Abstract
Conditional mutants for flagellar assembly (fla) provide a useful tool to study intraflagellar transport (IFT) at the molecular level, and provide a unique set of tools to analyze cilia. The analysis of IFT phenotypes of fla mutants at the permissive temperature by a quantitative image analysis approach identified four distinct phases of the IFT cycle and directly demonstrated structural and functional remodeling of IFT particles at both axonemal extremities. In addition, the genetic analysis of fla mutants reveal interesting interactions among genes involved in flagellar assembly that help to provide information about the structure and function of IFT particles and their motors. This chapter provides protocols to isolate, characterize, and identify conditional Chlamydomonas flagellar assembly mutants and their genes and to test genetic interactions among proteins encoded by these genes.
I. Introduction
Intraflagellar transport (IFT), the evolutionary conserved intracellular transport required for ciliary/flagellar assembly, maintenance, and signaling was first observed as a bidirectional movement of particles along Chlamydomonas flagella by using video-enhanced differential interference contrast (VE-DIC) light microscopy (Kozminski et al., 1993). Mutants in Chlamydomonas with conditional, temperature-sensitive defects in flagellar assembly (fla) retain flagella at the permissive temperature (21°C) but lose them at the restrictive temperature (32°C). Biochemical and microscopic analysis of fla10, which has a conditional mutation in the gene encoding one of the motor subunits of kinesin-2 (Walther et al., 1994), lead to the identification of IFT–protein complexes and uncovered the role of IFT in flagella-mediated signaling (Cole et al., 1998; Pan and Snell, 2002; Piperno and Mead, 1997; Piperno et al., 1996; Wang et al., 2006). Biochemical analysis of the Chlamydomonas flagella led to the identification of many of the proteins in the IFT particles and showed that they dissociate into two complexes called A and B (Cole et al., 1998; Piperno and Mead, 1997). Null alleles in Chlamydomonas IFT genes, which include bld1-1, bld1-2, and ift88-1, were instrumental in showing the essential role of IFT components in flagellar assembly (Brazelton et al., 2001; Pazour et al., 2000). Strains with these null alleles lack flagella and prevent a biochemical and microscopic approach to study the function of proteins encoded by their disrupted genes.
Temperature-sensitive mutants for flagellar assembly (fla) provide a useful tool to study IFT at the molecular level, and give Chlamydomonas a unique set of tools to analyze cilia (Adams et al., 1982; Huang et al., 1977; Iomini et al., 2001; Lux and Dutcher, 1991; Piperno et al., 1998). The analysis of IFT phenotypes of fla mutants at the permissive temperature by a quantitative image analysis approach called kymography identified four distinct phases of the IFT cycle and directly demonstrated structural and functional remodeling of IFT particles at both axonemal extremities. Finally, fla mutants reveal unique genetic interactions among genes involved in flagellar assembly that help to provide information about the structure and function of IFT particles and their motors.
This chapter provides protocols to isolate, characterize, and identify conditional Chlamydomonas mutants for flagellar assembly and to test genetic interactions among proteins encoded by these genes. This approach has helped to advance our understanding of ciliary biology by uncovering specific functions of individual components of the IFT machinery and providing new tools to identify new genes involved in IFT regulation.
II. Isolation of Aflagellate Strains
Many, but not all aflagellate strains, have defects in IFT components and motors. Some of these mutants come from screens for cells that fail to mate, as flagellar proteins are required for recognition between cells of opposite mating types (Goodenough et al., 2007). For example, BLD1 that encodes IFT52 was identified in this screen (Brazelton et al., 2001). An alternative approach is based on the observation that exogenous DNA integrates into the Chlamydomonas genome nonhomologously (Tam and Lefebvre, 1993). Thus, it has been used extensively for insertional mutagenesis. Insertional mutants have yielded aflagellate or very short, stumpy flagellar strains. Screening Southern blots using probes to IFT proteins or motors has identified many of these proteins, which include ift46, dhc1b-1, stf1-1, stf1-2, and d1blic (Hou et al., 2004, 2007; Pazour et al., 1999; Porter et al., 1999), which assemble stumpy or short flagella.
The selectable markers for insertional mutagenesis currently include ARG7, NIT1, BLE, and APHVIII, which confers arginine prototrophy, nitrate prototrophy, resistance to Zeocin, and resistance to paromomycin, respectively. The APHVIII gene has several advantages. It is a relatively small gene. Paromomycin is highly selective and is not mutagenic like Zeocin, which induces chromosome breaks if the transgene is not active immediately. Null alleles are useful in combination with conditional alleles for deciphering genetic interactions (Section VI).
Thermal asymmetric interlaced (TAIL) and adaptor PCR are two methods to identify the sites of insertion without the need to use a candidate gene for Southern blots.
1. Prepare genomic DNA (1 μg) from insertional mutant strains and wild-type parental strains by previously described techniques (Johnson and Dutcher, 1991) and digest with PmlI (50 U) and PvuII (10 U) overnight (16–18 h) to obtain blunt-ended fragments ~1–6 kb in length.
2. Verify the digestion by agarose gel (10 μl in a 2% gel) electrophoresis. The fragments are purified by column PCR purification and eluted with 30 μl H2O.
3. The adaptor (Table I) is prepared by annealing the plus and minus adaptor (25 μM each, in a thermocycler, set to decrease temperature from 95 to 4°C over 3 h (1°C every 2 min), and stored at 4°C.
Table I.
Primers for Identifying the Insertion Site
| Primer name | Primer sequence |
|---|---|
| TPA1 | CGGGAGTTGTTTGTCAAGGT |
| TPA2 | GTTTGTCAAGGTGGCAGCTC |
| TPA3 | GATTCCCGTACCTCGTGTTGT |
| TPA1R | ACCTTGACAAACAACTCCCG |
| TPA2R | GAGCTGCCACCTTGACAAAC |
| TPA3R | ACAACACGAGGTACGGGAATC |
| TPA4 | CTCAGAAGAACTCGTCCAACAG |
| TPA5 | CATCAGGTCCCTCAGAAGAACT |
| TPA6 | GTAAAACGCCAGCTTTTCCTC |
| TPA7 | GAACCACGGGTCCTCCTC |
| AD3 | WGTGNAGNANCANAGA |
| AD5 | STTGNTASTNCTNTGC |
| AD6 | WCAGNTGWTNGTNCTG |
| AD11 | NCASGAWAGNCSWCAA |
| Minus adaptor | 5' phosphorylation – 5' – ACCAGCCCGG – –3' C7 spacer arm – 3' amino modifier |
| Plus adaptor | 5' GTAATACGACTCACTATAGAGTACGCGTGGTCGACGGCCCGGGCTGGT |
| RIM3-1 | CGGTATCGGAGGAAAAGCTG |
| RIM3-2 | GCTGTTGGACGAGTTCTTCTG |
| AP1 | GTAATACGACTCACTATAGAGT |
| AP2 | ACTATAGAGTACGCGTGGT |
4. The blunt-end genomic DNA fragments (3 μl) are ligated to the adaptor in a 20 μl reaction containing the adaptor (1.25 μM) and T4 DNA Ligase (400 U) in T4 DNA Ligase buffer. Incubate the reaction at 16°C for 16 h and inactivate the enzyme by incubation at 80°C for 20 min. Dilute the reaction to a total of 90 μl (with H2O) before being used in PCR reactions.
5. Two nested PCR reactions are conducted using primers RIM3-1 and TPA3R, then, RIM3-2 and TPA1R for the iPCR (Table I). Four different nested primer sets are used for the adaptor-mediated PCR: RIM3-1, AP1, then, RIM3-2, AP2; TPA1, AP1, then, TPA3, TPA2; TPA3R, AP1, then, TPA1R, AP2; and TPA4, AP1, then, TPA6, AP2. PCR reactions are carried out in a 50-μl volume, using 2 μl of the above DNA preparation mixture and consisting of 0.4 mM dNTPs, 5% (v/v) dimethyl sulfoxide (DMSO), 0.4 μM primers, 0.24 μl KLENTaq-LA, in supplied buffer was used. The reactions use a touchdown PCR protocol.
6. Products are sequenced to determine the location of the insertion site. Approximately 60% of the mutants tested gave insertions that can be verified by mapping.
III. Isolation of Conditional Flagellar Assembly (fla) Mutants
The conditional class of fla mutants are isolated on the basis that they are motile at the permissive temperature of 21°C but lose their flagella at the restrictive temperature of 32°C. As is observed for many conditional mutants, fla mutants also display weaker, but specific mutant phenotypes at the permissive temperature that have been informative about the function of their gene products.
The total number of independent fla mutants in Chlamydomonas is currently 22 in 17 different loci (Table II). Some of these affect IFT and its regulation, and others may affect templating or docking functions of the basal bodies. Given the complexity of the IFT machinery and its regulation, it is not surprising that the number of loci identified has not reached saturation. Only three of the loci (FLA8, FLA10, and FLA17) have more than one allele.
Table II.
Phenotypes and Genes for the FLA Loci
| Allele | Gene | Protein | Mutation | Permissive temperature phenotypea | Linkageb group | Screenc | References |
|---|---|---|---|---|---|---|---|
| fla2-l | FLA2 | Unknown | Reduced retrograde IFT | VI | H | ||
| flai-l | FLA3 | Kinesin-associated protein | F753L | Not tested | X | H | Mueller et al. (2005) |
| fla4 | FLA4 | Unknown | No IFT defect | X | H | ||
| fla5 | FLA5 | Unknown | No IFT defect | XI | A | ||
| fla6 | LOST | ||||||
| flal (flalQ-l) | FLAlQ | Kinesin-2 motor subunit | N329K | Reduced anterograde IFT | XIX | H | Walther et al. (1994) |
| Fla8-1 | FLA8 | Kinesin-2 motor subunit | E21K | Reduced anterograde IFT | XII/XIII | A | Miller et al. (2005) |
| fla8-2 (flal) | FLA8 | Kinesin-2 motor subunit | F55S | Reduced anterograde IFT | XII/XIII | H | Miller et al. (2005) |
| fla9 | FLA9 | Unknown | No IFT defect | XIX | A | ||
| 5l9 flal0-l4 | FLAlQ | Kinesin-2 motor subunit | E24K | Not tested | XIX | A | Miller et al. (2005) |
| 544flalQ-l5 | FLAlQ | Kinesin-2 motor subunit | Unknown | Not tested | XIX | A | Lux and Dutcher (1991) |
| flall | FLAll | IFT172 | L1615P | Reduced retrograde IFT | XIX | A | Pedersen et al. (2005) |
| flal2 | FLAl2 | Unknown | No IFT defect | XIX | A | ||
| flali | FLA13 | Unknown | Not tested | ND | A | ||
| flal5 | FLAl5 | IFT144 | C1283R | Reduced retrograde IFT | XIV | P | Iomini et al. (2009) |
| flal6 | FLAll | IFT139 | Deletion of exons 17-19 | Reduced retrograde IFT | VI | P | Iomini et al. (2009) |
| flall-l | FLAll | IFT139 | Deletion of exons 17-19 | Reduced retrograde IFT | VI | P | Iomini et al. (2009) |
| flall-2 | FLAll | IFT139 | Deletion of exons 17-19 | Reduced retrograde IFT | VI | P | Iomini et al. (2009) |
| flal8 | FLAl8 | Unknown | Reduced anterograde IFT | ND | P | ||
| fla2l | FLA2l | Unknown | No IFT defect | ND | P | ||
| fla24 | FLA24 | Unknown | Reduced retrograde IFT | VI | P | ||
| fla2l | FLA2l | Unknown | Reduced anterograde IFT | ND | P | ||
| fla28 | FLA28 | Unknown | Reduced anterograde IFT | ND | P |
Permissive temperature phenotypes are from Iomini et al. (2001).
ND Map location is not determined.
Indicates the screen that found the mutants: H, Huang et al. (1977); A, Adams et al. (1982); P, Piperno et al. (1998).
A. Chemical Mutagenesis
The screen for fla mutants is based on the observation that aflagellated Chlamydomonas cells are unable to oppose gravity. When cells lacking flagella are grown in liquid culture they form a pellet. By contrast, wild-type cells swim and are distributed evenly throughout the culture medium. Because the nonswimmer phenotype could also derive from cells with paralyzed flagella, a secondary screen with light microscopy is needed to identify strains with paralyzed flagella. The fla mutants currently available were generated in three different screens (Adams et al., 1982; Huang et al., 1977; Iomini et al., 2001; Piperno et al., 1998).
B. Solutions
1. Stock Solution of N-methyl-N′-nitro-N Nitrosoguanidine
Dissolve 2 mg/ml N-methyl-N′-nitro-N Nitrosoguanidine (MNNG) in 0.02 M citrate buffer at pH 5 prepared in fume hood.
R medium for cell culturing is based Sager and Granick's method as described previously (Harris, 1989). M-N/5 medium used in mutagenesis is described below.
M-N/5 stock solutions For 500 ml
1. 10% sodium citrate 0.5 ml
2. Trace elements 1 ml
For 1 l of stock:
H3BO3 100 mg
ZnSO4·7H2O 100 mg
MnSO4·H2O 40 mg
CoCl2·6H2O 20 mg
CuSO4·5H2O 4 mg
NH4 molybdate·4H2O 15 mg
3. 1% FeCl3·6H2O 0.1 ml
4. 4% CaCl2·2H2O 0.1 ml
5. 10% MgSO4·7H2O 0.3 ml
6. 10% K2HPO4 2.6 ml
pH will be 7.8–8
Solutions for deflagellation
0.5 N acetic acid For 500 ml: 14.3 ml of glacial acetic in H2O
1 M KHCO3 For 500 ml: 50.06 g KHCO3 in H2O
C. Day 1. Mutagenesis
1. Grow one loop of Chlamydomonas wild-type cells 137+ (Strain CC-125) taken from a freshly streaked culture in two flasks containing 100 ml of R medium each for 3 days at 25°C under bright fluorescent light.
2. Collect cells by centrifugation at 350 × g for 15 min at 21°C.
3. Resuspend each pellet in 20 ml of 0.02 M citrate buffer at pH 5, count cell number with a hemocytometer, and repeat centrifugation. Resuspend in 50 ml 0.02 M citrate buffer at pH 5 at 106 cells/ml in three 50-ml polypropylene conical tubes. Cultures need to be maintained with sterile conditions.
4. Add MNNG to final concentration of 1, 5, or 10 μg/ml, respectively, to tubes and incubate in the dark for 30 min at 25°C.
5. Collect all cells by centrifugation at 350 × g for 15 min at 21°C and wash two times in 10 ml M-N/5 medium, resuspend cells to 106 cells/ml. Medium lacking nitrogen (N) induces gametogenesis and stops cell division. This prevents the isolation of identical, nonindependent alleles from the screening. To determine cell viability after exposure to MNNG, plate 100 μl of cells at the concentration of 5 × 103 cells/ml based on cell counts on an R plate and let plate grow for at least 3 days in bright light. Viability is ~70, ~60, and ~45% for cell cultures treated with 1, 5, or 10 μg/ml of MNNG, respectively.
6. Incubate. cells exposed to the mutagenic agent overnight in bright light at 21°C.
D. Day 2. Eliminate Swimming Cells at 32°C (First Cycle of Deflagellation)
1. Deflagellate cells by adding 0.5 N acetic acid to reduce the pH of the medium to 4.5. Quickly check a sample by phase microscopy to ensure that cells have lost their flagella than neutralize with 1 M KHCO3 solution to return the medium to a pH of ~7. Do not leave cells for more than 1–2 min at pH 4.5.
2. Collect all cells by centrifugation at 350 × g for 15 min at 21°C and discard the supernatant.
3. Resuspend cells in 30 ml of fresh M-N/5 medium prewarmed to 32°C in 50-ml polypropylene conical tubes.
4. Incubate the tubes at 32°C for 2 h to allow flagella to partially reassemble.
5. Centrifuge tubes at 230 × g for 5 min to allow all deflagellated cells to settle at the bottom of the tube. Wrap tubes with aluminum foil so that light enters only from the top 2 cm of the tube and continue to incubate at 32°C for 2 h. Cells that have assembled flagella swim and phototax to the lighted part of the tube. Cells without flagella remain at the bottom in the unlit part of the tube.
6. Remove and discard supernatant by aspiration from the top of the tube using a 25-ml pipette and leave 5 ml of medium.
7. Add 30 ml of fresh M-N/5 medium pre-warmed to 32°C, spin at 230 × g for 5 min and incubate for 2 h at 32°C.
8. Repeat step 5.
9. Remove and discard all the supernatant aspiring from the top of the tube and resuspend the pellet in 7 ml of fresh M-N/5 at 21°C, transfer to 16-mm × 125-mm sterile glass tubes with round bottom and incubate at 21°C in bright light overnight.
E. Day 3. Eliminate Nonconditional Nonswimming Cells
1. Wrap tubes at 21°C in foil as described in step 5 and continue incubation at 21°C for 1 h to enrich for swimming cells.
2. Transfer the top 5 ml to a new tube.
3. Deflagellate and collect all cells by centrifugation at 350 × g for 15 min at 21°C and discard the supernatant.
4. Resuspend cells in 5 ml of fresh M-N/5 medium prewarmed to 32°C using 16-mm × 125-mm glass tubes.
5. Spin at 230 × g for 5 min, wrap tubes with aluminum foil as described above and incubate at 32°C for 1 h.
6. Remove and discard all the supernatant aspiring from the top of the tube and resuspend the pellet in 3 mL of fresh M-N/5 at 21°C and incubate at 21°C in bright light overnight.
F. Day 4. Culture Single Cells on Solid Medium
1. Wrap tube at 21°C in foil as described above and continue incubation at 21°C for 1 h and transfer most of the supernatant to a new tube.
2. Incubate at 32°C for 2 h, wrap tubes in foil, and continue incubation at 32°C for 1 h.
3. Remove all the supernatant and resuspend the pellet in 1 ml of fresh M-N/5 medium.
4. Adjust volume to obtain a suspension of 5 × 103 cells/ml and plate 100 μl per R plate.
G. Day 10. Screen of Single-Cell Colonies
1. Incubate plates at 21°C for 4–6 days until colonies appear. Pick single colonies into 200 μl of R medium overnight at 21°C.
2. Screen for colonies that fail to swim after 4 h at 32°C.
3. Analyze cells under phase microscope for aflagellate versus paralyzed flagellar cells.
IV. Characterizing Intraflagellar Transport in fla Mutants
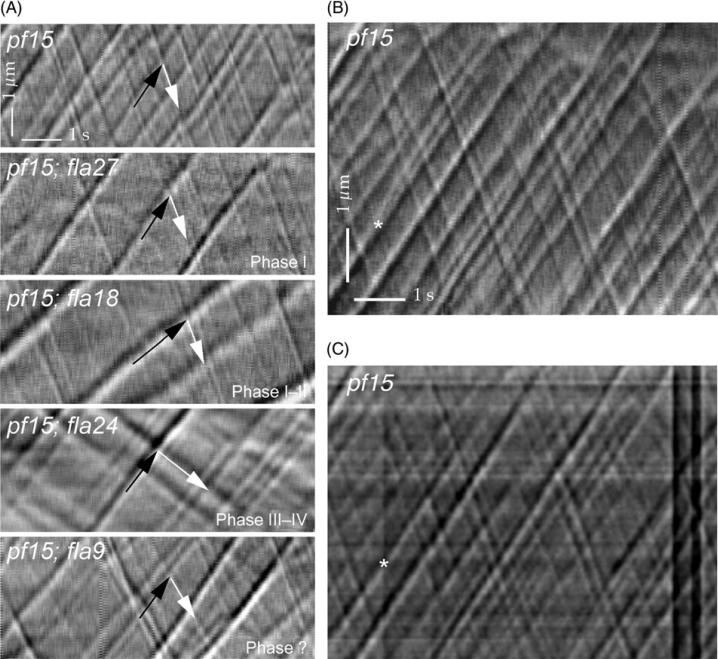
An accurate quantitative analysis of IFT in Chlamydomonas can be achieved by applying an image analysis approach called kymography to VE-DIC recordings, which allows visualizing IFT particle trajectories in two dimensions as a diagram representing space (y) and time (x) (Fig. 1). When this approach is applied to the analysis of flagella of wild-type cells, we find IFT particles moving toward the distal end of flagella are larger in size, slower in velocity, and less numerous than IFT particles moving toward the proximal end of the flagella suggesting a model in which at least four distinct phases of the IFT are recognizable (Iomini et al., 2001). Kymographs obtained from the analysis of fla mutant flagella at the permissive temperature lead to the identification of different classes of mutants that show specific defects in one or two consecutive phases of the IFT that are indicative of the function of the defective gene (see Table III).
Fig. 1.
(A) Kymographs of flagella from pf15, pf15; fla27, pf15; fla18, pf15; fla24, and pf15; fla9 cells. Vertical (y) and horizontal (x) axes represent distance from cell body (μm) and time of observation (s), respectively. Black and white arrows indicate traces formed by anterograde or retrograde IFT particles, respectively. The IFT phase that is defective in each recombinant is indicated (see Table III for further details). The strain pf15; fla9 and other double mutants (pf15; fla4, pf15; fla5, and pf15; fla21) behave similarly to each, and are not significantly different from pf15 at 21°C and are labeled as unknown (?). Velocity, frequency ratio, and overall particle frequency can be obtained from these images. (B, C) Kymographs of pf15 flagella analyzed by different methods. Kymograph in B was generated using the approach described previously (Iomini et al., 2001: Piperno et al., 1998). The kymograph in C was generated with MetaMorph (see text). Kymograph B has had noise removed while kymograph C has not. Lower levels of noise facilitate detection of particles trajectories. A bifurcation of a trajectory indicating a possible split of one particle or a sudden change in velocity of one of two particles moving together is resolved in B but not in C (*). Recordings of pf15 in B and C, and pf15; fla27, pf15; fla18, and pf15; fla24 in A are available as Supplemental movies 1, 2, 3, and 4, respectively (http://www.elsevierdirect.com/companions/9780123749734). Panel A is reprinted from Iomini et al. (2001).
Table III.
The Phases of Intraflagellar Transport
| Phase of IFT | Predicted phenotype of phase-defective mutants |
|---|---|
| I: Particles bind a cargo and change in structure and/or size in a position close to the basal bodies. | Defects in subunits of the particles or basal bodies. Accumulation of particles at proximal end of flagella. |
| II: Particles and cargo are transported to the distal end of flagella by an anterograde motor. | Defects in subunits of the particles or kinesin-2. Defective anterograde velocity of IFT particles. Accumulation of particles at proximal end of flagella. |
| III: Particles release the cargo and change their structure and/or size at the distal end of the flagella. | Defects in subunits of the particles or components of the axoneme or matrix at the flagellar tip. Accumulation of particles at the distal end of flagella. |
| IV: Particles are transported to a region close to the basal bodies by a retrograde motor. | Defects in subunits of the particles or cytoplasmic dynein. Defective retrograde velocity of IFT particles. Accumulation of particles at distal end or along flagella. |
A. VE-DIC Light Microscopy
Since imaging of IFT is only possible on paralyzed and straight flagella, recombinant strains between each of the fla mutants and a paralyzed flagella (pf) mutant that affects the axonemal central complex (pf15) were used.
1. Cells to be analyzed by VE-DIC microscopy are resuspended in R or M-N/5 liquid medium and cultured from 3 to 16 h at 21°C to allow complete flagellar assembly.
2. Cells were transferred to medium containing 0.25% low melting temperature agarose cooled to 30°C (SeaPlaque). A drop of this cell suspension was than delivered, using a Pasteur pipette, into a chamber created by a #1 (22 mm square) glass coverslip and a microscope slide held together by two parallel strips of a Scotch double-side adhesive tape and immediately cooled on a 4°C metallic plate for about 1 min.
3. Higher concentrations of low melting temperature agarose (up to 0.75%) can be utilized to inhibit flagellar motility when analyzing strains not available in the pf background (Mueller et al., 2005).
4. VE-DIC of IFT in living cells was carried out using a Zeiss Axiovert 35 (Carl Zeiss, Inc., Oberkochen, Germany) microscope with a 1.4 NA condenser, a Pan-Neofluar 100 ×/1.3 oil objective, and a 4× magnifier placed on the trinocular head in front of a video camera equipped with a Newvicon tube (Model C2400 Hamamatsu, Bridgewater, NJ). Transillumination light was provided by a mercury arc and filtered through a Zeiss standard green filter. Video images were acquired at a rate of 30 frames/s and stored directly on optical disks.
5. Calibration of pixel dimensions can be performed by use of a stage micrometer. In order to obtain an accurate angular alignment between the flagellar axis and the DIC axis, we mounted a home made rotating stage (similar to that used in polarizing microscopes) on the microscope. The slide was attached to the stage using grease and rotated so that the flagellum is perpendicular to the (x) axis and the cell body at the bottom of the image. Consequently, no reorientation of the images is required during image acquisition and analysis.
B. Digital Kymography
Methods for obtaining kymographs were described previously (Iomini et al., 2001; Piperno et al., 1998).
1. Each image in a video sequence was read from the optical disc recorder and digitized by a frame grabber. A 7-pixel-wide light intensity profile, or linescan, along the flagellum was obtained using a built-in function of the Image-1 software package (Universal Imaging Corporation, Dowingtown, PA).
2. Noise arises from several sources that include the presence of uneven background, light intensity fluctuations, and digitization noise. Since the noise interferes with particle trajectory detection, the data were subjected to singular value decomposition and principal component analysis (Golub and Reinsch, 1970; Malinowski, 1991; Press et al., 1992). Custom software that applies these procedures to each linescan in an automated fashion was developed and is available upon request. It averages across the flagellum. Commercial software such as MetaMorph generates kymographs from image stacks as well.
3. A second method uses an 8-s analog video obtained with a 30 frames/s camera. It was converted to 240 digital frames using a media converter (Sony, San Diego, CA, DVMC-DA2). The image stack was opened and processed using MetaMorph (Universal Imaging Corporation).
a. From the Stack menu, choose Kymograph. The Kymograph dialog box will appear.
b. Select the source image stack with the Source Stack image selector.
c. With the single line tool draw a line region across the area of interest in the image window. Because the Kymograph command draws the result image starting with the line region values in the topmost plane in the stack, you should verify that you are looking at the top plane in your stack; otherwise, your result image may appear to be inverted.
d. Select the All Planes check box.
e. Select the Line Width spin box and assign the value 7 or a value that cover the entire width of the flagellum.
f. Select Average as a gray-scale value.
g. Choose Create Kymograph and save the new image file.
4. Figure 1B and C shows kymographs obtained with noise reduction as described in step 2 and without noise reduction by MetaMorph on the same data set. Although the noise due to light fluctuation is completely removed when singular value decomposition and principal component analysis are applied, the two plots are comparable in terms of detection of IFT particles trajectories.
5. In these plots, moving particles appeared as diagonal ridges or streaks, whose slope was proportional to their velocity. The velocity of each particle was calculated from the slope of a line drawn manually along each of the diagonal ridges. A lower limit for the value of the frequency of IFT, expressed in particles/second, was estimated from the ratio of the total number of particles detected to the total observation time, equal to the number of linescans divided by 30, the video frame rate.
6. Kymographs obtained as Tiff files were filtered using Photoshop CS3 (Adobe Corp., San Jose, CA) by applying Gaussian blur (0.7) and unsharp mask (298%, 2.7 pixel radius, 1 threshold) (Dentler, 2005).
V. Genetic Characterization
A. Determining the Number of Genes Among a Collection of Mutants
Few mutants in Chlamydomonas are tested for complementation in diploid strains (Ebersold, 1967). Instead, loci are generally defined by recombination mapping.
1. Once a mutant phenotype is likely to be caused by a single mutation because it shows 2:2 segregation of the mutant and wild-type phenotypes in tetrads or in random progeny, pairwise crosses can be performed (Dutcher, 1995). It is often useful to backcross a mutagenized strain three to four times before further characterization to remove unlinked mutations.
2. The phenotypes of progeny from pair-wise crosses are analyzed. Tight linkage indicates the mutants are likely to be in the same locus (no recombinants out of 30–50 progeny). Complementation in diploids can be performed (see Section V.E and Dutcher et al., 1988).
B. Linkage Analysis
1. Linkage Group Determination
To determine linkage between candidate genes and flagellar assembly mutants or between a linkage group and flagellar assembly mutants, PCR-based markers can be used more efficiently than other phenotypic markers (Bowers et al., 2003; Kathir et al., 2003). DNA from progeny that pelleted at 32°C from tetrads of crosses of fla mutants × CC-1952 was analyzed for segregation of the mutant allele with respect to polymorphic alleles. CC-1952, also known as S1D2, is a highly polymorphic strain (Gross et al., 1988) that has approximately one change per 80 bp (Vysotskaia et al., 2001). Markers developed by low coverage sequencing of the S1D2 strain has provided over 12,000 possible markers to use for mapping using PCR amplification of meiotic progeny from a cross of the mutant and CC-1952 (Rymarquis et al., 2005). There are three classes of markers. The derived cleaved amplified polymorphisms (dCAPS) markers are amplified PCR products that produce differences between the strains after digestion with a restriction enzyme. The simple sequence repeats (SSR) markers are amplified PCR products that produce products that differ in length that result from differences in the size of repetitive elements that include GT, CA, TA, TCC, TGA, and TGG. The STS markers are sequence-tagged sites that use three primers for their analysis.
When mapping a new mutation, the first goal is to determine the linkage group. It is useful to determine if the mutant shows centromere linkage. Genes that are linked (<20 map units from the centromere) can be mapped with a set of primers that are linked to each of the 17 centromeres. If the gene is unlinked to its centromere, then it must be mapped to a linkage group using markers that are located in the middle of each of the 34 arms of the linkage groups.
2. Centromere Linkage
1. Isolate DNA from meiotic progeny from 10 tetrads that have scored for the flagellar phenotype. A very crude DNA preparation can be made in a 20-μl reaction. It contains 2 μl Vent Buffer (20 mM Tris–Cl pH 8.8, 10 mM KCl, 10 mM (NH4)SO4, 2 mM MgSO4, 0.1% Triton X-100 ), 1 μl proteinase K (20 mg/ml), 17 μl water, and ~105 cells. The mix is subjected to 58°C for 1 h and 95°C for 30 min. The mixture containing the DNA can be stored at 4°C for several months. A volume of 0.2 μl of the supernatant is used in 10-μl PCR reactions.
2. Perform PCR with a centromere-linked marker. HSP70A and TcTex1 are excellent markers as both are very close to their respective centromeres (<0.5 map units). If the fla mutant and the PCR marker are on different chromosomes, then the frequency of tetratype tetrads provides a way to determine the distance from FLA to its centromere. If the frequency is greater than 67%, then the FLA gene is unlinked to its centromere. If it is less, then the FLA gene is linked. The distance is described by the equation: Distance from FLA gene to its centromere = T × 100/2 (number of tetrads).
3. The centromere markers and PCR conditions using Taq polymerase are shown in Table IV.
Table IV.
Centromere-Linked Primers and PCR Conditions
| Primer 1 | Primer 2 | Anneal T (°C) | [Mg] (mM) | PCR product (bp) | Enzyme | Band sizesa |
|---|---|---|---|---|---|---|
| TcTexl | ||||||
| ATT GAC GTC TCG GAA GAG GA | ACC TCC TGT TGC ACC ATT TC | 55 | 3.5 | 400 | HaeIII | 400/500 |
| HSP70A | ||||||
| AGC TGC TGC AGG ACT TCT TC | GCT GGT TGT CGG AGT AGG TC | 55 | 2.7 | 400 | HaeIII | 180/200 |
The distinguishing band from the CC-125 parent is listed first and the CC-1952 parent is listed second.
4. If the FLA gene is close (<20% tetratypes) to the centromere-linked markers, then a single marker for each chromosome can be used to detect linkage. Markers can be picked from various sources (Bowers et al., 2003; Kathir et al., 2003; Rymarquis et al., 2005). If the FLA gene is more than 20 map units from the centromere, then a marker from the middle of each arm can be used for mapping. Mapping to a chromosome can generally be accomplished using 40–50 meiotic progeny that can be collected from dissected tetrads or random progeny.
5. Once linkage is detected, the next goal is to find markers that are tightly linked to the FLA gene. On an average, 1 map unit or 1% recombination is about 100–105 kb in length. Regions near the centromere or telomere appear to have less recombination. Mapping to an interval of 1–2 map units generally requires 300–500 progeny. This generates a small enough region to allow identification by transformation and rescue.
3. Linkage with Candidate Genes
Candidate genes provide a useful way to identify the gene more quickly than mapping across the entire genome. In the identification of FLA8, biochemical data suggested that it was missing kinesin-2 (Cole et al., 1998). Thus, it was straightforward to map fla8 and fla1 strains with respect to the kinesin-2 subunits in the genome (Miller et al., 2005). The flagellar assembly mutants that show defective retrograde IFT are another example. Five mutants (fla2, fla15, fla16, fla17, and fla24) show decreased retrograde velocities, an increased ratio of anterograde to retrograde particles and an accumulation of complex B proteins in the flagella (Iomini et al., 2001). Sequences for complex A proteins were used to make dCAP markers and each were mapped relative to each of these mutants.
1. Design dCAPs markers. If there are no markers that have been developed for a gene of interest, the 3′UTR or stretches of repeats that are at least 100 bp long provide the easiest ways to develop a new marker.
2. Sets of primers that produce a product of 200–400 bp are chosen.
3. Each primer is subjected to BLAST to the JGI genome browser to ask if the primer is unique in the genome at an expected value of 1e–1. If there are multiple hits, then new primers need to be picked.
4. If there is no size difference between PCR products for the mutant and the CC-1952 strain is observed, six to eight restriction enzymes that have GC-rich recognition sites are tested for one that produces size differences between the two parental strains (Fig. 2).
Fig. 2.

PCR products from meiotic progeny (1–9) of a cross of the mutant parent (P) by the CC-1952 (S1D1) strain. The primers span a repetitive GT region and the two strains have a size polymorphism. This marker is linked (9/9) to the mutant phenotype.
5. Linkage is tested in 40–50 meiotic progeny from a cross of the mutant by CC-1952. The progeny are generally chosen to have the mutant phenotype. If linkage is found, then the candidate gene is sequenced from the mutant.
6. Reversion of the mutant phenotype can be used to provide further evidence (Rymarquis et al., 2005; Iomini et al., 2009) or transformation and rescue.
C. Chlamydomonas Transformation and SHIRT
This protocol is modified from other protocols (Kovar et al., 2002; Shimogawara et al., 1998).
1. Chlamydomonas cells were inoculated in 100 ml liquid R medium for 3 days under continuous illumination with gentle shaking until cells reached a concentration of ~5 × 106 cells/ml.
2. Cells were collected by centrifugation and treated with autolysin for 0.5 h at room temperature to remove cell walls (Dutcher, 1995; Harris, 1989). Sterility must be maintained throughout the protocol.
3. Autolysin-treated cells were chilled on ice for 10 min before collected by centrifugation at 4°C at 1000 × g. Cells were gently resuspended on ice in R + 100 mM mannitol to the final concentration of ~4 × 108 cells/ml.
4. A volume of 250 μl of cells (~1 × 108 cells) was used for transformation with 6 μg of bacterial artificial chromosome (BAC) DNA or 1 μg plasmid DNA with or without (the addition of 1 μg of pSI103, which confers resistance to paromomycin (Sizova et al., 2001), for cotransformation).
5. Cells and DNA were added to an electroporation cuvette (4-mm gap, Bio-Rad, Hercules, CA) and incubated in a 16°C water bath for 5 min before electroporation, which was performed in a Bio-Rad Gene Pulser II with the following setting: 0.75 kV, 25 μF, and 50 . Cells were electroporated with one pulse and incubated at room temperature for 10 min before transfer to 50 ml R + 100 mM mannitol liquid medium and incubated overnight at room temperature with continuous illumination.
6. Cells were resuspended gently in 1 ml 25% cornstarch in R medium.
7. Cells are innoculated onto five R plates with 10 μg/ml paromomycin for cotransformation experiments. Colonies appear within 5–7 days at 25°C.
8. Without cotransformation, the cells are inoculated into 48 tubes (25 mm × 150 mm) with 20-ml R liquid medium. In liquid medium, the upper 5–10 ml of medium is transferred to new R liquid 4–5× over the course of 2 weeks. Cells are transferred before they reach a density of 5 × 106 to prevent cells entering stationary phase where they become aflagellated. Once swimming cells are detected, 50 μl of medium to plated to solid medium for single colonies. A single colony that is able to swim at the restrictive temperature is selected for further study from each tube.
9. Each rescued transformant is crossed to a wild-type strain (CC-124) to determine if the rescue is extragenic.
1. SHIRT Analysis
Because a BAC often contains many genes, rescue does not always reveal which gene is encoded by the locus of interest. One can subclone fragments of the BAC and transform with the smaller pieces. Another option is the Segregation of Heterozygosity in Rescued Transformants (SHIRT) method (Esparza, 2008). The SHIRT analysis provides a rapid method to determine which regions of BAC DNA are integrated nonhomologously into the genome and cosegregate with the rescued phenotype. It obviates the requirement for subcloning to determine the causative DNA for rescued transformants. It is rare in Chlamydomonas that the entire transforming DNA of a BAC or plasmid integrates into the genome, and independent transformants generally integrate nonidentical segments of the BAC or plasmid DNA (Dutcher and Trabuco, 1998; Myster et al., 1999).
1. For this analysis, multiple independent transformants are mated to the polymorphic strain CC-1952 and meiotic tetrad progeny are recovered.
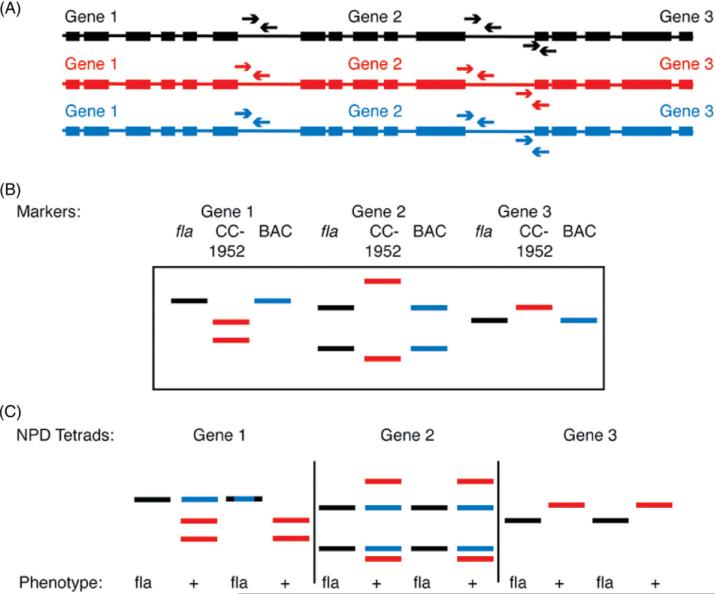
2. Nonparental ditype (NPD) tetrads are identified that have two mutant progeny (fla) and two wild-type progeny (FLA BAC DNA). A dCAPS marker is made for each gene in the BAC used for transformation (Fig. 3). As discussed above, markers predicted by Rymarquis et al. (2005) are tested first. If no predictions are available, new primers can be designed as described above.
Fig. 3.
Schematic diagram of SHIRT. SHIRT uses mapping and PCR-based markers to determine which parts of a BAC are responsible (cosegregate) for rescue of a mutant phenotype following transformation. (A) Diagram of three genes on a BAC from the mutant parent (black), the polymorphic mapping strain CC-1952 (red), and the BAC (blue). Primers for PCR are generally made to the 3′UTR of genes to be tested (arrows). (B) Bands of digested PCR products (dCAP markers) for the three genes from the three sources of DNA. The digested PCR products from the black and blue alleles cannot be distinguished from each other. (C) Three possible outcomes among NPD tetrads in which the mutant phenotype is observed in two of the four progeny (fla and +). The pattern with Gene 1 suggests that the BAC DNA is not responsible for rescue. The hybrid (Black and Blue) band indicates that the band is amplified from both the mutant and the BAC allele. The pattern with Gene 2 is consistent with the BAC DNA providing rescue. Further proof requires additional NPD tetrads with this pattern. The pattern with Gene 3 suggests that it is not integrated into the genome and therefore not responsible for rescue. This figure is reprinted from Iomini et al. (2009). (See Plate no. 6 in the Color Plate Section.)
3. In the progeny, from the cross in step 1, there are three alleles of each gene. One copy will be from the CC-1952 parent, one from the inserted BAC, and one from the mutant parent. Since the BAC library was made from the same strain as the mutant parent, the alleles contributed by the BAC and the mutant parent will possess the same polymorphism while the allele contributed by the CC-1952 parent will be different. PCR is performed for each marker the progeny that show an NPD segregation pattern for the flagellar phenotype and the rescue (Fig. 3).
4. The two aflagellate progeny should have only a copy of the parental allele and the two swimming progeny have the BAC and CC-1952 alleles (Fig. 3; see Gene 2 diagram), if the gene is responsible for rescue.
5. If the gene from the BAC is not integrated into the genome, then the PCR products will resemble the Gene 3 example (Fig. 3).
6. If the gene from the BAC does not rescue, the pattern may resemble Gene 1 and not cosegregate (Fig. 3C).
7. DNA was isolated from four NPD tetrads for each transformant and the segregation of genes from the BAC determined. Figure 4 shows the pattern for rescue of the fla15 mutant.
Fig. 4.
SHIRT analysis of the FLA15 transgene. PCR products from an NPD are shown. The parents (fla15 + BAC; CC-1952) are in lanes 1 and 2. The four meiotic progeny are in lanes 3–6. Lanes 3 and 6 are from cells aflagellate at 32°C and the band is consistent with the mutant allele. Lanes 4 and 5 are from flagellated cells and the bands are consistent with the wild-type FLA15 allele from the CC-1952 parent and the BAC contributed transgene.
D. Reversion of Flagellar Mutants Using Ultraviolet Light
1. Cultures of mutant cells were plated onto R medium at a density of ~2000 colonies per plate and grown for 4 days at 21°C. They were subjected to ultraviolet irradiation (70 J) and left in the dark for 18 h to prevent photoreactivation. An ultraviolet lamp that has been calibrated or a Stratagene UV Stratalinker 1800 La Jolla, CA can be used.
2. Approximately 40 mutagenized colonies are transferred to 20 ml of R medium in 25-mm × 150-mm tubes and placed at 32°C. A volume of 5 ml of supernatant was transferred to new tubes after 3 days and this was repeated four times.
3. When swimming cells are visible, an aliquot with ~3 × 102 cells is plated to achieve individual colonies and one colony from each tube with swimming cells is retained and scored for flagellar assembly.
4. Cross each swimming strain by wild-type cells to determine if it is intragenic (failure to recover the fla mutant phenotype) or extragenic (recovery of the fla mutant phenotype).
5. The intragenic revertants can be analyzed by a dCAPs marker if the mutant creates or destroys a restriction enzyme restriction site or by sequencing (Miller et al., 2005).
6. The outcome from reversion analysis is highly dependent on the starting allele and locus. This is illustrated by the outcomes of experiments with different genes and different alleles. Some alleles give only intragenic revertants or extragenic suppressors while others give both types of events (Table V). It appears to be allele specific and not locus specific.
Table V.
Outcomes for Isolating Revertants and Suppressors offla Mutants
| Gene | Number of intragenic events | Percent intragenic events | References |
|---|---|---|---|
| fla10-1 | 181 | 98.4 | Lux and Dutcher (1991) |
| fla10-14 | 22 | 32 | Lux and Dutcher (1991) |
| fla10-14 a | 19 | 100 | Miller et al. (2005) |
| fla10-15 | 13 | 21 | Lux and Dutcher (1991) |
| fla8-1 | 15 | 100 | Miller et al. (2005) |
| fla8-2 | 35 | 100 | Miller et al. (2005) |
| fla15-1 | 56 | 100 | Iomini et al. (2009) |
| fla17-2 | 24 | 0 | Iomini et al. (2009) |
Independent experiment.
7. Intragenic revertants can be used for correlating structure and function of the mutated protein (Miller et al., 2005).
E. Diploid Strain Construction and Analysis
Diploid strains are useful for determining if the mutations are recessive and can be selected by several different selective markers. Since less than 0.1% of zygotes become mitotically growing diploid strains, it is important to have a selection for them.
1. One selection uses the arg2 and arg7 alleles. These are alleles that show intra-allelic complementation in the arginosucinate lyase locus (ARG7). The two parents both carry an auxotrophic mutation and must be grown on medium containing arginine. Diploid strains are selected on medium lacking arginine. One disadvantage of this method arises from the acquisition of additional mutations that block further rounds of meiosis.
2. A second set of selective markers is nit2, AC17 and NIT2, ac17 strains. The diploid strains are selected on minimal medium lacking acetate with 2 mM KNO2 instead of ammonium in the medium. This method is often easy as many of the chemically induced flagellar mutants have the nit2, AC17 genotype. It has one disadvantage in that the nit2 parent shows some growth on the medium. Isolation of the diploid strains requires a second round of streaking onto selective medium to remove the parental strain.
VI. Characterization of Genetic Interactions
A. Synthetic Phenotypes
Synthetic phenotypes are often used to build networks of interactions in many cellular processes (Ooi et al., 2006). Many double mutant combinations of hypomorphic alleles for genes that act in the same pathway show synthetic phenotypes, but mutants in two independent pathways can also underlie synthetic phenotypes. In some cases, these alternatives can be distinguished by comparison of null alleles. Double mutants between various conditional flagellar assembly mutants suggest that this is a useful method.
1. Construct haploid strains with two mutations by crossing two fla mutations. Each of the haploid fla mutant strains assemble flagella at 21°C but not at 32°C.
2. Check for phenotype in the progeny with two mutations. In NPD tetrads, two of the progeny will be wild type and swimming as they have both wild-type alleles. The other two progeny will have both fla mutants. In some examples, the double mutants have a phenotype at the permissive temperature (Table VI). The appearance of a synthetic phenotype with partial loss of function alleles indicates that the genes act in the same pathway (Hereford and Hartwell, 1974).
Table VI.
Interpretations of Synthetic Phenotypes
| Phenotypic observation | Statement | Conclusions |
|---|---|---|
| fla15; fla17 double mutants are aflagellate at 21°C | Mutants in two complex A proteins increase the severity of the mutant phenotype | These proteins may interact in the IFT particle |
| fla15; flail double mutants are aflagellate at 21°C | Mutants in a complex A and a complex B protein increase the severity of the mutant phenotype | Complex A may interact with complex B through IFT172 and IFT144 |
| fla15; flalO double mutants are unaffected and flagellate at21°C | A mutant in complex A and kinesin-2 do not increase the severity of the phenotype at the permissive temperature | Complex A and kinesin-2 do not interact |
3. Additional conditional and null alleles would strengthen these arguments about the presence and absence of interactions (Iomini et al., 2009).
B. Second-Site Noncomplementation
Second-site noncomplementation, also known as dominant enhancement, is defined as the lack of complementation between two recessive mutations in two unlinked loci (Hawley and Gilliland, 2006). It can arise via a number of mechanisms, which include the creation of a poisonous interaction complex or the reduction in the dose of a complex formed by the two gene products.
1. Noncomplementation requires that the mutations be recessive. Diploid strains heterozygous for fla mutants must be tested for dominance. For example, diploid strains heterozygous for the fla2-1, fla11-1, fla15-1, fla17-1, or fla24-1 alleles are able to swim at 21 and 32°C; all of these mutations are recessive to the wild-type allele.
2. Diploid strains that are heterozygous for all pair-wise combinations of fla mutations are constructed. For the retrograde defects (Iomini et al., 2009) and for suppressors of pf10 (Dutcher et al., 1988), many combinations show second-site noncomplementation.
3. Many of the double heterozygous diploid strains (fla15, FLA15, fla17, FLA17) show a more extreme phenotype than the homozygous diploid strains at 21°C. This suggests that IFT particles are more compromised in these combinations at the permissive temperature. Again this provides evidence for interactions.
4. The availability of both conditional and null alleles would help in the analysis of these interactions. If null alleles show the phenotype, then the noncomplementation cannot arise from poisonous interactions. It would be likely to reflect a strict requirement for equal dosage of the two genes.
C. Two-Copy Suppression
The presence of multiple copies of a gene or overexpression of a gene can suppress the phenotype of mutations in a noncognate gene in Saccharomyces cerevisiae and Schizosaccharomyces pombe (Forsburg, 2001). This observation has been termed multicopy suppression and has been used extensively to find genes that act in the same pathway. Dosage suppressors may occur because the protein at the increased dosage helps to stabilize the mutant protein or the increased dosage protein may act downstream to bypass the requirement for the mutant protein. Multicopy suppression has not been sought or observed extensively in Chlamydomonas. Recently, we have found examples of it.
1. To observe two-copy suppression, mutants that have been rescued by a cognate transgene are crossed with other fla mutants. The strains can be genotyped with dCAPs markers. For example, two copies of the wild-type IFT144 gene (FLA15) can rescue the fla17-1 mutant phenotype at 32°C.
2. The pattern of rescue by two wild-type copies of a gene may provide information about the number of copies of a protein in a complex. For example, two copies of the IFT144 gene partially rescue the phenotype of fla11-1 cells, but do not rescue the mutant phenotypes of the fla2, fla8, fla10, or fla24 strains (Iomini et al., 2009). Two copies of the wild-type IFT139 gene (FLA17) rescue the fla15-1 mutant phenotype. Two copies of the IFT139 gene do not rescue the fla2, fla8, fla10, fla11, or fla24 mutant phenotypes.
3. To demonstrate that the rescue requires two wild-type copies, rescue is tested in a double mutant where there cannot be two wild-type copies of the gene in question. For example, a single wild-type copy of IFT139 or IFT144 rescues the aflagellate phenotype of the fla15; fla17 double mutant at 21°C, but not at 32°C. One interpretation is that two-copy suppression results from stabilization of the mutant gene product in the presence of an excess in the gene product from the noncognate transgene product together with the endogenous copy of the gene.
4. If two-copy suppression is not reciprocal (two copies of gene 1 rescues mutant 2 and two copies of gene 2 rescues mutant 1), it may suggest that there is more than one copy of one of the proteins is present in each IFT particle.
5. The availability of null alleles would be useful to ask if two copies are sufficient or whether the interaction requires the mutant gene product.
Supplementary Material
References
- Adams GM, Huang B, Luck DJ. Temperature-sensitive, assembly-defective flagella mutants of Chlamydomonas reinhardtii. Genetics. 1982;100:579–586. doi: 10.1093/genetics/100.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers AK, Keller JA, Dutcher SK. Molecular markers for rapidly identifying candidate genes in Chlamydomonas reinhardtii. Ery1 and ery2 encode chloroplast ribosomal proteins. Genetics. 2003;164:1345–1353. doi: 10.1093/genetics/164.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazelton WJ, Amundsen CD, Silflow CD, Lefebvre PA. The bld1 mutation identifies the Chlamydomonas osm-6 homolog as a gene required for flagellar assembly. Curr. Biol. 2001;11:1591–1594. doi: 10.1016/s0960-9822(01)00485-7. [DOI] [PubMed] [Google Scholar]
- Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J. Cell Biol. 1998;141:993–1008. doi: 10.1083/jcb.141.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentler W. Intraflagellar transport (IFT) during assembly and disassembly of Chlamydomonas flagella. J. Cell Biol. 2005;170:649–659. doi: 10.1083/jcb.200412021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutcher SK. Mating and tetrad analysis in Chlamydomonas reinhardtii. Methods Cell Biol. 1995;47:531–540. doi: 10.1016/s0091-679x(08)60857-2. [DOI] [PubMed] [Google Scholar]
- Dutcher SK, Gibbons W, Inwood WB. A genetic analysis of suppressors of the PF10 mutation in Chlamydomonas reinhardtii. Genetics. 1988;120:965–976. doi: 10.1093/genetics/120.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutcher SK, Trabuco EC. The UNI3 gene is required for assembly of basal bodies of Chlamydomonas and encodes delta-tubulin, a new member of the tubulin superfamily. Mol. Biol. Cell. 1998;9:1293–1308. doi: 10.1091/mbc.9.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersold WT. Chlamydomonas reinhardi: Heterozygous diploid strains. Science. 1967;157:447–449. doi: 10.1126/science.157.3787.447. [DOI] [PubMed] [Google Scholar]
- Esparza JM. Ph. D. Thesis. Washington University; St. Louis: 2008. Epsilon tubulin mutants affect basal body integrity, disrupt katanin localization and increase microtubule stability. [Google Scholar]
- Forsburg SL. The art and design of genetic screens: Yeast. Nat. Rev. Genet. 2001;2:659–668. doi: 10.1038/35088500. [DOI] [PubMed] [Google Scholar]
- Golub GH, Reinsch C. Singular value decomposition and least squares solutions. Numer. Math. 1970;14:403–420. [Google Scholar]
- Goodenough U, Lin H, Lee JH. Sex determination in Chlamydomonas. Semin. Cell Dev. Biol. 2007;18:350–361. doi: 10.1016/j.semcdb.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Gross CH, Ranum LP, Lefebvre PA. Extensive restriction fragment length polymorphisms in a new isolate of Chlamydomonas reinhardtii. Curr. Genet. 1988;13:503–508. doi: 10.1007/BF02427756. [DOI] [PubMed] [Google Scholar]
- Harris EH. The Chlamydomonas Sourcebook. Academic Press, Inc.; San Diego: 1989. [Google Scholar]
- Hawley RS, Gilliland WD. Sometimes the result is not the answer: The truths and the lies that come from using the complementation test. Genetics. 2006;174:5–15. doi: 10.1534/genetics.106.064550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hereford LM, Hartwell LH. Sequential gene function in the initiation of Saccharomyces cerevisiae DNA synthesis. J. Mol. Biol. 1974;84:445–461. doi: 10.1016/0022-2836(74)90451-3. [DOI] [PubMed] [Google Scholar]
- Hou Y, Pazour GJ, Witman GB. A dynein light intermediate chain, D1bLIC, is required for retrograde intraflagellar transport. Mol. Biol. Cell. 2004;15:4382–4394. doi: 10.1091/mbc.E04-05-0377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y, Qin H, Follit JA, Pazour GJ, Rosenbaum JL, Witman GB. Functional analysis of an individual IFT protein: IFT46 is required for transport of outer dynein arms into flagella. J. Cell Biol. 2007;176:653–665. doi: 10.1083/jcb.200608041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Rifkin MR, Luck DJ. Temperature-sensitive mutations affecting flagellar assembly and function in Chlamydomonas reinhardtii. J. Cell Biol. 1977;72:67–85. doi: 10.1083/jcb.72.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iomini C, Babaev-Khaimov V, Sassaroli M, Piperno G. Protein particles in Chlamydomonas flagella undergo a transport cycle consisting of four phases. J. Cell Biol. 2001;153:13–24. doi: 10.1083/jcb.153.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iomini C, Li L, Esparza JM, Dutcher SK. Retrograde IFT mutants identify complex A proteins with multiple genetic interactions in Chlamydomonas reinhardtii. Genetics. 2009;183 doi: 10.1534/genetics.109.101915. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DE, Dutcher SK. Molecular studies of linkage group XIX of Chlamydomonas reinhardtii: Evidence against a basal body location. J. Cell Biol. 1991;113:339–346. doi: 10.1083/jcb.113.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathir P, LaVoie M, Brazelton WJ, Haas NA, Lefebvre PA, Silflow CD. Molecular map of the Chlamydomonas reinhardtii nuclear genome. Eukaryotic Cell. 2003;2:362–379. doi: 10.1128/EC.2.2.362-379.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar JL, Zhang J, Funke RP, Weeks DP. Molecular analysis of the acetolactate synthase gene of Chlamydomonas reinhardtii and development of a genetically engineered gene as a dominant selectable marker for genetic transformation. Plant J. 2002;29:109–117. doi: 10.1046/j.1365-313x.2002.01193.x. [DOI] [PubMed] [Google Scholar]
- Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL. A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc. Natl. Acad. Sci. USA. 1993;90:5519–5523. doi: 10.1073/pnas.90.12.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lux FG, III, Dutcher SK. Genetic interactions at the FLA10 locus: Suppressors and synthetic phenotypes that affect the cell cycle and flagellar function in Chlamydomonas reinhardtii. Genetics. 1991;128:549–561. doi: 10.1093/genetics/128.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinowski ER. John Wiley & Sons Inc.; New York: 1991. Factor Analysis in Chemistry.. [Google Scholar]
- Miller MS, Esparza JM, Lippa AM, Lux FG, III, Cole DG, Dutcher SK. Mutant kinesin-2 motor subunits increase chromosome loss. Mol. Biol. Cell. 2005;16:3810–3820. doi: 10.1091/mbc.E05-05-0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller J, Perrone CA, Bower R, Cole DG, Porter ME. The FLA3 KAP subunit is required for localization of kinesin-2 to the site of flagellar assembly and processive anterograde intraflagellar transport. Mol. Biol. Cell. 2005;16:1341–1354. doi: 10.1091/mbc.E04-10-0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myster SH, Knott JA, Wysocki KM, O'Toole E, Porter ME. Domains in the 1alpha dynein heavy chain required for inner arm assembly and flagellar motility in Chlamydomonas. J. Cell Biol. 1999;146:801–818. doi: 10.1083/jcb.146.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi SL, Pan X, Peyser BD, Ye P, Meluh PB, Yuan DS, Irizarry RA, Bader JS, Spencer FA, Boeke JD. Global synthetic-lethality analysis and yeast functional profiling. Trends Genet. 2006;22:56–63. doi: 10.1016/j.tig.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Pan J, Snell WJ. Kinesin-II is required for flagellar sensory transduction during fertilization in Chlamydomonas. Mol. Biol. Cell. 2002;13:1417–1426. doi: 10.1091/mbc.01-11-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J. Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Witman GB. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J. Cell Biol. 1999;144:473–481. doi: 10.1083/jcb.144.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen LB, Miller MS, Geimer S, Leitch JM, Rosenbaum JL, Cole DG. Chlamydomonas IFT172 is encoded by FLA11, interacts with CrEB1, and regulates IFT at the flagellar tip. Curr. Biol. 2005;15:262–266. doi: 10.1016/j.cub.2005.01.037. [DOI] [PubMed] [Google Scholar]
- Piperno G, Mead K. Transport of a novel complex in the cytoplasmic matrix of Chlamydomonas flagella. Proc. Natl. Acad. Sci. USA. 1997;94:4457–4462. doi: 10.1073/pnas.94.9.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G, Mead K, Henderson S. Inner dynein arms but not outer dynein arms require the activity of kinesin homologue protein KHP1(FLA10) to reach the distal part of flagella in Chlamydomonas. J. Cell Biol. 1996;133:371–379. doi: 10.1083/jcb.133.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G, Siuda E, Henderson S, Segil M, Vaananen H, Sassaroli M. Distinct mutants of retrograde intraflagellar transport (IFT) share similar morphological and molecular defects. J. Cell Biol. 1998;143:1591–1601. doi: 10.1083/jcb.143.6.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter ME, Bower R, Knott JA, Byrd P, Dentler W. Cytoplasmic dynein heavy chain 1b is required for flagellar assembly in Chlamydomonas. Mol. Biol. Cell. 1999;10:693–712. doi: 10.1091/mbc.10.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press WH, Teukolski SA, Vetterling WT, Flannery BP. Numerical Recipes in C: The Art of Scientific Computing. Cambridge University Press; Cambridge, UK: 1992. [Google Scholar]
- Rymarquis LA, Handley JM, Thomas M, Stern DB. Beyond complementation. Map-based cloning in Chlamydomonas reinhardtii. Plant Physiol. 2005;137:557–566. doi: 10.1104/pp.104.054221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimogawara K, Fujiwara S, Grossman A, Usuda H. High-efficiency transformation of Chlamydomonas reinhardtii by electroporation. Genetics. 1998;148:1821–1828. doi: 10.1093/genetics/148.4.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sizova I, Fuhrmann M, Hegemann P. A Streptomyces rimosus aphVIII gene coding for a new type phosphotransferase provides stable antibiotic resistance to Chlamydomonas reinhardtii. Gene. 2001;277:221–229. doi: 10.1016/s0378-1119(01)00616-3. [DOI] [PubMed] [Google Scholar]
- Tam LW, Lefebvre PA. Cloning of flagellar genes in Chlamydomonas reinhardtii by DNA insertional mutagenesis. Genetics. 1993;135:375–384. doi: 10.1093/genetics/135.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vysotskaia VS, Curtis DE, Voinov AV, Kathir P, Silflow CD, Lefebvre PA. Development and characterization of genome-wide single nucleotide polymorphism markers in the green alga Chlamydomonas reinhardtii. Plant Physiol. 2001;127:386–389. [PMC free article] [PubMed] [Google Scholar]
- Walther Z, Vashishtha M, Hall JL. The Chlamydomonas FLA10 gene encodes a novel kinesin-homologous protein. J. Cell Biol. 1994;126:175–188. doi: 10.1083/jcb.126.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Pan J, Snell WJ. Intraflagellar transport particles participate directly in cilium-generated signaling in Chlamydomonas. Cell. 2006;125:549–562. doi: 10.1016/j.cell.2006.02.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.