Abstract
Problem
Communication between uterine epithelial cells and the underlying stromal fibroblasts is critical for proper endometrial function. Stromal fibroblast-derived growth factors have been shown to regulate epithelial immune functions. The purpose of this study was to determine whether keratinocyte growth factor (KGF) regulates uterine epithelial cell chemokine and antimicrobial secretion.
Method of study
Uterine epithelial cells were isolated from Balb/c mice and cultured in either 96-well plates or transwell inserts. Epithelial cells were treated with KGF, epidermal growth factor (EGF), or hepatocyte growth factor (HGF). Macrophage inflammatory protein 3α (MIP3α) and keratinocyte-derived chemokine (KC) levels were measured by ELISA.
Results
Keratinocyte growth factor stimulated the secretion of MIP3α and KC. The effects on MIP3α by KGF were specific because EGF and HGF had no effect. In contrast, KGF, EGF, and HGF had similar effects on KC. Furthermore, KGF administered to the apical side of epithelial cells had no effect on MIP3α or KC secretion, indicating that the KGF receptor is located on the basolateral surface of uterine epithelial cells.
Conclusion
We demonstrate that KGF plays a role in uterine epithelial cell secretion of MIP3α and KC, key immune mediators involved in the protection of mucosal surfaces in the female reproductive tract.
Keywords: Epithelial, immunology, KC, KGF, MIP3α, uterus
Introduction
The innate immune system is the first line of protection against pathogens that encounter and breach the surface of mucosal sites.1,2 In the female reproductive tract, epithelial cells act as sentinels by protecting women from invading pathogens that may initiate a reproductive infection, pelvic inflammatory disease, and in some cases lead to infertility and/or death.3 Furthermore, in the upper portion of the tract, uterine epithelial cells maintain a delicate balance between sensing pathogens present in the lumen while still remaining tolerant of sperm, blastocyst implantation, and an immunologically distinct fetal–placental unit.4 Uterine epithelial cells are connected by tight junctions; thus forming a physical barrier that prevents pathogens from accessing the underlying mucosa.3,5 In addition to providing a physical barrier, uterine epithelial cells actively participate in a number of other mucosal immune responses.6–14 Epithelial cells constitutively secrete cytokines, chemokines, and antimicrobials that are important for normal physiological processes such as tissue regeneration and remodeling of the endometrium during each reproductive cycle.15–17 Some of the cytokines, chemokines, and antimicrobials produced by epithelial cells include tumor necrosis factor α (TNFα), interleukin-8 (IL-8), macrophage inflammatory protein 3α (MIP3α), transforming growth factor β (TGFβ), beta-defensins, and secretory leukocyte protease inhibitor (SLPI).11,13,18–21 In addition to constitutive secretion, uterine epithelial cell secretion of cytokines, chemokines, and antimicrobials can be induced upon pathogen exposure.11–14 Epithelial cells express Toll-like receptors (TLRs) that recognize highly conserved molecular patterns shared by many microorganisms. Engagement of a TLR by its cognate ligand can lead to the induction of cytokines, chemokines, and antimicrobials.11–14,19,22,23
In the upper portion of the tract, the lining of the uterus is composed of a simple columnar epithelium that sits atop a stromal layer.5 Normal endometrial development and function are dependent on precise bidirectional communication between uterine epithelial cells and underlying stromal fibroblasts.24 This intricate communication begins during embryonic development and continues through adulthood.24,25 Importantly, uterine stromal fibroblasts influence the differentiation of epithelial cells in processes specific to each area of the tract.25–29 Regulation of epithelial cell proliferation and secretory activity by uterine stromal fibroblasts is accomplished through the release of soluble factors (i.e., growth factors) turnover of extracellular matrix and direct cell–cell contact. 24,30,31
Several growth factor and their receptors, including epidermal growth factor (EGF), insulin-like growth factor-I (IGF-I), hepatocyte growth factor (HGF), and keratinocyte growth factor (KGF), have been identified within the uterus and shown to be involved in stromal fibroblast–epithelial cell interactions. 32–34 KGF, also known as fibroblast growth factor (FGF)-7, has been found in many mucosal sites including the female reproductive tract and shown to induce proliferation of uterine and vaginal epithelial cells.35–39 More recently, KGF was found to play a key role in wound healing via re-epithelialization as well as protecting lung epithelium from oxidant damage.40–44 The KGF receptor (KGFR) is a transmembrane tyrosine kinase receptor expressed by epithelial cells.45–47 Owing to the specific ligand and receptor expression patterns, KGF is thought to function as a paracrine mediator of stromal fibroblast–epithelial cell interactions.36,48–50
Previous studies in our laboratory have demonstrated that HGF increases uterine epithelial cell transepithelial resistance (TER) while decreasing TNFα secretion.51 HGF neutralization of conditioned stromal fibroblast media (CSM) abolished the increase in TER but unexpectedly failed to reverse CSM effects on TNFα secretion. These findings led to the conclusion that HGF has a role in regulating the integrity of the uterine epithelial cell barrier but other soluble factors play a role in TNFα secretion.51 These results also prompted further examination of the effects of other growth factors, such as KGF, on epithelial cell immune function. Two molecules that are important in innate immunity of the female reproductive tract are MIP3α and IL-8.17,52–55 Because neutrophils and dendritic cells (DCs) play an important role in host protection through phagocytosis (neutrophils) and pathogen sensing (DCs),56–58 identifying the role and regulation of MIP3α and IL-8 becomes important. MIP3α, also known as CCL20, recruits immature DCs, memory T cells, and B cells by binding to CCR6.59–65 MIP3α functions not only as a chemokine, but also as an antimicrobial.55,66 Previously, our laboratory examined MIP3α secretion and found that polarized rat, mouse, and human uterine epithelial cells constitutively secrete MIP3α.19,52,55,67 Furthermore, mouse uterine epithelial cell secretion of MIP3α was increased following treatment with TLRs 1, 2, 6, 4, 7, and 9 agonists, while treatment with a TLR3 agonist had no effect.67 IL-8 primarily functions to recruit neutrophils, and to a lesser extent T cells.54,68–70 IL-8 is present in the human endometrium and has been shown to be involved in many aspects of normal human endometrial physiology including proliferation, angiogenesis, menstruation, implantation, cervical ripening, and parturition.54,71–73 IL-8 has not been identified in the rodent, but chemokines similar to IL-8 have been detected.74–76 KC (keratinocyte-derived chemokine) is a potent neutrophil chemoattractant that has come to be known as the functional mouse homolog of human IL-874,77–79 and is expressed in the rodent female reproductive tract.58,77,80–83 Recently, studies in our laboratory have demonstrated that mouse uterine epithelial cells constitutively secrete KC. In addition, treatment with TLR1/6, 2, and 4 agonists significantly increased KC secretion.11
Knowing that the secretion of chemokines and antimicrobials is essential in endometrial protection, the goal of this study was to determine whether KGF regulates mouse uterine epithelial cell chemokine and antimicrobial secretion. Specifically, our objectives were to determine whether KGF affects uterine epithelial cell secretion of MIP3α and KC and to compare the effects of KGF on uterine epithelial cells to those of EGF and HGF. We show that KGF stimulates uterine epithelial cell secretion of MIP3α and KC, suggesting that KGF plays a previously unrecognized role in female reproductive tract immune responses.
Material and methods
Animals
Female, sexually mature, 8-week-old Balb/c mice from the National Cancer Institute colony at Charles River Laboratories (Kingston, NY, USA) were housed at the Dartmouth Animal Resources Center and kept in a constant-temperature environment with controlled light–dark intervals of 12 hr each. Animals were allowed food and water ad libitum. At 10–12 weeks of age, animals were killed by CO2, and uteri were removed. Uteri were pooled from 15–30 animals at various stages of the estrous cycle. All procedures involving animals were conducted after approval from the Dartmouth College Institutional Animal Care and Use Committee.
Uterine Epithelial Cell Preparation
For isolation of epithelial cells, uteri were removed, cut open lengthwise, pooled, and incubated in a solution of 46,500 units trypsin (Sigma-Aldrich, St Louis, MO, USA) per 1 mL of 2.5% pancreatin (Invitrogen, Carlsbad, CA, USA) at 20 mL/g of tissue for 1 hr at 4°C followed by 1 hr at 22°C. Uteri were then transferred to ice-cold Hanks balanced salt solution (HBSS; Invitrogen) and vortexed with the Vortex-Genie on setting #6 (Scientific Industries Inc., Springfield, MA, USA) to release sheets of epithelial cells. Uterine tissues were rinsed, vortexed two more times, and the resulting cell suspensions combined. Epithelial cells were passed through a 20-μm-mesh nylon screen (Small Parts, Inc., Miami Lakes, FL, USA) to recover epithelial cell sheets. Epithelial cell sheets were removed from the 20-μm screen, centrifuged at 500 × g for 5 min, and resuspended in complete medium consisting of Dulbecco’s modified eagle medium (DMEM)/Ham F-12 nutrient mixed 1:1 (without phenol red; Invitrogen) containing 10% stripped fetal bovine serum (FBS; Hyclone, Logan, UT, USA) supplemented with 20 mM Hepes (Invitrogen), 2 mM L-glutamine (Mediatech, Herndon, VA, USA), and 100 μg/mL Primocin (Invivo-Gen, San Diego, CA, USA). This complete medium will be referred to as DMEM/F-12 + 10% stripped FBS. In some experiments, freshly isolated uterine epithelial cells were incubated in Cellgro Complete Medium (Mediatech) supplemented with 15 mM Hepes (Invitrogen) and 100 μg/mL Primocin (Invivo-Gen). This complete medium will be referred to as Cellgro. The purity of cell cultures was more than 99% epithelial cells as previously reported.84
Epithelial Cell Transwell Culture
For studies with polarized cells, epithelial cell sheets were seeded in the upper (apical) compartment of 10-mm-diameter Nunc tissue culture inserts with 0.4-μm pore membranes (Nalge Nunc, Rochester, NY, USA) coated with diluted Matrigel (1:4 dilution; growth factor reduced, without phenol red; BD Bio-sciences, Bedford, MA, USA). Uterine epithelial cells were seeded in a volume of 300 μL per insert at a ratio of 3–4 culture inserts per uterus. Inserts containing epithelial cells were placed in 24-well Nuclon plates (Nalge Nunc) containing 500 μL of DMEM/F-12 + 10% stripped FBS. Plates and inserts containing epithelial cells were incubated at 37°C with 5% CO2 for 5–7 days to allow cells to grow to confluence and form tight junctions (TER ≥ 2000 ohms/well). Medium was collected from the apical and basolateral compartments and replaced at 48-hr intervals.
Transepithelial Resistance Measurement
Transepithelial resistance of epithelial cells grown on inserts was monitored daily with an EVOM™ epithelial voltohmmeter and electrode (World Precision Instruments Inc., New Haven, CT, USA). Background TER of Matrigel-coated cell culture inserts was approximately 250 ohms/well. Epithelial cells were considered confluent and polarized when high TER (≥2000 ohms/well) was reached.
Epithelial Cell Fresh Preparation
For studies with freshly isolated epithelial cells, epithelial cell sheets were resuspended in Cellgro, and sheets were then sheared by passage through a 20-gauge needle resulting in the formation of a single cell suspension. Epithelial cells were counted using 0.4% trypan blue (Gibco/Invitrogen, Carlsbad, CA, USA), centrifuged at 400 × g for 8 min, resuspended in Cellgro at a final density of 2 × 105 cells/100 μL, and plated into 96-well tissue culture plates (Nalge Nunc). Epithelial cells were incubated overnight at 37°C with 5% CO2 prior to treatment.
Growth Factor Treatment
Recombinant human KGF (R&D Systems, Minneapolis, MN; PeproTech Inc., Rocky Hill, NJ, USA), HGF (R&D Systems; PeproTech, Inc.), and EGF (R&D Systems) were added to the basolateral compartment for 48 hr (unless otherwise noted) of polarized, uterine epithelial cells that had reached high TER (≥2000 ohms/well). For experiments with freshly isolated uterine epithelial cells, growth factors were added directly to the cells in 96-well plates.
Supernatant Collection & Chemokine Analysis
Following treatment, supernatants were collected and centrifuged at 10,000 × g for 5 min at 4°C, transferred, and stored at −80°C until assayed. Supernatants from either polarized or freshly prepared uterine epithelial cells were diluted prior to analysis for MIP3α and KC secretion by commercially available ELISA kits (R&D Systems). Supernatants collected from apical and basolateral compartments of polarized epithelial cells were diluted in media at 1:20 for MIP3α and 1:10 for KC detection. Supernatants from freshly isolated epithelial cells were diluted in Cellgro at 1:10 for both MIP3α and KC analysis. Standards were diluted into the appropriate media, and ELISAs were carried out according to manufacturer’s protocol.
Statistics
Data were calculated as the mean ± standard error of the mean. Prism 4 for Macintosh (GraphPad Software, Inc. La Jolla, CA, USA) was used to calculate t-tests and one-way repeated measures analysis of variance (ANOVA). When the statistical test indicated that there was a significant difference between means, Bonferroni post-tests were used to adjust P-values. A P-value of <0.05 was used as an indication of statistical significance.
Results
KGF Stimulated Freshly Isolated Uterine Epithelial Cell MIP3α and KC Secretion
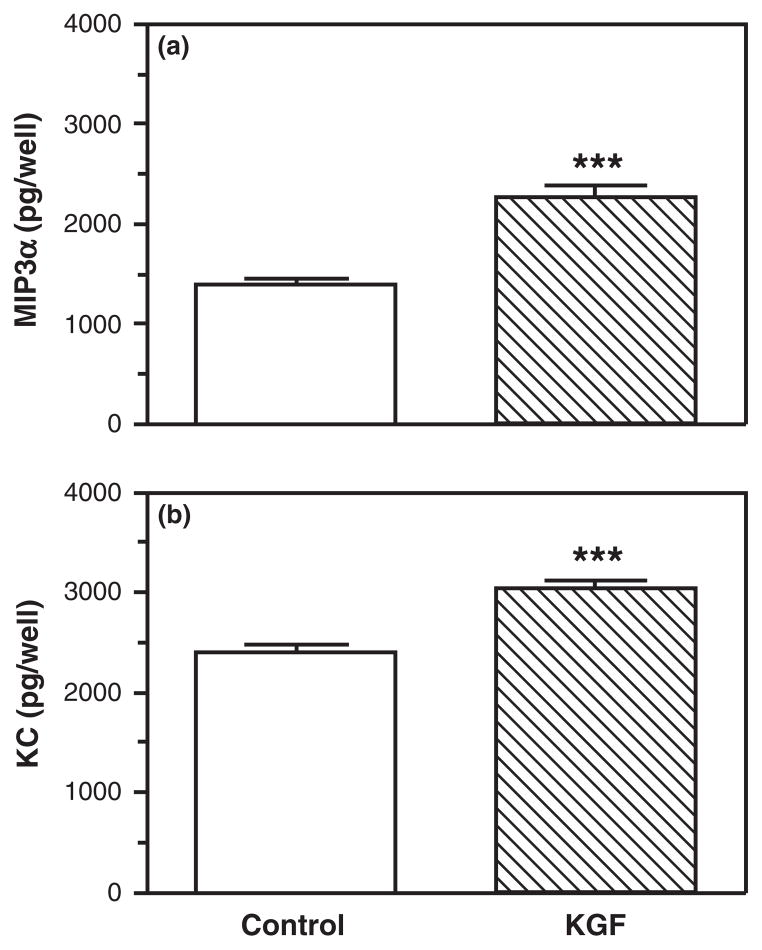
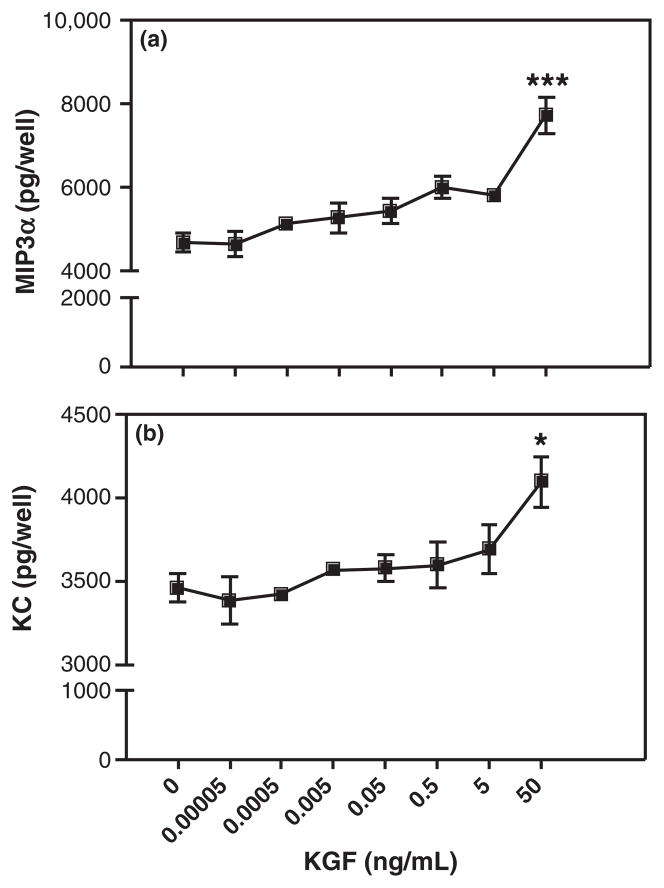
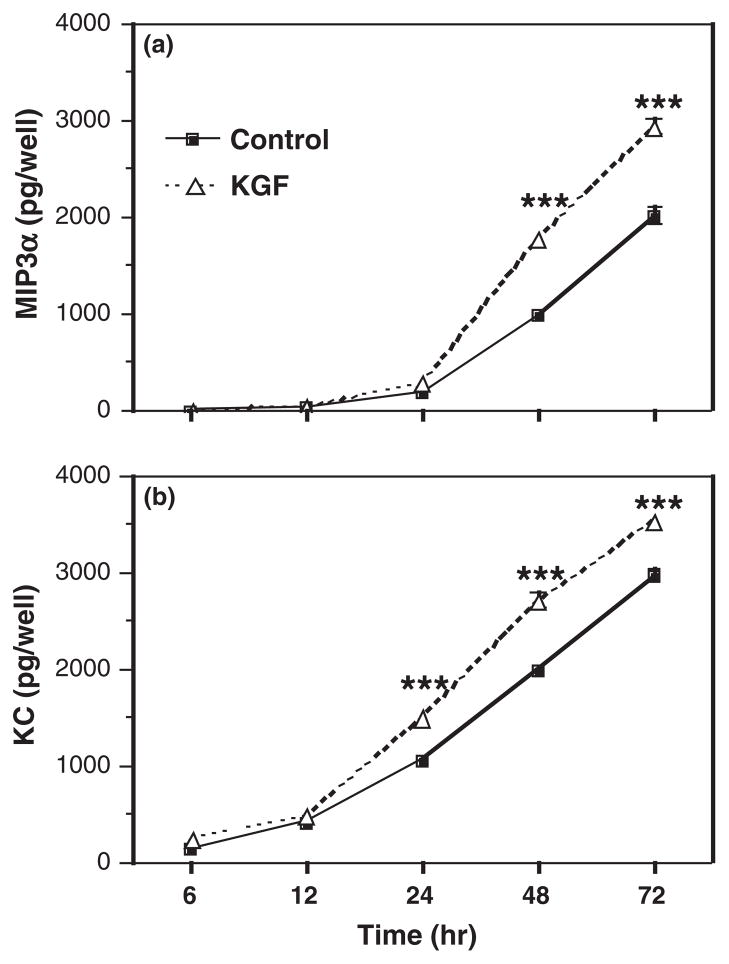
Growth factors have emerged as possible stromalderived mediators based on their ability to diffuse across the basement membrane and elicit rapid changes in cell morphology and function.32 One such growth factor, KGF, is a stromal-derived paracrine mediator known to increase epithelial cell proliferation in many tissues including mammary gland, uterus, and vagina.36,39,49,50 To test the hypothesis that KGF regulates uterine epithelial cell MIP3α and KC secretion, freshly isolated uterine epithelial cells were plated onto 96-well plates and treated with KGF (50 ng/mL) for 48 hr. As seen in Fig. 1, KGF treatment significantly increased both MIP3α (Fig. 1a) and KC (Fig. 1b) secretion by uterine epithelial cells. To further define the effects of KGF on MIP3α and KC release, dose–response and time course studies were carried out. Fig. 2 demonstrates that KGF treatment increased secretion of MIP3α (Fig. 2a) and KC (Fig. 2b) in a dose-dependent manner. KGF doses of 0.00005–5 ng/mL had no significant effect on MIP3α secretion; although there was a trend toward increased secretion as KGF dose increased. When epithelial cells were treated with 50 ng/mL of KGF, MIP3α secretion was significantly greater than controls. This same dose-dependent pattern was observed with KC secretion. As seen in Fig. 2b, increasing doses of KGF increased KC secretion, with 50 ng/mL, resulting in a statistically significant increase in KC levels. To investigate the time course of responsiveness of epithelial cells, epithelial cells were incubated with KGF (50 ng/mL), and secretions were collected at various time points. Fig. 3a demonstrates that MIP3α was undetectable prior to 24 hr but then increased significantly above controls at 48 and 72 hr. In contrast, when secretions were analyzed for KC (Fig. 3b), levels were detectable at 12 hr, and the effect of KGF was first measurable at 24 hr. This increase in KC, relative to controls, persisted at 48 and 72 hr. Overall, these findings indicate that freshly isolated epithelial cells secrete MIP3α and KC in a dose- and time-dependent manner in response to KGF treatment.
Fig. 1.
Keratinocyte growth factor (KGF) increases MIP3α and keratinocyte-derived chemokine (KC) secretion by freshly prepared mouse uterine epithelial cells. Freshly isolated mouse uterine epithelial cells were incubated overnight prior to treatment with control medium or KGF (50 ng/mL) for 48 hr. Supernatants were collected and analyzed for MIP3α (a) and KC (b). The results are shown as the mean + S.E.M. of eight separate experiments. ***MIP3α or KC significantly (P < 0.001) greater with KGF treatment than controls.
Fig. 2.
Keratinocyte growth factor (KGF) dose-dependently stimulates freshly prepared uterine epithelial cell release of MIP3α and keratinocyte-derived chemokine (KC). Freshly isolated mouse uterine epithelial cells were incubated overnight prior to treatment with KGF (0.00005–50 ng/mL) for 48 hr. Supernatants were collected and analyzed for MIP3α (a) and KC (b). The results are shown as the mean + S.E.M. ***MIP3α significantly (P < 0.001) or *KC significantly (P < 0.05) greater with KGF treatment than controls.
Fig. 3.
Time course of the effect of keratinocyte growth factor (KGF) on MIP3α and keratinocyte-derived chemokine (KC) secretion by uterine epithelial cells. Freshly isolated mouse uterine epithelial cells were incubated overnight prior to treatment with control medium or KGF (50 ng/mL) for 6, 12, 24, 48, or 72 hr. Supernatants were collected and analyzed for MIP3α (a) or KC (b). The results are shown as the mean + S.E.M. ***MIP3α or KC significantly (P < 0.001) greater with KGF treatment than controls.
Polarized Uterine Epithelial Cell Secretion of MIP3α and KC Increased in Response to KGF
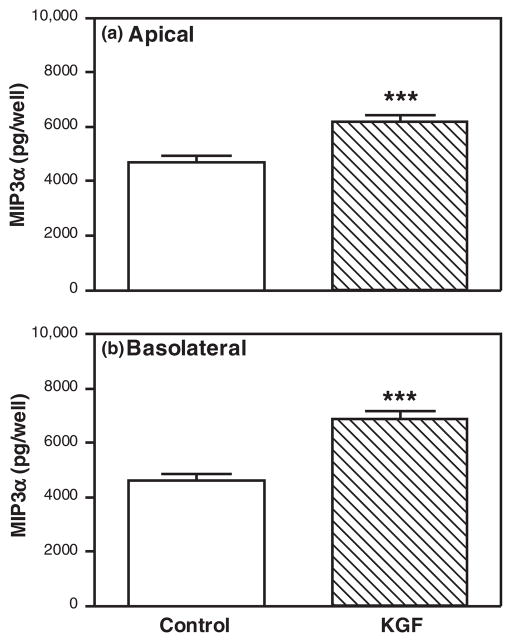
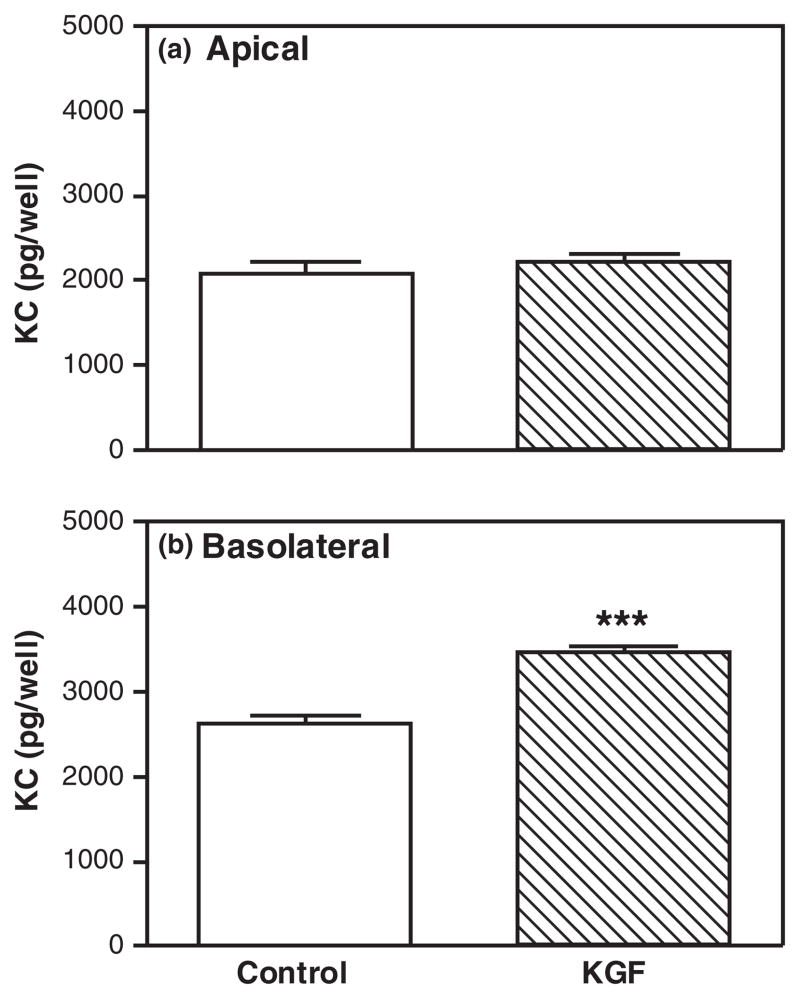
Having observed that KGF increased MIP3α and KC secretion by freshly isolated epithelial cells, we wanted to test the hypothesis that KGF would increase apical and basolateral secretion of both MIP3α and KC by polarized uterine epithelial cells. Polarized uterine epithelial cells (TER ≥ 2000 ohms/well) were treated with KGF (50 ng/mL) in the basolateral compartment for 48 hr. As seen in Figs 4 and 5 and consistent with previous data,11,85,86 polarized mouse uterine epithelial cells constitutively secreted MIP3α and KC into both apical and basolateral compartments (see control bars in Figs 4 and 5). Fig. 4 demonstrates that KGF significantly stimulated both the apical and the basolateral secretion of MIP3α. Moreover, these data indicate that MIP3α is released equally into apical and basolateral compartments. In contrast, KGF treatment induced only basolateral KC but had no effect on apical KC secretion relative to controls (Fig. 5). Whereas MIP3α was secreted bidirectionally, these findings indicate that KC is preferentially secreted into the basolateral compartment. Consistent with our findings with freshly prepared epithelial cells (Fig. 1), KGF stimulates MIP3α and KC secretion by polarized uterine epithelial cells.
Fig. 4.
Keratinocyte growth factor (KGF) increases apical and basolateral MIP3α secretion by polarized uterine epithelial cells. Mouse uterine epithelial cells were isolated and polarized on transwell cell culture inserts. Polarized epithelial cells were treated with control medium or KGF (50 ng/mL) in the basolateral compartment for 48 hr. Apical (a) and basolateral (b) supernatants were collected and analyzed for MIP3α. The results are shown as the mean + S.E.M. of 10 separate experiments. ***MIP3α significantly (P < 0.001) greater with KGF treatment than controls.
Fig. 5.
Keratinocyte growth factor (KGF) stimulates basolateral keratinocyte-derived chemokine (KC) secretion by polarized uterine epithelial cells. Mouse uterine epithelial cells were isolated and polarized on transwell cell culture inserts. Polarized epithelial cells were treated with control medium or KGF (50 ng/mL) in the basolateral compartment for 48 hr. Apical (a) and basolateral (b) supernatants were collected and analyzed for KC. The results are shown as the mean + S.E.M. of 10 separate experiments. ***KC significantly (P < 0.001) greater with KGF treatment than controls.
Specificity of KGF Effects on MIP3α and KC Secretion by Polarized Mouse Uterine Epithelial Cells
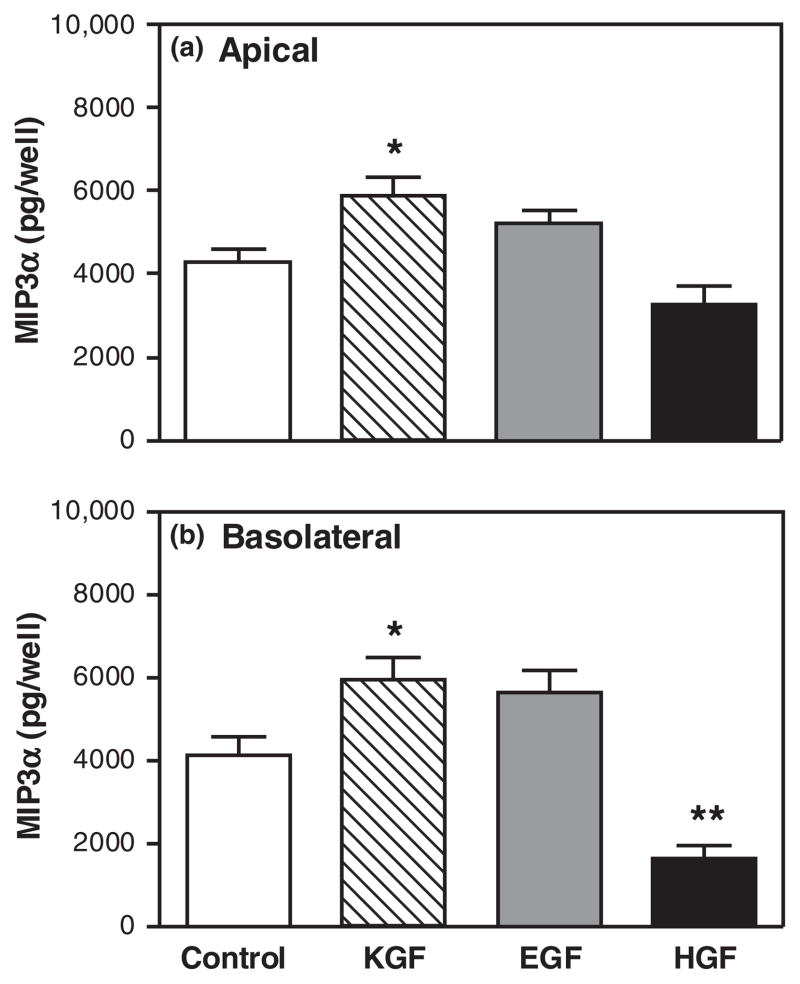
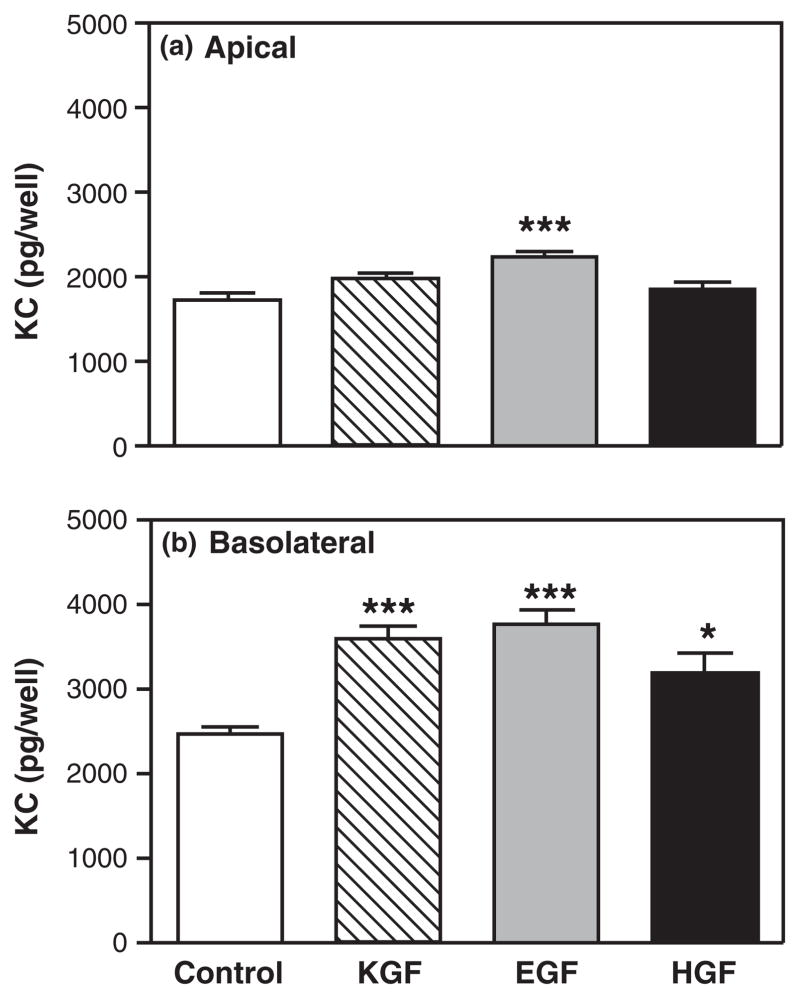
Numerous studies have shown that a number of growth factors are produced in the endometrium and contribute to homeostasis and growth through-out the reproductive cycle.32,49,87–93 To test the hypothesis that the effects of KGF on MIP3α and KC secretion were specific, polarized uterine epithelial cells were treated either with KGF (50 ng/mL), EGF (4 ng/mL), or HGF (50 ng/mL) in the basolateral compartment for 48 hr. Fig. 6 shows that KGF significantly increased MIP3α secretion both apically and basolaterally. In contrast, EGF had no effect on either apical and basolateral secretion of MIP3α. Interestingly, HGF had no effect on apical MIP3α secretion but significantly inhibited basolateral MIP3α secretion. When the same secretions were analyzed for KC levels, we unexpectedly found that all three growth factors examined had similar effects on KC secretion. As seen in Fig. 7, of those growth factors tested, only EGF significantly increased apical KC secretion. In contrast, basolateral KC secretion was significantly increased not only by KGF, but also in response to EGF and HGF. These findings indicate that the effects on MIP3α are specific to KGF, while effects on KC may be similar in response to other growth factors.
Fig. 6.
Specificity of keratinocyte growth factor (KGF) effect on MIP3α secretion. Mouse uterine epithelial cells were isolated and polarized on transwell cell culture inserts. Polarized epithelial cells were treated with control medium, KGF (50 ng/mL), epidermal growth factor (4 ng/mL), or HGF (50 ng/mL) in the basolateral compartment for 48 hr. Apical (a) and basolateral (b) supernatants were collected and analyzed for MIP3α. The results are shown as the mean + S.E.M. of four separate experiments. *MIP3α significantly (P < 0.05) greater with KGF treatment than with control medium. **MIP3α significantly (P < 0.01) less with HGF treatment than with control medium.
Fig. 7.
Specificity of keratinocyte growth factor (KGF) effect on keratinocyte-derived chemokine (KC) release. Mouse uterine epithelial cells were isolated and polarized on transwell cell culture inserts. Polarized epithelial cells were treated with control medium, KGF (50 ng/mL), epidermal growth factor (4 ng/mL), or HGF (50 ng/mL) in the basolateral compartment for 48 hr. Apical (a) and basolateral (b) supernatants were collected and analyzed for KC. The results are shown as the mean + S.E.M. of four separate experiments. *KC significantly (P < 0.05) greater with growth factor treatment than with control medium. ***KC significantly (P < 0.001) greater with growth factor treatment than with control medium.
KGF Receptor is Located on the Basolateral Surface of Polarized Mouse Uterine Epithelial Cells
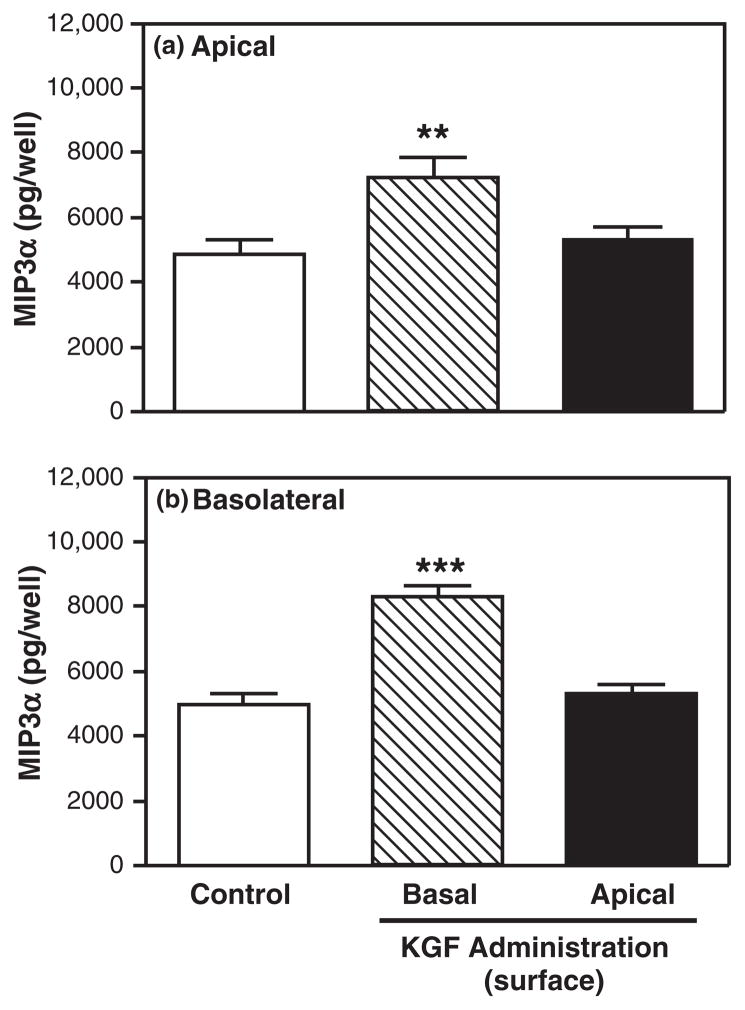
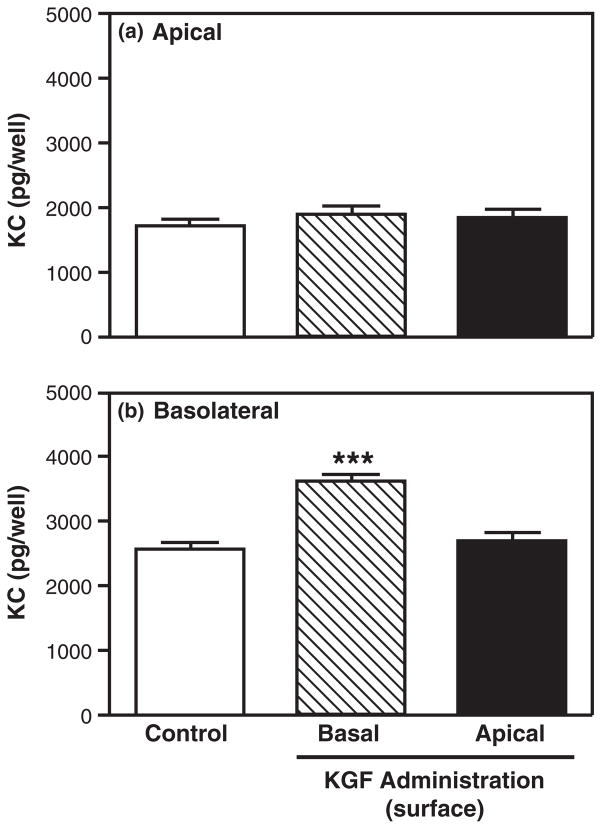
The receptor for KGF has been located on the basolateral surface of Caco-2 cells, an intestinal epithelial cell line.94 To determine whether mouse uterine epithelial cells express KGFR on the basolateral surface, polarized uterine epithelial cells were incubated with KGF placed either in the apical compartment or basolateral compartment for 48 hr prior to media collection and analysis. As seen in Fig. 8, when added to the basolateral surface, KGF significantly increased both apical and basolateral MIP3α secretion relative to control values. In contrast, when KGF was placed in the apical compartment at a concentration identical to that used in the basolateral compartment, no effect on either apical or basolateral MIP3α secretion was observed. As a part of these studies, we analyzed KC and found that basolateral addition of KGF significantly increased basolateral KC secretion (Fig. 9). Similar to that seen with MIP3α, apical KGF administration had no effect on either apical or basolateral KC secretion. Together, these data suggest that KGFR is located on the basolateral surface of polarized uterine epithelial cells.
Fig. 8.
Effect of keratinocyte growth factor (KGF) apical treatment on polarized uterine epithelial cell MIP3α secretion. Mouse uterine epithelial cells were isolated and polarized on transwell cell culture inserts. Polarized epithelial cells were treated with control medium, KGF (50 ng/mL) in the apical or basolateral compartment for 48 hr. Apical (a) and basolateral (b) supernatants were collected and analyzed for MIP3α. The results are shown as the mean + S.E.M. of two separate experiments. **MIP3α significantly (P < 0.01) greater with KGF treatment than with control medium. ***MIP3α significantly (P < 0.001) greater with KGF treatment than with control medium.
Fig. 9.
Effect of keratinocyte growth factor (KGF) apical treatment on keratinocyte-derived chemokine (KC) release by polarized uterine epithelial cells. Mouse uterine epithelial cells were isolated and polarized on transwell cell culture inserts. Polarized epithelial cells were treated with control medium, KGF (50 ng/mL) in the apical or basolateral compartment for 48 hr. Apical (a) and basolateral (b) supernatants were collected and analyzed for KC. The results are shown as the mean + S.E.M. of two separate experiments. ***KC significantly (P < 0.001) greater with KGF treatment than with control medium.
Discussion
The data presented in this study demonstrate that KGF, a stromal fibroblast-derived factor, affects mouse uterine epithelial cell secretion of both MIP3α and KC, key immune mediators involved in the protection of mucosal surfaces in the female reproductive tract. Our studies indicate that when added to the basolateral surface of polarized uterine epithelial cells, KGF stimulated apical and basolateral secretion of MIP3α as well as the basolateral secretion of KC. When added to the apical surface, KGF had no effect on either MIP3α or KC secretion, suggesting that the receptor for KGF is located on the basolateral surface of uterine epithelial cells. The effects on MIP3α by KGF are specific in that EGF and HGF had no effect on MIP3α secretion. In contrast, KGF, EGF, and HGF had similar effects on KC release by uterine epithelial cells. Overall, our findings suggest that female reproductive tract immune protection against bacterial, fungal, and viral pathogens may be indirectly mediated via secretions from underlying stromal fibroblasts.
KGF is a stromal fibroblast-secreted peptide that is a member of the FGF family.35,36 The production of KGF by stromal fibroblasts as well as the mitogenic, morphogenic, and motogenic effects of KGF on epithelial cells suggests that KGF functions as a paracrine mediator of stromal fibroblast–epithelial cell interactions.39,48–50,95 Others have shown that uterine and vaginal epithelial cell proliferation increases approximately 5-fold in neonatal mice following treatment with KGF.38 In the lung, KGF has been shown to influence epithelial cell secretory function. 43,83,96 When alveolar type II cells were treated with KGF, surfactant protein (SP)-A and SP-D expression and secretion increased.96 Other studies have investigated the role of KGF in neutrophil recruitment in the lung and found that KGF administration prior to acid aspiration injury decreases the secretion of KC into bronchoalveolar lavage fluid.83 Our studies extend these findings by demonstrating that KGF regulates the secretion of MIP3α and KC by primary mouse uterine epithelial cells. To the best of our knowledge, this is the first report demonstrating that in vitro treatment of uterine epithelial cells with KGF results in increased MIP3α and KC secretion.
This study compared KGF-induced MIP3α and KC secretion by non-polarized and polarized uterine epithelial cells to determine whether time in culture affects KGF responsiveness. Several studies have compared polarized epithelial cells with non-polarized cells under a variety of conditions and found functional differences. For example, in a study comparing polarized and non-polarized human endometrial monolayer culture systems on murine embryo development, two-cell embryos cultured on a polarized monolayer had a significantly higher developmental rate than those from non-polarized cells.97 When the KGFR, a transmembrane tyrosine kinase receptor expressed on epithelial cells,45–47 was measured in the mammary gland, epithelial cell expression of KGFR was greatest in freshly isolated cells with levels decreasing as cells were kept in culture.98 Our findings show no difference in KGF-induced chemokine secretion, suggesting that polarized uterine epithelial cells in culture for 5–7 days are as responsive to KGF as freshly isolated cells. Moreover, when receptor localization was determined by addition of KGF to apical versus basolateral surface of polarized cells, we found that basolateral but not apical administration altered MIP3α and KC secretion. These findings extend the immunohistochemical observations of Visco et al. who reported that the KGFR is located on the basolateral surface of intestinal epithelial cells.94
KGF treatment of freshly isolated epithelial cells for various lengths of time indicated that MIP3α and KC have different secretory profiles. Constitutive secretion of KC was detected sooner than MIP3α and the length of time for the effects of KGF to be observed was also different between MIP3α and KC. From a mechanistic standpoint, these data suggest that KC and MIP3α are regulated and/or processed differently in response to KGF. Previously, we found that cytokine secretion varies with the cytokine measured and the TLR agonist used.19 Time course studies of MIP3α and TNFα secretion in response to Pam3Cys and LPS (lipopolysaccharide; a component of the outer membrane of Gram-negative bacteria) indicated that MIP3α release peaked between 4 and 6 hr after treatment, whereas TNFα release was gradual over the length of the 12-hr incubation.19 Our findings that KGF increases KC and MIP3α at 24 and 48 hr, respectively, indicate that KGF regulates cytokine secretion by uterine epithelial cells via different mechanisms. Further studies are needed to determine the regulation (i.e., transcriptional, translational, and exocytic) of MIP3α and KC production and secretion by uterine epithelial cells.
Our results indicate that KGF regulates the apical and basolateral MIP3α secretion by uterine epithelial cells. Considering the dual functions of MIP3α as a pro-inflammatory chemokine and an antimicrobial, 55,62–66 MIP3α is an important molecule in both the innate and adaptive immune systems.99 MIP3α has antimicrobial effects against Escherichia coli, Pseudomonas aeruginosa, Moraxella catarrhalis, Streptococcus pyogenes, Enterococcus faecium, Staphylococcus aureus, Candida albicans, and HIV-1.55,66 The observed increase in apical secretion of MIP3α in response to KGF most likely relates to its function as an antimicrobial. 66,100 Having MIP3α available in the uterine lumen would allow for the direct killing of pathogens that are introduced into the tract at the time of mating, when pathogens are most likely to be present. KGF also stimulated the basolateral secretion of MIP3α, suggesting that MIP3α may have a role in the recruitment of immature DCs into the endometrium. DCs are present throughout the estrous cycle, with highest numbers present at estrus relative to other stages of the cycle.57 These findings suggest that MIP3α may play an important role in the implantation of a blastocyst.101,102 Synthesis of MIP3α can be induced in response to inflammatory mediators such as IL-1β and TNFα as well as LPS.19,67,103–105 In the female reproductive tract, MIP3α secretion by epithelial cells can be induced following treatment with pathogen-associated molecular patterns (PAMPs) or Escherichia coli.19,52,67 Our studies extend these findings by demonstrating that, in addition to PAMPs and bacteria, growth factors produced by underlying stromal fibroblasts regulate epithelial cell immune protection.
The data presented here demonstrate that KGF increases uterine epithelial cell basolateral KC secretion. Thus far, KC appears to function exclusively as a chemokine for neutrophils; therefore basolateral secretion of KC is most likely responsible for the infiltration of neutrophils into the endometrium.56,58,77,82 Others have shown that neutrophils are essential for remodeling and regeneration of the endometrium during each reproductive cycle in the human and rodent.16,56,58,106–109 Our data showing preferential secretion of KC into the basolateral compartment in response to KGF demonstrate the complexity of the immune system in the female reproductive tract and suggest a level of cell–cell communication that has not previously been appreciated.
Our studies indicate that the KGF-induced secretion of MIP3α is specific in that EGF had no effect on MIP3α secretion while HGF inhibited basolateral MIP3α. In contrast, EGF stimulated both apical and basolateral KC secretion, while HGF stimulated only basolateral KC secretion. Others have shown that KGF, EGF, and HGF have potent mitogenic effects on epithelial cells,87,110,111 but their actions on cytokine/chemokine secretion by uterine epithelial cells, to the best of our knowledge, have not been examined. Our study indicates that each growth factor has its own pattern of action on uterine epithelial cell secretion of innate immune molecules. Previously, our laboratory found that HGF placed in the basolateral compartment of polarized uterine epithelial cells increased TER and decreased TNFα secretion. 51 This study expands these findings by demonstrating that KGF and EGF also affect epithelial cell secretion of MIP3α and KC, both of which are critical to innate immune protection.
In summary, the findings presented from this study indicate that KGF is an important regulator of mouse uterine epithelial cell immune function. To the best of our knowledge, this is the first demonstration that KGF stimulates both apical and basolateral secretion of MIP3α and basolateral secretion of KC by polarized mouse uterine epithelial cells in vitro. It remains to be determined whether estradiol and/or progesterone regulates KGF and the extent to which KGF effects are critical to immune protection, successful fertilization, and reproduction.
Acknowledgments
The authors express their gratitude to Richard M. Rossoll for all of his assistance in these studies as well as to John V. Fahey, Ph.D. for his critical review of this manuscript. They also thank Dr James C. Leiter for his guidance with statistical analysis of the data. This work was supported by NIH research grant AI013541.
References
- 1.Janeway CA, Travers P, Walport M, Schlomchik MJ. Immunobiology: The Immune System in Health and Disease. 6. New York: Garland Science; 2005. [Google Scholar]
- 2.Vaerman J-P. The secretory immune system. Antibiot Chemother. 1987;39:41–50. [PubMed] [Google Scholar]
- 3.Wira CR, Grant-Tschudy KS, Crane-Godreau MA. Epithelial cells in the female reproductive tract: a central role as sentinels of immune protection. Am J Reprod Immunol. 2005;53:65–76. doi: 10.1111/j.1600-0897.2004.00248.x. [DOI] [PubMed] [Google Scholar]
- 4.Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev. 2005;206:306–335. doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 5.Dockery P. The fine structure of the mature human endometrium. In: Glasser SR, Aplin JD, Giudice LC, Tabibzadeh S, editors. The Endometrium. New York: Taylor & Francis; 2002. pp. 21–38. [Google Scholar]
- 6.Sullivan DA, Wira CR. Hormonal regulation of immunoglobulins in the rat uterus: uterine response to multiple estradiol treatments. Endocrinology. 1984;114:650–658. doi: 10.1210/endo-114-2-650. [DOI] [PubMed] [Google Scholar]
- 7.Wira CR, Rossoll RM. Antigen-presenting cells in the female reproductive tract: influence of the estrous cycle on antigen presentation by uterine epithelial and stromal cells. Endocrinology. 1995;136:4526–4534. doi: 10.1210/endo.136.10.7664673. [DOI] [PubMed] [Google Scholar]
- 8.Fahey JV, Wira CR. Effect of menstrual status on antibacterial activity and secretory leukocyte protease inhibitor production by human uterine epithelial cells in culture. J Infect Dis. 2002;185:1606–1613. doi: 10.1086/340512. [DOI] [PubMed] [Google Scholar]
- 9.Robertson SA, Mayrhofer G, Seamark RF. Uterine epithelial cells synthesize granulocyte-macrophage colony-stimulating factor and interleukin-6 in pregnant and nonpregnant mice. Biol Reprod. 1992;46:1069–1079. doi: 10.1095/biolreprod46.6.1069. [DOI] [PubMed] [Google Scholar]
- 10.Quayle AJ, Porter EM, Nussbaum AA, Wang YM, Brabec C, Yip KP, Mok SC. Gene expression, immunolocalization, and secretion of human defensin-5 in human female reproductive tract. Am J Pathol. 1998;152:1247–1258. [PMC free article] [PubMed] [Google Scholar]
- 11.Soboll G, Shen L, Wira CR. Expression of Toll-like receptors (TLR) and responsiveness to TLR agonists by polarized mouse uterine epithelial cells in culture. Biol Reprod. 2006;75:131–139. doi: 10.1095/biolreprod.106.050690. [DOI] [PubMed] [Google Scholar]
- 12.Soboll G, Schaefer TM, Wira CR. Effect of toll-like receptor (TLR) agonists on TLR and microbicide expression in uterine and vaginal tissues of the mouse. Am J Reprod Immunol. 2006;55:434–446. doi: 10.1111/j.1600-0897.2006.00381.x. [DOI] [PubMed] [Google Scholar]
- 13.Schaefer TM, Fahey JV, Wright JA, Wira CR. Innate immunity in the human female reproductive tract: antiviral response of uterine epithelial cells to the TLR3 agonist poly(I:C) J Immunol. 2005;174:992–1002. doi: 10.4049/jimmunol.174.2.992. [DOI] [PubMed] [Google Scholar]
- 14.Schaefer TM, Desouza K, Fahey JV, Beagley KW, Wira CR. Toll-like receptor (TLR) expression and TLRmediated cytokine/chemokine production by human uterine epithelial cells. Immunology. 2004;112:428–436. doi: 10.1111/j.1365-2567.2004.01898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robertson SA, Mayrhofer G, Seamark RF. Ovarian steroid hormones regulate granulocyte-macrophage colony-stimulating factor synthesis by uterine epithelial cells in the mouse. Biol Reprod. 1996;54:183–196. doi: 10.1095/biolreprod54.1.183. [DOI] [PubMed] [Google Scholar]
- 16.Jones RL, Hannan NJ, Kaitu’u TJ, Zhang J, Salamonsen LA. Identification of chemokines important for leukocyte recruitment to the human endometrium at the times of embryo implantation and menstruation. J Clin Endocrinol Metab. 2004;89:6155–6167. doi: 10.1210/jc.2004-0507. [DOI] [PubMed] [Google Scholar]
- 17.Shen L, Fahey JV, Hussey SB, Asin SN, Wira CR, Fanger MW. Synergy between IL-8 and GM-CSF in reproductive tract epithelial cell secretions promotes enhanced neutrophil chemotaxis. Cell Immunol. 2004;230:23–32. doi: 10.1016/j.cellimm.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Grant-Tschudy KS, Wira CR. Effect of oestradiol on mouse uterine epithelial cell tumour necrosis factoralpha release is mediated through uterine stromal cells. Immunology. 2005;115:99–107. doi: 10.1111/j.1365-2567.2005.02134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crane-Godreau MA, Wira CR. CCL20/macrophage inflammatory protein 3alpha and tumor necrosis factor alpha production by primary uterine epithelial cells in response to treatment with lipopolysaccharide or Pam3Cys. Infect Immun. 2005;73:476–484. doi: 10.1128/IAI.73.1.476-484.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fahey JV, Schaefer TM, Channon JY, Wira CR. Secretion of cytokines and chemokines by polarized human epithelial cells from the female reproductive tract. Hum Reprod. 2005;20:1439–1446. doi: 10.1093/humrep/deh806. [DOI] [PubMed] [Google Scholar]
- 21.Ghosh M, Shen Z, Fahey JV, Cu-Uvin S, Mayer K, Wira CR. Trappin-2/Elafin: a novel innate anti-HIV-1 molecule of the human female reproductive tract. Immunology. 2009;129:207–219. doi: 10.1111/j.1365-2567.2009.03165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kabelitz D, Medzhitov R. Innate immunity – cross-talk with adaptive immunity through pattern recognition receptors and cytokines. Curr Opin Immunol. 2007;19:1–3. doi: 10.1016/j.coi.2006.11.018. [DOI] [PubMed] [Google Scholar]
- 23.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Donjacour AA, Cunha GR. Stromal regulation of epithelial function. Cancer Treat Res. 1991;53:335–364. doi: 10.1007/978-1-4615-3940-7_16. [DOI] [PubMed] [Google Scholar]
- 25.Cunha GR. The dual origin of vaginal epithelium. Am J Anat. 1975;143:387–392. doi: 10.1002/aja.1001430309. [DOI] [PubMed] [Google Scholar]
- 26.Cunha GR. Stromal induction and specification of morphogenesis and cytodifferentiation of the epithelia of the Mullerian ducts and urogenital sinus during development of the uterus and vagina in mice. J Exp Zool. 1976;196:361–370. doi: 10.1002/jez.1401960310. [DOI] [PubMed] [Google Scholar]
- 27.Cunha GR, Bigsby RM, Cooke PS, Sugimura Y. Stromal-epithelial interactions in adult organs. Cell Differ. 1985;17:137–148. doi: 10.1016/0045-6039(85)90481-6. [DOI] [PubMed] [Google Scholar]
- 28.Bukovsky A, Caudle MR, Keenan JA, Upadhyaya NB, Van Meter SE, Wimalasena J, Elder RF. Association of mesenchymal cells and immunoglobulins with differentiating epithelial cells. BMC Dev Biol. 2001;1:11. doi: 10.1186/1471-213X-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cunha GR. Epithelial-stromal interactions in development of the urogenital tract. Int Rev Cytol. 1976;47:137–194. doi: 10.1016/s0074-7696(08)60088-1. [DOI] [PubMed] [Google Scholar]
- 30.Imagawa W, Pedchenko VK, Helber J, Zhang H. Hormone/growth factor interactions mediating epithelial/stromal communication in mammary gland development and carcinogenesis. J Steroid Biochem Mol Biol. 2002;80:213–230. doi: 10.1016/s0960-0760(01)00188-1. [DOI] [PubMed] [Google Scholar]
- 31.Arnold JT, Lessey BA, Seppala M, Kaufman DG. Effect of normal endometrial stroma on growth and differentiation in Ishikawa endometrial adenocarcinoma cells. Cancer Res. 2002;62:79–88. [PubMed] [Google Scholar]
- 32.Cooke PS, Buchanan DL, Kurita T, Lubahn DB, Cunha GR. Role of stromal-epithelial interactions in hormonal responses of the uterus. In: Glasser SR, Aplin JD, Giudice LC, Tabibzadeh S, editors. The Endometrium. New York: Taylor & Francis; 2002. pp. 151–166. [Google Scholar]
- 33.Cooke PS, Buchanan DL, Young P, Setiawan T, Brody J, Korach KS, Taylor J, Lubahn DB, Cunha GR. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci U S A. 1997;94:6535–6540. doi: 10.1073/pnas.94.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cunha GR, Young P. Role of stroma in oestrogen-induced epithelial proliferation. Epithelial Cell Biol. 1992;1:18–31. [PubMed] [Google Scholar]
- 35.Finch PW, Rubin JS, Miki T, Ron D, Aaronson SA. Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science. 1989;245:752–755. doi: 10.1126/science.2475908. [DOI] [PubMed] [Google Scholar]
- 36.Rubin JS, Osada H, Finch PW, Taylor WG, Rudikoff S, Aaronson SA. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc Natl Acad Sci USA. 1989;86:802–806. doi: 10.1073/pnas.86.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Housley RM, Morris CF, Boyle W, Ring B, Biltz R, Tarpley JE, Aukerman SL, Devine PL, Whitehead RH, Pierce GF. Keratinocyte growth factor induces proliferation of hepatocytes and epithelial cells throughout the rat gastrointestinal tract. J Clin Invest. 1994;94:1764–1777. doi: 10.1172/JCI117524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hom YK, Young P, Thomson AA, Cunha GR. Keratinocyte growth factor injected into female mouse neonates stimulates uterine and vaginal epithelial growth. Endocrinology. 1998;139:3772–3779. doi: 10.1210/endo.139.9.6182. [DOI] [PubMed] [Google Scholar]
- 39.Finch PW, Cunha GR, Rubin JS, Wong J, Ron D. Pattern of keratinocyte growth factor and keratinocyte growth factor receptor expression during mouse fetal development suggests a role in mediating morphogenetic mesenchymal-epithelial interactions. Dev Dyn. 1995;203:223–240. doi: 10.1002/aja.1002030210. [DOI] [PubMed] [Google Scholar]
- 40.Marchese C, Chedid M, Dirsch OR, Csaky KG, Santanelli F, Latini C, LaRochelle WJ, Torrisi MR, Aaronson SA. Modulation of keratinocyte growth factor and its receptor in reepithelializing human skin. J Exp Med. 1995;182:1369–1376. doi: 10.1084/jem.182.5.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savla U, Waters CM. Barrier function of airway epithelium: effects of radiation and protection by keratinocyte growth factor. Radiat Res. 1998;150:195–203. [PubMed] [Google Scholar]
- 42.Wu KI, Pollack N, Panos RJ, Sporn PH, Kamp DW. Keratinocyte growth factor promotes alveolar epithelial cell DNA repair after H2O2 exposure. Am J Physiol. 1998;275:L780–L787. doi: 10.1152/ajplung.1998.275.4.L780. [DOI] [PubMed] [Google Scholar]
- 43.Yano T, Mason RJ, Pan T, Deterding RR, Nielsen LD, Shannon JM. KGF regulates pulmonary epithelial proliferation and surfactant protein gene expression in adult rat lung. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1146–L1158. doi: 10.1152/ajplung.2000.279.6.L1146. [DOI] [PubMed] [Google Scholar]
- 44.Atabai K, Ishigaki M, Geiser T, Ueki I, Matthay MA, Ware LB. Keratinocyte growth factor can enhance alveolar epithelial repair by nonmitogenic mechanisms. Am J Physiol Lung Cell Mol Physiol. 2002;283:L163–L169. doi: 10.1152/ajplung.00396.2001. [DOI] [PubMed] [Google Scholar]
- 45.Miki T, Fleming TP, Bottaro DP, Rubin JS, Ron D, Aaronson SA. Expression cDNA cloning of the KGF receptor by creation of a transforming autocrine loop. Science. 1991;251:72–75. doi: 10.1126/science.1846048. [DOI] [PubMed] [Google Scholar]
- 46.Miki T, Bottaro DP, Fleming TP, Smith CL, Burgess WH, Chan AM, Aaronson SA. Determination of ligand-binding specificity by alternative splicing: two distinct growth factor receptors encoded by a single gene. Proc Natl Acad Sci USA. 1992;89:246–250. doi: 10.1073/pnas.89.1.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bottaro DP, Rubin JS, Ron D, Finch PW, Florio C, Aaronson SA. Characterization of the receptor for keratinocyte growth factor Evidence for multiple fibroblast growth factor receptors. J Biol Chem. 1990;265:12767–12770. [PubMed] [Google Scholar]
- 48.Pedchenko VK, Imagawa W. Estrogen treatment in vivo increases keratinocyte growth factor expression in the mammary gland. J Endocrinol. 2000;165:39–49. doi: 10.1677/joe.0.1650039. [DOI] [PubMed] [Google Scholar]
- 49.Pekonen F, Nyman T, Rutanen EM. Differential expression of keratinocyte growth factor and its receptor in the human uterus. Mol Cell Endocrinol. 1993;95:43–49. doi: 10.1016/0303-7207(93)90027-h. [DOI] [PubMed] [Google Scholar]
- 50.Yan G, Fukabori Y, Nikolaropoulos S, Wang F, McKeehan WL. Heparin-binding keratinocyte growth factor is a candidate stromal-to-epithelial-cell andromedin. Mol Endocrinol. 1992;6:2123–2128. doi: 10.1210/mend.6.12.1491693. [DOI] [PubMed] [Google Scholar]
- 51.Grant-Tschudy KS, Wira CR. Hepatocyte growth factor regulation of uterine epithelial cell transepithelial resistance and tumor necrosis factor alpha release in culture. Biol Reprod. 2005;72:814–821. doi: 10.1095/biolreprod.104.035618. [DOI] [PubMed] [Google Scholar]
- 52.Crane-Godreau MA, Wira CR. Effect of Escherichia coli and Lactobacillus rhamnosus on macrophage inflammatory protein 3 alpha, tumor necrosis factor alpha, and transforming growth factor beta release by polarized rat uterine epithelial cells in culture. Infect Immun. 2004;72:1866–1873. doi: 10.1128/IAI.72.4.1866-1873.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cremel M, Berlier W, Hamzeh H, Cognasse F, Lawrence P, Genin C, Bernengo JC, Lambert C, Dieu-Nosjean MC, Delezay O. Characterization of CCL20 secretion by human epithelial vaginal cells: involvement in Langerhans cell precursor attraction. J Leukoc Biol. 2005;78:158–166. doi: 10.1189/jlb.0305147. [DOI] [PubMed] [Google Scholar]
- 54.Arici A, Head JR, MacDonald PC, Casey ML. Regulation of interleukin-8 gene expression in human endometrial cells in culture. Mol Cell Endocrinol. 1993;94:195–204. doi: 10.1016/0303-7207(93)90168-j. [DOI] [PubMed] [Google Scholar]
- 55.Ghosh M, Shen Z, Schaefer TM, Fahey JV, Gupta P, Wira CR. CCL20/MIP3alpha is a novel anti-HIV-1 molecule of the human female reproductive tract. Am J Reprod Immunol. 2009;62:60–71. doi: 10.1111/j.1600-0897.2009.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Corbeil LB, Chatterjee A, Foresman L, Westfall JA. Ultrastructure of cyclic changes in the murine uterus, cervix, and vagina. Tissue Cell. 1985;17:53–68. doi: 10.1016/0040-8166(85)90015-1. [DOI] [PubMed] [Google Scholar]
- 57.Zarnani AH, Moazzeni SM, Shokri F, Salehnia M, Jeddi Tehrani M. Analysis of endometrial myeloid and lymphoid dendritic cells during mouse estrous cycle. J Reprod Immunol. 2006;71:28–40. doi: 10.1016/j.jri.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 58.Sonoda Y, Mukaida N, Wang JB, Shimada-Hiratsuka M, Naito M, Kasahara T, Harada A, Inoue M, Matsushima K. Physiologic regulation of postovulatory neutrophil migration into vagina in mice by a C-X-C chemokine(s) J Immunol. 1998;160:6159–6165. [PubMed] [Google Scholar]
- 59.Hieshima K, Imai T, Opdenakker G, Van Damme J, Kusuda J, Tei H, Sakaki Y, Takatsuki K, Miura R, Yoshie O, Nomiyama H. Molecular cloning of a novel human CC chemokine liver and activation-regulated chemokine (LARC) expressed in liver. Chemotactic activity for lymphocytes and gene localization on chromosome 2. J Biol Chem. 1997;272:5846–5853. doi: 10.1074/jbc.272.9.5846. [DOI] [PubMed] [Google Scholar]
- 60.Olson TS, Ley K. Chemokines and chemokine receptors in leukocyte trafficking. Am J Physiol Regul Integr Comp Physiol. 2002;283:R7–R28. doi: 10.1152/ajpregu.00738.2001. [DOI] [PubMed] [Google Scholar]
- 61.Baba M, Imai T, Nishimura M, Kakizaki M, Takagi S, Hieshima K, Nomiyama H, Yoshie O. Identification of CCR6, the specific receptor for a novel lymphocytedirected CC chemokine LARC. J Biol Chem. 1997;272:14893–14898. doi: 10.1074/jbc.272.23.14893. [DOI] [PubMed] [Google Scholar]
- 62.Fitzhugh DJ, Naik S, Caughman SW, Hwang ST. Cutting edge: C-C chemokine receptor 6 is essential for arrest of a subset of memory T cells on activated dermal microvascular endothelial cells under physiologic flow conditions in vitro. J Immunol. 2000;165:6677–6681. doi: 10.4049/jimmunol.165.12.6677. [DOI] [PubMed] [Google Scholar]
- 63.Perez-Canadillas JM, Zaballos A, Gutierrez J, Varona R, Roncal F, Albar JP, Marquez G, Bruix M. NMR solution structure of murine CCL20/MIP-3alpha, a chemokine that specifically chemoattracts immature dendritic cells and lymphocytes through its highly specific interaction with the beta-chemokine receptor CCR6. J Biol Chem. 2001;276:28372–28379. doi: 10.1074/jbc.M103121200. [DOI] [PubMed] [Google Scholar]
- 64.Hoover DM, Boulegue C, Yang D, Oppenheim JJ, Tucker K, Lu W, Lubkowski J. The structure of human macrophage inflammatory protein-3alpha/CCL20. Linking antimicrobial and CC chemokine receptor-6-binding activities with human betadefensins. J Biol Chem. 2002;277:37647–37654. doi: 10.1074/jbc.M203907200. [DOI] [PubMed] [Google Scholar]
- 65.Krzysiek R, Lefevre EA, Bernard J, Foussat A, Galanaud P, Louache F, Richard Y. Regulation of CCR6 chemokine receptor expression and responsiveness to macrophage inflammatory protein-3alpha/CCL20 in human B cells. Blood. 2000;96:2338–2345. [PubMed] [Google Scholar]
- 66.Yang D, Chen Q, Hoover DM, Staley P, Tucker KD, Lubkowski J, Oppenheim JJ. Many chemokines including CCL20/MIP-3alpha display antimicrobial activity. J Leukoc Biol. 2003;74:448–455. doi: 10.1189/jlb.0103024. [DOI] [PubMed] [Google Scholar]
- 67.Soboll G, Crane-Godreau MA, Lyimo MA, Wira CR. Effect of oestradiol on PAMP-mediated CCL20/MIP-3 alpha production by mouse uterine epithelial cells in culture. Immunology. 2006;118:185–194. doi: 10.1111/j.1365-2567.2006.02353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Larsen CG, Anderson AO, Appella E, Oppenheim JJ, Matsushima K. The neutrophil-activating protein (NAP-1) is also chemotactic for T lymphocytes. Science. 1989;243:1464–1466. doi: 10.1126/science.2648569. [DOI] [PubMed] [Google Scholar]
- 69.Matsushima K, Morishita K, Yoshimura T, Lavu S, Kobayashi Y, Lew W, Appella E, Kung HF, Leonard EJ, Oppenheim JJ. Molecular cloning of a human monocyte-derived neutrophil chemotactic factor (MDNCF) and the induction of MDNCF mRNA by interleukin 1 and tumor necrosis factor. J Exp Med. 1988;167:1883–1893. doi: 10.1084/jem.167.6.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baggiolini M, Walz A, Kunkel SL. Neutrophilactivating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kayisli UA, Mahutte NG, Arici A. Uterine chemokines in reproductive physiology and pathology. Am J Reprod Immunol. 2002;47:213–221. doi: 10.1034/j.1600-0897.2002.01075.x. [DOI] [PubMed] [Google Scholar]
- 72.Arici A, Seli E, Senturk LM, Gutierrez LS, Oral E, Taylor HS. Interleukin-8 in the human endometrium. J Clin Endocrinol Metab. 1998;83:1783–1787. doi: 10.1210/jcem.83.5.4754. [DOI] [PubMed] [Google Scholar]
- 73.Luk J, Seval Y, Kayisli UA, Ulukus M, Ulukus CE, Arici A. Regulation of interleukin-8 expression in human endometrial endothelial cells: a potential mechanism for the pathogenesis of endometriosis. J Clin Endocrinol Metab. 2005;90:1805–1811. doi: 10.1210/jc.2004-1813. [DOI] [PubMed] [Google Scholar]
- 74.Bozic CR, Gerard NP, von Uexkull-Guldenband C, Kolakowski LF, Jr, Conklyn MJ, Breslow R, Showell HJ, Gerard C. The murine interleukin 8 type B receptor homologue and its ligands. Expression and biological characterization. J Biol Chem. 1994;269:29355–29358. [PubMed] [Google Scholar]
- 75.Oquendo P, Alberta J, Wen DZ, Graycar JL, Derynck R, Stiles CD. The platelet-derived growth factor-inducible KC gene encodes a secretory protein related to platelet alpha-granule proteins. J Biol Chem. 1989;264:4133–4137. [PubMed] [Google Scholar]
- 76.Wolpe SD, Sherry B, Juers D, Davatelis G, Yurt RW, Cerami A. Identification and characterization of macrophage inflammatory protein 2. Proc Natl Acad Sci U S A. 1989;86:612–616. doi: 10.1073/pnas.86.2.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bozic CR, Kolakowski LF, Jr, Gerard NP, Garcia-Rodriguez C, von Uexkull-Guldenband C, Conklyn MJ, Breslow R, Showell HJ, Gerard C. Expression and biologic characterization of the murine chemokine KC. J Immunol. 1995;154:6048–6057. [PubMed] [Google Scholar]
- 78.Cochran BH, Reffel AC, Stiles CD. Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell. 1983;33:939–947. doi: 10.1016/0092-8674(83)90037-5. [DOI] [PubMed] [Google Scholar]
- 79.Introna M, Bast RC, Jr, Tannenbaum CS, Hamilton TA, Adams DO. The effect of LPS on expression of the early “competence” genes JE and KC in murine peritoneal macrophages. J Immunol. 1987;138:3891–3896. [PubMed] [Google Scholar]
- 80.Finn CA, Pope MD. Infiltration of neutrophil polymorphonuclear leucocytes into the endometrial stroma at the time of implantation of ova and the initiation of the oil decidual cell reaction in mice. J Reprod Fertil. 1991;91:365–369. doi: 10.1530/jrf.0.0910365. [DOI] [PubMed] [Google Scholar]
- 81.McColl SR, Clark-Lewis I. Inhibition of murine neutrophil recruitment in vivo by CXC chemokine receptor antagonists. J Immunol. 1999;163:2829–2835. [PubMed] [Google Scholar]
- 82.Wood GW, Hausmann EH, Kanakaraj K. Expression and regulation of chemokine genes in the mouse uterus during pregnancy. Cytokine. 1999;11:1038–1045. doi: 10.1006/cyto.1999.0513. [DOI] [PubMed] [Google Scholar]
- 83.Nemzek JA, Ebong SJ, Kim J, Bolgos GL, Remick DG. Keratinocyte growth factor pretreatment is associated with decreased macrophage inflammatory protein-2alpha concentrations and reduced neutrophil recruitment in acid aspiration lung injury. Shock. 2002;18:501–506. doi: 10.1097/00024382-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 84.Grant-Tschudy KS, Wira CR. Paracrine mediators of mouse uterine epithelial cell transepithelial resistance in culture. J Reprod Immunol. 2005;67:1–12. doi: 10.1016/j.jri.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 85.Crane-Godreau MA, Wira CR. Effects of estradiol on lipopolysaccharide and Pam3Cys stimulation of CCL20/macrophage inflammatory protein 3 alpha and tumor necrosis factor alpha production by uterine epithelial cells in culture. Infect Immun. 2005;73:4231–4237. doi: 10.1128/IAI.73.7.4231-4237.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Soboll G, Crane-Godreau MA, Lyimo MA, Wira CR. Effect of Oestradiol on PAMP mediated CCL20/MIP3alpha production by mouse uterine epithelial cells in culture. Immunology. 2006;118:185–194. doi: 10.1111/j.1365-2567.2006.02353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sugawara J, Fukaya T, Murakami T, Yoshida H, Yajima A. Hepatocyte growth factor stimulates proliferation, migration, and lumen formation of human endometrial epithelial cells in vitro. Biol Reprod. 1997;57:936–942. doi: 10.1095/biolreprod57.4.936. [DOI] [PubMed] [Google Scholar]
- 88.Lail-Trecker M, Gulati R, Peluso JJ. A role for hepatocyte growth factor/scatter factor in regulating normal and neoplastic cells of reproductive tissues. J Soc Gynecol Investig. 1998;5:114–121. doi: 10.1016/s1071-5576(97)00111-1. [DOI] [PubMed] [Google Scholar]
- 89.Hom YK, Young P, Wiesen JF, Miettinen PJ, Derynck R, Werb Z, Cunha GR. Uterine and vaginal organ growth requires epidermal growth factor receptor signaling from stroma. Endocrinology. 1998;139:913–921. doi: 10.1210/endo.139.3.5817. [DOI] [PubMed] [Google Scholar]
- 90.Ejskjaer K, Sorensen BS, Poulsen SS, Mogensen O, Forman A, Nexo E. Expression of the epidermal growth factor system in human endometrium during the menstrual cycle. Mol Hum Reprod. 2005;11:543–551. doi: 10.1093/molehr/gah207. [DOI] [PubMed] [Google Scholar]
- 91.Rutanen EM. Insulin-like growth factors in endometrial function. Gynecol Endocrinol. 1998;12:399–406. doi: 10.3109/09513599809012842. [DOI] [PubMed] [Google Scholar]
- 92.Slayden OD, Rubin JS, Lacey DL, Brenner RM. Effects of keratinocyte growth factor in the endometrium of rhesus macaques during the luteal-follicular transition. J Clin Endocrinol Metab. 2000;85:275–285. doi: 10.1210/jcem.85.1.6251. [DOI] [PubMed] [Google Scholar]
- 93.Siegfried S, Pekonen F, Nyman T, Ammala M. Expression of mRNA for keratinocyte growth factor and its receptor in human endometrium. Acta Obstet Gynecol Scand. 1995;74:410–414. doi: 10.3109/00016349509024400. [DOI] [PubMed] [Google Scholar]
- 94.Visco V, Belleudi F, Marchese C, Leone L, Aimati L, Cardinali G, Kovacs D, Frati L, Torrisi MR. Differential response to keratinocyte growth factor receptor and epidermal growth factor receptor ligands of proliferating and differentiating intestinal epithelial cells. J Cell Physiol. 2004;200:31–44. doi: 10.1002/jcp.10385. [DOI] [PubMed] [Google Scholar]
- 95.Zang XP, Pento JT. Keratinocyte growth factorinduced motility of breast cancer cells. Clin Exp Metastasis. 2001;18:573–580. doi: 10.1023/a:1011997317994. [DOI] [PubMed] [Google Scholar]
- 96.Xu X, McCormick-Shannon K, Voelker DR, Mason RJ. KGF increases SP-A and SP-D mRNA levels and secretion in cultured rat alveolar type II cells. Am J Respir Cell Mol Biol. 1998;18:168–178. doi: 10.1165/ajrcmb.18.2.2824. [DOI] [PubMed] [Google Scholar]
- 97.Baghaban Eslami Nejad MR, Rezazadeh Valojerdi M, Kazemi Ashtiani S. A comparison of polarized and non-polarized human endometrial monolayer culture systems on murine embryo development. J Exp Clin Assist Reprod. 2005;2:7. doi: 10.1186/1743-1050-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pedchenko VK, Imagawa WT. Mammogenic hormones differentially modulate keratinocyte growth factor (KGF)-induced proliferation and KGF receptor expression in cultured mouse mammary gland epithelium. Endocrinology. 1998;139:2519–2526. doi: 10.1210/endo.139.5.6007. [DOI] [PubMed] [Google Scholar]
- 99.Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. 2003;14:409–426. doi: 10.1016/s1359-6101(03)00049-2. [DOI] [PubMed] [Google Scholar]
- 100.Ghosh M, Shen Z, Schaefer TM, Fahey JV, Gupta P, Wira CR. CCL20/MIP3α is a novel anti-HIV-1 molecule of the human female reproductive tract. Am J Reprod Immunol. 2009;62:60–71. doi: 10.1111/j.1600-0897.2009.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zarnani AH, Moazzeni SM, Shokri F, Salehnia M, Jeddi-Tehrani M. Kinetics of murine decidual dendritic cells. Reproduction (Cambridge, England) 2007;133:275–283. doi: 10.1530/rep.1.01232. [DOI] [PubMed] [Google Scholar]
- 102.Plaks V, Birnberg T, Berkutzki T, Sela S, BenYashar A, Kalchenko V, Mor G, Keshet E, Dekel N, Neeman M, Jung S. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J Clin Invest. 2008;118:3954–3965. doi: 10.1172/JCI36682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reibman J, Hsu Y, Chen LC, Bleck B, Gordon T. Airway epithelial cells release MIP-3alpha/CCL20 in response to cytokines and ambient particulate matter. Am J Respir Cell Mol Biol. 2003;28:648–654. doi: 10.1165/rcmb.2002-0095OC. [DOI] [PubMed] [Google Scholar]
- 104.Starner TD, Barker CK, Jia HP, Kang Y, McCray PB., Jr CCL20 is an inducible product of human airway epithelia with innate immune properties. Am J Respir Cell Mol Biol. 2003;29:627–633. doi: 10.1165/rcmb.2002-0272OC. [DOI] [PubMed] [Google Scholar]
- 105.Hosokawa Y, Hosokawa I, Ozaki K, Nakae H, Matsuo T. Increase of CCL20 expression by human gingival fibroblasts upon stimulation with cytokines and bacterial endotoxin. Clin Exp Immunol. 2005;142:285–291. doi: 10.1111/j.1365-2249.2005.02912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Salamonsen LA, Zhang J, Brasted M. Leukocyte networks and human endometrial remodelling. J Reprod Immunol. 2002;57:95–108. doi: 10.1016/s0165-0378(02)00011-6. [DOI] [PubMed] [Google Scholar]
- 107.Salamonsen LA, Lathbury LJ. Endometrial leukocytes and menstruation. Hum Reprod Update. 2000;6:16–27. doi: 10.1093/humupd/6.1.16. [DOI] [PubMed] [Google Scholar]
- 108.Brasted M, White CA, Kennedy TG, Salamonsen LA. Mimicking the events of menstruation in the murine uterus. Biol Reprod. 2003;69:1273–1280. doi: 10.1095/biolreprod.103.016550. [DOI] [PubMed] [Google Scholar]
- 109.Senger PL. Pathways to Pregnancy and Parturition. Pullman: Current Conceptions Inc; 1999. [Google Scholar]
- 110.Rubin JS, Bottaro DP, Chedid M, Miki T, Ron D, Cheon G, Taylor WG, Fortney E, Sakata H, Finch PW, LaRochelle WJ. Keratinocyte growth factor. Cell Biol Int. 1995;19:399–411. doi: 10.1006/cbir.1995.1085. [DOI] [PubMed] [Google Scholar]
- 111.Tomooka Y, DiAugustine RP, McLachlan JA. Proliferation of mouse uterine epithelial cells in vitro. Endocrinology. 1986;118:1011–1018. doi: 10.1210/endo-118-3-1011. [DOI] [PubMed] [Google Scholar]