Abstract
Purpose
Retrospective studies suggest that primary breast cancers lacking estrogen receptor (ER) and progesterone receptor (PR) and not overexpressing human epidermal growth factor receptor 2 (HER2; triple-negative tumors) are particularly sensitive to DNA-damaging chemotherapy with alkylating agents.
Patients and Methods
Patients enrolled in International Breast Cancer Study Group Trials VIII and IX with node-negative, operable breast cancer and centrally assessed ER, PR, and HER2 were included (n = 2,257). The trials compared three or six courses of adjuvant classical cyclophosphamide, methotrexate, and fluorouracil (CMF) with or without endocrine therapy versus endocrine therapy alone. We explored patterns of recurrence by treatment according to three immunohistochemically defined tumor subtypes: triple negative, HER2 positive and endocrine receptor absent, and endocrine receptor present.
Results
Patients with triple-negative tumors (303 patients; 13%) were significantly more likely to have tumors > 2 cm and grade 3 compared with those in the HER2-positive, endocrine receptor–absent, and endocrine receptor–present subtypes. No clear chemotherapy benefit was observed in endocrine receptor–present disease (hazard ratio [HR], 0.90; 95% CI, 0.74 to 1.11). A statistically significantly greater benefit for chemotherapy versus no chemotherapy was observed in triple-negative breast cancer (HR, 0.46; 95% CI, 0.29 to 0.73; interaction P = .009 v endocrine receptor–present disease). The magnitude of the chemotherapy effect was lower in HER2-positive endocrine receptor–absent disease (HR, 0.58; 95% CI, 0.29 to 1.17; interaction P = .24 v endocrine receptor–present disease).
Conclusion
The magnitude of benefit of CMF chemotherapy is largest in patients with triple-negative, node-negative breast cancer.
INTRODUCTION
Recommended principles for the choice of therapies in operable breast cancer include the recognition of diverse subtypes of breast cancer and the identification of particular targets based on genetic signature and immunohistochemistry.1 Recent studies using DNA microarray profiling have led to classification of invasive breast cancer subgroups with common molecular features2–4 and the recognition that breast cancer is a heterogeneous entity.5,6 Several molecular subgroups have been proposed: human epidermal growth factor receptor 2 (HER2) –overexpressing/estrogen receptor (ER) –negative and progesterone receptor (PR) –negative tumors, basal-like (ER-, PR-, and HER2-negative disease), and luminal-like (ER and/or PR expression).3,4
An immunohistochemical (IHC) profile based on the degree of expression of ER, PR, and HER2 similarly identifies subgroups of breast cancer patients who will respond to different systemic neoadjuvant and adjuvant treatments.1,7 In the neoadjuvant setting, less benefit from the introduction of chemotherapy can be expected among patients whose tumors contain high levels of ER compared with those whose tumors are defined as endocrine receptor absent.8,9 Studies in the adjuvant setting in the node-positive population, showed that patients with ER-negative disease derived a greater benefit from modern improvements in chemotherapy regimens when compared with those with ER-positive disease.7,10 However, limited information is available in the adjuvant setting on the responsiveness to chemotherapy in the node-negative population.
The aim of this retrospective study was to evaluate the magnitude of the effect of adjuvant classical CMF (cyclophosphamide, methotrexate, and fluorouracil) chemotherapy in a large series of patients with node-negative breast cancer treated within the context of two adjuvant trials according to centrally reviewed IHC subtypes.
PATIENTS AND METHODS
Patients were enrolled in two randomized clinical trials conducted by the International Breast Cancer Study Group (IBCSG), which have been reported elsewhere.11,12 Briefly, between 1990 and 1999, IBCSG Trial VIII randomly assigned 1,109 assessable pre-/perimenopausal women with lymph node–negative breast cancer to sequential treatment with six 28-day courses of classical CMF chemotherapy followed by 18 monthly subcutaneous implants of goserelin, six 28-day courses of classical CMF alone, 24 monthly implants of goserelin alone, or no adjuvant treatment. The no-treatment arm was discontinued in 1992 after 46 patients had been assigned to that group. Tamoxifen was not prescribed. From 1988 to 1999, IBCSG Trial IX randomly assigned 1,669 eligible and assessable postmenopausal women with lymph node–negative breast cancer to sequential treatment with three 28-day courses of classical CMF chemotherapy followed by tamoxifen for 57 months or tamoxifen (20 mg/d) alone for 5 years. The primary end point was disease-free survival (DFS). Targeted anti-HER2 therapy was not used in these trials. The intention to perform separate analyses according to ER status was specified in the original protocol. In 1998, a protocol amendment for both trials restricted enrollment to patients with ER-positive tumors on the basis of evidence from other trials that ovarian ablation (in the case of Trial VIII) and tamoxifen alone (in the case of Trial IX) were not effective for patients with ER-negative tumors. Institutional review boards reviewed and approved the protocols, and informed consent was required according to the criteria established within the individual countries.
Central Pathology Review
Retrospective tissue collection was carried out in accordance with institutional guidelines and national laws. More than 80% of patients randomly assigned in Trials VIII and IX had archival tumor material available for review in the IBCSG Central Pathology Laboratory. Central pathology review was conducted without knowledge of patient treatment assignment or outcome. Expression of ER, PR, HER2, and Ki-67 labeling index (LI) in the primary tumors was determined by IHC.13,14 Ki-67 LI was assessed using mouse monoclonal antibody MIB-1 (1:200 dilution; Dako, Glostrup, Denmark); the percentage of cells that showed definite nuclear immunoreactivity with MIB-1 among 2,000 invasive neoplastic cells in randomly selected high-power fields (×400) at the periphery of the tumor was recorded.
Hormone receptor–absent status was defined as < 1% immunoreactive cells, in accordance with recent reports.1,7 Whole tumor sections were incubated with the specific primary mouse monoclonal antibodies to ER (clone 1D5; 1:100 dilution) or PR (clone 1A6; 1:800 dilution) (both from Dako). HER2 immunoreactivity was assessed using a HercepTest kit (Dako), as recommended by the manufacturer, and was scored for the intensity of immunostaining, the completeness of cell membrane staining, and the percentage of immunoreactive neoplastic cells by using a four-tier scale from 0 to 3+, as previously described.14 HER2-negative status was defined as immunostaining levels 0, 1+, and 2+, and HER2 overexpression was defined as intense and complete membrane staining of > 10% of the tumor cells.
Fluorescent in situ hybridization testing was not performed because of inadequate tumor sections. A patient's tumor was considered triple negative if ER and PR were both absent and HER2 status was negative. The tumor was classified as HER2 positive, endocrine receptor absent if HER2 was overexpressed and ER and PR were both absent, and as endocrine receptor present if either ER or PR or both were present. Patients with missing information regarding ER, PR, and HER2 status were excluded from this analysis (n = 521).
Statistical Analysis
The χ2 test and Fisher's exact test15 were used to evaluate associations between tumor subtype and menopausal status (trial), tumor size, tumor grade, and vessel invasion. The Kruskal-Wallis test16 was used to evaluate associations with age categories.
DFS was defined as the time from random assignment to the first failure (including relapse, second primary, or death). Time to relapse was defined as the time from random assignment to any breast cancer relapse (local, regional, or distant, including contralateral breast events). Overall survival (OS) was defined as the time from random assignment to death from any cause. Patients who were event free at the time of last follow-up were censored at that time.
Time to relapse was evaluated using cumulative incidence analysis and competing-risks regression analysis.17 For these analyses, second non-breast primaries and deaths without relapse were treated as competing events. Tumor subtype status was evaluated as a treatment-effect modifier after adjustment for other prognostic factors. The treatment comparison was chemotherapy versus no chemotherapy.
Separate models were run according to menopausal status (defined by trial population). Model-based hazard ratios (HRs) and 95% CIs for the chemotherapy treatment effect were estimated according to tumor subtype. Wald tests were used to determine the statistical significance of the interaction effect of treatment group and tumor subtype. DFS and OS were estimated using the product-limit method.18 The log-rank test19 was used to compare treatment groups in terms of DFS and OS.
RESULTS
This analysis was conducted on the 2,257 patients for whom IHC-defined tumor subtype was available (82.6% of the patients included in Trials VIII and IX). Baseline characteristic of patients with centrally assessed ER, PR, and HER2 and those of patients not included in the analysis due to unavailability of central review were comparable (Table 1), thus minimizing potential selection bias. A total of 303 patients (13%) had triple-negative breast cancer, 119 (5%) had HER2-positive, endocrine receptor–absent disease, and 1,835 (81%) had endocrine receptor–present disease. A total of 1,255 women (56%) were randomly assigned to receive chemotherapy, and the remaining 1,002 (44%) were assigned to no chemotherapy. The median follow-up was 11 years (maximum: 18.6 years).
Table 1.
Patient and Disease Characteristics
| Characteristic | Triple Negative (n = 303) |
HER2 Positive, Endocrine Receptor Absent (n = 119) |
Endocrine Receptor Present (n = 1,835) |
P* | Not Included† (n = 521) |
||||
|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | ||
| Menopausal status | |||||||||
| Premenopausal (IBCSG Trial VIII) | 136 | 45 | 39 | 33 | 737 | 40 | .07 | 195 | 37 |
| Postmenopausal (IBCSG Trial IX) | 167 | 55 | 80 | 67 | 1,098 | 60 | 326 | 63 | |
| Tumor size, cm | |||||||||
| ≤ 2 | 127 | 42 | 60 | 50 | 1,164 | 63 | < .001 | 336 | 64 |
| > 2 | 173 | 57 | 58 | 49 | 648 | 35 | 175 | 34 | |
| Unknown | 3 | 1 | 1 | 1 | 3 | 1 | 10 | 2 | |
| Tumor grade | |||||||||
| 1 | 5 | 2 | 2 | 2 | 363 | 20 | < .001 | 129 | 25 |
| 2 | 62 | 20 | 35 | 30 | 926 | 51 | 209 | 40 | |
| 3 | 236 | 78 | 78 | 66 | 538 | 29 | 164 | 31 | |
| Unknown | 0 | 0 | 4 | 3 | 8 | 0 | 19 | 4 | |
| Peritumoral vascular invasion | |||||||||
| Yes | 233 | 77 | 87 | 73 | 1,447 | 79 | .24 | 393 | 75 |
| No | 44 | 15 | 24 | 20 | 280 | 15 | 70 | 13 | |
| Unknown | 26 | 9 | 8 | 7 | 108 | 6 | 58 | 11 | |
| Age, years (mean ± SD) | 52.7 ± 10.1 | 55.0 ± 10.0 | 54.3 ± 10.0 | .03 | 54.1 ± 10.1 | ||||
| IBCSG Trial VIII | 43.6 ± 5.7 | 44.2 ± 6.4 | 44.4 ± 5.4 | .28 | 43.9 ± 5.9 | ||||
| IBCSG Trial IX | 60.1 ± 5.9 | 60.3 ± 6.4 | 60.9 ± 6.2 | .38 | 60.2 ± 6.5 | ||||
Abbreviations: HER2, human epidermal growth factor receptor 2; IBCSG, International Breast Cancer Study Group; SD, standard deviation.
P values compare the three tumor subtypes and are based on Fisher's exact test (menopausal status, tumor size, and peritumoral vascular invasion), the χ2 test (tumor grade), and the Kruskal-Wallis test (age).
Central review of estrogen receptor, progesterone receptor, and HER2 not available.
Tumors larger than 2 cm and grade 3 tumors were more commonly observed among patients with triple-negative disease, followed by those with HER2-positive, endocrine receptor–absent disease, and those with endocrine receptor–present disease, respectively (57% v 49% v 35%; P < .001; tumor > 2 cm and 78% v 68% v 29%; P < .001; grade 3).
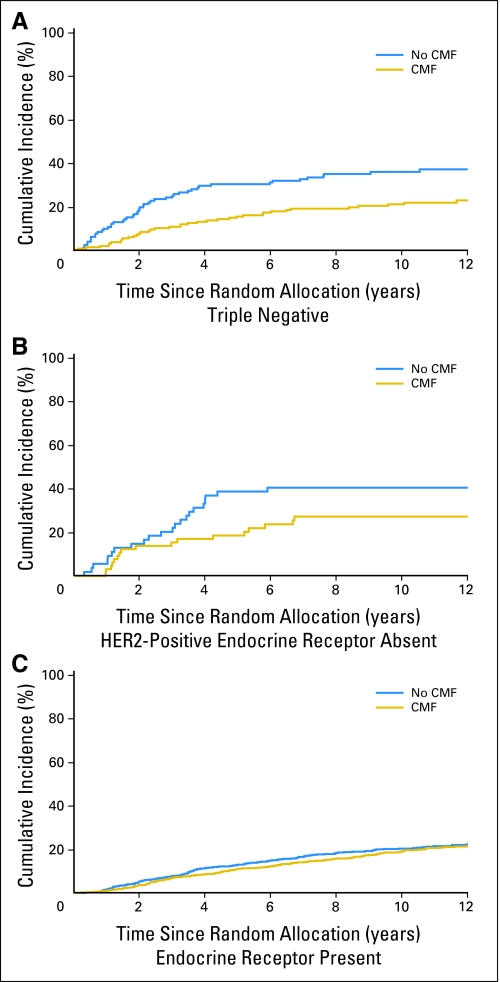
Table 2 shows the numbers of relapse events available for each subtype along with 10-year cumulative incidence rates. The cumulative incidence rates in patients with endocrine receptor–present disease were similar in those patients who received chemotherapy when compared with those allocated no chemotherapy (19% v 20%). By contrast, a large difference in terms of incidence of events for chemotherapy versus no chemotherapy was observed in patients with triple-negative (21% v 36%) or HER2-positive, endocrine receptor–absent subtypes (27% v 41%). Table 3 shows the results of multivariable competing-risk regression analyses predicting time to relapse. No clear effect was observed in the endocrine receptor–present subtype (HR, 0.90; 95% CI, 0.74 to 1.11). The magnitude of the chemotherapy effect was low both in patients with HER2-positive, endocrine receptor–present disease (220 patients [10%]; HR, 0.73; 95% CI, 0.42 to 1.25) and in those with HER2-negative, endocrine receptor–present disease (1,576 patients [71%]; HR, 0.93; 95% CI, 0.75 to 1.16). In contrast, a significant reduction in the HR for chemotherapy versus no chemotherapy was observed for the triple-negative subtype (HR, 0.46; 95% CI, 0.29 to 0.73; interaction P = .009 relative to endocrine receptor–present subtype). A smaller effect was observed for the HER2-positive, endocrine receptor–absent subtype (HR, 0.58; 95% CI, 0.29 to 1.17; interaction P = .24 relative to endocrine receptor–present subtype).
Table 2.
Cumulative Incidence Estimates of Relapse According to Tumor Subtype, Menopausal Status, and Treatment Group
| Treatment and Subtype | No. of Patients | No. of Relapse Events | 10-Year Cumulative Incidence of Relapse ± SE |
|---|---|---|---|
| All Patients | |||
| Triple negative | |||
| No CMF | 133 | 49 | 36 ± 4 |
| CMF | 170 | 38 | 21 ± 3 |
| HER2 positive, endocrine receptor absent | |||
| No CMF | 54 | 22 | 41 ± 7 |
| CMF | 65 | 17 | 27 ± 6 |
| Endocrine receptor present | |||
| No CMF | 815 | 171 | 20 ± 1 |
| CMF | 1,020 | 203 | 19 ± 1 |
| Premenopausal patients (IBCSG Trial VIII) | |||
| Triple negative | |||
| No CMF | 49 | 21 | 39 ± 7 |
| CMF | 87 | 25 | 27 ± 5 |
| HER2 positive, endocrine receptor absent | |||
| No CMF | 12 | 6 | 50 ± 15 |
| CMF | 27 | 11 | 43 ± 10 |
| Endocrine receptor present | |||
| No CMF | 263 | 69 | 27 ± 3 |
| CMF | 474 | 100 | 21 ± 2 |
| Postmenopausal patients (IBCSG Trial IX) | |||
| Triple negative | |||
| No CMF | 84 | 28 | 34 ± 5 |
| CMF | 83 | 13 | 15 ± 4 |
| HER2 positive, endocrine receptor absent | |||
| No CMF | 42 | 16 | 38 ± 8 |
| CMF | 38 | 6 | 16 ± 6 |
| Endocrine receptor present | |||
| No CMF | 552 | 102 | 17 ± 2 |
| CMF | 546 | 103 | 18 ± 2 |
Abbreviations: HER2, human epidermal growth factor receptor 2; IBCSG, International Breast Cancer Study Group; CMF, cyclophosphamide, methotrexate, and fluorouracil.
Table 3.
Group-Specific Treatment HRs for Relapse Based on Competing-Risks Regression
| Tumor Subtype | HR (CMF v No CMF) | 95% CI | P* | Overall Interaction P† |
|---|---|---|---|---|
| All patients | ||||
| Triple negative | 0.46 | 0.29 to 0.73 | .009 | |
| HER2 positive, endocrine receptor absent | 0.58 | 0.29 to 1.17 | .24 | |
| Endocrine receptor present | 0.90 | 0.74 to 1.11 | — | |
| .022 | ||||
| Premenopausal patients (IBCSG Trial VIII) | ||||
| Triple negative | 0.51 | 0.27 to 0.98 | .22 | |
| HER2 positive, endocrine receptor absent | 0.68 | 0.22 to 2.14 | .79 | |
| Endocrine receptor present | 0.80 | 0.59 to 1.08 | — | |
| .47 | ||||
| Postmenopausal patients (IBCSG Trial IX) | ||||
| Triple negative | 0.38 | 0.19 to 0.76 | .01 | |
| HER2 positive, endocrine receptor absent | 0.37 | 0.13 to 1.03 | .06 | |
| Endocrine receptor present | 1.01 | 0.77 to 1.33 | — | |
| .0091 |
NOTE. The treatment comparison was CMF (cyclophosphamide, methotrexate, and fluorouracil) chemotherapy v no CMF chemotherapy. Hazard ratio (HR) estimates are adjusted for peritumoral vascular invasion, tumor size, and tumor grade. For the “all patients” comparison, the HRs are also adjusted for menopausal status.
Abbreviations: HER2, human epidermal growth factor receptor 2; IBCSG, International Breast Cancer Study Group.
All P values are interaction P values that assess heterogeneity of HRs relative to the HR in the endocrine receptor–present group.
Overall interaction P value assesses heterogeneity across all three groups.
We conducted an exploratory analysis evaluating the magnitude of the chemotherapy effect in endocrine receptor–present disease and either HER2 positive and/or Ki-67 high (≥ 14%; 517 patients), and in endocrine receptor–present disease and not HER2 positive or Ki-67 high (1,028 patients), in accordance with recently reported data.7 The magnitude of the chemotherapy effect was not significant in both groups (HR, 0.96; 95% CI, 0.74 to 1.23 and HR, 0.83; 95% CI, 0.53 to 1.30, respectively).
We also looked at relative risk reduction with chemotherapy in the HER2-negative group (n = 2,046) versus the HER2-positive group (n = 361). The respective HRs for chemotherapy versus no chemotherapy (competing-risks regression analysis after adjustment for peritumoral vascular invasion, tumor size, tumor grade, and menopausal status) were 0.77 (95% CI, 0.64 to 0.93) and 0.70 (95% CI, 0.47 to 1.06). No significant interaction was detected between treatment and HER2 status (P = .68).
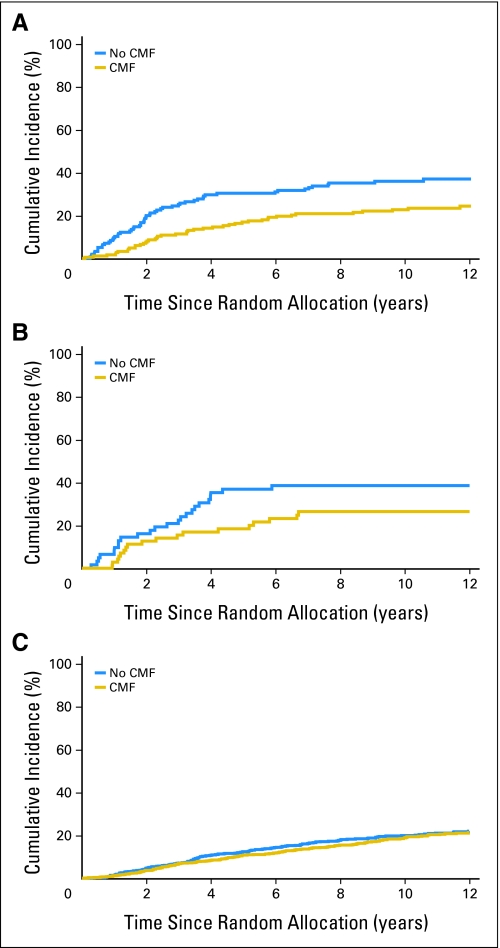
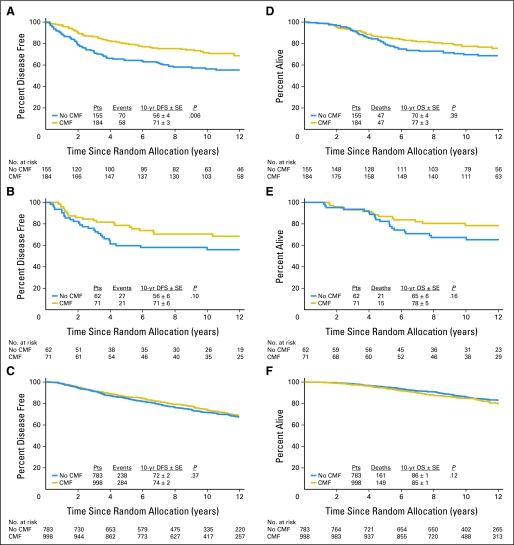
Figures 1 and 2 illustrate the estimated subtype-specific cumulative incidence of relapse over time according to treatment group for all patients (Fig 1) and for premenopausal patients (Figs 2A through 2C) and postmenopausal patients (Figs 2D through 2F). DFS and OS for each subtype according to treatment are shown in Figure 3. Chemotherapy significantly improved 10-year DFS in the triple-negative subtype (73% v 57%; P = .007), whereas no effect was observed in the endocrine receptor–present subtype (74% v 71%; P = .25).
Fig 1.
Estimated subtype-specific cumulative incidence of relapse over time according to treatment group (CMF [cyclophosphamide, methotrexate, and fluorouracil] chemotherapy v no CMF chemotherapy) for patients with (A) triple-negative, (B) human epidermal growth factor receptor 2 (HER2) –positive, endocrine receptor–absent, or (C) endocrine receptor–present tumors.
Fig 2.
Estimated subtype-specific cumulative incidence of relapse over time according to treatment group (CMF [cyclophosphamide, methotrexate, and fluorouracil] chemotherapy v no CMF chemotherapy) for premenopausal patients in International Breast Cancer Study Group (IBCSG) Trial VIII with (A) triple-negative tumors, (B) human epidermal growth factor receptor 2 (HER2) –positive, endocrine receptor–absent tumors, or (C) endocrine receptor–present tumors and for postmenopausal patients in IBCSG Trial IX with (D) triple-negative tumors, (E) HER2-positive, endocrine receptor–absent tumors, or (F) endocrine receptor–present tumors.
Fig 3.
Disease-free survival (DFS) and overall survival (OS) comparing patients assigned to CMF (cyclophosphamide, methotrexate, and fluorouracil) chemotherapy with patients assigned to no CMF chemotherapy with triple-negative (A,D), human epidermal growth factor receptor 2 (HER2) –positive, endocrine receptor–absent (B,E), and endocrine receptor–present (C,F) subtypes. Pts, patients; yr, year.
Our analysis defined ER and PR according to absent or present, using at least 1% of stained cells as the cutoff for hormone receptor present. We also reanalyzed using the more arbitrary but often used 10% cutoff to define hormone receptor positive (≥ 10%) and negative (< 10%). The results were similar and are included in the Appendix, in Appendix Tables A1-A4 (online only), and Appendix Figures A1-A3 (online only).
DISCUSSION
Recognition of factors predictive of response has become crucial for understanding treatment effects in the adjuvant treatment of breast cancer. Recent developments include increasing attention to predictive factors and to systemic therapies prescribed in homogeneous groups of patients according to the higher chance of response.1
Intrinsically different subtypes of breast cancer have been identified on the basis of genetic profile and IHC determination of selected targets.3,7,20 Given the paramount importance of using targeted therapies wherever possible for adjuvant treatment, timely, accurate, and reliable histopathologic assessment that identifies and quantifies the target is essential.
Emerging experimental and clinical studies have shown that steroid hormone receptor expression and epidermal growth factor receptor (EGFR; mainly HER2) pathways, alone or in combination, play key roles in the response to therapy in early breast cancer.21–23 We therefore present our results to explore the question of chemotherapy responsiveness in early breast cancer, focused on tumor subtypes defined according to accepted cutoffs for ER, PR, and HER2 that have known clinical relevance and are used in regular clinical practice for treatment decision making.
This study provides useful insights into the treatment of breast cancer because it is based on a large population of patients with node-negative breast cancer treated within the context of randomized clinical trials with standardized treatments and follow-up. The longer (> 10 years) median follow-up is particularly important in a population of node-negative patients. Moreover, a central pathology review using modern procedures gave a consistent and accurate assessment of the biologic features of primary breast cancers.
For several years, the correlation between the effects of adjuvant chemotherapy and degree of steroid hormone expression was studied, but the interpretation of these results remains controversial.24,25 The association between endocrine-responsiveness and outcome may be confounded in retrospective analyses by the large variation in chemotherapy regimens, patient features, methods, and cutoffs used for the determination of hormone receptors.
Recently published data from retrospective analyses of trial data from Cancer and Leukemia Group B26 found that the additional benefit of adding a taxane to adjuvant chemotherapy was not seen in patients with HER2-negative, ER-positive tumors, while the US Breast Cancer Intergroup review of 6,644 node-positive breast cancer patients who received adjuvant chemotherapy supports the value of extensive modern adjuvant chemotherapy in patients with tumors classified as ER negative.10 More recently, other authors reported that patients with ER-negative tumors (both triple negative and HER2 positive) showed a better response to taxane-containing chemotherapy than to chemotherapy that does not contain taxane,7 again supporting a possible increased chemosensitivity of those tumors that did not express steroid hormone receptors.
The results of this study are in line with these findings, supporting a larger effect for a particular chemotherapy regimen (classical CMF) in a specific tumor subtype: triple negative. However, this study differs from others already reported. Only a minority of published studies have a median follow-up exceeding 5 years, while we report results after a median of 11 years of follow-up. Such a prolonged follow-up is particularly important for the assessment of delayed events seen among patients with endocrine-responsive disease.27 Moreover, the present study focuses on a node-negative population, which has a lower metastatic potential. Finally, our analysis evaluates breast cancer subtype as a treatment-effect modifier for a comparison of chemotherapy versus no chemotherapy, an approach which is not commonly reported in the literature.
A potential limitation of this study might be related to the regimen most commonly used in the past (ie, classical CMF) and its duration (three courses in the postmenopausal population). However, there is evidence that CMF chemotherapy is not inferior to anthracycline-containing therapy in patients with HER2-negative breast cancer.28 In particular, in a study that compared two regimens with the same schedule and duration—cyclophosphamide, epirubicin, and fluorouracil (CEF) and classical CMF—patients whose tumors do not amplify or overexpress HER2 received virtually no benefit from CEF compared with CMF.29,30 More recently, a retrospective analysis from a randomized trial supports the hypothesis that anthracycline-containing adjuvant chemotherapy regimens may be inferior to adjuvant classical CMF in women with a core basal phenotype (negative for hormone receptors and HER2 and positive for CK5/6 or EGFR).30 In particular, in the anthracycline-containing chemotherapy (CEF) arm, patients with core basal tumors had worse survival outcome (HR, 1.8; log-rank P = .02) relative to the other biologic subtypes. Conversely, in the classical CMF arm, there was no significant difference (HR, 0.9; P = .7). The majority of the above-mentioned trials evaluated the magnitude of the effect of anthracycline-containing chemotherapy in a node-positive population. In this study, we showed that classical CMF chemotherapy significantly improved treatment outcome in a specific patient population characterized by a lower potential for metastatic disease such as a node-negative population.
Although no direct comparison with other regimens is available within this study, the impressive magnitude of the observed effect supports the hypothesis that the classical CMF regimen, originally formulated on the basis of the highly effective MOPP (mechlorethamine, vincristine, procarbazine, prednisone) regimen, may still represent a reasonable choice of treatment in selected patients. Moreover, long-term results and other information available to date show that the classical CMF regimen is safe31 and has little long-term toxicity.32 Whether newer forms of chemotherapy may be beneficial in this group of patients is one of the research priorities in this field.
There are several potential explanations for the different responses to chemotherapy according to tumor subtype. The triple-negative subtype is characterized by high expression of the proliferation cluster of genes.3 A higher proliferative index as measured by Ki-67 LI expression has been observed in this subtype compared with the endocrine-responsive subtype.33 Potential targets for chemotherapeutic agents are present in triple-negative tumors, the majority of which overexpress EGFR and endothelial growth factors,34 though the response to agents targeting EGFR in breast cancer has been disappointing.35 However, in vitro chemosensitivity studies have found that human cells lacking BRCA1, and to some extent other triple-negative cells, may be sensitive to drugs that cause double-strand breaks in DNA36 such as alkylating agents. Tumors with a high proliferation rate may also be particularly sensitive to the antimetabolites used in the CMF regimen.
The question of duration of adjuvant chemotherapy for breast cancer has been directly addressed in several trials.37–39 Most of these were small and therefore unsuitable for detecting differences of even modest magnitude. Furthermore, results in the node-negative population are lacking.
It should be emphasized that the tumor subtypes identified in this analysis include heterogeneous groups of tumors, and that the identification of further tumor subtypes amenable to targeted treatments represents a research priority.40 In this study, we explored the role of HER2 overexpression and Ki-67 expression in the definition of subgroups within the luminal population, as recently reported,7 but we were not able to identify subgroups with different responsiveness to chemotherapy. Possible reasons for these results include the low number of events registered in studies focusing on a node-negative population.
In conclusion, this study confirms that the efficacy of adjuvant systemic therapy for early breast cancer depends on variable features, including those of the tumor, the patient, and the treatment itself. We demonstrated that the magnitude of the effect of chemotherapy in early breast cancer significantly correlates with tumor subtypes identified by IHC. The results of this study provide substantial additional evidence to support the concept that further progress in the adjuvant treatment of breast cancer will require studies dedicated to specific niches of patients selected through the identification of treatment targets.
Acknowledgment
We thank the patients, pathologists, and investigators who contributed to these trials and are grateful to Ms Wei Xiao of the University of Vermont for data analysis.
Appendix
The statistical analysis for this study was repeated using the hormone receptor cutoff of < 10% to define an estrogen receptor (ER) –negative or progesterone receptor (PR) –negative cohort rather than, as was done in the original statistical analysis, using 0% to define an ER-absent cohort and > 0% to define an ER-present cohort. In the following replication of the Results, there are three subgroups: triple negative (human epidermal growth factor receptor 2 [HER2] negative, ER negative, PR negative); HER2 positive, endocrine nonresponsive (HER2 positive, ER negative, and PR negative); and endocrine responsive (ER positive or PR positive, any HER2).
Results
This analysis was conducted on the 2,253 patients for whom immunohistochemistry-defined subtype was available. A total of 339 patients (15%) had triple-negative disease; 133 (6%) had HER2-positive, endocrine-nonresponsive disease; and 1,781 (79%) had endocrine-responsive disease.
The following participants and authors were part of the International Breast Cancer Study Group:
Scientific Committee: A. Goldhirsch, A.S. Coates (Co-Chairs); Foundation Council: B. Thürlimann (President), M. Castiglione, A.S. Coates, J.P. Collins, H. Cortés Funes, R.D. Gelber, A. Goldhirsch, M. Green, A. Hiltbrunner, S.B. Holmberg, D.K. Hossfeld, I. Láng, J. Lindtner, F. Paganetti, C.-M. Rudenstam, R. Stahel, H.-J. Senn, A. Veronesi; Coordinating Center, Bern, Switzerland: M. Castiglione-Gertsch (CEO and Study Chair), A. Hiltbrunner (Director), G. Egli, M. Rabaglio, R. Maibach, R. Studer, B. Ruepp, E. Marbot; Pathology Office: R. Kammler (Head Pathology Coordinating Office), H.-R. Pauli, A. Aeschbacher, S. Oelhafen; Statistical Center, Harvard School of Public Health and Dana-Farber Cancer Institute, Boston, MA: R.D. Gelber (Group Statistician), K. Price (Director of Scientific Administration), A. Giobbie-Hurder, M. Regan, D. Zahrieh, S. Gelber, A. Keshaviah, Z. Sun, B. Cole, L. Nickerson; Data Management Center, Frontier Science and Technology Research Foundation, Amherst, NY; L. Blacher (Director), R. Hinkle (Trial Data Manager), S. Lippert, J. Celano; Pathology Office, European Institute of Oncology, Milan, Italy: G. Viale, E. Maiorano, M. Mastropasqua, S. Andrighetto, G. Peruzzotti, R. Ghisini, E. Scarano, P. Dell'Orto, B. Del Curto; Pathology Office, University of Glasgow, Scotland, United Kingdom: B. Gusterson, E. Mallon; The Ontario Cancer Treatment and Research Foundation, Toronto Sunnybrook Regional Cancer Centre, Toronto, Canada: K. Pritchard, D. Sutherland, C. Sawka, G. Taylor, R. Choo, C. Catzavelos, K. Roche, H. Wedad; National Institute of Oncology, Budapest, Hungary: I. Láng, E. Hitre, E. Juhos, I. Szamel, J. Toth, Z. Orosz, I. Peter; Centro di Riferimento Oncologico, Aviano, Italy: D. Crivellari, S. Monfardini, E. Galligioni, M.D. Magri, A. Veronesi, A. Buonadonna. S. Massarut, C. Rossi, E. Candiani, A. Carbone, T. Perin, R. Volpe, M. Roncadin, M. Arcicasa, F. Coran, S. Morassut; Spedali Civili and Fondazione Beretta, Brescia, Italy: E. Simoncini, G. Marini, P. Marpicati, M. Braga, P. Grigolato, L. Lucini; General Hospital, Gorizia, Italy: S. Foladore, L. Foghin, G. Pamich, C. Bianchi, B. Marino, A. Murgia, V. Milan; European Institute of Oncology, Milano, Italy: A. Goldhirsch, M. Colleoni, G. Martinelli, L. Orlando, F. Nolé, A. Luini, R. Orecchia, G. Viale, G. Renne, G. Mazzarol, F. Peccatori, F. de Braud, A. Costa, S. Zurrida, P. Veronesi, V. Sacchini, V. Galimberti, M. Intra, S. Cinieri, G. Peruzzotti, U. Veronesi; Ospedale Infermi, Rimini, Italy: A. Ravaioli, D. Tassinari, G. Oliverio, F. Barbanti, P. Rinaldi, L. Gianni, G. Drudi; Ospedale S. Eugenio, Roma, Italy: M. Antimi, M. Minelli, V. Bellini, R. Porzio, E. Pernazza, G. Santeusanio, L.G. Spagnoli; Ospedale S. Bortolo, Vicenza, Italy: M. Magazu, V. Fosser, P. Morandi, G. Scalco, M. Balli, E.S.G. d'Amore, S. Meli, G. Torsello; The Institute of Oncology, Ljubljana, Slovenia: J. Lindtner, D. Erzen, E. Majdic, B. Stabuc, A. Plesnicar, R. Golouh, J. Lamovec, J. Jancar, I. Vrhovec, M. Kramberger; Groote Schuur Hospital and University of Cape Town, Cape Town, Republic of South Africa: D.M. Dent, A. Gudgeon, E. Murray, G. Langman, I.D. Werner, P. Steynor, J. Toop, E. McEvoy; Sandton Oncology Center, Johannesburg, Republic of South Africa: D. Vorobiof, M. Chasen, G. Fotheringham, G. de Muelenaere, B. Skudowitz, C. Mohammed, A. Rosengarten, C. Thatcher; Madrid Breast Cancer Group, Madrid, Spain: H. Cortés-Funes, C. Mendiola, J. Hornedo, R. Colomer, F. Cruz Vigo, P. Miranda, A. Sierra, F. Martinez-Tello, A. Garzon, S. Alonso, A. Ferrero; West Swedish Breast Cancer Study Group, Göteborg, Sweden: C.M. Rudenstam, M. Suurküla, Ö. Sjukhuset, G. Havel, S. Persson, J.H. Svensson, G. Östberg, S.B. Holmberg, A. Wallgren, S. Ottosson-Lönn, R. Hultborn, G. Colldahl-Jäderström, E. Cahlin, J. Mattsson, L. Ivarsson, O. Ruusvik, L.G. Niklasson, S. Dahlin, G. Karlsson, B. Lindberg, A. Sundbäck, S. Bergegårdh, H. Salander, C. Andersson, M. Heideman, Y. Hessman, O. Nelzén, G. Claes, T. Ramhult, A. Kovacs, P. Liedberg, L. Larsson, A. Nissborg, Z. Einbeigi, L. Klint, G. Lengstrand, P. Karlsson; Swiss Group for Clinical Cancer Research member institutions: Inselspital, Bern, Switzerland: M.F. Fey, M. Castiglione-Gertsch, E. Dreher, H. Schneider, S. Aebi, J. Ludin, G. Beck, A. Haenel, J.M. Lüthi, L. Mazzucchelli, J.P. Musy, H.J. Altermatt, M. Nandedkar, K. Buser; Kantonsspital, St. Gallen, Switzerland: H.J. Senn, B. Thürlimann, Ch. Oehlschlegel, G. Ries, M. Töpfer, U. Lorenz, O. Schiltknecht, B. Späti, A. Ehrsam, M. Bamert, W.F. Jungi; Istituto Oncologico della Svizzera Italiana, Bellinzona, Switzerland: F. Cavalli, O. Pagani, H. Neuenschwander, L. Bronz, C. Sessa, M. Ghielmini, T. Rusca, P. Rey, J. Bernier, E. Pedrinis, T. Gyr, L. Leidi, G. Pastorelli, G. Caccia, A. Goldhirsch; Kantonsspital, Basel, Switzerland: R. Herrmann, C.F. Rochlitz, J.F. Harder, S. Bartens, U. Eppenberger, J. Torhorst, H. Moch; Hôpital des Cadolles, Neuchâtel, Switzerland: D. Piguet, P. Siegenthaler, V. Barrelet, R.P. Baumann, B. Christen; University Hospital, Zürich, Switzerland: B. Pestalozzi, C. Sauter, D. Fink, M. Fehr, U. Haller, U. Metzger, P. Huguenin, R. Caduff; Centre Hospitalier Universitaire Vandois, Lausanne, Switzerland: L. Perey, S. Leyvraz, P. Anani, F. Gomez, D. Wellman, G. Chapuis, P. De Grandi, P. Reymond, M. Gillet, J.F. Delaloye, C. Genton, M. Fiche; Hôpital Cantonal, Geneva, Switzerland: P. Alberto, H. Bonnefoi, P. Schäfer, F. Krauer, M. Forni, M. Aapro, R. Egeli, R. Megevand, E. Jacot-des-Combes, A. Schindler, B. Borisch, S. Diebold, M. Genta, M. Pelte; Kantonsspital Graubünden, Chur, Switzerland: F. Egli, P. Forrer, A. Willi, R. Steiner, J. Allemann, T. Rüedi, A. Leutenegger, U. Dalla Torre, H. Frick; Australian New Zealand Breast Cancer Trials Group member institutions: Operations Office, University of Newcastle, Newcastle, Australia: J.F. Forbes, D. Lindsay; The Cancer Council Victoria (previously Anti-Cancer Council of Victoria), Clinical Trials Office, Melbourne, Australia: J. Collins, R. Snyder, B. Brown, E. Abdi, H. Armstrong, A. Barling, R. Basser, P. Bhathal, W.I. Burns, M. Chipman, J. Chirgwin, I. Davis, R. Drummond, D. Finkelde, P. Francis, D. Gee, G. Goss, M. Green, P. Gregory, J. Griffiths, S. Hart, D. Hastrich, M. Henderson, R. Holmes, P. Jeal, D. Joseph, P. Kitchen, P. Kostos, G. Lindeman, B. Mann, R. McLennan, L. Mileshkin, P. Mitchell, C. Murphy, S. Neil, I. Olver, M. Pitcher, A. Read, D. Reading, R. Reed, G. Richardson, A. Rodger, I. Russell, M. Schwarz, S. Slade, R. Stanley, M. Steele, J. Stewart, C. Underhill, J. Zalcberg, A. Zimet, C. Dow, R. Valentine; Flinders Medical Centre, Bedford Park, South Australia: T. Malden; Mount Hospital, Perth, Western Australia: G. Van Hazel; Calvary Mater Newcastle, Newcastle, Australia: J.F. Forbes, S. Braye, J. Stewart, D. Jackson, R. Gourlay, J. Bishop, S. Cox, S. Ackland, A. Bonaventura, C. Hamilton, J. Denham, P. O'Brien, M. Back, S. Brae, R. Muragasu; Prince of Wales, Randwick, NSW, Australia: M. Friedlander, B. Brigham, C. Lewis; Royal Adelaide Hospital, Adelaide, Australia: I.N. Olver, D. Keefe, M. Brown, P.G. Gill, A. Taylor, E. Yeoh, E. Abdi, J. Cleary, F. Parnis; Sir Charles Gairdner Hospital, Nedlands, Western Australia: M. Byrne, G. Van Hazel, J. Dewar, M. Buck, G. Sterrett, D. Ingram, D. Hastrich, D. Joseph, F. Cameron, K.B. Shilkin, P. Michell, J. Sharpio, G. Harloe, J. Lewis, B. Snowball, P. Garcia Webb, J. Harvey, W.D. De Boer, P. Robbins, N. Buxton, M.N.I. Walters; University of Sydney, Dubbo Base Hospital and Royal Prince Alfred Hospital, Sydney, Australia: J. Beith, M.H.N. Tattersall, A.S. Coates, F. Niesche, R. West, S. Renwick, J. Donovan, P. Duval, R.J. Simes, A. Ng, D. Glenn, R.A. North, R.G. O'Connor, M. Rice, G. Stevens, J. Grassby, S. Pendlebury, C. McLeod, M. Boyer, A. Sullivan, J. Hobbs, D. Lind, J. Grace, P. McKenzie; W.P. Holman Clinic, Launceston, Australia: D. Boadle, T. Brain, I. Byard, D. Byram; Auckland Breast Cancer Study Group, Auckland, New Zealand: V.J. Harvey, R.G. Kay, P. Thompson, D. Porter, C.S. Benjamin, A. Bierre, M. Miller, B. Hochstein, A. Lethaby, J. Webber, J.P. Allen, M. Allon, J.F. Arthur, M. Gurley, P. Symmans, M. Christie, A.R. King; Waikato Hospital, Hamilton, New Zealand: I. Kennedy, G. Round, J. Long.
Fig A1.
Estimated subtype-specific cumulative incidence of relapse over time according to treatment group (CMF [cyclophosphamide, methotrexate, and fluorouracil] chemotherapy v no CMF chemotherapy) for patients with (A) triple-negative, (B) human epidermal growth factor receptor 2–positive, endocrine-nonresponsive, or (C) endocrine-responsive tumors.
Fig A2.
Estimated subtype-specific cumulative incidence of relapse over time according to treatment group (CMF [cyclophosphamide, methotrexate, and fluorouracil] chemotherapy v no CMF chemotherapy) for premenopausal patients in International Breast Cancer Study Group (IBCSG) Trial VIII with (A) triple-negative, (B) human epidermal growth factor receptor 2 (HER2) –positive, endocrine-nonresponsive, or (C) endocrine-responsive tumors and for postmenopausal patients in IBCSG Trial IX with (D) triple-negative, (E) HER2-positive, endocrine-nonresponsive, or (F) endocrine-responsive tumors.
Fig A3.
Disease-free survival (DFS) and overall survival (OS) comparing patients assigned to CMF (cyclophosphamide, methotrexate, and fluorouracil) chemotherapy with patients assigned to no CMF chemotherapy for triple-negative (A,D), human epidermal growth factor receptor 2–positive, endocrine-nonresponsive (B,E), and endocrine-responsive (C,F) subtypes. yr, year; Pts, patients.
Table A1.
Baseline Characteristics of Patients With Centrally Assessed ER, PR, and HER2 and Patients Not Included in the Analysis
| Characteristic | Patients With Missing Receptor Information (n = 519) |
Patients Included in Analysis (n = 2,257) |
P* | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Menopausal status | |||||
| Premenopausal (IBCSG Trial VIII) | 196 | 38 | 912 | 40 | .27 |
| Postmenopausal (IBCSG Trial IX) | 323 | 62 | 1,345 | 60 | |
| Tumor size, cm | |||||
| ≤ 2 | 336 | 65 | 1,351 | 60 | .030 |
| > 2 | 173 | 33 | 879 | 39 | |
| Unknown | 10 | 2 | 27 | 1 | |
| Tumor grade | |||||
| 1 | 129 | 25 | 370 | 16 | < .001 |
| 2 | 209 | 40 | 1,023 | 45 | |
| 3 | 162 | 31 | 852 | 38 | |
| Unknown | 19 | 4 | 12 | 1 | |
| Vessel invasion | |||||
| Yes | 391 | 75 | 1,767 | 78 | < .001 |
| No | 70 | 13 | 348 | 15 | |
| Unknown | 58 | 11 | 142 | 6 | |
| Chemotherapy | |||||
| Yes | 284 | 55 | 1,255 | 56 | .73 |
| No | 235 | 45 | 1,002 | 44 | |
| Age, years (mean ± SD) | 54.1 ± 10.3 | 54.1 ± 10.0 | .96 | ||
| IBCSG Trial VIII | 43.9 ± 6.0 | 44.3 ± 5.5 | .44 | ||
| IBCSG Trial IX | 60.2 ± 6.5 | 60.8 ± 6.1 | .20 | ||
Abbreviations: ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; IBCSG, International Breast Cancer Study Group; SD, standard deviation.
P values are based on Fisher's exact test for all characteristics except for age, which was evaluated using the Wilcoxon rank-sum test.
Table A2.
Patient and Disease Characteristics
| Characteristic | Triple Negative (n = 339) |
HER2 Positive, Endocrine Nonresponsive (n = 133) |
Endocrine Responsive (n = 1,781) |
P* | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Menopausal status | |||||||
| Premenopausal (IBCSG Trial VIII) | 155 | 46 | 47 | 35 | 708 | 40 | .06 |
| Postmenopausal (IBCSG Trial IX) | 184 | 54 | 86 | 65 | 1,073 | 60 | |
| Tumor size, cm | |||||||
| ≤ 2 | 138 | 41 | 67 | 51 | 1,143 | 65 | < .001 |
| > 2 | 198 | 59 | 65 | 49 | 615 | 35 | |
| Unknown | 3 | 1 | 3 | ||||
| Tumor grade | |||||||
| 1 | 8 | 2 | 2 | 2 | 360 | 20 | < .001 |
| 2 | 70 | 21 | 39 | 29 | 912 | 51 | |
| 3 | 261 | 77 | 88 | 66 | 501 | 28 | |
| Unknown | 0 | 0 | 4 | 3 | 8 | 0 | |
| Peritumoral vascular invasion | |||||||
| Yes | 263 | 78 | 94 | 71 | 1,408 | 79 | .09 |
| No | 49 | 14 | 29 | 22 | 268 | 15 | |
| Unknown | 27 | 8 | 10 | 8 | 105 | 6 | |
| Age, years (mean ± SD) | 52.5 ± 10.2 | 54.4 ± 10.0 | 54.4 ± 9.9 | < .001 | |||
| IBCSG Trial VIII | 43.4 ± 5.9 | 43.3 ± 6.4 | 44.4 ± 5.4 | .09 | |||
| IBCSG Trial IX | 60.0 ± 5.8 | 60.4 ± 6.5 | 60.9 ± 6.2 | .26 | |||
Abbreviations: HER2, human epidermal growth factor receptor 2; IBCSG, International Breast Cancer Study Group; SD, standard deviation.
P values are based on Fisher's exact test for menopausal status and tumor size, χ2 test for tumor grade and peritumoral vascular invasion, and Kruskal-Wallis test for age.
Table A3.
Cumulative Incidence Estimates of Relapse According to Tumor Subtype, Menopausal Status, and Treatment Group
| Treatment and Subtype | No. of Patients | No. of Relapse Events | 10-Year Cumulative Incidence of Relapse ± SE |
|---|---|---|---|
| All patients | |||
| Triple negative | |||
| No CMF | 155 | 57 | 36 ± 4 |
| CMF | 184 | 44 | 23 ± 3 |
| HER2 positive, endocrine nonresponsive | |||
| No CMF | 62 | 24 | 39 ± 6 |
| CMF | 71 | 18 | 27 ± 5 |
| Endocrine responsive | |||
| No CMF | 783 | 161 | 20 ± 2 |
| CMF | 998 | 196 | 19 ± 1 |
| Premenopausal patients (IBCSG Trial VIII) | |||
| Triple negative | |||
| No CMF | 58 | 25 | 40 ± 7 |
| CMF | 97 | 28 | 27 ± 5 |
| HER2 positive, endocrine nonresponsive | |||
| No CMF | 17 | 8 | 47 ± 13 |
| CMF | 30 | 11 | 39 ± 10 |
| Endocrine responsive | |||
| No CMF | 248 | 63 | 26 ± 3 |
| CMF | 460 | 97 | 21 ± 2 |
| Postmenopausal patients (IBCSG Trial IX) | |||
| Triple negative | |||
| No CMF | 97 | 32 | 34 ± 5 |
| CMF | 87 | 16 | 18 ± 4 |
| HER2 positive, endocrine nonresponsive | |||
| No CMF | 45 | 16 | 36 ± 7 |
| CMF | 41 | 7 | 18 ± 6 |
| Endocrine responsive | |||
| No CMF | 535 | 98 | 17 ± 2 |
| CMF | 538 | 99 | 17 ± 2 |
Abbreviations: CMF, cyclophosphamide, methotrexate, and fluorouracil; IBCSG, International Breast Cancer Study Group.
Table A4.
Group-Specific Treatment HRs for Relapse Based on Competing-Risks Regression
| Tumor Subtype | HR (CMF v no CMF) | 95% CI | Interaction P* | Overall Interaction P† |
|---|---|---|---|---|
| All patients | ||||
| Triple negative | 0.51 | 0.33 to 0.78 | .02 | |
| HER2 positive, endocrine nonresponsive | 0.63 | 0.32 to 1.25 | .33 | |
| Endocrine responsive | 0.90 | 0.73 to 1.10 | — | |
| .048 | ||||
| Premenopausal patients (IBCSG Trial VIII) | ||||
| Triple negative | 0.51 | 0.28 to 0.94 | .16 | |
| HER2 positive, endocrine nonresponsive | 0.69 | 0.23 to 2.07 | .76 | |
| Endocrine responsive | 0.82 | 0.61 to 1.12 | — | |
| .38 | ||||
| Postmenopausal patients (IBCSG Trial IX) | ||||
| Triple negative | 0.47 | 0.25 to 0.88 | .04 | |
| HER2 positive, endocrine nonresponsive | 0.46 | 0.17 to 1.24 | .15 | |
| Endocrine responsive | 0.98 | 0.74 to 1.28 | — | |
| .053 |
NOTE. The treatment comparison was CMF (cyclophosphamide, methotrexate, and fluorouracil) chemotherapy v no CMF chemotherapy. Hazard ratio (HR) estimates are adjusted for peritumoral vascular invasion, tumor size, and tumor grade. For the “all patients” comparison, the HRs are also adjusted for menopausal status.
All P values are interaction P values that assess heterogeneity of HRs relative to the HR in the endocrine-responsive group.
The overall interaction P assesses heterogeneity across all three groups.
Footnotes
Written on behalf of the International Breast Cancer Study Group.
Supported in part by the Swiss Group for Clinical Cancer Research; Cancer Research Switzerland/Oncosuisse; Frontier Science and Technology Research Foundation; The Cancer Council Australia; Australian New Zealand Breast Cancer Trials Group; Grant No. CA-75362 from the National Cancer Institute, National Institutes of Health, Bethesda, MD; Swedish Cancer Society; Foundation for Clinical Research of Eastern Switzerland; grants from the National Health and Medical Research Council of Australia; and Breakthrough Breast Cancer (B.A.G.).
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Marco Colleoni, Giuseppe Viale, Aron Goldhirsch, Barry A. Gusterson
Administrative support: Karen N. Price, Aron Goldhirsch
Provision of study materials or patients: Marco Colleoni, Giuseppe Viale, Eugenio Maiorano, Mauro G. Mastropasqua, Diana Crivellari, Aron Goldhirsch, Alan S. Coates, Barry A. Gusterson
Collection and assembly of data: Meredith M. Regan, Diana Crivellari, Barry A. Gusterson
Data analysis and interpretation: Marco Colleoni, Bernard F. Cole, Meredith M. Regan, Richard D. Gelber, Barry A. Gusterson
Manuscript writing: Marco Colleoni, Bernard F. Cole, Giuseppe Viale, Meredith M. Regan, Karen N. Price, Diana Crivellari, Alan S. Coates, Barry A. Gusterson
Final approval of manuscript: Marco Colleoni, Bernard F. Cole, Giuseppe Viale, Meredith M. Regan, Karen N. Price, Eugenio Maiorano, Mauro G. Mastropasqua, Diana Crivellari, Richard D. Gelber, Aron Goldhirsch, Alan S. Coates, Barry A. Gusterson
REFERENCES
- 1.Goldhirsch A, Ingle JN, Gelber RD, et al. Thresholds for therapies: Highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer. Ann Oncol. 2009;20:1319–1329. doi: 10.1093/annonc/mdp322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 3.Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sørlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yehiely F, Moyano JV, Evans JR, et al. Deconstructing the molecular portrait of basal-like breast cancer. Trends Mol Med. 2006;12:537–544. doi: 10.1016/j.molmed.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Rakha EA, El-Sayed ME, Green AR, et al. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109:25–32. doi: 10.1002/cncr.22381. [DOI] [PubMed] [Google Scholar]
- 7.Hugh J, Hanson J, Cheang MC, et al. Breast cancer subtypes and response to docetaxel in node-positive breast cancer: Use of an immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol. 2009;27:1168–1176. doi: 10.1200/JCO.2008.18.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colleoni M, Viale G, Zahrieh D, et al. Expression of ER, PgR, HER1, HER2, and response: A study of preoperative chemotherapy. Ann Oncol. 2008;19:465–472. doi: 10.1093/annonc/mdm509. [DOI] [PubMed] [Google Scholar]
- 9.Kaufmann M, Hortobagyi GN, Goldhirsch A, et al. Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: An update. J Clin Oncol. 2006;24:1940–1949. doi: 10.1200/JCO.2005.02.6187. [DOI] [PubMed] [Google Scholar]
- 10.Berry DA, Cirrincione C, Henderson IC, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006;295:1658–1667. doi: 10.1001/jama.295.14.1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.International Breast Cancer Study Group (IBCSG) Endocrine responsiveness and tailoring adjuvant therapy for postmenopausal lymph node-negative breast cancer: A randomized trial. J Natl Cancer Inst. 2002;94:1054–1065. doi: 10.1093/jnci/94.14.1054. [DOI] [PubMed] [Google Scholar]
- 12.International Breast Cancer Study Group. Castiglione-Gertsch M, O'Neill A, et al. Adjuvant chemotherapy followed by goserelin versus either modality alone for premenopausal lymph node-negative breast cancer: A randomized trial. J Natl Cancer Inst. 2003;95:1833–1846. doi: 10.1093/jnci/djg119. [DOI] [PubMed] [Google Scholar]
- 13.Viale G, Regan MM, Maiorano E, et al. Chemoendocrine compared with endocrine adjuvant therapies for node-negative breast cancer: Predictive value of centrally reviewed expression of estrogen and progesterone receptors—International Breast Cancer Study Group. J Clin Oncol. 2008;26:1404–1410. doi: 10.1200/JCO.2007.10.6393. [DOI] [PubMed] [Google Scholar]
- 14.Viale G, Regan MM, Mastropasqua MG, et al. Predictive value of tumor Ki-67 expression in two randomized trials of adjuvant chemoendocrine therapy for node-negative breast cancer. J Natl Cancer Inst. 2008;100:207–212. doi: 10.1093/jnci/djm289. [DOI] [PubMed] [Google Scholar]
- 15.Fisher RA. The logic of inductive inference. J R Stat Soc. 1935;98:39–84. [Google Scholar]
- 16.Kruskal WH, Wallis WA. Use of ranks in one-criterion variance analysis. J Am Stat Assoc. 1952;47:583–621. [Google Scholar]
- 17.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 19.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient: II. Analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Regan MM, Viale G, Mastropasqua MG, et al. Re-evaluating adjuvant breast cancer trials: Assessing hormone receptor status by immunohistochemical versus extraction assays. J Natl Cancer Inst. 2006;98:1571–1581. doi: 10.1093/jnci/djj415. [DOI] [PubMed] [Google Scholar]
- 21.Barrett-Lee PJ. Growth factor signalling in clinical breast cancer and its impact on response to conventional therapies: A review of chemotherapy. Endocr Relat Cancer. 2005;12(suppl 1):S125–S133. doi: 10.1677/erc.1.01024. [DOI] [PubMed] [Google Scholar]
- 22.Arpino G, Weiss H, Lee AV, et al. Estrogen receptor-positive, progesterone receptor-negative breast cancer: Association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst. 2005;97:1254–1261. doi: 10.1093/jnci/dji249. [DOI] [PubMed] [Google Scholar]
- 23.Guarneri V, Broglio K, Kau SW, et al. Prognostic value of pathologic complete response after primary chemotherapy in relation to hormone receptor status and other factors. J Clin Oncol. 2006;24:1037–1044. doi: 10.1200/JCO.2005.02.6914. [DOI] [PubMed] [Google Scholar]
- 24.Roché H, Fumoleau P, Spielmann M, et al. Sequential adjuvant epirubicin-based and docetaxel chemotherapy for node-positive breast cancer patients: The FNCLCC PACS 01 Trial. J Clin Oncol. 2006;24:5664–5671. doi: 10.1200/JCO.2006.07.3916. [DOI] [PubMed] [Google Scholar]
- 25.Martin M, Pienkowski T, Mackey J, et al. Adjuvant docetaxel for node-positive breast cancer. N Engl J Med. 2005;352:2302–2313. doi: 10.1056/NEJMoa043681. [DOI] [PubMed] [Google Scholar]
- 26.Hayes DF, Thor AD, Dressler LG, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007;357:1496–1506. doi: 10.1056/NEJMoa071167. [DOI] [PubMed] [Google Scholar]
- 27.Saphner T, Tormey DC, Gray R. Annual hazard rates of recurrence for breast cancer after primary therapy. J Clin Oncol. 1996;14:2738–2746. doi: 10.1200/JCO.1996.14.10.2738. [DOI] [PubMed] [Google Scholar]
- 28.Gennari A, Sormani MP, Pronzato P, et al. HER2 status and efficacy of adjuvant anthracyclines in early breast cancer: A pooled analysis of randomized trials. J Natl Cancer Inst. 2008;100:14–20. doi: 10.1093/jnci/djm252. [DOI] [PubMed] [Google Scholar]
- 29.Pritchard KI, Shepherd LE, O'Malley FP, et al. HER2 and responsiveness of breast cancer to adjuvant chemotherapy. N Engl J Med. 2006;354:2103–2111. doi: 10.1056/NEJMoa054504. [DOI] [PubMed] [Google Scholar]
- 30.Cheang MC, Chia SK, Tu D, et al. Anthracyclines in basal breast cancer: The NCIC-CTG trial MA5 comparing adjuvant CMF to CEF. J Clin Oncol. 2009;27(suppl):11s. abstr 519. [Google Scholar]
- 31.Goldhirsch A, Coates AS, Colleoni M, et al. Adjuvant chemoendocrine therapy in postmenopausal breast cancer: Cyclophosphamide, methotrexate, and fluorouracil dose and schedule may make a difference—International Breast Cancer Study Group. J Clin Oncol. 1998;16:1358–1362. doi: 10.1200/JCO.1998.16.4.1358. [DOI] [PubMed] [Google Scholar]
- 32.Colleoni M, Price KN, Castiglione-Gertsch M, et al. Mortality during adjuvant treatment of early breast cancer with cyclophosphamide, methotrexate, and fluorouracil—International Breast Cancer Study Group. Lancet. 1999;354:130–131. doi: 10.1016/s0140-6736(99)02015-2. [DOI] [PubMed] [Google Scholar]
- 33.Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki-67 in early breast cancer. J Clin Oncol. 2005;23:7212–7220. doi: 10.1200/JCO.2005.07.501. [DOI] [PubMed] [Google Scholar]
- 34.Viale G, Rotmensz N, Maisonneuve P, et al. Invasive ductal carcinoma of the breast with the “triple-negative” phenotype: Prognostic implications of EGFR immunoreactivity. Breast Cancer Res Treat. 2009;116:317–328. doi: 10.1007/s10549-008-0206-z. [DOI] [PubMed] [Google Scholar]
- 35.Gusterson BA, Hunter KD. Should we be surprised at the paucity of response to EGFR inhibitors? Lancet Oncol. 2009;10:522–527. doi: 10.1016/S1470-2045(09)70034-8. [DOI] [PubMed] [Google Scholar]
- 36.James CR, Quinn JE, Mullan PB, et al. BRCA1, a potential predictive biomarker in the treatment of breast cancer. Oncologist. 2007;12:142–150. doi: 10.1634/theoncologist.12-2-142. [DOI] [PubMed] [Google Scholar]
- 37.International Breast Cancer Study Group. Duration and reintroduction of adjuvant chemotherapy for node-positive premenopausal breast cancer patients. J Clin Oncol. 1996;14:1885–1894. doi: 10.1200/JCO.1996.14.6.1885. [DOI] [PubMed] [Google Scholar]
- 38.Sauerbrei W, Bastert G, Bojar H, et al. Randomized 2 x 2 trial evaluating hormonal treatment and the duration of chemotherapy in node-positive breast cancer patients: An update based on 10 years' follow-up—German Breast Cancer Study Group. J Clin Oncol. 2000;18:94–101. doi: 10.1200/JCO.2000.18.1.94. [DOI] [PubMed] [Google Scholar]
- 39.Colleoni M, Gelber S, Simoncini E, et al. Effects of a treatment gap during adjuvant chemotherapy in node-positive breast cancer: Results of International Breast Cancer Study Group (IBCSG) Trials 13-93 and 14-93. Ann Oncol. 2007;18:1177–1184. doi: 10.1093/annonc/mdm091. [DOI] [PubMed] [Google Scholar]
- 40.Gusterson B. Do ‘basal-like’ breast cancers really exist? Nat Rev Cancer. 2009;9:128–134. doi: 10.1038/nrc2571. [DOI] [PubMed] [Google Scholar]