 and T2 contrast and noncontrast MRIs were repeated every 8 weeks during treatment, and clinical and laboratory tests were repeated every 2 weeks. Toxicities were evaluated during each cycle and graded according to CTCAE 3.0.
Treatment Response Evaluation
Response to therapy was evaluated after at least 2 cycles of study treatment using the MacDonald criteria.40 These criteria use the largest cross-sectional area of the postcontrast images, neurological status, and corticosteroid dose. Complete response (CR) was defined as complete disappearance of measurable disease by MRI, partial response (PR) as >50% decrease in the area of enhancement, and PD as >25% increase in the area of enhancement, appearance of a new lesion, or deterioration in clinical status, likely secondary to tumor progression. Patients with CR or PR had to be on the same or decreased steroid dose and have stable or improved neurological findings. Stable disease (SD) is defined for patients not fulfilling CR, PR, or PD criteria.
Immunohistochemistry
Surgical specimens were routinely formalin-fixed and paraffin-embedded. Histological sections (4 µm) were stained with TissuGnost monoclonal mouse EGFR antibody (E 30; 1:200 dilution, Merck KgaA, Darmstadt, Germany). Briefly, tissue sections were deparaffinized and rehydrated followed by pretreatment in a microwave oven for 15 minutes at 95°C in tris-ethylene glycol tetraacetic acid buffer pH 9. Subsequently, staining was performed using a DAKO Autostainer (DAKO, Copenhagen, Denmark), allowing primary antibody to be incubated for 30 minutes at room temperature (RT). After washing with phosphate-buffered saline (PBS), sections were incubated with DAKO antimouse Envision+ System labeled with HRP (K4001, DAKO) for 30 minutes at RT and washed with PBS. DAKO Liquid DAB+ Substrate Chromogen System (K3468, DAKO) was applied for 10 minutes and sections washed with PBS. Sections were counterstained with hematoxylin.
Evaluation of the slides was performed independently and under blind conditions by H.B. (neuropathologist) and B.H. (MD, PhD researcher). EGFR labeling of tumor cells was scored semiquantitatively on a scale from 0 to 3 (0 = 0%; 1 = 1%–10%; 2 = 11%–50%; 3 = >50% cells stained positive).
Statistical Considerations
The primary endpoint of this study was 6-month progression-free survival (PFS). Yung et al.3 reported a median PFS of 3 months with a 6-month PFS of 21% (95% CI: 13%–29%) among patients with first-relapse GBM who were treated with temozolomide. These data were used as the historical basis for the design of our phase II study. With 43 included patients and an assumed median PFS of historical controls of 3 months, there will be an approximate 80% power to detect an improvement of 2 months, and an approximate 60% power to detect an improvement of 1.4 months. If the median PFS from our trial is 3 months, the lower 95% CI will be approximately 2 months. We estimated 6-month PFS, time-to-progression (TTP), overall survival (OS), and associated 95% CIs using SPSS software, version 15.0 (Chicago, Illinois) and Kaplan–Meier methodology. The log-rank test was performed to compare survival in responders vs nonresponders. Pearson χ2 and Fischer's exact tests were used for correlation between the EGFR level and response. Probability values (P values) <.05 were considered statistically significant. The Kaplan–Meier methodology was used for correlation between EGFR and survival.
Results
Patient Characteristics
Forty-three patients were enrolled from August 2006 to February 2008. Baseline characteristics of the patients are shown in Table 1. All patients had histologically verified primary GBM and had received standard treatment,1 after which they showed progressive or recurrent disease within 6 months. Median study treatment duration was 14 weeks (range: 2–84 weeks).
Table 1.
Patient characteristics of the ITT population
| Characteristic | Cetuximab/bevacizumab/irinotecan (n = 43) |
|
|---|---|---|
| Number | Percentage | |
| Gender | ||
| Male | 25 | 58 |
| Female | 18 | 42 |
| Age (y) | ||
| Median | 54 | |
| Range | 23–70 | |
| WHO performance status | ||
| 0 | 9 | 21 |
| 1 | 26 | 60 |
| 2 | 8 | 19 |
| Reoperation before study treatment | ||
| Yes | 12 | 28 |
| No | 31 | 72 |
| Site of treatment | ||
| Copenhagen | 32 | 74 |
| Aalberg | 9 | 21 |
| Odense | 3 | 7 |
| Concomitant medications | ||
| EIAED | 6 | 14 |
| Non-EIAED | 37 | 86 |
| Corticosteroids | 35 | 81 |
| Time from diagnosis until starting study treatment (d) | ||
| Median | 266 | |
| Range | 164–937 | |
| Time from first recurrence until starting study treatment (d) | ||
| Median | 59 | |
| Range | 15–162 | |
Abbreviations: EIAED, enzyme-inducing antiepileptic drug; non-EIAED, non-enzyme-inducing antiepileptic drug; ITT, intention-to-treat.
Response Rate
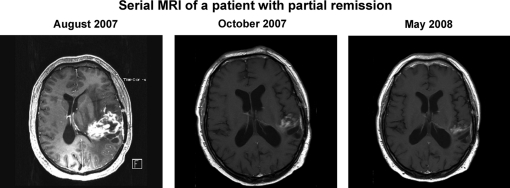
The response rate of all patients based on intention-to-treat (ITT) (CR + PR) was 26% (95% CI: 14%–41%; Table 2). Eleven of the patients included (n = 43) went off study prior to MRI evaluation due to early deterioration or severe adverse events leading to early discontinuation of the treatment. Among evaluable patients (n = 32), best response was recorded after 2–4 treatment cycles. Both patients with CR had minor tumor load at the initiation of study treatment. Figure 1 shows serial MRI for a patient with PR.
Table 2.
Response in patients intended to treat
| Characteristic | Cetuximab/bevacizumab/irinotecan (n = 43) |
|
|---|---|---|
| Number of patients | Percentage | |
| ORR: CR + PR | 11 | 26 (95% CI: 14%–41%) |
| CR | 2 | 5 |
| PR | 9 | 21 |
| SD | 17 | 40 |
| PD | 4 | 9 |
| Not evaluable | 11 | 26 |
Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; ORR, objective response rate.
Fig. 1.
MRI scan of a 64-year-old man with a PR and a TTP of 342 days. The patient initiated treatment within 4 weeks of the MRI scan originating from August 2007.
Progression-Free Survival
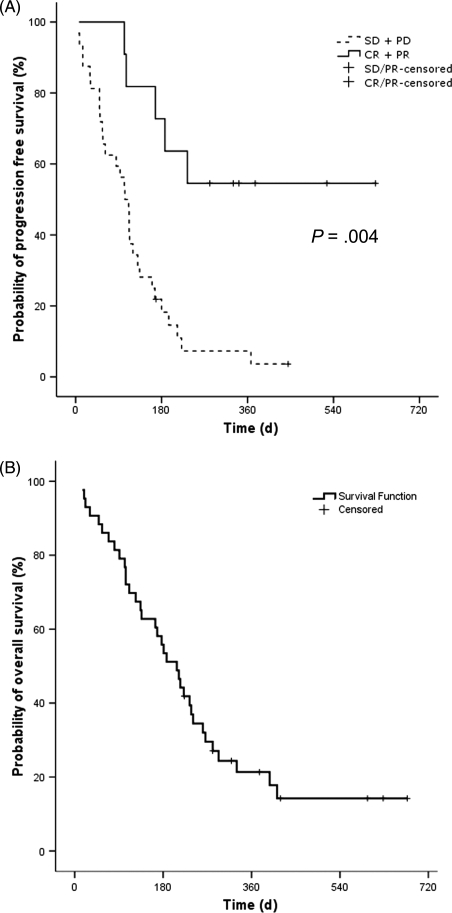
Median follow-up time was 15 months (range: 7–25 months) and median PFS was 16 weeks (95% CI: 13–20 weeks). The 6-month PFS was 33% (95% CI: 19%–48%). Of the 2 patients with CR, 1 had 24 weeks to tumor progression and the other had not progressed at the time of study evaluation, 90 weeks after initiating study treatment. Figure 2A shows the Kaplan–Meier PFS plot, illustrating TTP for those with CR + PR vs SD + PD, which showed a significant difference between these groups (P < .004).
Fig. 2.
Kaplan–Meier estimates showing TTP for evaluable patients (n = 32) (A) and OS for the ITT population (n = 43) (B).
Overall Survival
Median OS as estimated by the Kaplan–Meier analysis (Fig. 2B) was 30 weeks (95% CI: 23–37 weeks). One patient with CR, 4 patients with PR, and 2 patients with SD were still alive at the time of study evaluation.
EGFR Expression
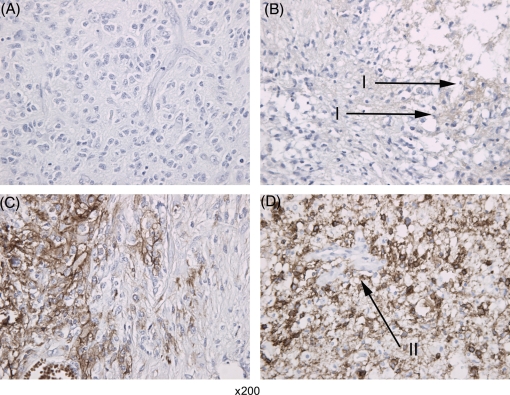
EGFR expression was determined for 39 of the 43 patients included, of which 2 were missing in the evaluable group of patients (n = 32). Of the 11 patients with CR and PR, 8 had <10% and 3 had >50% EGFR expression. Of the 19 patients with SD or PR, 13 had <10%, 4 had 11%–50%, and 2 had >50% EGFR expression. Kaplan–Meier methodology showed no correlation between EGFR expression and survival, and no significant correlation was found between EGFR expression and response using the Pearson χ2 and Fischer's exact tests (data not shown). Figure 3 shows examples of EGFR staining.
Fig. 3.
Examples of EGFR expression by immunohistochemistry scored semiquantitatively on a scale from 0 to 3. (A) 0 = 0%; (B) 1 = 1%–10%; (C) 2 = 11%–50%; (D) 3 = >50% cells stained positive. Arrowheads I showing positive EGFR staining. Arrowhead II showing a vessel, not staining for EGFR.
Tolerability
Adverse events are summarized in Table 3. Six patients discontinued study treatment: one each for multiple pulmonary embolisms, lacunar infarction, severe skin toxicity which needed plastic surgery, pneumonia resulting in >2 weeks treatment suspension, suspicion of interstitial lung disease which normalized after discontinuation of treatment, and infection in a scalp scar originating from a reoperation procedure causing intracerebral air embolism and eventual death. Furthermore, 1 patient had cardiac arrest on day 24 and died the following day: autopsy showed acute pulmonary edema and no sign of intracerebral, cardiac or pulmonary bleeding, or thrombosis. Possible cause of death was epileptic seizure resulting in cerebral-triggered cardiac arrest, not related to study therapy. Three patients developed deep-vein thrombosis, all continued study treatment after initiation of a low-molecular–weight heparin, although one of these experienced grade 3 GI bleeding of unknown origin but continued study treatment after recovery.
Table 3.
Adverse events in the ITT population
| Adverse event | Cetuximab/bevacizumab/irinotecan (n = 43) |
|
|---|---|---|
| Grade 1/2 (No. [%]) | Grade 3/4 (No. [%]) | |
| Nausea | 13 (30) | 0 |
| Vomiting | 5 (12) | 1 (2) |
| Diarrhea | 14 (9) | 3 (7) |
| Stomatitis | 12 (28) | 0 |
| Constipation | 16 (37) | 1 (2) |
| Loss of appetite | 6 (14) | 1 (2) |
| Fatigue | 22 (51) | 0 |
| Neutropenia | 5 (12) | 2 (5) |
| Fever | 5 (12) | 0 |
| Infection | 9 (21) | 6 (14) |
| Thrombosis | 0 | 4 (9) |
| CNS hemorrhage | 1 (2) | 0 |
| Skin reaction | 26 (60) | 3 (7) |
| Bleeding | 6 (14) | 2 (5) |
| Interstitial lung disease | 0 | 1 (2) |
Abbreviation: ITT, intention-to-treat.
Three patients experienced grade 3 or 4 allergic reactions during the first cetuximab administration despite premedication and all continued on study without cetuximab according to study protocol. Cetuximab is known for its skin toxicity: 12 patients had grade 1, 14 had grade 2, and 3 had grade 3 skin toxicity. Of the latter, one discontinued study treatment and the other two continued on study treatment without cetuximab. No patient developed grade 4 hematologic toxicity or grade 4 nonhematological toxicity except as noted above. Three patients developed arterial hypertension during study treatment and all were treated with appropriate antihypertensive medication and continued study treatment.
Discussion
We report the first phase II trial of irinotecan and bevacizumab in combination with cetuximab for the treatment of recurrent primary GBM. This study demonstrates that cetuximab, bevacizumab plus irinotecan, has an acceptable safety profile and induces a considerable number of clinically relevant, durable responses. For the ITT population, 6-month PFS was 33% (95% CI: 19%–48%), being 73% and 25% for those with CR/PR and SD/PD, respectively, and median PFS was 16 weeks (95% CI: 13–20 weeks). The response rate was 26% (95% CI: 14%–41%). The initial sample size of 43 patients was reduced to 32 evaluable patients; patients with recurrent GBM are particularly vulnerable and since the first MRI evaluation was performed after 8 weeks, 11 patients discontinued within this time due to early deterioration or severe adverse events. However, these patients were included in all ITT analyses.
The efficacy of bevacizumab plus irinotecan for recurrent high-grade glioma was first shown by Stark-Vance,6 who found a response rate of 43% among 21 patients. In our study, we only included patients with primary GBM, which may explain our lower response rate. Our results are comparable with those obtained with the combination of bevacizumab and chemotherapy in high-grade glioma patients by Norden et al.,26 Guiu et al.,41 and Poulsen et al.,5 who showed response rates of 34%, 36%, and 25%, respectively. Bevacizumab was combined with irinotecan in the latter 2 studies. Our results are not comparable with those of Vredenburgh et al.,4 who showed a response rate of 63% and median OS of 40 weeks with bevacizumab plus irinotecan in patients with recurrent malignant glioma. However, 6-month PFS was 32% in their study, which is comparable with our result. In our study, 9 of the 17 patients with SD (53%) had tumor reduction between 25% and 48%, which clearly indicates a clinical benefit.
EGFR is known to be amplified and/or overexpressed in 35%–45% of primary GBM tumors,9–12 and 40% of GBM tumors with EGFR amplification express the mutant EGFRvIII receptor which induces ligand-independent constitutive activation.12,42 Accordingly, targeting EGFR and EGFRvIII and their down-stream pathways has been of considerable interest in the search for new treatments of high-grade glioma. Moreover, EGFR activation can increase VEGF production in glioma cell lines,43 and EGFR inhibition by cetuximab reduces the VEGF production of both in vitro and in vivo in various cancer cell lines.35–37 In addition, it has been shown that cetuximab reduces the level of hypoxia-inducible factor-1 alpha (HIF-1α), which is a transcriptional regulator of VEGF expression.44 Necrosis and hypoxia are mandatory in GBM and hypoxia leads, among other factors, to stabilization of HIF-1α and HIF-2α subunits that initiate VEGF transcription.45 Thus, combining inhibition of EGFR and VEGFR (by inhibiting binding of the ligand VEGF) might be expected to have a beneficial effect in primary GBM. In anticipation of EGFR being an essential target in the treatment of high-grade glioma, gefitinib and erlotinib have been studied as single-agents or in combination with chemotherapy, radiotherapy, and other targeted therapies, but with only modest effect.13–17 Cetuximab in combination with radiotherapy has been shown to reduce the viability of EGFR-amplified glioma cell lines both in vitro and in vivo and has been shown to bind EGFRvIII and induce internalization of the receptor.20,21 In addition, cetuximab has been shown to induce 40%–50% inhibition of cell proliferation in vitro.46 The use of cetuximab for high-grade glioma patients has been limited. However, Belda-Iniesta et al.34 showed some durable responses when using cetuximab in 3 patients with recurrent GBM who remained clinically and radiologically stable for 14, 13, and 11 months, respectively. These 3 patients all had positive EGFR staining. In our study, EGFR were overexpressed in 11 (37%) of evaluable patients and the expression was not correlated with response or survival.
Cetuximab has shown to be ineffective when treating colon cancer patients with K-ras mutations;47 however, K-ras mutations are not very common in GBM.48 The lack of an improved response rate when combining cetuximab with bevacizumab and irinotecan might be caused by mutations in the tumor suppressor gene, phosphatase, and tensin homolog (PTEN). Importantly, PTEN mutations occur in 20%–40% of GBM tumors and have been shown by other groups to mediate resistance to anti-EGFR treatment.17,49 Thus, it would appear that EGFR is not of such pivotal importance for maintenance of glioma tumor growth as had been expected previously, despite the fact that EGFR is often found to be overexpressed and/or amplified in primary GBM.
At the time of initiation of our trial, there were no data showing the effect of bevacizumab alone vs the combination of bevacizumab and irinotecan in patients with GBM. Subsequently, Cloughesy et al.50 have shown that 6-month PFS (50% vs 35%) and response rate (33% vs 20%) are not significantly higher in patients treated with bevacizumab and irinotecan when compared with those receiving bevacizumab alone, respectively. Moreover, a recent study by Kreisl et al.51 showed that single-agent bevacizumab resulted in significant activity in heavily pretreated patients with GBM with a 6-month PFS of 29% and a response rate of 35%, without benefit from the addition of irinotecan at progression. These results are comparable with the 6-month PFS and response rate found in our study (33% and 26%, respectively). On the basis of the results of the studies by Cloughesy et al.50 and Kreisl et al.,51 bevacizumab has now been approved by the US Food and Drug Administration as single-agent treatment for patients with progressive GBM following prior therapy.
In contrast to small-molecule TKIs, cetuximab is a large molecule, which will possibly not cross the intact BBB. However, this should also be the case with bevacizumab, and the significant clinical benefit of this agent may be related to the fact that BBB is not intact in areas of active tumor cells.52 We found it appropriate to add cetuximab to a backbone of bevacizumab and irinotecan, since it has been shown in 2 phase I studies that combination of erlotinib or gefitinib with irinotecan induced dose-limiting diarrhea.31,32 Other new interesting agents also interfere with vascularization. Cilengitide is an integrin inhibitor with clinical activity in recurrent GBM.53 AZD2171 (cediranib) is a multitargeted TKI that blocks VEGFR-1, VEGFR-2, and VEGFR-3 signaling that showed a response rate of 56% as single-agent therapy in recurrent GBM.54 Both these agents are now being tested in phase III trials for GBM.
In our study, 11 patients were not evaluable for response. This indicates that patient selection is very important when evaluating new regimens in GBM, because these patients are vulnerable due to immobilization, and minor changes in the primary tumor may result in global alteration and severe deterioration.
In conclusion, cetuximab in combination with bevacizumab and irinotecan in patients with recurrent GBM was found to be a well-tolerated regimen, except for skin toxicity, with an encouraging response rate, including 2 patients with CR. However, the response rate does not appear to be superior with the addition of cetuximab to that which can be obtained with single-agent bevacizumab or the combination of bevacizumab plus irinotecan. Consequently, there would appear to be no rationale for adding cetuximab to the bevacizumab-based regimens in recurrent high-grade glioma in the future.
Conflict of interest statement. U.L. has previously received a research grant from Roche A/S and Merck and has received speaking fees from the same companies.
Funding
This study was supported by the Copenhagen University Hospital and the Danish National Board of Health (journal number: 2006-12103-254).
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 3.Yung WK, Albright RE, Olson J, et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer. 2000;83:588–593. doi: 10.1054/bjoc.2000.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 5.Poulsen HS, Grunnet K, Sorensen M, et al. Bevacizumab plus irinotecan in the treatment patients with progressive recurrent malignant brain tumours. Acta Oncol. 2009;48:52–58. doi: 10.1080/02841860802537924. [DOI] [PubMed] [Google Scholar]
- 6.Stark-Vance V. Bevacizumab and CPT-11 in the treatment of relapsed malignant glioma [abstract 342] Neuro-Oncology. 2005;7:369. [Google Scholar]
- 7.Kleihues P, Ohgaki H. Primary and secondary glioblastomas: from concept to clinical diagnosis. Neuro-Oncology. 1999;1:44–51. doi: 10.1093/neuonc/1.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kita D, Yonekawa Y, Weller M, Ohgaki H. PIK3CA alterations in primary (de novo) and secondary glioblastomas. Acta Neuropathol. 2007;113:295–302. doi: 10.1007/s00401-006-0186-1. [DOI] [PubMed] [Google Scholar]
- 9.Ohgaki H, Dessen P, Jourde B, et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64:6892–6899. doi: 10.1158/0008-5472.CAN-04-1337. [DOI] [PubMed] [Google Scholar]
- 10.Ekstrand AJ, James CD, Cavenee WK, Seliger B, Pettersson RF, Collins VP. Genes for epidermal growth factor receptor, transforming growth factor alpha, and epidermal growth factor and their expression in human gliomas in vivo. Cancer Res. 1991;51:2164–2172. [PubMed] [Google Scholar]
- 11.Smith JS, Tachibana I, Passe SM, et al. PTEN mutation, EGFR amplification, and outcome in patients with anaplastic astrocytoma and glioblastoma multiforme. J Natl Cancer Inst. 2001;93:1246–1256. doi: 10.1093/jnci/93.16.1246. [DOI] [PubMed] [Google Scholar]
- 12.Frederick L, Wang XY, Eley G, James CD. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 2000;60:1383–1387. [PubMed] [Google Scholar]
- 13.Rich JN, Reardon DA, Peery T, et al. Phase II trial of gefitinib in recurrent glioblastoma. J Clin Oncol. 2004;22:133–142. doi: 10.1200/JCO.2004.08.110. [DOI] [PubMed] [Google Scholar]
- 14.Lassman AB, Rossi MR, Razier JR, et al. Molecular study of malignant gliomas treated with epidermal growth factor receptor inhibitors: tissue analysis from North American brain tumor consortium trials 01–03 and 00–01. Clin Cancer Res. 2005;11:7841–7850. doi: 10.1158/1078-0432.CCR-05-0421. [DOI] [PubMed] [Google Scholar]
- 15.Franceschi E, Cavallo G, Lonardi S, et al. Gefitinib in patients with progressive high-grade gliomas: a multicentre phase II study by Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO) Br J Cancer. 2007;96:1047–1051. doi: 10.1038/sj.bjc.6603669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haas-Kogan DA, Prados MD, Tihan T, et al. Epidermal growth factor receptor, protein kinase B/Akt, and glioma response to erlotinib. J Natl Cancer Inst. 2005;97:880–887. doi: 10.1093/jnci/dji161. [DOI] [PubMed] [Google Scholar]
- 17.Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 18.Li S, Schmitz KR, Jeffrey PD, et al. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell. 2005;7:301–311. doi: 10.1016/j.ccr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein NI, Prewett M, Zuklys K, Rockwell P, Mendelsohn J. Biological efficacy of a chimeric antibody to the epidermal growth factor receptor in a human tumor xenograft model. Clin Cancer Res. 1995;1:1311–1318. [PubMed] [Google Scholar]
- 20.Eller JL, Longo SL, Hicklin DJ, Canute GW. Activity of anti-epidermal growth factor receptor monoclonal antibody C225 against glioblastoma multiforme. Neurosurgery. 2002;51:1005–1013. doi: 10.1097/00006123-200210000-00028. [DOI] [PubMed] [Google Scholar]
- 21.Eller JL, Longo SL, Kyle MM, Bassano D, Hicklin DJ, Canute GW. Anti-epidermal growth factor receptor monoclonal antibody cetuximab augments radiation effects in glioblastoma multiforme in vitro and in vivo. Neurosurgery. 2005;56:155–162. doi: 10.1227/01.neu.0000145865.25689.55. [DOI] [PubMed] [Google Scholar]
- 22.Combs SE, Schulz-Ertner D, Roth W, Herold-Mende C, Debus J, Weber KJ. In vitro responsiveness of glioma cell lines to multimodality treatment with radiotherapy, temozolomide, and epidermal growth factor receptor inhibition with cetuximab. Int J Radiat Oncol Biol Phys. 2007;68:873–882. doi: 10.1016/j.ijrobp.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Salmaggi A, Eoli M, Frigerio S, et al. Intracavitary VEGF, bFGF, IL-8, IL-12 levels in primary and recurrent malignant glioma. J Neurooncol. 2003;62:297–303. doi: 10.1023/a:1023367223575. [DOI] [PubMed] [Google Scholar]
- 24.Godard S, Getz G, Delorenzi M, et al. Classification of human astrocytic gliomas on the basis of gene expression: a correlated group of genes with angiogenic activity emerges as a strong predictor of subtypes. Cancer Res. 2003;63:6613–6625. [PubMed] [Google Scholar]
- 25.Eskens FA, Sleijfer S. The use of bevacizumab in colorectal, lung, breast, renal and ovarian cancer: where does it fit? Eur J Cancer. 2008;44:2350–2356. doi: 10.1016/j.ejca.2008.07.042. [DOI] [PubMed] [Google Scholar]
- 26.Norden AD, Drappatz J, Muzikansky A, et al. An exploratory survival analysis of anti-angiogenic therapy for recurrent malignant glioma. J Neurooncol. 2008;92:149–155. doi: 10.1007/s11060-008-9745-8. [DOI] [PubMed] [Google Scholar]
- 27.Prados MD, Lamborn K, Yung WK, et al. A phase 2 trial of irinotecan (CPT-11) in patients with recurrent malignant glioma: a North American Brain Tumor Consortium study. Neuro-Oncology. 2006;8:189–193. doi: 10.1215/15228517-2005-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cloughesy TF, Filka E, Kuhn J, et al. Two studies evaluating irinotecan treatment for recurrent malignant glioma using an every-3-week regimen. Cancer. 2003;97(suppl 9):2381–2386. doi: 10.1002/cncr.11306. [DOI] [PubMed] [Google Scholar]
- 29.Vredenburgh JJ, Desjardins A, Reardon DA, Friedman HS. Experience with irinotecan for the treatment of malignant glioma. Neuro-Oncology. 2008;11:80–91. doi: 10.1215/15228517-2008-075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raymond E, Fabbro M, Boige V, et al. Multicentre phase II study and pharmacokinetic analysis of irinotecan in chemotherapy-naive patients with glioblastoma. Ann Oncol. 2003;14:603–614. doi: 10.1093/annonc/mdg159. [DOI] [PubMed] [Google Scholar]
- 31.Messersmith WA, Laheru DA, Senzer NN, et al. Phase I trial of irinotecan, infusional 5-fluorouracil, and leucovorin (FOLFIRI) with erlotinib (OSI-774): early termination due to increased toxicities. Clin Cancer Res. 2004;10:6522–6527. doi: 10.1158/1078-0432.CCR-04-0746. [DOI] [PubMed] [Google Scholar]
- 32.Hofheinz RD, Kubicka S, Wollert J, Arnold D, Hochhaus A. Gefitinib in combination with 5-fluorouracil (5-FU)/folinic acid and irinotecan in patients with 5-FU/oxaliplatin-refractory colorectal cancer: a phase I/II study of the Arbeitsgemeinschaft fur Internistische Onkologie (AIO) Onkologie. 2006;29:563–567. doi: 10.1159/000096449. [DOI] [PubMed] [Google Scholar]
- 33.Saltz LB, Lenz HJ, Kindler HL, et al. Randomized phase II trial of cetuximab, bevacizumab, and irinotecan compared with cetuximab and bevacizumab alone in irinotecan-refractory colorectal cancer: the BOND-2 study. J Clin Oncol. 2007;25:4557–4561. doi: 10.1200/JCO.2007.12.0949. [DOI] [PubMed] [Google Scholar]
- 34.Belda-Iniesta C, Carpeño JC, Saenz EC, Gutiérrez M, Perona R, Barón MB. Long term responses with cetuximab therapy in glioblastoma multiforme. Cancer Biol Ther. 2006;5:912–914. doi: 10.4161/cbt.5.8.3118. [DOI] [PubMed] [Google Scholar]
- 35.Ciardiello F, Bianco R, Damiano V, et al. Antiangiogenic and antitumor activity of anti-epidermal growth factor receptor C225 monoclonal antibody in combination with vascular endothelial growth factor antisense oligonucleotide in human GEO colon cancer cells. Clin Cancer Res. 2000;6:3739–3747. [PubMed] [Google Scholar]
- 36.Perrotte P, Matsumoto T, Inoue K, et al. Anti-epidermal growth factor receptor antibody C225 inhibits angiogenesis in human transitional cell carcinoma growing orthotopically in nude mice. Clin Cancer Res. 1999;5:257–265. [PubMed] [Google Scholar]
- 37.Petit AM, Rak J, Hung MC, et al. Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol. 1997;151:1523–1530. [PMC free article] [PubMed] [Google Scholar]
- 38.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.European Medicines Agency (EMEA) ICH Topic E 6 (R1): Guideline for Good Clinical Practice. http://www.emea.europa.eu/pdfs/human/ich/013595en.pdf . Accessed July 13, 2009. [Google Scholar]
- 40.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 41.Guiu S, Taillibert S, Chinot O, et al. Bevacizumab/irinotecan. An active treatment for recurrent high grade gliomas: preliminary results of an ANOCEF Multicenter Study. Rev Neurol (Paris) 2008;164:588–594. doi: 10.1016/j.neurol.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 42.Wikstrand CJ, McLendon RE, Friedman AH, Bigner DD. Cell surface localization and density of the tumor-associated variant of the epidermal growth factor receptor, EGFRvIII. Cancer Res. 1997;57:4130–4140. [PubMed] [Google Scholar]
- 43.van Cruijsen H, Giaccone G, Hoekman K. Epidermal growth factor receptor and angiogenesis: opportunities for combined anticancer strategies. Int J Cancer. 2005;117:883–888. doi: 10.1002/ijc.21479. [DOI] [PubMed] [Google Scholar]
- 44.Luwor RB, Lu Y, Li X, Mendelsohn J, Fan Z. The antiepidermal growth factor receptor monoclonal antibody cetuximab/C225 reduces hypoxia-inducible factor-1 alpha, leading to transcriptional inhibition of vascular endothelial growth factor expression. Oncogene. 2005;24:4433–4441. doi: 10.1038/sj.onc.1208625. [DOI] [PubMed] [Google Scholar]
- 45.Keith B, Simon MC. Hypoxia-inducible factors, stem cells, and cancer. Cell. 2007;129:465–472. doi: 10.1016/j.cell.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel D, Lahiji A, Patel S, et al. Monoclonal antibody cetuximab binds to and down-regulates constitutively activated epidermal growth factor receptor vIII on the cell surface. Anticancer Res. 2007;27:3355–3366. [PubMed] [Google Scholar]
- 47.Lièvre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–3995. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 48.Lymbouridou R, Soufla G, Chatzinikola AM, Vakis A, Spandidos DA. Down-regulation of K-ras and H-ras in human brain gliomas. Eur J Cancer. 2009;45:1294–1303. doi: 10.1016/j.ejca.2008.12.028. [DOI] [PubMed] [Google Scholar]
- 49.Bianco R, Shin I, Ritter CA, et al. Loss of PTEN/MMAC1/TEP in EGF receptor-expressing tumor cells counteracts the antitumor action of EGFR tyrosine kinase inhibitors. Oncogene. 2003;18:2812–2822. doi: 10.1038/sj.onc.1206388. [DOI] [PubMed] [Google Scholar]
- 50.Cloughesy TF, Prados MD, Wen PY, et al. A phase II, randomized, non-comparative clinical trial of the effect of bevacizumab (BV) alone or in combination with irinotecan (CPT) on 6-month progression free survival (PFS6) in recurrent, treatment-refractory glioblastoma (GBM) [abstract 2010b] J Clin Oncol. 2008;26(suppl.) [Google Scholar]
- 51.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2008;27:740–745. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deeken JF, Löscher W. The blood-brain barrier and cancer: transporters, treatment, and Trojan horses. Clin Cancer Res. 2007;13:1663–1674. doi: 10.1158/1078-0432.CCR-06-2854. [DOI] [PubMed] [Google Scholar]
- 53.Reardon DA, Fink KL, Mikkelsen T, et al. Randomized phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J Clin Oncol. 2008;26:5610–5617. doi: 10.1200/JCO.2008.16.7510. [DOI] [PubMed] [Google Scholar]
- 54.Batchelor T, Sorensen AG, Ancukiewicz M, et al. A phase II trial of AZD2171 (cediranib), an oral pan-VEGF receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma [abstract 2001] J Clin Oncol. 2007;25(suppl.) doi: 10.1200/JCO.2009.26.3988. [DOI] [PMC free article] [PubMed] [Google Scholar]