Abstract
Background & Aims
Progressive familial intrahepatic cholestasis (PFIC) with normal serum levels of gamma-glutamyltranspeptidase can result from mutations in ATP8B1 (encoding familial intrahepatic cholestasis 1 [FIC1]) or ABCB11 (encoding bile salt export pump [BSEP]). We evaluated clinical and laboratory features of disease in patients diagnosed with PFIC, who carried mutations in ATP8B1 (FIC1 deficiency) or ABCB11 (BSEP deficiency). Our goal was to identify features that distinguish presentation and course of these 2 disorders, thus facilitating diagnosis and elucidating the differing consequences of ATP8B1 and ABCB11 mutations.
Methods
A retrospective multi-center study was conducted, using questionnaires and chart review. Available clinical and biochemical data from 145 PFIC patients with mutations in either ATP8B1 (61 “FIC1 patients”) or ABCB11 (84 “BSEP patients”) were evaluated.
Results
At presentation, serum aminotransferase and bile salt levels were higher in BSEP patients; serum alkaline phosphatase values were higher, and serum albumin values were lower, in FIC1 patients. Elevated white blood cell counts, and giant or multinucleate cells at liver biopsy, were more common in BSEP patients. BSEP patients more often had gallstones and portal hypertension. Diarrhea, pancreatic disease, rickets, pneumonia, abnormal sweat tests, hearing impairment, and poor growth were more common in FIC1 patients. Among BSEP patients, the course of disease was less rapidly progressive in patients bearing the D482G mutation.
Conclusions
Severe forms of FIC1 and BSEP deficiency differed. BSEP patients manifested more severe hepatobiliary disease, while FIC1 patients showed greater evidence of extrahepatic disease.
Keywords: cholestasis, genetics, transport protein, pediatrics, P-type ATPase, ATP binding cassette protein, ATP8B1, FIC1, ABCB11, BSEP
INTRODUCTION
Mutations in ATP8B1 or ABCB11 can result in hereditary cholestasis [1–3]. ATP8B1 encodes FIC1 (familial intrahepatic cholestasis 1), a widely expressed membrane P-type ATPase [1]. FIC1 may function as an aminophospholipid flippase, transferring phosphatidylserine from the outer to the inner leaflet of the plasma membrane [4–5]. ABCB11, expressed only in the liver, encodes the bile salt export pump (BSEP), responsible for the bulk of conjugated bile acid (BA) transport from hepatocytes into biliary canaliculi [6–9]. A spectrum of disease severity is seen in both FIC1 and BSEP deficiencies [10–12]. Severe FIC1 or BSEP deficiency is termed ‘low-γGT’ progressive familial intrahepatic cholestasis (low-γGT PFIC), as gamma-glutamyl transpeptidase (γGT) activity in serum typically remains within normal ranges and is low relative to the degree of cholestasis.
Before identification of ATP8B1 and ABCB11, low-γGT PFIC was considered a single disease entity [13–14]. Genetic analysis now permits FIC1 and BSEP deficiencies to be studied individually. Case series have suggested differences between the 2 phenotypes; however, these featured modest numbers of patients, often not distinguished genetically [15–23]. To perform a comprehensive evaluation of genotype-phenotype correlations in these 2 diseases, we assembled a cohort of 145 patients with low-γGT PFIC in whom mutational analysis of ATP8B1 and ABCB11 established the diagnosis of FIC1 deficiency (FIC1 patients, N = 61) or BSEP deficiency (BSEP patients, N = 84).
Here we report findings at presentation and during the course of disease before surgical intervention (partial external biliary diversion [PEBD], ileal exclusion [IE], and/or orthotopic liver transplantation [OLT]). We report differences in symptoms and laboratory findings that distinguish severe FIC1 deficiency from severe BSEP deficiency. Our results also indicate that while BSEP deficiency is primarily a liver disease, FIC1 deficiency is a multisystem disorder.
PATIENTS AND METHODS
Patients were enrolled through centers with study protocols approved by the appropriate committees, including University of California, San Francisco, CA, USA; King’s College Hospital, London, UK; Children’s Memorial Health Institute, Warsaw, Poland; Children’s Memorial Hospital, Chicago, IL, USA; Université Catholique de Louvain and Cliniques St Luc, Louvain, Belgium; and Karolinska University Hospital, Huddinge, Sweden. Informed consent was obtained from patients, parents, or guardians.
Patients were enrolled from multiple sites in North America (48 patients), Europe (91 patients, including 32 from Poland), and the Middle East (6 patients). Partial phenotypic descriptions of some patients are published [12, 15, 17–18, 21–22, 24–37]. Subjects carried a clinical diagnosis of low-γGT PFIC and had mutation(s) in ATP8B1 or ABCB11 [1–2, 10, 12, 15, 18, 22, 30, 33, 38]. Clinical and biochemical data were collected using a questionnaire and by chart review. Enrollment was closed in 2004. We analyzed findings at presentation and during the course of disease until surgical intervention. Evidence of hearing impairment and sweat test results were reported regardless of surgery.
Data collection was retrospective; not all requested data were available for every participant. For each variable, the number of subjects (‘N’) from whom data were available is indicated. For clinical chemistry test results at presentation, the earliest available measurement within the 1st year of life was used. Median value per patient per age bin was used for evaluation of transaminases and ALP over pre-surgical course of disease. Most continuous variables were transformed into multiples (fold) of upper or lower limit of normal range (FULN or FLLN) to adjust for differences in reference ranges among sites. Serum bilirubin values were converted to mg/dl without normalizing. Serum alkaline phosphatase (ALP) values were not normalized, as age-specific normal ranges were not available for all centers; absolute values in International Units (IU) per liter were used. One center reported ALP values as microkat/L; these values were multiplied by 59 to convert to IU/L. White blood cell count (WBC) and platelet levels were grouped into 3 categories: low, normal, or high. Birth-weights were normalized to the sex-specific median for full-term births. Height and weight were evaluated based on the latest data during the study period.
To normalize distributions, most continuous variables were log-transformed before analysis. Analyses were performed using Statistical Analysis System version 9 (SAS Institute, Cary, NC, USA) or Graphpad Prism and QuickCalcs (Graphpad Software, Inc, La Jolla, CA, USA). Continuous dependent variables were analyzed using linear regression. Where non-normal distribution of residuals was encountered, the bias-corrected accelerated bootstrapping method was used to obtain valid confidence intervals, with p-values defined as one minus the highest confidence level for which the interval excluded an effect of zero [39]. The Wilcoxon signed-rank test was used for comparison of birth-weight distribution to normal expectation. For comparison of transaminase and ALP values in different age ranges between FIC1 and BSEP deficiencies, the t-test was used. For categorical variables, logistic regression or Fisher’s Exact test was employed.
We report nominal p-values without adjustment for multiple testing. Given the biological relationships among the parameters examined, coherent sets of findings often reinforce rather than detract from one another, making such adjustment unhelpful [40–43].
We tested the robustness to potential confounding factors of findings with small p-values using bivariate and multivariate analyses (where computationally feasible), factoring in sex, year of birth, and presence of older affected siblings.
The primary comparison was between FIC1 and BSEP deficiency. We also performed exploratory analyses to evaluate whether BSEP patients carrying 1 or 2 copies of the common European D482G (c.1445A>G) and/or E297G (c.890A>G) mutations differed from other BSEP patients, and whether FIC1 patients carrying G308V differed from other FIC1 patients.
For clinical findings, we used a 3-way categorization of ‘FIC1’ versus ‘D482G BSEP’ (BSEP patients carrying D482G on one or both of their ABCB11 alleles) versus ‘non-D482G BSEP’ (BSEP patients not carrying D482G). Since biochemical data were available for relatively few D482G-bearing patients, 3-way analyses were not performed, although in some figures the 2 BSEP subgroups are shown separately for qualitative comparison. The D482G and non-D482G BSEP subgroups do not always sum to the total N for ‘all BSEP’, because we could not sub-classify 3 BSEP patients in whom only one mutation was detected and presence of D482G on the 2nd allele was not ruled out; they were excluded from this analysis.
To build a model predictive of FIC1 versus BSEP deficiency, we performed univariate and multivariate logistic regression. Only variables for which values were available for a substantial number of patients were tested. Given the high correlation (0.78) between AST and ALT measured in the same patient on the same date, we defined the variable sAT (serum aminotransferase) as the FULN for ALT, or for AST, if ALT was not available. Stepwise logistic regression was used to build a predictive model, with the p-value cutoff for entry selected by cross-validation [39]. All cutoffs ≤0.02 resulted in the same model, with sAT as the only predictor (p <0.0001). Cross-validation suggested that this cutoff produced the best prediction.
We analyzed transplant-free survival, surgery-free survival, and age at report of cirrhosis using Kaplan-Meier curves and Cox proportional hazards models. For age of cirrhosis, patients were censored at age of surgery or age of death, if free of cirrhosis at that time.
RESULTS
Genetic and demographic features
Sixty-one patients carried mutation(s) in ATP8B1, and 84 patients carried mutation(s) in ABCB11 (Supplementary Table 1). One of 2 ‘common’ ABCB11 mutations (D482G or E297G) was identified on one or both alleles in 51 BSEP patients (61%) [2, 12]. Demographic features (Table 1) of the FIC1 and BSEP deficiency cohorts were similar. Complete or partial clinical data were available for all 145 patients, and laboratory results, for 140 patients. Clinical data at presentation were available for 139 patients; laboratory results in the 1st year of life were available for 74 patients (32 FIC1, 42 BSEP).
Table 1.
Demographic features of the study cohort of 145 patients.
| Feature | ATP8B1 (FIC1) | ABCB11 (BSEP) | p* |
|---|---|---|---|
| Year of birth: median (full range) | 1994 (1976–2002) | 1993 (1969–2003) | 0.75 |
| Female (%) (tally) | 64% (39/61) | 49% (41/84) | 0.072 |
| % with older affected sibling (tally) | 25% (15/61) | 15% (13/84) | 0.17 |
| Data collected until Age (years): median (interquartile range) | 4.4 (1.7–9.2) | 3.6 (2.0–8.9) | 0.91 |
Linear and logistic regression were used, and Mann-Whitney test for age due to non-normality . ‘Age’ is the age at first surgical intervention (PBD, OLT or IE), or in the absence of surgery, the age at last follow-up.
Since functional studies have suggested that the BSEP D482G and E297G mutations may not completely abolish protein function [9, 44–47], we performed exploratory analyses stratified by mutation. Our results suggested that phenotypic differences exist between BSEP patients with and without D482G (data not shown); those differences that attained statistical significance are discussed below.
Clinical features at presentation
Birth
Four FIC1 and 2 BSEP patients were from twin pregnancies. Four of 46 singleton pregnancies (8.7%) of FIC1 patients and 3 of 78 singleton pregnancies (3.8%) of BSEP patients were delivered prematurely (<36 weeks gestation). Normalized, sex-specific birth-weights of full-term FIC1 and BSEP patients from singleton births were similar (Table 2). For all patient groups, birth-weights trended below expectation; this only reached statistical significance in non-D482G BSEP patients (p <0.0003, 95% CI 0.88–0.96).
Table 2.
Presentation symptoms.
| Feature | FIC1 | BSEP |
p* FIC1 vs All BSEP |
||
|---|---|---|---|---|---|
| All BSEP | D482G |
non D482G |
|||
|
Birthweight#, median (interquartile range) |
0.97 (0.88–1.09) N=42 |
0.93 (0.87–1.02) N= 70 |
0.95 (0.89–1.05) N=19 |
0.93 (0.87–1.00) N=48 |
0.23 |
|
Age (months) at Onset, median (interquartile range) |
2.0 (0.5–2.0) N=53 |
2.0 (1.0–4.5) N=79 |
3.0 (1.3 –5.0) N=18 |
1.8 (0.4–4.0) N=58 |
0.059 |
|
Symptoms initially intermittent |
25/56 (45%) |
46/81 (57%) |
13/21 (62%) |
30/57 (53%) |
0.17* |
| Jaundice | 42/54 (78%) |
57/82 (70%) |
10/21 (48%) |
45/58 (78%) |
0.29 |
| Diarrhea | 11/54 (20%) |
5/82 (6%) |
2/21 (10%) |
3/58 (5%) |
0.017 |
|
Manifestations of vitamin deficiency |
2/54 (4%) |
10/82 (12%) |
4/21 (19%) |
6/58 (10%) |
0.11 |
Linear and logistic regression, or Fisher Exact test(*), were used..
Birthweight divided by sex-specific 50th percentile.
Bolded entries in the D482G column indicate that results for D482G BSEP patients differ from one or both other groups (as described in the text).
Onset
One BSEP patient had a reported age of onset (66 months) much later than all other patients (all ≤12 months) and was excluded from analysis of onset age [18, 25]. Onset by 3 months was reported in most patients (FIC1: 85%; D482G BSEP: 67%; non-D482G BSEP: 71%). Median age at onset was similar in FIC1 and BSEP deficiency, although there was a trend (Table 2) toward earlier reported onset in FIC1 deficiency (onset 0.85 months earlier, 95% CI = −1.65 to +0.02 months).
Symptoms reported at presentation
Symptoms were intermittent early in disease course in 52% (71/137) of patients. Jaundice was the most common presentation symptom (73% of patients). Jaundice was less frequent in patients bearing BSEP D482G (48%) than in patients carrying other BSEP mutations (78%; p = 0.013) or in FIC1 patients (78%; p = 0.014) (Table 2). Diarrhea was more frequent in FIC1 than in BSEP patients (Table 2; OR = 3.9, 95% CI = 1.3–12.1). Twelve patients (9%; 2 FIC1 and 10 BSEP) presented with clinical manifestations of vitamin deficiency, including coagulopathy (10 patients), rickets (2 patients), and/or seizures (3 patients). Clinically manifest vitamin deficiency was more common in BSEP patients bearing D482G than in FIC1 patients (p = 0.047). Nine of these 12 children were male (p = 0.035, male versus female, OR = 4.3, 95% CI = 1.11–16.6). Seven of the twelve (3 D482G BSEP patients, 3 other BSEP patients, and 1 FIC1 patient) presenting with manifestations of vitamin deficiency were not clinically jaundiced.
Laboratory studies at presentation
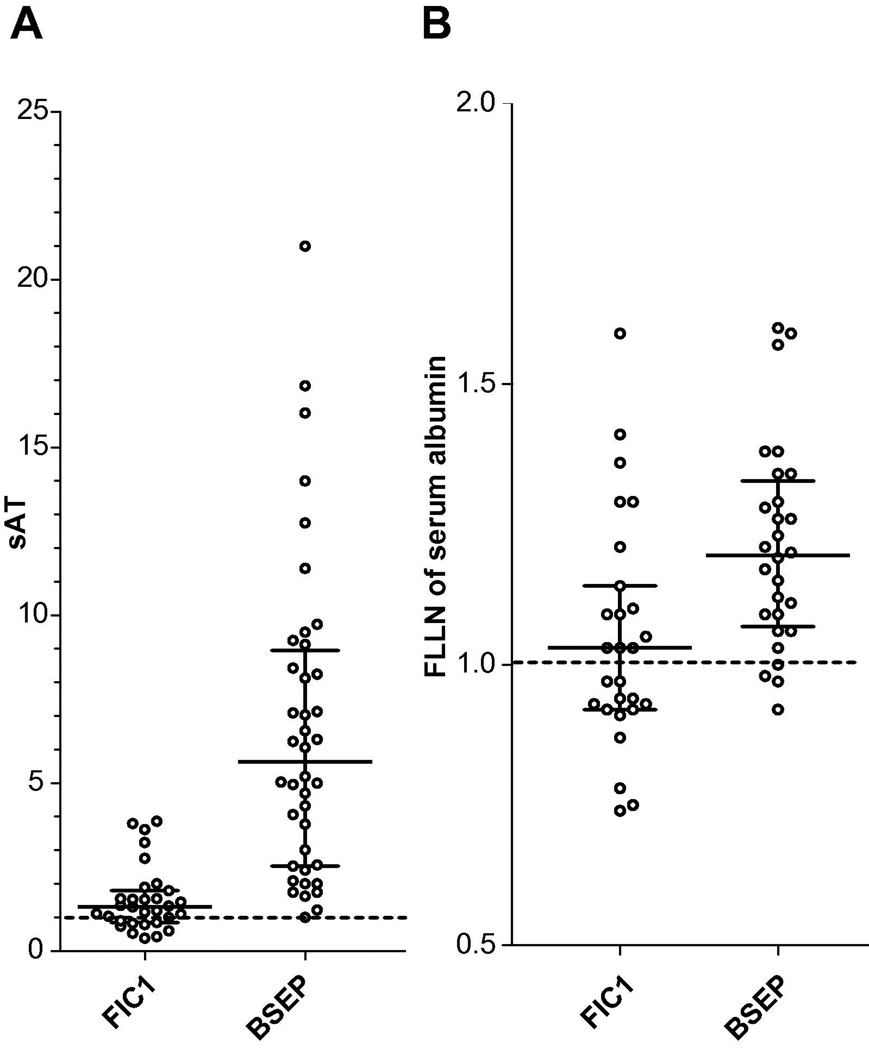
To assess biochemical features of disease at presentation, we analyzed the earliest reported value for each patient within the 1st year of life. Serum AST and ALT activities were significantly higher in BSEP deficiency than in FIC1 deficiency (Table 3, Fig. 1A); AST was estimated to be 3.7-fold higher (95% CI 2.5–5.6), and ALT 3.8-fold higher (95% CI 2.7–5.6). Six (22%) FIC1 patients, but only 1 BSEP patient, had normal AST (p = 0.11, Fisher’s Exact test). A different BSEP patient had normal ALT; 30% of FIC1 patients did (p = 0.005, Fisher’s Exact test). ALP values were 66% higher (95% CI 9.9–151%) in FIC1 than BSEP patients (Table 3). Serum albumin levels were 14% lower (95% CI 6.0–21%) in FIC1 patients than in BSEP patients (Table 3); approximately half of the FIC1 patients had abnormally low serum albumin levels while few BSEP patients did (Fig. 1B).
Table 3.
Laboratory Studies at Presentation.
| Serum assay | FIC1 | BSEP | p |
|---|---|---|---|
|
AST (FULN) |
1.40 (1.11–2.19) N=27 |
5.33 (4.06–10.50) N=29 |
<0.0001 |
|
ALT (FULN) |
1.25 (0.85–1.80) N=30 |
5.63 (2.41–9.13) N=34 |
<0.0001 |
|
ALP (IU/l) |
851 (496–1506) N=30 |
482 (298–1106) N=36 |
0.017 |
|
γGT (FULN) |
0.55 (0.37–0.74) N=28 |
0.42 (0.28–0.70) N=39 |
0.26 |
|
Albumin (FLLN) |
1.03 (0.92–1.14) N=27 |
1.19 (1.07–1.31) N=28 |
0.0014 |
|
Total Protein (FLLN) |
1.19 (0.96–1.30) N=19 |
1.11 (1.01–1.22) N=16 |
0.74 |
|
Bilirubin, total (mg/dl) |
6.48 (3.53–15.10) N=32 |
6.20 (4.71–7.70) N=40 |
0.10 |
|
Bilirubin, direct (mg/dl) |
3.35 (1.94–11.15) N=28 |
4.60 (2.94 –6.01) N=27 |
0.33 |
|
Hemoglobin (FLLN) |
1.06 (0.97–1.10) N=22 |
1.00 (0.93–1.06) N=26 |
0.41 |
|
Hematocrit (FLLN) |
0.97 (0.79–1.06) N=21 |
0.93 (0.88–1.07) N=12 |
0.59 |
|
WBC above normal (% of patients) |
33% N=24 |
78% N=18 |
0.0064 |
|
Platelets above normal (% of patients) |
75% N=24 |
58% N=26 |
0.20 |
|
Prothrombin time (FULN) |
1.00 (0.81–1.16) N=19 |
0.81 (0.77–1.09) N=15 |
0.89 |
|
Cholesterol (FULN) |
0.81 (0.69–0.98) N=19 |
1.08 (0.81–1.20) N=33 |
0.059 |
|
Triglycerides (FULN) |
1.40 (1.11–1.85) N=13 |
1.47 (1.10–2.32) N=22 |
0.77 |
|
SBA (FULN) |
17.06 (9.00–25.17) N=13 |
31.55 (19.71–49.43)N=23 |
0.037 |
Results are from earliest available measurement, within the first year of life. For continuous variables, medians, interquartile ranges, and sample sizes are shown. Linear regression was used to obtain p-values, along with the estimated fold- or percentage differences reported in the text. For WBC and platelets, percent of patients is shown.
Figure 1. The distribution of biochemical data at presentation. Medians and interquartile ranges are shown.
A: Serum transaminase values shown as sAT: defined as FULN of ALT if available, otherwise FULN of AST (FULN, fold of the upper limit of the normal range). For FIC1 deficiency, N = 31; for BSEP deficiency, N = 40. B: Serum albumin values (FLLN, fold lower limit of normal range). For FIC1 deficiency, N = 27; for BSEP deficiency, N = 28. The horizontal dashed line marks the upper (A) or lower (B) limit of normal.
Elevated WBC counts were more common in BSEP patients than in FIC1 patients (OR = 7.1, 95% CI = 1.72 to 25). No patient had a WBC count below the normal range. Serum cholesterol values trended higher in BSEP patients (Table 3, estimated 20% higher in BSEP deficiency, 95% CI 47% higher to 1% lower). While 58% of BSEP patients had elevated cholesterol values, only 21% of FIC1 patients did (p = 0.019, Fisher exact test). Serum BA values were elevated in all patients, the lowest recorded value being 5-fold elevated. Values for sBA in FIC1 deficiency were estimated as only 50% (95% CI 4–70%) of those in BSEP deficiency.
Liver biopsy results were available for 62 patients in the 1st year of life. Giant or multinucleate cells were reported in 24/33 (73%) BSEP patients and 2/29 (7%) FIC1 patients (p <0.0001). Two of six (33%) BSEP patients with D482G and 22/27 (81%) without D482G were reported to have giant cells (p = 0.034).
Distinguishing FIC1 from BSEP deficiency at presentation
Rapid and early differential diagnosis between FIC1 and BSEP disease would be facilitated by development of a diagnostic model valid at presentation. We used logistic regression to identify which laboratory variables help to distinguish FIC1 from BSEP deficiency at presentation. The strongest predictive effects were seen with ALT (p <0.0001; AROC 0.91) and AST (p <0.0001; AROC 0.89). Significant but weaker predictive effects were observed with 3 other serum analytes: albumin (p = 0.0084; AROC 0.74), sBA (p = 0.024; AROC 0.77), and ALP (p = 0.024; AROC 0.66) values.
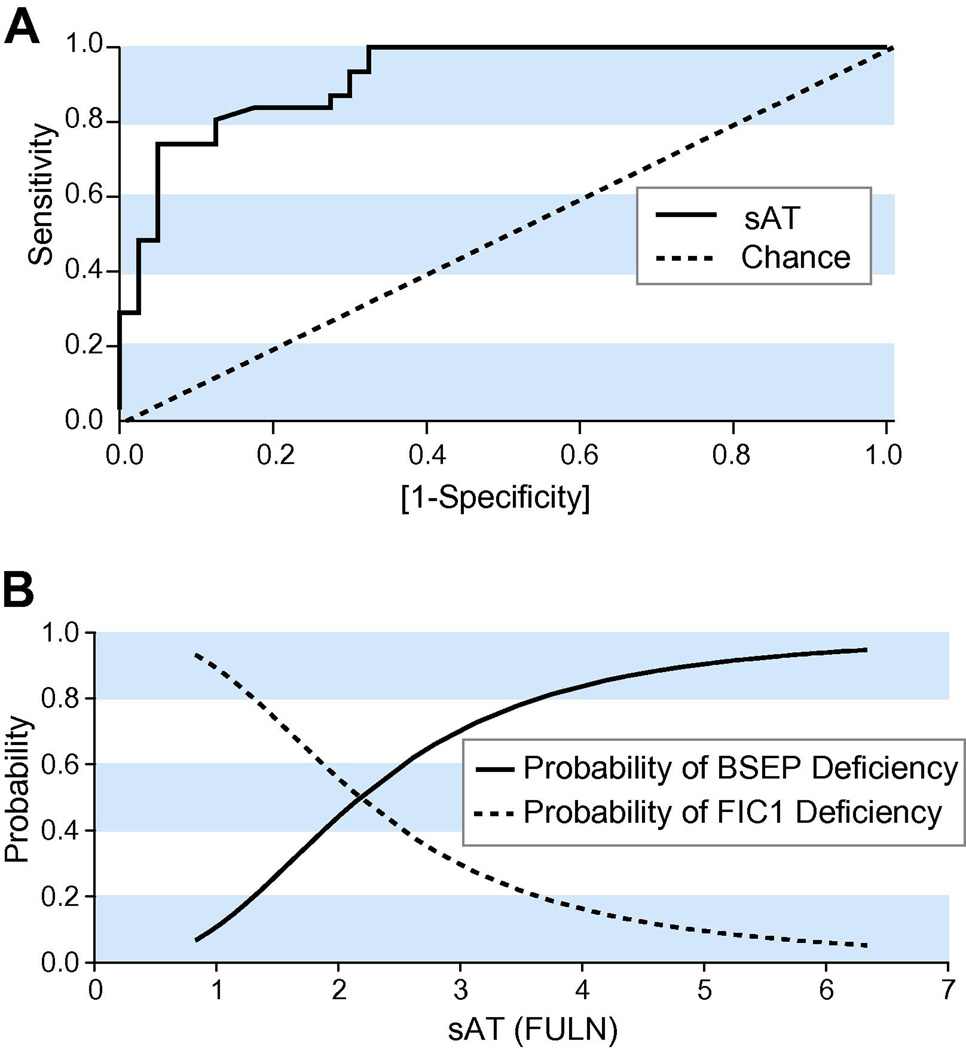
Given that some centers report either AST or ALT, and that AST and ALT are highly correlated, we created a diagnostic model using sAT, defined as FULN of ALT, or of AST, if ALT was not available. The sAT-based model had an AROC of 0.92 and p <0.0001 for FIC1 vs. BSEP deficiency (Fig. 2A). The ‘break-even point,’ where a patient is equally likely to have FIC1 or BSEP deficiency, is at sAT = 2.2 (95% CI 1.7–2.9) (Fig. 2B). To optimize the model we then evaluated, by cross-validation, the effect of addition of other variables, including albumin, sBA, ALP, and the best distinguishing clinical feature (diarrhea). Since addition of these variables did not improve cross-validated disease prediction in this dataset, there appeared to be no benefit to incorporating them into the model based only on sAT.
Figure 2. Discriminating FIC1 and BSEP deficiencies.
A: ROC curve for prediction of FIC1 versus BSEP deficiency. The curves for sAT (serum aminotransferase activity, expressed as FULN) versus chance are shown. B: Probability of FIC1 versus BSEP deficiency. The predicted probability of FIC1 versus BSEP deficiency is obtained from 1/(1+e[1.88×log2SAT−2.11]). All values of sAT <2 collectively confer a sensitivity of 81% (95% CI of 63–93%) and specificity of 88% (95% CI of 73–96%) for FIC1 deficiency. Similarly, all values of sAT >2 collectively have sensitivity of 88% (95% CI of 73–96%) and specificity of 81% (95% CI of 63–93%) for BSEP deficiency.
Disease Course
Hepatobiliary disease (Table 4)
Table 4.
Features of disease course.
| Feature | FIC1 | BSEP |
p FIC1 vs All BSEP |
||
|---|---|---|---|---|---|
| All BSEP | D482G | non D482G |
|||
| Gallstones | 0/61 (0%) |
27/84 (32%) |
8/21 (38%) |
17/60 (28%) |
< 0.0001 |
| HCC | 0/61 (0%) |
4/84 (5%) |
0/21 (0%) |
4/60 (7%) |
0.14 |
|
Portal Hypertension |
15/61 (25%) |
30/84 (36%) |
3/21 (14%) |
27/60 (45%) |
0.20 |
| Diarrhea | 37/61 (61%) |
17/84 (20%) |
4/21 (19%) |
13/60 (22%) |
<0.0001 |
|
Pancreatic Disease |
7/61 (12%) |
1/84 (1%) |
1/21 (5%) |
0/60 (0%) |
0.0099 |
| Rickets | 28/61 (46%) |
10/84 (12%) |
1/21 (5%) |
8/60 (13%) |
< 0.0001 |
| Pneumonia | 8/61 (13%) |
1/84 (1%) |
1/21 (5%) |
0/60 (0%) |
0.0044 |
| Hearing Loss* | 19/61 (31%) |
0/83 (0%) |
0/21 (0%) |
0/59 (0%) |
< 0.0001 |
| Failure to thrive | 46/51 (90%) |
46/78 (59%) |
15/21 (71%) |
31/55 (56%) |
0.0001 |
|
Height < 3rd percentile |
33/39 (85%) |
32/65 (49%) |
8/19 (42%) |
21/43 (49%) |
0.0003 |
|
Weight <3rd percentile |
23/41 (56%) |
20/68 (29%) |
2/16 (13%) |
16/49 (33%) |
0.0083 |
Fisher exact test was used.
Data on hearing loss are reported irregardless of surgery. Findings on other features are those prior to surgery (PBD, IE, or OLT). ).
Bolded entries in the ‘Portal Hypertension’ row indicate that results for non-D482G BSEP patients differ from D482G BSEP and FIC1 patients (as described in the text).
While no FIC1 patients were diagnosed with gallstones, 32% of BSEP patients were. Four BSEP patients, all without D482G, were diagnosed with hepatocellular carcinoma (HCC). Overall, 31% of patients were diagnosed with portal hypertension (PH); BSEP patients without D482G developed PH more frequently than those bearing D482G (p = 0.017), and than FIC1 patients (p = 0.022). Twenty-four percent of patients were diagnosed with cirrhosis (median age 4.4y, interquartile range 2.3–6.5y); BSEP patients bearing the D482G mutation survived to an older age without diagnosis of cirrhosis than did other BSEP patients (p = 0.029, hazard ratio = 0.19, 95% CI = 0.04–0.84).
Medications
Reported data on medication use and effect upon pruritus (improvement versus no improvement) were analyzed. Response to ursodeoxycholate (UDCA), cholestyramine, and rifampicin may have been better for BSEP than FIC1 disease (OR 1.9, 3.1, and 2.6), but no differences reached statistical significance (p = 0.19, 0.14, 0.091); phenobarbitol was used by too few, with positive response in too few patients (only 4), to permit meaningful analysis. Overall, UDCA was the most commonly used drug, whereas rifampicin was reported to improve pruritus in a greater proportion of patients to whom it was administered (Supplementary Table 2).
Extrahepatic findings (Table 4)
Diarrhea, pancreatic disease (BSEP: 1 pancreatitis; FIC1: 2 pancreatitis, 5 pancreatic insufficiency), rickets, and pneumonia were all more frequently reported in FIC1 patients than in BSEP patients. Hearing impairment was reported exclusively in FIC1 patients.
Growth
FIC1 patients demonstrated worse growth than did BSEP patients, with greater proportions reported to be failing to thrive and documented to be below the 3rd centiles for weight and height (Table 4).
Laboratory findings
Sweat tests were performed in 19 patients with FIC1 deficiency and 23 with BSEP deficiency. Six (32%) FIC1 patients and 1 BSEP patient had an abnormal result (p = 0.034).
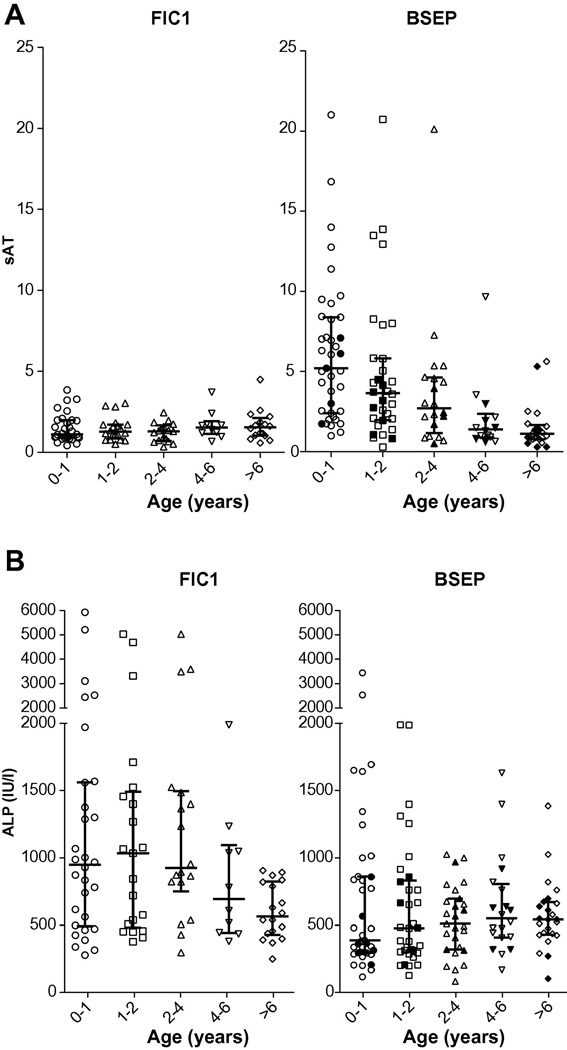
Of the variables that differed between FIC1 and BSEP at presentation, sufficient data were available for sAT and ALP to allow evaluation over time. For both, the differences were present for age bins up to 4 years. The trends toward a decrease with time of sAT in BSEP, and ALP in FIC1, meant that in later age bins the differences between diseases were attenuated (Figure 3).
Figure 3. Serum transaminase and ALP values over time.
Medians and interquartile ranges for the indicated age bins are shown. For BSEP, filled shapes represent data from patients bearing D482G, and open shapes, all other patients. (A) Serum transaminase values, expressed as sAT. P-values (t-test) from comparison of log-transformed data from BSEP and FIC1 disease: age 0–1: <0.0001; age 1–2: = 0.0001; age 2–4: = 0.0015; age 4–6: = 0.95; age >6: = 0.30. (B) ALP values. P-values (t-test) from comparison of log-transformed data from BSEP and FIC1 disease: age 0–1: = 0.0017; age 1–2: = 0.0013; age 2–4: = 0001; age 4–6: = 0.20; age >6: = 0.53.
Surgery-free survival
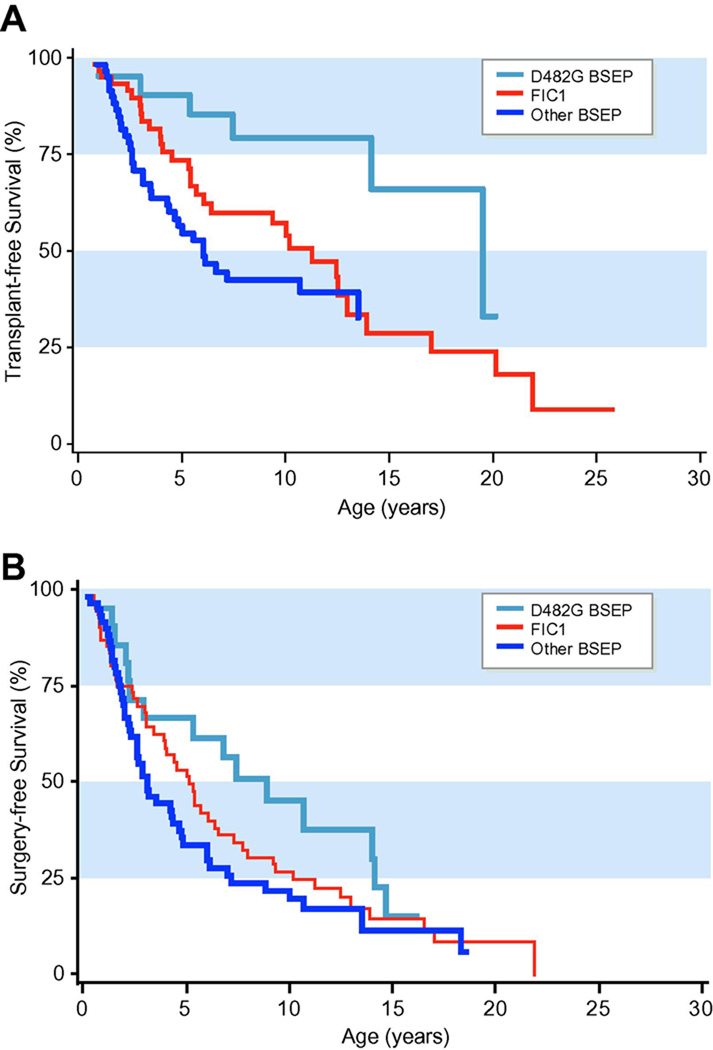
By the end of the study period, 75% (109/145) of patients had undergone surgical intervention (PBD, IE, and/or OLT). Sixty-four patients had undergone OLT. Of those patients who did not undergo surgical intervention, 7 died (5 FIC1 and 2 BSEP patients without D482G). BSEP patients carrying the D482G mutation survived to a greater age without OLT than did other BSEP patients (p = 0.0092, hazard ratio = 0.31, 95% CI = 0.13–0.75) or FIC1 patients (p = 0.045, hazard ratio=0.41, 95% CI = 0.17–0.98) (Figure 4A). The probability of any form of surgery was less in BSEP patients with D482G than in others, and this bordered on significance (p = 0.050, hazard ratio = 0.56, 95% CI = 0.31–1.00) (Fig. 4B).
Figure 4. Kaplan-Meier analysis for survival is shown.
In each case FIC1, D482G-BSEP and other BSEP are plotted. Fig.4A shows survival without OLT. 26/61 (43%) FIC1 patients, and 38/84 (45%) BSEP patients underwent OLT. Among BSEP patients, 5/21 (24%) with D482G and 30/60 (50%) without D482G underwent OLT. Fig. 4B shows survival without OLT, PEBD or IE.
DISCUSSION
FIC1 and BSEP deficiencies are the most common forms of persistent neonatal cholestasis with normal serum γGT levels. Detailed comparison of phenotypes in patients with confirmed genetic diagnoses is now possible. Here we report analysis of clinical and laboratory findings at presentation and over the course of disease before surgical intervention in these 2 disorders. This study necessarily has limitations due to its retrospective design and to variation in practices and reporting among clinical centers. However, this cohort is by far the largest in which genetically informed analysis has been conducted, and the 1st allowing systematic comparison of FIC1 and BSEP deficiencies; consequently, we have been able to demonstrate multiple clinically important differences in disease course between the 2 disorders. Our findings may facilitate timely diagnosis and inform clinical management, as well as help to elucidate the pathophysiological mechanisms underlying the 2 disorders.
With a few exceptions, presentation symptoms did not differ substantially between BSEP and FIC1 deficiency. Presentation with diarrhea was more common in FIC1 deficiency, consistent with FIC1 expression in the intestine [1,11,48]. Seven patients (mostly BSEP) presented with clinically manifest vitamin deficiency in the absence of jaundice, confirming that this complication of cholestasis can manifest in the absence of icterus. Laboratory investigation identifies differences between the 2 conditions at presentation. Giant or multinucleate hepatocytes were infrequent in liver biopsies from children with FIC1 deficiency. This contrasts with many other causes of cholestasis in young children, including BSEP deficiency. Values of sBA, ALT, AST, and cholesterol were higher in BSEP than FIC1 patients. These findings may reflect greater abnormalities in canalicular BA transport and intracellular BA retention in BSEP deficiency. WBC counts were also more likely to be elevated in BSEP patients than in FIC1 patients. By contrast, serum ALP values were higher in FIC1. As both FIC1 and ALP are expressed in tissues besides the liver, this finding may reflect plasma membrane destabilization in multiple tissues. Albumin values were lower in FIC1 patients, which may reflect decreased synthesis or increased loss.
Of the features evaluated, serum ALT and AST values differed most dramatically between FIC1 and BSEP deficiencies, yielding a predictive model that can facilitate differential diagnosis at presentation; addition of other serum biochemical and clinical variables that differed between FIC1 and BSEP to the transaminase-based model did not appear to improve discrimination between the 2 disorders. This model may be particularly useful in combination with histopathological and immunohistochemical studies in prioritizing genes for testing [12,17].
The course of disease differs between FIC1 and BSEP deficiencies. BSEP patients were more likely to develop gallstones. All 4 study participants who developed HCC were BSEP patients, a trend consistent with previous reports of HCC in BSEP deficiency [12]. The incidence of HCC in BSEP appears lower in the current cohort, which may reflect the smaller proportion of patients with 2 protein truncating mutations in the current study; such patients are known to be at increased risk [12].
FIC1 patients were more likely to manifest extrahepatic disease, including diarrhea, pancreatic disease, rickets, pneumonia, hearing impairment, abnormal sweat test results, and poor growth. These findings are consistent with the broad tissue distribution of FIC1 expression, and mark FIC1 deficiency as a multisystem disorder.
BSEP deficiency was considered both as one condition and as stratified by mutation when analyzing clinical findings. The higher incidence of presentation without jaundice but with vitamin deficiency, and lower frequency of giant cells upon biopsy, in “D482G BSEP” suggests a more insidious onset; however, the overall clinical picture at presentation does not differ substantially among the BSEP mutation subclasses. Some features of the disease course in BSEP patients with D482G suggested a more slowly progressing disease, consistent with data suggesting that the D482G BSEP protein retains some function [9, 45–46]; when compared to other BSEP patients, those bearing D482G developed portal hypertension less frequently, developed cirrhosis at an older age, and required OLT at an older age. For most disease features, the results of comparison between FIC1 and BSEP patients were similar whether BSEP patients with D482G were included or excluded. An exception was portal hypertension, where FIC1 patients had an intermediate phenotype between BSEP D482G and non-D482G patients. For some disease features, we had wide confidence intervals for the estimated differences between BSEP mutational subgroups, which implies little evidence for or against potentially important differences.
We evaluated clinical and biochemical features in the largest cohort of genetically characterized PFIC patients yet assembled. We found that severe FIC1 deficiency differed from severe BSEP deficiency both at presentation and as the disease progressed; BSEP patients manifested biochemical evidence of greater hepatobiliary injury (higher ALT and AST values), more severely impaired bile salt handling (higher sBA and cholesterol values at presentation and gallstone disease), and a more progressive disease course (portal hypertension). FIC1 patients showed stronger clinical and laboratory evidence of extrahepatic disease. These findings are of clinical utility and suggest differences in disease pathophysiology.
Supplementary Material
Acknowledgements
We thank C. Jin for assistance with data analysis, J. Vargas for assistance with data compilation, and the following individuals for contribution of patient data: A. Bourgois, B. Dahms, A. Devenyi, K. Foster, M. Gadomski, I. Gonçalves, M. Hadchouel, P. Harren, D. Harris, S. Horslen, S. Kelly, J. Kokkonen, S. Lidofsky, J. Lokar, P. McKiernan, S. Nikkel, J. Peters, D. Piccoli, C. Potter, P. Putnam, S. Radwal, E. Rand, R. Redline, E. Roberts, H. Sharp, Y. Sims, R. Squires, B. Stahulak, T. Stephen, E. Sturm, L. Szőnyi, and S. Tifft. We also thank the families who participated in this study.
Funding: This work was supported by National Institutes of Health (NIH) grants R01 DK50697 (LNB), U54 DK078377 (Cholestatic Liver Disease Consortium/RJ Sokol), U01 DK62500 and U01DK062453 (Childhood Liver Disease Research and Education Network/PR and RJ Sokol) and the P30 DK26743 (UCSF Liver Center); by Guy’s and St Thomas’ Charity and the Children’s Liver Disease Foundation (JAB, SS, and RJT); by the Polish American Foundation for Medical Education, Chicago, IL (PC); by University Medical Center Utrecht and Utrecht University (AMvE); and by St. Luc Pediatric Clinical Investigation Centre (ES). Statistical analysis was facilitated by NIH grant NCRR UCSF-CTSI UL1 RR024131.
Abbreviations
- ABCB11
ATP binding cassette, sub-family B, member 11
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AROC
area under the ROC (receiver operating characteristic) curve
- AST
aspartate aminotransferase
- ATP8B1
ATPase, class I, type 8B, member 1
- BA
bile acids
- BRIC
benign recurrent intrahepatic cholestasis
- BSEP
bile salt export pump
- FIC1
familial intrahepatic cholestasis 1
- FLLN
fold lower limit of normal range
- FULN
fold upper limit of normal range
- γGT
gamma-glutamyltranspeptidase
- IE
ileal exclusion
- N
sample size
- OLT
orthotopic liver transplantation
- OR
odds ratio
- PEBD
partial external biliary diversion
- PFIC
progressive familial intrahepatic cholestasis
- sAT
serum aminotransferase activity
- sBA
serum bile acids
- WBC
white blood cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Dr. Steven Lobritto was on the Speakers’ Bureau of TAP Pharmaceuticals during the development of this manuscript, however, he is no longer on the speakers Bureau of TAP pharmaceuticals. No other conflicts of interest exist.
REFERENCES
- 1.Bull LN, van Eijk MJ, Pawlikowska L, DeYoung JA, Juijn JA, Liao M, et al. A gene encoding a P-type ATPase mutated in two forms of hereditary cholestasis. Nat Genet. 1998;18:219–224. doi: 10.1038/ng0398-219. [DOI] [PubMed] [Google Scholar]
- 2.Strautnieks SS, Bull LN, Knisely AS, Kocoshis SA, Dahl N, Arnell H, et al. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet. 1998;20:233–238. doi: 10.1038/3034. [DOI] [PubMed] [Google Scholar]
- 3.Knisely AS, Bull L, Shneider BL. Low-γGT familial intrahepatic cholestasis. Gene Reviews: Clinical Genetic Information Resource. 2008 [Google Scholar]
- 4.Ujhazy P, Ortiz D, Misra S, Li S, Moseley J, Jones H, et al. Familial intrahepatic cholestasis 1: Studies of localization and function. Hepatology. 2001;34:768–775. doi: 10.1053/jhep.2001.27663. [DOI] [PubMed] [Google Scholar]
- 5.Paulusma CC, Folmer DE, Ho-Mok KS, de Waart DR, Hilarius PM, Verhoeven AJ, et al. ATP8B1 requires an accessory protein for endoplasmic reticulum exit and plasma membrane lipid flippase activity. Hepatology. 2008;47:268–278. doi: 10.1002/hep.21950. [DOI] [PubMed] [Google Scholar]
- 6.Green RM, Hoda F, Ward KL. Molecular cloning and characterization of the murine bile salt export pump. Gene. 2000;241:117–123. doi: 10.1016/s0378-1119(99)00460-6. [DOI] [PubMed] [Google Scholar]
- 7.Wang R, Salem M, Yousef IM, Tuchweber B, Lam P, Childs SJ, et al. Targeted inactivation of sister of P-glycoprotein gene (spgp) in mice results in nonprogressive but persistent intrahepatic cholestasis. Proc Natl Acad Sci U S A. 2001;98:2011–2016. doi: 10.1073/pnas.031465498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akita H, Suzuki H, Ito K, Kinoshita S, Sato N, Takikawa H, et al. Characterization of bile acid transport mediated by multidrug resistance associated protein 2 and bile salt export pump. Biochim Biophys Acta. 2001;1511:7–16. doi: 10.1016/s0005-2736(00)00355-2. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Soroka CJ, Boyer JL. The role of bile salt export pump mutations in progressive familial intrahepatic cholestasis type II. J Clin Invest. 2002;110:965–972. doi: 10.1172/JCI15968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klomp LW, Vargas JC, van Mil SW, Pawlikowska L, Strautnieks SS, van Eijk MJ, et al. Characterization of mutations in ATP8B1 associated with hereditary cholestasis. Hepatology. 2004;40:27–38. doi: 10.1002/hep.20285. [DOI] [PubMed] [Google Scholar]
- 11.van Mil S, van der Woerd WL, van der Brugge G, Sturm E, Jansen PL, Bull L, et al. Benign recurrent intrahepatic cholestasis type 2 is caused by mutations in ABCB11. Gastroenterology. 2004;127:379–384. doi: 10.1053/j.gastro.2004.04.065. [DOI] [PubMed] [Google Scholar]
- 12.Strautnieks SS, Byrne JA, Pawlikowska L, Cebecauerova D, Rayner A, Dutton L, et al. Severe bile salt export pump deficiency: 82 different ABCB11 mutations in 109 families. Gastroenterology. 2008;134:1203–1214. doi: 10.1053/j.gastro.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 13.Alonso EM, Snover DC, Montag A, Freese DK, Whitington PF. Histologic pathology of the liver in progressive familial intrahepatic cholestasis. J Pediatr Gastroenterol Nutr. 1994;18:128–133. doi: 10.1097/00005176-199402000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Whitington PF, Freese DK, Alonso EM, Schwarzenberg SJ, Sharp HL. Clinical and biochemical findings in progressive familial intrahepatic cholestasis. J Pediatr Gastroenterol Nutr. 1994;18:134–141. doi: 10.1097/00005176-199402000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Lykavieris P, van Mil S, Cresteil D, Fabre M, Hadchouel M, Klomp L, et al. Progressive familial intrahepatic cholestasis type 1 and extrahepatic features: no catch-up of stature growth, exacerbation of diarrhea, and appearance of liver steatosis after liver transplantation. J Hepatol. 2003;39:447–452. doi: 10.1016/s0168-8278(03)00286-1. [DOI] [PubMed] [Google Scholar]
- 16.Chen HL, P.S C, Hsu HC, Ni YH, Hsu HY, Lee JH, et al. FIC1 and BSEP defects in Taiwanese patients with chronic intrahepatic cholestasis with low alpha-glutamyltranspeptidase levels. J Pediatr. 2002;140:119–124. doi: 10.1067/mpd.2002.119993. [DOI] [PubMed] [Google Scholar]
- 17.Bull LN, Carlton VE, Stricker NL, Baharloo S, DeYoung JA, Freimer NB, et al. Genetic and morphological findings in progressive familial intrahepatic cholestasis (Byler disease [PFIC-1] and Byler syndrome): evidence for heterogeneity. Hepatology. 1997;26:155–164. doi: 10.1002/hep.510260121. [DOI] [PubMed] [Google Scholar]
- 18.Jansen PLM, Strautnieks SS, Jacquemin E, Hadchouel M, Sokal E, Hooiveld GJ, et al. Hepatocanalicular bile salt export pump deficiency in patients with progressive familial intrahepatic cholestasis. Gastroenterology. 1999;117:1370–1379. doi: 10.1016/s0016-5085(99)70287-8. [DOI] [PubMed] [Google Scholar]
- 19.Wanty C, Joomye R, Van Hoorebeek N, Paul K, Otte JB, Reding R, et al. Fifteen years single center experience in the management of progressive familial intrahepatic cholestasis of infancy. Acta Gastroenterol Belg. 2004;67:313–319. [PubMed] [Google Scholar]
- 20.Oshima T, Ideda K, Takasaka T. Sensorineural hearing loss associated with Byler disease. Tohoku J Exp Med. 1999;187:83–88. doi: 10.1620/tjem.187.83. [DOI] [PubMed] [Google Scholar]
- 21.Walkowiak J, Jankowska I, Pawlowska J, Bull L, Herzig KH, Socha J. Normal pancreatic secretion in children with progressive familial intrahepatic cholestasis type 1. Scand J Gastroenterol. 2006;41:1480–1483. doi: 10.1080/00365520600842344. [DOI] [PubMed] [Google Scholar]
- 22.Walkowiak J, Jankowska I, Pawlowska J, Strautnieks S, Bull L, Thompson R, et al. Exocrine pancreatic function in children with progressive familial intrahepatic cholestasis type 2. J Pediatr Gastroenterol Nutr. 2006;42:416–418. doi: 10.1097/01.mpg.0000218154.26792.6a. [DOI] [PubMed] [Google Scholar]
- 23.Egawa H, Yorifuji T, Sumazaki R, Kimura A, Hasegawa M, Tanaka K. Intractable diarrhea after liver transplantation for Byler's disease: Successful treatment with bile adsorptive resin. Liver Transpl. 2002;8:714–716. doi: 10.1053/jlts.2002.34384. [DOI] [PubMed] [Google Scholar]
- 24.Whitington PF, Whitington GL. Partial external diversion of bile for the treatment of intractable pruritus associated with intrahepatic cholestasis. Gastroenterology. 1988;95:130–136. doi: 10.1016/0016-5085(88)90301-0. [DOI] [PubMed] [Google Scholar]
- 25.Jacquemin E, Dumont M, Bernard O, Erlinger S, Hadchouel M. Evidence for defective primary bile acid secretion in children with progressive familial intrahepatic cholestasis (Byler disease) Eur J Pediatr. 1994;153:424–428. doi: 10.1007/BF01983406. [DOI] [PubMed] [Google Scholar]
- 26.Emond JC, Whitington PF. Selective surgical management of progressive familial intrahepatic cholestasis (Byler's disease) J Pediatr Surg. 1995;30:1635–1641. doi: 10.1016/0022-3468(95)90440-9. [DOI] [PubMed] [Google Scholar]
- 27.Bourke B, Goggin N, Walsh D, Kennedy S, Setchell KD, Drumm B. Byler-like familial cholestasis in an extended kindred. Arch Dis Child. 1996;75:223–227. doi: 10.1136/adc.75.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ismail H, Kalicinski P, Markiewicz M, Jankowska I, Pawlowska J, Kluge P, et al. Treatment of progressive familial intrahepatic cholestasis: liver transplantation or partial external biliary diversion. Pediatr Transplant. 1999;3:219–224. doi: 10.1034/j.1399-3046.1999.00046.x. [DOI] [PubMed] [Google Scholar]
- 29.Eiberg H, Nielsen IM. Linkage of cholestasis familiaris groenlandica/Byler-like disease to chromosome 18. Int J Circumpolar Health. 2000;59:57–62. [PubMed] [Google Scholar]
- 30.Klomp LW, Bull LN, Knisely AS, van Der Doelen MA, Juijn JA, Berger R, et al. A missense mutation in FIC1 is associated with greenland familial cholestasis. Hepatology. 2000;32:1337–1341. doi: 10.1053/jhep.2000.20520. [DOI] [PubMed] [Google Scholar]
- 31.Fischler B, Papadogiannakis N, Nemeth A. Clinical aspects on neonatal cholestasis based on observations at a Swedish tertiary referral centre. Acta Paediatr. 2001;90:171–178. doi: 10.1080/080352501300049361. [DOI] [PubMed] [Google Scholar]
- 32.Kalicinski PJ, Ismail H, Jankowska I, Kaminski A, Pawlowska J, Drewniak T, et al. Surgical treatment of progressive familial intrahepatic cholestasis: comparison of partial external biliary diversion and ileal bypass. Eur J Pediatr Surg. 2003;13:307–311. doi: 10.1055/s-2003-43570. [DOI] [PubMed] [Google Scholar]
- 33.Chen CM, Ananthanarayanan M, Emre S, Neimark E, Bull L, Knisely AS, et al. Progressive Familial Intrahepatic Cholestasis, Type 1, is associated with decreased farnesoid X receptor activity. Gastro. 2004;126:756–764. doi: 10.1053/j.gastro.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 34.Knisely AS, Strautnieks SS, Meier Y, Stieger B, Byrne JA, Portmann BC, et al. Hepatocellular carcinoma in ten children under five years of age with bile salt export pump deficiency. Hepatology. 2006;44:478–486. doi: 10.1002/hep.21287. [DOI] [PubMed] [Google Scholar]
- 35.Wachters-Hagedoorn RE, Porte RJ, Gouw AS, Bijleveld CM, Verkade HJ, Sturm E. [Two sisters with progressive familial intrahepatic cholestasis] Ned Tijdschr Geneeskd. 2004;148:1788–1792. [PubMed] [Google Scholar]
- 36.Jankowska I, Pawlowska J, Ismail H, Bull L, Kluge P, Burda-Muszyńska B, et al. Trudności w diagnostyce i leczeniu postepujacej rodzinnej cholestazy wewnatrzwatrobowwej. In: Socha J, Ryżko J, editors. Kazuistyka gastroenterologiczna u dzieci. Warsaw: Lekarskie PZWL; 2000. pp. 165–172. [Google Scholar]
- 37.Arnell H, Bergdahl S, Papadogiannakis N, Nemeth A, Fischler B. Preoperative observations and short-term outcome after partial external biliary diversion in 13 patients with progressive familial intrahepatic cholestasis. J Pediatr Surg. 2008;43:1312–1320. doi: 10.1016/j.jpedsurg.2007.10.055. [DOI] [PubMed] [Google Scholar]
- 38.Byrne JA, Strautnieks SS, Ihrke G, Pagani F, Knisely AS, Linton KJ, et al. Missense mutations and single nucleotide polymorphisms in ABCB11 impair BSEP processing and function or disrupt pre-mRNA splicing. Hepatology. 2009;49:553–567. doi: 10.1002/hep.22683. [DOI] [PubMed] [Google Scholar]
- 39.Efron B, Tibshirani R. An Introduction to the Bootstrap. London: Chapman and Hall; 1993. pp. 178–188.pp. 214–218.pp. 239–241.pp. 398–403. [Google Scholar]
- 40.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- 41.Savitz DA, Olshan AF. Multiple comparisons and related issues in the interpretation of epidemiologic data. Am J Epidemiol. 1995;142:904–908. doi: 10.1093/oxfordjournals.aje.a117737. [DOI] [PubMed] [Google Scholar]
- 42.Perneger TV. What's wrong with Bonferroni adjustments. Bmj. 1998;316:1236–1238. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bacchetti P. Peer review of statistics in medical research: the other problem. Bmj. 2002;324:1271–1273. doi: 10.1136/bmj.324.7348.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noe J, Kullak-Ublick GA, Jochum W, Stieger B, Kerb R, Haberl M, et al. Impaired expression and function of the bile salt export pump due to three novel ABCB11 mutations in intrahepatic cholestasis. J Hepatol. 2005;43:536–543. doi: 10.1016/j.jhep.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 45.Hayashi H, Takada T, Suzuki H, Akita H, Sugiyama Y. Two common PFIC2 mutations are associated with the impaired membrane trafficking of BSEP/ABCB11. Hepatology. 2005;41:916–924. doi: 10.1002/hep.20627. [DOI] [PubMed] [Google Scholar]
- 46.Plass JR, Mol O, Heegsma J, Geuken M, de Bruin J, Elling G, et al. A progressive familial intrahepatic cholestasis type 2 mutation causes an unstable, temperature-sensitive bile salt export pump. J Hepatol. 2004;40:24–30. doi: 10.1016/s0168-8278(03)00483-5. [DOI] [PubMed] [Google Scholar]
- 47.Mochizuki K, Kagawa T, Numari A, Harris MJ, Itoh J, Watanabe N, et al. Two N-linked glycans are required to maintain the transport activity of the bile salt export pump (ABCB11) in MDCK II cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G818–G828. doi: 10.1152/ajpgi.00415.2006. [DOI] [PubMed] [Google Scholar]
- 48.van Mil SW, van Oort MM, van den Berg IE, Berger R, Houwen RH, Klomp LW. Fic1 is expressed at apical membranes of different epithelial cells in the digestive tract and is induced in the small intestine during postnatal development of mice. Pediatr Res. 2004;56:981–987. doi: 10.1203/01.PDR.0000145564.06791.D1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.