Abstract
Kruppel-like factor 9 (KLF9) is a zinc finger transcription factor that regulates estrogen and progesterone action by modulating the activity of progesterone receptor (PGR). The transition from proliferative to secretory endometrial epithelium involves loss of estrogen receptor/PGR expression and loss of direct response to sex steroids. HOXA10 partially mediates progesterone responsiveness in the endometrium. Here, we demonstrate that HOXA10 directly regulates KLF9 in endometrial epithelial cells and not in stromal cells. Immunohistochemistry performed on endometrial tissue obtained from normal, reproductive-age women revealed that KLF9 expression was decreased in the secretory phase of the menstrual cycle compared to the proliferative phase. In vitro, HOXA10 transfection of human endometrial epithelial cells (Ishikawa), but not stromal cells (HESC), resulted in a greater than 50% decrease in KLF9 mRNA and protein expression. Reporter constructs driven by the KLF9 promoter were repressed by cotransfection with HOXA10. Electrophoretic mobility shift assay was used to demonstrate direct binding of HOXA10 to the KLF9 promoter. Targeted mutation of the HOXA10-binding site in the KLF9 promoter resulted in loss of HOXA10 binding and loss of repression by HOXA10 in reporter assays. HOXA10 directly and selectively repressed KLF9 expression in endometrial epithelial cells. HOXA10 repression of KLF9 likely contributes to the loss of sex steroid responsiveness in secretory-phase endometrial epithelium.
Keywords: endometrium,; female reproductive tract,; gene regulation,; HOXA10,; implantation,; Kruppel-like factor 9,; uterus
Kruppel-like factor 9 (KLF9) is directly regulated by HOXA10 in endometrial epithelial cells, and not in stromal cells, thereby mediating human endometrial gene expression for optimal fertility.
INTRODUCTION
Kruppel-like factor 9 (KLF9) is a member of the Kruppel-like family of mammalian zinc finger-containing transcription factors [1]. Klf9 was first identified as a transcriptional repressor of the rat Cyp1a1 (previously P-4501A1) gene, and was originally named basic transcription element-binding protein 1 (Bteb1) [2]. Klf9 mRNA is ubiquitously expressed in various tissues, including the brain, testis, kidney, and liver. It is also expressed in mammalian uterine endometrium, and its mRNA abundance varies with cell type [3]. In porcine endometrium of pregnancy, KLF9 is expressed in luminal and glandular epithelium and in stromal cells. Progesterone plays an essential role in uterine endometrial growth and differentiation; KLF9 is expressed in the endometrium during early pregnancy, coincident with progesterone receptor (PGR) [4, 5]. It directly interacts with PGR to mediate progesterone-responsive gene expression in endometrial epithelial cells [4–6]. Estrogen is also a critical regulator of endometrial epithelial cells that acts through estrogen receptor (ESR) to promote cell proliferation. KLF9 has been identified as a transcriptional repressor of ESR1 signaling in Ishikawa endometrial adenocarcionoma cells [7]. Conversely, endometrial stromal KLF9 is required for estrogen-induced stromal-epithelial communication and endometrial epithelial cell proliferation [8]. Targeted mutation of Klf9 results in subfertility, uterine hypoplasia, and partial progesterone resistance [9].
Hox genes were first recognized as an evolutionarily conserved family of transcription factors critical to the control of early embryonic development. HOXA10 encodes a transcription factor and is expressed in both the embryonic and adult reproductive tracts, predominantly in the uterus [10]. Estrogen and progesterone each regulate HOXA10 expression in both the embryonic and adult reproductive tracts [11, 12]. The regulation of HOXA10 by sex steroids is direct, as a result of either ESR or PGR binding to their respective regulatory elements within the HOXA10 gene 5′ regulatory region [11, 13, 14]. HOXA10 expression varies in the adult mouse and human uterine endometrium during the mouse estrous cycle or human menstrual cycle. Its expression dramatically increases in the glandular epithelium in the midsecretory phase, the time of implantation. Differential regulation of HOXA10 in the human endometrium leads to differentiation of this tissue [11]. HOXA10 expression is required for endometrial receptivity and affects embryo implantation. Targeted mutation of Hoxa10 results in infertility in female mice. However, few target genes of HOXA10 regulation are known.
Both HOXA10 and KLF9 regulate growth of human endometrium, which is composed of stromal and epithelial cells that respond to sex steroids during the menstrual cycle. Whereas stromal cells maintain sex steroid receptor expression through the menstrual cycle, the epithelial cells lose expression of sex steroid receptors in the secretory phase [15–17] and are not directly sex steroid responsive. Stromal-epithelial communication may indirectly drive the epithelial response to sex steroids in the secretory phase; however, the mechanisms that underlie epithelial loss of steroid responsiveness are not well characterized. Both HOXA10 and KLF9 are required for optimal endometrial function and may modulate sex steroid responsiveness in this tissue.
Previously, we reported that HOXA10 partially mediates progesterone responsiveness in the endometrium [18]. Because HOXA10 does not regulate the PGR directly, we hypothesized that it might regulate PGR cofactors, such as KLF9. Both HOXA10 and KLF9 are essential for optimal uterine development and embryo implantation, suggesting possible interaction in a single signaling pathway. The factors that regulate KLF9 expression are not characterized. In the present study, we demonstrate that HOXA10 regulates KLF9 in human endometrial cells.
MATERIALS AND METHODS
Immunohistochemistry
Samples of endometrium were collected throughout the menstrual cycle from six normally cycling, reproductive-age women (age, 28–40 yr), who were not using hormonal therapy, under a Human Investigation Committee-approved protocol and with informed consent. Three samples were obtained from the proliferative phase and three from the secretory phase. Formalin-fixed, paraffin-embedded tissues were cut into sections (thickness, 5 μm), mounted on coated slides. Slides were deparaffinized and rehydrated through a serious of xylene and ethanol washes, followed by permeabilization in 95% cold ethanol. After a 5-min rinse in distilled water, an antigen-presenting step was performed by steaming the slides in 0.01 mM sodium citrate buffer for 20 min, followed by removal of the staining jar from the steam chamber and cooling for 20 min. Slides were rinsed for 5 min in PBS with 0.1% Tween-20 (PBST), and sections were circumscribed with a hydrophobic pen. Endogenous peroxidase was quenched with 3% hydrogen peroxide for 5 min, followed by a 5-min PBST wash. Nonspecific binding was blocked with 1.5% normal horse serum in PBST for 1 h at room temperature. Slides were then incubated in the primary antibody overnight at 4°C with polyclonal HOXA10 antibody at a dilution of 1:200 or with polyclonal BTEB1 antibody at a dilution of 1:200. All primary antibodies were purchased from Santa Cruz Biotechnology: HOXA10 (sc-17159) and BTEB1 (sc-12996). Normal goat immunoglobulin (Ig) G (Santa Cruz Biotechnology) was used as a negative control. Biotinylated secondary antibodies were purchased from Vector Laboratories. Horse anti-goat secondary antibody (3.5 μg/ml) for HOXA10 and KLF9 were applied for 1 h at room temperature. Slides were washed in 1× PBST, incubated in ABC Elite (Vector Laboratories) for 15 min at room temperature, washed in 1× PBST, and incubated for 5 min in diaminobenzidine (400 mg/ml; Vector Laboratories). A 15-sec exposure to hematoxylin was used as a counterstain. For each individual primary antibody, all slides were processed simultaneously. Slides were dehydrated through 3-min ethanol and xylene washes and mounted with Permount (Fisher Scientific). Expression and localization differences were evaluated and scored by two different observers, who were blinded to the tissue origin, using an Olympus BX 40 light microscope and the H-SCORE system [19]. The H-SCORE, representing levels of staining intensity and distribution, is calculated using the following equation: H-SCORE = Pi (I + 1), where I is the intensity of staining with a value of 1, 2, or 3 (weak, moderate, or strong, respectively) and Pi is the percentage of stained cells for each intensity, varying from 0 to 100%. H-SCORE data were analyzed by Mann-Whitney rank sum test.
Cell Culture
The human endometrial stromal cells line HESC was a generous gift of Dr. Charles J. Lockwood (Yale University, New Haven, CT) [20]. The human endometrial adenocarcinoma cell line Ishikawa was a generous gift of Dr. Richard Hochberg (Yale University). HESC cells were maintained in a phenol red-free Dulbecco modified Eagle medium/Ham F-12 (Sigma) supplemented with 10% charcoal-stripped calf serum, 1% penicillin/streptomycin, and 1% sodium pyruvate. Ishikawa cells were maintained in minimum essential medium (Sigma) supplemented with 10% charcoal-stripped calf serum, 1% penicillin/streptomycin, and 1% sodium pyruvate.
Transient Gene Transfection in Human Cell Lines
HOXA10 cDNA was cloned into the EcoRI site of pcDNA3.1(+) (Invitrogen). PcDNA3.1(+) without the HOXA10 insert was used as a control (Invitrogen). SiGenome duplex HOXA10 siRNA (catalog no. D-006336–01) and control nontargeting siRNA (catalog no. D-001210–02) were purchased from Dharmacon.
HESC, grown to 60–70% confluence, were transfected using TransIT-LT1 (Mirus Bio) with either pcDNA3.1(+)/HOXA10 (4.0 μg for a six-well plate, 12 μg for a 10-cm dish) or HOXA10 siRNA (20 μM for a six-well plate, 60 μM for a 10-cm dish), using empty pcDNA3.1(+) or nonspecific siRNA as respective control. Ishikawa cells, grown to 45–55% confluence, were transfected using Lipofectamine 2000 (Invitrogen) with either pcDNA3.1(+)/HOXA10 (4.0 μg for a six-well plate, 12 μg for a 10-cm dish) or HOXA10 siRNA (20 μM for a six-well plate, 60 μM for a 10-cm dish), using empty pcDNA3.1(+) or nonspecific siRNA as respective control. After 4 h the media were changed, and cells were incubated for an additional 20 h in OPTI-MEM I Reduced Serum Medium (Invitrogen), without serum or antibiotics. Forty-eight hours posttransfection, total RNA and protein were isolated. All transfections were performed in triplicate.
Real-Time PCR
Quantitative real-time RT-PCR was performed using iScript cDNA Synthesis Kit and iQ SYBR Green Supermix (Bio-Rad). RNA was reverse-transcribed for 30 min at 42°C. PCR was performed for 45 cycles at 95°C for 15 sec, 61°C for 20 sec, and 72°C for 25 sec. HOXA10 and actin, beta (ACTB) primers were used as previously described [21]. KLF9 primers were 5′-ACAGTGGCTGTGGGAAAGTC-3′ (forward) and 5′-AACTGCTTTTCCCCAGTGTG-3′ (reverse); these primers spanned the KLF9 intron and yielded a PCR product of 166 bp. Expression was normalized to the expression of ACTB from the same sample. Melting-curve analysis was conducted to determine the specificity of the amplified products and to ensure the absence of primer-dimer formation. All products obtained yielded the predicted melting temperature. Analysis of relative gene expression data used 2−ΔΔCt method [22]. The Ct values were converted to the term 2−Ct. Group means were evaluated by t-test (n ≥ 3). Differences of P ≤ 0.05 were considered to be significant.
Western Blot Analysis
Whole protein was extracted from HESC and Ishikawa cells using Nuclear Extract Kit (Activemotif) according to manufacturer's protocol but using whole-protein extract. Equal amounts of protein (60 μg for both HOXA10 and KLF9) were electrophoresed through 4–15% polyacrylamide gels (Bio-Rad) at 160 V for 70 min and then transferred onto Immun-Blot polyvinylidene difluoride membranes (Bio-Rad) in transfer buffer (25 mM Tris, 192 mM glycine, and 20% methanol) at 100 V for 1 h. After incubation in blocking buffer (1× PBS, 0.2% Tween 20, and 5% milk), the membrane was incubated with goat polyclonal HOXA10 antibody (sc-17159) diluted 1:200, polyclonal BTEB1 antibody (sc-12996) diluted 1:200 overnight at 4°C, and mouse monoclonal alpha-tubulin antibody (sc-8035; Santa Cruz Biotechnology) diluted 1:1000 at room temperature for 1 h. After washing, the membranes were incubated with biotinylated horse anti-goat secondary antibody or goat anti-mouse secondary antibody (Vector Laboratories) diluted in the blocking buffer (3.5 μg/ml) at room temperature for 1 h. The membranes were incubated in ABC Elite and then stained by diaminobenzidine.
Construction of Plasmid for Promoter Analysis and In Vitro Mutagenesis
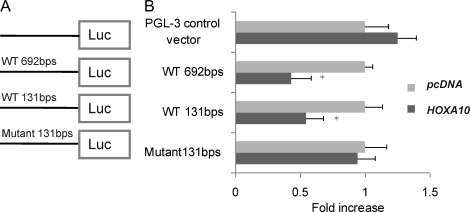
After sequence analysis of the 5′ region of the KLF9, a 692-bp sequence upstream of the KLF9 ATG was identified as containing several putative HOX-binding sites, amplified by PCR, and cloned into pGL3-control vector (Promega). The fragment was generated by PCR from human genomic DNA (Promega) using KLF9 5′ regulatory region-specific primers with added restriction sites. The sequences of the primers were as follows: 5′-GCCTCGAGCATTTGTGTTTATTCTTGGAC-3′ and 5′-CGGGGTACCATTTCGCAGAATCCCATCAC-3′. PCR was performed as follows: 95°C for 60 sec, 56°C for 60 sec, and 72°C for 90 sec for 35 cycles. The primers were designed using the GenBank database (AL162390). After identifying a 131-bp regulatory region within the previously described 692-bp 5′ regulatory region as described below, it was also subcloned into pGL3-control vector. The 131-bp region containing mutations of seven putative HOXA10-binding sites in this sequence were also cloned into pGL3-control vector (Promega). Both wild and mutant sense and antisense DNA were synthesized by the W.M. Keck Oligonucleotide Laboratory at Yale University. The two strands were heated up to 100°C for 5 min and annealed in room temperature overnight. (The sites of the mutations in the 131 bp are shown in Fig. 6.)
FIG. 6.
Putative HOXA10 binding sites drove luciferase activity in response to HOXA10. A) A schematic representation of 5′ region in KLF9 gene. B) In transactivation assays, the 692-bp pGL3-control-KLF9 sequence resulted in a 43% decrease in luciferase expression induced by HOXA10 in Ishikawa cells. The smaller, 131-bp segment containing the HOXA10-binding sites cloned into pGL3-control resulted in a 54.7% decrease in luciferase expression induced by HOXA10 in Ishikawa cells. No decrease in luciferase activity driven by HOXA10 was observed using the 131-bp mutant pGL3-control-KLF9 sequence in Ishikawa cells that lack the HOXA10-binding sites. *P < 0.05.
Transfection and Luciferase Assays
Ishikawa cells, grown to 45–55% confluence, were transfected using Lipofectamine 2000 with 0.4 μg of either pGL3-control-692bp KLF9 Promoter or pGL3-control-131bp KLF9 Promoter or pGL3-control-131bp KLF9 mutated Promoter in 24-well plates. (The construct design is indicated in Fig. 6A.) Each group of cells was cotransfected with either 1.0 μg of pcDNA3.1(+)/HOXA10 vector or empty pcDNA3.1(+) vector. All cells were cotransfected with 10 ng of pRL-TK to control for transfection efficiency. After 4 h, the media were changed, and 48 h posttransfection, the cells were rinsed with cold PBS and lysed with 1× Reporter Lysis Buffer (Promega). The lysate was collected after two freeze/thaw cycles. Luciferase activity was measured using the luciferase reagent kit (Promega). Transfections were performed in duplicate, and experiments were repeated three times. The pGL3-control vector was used as a control.
Electrophoretic Mobility Shift Assay
Complementary oligonucleotides corresponding to the sequences shown in Figure 6 were annealed and end labeled with 32P-dATP (PerkinElmer Life Sciences) using T4 polynucleotide kinase (New England BioLabs) and purified with MicroSpin G-25 columns (Amersham Pharmacia Biotech). The site of mutation in the 131-bp probe is shown in Figure 6. Nuclear extract was obtained from Ishikawa cells using Nuclear Extract Kit according to the manufacturer's protocol. Binding reactions were performed as previously described [21]. In brief, 25 μl of mixture of 10 μg of nuclear extract and 80 000 cpm of 32P-labeled oligonucleotides were incubated for 40 min at 37°C. The resultant protein-DNA complexes were separated on a 5% polyacrylamide gel (acrylamide:bisacrylamide, 29:1) for 3 h at 180 V in 0.5× TBE buffer (1× TBE: 50 mM Tris, 50 mM boric acid, and 1 mM ethylenediaminetetra-acetic acid) at 4°C. To confirm the identity of the HOXA10-binding site in the shifted complex, 10 μg of nuclear extract protein were incubated with 10 μg of goat polyclonal HOXA10 antibody (sc-17159) or control IgG at 4°C overnight, followed by a 40-min incubation with labeled oligonucleotides at 37°C. For competition, 50x cold unlabeled probe was added into the reaction. The gel was dried under vacuum at 80°C, exposed from overnight to 2 days on X-OMAT film (Kodak), and subsequently developed.
Statistical Analysis
Expression of RNA was compared using the t-test. The H-SCORE was compared using the Mann-Whitney test. Differences of P ≤ 0.05 were considered to be significant.
RESULTS
KLF9 Protein Expression in Human Cycling Endometrial Tissue
To determine the expression pattern of KLF9 through the menstrual cycle, we performed immunohistochemical analysis on human endometrial samples collected from women in the proliferative and secretory phase. KLF9 protein was found in the nucleus of both stromal and glandular cells of human endometrium. Immunohistochemical results showed KLF9 expression was relatively high in the proliferative phase and lower in the secretory phase in glandular epithelial cells of human cycling endometrium (Fig. 1). In contrast, stromal KLF9 expression was lower than seen in epithelial cells and only minimally decreased between the proliferative and secretory phase. The expression was also evaluated by the semiquantitative H-SCORE (Table 1). This analysis confirmed that epithelial cell secretory-phase expression was reduced to approximately 50% of that in the proliferative-phase glands, whereas stromal cell expression was not decreased to a significant level.
FIG. 1.
KLF9 protein expression in human cycling endometrium tissue. KLF9 protein was expressed in nuclei of both stromal and glandular cells in human endometrium. A) Immunohistochemical results show KLF9 expression was relatively high in the proliferative phase in glandular epithelial cells. B) KLF9 expression was lower in the secretory phase in glandular epithelial cells. Original magnification ×600; bar = 50 μm.
TABLE 1.
H-SCORE analysis (mean ± SEM).

HOXA10 Regulated KLF9 mRNA Expression in Human Endometrial Cell Lines
To determine whether HOXA10 can regulate KLF9 expression in human endometrium, the human endometrial stromal cell line HESC and the human endometrial epithelial cell line Ishikawa were transfected with pcDNA3.1(+)/HOXA10 vector. We have previously described HOXA10 gene expression in HESC and Ishikawa cells [21]. HESC cells are a telomerase immortalized endometrial stromal cell line, and Ishikawa cells are the best characterized well-differentiated and sex steroid-responsive endometrial epithelial cell line. Transfection efficiency was similar in the two cell lines. Quantitative reverse transcriptase-polymerase chain reaction (QRT-PCR) results demonstrated that HOXA10 gene expression increased more than 1000-fold after transfection of HESC and Ishikawa cells with the pcDNA-HOXA10 vector (Fig. 2, A and C). HOXA10 mRNA expression was decreased to 27% of pretreatment level after transfection of Ishikawa cells with HOXA10 siRNA and to approximately 70% of pretreatment level after transfection of HESC cells with HOXA10 siRNA (Fig. 2, B and D). PcDNA3.1/HOXA10 transfection decreased KLF9 mRNA expression to 45% of pretreatment level (P < 0.02) in Ishikawa cells, but no change in KLF9 mRNA expression was seen after HOXA10 siRNA transfection in these cells (Fig. 3, A and B). QRT-PCR results show that KLF9 mRNA expression was decreased to a lesser extent in HOXA10-transfected HESC cells than in Ishikawa cells; the difference in HESC cells was not statistically significant (P = 0.28) (Fig. 3C). No difference was noted between HOXA10 siRNA and control siRNA treatment groups (Fig. 3D).
FIG. 2.
HOXA10 mRNA expression in human endometrial cell lines. A) Real-time PCR results show that HOXA10 expression was increased more than 1000-fold (P < 0.05) after transfection with pcDNA3.1/HOXA10 vector in Ishikawa cells. B) HOXA10 expression was decreased to 27% (P < 0.05) of pretreatment level after transfection with HOXA10 siRNA in Ishikawa cells. C) Similarly, real-time PCR results show HOXA10 expression was increased more than 1000-fold (P < 0.05) after transfection with pcDNA3.1/HOXA10 vector in HESC cells. D) HOXA10 expression was decreased to approximately 70% (P < 0.05) of pretreatment level after transfection with HOXA10 siRNA in HESC cells. *P < 0.05.
FIG. 3.
HOXA10 regulated KLF9 mRNA expression in human endometrium cell line. A) Real-time PCR results show that pcDNA3.1/HOXA10 transfection decreased KLF9 mRNA expression to 44% of pretreatment level (P < 0.05) in Ishikawa cells. B) No change was found in KLF9 mRNA expression after transfection with HOXA10 siRNA in Ishikawa cells. C) Real-time PCR results show that pcDNA3.1/HOXA10 transfection decreased KLF9 mRNA expression to 52% of pretreatment level in HESC cells; however, this did not reach statistical significance. D) No increase in KLF9 mRNA expression was found after transfection of HOXA10 siRNA in HESC cells. *P < 0.05.
HOXA10 Regulated KLF9 Protein Expression in Human Endometrial Cell Lines
Western blot analysis demonstrated that HOXA10 protein expression increased after transfection with pcDNA-HOXA10 vector and decreased after transfection with HOXA10 siRNA in the HESC and Ishikawa cell lines (Fig. 4). Results of Western blot analysis showed that pcDNA/HOXA10 transfection also decreased KLF9 protein expression in Ishikawa cells; however, no change in KLF9 protein expression was seen after HOXA10 siRNA transfection in these cells (Fig. 5A). No change was found in KLF9 protein expression in HESC cells after pcDNA3.1/HOXA10 transfection or HOXA10 siRNA transfection (Fig. 5B).These results confirmed the regulatory relationship demonstrated in human Ishikawa cell and HESC mRNA expression.
FIG. 4.
HOXA10 protein expression in human endometrial cell lines. A) Western blot results demonstrated that HOXA10 gene expression increased after transfection with the pcDNA-HOXA10 vector and decreased after transfection with HOXA10 siRNA in Ishikawa cells. B) Western blot results demonstrated that HOXA10 gene expression increased after transfection with the pcDNA-HOXA10 vector and decreased after transfection HOXA10 siRNA in the HESC cell line as well.
FIG. 5.
HOXA10 regulated KLF9 protein expression in human endometrial cell lines. A) Western blot results show that pcDNA/HOXA10 transfection decreased KLF9 protein expression in Ishikawa cells. No change was observed in KLF9 protein expression after HOXA10 siRNA transfection. B) Western blot results show no change in KLF9 protein expression in HESC cells after the pcDNA3.1/HOXA10 transfection or HOXA10 siRNA transfection. The results are each representative of three separate experiments using each cell type.
Regulation of KLF9 Promoter Activity by HOXA10
To determine whether HOXA10 directly regulated transcription of KLF9 gene expression, Ishikawa cells were transfected with an artificial reporter construct containing the KLF9 promoter and 5′ regulatory region. Approximately 700 bp of the KLF9 5′ sequence upstream of ATG was amplified by PCR and cloned into pGL3-control vector (Promega) (Fig. 6A). We performed sequence analysis of the KLF9 gene promoter and identified seven putative HOXA10-binding sites that were located within the previously described, 692-bp 5′ regulatory region. These putative HOXA10-binding sites sequences, which were clustered to a 131-bp region, were cloned into pGL3-control. The same region with the mutated HOXA10-binding site sequences was also cloned into pGL3-control. Cells were cotransfected pGL3-control-KLF9 Promoter and pcDNA3.1(+)/HOXA10. The pRL-TK vector was cotransfected as a control for transfection efficiency and used for normalization. In transactivation assays, expression driven by the 692-bp pGL3-control-KLF9 sequence was decreased by 43% when HOXA10 was cotransfected in Ishikawa cells. The smaller, 131-bp pGL3-control-KLF9 sequence resulted in a 55.3% decrease in luciferase expression induced by HOXA10 in Ishikawa cells. No effect of HOXA10 was found on luciferase activity driven by the 131-bp mutant pGL3-control-KLF9 sequence in Ishikawa cells. Results are presented as an average of three experiments and are statistically significant (P < 0.05) (Fig. 6B). The results demonstrate the existence of a putative HOXA10-binding site in the KLF9 promoter sequence.
HOXA10-Binding Site Located in KLF9 Promoter
To determine whether HOXA10 can bind KLF9 promoter, electrophoretic mobility shift assays (EMSAs) were performed as shown in Figure 7. Double-stranded, wild and mutant, 131-bp probes of KLF9 promoter (−594/−463) were incubated with nuclear extract from Ishikawa cells. A specific complex from Ishikawa nuclear extract was observed with the wild-type 131-bp probe. In contrast, no shifted complex was observed using the mutant 131-bp probe, which had mutations of several putative HOXA10-binding sites with the following sequences: ATTA, TTAT, and AATA. These results were consistent with the activity driven by the putative HOXA10-binding sites in the luciferase reporter assay. To confirm that the shifted complex included HOXA10, anti-HOXA10 antibody was used in the EMSA. A supershift (DNA-protein-antibody) complex from Ishikawa cells nuclear extract was observed using the 131-bp probe. No supershifted complex was observed using the mutated 131-bp probe or using the wild-type probe and non-specific IgG.
FIG. 7.
EMSA showing the HOXA10-binding to the KLF9 promoter. Nuclear extract was obtained from Ishikawa cells for use in EMSA. Lanes 1 and 2 demonstrate the 32P-labeled, 131-bp, wild-type and mutated probes, respectively. Lane 3 demonstrates the interaction of 32P-labeled, wild-type probe with nuclear extract from Ishikawa cells; significant binding is observed. Lane 4 demonstrates loss of binding to the mutated probe. Lane 5 demonstrates the cold unlabeled competing probe and HOXA10 polyclonal antibody added into the reaction of labeled wild probe with nuclear extract from Ishikawa cells. Lane 6 demonstrated further retarded mobility of the shifted complex (supershift) using 131-bp wild-type probe, HOXA10 polyclonal antibody, and Ishikawa nuclear extract. The arrow indicates the supershifted complex. Results are representative of seven independent experiments.
DISCUSSION
KLF9 is expressed in human endometrium. It is essential for optimal uterine development and fertility [9, 23]. HOXA10 is also expressed in both the embryonic and the adult reproductive tracts. Its expression is required for endometrial receptivity and affects embryo implantation [10, 11, 24–26]. Both KLF9 and HOXA10 play important roles in uterine endometrial function. HOXA10 expression varies in the adult mouse and human uterine endometrium during the mouse estrous cycle or human menstrual cycle. We have previously shown that human endometrial HOXA10 expression is lower in the proliferative phase than in the secretary phase. KLF9 expression has been reported in mammalian endometrium and in human endometrial cancer cell lines, such as Ishikawa cells and HEC-1-A cells [3, 6, 8, 27]. In the present study, immunohistochemistry results show that KLF9 was expressed in both stromal and glandular cells in the human endometrium. In contrast to HOXA10, KLF9 expression during the proliferative phase is higher than that during the secretary phase in glandular cells.
In the present study, we demonstrate a regulatory relationship between these two proteins. In Ishikawa cells, KLF9 expression was decreased after HOXA10 transfection. The decreased KLF9 expression after an increase in HOXA10 parallels the in vivo data, where KLF9 expression decreases in the secretory phase as HOXA10 expression increases. In stromal cells, KLF9 mRNA expression was not significantly altered by HOXA10, correlating with the absence of significant change in the menstrual cycle in vivo. These results suggest that HOXA10 regulates KLF9 expression in endometrial epithelial cells and that this regulation likely is direct. In the KLF9 promoter region, we identified seven HOXA10-binding sites, which were located within 131 bp (−594/−463) of the start of transcription. We demonstrate that these HOXA10-binding sites in the KLF9 promoter directly regulate KLF9 expression.
Progesterone exerts its actions in uterine endometrium through activation of the PGR. HOXA10 is induced by progesterone and, in turn, mediates the effects of progesterone/PGR [18]. Direct targets of HOXA10 transcriptional regulation are not necessarily directly regulated by progesterone, implying that HOXA10 may mediate progesterone action on some genes. HOXA10 directly regulates several known progesterone-responsive genes, including beta3-integrin subunit, EMX2, insulin-like growth factor binding protein 1 (IGFBP1), and the prostaglandin E receptors PTGER3 (EP3) and PTGER4 (EP4) [28–32]. KLF9 expression is down-regulated by HOXA10 in Ishikawa cells. KLF9 is a PGR-interacting protein; therefore, diminished KLF9 driven by HOXA10 may lead to diminished progesterone responsiveness of PGR/KLF9 target genes in the endometrial epithelium. This down-regulation of KLF9 parallels the down-regulation of secretary-phase PGR in endometrial epithelial cells. HOXA10 regulation of KLF9 is also cell-type specific and was not seen in stromal cells or nonuterine cell types. HOXA10-driven KLF9 repression may contribute the well-characterized, decreased responsiveness of endometrial epithelium to progesterone in the secretory phase.
HOXA10 regulates KLF9 in a common pathway that mediates human endometrial gene expression and is essential for optimal fertility. As a transcription factor, HOXA10 down-regulates KLF9 expression by binding and interacting with a HOXA10-binding site in the KLF9 promoter. HOXA10 and KLF9 together likely regulate progesterone action in endometrial epithelial cells. The loss of epithelial progesterone responsiveness and the shift to paracrine regulation from the stroma is essential to the establishment of uterine receptivity. In the present study, we demonstrate that this process involves not only loss of epithelial PGR but also loss of the PGR coregulator KLF9. HOXA10 may serve to coordinate loss of PGR cofactors concomitant with loss of PGR, assuring the switch from sex steroid response to stromal control of epithelial function.
Footnotes
Supported by National Institutes of Health Grant HD36887 and HD52668.
REFERENCES
- Dang DT, Pevsner J, Yang VW.The biology of the mammalian Krüppel-like family of transcription factors. Int J Biochem Cell Biol 2000; 32: 1103–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imataka H, Sogawa K, Yasumoto K, Kikuchi Y, Sasano K, Kobayashi A, Hayami M, Fujii-Kuriyama Y.Two regulatory proteins that bind to the basic transcription element (BTE), a GC box sequence in the promoter region of the rat P-4501A1 gene. EMBO J 1992; 11: 3663–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Michel FJ, Wing A, Simmen FA, Simmen RC.Cell-type expression, immunolocalization, and deoxyribonucleic acid-binding activity of basic transcription element binding transcription factor, an Sp-related family member in porcine endometrium of pregnancy. Biol Reprod 1994; 57: 707–714. [DOI] [PubMed] [Google Scholar]
- Zhang D, Zhang X-L, Michel FJ, Blum JL, Simmen FA, Simmen RC.Direct interaction of the Krüppel-like Family (KLF) member, BTEB1 and progesterone receptor mediates progesterone-responsive gene expression in endometrial epithelial cells. Endocrinology 2002; 141: 62–73. [DOI] [PubMed] [Google Scholar]
- Zhang X-L, Zhang D, Michel FJ, Blum JL, Simmen FA, Simmen RC.Selective interactions of KLF9/BTEB1 with progesterone receptor isoforms A and B determine transcriptional activity of progesterone-responsive genes in endometrial epithelial cells. J Biol Chem 2003; 278: 21474–21482. [DOI] [PubMed] [Google Scholar]
- Velarde MC, Iruthayanathan M, Eason RR, Zhang D, Simmen FA, Simmen RC.Progesterone receptor transactivation of the secretory leukocyte protease inhibitor gene in Ishikawa endometrial epithelial cells involves recruitment of Krüppel-like factor 9/basic transcription element binding protein-1. Endocrinology 2006; 147: 1969–1978. [DOI] [PubMed] [Google Scholar]
- Velarde MC, Zeng Z, McQuown JR, Simmen FA, Simmen RC.Kruppel-like factor 9 is a negative regulator of ligand-dependent estrogen receptor alpha signaling in Ishikawa endometrial adenocarcinoma cells. Mol Endocrinol 2007; 21: 2988–3001. [DOI] [PubMed] [Google Scholar]
- Pabona JM, Velarde MC, Zeng Z, Simmen FA, Simmen RC.Nuclear receptor coregulator Krüppel-like factor 9 and prohibitin 2 expression in estrogen-induced epithelial cell proliferation in the mouse uterus. J Endocrinol 2009; 200: 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmen RC, Eason RR, McQuown JR, Linz AL, Kang TJ, Chatman L, Jr, Till SR, Fujii-Kuriyama Y, Simmen FA, Oh SP.Subfertility, uterine hypoplasia, and partial progesterone resistance in mice lacking the Kruppel-like factor 9/basic transcription element-binding protein-1 (Bteb1) gene. J Biol Chem 2004; 279: 29286–29294. [DOI] [PubMed] [Google Scholar]
- Taylor HS, Vanden Heuvel GB, Igarashi PA.Conserved Hox axis in the mouse and human female reproductive system: late establishment and persistent adult expression of the Hoxa cluster genes. Biol Reprod 1997; 57: 1338–1345. [DOI] [PubMed] [Google Scholar]
- Taylor HS, Arici A, Olive D, Igarashi P.HOXA10 is expressed in response to sex steroids at the time of implantation in the human endometrium. J Clin Invest 1998; 101: 1379–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block K, Kardana A, Igarashi P, Taylor HS.In utero diethylstilbestrol (DES) exposure alters Hox gene expression in the developing mullerian system. FASEB J 2000; 14: 1101–1108. [DOI] [PubMed] [Google Scholar]
- Taylor HS, Igarashi P, Olive DL, Arici A.Sex steroids mediate HOXA11 expression in the human peri-implantation endometrial. J Clin Endocrinol Metab 1999; 84: 1129–1135. [DOI] [PubMed] [Google Scholar]
- Akbas GE, Song J, Taylor HS.A HOXA10 estrogen response element (ERE) is differentially regulated by 17beta-estradiol and diethylstilbestrol (DES). J Mol Biol 2004; 340: 1013–1023. [DOI] [PubMed] [Google Scholar]
- Bombail V, MacPherson S, Critchley HO, Saunders PT.Estrogen receptor related beta is expressed in human endometrium throughout the normal menstrual cycle. Hum Reprod 2008; 23: 2782–2790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylonas I, Jeschke U, Shabani N, Kuhn C, Balle A, Kriegel S, Kupka MS, Friese K.Normal and malignant human endometrium express immunohistochemically estrogen receptor alpha (ER-alpha), estrogen receptor beta (ER-beta) and progesterone receptor (PR). Acta Histochem 2004; 106: 245–252. [DOI] [PubMed] [Google Scholar]
- Press MF, Udove JA, Greene GL.Progesterone receptor distribution in the human endometrium. Analysis using monoclonal antibodies to the human progesterone receptor. Am J Pathol 1988; 131: 112–124. [PMC free article] [PubMed] [Google Scholar]
- Daftary GS, Taylor HS.Pleiotropic effects of Hoxa10 on the functional development of peri-implantation endometrium. Mol Reprod Dev 2004; 67: 8–14. [DOI] [PubMed] [Google Scholar]
- Lessey BA, Castelbaum AJ, Sawin SW, Buck CA, Schinnar R, Bilker W, Strom BL.Aberrant integrin expression in the endometrium of women with endometriosis. J Clin Endocrinol Metab 1994; 79: 643–649. [DOI] [PubMed] [Google Scholar]
- Krikun G, Mor G, Alvero A, Guller S, Schatz F, Sapi E, Rahman M, Caze R, Qumsiyeh M, Lockwood CJ.A novel immortalized human endometrial stromal cell line with normal progestational response. Endocrinology 2004; 145: 2291–2296. [DOI] [PubMed] [Google Scholar]
- Du H, Daftary GS, Lalwani SI, Taylor HS.Direct regulation of HOXA10 by 1,25-(OH)2D3 in human myelomonocytic cells and human endometrial stromal cells. Mol Endocrinol 2005; 19: 2222–2233. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD.Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta DeltaC(T)) method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- Velarde MC, Geng Y, Eason RR, Simmen FA, Simmen RC.Null mutation of Kruppel-like factor 9/basic transcription element binding protein-1 alters peri-implantation uterine development in mice. Biol Reprod 2005; 73: 472–481. [DOI] [PubMed] [Google Scholar]
- Bagot CN, Kliman HJ, Taylor HS.2001 Maternal Hoxa10 is required for pinopod formation in the development of mouse uterine receptivity to embryo implantation. Dev Dyn 2001; 222: 538–544. [DOI] [PubMed] [Google Scholar]
- Bagot CN, Troy PJ, Taylor HS.Alteration of maternal Hoxa10 expression by in vivo gene transfection affects implantation. Gene Ther 2000; 7: 1378–1384. [DOI] [PubMed] [Google Scholar]
- Taylor HS, Daftary GS, Selam B.Endometrial HOXA10 expression after controlled ovarian hyperstimulation with recombinant follicle-stimulating hormone. Fertil Steril 2003; 80(suppl 2):839–843. [DOI] [PubMed] [Google Scholar]
- Simmen FA, Su Y, Xiao R, Zeng Z, Simmen RC.The Krüppel-like factor 9 (KLF9) network in HEC-1-A endometrial carcinoma cells suggests the carcinogenic potential of dysregulated KLF9 expression. Reprod Biol Endocrinol 2008; 6: 41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H, Ma L, Ma WG, Maas RL, Dey SK.Hoxa-10 regulates uterine stromal cell responsiveness to progesterone during implantation and decidualization in the mouse. Mol Endocrinol 1999; 13: 1005–1017. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Taylor HS, Akbas GE, Foucher I, Trembleau A, Jaffe RC, Fazleabas AT, Unterman TG.Regulation of insulin-like growth factor binding protein-1 promoter activity by FKHR and HOXA10 in primate endometrial cells. Biol Reprod 2003; 68: 24–30. [DOI] [PubMed] [Google Scholar]
- Daftary GS, Troy PJ, Bagot CN, Young SL, Taylor HS.Direct regulation of beta3-integrin subunit gene expression by HOXA10 in endometrial cells. Mol Endocrinol 2002; 16: 571–579. [DOI] [PubMed] [Google Scholar]
- Troy PJ, Daftary GS, Bagot CN, Taylor HS.Transcriptional repression of peri-implantation EMX2 expression in mammalian reproduction by HOXA10. Mol Cell Biol 2003; 23: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor H, Fei X.Emx2 regulates mammalian reproduction by altering endometrial cell proliferation. Mol Endocrinol 2005; 19: 2839–2846. [DOI] [PubMed] [Google Scholar]