Abstract
Cell invasion through basement membrane (BM) during development, immune surveillance, and metastatic cancer remains poorly understood. We have completed the first in vivo screen for regulators of cell invasion through BM, using the simple model of C. elegans anchor cell invasion, and identified 99 genes that promote invasion, including the chaperonin complex cct. Notably, most of these genes have not been previously implicated in cell invasive behavior. We further characterized members of the cct complex and 11 other genes, determining the distinct aspects of the invasive cascade that they regulate, including formation of a specialized invasive cell membrane and its ability to breach the BM. Suggesting a shared genetic program underlies cell invasion, siRNA knockdown of the human orthologs of cct-5 and lit-1, both previously unknown pro-invasive genes, reduced the invasiveness of metastatic carcinoma cells. Our results reveal the genetic underpinnings of cell invasion and provide new potential therapeutic targets to limit this behavior.
Introduction
The ability of cells to traverse the barriers imposed by basement membrane (BM), the dense sheet-like extracellular matrix that surrounds most tissues, is a critical cellular behavior that occurs during both normal ontogeny and immune system function (1). For example, trophoblast cells breach the endometrium BM to establish the placenta (2) and leukocytes cross the perivascular BM to reach sites of infection and injury (3). Uncontrolled cell-invasive activity is also associated with various human diseases, most notably cancer, where transformed cells are thought to hijack developmental invasive programs to metastasize (4, 5). Despite its importance in both development and human disease, cell invasion through BM remains poorly understood (1, 3, 6–8). The majority of the work on cell invasion has been limited to in vitro models, which do not reflect the in vivo microenvironment and endogenous BM architecture (9, 10). Although recent advances in imaging technology are providing new insights into cell invasion in vertebrates (11), it remains challenging to perform functional perturbations in these models and simultaneously visualize the complex, dynamic process of cell invasion through BM.
Anchor cell (AC) invasion in C. elegans is a simple model of cell invasion through BM that combines forward genetics with single-cell visual analysis. During C. elegans larval development, the AC, a specialized gonadal cell, breaches the gonadal and ventral epidermal BMs to contact the central primary-fated vulval precursor cells (1° VPCs), initiating uterine-vulval connection (12, 13). AC invasion is a regulated and robust process, which occurs invariantly before the P6.p four-cell stage in wild-type animals (Fig. 1A) (12). During the L2/L3 molt (approximately six hours before invasion), a specialized invasive cell membrane, rich in F-actin and actin-regulators, is established within the AC through coordination of netrin (14) and integrin (15) signaling at the interface of the AC and BM (Fig. 1A). AC invasion is stimulated by an unidentified chemotactic cue secreted by the underlying 1° VPCs (Fig. 1A). The ability of the AC to breach the underlying BMs in response to this cue is dependent upon two oncogenes, the bZIP transcription factor (TF) fos-1a (13) and zinc finger TF, egl-43L, the C. elegans ortholog of vertebrate EVI1 and MEL paralogs (16, 17). Together these TFs regulate the expression of the zinc metalloproteinase, zmp-1, as well as other pro-invasive targets in the AC (13).
Fig. 1.
A summary of C. elegans AC invasion and invasion defects following RNAi depletion. (A) Schematic representation of the known mechanisms underlying AC invasion. At the L2/L3 molt (P6.p 1-cell stage; left), approximately six hours before invasion, UNC-6 (netrin) secretion (yellow arrows) from the ventral nerve cord (VNC) and integrin signaling polarize the AC’s basal cell membrane by recruiting F-actin, actin regulators, and the netrin receptor (UNC-40; orange), towards the juxtaposed basement membranes (BM, shown in green) (14, 15). During the mid-to-late L3 stage (P6.p 2-cell stage; middle), an unidentified cue from the 1° VPCs (blue arrows) stimulates invasive protrusions from the AC that require the activity of the transcription factors FOS-1A and EGL-43L to breach the BM. During the late L3 stage (P6.p 4-cell stage), the AC contacts the P6.p granddaughters, initiating the connection between the developing uterine and the vulval epithelium. (B) Nomarski (left), corresponding fluorescence image (middle), and overlay (right). Anterior is left and ventral is down. In wild-type or worms fed control RNAi (L4440 empty vector; top panel), the AC (magenta, zmp-1>mCherry; white arrowhead) breaches the BM (green, LAM-1::GFP) and contacts the central primary fated P6.p granddaughters (bracket; P6.p 4-cell stage). In contrast, after RNAi mediated knockdown of hda-1 and cdc-37 (middle and bottom panels, respectively), the BMs remain intact following a failure in AC invasion (table S3). Scale bar, 5 μm.
Towards the goal of comprehensively identifying regulators of cell invasion through BM in vivo, we have performed a focused whole-genome RNA interference (RNAi) screen. Here, we report 99 regulators of AC invasion, most of which have not been previously implicated in invasion or metastasis. We have further characterized the most robust pro-invasive genes, including members of the cct complex and 11 others, encompassing both known oncogenes and previously unknown regulators of cell invasion. Notably, small interfering RNA (siRNA) knockdown of two of these pro-invasive genes reduced the invasiveness of metastatic carcinoma cells, suggesting that our approach has identified conserved regulators that might be potential therapeutic targets in halting cancer progression.
Results
An RNAi screen identifies 99 regulators of AC invasion
A defect in AC invasion disrupts uterine-vulval attachment and results in an easily observed Protruded vulva (Pvl) and Egg-laying defective (Egl) phenotype. We have taken advantage of data collected from a number of whole-genome RNAi screens that have identified the complement of genes giving a Pvl or Egl phenotype following RNAi depletion (18–20). Using these data, we examined 539 genes that produce a Pvl and Egl phenotype after RNAi knockdown using Nomarski optics at the time of AC invasion (table S1). We identified 99 genes whose reduction results in a failure of the AC to breach the BM, as evidenced by an unbroken phase-dense line underneath the AC (Fig. 1B and tables S2 and S3). 95% (94/99) of these genes have human orthologs as determined by BLASTP analysis, of which 90% (85/94 genes) have not been previously implicated in cell invasion or cancer metastasis (table S2). Validating the specificity and rigor of this approach, components of genetic pathways known to promote AC invasion, including the TFs fos-1a (13) and egl-43L (16, 17); the netrin receptor unc-40 (14); and the integrin α subunit ina-1 (15) were identified (Table 1 and table S2).
Table 1.
A subset of genes for which RNAi depletion inhibits AC invasion. Genes in bold are known regulators of cell invasion or metastasis. Genes marked with an asterisk have been previously implicated in AC invasion. The (†) denotes members of the cct complex selected for further characterization. AC invasion defect is listed as the percent average defect from multiple trials (minimum of two) at the P6.p 4-cell stage of 1° VPC division. See tables S2 and S3 for a complete list of pro-invasive genes and the penetrance of their AC invasion defect by RNAi.
| Gene Public Name | Sequence Name (Gene) | CDS Description | Homolog BLASTP e-value | Homologous Protein | Homolog Description | AC invasion defect (%) |
|---|---|---|---|---|---|---|
| Transcription Factors | ||||||
|
| ||||||
| hbl-1 | F13D11.2 | C2H2-type zinc finger | 1.10E-20 | ENSEMBL:ENSP00000340749 | Isoform 2 of Zinc finger protein 124 | 42% |
| *fos-1a13,28 | F29G9.4 | BZIP transcription factor | 0.000000017 | VG:OTTHUMP00000200848 | FOS-like antigen 2 | 45% |
| mep-1 | M04B2.1 | zinc-finger protein | 45% | |||
| unc-62 | T28F12.2 | homeodomain transcription factor | 7.10E-79 | ENSEMBL:ENSP00000326296 | Isoform Meis2C of Homeobox protein Meis2 | 35% |
| *egl-43L 16,17,29 | R53.3 | Zinc finger, C2H2 type (4 domains) | 9.10E-47 | ENSEMBL:ENSP00000367643 | PRDM16 (EVI1/MEL) | 33% |
|
| ||||||
| Chromatin remodification and architecture | ||||||
|
| ||||||
| hda-121,32 | C53A5.3 | Yeast RPD3 protein like | 2.80E-170 | ENSEMBL:ENSP00000362649 | Histone deacetylase 1 | 57% |
|
| ||||||
| Protein synthesis/degredation/folding | ||||||
|
| ||||||
| cct-1 | T05C12.7 | TCP-1 like chaperonin | 3.70E-196 | ENSEMBL:ENSP00000317334 | T-complex protein 1 subunit alpha | 88% |
| †cct-2 | T21B10.7 | TCP-1 like chaperonin | 5.11E-183 | ENSEMBL:ENSP00000299300 | T-complex protein 1 subunit beta | 80% |
| cct-4 | K01C8.10 | TCP-1 like chaperonin | 4.30E-179 | ENSEMBL:ENSP00000377958 | T-complex protein 1 subunit delta | 81% |
| †cct-5 | C07G2.3 | TCP-1 like chaperonin | 1.90E-203 | ENSEMBL:ENSP00000280326 | T-complex protein 1 subunit epsilon | 86% |
| †cct-6 | F01F1.8 | TCP-1 like chaperonin | 8.39E-183 | ENSEMBL:ENSP00000275603 | T-complex protein 1 subunit zeta | 79% |
| †cct-7 | T10B5.5 | TCP-1 like chaperonin | 7.10E-188 | ENSEMBL:ENSP00000258091 | T-complex protein 1 subunit eta | 83% |
| cct-8 | Y55F3AR.3 | TCP-1 like chaperonin | 3.60E-166 | ENSEMBL:ENSP00000286788 | T-complex protein 1 subunit theta | 81% |
| cdc-3722,33 | W08F4.8 | Hsp90 co-chaperone | 2.00E-73 | ENSEMBL:ENSP00000222005 | Hsp90 co-chaperone Cdc37 | 44% |
|
| ||||||
| Signaling and membrane trafficking | ||||||
|
| ||||||
| *ina-115,31 | F54G8.3 | Integrin alpha chain | 2.00E-83 | ENSEMBL:ENSP00000264107 | Isoform Alpha-6X1A of Integrin alpha-6 precursor | 30% |
| *unc-4014,30 | T19B4.7 | membrane protein | 1.30E-164 | ENSEMBL:ENSP00000304146 | Netrin receptor DCC precursor | 58% |
| lit-1 | W06F12.1 | serine/threonine kinase (CDC2/CDC28 subfamily) | 2.50E-144 | ENSEMBL:ENSP00000262393 | nemo-like kinase | 31% |
|
| ||||||
| Metabolism | ||||||
|
| ||||||
| T03F1.8 | T03F1.8 | guanylate kinase | 1.30E-51 | ENSEMBL:ENSP00000317659 | Guanylate kinase | 41% |
| C48A7.2 | C48A7.2 | phosphate permease | 1.90E-105 | ENSEMBL:ENSP00000272542 | Sodium-dependent phosphate transporter 1 | 59% |
|
| ||||||
| Nematode-specific gene with unknown function | ||||||
|
| ||||||
| F28F8.5 | F28F8.5 | 30% | ||||
|
| ||||||
| Uncharacterized conserved genes | ||||||
|
| ||||||
| T20B12.1 | T20B12.1 | 1.30E-90 | ENSEMBL:ENSP00000313953 | tetratricopeptide repeat domain 27 | 45% | |
| cacn-1 | W03H9.4 | 4.10E-112 | ENSEMBL:ENSP00000221899 | Isoform 2 of Uncharacterized protein C19orf29 | 32% | |
11 pro-invasive genes and the cct complex regulate the AC’s ability to breach BM
To focus on the most crucial regulators of AC invasion, we further characterized genes whose RNAi depletion gave a robust AC invasion defect, which we set at ≥30%, a degree of penetrance similar to invasion defects resulting from RNAi directed against members of known AC invasion pathways, including FOS-1A and EGL-43L activity as well as INA-1 and UNC-40 signaling (Table 1 and tables S2 and S3). This list includes genes with human orthologs that have been implicated directly in cell invasion and metastasis [hda-1, a histone deactylase (21), and cdc-37, a co-chaperone of hsp90 (22)] as well as previously unknown regulators (mep-1, C48A7.2, cacn-1, F28F8.5, T20B12.1, lit-1, T03F1.8, hbl-1, unc-62, and the cct complex) (Table 1). The genes whose RNAi knockdown gave the most robust AC invasion defect (>79%) included seven of the eight member cct chaperonin complex (Table 1 and tables S2 and S3). Because AC invasion defects were similar for all cct genes tested (Table 1 and table S2), we further characterized several members of this complex, cct-2, cct-5, cct-6, and cct-7 (henceforth referred to in the text as cct). Consistent with our RNAi results, AC invasion defects were detected in putative null alleles for mep-1, hda-1, C48A7.2, and cct-6 (table S4 and fig. S1). For genes lacking putative null alleles or whose loss-of-function prevented animals from surviving to the time of AC invasion, we observed AC invasion defects using multiple dsRNA constructs, ruling out off-target RNAi effects (23) (table S4). To verify that the observed AC invasion defect was specifically because of failure to breach the underlying BM, we examined the integrity of laminin, a key structural component of BM. Following RNAi depletion targeting cct and the remaining 11 genes, LAM-1::GFP (laminin) remained intact under the AC after a failure to invade, confirming that the invasion defects were due to an inability to break through the BM (Fig. 1B and fig. S2).
Invasion is promoted by nine genes and the cct complex which function in the AC post-specification
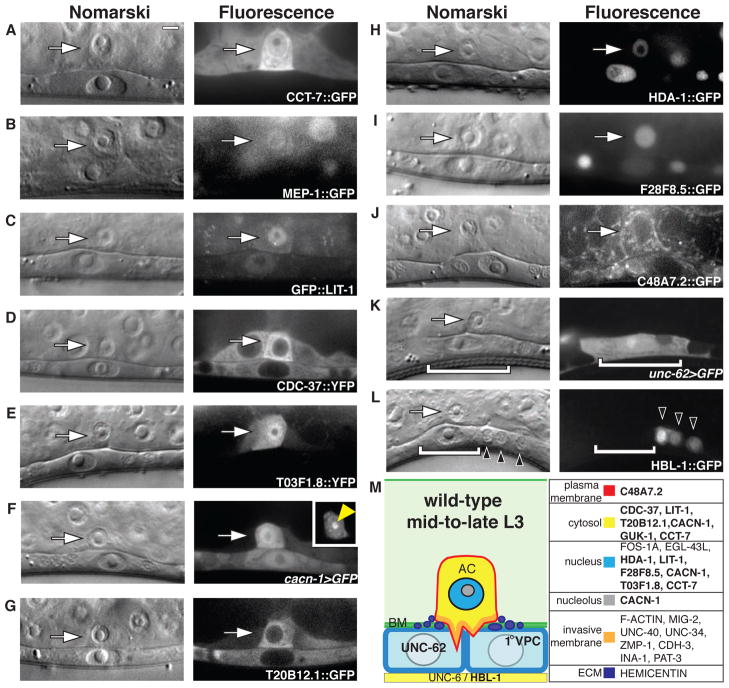
To understand how these newly identified genes regulate invasion, we first examined transcriptional and translational GFP reporters for localization and abundance. We found that the cct complex and six other genes – mep-1, a zinc finger TF; lit-1, an ortholog of nemo-like kinase (NLK); cdc-37; T03F1.8, a guanylate kinase; and two uncharacterized conserved proteins, cacn-1 and T20B12.1 – were up-regulated in the AC before or during the time of invasion (Fig. 2, A to G and figs. S3 and S4), similar to other known regulators of AC invasion [such as fos-1a, zmp-1, egl-43L, and pat-3 (the integrin β subunit)] (13, 15–17). Additionally, we identified three genes – hda-1; F28F8.5, an uncharacterized nematode-specific gene; and C48A7.2, a sodium and phosphate transporter – which showed localization in most cells, including the AC (Fig. 2, H to J and figs. S3 and S4). hbl-1 and unc-62 (the C. elegans orthologs of the TFs hunchback and homothorax, respectively) were not detected in the AC prior to or during the time of invasion, but were expressed in the underlying vulval cells (unc-62>GFP) or in the ventral nerve cord and underlying vulval cells at earlier stages of development (hbl-1::GFP) (Fig. 2, K and L and fig. S5). The absence of localization in the AC and expression in the vulval cells suggested that hbl-1 and unc-62 might act in VPCs to promote invasion. A summary of the localization of the proteins encoded by newly identified pro-invasive genes is shown in Fig. 2M.
Fig. 2.
Transgene reporter localization of newly identified genes that regulate AC invasion. Nomarksi image, left; corresponding fluorescence image, right at the P6.p 1-cell stage. Anterior is left and ventral is down. All images are confocal z-slices except (C) and (D), which are wide-field fluorescence images. (A to J) Translational (::) and transcriptional (>) reporter constructs for the cct complex (as shown by cct-7::GFP) and nine of the 11 newly identified pro-invasive genes showed AC-enriched (arrow) GFP localization in various subcellular compartments before and during AC invasion. (K) A transcriptional reporter for unc-62 (unc-62>GFP) showed VPC expression before and during the time of AC invasion (white brackets). (L) A translational reporter for HBL-1 (hbl-1::GFP) was detected in the cell bodies of the ventral nerve cord (VNC; black arrowheads) before and during the time of AC invasion. Although HBL-1::GFP is not localized to the VPCs at the time of invasion, it is expressed in VPCs hours before invasion (fig. S5). (M) Summary diagram of the subcellular localization of proteins in the AC, underlying 1° VPCs, and VNC during invasion. Genes identified in this study are bolded. Scale bar, 5 μm.
AC invasion relies on the proper specification of the AC and underlying 1° VPCs, which generate the invasive cue (12). To determine if these newly identified genes regulate invasion by causing defects in AC or 1° VPC cell specification, we examined the expression of lin-3>GFP and egl-17>GFP, markers of AC and 1° VPC cell fate, respectively (24, 25). lin-3>GFP was detected in the AC following RNAi-mediated depletion of the cct complex and the remaining 11 genes, indicating that AC specification was normal despite reduction of activity of these genes (fig. S6). The expression of egl-17>GFP in the underlying vulval cells was lost only after depletion of unc-62 by RNAi, consistent with a role for unc-62 in regulating invasion by controlling 1° VPC specification (fig. S7). Additionally, RNAi knockdown of hbl-1 resulted in precocious egl-17>GFP expression and division of the VPCs (figs. S5B and S7), a heterochronic phenotype that leads to inability of the AC to respond to the early release of the vulval cue (12). Taken together with the transgene localization of unc-62>GFP and hbl-1::GFP, these results suggest that unc-62 and hbl-1 promote AC invasion by regulating 1° VPC specification, and that the remaining nine pro-invasive genes and cct complex appear to influence post-specification aspects of the invasive process.
To further examine the function of this subset of genes from our screen that promote invasion, we utilized a C. elegans strain in which only uterine tissue, including the AC, is sensitive to RNAi (15) (table S5). RNAi depletions targeting cct and nine other genes, all of which showed transgene localization in the AC (Fig. 2, A to J), blocked invasion (table S5). Because uterine cells do not contribute to AC invasion (12), an AC invasion defect in this background indicates that the gene functions in the AC. In contrast, RNAi targeting hbl-1 and unc-62, which were not expressed in the AC (Fig. 2, K and L) showed normal invasion in the uterine-specific RNAi sensitive background (table S5), consistent with the data above suggesting that these genes function within the vulval cells. We conclude that most of the genes identified in our screen that block invasion after reduction of activity function within the AC.
Genes that function within the AC regulate multiple aspects of invasion
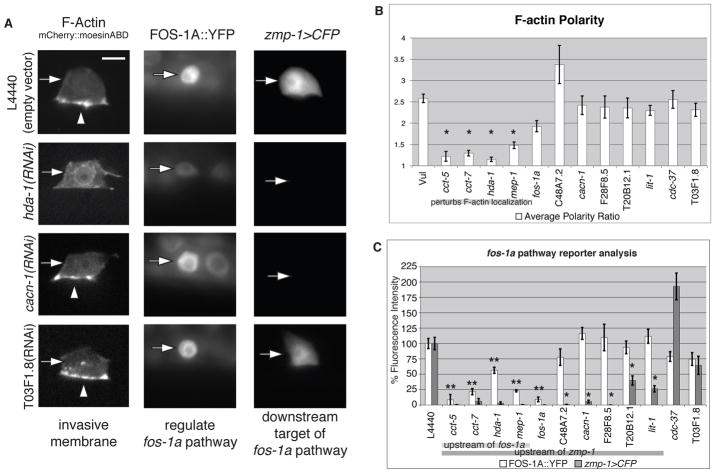
Before invasion, netrin and integrin signaling in the AC establish a specialized F-actin rich invasive cell membrane that contacts the underlying BM (14, 15). To determine whether any of the pro-invasive genes that function in the AC regulate the formation of this invasive cell membrane domain, we used a probe containing the F-actin-binding domain of the moesin gene (mCherry::moeABD) to visualize F-actin at the invasive membrane (14). RNAi targeting cct, hda-1, or mep-1 resulted in loss of polarized F-actin, indicating a failure to form the invasive cell membrane (Fig. 3, A and B, and fig. S8). Consistent with this finding, both the cct complex (26) and histone deacetylases (HDACs) (21, 27) regulate the actin cytoskeleton and cell motility in mammalian cells. To determine whether these four genes regulate invasive membrane formation through interactions with netrin or integrin signaling, we examined the localization of the netrin receptor (UNC-40::GFP) and integrin β subunit PAT-3 (PAT-3::GFP following RNAi knockdown. Localization of the netrin receptor (fig. S9) or of integrin (fig. S10) at the invasive membrane was not altered following the reduction of cct, hda-1, or mep-1, suggesting that these genes function independently of netrin and integrin receptor localization to regulate the polarization of the F-actin cystoskeleton.
Fig. 3.
Newly identified regulators of AC invasion function within established as well as previously uncharacterized pathways to promote invasion. (A) Confocal z-slice fluorescence images of F-actin probe (left; mCherry::moeABD), and wide-field fluorescence images of FOS-1A::YFP (middle) and zmp-1>CFP (right), in empty vector control and hda-1, cacn-1, and T03F1.8 RNAi depleted animals at the time of invasion. A single factor ANOVA followed by Tukey’s HSD test for significance was used in all cases. For this and all subsequent figures (n) refers to the number of animals per genotype. The top panel shows localization or expression for all three reporters in wild-type ACs. RNAi depletion of hda-1 resulted in a loss of F-actin at the invasive membrane (n = 18; P < 0.05) and reduced FOS-1A::YFP fluorescence in the AC to 57% of that in wild-type animals (n = 11; P < 0.05). In contrast, RNAi depletion of cacn-1 (n = 11) or T03F1.8 (n = 17) did not affect polarization of F-actin at the invasive front (arrowhead) or significantly alter FOS-1A::YFP fluorescence in the AC (n ≥ 10; P > 0.05). zmp-1>CFP expression was significantly reduced after RNAi depletion of hda-1 or cacn-1 (n = 12 for both; P < 0.05), but not of T03F1.8 (n = 10; P > 0.05). (B and C) Quantification of an F-actin probe (B) and FOS-1A::YFP/zmp-1>CFP (C) in Vulvaless (Vul), empty vector control (L4440), and RNAi depleted animals in which the AC failed to invade (n ≥ 10 animals for each RNAi depletion). *, P < 0.05. (B) RNAi depletion of the cct complex, hda-1, and mep-1 blocked polarization of F-actin at the invasive membrane (grey bar). In Vul animals, AC invasion is blocked, but polarity is maintained (14). Knockdown of the other genes caused defects in AC invasion, though the ACs were still polarized. Loss of C48A7.2 resulted in reduced AC size, which could account for an F-actin polarity ratio that is greater than in Vul control animals. (C) RNAi knockdown of the cct complex, hda-1, and mep-1 down-regulated FOS-1A::YFP (P < 0.05; white bars) and its downstream target, zmp-1>CFP (P < 0.05; black bars) in the AC (denoted by the double asterisks). RNAi targeting five genes (C48A7.2, cacn-1, F28F8.5, T20B12.1, and lit-1) reduced zmp-1>CFP expression in the AC (P < 0.05), but did not affect FOS-1A::YFP expression (denoted by the single asterisk). cdc-37 and T03F1.8 RNAi depletion did not significantly down-regulate either fos-1a pathway reporter (P > 0.05). Although cdc-37(RNAi) increased zmp-1>CFP expression, we have previously shown that increased zmp-1 expression does not alter invasion (13). Error bars report the standard error of the mean (SEM). Scale bar, 5 μm.
Independent of invasive membrane formation, the ability of the AC to breach the underlying BM relies upon at least two TFs, fos-1a and egl-43L, which function in the AC to regulate the expression of downstream targets, including the zinc metalloproteinase zmp-1 (13, 16, 17). To determine whether any of the genes functioning within the AC regulate the fos-1a–egl-43L pathway, we quantified the fluorescence of a translational reporter for fos-1a (fos-1a::YFP) and a transcriptional reporter for zmp-1 (zmp-1>CFP). Loss of five of the genes (C48A7.2, cacn-1, F28F8.5, T20B12.1, and lit-1) reduced the expression of zmp-1>CFP, but not the abundance of FOS-1A::YFP, and loss of two genes (cdc-37 and T03F1.8) did not down-regulate either reporter (Fig. 3, A and C, and figs. S11 and S12). RNAi depletion of cct, hda-1, and mep-1 decreased both FOS-1A::YFP abundance and zmp-1>CFP expression. These results suggest that HDACs may play a conserved role in the transcriptional regulation of fos family oncogenes during invasion, because in mammalian cell culture, HDAC inhibition represses invasion in v-fos transformed cells and leads to the re-expression of normally suppressed genes (21). Notably, the cct complex, hda-1, and mep-1 also perturbed F-actin polarity, revealing a set of upstream regulators that control both fos-1a dependent BM removal and invasive membrane formation (13–15).
Of the five genes that promoted zmp-1>CFP expression, only the abundance of the translational reporter for lit-1 (GFP::lit-1) was down-regulated by fos-1 RNAi depletion, indicating that lit-1 is a target of fos-1a (fig. S13 and S14A). Additionally, lit-1 RNAi depletion did not affect FOS-1A::YFP abundance or egl-43L>GFP expression in the AC (fig. S14), suggesting it acts downstream of fos-1a and egl-43L, but upstream of zmp-1 to promote AC invasion (Fig. 4A). The remaining four genes that promote zmp-1>CFP expression appear to act parallel to the fos-1a pathway, because fos-1 RNAi depletions did not affect their abundance (fig. S13). Taken together, these experiments identify three main groups of genes that promote AC invasion: (i) upstream regulators controlling both the establishment of the invasive membrane and BM removal through the fos-1a pathway (cct complex members, mep-1, and hda-1); (ii) genes that are downstream (lit-1) or parallel (C48A7.2, F28F8.5, T20B12.1, and cacn-1) to the fos-1a pathway; and (iii) two genes (cdc-37 and T03F1.8) that fail to regulate either invasive membrane formation or components of the fos-1a pathway, suggesting that they control distinct aspects of AC invasion (Fig. 4A).
Fig. 4.

Identification of conserved regulators of cell invasion through BM. (A) Newly identified AC invasion promoting genes were mapped onto pre-existing molecular pathways governing AC invasion. Two transcription factors, hbl-1 and unc-62, appear to indirectly affect invasion by regulating 1° VPC specification. Upstream regulators (hda-1, mep-1, and the cct complex; black box) control both the establishment of the AC invasive membrane and the fos-1a transcriptional pathway underlying BM removal. Additionally, genes were identified that function within (lit-1) and parallel to (cacn-1, F28F8.5, T20B12.1, C48A7.2) the fos-1a pathway. Lastly, we have identified two genes, cdc-37 and T03F1.8, that act in distinct aspects of AC invasion beyond the establishment of the invasive membrane and fos-1a pathway. (B to F) CCT5 and NLK are required for tissue-invasive activity of breast and colon tumor cells in vivo. GFP-labelled MDA-MB-231 cells were transfected with a control siRNA (SCR) or siRNA directed against CCT5 (siCCT5) or NLK (siNLK). The transfectants were cultured for 3 days on top of the CAM of 11-day-old chicks. Chick BM and cell nuclei in cross-sections were visualized by staining with an antibody directed against type IV collagen (red) and the nuclear stain, 4,6-diamidino-2-phenylindole (DAPI; blue), respectively. (B) Cross section of chick CAM in the absence of overlying cancer cells shows the ectodermal BM (white arrowheads) at the CAM surface (position indicated by the black arrow). Vascular structures, which are surrounded by type IV collagen, can also be observed along with the endodermal BM along the lower edge of the CAM. (C) GFP-labeled MDA-MB-231 cells electroporated with scrambled siRNA (Scr) breach the chick BM. Invading cancer cells are outlined with white dashed line. Areas of BM degradation are demarcated with yellow arrows. White arrowheads mark areas with intact BM. (D and E) MDA-MB-231 cells electroporated with siRNAs directed against CCT5 or NLK fail to breach the BM. (F) Invasion is quantified as the number of breast (MDA-MD-231) and colon (HCT116) tumor cells (106) that cross the CAM surface (mean Inv ± SEM of 3 or more experiments) following siRNA knockdown of CCT5 and NLK (*, P < 0.001) compared to control (Scr) siRNA treatment, using a single factor ANOVA followed by Tukey’s HSD test for significance. Scale bar, 100 μm.
cct-5/CCT5 and lit-1/NLK are required for invasion in carcinoma cells
The human orthologs of genes known to promote AC invasion [fos-1a/FOS family members (28), egl-43L/EVI-1 (29), netrin (30), and integrin (31)] regulate the tissue-invasive activity of mammalian cells. Similarly, several of the human orthologs to genes identified in our screen have been directly implicated in controlling cell invasion or metastasis, including HDA1 and CDC37, both of which are targets of anti-cancer therapeutics (32, 33). To determine whether any of the other newly identified genes also regulate the invasive behaviour of mammalian cells, we used siRNA to knock down human orthologs of two genes, cct-5/CCT5 and lit-1/NLK, in transformed human breast cancer cells (MDA-MB-231) and colon carcinoma cells (HCT116) explanted onto a chick chorioallantoic membrane (CAM), an ex vivo system used to assay the ability of cells to cross an endogenous BM (34) (Fig. 4B). Though not linked previously to tissue-invasive activity, members of the CCT/TCP-1 complex are thought to function as chaperones for various proteins, including actin and tubulin monomers (26), and NLK is a negative regulator of the canonical Wnt signaling pathway (35). Following electroporation with siRNA scrambled controls, breast and colon carcinoma cells were able to breach the BM of the chick CAM (Fig. 4C). Strikingly, siRNA-mediated knockdown of either CCT5 or NLK reduced invasion in both cancer cell lines, leaving the BM intact under the proliferating tumor mass (Fig. 4, D and E, and fig. S15, B and C). siRNA-mediated depletion of CCT5 and NLK did not significantly affect breast carcinoma cell proliferation, migration, or apoptosis (fig. S15, E and F), suggesting that these genes are specifically affecting the BM transmigration activity of the cancer cells. Taken together, these results suggest that the genes identified that control AC invasion might also be components of a conserved mechanism used by mammalian cells to breach BM barriers.
Discussion
BM invasion is a critical process that occurs during development, immune system surveillance, and the spread of metastatic cancer. Here, we report the identification of 99 genes that promote AC invasion during C. elegans larval development, the first (to our knowledge) loss-of-function screen to identify cell invasion regulators in vivo. We have identified genes that were up-regulated in the AC (cdc-37 and lit-1) as well as several that were ubiquitously expressed (hda-1, F28F8.5, and C48A7.2); critically important genes that might be missed in expression-based studies to identify genes that regulate invasion. Remarkably, 90% of the pro-invasive genes that have human orthologs have not been previously implicated in cell invasion or cancer metastasis. We further characterized the most robust regulators of AC invasion, identifying lit-1 as a new member of the fos-1a pathway as well as a set of genes (cct complex, hda-1, and mep-1) that function to regulate multiple aspects of invasion, including the establishment of a specialized invasive membrane (14, 15) and the ability to remove underlying BM (13).
The basement membrane, which is an interwoven network of extracellular matrix molecules, is an ancient metazoan structure underlying the basal surface of epithelia and endothelia. The predominant components of the BM that provide structural support and barrier function are the meshwork of type IV collagen and laminin, which are evolutionarily conserved from sponges to humans (8, 36). Similar to other cell biological processes (such as stem cell determination and maintenance, apopotosis, regulation of cell division, and epithelial to mesenchymal transition), it has been suggested that the genetic networks controlling cell invasion during development are also conserved and redeployed during tumor invasion (1, 8, 34, 36, 37). Here, we provide additional evidence for the conservation of cell invasion programs during development and disease showing that the human orthologs of several newly identified regulators of AC invasion, including the cct complex and an intracellular kinase, lit-1, also function during carcinoma cell invasion. This suggests that much of the required invasion machinery is shared, and that the regulators of cell invasion identified here might offer potent new therapeutic targets to modulate cell invasive behavior in development and human diseases such as cancer.
Materials and Methods
Worm handling and strains
Wild-type nematodes were strain N2. Strains were reared at 15°C, 20°C, and 25°C using standard conditions (38). In the text and figures we designate linkage to a promoter using the (>) symbol and linkages that fuse open reading frames using the (::) annotation (14). Vulvaless animals were created using the strain lin-3(n1059)/lin-3(n378) (12). The following transgenes and alleles were used for experiments performed in this paper: qyIs42[pat-3::GFP, ina-1], qyIs49[T03F1.8::YFP], qyIs67[cdh-3>unc-40::GFP], qyIs68[cdh-3>unc-40::GFP], qyIs69[C48A7.2::YFP], qyIs72[cdc-37::YFP], qyIs91[egl-43L::GFP], qyIs93[hda-1::GFP], qyIs96[cacn-1>GFP], qyIs99[unc-62>GFP], qyIs100[T20B12.1::GFP], qyIs102[fos-1a>rde-1], qyIs114[cdh-3>cacn-1::GFP], qyEx171[cct-7::GFP], UL906[F28F8.5::GFP], sEx10433[cct-2>GFP], sEx12510[cct-7>GFP], ctIs39[hbl-1::GFP], neEx1[lit-1::GFP], cgc5338Is1mep-1::GFP]; LGI: ayIs4[egl-17>GFP], dpy-5(e907); LGII: cacn-1(tm3042), cacn-1(tm(3126), cct-2(ok3438), qyIs17[zmp-1>mCherry], rrf-3(pk1426), rol-6(n1270); LGIII: cct-6(ok2904), lit-1(ok649), unc-119(ed4), syIs107[lin-3>GFP]; LGIV: eri-1(mg366), mep-1(n3702), mep-1(q660), mep-1(ok421), syIs68[zmp-1>CFP], dpy-20(e1282), lin-3(n1059)/lin-3(n378); LGV: hda-1(e1795), hda-1(ok1595), rde-1(ne219), qyIs50[cdh-3>mCherry::moeABD]; LGX: hbl-1(mg285), hbl-1(ve18), lin-15B(n744), syIs123[fos-1a::YFP], qyIs66[cdh-3>unc-40::GFP], qyIs7[lam-1::GFP].
Molecular biology and generation of transgenic animals
Translational reporter constructs fusing coding sequences for GFP to cDNAs encoding UNC-40, laminin, and the actin-binding domain of moesin have been described previously (14, 15). We utilized PCR fusion (13) to generate promoter>GFP and PROTEIN::GFP constructs to the genes listed in table S6. AC-specific promoter fusions were generated using the cdh-3mk62–63 AC-specific regulatory element (13). Templates and specific primer sets for each promoter and reporter gene are listed in table S7. Transgenic worms were created by transformation with co-injection markers pPDMM016B (unc-119+) into the germline of unc-119(ed4) worms. These expression constructs were injected with EcoRI-digested salmon sperm DNA and pBSSK- DNA at 50–100 ng/μl serving as carrier DNA along with serial dilutions of the GFP fusion construct in order to optimize expression levels and avoid toxicity. Transgenic extrachromosomal (Ex) lines and integrated strains (Is) generated in this study are listed in the table S6. Integrated strains were generated as described previously (13).
RNA interference
Double stranded RNA (dsRNA) for the 539 genes listed in table S1 was delivered by feeding to rrf-3(pk1426) worms, using the RNAi protocol that was originally used to produce a Pvl or Egl phenotype (L4 or L1 plating) (18–20). RNAi vectors from the Ahringer (18) and Vidal (20) dsRNA libraries were sequenced to verify the correct insert. 100 bp dsRNA constructs were designed and cloned into L4440 to minimize any potential off-target RNAi affects using the web portal dsCheck (http://dscheck.rnai.jp/) (table S4) and fed to rrf-3(pl1426); lam-1::GFP worms to verify AC invasion defects. Uterine-specific RNAi sensitivity was generated by restoring RDE-1 protein to the cells of the somatic gonad under the control of the fos-1a promoter in rde-1(ne219) mutant animals, using rde-1(ne219); fos-1a>rde-1; rrf-3(pk1426) worms (15).
Image acquisition, processing, and analyses
Images were acquired using a Zeiss AxioImager A1 microscope with a 100x plan-apochromat objective and a Zeiss AxioCam MRm CCD camera, controlled by Zeiss Axiovision software (Zeiss Microimaging), or using a Yokogawa spinning disk confocal mounted on a Zeiss AxioImager A1 microscope using IVision software (Biovision Technologies). Images were processed in ImageJ (NIH Image) and overlaid using Photoshop CS3 (Adobe Systems).
AC invasion scoring, polarity and fluorescence intensity measurements
AC invasion was evaluated in relation to the timing of P6.p descendant divisions, the L3 molt, gonad reflection, and ventral uterine (VU) cell divisions (13). For the initial RNAi screen, all 539 genes (table S1) targeted by feeding RNAi were scored using Nomarski optics for AC invasion defects and the production of adult Pvl and Egl phenotypes to confirm the effectiveness of the RNAi clone used. At least two independent dsRNA feeding experiments were performed for those genes that showed an AC invasion defect (defined as a minimum of two out of nine animals having a block in AC invasion) upon RNAi depletion (tables S2 and S3). Polarity measurements and ratios for F-actin (mCherry::moeABD) and UNC-40::GFP in wild-type, mutant, and RNAi-targeted strains were determined using ImageJ (NIH Image) v.1.4 by comparing the average fluorescence intensity from five-pixel-wide linescans drawn along the invasive and non-invasive membranes of mCherry::moeABD and UNC-40::GFP in wild-type and RNAi depleted animals. A polarity ratio was generated by dividing the fluorescence density of the invasive membrane by the fluorescence density of the non-invasive membrane (n > 10 animals were scored for each genotype with an observable defect in AC invasion at the P6.p 4-cell stage) (14, 15). Fluorescence intensity measurements of AC GFP abundance for translational reporters (fos-1a::YFP (syIs123), T20B12.1::GFP (qyIs100), GFP::lit-1 (neEx1), F28F8.5::GFP (UL906), cdc-37::YFP (qyIs72), and T03F1.8::YFP (qyIs49) and AC GFP expression of transcriptional reporters zmp-1>CFP (qyIs17), egl-43L>GFP (qyIs91), cacn-1>GFP (qyIs96) was determined using ImageJ (NIH Image) v.1.4 (15) (n > 10 animals for each with an observable defect in AC invasion). In all cases, except quantification of GFP::LIT-1 abundance (by an unpaired Student’s t-test), a single factor ANOVA, followed by Tukey’s HSD test was used to determine statistical significance of changes in GFP abundance or polarity.
Tissue culture, siRNA eletroporation and CAM invasion assay
Human breast cancer cells (MDA-MB-231) or colon cancer cells (HCT116), marked with GFP, were grown in DMEM with 10% FBS. Cells were transfected with 50 pmol each of control, CCT5, or NLK siRNA (Invitrogen) by electroporation using Amaxa Cell Line Nucleofector Kit V according to the manufacturer’s instructions (Lonza). Silencing efficiency was established 48 hours post-electroporation by RT-PCR (fig. S15A). Transfected cells (≈ 1x106) were cultured atop the chorioallantoic membrane (CAM) of 11 d-old chicken embryos for 3 days as described previously (34, 39). Invasion was monitored in cross-sections of the fixed CAM by fluorescent microscopy with BM integrity assessed with a chick-specific mouse monoclonal antibody directed against type IV collagen (provided by J. Fitch and T. Linsenmayer, Tufts University). Invasion is expressed as the mean number of tumor cells (106) below the CAM surface; statistical significance was calculated by a single factor ANOVA followed by Tukey’s HSD test. CAM invasion results obtained with a pool of CCT5 siRNAs or a single NLK siRNA were confirmed with at least two individual CCT5 siRNAs or a second independent siRNA directed against NLK (fig. S15B). Cancer cell migration, proliferation, and apoptosis (fig. S15, E and F) were quantified as described (34, 39).
Supplementary Material
Fig. S1. Putative null alleles of mep-1, hda-1, cct-6, C48A7.2, and hbl-1 show AC invasion defects.
Fig. S2. LAM-1::GFP (laminin) is intact following RNAi targeting the cct complex and 11 other pro-invasive genes at the P6.p 4-cell stage of VPC division
Fig. S3. Transgene reporter localization of newly identified pro-invasive genes at the P6.p 2-cell stage of VPC division
Fig. S4. Transgene reporter localization of newly identified pro-invasive genes at the P6.p 4-cell stage of VPC division
Fig. S5. hbl-1 functions in VPC specification to promote AC invasion
Fig. S6. The AC is correctly specified, as shown by lin-3>GFP, following RNAi depletion of the newly identified regulators of AC invasion
Fig. S7. 1° VPC specification following RNAi depletion of newly identified AC invasion genes
Fig. S8. Identification of regulators of invasive membrane formation
Fig. S9. Regulators of invasive membrane formation function independently of netrin receptor localization
Fig. S10 Regulators of invasive membrane formation function independently of integrin receptor localization
Fig. S11. Identification of regulators of the fos-1a pathway
Fig. S12. Identification of genes that act within or parallel to the fos-1a pathway
Fig. S13. fos-1a RNAi depletion identifies lit-1 as a new member of the fos-1a pathway
Fig. S14. lit-1 functions downstream of fos-1a and egl-43L during AC invasion
Fig. S15. CCT5 and NLK siRNA-mediated knockdown specifically affect BM transmigration activity
table S1. 539 Pvl and Egl genes targeted by RNAi for AC invasion defects
table S2. Identification of 99 regulators of AC invasion
table S3. RNAi depletion of 99 genes results in an AC invasion defect
table S4. Timing and degree of AC invasion into the vulval epithelium: mutant analysis and off-target RNAi controls
table S5. Timing and degree of AC invasion into the vulval epithelium: uterine-specific RNAi
table S6. Extrachromosomal array and integrated strain generation
table S7. Primer sequences and templates used for PCR fusions and restriction enzyme cloning
Summary.
Regulators of basement membrane transmigration are conserved between C. elegans development and human cancer cell invasion.
Acknowledgments
We are grateful to P. Sternberg in whose laboratory this study was initiated, E. Cram for cacn-1(tm3042) and cacn-1(tm3082), A. Puoti for the mep-1::GFP strain, I. Hope for the F28F8.5::GFP strain, N. Poulson for the cdc-37::GFP strain, F. Mason for T03F1.8::GFP strain, A. Hajnal for egl-43L primers, C. Hu for help with the CAM assay, J. Ziel for help with statistical analyses, the Caenorhabditis Genetics Center for providing additional strains, and E. Hagedorn, M. Yang, J. Ziel, N. Matus, and D. Killebrew for comments on the manuscript.
Funding: D.Q.M. is a Robert Black Fellow of the Damon Runyon Cancer Research Foundation (DRG-1949-07). This work was supported by NIH Grant CA088308 and the Breast Cancer Research Foundation to S.J.W., and a Basil O’Connor Award, Pew Scholars Award and NIH Grants GM079320 and K01 CA098316-01 to D.R.S.
Footnotes
Author contributions: D.Q.M., S.D., S.J.W., and D.R.S. participated in the experimental design; D.Q.M., X.L., S.D., D.A., Q.C., and D.R.S. participated in data acquisition and analysis; D.Q.M., S.J.W., and D.R.S. wrote the manuscript.
References and Notes
- 1.Rowe RG, Weiss SJ. Breaching the basement membrane: who, when and how? Trends Cell Biol. 2008 Nov;18:560–574. doi: 10.1016/j.tcb.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Duc-Goiran P, Mignot TM, Bourgeois C, Ferre F. Embryo-maternal interactions at the implantation site: a delicate equilibrium. Eur J Obstet Gynecol Reprod Biol. 1999 Mar;83:85–100. doi: 10.1016/s0301-2115(98)00310-8. [DOI] [PubMed] [Google Scholar]
- 3.Yadav R, Larbi KY, Young RE, Nourshargh S. Migration of leukocytes through the vessel wall and beyond. Thromb Haemost. 2003 Oct;90:598–606. doi: 10.1160/TH03-04-0220. [DOI] [PubMed] [Google Scholar]
- 4.Stratton MR, Campbell PJ, Futreal PA. The cancer genome. Nature. 2009 Apr 9;458:719–724. doi: 10.1038/nature07943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta GP, Massague J. Cancer metastasis: building a framework. Cell. 2006 Nov 17;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Podbilewicz B. How does a cell anchor and invade an organ? Developmental cell. 2003 Jul;5:5–7. doi: 10.1016/s1534-5807(03)00202-8. [DOI] [PubMed] [Google Scholar]
- 7.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000 Jan 7;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 8.Srivastava A, Pastor-Pareja JC, Igaki T, Pagliarini R, Xu T. Basement membrane remodeling is essential for Drosophila disc eversion and tumor invasion. Proceedings of the National Academy of Sciences of the United States of America. 2007 Feb 20;104:2721–2726. doi: 10.1073/pnas.0611666104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Even-Ram S, Yamada KM. Cell migration in 3D matrix. Curr Opin Cell Biol. 2005 Oct;17:524–532. doi: 10.1016/j.ceb.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 10.Sabeh F, Shimizu-Hirota R, Weiss SJ. Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. The Journal of cell biology. 2009 Apr 6;185:11–19. doi: 10.1083/jcb.200807195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sahai E. Illuminating the metastatic process. Nat Rev Cancer. 2007 Oct;7:737–749. doi: 10.1038/nrc2229. [DOI] [PubMed] [Google Scholar]
- 12.Sherwood DR, Sternberg PW. Anchor cell invasion into the vulval epithelium in C. elegans. Developmental cell. 2003 Jul;5:21–31. doi: 10.1016/s1534-5807(03)00168-0. [DOI] [PubMed] [Google Scholar]
- 13.Sherwood DR, Butler JA, Kramer JM, Sternberg PW. FOS-1 promotes basement-membrane removal during anchor-cell invasion in C. elegans. Cell. 2005 Jun 17;121:951–962. doi: 10.1016/j.cell.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 14.Ziel JW, Hagedorn EJ, Audhya A, Sherwood DR. UNC-6 (netrin) orients the invasive membrane of the anchor cell in C. elegans. Nat Cell Biol. 2009 Feb;11:183–189. doi: 10.1038/ncb1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagedorn EJ, Yashiro H, Ziel JW, Ihara S, Wang Z, Sherwood DR. Integrin acts upstream of netrin signaling to regulate formation of the anchor cell’s invasive membrane in C. elegans. Developmental cell. 2009 Aug;17:187–198. doi: 10.1016/j.devcel.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang BJ, Meruelo AD, Sternberg PW. C. elegans EVI1 proto-oncogene, EGL-43, is necessary for Notch-mediated cell fate specification and regulates cell invasion. Development (Cambridge, England) 2007 Feb;134:669–679. doi: 10.1242/dev.02769. [DOI] [PubMed] [Google Scholar]
- 17.Rimann I, Hajnal A. Regulation of anchor cell invasion and uterine cell fates by the egl-43 Evi-1 proto-oncogene in Caenorhabditis elegans. Developmental biology. 2007 Aug 1;308:187–195. doi: 10.1016/j.ydbio.2007.05.023. [DOI] [PubMed] [Google Scholar]
- 18.Simmer F, Moorman C, van der Linden AM, Kuijk E, van den Berghe PV, Kamath RS, Fraser AG, Ahringer J, Plasterk RH. Genome-wide RNAi of C. elegans using the hypersensitive rrf-3 strain reveals novel gene functions. PLoS biology. 2003 Oct;1:E12. doi: 10.1371/journal.pbio.0000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamath RS, Fraser AG, Dong Y, Poulin G, Durbin R, Gotta M, Kanapin A, Le Bot N, Moreno S, Sohrmann M, Welchman DP, Zipperlen P, Ahringer J. Systematic functional analysis of the Caenorhabditis elegans genome using RNAi. Nature. 2003 Jan 16;421:231–237. doi: 10.1038/nature01278. [DOI] [PubMed] [Google Scholar]
- 20.Rual JF, Ceron J, Koreth J, Hao T, Nicot AS, Hirozane-Kishikawa T, Vandenhaute J, Orkin SH, Hill DE, van den Heuvel S, Vidal M. Toward improving Caenorhabditis elegans phenome mapping with an ORFeome-based RNAi library. Genome Res. 2004 Oct;14:2162–2168. doi: 10.1101/gr.2505604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGarry LC, Winnie JN, Ozanne BW. Invasion of v-Fos(FBR)-transformed cells is dependent upon histone deacetylase activity and suppression of histone deacetylase regulated genes. Oncogene. 2004 Jul 8;23:5284–5292. doi: 10.1038/sj.onc.1207687. [DOI] [PubMed] [Google Scholar]
- 22.Zhang T, Hamza A, Cao X, Wang B, Yu S, Zhan CG, Sun D. A novel Hsp90 inhibitor to disrupt Hsp90/Cdc37 complex against pancreatic cancer cells. Mol Cancer Ther. 2008 Jan;7:162–170. doi: 10.1158/1535-7163.MCT-07-0484. [DOI] [PubMed] [Google Scholar]
- 23.Naito Y, Yamada T, Matsumiya T, Ui-Tei K, Saigo K, Morishita S. dsCheck: highly sensitive off-target search software for double-stranded RNA-mediated RNA interference. Nucleic Acids Res. 2005 Jul 1;33:W589–591. doi: 10.1093/nar/gki419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang C, Newman AP, Sternberg PW. Reciprocal EGF signaling back to the uterus from the induced C. elegans vulva coordinates morphogenesis of epithelia. Curr Biol. 1999 Mar 11;9:237–246. doi: 10.1016/s0960-9822(99)80112-2. [DOI] [PubMed] [Google Scholar]
- 25.Burdine RD, Branda CS, Stern MJ. EGL-17(FGF) expression coordinates the attraction of the migrating sex myoblasts with vulval induction in C. elegans. Development (Cambridge, England) 1998 Mar;125:1083–1093. doi: 10.1242/dev.125.6.1083. [DOI] [PubMed] [Google Scholar]
- 26.Grantham J, Brackley KI, Willison KR. Substantial CCT activity is required for cell cycle progression and cytoskeletal organization in mammalian cells. Exp Cell Res. 2006 Jul 15;312:2309–2324. doi: 10.1016/j.yexcr.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Yuan Z, Zhang Y, Yong S, Salas-Burgos A, Koomen J, Olashaw N, Parsons JT, Yang XJ, Dent SR, Yao TP, Lane WS, Seto E. HDAC6 modulates cell motility by altering the acetylation level of cortactin. Molecular cell. 2007 Jul 20;27:197–213. doi: 10.1016/j.molcel.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasegawa H, Senga T, Ito S, Iwamoto T, Hamaguchi M. A role for AP-1 in matrix metalloproteinase production and invadopodia formation of v-Crk-transformed cells. Exp Cell Res. 2009 May 1;315:1384–1392. doi: 10.1016/j.yexcr.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 29.Mitani K. Molecular mechanisms of leukemogenesis by AML1/EVI-1. Oncogene. 2004 May 24;23:4263–4269. doi: 10.1038/sj.onc.1207777. [DOI] [PubMed] [Google Scholar]
- 30.Rodrigues S, De Wever O, Bruyneel E, Rooney RJ, Gespach C. Opposing roles of netrin-1 and the dependence receptor DCC in cancer cell invasion, tumor growth and metastasis. Oncogene. 2007 Aug 16;26:5615–5625. doi: 10.1038/sj.onc.1210347. [DOI] [PubMed] [Google Scholar]
- 31.White DE, Muller WJ. Multifaceted roles of integrins in breast cancer metastasis. Journal of mammary gland biology and neoplasia. 2007 Sep;12:135–142. doi: 10.1007/s10911-007-9045-5. [DOI] [PubMed] [Google Scholar]
- 32.Drummond DC, Noble CO, Kirpotin DB, Guo Z, Scott GK, Benz CC. Clinical development of histone deacetylase inhibitors as anticancer agents. Annual review of pharmacology and toxicology. 2005;45:495–528. doi: 10.1146/annurev.pharmtox.45.120403.095825. [DOI] [PubMed] [Google Scholar]
- 33.Gray PJ, Jr, Prince T, Cheng J, Stevenson MA, Calderwood SK. Targeting the oncogene and kinome chaperone CDC37. Nat Rev Cancer. 2008 Jul;8:491–495. doi: 10.1038/nrc2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ota I, Li XY, Hu Y, Weiss SJ. Induction of a MT1-MMP and MT2-MMP-dependent basement membrane transmigration program in cancer cells by Snail1. Proceedings of the National Academy of Sciences of the United States of America. 2009 Dec 1;106:20318–20323. doi: 10.1073/pnas.0910962106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rocheleau CE, Yasuda J, Shin TH, Lin R, Sawa H, Okano H, Priess JR, Davis RJ, Mello CC. WRM-1 activates the LIT-1 protein kinase to transduce anterior/posterior polarity signals in C. elegans. Cell. 1999 Jun 11;97:717–726. doi: 10.1016/s0092-8674(00)80784-9. [DOI] [PubMed] [Google Scholar]
- 36.Aouacheria A, Geourjon C, Aghajari N, Navratil V, Deleage G, Lethias C, Exposito JY. Insights into early extracellular matrix evolution: spongin short chain collagen-related proteins are homologous to basement membrane type IV collagens and form a novel family widely distributed in invertebrates. Mol Biol Evol. 2006 Dec;23:2288–2302. doi: 10.1093/molbev/msl100. [DOI] [PubMed] [Google Scholar]
- 37.Rowe RG, Li XY, Hu Y, Saunders TL, Virtanen I, Garcia de Herreros A, Becker KF, Ingvarsen S, Engelholm LH, Bommer GT, Fearon ER, Weiss SJ. Mesenchymal cells reactivate Snail1 expression to drive three-dimensional invasion programs. The Journal of cell biology. 2009 Feb 9;184:399–408. doi: 10.1083/jcb.200810113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974 May;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sabeh F, Ota I, Holmbeck K, Birkedal-Hansen H, Soloway P, Balbin M, Lopez-Otin C, Shapiro S, Inada M, Krane S, Allen E, Chung D, Weiss SJ. Tumor cell traffic through the extracellular matrix is controlled by the membrane-anchored collagenase MT1-MMP. The Journal of cell biology. 2004 Nov 22;167:769–781. doi: 10.1083/jcb.200408028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Putative null alleles of mep-1, hda-1, cct-6, C48A7.2, and hbl-1 show AC invasion defects.
Fig. S2. LAM-1::GFP (laminin) is intact following RNAi targeting the cct complex and 11 other pro-invasive genes at the P6.p 4-cell stage of VPC division
Fig. S3. Transgene reporter localization of newly identified pro-invasive genes at the P6.p 2-cell stage of VPC division
Fig. S4. Transgene reporter localization of newly identified pro-invasive genes at the P6.p 4-cell stage of VPC division
Fig. S5. hbl-1 functions in VPC specification to promote AC invasion
Fig. S6. The AC is correctly specified, as shown by lin-3>GFP, following RNAi depletion of the newly identified regulators of AC invasion
Fig. S7. 1° VPC specification following RNAi depletion of newly identified AC invasion genes
Fig. S8. Identification of regulators of invasive membrane formation
Fig. S9. Regulators of invasive membrane formation function independently of netrin receptor localization
Fig. S10 Regulators of invasive membrane formation function independently of integrin receptor localization
Fig. S11. Identification of regulators of the fos-1a pathway
Fig. S12. Identification of genes that act within or parallel to the fos-1a pathway
Fig. S13. fos-1a RNAi depletion identifies lit-1 as a new member of the fos-1a pathway
Fig. S14. lit-1 functions downstream of fos-1a and egl-43L during AC invasion
Fig. S15. CCT5 and NLK siRNA-mediated knockdown specifically affect BM transmigration activity
table S1. 539 Pvl and Egl genes targeted by RNAi for AC invasion defects
table S2. Identification of 99 regulators of AC invasion
table S3. RNAi depletion of 99 genes results in an AC invasion defect
table S4. Timing and degree of AC invasion into the vulval epithelium: mutant analysis and off-target RNAi controls
table S5. Timing and degree of AC invasion into the vulval epithelium: uterine-specific RNAi
table S6. Extrachromosomal array and integrated strain generation
table S7. Primer sequences and templates used for PCR fusions and restriction enzyme cloning