Abstract
Ocular gene therapy is becoming a well-established field. Viral gene therapies for the treatment of Leber’s congentinal amaurosis (LCA) are in clinical trials, and many other gene therapy approaches are being rapidly developed for application to diverse ophthalmic pathologies. Of late, development of non-viral gene therapies has been an area of intense focus and one technology, polymer-compacted DNA nanoparticles, is especially promising. However, development of pharmaceutically and clinically viable therapeutics depends not only on having an effective and safe vector but also on a practical treatment strategy. Inherited retinal pathologies are caused by mutations in over 220 genes, some of which contain over 200 individual disease-causing mutations, which are individually very rare. This review will focus on both the progress and future of nanoparticles and also on what will be required to make them relevant ocular pharmaceutics.
Keywords: Gene Therapy, Nanoparticles, Retina
1. Introduction
Chronic, degenerative diseases of the retina lack curative treatments, and their blinding effects can seriously limit the quality of life. Among these diseases are several for which causative genes have been clearly identified including retinitis pigmentosa (RP), Leber’s congential amaurosis (LCA), some forms of macular degeneration (MD), and ciliopathies such as Bardet-Biedl syndrome (BBS). Other diseases have both genetic and environmental components including age-related macular degeneration (ARMD) and glaucoma.
Treatments for chronic retinal diseases have historically been limited by several factors. Many drugs cannot pass through the cornea/sclera when administered topically, and the eye is protected from the bloodstream by the blood-retina barrier so that ocular bioavailability after systemic administration is typically quite low. Some exceptions do exist; notable examples include intraocular pressure lowering drugs (administered topically) for the treatment of glaucoma and vitamin A (administered systemically) for the treatment of visual defects associated with chromophore deficiencies. As a result of these limitations, alternative administration procedures have been sought to deliver drugs to the inside of the eye and advancements have made in the use of intravitreal or subretinal injections; however, these methods are quite invasive and repeated treatments for a chronic disease are difficult and undesirable. Finally, for many retinal diseases, the onset of phenotype often follows the onset of degeneration, making regeneration a requisite for curative treatments.
Advancements in drug delivery, the generation of controlled/sustained release drugs, and an improved understanding of the pathogenesis of many degenerative retinal diseases have helped to significantly advance the field. Recently developed drugs include peptide-based, antibody-based, and small molecule therapeutics. Virally delivered gene therapeutics are in clinical trials (Bainbridge, J.W. et al. 2008; Cideciyan, A.V. et al. 2008; Bressler, S.B. 2009). Research into genetic therapies in particular is expanding and maturing. Herein we discuss the development and application of safe, effective, non-viral (primarily nanoparticle)–based gene therapies for ocular use. In addition, we provide thoughts and strategies to help translate those vectors into clinical use.
1.1. Ocular gene therapy
The eye is an excellent target for the development of genetic therapies. One obvious advantage is that therapy on the genetic level addresses the source of the problem, not just the symptoms, and the option for local delivery may improve effectiveness without systemic toxicity. Many debilitating monogenic retinal diseases are well characterized, with identified genes and mutations. Animal models containing loss-of-function and gain-of-function mutations are available, as are models containing mutations in structural, functional, and developmental genes, and models for testing the effects of genetic therapies on wound healing and surgical interventions enabling researchers to test therapies designed to target a variety of disease categories (Nour, M. et al. 2003; Wilson, J.H. et al. 2003; Mohan, R.R. et al. 2005; Chang, B. et al. 2006; Farjo, R. et al. 2006; den Hollander, A.I. et al. 2008; Baehr, W. et al. 2009). The same barriers that make ocular administration difficult also make the eye relatively immune-privileged. Intraocularly-delivered drugs are far less likely to induce severe immune responses than their systemically-delivered counterparts, and intraocularly administered drugs usually have low systemic bioavailability and low volume of distribution (Andrieu-Soler, C. et al. 2006). Ideally, gene therapy vectors are taken into the target cells of interest where the genetic material is protected in the nucleus and can continually express its gene product without the requirement for regular, repeated dosing. From a drug development standpoint, the eye is also an ideal tissue. The presence of an internal control (contralateral untreated eye) and the availability of direct non-invasive measures of retinal/visual function in model organisms such as fundus photography, electroretinography, light-induced pupillary size measurements, optical coherence tomography, and optomotor behavior testing make assessing the alleviation of disease symptoms straightforward.
Several barriers to clinically viable retinal gene therapy exist. The human retina is composed primarily of highly differentiated neuronal cells and is post-mitotic from birth. Some gene therapy vectors do not transfect post-mitotic or neuronal cells well. In addition, many inherited retinal degenerations do not present with a detectable phenotype until mid-life and after significant cell death has already occurred (Boon, C.J. et al. 2008). Curative treatments at this stage would need to be capable not only of rescuing the disease causing defect but also of stimulating neuronal regeneration, a highly ambitious combination. More typically, gene therapies are designed to retard or prevent further degeneration and to improve structure/function in remaining cells. While this goal is worthy and decidedly more easily achieved, it cannot always be expected to restore complete vision. Finally, over 220 retinal disease-causing genes and loci have been identified (http://www.sph.uth.tmc.edu/retnet/disease.htm). Some of these genes, for example rhodopsin and retinal degeneration slow (RDS) contain numerous individual disease-causing mutations (>200 and >90, respectively) (http://www.retina-international.org/sci-news/rdsmut.htm, (Chadderton, N. et al. 2009)). Even though most of them are extremely rare, collectively they affect a substantial number of individuals. Traditional gene replacement strategies for autosomal dominant RP and X-linked RP would require at least one different treatment for each gene. For autosomal dominant RP with gain-of-function mutations, more complex genetic strategies will be required to knock-out the mutant allele and replace a wild-type allele. Under current conditions, the cost-benefit ratio for so many treatments is unlikely to be favorable. Alternative strategies for therapy design that take these issues into account must therefore be considered.
1.2. Genetic basis of retinal diseases
Retinal diseases can be broadly classified as (i) those that are clearly genetic having identified clear inheritance patterns and/or isolated causative genes, (ii) those that have a genetic component and significant contribution from environmental factors, and (iii) those with little or no genetic component. All three types may be treated with gene therapies, although different approaches may be required. Monogenic retinal diseases are most commonly targeted in gene therapy studies. Disease-causing mutations have been identified in almost all members of the canonical phototransduction cascade, including rod and cone opsins, rod and cone phosphodiesterase, rod and cone cyclic nucleotide gated channel, and retinoid visual cycle (http://www.sph.uth.tmc.edu/retnet/disease.htm). Depending on the gene and cell, these mutations can cause retinal pigment epithelial (RPE) and/or rod- or cone-dominant photoreceptor degeneration. Mutations in structural genes are also common. Mutations in the ciliary BBS proteins cause multi-organ diseases, including Usher syndrome and Bardet-Biedl syndrome, both of which include a severe ophthalmic component. Similarly, mutations in the photoreceptor-specific structural tetraspanin RDS (retinal degeneration slow) cause rod- and cone- dominant degenerative pathologies (http://www.sph.uth.tmc.edu/retnet/disease.htm).
Diseases affecting rods typically present with an RP phenotype, and cone-dominant diseases present as a macular dystrophy or degeneration. About 50–60% of RP patients have an identified causative genetic mutation with inheritance being dominant, recessive, x-linked, or digenic (Hartong, D.T. et al. 2006; Shintani, K. et al. 2009). The primary symptoms of RP usually begin with reductions in night vision and peripheral vision. In some patients this is followed by late-onset cone degeneration and defects in central vision, but others have cone and rod-degeneration occurring simultaneously. In animal models, reduced scotopic electroretinogram (ERG), defects in the rod outer segment (OS) structure and death/degeneration of rod cells are the most common signs of RP (Cheng, T. et al. 1997). Current treatments for RP are limited and usually consist of the usage of vitamin A palmitate supplements (Berson, E.L. et al. 1993; Sibulesky, L. et al. 1999) or use of devices to assist in living with reduced visual fields (Shintani, K. et al. 2009).
Inherited macular or pattern dystrophies vary widely in presentation and are primarily diagnosed based on fundus appearance. They are often associated with reduced central vision, reduced visual fields, difficulty in close work, and choroidal neovascularization. In rodents without maculas, the disease often presents with cone degeneration/dystrophy and decreased photopic ERG (Ding, X.Q. et al. 2004). Mutations in cone phototransduction genes, particularly the cone cyclic nucleotide gated channels, cause achromatopsia in some patients (Wissinger, B. et al. 2001; Kohl, S. et al. 2005). While macular degeneration/dystrophy can be caused by mutations in cone genes, such as cone opsins or cone phosphodiesterase (Nathans, J. et al. 1992; Thiadens, A.A. et al. 2009), macular disease-causing mutations are also found in proteins involved in the visual cycle and chromophore processing, often expressed in the retinal pigment epithelium (RPE). These mutations often present in humans with a macular phenotype since cones of the macula are tightly packed and most easily affected when their support source, the adjacent RPE, is damaged. Defects in RPE-specific genes can also cause RP, LCA, Best’s disease or multiple other clinical presentations with differeing symptoms and fundus appearance, resulting from differing responsible genes (Forsman, K. et al. 1992; den Hollander, A.I. et al. 2008). All of these diseases vary widely in time of onset/phenotype presentation and often have inconsistent genotype/phenotype correlations.
Treatment of macular degenerations and dystrophies may involve the use of antioxidant vitamin supplements, such as vitamins A, C, and E (http://www.nei.nih.gov/amd/), (Shintani, K. et al. 2009)), and clinical trials are ongoing using, complement inhibitors (http://www.clinicaltrials.gov/, NCT00935883) or inhibitors of vitamin A metabolism (http://www.clinicaltrials.gov/ NCT00346853). If choroidal neovascularization is a disease feature, pharmacological treatment with injectable anti-angiogenics or surgical/laser treatment can be used. Experimental treatments for RP and MD include the use of gene therapy, transplanting stem cells, or the use of retinal prosthetics (Shintani, K. et al. 2009). Traditional gene replacement therapy and gene knockdown therapy, which eliminates or reduces expression of a gain-of-function allele, are the most common gene therapy approaches for the treatment of RP and MD.
Diseases with both genetic and environmental components are much more common than the monogenic diseases. For example, two of the most prevalent eye diseases, ARMD and glaucoma, have a genetic component but are not simply inherited. Complement factor H mutations among others are linked to ARMD, while glaucoma is linked to mutations in myocilin among other proteins. However, since the genetic component is generally considered to be incompletely causative or only present in a small number of cases, traditional gene replacement may not be particularly successful. Gene therapies for these diseases, and also for diseases without any known genetic component, most often take the form of delivery of a non-mutated, protective gene. This gene is often a neurotrophic factor or anti-angiogenic factor designed to improve overall retinal health (Lebrun-Julien, F. et al. 2008; Zhang, M. et al. 2009). While this approach may not be directly curative, it is more widely applicable and can target a larger variety of disease etiologies. However, one of the major limitations for these non-monogenic degenerative disorders is an incomplete understanding of the disease process. Successful treatment of such diseases may require concurrent administration of multiple therapies, either genetic or otherwise, some of which may not yet be theoretically apparent.
Development of a successful genetic therapy of either type is substantially more involved than was initially believed when the first studies of this kind were undertaken. While viral gene therapies for the treatment of retinal diseases are thriving (Acland, G.M. et al. 2005; Bainbridge, J.W. et al. 2008; Cideciyan, A.V. et al. 2009), they still exhibit some limitations (Thomas, C.E. et al. 2003). One thing has become abundantly clear as the field has evolved: the one-size-fits-all approach is not likely to be successful. The development of alternative therapies is both prudent and practical.
2. Specific considerations for the design of ocular gene therapies
2.1. Methods of delivery
Delivery to the eye can be achieved numerous ways. The easiest and least invasive method is surface instillation, but this method is virtually ineffective for delivery of genetic material to the posterior segment and intraocular space (Andrieu-Soler, C. et al. 2006), although it may be quite effective for treatment of the cornea (Mohan, R.R. et al. 2005). Corneal penetration is very limited and clearance from the ocular surface is typically rapid. Surface instillation is more suitable as a delivery method for corneal or conjunctival conditions rather than for retinal gene delivery. Sub-conjunctival delivery is more invasive and still suffers from the same limitations as surface instillation in terms of access to the retina and posterior segment (Andrieu-Soler, C. et al. 2006). Exploration of intravenous delivery for ocular drugs is being investigated (Singh, S.R. et al. 2009), but the blood-retinal barrier coupled with the large systemic volume of distribution has historically limited the effectiveness of this method. Intravitreal and subretinal injections are generally considered to be the most effective ways of delivering material to the back of the eye. Gene delivery vectors (viruses, liposomes, nanoparticles) have different abilities to penetrate the retina after intravitreal injection (Conley, S.M. et al. 2008). Intravitreal injection may be optimal for delivery to retinal ganglion cells and inner retinal interneurons, but subretinal injection has been shown to be more effective in most cases for delivery to the outer retina, specifically photoreceptors and RPE (Andrieu-Soler, C. et al. 2006; Farjo, R. et al. 2006). Several approved ARMD drugs are already delivered via intravitreal injection, and subretinal injection is being employed in the current ongoing gene therapy trials for RPE65-associated LCA (Bainbridge, J.W. et al. 2008; Cideciyan, A.V. et al. 2008; Bressler, S.B. 2009).
2.2. Repeated dosing and persistence of expression
While subretinal and intravitreal injections are both clinically viable delivery options, they are relatively invasive. Patients are subjected to increased risk of severe endophthalmitis and retinal detachment, potentially resulting in vision loss. Consideration should be given when designing a gene therapy vector to minimizing the necessity of repeat dosing. Research into controlled release vectors and sustained delivery devices is ongoing; an implantable device for prolonged vitreal release of the antiviral gancyclovir has been approved (Kuno, N. et al. 2010). Currently, drugs approved for treatment of chronic ocular diseases have to be administered repeatedly. For example, the anti-VEGF aptamer pegaptanib (Macugen) has a prescribed dosing regimen of every six weeks and the anti-VEGF monoclonal antibody ranibizumab (Lucentis) has a recommended dosing regimen of every 28 days.
The cells of the retina are post-mitotic, and degenerative retinal diseases are chronic in nature. One of the benefits of an ideal gene therapy vector would be long-term gene expression without the need for repeat dosing. This means that the primary concerns when testing gene expression vectors must be the expression levels and phenotypic rescue, and also the persistence of expression. Non-viral ocular gene therapy studies have not typically examined long-term expression (Andrieu-Soler, C. et al. 2006). Plenty of reports are available on in vitro and in vivo gene expression after 2 days, or even up to one month, but few have studied longer-term expression (Andrieu-Soler, C. et al. 2006; Conley, S.M. et al. 2008).
2.3. Age of onset and genotype/phenotype correlations
Two final specific concerns for the development of gene therapies for ocular degenerative diseases are the variation in age of disease onset and the inconsistency in genotype/phenotype correlations. Ideally, a genetic therapy would be well-tolerated and last for the life of a patient. For example, a genetic screening test would identify a causative mutation at birth or in young childhood, the treatment would be delivered before the onset of degeneration, and the condition would be prevented completely. However, current therapies, even those classified as long-lasting ones may eventually fail, and the risk associated with the subretinal procedure is not inconsequential. While such a risk may be justified in a blinded or degenerating eye, it would be hard to justify the use of such a treatment in a healthy eye. Furthermore, incomplete penetrance has been observed in many cases; as one example, family members sharing the same disease mutation range in RDS (deletion of codon 153/154) exhibit phenotypes ranging from no visible abnormalities to RP to pattern dystrophy (Weleber, R.G. et al. 1993). This phenotypic variability further reduces the desirability of treatment based solely on genotype. However, treatment after onset of diseases symptoms usually means cell loss has already begun. Age of onset also varies considerably; some retinal diseases such as LCA usually have a severe, early onset often with blindness by 1 year (den Hollander, A.I. et al. 2008). Other diseases exhibit a much later onset; for example, patients with MD associated with the R172W mutation in the RDS gene often exhibit no disease phenotype until the 3–4th decade of life (Piguet, B. et al. 1996).
2.4. Barriers to effective transfection
The first step in efficient transfection is delivery to the site of interest. Subretinal injection is generally efficient at accomplishing delivery to the outer retina, but often the extent of vector delivery is limited to the putative region of temporary retinal detachment. The vectors do not diffuse laterally through the entirety of the subretinal space and transgene expression is therefore regional in nature (Sarra, G.M. et al. 2001). The second step is cellular uptake of DNA. Viral tropisms are well-characterized, but several serotypes have been identified that readily transfect retinal cells (Surace, E.M. et al. 2008). Several non-viral methods exhibit efficient passage through the plasma membrane either through receptor-mediated uptake or traditional endocytic pathways, which are further discussed below (Chen, X. et al. 2008; del Pozo-Rodriguez, A. et al. 2008). In spite of this success, many non-viral delivery methods fail at the next step: gene expression. Efficient gene expression requires the vectors to efficiently travel through the cytoplasm and through the nuclear membrane. Passage through the nuclear membrane is either active (receptor mediated) or passive (particles smaller than 25 nm are generally thought to be able to penetrate the nuclear pore complexes) (Liu, G. et al. 2003). In many cases, non-viral vectors are readily taken up into cells but are not well expressed (Hoffman, E.A. et al. 2005). The vectors may have difficulties escaping from the endocytic/lysosomal pathway, they may be degraded by cytoplasmic DNases, or they may not be able to get into the nucleus. Receptor-mediated transport directly to the nucleus and inclusion of a nuclear targeting peptide can help alleviate this issue (Rhee, M. et al. 2006; Chen, X. et al. 2008). Finally, once in the nucleus, the ideal delivery vehicle releases the DNA and subsequent level of gene expression, tissue specificity, and persistence of expression depends on plasmid characteristics.
2.5. The ideal non-viral gene delivery vector
The vector should be taken up extensively and efficiently in the tissue of interest, with minimal ectopic uptake or expression. Levels of gene expression should be high enough to promote phenotypic improvement, without causing over-expression induced toxicity. For chronic disease treatment, gene expression should start rapidly after treatment delivery and persist throughout the life of the organism. Expression of the exogenously delivered gene should correct the disease phenotype and prevent further degeneration. The vector should be able to be delivered by a safe non-invasive method. Finally, the vector should be well tolerated and should not cause a significant immune response, inflammation, integrational toxicity, or other adverse physiological outcomes. While many viral-based systems meet some of these criteria, the focus here will be on efforts to design effective non-viral delivery vectors.
3. Non-viral gene therapy options
3.1. Naked DNA
Non-viral gene therapies consist of oligonucleotides (usually DNA) delivered either alone or complexed with a chemical agent and with or without the assistance of a physical method such as electroporation (Wells, D.J. ; Wells, D.J. 2004). The most basic form of non-viral gene therapy is naked, unpackaged plasmid DNA. However, it is well established that plasmid DNA possesses very little ability to transfect mammalian cells, with the exception of muscle cells, without physical or chemical assistance (Andrieu-Soler, C. et al. 2006). Several studies investigating the ability of plasmid DNA to transfect ocular/retinal cells have reported negative results with little-to-no transfection or gene expression (Dezawa, M. et al. 2002; Andrieu-Soler, C. et al. 2006; Cai, X. et al. 2009).
3.2. Liposomes
Often, DNA is complexed with cationic lipids to form liposomes. Cationic lipids are electrostatically favorable for complexing negatively charged DNA, but often small amounts of neutrally charged lipids are incorporated as well. For example, 1,2,-dioleoyl-3-phosphatidylethanolamine (DOPE) is often included as it can destabilize lysosomes and thereby allow the transfected DNA to be released into the cytoplasm (Naik, R. et al. 2009). Liposomes/lipoplex vectors are usually self-assembling and biodegradable. They have the advantage of more readily penetrating cell membranes than naked DNA, but exhibit significant variability in transfection efficiency and are usually quickly silenced. For example, we have demonstrated that in primary cultures of trabecular meshwork cells exposed to lipid-based gene delivery complexes (lipofectamine) almost 100% of the cells take the particles into the cytoplasm, but that only ~4% of these cells express the vector (Hoffman, E.A. et al. 2005). Part of this discrepancy is a result of cellular DNases since inhibition of DNase I doubles the transfection efficiency (~8%). Clearly, other factors such as difficulty in permeating the nuclear membrane also inhibit efficient gene expression. Liposomes have been commonly used in the eye in vivo with mixed results and current efforts in this direction are focused on improving transfection efficiency and duration of expression (Naik, R. et al. 2009).
3.3. Solid lipid nanoparticles
A newer type of lipid based gene carrier is solid-lipid nanoparticles. Compared to traditional liposomes, these particles are reportedly easier to make in large quantities and are ~200 nm in diameter (Bondi, M.L. et al. 2010). Thus far the only reports of delivery of these particles to ocular tissue studied their ability to direct gene expression in the transformed human RPE cells ARPE-19 cell line (del Pozo-Rodriguez, A. et al. 2008). The authors reported that the particles were taken up via clathrin-mediated endocytosis, but the ARPE-19 cells did not transfect well-with ~2.5% transfection efficiency, so it is evident that additional research will be needed before these lipid-based particles are clinically useful vectors.
3.4. Other nanoparticles
Nanoparticles can take many forms; technically anything smaller than 1 µm in diameter is a nanoparticle, although particles reported to be successful for gene delivery usually have a hydrodynamic diameter of less than 400 nm. Generally, smaller particles are considered to be more efficient; certainly very small particles (<25 nm) have the potential to efficiently pass through the pores in the nuclear membrane, thus overcoming a significant barrier to successful transfection (Liu, G. et al. 2001).
Nanoparticles have also been used extensively for ocular applications other than gene delivery. For example, cerium oxide nanoparticles have been used to alleviate oxidative stress in models of light induced retinal degeneration (Chen, J. et al. 2006) and polymeric nanoparticles are frequently used for delivery of pharmaceuticals. Gene delivery nanoparticles usually consist of a peptide or polymer base that condenses or encapsulates the DNA of interest. Some of the best explored include polylactide (PLA)- and polylactide co-glycolide (PLGA)-based particles and particles condensed with cationic polypeptides such as polylysine (for examples, see (Ziady, A.G. et al. 2003; Bejjani, R.A. et al. 2005; Munier, S. et al. 2005; Farjo, R. et al. 2006; Cun, D. et al. 2010; Zhong, Q. et al. 2010)).
4. Polypeptide based nanoparticles
4.1. Principles of DNA condensation
The formation of polypeptide-based nanoparticles relies on the principles of DNA condensation. In spite of the observation that cells expend significant energy keeping DNA condensed enough to fit inside the nucleus, it has been repeatedly demonstrated that DNA will condense or compact under the right conditions. The biophysics of this process has been extensively studied and excellently reviewed (Bloomfield, V.A. 1997). The inclusion of multivalent cation condensing agents is one of the requirements due to the highly negatively charged nature of the DNA backbone. These agents can vary from organic polyamines to inorganic polycations to polypeptides such as polylysine, which is used in some of the therapeutic nanoparticles currently in use. The approximate minimum length for DNA condensation is 400 bp because of a combination of physical factors (Widom, J. et al. 1980). Initial electron microscopy experiments using T7 phage DNA and the polycation spermine demonstrated that DNA usually condenses into a toroid or spherical shape approximately 50–70 nm in diameter (see Fig. 1B) (Wilson, R.W. et al. 1979), which is significant compression considering the length of T7 DNA is estimated to be 14 µm (Chattoraj, D.K. et al. 1978). This spherical shape can be modified if the condensing agent has some non-polar characteristics. Condensing agents generally work by three methods: (i) by neutralizing repulsive charges on the DNA phosphates, (ii) by making the interactions between the DNA and the solvent less favorable while enhancing attractive molecular forces, and (iii) by causing localized DNA bending which can encourage condensation (Bloomfield, V.A. 1997).
Figure 1. Relative sizes of various nanoparticle-based delivery vectors.
Shown are scale depictions of the relative sizes of different non-viral vectors. Sizes are either hydrodynamic diameters (HD) or measured by electron microscopy (EM) or dynamic light scattering (DLS). (A) Plasmid DNA [DLS ~1200 nm] (Parker Read, S. et al. 2010). (B) Toroidal, spermine compacted phage DNA [HD ~50 nm] (Wilson, R.W. et al. 1979). (C) CK30-PEG trifluoroacetate ellipsoid-shaped nanoparticle [EM ~22 × 50 nm] (Farjo, R. et al. 2006). (D) CK30-PEG acetate rod-shaped nanoparticle [EM ~8–11 × 200 nm] (Farjo, R. et al. 2006). (E) PEG-POD spherical nanoparticle [DLS ~130 nm] (Parker Read, S. et al. 2010). (F,G) Untargeted and RGD targeted PLGA nanoparticles [HD ~220–420 nm] (Singh, S.R. et al. 2009).
DNA condensation occurs only when the free energy contributions from forces that resist condensation are overcome by those that favor it. Two major negative energy contributions resist condensation. First, entropy is lost during condensation as the disordered, mixed state is replaced by a state in which the DNA is compacted and the solvent (water) is excluded. Second, DNA bending, which is required for condensation, will be thermodynamically unfavored, except in some cases of multivalent cation binding (Bloomfield, V.A. 1997). Two major forces can be considered to contribute energy toward condensation under the proper conditions. In the presence of multivalent cations of at least +3 charge, both electrostatic and hydration forces can contribute positively towards condensation (Bloomfield, V.A. 1997). It has been empirically and theoretically determined that approximately 90% of the DNA’s negative charge must be neutralized by either the condensing agent or the monovalent cations in solution for condensation to occur (Wilson, R.W. et al. 1979). However, the remaining 10% is still quite substantial and two theories have been proposed to account for the molecular attraction that must overcome this charge and accompany condensation. First, positive electrostatic attractive forces may be generated by the formation of correlated ionic fluctuations arising as a result of induced dipole interactions between adjacent macromolecules or may arise as a result of the formation of complementary pseudo-2D ionic lattices (Bloomfield, V.A. 1997). These forces are thought to contribute to stable condensed forms provided the counterions have at least a +3 charge.
The second attractive force thought to be at work during the process of DNA condensation is hydration force (Bloomfield, V.A. 1997). Although hydration forces can be either attractive or repulsive, the net effect of hydration on DNA condensation appears to be attractive. This force can be understood thermodynamically; as counterions bind DNA, water molecules are released or rearranged and entropy on a molecular level increases, thus favoring the process. It can also be understood structurally; DNA in solution will have a layer of polarized water molecules surrounding its negatively charged phosphate groups, contributing to the repulsive forces. As the counterions near, the water molecules can reorganize into a complementary pattern and create localized regions of molecular attraction.
In addition to their role in neutralizing negative charge and promoting net attractive forces on a molecular level, the condensing polycations may also play a structural role in promoting DNA bending. It has been shown, at least for some polycations, that they are mobile along the DNA backbone and can reside either in the minor DNA groove, wherein they nestle without causing structural alterations, or in the major groove where they will contribute to localized DNA bending (Bloomfield, V.A. 1997).
4.2. Early application of DNA condensation to the formulation of nanoparticles for gene therapy
Utilizing DNA compaction to generate gene therapy vectors was a promising idea. The size of the initially characterized compacted DNA toroids were ~50 nm (Figure 1B) (Wilson, R.W. et al. 1979), which was small enough be potentially useful for gene therapy. Although the ideal size should be smaller, the packaging capacity was theoretically quite large. However, significant effort remained to be invested to transition from a biophysical observation of molecular behavior to a practical gene delivery strategy.
Polylysine is positively charged and reasonably non-antigenic and was chosen for nanoparticle compaction. The spermine condensation studies, reporting toroidal, 50–70 nm particles, were done in low salt conditions. Initial compaction studies using polylysine increased salt concentrations to ~1 M. Lysine was added to DNA at high concentrations, and at a 1:1 molar charge ratio of polylysine:DNA cooperative binding of the polylysine to the DNA led to the formation of a condensed particle either ellipsoid (diameter of 15–30 nm) or rod-like in shape (length, ~100 nm) (Perales, J.C. et al. 1994; Perales, J.C. et al. 1994; Perales, J.C. et al. 1997; Liu, G. et al. 2001). Varying the length of the polylysine, the concentration of NaCl in solution, the molar charge ratio of polylysine:DNA, and the substitution of the polylysines could alter the shape of the particles, the size of the particles, the percent of the DNA condensed, and the likelihood of particle aggregation (Liu, G. et al. 2001).
The compacted complexes or nanoparticles formed by cooperative binding are significantly more resistant to degradation by cellular DNases than either free DNA or the larger, intermediate complexes of DNA that is non-cooperatively bound to polylysine. In early in vitro experiments, the polylysine was galactosylated to target the particles to hepatocytes, which express the asialoglycoprotein receptor (Perales, J.C. et al. 1994; Perales, J.C. et al. 1994; Perales, J.C. et al. 1997). When HuH-7 cells (a human hepatoma cell line) were exposed to these particles, uptake was only observed for particles in the 30 nm range (Liu, G. et al. 2001). In spite of the promise of these particles, delivery in ~1M NaCl is not ideal for human use, and the particles precipitated at low-salt concentrations. Even at 1M NaCl, they could form large aggregates and were not homogeneous, prompting further research into compaction methodology.
4.3. Low salt particles and the introduction of PEGylation
To overcome the limitations of the high-salt particles, subsequent research in nanoparticle synthesis done by our collaborators at Copernicus Therapeutics involved significant alterations in the compaction procedure. Particle compaction is achieved at low salt concentrations or in water under very precise conditions. DNA is added in aliquots to a solution of lysine peptides, sometimes coupled to polyethylene glycol (PEG) (Liu, G. et al. 2003). The particles are experimentally demonstrated to be unimolecular with respect to DNA. With the addition of PEGylated polylysine, these particles are charge neutral with a ζ potential near zero or slightly negative, which is important since positively charged particles can activate the complement cascade (Thakor, D. et al. 2007; Sun, W. et al. 2009). When properly compacted and processed, these DNA nanoparticles are homogeneous in size and shape, consist only of compacted DNA, do not form aggregates, are colloidally stable in physiological salt concentrations, and protect plasmid DNA from DNAse digestion. The particle size depends on the size of the plasmid compacted, but a ~5 kb plasmid has minor and major diameters of ~24×~35 nm, respectively, when formulated as ellipsoidal nanoparticles (Liu, G. et al. 2003; Fink, T.L. et al. 2006). Moreover, the shape of these DNA nanoparticles can be specifically altered by changing the counterion of the lysine peptide prior to DNA mixing (Fink, T.L. et al. 2006). For example, when trifluoroacetate or acetate are the lysine counterions, ellipsoidal or rod-like compacted DNA nanopaticles are forumulated (Fink, T.L. et al. 2006). Other counterions, such as chloride and bicarbonate also affect nanoparticle shape and other properties(Kowalczyk, T. et al. 2001). Importantly, a panel of quality control assays with formal end-release specifications have been developed at Copernicus that address FDA manufacturing guidelines and which assure reproducible and standardized formulations of compacted DNA nanoparticles, as is appropriate for human clinical trials (Konstan, M.W. et al. 2004).
PEGylation is defined as the addition of repeating polyethylene glycol (PEG) moieties to proteins or polymers. Such changes can alter the target in a variety of ways, not least of which is by increasing hydrophilicity and altering electrostatic binding properties (Pisal, D.S. et al. 2010). The bulky nature of PEGylated compounds can also help protect them from degradation by cellular enzymes, thus increasing complex stability. The chemistry of PEGylation has changed over time and has developed to the point that polydisperse mixtures of PEG can be separated into much narrower size ranges than previously, with very few impurities, such that more uniform PEGylation can be achieved. Current techniques for PEGylation of polylysine for the generation of nanoparticles involve mixing a purified 30-mer of polylysine terminating with a single cysteine (CK30) and methoxy-PEG10K-maleimide (Liu, G. et al. 2003). The maleimide groups react more efficiently with the sulfhydryl group of the cysteine than they do with the free amines of the lysine thus enabling the formation of a specific, covalently modified, PEGylated CK30 peptide.
PEGylating the polypeptide enables unimolecularly compacted DNA to be colloidally stable in physiological salt concentrations for extended periods of time without significant aggregation (Ziady, A.G. et al. 2003). If bifunctional PEGylation is required, for example when a compacting peptide and a targeting peptide need to be conjugated to the PEG, PEG-[OPSS]2 (ortho-pyridyl disulfide) can be used instead of PEG-maleimide (Sun, W. et al. 2009). In addition to improving stability, PEGylation also enhances transfection efficiency of polylysine-based nanoparticles. When CK30 compacted nanoparticles were delivered to murine airways, no significant reporter gene expression was detected in airway tissue. In contrast, substantial luciferase activity was measured when CK30-PEG was used for compaction, supporting the hypothesis that PEGylation can improve transfection efficiency (Ziady, A.G. et al. 2003).
As in the case with the high-salt particles, successful nanoparticle driven gene expression with the low-salt particles depended on particle size. When particles were injected into the cytoplasm of HuH-7 cells, nuclear uptake was only observed for particles with minor diameters of 25 nm or less, suggesting facilitated transport through nuclear pores occurred (Liu, G. et al. 2003). To further test the hypothesis that improvements in nanoparticle mediated transfection efficiency are in part due to enhanced nuclear uptake, compacted and uncompacted DNA was injected into the cytoplasm. Reporter gene expression levels were 10-fold higher for compacted DNA than for uncompacted DNA, and compacted DNA transgene expression was prevented by wheat germ agglutinin, a nuclear pore blockade agent (Liu, G. et al. 2003). These compacted particles were able to transfect both dividing and non-dividing cultured cells (Liu, G. et al. 2003), which is beneficial since many cells have slow division rates and neuronal and retinal cells are post-mitotic. These low-salt particles are the basis for the particles in use currently and their successful application is discussed in section 5.
4.4. Capacity of DNA nanoparticles
One of the traditional limitations of the otherwise successful AAV vectors has been the small cargo capacity (~4.6 kB). Ideally, effective nanoparticle vectors would be able to carry a larger cargo than that. Since efficient nanoparticle nuclear uptake is size limited based on in vitro microinjection studies (Liu, G. et al. 2003), an understanding of the effects of vector size on nanoparticle size and transfection efficiency is critical. Both ellipsoid and rod-shaped CK30-PEG nanoparticles were generated using plasmids of 5.3 kbp, 9.7 kbp, and 20.2 kbp, each carrying a luciferase expression vector (Fink, T.L. et al. 2006). Average sizes for the resulting ellipsoid nanoparticles were 22 × 50, 30 × 53, and 32 × 58 nm, respectively (minor × major diameter) while average sizes for the resulting rod-shaped particles were 8.4 × 184, 8.3 × 393, and 11.3 × 537 nm, respectively (Figure 1C–D). In all instances, the minor diameters of the rod-shaped nanoparticles were sufficiently small to enable passage through the nuclear pore (Fink, T.L. et al. 2006). In contrast to initial in vitro microinjection results in which particles greater than 25 nm in minimum diameter had poor expression, all six size and formulation combinations of nanoparticles had equivalent transfection efficiency when delivered to the murine lung in vivo. This difference in transfection efficiencies based on diameter size is possibly attributable to differences in nanoparticle uptake and trafficking between in vitro and in vivo conditions. These encouraging results indicate that nanoparticles have significant compaction capacity and may be capable of delivering multiple genes or increased regulatory sequences if necessary.
4.5. CK30-PEG nanoparticle trafficking
4.5.1. Time course of nanoparticle uptake
Initial time course experiments on the trafficking pathways of CK30-PEG nanoparticles revealed that rhodamine-labeled CK30-PEG nanoparticles were detected in the cytoplasm of cultured airway epithelial cells within 15 minutes after application. Between 15–30 minutes after application, the majority of nanoparticles were detected in the nucleus, with concentration in the nucleolus evident by 1 h post-treatment (Chen, X. et al. 2008). Nanoparticle-mediated reporter gene expression was observed by 18 h post-treatment; however, time points between 1 h and 18 h were not reported so it is not clear how early gene expression can be detected. The transfection efficiency benefits of nanoparticles over naked DNA are clearly related to uptake and trafficking to the nucleus. When nuclei were microinjected with either naked DNA or similar nanoparticles, gene expression was equivalent (Liu, G. et al. 2003).
To determine how quickly nanoparticle-driven gene expression occurs in the eye, we performed subretinal injections of CK30-PEG compacted nanoparticles on a set of rds+/− animals at post-natal day (P) 5, and then examined the time course of gene expression. The nanoparticles carried the normal mouse peripherin/RDS transgene (NMP) under the control of the interphotoreceptor retinoid binding protein promoter (IRBP) and were previously shown to drive significant levels of gene expression by post injection (PI-) day 2 (Farjo, R. et al. 2006). By 2h PI, 1 of 4 injected eyes had a gene expression level above background levels expressed in the uninjected contralateral eye. In the samples collected at 8 h PI, 3 of 4 injected eyes had elevated levels of gene expression. By 2 days PI, all three injected eyes had elevated transgene expression levels and at seven days, 4 of 5 injected eyes expressed elevated levels of RDS (unpublished data). In contrast, naked DNA did not drive significant mRNA expression. The variability in the time of onset of expression may be due to variations in the precise location of injection; the subretinal space in the P5 retina is poorly defined and some injections may not fully penetrate the neural retina. It is also not known how soon transgene-driven protein expression will follow the expression of transgene message, although abundant protein is detected by PI-2. Regardless,CK30 PEG nanoparticles were taken up into retinal cells, transferred to the nucleus and transcribed into mRNA by within 2–8 h after subretinal injection on P5.
4.5.2. Role of cell surface nucleolin
It has been demonstrated that CK30-PEG nanoparticles can successfully transfect the brain, eye, and lung without assistance from methodologies commonly used to transport exogenous nucleotides through the plasma membrane such as lipofectamine or calcium phosphate. This successful nanoparticle transfection is not observed for all cell types, suggesting the possibility of a specific, active transport mechanism. Often, expression vectors are taken up into the endosomal/lysosomal pathway, either via clathrin-mediated or clathrin-independent endocytosis (del Pozo-Rodriguez, A. et al. 2008). Successful transgene expression after this process requires the vector to somehow escape from this degradative pathway and then proceed to the nucleus.
Some have proposed that CK30-PEG nanoparticles are not processed by this pathway. In vitro experiments have shown that CK30 PEG nanoparticles do not co-localize with early endosomal or lysosomal markers, supporting the hypothesis that they are not trafficked via clathrin-mediated endocytosis (Chen, X., and Davis, P.B 2006; Chen, X. et al. 2008). On the contrary, nanoparticles in both the cytoplasm and nucleus co-localize with the protein nucleolin (Chen, X. et al. 2008). Although this protein is ubiquitously expressed and is involved in rDNA transcription and mRNA metabolism, it is only expressed on the plasma membrane of certain cell types, including lung epithelial cells (Chen, X., and Davis, P.B 2006). Biochemical experiments demonstrated that CK30 PEG nanoparticles can bind cell-surface nucleolin specifically and with high affinity. Uptake of a nucleolin monoclonal antibody followed the same pathway as the nanoparticles and did not colocalize with any endosomal/lysosomal markers, supporting the hypothesis that nucleolin-mediated trafficking is independent of the endosomal/lysosomal pathway (Chen, X. et al. 2008). Furthermore, the role of nucleolin as a nanoparticle binding partner was solidified by experiments in which nucleolin levels were knocked down either by siRNA or by serum starvation, and specific reductions were observed in nanoparticle transfection efficiency but not lipofectamine transfection efficiency. Finally, co-delivery of free extracellular nucleolin and nanoparticles competitively inhibited nanoparticle uptake (Chen, X. et al. 2008). These results suggest that nucleolin is a cell surface receptor for CK30-PEG nanoparticles. More importantly, nucleolin is known to shuttle between cellular compartments, including the plasma membrane and the nucleus, without use of traditional pathways. Nucleolin-mediated nanoparticle trafficking thereby bypasses or enhances passage through several steps that can be limiting factors for traditional transfections based on clathrin-mediated endocytosis, including escape from the endosomal pathway, cytoplasmic diffusion of delivered DNA, extensive exposure to cellular DNases, and access to the nucleus. Active transport of nanoparticles across the nuclear membrane by nucleolin may partially explain why slight increases in particle diameter do not severely affect transfection efficiency.
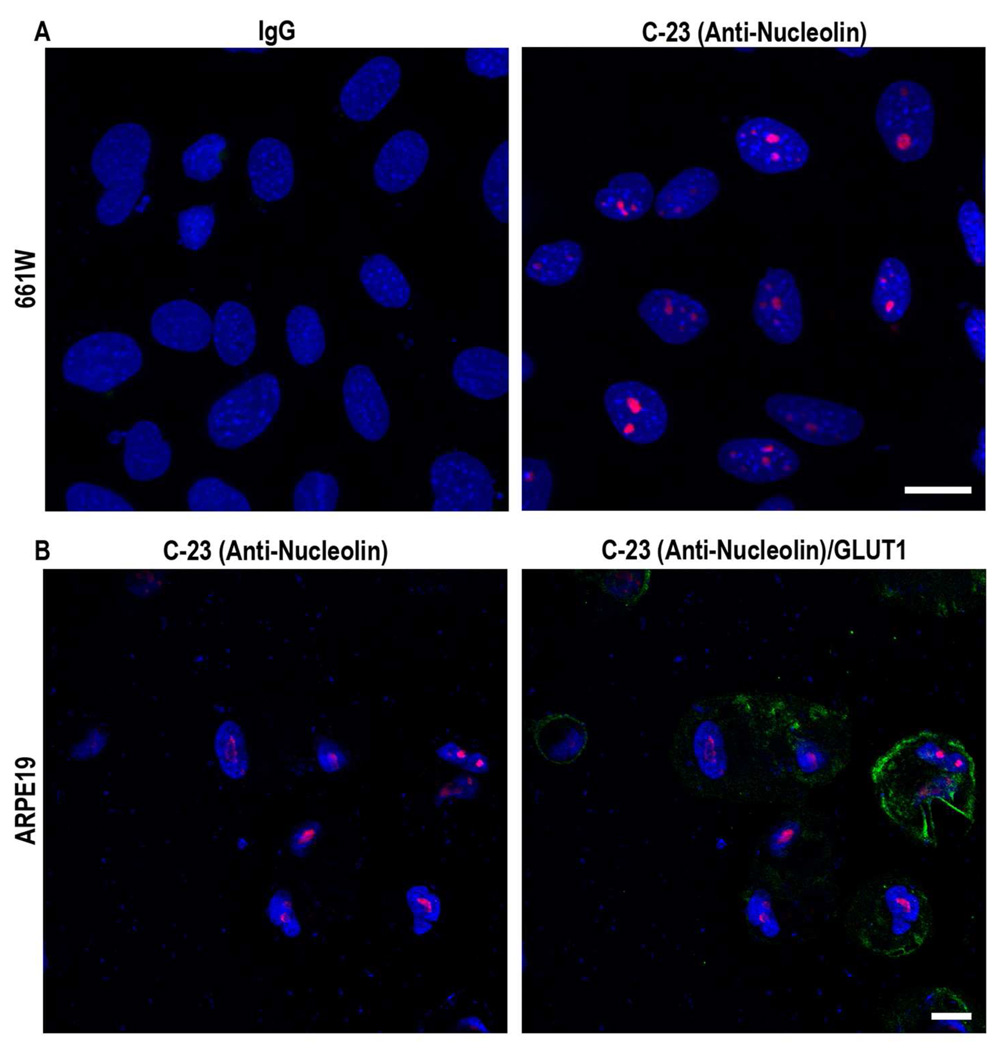
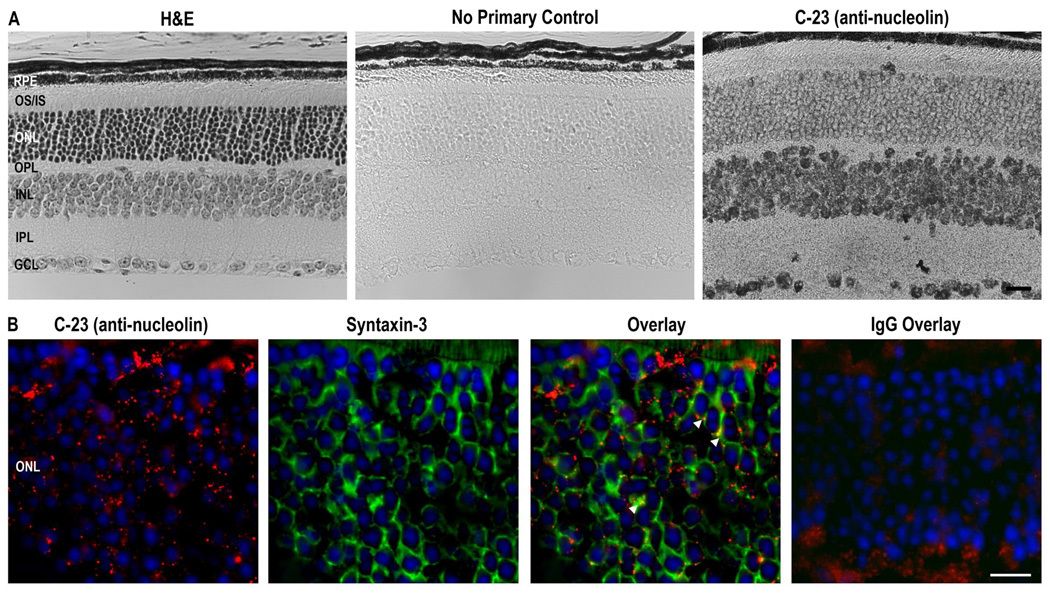
To study nanoparticle trafficking in the eye, we exposed two transformed ocular cell lines, 661W (transformed cone-derived cells) and ARPE19 (transformed RPE cells) to CK30-PEG nanoparticles containing a GFP reporter gene. In contrast to our results in vivo (Farjo, R. et al. 2006; Cai, X. et al. 2009) these cells did not efficiently express the nanoparticles (not shown). Subsequent immunocytochemistry revealed that these transformed cell lines do not express cell-surface nucleolin (Figure 2), although the protein is expressed inside the nucleus (red). These results provide additional, albeit indirect, support for the hypothesis that efficient nanoparticle uptake/trafficking is dependent on the presence of nucleolin on the plasma membrane. Since nanoparticles injected into the eye efficiently drive gene expression, we hypothesized that retinal cells, including photoreceptors, may express nucleolin on their surface. Immunohistochemistry on wild-type (WT) retinal sections (Figure 3A) revealed that nucleolin is expressed in the retina as expected. Significant nucleolin expression was detected in ganglion cell nuclei, and inner retinal cell nuclei, and in the perinuclear region of outer nuclear layer (ONL) of photoreceptor cells. It is also possible that RPE nuclei express nucleolin but the pigmentation in that layer made it difficult to confirm. To determine whether the perinuclear staining observed in the ONL was associated with the plasma membrane, immunofluorescent co-labeling with anti-nucleolin and the plasma membrane marker anti-syntaxin 3 was performed. Although the nucleolin antibody was not optimized for immunofluorescence, we observed punctate perinuclear anti-nucleolin labeling (Figure 3B, red), which co-localized with the plasma membrane marker (green-arrows), suggesting that nucleolin was expressed on the surface of photoreceptor cells.
Figure 2. Nucleolin is not expressed on the plasma membrane of cultured ocular cells.
Transformed cone-derived 661W cells (A) and transformed human RPE ARPE-19 cells (B) were fixed in 4% paraformaldehyde and stained with either mouse monoclonal C-23 MS-3 anti-nucleolin (red) or the plasma membrane marker rabbit polyclonal anti-GLUT1 (green) as indicated. 661W and ARPE-19 cells only expressed nucleolin within the nucleus. Co-labeling with GLUT1 did not reveal any expression of nucleolin on the plasma membrane. Scale bar, 10 µm.
Figure 3. Nucleolin is expressed in the murine retina.
Paraffin-embedded (A) or frozen (B) retinal sections from postnatal day (P) 30 wild-type (WT) mice were fixed in 4% paraformaldehyde. (A) Sections were stained with H&E, no primary antibody, or immunoreacted with C-23 MS-3 monoclonal anti-nucleolin antibody (1:5). The secondary antibody was gold-conjugated goat-anti-mouse IgG (5 nm gold particles, 1:1000). Nucleolin is expressed in the outer nuclear layer (ONL), inner nuclear layer (INL), and ganglion cell layer (GCL). (B) Sections were stained with rabbit polyclonal anti-syntaxin-3 (−1:500, green) to label photoreceptor plasma membrane and anti-nucleolin C-23 F-18 goat polyclonal antibody (1:5, red). Shown is a single plane from a confocal micrograph. Punctate co-labeling (yellow-arrows) suggests that nucleolin is expressed on the plasma membrane of photoreceptors. RPE, retinal pigment epithelium; OS/IS, outer/inner segments; OPL, outer plexiform layer; IPL, inner plexiform layer. Scale Bar, 20 µm.
4.5.3. Incorporation of Targeting Ligands
CK30-PEG nanoparticles appear to be trafficked by a nucleolin-mediated pathway. Incorporation of targeting ligands can help improve tissue specificity, and targeting might assist with uptake and trafficking when cells do not express cell surface nucleolin. Early targeting was achieved by covalent binding to the polylysine side chains, although currently targeting ligands are bound to the particles via the use of bi-functional PEG (Sun, W. et al. 2009). Various targeting ligands have been incorporated into polylysine-compacted DNA nanoparticles. Galactosylated polylysine was used to target particles to the asialoglycoprotein receptor expressed on human hepatoma (HuH-7) cells (Liu, G. et al. 2001). Co-exposure with the alternate ligand asialofetuin-ALF for this receptor inhibited nanoparticle-driven reporter gene expression by 70–90%, confirming the role of that receptor in uptake of the targeted particles. In most cases, efficient transfection was only observed for 15–30 nm particles, and ALF-mediated inhibition of transfection was only observed with these small particles (Liu, G. et al. 2001). This suggests that the small amount of residual transfection observed with the larger particles is not due to receptor-mediated uptake.
More often, however, targeting complexes are peptides rather than sugars. These peptides either target a specific receptor or fall into a class of cell-penetrating peptides, which are receptor independent. Common examples of cell targeting peptides, which have been used to target various types of nanoparticles include RGD peptides, which bind to integrin receptors; transferrin, an iron-binding peptide, and the CD13 binding peptide, all of which have been used to target PEG-PEI (polyethyleneimine) nanoparticles to various tissues (Juliano, R.L. et al. 2009). Cell penetrating peptides translocate through the plasma membrane without the assistance of cell surface receptors. In some instances, the peptide structure is α-helical, contains hydrophilic amino acids such as arginine and lysine, or contains a hydrophobic core. These features are hypothesized to contribute to the ability of the peptide to penetrate the plasma membrane.
Non-PEGylated lysine 100-mer–based nanoparticles have incorporated the synthetic targeting peptide C1315 (Ziady, A.G. et al. 1997; Ziady, A.G. et al. 1998), which targets the serpin enzyme comlex receptor (SEC-R) expressed on hepatocytes, macrophages, neurons, and airway epithelial cells (Ziady, A.G. et al. 2004). Experiments using the targeting peptide on the lysine side chain endeavored to determine the degree of substitution which would give the optimal transfection efficiency. Expression of the reporter gene was best when substitution is ~8–11 ligands per nanoparticle. This represents very low rates of polylysine substitution, and particles for which lysine substitution was higher yielded little or no gene expression (Ziady, A.G. et al. 1998). A second peptide targeting the SEC-R (Ziady, A.G. et al. 1997) has also been hypothesized to be a cell-penetrating peptide (Rhee, M. et al. 2006). When the peptide C105Y was added to nanoparticles at a 1:200 (molar ratio ligand:lysine) and delivered intravenously to mice, reporter gene expression was observed in the lung, liver, spleen, and tissue macrophages (Ziady, A.G. et al. 2004). C105Y-targeted particles containing the cystic fibrosis transmembrane regulatory (CFTR) gene have also been used to correct the electrical defect in nasal epithelial cells of mice lacking CFTR (Ziady, A.G. et al. 2002). Like untargeted nanoparticles, C105Y peptide is rapidly taken up by cells and transported to the nucleus. Live cell imaging studies detected free C105Y peptide in the nucleus as soon as 3 min after delivery to the surface of HuH-7 cells (Rhee, M. et al. 2006). To determine whether C105Y-mediated nanoparticle uptake is receptor-mediated or results from its cell penetrating properties, experiments were performed with 2 different fluorescently labeled forms of the peptide. Neither C105Y in which the amino acid order has been altered nor the D-isomer of C105Y interacted with SEC-R, but both were taken into the cell (Rhee, M. et al. 2006). Interestingly, SEC-R–mediated uptake occurred via clathrin-associated endocytosis, but uptake of C105Y did not occur through either the clathrin or caveolin mediated pathways (Rhee, M. et al. 2006). C105Y is found on intracellular membrane-bound vesicles, suggesting some form of endocytic, albeit non-degradative, uptake (Rhee, M. et al. 2006).
These results indicate that incorporation of a minimal number of targeting peptides efficiently promotes gene expression and suggest that incorporation of a single targeting peptide bi-functional PEGylation (Sun, W. et al. 2009) on each PEG-CK30 would be sufficient to promote efficient receptor-mediated uptake. The ability to target nanoparticles to specific cell types, and their subsequent uptake and rapid, non-degradative trafficking to the nucleus is an exciting option for increasing the tissue specificity of transfection and for enhancing the transfection of cell types that do not express cell-surface nucleolin.
4.6. Synthesis of CK30-PEG10K nanoparticles
For a review of the process for synthesis of the CK30-PEG nanoparticles, the reader is referred to (Liu, G. et al. 2003; Sun, W. et al. 2009); the process will be briefly reviewed herein.
4.6.1. Synthesis of CK30-PEG
The first step is the synthesis of the compaction peptide CK30. This process is carried out using solid-phase peptide synthesis and Fmoc chemistry. Although the procedures for synthesis are well established, significant optimization is required for the generation of a pure product at high yields. Peptides are built in the C to N direction, one amino acid at a time via Fmoc coupling. After the peptide is generated, it is cleaved from the resin and purified on a reverse-phase column usually with trifluoroacetate as the lysine counterion. This process can be used to generate targeting peptides as well as compacting peptides. After generation of the CK30, incubation overnight under specific conditions with PEG-maleimide leads to the formation of PEGylated CK30, which can be purified on an ion exchange column. If desired, the counterion can be switched to acetate by gel filtration (Liu, G. et al. 2003). If incorporation of a targeting peptide (in addition to the compaction peptide) is desired, bifunctional PEG (PEG-[OPSS]2) instead of PEG-maleimide should be used. This enables the PEG polymers to bind both the targeting and compaction peptides (Sun, W. et al. 2009).
4.6.2. Synthesis of CK30-PEG nanoparticles
The plasmid DNA used in compaction can be synthesized by normal methods; however, it is important that it be purified and relatively free of bacterial endotoxins to prevent an immune response. To make the nanoparticles, the DNA is slowly added to a vortexing mixture of CK30-PEG to a final molar charge ratio of 2:1 (amine:phosphate) (Liu, G. et al. 2003)`. The compacted DNA is filtered, solvent exchanged to saline and concentrated. If targeted particles are desired, the targeting ligand is then reacted with the compacted nanoparticles to add the additional peptide to the bifunctional PEG (Sun, W. et al. 2009).
4.6.3. Characterization of CK30-PEG nanoparticles
Particle consistency is paramount. Extensive characterization of nanoparticles is required before use as variations in size and shape can affect efficiency. Sedimentation analysis, an indicator for aggregates, involves characterization of the DNA concentration before and after brief centrifugation of the particles. Undesirable aggregated particles, if present, will spin out and decrease the DNA concentration. Turbidity parameter analysis is also straightforward; it involves generating a UV light scattering curve for the nanoparticles and fitting the rate of decay of scattering signal to Raleigh’s law. Unaggregated DNA nanoparticles will have a turbidity parameter slope of approximately −4 (Liu, G. et al. 2003; Ziady, A.G. et al. 2003). To further study the size and shape of the nanoparticles, electron microscopy can be performed. Since the function of the particles relies in part on their charge, dynamic light scattering can be used to test the zeta potential of the particles. Stability of the particles can be assessed by DNase 1 treatment, or incubation in serum and then trypsinization and agarose gel electrophoresis. Acceptable parameters for all these tests are published (Liu, G. et al. 2003; Ziady, A.G. et al. 2003; Sun, W. et al. 2009). Successfully prepared particles are stable for up to 3 years at 4°C when handled properly (Ziady, A.G. et al. 2003).
5. Application of CK30-PEG nanoparticles
5.1. Delivery of CK30-PEG nanoparticles to the lung
Initial studies tested the ability of CK30-PEG nanoparticles carrying the luciferase gene under the control of the CMV enhancer/promoter or the CMV enhancer/EIF-1 promoter (Ziady, A.G. et al. 2003). Nanoparticles were administered intranasally or intratracheally. Luciferase enzyme activity was dose-dependent from 10–100 µg nanoparticle concentration and plateaued from100 to 300 µg 2 days after instillation. Expression dropped off considerably over the course of approximately 2 weeks, although it did remain above background at post-injection day (PI)-12. This result was not unexpected since the CMV promoter is well-described to undergo transcriptional silencing. Some animals were given with nanoparticles condensed with CK30 without PEG (Ziady, A.G. et al. 2003). These animals did not show any appreciable luciferase expression in common with animals dosed with naked uncompacted DNA. No significant nanoparticle-mediated luciferase expression was observed in any tissues other than the lung and trachea. Within the lung, nanoparticle mediated gene expression was detected in the epithelial cells of small airways, and in medium airways and blood vessels. Expression was detected in multiple airways, but was usually patchy; ~50% of cells were transfected (Ziady, A.G. et al. 2003).
Complementary safety studies on the delivery of these nanoparticles to the murine lung demonstrated that they are reasonably safe and well tolerated (Ziady, A.G. et al. 2003). Mice were dosed intranasally with 10 or 100 µg of nanoparticles or various controls including saline, naked DNA, bacterial genomic DNA, and lipofectin-complexed DNA. No systemic inflammatory responses to the nanoparticles was reported. Within the lung, immune responses to low doses of nanoparticles were no different than those to saline infusion. At the 100 µg dose, trace-to-mild levels of mononuclear infiltrates were observed on post-injection day 2 (PI-2) with partial resolution by PI-10 and complete resolution by PI-28. Animals in this treatment group also had slightly elevated levels of bronchoalveolar KC (murine IL-8) and IL-6 1 to 2 days PI. This very modest immune response was significantly less than that observed in response to instillation of the positive control E. coli genomic DNA and equivalent to that incited by delivery of 20X less lipofectin conjugated DNA (Ziady, A.G. et al. 2003).
These promising preliminary results prompted the onset of a phase I/II clinical trial for nanoparticle-mediated delivery of the cystic fibrosis transmembrane regulatory (CFTR) gene to the nasal mucosa of cystic fibrosis patients (Konstan, M.W. et al. 2004). The primary endpoint for the trial was safety and tolerability, with secondary functional outcomes. Patients were given 1 of 3 doses (0.8, 2.67, or 8.0 mg) of nanoparticles containing the CMV promoter and the CFTR gene or placebo (saline) via nasal infusion into the right or left nares. The treatments were well-tolerated. No reportable adverse events were recorded and non-reportable adverse events were not thought to be related to the nanoparticles. At 3 and 13 days post-treatment, nasal epithelial cells were collected and analyzed for levels of nanoparticle DNA and message. On both days, nanoparticle DNA but not nanoparticle-driven CFTR message was detected. To determine whether the nanoparticles were able to provide any phenotypic improvement, recordings of nasal potential difference (NPD) responses to isoproterenol were performed both before and at various time points after treatment. NPD responses to isoproterenol are a measure of how much CFTR chloride current and therefore channel is present. Compared to healthy control participants, CFTR patients typically have very little response to isoproterenol. After nanoparticle treatment, 8 of the 12 study participants demonstrated improved NPD responses to isoproterenol. These improvements were independent of the nanoparticle dosage received and usually occurred between PI-1 and PI-7. One patient had an improvement on PI-28 (Konstan, M.W. et al. 2004). These results are not inconsistent with an absence of detectable transgenic CFTR mRNA; the sensitivity of the RT-PCR assays requires almost 100% reconstitution of CFTR channels to exceed the limit of detection while only 3–6% channel reconstitution appears to be sufficient for recovery of the NPD isoproterenol response (Konstan, M.W. et al. 2004). These encouraging results indicate that nanoparticles were well-tolerated and are capable of providing biologically meaningful endpoints.
5.2. Delivery of CK30-PEG nanoparticles to the brain
Most non-viral genetic therapies have had limited success transfecting the brain. Several studies examined the brain transfectivity of naked DNA and PEI- or lipid-based nanoparticles in the 100-nm range but reported little persistent gene expression (Schwartz, B. et al. 1996; Nimesh, S. et al. 2006; Oh, S. et al. 2007). It has been hypothesized that this was at least in part due to their relatively larger size and consequent inefficient diffusion through the brain extracellular matrix (Thorne, R.G. et al. 2006), since newer, smaller silica-based particles have yielded better transfection efficiency (Bharali, D.J. et al. 2005). Initial proof-of-principle experiments with CK30-PEG nanoparticles utilized the pZeo eGFP reporter vector which has eGFP under the control of the CMV promoter. The pZeo eGFP vector was delivered in either compacted or uncompacted (naked plasmid) form by intracerebral injection into the left striatum of adult rats. GFP expression was found in both neurons and glia on PI-4 but very few neurons continued to express the transgene by PI-21 (Yurek, D.M. et al. 2009). No expression was ever detected in animals injected with uncompacted plasmid. When a luciferase vector was used, a similar decrease in overall transgene expression was observed prompting the authors to use the polyubiquitin C (UbC) promoter, which is a promoter less prone to silencing. This approach decidedly reduced silencing and significant gene expression was observed up to eleven weeks PI (the duration of the study) (Yurek, D.M. et al. 2009). In similar studies, direct injection of a compacted UbC luciferase plasmid generated undiminished luciferase activity in the mouse brain for 1 year (Kaytor, M.D. et al. 2009). For the most part, expression was limited to the area of injection although some expression was also observed in the corpus callosum, suggesting the nanoparticles may have some migratory potential. Importantly minimal immune response was detected; low levels of expression of markers for macrophages/microglia and cytotoxic T-cells were observed but were limited to the injection tract and were not different between nanoparticle- and sham-injected animals. Subsequent experiments demonstrated that nanocompacted therapeutic genes such as glial cell line-derived neurotrophic factor (GDNF) can also be delivered similarly and successfully expressed in the rat brain (Yurek, D.M. et al. 2009). These experiments demonstrate that nanoparticles can be successfully delivered and their cargo genes safely and persistently expressed in both neuronal and glial brain tissue.
More recently, these particles have been used to deliver therapeutic GDNF in a Parkinson’s treatment model. Rats were given a unilateral 6-hydroxydopamine lesion, which generates a Parkinson’s-like condition. One currently explored treatment modality is to graft in embryonic ventral mesencephalic tissue to replace the lost dopaminergic neurons (Andereggen, L. et al. 2009). This approach, however, has been relatively unsuccessful in clinical trials. The lack of success has been attributed to the death of grafted cells shortly after implantation (Sortwell, C.E. et al. 2001) because of a lack of appropriate growth factors in the adult brain. This hypothesis is supported by the observation that supplementation with GDNF can help preserve cells (Kirik, D. et al. 2000; Yurek, D.M. et al. 2009). To determine the ability of GDNF expressing nanoparticles to mediate improvement in the post-graft phenotype, 5 weeks after the generation of the Parkinson’s lesion, GDNF-expressing nanoparticles were delivered to the affected area (Yurek, D.M. et al. 2009). One week later, the tissue graft was performed. Compared to non-treated animals, the nanoparticle-treated animals expressed significantly more GDNF on the injected side of the brain. Furthermore, statistically significant improvements in motor behavior as measured by spontaneous forelimb activity and attenuation of adverse rotational behavior were observed in graft recipients which had received nanoparticles compared with those rats who had received grafts and saline. These functional improvements correlated with structural improvements. Compared with graft-only recipients, nanoparticle pre-treated animals had significantly more TH+ cells and more fiber outgrowths from the graft (Yurek, D.M. et al. 2009).
5.3. Delivery of CK30-PEG nanoparticles to the eye
5.3.1. Reporter gene expression in the eye
CK30-PEG nanoparticles can also be successfully delivered and expressed in the eye. Initial experiments used nanoparticles carrying the pZeo eGFP plasmids, in which eGFP is transcriptionally-controlled by the CMV promoter. In initial experiments conducted in the eye, two different formulations were used: the rod-shaped acetate compacted particles similar to those used in the brain experiments and ellipsoid particles compacted with trifluoroacetate as the lysine counterion (Farjo, R. et al. 2006). Injection site and level of expression in the eye varied depending on the location of the delivery and the particle used. After intravitreal injection, both nanoparticles drove extremely high levels of gene expression in the lens of P30 WT mice. Only modest expression was found in the retina and very little expression was detected in the pigment epithelia/choroid/sclera (PECS). Furthermore, retinal expression was higher with the trifluoroacetate particles than with the acetate ones. In contrast, after subretinal injection, expression in the retina and PECS was substantially higher than in the lens, and the acetate particles generated the highest gene expression levels. Gene expression was observed in the ONL of photoreceptor cells, RPE, optic nerve head, and in the extra-ocular muscles. Expression lasted less than 7 days, which is consistent with the well-known effect of the CMV promoter to down-regulate. Interestingly it was observed that varying the quantity of material delivered enabled gene expression levels to be titrated to mimic endogenous levels of various ocular proteins (Farjo, R. et al. 2006). Naked DNA did not drive significant transgene expression.
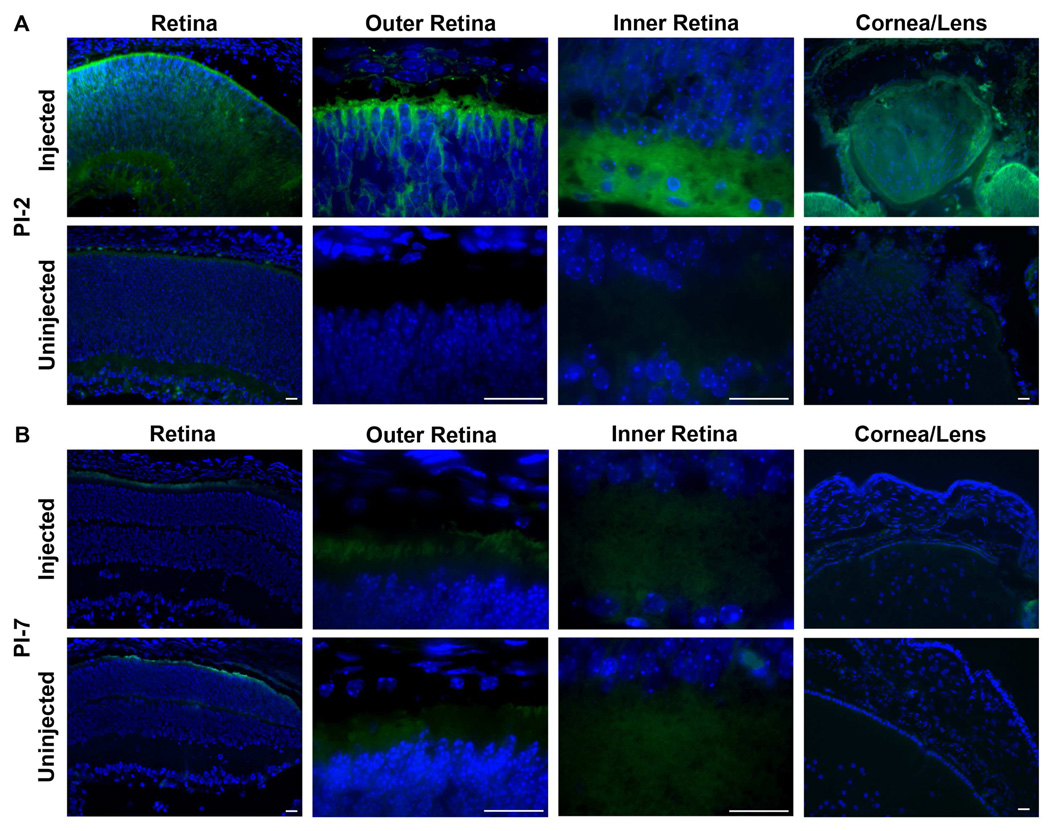
To determine whether nanoparticles could be successfully delivered and expressed in the developing, neonatal mouse eye, 300 nl of pZeo-GFP nanoparticles (4 µg/µl) were subretinally injected into post natal day 5 WT eyes (Figure 4). On PI-2, GFP expression was observed in the outer retina, inner retina, and ganglion cell layer (Figure 4A). Some expression may be seen in RPE cells, although the pigmentation of this cell layer makes it difficult to determine. Expression is also seen in the cornea and lens. By PI-7, expression is significantly reduced (Figure 4B), which is similar to results of nanoparticle injection in the adult eye, and most likely because of the silencing of the CMV promoter. Naked DNA- and saline-injected eyes do not exhibit any GFP fluorescence (Figure 4C). No expression of the transgene was found in the uninjected contralateral eyes. The expression of GFP in the cornea and lens after subretinal injection at P5 is likely because of the small size and the developing nature of the P5 eye and suggests that tissue-specific promoters and/or genes may need to be selected if concerns about ectopic expression are an issue.
Figure 4. CK30-PEG nanoparticles drive GFP expression after subretinal injection at postnatal day 5.
Wild-type (WT) mice underwent subretinal injection on postnatal day (P)5 with CK30-PEG nanoparticles containing the pZEO-GFP vector which incorporates the CMV promoter. Retinal sections were stained with DAPI and imaged. (A) On post-injection day (PI)-2, GFP expression was detected throughout the retina and in the cornea and lens. (B) On PI-7 no significant expression was detected, likely due to CMV promoter down-regulation. Scale bar, 25 µm.
5.3.2. Rescue of a RP model using CK30-PEG nanoparticles
Subsequent studies have investigated the ability of the CK30-PEG nanoparticles delivered by subretinal injection to mediate phenotypic rescue of retinal degenerative phenotypes and to drive more persistent, long-term expression. This work has utilized the rds+/− mouse model of autosomal dominant RP (ADRP) (Cheng, T. et al. 1997). This mouse exhibits early onset, slow rod degeneration that is followed by late-onset cone degeneration, and carries classic ADRP symptoms similar to those seen in patients with RDS mutations (Cheng, T. et al. 1997; Farjo, R. et al. 2006). This model is a loss-of-function model in which the disease phenotype arises from haploinsufficiency. We showed that genetic supplementation (via transgenesis) with WT RDS is capable of rescuing the phenotype (Nour, M. et al. 2004; Nour, M. et al. 2008) suggesting it would be a good model to test nanoparticles. The nanoparticles used for these studies were compacted with acetate as the lysine counterion forming rods, and they contained an expression cassette which had 1 of 3 promoters preceding the normal murine RDS cDNA (termed NMP) (Cai, X. et al. 2009; Cai, X. et al. 2009). The first promoter derived from the interphotoreceptor retinoid binding protein (IRBP) gene is known to drive gene expression in both rods and cones (Liou, G.I. et al. 1991; Yokoyama, T. et al. 1992). The second, the mouse opsin promoter (MOP), has been shown to drive very high levels of gene expression in rods with some minimal basal activity in cones, and was chosen because the primary early defect in rds+/− mice occurs in rods (Flannery, J.G. et al. 1997; Quiambao, A.B. et al. 1997). The final promoter chosen was the ubiquitously expressed chicken-beta actin (CBA) promoter.
After subretinal injection in P5 rds+/− mice, all 3 promoters drove high levels of gene expression, RDS levels (message and protein) in injected eyes stabilized at levels approximately 2 fold higher than RDS levels in uninjected, saline-injected, or uncompacted naked plasmid–injected eyes. These levels were stable for the 4-month study period(Cai, X. et al. 2009; Cai, X. et al. 2009). Encouragingly, our ongoing studies have demonstrated that when injected in this murine model, IRBP-NMP nanoparticles are capable of driving gene expression for up to 15 months, which is longest time point examined (unpublished data). Gene expression was observed in the rod and cone OS, which is the proper subcellular localization for RDS. No ectopic expression was observed elsewhere in the retina and only minimal expression observed in the RPE (Cai, X. et al. 2009). The lack of ectopic expression after delivery of CBA nanoparticles was surprising, but can be attributed to the cell-specific nature of the RDS transgene. RDS is a structural protein critical for the formation of OS. In cells that lack those structures, any RDS produced would likely be rapidly degraded.
Gene expression was detected throughout the retina, not just near the site of injection. Importantly, delivery of all three particles led to significant improvement in retinal structure as measured by histology/EM and expression of photoreceptor proteins, function as measured by ERG (Cai, X. et al. 2009; Cai, X. et al. 2009), and visual behavior as measured by optomotor tracking response (Cai, X. et al. 2009). These improvements persisted for 4 months PI. Structural improvement was more pronounced on the injected side of the eye. Even when gene expression was driven by the rod-dominant MOP promoter, improvements in cone function was significantly more pronounced than improvements in rod function, regardless of the promoter used. In a parallel study, MOP-NMP nanoparticles were delivered on P21 instead of P5 (Cai, X. et al. 2009). While the overall expression profile was decidedly similar after treatment on P5 or P21, including the location, duration and levels of transgene activity, rescue was significantly less pronounced after P21 injection (Cai, X. et al. 2009).
Several important observations have arisen out of this work, demonstrating the need for additional research and development. First, we noticed that compared to WT eyes, rds+/− eyes were slower to heal and regain function after subretinal injection of saline. This process is likely due to the ongoing degeneration or damage that occurs in rds+/− eyes, and its clinical implications are worth considering. Second, our observation that cones were more easily rescued than rods is likely due to a combination of two factors. RDS is differentially required by rods and cones; rods have a higher demand for RDS than cones. Thus, the rescue of rods may require higher expression levels than the rescue of cones, and rods begin to degenerate early in rds+/− eyes while cones degenerate later so treatment delivery, especially on P21, occurs after degeneration has already begun for rods while cones are essentially normal. This highlights the idea that it is easier to develop a preventative cure than a regenerative one, but underscores the difficulties that may arise from treating human patients whose onset of degeneration may precede presentation of the phenotype and clinical diagnosis. Results from our experiments with different treatment ages support this concept: treatment of the neonatal, which is still differentiating and not yet degenerating, rds+/− retina was more successful than treatment of the juvenile, post-mitotic P21 retina, although this difference was not because of differential transgene uptake or expression. Unfortunately, this treatment paradigm is not possible in the human retina, which is post-mitotic from birth.
5.3.3. Delivery of CK30-PEG nanoparticles to the RPE
While photoreceptors are the defective cell type in a large portion of inherited retinal degenerations, RPE-based diseases are also common. Deficiencies in visual cycle isomerohydrolase, RPE65, in the RPE lead to chromophore deficiency and the development of LCA. We generated CK30-PEG nanoparticles containing the RPE65 gene under the control of three different promoters: CMV, CBA, and the RPE-specific vitelliform macular dystrophy 2 (VMD2) promoter. Rpe65−/− animals underwent subretinal injection on P5, and mRNA levels were examined by real-time PCR on PI-2, PI-7, and PI-30. As we had previously observed, naked DNA did not drive appreciable gene expression. All three of the nanoparticles generated expression at PI-2, but by PI-30 levels in nanoparticle injected eyes had returned to baseline levels (unpublished data). This divergence in the ability of nanoparticles to drive long-term expression in photoreceptors and RPE is prompting investigation into vector engineering strategies designed to enhance long-term gene expression.
6. Toxicity of CK30-PEG nanoparticles
6.1. Lack of ocular toxicity
Of critical relevance to the development of nanoparticles for clinical ocular use is their ability to be well-tolerated. Toxicology data from animal and human lung studies indicate the particles were safe (Ziady, A.G. et al. 2003). WT mice exhibited full functional recovery after delivery of nanoparticles (Farjo, R. et al. 2006), suggesting that the nanoparticles were fairly non-toxic. Only surgically-associated minor toxic responses were reported from studies involving the brain (Yurek, D.M. et al. 2009). To understand the the ocular immune response to nanoparticles, P22 WT animals were subretinally injected with 0.3 µg, 1.0 µg or 3.0 µg pZEO eGFP CK30-PEG nanoparticles. Immune reactivity was evaluated at 1, 2, 4, or 7 days PI (Ding, X.Q. et al. 2009). No infiltration of polymorphonuclear neutrophils, lymphocytes, or macrophages was detected. We also examined expression of the pro-inflammatory chemokines IL-8 (known as KC in mice) TNF-α, and monocyte chemotactic protein 1 (MCP-1). We observed a transient increase in KC mRNA levels and in MCP-1 mRNA and protein levels on PI-1, which returned to baseline by PI-2. This increase was recorded in both nanoparticle-treated and saline-injected controls making this response likely to be a surgical artifact rather than a toxic effect of the nanoparticles. We did not detect any alterations in TNF-α levels (Ding, X.Q. et al. 2009). These results demonstrate that CK30-PEG nanoparticles are remarkably well-tolerated in the WT murine eye and do not induce significant inflammatory responses.
Previously, we observed that rds+/− eyes were more sensitive to subretinal injection–related damage than WT eyes and did not exhibit functional recovery, as assessed by ERG, as soon as WT (Nour, M. et al. 2003; Cai, X. et al. 2009). This is likely because of the structural features that accompany degeneration in that rds+/− eyes, but we could not rule out the possibility that rds+/− eyes undergoing degeneration may be more susceptible to nanoparticle-induced immune responses. To that end, we performed similar toxicologic profiling of rds+/− mice injected with nanoparticles. In these studies, animals were injected on either P5 or P22 with MOP-NMP CK30-PEG nanoparticles (Cai, X. et al. 2009). Compared to uninjected controls, rds+/− mice had similar levels of IL-6 and TNF-α mRNA levels on PI-2 or PI-30. No signs of macrophage infiltration were detected in nanoparticle dosed rds+/− eyes (Cai, X. et al. 2009). These data support the idea that nanoparticles are safe and well-tolerated after subretinal injection.
6.2. Lack of systemic toxicity and insertional mutagenesis
There is no evidence that subretinal delivery of nanoparticles leads to appreciable distribution outside the eye. No nanoparticle expression is observed in the contralateral uninjected eye of treated animals at either the protein or message level. Protein and message levels for both reporter genes and therapeutic genes in uninjected contralateral eyes are equivalent to those seen in uninjected animals (Farjo, R. et al. 2006; Cai, X. et al. 2009). In contrast to some forms of adeno-associated virus (AAV), nanoparticle expression in the brain after subretinal injection is not observed (unpublished data), further suggesting that there is no anterograde transport of nanoparticles to the brain. Gross measures of toxicity, such as body size, activity, and grooming habits were no different between nanoparticle- and saline-injected controls. Furthermore, nanoparticles administered to the murine lung do not elicit systemic toxicity. Serum chemistry, IL-6 levels, and serum complement levels were simlar in nanoparticle-treated mice and untreated mice, supporting the data on a lack of systemic toxicity (Ziady, A.G. et al. 2003). Similarly, no systemic toxicity in the cystic fibrosis clinical trial was attributed to the DNA nanoparticles (Konstan, M.W. et al. 2004). This is in stark contrast to the inflammatory syndrome observed after airway delivery of lipid-based complexes to human and murine lungs (Freimark, B.D. et al. 1998; Alton, E.W. et al. 1999; Ruiz, F.E. et al. 2001). While future studies are needed to fully examine potential systemic immune responses to subretinally delivered nanoparticles, current evidence suggests that any systemic toxicities will be minimal.
Insertional mutagenesis has been a concern with some previous viral vectors. Traditionally, non-viral vectors lack the capacity for genomic integration, which is considered a limitation since some have hypothesized that this lack contributes to a short duration of transgene expression and loss of transgene during cell division. Integrating non-viral vectors most often incorporate a system of transposases to enhance targeted integration. Since the CK30-PEG nanoparticles currently in use incorporate neither these transposase systems nor viral integrases, they are hypothesized to remain episomal. Studies investigating this issue are currently in progress.
7. Use of other nanoparticles in the eye
Studies utilizing the CK30-PEG nanoparticles for the treatment of RPE-based diseases and for the treatment of other photoreceptor diseases are ongoing, but other nanoparticles have also been tested for use in the retina. Many of them are still in the preliminary phases of development but show some promising characteristics.
7.1. Other polypeptide-based particles
Researchers have identified a cationic polypeptide, which shares features of the glycosaminoglycan-binding domain of fibroblast growth factor. This peptide termed POD was capable of binding to the cell surface and traversing the plasma membrane of human embryonic retinoblasts in vitro, and ganglion, photoreceptor, and RPE cells in vivo (Parker Read, S. et al. ; Johnson, L.N. et al. 2008; Johnson, L.N. et al. 2009). This peptide also has DNA condensation capabilities, and under defined conditions generates discrete spherical nanoparticles with a hydrodynamic diameter of approximately 130 nm (Figure 1E). The size of these particles is large compared to the CK30PEG nanoparticles formulated at Copernicus, but the particles are substantially compacted compared to the naked DNA with a hydrodynamic diameter ~1200 nm (Figure 1A) (Parker Read, S. et al. 2010). The stability of these particles and their resistance to cellular DNAses increased when condensed with PEG-POD, and the nanoparticles readily penetrated RPE cells. These PEG-POD nanoparticles were capable of driving high level expression of the reporter gene luciferase after subretinal injection into adult mice and did not cause any permanent decrease in retinal function (Parker Read, S. et al. 2010). The authors did not extend their experiments past 48 hours so the long-term gene expression profile of this nanoparticle remains to be defined. While much further development remains to be done before these particles can be considered an effective gene delivery vector for the treatment of chronic conditions, these preliminary results are noteworthy and suggest that various nanoparticle formulations have potential for therapy of ocular diseases.
7.2. Polylactide (PLA) and polylactide co-glycolide (PLGA) nanoparticles
PLA and polylactide co-glycolide PLGA nanoparticles are widely used for drug delivery. The degradation of microspheres composed of these polymers can be altered by changing the molecular weight and composition of the complexes (Pisal, D.S. et al. 2010). This enables sustained drug delivery, which is advantageous for the treatment of chronic diseases. However, the majority of these complexes are too large to be particularly effective for intracellular gene delivery. The generation of sub-micron– sized PLA and PLGA nanoparticles has helped to remedy this. Although these particles are too large (~140 nm) to pass through the nuclear pores, it may be possible to develop them into successful ocular gene delivery vehicles. Preliminary studies have demonstrated that they are reasonably non-toxic and that they are capable of trans-retinal passage after intravitreal delivery and are subsequently taken into RPE cells both in vitro and in vivo (Bejjani, R.A. et al. 2005). Although these experiments used only particles loaded with dye, they did persist in the outer retina for up to four months and are capable of delivering nucleic acids. Uptake into outer retinal cells after intravitreal administration is a substantial advantage if gene expression profiles are favorable.
Modified PLGA nanoparticles have been used for gene delivery. They have been shown to transfect Langerhans cells and drive gene expression after transdermal delivery (Lee, P.W. et al.). In addition, targeted PLGA nanoparticles ~220–420 nm in diameter (Figure 1G) containing the anti-VEGF intraceptor plasmid and labeled with an RGD peptide or transferrin have recently been shown to drive gene expression in a laser-induced choroidal neovascularization model after intravenous (IV) administration (Singh, S.R. et al. 2009). The success of IV delivery in this case is due to the upregulation of the integrin receptor αVβ3, which recognizes the RGD peptide, and increased iron accumulation, which is mediated by transferrin on newly forming blood vessels in choroidal neovascularization and ARMD. Little gene expression in the eye was observed when non-targeted particles were delivered by IV nor in the non-laser treated contralateral (i.e. no neovascularization) eye. These observations mean that IV delivery is not likely to be particularly effective for the treatment of photoreceptor- or RPE-affiliated retinal degenerative diseases unless they are associated with leaky blood vessels; however, RGD targeted nanoparticles were taken up and expressed both in vascular endothelial cells and RPE cells, due to the expression of αVβ3 on RPE cells. Although no extensive time course was carried out to study the persistence of transgene expression, targeted PLGA nanoparticles were clearly capable of transfecting some retinal cells and driving gene expression. Subretinal or intravitreal administration may lead to significant levels of gene expression. Another benefit of the use of PLGA/PLA for gene delivery is that these compounds are already FDA approved for the delivery of some ocular drugs, which should facilitate their approval for additional applications (Raju, H.B. et al. 2008).
8. Vector engineering to enhance efficiency
For ocular gene therapy to be successful, two components are needed: the nucleic acid and the complexing method, coating, or delivery vehicle. CK30-PEG (and POD-PEG) nanoparticles are excellent delivery candidates, but enhancements in the persistence of gene expression are still needed for some applications to be clinically relevant. Long term transgene expression in the eye when delivered as CK30-PEG nanoparticles has been demonstrated for some transgenes (Mark Cooper personal communication), and additional expression vector engineering strategies can be helpful to facilitate long-term transgene expression.
8.1. Vector integration
One of the limitations of some viral vectors has been random genomic integration leading to insertional mutagenesis and toxicity. One of the earliest high profile setbacks for gene therapy arose from insertional mutagenesis of a vector designed to treat severe X-linked immuno-deficiency. Integration led to trans-activation of cellular proto-oncogenes resulting in fatal leukemias in study participants (Hacein-Bey-Abina, S. et al. 2002; Hacein-Bey-Abina, S. et al. 2003). As a result, the tendency of non-viral vectors to stay episomal has been considered beneficial; however, some transgenes that stay episomal may not continue to be expressed and are not properly passed on to daughter cells if the transfected population is replicating. Therefore, in some cases, targeted integration may be useful.
8.1.1. Sleeping Beauty Transposon-Transposase
Two popular methods have been developed to promote more specific genomic integration of nonviral vectors. The first is the Sleeping Beauty Transposon Transposase (SBTT) system. A transposon is a sequence of DNA, which retains the ability to migrate from one chromosomal locus or piece of episomal DNA to another. This transition is mediated by a transposase enzyme. For gene therapy applications, the delivery vector contains the transposase expression cassette, which is flanked by transposon sequences, or consists of two separate plasmids. One plasmid contains the enzyme and the other, the transgene. The two plasmid approach is reportedly less efficient (Izsvak, Z. et al. 2009).
Integration is not sequence specific since the transposase does not recognize a single or defined set of consensus sequences. Insertion has been shown to be non-random, with some preference for microsatellite repeats, which are often found in non-coding DNA. Insertion does not tend to occur nearly as often in active genes as viral integration. Extensive characterization of the integration process has demonstrated that the integration site is most likely determined by the physical structure of the region (Liu, G. et al. 2005). Optimum integration and expression occurs within a very narrow range of transposase concentrations, but does not appear to be related to the orientation of the transgene expression cassette within the flanking transposons (Izsvak, Z. et al. 2009). Transposition is not, however, 100%. Recently, efforts have been made to increase the transposition efficiency by utilizing hyperactive transposases generated by systematic mutagenesis based on phylogenetic analysis. One hyperactive transposase, termed HSB17, was observed to be 17 times more active than the original transposase and has been successfully used to increase integration efficiency and subsequent gene expression in hematopoietic progenitor cells (Baus, J. et al. 2005). Although the use of these newer transposases appears to increase integration efficiency, there is also concern about post-integrative gene silencing and integration-mediated activation of other nearby genes. Integration may have led to activation of distal genes, although this has been attributed to the presence of activating factors in the expression cassette and not to the integration per se (Zhu, J. et al.). In addition, the integrated transgene may be hypermethylated at CpG sites, which correlates with decreased transgene expression, suggesting that exclusion of CpG islands from future expression cassettes may be a valuable approach (Zhu, J. et al.). The system has been used to successfully drive persistent gene expression in the lung and liver for as long as 3 to 5 months and has also been successful in driving integration in hematopoietic stem/progenitor cells and peripheral blood lymphocytes (Mikkelsen, J.G. et al. 2003; Belur, L.R. et al. 2007; Podetz-Pedersen, K.M. et al. 2009; Sumiyoshi, T. et al. 2009). Phase I/II clinical trials have begun in which a chimeric antigen receptor designed to redirect the specificity of T-cells is delivered using an SBTT vector system (Hackett, P.B. et al. 2010), the results of which will be eagerly awaited. Vectors containing SBTT sequences can theoretically be incorporated into viral or non-viral delivery methodology provided sufficient packaging capacity is available. Such vectors have already been successfully delivered as polyethylenimine nanoparticles to the murine liver and lung (Podetz-Pedersen, K.M. et al. 2009).
8.1.2. ΦC31 Integrase
The second integrating system popular for incorporation into non-viral vectors is the ΦC31 integrase system. The ΦC31 integrase system comprises a bacteriophage integrase delivered in a plasmid along with a transgene flanked by attB sites (Chalberg, T.W. et al. 2005). The ΦC31 system is considered to be site specific and natively induces recombination between attB sites and attP sites in the genome. In mammalian cells, the recombination is observed at a limited number of pseudo attP sites which share some limited sequence similarity (Chalberg, T.W. et al. 2006; Ehrhardt, A. et al. 2006). Simlar to the SBTT system, sequence is not thought to be the only factor determining integration site. The physical structure of the DNA is thought to also influence the probability of recombination. Significantly fewer integration sites are observed in the mammalian genome for the ΦC31 system compared to the SBTT system, and they are not observed to be in close proximity to cancer-associated genes, suggesting a low probability of insertionally-induced carcinogenesis (Ehrhardt, A. et al. 2005). The biggest limiting factor associated with the use of the ΦC31 system has been that significant chromosomal rearrangements and deletions have been observed along with integration of the transgene. This is thought to result from recombination between the transgene and the chosen chromosome and also recombination between pseudo attP sites on multiple chromosomes (Ehrhardt, A. et al. 2006). Alterations to the integrase have also been used to increase integration efficiency. For the ΦC31 system, a C-terminal nuclear localization sequence (NLS) has been to the integrase to enhance its transport from the cytosol to the nucleus where recombination occurs but use of this new construct has resulted in conflicting data (Liu, S. et al. ; Woodard, L.E. et al. 2009). In contrast, systematic mutagenesis has generated integrase variants with both increased integration efficiency and specificity. Significant post-integration silencing has also been observed as a result of both increased methylation and alterations in histone structure depending on the cell type (Aneja, M.K. et al. 2009). The ΦC31 system has been used to successfully drive transgene expression in frog, mice, rat, and bovine systems, and in the rat retina, in the murine lung and liver, and in human fibroblasts (Olivares, E.C. et al. 2002; Chalberg, T.W. et al. 2005; Liu, J. et al. 2006; Allen, B.G. et al. 2009; Aneja, M.K. et al. 2009; Schetelig, M.F. et al. 2009).
Although the integration techniques discussed above are considered to be significantly safer than those typically associated with retroviruses, they still carry some risk of insertional mutagenesis, because of the relatively high number of insertion sites created using SBTT and the high probability of chromosomal recombination or deletion caused by the ΦC31 system. Methods to increase persistence of expression for episomal vectors are being explored. Some include the development of vectors containing nuclear matrix attachment regions to help keep vectors in transcriptionally active regions, the development of self replicating vectors, and the development of vectors without regions prone to epigenetic silencing.
8.2. Maintenance of expression of episomal vectors
If incorporation of integrating systems is not desired, several methods exist to maximize duration and levels of transgene expression. Delivered genes typically include a coding region for the therapeutic or reporter gene of interest coupled with 3’ and 5’ regulatory regions. Because endogenous gene expression is controlled not only by proximal regulatory sequences but also by sequences that are often several hundred kilobases away from the coding region, it is not usually possible to reproduce the intact gene in delivery vectors. Once the delivered DNA reaches the nucleus, many epigenetic factors can influence subsequent expression. These include chromatin structure and the histone code, organization of the nucleus and location of the episomal vector therein, and the structure of the nuclear matrix and architecture (Jackson, D.A. et al. 2006). Vector modifications based on an understanding of these issues, and using endogenous or synthetic nucleotide elements, can be incorporated into plasmids to generate targeted, regulated “mini-genes” with optimized expression.
8.2.1. Minimizing silencing
Gene silencing is a complex issue that exhibits extraordinary tissue- and vector-based variability. Several mechanisms are thought to underlie gene silencing under certain conditions. Silencing is typically defined as a decrease in the transcription of available DNA and not as a decrease in expression because of vector loss or post-translational degradation. Transcriptional gene silencing can arise from the formation of non-transcribed heterochromatin. Experimentally, heterochromatin and euchromatin can be differentiated by the pattern of modification of their histones (for a review see (Jenuwein, T. et al. 2001; Richards, E.J. et al. 2002;Fischle, W. et al. 2003)). Modification of these histones alters the packaging and condensation of the DNA and silences regions of expressed euchromatin or activates repressed regions of heterochromatin. In many cases, the histone-modifying deacetylases and methyltransferases work together to spread heterochromatin thus stably repressing entire genetic loci (Richards, E.J. et al. 2002). This spreading process can be limited by the presence of insulating barrier regions such as inverted repeats (Noma, K. et al. 2001). In the case of transgenes, incorporation into heterochromatin after random integration is often cited as a cause for silencing, which can be overcome by incorporating insulating units. Several regulatory sequences that have insulating properties have been described (Bell, A.C. et al. 2001) including scaffold/nuclear matrix attachment regions (S/MARs) (Jackson, D.A. et al. 2006).
Heterochromatin spreading can cause episomal transgene silencing (Riu, E. et al. 2007). It has been established that some prokaryotic plasmid backbone sequences are associated with heterochromatin-like histone patterns (Suzuki, M. et al. 2006; Riu, E. et al. 2007) and spreading of these regions is thought to underlie one mechanism for vector silencing. Data supporting this hypothesis come from several experiments. One experiment demonstrated that transgene silencing was partially inhibited by the incorporation of chicken cHS4-insulating regions on either end of the plasmid sequence, thus flanking the expression cassette (Chen, Z.Y. et al. 2008). Another experiment delivered plasmid DNA in one of three forms: intact, after a linearizing single cut, or after two cuts (yielding only the expression cassette). Expression of two different genes from multiple different vectors was studied, and in all cases, persistent gene expression up to 90 days was observed only in animals that received the purified expression cassette (Chen, Z.Y. et al. 2004). In subsequent tests, a plasmid containing the human clotting factor IX (FIX) flanked by two I-SceI restriction sites was co-delivered with a plasmid containing the I-SceI endonuclease, resulting in the generation of circles containing bacterial backbone and circles containing only the expression cassette. Mice receiving both vectors exhibited transgene expression for 8 months. Mice who received only the FIX plasmid, but not the I-SceI plasmid had no persistent gene expression, providing further support that a covalent attachment between the bacterial sequence and the expression cassette is required for heterochromatin spreading–based silencing (Riu, E. et al. 2005).
Delivery of linear DNA is possible, but producing large amounts of highly purified expression cassette can be difficult. Also, delivery of multiple vectors to facilitate in vivo recombination is hard to control. To facilitate delivery of plasmids containing only the expression cassette, termed minicircles, vectors were designed that contained the expression cassette of interest flanked by ΦC31 integrase recognition sequences (attP/B) and the ΦC31 integrase expression cassette under the control of a bacterial promoter (Chen, Z.Y. et al. 2003). The E. coli containing the plasmid were cultured under standard conditions then expression of the integrase was induced by L-arabinose thus stimulating site-specific recombination. The minicircles were then purified by removal of the bacterial backbone and used for delivery (Chen, Z.Y. et al. 2005; Mayrhofer, P. et al. 2008). Similar to linear expression cassettes, these vectors drove persistent gene expression in mice in vivo, (Chen, Z.Y. et al. 2003; Chen, Z.Y. et al. 2005). Minicircles have been shown to drive gene expression in skin (Yoon, C.S. et al. 2009) and skeletal muscle (Chang, C.W. et al. 2008; Stenler, S. et al. 2009) in addition to liver (Chen, Z.Y. et al. 2003). In addition, intramyocardial injection of minicircle vectors containing reporter genes drove gene expression in cardiomyocytes for up to 12 weeks. When minicircles carrying the therapeutic gene HIF-1α were delivered to the heart, they mediated improcement in a murine model of myocardial infarction (Huang, M. et al. 2009). These data suggest that delivery of minicircle vectors may be a way to overcome plasmid-mediated gene silencing.
The role of CpG methylation in silencing is a controversial one. Silencing of transgenes delivered by lipofectamine was eliminated by treatment with the DNA methyltransferase inhibitor 5-azacytidine (Hong, K. et al. 2001) in in vitro studies in C3A human hepatoblastoma cells, suggesting that methylation may be associated with silencing. Similarly, when murine embryonic stem cells were transduced with lentiviral vectors, silencing correlated with promoter region hypermethylation and alterations in histone structure, suggesting that methylation changes may play a role in signaling chromatin alterations (He, J. et al. 2005). In other studies, insertion of an S/MAR region upstream of the expression cassette inhibited promoter methylation and gene silencing (Jenke, A.C. et al. 2004; Argyros, O. et al. 2008). In comparison experiments in the lung, levels of expression with polyethyleneimine based CpG-free vectors were higher than with standard vectors and elicited lower levels of inflammation because of a reduction in the number of unmethylated CpG islands (Hyde, S.C. et al. 2008). On the other hand, Mark Kay’s group studied gene expression in the murine liver after delivery of plasmids depleted of CpG islands, or delivery of linear expression cassettes and linearized plasmids, containing bacterial backbone and either methylated or unmethylated cytosines, which are known to concatemerize in vivo (Chen, Z.Y. et al. 2008). They reported that the methylation status of the bacterial backbone was not relevant to gene expression: both methylated and unmethylated concatemers were silenced rapidly. The authors confirmed that this did not result from methylation of the unmethylated DNA after transfection, and further confirmation was provided by their subsequent observation that depletion of CpGs from the bacterial backbone did not eliminate silencing (Chen, Z.Y. et al. 2008). These straightforward experiments suggest that CpG islands and cytosine methylation in the liver do not control silencing of vectors containing HAAT and HFIX under the control of the RSV promoter (Chen, Z.Y. et al. 2008). It is not clear from the literature what the distinct roles of promoter methylation and heterochromatin spreading may be. Results appear to vary in a tissue- and vector-specific manner, and future studies will need to address this further.
8.2.2. Incorporation of nuclear targeting
The nucleus is not a homogeneous compartment and gene expression requires that the region of the chromosome of interest or the episomal vector be able to access transcriptionally active sites and ideally avoid sites where no transcription is occurring. It has been shown that RNA polymerase and transcription complexes are associated with the nuclear cytoskeleton (Cook, P.R. 1999) and it is hypothesized that these complexes have a comparatively fixed location within the nucleus while the chromatin will be in motion (Cook, P.R. 1999). The nuclear cytoskeleton is composed of a filamentous laminar layer that is adjacent to the inner nuclear membrane and an internal network. The nuclear matrix is a fibrogranular structure containing both filamentous nucleoskeletal proteins such as lamin filaments and a nuclear isoform of β-actin and scaffolding proteins designed to bind DNA/RNA such as scaffolding factors A and B (SAF-A/B) and attachment region binding protein (Jackson, D.A. et al. 2006).
These scaffold proteins, particularly SAF-B, are known to interact with specific DNA elements called scaffold/matrix attachment regions (S/MARs) to form chromatin loops (Jackson, D.A. et al. 2006; Harraghy, N. et al. 2008). S/MARs are thought to promote gene transcription both by modulating superhelical stress (Bode, J. et al. 2000) using their AT rich regions (Jenke, A.C. et al. 2004) and by targeting domains to transcriptionally active regions of the nucleus (Jackson, D.A. et al. 2006). Furthermore, evidence suggests that vectors containing S/MAR regions are likely to be associated with modified histones characteristic of active chromatin (Fernandez, L.A. et al. 2001; Rupprecht, S. et al. 2009). Finally, there is also some evidence that inclusion of an S/MAR region may provide an insulating effect by inhibiting promoter region methylation and silencing as seen for CMV and HAAT promoters (Jenke, A.C. et al. 2004; Argyros, O. et al. 2008). The role of methylation in gene silencing and the reported effects of inclusion of S/MARs on silencing of the CMV promoter are variable.
The S/MAR region of the human β-interferon gene is one of the most well-characterized (Bode, J. et al. 1992; Jenke, A.C. et al. 2004) and can be incorporated into gene therapy plasmids. S/MAR elements have been used in both viral and non-viral vectors for gene therapy, and efficacy of these vectors for promoting enhanced duration of expression was variable (Argyros, O. et al. 2008; Harraghy, N. et al. 2008). The effects were both tissue and cell type-specific and dependent on the other components of the vector like promoter regions and bacterial sequences. Inclusion of the S/MAR did not provide benefit in some instances, while substantial improvements in the gene expression profile were observed for others. For example, when an S/MAR was introduced into an HSV-1 vector and injected into the rat brain, neuronal expression was observed, but the S/MAR did not attenuate the traditional HSV-1-associated silencing (Makarova, O. et al. 1996). In stark contrast, hydrodynamic delivery of a naked vector containing this S/MAR region, the HAAT promoter, and the luciferase gene to the murine liver resulted in elevated levels of reporter gene expression for 6 months after treatment while self-replicating plasmids and non-replicating plasmids that lacked S/MARs were effectively silenced within 1 week (Argyros, O. et al. 2008). S/MAR containing vectors have also been used to drive transgene expression in hematopoietic stem cells (Papapetrou, E.P. et al. 2006) and T-cells (Cooper, L.J. et al. 2004) with variable levels of efficiency.
Work using S/MAR regions in non-viral therapies for ocular applications is ongoing. Results from neuronal cells suggest that S/MARs may not be particularly helpful for promoting gene expression in photoreceptors or retinal interneurons, although the use of viral vectors in brain studies may not be applicable to the non-viral vectors currently in use. Of note, long-term (1 year) expression in the mouse striatum (Kaytor et al, 2009) was achieved following delivery of CK30-PEG nanoparticles containing a luciferase plasmid incorporating a polyubiquitin C promoter and two S/MAR domains flanking the eukaryotic cassette. Interestingly, the tyrosinase gene, which is highly expressed in RPE during development and is involved in the formation of melanin pigment, has an S/MAR in its 5’ region (Porter, S.D. et al. 1999). This S/MAR has been shown to confer cell type–specific position independence on transgene transcription. Multiple transgenic mouse lines containing the human tyrosinase cDNA, promoter region, and S/MAR express the transgene in retinal melanocytes including RPE while transgenes were not expressed or were found at very low levels in lines which the transgene was only composed of the cDNA and promoter region (Porter, S.D. et al. 1999). These data provide indirect evidence to suggest that the RPE may be amenable to S/MAR-mediated improvements in gene expression, which is particularly exciting given the need to improve nanoparticle-mediated RPE expression.
8.2.3. Self-replicating vectors
A chronic problem with non-viral and non-integrating plasmids is vector loss during cell division and subsequent decreases in gene expression. To overcome this issue, significant effort has been invested in incorporating features that promote replication into non-viral vectors. These include viral and eukaryotic replicons or minimal parts thereof (Mazda, O. 2002; Lipps, H.J. et al. 2003; Jenke, A.C. et al. 2004). Incorporation of effective mammalian origins of replication has been difficult as it is well established that selection of these relies on epigenetic factors such as nuclear organization and chromosome structure rather than on specific DNA sequences (Gilbert, D.M. 2002; Gilbert, D.M. 2004). Mammalian replication initiation zones can bind to the nuclear matrix before replication begins and inclusion of S/MARs can confer replicability in some cell lines (Piechaczek, C. et al. 1999; Schaarschmidt, D. et al. 2004; Papapetrou, E.P. et al. 2006). Several effective self-replicating vectors have been developed for testing as gene delivery tools, but the post-mitotic nature of mature retinal cells means that vector loss as a result of cell division is not likely to be a primary mechanism for lack of gene expression; therefore, use of self-replicating vectors is unlikely to promote improved gene expression in the retina. Dr. Hans Lipps’ group (Jackson, D.A. et al. 2006) provides an excellent, more in depth discussion of this issue.
9. Incorporation of physical methods and non-invasive imaging into non-viral gene delivery
Physical methods have been incorporated into gene delivery approaches for one of two reasons. The first was to increase or target uptake of the vector. The second and more recent was for improved in vivo imaging and diagnostics. Electroporation is by far the most common methodology; other physical methods include ultrasound, magnetofection, iontophoresis, and hydrodynamic delivery (Andrieu-Soler, C. et al. 2006; Andrieu-Soler, C. et al. 2006; Conley, S.M. et al. 2008). These methods all induce temporary holes in the plasma membrane, allowing vectors to be taken up more efficiently. While this issue is of great significance for vectors with relatively low transfection efficiencies such as naked DNA and many lipid-based gene carrier systems, cell uptake does not appear to be the limiting factor for persistent gene expression and rescue in cases of ocular delivery of polymeric- or polypeptide-based nanoparticles (Cai, X. et al. 2009; Cai, X. et al. 2009).
Non-invasive imaging modalities have improved the ability to track and image delivered nanoparticles. Long-term study of treated animals is costly and time consuming when multiple animals have to be sacrificed at every study timepoint. Recent advances in rodent and small animal imaging technology have made real-time, in vivo analysis possible, providing the delivery vector can be tracked. Three imaging methods are currently popular. Bioluminescent imaging (BLI) is used to track expression of bioluminescent genes such as luciferase, and has been used to evaluate luicferase activity following CK30-PEG nanoparticle delivery to the brain, eye, and lung (Kaytor, M.D. et al. 2009; Sun, W. et al. 2009; Yurek, D.M. et al. 2009). Positron emission tomography (PET) and small animal magnetic resonance imaging (MRI) can be used to track the actual particles, if the proper contrast agents are incorporated during particle synthesis (Sun, W. et al. 2009). Furthermore, BLI and PET can be used to track the quantity of particles or level of gene expression in given locations. Finally, the special resolution of small animal MRI is now around 100 µm, so detection can be highly specific. Successful tracking of gene expression levels using BLI requires that the plasmid that is compacted by the nanoparticle or any other gene delivery vector contain a bioluminescent gene. On the other hand, MRI and PET require the incorporation of metal-chelating agents such as the macromolecular chelator DOTA into the generation of the compacting peptide. DOTA amines can be incorporated into CK30 peptide synthesis (C-DOTA-K30) and then delivered along with metal contrast agents such as gadolinium (Gd) (Sun, W. et al. 2009). In conjunction with BLI, these imaging technologies can convey information about both where the delivered particles are, and where they are being expressed. More recently developed imaging technologies, which utilize different metals, can be used to detect changes in pH (Querol, M. et al. 2008). These techniques can enable determination of not only the tissue distribution of delivered nanoparticles but also the cellular compartments in which they are taken. These new applications can serve both to enhance our knowledge of the pharmacological properties of nanoparticles and provide a new diagnostic application for them.
10. Options for streamlining therapeutic design for retinal gene therapy
In spite of the multitude of good gene delivery vehicles currently available, a serious lingering concern is how to target the vast array of retinal disease-causing mutations in a cost-effective manner. Since many disease-causing genes contain multiple disease-causing gain-of-function mutations, an approach to minimize the number of different delivery vectors required for each gene is mutation-independent knockdown of the endogenous allele coupled with delivery of an RNAi-resistant WT allele. Delivery of shRNA is a common method for treating gain-of-function mutations and can be packaged in almost any gene delivery vehicle including CK30-PEG nanoparticles (Mark Cooper, personal communication). Although this dual approach is elegant, practical application is complex and requires extensive optimization. Initial experiments in cultured retinal explants identified an shRNA that effectively knocked down endogenous rhodopsin levels by more than 75% in 88% of transfected cells (Kiang, A.S. et al. 2005). Furthermore, a normally translated mutant rhodopsin construct was designed which was significantly more resistant to the shRNA than the endogenous opsin. Recently this preliminary work was followed up by work in a transgenic rhodopsin murine model of dominant RP (the Pro347Ser mouse). The authors demonstrated that their shRNA can mediate significant suppression in vivo and that retinal structure and function can be improved when the WT replacement gene is delivered by transgenesis (i.e. P347S mouse on a WT Rho+/+ background) (Chadderton, N. et al. 2009). The next step will be actual dual delivery of the knockdown and replacement therapies in vivo as proof-of-principle for this exciting idea.
Treatment of recessive diseases or dominantly inherited diseases caused by haploinsufficiency is theoretically much more straightforward, requiring only gene supplementation. However, the vast number of individual disease causing genes still makes a broadly applicable therapeutic option desirable. An approach to address the multitude of disease-causing genes has been the delivery of neurotrophic factors. Ideally, this approach is independent of the disease mutation and the same vector might be useful for the treatment of multiple forms of degeneration. Brain derived neurotrophic factor (BDNF), ciliary derived neurotrophic factor (CNTF), and glial cell line-derived neurotrophic factor (GDNF) have all been tested. BDNF was recently delivered by subretinal injection followed by electroporation to the retinas of Royal College of Surgeons rats (a model of early onset RP) (Zhang, M. et al. 2009). The authors reported improvements in the number of rows of photoreceptor nuclei and reductions in the number of TUNEL-positive cells in treated animals, suggesting that BDNF may have the ability to improve photoreceptor survival in a degenerative eye mouse model. Similar improvements in photoreceptor survival were reported when GDNF-secreting stem cells (Gregory-Evans, K. et al. 2009) were injected into a murine transgenic rhodopsin murine model of RP, although the stem cells themselves induced some adverse events. Improvements were also reported when AAV-containing the GDNF gene was injected into an RDS RP model (Buch, P.K. et al. 2006). Results with CNTF have been mixed; while CNTF treatment was reported to enhance retinal survival, it was also shown to be associated with decreases in retinal function as measured by ERG (Schlichtenbrede, F.C. et al. 2003; Buch, P.K. et al. 2006). These results suggest that delivery of neurotrophic factors can delay degeneration. However, it is not clear what the ultimate result of such a delay will be. In many cases, functional deficits in vision may still be expected to arise due to the underlying defect. Combination therapy with a neurotrophic factor and a gene replacement vector has been tried with co-delivery of CNTF and RDS (Schlichtenbrede, F.C. et al. 2003). Unfortunately, the negative effects of CNTF cancelled out any positive benefits of gene supplementation; perhaps a different neurotrophic factor will provide beneficial effects. This dual approach, however, does not address the issue of the vast number of retinal disease-causing genes.
11. Concluding remarks
Polypeptide-based nanoparticles, particularly CK30-PEG, are becoming excellent candidates for retinal gene delivery vehicles. They are highly and persistently expressed throughout the retina and are safe and well-tolerated. Incorporation of vector-engineering strategies designed to enhance plasmid expression profiles may help improve phenotypic rescue in rod and cone photoreceptors and in RPE cells. The ability to target nanoparticles to different cell types and/or regions of the cell make them customizable for diverse applications. The remaining concerns facing the use of these vectors are how to efficiently target the vast array of disease genes, and how to cure genetic diseases diagnosed after the onset of degeneration.
Acknowledgments
The preparation of this manuscript was supported by NIH EY010609 & EY018656 (MIN) and EY018512 (SMC) and the Foundation Fighting Blindness. We thank Dr. Mark Cooper for helpful comments on the manuscript, and Dr. Heidi Stricker and Mr. Michael Stuck for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acland GM, Aguirre GD, Bennett J, et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther. 2005;12:1072–1082. doi: 10.1016/j.ymthe.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen BG, Weeks DL. Bacteriophage phiC31 integrase mediated transgenesis in Xenopus laevis for protein expression at endogenous levels. Methods Mol Biol. 2009;518:113–122. doi: 10.1007/978-1-59745-202-1_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alton EW, Stern M, Farley R, et al. Cationic lipid-mediated CFTR gene transfer to the lungs and nose of patients with cystic fibrosis: a double-blind placebo-controlled trial. Lancet. 1999;353:947–954. doi: 10.1016/s0140-6736(98)06532-5. [DOI] [PubMed] [Google Scholar]
- Andereggen L, Meyer M, Guzman R, et al. Effects of GDNF pretreatment on function and survival of transplanted fetal ventral mesencephalic cells in the 6-OHDA rat model of Parkinson's disease. Brain Res. 2009;1276:39–49. doi: 10.1016/j.brainres.2009.04.021. [DOI] [PubMed] [Google Scholar]
- Andrieu-Soler C, Bejjani RA, de Bizemont T, et al. Ocular gene therapy: a review of nonviral strategies. Mol Vis. 2006;12:1334–1347. [PubMed] [Google Scholar]
- Andrieu-Soler C, Doat M, Halhal M, et al. Enhanced oligonucleotide delivery to mouse retinal cells using iontophoresis. Mol Vis. 2006;12:1098–1107. [PubMed] [Google Scholar]
- Aneja MK, Geiger J, Imker R, et al. Optimization of Streptomyces bacteriophage phi C31 integrase system to prevent post integrative gene silencing in pulmonary type II cells. Exp Mol Med. 2009;41:919–934. doi: 10.3858/emm.2009.41.12.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyros O, Wong SP, Niceta M, et al. Persistent episomal transgene expression in liver following delivery of a scaffold/matrix attachment region containing non-viral vector. Gene Ther. 2008;15:1593–1605. doi: 10.1038/gt.2008.113. [DOI] [PubMed] [Google Scholar]
- Baehr W, Frederick JM. Naturally occurring animal models with outer retina phenotypes. Vision Res. 2009;49:2636–2652. doi: 10.1016/j.visres.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bainbridge JW, Ali RR. Success in sight: The eyes have it! Ocular gene therapy trials for LCA look promising. Gene Ther. 2008;15:1191–1192. doi: 10.1038/gt.2008.117. [DOI] [PubMed] [Google Scholar]
- Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Baus J, Liu L, Heggestad AD, et al. Hyperactive transposase mutants of the Sleeping Beauty transposon. Mol Ther. 2005;12:1148–1156. doi: 10.1016/j.ymthe.2005.06.484. [DOI] [PubMed] [Google Scholar]
- Bejjani RA, BenEzra D, Cohen H, et al. Nanoparticles for gene delivery to retinal pigment epithelial cells. Mol Vis. 2005;11:124–132. [PubMed] [Google Scholar]
- Bell AC, West AG, Felsenfeld G. Insulators and boundaries: versatile regulatory elements in the eukaryotic. Science. 2001;291:447–450. doi: 10.1126/science.291.5503.447. [DOI] [PubMed] [Google Scholar]
- Belur LR, Podetz-Pedersen K, Frandsen J, et al. Lung-directed gene therapy in mice using the nonviral Sleeping Beauty transposon system. Nat Protoc. 2007;2:3146–3152. doi: 10.1038/nprot.2007.460. [DOI] [PubMed] [Google Scholar]
- Berson EL, Rosner B, Sandberg MA, et al. A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Arch Ophthalmol. 1993;111:761–772. doi: 10.1001/archopht.1993.01090060049022. [DOI] [PubMed] [Google Scholar]
- Bharali DJ, Klejbor I, Stachowiak EK, et al. Organically modified silica nanoparticles: a nonviral vector for in vivo gene delivery and expression in the brain. Proc Natl Acad Sci U S A. 2005;102:11539–11544. doi: 10.1073/pnas.0504926102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield VA. DNA condensation by multivalent cations. Biopolymers. 1997;44:269–282. doi: 10.1002/(SICI)1097-0282(1997)44:3<269::AID-BIP6>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Bode J, Benham C, Knopp A, et al. Transcriptional augmentation: modulation of gene expression by scaffold/matrix-attached regions (S/MAR elements) Crit Rev Eukaryot Gene Expr. 2000;10:73–90. [PubMed] [Google Scholar]
- Bode J, Kohwi Y, Dickinson L, et al. Biological significance of unwinding capability of nuclear matrix-associating DNAs. Science. 1992;255:195–197. doi: 10.1126/science.1553545. [DOI] [PubMed] [Google Scholar]
- Bondi ML, Craparo EF. Solid lipid nanoparticles for applications in gene therapy: a review of the state of the art. Expert Opin Drug Deliv. 2010;7:7–18. doi: 10.1517/17425240903362410. [DOI] [PubMed] [Google Scholar]
- Boon CJ, den Hollander AI, Hoyng CB, et al. The spectrum of retinal dystrophies caused by mutations in the peripherin/RDS gene. Prog Retin Eye Res. 2008;27:213–235. doi: 10.1016/j.preteyeres.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Bressler SB. Introduction: Understanding the role of angiogenesis and antiangiogenic agents in age-related macular degeneration. Ophthalmology. 2009;116:S1–S7. doi: 10.1016/j.ophtha.2009.06.045. [DOI] [PubMed] [Google Scholar]
- Buch PK, MacLaren RE, Duran Y, et al. In contrast to AAV-mediated Cntf expression, AAV-mediated Gdnf expression enhances gene replacement therapy in rodent models of retinal degeneration. Mol Ther. 2006;14:700–709. doi: 10.1016/j.ymthe.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Cai X, Conley SM, Nash Z, et al. Gene delivery to mitotic and postmitotic photoreceptors via compacted DNA nanoparticles results in improved phenotype in a mouse model of retinitis pigmentosa. FASEB J. 2009 doi: 10.1096/fj.09-139147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Nash Z, Conley SM, et al. A partial structural and functional rescue of a retinitis pigmentosa model with compacted DNA nanoparticles. PLoS One. 2009;4:e5290. doi: 10.1371/journal.pone.0005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadderton N, Millington-Ward S, Palfi A, et al. Improved retinal function in a mouse model of dominant retinitis pigmentosa following AAV-delivered gene therapy. Mol Ther. 2009;17:593–599. doi: 10.1038/mt.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalberg TW, Genise HL, Vollrath D, et al. phiC31 integrase confers genomic integration and long-term transgene expression in rat retina. Invest Ophthalmol Vis Sci. 2005;46:2140–2146. doi: 10.1167/iovs.04-1252. [DOI] [PubMed] [Google Scholar]
- Chalberg TW, Portlock JL, Olivares EC, et al. Integration specificity of phage phiC31 integrase in the human genome. J Mol Biol. 2006;357:28–48. doi: 10.1016/j.jmb.2005.11.098. [DOI] [PubMed] [Google Scholar]
- Chang B, Dacey MS, Hawes NL, et al. Cone photoreceptor function loss-3, a novel mouse model of achromatopsia due to a mutation in Gnat2. Invest Ophthalmol Vis Sci. 2006;47:5017–5021. doi: 10.1167/iovs.05-1468. [DOI] [PubMed] [Google Scholar]
- Chang CW, Christensen LV, Lee M, et al. Efficient expression of vascular endothelial growth factor using minicircle DNA for angiogenic gene therapy. J Control Release. 2008;125:155–163. doi: 10.1016/j.jconrel.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chattoraj DK, Gosule LC, Schellman A. DNA condensation with polyamines. II. Electron microscopic studies. J Mol Biol. 1978;121:327–337. doi: 10.1016/0022-2836(78)90367-4. [DOI] [PubMed] [Google Scholar]
- Chen J, Patil S, Seal S, et al. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nat Nanotechnol. 2006;1:142–150. doi: 10.1038/nnano.2006.91. [DOI] [PubMed] [Google Scholar]
- Chen X, Davis PB. Compacted DNA nanoparticles transfect cells by binding to cell surface nucleolin. Mol Ther. 2006:13. [Google Scholar]
- Chen X, Kube DM, Cooper MJ, et al. Cell surface nucleolin serves as receptor for DNA nanoparticles composed of pegylated polylysine and DNA. Mol Ther. 2008;16:333–342. doi: 10.1038/sj.mt.6300365. [DOI] [PubMed] [Google Scholar]
- Chen ZY, He CY, Ehrhardt A, et al. Minicircle DNA vectors devoid of bacterial DNA result in persistent and high-level transgene expression in vivo. Mol Ther. 2003;8:495–500. doi: 10.1016/s1525-0016(03)00168-0. [DOI] [PubMed] [Google Scholar]
- Chen ZY, He CY, Kay MA. Improved production and purification of minicircle DNA vector free of plasmid bacterial sequences and capable of persistent transgene expression in vivo. Hum Gene Ther. 2005;16:126–131. doi: 10.1089/hum.2005.16.126. [DOI] [PubMed] [Google Scholar]
- Chen ZY, He CY, Meuse L, et al. Silencing of episomal transgene expression by plasmid bacterial DNA elements in vivo. Gene Ther. 2004;11:856–864. doi: 10.1038/sj.gt.3302231. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Riu E, He CY, et al. Silencing of episomal transgene expression in liver by plasmid bacterial backbone DNA is independent of CpG methylation. Mol Ther. 2008;16:548–556. doi: 10.1038/sj.mt.6300399. [DOI] [PubMed] [Google Scholar]
- Cheng T, Peachey NS, Li S, et al. The effect of peripherin/rds haploinsufficiency on rod and cone photoreceptors. J Neurosci. 1997;17:8118–8128. doi: 10.1523/JNEUROSCI.17-21-08118.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Aleman TS, Boye SL, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci U S A. 2008;105:15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cideciyan AV, Hauswirth WW, Aleman TS, et al. Human RPE65 gene therapy for Leber congenital amaurosis: persistence of early visual improvements and safety at 1 year. Hum Gene Ther. 2009;20:999–1004. doi: 10.1089/hum.2009.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley SM, Cai X, Naash MI. Nonviral ocular gene therapy: assessment and future directions. Curr Opin Mol Ther. 2008;10:456–463. [PMC free article] [PubMed] [Google Scholar]
- Cook PR. The organization of replication and transcription. Science. 1999;284:1790–1795. doi: 10.1126/science.284.5421.1790. [DOI] [PubMed] [Google Scholar]
- Cooper LJ, Topp MS, Pinzon C, et al. Enhanced transgene expression in quiescent and activated human CD8+ T cells. Hum Gene Ther. 2004;15:648–658. doi: 10.1089/1043034041361217. [DOI] [PubMed] [Google Scholar]
- Cun D, Foged C, Yang M, et al. Preparation and characterization of poly(DL-lactide-co-glycolide) nanoparticles for siRNA delivery. Int J Pharm. 2010;390:70–75. doi: 10.1016/j.ijpharm.2009.10.023. [DOI] [PubMed] [Google Scholar]
- del Pozo-Rodriguez A, Delgado D, Solinis MA, et al. Solid lipid nanoparticles for retinal gene therapy: transfection and intracellular trafficking in RPE cells. Int J Pharm. 2008;360:177–183. doi: 10.1016/j.ijpharm.2008.04.023. [DOI] [PubMed] [Google Scholar]
- den Hollander AI, Roepman R, Koenekoop RK, et al. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog Retin Eye Res. 2008;27:391–419. doi: 10.1016/j.preteyeres.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Dezawa M, Takano M, Negishi H, et al. Gene transfer into retinal ganglion cells by in vivo electroporation: a new approach. Micron. 2002;33:1–6. doi: 10.1016/s0968-4328(01)00002-6. [DOI] [PubMed] [Google Scholar]
- Ding XQ, Nour M, Ritter LM, et al. The R172W mutation in peripherin/rds causes a cone-rod dystrophy in transgenic mice. Hum Mol Genet. 2004;13:2075–2087. doi: 10.1093/hmg/ddh211. [DOI] [PubMed] [Google Scholar]
- Ding XQ, Quiambao AB, Fitzgerald JB, et al. Ocular delivery of compacted DNA-nanoparticles does not elicit toxicity in the mouse retina. PLoS One. 2009;4:e7410. doi: 10.1371/journal.pone.0007410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt A, Engler JA, Xu H, et al. Molecular analysis of chromosomal rearrangements in mammalian cells after phiC31-mediated integration. Hum Gene Ther. 2006;17:1077–1094. doi: 10.1089/hum.2006.17.1077. [DOI] [PubMed] [Google Scholar]
- Ehrhardt A, Xu H, Huang Z, et al. A direct comparison of two nonviral gene therapy vectors for somatic integration: in vivo evaluation of the bacteriophage integrase phiC31 and the Sleeping Beauty transposase. Mol Ther. 2005;11:695–706. doi: 10.1016/j.ymthe.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Farjo R, Naash MI. The role of Rds in outer segment morphogenesis and human retinal disease. Ophthalmic Genet. 2006;27:117–122. doi: 10.1080/13816810600976806. [DOI] [PubMed] [Google Scholar]
- Farjo R, Skaggs J, Quiambao AB, et al. Efficient non-viral ocular gene transfer with compacted DNA nanoparticles. PLoS One. 2006;1:e38. doi: 10.1371/journal.pone.0000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez LA, Winkler M, Grosschedl R. Matrix attachment region-dependent function of the immunoglobulin mu enhancer involves histone acetylation at a distance without changes in enhancer occupancy. Mol Cell Biol. 2001;21:196–208. doi: 10.1128/MCB.21.1.196-208.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink TL, Klepcyk PJ, Oette SM, et al. Plasmid size up to 20 kbp does not limit effective in vivo lung gene transfer using compacted DNA nanoparticles. Gene Ther. 2006;13:1048–1051. doi: 10.1038/sj.gt.3302761. [DOI] [PubMed] [Google Scholar]
- Fischle W, Wang Y, Allis CD. Histone and chromatin cross-talk. Curr Opin Cell Biol. 2003;15:172–183. doi: 10.1016/s0955-0674(03)00013-9. [DOI] [PubMed] [Google Scholar]
- Flannery JG, Zolotukhin S, Vaquero MI, et al. Efficient photoreceptor-targeted gene expression in vivo by recombinant adeno-associated virus. Proc Natl Acad Sci U S A. 1997;94:6916–6921. doi: 10.1073/pnas.94.13.6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsman K, Graff C, Nordstrom S, et al. The gene for Best's macular dystrophy is located at 11q13 in a Swedish family. Clin Genet. 1992;42:156–159. doi: 10.1111/j.1399-0004.1992.tb03229.x. [DOI] [PubMed] [Google Scholar]
- Freimark BD, Blezinger HP, Florack VJ, et al. Cationic lipids enhance cytokine and cell influx levels in the lung following administration of plasmid: cationic lipid complexes. J Immunol. 1998;160:4580–4586. [PubMed] [Google Scholar]
- Gilbert DM. Replication timing and transcriptional control: beyond cause and effect. Curr Opin Cell Biol. 2002;14:377–383. doi: 10.1016/s0955-0674(02)00326-5. [DOI] [PubMed] [Google Scholar]
- Gilbert DM. In search of the holy replicator. Nat Rev Mol Cell Biol. 2004;5:848–855. doi: 10.1038/nrm1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory-Evans K, Chang F, Hodges MD, et al. Ex vivo gene therapy using intravitreal injection of GDNF-secreting mouse embryonic stem cells in a rat model of retinal degeneration. Mol Vis. 2009;15:962–973. [PMC free article] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Le Deist F, Carlier F, et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N Engl J Med. 2002;346:1185–1193. doi: 10.1056/NEJMoa012616. [DOI] [PubMed] [Google Scholar]
- Hacein-Bey-Abina S, Von Kalle C, Schmidt M, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]
- Hackett PB, Largaespada DA, Cooper LJ. A Transposon and Transposase System for Human Application. Mol Ther. 2010 doi: 10.1038/mt.2010.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harraghy N, Gaussin A, Mermod N. Sustained transgene expression using MAR elements. Curr Gene Ther. 2008;8:353–366. doi: 10.2174/156652308786071032. [DOI] [PubMed] [Google Scholar]
- Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- He J, Yang Q, Chang LJ. Dynamic DNA methylation and histone modifications contribute to lentiviral transgene silencing in murine embryonic carcinoma cells. J Virol. 2005;79:13497–13508. doi: 10.1128/JVI.79.21.13497-13508.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman EA, Conley SM, Stamer WD, et al. Barriers to productive transfection of trabecular meshwork cells. Mol Vis. 2005;11:869–875. [PubMed] [Google Scholar]
- Hong K, Sherley J, Lauffenburger DA. Methylation of episomal plasmids as a barrier to transient gene expression via a synthetic delivery vector. Biomol Eng. 2001;18:185–192. doi: 10.1016/s1389-0344(01)00100-9. [DOI] [PubMed] [Google Scholar]
- Huang M, Chen Z, Hu S, et al. Novel minicircle vector for gene therapy in murine myocardial infarction. Circulation. 2009;120:S230–S237. doi: 10.1161/CIRCULATIONAHA.108.841155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde SC, Pringle IA, Abdullah S, et al. CpG-free plasmids confer reduced inflammation and sustained pulmonary gene expression. Nat Biotechnol. 2008;26:549–551. doi: 10.1038/nbt1399. [DOI] [PubMed] [Google Scholar]
- Izsvak Z, Chuah MK, Vandendriessche T, et al. Efficient stable gene transfer into human cells by the Sleeping Beauty transposon vectors. Methods. 2009;49:287–297. doi: 10.1016/j.ymeth.2009.07.001. [DOI] [PubMed] [Google Scholar]
- Jackson DA, Juranek S, Lipps HJ. Designing nonviral vectors for efficient gene transfer and long-term gene expression. Mol Ther. 2006;14:613–626. doi: 10.1016/j.ymthe.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Jenke AC, Scinteie MF, Stehle IM, et al. Expression of a transgene encoded on a non-viral episomal vector is not subject to epigenetic silencing by cytosine methylation. Mol Biol Rep. 2004;31:85–90. doi: 10.1023/b:mole.0000031363.35839.46. [DOI] [PubMed] [Google Scholar]
- Jenke AC, Stehle IM, Herrmann F, et al. Nuclear scaffold/matrix attached region modules linked to a transcription unit are sufficient for replication and maintenance of a mammalian episome. Proc Natl Acad Sci U S A. 2004;101:11322–11327. doi: 10.1073/pnas.0401355101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Johnson LN, Cashman SM, Kumar-Singh R. Cell-penetrating peptide for enhanced delivery of nucleic acids and drugs to ocular tissues including retina and cornea. Mol Ther. 2008;16:107–114. doi: 10.1038/sj.mt.6300324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson LN, Cashman SM, Read SP, et al. Cell penetrating peptide POD mediates delivery of recombinant proteins to retina, cornea and skin. Vision Res. 2009 doi: 10.1016/j.visres.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano RL, Alam R, Dixit V, et al. Cell-targeting and cell-penetrating peptides for delivery of therapeutic and imaging agents. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2009;1:324–335. doi: 10.1002/wnan.4. [DOI] [PubMed] [Google Scholar]
- Kaytor MD, Weatherspoon MR, Green KJ, et al. In Vivo delivery of nucleic acid to the brain using DNA nanoparticles. Mol Ther. 2009;17:S519. [Google Scholar]
- Kiang AS, Palfi A, Ader M, et al. Toward a gene therapy for dominant disease: validation of an RNA interference-based mutation-independent approach. Mol Ther. 2005;12:555–561. doi: 10.1016/j.ymthe.2005.03.028. [DOI] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Bjorklund A, et al. Long-term rAAV-mediated gene transfer of GDNF in the rat Parkinson's model: intrastriatal but not intranigral transduction promotes functional regeneration in the lesioned nigrostriatal system. J Neurosci. 2000;20:4686–4700. doi: 10.1523/JNEUROSCI.20-12-04686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl S, Varsanyi B, Antunes GA, et al. CNGB3 mutations account for 50% of all cases with autosomal recessive achromatopsia. Eur J Hum Genet. 2005;13:302–308. doi: 10.1038/sj.ejhg.5201269. [DOI] [PubMed] [Google Scholar]
- Konstan MW, Davis PB, Wagener JS, et al. Compacted DNA nanoparticles administered to the nasal mucosa of cystic fibrosis subjects are safe and demonstrate partial to complete cystic fibrosis transmembrane regulator reconstitution. Hum Gene Ther. 2004;15:1255–1269. doi: 10.1089/hum.2004.15.1255. [DOI] [PubMed] [Google Scholar]
- Kowalczyk T, Pasumarthy M, Gedeon CR, et al. Type of polylysine counterion influences morphology and biological function of condensed DNA. Mol Ther. 2001;3:S359. [Google Scholar]
- Kuno N, Fujii S. Biodegradable intraocular therapies for retinal disorders: progress to date. Drugs Aging. 2010;27:117–134. doi: 10.2165/11530970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Lebrun-Julien F, Di Polo A. Molecular and cell-based approaches for neuroprotection in glaucoma. Optom Vis Sci. 2008;85:417–424. doi: 10.1097/OPX.0b013e31817841f7. [DOI] [PubMed] [Google Scholar]
- Lee PW, Hsu SH, Tsai JS, et al. Multifunctional core-shell polymeric nanoparticles for transdermal DNA delivery and epidermal Langerhans cells tracking. Biomaterials. 31:2425–2434. doi: 10.1016/j.biomaterials.2009.11.100. [DOI] [PubMed] [Google Scholar]
- Liou GI, Matragoon S, Yang J, et al. Retina-specific expression from the IRBP promoter in transgenic mice is conferred by 212 bp of the 5'-flanking region. Biochem Biophys Res Commun. 1991;181:159–165. doi: 10.1016/s0006-291x(05)81395-6. [DOI] [PubMed] [Google Scholar]
- Lipps HJ, Jenke AC, Nehlsen K, et al. Chromosome-based vectors for gene therapy. Gene. 2003;304:23–33. doi: 10.1016/s0378-1119(02)01215-5. [DOI] [PubMed] [Google Scholar]
- Liu G, Geurts AM, Yae K, et al. Target-site preferences of Sleeping Beauty transposons. J Mol Biol. 2005;346:161–173. doi: 10.1016/j.jmb.2004.09.086. [DOI] [PubMed] [Google Scholar]
- Liu G, Li D, Pasumarthy MK, et al. Nanoparticles of compacted DNA transfect postmitotic cells. J Biol Chem. 2003;278:32578–32586. doi: 10.1074/jbc.M305776200. [DOI] [PubMed] [Google Scholar]
- Liu G, Molas M, Grossmann GA, et al. Biological properties of poly-L-lysine-DNA complexes generated by cooperative binding of the polycation. J Biol Chem. 2001;276:34379–34387. doi: 10.1074/jbc.M105250200. [DOI] [PubMed] [Google Scholar]
- Liu J, Jeppesen I, Nielsen K, et al. Phi c31 integrase induces chromosomal aberrations in primary human fibroblasts. Gene Ther. 2006;13:1188–1190. doi: 10.1038/sj.gt.3302789. [DOI] [PubMed] [Google Scholar]
- Liu S, Ma J, Wang W, et al. Mutational analysis of highly conserved residues in the phage phiC31 integrase reveals key amino acids necessary for the DNA recombination. PLoS One. 5:e8863. doi: 10.1371/journal.pone.0008863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova O, Gorneva G, Wu F, et al. Incorporation of nuclear matrix attachment regions into the herpes simplex virus type 1 genome does not induce long-term expression of a foreign gene during latency. Gene Ther. 1996;3:829–833. [PubMed] [Google Scholar]
- Mayrhofer P, Blaesen M, Schleef M, et al. Minicircle-DNA production by site specific recombination and protein-DNA interaction chromatography. J Gene Med. 2008;10:1253–1269. doi: 10.1002/jgm.1243. [DOI] [PubMed] [Google Scholar]
- Mazda O. Improvement of nonviral gene therapy by Epstein-Barr virus (EBV)-based plasmid vectors. Curr Gene Ther. 2002;2:379–392. doi: 10.2174/1566523023347814. [DOI] [PubMed] [Google Scholar]
- Mikkelsen JG, Yant SR, Meuse L, et al. Helper-Independent Sleeping Beauty transposon-transposase vectors for efficient nonviral gene delivery and persistent gene expression in vivo. Mol Ther. 2003;8:654–665. doi: 10.1016/s1525-0016(03)00216-8. [DOI] [PubMed] [Google Scholar]
- Mohan RR, Sharma A, Netto MV, et al. Gene therapy in the cornea. Prog Retin Eye Res. 2005;24:537–559. doi: 10.1016/j.preteyeres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Munier S, Messai I, Delair T, et al. Cationic PLA nanoparticles for DNA delivery: comparison of three surface polycations for DNA binding, protection and transfection properties. Colloids Surf B Biointerfaces. 2005;43:163–173. doi: 10.1016/j.colsurfb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Naik R, Mukhopadhyay A, Ganguli M. Gene delivery to the retina: focus on non-viral approaches. Drug Discov Today. 2009;14:306–315. doi: 10.1016/j.drudis.2008.09.012. [DOI] [PubMed] [Google Scholar]
- Nathans J, Merbs SL, Sung CH, et al. Molecular genetics of human visual pigments. Annu Rev Genet. 1992;26:403–424. doi: 10.1146/annurev.ge.26.120192.002155. [DOI] [PubMed] [Google Scholar]
- Nimesh S, Goyal A, Pawar V, et al. Polyethylenimine nanoparticles as efficient transfecting agents for mammalian cells. J Control Release. 2006;110:457–468. doi: 10.1016/j.jconrel.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Noma K, Allis CD, Grewal SI. Transitions in distinct histone H3 methylation patterns at the heterochromatin domain boundaries. Science. 2001;293:1150–1155. doi: 10.1126/science.1064150. [DOI] [PubMed] [Google Scholar]
- Nour M, Ding XQ, Stricker H, et al. Modulating expression of peripherin/rds in transgenic mice: critical levels and the effect of overexpression. Invest Ophthalmol Vis Sci. 2004;45:2514–2521. doi: 10.1167/iovs.04-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nour M, Fliesler SJ, Naash MI. Genetic supplementation of RDS alleviates a loss-of-function phenotype in C214S model of retinitis pigmentosa. Adv Exp Med Biol. 2008;613:129–138. doi: 10.1007/978-0-387-74904-4_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nour M, Naash MI. Mouse models of human retinal disease caused by expression of mutant rhodopsin. A valuable tool for the assessment of novel gene therapies. Adv Exp Med Biol. 2003;533:173–179. doi: 10.1007/978-1-4615-0067-4_22. [DOI] [PubMed] [Google Scholar]
- Nour M, Quiambao AB, Peterson WM, et al. P2Y(2) receptor agonist INS37217 enhances functional recovery after detachment caused by subretinal injection in normal and rds mice. Invest Ophthalmol Vis Sci. 2003;44:4505–4514. doi: 10.1167/iovs.03-0453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh S, Pluhar GE, McNeil EA, et al. Efficacy of nonviral gene transfer in the canine brain. J Neurosurg. 2007;107:136–144. doi: 10.3171/JNS-07/07/0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivares EC, Hollis RP, Chalberg TW, et al. Site-specific genomic integration produces therapeutic Factor IX levels in mice. Nat Biotechnol. 2002;20:1124–1128. doi: 10.1038/nbt753. [DOI] [PubMed] [Google Scholar]
- Papapetrou EP, Ziros PG, Micheva ID, et al. Gene transfer into human hematopoietic progenitor cells with an episomal vector carrying an S/MAR element. Gene Ther. 2006;13:40–51. doi: 10.1038/sj.gt.3302593. [DOI] [PubMed] [Google Scholar]
- Parker Read S, Cashman SM, Kumar-Singh R. A poly(ethylene) glycolylated peptide for ocular delivery compacts DNA into nanoparticles for gene delivery to post-mitotic tissues in vivo. J Gene Med. 12:86–96. doi: 10.1002/jgm.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker Read S, Cashman SM, Kumar-Singh R. A poly(ethylene) glycolylated peptide for ocular delivery compacts DNA into nanoparticles for gene delivery to post-mitotic tissues in vivo. J Gene Med. 2010;12:86–96. doi: 10.1002/jgm.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perales JC, Ferkol T, Beegen H, et al. Gene transfer in vivo: sustained expression and regulation of genes introduced into the liver by receptor-targeted uptake. Proc Natl Acad Sci U S A. 1994;91:4086–4090. doi: 10.1073/pnas.91.9.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perales JC, Ferkol T, Molas M, et al. An evaluation of receptor-mediated gene transfer using synthetic DNA-ligand complexes. Eur J Biochem. 1994;226:255–266. doi: 10.1111/j.1432-1033.1994.tb20049.x. [DOI] [PubMed] [Google Scholar]
- Perales JC, Grossmann GA, Molas M, et al. Biochemical and functional characterization of DNA complexes capable of targeting genes to hepatocytes via the asialoglycoprotein receptor. J Biol Chem. 1997;272:7398–7407. doi: 10.1074/jbc.272.11.7398. [DOI] [PubMed] [Google Scholar]
- Piechaczek C, Fetzer C, Baiker A, et al. A vector based on the SV40 origin of replication and chromosomal S/MARs replicates episomally in CHO cells. Nucleic Acids Res. 1999;27:426–428. doi: 10.1093/nar/27.2.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet B, Heon E, Munier FL, et al. Full characterization of the maculopathy associated with an Arg-172-Trp mutation in the RDS/peripherin gene. Ophthalmic Genet. 1996;17:175–186. doi: 10.3109/13816819609057891. [DOI] [PubMed] [Google Scholar]
- Pisal DS, Kosloski MP, Balu-Iyer SV. Delivery of therapeutic proteins. J Pharm Sci. 2010 doi: 10.1002/jps.22054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podetz-Pedersen KM, Bell JB, Steele TW, et al. Gene Expression in Lung and Liver After Intravenous Infusion of Polyethylenimine Complexes of Sleeping Beauty Transposons. Hum Gene Ther. 2009 doi: 10.1089/hum.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter SD, Hu J, Gilks CB. Distal upstream tyrosinase S/MAR-containing sequence has regulatory properties specific to subsets of melanocytes. Dev Genet. 1999;25:40–48. doi: 10.1002/(SICI)1520-6408(1999)25:1<40::AID-DVG5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Querol M, Bogdanov A., Jr Environment-sensitive and enzyme-sensitive MR contrast agents. Handb Exp Pharmacol. 2008:37–57. doi: 10.1007/978-3-540-77496-9_3. [DOI] [PubMed] [Google Scholar]
- Quiambao AB, Peachey NS, Mangini NJ, et al. A 221-bp fragment of the mouse opsin promoter directs expression specifically to the rod photoreceptors of transgenic mice. Vis Neurosci. 1997;14:617–625. doi: 10.1017/s095252380001258x. [DOI] [PubMed] [Google Scholar]
- Raju HB, Goldberg JL. Nanotechnology for Ocular Therapeutics and Tissue Repair. Expert Reviews in Ophthalmology. 2008;3:431–436. [Google Scholar]
- Rhee M, Davis P. Mechanism of uptake of C105Y, a novel cell-penetrating peptide. J Biol Chem. 2006;281:1233–1240. doi: 10.1074/jbc.M509813200. [DOI] [PubMed] [Google Scholar]
- Richards EJ, Elgin SC. Epigenetic codes for heterochromatin formation and silencing: rounding up the usual suspects. Cell. 2002;108:489–500. doi: 10.1016/s0092-8674(02)00644-x. [DOI] [PubMed] [Google Scholar]
- Riu E, Chen ZY, Xu H, et al. Histone modifications are associated with the persistence or silencing of vector-mediated transgene expression in vivo. Mol Ther. 2007;15:1348–1355. doi: 10.1038/sj.mt.6300177. [DOI] [PubMed] [Google Scholar]
- Riu E, Grimm D, Huang Z, et al. Increased maintenance and persistence of transgenes by excision of expression cassettes from plasmid sequences in vivo. Hum Gene Ther. 2005;16:558–570. doi: 10.1089/hum.2005.16.558. [DOI] [PubMed] [Google Scholar]
- Ruiz FE, Clancy JP, Perricone MA, et al. A clinical inflammatory syndrome attributable to aerosolized lipid-DNA administration in cystic fibrosis. Hum Gene Ther. 2001;12:751–761. doi: 10.1089/104303401750148667. [DOI] [PubMed] [Google Scholar]
- Rupprecht S, Lipps HJ. Cell cycle dependent histone dynamics of an episomal non-viral vector. Gene. 2009;439:95–101. doi: 10.1016/j.gene.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Sarra GM, Stephens C, de Alwis M, et al. Gene replacement therapy in the retinal degeneration slow (rds) mouse: the effect on retinal degeneration following partial transduction of the retina. Hum Mol Genet. 2001;10:2353–2361. doi: 10.1093/hmg/10.21.2353. [DOI] [PubMed] [Google Scholar]
- Schaarschmidt D, Baltin J, Stehle IM, et al. An episomal mammalian replicon: sequence-independent binding of the origin recognition complex. EMBO J. 2004;23:191–201. doi: 10.1038/sj.emboj.7600029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schetelig MF, Scolari F, Handler AM, et al. Site-specific recombination for the modification of transgenic strains of the Mediterranean fruit fly Ceratitis capitata. Proc Natl Acad Sci U S A. 2009;106:18171–18176. doi: 10.1073/pnas.0907264106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichtenbrede FC, MacNeil A, Bainbridge JW, et al. Intraocular gene delivery of ciliary neurotrophic factor results in significant loss of retinal function in normal mice and in the Prph2Rd2/Rd2 model of retinal degeneration. Gene Ther. 2003;10:523–527. doi: 10.1038/sj.gt.3301929. [DOI] [PubMed] [Google Scholar]
- Schwartz B, Benoist C, Abdallah B, et al. Gene transfer by naked DNA into adult mouse brain. Gene Ther. 1996;3:405–411. [PubMed] [Google Scholar]
- Shintani K, Shechtman DL, Gurwood AS. Review and update: current treatment trends for patients with retinitis pigmentosa. Optometry. 2009;80:384–401. doi: 10.1016/j.optm.2008.01.026. [DOI] [PubMed] [Google Scholar]
- Sibulesky L, Hayes KC, Pronczuk A, et al. Safety of <7500 RE (<25000 IU) vitamin A daily in adults with retinitis pigmentosa. Am J Clin Nutr. 1999;69:656–663. doi: 10.1093/ajcn/69.4.656. [DOI] [PubMed] [Google Scholar]
- Singh SR, Grossniklaus HE, Kang SJ, et al. Intravenous transferrin, RGD peptide and dualtargeted nanoparticles enhance anti-VEGF intraceptor gene delivery to laser-induced CNV. Gene Ther. 2009;16:645–659. doi: 10.1038/gt.2008.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sortwell CE, Camargo MD, Pitzer MR, et al. Diminished survival of mesencephalic dopamine neurons grafted into aged hosts occurs during the immediate postgrafting interval. Exp Neurol. 2001;169:23–29. doi: 10.1006/exnr.2001.7644. [DOI] [PubMed] [Google Scholar]
- Stenler S, Andersson A, Simonson OE, et al. Gene Transfer to Mouse Heart and Skeletal Muscles Using a Minicircle Expressing Human Vascular Endothelial Growth Factor. J Cardiovasc Pharmacol. 2009 doi: 10.1097/FJC.0b013e318194234e. [DOI] [PubMed] [Google Scholar]
- Sumiyoshi T, Holt NG, Hollis RP, et al. Stable transgene expression in primitive human CD34+ hematopoietic stem/progenitor cells, using the Sleeping Beauty transposon system. Hum Gene Ther. 2009;20:1607–1626. doi: 10.1089/hum.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Ziady AG. Real-time imaging of gene delivery and expression with DNA nanoparticle technologies. Methods Mol Biol. 2009;544:525–546. doi: 10.1007/978-1-59745-483-4_33. [DOI] [PubMed] [Google Scholar]
- Surace EM, Auricchio A. Versatility of AAV vectors for retinal gene transfer. Vision Res. 2008;48:353–359. doi: 10.1016/j.visres.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Kasai K, Saeki Y. Plasmid DNA sequences present in conventional herpes simplex virus amplicon vectors cause rapid transgene silencing by forming inactive chromatin. J Virol. 2006;80:3293–3300. doi: 10.1128/JVI.80.7.3293-3300.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakor D, Spigelman I, Tabata Y, et al. Subcutaneous peripheral injection of cationized gelatin/DNA polyplexes as a platform for non-viral gene transfer to sensory neurons. Mol Ther. 2007;15:2124–2131. doi: 10.1038/sj.mt.6300256. [DOI] [PubMed] [Google Scholar]
- Thiadens AA, den Hollander AI, Roosing S, et al. Homozygosity mapping reveals PDE6C mutations in patients with early-onset cone photoreceptor disorders. Am J Hum Genet. 2009;85:240–247. doi: 10.1016/j.ajhg.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346–358. doi: 10.1038/nrg1066. [DOI] [PubMed] [Google Scholar]
- Thorne RG, Nicholson C. In vivo diffusion analysis with quantum dots and dextrans predicts the width of brain extracellular space. Proc Natl Acad Sci U S A. 2006;103:5567–5572. doi: 10.1073/pnas.0509425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weleber RG, Carr RE, Murphey WH, et al. Phenotypic variation including retinitis pigmentosa, pattern dystrophy, and fundus flavimaculatus in a single family with a deletion of codon 153 or 154 of the peripherin/RDS gene. Arch Ophthalmol. 1993;111:1531–1542. doi: 10.1001/archopht.1993.01090110097033. [DOI] [PubMed] [Google Scholar]
- Wells DJ. Electroporation and ultrasound enhanced non-viral gene delivery in vitro and in vivo. Cell Biol Toxicol. 26:21–28. doi: 10.1007/s10565-009-9144-8. [DOI] [PubMed] [Google Scholar]
- Wells DJ. Gene therapy progress and prospects: electroporation and other physical methods. Gene Ther. 2004;11:1363–1369. doi: 10.1038/sj.gt.3302337. [DOI] [PubMed] [Google Scholar]
- Widom J, Baldwin RL. Cation-induced toroidal condensation of DNA studies with Co3+(NH3)6. J Mol Biol. 1980;144:431–453. doi: 10.1016/0022-2836(80)90330-7. [DOI] [PubMed] [Google Scholar]
- Wilson JH, Wensel TG. The nature of dominant mutations of rhodopsin and implications for gene therapy. Mol Neurobiol. 2003;28:149–158. doi: 10.1385/MN:28:2:149. [DOI] [PubMed] [Google Scholar]
- Wilson RW, Bloomfield VA. Counterion-induced condesation of deoxyribonucleic acid. a light-scattering study. Biochemistry. 1979;18:2192–2196. doi: 10.1021/bi00578a009. [DOI] [PubMed] [Google Scholar]
- Wissinger B, Gamer D, Jagle H, et al. CNGA3 mutations in hereditary cone photoreceptor disorders. Am J Hum Genet. 2001;69:722–737. doi: 10.1086/323613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodard LE, Hillman RT, Keravala A, et al. Effect of nuclear localization and hydrodynamic delivery-induced cell division on phiC31 integrase activity. Gene Ther. 2009 doi: 10.1038/gt.2009.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama T, Liou GI, Caldwell RB, et al. Photoreceptor-specific activity of the human interphotoreceptor retinoid-binding protein (IRBP) promoter in transgenic mice. Exp Eye Res. 1992;55:225–233. doi: 10.1016/0014-4835(92)90186-v. [DOI] [PubMed] [Google Scholar]
- Yoon CS, Jung HS, Kwon MJ, et al. Sonoporation of the minicircle-VEGF(165) for wound healing of diabetic mice. Pharm Res. 2009;26:794–801. doi: 10.1007/s11095-008-9778-x. [DOI] [PubMed] [Google Scholar]
- Yurek DM, Flectcher AM, Kowalczyk TH, et al. Compacted DNA nanoparticle gene transfer of GDNF to the rat striatum enhances the survival of grafted fetal dopamine neurons. Cell Transplant. 2009;18:1183–1196. doi: 10.3727/096368909X12483162196881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurek DM, Fletcher AM, Smith GM, et al. Long-term transgene expression in the central nervous system using DNA nanoparticles. Mol Ther. 2009;17:641–650. doi: 10.1038/mt.2009.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Mo X, Fang Y, et al. Rescue of photoreceptors by BDNF gene transfer using in vivo electroporation in the RCS rat of retinitis pigmentosa. Curr Eye Res. 2009;34:791–799. doi: 10.1080/02713680903086018. [DOI] [PubMed] [Google Scholar]
- Zhong Q, Chinta DM, Pamujula S, et al. Optimization of DNA delivery by three classes of hybrid nanoparticle/DNA complexes. J Nanobiotechnology. 2010;8:6. doi: 10.1186/1477-3155-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Park CW, Sjeklocha L, et al. High-Level Genomic Integration, Epigenetic Changes, and Expression of Sleeping Beauty Transgene. Biochemistry. doi: 10.1021/bi9016846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziady AG, Ferkol T, Gerken T, et al. Ligand substitution of receptor targeted DNA complexes affects gene transfer into hepatoma cells. Gene Ther. 1998;5:1685–1697. doi: 10.1038/sj.gt.3300777. [DOI] [PubMed] [Google Scholar]
- Ziady AG, Gedeon CR, Miller T, et al. Transfection of airway epithelium by stable PEGylated poly-L-lysine DNA nanoparticles in vivo. Mol Ther. 2003;8:936–947. doi: 10.1016/j.ymthe.2003.07.007. [DOI] [PubMed] [Google Scholar]
- Ziady AG, Gedeon CR, Muhammad O, et al. Minimal toxicity of stabilized compacted DNA nanoparticles in the murine lung. Mol Ther. 2003;8:948–956. doi: 10.1016/j.ymthe.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Ziady AG, Kelley TJ, Milliken E, et al. Functional evidence of CFTR gene transfer in nasal epithelium of cystic fibrosis mice in vivo following luminal application of DNA complexes targeted to the serpin-enzyme complex receptor. Mol Ther. 2002;5:413–419. doi: 10.1006/mthe.2002.0556. [DOI] [PubMed] [Google Scholar]
- Ziady AG, Kim J, Colla J, et al. Defining strategies to extend duration of gene expression from targeted compacted DNA vectors. Gene Ther. 2004;11:1378–1390. doi: 10.1038/sj.gt.3302299. [DOI] [PubMed] [Google Scholar]
- Ziady AG, Perales JC, Ferkol T, et al. Gene transfer into hepatoma cell lines via the serpin enzyme complex receptor. Am J Physiol. 1997;273:G545–G552. doi: 10.1152/ajpgi.1997.273.2.G545. [DOI] [PMC free article] [PubMed] [Google Scholar]