Abstract
Formation of neural circuits depends on stable contacts between neuronal processes, mediated by interaction of cell adhesion molecules, including N-cadherin. In the present study, we found that activity-dependent dendrite arborization specifically requires N-cadherin–mediated extracellular neuron–neuron interaction, because the enhancement did not occur for neurons cultured in isolation or plated on an astrocyte monolayer and was abolished by a recombinant soluble N-cadherin ectodomain. Furthermore, depolarization elevated the level of membrane-associated cadherin/catenin complexes and surface N-cadherin. Importantly, surface N-cadherin elevation is specifically required for the maintenance of nascent dendrite arbors. Through loss- and gain-of-function approaches, we showed that N-cadherin-mediated dendrite growth requires association of the cadherin/catenin complex with the actin cytoskeleton. In summary, these results identify a previously unexplored and specific function for activity-induced, N-cadherin–mediated neuron–neuron contacts in the maintenance of dendrite arbors.
Keywords: cell adhesion molecule, catenin, actin
Dendrite growth and development are regulated by a combination of intrinsic programs and extrinsic signals, including neuronal activity, neurotrophins, morphogens, guidance cues, and cell adhesion molecules (CAMs), such as classical and seven-pass transmembrane cadherins (1 –4). The establishment and maintenance of synaptic contacts between axons and dendrites also depend on CAMs, including neurexins/neurligins, EphB/ephrin-Bs, Neural-cadherin (N-cadherin), Ig superfamily members, and leucine-rich repeat containing synaptic adhesion molecules (5, 6). Importantly, the processes of axon/dendrite development and synapse formation are tightly correlated and regulated by neuronal activity (1, 7).
N-cadherin is a transmembrane CAM that interacts in a homophilic Ca2+-dependent manner through its extracellular ectodomains (8). Together with β-catenin and αN-catenin, it forms the cadherin/catenin complex, a main complex linking the extracellular environment to the actin cytoskeleton (9). The cadherin/catenin complex is present at high levels in both axons and dendrites (10), forming adherens junctions in epithelial cells and synaptic junctions in neurons.
In the present study, we examined the function of N-cadherin–mediated cell-cell interaction in the stabilization of dendritic arbors and in activity-dependent enhancement of dendritogenesis. Using a soluble N-cadherin ectodomain, we demonstrated a requirement for N-cadherin–mediated extracellular interaction in activity-dependent dendrite growth. Furthermore, by plating neurons in isolation, we showed that cell–cell contact is required for activity and N-cadherin–dependent dendrite growth. Finally, we showed that neuronal activity elevated surface N-cadherin level, an effect required for the maintenance of dendrite arbors. Together, these results identify a previously unexplored and specific function for activity-induced elevation of surface N-cadherin in the maintenance of dendrite arbors.
Results
Critical Role of N-Cadherin–Mediated Neuron–Neuron Interaction in Dendrite Development.
To specifically disrupt N-cadherin–mediated extracellular interaction, we generated a construct expressing a secreted, soluble form of the first extracellular ectodomain of N-cadherin, EC1 (Fig. 1A), a domain essential for cadherin-mediated interaction between adjoining cells (8). Immunoprecipitation assay showed that secreted EC1 can be detected in the medium of transfected day in vitro (DIV) 8 neurons (Fig. 1B). Further biochemical and immunocytochemical assays showed that it interacted highly with N-cadherin, and very weakly if at all, with E-cadherin (Fig. S1 A–C). Transient overexpression of EC1 from DIV 6 to 8 in hippocampal neurons significantly reduced their dendrite complexity (Fig. 1C), as measured by total dendritic branch length (TDBL) (Fig. 1D) and total dendritic branch tip number (TDBTN) (Fig. 1E), an effect consistent over 12 sister cultures (Fig. 1F). EC1 expressed in HeLa cells also reduced the dendrite arbors of nearby cocultured neurons (Fig. S1 D and E). Furthermore, when purified recombinant EC1 protein was added to the medium of GFP-expressing neurons from DIV 6 to 8, it reduced dendrite growth in a dose-dependent fashion, both in regular culture medium (Fig. 1 G–I) and following depolarization with elevated extracellular K+ (14 mM) (Fig. 1 J–L), a manipulation that mimicked increased neural activity (11, 12). As controls, addition of BSA or denatured EC1 (b-EC1) did not affect dendrite morphology (Fig. 1 G–L). EC1 also effectively blocked the dendrite-promoting effects of other activity-mimicking manipulations, including kainic acid or picrotoxin treatment, as well as brain-derived neurotrophic factor (BDNF) application (Fig. S1 F–H). The effect of EC1 protein in reducing TDBTN and blocking activity-induced changes was also significant for older neurons treated with EC1 protein at DIV 14 to 16 (Fig. S1I). Consistent with the functioning of the cadherin/catenin complex as a single unit that requires N-cadherin–mediated extracellular interaction, the dendrite-promoting effects of β-/αN-catenin or N-cadherin overexpression were effectively blocked by EC1 coexpression at DIV 6 to 8 (Fig. S1 J and K). These results demonstrate that the EC1 domain of N-cadherin plays a critical role in activity and cadherin/catenin-dependent dendrite development.
Fig. 1.
N-cadherin-mediated extracellular interaction is required for activity-dependent dendrite growth. (A) EC1 and full-length N-cadherin. EC, extracellular domain; ss, signal peptide; TM, transmembrane domain. (B) Immunoprecipitation (IP) of EC1-myc specifically from EC1-transfected neurons. Ctrl immunoblots (IB) show equivalent level of IgG in IP samples, and of tubulin in whole-cell samples. (C) Representative images of neurons transfected with GFP only (Ctrl) or EC1. (D) Cumulative probability distribution and quantitation of normalized TDBL for EC1 (0.71 ± 0.04, P < 0.001). (E) Cumulative probability distribution and quantitation of normalized TDBTN for EC1 (0.83 ± 0.02, P < 0.001). (F) Change in TDBTN consistent over 12 sister cultures. (G) Images of neurons incubated with EC1 protein, concentration/mL as indicated. (H) Quantitation of TDBTN: 0.2 μg EC1 (0.94 ± 0.03), 1 μg EC1 (0.81 ± 0.03, P < 0.001), 5 μg EC1 (0.80 ± 0.03, P < 0.001), BSA (0.99 ± 0.03), and b-EC1 (0.95 ± 0.03). (I) Quantitation of TDBL: 0.2 μg EC1 (0.99 ± 0.05), 1 μg EC1 (0.72 ± 0.04, P < 0.001), 5 μg EC1 (0.76 ± 0.05, P < 0.01), BSA (1.03 ± 0.04), and b-EC1 (1.03 ± 0.06). (J) Images of neurons incubated with K+ and EC1. (K) Quantitation of TDBTN: K+ (1.25 ± 0.04, P < 0.001), K+ + 0.2 μg EC1 (1.21 ± 0.03, P < 0.001), K+ + 1 μg EC1 (0.99 ± 0.04), K+ + 5 μg EC1 (0.79 ± 0.03, P < 0.001 vs. Ctrl, P < 0.001 vs. K+), and K+ + BSA (1.30 ± 0.03, P < 0.001). (L) Quantitation of TDBL: K+ (1.52 ± 0.08, P < 0.001), K+ + 0.2 μg EC1 (1.47 ± 0.07, P < 0.001), K+ + 1 μg EC1 (1.03 ± 0.04), K+ + 5 μg EC1 (0.70 ± 0.04, P < 0.01 vs. Ctrl, P < 0.001 vs. K+), and K+ + BSA (1.66 ± 0.06, P < 0.001). In this and all subsequent figures, number of neurons as indicated in bar graphs; more detailed quantitation is in Table S1. *P < 0.05, **P < 0.01, ***P < 0.001. (Scale bar, 20 μm.)
To test whether N-cadherin–mediated interaction between neighboring cells is required for activity-dependent dendrite growth, we plated isolated hippocampal neurons at low density. Under these conditions, neither EC1 protein nor K+ modulated dendrite growth when applied DIV 1 to 3 (Fig. 2 A–C). This finding was not affected by plating the neurons on an astrocyte feeder layer (Fig. 2 D–F), indicating no roles for neuron-astrocyte interaction in cadherin- or activity-dependent dendrite growth. However, astrocytes did contribute to activity-independent neurite growth, as neurons plated on astrocytes had 34% more dendrites compared with sister cultures plated alone. As a positive control, in DIV 3 neurons plated at high density, EC1 protein reduced TDBTN/TDBL and completely blocked the effect of K+ in promoting dendrite arborization (Fig. 2 G–I). Similarly, overexpression of N-cadherin in low-density culture did not affect dendrite growth (Fig. 2 J and K), although its expression in high-density culture significantly promoted it (Fig. 2 L and M). Together, these results demonstrate that N-cadherin–mediated neuron–neuron interaction is required for activity and N-cadherin–dependent dendrite growth.
Fig. 2.
Requirement for neuron–neuron interaction in activity and N-cadherin-dependent dendrite growth. (A) Images of DIV 3 neurons plated at low density, with no contacting neighbors (MAP2 channel). (B) Quantitation of TDBTN: EC1 (1.11 ± 0.06), K+ (1.03 ± 0.04), and K+ + EC1 (1.05 ± 0.05). (C) Quantitation of TDBL: EC1 (1.06 ± 0.07), K+ (1.02 ± 0.05), and K+ + EC1 (1.04 ± 0.07). (D) Images of low-density neurons plated on astrocytes (weak background-like MAP2 staining). (E) Quantitation of TDBTN: EC1 (0.95 ± 0.04), K+ (0.99 ± 0.05), and K+ + EC1 (1.03 ± 0.05). (F) Quantitation of TDBL: EC1 (1.03 ± 0.06), K+ (1.02 ± 0.08), and K+ + EC1 (1.08 ± 0.06). (G) Images of neurons plated at high density. (H) Quantitation of TDBTN: EC1 (0.79 ± 0.05, P < 0.05), K+ (1.39 ± 0.07, P < 0.01), and K+ + EC1 (0.81 ± 0.04, P < 0.05). (I) Quantitation of TDBL: EC1 (0.69 ± 0.05, P < 0.05), K+ (1.47 ± 0.10, P < 0.001), and K+ + EC1 (0.68 ± 0.06, P < 0.05). (J and K) N-cadherin overexpression in low density cultures does not affect TDBTN (1.07 ± 0.06, P = 0.39) or TDBL (1.01 ± 0.06, P = 0.91). (L and M) N-cadherin overexpression in high density cultures significantly increased TDBTN (1.46 ± 0.07, P < 0.001) and TDBL (1.54 ± 0.11, P < 0.001). *P < 0.05, **P < 0.01. (Scale bars in A, D, and G, 20 μm.)
Concurrent Requirement for Actin Dynamics and N-Cadherin–Dependent Cell Adhesion During Dendrite Growth.
Recent studies showed suppression of Arp2/3-mediated actin polymerization by α-catenin in vitro, indicating mutual competition for F-actin binding (13, 14). To further examine the relationship between the cadherin/catenin complex and actin dynamics during dendrite development, we reduced endogenous Arp3 level by RNA interfence (RNAi) from DIV 6 to 8. This manipulation reduced TDBTN, an effect fully rescued by overexpression of RNAi-resistant human Arp3 (h-Arp3). In contrast, Arp3-RNAi completely blocked the effects of K+ treatment, N-cadherin, or αN-catenin overexpression (Fig. 3A). These results suggest that the dendrite-promoting effect of neuronal activity and the cadherin/catenin complex requires Arp3-dependent F-actin assembly. Consistently, pharmacological and molecular manipulations that interfere with actin dynamics also blocked activity- and cadherin/catenin-dependent dendrite growth (Fig. S2 A and B).
Fig. 3.
Interplay between the cadherin/catenin complex and actin dynamics during dendrite development. (A) Quantitation: Arp3-RNAi (0.75 ± 0.02), K+ (1.23 ± 0.03), K+ + Arp3-RNAi (0.75 ± 0.03), N-cad (1.23 ± 0.03), N-cad + Arp3-RNAi (0.74 ± 0.03), αN-cat (1.15 ± 0.03), αNcat + Arp3-RNAi (0.73 ± 0.03), h-Arp3 (1.25 ± 0.03), and h-Arp3 + Arp3-RNAi (1.25 ± 0.04). P < 0.01 or P < 0.001 for all conditions vs. Ctrl. (B) Quantitation: Arp3 (1.23 ± 0.03), Arp3 + EC1 (0.79 ± 0.03), WAVE2 (1.30 ± 0.04), WAVE2 + EC1 (0.81 ± 0.03), WASP (1.28 ± 0.03), WASP + EC1 (0.83 ± 0.03), VASP (1.22 ± 0.03), VASP + EC1 (0.78 ± 0.02), and EC1 alone (0.78 ± 0.01). P < 0.001 for all conditions vs. Ctrl. (C) Quantitation: Ncad-AD (1.33 ± 0.03), Ncad-RNAi (0.83 ± 0.02), Ncad-RNAi + Ncad-AD (1.22 ± 0.03), βcat-RNAi (0.85 ± 0.02), βcat-RNAi + Ncad-AD (1.26 ± 0.04), αNcat-RNAi (0.82 ± 0.03), αNcat-RNAi + Ncad-AD (1.43 ± 0.05), EC1 (0.81 ± 0.03), and EC1 + Ncad-AD (0.85 ± 0.03). P < 0.01 or P < 0.001 for all conditions vs. Ctrl. **P < 0.01; ***P < 0.001.
Having shown that Arp3 overexpression is sufficient to induce dendritogenesis (Fig. 3 A and B), we asked whether other F-actin assembly-promoting factors have similar effects. As shown in Fig. 3B, overexpression of Wiskott-Aldrich syndrome protein (WASP) or WASP family verprolin-homologus protein 2 (WAVE2) (15), both activators of Arp3, as well as overexpression of the vasodilator-stimulated phosphoprotein (VASP) (16), a protein that prevents the capping of F-actin barbed ends, all promoted dendritogenesis. Importantly, these actin-dependent dendrite growth effects all required cadherin-dependent extracellular interaction, as they were effectively blocked by EC1 coexpression (Fig. 3B). We note that although multiple regulators of F-actin assembly could promote dendrite growth, not all such proteins are effective. For example, vinculin, an F-actin binding protein with homology to αN-catenin (17), did not affect TDBTN (Fig. S2C).
A Chimeric Protein Mimics the Dendritogenic Effect of the Cadherin/Catenin Complex.
Because the dendrite-promoting effects of F-actin assembly factors require N-cadherin–dependent cell adhesion, we asked whether the chimeric protein “Ncad-AD,” consisting of the extracellular and transmembrane domains of N-cadherin fused to the actin binding domain of αN-catenin (18), can mimic the dendritogenic effect of the entire cadherin/catenin complex. Indeed, overexpressing Ncad-AD increased dendrite growth and fully prevented the dendrite-reducing effect of lowering endogenous N-cadherin, β-catenin, or αN-catenin levels with RNAi (Fig. 3C), showing that Ncad-AD can function in place of the cadherin/catinin complex. Consistent with the notion that Ncad-AD mimics the endogenous function of N-cadherin and depends on cell–cell interaction, coexpression of Ncad-AD did not prevent the dendrite reducing effect of EC1 (Fig. 3C). These results suggest that the main function of the cadherin/catenin complex during dendrite development is to anchor F-actin to N-cadherin mediated cell-cell contacts.
Activity Increased the Level of Membrane-Associated Cadherin/Catenin Complex.
Because gain- and loss-of-function experiments demonstrated that N-cadherin, β-catenin, αN-catenin, and Arp3 are all required for activity- and cadherin-dependent enhancement of dendrite growth (Figs. 1 J–L and 3 A and C, and Fig. S2 D and E), we tested whether elevating neuronal activity altered the endogenous level of these proteins in DIV 8 neurons. We found increased membrane-associated levels of N-cadherin, β-catenin, αN-catenin, and Arp3 after 4 h K+ treatment (Fig. 4 A and B, and Fig. S3). The increase in N-cadherin, β-catenin, and αN-catenin was still detected after 48 h of high K+ treatment (Fig. 4 A and B, and Fig. S3 A–D), but did not persist for Arp3 (Fig. S3 E and F). Thus, activity promotes membrane association of all endogenous cadherin/catenin complex components and, more transiently, Arp3.
Fig. 4.
Neuronal activity increased surface and membrane levels of endogenous N-cadherin. (A) Blot of membrane N-cadherin following K+ treatment in culture. (B) Quantitation: 4 h K+ (1.87 ± 0.24, P < 0.05) and 48 h K+ (2.13 ± 0.28, P < 0.05). (C) Blot of membrane N-cadherin following KA injection in vivo. (D) Quantitation: 4 h KA (1.57 ± 0.12, P < 0.05) and 48 h KA (2.11 ± 0.41, P < 0.05). (E) Blot of surface biotinylated N-cadherin following K+ treatment. (F) Quantitation: 4 h K+ (2.31 ± 0.36, P < 0.05) and 48 h K+ (2.43 ± 0.46, P < 0.05). (G) Blot of whole-cell sample for E. (H) Quantitation: 4 h K+ (0.91 ± 0.08) and 48 h K+ (1.03 ± 0.04). Number of independent sample preparations as indicated in bar graphs. *P < 0.05.
To determine whether activity-dependent increases in membrane N-cadherin could be observed following in vivo manipulation, we systemically injected juvenile rats (P 6–8) with kainic acid (KA), a manipulation that at low dose induces mild seizures and synaptic potentiations (19, 20). As shown in Fig. 4 C and D, N-cadherin protein level increased significantly in the hippocampal membrane fraction of rats treated with KA for 4 or 48 h. A caveat of analyzing samples from the membrane fraction is that it also includes membranes of some intracellular components. Thus, we further verified our results using surface biotinylation assays in DIV 8 neurons. We showed that both 4- and 48-h K+ treatment significantly increased surface biotinylated N-cadherin (Fig. 4 E and F), without affecting the total cellular pool (Fig. 4 G and H). In summary, elevated neuronal activity significantly increased the surface level of N-cadherin, an effect that likely also occurs in vivo.
N-Cadherin Is Specifically Required for Dendrite Maintenance.
Because increased neuronal activity can up-regulate surface N-cadherin level within 4 h, we further investigated short-term effects of neuronal activity on dendritogenesis. We found in DIV 8 neurons that 4 h K+ (pulse) reliably increased TDBTN and persisted for at least an additional 4 h following K+ washout (chase) (Fig. 5 A–C). Interestingly, unlike its complete block of long-term activity-induced dendrite growth (Fig. 1 J–L), EC1 protein application during the 4 h K+ treatment did not inhibit K+-induced dendrite growth (Fig. 5D). Similarly, addition of Ncad-Fc, consisting of the extracellular domain of N-cadherin fused to Fc, also did not affect K+-induced increase in TDBTN (Fig. 5D). In contrast, addition of latrunculin A or jasplakinolide, drugs that interefere with actin dynamics, completely blocked K+-induced dendrite growth (Fig. 5D). Because 4 h is the shortest window in which we reliably detect changes in dendrite growth, we arbitrarily termed this the “early phase” of dendrite growth, a process that requires actin dynamics, but not N-cadherin–dependent interaction.
Fig. 5.
Requirement of N-cadherin for the maintenance of activity-dependent dendrite growth. (A) Schematic of the paradigm used. (B) Images of neurons treated with K+. (Scale bar, 20 μm.) (C) Quantitation: “K+-pulse” (1.54 ± 0.04, P < 0.001) and “K+-chase” (1.53 ± 0.04, P < 0.001). (D) Quantitation for early-phase treatments: K+ (1.42 ± 0.03, P < 0.001), K+ + LatA (1.07 ± 0.03, P > 0.05 vs. Ctrl, P < 0.001 vs. K+), K+ + Jasp (1.02 ± 0.03, P > 0.05 vs. Ctrl, P < 0.001 vs. K+), K+ + EC1 (1.29 ± 0.04, P < 0.001), K+ + Ctrl P. (1.31 ± 0.04, P < 0.001), K+ + Ncad-Fc (1.33 ± 0.05, P < 0.001), and K+ + Ctrl A. (1.27 ± 0.04, P < 0.001). (E) Quantitation for late-phase treatments: K+ (1.34 ± 0.04, P < 0.001), EC1 (0.98 ± 0.03), K+ + EC1 (1.02 ± 0.02), Ctrl P. (0.94 ± 0.03), K+ + Ctrl P. (1.30 ± 0.04, P < 0.001), Ncad-Fc (0.95 ± 0.03), K+ + Ncad-Fc (1.00 ± 0.02), Ctrl A. (1.02 ± 0.04), and K+ + Ctrl A. (1.29 ± 0.03, P < 0.001). (F) Corresponding TDBL: K+ (1.13 ± 0.04, P < 0.05), K+ + EC1 (0.99 ± 0.03), K+ + Ctrl P. (1.15 ± 0.03, P < 0.01), K+ + Ncad-Fc (0.94 ± 0.03), and K+ + Ctrl A. (1.16 ± 0.03, P < 0.01). (G) Quantitation of TDBTN: K+-pulse (1.28 ± 0.03, P < 0.001), K+-chase (1.28 ± 0.02, P < 0.001), Ncad-RNAi (0.83 ± 0.02, P < 0.001), Ncad-RNAi + K+-pulse (1.09 ± 0.03), and Ncad-RNAi + K+-chase (0.94 ± 0.03). (H) Corresponding TDBL: K+-pulse (1.17 ± 0.03, P < 0.01), K+-chase (1.16 ± 0.04, P < 0.01), Ncad-RNAi (0.67 ± 0.03, P < 0.001), Ncad-RNAi + K+-pulse (0.81 ± 0.04, P < 0.01), and Ncad-RNAi + K+-chase (0.74 ± 0.03, P < 0.001). (I) Rate of dendrite extension in live imaging experiments for: Ctrl (212.8 ± 26.5) then K+ (390.6 ± 46.0, P < 0.001); Ctrl (166.4 ± 25.0) then “K+ + EC1” (394.7 ± 78.7, P < 0.01); K+ (240.8 ± 37.3) then Ctrl chase (132.0 ± 27.8, P < 0.05), and K+ (251.2 ± 28.1) then EC1 chase (105.3 ± 20.3, P < 0.01). (J) Corresponding rate of dendrite retraction for: Ctrl (114.3 ± 19.9) then K+ (93.0 ± 16.0, P = 0.29); Ctrl (82.8 ± 14.5) then “K+ + EC1” (79.6 ± 20.5, P = 0.87); K+ (90.5 ± 25.7) then Ctrl chase (89.2 ± 13.6, P = 0.96), and K+ (78.8 ± 19.1) then EC1 chase (242.7 ± 20.5, P < 0.001). *P < 0.05; **P < 0.01; ***P < 0.001.
The lack of EC1 effect during short-term K+ treatment, in contrast to its complete block of the longer term (48 h) effect of increased neuronal activity on dendrite growth, prompted us to examine the hypothesis that N-cadherin–mediated cell–cell interaction is not required for the initiation of dendrite extension, but for its maintenance. Because the effects of K+ last for an additional 4 h after replacement of high K+ medium with regular medium (Fig. 5C), we used this paradigm for studying the “late phase” or maintenance of dendrite growth. Indeed, if early phase K+ treatment is followed with late phase EC1 application in regular culturing medium, activity-induced increases in TDBTN/TDBL were completely abolished (Fig. 5 E and F), demonstrating a specific requirement of N-cadherin–mediated cell–cell interaction in the maintenance of nascent dendrites. Similarly, Ncad-Fc also completely abolished the dendrite growth promoting effects of high K+ (Fig. 5 E and F), though addition of a control protein (Ctrl P.) or control antibody (Ctrl A.) did not. In complementary experiments, we showed that the maintenance of dendrites during the late phase is reduced in neurons transfected with Ncad-RNAi (Fig. 5 G and H). Consistent with the notion that N-cadherin mediates its effects by anchoring F-actin to the cell surface, actin dynamics are also required during the late phase (Fig. S4 A–C). Together, these results demonstrate that N-cadherin–mediated neuron–neuron interaction is specifically required for the maintenance of nascent dendrites.
We further analyzed the effects of K+ and EC1 protein on dendrite extension and retraction in live imaging experiments in DIV 8 neurons. We showed that K+ treatment for 4 h (during the early phase) significantly increased the rate of dendrite extension without altering the rate of dendrite retraction, compared with a previous 4 h under control conditions (black bar vs. gray bar, Fig. 5 I and J, and Fig. S4 D–F). This effect of high K+ was not affected by EC1 coapplication (Fig. 5 I and J). However, when EC1 was applied during the late phase of dendrite growth, as a chase to 4-h high K+ treatment, the rate of dendrite retraction was significantly increased, compared with controls without EC1 protein (Fig. 5J and Fig. S4F). These results provide further evidence for N-cadherin–mediated neuron–neuron interaction in the maintenance and stabilization of dendrite arbors.
Activity-Dependent Regulation of Surface N-Cadherin Expression.
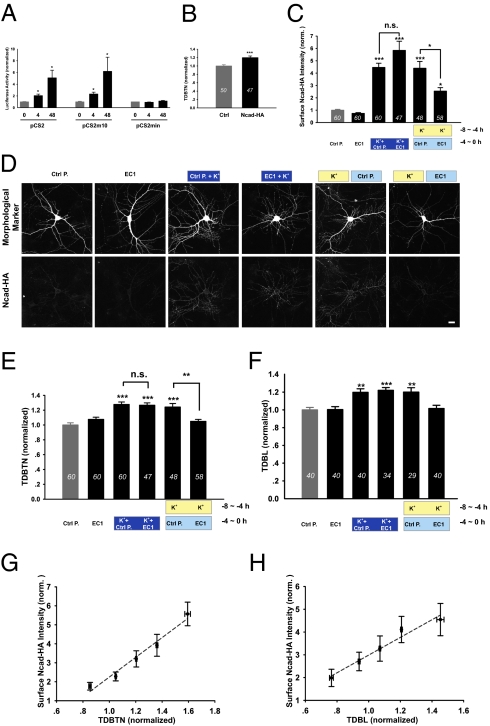
Having demonstrated that activity increased surface N-cadherin (Fig. 4) and that N-cadherin-mediated extracellular interaction is required for the maintainance of newly formed dendrites (Fig. 5 E–J), we further examined whether activity-induced increase in surface N-cadherin correlated with activity-induced dendrite growth. To separate the effect of neuronal activity on the surface delivery of N-cadherin from its potential effects on gene expression, we constructed a vector expressing an extracellularly epitope-tagged N-cadherin (Ncad-HA), under an activity-independent promoter (Fig. 6 A and B). Luciferase assays showed that the modified promoter in the pCS2min vector, with all cAMP response elements and SP1 sites mutated or deleted, did not respond to neuronal activity at 4 or 48 h after K+ treatment (Fig. 6A). In contrast, luciferase activity under the pCS2 parent vector or the pCS2m10 vector (10 cAMP response elements mutated) was significantly elevated by K+ treatment at both time points (Fig. 6A). Overexpression of Ncad-HA, under the activity-independent pCS2min promoter, significantly increased dendrite growth (Fig. 6B), similar to the effect of wildtype N-cadherin overexpression (Fig. 3A), demonstrating that the tag did not interfere with N-cadherin function.
Fig. 6.
Activity-induced increase in surface N-cadherin level depends on its extracellular interaction. (A) Luciferase activity for neurons transfected with pCS2 (4 h K+: 2.05 ± 0.03, P < 0.05; 48 h K+: 5.09 ± 1.30, P < 0.05), pCS2m10 (4 h K+: 2.34 ± 0.32, P < 0.05; 48 h K+: 6.19 ± 2.42, P < 0.05), or pCS2min (4 h K+: 0.92 ± 0.05; 48 h K+: 1.13 ± 0.10). (B) Ncad-HA increased TDBTN (1.20 ± 0.04, P < 0.001). (C) Surface Ncad-HA intensity (normalized to neuron area measured in GFP channel): EC1 (0.74 ± 0.06), K+-pulse + Ctrl P. (4.46 ± 0.34, P < 0.001), K+-pulse + EC1 (5.84 ± 0.75, P < 0.001), K+-chase + Ctrl P. (4.39 ± 0.55, P < 0.001), and K+-chase + EC1 (2.54 ± 0.28, P < 0.05 vs. Ctrl, P < 0.05 vs. K+-chase + Ctrl P.). (D) Images of neurons cotransfected with GFP and Ncad-HA, and surface labeled for N-cadherin. (Scale bar, 20 μm.) (E) TDBTN: EC1 (1.08 ± 0.03), K+-pulse + Ctrl P. (1.28 ± 0.03, P < 0.001), K+-pulse + EC1 (1.27 ± 0.03, P < 0.001), K+-chase + Ctrl P. (1.24 ± 0.05, P < 0.001), and K+-chase + EC1 (1.05 ± 0.03, n.s. vs. Ctrl, P < 0.01 vs. K+-chase + Ctrl P.). (F) TDBL: EC1 (1.00 ± 0.03), K+-pulse + Ctrl P. (1.20 ± 0.04, P < 0.01), K+-pulse + EC1 (1.22 ± 0.03, P < 0.001), K+-chase + Ctrl P. (1.20 ± 0.05, P < 0.01), and K+-chase + EC1 (1.02 ± 0.04, n.s. vs. Ctrl, P < 0.01 vs. K+-chase + Ctrl P.). (G) Plot of surface Ncad-HA intensity vs. TDBTN for all neurons, linear regression is dotted line (r 2 = 0.97, P < 0.005). (H) Plot of surface Ncad-HA intensity vs. TDBL, linear regression is dotted line (r 2 = 0.96, P < 0.005). *P < 0.05; **P < 0.01; ***P < 0.001.
Using HA antibodies to surface-label N-cadherin in DIV 8 neurons transfected with pCS2min-Ncad-HA, we found that 4 h K+ increased surface N-cadherin level, an effect not affected by EC1 coapplication (Fig. 6 C and D). However, when neurons were pulsed with 4 h K+ and chased for an additional 4 h with EC1 protein during the late phase, surface Ncad-HA intensity and TDBL/TDBTN were significantly reduced, compared with that found using control protein (Fig. 6 C–F). This reduction in surface N-cadherin protein after EC1 incubation is consistent with a requirement of extracellular N-cadherin–mediated homophilic interaction/achoring for its surface stabilization. Importantly, plotting surface Ncad-HA level against TDBTN/TDBL for all neurons, we observed correlated linear relationships (Fig. 6 G and H). As further evidence that the observed increase in surface Ncad-HA level is not caused by increased translation, the presence of protein synthesis inhibitors did not affect the K+-induced increase in surface Ncad-HA (Fig. S5). Thus, activity-induced increase in surface N-cadherin is specifically required for the maintenance of newly formed dendrites.
Discussion
Using a variety of newly generated tools, we demonstrated that the enhanced dendrite growth induced by increased neuronal activity requires neuron–neuron interactions mediated by N-cadherin. We showed that this extracellular action of N-cadherin requires functional interaction of the cadherin/catenin complex with the dynamic actin cytoskeleton. Furthermore, we found that neuronal activity promotes dendritogenesis by elevating surface N-cadherin level, a process specifically required for the maintenance of nascent dendrite arbors. Together, these results point to the critical role of N-cadherin–mediated cell–cell contact in stabilizing dendrite arbors during activity-dependent development.
Function of N-Cadherin–Mediated Neuron–Neuron Interaction.
Mounting evidence suggests that dendrite arborization is highly regulated by homo- and heterotypic cell–cell interactions, as exemplified by the tiling of dendrite arbors mediated by Down syndrome cell-adhesion molecule (21), and by the growing list of CAMs shown to regulate dendrite growth (1 –4). In the present study, we used EC1, a soluble N-cadherin ectodomain, to demonstrate a requirement for N-cadherin mediated extracellular interaction in activity-dependent dendrite growth (Fig. 1 J–L, and Fig. S1 G–I). We further demonstrated that this N-cadherin-dependent dendrite growth requires direct contact between neuronal processes, as neurons plated at low density, such that the axons and dendrites of neighboring cells do not contact, did not respond to high K+, EC1 protein or N-cadherin overexpression (Fig. 2 A–F, J, and K). Because overexpression of members of the cadherin/catenin complex also promotes axonal outgrowth (22 –26), a simple interpretation is that N-cadherin mediates activity-dependent neurite outgrowth by promoting extracellular contacts between axons and dendrites.
Relationship Between Dendrite Stabilization and Synaptogenesis.
Because dendrite growth and synapse formation occur concurrently during development, these processes may be coordinated and interdependent (27). In fact, live imaging experiments demonstrated that synapse formation stabilizes dendritic and axonal arbors (28 –30). Our results, demonstrating a requirement for N-cadherin-mediated neuron–neuron interaction specifically in the maintenance of newly formed dendritic arbors, suggest that N-cadherin may be one of the CAMs coordinating neurite growth and synapse formation. Consistently, newly formed dendrite abors induced by high K+ have morphological synapses (Fig. S6), and N-cadherin can be recruited to synapses (10, 31 –34).
Cadherin/Catenin Complex and Actin Assembly.
Previous in vitro assays showed that α-catenin can suppress the actin polymerizing effects of Arp3 and was interpreted as direct competition between α-catenin and the Arp 2/3 complex for binding to actin filaments (13). However, our results showed that they function in the same direction to regulate dendrite development (Fig. 3 A and B), and that neuronal activity promotes the membrane association of both proteins (Fig. S3 C–F). Furthermore, the effect of αN-catenin overexpression on promoting dendrite growth depends on endogenous Arp3 (Fig. 3A). Thus, regardless of the molecular dynamics adjacent to the neuronal surface membrane, overall, Arp3-mediated actin assembly cooperates with αN-catenin and other cadherin/catenin complex components to promote dendrite growth.
As further evidence for cooperation between the cadherin/catenin complex and F-actin in mediating dendrite growth, we showed that overexpression of Ncad-AD, consisting of the extracellular domain of N-cadherin fused to the actin binding domain of αN-catenin, effectively promoted dendrite growth in neurons with lowered endogenous level of N-cadherin, β-catenin, or αN-catenin (Fig. 3C). Importantly, although the effect of Ncad-AD overexpression on promoting dendrite growth was extremely robust, it still depended on N-cadherin mediated neuron–neuron interaction, because EC1 coexpression completely abolished its effect. Taken together, these results demonstrate that memebrane anchoring of F-actin to N-cadherin-mediated cell-cell contacts is required for dendrite growth.
Activity-Dependent Regulation of Surface N-Cadherin Delivery and Stabilization.
Having demonstrated that N-cadherin–mediated neuron–neuron interaction and its association with F-actin are both required for activity-dependent dendrite growth, an important question is how neuronal activity regulates surface N-cadherin level. Using biochemical assays, we showed that high K+ significantly and persistently elevated surface N-cadherin (Fig. 4), consistent with previous reports in older neuronal cultures (34, 35). Furthermore, because the activity-induced increase in surface N-cadherin occurred when it was expressed under an activity-independent promoter and in the presence of protein synthesis inhibitors (Fig. 6 and Fig. S5), this process likely occurs through posttranslational changes, including increased net surface delivery and increased surface stabilization.
The most extensively studied example of activity-dependent surface delivery of membrane proteins is AMPA receptor, inserted into synaptic sites following long-term potentiation induction and sensory experience (36, 37). The supply of surface-delivered AMPA receptors is thought to derive from the recycling endosome (38), a reserved pool that holds recycled receptors from the cell membrane and newly synthesized proteins from the Golgi apparatus (39, 40). Because the C-terminal region of N-cadherin is important for its regulated endocytosis following NMDA treatment (34), and the recycling endosome is shown to be important in the regulated trafficking of E-cadherin (40), similar mechanisms could regulate activity-dependent surface delivery of N-cadherin during dendrite development.
In contrast to a more speculative understanding of activity-regulated trafficking of N-cadherin, its activity-dependent stabilization is more straightforward. Because N-cadherin can homophilically interact with the extracellular domain of N-cadherin on juxta-opposed membranes, this interaction could stabilize its membrane level. Consistently, we showed that EC1 protein addition during the late phase following K+ treatment reduced surface N-cadherin, an effect correlated with reduced TDBTN and increased dendrite retraction (Figs. 5J and 6 C–H). Thus, stabilization of surface N-cadherin via homophilic interactions between juxta-opposed membranes is likely to be an important mechanism for maintaining surface N-cadherin.
Based on our results, we propose a two-step model for activity-induced dendrite growth (Fig. S7). During the early phase, neuronal activity increases actin dynamics (1, 3) and surface delivery of N-cadherin (Figs. 4A and 6 C and D), resulting in enhanced dendrite growth (Fig. 5 B–F). During the ensuing late phase, surface N-cadherins form homophilic interactions with N-cadherins on opposing membranes, and intracellular links to the actin cytoskeleton through the cadherin/catenin complex, stabilizing the newly formed dendritic arbors (Figs. 5 B–F and 6 C–F). Together, these molecular interactions mediate the effect of neuronal activity on promoting dendrite growth, an important aspect of coordinated neural circuit development.
Materials and Methods
Details of materials and methods are in SI Materials and Methods. The use and care of animals in this study follows the guidelines of the Institutional Animal Care and Use Committee of the Institute of Neuroscience, Chinese Academy of Sciences.
Hippocampal Neuronal Culture Preparation and Pharmacological Treatments.
Hippocampal neuronal cultures, plated at 50,000 cells/cm2, were prepared from P0 Sprague-Dawley rat pups, as previously described (12). For low-density cultures, neurons were plated at 3,000 cells/cm2. Pharmacological treatments included 10 mM KCl, Ncad-Fc (1 μg/mL, R&D Systems), α-GFP (1 μg/mL, Chemicon), α-Fc (1 μg/mL, Jackson ImmunoResearch), EC1 (5 μg/mL), boiled EC1 (5 μg/mL, 95 °C for 5 min), or BSA (5 μg/mL; New England Biolabs, Inc.).
Supplementary Material
Acknowledgments
We thank Drs. D. Benson, S. Craig, S.-M. Duan, E. Fuchs, J. Gettemans, M. Kirschner, Z.-G. Luo, R. Kemler, G. Scita, F. van Roy, O. Weiner, M. Yamada, and J. -Q. Zhou for constructs and antibodies, Z.-T. Geng, W. Wang, and Z.-F. Wan for neuronal culturing, and Drs. M.-M. Poo, Z.-G. Luo, and members of the Yu laboratory for suggestions and discussions, and Dr. Poo for comments on the manuscript. This work was supported by Grants 2006CB806600 and 2006CB943903 from the Ministry of Science and Technology , 30721004 from the National Science Foundation China, and the Hundred Talent Program and Grant KSCX2-YW-R-103 from the Chinese Academy of Sciences.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1003480107/-/DCSupplemental.
References
- 1.Chen Y, Ghosh A. Regulation of dendritic development by neuronal activity. J Neurobiol. 2005;64:4–10. doi: 10.1002/neu.20150. [DOI] [PubMed] [Google Scholar]
- 2.Parrish JZ, Emoto K, Kim MD, Jan YN. Mechanisms that regulate establishment, maintenance, and remodeling of dendritic fields. Annu Rev Neurosci. 2007;30:399–423. doi: 10.1146/annurev.neuro.29.051605.112907. [DOI] [PubMed] [Google Scholar]
- 3.Urbanska M, Blazejczyk M, Jaworski J. Molecular basis of dendritic arborization. Acta Neurobiol Exp (Warsz) 2008;68:264–288. doi: 10.55782/ane-2008-1695. [DOI] [PubMed] [Google Scholar]
- 4.Ye B, Jan YN. The cadherin superfamily and dendrite development. Trends Cell Biol. 2005;15:64–67. doi: 10.1016/j.tcb.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Dalva MB, McClelland AC, Kayser MS. Cell adhesion molecules: Signalling functions at the synapse. Nat Rev Neurosci. 2007;8:206–220. doi: 10.1038/nrn2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McAllister AK. Dynamic aspects of CNS synapse formation. Annu Rev Neurosci. 2007;30:425–450. doi: 10.1146/annurev.neuro.29.051605.112830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cline H, Haas K. The regulation of dendritic arbor development and plasticity by glutamatergic synaptic input: A review of the synaptotrophic hypothesis. J Physiol. 2008;586:1509–1517. doi: 10.1113/jphysiol.2007.150029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez TD, Nelson WJ. In: Cell Adhesion. Behrens J, Nelson WJ, editors. Berlin: Springer; 2004. pp. 3–22. [Google Scholar]
- 9.Nelson WJ. Regulation of cell-cell adhesion by the cadherin-catenin complex. Biochem Soc Trans. 2008;36:149–155. doi: 10.1042/BST0360149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benson DL, Tanaka H. N-cadherin redistribution during synaptogenesis in hippocampal neurons. J Neurosci. 1998;18:6892–6904. doi: 10.1523/JNEUROSCI.18-17-06892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Redmond L, Kashani AH, Ghosh A. Calcium regulation of dendritic growth via CaM kinase IV and CREB-mediated transcription. Neuron. 2002;34:999–1010. doi: 10.1016/s0896-6273(02)00737-7. [DOI] [PubMed] [Google Scholar]
- 12.Yu X, Malenka RC. Beta-catenin is critical for dendritic morphogenesis. Nat Neurosci. 2003;6:1169–1177. doi: 10.1038/nn1132. [DOI] [PubMed] [Google Scholar]
- 13.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takenawa T, Suetsugu S. The WASP-WAVE protein network: Connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol. 2007;8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- 16.Drees F, Gertler FB. Ena/VASP: proteins at the tip of the nervous system. Curr Opin Neurobiol. 2008;18:53–59. doi: 10.1016/j.conb.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ziegler WH, Liddington RC, Critchley DR. The structure and regulation of vinculin. Trends Cell Biol. 2006;16:453–460. doi: 10.1016/j.tcb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Nagafuchi A, Ishihara S, Tsukita S. The roles of catenins in the cadherin-mediated cell adhesion: functional analysis of E-cadherin-alpha catenin fusion molecules. J Cell Biol. 1994;127:235–245. doi: 10.1083/jcb.127.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lothman EW, Collins RC. Kainic acid induced limbic seizures: Metabolic, behavioral, electroencephalographic and neuropathological correlates. Brain Res. 1981;218:299–318. doi: 10.1016/0006-8993(81)91308-1. [DOI] [PubMed] [Google Scholar]
- 20.Ben-Ari Y, Represa A. Brief seizure episodes induce long-term potentiation and mossy fibre sprouting in the hippocampus. Trends Neurosci. 1990;13:312–318. doi: 10.1016/0166-2236(90)90135-w. [DOI] [PubMed] [Google Scholar]
- 21.Hattori D, Millard SS, Wojtowicz WM, Zipursky SL. Dscam-mediated cell recognition regulates neural circuit formation. Annu Rev Cell Dev Biol. 2008;24:597–620. doi: 10.1146/annurev.cellbio.24.110707.175250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bekirov IH, Nagy V, Svoronos A, Huntley GW, Benson DL. Cadherin-8 and N-cadherin differentially regulate pre- and postsynaptic development of the hippocampal mossy fiber pathway. Hippocampus. 2008;18:349–363. doi: 10.1002/hipo.20395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bixby JL, Zhang R. Purified N-cadherin is a potent substrate for the rapid induction of neurite outgrowth. J Cell Biol. 1990;110:1253–1260. doi: 10.1083/jcb.110.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doherty P, Rowett LH, Moore SE, Mann DA, Walsh FS. Neurite outgrowth in response to transfected N-CAM and N-cadherin reveals fundamental differences in neuronal responsiveness to CAMs. Neuron. 1991;6:247–258. doi: 10.1016/0896-6273(91)90360-c. [DOI] [PubMed] [Google Scholar]
- 25.Esch T, Lemmon V, Banker G. Differential effects of NgCAM and N-cadherin on the development of axons and dendrites by cultured hippocampal neurons. J Neurocytol. 2000;29:215–223. doi: 10.1023/a:1026515426303. [DOI] [PubMed] [Google Scholar]
- 26.Yu X, Malenka RC. Multiple functions for the cadherin/catenin complex during neuronal development. Neuropharmacology. 2004;47:779–786. doi: 10.1016/j.neuropharm.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 27.Vaughn JE, Barber RP, Sims TJ. Dendritic development and preferential growth into synaptogenic fields: A quantitative study of Golgi-impregnated spinal motor neurons. Synapse. 1988;2:69–78. doi: 10.1002/syn.890020110. [DOI] [PubMed] [Google Scholar]
- 28.Meyer MP, Smith SJ. Evidence from in vivo imaging that synaptogenesis guides the growth and branching of axonal arbors by two distinct mechanisms. J Neurosci. 2006;26:3604–3614. doi: 10.1523/JNEUROSCI.0223-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niell CM, Meyer MP, Smith SJ. In vivo imaging of synapse formation on a growing dendritic arbor. Nat Neurosci. 2004;7:254–260. doi: 10.1038/nn1191. [DOI] [PubMed] [Google Scholar]
- 30.Ruthazer ES, Li J, Cline HT. Stabilization of axon branch dynamics by synaptic maturation. J Neurosci. 2006;26:3594–3603. doi: 10.1523/JNEUROSCI.0069-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jontes JD, Emond MR, Smith SJ. In vivo trafficking and targeting of N-cadherin to nascent presynaptic terminals. J Neurosci. 2004;24:9027–9034. doi: 10.1523/JNEUROSCI.5399-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elste AM, Benson DL. Structural basis for developmentally regulated changes in cadherin function at synapses. J Comp Neurol. 2006;495:324–335. doi: 10.1002/cne.20876. [DOI] [PubMed] [Google Scholar]
- 33.Togashi H, et al. Cadherin regulates dendritic spine morphogenesis. Neuron. 2002;35:77–89. doi: 10.1016/s0896-6273(02)00748-1. [DOI] [PubMed] [Google Scholar]
- 34.Tai CY, Mysore SP, Chiu C, Schuman EM. Activity-regulated N-cadherin endocytosis. Neuron. 2007;54:771–785. doi: 10.1016/j.neuron.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 35.Murase S, Mosser E, Schuman EM. Depolarization drives beta-Catenin into neuronal spines promoting changes in synaptic structure and function. Neuron. 2002;35:91–105. doi: 10.1016/s0896-6273(02)00764-x. [DOI] [PubMed] [Google Scholar]
- 36.Kessels HW, Malinow R. Synaptic AMPA receptor plasticity and behavior. Neuron. 2009;61:340–350. doi: 10.1016/j.neuron.2009.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 38.Park M, Penick EC, Edwards JG, Kauer JA, Ehlers MD. Recycling endosomes supply AMPA receptors for LTP. Science. 2004;305:1972–1975. doi: 10.1126/science.1102026. [DOI] [PubMed] [Google Scholar]
- 39.Futter CE, Connolly CN, Cutler DF, Hopkins CR. Newly synthesized transferrin receptors can be detected in the endosome before they appear on the cell surface. J Biol Chem. 1995;270:10999–11003. doi: 10.1074/jbc.270.18.10999. [DOI] [PubMed] [Google Scholar]
- 40.Lock JG, Hammond LA, Houghton F, Gleeson PA, Stow JL. E-cadherin transport from the trans-Golgi network in tubulovesicular carriers is selectively regulated by golgin-97. Traffic. 2005;6:1142–1156. doi: 10.1111/j.1600-0854.2005.00349.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.